Abstract
A causal link between hematopoietic stem/progenitor cell (HSPC) dysfunction and DNA damage accrual has been proposed. Clinically relevant strategies to maintain genome integrity in these cells are needed. Here we report that eltrombopag, a small molecule agonist of the thrombopoietin (TPO) receptor used in the clinic, promotes DNA double strand break (DSB) repair in human HSPCs. We demonstrate that eltrombopag specifically activates the classical non-homologous end joining (C-NHEJ) DNA repair mechanism, a pathway known to support genome integrity. Eltrombopag-mediated DNA repair results in enhanced genome stability, survival and function of primary human HSPCs, as demonstrated in karyotyping analyses, colony forming unit assays and after transplantation in immunodeficient NSG mice. Eltrombopag may offer a new therapeutic modality to protect human HSPCs against genome insults.
Keywords: Hematopoietic stem cells, Eltrombopag, Thrombopoietin, DNA repair
Introduction
Maintenance of genome integrity is crucial to preserve the function of long-term repopulating hematopoietic stem and progenitor cells (HSPCs) for lifelong blood production. DNA double strand breaks (DSBs) are well-studied cytotoxic DNA alterations that may arise from exposure to cancer therapies, such as ionizing radiation (IR) and chemotherapeutic agents, or from endogenous products of metabolic activities, such as reactive oxygen species (ROS).
Repair of DNA DSBs uses two primary mechanisms: homologous recombination (HR) and non-homologous end joining (NHEJ)1,2. In HSPCs, NHEJ is the prominent repair mechanism3–5. This pathway is generally considered error-prone, in contrast to the error-free HR mechanism. However, this view has been challenged by the recent description of an alternative NHEJ (alt-NHEJ) mechanism, distinct from the classical or canonical NHEJ (C-NHEJ) pathway6. The classical form of NHEJ is a fast operating process. The DNA-dependent protein kinase (DNA-PK) and ligase IV/XRCC4/XLF complexes are principal enzymatic components of this pathway7,8. In contrast, alt-NHEJ repairs DNA DSBs with much slower kinetics and the enzymatic requirements are distinct from those implicated in C-NHEJ. The alt-NHEJ mechanism is considered to be a backup pathway, gaining functional relevance in DNA DSB repair when C-NHEJ fails9–12. This alternative pathway is highly mutagenic13–15, accounting for the reported fallibility of NHEJ16–18. In contrast, C-NHEJ is associated with more limited and infrequent alterations in sequence at the repair junctions and is considered to be of critical importance in maintaining genome stability16,18,19.
Recent investigations have uncovered a specific function of thrombopoietin (TPO), a primary regulator of HSPC survival, in promoting C-NHEJ-mediated DNA DSB repair in these cells20,21. Short exposure of murine HSPCs to TPO prior to low-dose IR protected cells against genome instability and partially rescued their loss of function. These data implied that activating the TPO signaling axis in HSPCs prior to therapy with anticancer agents may offer clinical benefits by reducing the risks of HSPC injury and secondary hematological malignancies. Recombinant TPO is not available for clinical use, but the synthetic small molecule mimetic of TPO, eltrombopag, was recently shown to stimulate multipotent long-term repopulating HSPCs in patients with bone marrow (BM) failure22,23, resulting in persistent trilineage hematopoiesis. In this study, we investigated whether eltrombopag may also promote DNA repair in human HSPCs.
Materials and Methods
An overview of study design is found in Figure 1A, and methods are detailed in Supplements.
Figure 1 |. Eltrombopag promotes the C-NHEJ DNA DSB repair mechanism in human HSPCs.
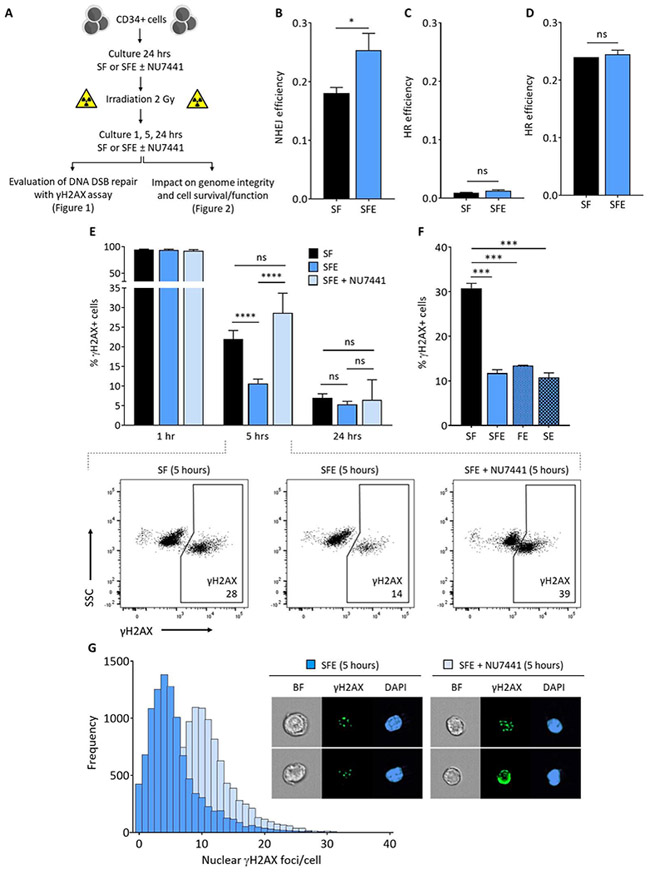
(A) Experimental procedure. Human CD34+ cells were collected by apheresis of normal volunteers after G-CSF mobilization. The γH2AX assay was used to evaluate DNA DSB repair at 1, 5 and 24 hours after 2Gy IR, following a 24-hour culture in the presence of early-acting cytokines (SCF and Flt3L, designated SF), or SF supplemented with eltrombopag (SFE) with and without DNA-PK inhibitor (NU7441). Mock irradiated cells were processed and cultured following the same procedure, but not exposed to IR. Results of DNA DSB repair activity and underlying mechanisms are presented in Figure 1, and data demonstrating the impact of DSB repair on genome integrity, cell survival and function are shown in Figure 2. (B) Efficiency of NHEJ DNA repair in human HSPCs cultured with SF or SFE for 24 hours before IR and electroporation of reporter plasmids (n=3). (C) Efficiency of HR DNA repair in human HSPCs cultured for 96 hours to enhance cell cycling before IR and electroporation of reporter plasmids (n=3). (D) Efficiency of HR DNA repair in actively cycling c-MPL expressing HEL 92.1.7 cells cultured for 96 hours before IR and electroporation of reporter plasmids (n=3). (E) Summary of H2AX phosphorylation (γH2AX) at 1, 5 and 24 hours after 2Gy IR of human HSPCs cultured with SF, SFE or SFE + NU7441 (n=24, from 13 independent donors). Insets show representative γH2AX immunostaining flow cytometry plots indicating that addition of the DNA-PK inhibitor NU7441 during culture completely abrogated the enhanced kinetics of DNA DSB repair observed at 5 hours with eltrombopag in HSPCs compared with the SF control group (bottom). The flow cytometry gating strategy is shown in Figure S3A. (F) Summary percentages of γH2AX+ cells at 5 hours post-IR of human HSPCs cultured in SF, SFE, FE (Flt3L and eltrombopag but no SCF), or SE (SCF and eltrombopag but no Flt3L) (n=3). (G) Frequencies of nuclear γH2AX foci detected per cell at 5 hours post-IR using multispectral imaging flow cytometry in human HSPCs cultured with SFE in the presence or absence of NU7441. A representative of 3 experiments is shown. Insets show bright field (BF) images, γH2AX foci and DAPI nuclear staining of representative cells. In panels (B) to (F), results are displayed as mean ± SEM; * signifies p<0.05, *** p<0.001, **** p<0.0001, and ns-not significant, by unpaired Student’s t-tests.
Evaluation of DNA DSB repair
CD34+ cells were obtained from healthy individuals after informed consent under an IRB-approved clinical protocol. Cells were cultured for 24 hours in the presence of cytokines, SCF and Flt3L (SF) or SF supplemented with eltrombopag (SFE) with or without DNA-PK inhibitor (NU7441), prior to induction of DNA DSBs by exposure to low dose (2Gy) IR. To assess the kinetics of DNA repair, cultures were continued for an additional 1, 5 and 24 hours after IR, and cells were stained with anti-phospho-histone (H2A.X) prior to data collection by flow cytometry. To distinguish between NHEJ and HR, CD34+ cells were electroporated with either NHEJ-GFP or HR-GFP reporter plasmids24, and tdTomato plasmids as internal control. Efficiency of DNA repair was expressed as the ratio of GFP+tdTomato+ cells to total tdTomato+ cells. For multispectral imaging flow cytometry, cells were incubated overnight with H2A.X and stained with DAPI prior to capture on ImageStream.
Evaluation of cellular genome integrity, survival and function
To assess genome integrity, chromosomal aberrations were blindly examined in CD34+ cell metaphase spreads. To quantify cellular survival, cells were stained with Annexin V/viability dye. Gene expression analysis was performed using a custom Qiagen RT2 Profiler PCR Array in CD34+ cells collected 5 hours after IR. To measure progenitor function, CD34+ cells were plated in MethoCult Medium and colonies were scored after 10–14 days. To assess long-term repopulating potential, HSPCs were injected i.v. in sublethally irradiated NSG mice on a study protocol approved by the NHLBI ACUC. BM cells were stained with anti-CD45-PE to quantify human cell engraftment 12 weeks post-transplantation.
Results and discussion
To assess whether eltrombopag promotes DNA DSB repair in human HSPCs and to identify the pathways involved, CD34+ cells obtained from healthy individuals were first cultured for 24 hours in the presence or absence of eltrombopag, exposed to low dose (2Gy) IR, and transfected with NHEJ or HR DNA DSB repair reporter plasmids24. Eltrombopag significantly improved NHEJ activity after IR (Figure 1B and Figure S1). Because DNA DSBs are repaired by HR only in the late S/G2 phase of the cell cycle, we examined the efficiency of HR in actively dividing CD34+ cells (Figure 1C) and c-MPL-expressing HEL 92.1.7 cells (Figure 1D). Eltrombopag did not promote HR activity after IR in these cells.
To distinguish between the fast-operating C-NHEJ and slower kinetics alt-NHEJ repair mechanisms, CD34+ cells were cultured and irradiated as above, and then assessed for changes in H2AX phosphorylation (γH2AX), an indicator of IR-induced DSBs, at various times after IR. Maximal percentages of H2AX phosphorylation was observed 1 hour after IR for both culture conditions, indicating that eltrombopag does not prevent DNA DSB formation in HSPCs (Figure 1E). Five hours after IR, most cells cultured with eltrombopag had resolved DNA DSBs, while 2-fold higher percentages of γH2AX+ cells persisted in the control group (Figure 1E). The observed effect was specific to eltrombopag; removal of other cytokines did not prevent DNA repair (Figure 1F). By 24 hours post-IR, the control group had similarly resolved DNA DSBs, implying that DNA repair occurred via a slower-acting pathway in the absence of eltrombopag (Figure 1E). Similar results were obtained when cultures were supplemented with TPO (Figure S2).
To confirm that eltrombopag enhances repair of DNA DSBs in irradiated human HSPCs by promoting a pathway with fast kinetics, we inhibited DNA-PK, a kinase specific to the fast-operating C-NHEJ pathway7. Addition of the DNA-PK inhibitor NU7441 during culture had no impact on DNA DSB formation measured at 1 hour or on DNA repair at 24 hours, but completely abrogated the enhanced kinetics of DNA DSB repair observed at 5 hours with eltrombopag in HSPCs (Figure 1E). To further corroborate these findings, we examined nuclear γH2AX foci at single cell resolution using multispectral imaging flow cytometry. The frequencies of γH2AX foci detected per HSPC at 5 hours post-IR in the presence of eltrombopag were also found to be markedly increased when NU7441 was added during culture (Figure 1G). These results establish that eltrombopag enhances the baseline fast-operating DNA-PK-dependent C-NHEJ DNA repair mechanism measured at 5 hours in human HSPCs. In contrast, in the absence of eltrombopag more cells resolve DNA DSBs using the slower, DNA-PK-independent and error-prone alt-NHEJ mechanism measured at 24 hours.
We next investigated the impact of promoting C-NHEJ repair on genome integrity, cell survival and function of irradiated HSPCs. Metaphases prepared from irradiated HSPCs cultured with eltrombopag showed significantly fewer chromosomal aberrations than irradiated cells cultured in the absence of eltrombopag, and only minimally increased aberrations relative to cultured HSPCs not exposed to IR (Figure 2A-B). Addition of eltrombopag during culture was also associated with a substantial decrease in apoptosis (measured at 5 hours, Figure 2C-D) and enhanced survival (measured at 24 hours, Figure 2E-F) of irradiated HSPCs compared with control groups. When C-NHEJ DNA repair was inhibited with NU7441, the cell survival benefit observed with eltrombopag was abolished (Figure 2C-F). To corroborate these findings, we identified 128 genes involved in DNA damage signaling, cell cycle regulation, apoptosis or DNA repair (Table SI), and compared their expression by RT-qPCR arrays in irradiated HSPCs cultured with or without eltrombopag. Five hours after IR, cells cultured with eltrombopag showed a significant (3 to 6-fold) decrease in expression of four pro-apoptotic and growth-inhibiting genes, namely GADD45G, BBC3, PPM1D and CCNO, compared with HSPCs cultured without eltrombopag (Figure 2G). To evaluate the functional relevance of these findings, cells were assessed in CFU progenitor assays and by transplantation into NSG mice. In CFU assays, irradiated HSPCs cultured with eltrombopag yielded 3- to 4-fold more CFUs than control groups (Figure 2H). In xenograft assays, a two-fold increase in human cell engraftment was observed 3 months after transplantation of IR-exposed HSPCs cultured in the presence of eltrombopag compared with control groups (Figure 2I).
Figure 2 |. Eltrombopag-mediated DNA DSB repair results in enhanced genome stability, survival and function of human HSPCs.
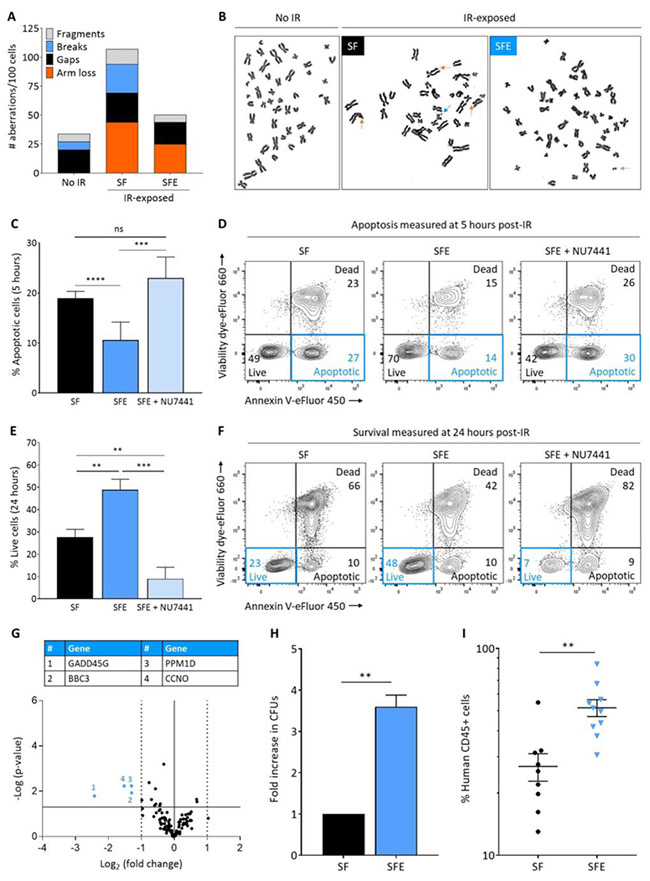
(A) Quantification of chromosomal aberrations on metaphase spreads of human CD34+ cells cultured as described in Figure 1A with SF or SFE prior to 2Gy IR. No IR control CD34+ cells were subjected to the same culture conditions with SFE, but not exposed to IR. Chromosomal aberrations observed in the no IR group result from the prolonged ex vivo cultures required for karyotyping analyses. (B) Representative metaphase spreads of human CD34+ cells showing chromosomal aberrations marked with arrows. Color of each arrow refers to a specific aberration, as defined in legend of Figure 2A. (C) Summary of percentages of apoptotic cells at 5 hours post-IR of human HSPCs cultured with SF, SFE or SFE + NU7441 (n=15, from 11 independent donors). (D) Representative cell viability flow cytometry plots obtained by dual annexin V/viability dye immunostaining at 5 hours post-IR of human HSPCs cultured with SF, SFE or SFE + NU7441. (E) Summary of percentages of live cells at 24 hours post-IR of human HSPCs cultured with SF, SFE or SFE + NU7441 (n=15, from 11 independent donors). (F) Representative cell viability flow cytometry plots obtained by dual annexin V/viability dye immunostaining at 24 hours post-IR of human HSPCs cultured with SF, SFE or SFE + NU7441. The flow cytometry gating strategy is shown in Figure S3B. (G) Volcano plot showing gene expression data at 5 hours post-IR of human HSPCs cultured with SFE compared with SF (n=3). The X and Y axes show gene expression fold-change of SFE vs SF and statistical significance, respectively. Each point represents an individual gene. The solid horizontal line shows where p = 0.05; points above the line have p < 05 and points below the line have p > 0.05. The solid vertical line shows where fold-change = 1 and the dashed vertical lines show where fold-change is > 2-fold. The colored numbered points indicate genes-of-interest that display both > 2-fold changes in expression and high statistical significance. All results were normalized based on GAPDH expression. (H) Fold increase in colony forming units (CFUs) counted 10-14 days affter plating human HSPCs cultured and irradiated as previously described; data were normalized to the SF control group. (I) Human cell engrafftment as measured by human CD45-expressing cells in the bone marrow of immunodeficient recipient (NSG) mice 12 weeks after transplantation of human HSPCs cultured and irradiated as previously described. Each dot represents an individual mouse (n = 19 mice). In panels (C), (E), (H) and (I), results are displayed as mean ± SEM; ** signifies p<0.01, *** p<0.001, **** p<0.0001, and ns-not significant, by unpaired Student’s t-tests.
In conclusion, this study builds on the previous demonstration that TPO promotes DNA repair in murine HSPCs and extends this finding to a clinically-relevant c-MPL agonist, eltrombopag, in human progenitors and cells with long-term repopulating capacity. The resulting enhanced genome stability, cell survival and function indicate that eltrombopag may offer a new therapeutic modality for the prevention of HSPC injury induced by IR in cancer therapy, and could have implications for the treatment of genome instability syndromes such as Fanconi anemia25. Given concerns raised that eltrombopag might promote clonal evolution, close monitoring is warranted in prospective clinical trials.
Supplementary Material
Highlights.
Eltrombopag promotes C-NHEJ DNA repair in human HSPCs.
Eltrombopag-mediated DNA repair results in enhanced HSPC genome stability.
Eltrombopag-mediated DNA repair promotes HSPC survival and function.
Eltrombopag may prevent HSPC injury induced by IR in cancer therapy.
Eltrombopag may offer a new therapeutic modality for genome instability syndromes.
Acknowledgments
The authors thank David Stroncek, MD and the NIH Department of Transfusion Medicine (DTM) and Cell Processing Section (CPS) staff for apheresis, selection and cryopreservation of human CD34+ cells; Kinneret Broder, RN, Therese Intrater, RN, Debbie Draper, RN, Tatyana Worthy, RN, and the outpatient clinic nursing staff for recruiting normal volunteers and providing G-CSF administration teaching to healthy subjects; Crystal Thomas, DVM, James Hawkins, DVM and the mouse core facility staff for excellent animal care; Keyvan Keyvanfar for technical support with flow cytometry; J. Philip McCoy, PhD, Venina Dominical, PhD and the NHLBI flow cytometry core staff for their contribution to multispectral imaging flow cytometry; Xin Chen, PhD for assistance with biostatistical analyses; Vera Gorbunova, PhD (University of Rochester, NY) for providing NHEJ and HR GFP reporter plasmids; Neal S. Young, MD, Cynthia E. Dunbar, MD, and Juan Pablo Ruiz, PhD for helpful discussions and reviewing the manuscript. This work was supported by the intramural research program of the National Heart, Lung and Blood Institute (NHLBI) of the NIH, and a Cooperative Research and Development Agreement (CRADA) between NHLBI and GlaxoSmithKline/Novartis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interests
GlaxoSmithKline and Novartis, the manufacturers of eltrombopag, provided research grade drug and research funding to the NHLBI under a Cooperative Research and Development Agreement (CRADA).
References
- 1.Jackson SP & Bartek J The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukas J & Lukas C Molecular biology. Shielding broken DNA for a quick fix. Science 339, 652–653 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 7, 174–185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447, 686–690 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Betermier M, Bertrand P & Lopez BS Is non-homologous end-joining really an inherently error-prone process? PLoS Genet 10, e1004086 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber MR The mechanism of human nonhomologous DNA end joining. J Biol Chem 283, 1–5 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Mladenov E & Iliakis G Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutat Res 711, 61–72 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Corneo B, et al. Rag mutations reveal robust alternative end joining. Nature 449, 483–486 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Grabarz A, Barascu A, Guirouilh-Barbat J & Lopez BS Initiation of DNA double strand break repair: signaling and single-stranded resection dictate the choice between homologous recombination, non-homologous end-joining and alternative end-joining. Am J Cancer Res 2, 249–268 (2012). [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, et al. Biochemical evidence for Ku-independent backup pathways of NHEJ. Nucleic Acids Res 31, 5377–5388 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, et al. DNA ligase III as a candidate component of backup pathways of nonhomologous end joining. Cancer Res 65, 4020–4030 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Boboila C, et al. Alternative end-joining catalyzes robust IgH locus deletions and translocations in the combined absence of ligase 4 and Ku70. Proc Natl Acad Sci U S A 107, 3034–3039 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Difilippantonio MJ, et al. Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J Exp Med 196, 469–480 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Difilippantonio MJ, et al. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature 404, 510–514 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson DO, et al. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci USA 97, 6630–6633 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guirouilh-Barbat J, et al. Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol Cell 14, 611–623 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Iliakis G, et al. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet Genome Res 104, 14–20 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Burma S, Chen BP & Chen DJ Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 5, 1042–1048 (2006). [DOI] [PubMed] [Google Scholar]
- 20.de Laval B, et al. Thrombopoietin-increased DNA-PK-dependent DNA repair limits hematopoietic stem and progenitor cell mutagenesis in response to DNA damage. Cell Stem Cell 12, 37–48 (2013). [DOI] [PubMed] [Google Scholar]
- 21.de Laval B, et al. Thrombopoietin promotes NHEJ DNA repair in hematopoietic stem cells through specific activation of Erk and NF-kappaB pathways and their target, IEX-1. Blood 123, 509–519 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olnes MJ, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med 367, 11–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsley DM, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. NEngl J Med 376, 1540–1550 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao Z, Jiang Y, Liu X, Seluanov A & Gorbunova V DNA repair by homologous recombination, but not by nonhomologous end joining, is elevated in breast cancer cells. Neoplasia 11, 683–691 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagby G Recent advances in understanding hematopoiesis in Fanconi Anemia. F1000Res 7, 105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


