Abstract
Objective:
Cancer mortality inequity among persons of African Ancestry is remarkable. Yet, Black inclusion in cancer biology research is sorely lacking and warrants urgent attention. Epidemiologic research linking African Ancestry and the African Diaspora to disease susceptibility and outcomes is critical for understanding the significant and troubling health disparities among Blacks. Therefore, in a cohort of diverse Blacks, this study examined differences in genetic ancestry informative markers (AIMs) in the DNA repair pathway and the cancer related biomarker 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL).
Methods:
Participants completed a questionnaire and provided bio-specimens. AIMs in or around DNA repair pathway genes were analyzed to assess differences in minor allele frequency (MAF) across the 3 ethnic subgroups. NNAL concentration in urine was measured among current smokers.
Results:
To date the cohort includes 852 participants, 88.3% being Black. Of the 752 Blacks, 51.3% were US-born, 27.8% were Caribbean-born, and 19.6% were Africa-born. Current and former smokers represented 14.9% and 10.0%, respectively. US-born Blacks were more likely to be smokers and poor metabolizers of NNAL. Two-way hierarchical clustering revealed MAF of AIMs differed across the 3 ethnic subgroups.
Conclusion:
Our findings are consistent with the emerging literature demonstrating Black heterogeneity underscoring African Ancestry genetic subgroup differences— specifically relevant to cancer. Further investigations, with data harmonization and sharing, are urgently needed to begin to map African Ancestry cancer biomarkers as well as race, and race by place\region comparative biomarkers to inform cancer prevention and treatment in the era of precision medicine.
Keywords: Disparities, prevention, cancer screening, African-American, Afro-Caribbean, African, immigrant health, race/ethnicity, health care, diversity, genetics, health behaviors
Introduction
The association between tobacco use, specifically cigarette smoking, and cancer risk and mortality has been long established (Ubell 1954; Hammond and Horn 1954; Doll and Hill 1954; Schairer and Schöniger 1944; Müller 1940). The Centers for Disease Control and Prevention (CDC) reported that 443,000 deaths in the US will be caused by smoking or exposure to second hand smoke (Centers for Disease Control and Prevention 2011b). Further, for 2017 an estimated 190,500 cancer deaths stemming from tobacco use (Sawyers et al. 2013). Cigarette smoking has been associated with 18 different anatomical sites, including cancers of the head and neck, the urogenital, digestive and hematopoietic systems, and the lung and bronchus (Arteaga et al. 2014). In the US, the prevalence of smoking in adults 18 years and older is ~16%; the prevalence for Blacks and Whites is similar, about 16.5% and 16.6%, respectively (Jamal et al. 2018; National Center for Health Statistics 2016). Yet, profound disparities exist in overall cancer survival and cancer mortality, where the mortality rate is 24% higher in Black men and 14% higher in Black women when compared to their White counterpart (C. DeSantis et al. 2016). Assessing the cause of this disparity is multi-factorial, however the need for epidemiologic research linking African Ancestry and the African Diaspora to disease susceptibility and outcomes is critical for understanding the significant health disparities among Blacks.
In the United States (US), the term African-American (AA) is used to classify an individual who self-identifies as a person with African ancestral origins (Bhopal 2004). The 2010 census reported that AAs constitute about 12.6% of the total population (n= 38,929,319); where foreign born Black individuals represent 12.1% of the AA population (Grieco et al. 2012; Humes, Jones, and Ramirez 2010). In 2017 The American Cancer Society again documented that AA have the greatest cancer burden with an estimated 189,910 incident cancer cases and 69,410 cancer related deaths among AAs (C. E. DeSantis et al. 2016). The undue U.S. Black cancer burden and the significant representation of immigrants of African ancestry among this population warrants diversity inclusion, representation and examination in cancer research.
Despite the current advances in research and medical practice, the disproportionate burden of chronic disease in US Black populations remain consistent. In general, large prospective cohort studies and randomized controlled trials have involved predominantly White study populations with little involvement of minority racial groups (Oglesby 1976; Garfinkel and Heath Jr. 1982; Rimm et al. 1991; Zhang et al. 2000; Taioli et al. 2013; C. C. Ragin et al. 2010; Belanger et al. 1978). Within the last two decades, a number of cohorts have been established attempting to over sample for the AA population (Kolonel et al. 2000; L. Rosenberg, Adams-Campbell, and Palmer 1972; Sempos, Bild, and Manolio 1999) with the Southern Community Cohort Study (SCCS) being established most recently (Signorello et al. 2005). The SCCS, initiated in 2001, currently has ~85,000 adults recruited from southeastern U.S. and has a large biorepository and the largest representation of AAs (two-thirds of its entire study population) among existing U.S. cohorts. However, none of these cohorts have assessed the diversity within the AA population neither have any of these cohorts involved the AA populations in the northeastern region of the US, where there is a large representation of foreign born Black individuals (Grieco et al. 2012).
We and others have shown that within-group differences in overall health, health behaviors, and health outcomes exist among the US Black population (J Fang, Madhavan, and Alderman 1996; Pinheiro et al. 2016; Creque et al. 2010; Cobran et al. 2014; C. Ragin et al. 2011; Consedine et al. 2015; J Fang, Madhavan, and Alderman 1997; Mutetwa et al. 2010). For example, Pinheiro et al. (2016) analyzed cancer mortality rates in Caribbean and US-born Florida residents. The authors concluded that overall risk of cancer related death was significantly higher in US-born Blacks than Caribbean-born persons.
When looking at genetics, extensive work has been done to examine genetic variation and disease risk in the Black population (Fernandez et al. 2015; Irizarry-Ramírez et al. 2017; Conti et al. 2017; Huo et al. 2016; Chen et al. 2013; Huo et al. 2012; Wang et al. 2017). However, few studies have assessed within race differences. One notable study conducted by Murphy et al., showed overt differences in the prevalence of 8q24 risk alleles in men of African ancestry and compared the prevalence of SNPs in this region among men from Jamaica, Nigeria and Cameroon. Specifically, Murphy reported that rs6983267, rs7008482, and rs7000448 had the highest prevalence in West African men and rs6983561, rs7008482, and rs16901979 were significantly associated with prostate cancer risk in West African men but not in Caribbean men in the study (Murphy et al. 2012). This work suggests that germline differences exist and should be further examined within this population.
With the need for an ethnically diverse prospective cohort of AAs, we have established the Cancer Prevention Project of Philadelphia (CAP3), a registry of clinic and population-based controls, who are residents of the Philadelphia metropolitan area, New Jersey, Delaware and New York metropolitan area. The CAP3 study serves as a resource for nested research studies addressing racial disparities in cancer. The data collected support multi-level investigations of epidemiological, behavioral, genetic and other molecular factors. This cohort also serves as resource for cancer control through the investigation of multi-level factors related to health behaviors such as cancer screenings and anthropometry; these data will be reported elsewhere.
Specifically, in a diverse African Ancestry human sample, this investigation focused on smoking and tobacco genotoxicity. We chose to examine ancestry informative markers (AIMs) along the DNA repair pathway because of the relationship between tobacco exposure and DNA damage. Carcinogens found in tobacco smoke covalently bind to DNA to form adducts which persist if they escape DNA repair. Subsequently, leading to miscoding during DNA replication, resulting in a permanent mutation and causing defects in DNA and the DNA repair pathway; this can promote cancer cell growth (Kelley, Logsdon, and Fishel 2014). This paper describes our methodology, basic demographics of our study population to date, genetics and select biomarker data.
Methods
This study was approved by the Institutional Review Board at Fox Chase Cancer Center and participants provided informed consent.
CAP3 recruitment
During this first phase of the study, CAP3 recruits approximately 200 participants annually, with >75% being of African descent. Our study recruitment is predominantly conducted in the Philadelphia metropolitan area, an urban setting where the Black community is the largest minority group (~43% of the total population) consisting of US-born Black, African and Caribbean communities (“Quick Facts: Philadelphia County, Pennsylvania” 2015). The study also captures Black individuals who reside in adjoining metropolitan areas such as New Jersey, Delaware and New York City. Cohort enrollment is limited to English speakers over the age of 18, who do not knowingly have a cancer diagnosis at the time of study enrollment; the presence of other comorbidities, i.e. hypertension, diabetes, etc., does not affect study eligibility.
Participants were recruited from health fairs, community centers, places of worship and senior centers across Philadelphia County. Community-based recruitment was facilitated by establishing a network of partnerships with key community-based organizations (CBO) that were already providing health promotion services to minority and immigrant populations in Philadelphia. This network is informed by community engagement principles including bi-directional communication and learning, and building community trust and capacity for community health improvements (B. Israel et al. 2013; B. A. Israel et al. 1998, 2010). A number of organizations have joined and the network continues to grow.
Clinic-based recruitment activities also took place in both primary and specialized care clinics associated with Temple University Health System (TUHS). All participants were consented. Participation included completing a face-to-face interviewer administered questionnaire --either on paper or electronically using tablets –based on the number of participants at each venue. At the completion of questionnaire administration, participants were then asked to provide mouthwash/buccal and urine samples.
Questionnaire
The study questionnaire used items adapted from the 2011 Behavioral Risk Factors Surveillance System Questionnaire and the National Health and Nutrition Examination Survey, both developed by the CDC (National Center for Health Statistics 1999; Centers for Disease Control and Prevention 2011a). Questions pertaining to family history of cancer and other comorbidities, medication use, and improvements to the alcohol, and female health history, were adapted from the SCCS (Signorello et al. 2005). The questionnaire collects information on demographics; tobacco use; food frequency; alcohol consumption; physical activity; personal medical history; family medical history; reproductive history (for women); medication use; emotional well-being and social support; religion/spirituality; health insurance; use of medical and cancer screening services; perceptions of genetic screening; lung health; oral health; and ancestry information. Anthropometry was obtained, and demographic characteristics were assessed with items on age, height, weight, level of education, marital status, occupation, total household income, race, current zip code, country of birth, country of parental origin, and length of time in the US (if foreign born). Although data were collected for these measures, this analysis will only focus on select demographics, tobacco use, the tobacco related biomarker NNAL, and genetic data related to ancestry. All other data previously mentioned data will be reported elsewhere.
Bio-specimen collection and handling
Mouthwash/buccal samples were collected after the study questionnaire was administered. Study participants were supplied with a 50 mL Falcon™ tube and a 30 mL specimen cup containing 10mL of Scope and were instructed to gargle while tilting their heads back for 30-60 seconds before spitting it back into the 50 mL tube. Participants were required to have refrained from eating and drinking at least 30 minutes prior to performing the mouthwash rinses. Mouthwash samples were then transported at 4ºCelsius (on ice packs) to the laboratory where they were processed. Samples were centrifuged, and the mouthwash supernatant was stored at −20ºC for future applications. DNA extraction was performed from the oral exfoliated cell pellet using the QIAGEN PureGene DNA extraction protocol (Qiagen 2014). DNA quality was then assessed, and all samples were tested for beta globin.
Urine collection began in year two of recruitment and was used to test for biomarkers related to tobacco metabolites. Study staff instructs participants on how to conduct a clean catch urine collection (Linda J, Vorvic; David, Zieve ; Ogilvie 2016) and the participant self-collected in the nearest restroom. Urine samples were transported to the laboratory, at 4ºC, where they were aliquoted and stored at −80°C.
NNAL measurement
The association between tobacco use and its role in the etiology of certain cancers is well established (Sasco, Secretan, and Straif 2004; Cresanta 1992; Boyle 1997; S. Park et al. 2014; Poirier et al. 2016; W. Watson and Cokte 1955; Hammond 1954; Wynder 1954; W. L. Watson and Conte 1954). Work has been done to examine biomarkers related to tobacco smoke exposure and lung cancer risk, specifically 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) which has been correlated to lung cancer risk (Centers for Disease Control and Prevention 2016; Yuan et al. 2014; Ashley et al. 2010; Sarkar, Wang, and Liang 2012; Church et al. 2009; Yuan et al. 2009). NNAL is a tobacco-specific biomarker that can be measured in urine using well established standardized laboratory protocols. The biomarker assay is sensitive enough to detect tobacco exposure in smokers and in second-hand smokers and is also a specific tobacco metabolite, in that it is not a dietary component and is only found in the environment when there is tobacco smoke present. Racial differences in the metabolic efficiency of NNAL has also been examined where AAs have been shown to have higher levels of NNAL in urine when compared to Whites (Richie et al. 1997; Muscat et al. 2005).
Urine samples from current smokers were identified and analyzed for free and total NNAL quantitative measurements, the details of the experimental procedures were previously described by Bhat et al. (Bhat et al. 2011) Prior to extraction, known amount of [2H5]-NNAL was spiked in individual urine samples as the recovery standard. Eight NNAL calibration standards spanning from 60 to 10000 pg/mL were freshly prepared for every sample set analysis. Calibration curves were constructed using a linear regression. Based on the integrated peak area and calibration curves, free NNAL and total NNAL concentrations in the samples were determined in pg/mL. This assay demonstrated sufficient sensitivity to detect low ranges of NNAL found in smokers’ urine and made it possible to work with smaller volumes of urine.
For current smokers, NNAL glucuronidation ratios were calculated using the following formula: (total NNAL- free NNAL)/free NNAL. Participants were categorized as poor metabolizers (ratio <2), intermediate metabolizers (ratio between 2 and 5), and extensive metabolizers (ratio >5) phenotypes. Summary statistics were also calculated for each metabolizer category.
Ancestry informative markers and validation of computational pipeline
Using the 1000 Genomes Project data, a computational pipeline was built to perform a systematic genome-wide survey that compared allelic differences within the genes of African and European ancestry populations. All single nucleotide polymorphisms (SNP) data from the 1000 Genomes Project was downloaded via the National Center for Biotechnology Information (NCBI) as variant call format (VCF) files, one file for each chromosome. A combination of VCF tools, perl scripts, and population genomic software, such as Plink, was used to extract populations of interest [African (YRI, GWD, MSL, ESN, and LWK) and European (CEU, TSI, FIN, GBR, and IBS)]. Using a sliding windows approach and ancestry informativeness calculations (N. A. Rosenberg et al. 2003), SNPs that highly differentiate African from European ancestry populations via ancestry informativeness estimates (In > 0.25) were identified. This enabled the identification of SNPs that are unique to populations of African ancestry. The Ancestry Informative Markers (AIMs) located in or around (± 1MB) DNA damage response genes (n=162) identified through this computational method were validated in a subset of the study cohort (n=573). The frequencies of these AIMs were compared with the frequencies reported for African Americans (ASW) and Caucasian Americans (CEU) in the 1000 Genomes Project. Spearman’s rho was calculated to assess differences between our sample and the 1000 Genomes populations.
Minor allele frequencies (MAF) were calculated for each AIM. The direct counting method was used to determine allele frequency. To examine genetic diversity between Black sub-groups, a heat map of MAF of AIMs in the DNA repair pathway genes was generated using two-way hierarchical clustering based on Euclidean distance as the metric based on average linkage. The heat map identifies sub-groups of AIMs with similar frequency profiles across sub-populations of Blacks as indicated by the homogeneous coloring. Analyses were performed using R statistical software (version 3.3.3).
Statistical analyses
Descriptive analyses were performed to summarize demographic information for the cohort. The Black population was stratified into three groups based on self-reported country of birth and parental country of birth. In this report we will refer to AAs as individuals born in the US with parents born in the US, Afro-Caribbeans (ACs) as individuals born in the US or Caribbean with parents born in the Caribbean, and Africans (AFs) as individuals born in the US or Africa with parents born in Africa. Participants where parental country of origin was missing were classified by their country of birth; this represented <10% of the study population. These analyses were done using STATA version 13.1 (Stata LP, College Station, TX, USA).
Results
Demographics
To date 852 individuals have been recruited, with questionnaire and bio-specimen data (buccal sample N=852; urine sample N=288). Most study participants were female (66.3%; n=565). Mean age for the total population was 47.61 years (SD ± 15.31; range: min: 18, max: 85), with 23.5% falling within the 50-59 age-group category. The remaining descriptive statistics for the study population are presented in Table 1. In respect to race, 88.3% (n=752) self-reported as Black. Within this sub-group 51.3% of respondents were AA, 27.8% were AC, and 19.6% were AF; the remaining Black respondents were from other regions of the world (~1%). Mean length of time in the US, for foreign-born, Black study participants, was approximately 16 years (SD ± 11.97; range: min: 0 max: 55).
Table 1.
Descriptive Characteristics Stratified By Sex
| Male n = 287 N (%) |
Female n = 565 N (%) |
Total n= 852 N (%) |
p-Value | |
|---|---|---|---|---|
| Age Group (Years) | 0.026 | |||
| 18-29 | 49 (17.1) | 80 (14.2) | 129 (15.1) | |
| 30-39 | 39 (13.6) | 103 (18.2) | 142 (16.7) | |
| 40-49 | 76 (26.5) | 100 (17.7) | 176 (20.7) | |
| 50-59 | 61 (21.2) | 137 (24.3) | 198 (23.2) | |
| 60-69 | 42 (14.6) | 89 (15.7) | 131 (15.4) | |
| 70-79 | 13 (4.5) | 41 (7.3) | 54 (6.3) | |
| 80-89 | 6 (2.1) | 8 (1.4) | 14 (1.6) | |
| Missing | 1 (0.4) | 7 (1.2) | 8 (0.9) | |
| Race | 0.155 | |||
| White | 22 (7.7) | 38 (6.7) | 60 (7.0) | |
| Black or African American | 254 (88.5) | 498 (88.1) | 752 (88.3) | |
| Asian | 8 (2.8) | 7 (1.2) | 15 (1.8) | |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 1 (0.2) | 1 (0.1) | |
| American Indian or Alaska Native | 1 (0.3) | 2 (0.4) | 3 (0.4) | |
| Other | 2 (0.7) | 11 (2.0) | 13 (1.5) | |
| Missing | 0 (0.0) | 8 (1.4) | 8 (0.9) | |
| Marital Status | 0.001 | |||
| Married or Member of Unmarried Couple | 142 (49.5) | 224 (39.6) | 366 (43.0) | |
| Divorced, Widowed or Separated | 42 (14.6) | 143 (25.3) | 185 (21.7) | |
| Never Married | 102 (35.5) | 192 (34.0) | 294 (34.5) | |
| Missing | 1 (0.4) | 6 (1.1) | 7 (0.8) | |
| Income | 0.005 | |||
| >$10,000 -$14,999 | 40 (13.9) | 80 (14.2) | 120 (14.1) | |
| $15,000 to $24,999 | 44 (15.3) | 126 (22.3) | 170 (20.0) | |
| $25,000 to $49,999 | 85 (29.6) | 141 (25.0) | 226 (26.5) | |
| $50,000+ | 75 (26.1) | 103 (18.2) | 178 (20.9) | |
| Don’t Know/Missing | 43 (15.0) | 115 (20.3) | 151 (18.5) | |
| Education | 0.355 | |||
| Never Completed High School | 30 (10.5) | 62 (11.0) | 92 (10.8) | |
| High School Graduate | 84 (29.3) | 155 (27.4) | 239 (28.0) | |
| Some College | 60 (20.9) | 141 (25.0) | 201 (23.6) | |
| College Graduate | 101 (35.2) | 196 (34.7) | 297 (34.9) | |
| Graduate School | 11 (3.8) | 10 (1.8) | 21 (2.5) | |
| Missing | 1 (0.3) | 1 (0.2) | 2 (0.2) | |
Smoking and Tobacco Metabolism
Smoking behavior
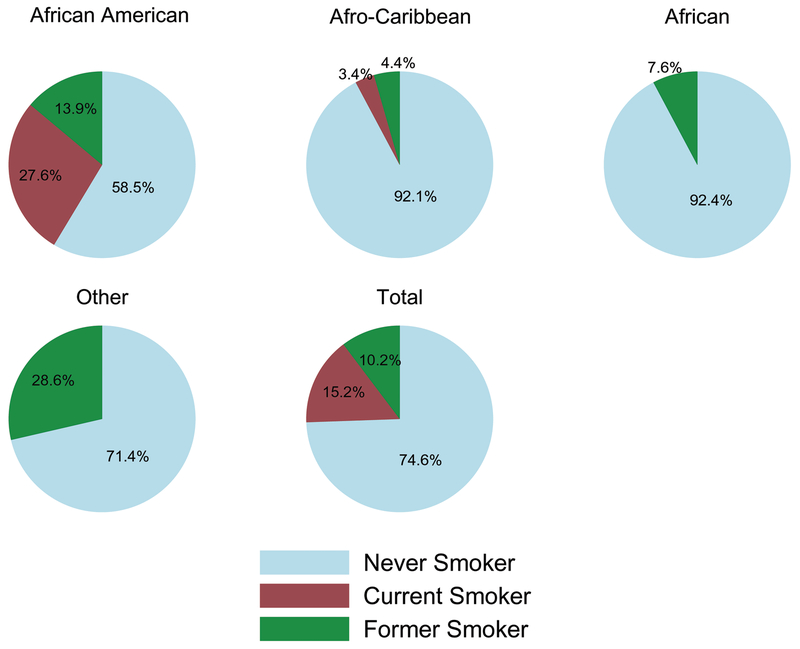
The prevalence of smoking for Black participants was 14.9%; 10.0% were former smokers. Mean age at first cigarette for current and former smokers was 16.72 years (SD ± 5.32). AAs had a higher percentage of current smokers than any other ethnic sub-group (p<0.001; Figure 1). There were no current smokers of African origin. For immigrants, although small in number we observed a significant relationship between length of time in the US and ever smoking. Immigrants had a 4% increase in odds of ever smoking as length of time in the US increases.
Figure 1.
Smoking Status of Blacks by Ethnic Subgroup
*Other category includes a combination of participants from Europe, Asia and other regions not otherwise categorized.
NNAL metabolism
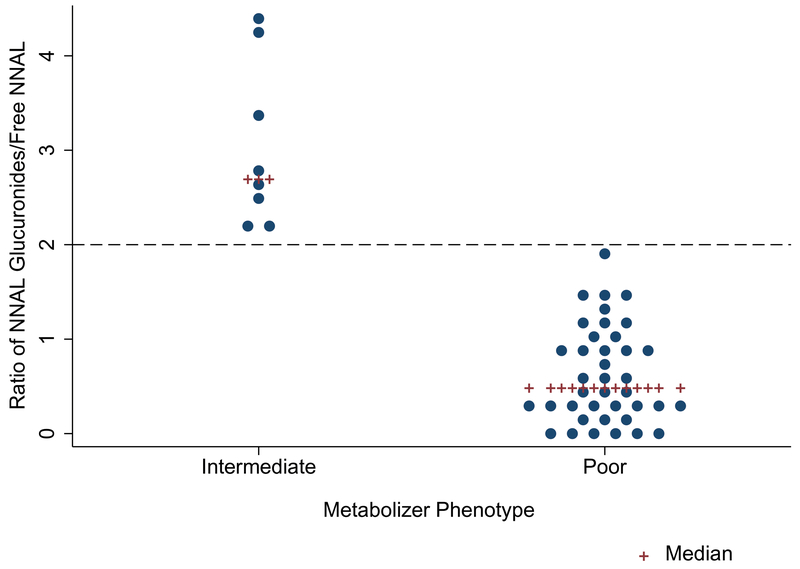
Smokers with urine available for analysis were processed to determine their efficiency in metabolizing NNAL (n=47, all Black participants). Our analysis revealed that this subset contained two metabolizer phenotypes, poor metabolizers (n=39) with a ratio of NNAL glucuronides/free NNAL <2 (median: 0.48, mean= 0.64 ± 0.51) and intermediate metabolizers (n=8) with a ratio between 2 and 5 (median: 2.7, mean = 3.05 ± 0.87) (Figure 2).
Figure 2.
Metabolizer Phenotype Distribution for Current Smokers
Second-hand smoke exposure
Second-hand smoke exposure was predominantly observed among AAs. Approximately 17.1% of AAs, 1.9% of ACs, and 0% of AFs reported allowing smoking in their home. For respondents that lived with a family member that smoked (N=33), 60.6% were AA, 30.3% were AC, and 6.1% were AF.
Differences in minor allele frequency of select DNA repair genes
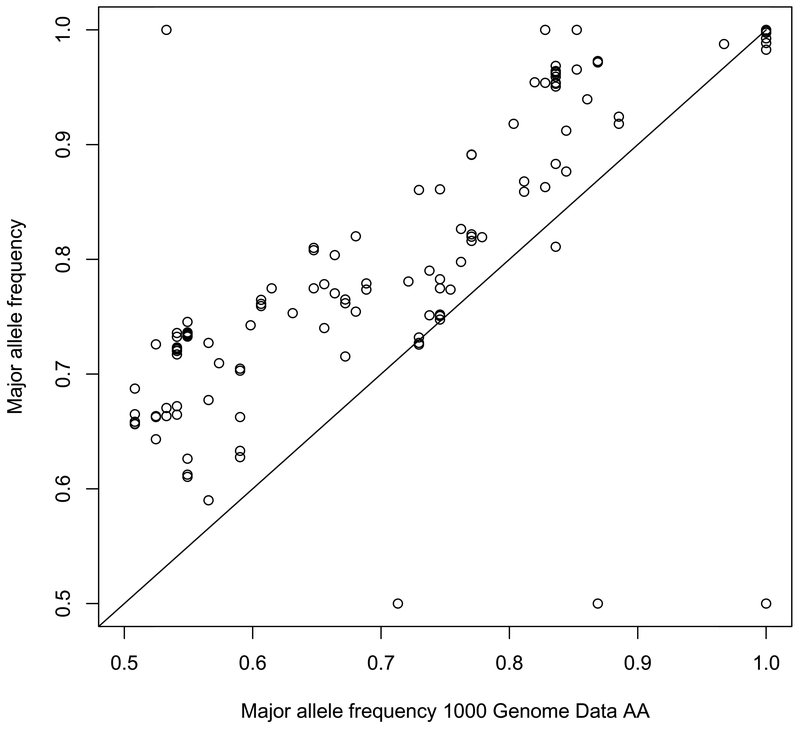
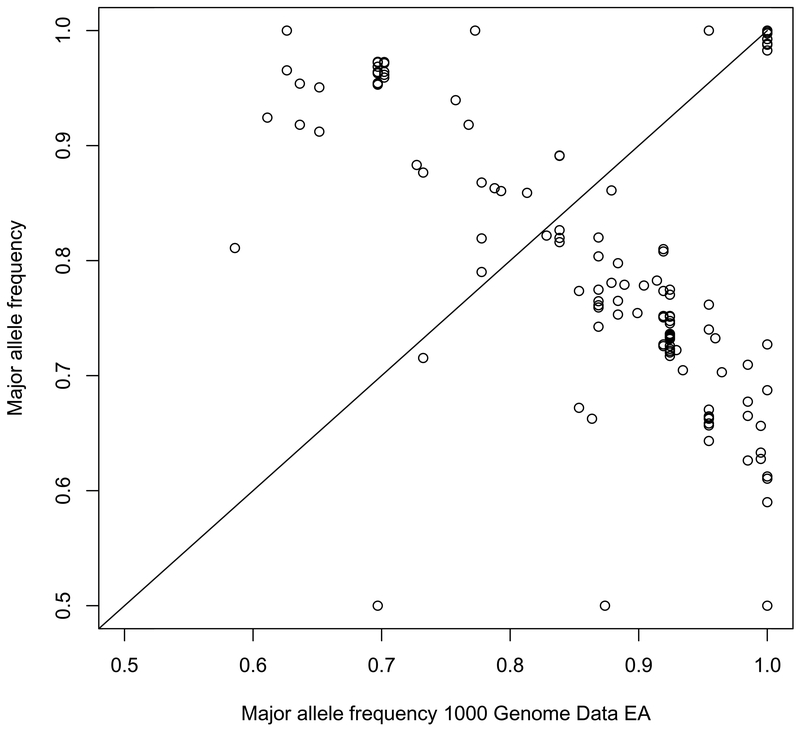
Results were authenticated and confirmed that the minor allele frequencies of AIMs in this sample set were similar to what has been reported for the AAs in the 1000 Genomes project (Spearman’s rho, 0.7997) and differ significantly from the frequencies reported for Whites in the 1000 Genomes project (Spearman’s rho, −0.3210) (Figures 3 and 4).
Figure 3.
Minor Allele Frequency: CAP3 vs. 1000Genomes African Population
Figure 4.
Minor Allele Frequency: CAP3 vs. 1000Genomes European Population
Two-way hierarchical clustering identified 3 distinct ethnic groups (y-axis) based on MAF of AIMs located (± 1MB) in or around DNA repair genes (Figure 5). The cluster analysis grouped AAs into one cluster and participants born in Africa and the Caribbean into a second cluster. Our analysis also revealed that the second cluster had two distinct groups which represented those born in Africa and the Caribbean, confirming overall, that MAFs of AIMs in or around DNA repair genes differed across the 3 ethnic subgroups.
Figure 5.
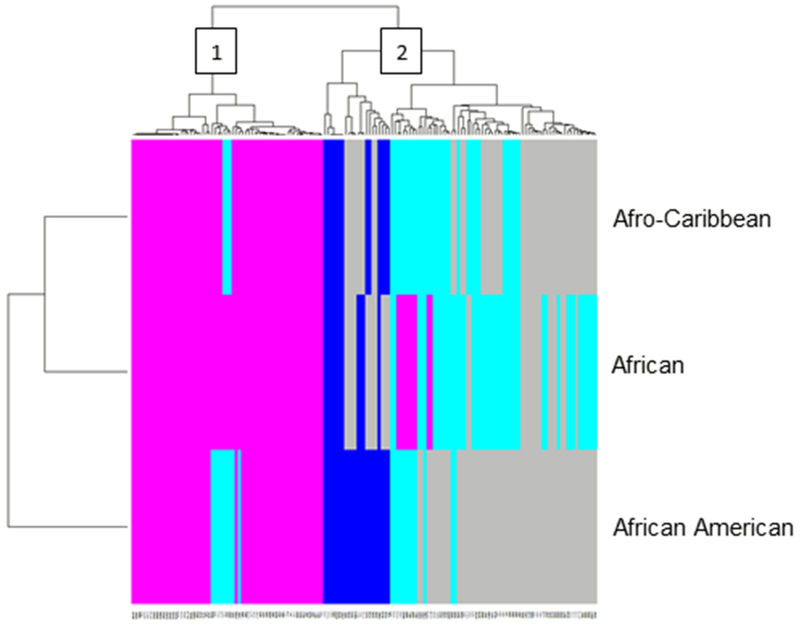
Two-way hierarchical clustering of minor allele frequencies for AIMs in the DNA repair pathway across three different populations of African descent.
There was also distinct clustering among these AIMs. We identified 2 clusters where one group of AIMs had MAFs that were more homogenous among the three ethnic groups (cluster 1), and another group of AIMs had MAFs that were more heterogeneous among the three ethnic groups (cluster 2).
Discussion
During our feasibility phase, CAP3 has successfully recruited over 700 individuals of African descent. We will continue to target this population in other cities in the northeast region of the US, displaying that the recruitment of Blacks for research studies is possible and that there is heterogeneity in this population.
Most studies of Blacks have assumed homogeneity within this group; this analysis identified genetic differences that distinctly identified three ethnic subgroups within race. Cluster analysis identified 3 distinct ethnic groups based on MAF among AIMs in the DNA repair pathway. The independent clustering of the 3 ethnic subgroups, suggests that there may be differences in the way each group responds to agents that cause DNA damage, which in turn effects cancer development and outcomes. However, future studies need to be done to examine and confirm this hypothesis.
We also provided preliminary data illustrating that blacks, within this cohort, were, predominantly, poor metabolizers of tobacco. It was observed that smokers in this cohort were either intermediate or poor metabolizers of tobacco, which is consistent with the existing literature (S. L. Park et al. 2015). Park et al, provide NNAL glucuronidation ratios and NNAL levels, which allowed for the subsequent calculation of NNAL metabolizer phenotypes. Based on their data, AAs were also more likely to be poor metabolizers of NNAL. This finding supplements the current literature on NNAL metabolism and evidence as to why AAs may have a higher risk of developing lung cancer.
The majority of current smokers in this cohort were US-born Blacks. Further examination of factors contributing to this difference in smoking behavior between subgroups might provide new insight for tailoring effective smoking cessation interventions.
The CAP3 cohort allowed us to describe the health of the Black population within Philadelphia; however, there is still much work to be done. As mentioned in our methods, we have collected urine and buccal specimens to conduct genetic and other biomarker laboratory analysis. We can conduct multi-level analyses examining the multiple contributions of behavior and biology. Future analyses of the built environment data already collected from this cohort may also provide further multi-level analyses within the Black community to identify factors that impact health.
Although recruitment for CAP3 in this first phase has been a success, there are quite a few limitations that must be discussed. First, our findings may not be generalizable to the general population. The intention of the research team was to oversample for participants of African ancestry, however the findings that we report are unique to this study population and cannot be compared to national data because national statistics by the ethnic sub-groups described in this report are limited (Ford, Narayan, and Mehta 2016; Jing Fang, Ayala, and Loustalot 2012; Mehta et al. 2015). It must also be noted that while we aimed to capture a broad range of population-based controls, our methodology for recruitment was somewhat limited because we utilized health fairs and hospital clinics to recruit most of our study participants. By using this methodology, we may have introduced Berksonian bias to the study as these individuals recruited from health fairs may be more likely to be aware of their health status and participate in this type of research. To reduce this potential bias, moving forward, we intend to use sampling methods similar to NHANES and BRFSS, i.e. random digit dialing, to expand our cohort demographic.
The CAP3 questionnaire is extensive and administered by trained research personnel to ensure consistency across administration. This methodology on survey administration has allowed us to have high-response rates for the majority of the data collected. In spite of the sensitivity of some our questions, our rates of completeness for the majority of survey items is high (Table 1). For sensitive questions, such as household income, which had the highest percent of missing data, we will conduct focus-groups to develop a supplemental questionnaire of ‘sensitive’ items that will be self-administered. We hope that this will put participants at ease and reduce item non-response within this study. Currently, the survey instrument is only offered to English-speakers, which may eliminate a subset of our target population that only speak French, Spanish, Creole or native African languages. We are currently working with our network of CBOs affiliated with our populations of interest and encounter individuals that speak the previously mentioned languages, to translate the survey instrument. We will train staff at the CBO in survey administration and confirm that they can administer the questionnaire in the appropriate language. The ultimate goal is to be able to recruit all individuals of African ancestry without language limitations.
Lastly, data collected for the cohort, at the time of analysis, was cross-sectional in nature and study participants had not yet been re-contacted for follow-up to assess the development of disease. As previously mentioned, study participants did not knowingly have cancer at the time of enrollment, which could be due to the lack of screening. For participants that have received screening, we request permission to receive recent test results. Other than this subset, there is no way for research staff to know if participants have cancer or are in a state of ‘good’ health at the time of enrollment. We hope to mimic current practices of the SCCS and collect social security information, as well as detailed contact information for study participants, to follow them over time. Social security numbers will allow us to, at the very minimum, retrieve death information from the national death index if participants are lost to follow-up or if participants did not consent to be re-contacted.
In summary, the establishment of CAP3 was an ambitious undertaking with limited funding, seeking to increase the enrollment of a heterogeneous group of Blacks in research studies. Although we do not have the numbers as large as other studies (Signorello et al. 2005) we have uniquely oversampled for underrepresented immigrant populations. With the strong community partnership network that we have established, we will continue to expand and increase recruitment of these populations in other urban and rural settings.
Acknowledgements
The authors extend gratitude to Jasmine Campbell, Aliyah Wakil, Helina Tadesse, Shasnettay Warner, Barbara Jerome, Bukola Sowunmi, Monisola Malomo, George Koshy, Danielle Stephenson, Ami Rao, Isha Rao and Getachew Temesgen for assisting with data collection, and data entry. Thank you to the Blair Lab and Clementina Mesaros, PhD at the University of Pennsylvania, who shared valuable experiences regarding NNAL extraction of our urine samples.
Funding statement: This work was supported in part by grants RSG-14-033-01-CPPB from the American Cancer Society and CA006927 from the National Cancer Institute, an appropriation from the Commonwealth of Pennsylvania as well as institutional support from the Cancer Prevention Program at Fox Chase Cancer Center.
Footnotes
Disclosure statement: The authors declare no conflict of interest.
References
- Arteaga Carlos L, Adamson Peter C, Engelman Jeffrey A, Foti Margaret, Gaynor Richard B, Hilsenbeck Susan G, Limburg Paul J, et al. 2014. “AACR Cancer Progress Report 2014.” Clinical Cancer Research : An Official Journal of the American Association for Cancer Research 20 (19 Suppl). NIH Public Access:S1–112. 10.1158/1078-0432.CCR-14-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley David L, O’Connor Richard J, Bernert John T, Watson Clifford H, Polzin Gregory M, Jain Ram B, Hammond David, et al. 2010. “Effect of Differing Levels of Tobacco-Specific Nitrosamines in Cigarette Smoke on the Levels of Biomarkers in Smokers.” Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 19 (6):1389–98. 10.1158/1055-9965.EPI-10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger CF, Hennekens CH, Rosner B, and Speizer FE. 1978. “The Nurses’ Health Study.” The American Journal of Nursing 78 (6):1039–40. [PubMed] [Google Scholar]
- Bhat Showket H, Gelhaus Stacy L, Mesaros Clementina, Vachani Anil, and Blair Ian A. 2011. “A New Liquid Chromatography/Mass Spectrometry Method for 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) in Urine.” Rapid Communications in Mass Spectrometry : RCM 25 (1). NIH Public Access:115–21. 10.1002/rcm.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal R 2004. “Glossary of Terms Relating to Ethnicity and Race: For Reflection and Debate.” Journal of Epidemiology and Community Health 58 (6). BMJ Publishing Group Ltd:441–45. 10.1136/JECH.2003.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P 1997. “Cancer, Cigarette Smoking and Premature Death in Europe: A Review Including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996.” Lung Cancer (Amsterdam, Netherlands) 17 (1):1–60. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2011a. “2011 Behavioral Risk Factor Surveillance System Questionnaire.” 2011. https://www.cdc.gov/brfss/questionnaires/pdf-ques/2011brfss.pdf.
- Centers for Disease Control and Prevention. 2011b. “Adult Smoking in the US.” CDC Vital Signs . 2011. https://www.cdc.gov/vitalsigns/adultsmoking/index.html. [Google Scholar]
- Centers for Disease Control and Prevention. 2016. “Biomonitoring Summaries - NNAL.” 2016. https://www.cdc.gov/biomonitoring/NNAL_BiomonitoringSummary.html.
- Chen Fang, Chen Gary K, Stram Daniel O, Millikan Robert C, Ambrosone Christine B, John Esther M, Bernstein Leslie, et al. 2013. “A Genome-Wide Association Study of Breast Cancer in Women of African Ancestry.” Human Genetics 132 (1). NIH Public Access:39–48. 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church Timothy R, Anderson Kristin E, Caporaso Neil E, Geisser Mindy S, Le Chap T, Zhang Yan, Benoit Adam R, Carmella Steven G, and Hecht Stephen S. 2009. “A Prospectively Measured Serum Biomarker for a Tobacco-Specific Carcinogen and Lung Cancer in Smokers.” Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 18 (1). NIH Public Access:260–66. 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobran Ewan K., Wutoh Anthony K., Lee Euni, Odedina Folakemi T., Ragin Camille, Aiken William, and Godley Paul A.. 2014. “Perceptions of Prostate Cancer Fatalism and Screening Behavior between United States-Born and Caribbean-Born Black Males.” Journal of Immigrant and Minority Health 16 (3):394–400. 10.1007/s10903-013-9825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consedine Nathan S, Tuck Natalie L, Ragin Camille R, and Spencer Benjamin A. 2015. “Beyond the Black Box: A Systematic Review of Breast, Prostate, Colorectal, and Cervical Screening among Native and Immigrant African-Descent Caribbean Populations.” Journal of Immigrant and Minority Health 17 (3):905–24. 10.1007/s10903-014-9991-0. [DOI] [PubMed] [Google Scholar]
- Conti David V, Wang Kan, Sheng Xin, Bensen Jeannette T, Hazelett Dennis J, Cook Michael B, Ingles Sue A, et al. 2017. “Two Novel Susceptibility Loci for Prostate Cancer in Men of African Ancestry.” JNCI: Journal of the National Cancer Institute 109 (8). 10.1093/jnci/djx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creque Ashley, Taioli Emanuela, Attong-Rogers Alison, and Ragin Camille. 2010. “Disparities in Uterine Cancer Survival in a Brooklyn Cohort of Black Women.” Future Oncology (London, England) 6 (2):319–27. 10.2217/fon.09.169. [DOI] [PubMed] [Google Scholar]
- Cresanta JL. 1992. “Epidemiology of Cancer in the United States.” Primary Care 19 (3):419–41. [PubMed] [Google Scholar]
- DeSantis Carol E., Siegel Rebecca L., Sauer Ann Goding Miller Kimberly D., Fedewa Stacey A., Alcaraz Kassandra I., and Jemal Ahmedin. 2016. “Cancer Statistics for African Americans, 2016: Progress and Opportunities in Reducing Racial Disparities.” CA: A Cancer Journal for Clinicians 66 (4):290–308. 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- DeSantis Carol, Siegel MPH Rebecca, Jemal MPH Ahmedin, Alcaraz Kassandra, Bertaut Tracie, Fedewa Stacey, Gansler Ted, et al. 2016. “Cancer Facts & Figures for African Americans 2016-2018.”
- Doll R, and Hill AB. 1954. “The Mortality of Doctors in Relation to Their Smoking Habits; a Preliminary Report.” British Medical Journal 1 (4877). British Medical Journal Publishing Group:1451–55. 10.1136/BMJ.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Madhavan S, and Alderman MH. 1996. “The Association between Birthplace and Mortality from Cardiovascular Causes among Black and White Residents of New York City.” The New England Journal of Medicine 335 (21):1545–51. 10.1056/NEJM199611213352101. [DOI] [PubMed] [Google Scholar]
- Fang J, Madhavan S, and Alderman MH. 1997. “Influence of Nativity on Cancer Mortality among Black New Yorkers.” Cancer 80 (1):129–35. [DOI] [PubMed] [Google Scholar]
- Fang Jing, Ayala Carma, and Loustalot Fleetwood. 2012. “Association of Birthplace and Self-Reported Hypertension by Racial/Ethnic Groups among US Adults – National Health Interview Survey, 2006–2010.” Journal of Hypertension 30 (12):2285–92. 10.1097/HJH.0b013e3283599b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Pedro, Salie Muneeb, du Toit Danielle, and van der Merwe Andre. 2015. “Analysis of Prostate Cancer Susceptibility Variants in South African Men: Replicating Associations on Chromosomes 8q24 and 10q11.” Prostate Cancer 2015:1–6. 10.1155/2015/465184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford Nicole D., Narayan K.M. Venkat, and Mehta Neil K.. 2016. “Diabetes among US- and Foreign-Born Blacks in the USA.” Ethnicity & Health 21 (1):71–84. 10.1080/13557858.2015.1010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel Lawrence;, and Heath Clark W. Jr. 1982. “Cancer Prevention Study II. The American Cancer Society Prospective Study.” Stat Bull Metrop Insur Co. 73 (4):21–29. [PubMed] [Google Scholar]
- Grieco Elizabeth M., Acosta Yesenia D., Patricia de la Cruz G, Gambino Christine, Gryn Thomas, Larsen Luke J., Trevelyan Edward N., And, and Walters Nathan P.. 2012. “American Community Survey Reports The Foreign-Born Population in the United States: 2010.”
- Hammond EC. 1954. “Epidemiologic Studies on Smoking in Relation to Lung Cancer.” Pennsylvania Medical Journal (1928) 57 (11):1084–87. [PubMed] [Google Scholar]
- Hammond EC, and Horn D. 1954. “The Relationship between Human Smoking Habits and Death Rates: A Follow-up Study of 187,766 Men.” Journal of the American Medical Association 155 (15):1316–28. [DOI] [PubMed] [Google Scholar]
- Humes Karen R, Jones Nicholas A, and Ramirez Roberto R. 2010. “Overview of Race and Hispanic Origin: 2010 2010 Census Briefs.”
- Huo Dezheng, Feng Ye, Haddad Stephen, Zheng Yonglan, Yao Song, Han Yoo-Jeong, Ogundiran Temidayo O., et al. 2016. “Genome-Wide Association Studies in Women of African Ancestry Identified 3q26.21 as a Novel Susceptibility Locus for Oestrogen Receptor Negative Breast Cancer.” Human Molecular Genetics 25 (21):ddw305. 10.1093/hmg/ddw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Dezheng, Zheng Yonglan, Ogundiran Temidayo O., Adebamowo Clement, Nathanson Katherine L., Domchek Susan M., Rebbeck Timothy R., et al. 2012. “Evaluation of 19 Susceptibility Loci of Breast Cancer in Women of African Ancestry.” Carcinogenesis 33 (4):835–40. 10.1093/carcin/bgs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry-Ramírez Margarita, Kittles Rick A., Wang Xuemei, Salgado-Montilla Jeannette, Nogueras-González Graciela M., Sánchez-Ortiz Ricardo, Guerrios Lourdes, et al. 2017. “Genetic Ancestry and Prostate Cancer Susceptibility SNPs in Puerto Rican and African American Men.” The Prostate 77 (10):1118–27. 10.1002/pros.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Parker EA, Eng E, and Schulz AJ, eds. 2013. Methods in Community-Based Participatory Research for Health. 2nd ed. San Franciso, CA: Jossey-Bass. [Google Scholar]
- Israel Barbara A., Coombe Chris M., Cheezum Rebecca R., Schulz Amy J., McGranaghan Robert J., Lichtenstein Richard, Reyes Angela G., Clement Jaye, and Burris Akosua. 2010. “Community-Based Participatory Research: A Capacity-Building Approach for Policy Advocacy Aimed at Eliminating Health Disparities.” American Journal of Public Health 100 (11):2094–2102. 10.2105/AJPH.2009.170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Barbara A, Schulz Amy J, Parker Edith A, and Becker Adam B. 1998. “Review of Community-Based Research: Assessing Partnership Approaches to Improve Public Health.” Annu. Rev. Public Health 19:173–202. [DOI] [PubMed] [Google Scholar]
- Jamal Ahmed, Phillips Elyse, Gentzke Andrea S., Homa David M., Babb Stephen D., King Brian A., and Neff Linda J.. 2018. “Current Cigarette Smoking Among Adults — United States, 2016.” MMWR. Morbidity and Mortality Weekly Report 67 (2):53–59. 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley Mark R, Logsdon Derek, and Fishel Melissa L. 2014. “Targeting DNA Repair Pathways for Cancer Treatment: What’s New?” Future Oncology (London, England) 10 (7). NIH Public Access:1215–37. 10.2217/fon.14.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, and Nagamine FS. 2000. “A Multiethnic Cohort in Hawaii and Los Angeles: Baseline Characteristics.” American Journal of Epidemiology 151 (4):346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linda J, Vorvic; David Zieve; Ogilvie Isla. 2016. “Clean Catch Urine Sample.” MedlinePlus Medical Encyclopedia. 2016. https://medlineplus.gov/ency/article/007487.htm. [Google Scholar]
- Mehta Neil K., Elo Irma T., Ford Nicole D., and Siegel Karen R.. 2015. “Obesity among U.S.- and Foreign-Born Blacks by Region of Birth.” American Journal of Preventive Medicine 49 (2):269–73. 10.1016/j.amepre.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller Franz Hermann. 1940. “Tabakmißbrauch Und Lungencarcinom.” Zeitschrift Für Krebsforschung 49 (1). Springer-Verlag:57–85. 10.1007/BF01633114. [DOI] [Google Scholar]
- Murphy Adam B., Ukoli Flora, Freeman Vincent, Bennett Frankly, Aiken William, Tulloch Trevor, Coard Kathleen, Angwafo Fru, and Kittles Rick A.. 2012. “8q24 Risk Alleles in West African and Caribbean Men.” The Prostate 72 (12):1366–73. 10.1002/pros.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat Joshua E., Djordjevic Mirjana V., Colosimo Stephen, Stellman Steven D., and Richie John P.. 2005. “Racial Differences in Exposure and Glucuronidation of the Tobacco-Specific Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone (NNK).” Cancer 103 (7):1420–26. 10.1002/cncr.20953. [DOI] [PubMed] [Google Scholar]
- Mutetwa Batsirai, Taioli Emanuela, Attong-Rogers Alison Layne Penelope, Roach Veronica, and Ragin Camille. 2010. “Prostate Cancer Characteristics and Survival in Males of African Ancestry According to Place of Birth: Data from Brooklyn-New York, Guyana, Tobago and Trinidad.” The Prostate 70 (10):1102–9. 10.1002/pros.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. 1999. “National Health and Nutrition Examination Survey 1999–2016 Survey Content Brochure.”
- National Center for Health Statistics. 2016. “National Health Interview Survey.” Public-Use Data File and Documentation. 2016. http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- Oglesby Paul. 1976. “The Multiple Risk Factor Intervention Trial (MRFIT).” JAMA 235 (8). American Medical Association:825. 10.1001/jama.1976.03260340031016. [DOI] [PubMed] [Google Scholar]
- Park SL, Carmella SG, Ming X, Vielguth E, Stram DO, Le Marchand L, and Hecht SS. 2015. “Variation in Levels of the Lung Carcinogen NNAL and Its Glucuronides in the Urine of Cigarette Smokers from Five Ethnic Groups with Differing Risks for Lung Cancer.” Cancer Epidemiology Biomarkers & Prevention 24 (3):561–69. 10.1158/1055-9965.EPI-14-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Sohee, Jee Sun Ha Shin Hai-Rim, Park Eun Hye, Shin Aesun, Jung Kyu-Won, Hwang Seung-Sik, et al. 2014. “Attributable Fraction of Tobacco Smoking on Cancer Using Population-Based Nationwide Cancer Incidence and Mortality Data in Korea.” BMC Cancer 14 (1):406. 10.1186/1471-2407-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro Paulo S, Callahan Karen E, Ragin Camille, Hage Robert W, Hylton Tara, and Kobetz Erin N. 2016. “Black Heterogeneity in Cancer Mortality: US-Blacks, Haitians, and Jamaicans.” Cancer Control : Journal of the Moffitt Cancer Center 23 (4):347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier AE, Grundy A, Khandwala F, Tamminen S, Friedenreich CM, and Brenner DR. 2016. “Cancer Incidence Attributable to Tobacco in Alberta, Canada, in 2012.” CMAJ Open 4 (4):E578–87. 10.9778/cmajo.20150069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiagen. 2014. Gentra® Puregene® Handbook. 4th ed. [Google Scholar]
- “Quick Facts: Philadelphia County, Pennsylvania.” 2015. US Census Bureau. 2015. http://www.census.gov/quickfacts/table/RHI225215/42101,00#headnote-js-a. [Google Scholar]
- Ragin Camille C, Langevin Scott, Rubin Scott, and Taioli Emanuela. 2010. “Review of Studies on Metabolic Genes and Cancer in Populations of African Descent.” Genetics in Medicine 12 (1):12–18. 10.1097/GIM.0b013e3181c8e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin Camille, Mutetwa Batsirai, Attong-Rogers Alison, Roach Veronica, and Taioli Emanuela. 2011. “Geographic and Outcome Variation among Black Men Diagnosed with Prostate Cancer.” Infectious Agents and Cancer 6 Suppl 2 (Suppl 2):S2. 10.1186/1750-9378-6-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP, Carmella SG, Muscat JE, Scott DG, Akerkar SA, and Hecht SS. 1997. “Differences in the Urinary Metabolites of the Tobacco-Specific Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone in Black and White Smokers.” Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 6 (10):783–90. [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, and Stampfer MJ. 1991. “Prospective Study of Alcohol Consumption and Risk of Coronary Disease in Men.” Lancet (London, England) 338 (8765):464–68. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Adams-Campbell L, and Palmer JR. 1972. “The Black Women’s Health Study: A Follow-up Study for Causes and Preventions of Illness.” Journal of the American Medical Women’s Association (1972) 50 (2):56–58. [PubMed] [Google Scholar]
- Rosenberg Noah A., Li Lei M., Ward Ryk, and Pritchard Jonathan K.. 2003. “Informativeness of Genetic Markers for Inference of Ancestry.” The American Journal of Human Genetics 73 (6):1402–22. 10.1086/380416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar Mohamadi, Wang Jingzhu, and Liang Qiwei. 2012. “Metabolism of Nicotine and 4-(Methylnitrosamino)-l-(3-Pyridyl)-Lbutanone (NNK) in Menthol and Non-Menthol Cigarette Smokers.” Drug Metabolism Letters 6 (3):198–206. [DOI] [PubMed] [Google Scholar]
- Sasco AJ, Secretan MB, and Straif K. 2004. “Tobacco Smoking and Cancer: A Brief Review of Recent Epidemiological Evidence.” Lung Cancer 45 (August):S3–9. 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- Sawyers CL, Abate-Shen C, Anderson KC, Barker A, Baselga J, Berger NA, Foti M, et al. 2013. “AACR Cancer Progress Report 2013.” Clinical Cancer Research 19 (20_Supplement):S1–98. 10.1158/1078-0432.CCR-13-2107. [DOI] [PubMed] [Google Scholar]
- Schairer E, and Schöniger E. 1944. “Lungenkrebs Und Tabakverbrauch.” Zeitschrift Für Krebsforschung 54 (4). Springer-Verlag:261–69. 10.1007/BF01628727. [DOI] [Google Scholar]
- Sempos CT, Bild DE, and Manolio TA. 1999. “Overview of the Jackson Heart Study: A Study of Cardiovascular Diseases in African American Men and Women.” The American Journal of the Medical Sciences 317 (3):142–46. [DOI] [PubMed] [Google Scholar]
- Signorello Lisa B, Hargreaves Margaret K, Steinwandel Mark D, Zheng Wei, Cai Qiuyin, Schlundt David G, Buchowski Maciej S, Arnold Carolyne W, McLaughlin Joseph K, and Blot William J. 2005. “Southern Community Cohort Study: Establishing a Cohort to Investigate Health Disparities.” Journal of the National Medical Association 97 (7). National Medical Association:972–79. [PMC free article] [PubMed] [Google Scholar]
- Taioli Emanuela, Sears Vestra, Watson Alexis, Flores-Obando Rafael E., Jackson Maria D., Ukoli Flora A., de Syllos Cólus Ilce M., et al. 2013. “Polymorphisms in CYP17 and CYP3A4 and Prostate Cancer in Men of African Descent.” The Prostate 73 (6):668–76. 10.1002/pros.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubell Earl. 1954. “Cigarette-Lung Cancer Link Proved, Dr. Hammond Says.” New York Herald Tribune, October 20, 1954. [Google Scholar]
- Wang Shengfeng, Huo Dezheng, Ogundiran Temidayo O, Ojengbede Oladosu, Zheng Wei, Nathanson Katherine L, Nemesure Barbara, Ambs Stefan, Olopade Olufunmilayo I, and Zheng Yonglan. 2017. “Association of Breast Cancer Risk and the MTOR Pathway in Women of African Ancestry in ‘The Root’ Consortium.” Carcinogenesis 38 (8):789–96. 10.1093/carcin/bgx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson William L., and Conte Alexander J.. 1954. “Smoking and Lung Cancer.” Cancer 7 (2). Wiley Subscription Services, Inc., A Wiley Company:245–49. . [DOI] [PubMed] [Google Scholar]
- Watson WL, and Cokte AJ. 1955. “Lung Cancer and Smoking.” The American Journal of Surgery 89 (2):447–56. [DOI] [PubMed] [Google Scholar]
- Wynder EL. 1954. “Tobacco as a Cause of Lung Cancer, with Special Reference to the Infrequency of Lung Cancer among Non-Smokers.” Pennsylvania Medical Journal (1928) 57 (11):1073–83. [PubMed] [Google Scholar]
- Yuan Jian-Min, Butler Lesley M, Stepanov Irina, and Hecht Stephen S. 2014. “Urinary Tobacco Smoke-Constituent Biomarkers for Assessing Risk of Lung Cancer.” Cancer Research 74 (2). NIH Public Access:401–11. 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Jian-Min, Koh Woon-Puay, Murphy Sharon E, Fan Yunhua, Wang Renwei, Carmella Steven G, Han Shaomei, et al. 2009. “Urinary Levels of Tobacco-Specific Nitrosamine Metabolites in Relation to Lung Cancer Development in Two Prospective Cohorts of Cigarette Smokers.” Cancer Research 69 (7). NIH Public Access:2990–95. 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SM, Willett WC, Hernán MA, Olek MJ, and Ascherio A. 2000. “Dietary Fat in Relation to Risk of Multiple Sclerosis among Two Large Cohorts of Women.” American Journal of Epidemiology 152 (11):1056–64. [DOI] [PubMed] [Google Scholar]