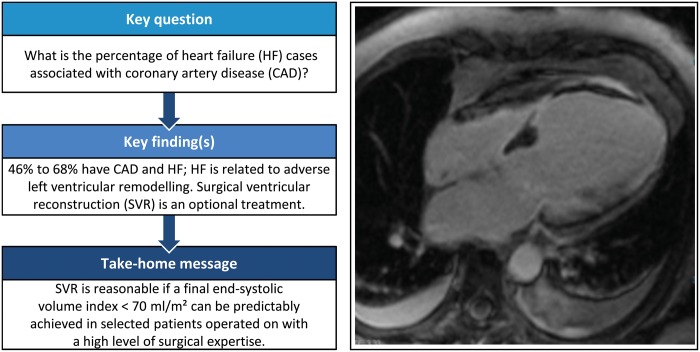
Summary
An increase in left ventricular volume after a myocardial infarction is a key component of the adverse remodelling process leading to chamber dysfunction, heart failure and an unfavourable outcome. Hence, the therapeutic strategies have been designed to reverse the remodelling process by medical therapy, devices or surgical strategies. Surgical ventricular reconstruction primarily combined with myocardial revascularization has been introduced as an optional intervention aimed to reduce the left ventricle through resection of the scar tissue and is recommended in selected patients with predominant heart failure symptoms, and with myocardial scarring and moderate left ventricular remodelling. This review outlines the rationale and the technique for reconstructing the left ventricle and the possible indications for using that technique, based on experiences from the centre with the largest international experience. The major contributions in the literature are briefly discussed.
Keywords: Left ventricular remodelling, Surgical ventricular reconstruction, Heart failure
INTRODUCTION
Despite advances in diagnosis and treatment, heart failure (HF) remains a substantial cause of death and disability [1, 2], driven mainly by the causal role of coronary artery disease (CAD) in the development of left ventricular (LV) dysfunction [3, 4]. The improved survival after myocardial infarction (MI) has led to an increase in the percentage of people at risk of developing HF: 22% of men and 46% of women develop HF within 6 years after acute MI. HF, in turn, is related to adverse remodelling, defined as an increase in LV volume and changes in geometry, standing therefore as the main target of the possible therapeutic interventions [5]. To this end, surgical treatments include myocardial revascularization, mitral valve repair or replacement and surgical ventricular reconstruction (SVR) combined depending on the specific conditions [6].
SVR is considered a possible therapeutic strategy designed to reduce the left ventricle through resection of the scar tissue. It is recommended in selected patients with HF in centres with expertise.
POSTINFARCT LEFT VENTRICULAR ADVERSE REMODELLING AND THE RATIONALE TO REVERSE IT
LV adverse remodelling refers to an alteration in the ventricular architecture as a response to a myocardial injury, and it is associated with increased mass and volume and altered geometry affecting cardiac function [7, 8]. LV adverse remodelling involves both the infarcted and non-infarcted myocardium as a consequence of increased wall stress [9], and, on a histological level, is driven by a combination of myocyte hypertrophy and apoptosis and differentiation of pre-existing local fibroblasts in myofibroblasts that, when activated, serve as the primary collagen-producing cells promoting fibrosis within the scar (i.e. replacement fibrosis) [10]. The subsequent evolution is related to the balance between extracellular matrix synthesis and degradation, meaning that poor extracellular matrix maintenance is responsible for scar expansion, thinning of the infarcted zone and initial dilatation in patients who are likely to develop HF [10]. In the early stage, LV dilatation might function as a compensatory mechanism in an effort to maintain stroke volume because the ejection fraction (EF) declines after loss of contractile tissue [9]. However, most often the remodelling process involves the remote, non-infarcted regions, mainly driven by eccentric hypertrophy, which in turn is responsible for increased mass, further chamber enlargement and geometric distortion [10] (Fig. 1). The decline in global ventricular function is related to a reduced performance of the hypertrophied myocytes, along with increased neurohormonal activation, extracellular matrix remodelling and collagen synthesis within the non-infarcted zone that may foster further LV dilatation and concomitant onset of signs and symptoms of congestive HF [7–10].
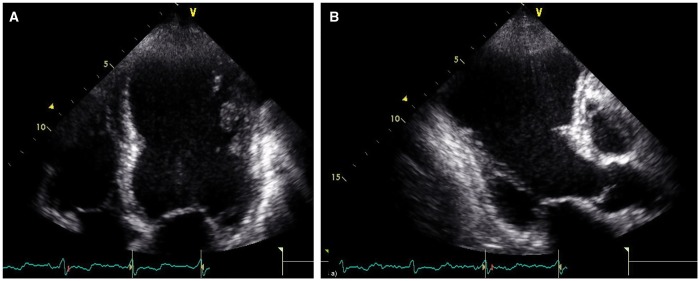
Figure 1:
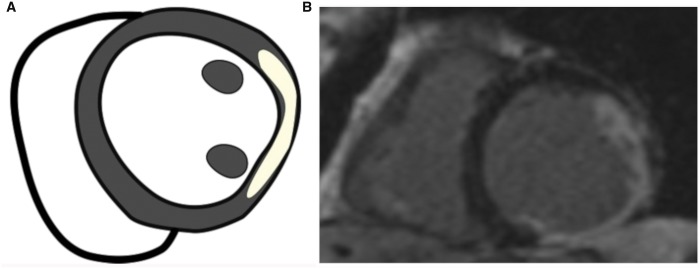
Anterior left ventricular remodelling following a previous anterior myocardial infarction in echocardiographic apical 4-chamber (A) and 3-chamber views (B) showing extensive involvement of the anterior septum, the posterior septum and the apex.
The goal of ventricular reconstruction is to resect the scar tissue, with concomitant reduction of the ventricle to a more physiological volume and to an elliptical shape. The reduction in chamber radius decreases myocardial systolic and diastolic wall stresses according to Laplace’s law and has the potential to induce reverse remodelling, which in turn enhances cardiac function [11].
When indicated, coronary artery bypass grafting (CABG) combined with SVR is used to treat the underlying CAD. It should be noted that the regions far from the scar tissue, the above-mentioned remote regions, may be ischaemic but viable, depending on the extent of the CAD. In this case, a myocardial revascularization that is as complete as possible has the potential to promote functional recovery.
Finally, secondary mitral regurgitation (MR) may occur in the setting of postinfarct LV adverse remodelling as a result of chamber dilatation and distortion (Fig. 2), both of which promote displacement of the papillary muscles with leaflet tethering and annular dilatation, depending on the site of the remodelling (anterior versus posterior) [12]. SVR either allows the surgeon to repair the mitral valve at the time of the LV opening or the procedure per se has the potential of improving valve competence by reducing LV volumes and papillary muscle distance or of decreasing internal diameters (especially in case of posterior remodelling) [13, 14].
Figure 2:
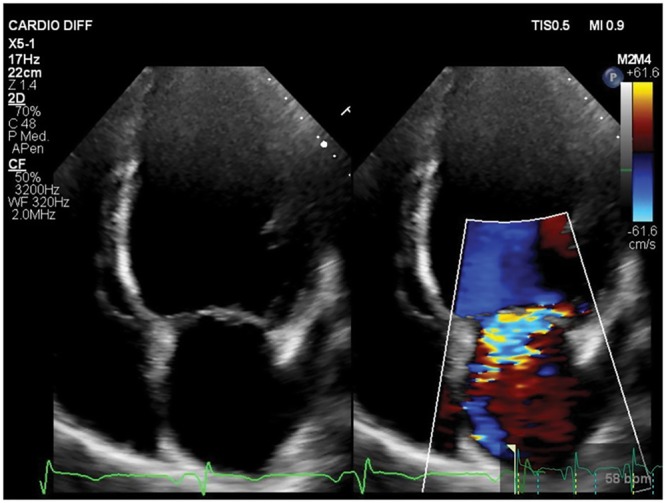
Ischaemic mitral regurgitation. Apical 4-chamber view showing ischaemic mitral regurgitation. The left ventricular internal diameter is increased, and the shape is spherical due to the presence of mitral regurgitation.
SURGICAL VENTRICULAR RECONSTRUCTION TECHNIQUE
The surgical procedure has been described in detail in Ref. [15]. The operation is performed under total cardiac arrest with antegrade crystalloid cardioplegia. First, coronary revascularization is performed with both internal mammary artery and sequential venous grafts, when indicated [15].
The surgical technique for the reconstruction of the ventricle has been refined over more than 20 years of experience and today the approach is tailored mainly according to the site of the remodelling and the occurrence of MR.
The technique for anterior remodelling
The ventricle is opened at the middle of the scar on the anterior wall, with an incision parallel to the left anterior descending artery, starting from the mid portion and proceeding towards the apex. Thrombi are removed if present. After a careful identification of the transitional zone between scarred and non-scarred tissue, a mannequin (TRISVRTM, Chase Medical, Richardson, TX, USA) is inserted into the LV chamber and filled with saline. The mannequin was introduced in 2001 to make the surgical procedure as standardized as possible. The mannequin is available in different sizes ranging from 80 ml to 150 ml. The selection of the appropriate size depends on the body surface area of the patient and on the degree of dilatation at the base of the ventricle. To this purpose, the internal LV diameter measured above the papillary muscles and below the mitral valve at end-diastole must be previously indicated to the surgeon. The mannequin has a conical shape: the base is positioned below the mitral annulus and the apex, towards the portion of the cavity that should be excluded. The mannequin is useful as a shaper to reconstruct the apex and as a sizer to preserve the long axis of the ventricle to between 7.5 cm and 8.5 cm. Indeed, the excessive shortening of the longitudinal axis can cause sphericalization of the residual cavity, which in turn is associated with a worsening of diastolic function and recurrence or new occurrence of MR at follow-up. If the remodelling process also involves the inferior wall, a plication of the distal portion is performed to optimize the shape and the position of the new apex. The exclusion of scar tissue is performed through an endoventricular circular suture passed in the transitional zone. The final shape of the ventricle is expected to be elliptical (Fig. 3). The mannequin is deflated and removed before the ventricle is closed. The opening is closed with a direct suture if it is less than 3 cm or, until recently, with a synthetic patch if it is greater than 3 cm. In the last 3 years, the patch was discontinued mainly because the pattern of LV remodelling has changed, probably as a consequence of the increasing numbers of percutaneous interventions in the acute setting of an MI: the classical dyskinetic aneurysm with a clear zone of transition between scarred and remote, non-infarcted myocardium almost disappeared along with increased evidence of more eccentric LV shapes with large akinetic regions. In the latter case, the shaper is crucial in guiding the LV reconstruction; avoiding the patch allows the surgeon to optimize the final shape.
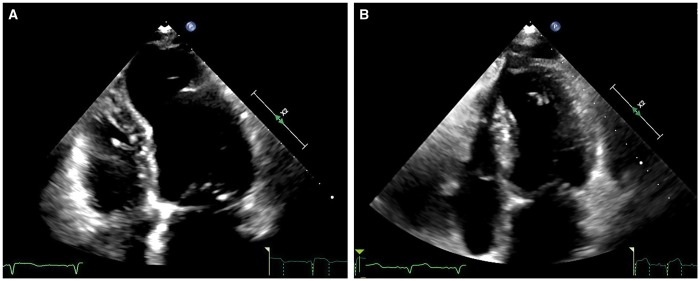
Figure 3:
Left ventricle before (A) and after (B) surgical remodelling in echocardiographic apical 4-chamber view.
In the presence of significant MR, the mitral valve is repaired through the ventricular opening with a double-armed suture running from 1 trigone to the other, embedding the 2 arms in the posterior anulus of the mitral valve. Usually, the suture is reinforced with a Teflon strip to avoid tears of the posterior leaflet of the valve. When the LV opening is not big enough to have a good exposure of the mitral valve, the surgeon may consider a restrictive mitral annuloplasty with a ring implant as described in detail in Ref. [24].
However, in presence of extensive anterior LV remodelling with an internal diameter greater than 65 mm, valve replacement is sometimes preferred to reduce the rate of recurrence of MR.
The technique for posterior remodelling
Posterior remodelling can occur either with the most common aneurysm that produces a bulging of the inferior wall or with a more global LV dilatation, which occurs as a consequence of an extended scar, usually included either between the 2 papillary muscles or between the posteromedial papillary muscle and the posterior septum (Figs 4 and 5, respectively). The classical aneurysm is excluded with a patch to close the neck of the dilatation. In presence of a more extensive posterior remodelling, the surgical approach is more complex, as we described in detail in Ref. [15].
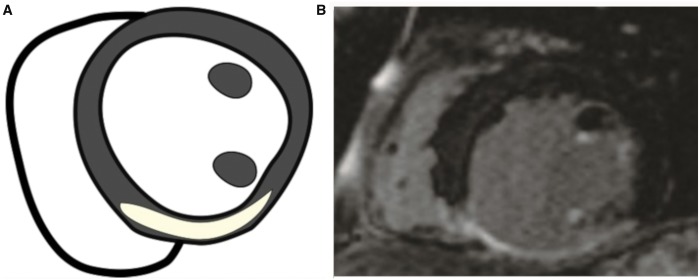
Figure 4:
Posterior remodelling. (A) Schematic; (B) at the late gadolinium enhancement-cardiac magnetic resonance (short axis), the scarred dilatation appears between the posteromedial papillary muscle and the septum.
Figure 5:
Posterior remodelling. (A) Schematic; (B) at the late gadolinium enhancement-cardiac magnetic resonance (short axis), the scarred dilatation appears mainly between the two papillary muscles.
PATIENT SELECTION: THE FIRST STEP TOWARDS THE RIGHT INDICATION
The decision to carry out the SVR stems from an accurate clinical evaluation of patients who have had a previous MI, combined with an imaging and laboratory workup designed to obtain accurate measurements of the LV geometric and haemodynamic parameters, a complete evaluation of the mitral valve apparatus, assessment of the scar tissue and of the viability of regions remote from the scar and measurements of circulating biomarkers.
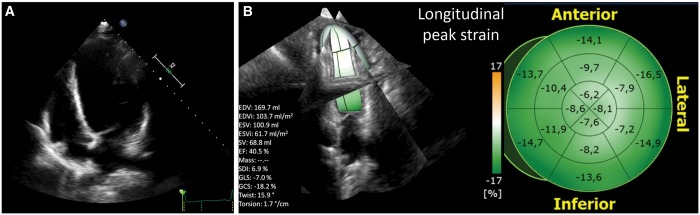
A complete echocardiographic evaluation is the first step in patient selection, providing accurate information about wall thickness, systolic and diastolic chamber dimensions and volumes and global cardiac function [16]. Deformation imaging using 2-dimensional speckle-tracking echocardiography has emerged as a powerful technique for quantifying myocardial function [17]. Whereas the EF suffers from the multicollinearity as a parameter derived from volumes, myocardial strain, assessed by STE, has been found to improve prognostic risk stratification [17]. Furthermore, in recent years, 3-dimensional speckle-tracking echocardiography has been implemented for measuring 3-dimensional strain [18] and has emerged as a more physiological tool to analyse the complexity of LV mechanics, with the potential to become the gold standard for assessing LV systolic function by echocardiography in the near future (Fig. 6). However, a major limitation of 2-dimensional speckle-tracking echocardiography and 3-dimensional speckle-tracking echocardiography is their reliance on a good acoustic window and quality data sets and on patient cooperation for breath holding, limiting its feasibility in a significant proportion of routine patients.
Figure 6:
Three-dimensional echocardiographic speckle-tracking analysis (TomTec Imaging Systems GmbH, Unterschleissheim, Germany) in a patient with previous anterior myocardial infarction, extensive remodelling (A) and a markedly impaired longitudinal deformation (B). Global 3-dimensional longitudinal strain measured by 3-dimensional speckle-tracking is −7% (lower limit of normality is −15%).
Cardiac magnetic resonance (CMR) is nowadays the gold standard imaging technique for planning the surgical procedure when not contraindicated [19]. CMR permits the assessment of LV and right ventricular structures and function and has the advantage of being able to examine ventricles of any size and shape. CMR has the additional ability to detect myocardial scar with late gadolinium enhancement (LGE) and to assess the thickness and function of the remaining non-enhanced myocardial tissue, which may be ischaemic but viable, likely for functional recovery after CABG or non-ischaemic but dysfunctional because of the increased wall stress and likely for functional improvement after volume reduction obtained through SVR [20]. Lastly, CMR has the potential to predict the lack of LV functional recovery after SVR when myocardial fibrosis is also detected in the remote regions, especially at the level of the basal segments [21]. In agreement with this observation, our group recently showed that the presence of LGE in the proximal anterior LV segments is associated with a poor response to SVR [meaning a higher postoperative LV end-systolic volume index (LVESVI)], which in turn affects survival, whereas the LGE extension is apparently not associated with the outcome [22].
Major limitations at this time include the need to exclude patients with pacemakers or devices for cardiac resynchronization therapy and the poor quality of images in patients with significant arrhythmia or severe shortness of breath.
According to our experience, the indications for SVR—one does not exclude the other—may be summarized as follows:
Previous anterior or posterior MI;
LVESVI >60 ml/m2: small ventricles have a higher likelihood for diastolic function worsening [23];
Regional LV asynergy, either dyskinetic or akinetic: in the presence of severe and diffuse LV asynergy, the procedure should be performed only if regions remote from the scar show detectable contraction, as indicated by CMR when feasible [24];
Predominant HF symptoms [New York Heart Association (NYHA) functional class III/IV] or in the presence of ventricular arrhythmias and/or angina needing surgical revascularization if the previous conditions are present [24].
Suggested contraindications
WHAT WE HAVE LEARNED FROM THE PAST
Prior to the year 2000, the largest contribution in the literature came from Dor’s group, who showed that SVR improves LV systolic function, NYHA functional class and survival by a reduction in ventricular volume and an increase in EF, not only in patients with classic dyskinetic aneurysm but also in those with dilated ischaemic cardiomyopathy and severe LV dysfunction [27]. After that, a large number of reports, resulting mainly from observational studies, have shown that SVR is effective and relatively safe, with a favourable 5-year outcome [26, 28–30].
The STICH trial was specifically designed to investigate the role of CABG combined or not with SVR in patients with HF with LV systolic dysfunction (defined as EF ≤35%) [31]. The trial did not show an additional survival benefit in the SVR group, although a significantly greater reduction in LVESVI was noted with the combined procedure. The relatively small percentage in LVESVI reduction observed in the combined group raised concerns about the extent of the SVR procedure that was used in this trial, meaning either that the selected ventricles were too small or that the volume reduction was inadequate. Our group hypothesized that the results of the STICH trial were related to a poor reduction in volume, provided that the preoperative volume had been properly selected, without changing the outcome between the 2 groups [32]. Witkowski et al. [33] confirmed the importance of a ‘target volume’ to be achieved with this surgery; they showed that a residual postsurgical LVESVI of at least 60 ml/m2 was independently associated with a favourable outcome. Later, a post hoc analysis from the STICH trial showed that a postoperative LVESVI of 70 ml/m2 or lower resulted in improved survival compared with CABG alone [34]. In agreement with these results, the just-released guidelines on myocardial revascularization (European Society of Cardiology/European Association for Cardio-Thoracic Surgery), recommend SVR at the time of CABG in selected patients operated on in centres with a high level of surgical expertise (class of recommendation IIb; level of evidence B) [35]. The STICH trial was strongly criticized [36]. In short, we believe that the main limitation of this trial relates to the heterogeneous population of patients enrolled, with moderate symptoms of either angina and HF, small volumes, without clear evidence of scar tissue (LGE/magnetic resonance imaging was not mandatory), more representative of the real world of ischaemic patients with low EF.
Surgical ventricular reconstruction and mitral valve surgery
Secondary MR may occur as part of the LV remodelling process and may adversely affect the prognosis [37, 38] (Fig. 2). As a disease of the left ventricle, chronic ischaemic MR occurs more frequently in patients with inferior MI (50–60% vs 20–25% of patients with anterior MI), and for those with postinfarct congestive HF, life expectancy is significantly reduced [38]. In our centre, we perform MV repair at the time of SVR and CABG in about 30% of patients [39]. According to our experience, in patients with previous inferior MI, MR is mainly related to a localized inferobasal LV remodelling, which in turn causes lateral displacement of the posteromedial papillary muscle, increase in the internal diameter and mitral valve posterior leaflet tethering. Conversely, in patients with previous anterior MI, MR occurs mainly in the setting of global LV dilatation and severe dysfunction, reflecting a more advanced stage of disease, with apical displacement of pupillary muscles causing tethering of both mitral valve leaflets [15]. From a retrospective analysis, we showed that preoperative severe MR was an independent predictor of late mortality in the anterior group but not in the posterior one [40]. However, regardless of the specific mechanism, we found an acceptable long-term survival for this challenging population, despite a high operative mortality rate (14.3%), still comparable with other series reported in the literature [39].
NEW INSIGHTS
N-terminal pro-B-type natriuretic peptide profile and the challenge of surgical reverse remodelling
Natriuretic peptides are increased in patients with HF and correlate well with ventricular wall stress and the severity of the HF. The concept behind SVR is that the exclusion of the infarcted scar tissue from the LV cavity and the related reduction of the volume improve cardiac function through a reduction of LV wall stress of the remote region [11, 27, 30]. To verify a possible role of natriuretic peptides in supporting the surgical procedure or in monitoring patients even after surgery, we recently investigated the role of serial measurements of N-terminal pro-B-type natriuretic peptide levels in patients with ischaemic HF undergoing SVR as reported in detail in Ref. [41]. We showed that the concentration of the biomarker significantly decreased at the postoperative follow-up examination, along with volume reduction and EF improvement [41]. Furthermore, time-varying N-terminal pro-B-type natriuretic peptide levels (either increasing or decreasing) were associated with changes in the risk of HF-related hospitalization or the occurrence of all-cause death at follow-up. In agreement with this observation, we previously showed that N-terminal pro-B-type natriuretic peptide concentration decreased significantly after surgery in patients with a more effective LV reduction, which in turn was related to the absence of scarring in the proximal anterior LV segments [22]. Future studies are needed to better understand the relationship between scar location, volume reduction, wall stress improvement, peptide modulation and their reciprocal influence on the outcome.
FUTURE DIRECTIONS
Currently, novel endovascular therapies designed to reduce LV volumes, thereby improving LV wall stress and cardiac performance, are being proposed as an option for patients with ischaemic HF who have not responded to the currently recommended treatment. Among these, the largest experience to date is with the Parachute device (CardioKinetix Inc., CA, USA), which is implanted through a transfemoral retrograde transaortic approach in the LV and is devised to exclude the akinetic scar in the previous anterior MI. The Parachute device was shown to consistently reduce LVESVI and symptoms at 1 year and to improve exercise tolerance [42, 43]. Larger series are needed to make robust conclusions.
CONCLUSIONS
SVR was strongly criticized after the results from the STICH trial that called into the question its added value in this high-risk population of patients with ischaemic HF. American surgeons are reluctant to use the procedure, which has been substantially abandoned. Indeed, several limitations have led to significant clinical uncertainty in making such results generalizable. The STICH investigators enrolled a small percentage of the eligible population (about 20%), not fully representative of daily clinical practice, sharing this limitation with most of the randomized clinical trials. Furthermore, in the STICH trial the concomitant registry designed to follow eligible patients who were not randomized was abandoned straightaway. Lastly, the final decision to randomize patients to SVR has been left to surgeons with different degrees of surgical expertise.
While waiting for further deep analysis of the STICH data, the 2018 Task Force of the European Society of Cardiology/European Association for Cardio-Thoracic Surgery still recommends SVR at the time of CABG in selected patients operated on in centres with a high level of surgical expertise [35].
Funding
This paper was published as part of a supplement supported by an educational grant from Medtronic. This work was partially supported by Ricerca Corrente funding from the Italian Ministry of Health to IRCCS Policlinico San Donato.
Conflict of interest: none declared.
REFERENCES
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 2. Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF.. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006;114:1202–13. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC. et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelfriet PM, Hoogenveen RT, Boshuizen HC, Van Baal PH.. To die with or from heart failure: a difference that counts: is heart failure underrepresented in national mortality statistics? Eur J Heart Fail 2011;13:377–83. [DOI] [PubMed] [Google Scholar]
- 5. Felker GM, Shaw LK, O'Connor CM.. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002;39:210–18. [DOI] [PubMed] [Google Scholar]
- 6. Zannad F, Agrinier N, Alla F.. Heart failure burden and therapy. Europace 2009;11 (Suppl 5):v1–9. [DOI] [PubMed] [Google Scholar]
- 7. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE.. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011;4:98–108. [DOI] [PubMed] [Google Scholar]
- 8. Cohn JN, Ferrari R, Sharpe N.. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000;35:569–82. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Braunwald E.. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990;81:1161–72. [DOI] [PubMed] [Google Scholar]
- 10. van den Borne SWM, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J.. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 2010;7:30–7. [DOI] [PubMed] [Google Scholar]
- 11. Di Donato M, Sabatier M, Toso A, Barletta G, Baroni M, Dor V. et al. Regional myocardial performance of non-ischaemic zones remote from anterior wall left ventricular aneurysm. Effects of aneurysmectomy. Eur Heart J 1995;16:1285–92. [DOI] [PubMed] [Google Scholar]
- 12. Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M. et al. Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation . J Am Coll Cardiol 2008;51:476–86. [DOI] [PubMed] [Google Scholar]
- 13. Menicanti L, Di Donato M, Frigiola A, Buckberg G, Santambrogio C, Ranucci M. et al. RESTORE Group. Ischemic mitral regurgitation: intraventricular papillary muscle imbrication without mitral ring during left ventricular restoration. J Thorac Cardiovasc Surg 2002;123:1041–50. [DOI] [PubMed] [Google Scholar]
- 14. Di Donato M, Castelvecchio S, Brankovic E, Santambrogio C, Montericcio V, Menicanti L.. Effectiveness of surgical ventricular restoration in patients with dilated ischemic cardiomyopathy and unrepaired mild mitral regurgitation. J Thorac Cardiovasc Surg 2007;134:1548–53. [DOI] [PubMed] [Google Scholar]
- 15. Castelvecchio S, Garatti A, Menicanti L.. Addressing the left ventricle in functional mitral regurgitation: In: KMJ Chan. (ed). Functional Mitral and Tricuspid Regurgitation. Springer International Publishing, Cham, Switzerland, 2017, 115–28. [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 17. Abate E, Hoogslag GE, Antoni ML, Nucifora G, Delgado V, Holman E. et al. Value of three-dimensional speckle-tracking longitudinal strain for predicting improvement of left ventricular function after acute myocardial infarction. Am J Cardiol 2012;110:961–7. [DOI] [PubMed] [Google Scholar]
- 18. Shin S-H, Suh YJ, Baek Y-S, Lee M-J, Park S-D, Kwon S-W. et al. Impact of area strain by 3D speckle tracking on clinical outcome in patients after acute myocardial infarction. Echocardiography 2016;33:1854–9. [DOI] [PubMed] [Google Scholar]
- 19. Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S.. The role of cardiovascular Magnetic Resonance Imaging in heart failure. J Am Coll Cardiol 2009;54:1407–24. [DOI] [PubMed] [Google Scholar]
- 20. Dor V, Sabatier M, Di Donato M, Maioli M, Toso A, Montiglio F.. Late hemodynamic results after left ventricular patch repair associated with coronary grafting in patients with postinfarction akinetic or dyskinetic aneurysm of the left ventricle. J Thorac Cardiovasc Surg 1995;110:1291–301. [DOI] [PubMed] [Google Scholar]
- 21. Takeda K, Matsumiya G, Hamada S, Sakaguchi T, Miyagawa S, Yamauchi T. et al. Left ventricular basal myocardial scarring detected by delayed enhancement magnetic resonance imaging predicts outcomes after surgical therapies for patients with ischemic mitral regurgitation and left ventricular dysfunction. Circ J 2011;75:148–56. [DOI] [PubMed] [Google Scholar]
- 22. Castelvecchio S, Careri G, Ambrogi F, Camporeale A, Menicanti L, Secchi F. et al. Myocardial scar location as detected by cardiac magnetic resonance is associated with the outcome in heart failure patients undergoing surgical ventricular reconstruction. Eur J Cardiothorac Surg 2018;53:143–9. [DOI] [PubMed] [Google Scholar]
- 23. Castelvecchio S, Menicanti L, Ranucci M, Di Donato M.. Impact of surgical ventricular restoration on diastolic function: implications of shape and residual ventricular size. Ann Thorac Surg 2008;86:1849–54. [DOI] [PubMed] [Google Scholar]
- 24. Castelvecchio S, Garatti A, Gagliardotto PV, Menicanti L.. Surgical ventricular reconstruction for ischaemic heart failure: state of the art. Eur Heart J Suppl 2016;18:E8–14. [DOI] [PubMed] [Google Scholar]
- 25. Garatti A, Castelvecchio S, Di Mauro M, Bandera F, Guazzi M, Menicanti L.. Impact of right ventricular dysfunction on the outcome of heart failure patients undergoing surgical ventricular reconstruction. Eur J Cardiothorac Surg 2015;47:333–40. [DOI] [PubMed] [Google Scholar]
- 26. Menicanti L, Castelvecchio S, Ranucci M, Frigiola A, Santambrogio C, de Vincentiis C. et al. Surgical therapy for ischemic heart failure: single-center experience with surgical anterior ventricular restoration. J Thorac Cardiovasc Surg 2007;134:433–41. [DOI] [PubMed] [Google Scholar]
- 27. Di Donato M, Sabatier M, Dor V, Toso A, Maioli M, Fantini F.. Akinetic versus dyskinetic postinfarction scar: relation to surgical outcome in patients undergoing endoventricular circular patch plasty repair. J Am Coll Cardiol 1997;29:1569–75. [DOI] [PubMed] [Google Scholar]
- 28. Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, Di Donato M. et al. RESTORE Group. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol 2004;44:1439–45. [DOI] [PubMed] [Google Scholar]
- 29. Menicanti L, Castelvecchio S.. Left ventricular reconstruction concomitant to coronary artery bypass grafting: when and how? Curr Opin Cardiol 2011;26:523–7. [DOI] [PubMed] [Google Scholar]
- 30. Di Donato M, Toso A, Dor V, Sabatier M, Barletta G, Menicanti L. et al. RESTORE Group. Surgical ventricular restoration improves mechanical intraventricular dyssynchrony in ischemic cardiomyopathy. Circulation 2004;109:2536–43. [DOI] [PubMed] [Google Scholar]
- 31. Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM. et al. STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Donato M, Castelvecchio S, Menicanti L.. End-systolic volume following surgical ventricular reconstruction impacts survival in patients with ischemic dilated cardiomyopathy. Eur J Heart Fail 2010;12:375–81. [DOI] [PubMed] [Google Scholar]
- 33. Witkowski TG, ten Brinke EA, Delgado V, Ng AC, Bertini M, Marsan NA. et al. Surgical ventricular restoration for patients with ischemic heart failure: determinants of two-year survival. Ann Thorac Surg 2011;91:491–8. [DOI] [PubMed] [Google Scholar]
- 34. Michler RE, Rouleau JL, Al-Khalidi HR, Bonow RO, Pellikka PA, Pohost GM. et al. STICH Trial Investigators. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2013;146:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. 2018. ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2019;55:4--90. [DOI] [PubMed] [Google Scholar]
- 36. Buckberg GD, Athanasuleas CL, Wechsler AS, Beyersdorf F, Conte JV, Stro JE.. The STICH trial unravelled. Eur J Heart Fail 2010;12:1024–7. [DOI] [PubMed] [Google Scholar]
- 37. Bouma W, van der Horst ICC, Wijdh-den Hamer IJ, Erasmus ME, Zijlstra F, Mariani MA. et al. Chronic ischaemic mitral regurgitation. Current treatment results and new mechanism-based surgical approaches. Eur J Cardiothorac Surg 2010;37:170–85. [DOI] [PubMed] [Google Scholar]
- 38. Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A. et al. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg 2003;125:135–43. [DOI] [PubMed] [Google Scholar]
- 39. Castelvecchio S, Parolari A, Garatti A, Gagliardotto P, Mossuto E, Canziani A. et al. Surgical ventricular restoration plus mitral valve repair in patients with ischaemic heart failure: risk factors for early and mid-term outcomes. Eur J Cardiothorac Surg 2016;49:e72–8. [DOI] [PubMed] [Google Scholar]
- 40. Garatti A, Castelvecchio S, Bandera F, Guazzi M, Menicanti L.. Surgical ventricular restoration: is there any difference in outcome between anterior and posterior remodeling? Ann Thorac Surg 2015;99:552–9. [DOI] [PubMed] [Google Scholar]
- 41. Castelvecchio S, Baryshnikova E, Pina IL, Ambrogi F, Milani V, Tramarin R. et al. Longitudinal profile of NT-proBNP levels in ischemic heart failure patients undergoing surgical ventricular reconstruction: the Biomarker Plus study. Int J Cardiol 2018;260:24–30. [DOI] [PubMed] [Google Scholar]
- 42. Schafer U. Left ventricular partitioning in systolic heart failure subjects: addressing a mechanistic void with current therapies. EuroIntervention 2016;12 (Suppl X):X93–6. [DOI] [PubMed] [Google Scholar]
- 43. Thomas M, Nienaber CA, Ince H, Erglis A, Vukcevic V, Schäfer U. et al. Percutaneous ventricular restoration (PVR) therapy using the parachute device in 100 subjects with ischaemic dilated heart failure: one-year primary endpoint results of PARACHUTE III, a European trial. EuroIntervention 2015;11:710–17. [DOI] [PubMed] [Google Scholar]