Abstract
Background:
Triclosan exposure may decrease circulating thyroxine levels or cause neuron apoptosis, which in turn may adversely affect neurodevelopment. However, few studies have examined the association of early life triclosan exposure with child behavior.
Objective:
To quantify the association between early-life triclosan exposure and child behavior at age 8-years in 202 mother-child pairs from the HOME study (Cincinnati, OH; enrolled: 2003–2006).
Methods:
We quantified urinary triclosan concentrations up to 3 times in mothers (16-weeks, 26-weeks, and delivery) and up to 6 times in children (1, 2, 3, 4, 5, and 8 years). Parents rated children’s problem behaviors at age 8-years using the Behavioral Assessment System for Children-2 (BASC-2). Adjusting for covariates and accounting for exposure measurement error, we estimated changes in behavior problem scores per 10-fold increase in mean gestational and childhood triclosan concentrations. In addition, we estimated sex-specific associations.
Results:
Child sex modified the association of gestational and childhood triclosan with several BASC-2 scales (sex x triclosan p-values<0.2). In boys, increasing gestational triclosan was associated with higher behavioral symptom index (β: 4.5; 95% CI: 1.0, 8.1), externalizing problems (β: 5.0; 95% CI: 1.2, 9.0), attention problem (β: 6.6; 95% CI: 2.4, 11), hyperactivity (β: 6.4; 95% CI: 2.1, 11), and somatization (β: 3.8; 95% CI: 0.3, 7.3) scores. In contrast, triclosan-BASC-2 associations in girls were generally null and not statistically significant. We observed similar patterns of associations between childhood triclosan and these same behavioral scores; however, their magnitude decreased substantially after adjusting for gestational triclosan and associations were not statistically significant.
Conclusion:
In this cohort, increasing gestational and childhood urinary triclosan concentrations were associated with higher behavior problem scores in 8-year old boys, but not girls.
Keywords: child behavior, childhood, epidemiology, neurodevelopment, pregnancy, triclosan
1. Introduction
Triclosan, a man-made antimicrobial compound that was used in some soaps, and continues to be used in some toothpastes, cosmetics, and cleaning products, is frequently detected in the urine of pregnant women and children in North America (Calafat et al., 2008; Stacy et al., 2017a; Woodruff et al., 2011). In 2016 and 2017, the FDA ruled that triclosan could no longer be used in over-the-counter (OTC) consumer antiseptic products, due to concerns regarding its safety (FDA, 2016; Food and Drug Adminnistration, 2016).
Studies in laboratory animals and humans suggest that early-life triclosan exposure disrupts thyroid hormone homeostasis in pregnancy, infancy, and childhood (Braun et al., 2017b; Johnson et al., 2016; Wang et al., 2017). During gestation, thyroid hormones transferred from the mother to the embryo and fetus are critical for proper neurodevelopment (Escobar et al., 2004); even small differences in thyroid hormone concentrations during pregnancy have been associated with adverse neurodevelopmental outcomes in children (Haddow et al., 1999; Henrichs et al., 2013; Heyer and Meredith, 2017; Korevaar et al., 2016). Furthermore, evidence from in vitro studies suggests that triclosan exposure may induce apoptotic signaling pathways in neocortical neurons (Szychowski et al., 2016, 2015).
Despite the potential for triclosan to adversely impact neurodevelopment, previous epidemiological studies are inconclusive. In the HOME (Health Outcomes and Measures of the Environment) Study, we previously found no association between triclosan and visual-spatial abilities (Braun et al., 2017a). In contrast, we found that triclosan concentrations at birth were inversely associated with child IQ at age 8 years for children in this same study (Jackson-Browne et al., 2018). We are aware of two studies assessing associations between early life triclosan exposure and parent-reported child behavior, which have yielded inconclusive results. In the MIREC study, a Canadian pregnancy-birth cohort, gestational urinary triclosan concentrations were not associated with higher behavior problem scores in 3 year-olds (Etzel et al., 2018). In the EDEN cohort of French mother-son pairs, 2nd trimester urinary triclosan concentrations were associated with higher behavior problem scores in boys at age 3 years (Philippat et al., 2017). Both studies assessed triclosan exposure once during pregnancy in either the first (MIREC) or second (EDEN) trimester, but not during other periods of potential susceptibility (i.e., childhood). Moreover, prior studies examining associations between triclosan and neurodevelopment did not account for triclosan exposure misclassification. Given that urinary triclosan concentrations are variable within individuals due to triclosan’s short half-life (~21 hours) and the episodic nature of exposure, exposure misclassification may attenuate associations between triclosan and neurodevelopmental outcomes (Arbuckle et al., 2015; Bertelsen et al., 2014; Calafat et al., 2008; Koeppe et al., 2013; Messerlian et al., 2018; Stacy et al., 2017a).
To better understand the potential effects of early life triclosan exposure on child behavior, we used a prospective pregnancy and birth cohort study to determine if repeated gestational and childhood urinary triclosan concentrations were associated with higher parent-reported behavior problems in children at age 8 years, accounting for measurement error in triclosan exposure.
2. Methods
2.1. Study Participants
We used data from mother-child pairs enrolled in the HOME Study, a prospective pregnancy and birth cohort in Cincinnati, Ohio that was designed to examine the health effects of early-life environmental exposures (Braun et al., 2016). We recruited women from nine prenatal care clinics from March 2003 to January 2006. Eligibility criteria included being ≥18 years old, 16±3 weeks pregnant, living in a home built before 1978, planning to live in the greater Cincinnati area for the next year, planning to continue prenatal care and deliver at participating hospitals, and no history of thyroid disorders, or diagnosis of diabetes, bipolar disorder, schizophrenia, or cancer resulting in radiation treatment or chemotherapy. Of 1,263 eligible women, 468 agreed to participate; we excluded children with congenital or genetic anomalies (n=2). Among 389 mothers who remained in the study until delivering singleton infants, those missing all triclosan measurements (n=5), neurobehavioral assessments at age 8 years due to loss to follow-up (n=170), and covariates (n=12) were also excluded from analyses. Our primary analysis included 202 mother-child pairs (52% of all mothers who delivered singletons) who had at least one prenatal and postnatal triclosan measurement, an 8-year neurobehavioral assessment, and all covariates.
The institutional review boards (IRBs) at Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals approved this study. The Centers for Disease Control and Prevention (CDC) and Brown University deferred to the CCHMC IRB as the IRB of record. All women provided informed consent for themselves and their children
2.2. Urinary Triclosan Concentrations
Women provided up to two single urine samples at their prenatal care clinic visits around 16- and 26-weeks of pregnancy and another at the delivery hospital, usually within 48 hours after delivery. We also collected single urine samples from children during annual clinic or home visits from age 1–5 years, and again at age 8 years. Maternal and child urine samples were collected at convenient times (i.e., randomly). Urine samples were collected from 2003 through 2014 and stored at −20°C until shipment to the CDC, where they were stored at or below −20°C until analysis. We analyzed maternal samples between 2007–2009; childhood samples were analyzed between 2010–2012 and in 2015. For urine sample collection, storage, and analysis, we followed previously described protocols to minimize external contamination (Jackson-Browne et al., 2018; Stacy et al., 2017a; Ye et al., 2013). The concentrations of total (free plus conjugated) triclosan were measured using online solid phase extraction-liquid chromatography isotope-dilution tandem mass spectrometry methods (Kuklenyik et al., 2005). The limit of detection (LOD) was 2.3 ng/mL for all gestational maternal urine samples and child urine samples collected at the 1–5-year visits and 1.0 ng/mL for child urine samples collected at the 8-year visit. Concentrations below the LOD were assigned a value of LOD/√2 (Hornung and Reed, 1990). To account for urine dilution, we measured urinary creatinine concentrations using previously described methods (Larsen, 1972).
2.3. Child Behavior Problems
We assessed children’s behavior problems at age 8 years via parent-report using the Behavioral Assessment System for Children-2 (BASC-2). The BASC-2 is a valid, reliable, 160-item assessment of a child’s adaptive and problem behaviors in community and home settings (Kamphaus, 2015). We analyzed three composite scales from the BASC-2: behavioral symptoms index (BSI), externalizing problems, and internalizing problems. In addition, we evaluated nine clinical subscales of problem behaviors on the BASC-2. The BSI reflects the child’s overall level of problem behaviors that includes aggression, atypicality, attention problems, depression, hyperactivity, and withdrawal subscales. The externalizing problem composite scale measures disruptive behavior problems including aggression, conduct problems, and hyperactivity subscales. Finally, the internalizing problem composite scale assesses inwardly directed behaviors including anxiety, depression, and somatization subscales. Higher scores on these BASC-2 scales indicate more problem behaviors.
2.4. Covariates
Using a directed acyclic graph, we considered adjusting for variables associated with gestational or childhood urinary triclosan concentrations and child behavior in previous studies, or those believed to confound relations between other environmental toxicants and neurodevelopment (Figure S1) (Bellinger, 2004; Braun et al., 2011; Stacy et al., 2017a, 2017b; Textor et al., 2016). We did not include variables identified as potential mediators or colliders in regression models (Rothman et al., 2008). Children’s sex was abstracted from medical records, while the remaining sociodemographic covariates (child’s race/ethnicity, mother’s education, household income, and marital status) were obtained using standardized interviews. To assess gestational exposure to tobacco smoke, we calculated the mean concentration of cotinine, a metabolite of nicotine, measured in maternal serum samples collected during pregnancy or at birth. We administered the Home Observation for Measurement of the Environment Inventory during home visits at age 1-year to assess the caregiving environment (Bradley et al., 2003). We also considered maternal depression and attention-deficit/hyperactivity disorder (ADHD) behaviors, which were assessed with the Beck Depression Inventory-II (BDI-II) (Beck et al., 1997) and Conners’ Adult ADHD Rating Scale (CAARS) (Conners et al., 1999) at the baseline and 8-year study visit, respectively. Higher scores on the BDI-II and CAARS indicate more depressive and ADHD-like symptoms, respectively.
2.5. Statistical Analysis
We began by calculating the geometric mean of log10-transformed gestational (16 and 26-week, and delivery samples) and childhood (ages 1–5 and 8-year samples) urinary triclosan concentrations according to covariates. We also calculated the mean and standard deviation of BSI, externalizing, and internalizing problem scores according to covariates.
2.5.1. Exposure Measurement Error
To account for triclosan exposure measurement error, we used distinct regression-calibration approaches for the gestational and childhood measures. Specifically, we used a random intercept model (Carroll et al., 2006), where subject-specific replicates were used to estimate true geometric mean gestational urinary triclosan concentrations (log10-transformed and creatinine adjusted) across three measurement occasions during the gestational period (16 and 26 weeks and at delivery).
Our model assumes that triclosan observations collected on the ith mother during the jth measurement occasion during gestation are distributed as Yij = μi +εij, where and . Under this model, the reliability of observed geometric mean triclosan concentration for the ith mother across gestation, , is given , where and are the between-subject and within-subject variance components respectively, and ni = 1–3 is the number of available triclosan measures. In our regression model for the BASC-2 outcomes we replaced by , its best linear unbiased predictor (BLUP) from a linear mixed effects (LME) model, estimated using the NLME package in R (Pinheiro and Bates, 2000). Under a random intercept model, the BLUP has a closed form given by is the generalized least squares (GLS) estimate of the geometric mean gestational triclosan concentration across all three measurement periods for the entire HOME Study sample. From this formula, is a weighted average of and , with observed geometric mean concentrations from subjects with just one to two gestational measures shrunk more towards the grand mean than those from subjects with complete data, because of their lower reliability. Further, if we rewrite , we see that we end up regressing BASC-2 scores not on , but on instead. Were all subjects contribute triclosan measures on all three gestational measurement occasions, their means would all be equally reliable and the estimate of the triclosan regression coefficient in the BASC-2 outcome model would change from β to β/R where . This is exactly the correction for dis-attenuation suggested by psychometric theory for the geometric mean of n imperfect, but equally reliable, exposure measures (Spearman, 1904).
The above calibration approach is appropriate when geometric mean triclosan concentrations show no time trends across measurement occasions. This was the case for gestational triclosan, but not for childhood measures, where our previous data showed that triclosan changed quadratically with child age (Stacy et al., 2017a). Therefore, a more complex linear mixed effects model was used to account for measurement error in childhood triclosan measures. Models included linear and quadratic fixed effects of time, random intercepts, and random linear time slopes. Under an unstructured between-subject covariance matrix for the random effects (ΣB) and a spherical covariance matrix for the within-subject residuals (whose dimension ni depends on the number of available measurement occasions for each subject), we can show that the previous shrinkage formula generalizes to one that replaces the scalar reliability measure for the gestational geometric mean with a subject-specific reliability . This subject-specific reliability matrix differentially shrinks the observed triclosan concentrations towards the GLS estimates of the occasion-specific grand means. The amount of shrinkage depends on the number and timing of measurement occasions for each subject as captured by the matrix Zi (details available upon request). Having estimated a subject-specific triclosan concentration trajectory for each HOME study participant, we then calculated the average concentration during the childhood period by integrating the resulting quadratic polynomial in closed form. We then divided the area under the curve by the length of exposure for each subject. The exact date of the 8-year visit was used to determine the childhood exposure interval.
It should be noted that regression calibration methods use an estimate from a first-stage regression model as the “true” triclosan concentration. It is, therefore, important to propagate this uncertainty to the second stage by appropriately inflating the standard errors for the outcome model. We accomplished this by simulating the joint distribution of the BLUPs via a parametric bootstrap. We subsequently employed these replicates as covariates in a succession of outcome models whose output was combined using Rubin’s multiple imputation rules (Rubin, 1987).
2.5.2. Regression Model Analyses
All statistical analyses and figures were completed using R (R Development Core Team, 2015). We used normal linear regression models to analyze BASC-2 T-scores, as our outcome, with geometric mean (GM) measurement error corrected log10-transformed gestational/childhood creatinine-standardized urinary triclosan concentrations, as our main exposure variable. In all regression models, we adjusted for child sex (female or male), race/ethnicity (Black-non-Hispanic, While-non-Hispanic, or other), maternal education (less than high school diploma, high school graduate, or some college or more), household income (continuous), marital status (married or unmarried), gestational log10-transformed serum cotinine (continuous), caregiving environment scores (continuous), BDI-II scores (continuous) and CAARS scores (continuous). In addition, we replaced model-based standard errors with robust versions at each bootstrap iteration, calculated via the SANDWICH package in R (Zeileis, 2006, 2004) to deal with possible non-normality in the outcome distributions.
Because we previously observed that child sex modified the associations between child behavior and other environmental chemical exposures in this cohort (Braun et al., 2011, 2009; Stacy et al., 2017b), we included sex x triclosan interaction terms in regression models to formally test for modification by sex at the p <0.2 threshold (Greenland et al., 2016; Vanderweele, 2009). If we found that sex did modify our associations, we then conducted sex-stratified regression analyses for all BASC-2 outcomes to estimate sex-specific effects.
2.5.3. Secondary Regression Model Analyses
Because household sources of triclosan exposure may be shared over time by both mothers and children, the association between gestational urinary triclosan concentrations and children’s behavior problems could be confounded by childhood urinary triclosan concentrations and vice-versa. Thus, we performed additional analyses with both gestational and childhood urinary triclosan concentrations together in the same regression model. In addition, we previously found that gestational urinary bisphenol A (BPA) concentrations had a weak positive association with triclosan concentrations (Stacy et al., 2017a) and modest positive association with higher girl’s behavioral outcomes in the HOME Study (Stacy et al., 2017b). Therefore, we performed sensitivity analyses further adjusting for gestational BPA concentrations in regression models.
2.5.4. Windows of Susceptibility Analyses
We investigated potential periods of heightened susceptibility to triclosan using a multiple-informants model. This approach utilizes information gathered from multiple individuals or sources to measure the same construct and can be used when there are repeated environmental exposure biomarker measures at different time points that serve as informants (Sánchez et al., 2011). Using multivariable linear regression with Generalized Estimating Equations (GEE), we simultaneously estimated the associations between BASC-2 composite (BSI, externalizing problem, and internalizing problem) scores and log10-transformed creatinine-standardized urinary triclosan concentrations at 9 time points; 3 during gestation (16 and 26-weeks and at delivery) and 6 during childhood (ages 1, 2, 3, 4, 5, and 8 years). In addition, to test for unique windows of susceptibility, we examined the interaction between log10-transformed creatinine-standardized triclosan concentration and exposure window. Due to the low power of the omnibus interaction test (Greenland et al., 2016), we employed a more liberal significance threshold for this test, regarding p-values < 0.2 as suggestive evidence that associations differed by exposure window.
3. Results
3.1. HOME Study participant characteristics.
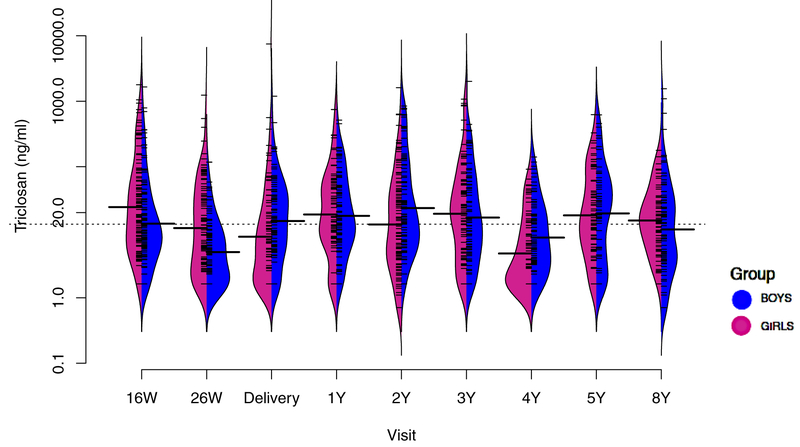
Sociodemographic characteristics were similar between included and excluded HOME study participants (Table S1). Median gestational triclosan concentrations decreased slightly between 16-weeks and delivery, from 18 to 13 ng/mL, whereas median childhood triclosan concentrations increased, from 4 to 17 ng/mL, between ages 1 to 4 years and then decreased slightly to 10 ng/mL at age 8 years (Figure 1, Table S2). Average gestational and childhood concentrations were 17 and 11 ng/mL, respectively (Table 1) and weakly correlated (r = 0.24; 95% CI: 0.11–0.37).
Figure 1.
Beanplots of urinary triclosan concentrations (ng/ml) from 16-weeks of pregnancy until age 8- years among mother-child pairs from the HOME Study: stratified by child sex.
N for each visit= 206, 202, 189, 183, 162, 165, 131, 160, and 202, respectively.
Abbreviations: W= weeks of gestation, Y= age in years.
Dotted line represents overall geometric mean uncreatinine-standardized-log10-transformed triclosan concentrations.
Solid line in each bean plot represents the geometric mean uncreatinine-standardized-log10-transformed urinary triclosan concentration for that visit.
Fringes within each plot represent individual observations and shaded area is the density function of triclosan concentrations.
Table 1.
Unadjusted GM gestational and childhood urinary triclosan concentrations (ng/ml) and mean 8-year BASC-2 composite scores according to sociodemographic, caregiving, and maternal factors among mother-child pairs in the HOME Study.
| Characteristics | N (%) | GM Maternal Triclosana (GSD) |
GM Child Triclosana (GSD) |
Mean Child BSI (SD) |
Mean Child Externalizing (SD) |
Mean Child Internalizing (SD) |
|---|---|---|---|---|---|---|
| Overall | 202 | 17 (3.6) | 11 (2.7) | 50 (9) | 49 (9) | 48 (9) |
| Child Sex | ||||||
| Female | 112 (55) | 16 (3.2) | 12 (2.8) | 48 (9) | 48 (9) | 49 (9) |
| Male | 90 (45) | 20 (3.9) | 11 (2.6) | 51 (9) | 51 (10) | 47 (8) |
| Child Race | ||||||
| White/Non-Hispanic | 126 (62) | 17 (3.8) | 12 (2.6) | 49 (8) | 49 (9) | 49 (9) |
| Black/Non-Hispanic | 65 (32) | 17 (3.1) | 10 (3.1) | 50 (10) | 50 (11) | 47 (9) |
| Other | 11 (6) | 19 (3.7) | 9.4 (2.1) | 48 (8) | 47 (8) | 49 (8) |
| Maternal Education | ||||||
| less than HS | 47 (23) | 16 (2.7) | 7.4 (2.5) | 50 (9) | 49 (10) | 48 (9) |
| HS | 58 (29) | 17 (3.5) | 12 (2.4) | 50 (10) | 51 (11) | 49 (9) |
| College+ | 97 (48) | 18 (4.0) | 13 (2.9) | 49 (8) | 49 (8) | 48 (9) |
| Income ($/year) | ||||||
| <$20K | 51 (25) | 15 (2.7) | 8.2 (2.4) | 51 (10) | 51 (12) | 48 (10) |
| $20–40K | 31 (15) | 18 (3.5) | 9.9 (2.7) | 49 (8) | 49 (10) | 46 (8) |
| $40–80K | 66 (33) | 17 (4.1) | 13 (2.6) | 49 (9) | 49 (8) | 48 (9) |
| >$80K | 54 (27) | 20 (3.8) | 14 (3.0) | 49 (8) | 48 (8) | 50 (8) |
| Marital Status | ||||||
| Married | 129 (63) | 18 (4.1) | 12 (2.7) | 49 (9) | 49 (9) | 49 (8) |
| Unmarried | 73 (37) | 15 (2.7) | 9.2 (2.7) | 50 (9) | 50 (10) | 48 (10) |
| Caregiving Score | ||||||
| <35 | 41 (20) | 15 (2.9) | 10 (3.2) | 50 (8) | 50 (9) | 48 (8) |
| 35–40 | 53 (26) | 16 (3.4) | 10 (2.5) | 50 (10) | 51 (12) | 48 (11) |
| >40 | 108 (54) | 18 (3.8) | 12 (2.6) | 49 (9) | 51 (12) | 49 (8) |
| Maternal Depressive Symptoms | ||||||
| Minimal | 166 (82) | 18 (3.8) | 11 (2.5) | 49 (9) | 48 (9) | 47 (8) |
| Mild | 21 (10) | 19 (2.5) | 12 (4.6) | 52 (10) | 52 (10) | 51 (12) |
| Moderate/Severe | 15 (7) | 12 (2.3) | 13 (2.1) | 56 (10) | 57 (12) | 53 (9) |
| Maternal ADHD Symptoms | ||||||
| <40 | 51 (25) | 22 (4.4) | 10 (2.5) | 44 (7) | 44 (8) | 44 (8) |
| 40–45 | 50 (25) | 19 (3.5) | 10 (2.9) | 48 (9) | 49 (7) | 47 (9) |
| 46–52 | 54 (27) | 17 (3.2) | 11 (2.6) | 51 (8) | 51 (8) | 50 (7) |
| >52 | 47 (23) | 13 (3.1) | 14 (2.9) | 55 (9) | 55 (11) | 53 (9) |
| Serum Cotinine | ||||||
| Unexposed | 89 (44) | 17 (3.4) | 11 (2.7) | 51 (9) | 50 (10) | 49 (9) |
| SHS | 94 (47) | 18 (3.8) | 11 (2.7) | 49 (9) | 49 (9) | 49 (9) |
| Active | 19 (9) | 13 (2.9) | 11 (2.6) | 47 (9) | 46 (9) | 44 (7) |
Abbreviations: N= Total number of participants, GM= Geometric mean calculated by averaging uncreatinine-standardized-log10-transformed maternal (16 and 26 week and delivery) and child (age 1–8 years) urinary triclosan concentrations (includes LOD substituted concentrations), GSD= Geometric Standard Deviation, ADHD= Attention Deficiency Hyperactivity Disorder, and SHS-secondhand smoke.
Caregiving score and maternal depression were administered at the 1-year visit; maternal ADHD scores were administered at the 8-year visit. Higher caregiving scores indicate greater quality and quantity of caregiving environment.
Higher maternal depression and ADHD values indicate greater severity of symptoms.
Serum cotinine definitions: Unexposed <0.015, SHS= (0.015 - <3.0) ng/mL, Active ≥ 3 ng/mL (active smoking was chosen based on results from the 1999–2004 NHANES which compared self-reported smoking status and serum cotinine levels among a representative sample of the US population) (Benowitz et al., 2009).
Geometric mean triclosan concentrations were lower in boys than girls at 16- and 26-weeks; similar to girls at 1-, 3-, and 5-years, but higher at delivery and at ages 2-, 4-, and 8-years of age (Figure 1). Gestational triclosan concentrations were higher in mothers who delivered boys, were college graduates and married, and had higher incomes and caregiving scores, and had lower serum cotinine concentrations, BDI-II scores, and CAARS scores (Table 1). In contrast, childhood triclosan concentrations did not differ by child sex, race and ethnicity, or maternal serum cotinine concentrations or BDI-II scores but were higher in children of mothers who were married and had higher education, higher income, and higher CAARS scores (Table 1).
Mean BSI, externalizing, and internalizing problem scores were 50, 49, and 48, respectively; scores did not substantially differ by child sex and race/ethnicity, or by maternal education, income, caregiving scores, and cotinine. BSI, externalizing, and internalizing problem scores were higher among children born to women with higher BDI-II and CAARS scores than women with lower BDI-II and CAARS scores (Table 1).
3.2. Urinary triclosan measurement error models.
For gestational triclosan, the reliability of creatinine-standardized-log10-transformed urinary triclosan concentrations was quite high, with approximately equal between- and within- subject variance components (,). This resulted in ICCs= 0.53, 0.69, 0.77 for subjects with 1, 2, and 3 observations, respectively, and a significant attenuation of the average exposure among subjects with one or more missing gestational-specific exposures. For childhood exposures, measurement error correction was more complicated due to the presence of between-person variation in both intercepts and linear time slopes (, , , ), as well as of significant fixed quadratic time effects (p<0.001). Here, the time axis corresponded to child’s age in month at each urinary triclosan measurement occasion, standardized by the mean (45.6) and standard deviation (28.6) of the overall sample.
3.3. Gestational urinary triclosan concentrations and BASC-2 scores.
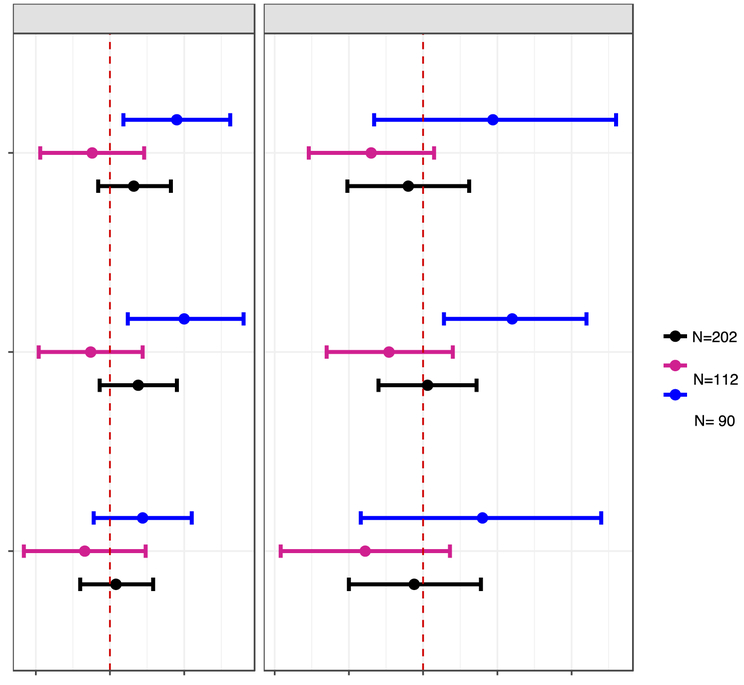
After adjustment for potential confounders, gestational triclosan concentrations were positively associated with higher BSI, externalizing, and internalizing scores (β’s= 1.6, 1.9, and 0.4, respectively), although all these associations did not reach statistical significance (Figure 2, Tables S5). Child sex modified the associations of gestational triclosan concentrations with all BASC-2 composite scales (gestational triclosan x sex p-values <0.2, Table S7). In boys, a 10-fold increase in gestational triclosan was associated with 4.5 (95% CI: 0.9, 8.1), 5.0 (95% CI: 1.2, 9.0), and 2.2 (95% CI: −1.1, 5.5) higher BSI, externalizing, and internalizing scores, respectively; associations among girls were not statistically significant (Figure 2, Table S5).
Figure 2.
Adjusted difference in child BASC-2 composite scale scores at age 8 years with a 10-fold increase in gestational and childhood triclosan concentrations.
Abbreviations: GM= Geometric mean, BASC-2= Behavioral Assessment for Children-2, BDI-II= Beck Depression Inventory-II, ADHD= attention deficit-hyperactivity disorder.
Separate gestational and childhood GM (measurement error corrected creatinine-standardized log10-transformed) urinary triclosan concentration linear regression models adjusted for log10-transformed serum cotinine concentrations (continuous), child sex (male vs. female)*, child race/ethnicity (White-non-Hispanic, Black-non, Hispanic, and other), household income (continuous), marital status (married vs. unmarried), maternal education (less than high school, high school graduate, and some college or greater), caregiving environment scores (continuous), BDI-II scores (continuous), and maternal ADHD scores (continuous).
* Removed in sex-stratified models.
Error bars are 95% Confidence Intervals.
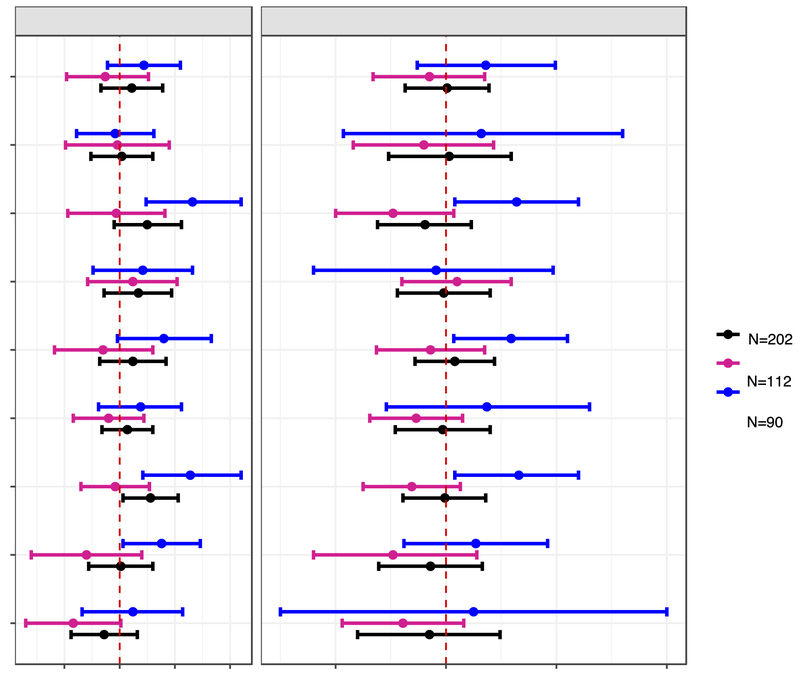
After adjustment for potential confounders, gestational triclosan concentrations were positively associated with all BASC-2 clinical subscale scores among all children, except for withdrawal scores (β: −1.4; 95% CI: −4.4, 1.6); all triclosan-BASC-2 associations were not statistically significant, except for hyperactivity (β: 2.8; 95% CI: 0.3, 5.3) (Figure 3, Table S5). Child sex modified the associations of gestational triclosan with aggression, attention, hyperactivity, somatization, and withdrawal problem scores (gestational triclosan x sex p-values <0.2, Table S7). In boys, a 10-fold increase in gestational triclosan was associated with 6.6 (95% CI: 2.4, 11), 6.4 (95% CI: 2.2, 11), and 3.8 (95% CI: 0.3, 7.3) point higher attention, hyperactivity, and somatization scores, respectively; in girls, these associations were negative and did not reach statistical significance (Figure 3, Table S5).
Figure 3.
Adjusted difference in child BASC-2 clinical subscale scores at age 8 years with a 10-fold increase in gestational and childhood
Abbreviations: GM= Geometric mean, BASC-2= Behavioral Assessment for Children-2, BDI-II= Beck Depression Inventory-II, ADHD= attention deficit-hyperactivity disorder.
Separate gestational and childhood GM (measurement error corrected creatinine-standardized log10-transformed) urinary triclosan concentration linear regression models adjusted for log10-transformed serum cotinine concentrations (continuous), child sex (male vs. female)*, child race/ethnicity (White-non-Hispanic, Black-non, Hispanic, and other), household income (continuous), marital status (married vs. unmarried), maternal education (less than high school, high school graduate, and some college or greater), caregiving environment scores (continuous), BDI-II (continuous), and ADHD (continuous).
* Removed in sex-stratified models.
Error bars are 95% Confidence Intervals.
3.4. Childhood urinary triclosan concentrations and BASC-2 scores.
Childhood triclosan concentrations were not associated with BASC-2 composite scores. However, child sex modified associations between childhood triclosan concentrations and BASC-2 composite scales (childhood triclosan x sex p-values < 0.2, Table S7). In boys, increasing childhood triclosan concentrations were associated with 4.7 (95% CI: −3.3, 13), 6.1 (95% CI: 1.4, 11), and 4.0 (95% CI: −4.2, 12) higher BSI, externalizing, and internalizing scores, respectively (Figure 2, Table S6). Associations between childhood triclosan and BASC-2 composite scales among girls were negative and more precise (narrower 95% CI) than associations among boys (Figure 2). Childhood triclosan concentrations were not associated with BASC-2 clinical subscale scores (Figure 3, Table S6). Child sex modified the associations of childhood triclosan with aggression, attention problems, conduct problems, depression, hyperactivity, and somatization scores (childhood triclosan x sex p-values <0.2, Table S7). In boys, each 10-fold increase in childhood triclosan concentrations was associated with 2.7 to 6.6 higher point scores on these clinical subscales (Figure 3, Table S6). In girls, we observed negative associations between childhood triclosan and all BASC-2 clinical subscale scores, except atypicality (β: 1.0; 95% CI: −4.0, 5.9); all 95% CIs for clinical subscale point estimates included the null value (Figure 3, Table S6).
3.5. Secondary Analyses: Joint gestational and childhood triclosan model and gestational BPA model results.
After adjusting for both gestational and childhood triclosan concentrations in the same model, the magnitude of the associations between triclosan concentrations and BASC-2 scores among boys were substantially attenuated, but less so for gestational triclosan compared to childhood triclosan (Table S8). For instance, after joint adjustment for both triclosan measures, the effect estimate for the association between gestational triclosan and externalizing problems in boys was attenuated from 5.0 to 4.1, whereas the estimate for childhood triclosan was attenuated from 6.1 to 3.6. Generally, the 95% CI’s in the jointly adjusted regression model included the null value and were less precise compared to models not adjusting for both measurements. However, associations of gestational triclosan with attention problem and hyperactivity scores remained significant among all children and boys (Table S8). Furthermore, many sex x triclosan p-values were no longer significant at the 0.2 level for gestational and childhood triclosan after joint adjustment (Table S9).
After adjusting for gestational BPA concentrations, the magnitude of the associations between triclosan concentrations and BASC-2 scores did not substantially change (Tables S10). In addition, all associations between triclosan concentrations and BASC-2 scores that were modified by child sex, remained significant at the 0.2 level after adjusting for gestational BPA concentrations (Table S7).
3.6. Window of susceptibility results.
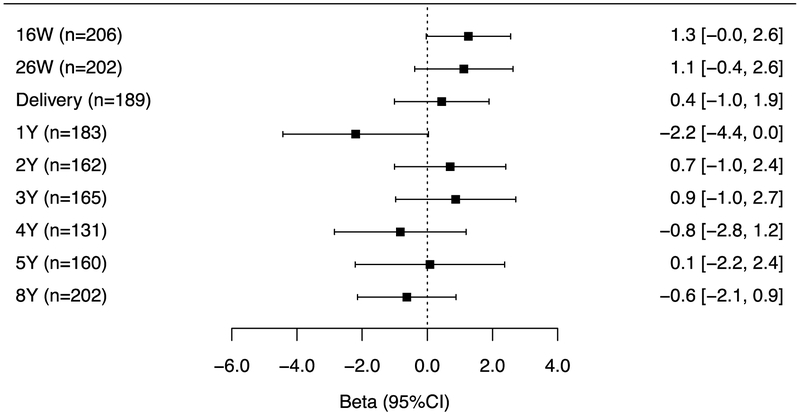
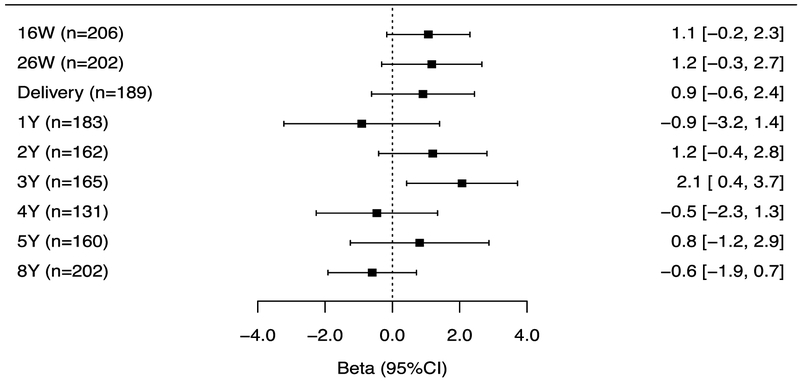
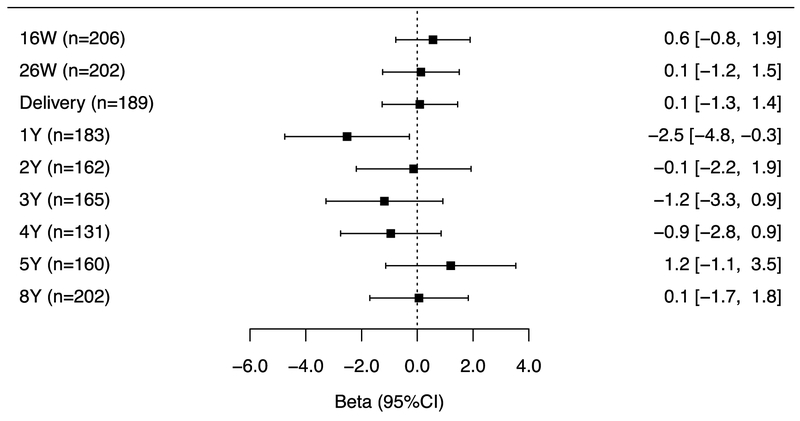
The associations of repeated gestational and childhood (log10-transformed-creatinine-standardized) triclosan concentrations with BSI and externalizing, but not internalizing problem scores varied by the timing of exposure measurement (triclosan x exposure period interaction p-value = 0.06, 0.06, and 0.28, respectively). Associations for measures during gestation and at delivery were consistently positive with all BASC-2 composite scores but the associations did not reach statistical significance (Figures 4A–C). During childhood, no individual triclosan concentrations were statistically significantly associated with BSI scores. However, triclosan concentrations at age 1 year was associated with lower internalizing scores, whereas age 3-year triclosan concentrations were associated with higher externalizing problem scores (β: 2.1; 95% CI: 0.4, 3.7) (Figures 4B–C).
Figure 4A.
Adjusted differences in child BSI scores at age 8-years per 10-fold increase in gestational and childhood triclosan concentrations.
Figure 4B.
Adjusted differences in child externalizing scores at age 8-years per 10-fold increase in gestational and childhood triclosan concentrations.
4. Discussion
In this cohort, we found that triclosan concentrations during gestation and the first 8 years of childhood were associated with more externalizing behavior problems in boys, but not girls. However, associations between childhood triclosan and externalizing behaviors were substantially attenuated after adjustment for gestational triclosan. In secondary analyses, repeated triclosan concentrations during gestation and at delivery were consistently positively associated with externalizing behaviors but these associations were not statistically significant. In contrast, repeated childhood triclosan concentrations were generally not associated with externalizing behaviors and more heterogeneous with regard to the direction and magnitude of associations.
The biological pathways by which triclosan exposure influences neurodevelopment during gestation or childhood is unknown. We speculate that early life triclosan exposures may affect child behavior by interfering with thyroid hormone homeostasis. A recent review of thyroid function and ADHD found some evidence for an association between maternal hypothyroidism and ADHD-related behaviors (i.e. inattention and hyperactivity), while there was insufficient evidence for an association between neonatal hypothyroidism and ADHD-related behaviors (Drover et al., 2019). In rodents, triclosan exposure during the gestational and postnatal periods reduces thyroxine (T4) concentrations during pregnancy, infancy, and adulthood (Johnson et al., 2016; Louis et al., 2017). In humans, recently published findings in our group and a Chinese cohort, found that increasing urinary triclosan concentrations during gestation or at delivery was associated with decreased maternal or neonatal thyroid hormone concentrations (Braun et al., 2017b; Wang et al., 2017). Decreased T4 concentrations in pregnancy and early childhood have also been shown to be an important indicator of developmental neurotoxicity; reductions in thyroid hormones in both periods have consistently been associated with poorer neurodevelopmental outcomes in both human and animal studies (Klein et al., 2001; Lazarus, 1999; Murphy et al., 2015). Another potential biological pathway that triclosan could affect neurodevelopment involves the induction of neocortical neuron apoptosis. In vitro studies show that triclosan exposure activates apoptotic signaling pathways and induces apoptosis through the aryl hydrocarbon receptor (AhR) via reduced transcriptional activity of AhR (Szychowski et al., 2016, 2015).
Prior epidemiologic studies examining early-life triclosan exposure and neurobehavioral outcomes in children have yielded inconclusive findings (Etzel et al., 2018; Philippat et al., 2017). Similar to our findings, in the EDEN study of French boys, 2nd trimester urinary triclosan concentrations were associated with higher externalizing behavior problem scores (Philippat et al., 2017). However, in the MIREC study, 1st trimester urinary triclosan concentrations were not associated with neurobehavioral outcomes at age 3 years (Etzel et al., 2018).
Several possible reasons may contribute to the difference in results of the present and prior studies. First, while discrepancies across these two prior studies could arise because they measured triclosan at different times of pregnancy, results from the present study do not suggest that the association of gestational triclosan concentrations and child behavior problems differ across the 2nd and 3rd trimesters. Second, it is possible that associations between urinary triclosan concentrations and child neurobehavior could differ if the outcomes were measured at different ages. Our current study was conducted at age 8-years but these prior studies were both conducted at earlier ages. However, we previously found that many aspects of parent-reported child behavior had fair to excellent reproducibility during the first 8 years of life (Braun et al., 2017c). Finally, while previous studies and the present one measured triclosan concentrations in the late 1st to 3rd trimester, prior studies only had a single prenatal triclosan measurement (Etzel et al., 2018; Philippat et al., 2017). Therefore, there is more potential for exposure misclassification in these prior studies. Our study had up to 3 measurements from the 2nd trimester through delivery and up to 6 measurements between ages 1 and 8 years. We utilized these repeated measures to account for triclosan exposure measurement error while accounting for variable missingness in the number of children’s urine samples and to identify susceptible periods of development to triclosan exposure.
Our current study found evidence of effect modification by child sex, however, the MIREC study did not find compelling evidence of sex modification and the study from France only assessed the association between triclosan and behavior problems in boys and thus could not test potential effect modification by child sex (Etzel et al., 2018; Philippat et al., 2017). Our sex-specific associations may be related to the potential effects of triclosan on gonadal hormones, which are known to influence sexually dimorphic behaviors (Cohen-Bendahan et al., 2005). In male rats, triclosan induces anti-androgenic effects by reducing luteinizing hormone, follicle-stimulating hormone, and cholesterol concentrations (Kumar et al., 2009; Rodríguez and Sanchez, 2010). A recent epidemiological study reported that urinary triclosan concentrations during pregnancy were associated with increased cord serum testosterone concentrations and that these associations were stronger in boys than girls (Wang et al., 2018), but a cross-sectional NHANES study of children at ages 6–19 years did not find an association between triclosan and testosterone in either sex (Scinicariello and Buser, 2016).
Our study has some strengths and limitations. One strength was our use of regression calibration to account for both triclosan measurement error and variation in the number of missing triclosan measurements. In addition, we used up to 9 repeated urinary triclosan concentrations from 16-week gestation through age 8 years to identify potential periods of heightened susceptibility. While we used regression-calibration to minimize the impact of triclosan exposure measurement error in our primary analyses, there is the potential for non-differential exposure misclassification to attenuate observed associations in our analysis of periods of heightened susceptibility given that we previously reported that triclosan concentrations had fair reproducibility during gestation (intraclass correlation coefficients [ICC]~0.5) and poor reproducibility during childhood (ICC~0.2–0.4) (Stacy et al., 2017a).
Second, we adjusted for an extensive array of covariates that may affect child behavior, including maternal ADHD behaviors and depressive symptoms. Still, residual confounding from unknown or unmeasured factors is always a possibility. For instance, gestational BPA concentrations were correlated with triclosan and behavior in this cohort (Stacy et al., 2017a, 2017c). However, our associations were not materially different when we adjusted for gestational BPA concentrations. Other environmental chemicals have been associated with behavioral outcomes in the HOME study, including polybrominated diphenyl ethers (Vuong et al., 2018, 2017; Zhang et al., 2017) and perfluoralkyl substances (Vuong et al., 2016). However, these were weakly correlated (r’s <0.3) with triclosan concentrations (Kalloo et al., 2018). Interestingly, the magnitude of the association between gestational triclosan and externalizing problems among boys in the current study (β = 5.0; 95% CI: 1.2–9.0) was comparable to the magnitude of association between gestational BPA and externalizing problems among girls in this cohort (β = 6.2, 95% CI: 0.8, 11.6) (Stacy et al., 2017c).
Third, there is the potential for type I error associated with multiple comparisons, since we measured associations between multiple triclosan measures and several behavioral outcomes. However, traditional methods of correcting for multiple comparisons do not account for the correlated nature of the outcomes and have low statistical power and accuracy (Gelman et al., 2012; Rothman, 1990). Moreover, the consistency of our associations between triclosan concentrations and several related behavioral domains reduces the likelihood that these results are spurious. Fourth, our sample size was modest, especially in sex-stratified analyses, which may have reduced our ability to precisely estimate associations. Thus, future studies with larger sample sizes are needed to confirm these associations. Finally, our findings may not be generalizable to other populations. However, urinary triclosan concentrations in the analyses cohort were similar to the original HOME study cohort and previous U.S. studies, including those from the NHANES (Philippat et al., 2015; Teitelbaum et al., 2008; Wolff et al., 2010; Woodruff et al., 2011).
In conclusion, both gestational and childhood triclosan concentrations were associated with several dimensions of externalizing behaviors, including hyperactivity and attention problems, at age 8 years among boys in this cohort. Given that these behaviors are core features of ADHD, future studies could examine clinical ADHD diagnosis in relation to triclosan exposure. Our results suggest that gestational triclosan exposures may be more strongly associated with these behaviors than childhood exposures. Future studies examining the neurotoxicity of early life triclosan exposure could also consider using statistical approaches to account for measurement error and examine internalizing problems during adolescence, when these traits tend to manifest.
Supplementary Material
Figure 4C.
Adjusted differences in child internalizing scores at age 8-years per 10-fold increase in gestational and childhood triclosan concentrations.
Abbreviations: BSI= behavioral symptoms index, BASC-2= Behavioral Assessment for Children-2, BDIII= Beck Depression Inventory-II, ADHD= attention deficit-hyperactivity disorder.
Multiple informant regression models of (creatinine-standardized-log10-transformed) urinary triclosan concentration and BASC-2 composite linear regression models adjusted for log10-transformed serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (White-non-Hispanic, Black-non, Hispanic, and other), household income (continuous), marital status (married vs. unmarried), maternal education (less than high school, high school graduate, and some college or greater), caregiving environment scores (continuous), BDI-II (continuous), and ADHD (continuous). Error bars are 95% Confidence Intervals.
Urinary triclosan x exposure period interaction p-value= 0.06, 0.06, and 0.28, respectively.
Highlights.
We measured urinary triclosan concentrations up to twice during pregnancy, at delivery, and up to 6 times in children from ages 1–8-years.
We assessed children’s problem behaviors at age 8 years by parent-report.
We used a regression-calibration approach to account for triclosan exposure measurement error.
Gestational and childhood triclosan concentrations were positively associated with externalizing problem, attention, and hyperactivity scores in boys, but not girls.
Acknowledgements:
This work was supported by NIEHS grants R01 ES024381, R01 ES020349, P01 ES011261, and R01 ES014575. We acknowledge the technical assistance of A. Calafat, X. Ye, Z. Zhou, P. Dwivedi, and J. Tao (Centers for Disease Control and Prevention CDC) in measuring the urinary triclosan concentrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Joseph M. Braun was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures.
References
- Arbuckle TE, Marro L, Davis K, Fisher M, Ayotte P, Bélanger P, Dumas P, LeBlanc A, Bérubé R, Gaudreau É, Provencher G, Faustma EM, Vigoren E, Ettinge AS, Dellarco M, MacPherson S, Fraser WD, 2015. Exposure to free and conjugated forms of bisphenol a and triclosan among pregnant women in the MIREC cohort. Environ. Health Perspect 123, 277–284. 10.1289/ehp.1408187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Guth D, Steer RA, Ball R, 1997. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav. Res. Ther 35, 785–791. 10.1016/S0005-7967(97)00025-9 [DOI] [PubMed] [Google Scholar]
- Bellinger DC, 2004. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ. Res 95, 394–405. 10.1016/j.envres.2003.07.013 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J, 2009. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol 169, 236–248. 10.1093/aje/kwn301 [DOI] [PubMed] [Google Scholar]
- Bertelsen RJ, Engel SM, Jusko T.a, Calafat AM, Hoppin J.a, London SJ, Eggesbø M, Aase H, Zeiner P, Reichborn-Kjennerud T, Knudsen GP, Guidry VT, Longnecker MP, 2014. Reliability of triclosan measures in repeated urine samples from Norwegian pregnant women. J. Expo. Sci. Environ. Epidemiol 1–5. 10.1038/jes.2013.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, Corwyn RF, 2003. The Child Care HOME Inventories: Assessing the quality of family child care homes. Early Child. Res. Q 18, 294–309. 10.1016/S0885-2006(03)00041-3 [DOI] [Google Scholar]
- Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, Yolton K, Lanphear BP, 2017a. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 58, 75–83. 10.1016/j.neuro.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Hoofnagle A, Papandonatos GD, Jackson-Browne M, Hauser R, Romano ME, Karagas MR, Yolton K, Thomas Zoeller R, Lanphear BP, 2017b. Associations of early life urinary triclosan concentrations with maternal, neonatal, and child thyroid hormone levels. Horm. Behav 10.1016/j.yhbeh.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP, 2011. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics 128, 873 LP–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP, 2016. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int. J. Epidemiol 1–10. 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP, 2009. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect 117, 1945–52. 10.1289/ehp.0900979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, Lanphear BP, Chen A, 2017c. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 62, 192–199. 10.1016/j.neuro.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ. Health Perspect 116, 303–307. 10.1289/ehp.10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R, Ruppert D, Stefanski L, Crainiceanu C, 2006. Measurement Error in Nonlinear Models, C&H/CRC Monographs on Statistics & Applied Probability. Chapman and Hall/CRC; 10.1201/9781420010138 [DOI] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA, 2005. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci. Biobehav. Rev 29, 353–384. 10.1016/j.neubiorev.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, Sparrow E, 1999. Self-ratings of ADHD symptoms in adults I: Factor structure and normative data. J. Atten. Disord 3, 141–151. 10.1177/108705479900300303 [DOI] [Google Scholar]
- Drover SSM, Villanger GD, Aase H, Skogheim TS, Longnecker MP, Zoeller RT, Reichborn-Kjennerud T, Knudsen GP, Zeiner P, Engel SM, 2019. Maternal Thyroid Function During Pregnancy or Neonatal Thyroid Function and Attention Deficit Hyperactivity Disorder. Epidemiology 30, 130–144. 10.1097/EDE.0000000000000937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar G.M. de, Obregón MJ, Rey FE, 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract. Res. Clin. Endocrinol. Metab 18, 225–248. [DOI] [PubMed] [Google Scholar]
- Etzel T, Muckle G, Arbuckle TE, Fraser WD, Ouellet E, Séguin JR, Lanphear B, Braun JM, 2018. Prenatal urinary triclosan concentrations and child neurobehavior. Environ. Int 114, 152–159. 10.1016/j.envint.2018.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2016. Consumer Updates - 5 Things to Know About Triclosan [WWW Document]. U.S. Food Drug Adm; URL https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm205999.htm (accessed 7.26.18). [Google Scholar]
- Food and Drug Adminnistration, H., 2016. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final rule. Fed. Regist 81, 61106–30. [PubMed] [Google Scholar]
- Gelman A, Hill J, Yajima M, 2012. Why We (Usually) Don’t Have to Worry About Multiple Comparisons. J. Res. Educ. Eff 5, 189–211. 10.1080/19345747.2011.618213 [DOI] [Google Scholar]
- Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG, 2016. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur. J. Epidemiol 31, 337–350. 10.1007/s10654-016-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ, 1999. Maternal Thyroid Deficiency during Pregnancy and Subsequent Neuropsychological Development of the Child. N. Engl. J. Med 341, 549–555. 10.1056/NEJM199908193410801 [DOI] [PubMed] [Google Scholar]
- Henrichs J, Ghassabian A, Peeters RP, Tiemeier H, 2013. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin. Endocrinol. (Oxf) 79, 152–162. 10.1111/cen.12227 [DOI] [PubMed] [Google Scholar]
- Heyer DB, Meredith RM, 2017. Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 58, 23–41. 10.1016/j.neuro.2016.10.017 [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, Braun JM, 2018. Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children. Environ. Health Perspect 126, 057001 10.1289/EHP2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, Campbell M, Donald JM, Sen S, Bero L, Zeise L, Woodruff TJ, 2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ. Int 92–93, 716–728. 10.1016/j.envint.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloo G, Wellenius GA, McCandless L, Calafat AM, Sjodin A, Karagas MR, Chen A, Yolton K, Lanphear BP, Braun JM, 2018. Profiles and Predictors of Environmental Chemical Mixture Exposure among Pregnant Women: The HOME Study. Environ. Sci. Technol acs.est.8b02946. 10.1021/acs.est.8b02946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphaus RW, 2015. Behavior Assessment System for Children, Second Edition (BASC-2), in: The Encyclopedia of Clinical Psychology. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 1–6. 10.1002/9781118625392.wbecp447 [DOI] [Google Scholar]
- Klein RZ, Sargent JD, Larsen PR, Waisbren SE, Haddow JE, Mitchell ML, 2001. Relation of severity of maternal hypothyroidism to cognitive development of offspring. J. Med. Screen 8, 18–20. 10.1136/jms.8.1.18 [DOI] [PubMed] [Google Scholar]
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD, 2013. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci. Total Environ 445–446, 299–305. 10.1016/j.scitotenv.2012.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, Steegers EAP, Visser TJ, White T, Tiemeier H, Peeters RP, 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43. 10.1016/S2213-8587(15)00327-7 [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Ye X, Calafat AM, 2005. Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for the Determination of Nine Environmental Phenols in Urine. Anal. Chem 77, 5407–5413. 10.1021/ac050390d [DOI] [PubMed] [Google Scholar]
- Kumar V, Chakraborty A, Kural MR, Roy P, 2009. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol 27, 177–185. 10.1016/J.REPROTOX.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Larsen K, 1972. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta 41, 209–217. [DOI] [PubMed] [Google Scholar]
- Lazarus JH, 1999. Thyroid hormone and intellectual development: a clinician’s view. Thyroid 9, 659–660. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, Stoker TE, 2017. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. J. Toxicol. Environ. Heal. - Part A Curr. Issues 80, 236–249. 10.1080/15287394.2017.1287029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Mustieles V, Minguez-Alarcon L, Ford JB, Calafat AM, Souter I, Williams PL, Hauser R, 2018. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ. Int 114, 60–68. 10.1016/J.ENVINT.2018.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NC, Diviney MM, Donnelly JC, Cooley SM, Kirkham CH, Foran AM, Breathnach FM, Malone FD, Geary MP, 2015. The effect of maternal subclinical hypothyroidism on IQ in 7- to 8-year-old children: A case-control review. Aust. New Zeal. J. Obstet. Gynaecol 55, 459–463. 10.1111/ajo.12338 [DOI] [PubMed] [Google Scholar]
- Philippat C, Bennett D, Calafat AM, Picciotto IH, 2015. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environ. Res 140, 369–376. 10.1016/j.envres.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R, EDEN Mother–Child Study Group, the E.M.S., 2017. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ. Health Perspect 125, 097014 10.1289/EHP1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, 2015. R: A language and environment for statistical computing. Rodríguez, P.E.A., Sanchez, M.S., 2010. Journal of Toxicology and Environmental Health, Part A Maternal Exposure to Triclosan Impairs Thyroid Homeostasis and Female Pubertal Development in Wistar Rat Offspring. J. Toxicol. Environ. Heal. Part A 73, 1678–1688. 10.1080/15287394.2010.516241org/10.1080/15287394.2010.516241 [DOI] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No Adjustments Are Needed for Multiple Comparisons. Epidemiology 1, 43–46. [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL, 2008. Modern Epidemioogy, 3rd ed. ed. Philadelphia : Wolters Kluwer Health/Lippincott Williams & Wilkins, c2008. [Google Scholar]
- Rubin DB, 1987. Multiple Imputation for Nonresponse in Surveys, Wiley Series in Probability and Statistics. John Wiley & Sons, Inc., Hoboken, NJ, USA: 10.1002/9780470316696 [DOI] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM, 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ. Health Perspect 119, 409–415. 10.1289/ehp.1102453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, 2016. Serum Testosterone Concentrations and Urinary Bisphenol A, Benzophenone-3, Triclosan, and Paraben Levels in Male and Female Children and Adolescents: NHANES 2011–2012. Environ. Health Perspect 124, 1898–1904. 10.1289/EHP150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C, 1904. The proof and measurement of association between two things. Ameri J Psychol 5, 72–101. 10.2307/1412159 [DOI] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Etzel T, Papandonatos G, Calafat AM, Chen A, Hauser R, Lanphear BP, Sathyanarayana S, Ye X, Yolton K, Braun JM, 2017a. Patterns, Variability, and Predictors of Urinary Triclosan Concentrations during Pregnancy and Childhood. Environ. Sci. Technol 51, 6404–6413. 10.1021/acs.est.7b00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, Braun JM, 2017b. Early life bisphenol A exposure and neurobehavior at 8 years of age: Identifying windows of heightened vulnerability. Environ. Int 107, 258–265. 10.1016/j.envint.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, Braun JM, 2017c. Early life bisphenol A exposure and neurobehavior at 8 years of age: Identifying windows of heightened vulnerability. Environ. Int 107, 258–265. 10.1016/J.ENVINT.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski KA, Sitarz AM, Wojtowicz AK, 2015. Triclosan induces Fas receptor-dependent apoptosis in mouse neocortical neurons in vitro. Neuroscience 284, 192–201. 10.1016/j.neuroscience.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Wnuk A, Kajta M, Wójtowicz AK, 2016. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ. Res 151, 106–114. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS, 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res 106, 257–269. 10.1016/j.envres.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH, 2016. Robust causal inference using directed acyclic graphs: The R package “dagitty.” Int. J. Epidemiol 45, 1887–1894. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- Vanderweele TJ, 2009. On the distinction between interaction and effect modification. Epidemiology 20, 863–871. 10.1097/EDE.0b013e3181ba333c [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A, 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm. Behav 101, 94–104. 10.1016/J.YHBEH.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjödin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, Chen A, 2016. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ. Res 147, 556–564. 10.1016/J.ENVRES.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Xie C, Webster GM, Sjödin A, Braun JM, Dietrich KN, Lanphear BP, Chen A, 2017. Childhood polybrominated diphenyl ether (PBDE) exposure and neurobehavior in children at 8 years. Environ. Res 158, 677–684. 10.1016/J.ENVRES.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chen L, Zhao S, Hu Y, Zhou Y, Gao Y, Wang W, Zhang J, Tian Y, 2018. Impacts of prenatal triclosan exposure on fetal reproductive hormones and its potential mechanism. Environ. Int 111, 279–286. 10.1016/J.ENVINT.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Wang X, Ouyang F, Feng L, Wang X, Liu Z, Zhang J, 2017. Maternal Urinary Triclosan Concentration in Relation to Maternal and Neonatal Thyroid Hormone Levels : A Prospective Study. Environ. Health Perspect 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, Kushi LH, Erdmann C, Hiatt RA, Rybak ME, Calafat AM, Centers, the B.C. and E.R., 2010. Investigation of Relationships between Urinary Biomarkers of Phytoestrogens, Phthalates, and Phenols and Pubertal Stages in Girls. Environ. Health Perspect 118, 1039–1046. 10.1289/ehp.0901690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect 119, 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM, 2013. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ. Health Perspect 121, 283–286. 10.1289/ehp.1206093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeileis A, 2006. Object-Oriented Computation of Sandwich Estimators. J. Stat. Softw 16 10.18637/jss.v016.i09 [DOI] [Google Scholar]
- Zeileis A, 2004. Econometric Computing with HC and HAC Covariance Matrix Estimators. J. Stat. Softw 11 10.18637/jss.v011.i10 [DOI] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjödin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A, 2017. Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environ. Health Perspect. 125, 746–752. 10.1289/EHP478 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.