Abstract
Ligands and receptors of the tumor necrosis factor (TNF) superfamily regulate immune responses and homeostatic functions with potential diagnostic and therapeutic implications. Kidney disease represents a global public health problem, whose prevalence is rising worldwide, due to the aging of the population and the increasing prevalence of diabetes, hypertension, obesity, and immune disorders. In addition, chronic kidney disease is an independent risk factor for the development of cardiovascular disease, which further increases kidney-related morbidity and mortality. Recently, it has been shown that some TNF superfamily members are actively implicated in renal pathophysiology. These members include TNF-related apoptosis-inducing ligand (TRAIL), its decoy receptor osteoprotegerin (OPG), and TNF-like weaker inducer of apoptosis (TWEAK). All of them have shown the ability to activate crucial pathways involved in kidney disease development and progression (e.g. canonical and non-canonical pathways of the transcription factor nuclear factor-kappa B), as well as the ability to regulate cell proliferation, differentiation, apoptosis, necrosis, inflammation, angiogenesis, and fibrosis with double-edged effects depending on the type and stage of kidney injury. Here we will review the actions of TRAIL, OPG, and TWEAK on diabetic and non-diabetic kidney disease, in order to provide insights into their full clinical potential as biomarkers and/or therapeutic options against kidney disease.
Keywords: biomarkers, cardiovascular risk, diabetic kidney disease, non-diabetic kidney disease, TRAIL and OPG, TWEAK
Introduction
The tumor necrosis factor (TNF) superfamily consists of ligands and receptors sharing a key regulatory role in the immune system development and functions, as confirmed by evolutionary studies suggesting that the expansion of this family took place together with the fine-tuning of adaptive immunity [1]. In addition, it has been shown that TNF-superfamily signaling modulates hematopoiesis and morphogenesis as well as different disease states, including cancer and diabetes [1–3].
TNF-superfamily ligands are type II homo-heterotrimeric transmembrane proteins, with a molecular structure characterized by the presence of a TNF Homology Domain, which binds to cystein-rich regions of specific TNF-superfamily receptors [3]. In addition, proteolytic processes, such as those mediated by ADAM, matrylisin, subtilisin-like furin family, and perhaps cysteine proteases, can release soluble TNF superfamily ligands by cleavage of their membrane-bound forms [3]. Likewise, also the receptors of this family, which are mostly type I–III transmembrane proteins, can be present as soluble molecules, due to either a missing transmembrane domain (such as in the case of osteoprotegerin), or proteolytic cleavage, or alternative splicing events [3]. All the main structural characteristics of TNF-superfamily signaling have been recently reviewed by Vanamee and Faustman [4]. In short, the basic signaling unit consists of a trimeric ligand and three receptors, each binding at the interface of two ligand monomers [4]. In addition, TNF-superfamily receptor resting and activation states depend on clustering processes at the cell surface. Overall, due to the complexity of TNF-superfamily signaling, ligands and receptors exert pleiotropic peripheral effects, such that some TNF-superfamily members are studied in different disease states [5]. These members include TNF-related apoptosis-inducing ligand (TRAIL), osteoprotegerin (OPG), and TNF-like weaker inducer of apoptosis (TWEAK).
The first of them, TRAIL, whose peripheral actions epitomize the complexity of the entire superfamily [4], has attracted much attention as a potential anti-cancer therapeutic target. TRAIL has the ability to induce apoptosis in tumor cells while preserving the normal ones, thanks to the activation of survival/proliferative and autophagy pathways [6–10]. This ability to selectively induce different/opposite cellular responses seems to be due to the high number of receptors that can differentially mediate TRAIL signaling, as well as to the balance with other circulating enzymes and cytokines, such as OPG. During the last two decades, several groups have addressed the role of TRAIL not only in cancer but also in other non-neoplastic diseases. Along with other Authors, we have found an inverse correlation between circulating TRAIL and the presence or the adverse prognosis of different pathological conditions including cardiovascular disease (CVD), type 1 and type 2 diabetes, and neurological disorders [11–21]. In addition, our results have provided insights into the therapeutic potential of recombinant TRAIL administration [22–26]. The second TNF-superfamily member, OPG, is a molecule that was initially identified as a bone resorption inhibitor, as it prevented the binding of receptor activator of nuclear factor kappa-Β (RANK) to its ligand RANKL [27–29]. Further studies showed that OPG was a decoy receptor (DcR) also for TRAIL [30], and that it could have additional direct effects on the vasculature and the immune system, independent of its binding to RANKL or TRAIL. Today, also OPG has emerged as a TNF-superfamily member with important effects on acute and chronic cardio-metabolic diseases [31], which might partly depend on the OPG/TRAIL ratio [32]. Third, TWEAK is another TNF-superfamily member with clinical potential. TWEAK was discovered at the same time of TRAIL and OPG [33] and it has recently gained much attention because of its ability to activate canonical and non-canonical pathways of the transcription factor nuclear factor-kappa B (NF-κB) leading to cell proliferation, differentiation, apoptosis, necrosis, inflammation, angiogenesis, and fibrosis [34].
Due to their belonging to the same family, in this review we will focus on the actions of TRAIL, OPG, and TWEAK on the kidney, in order to provide insights into their full clinical potential as biomarkers and/or therapeutic options against kidney disease. Kidney disease represents a global public health problem, whose prevalence is rising worldwide, due to the aging of the population and the increasing prevalence of diabetes, hypertension, obesity, and immune disorders [35]. It is estimated that some 10–15% of the population is affected by chronic kidney disease (CKD), whose end stage requires to be treated by dialysis or kidney transplantation. In addition, CKD is an independent risk factor for the development of CVD, which further increases kidney-related morbidity and mortality [35].
Overview on TRAIL, OPG, and TWEAK biology
TRAIL biology
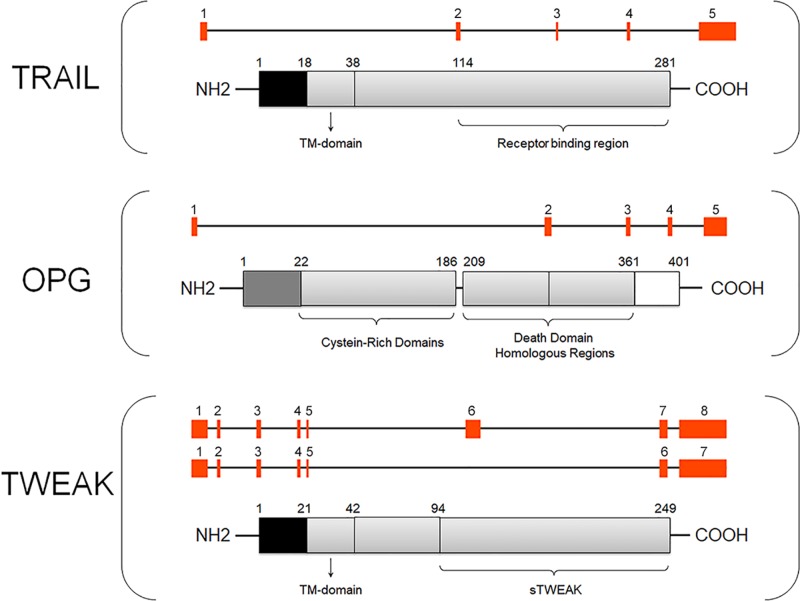
Since its discovery dating back to the nineties [36,37], TRAIL (also known as Apo-2 Ligand, CD253, or TNF-SF10) has emerged as a highly pleiotropic cytokine. Encoded by a gene located on chromosome 3 at position 3q26, human TRAIL is a 281 amino acid type II transmembrane protein (291 in the murine form) that can be cleaved and self-organize into soluble homotrimers, which are the ligand biologically active forms (Figure 1). Wiley and colleagues have reported that many tissues, as well as immune system cells, constitutively express significant levels of TRAIL, suggesting that TRAIL – unlike other TNF superfamily members – must not be cytotoxic to most tissues in physiological conditions [36]. The key biological feature of TRAIL is indeed its ability to selectively induce apoptosis in cancer/transformed cells while sparing the normal ones [38]. The mechanisms underlying this sort of ‘TRAIL resistance’ in normal (healthy) cells are still unclear, but scientific evidence suggests that this phenomenon might depend on the local conditions, such as inflammatory and/or pro-oxidative states [7,39,40]. For instance, it has been shown that oxidative stress promoted the redistribution of TRAIL receptors in membrane proteins (rafts), which could facilitate TRAIL signal transduction [7,41,42].
Figure 1. Graphical structure of TRAIL/OPG/TWEAK genes and proteins.
The picture shows a schematic representation of TRAIL/OPG/TWEAK genes (GENATLAS) where exons are indicated by red squares. For TWEAK, a transcript variant representing a shorter transcript encoding the functional protein is also shown. Each gene is shown with the respective schematic protein reporting the main relevant structural domains. Note: figures not drown to scale. Abbreviation: TM, transmembrane.
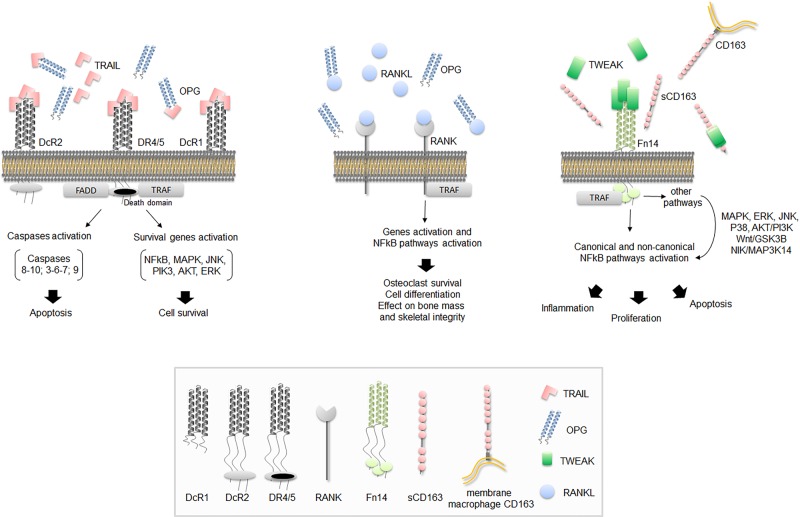
In cancer/transformed cells, TRAIL generally triggers the apoptotic cascade by binding to its two specific type I transmembrane death receptors (DRs) (TRAIL-R1/DR4 and TRAIL-R2/DR5), which harbor an intracellular death domain (DD) that induces caspase activation leading to cell death [43]. The details of the single intracellular steps leading to apoptosis, as well as all the anti-cancer therapeutic strategies targeting DRs, have been reviewed elsewhere [44,45]. In addition to the DRs, TRAIL can bind two transmembrane decoy receptors (DcRs), DcR1 (also known as TRAIL-R3) and DcR2 (also known as TRAIL-R4), which are unable to transmit the apoptotic signal since they do not contain any functional DD (i.e. DcR1 lacks the DD and DcR2 has only a truncated DD) (Figure 2A) [30,46]. Moreover, TRAIL can bind to soluble OPG (also known as TNF receptor superfamily member 11B, or TNFRSF11B), which lacks both transmembrane and cytoplasmic residues, such that it acts as a soluble DcR for TRAIL (Figure 2A) [30]. Overall, by binding to its receptors, TRAIL can activate not only apoptosis but also additional cell death pathways, such as necroptosis [47,48] and autophagy [49,50], as well as survival/proliferative and proinflammatory pathways, including those relying on NF-κB, ERK1/ERK2, and Akt signaling (Figure 2A) [51–54]. Although some mediators of TRAIL signaling have been identified, such as caspases and RIPK (receptor-interacting protein kinases), which act as apoptotic and necroptotic regulators [55], it remains to be fully elucidated how TRAIL could activate alternative pathways leading to opposite peripheral effects. So far, TRAIL multifaceted biology seems to be related not only to its complex receptor system, which can be influenced by local stimuli, but also to other conditions, such as the balance between metalloproteinase-2 and tissue inhibitor of metalloprotease-2, which seems to mediate the clearance of circulating TRAIL [56]. In addition, TRAIL biology is unavoidably linked to that of its DcR OPG.
Figure 2. Schematic representation of TRAIL/OPG/TWEAK signaling pathways.
The figure shows a schematic representation of the key pathways mediated by TRAIL/OPG/TWEAK. In (A) TRAIL-mediated apoptosis (intrinsic and extrinsic pathways) and cell survival signaling after interaction between TRAIL and DR4/5 are shown. In the figure, DcR1 and DcR2 are membrane-bounded TRAIL DcRs while OPG acts as soluble DcR for TRAIL. In (B) OPG takes place in the OPG/RANK/RANKL axis as DcR for RANKL. In (C) TWEAK-mediated pathways by interaction with its membrane bounded Fn14 receptor are shown. Soluble CD163 acts as TWEAK DcR. The monocyte/macrophage scavenger membrane receptor CD163 able to bind and internalize TWEAK is also shown. FADD is Fas-associated DD. TRAF is TNF receptor-associated factor. The figure legend shows a schematic representation of all ligands and receptors.
In healthy subjects, the blood levels of TRAIL are in the picogram (per milliliter) range [57] with reported physiological fluctuations due to age and gender [20], and significant changes in several pathological conditions. For example, along with other authors, we have found that TRAIL levels were significantly decreased in patients affected by CVD, such as acute myocardial infarction and coronary syndromes [58,59], as well as in patients with diabetes [17,19]. In addition, clinical studies have shown that there was an inverse correlation between circulating TRAIL and C-reactive protein [59], and between TRAIL and adverse cardio-metabolic outcomes [17,60,61], supporting a prognostic role for TRAIL. Several works carried out in animal models of cardio-metabolic diseases have attempted to understand if TRAIL had a causative role in cardio-metabolic disease development and progression. In this setting, we reported that TRAIL administration had protective effect against atherosclerosis, type 1 and type 2 diabetes, and non-alcoholic fatty liver disease, via the regulation of innate and adaptive immune system [22,25,26,62,63]. Nevertheless, other studies focusing on other tissues/conditions, such as Alzheimer disease and ischemic stroke, showed that TRAIL expression and signaling increased in disease states of the central nervous system (CNS) and promoted their progression [14,59]. It can be speculated that these conflicting effects might be due to the fact that TRAIL is not normally expressed in the CNS [36], while it is in the majority of the other organs and tissues [36].
OPG biology
OPG was firstly described as a glycoprotein involved in bone resorption regulation [27–29], and then it emerged as a TNF-superfamily member with multiple implications. The OPG gene, which is located on chromosome 8 at position 8q24 [27], encodes a circulating 401 amino acid glycoprotein with an N-terminal fragment harboring four tandem cysteine-rich motifs and a C-terminal fragment that seems to function as a linker of OPG monomers (Figure 1) [27]. The protein can indeed be detected as a free monomer of 60 kDa or as a homodimer of 120 kDa, which is reported as the most biologically active form [27]. OPG is generally released by several cell types, such as vascular smooth muscle cells, endothelial cells, osteoblasts and immune cells [27,64] and it can have either direct or indirect tissue effects that are due to its binding to RANKL (receptor activator of nuclear factor kappa-Β ligand) and/or TRAIL. By binding to RANKL [65], and inhibiting the interaction between RANKL and its membrane receptor RANK, OPG prevents bone remodeling (Figure 2B) [66,67], which represents the rationale behind its current use in patients with osteoporosis [66]. On the other hand, by binding to TRAIL, OPG inevitably affects/modulates TRAIL effects. Finally, by virtue of its heparin-binding domain, OPG can also interact directly with heparin sulfate proteoglycans, which are expressed at the cell surface, whereby it mediates additional ligand-independent effects that are still largely unexplored [68,69]. Not surprisingly, OPG biology lies at the intersection of different pathways, such as those regulating the vessels and the bone [31].
As reported for TRAIL, the blood levels of OPG are in the picogram (per milliliter) range with reported physiological fluctuations due to age and gender as well the blood-group, with higher circulating levels associated with non-zero blood groups [70]. OPG circulating levels change in several diseases. For example, along with other authors, we have found that OPG levels were significantly increased in patients affected by atherosclerosis [71], heart failure [72], as well as metabolic disturbances and diabetes [32,73]. In addition, OPG levels were significantly associated with markers of vascular damage [32]. Consistent with these results, several studies have shown that OPG is an independent risk factor for cardiovascular (CV) morbidity and mortality [71,74,75], such that OPG is considered a biomarker of CVD onset and progression [76]. Nevertheless, it remains to be clarified whether OPG has any causative/pathogenic role in vascular damage, as the studies carried out so far have provided conflicting results [16,31,32].
TWEAK biology
TWEAK (also known as TNFSF12 or Apo3L) was firstly described in 1997, when Chicheportiche and colleagues carried out a macrophage cDNA library screening and found that one of the cDNAs was a TNF family member, which, like other members of the TNF-superfamily, was able to induce apoptosis even though in a weaker and non-DD-dependent fashion [33]. Encoded by a gene located on chromosome 17 at position 3q26, TWEAK is a 249 amino acid type II transmembrane protein that can be cleaved by a furin endoprotease, with the generation of a 18 kDa soluble protein (156 amino acids), which is the most common form of TWEAK ligand [33] (Figure 1). Interestingly, both soluble and membrane TWEAK seem to activate different intracellular signaling pathways, leading to different biological responses [34]. So far, two receptors of TWEAK have been identified: the transmembrane fibroblast growth factor-inducible molecule 14 (Fn14) [77], which is the only receptor mediating TWEAK actions/effects, and the macrophage-derived scavenger receptor CD163. CD163 is expressed by monocytes and macrophages as a type I transmembrane protein and as a soluble protein (sCD163), which can both act as substitute receptors in the cells lacking Fn14 [78]. Nevertheless, the existence of other TWEAK receptors has been speculated [79]. Like TRAIL and OPG, soluble TWEAK is expressed by several cells of the immune system, such as monocytes, natural killer (NK) cells, and dendritic cells [80], where it seems to promote the switch from innate to adaptive immunity responses [80]. Additional non-hematopoietic sources of TWEAK are represented by endothelial and smooth muscle cells [81]. Generally, the activation of the TWEAK/Fn14 pathway is strictly dependent on Fn14 up-regulation. In normal (healthy) conditions, both TWEAK and Fn14 appear to be expressed at low levels; by contrast, in various disease conditions, including autoimmune and inflammatory/neuro-inflammatory diseases [82–87] as well as several types of cancer [88–93], both Fn14 and TWEAK expressions are up-regulated.
At the molecular level, TWEAK/Fn14 signaling initiates with TWEAK binding to the extracellular domain of its receptor, then the signal is transduced by TNF receptor-associated factors (TRAFs), which mediate the activation of both canonical and non-canonical NF-κB pathways (NF-κB1 and NF-κB2, respectively) [94–97] (Figure 2C). Apart from NF-κB, TWEAK can activate other pathways, such as mitogen-activated protein kinase (MAPK) with ERK, JNK and p38 signaling, as well as AKT/PI3K, Wnt/GSK3B, and NIK/MAP3K14 pathways (Figure 2C). As expected, TWEAK-mediated biological effects span from cell survival/proliferation, migration/invasion, cell apoptosis, and angiogenic activity, to the overexpression of inflammatory cytokines and inflammation as well as modulation of cell differentiation pathways. In addition, TWEAK seems to interact with Dicer (a ribonuclease involved processing microRNA precursors into microRNAs), reducing its pre-microRNA processing activity and thus affecting microRNA-regulated gene expression [98]. In general, the multiple (and sometimes opposite) effects of TWEAK seem to be due to different types of activation with a ‘transient’ activation involved into reparative/regenerative processes and an ‘over/sustained activation’ responsible for progressive tissue damage and cancer [99].
TRAIL, OPG, and TWEAK in the normal kidney
Several TNF superfamily members, such as TNF, Fas ligand, as well as TRAIL, OPG, and TWEAK are expressed in the human kidney [27,33,100–102]. Their sources include not only intrinsic kidney cells, but also the vessels [16,103], as well as infiltrating macrophages or lymphocytes. In general, given that knockout mice lacking the gene for TRAIL, OPG, or TWEAK do not exhibit signs of kidney pathology, it has been argued that these ligands are not strictly required for kidney development and physiology [80,104,105].
When looking at intrinsic kidney cells, studies on the tissue distribution of TRAIL and its receptors have shown that – in healthy conditions – TRAIL is expressed by the renal tubules, but not by the glomeruli [100]. In particular, Spierings and colleagues found that TRAIL, TRAIL-R1, and TRAIL-R2 were expressed in the tubuli contorti, while TRAIL-R2 was expressed in the Henle’s loop cells [106]. In vitro experiments on tubular cells have shown that TRAIL has different effects ranging from apoptotic [100] to proliferative actions [107], depending on local conditions. Also OPG has been found expressed in the kidney, including kidney samples [27,101], cultured tubular cells [100], and urinary exosome-like vesicles [108]. In particular, Benito-Martin and colleagues found that cultured human proximal tubular epithelial cells constitutively secreted exosome-like vesicles containing OPG [108], hypothesizing that tubular cell exosomes might have a regulatory role in matrix deposition, inflammation, and apoptosis. By contrast, exosome-like vesicles did not contain TRAIL or TWEAK [108].
As compared with TRAIL and OPG, experimental/animal studies have shown that TWEAK and its receptor Fn14 are constitutively expressed not only by tubular but also by glomerular cells [109,110], such as mesangial cells and podocytes [110,111]. In normal conditions, TWEAK and Fn14 expression is rather low but it becomes significant in the presence of tissue damage/inflammatory processes [111,112]. This is consistent with experimental and human studies, showing that Fn14 was not expressed in the normal kidney, but its expression together with that of TWEAK greatly increased in diverse forms of acute kidney injury and CKD, such as proteinuric and non-proteinuric, immune- and non-immune-mediated kidney disease [113]. In addition, experimental studies have shown that, as many other TNF-superfamily members, such as TRAIL, TWEAK can induce different cellular responses, including proliferation [114], inflammation [110], as well as apoptosis [115], depending on the milieu conditions.
Acute kidney injury and chronic kidney disease
As a premise to the studies investigating the actions of TRAIL, OPG, and TWEAK on kidney disease, it has to be said that the loss of kidney function can be acute (<90 days) or chronic (>90 days) [116], and it can have several causes that contribute and/or cause local activation of common damaging pathways such as inflammation, fibrosis, and cell death (with apoptosis, necroptosis, and necrosis) [117,118]. Acute kidney injury (also referred to as acute kidney disease when it lasts more than 7 and less than 90 days) has an estimated incidence rate of 20–30% [119]. Classically, the causes of AKI/AKD can be classified based on the mechanisms leading to kidney damage. These include pre-renal (e.g. reduced kidney perfusion), intra-renal (direct renal parenchimal injury), and post-renal causes (e.g. obstruction of urinary tract). The etiology can significantly vary in different regions of the world [116]. In high-income countries AKI/AKD is generally seen in adults with multiple comorbidities – especially diabetes and CVD – who are exposed to nephrotoxins, such as radiocontrast media, antibiotics, or to infections, and/or dehydration. In low- and middle-income countries AKI/AKD affects younger patients and it is most often due to episodes of infection, diarrhea, and/or obstetric complications [116].
It has to be noted that an episode of AKI/AKD is not only associated with short-term but also with long-term adverse outcomes, such as incomplete or non-recovery of kidney function with development of CKD and a reduction in survival. Apart from AKI/AKD, the most common contributing factors to CKD include genetic abnormalities and ageing, while the most common underlying diseases associated with CKD are diabetes [120] and hypertension [117]. However, CKD can be also due to infectious diseases, autoimmune diseases (such as lupus), and medications. Overall, it is estimated that CKD affects 10-15% of the population. As CKD progresses, it may require renal replacement therapies, such as peritoneal dialysis, hemodialysis, or kidney transplantation with severe consequences on patient survival. It has in fact been reported that life expectancy is one-third of that of the general population for patients on dialysis, while for patients receiving a kidney transplant is 45–85% of that of the general population [117]. Given that one of the most common cause of AKI/AKD/CKD is diabetes and that diabetes accounts for 33% of patients initiating renal replacement therapies worldwide [120], we will divide the studies on the actions of TRAIL, OPG, and TWEAK into those focusing on non-diabetic and those focusing on diabetic kidney disease.
TRAIL, OPG, and TWEAK in non-diabetic kidney disease
TRAIL in non-diabetic kidney disease
Most of the works on TRAIL have explored its anti-cancer activity for the treatment of renal carcinoma. As a result, the full significance of TRAIL on non-neoplastic kidney disease has not been fully elucidated yet. Overall, experimental and clinical studies have demonstrated that TRAIL is up-regulated in different kidney diseases, ranging from diabetic nephropathy [100] to non-diabetic conditions, which include lupus nephritis [107], minimal-change nephrotic syndrome (MCNS) [121], rejected kidney transplant, as well as acute kidney injury [122], or renal ischemia reperfusion injury [123]. Nevertheless, in these studies, TRAIL up-regulation was not necessarily followed by an increase in circulating TRAIL. For example, although TRAIL was up-regulated on mononuclear cells in patients with MCNS, circulating TRAIL levels did not change between the groups. In addition, in patients with autosomal dominant polycystic kidney disease, circulating TRAIL was actually lower than in healthy volunteers [124]. This has been ascribed to the fact that TRAIL is widely expressed and its circulating levels might come from different tissue sources outside the kidney. Other explanations include a tissue uptake of TRAIL by its specific receptors (including the DcR OPG). Consistent with this hypothesis, TRAIL receptors were found up-regulated in kidney biopsies of various glomerular diseases [125]. Interestingly, in a recent paper evaluating the association between a vast number of proteins and kidney function, TRAIL-R2 turned out to be the protein most strongly associated with kidney function decline [126].
Interestingly, Liabeuf and colleagues have recently reported that circulating TRAIL levels are inversely associated with the mortality risk in CKD patients [127], suggesting a protective role for TRAIL. Consistent with this, in vitro experiments on primary renal proximal tubular epithelial cells showed that TRAIL did not stimulate apoptosis, but rather proliferation [107]. By contrast, blockade of TRAIL ameliorated tissue damage in two animal models of kidney disease, namely renal ischemia reperfusion injury [123], and burn-related acute kidney injury [122]. These contradictory findings between human and animal studies might be partly due to a different regulation and function of TRAIL between humans and mice, as well as in different settings (i.e. AKI or CKD) [102]. In any case, to date, it has not been clarified if TRAIL up-regulation at a tissue level is harmful or protective, and/or if TRAIL could be used as a biomarker or as a therapeutic target in patients with CKD.
OPG in non-diabetic kidney disease
On the other hand, there is growing interest in OPG as a biomarker in patients with CKD (Table 1). Several clinical studies have shown that OPG increases in CKD [128,129], including patients undergoing renal replacement therapy [130]. The only ‘exception to the rule’ seems to be nephrotic syndrome, where Mohamed and colleagues found that OPG levels were significantly lower as compared with control patients, which might have been due to either protein loss or steroid effect that are typical of this condition [131]. Nevertheless, along with other Authors, we have recently reported that there is a significant inverse association between OPG and renal function [132]. Consistent with these data, high levels of OPG have been associated with long-term risk of renal decline, renal disease hospitalizations, and/or deaths in elderly women [133]. Similar results, which will be summarized in the following paragraphs, have been obtained in patients with diabetic kidney disease. Given that in one of our previous works we found that OPG administration was associated with proinflammatory and pro-oxidative renal changes, it can be speculated that OPG might be not only a risk marker but also a risk factor of CKD [132].
Table 1. Studies including the TNF-superfamily triad members TRAIL/OPG/TWEAK as biomarkers and/or therapeutic targets in renal pathological conditions (reported on www.clinicaltrials.gov).
| Disease pathological conditions | Study type | TNF-superfamily member | Intervention(s) | Phase | Status | Study ID |
|---|---|---|---|---|---|---|
| CKD | Int. | OPG levels (biomarker) | Plasma OPG level; FGF-23 level; vascular calcification score | na | R | NCT02808572 |
| CKD | Int. | OPG levels (biomarker) | Plasma OPG level; FGF-23 level; vascular calcification score | na | R | NCT02813642 |
| Renal failure, haemodialysis | Int. | OPG levels (biomarker) | Low molecular weigth heparin; unfractioned heparin | na | U | NCT00669721 |
| Vitamin D Def., vascular calcification, ESRF, peritoneal dialysis | Int. | OPG levels (biomarker) | Cholecalciferol; placebo | IV | A | NCT02598635 |
| Chronic renal failure | Obs. | OPG levels (biomarker) | Identification of CV risk factors linked to renal failure progression | na | Unk | NCT00608998 |
| Renal transplant | Int. | OPG levels (biomarker) | Zortress, everolimus | na | With. | NCT01612299 |
| Renal insufficiency, CKD, hyperphosphatemia bone diseases | Int. | OPG levels (biomarker) | Lanthanum carbonate; calcium carbonate | IV | E | NCT02237534 |
| Kidney failure, chronic haemodialysis | Int. | OPG levels (biomarker) | Apabetalone; placebos | I-II | A* | NCT03160430 |
| Clear-cell, metastatic renal cell carcinoma, bone metastases | Int. | OPG levels (biomarker) | XOFIGO radium-223 dichloride | I-II | A | NCT02880943 |
| Chronic kidney failure; inflammation vascular calcification | Obs. | OPG levels (biomarker) | Vascular calcification’s risk factors in haemodialysis patients | na | C | NCT00694824 |
| Fabry disease | Int. | OPG levels (biomarker) | RVX000222 | I-II | A | NCT03228940 |
| Osteoporosis and CKD | Int. | OPG levels (biomarker) | Denosumab; placebo | IV | A* | NCT02792413 |
| Renal cell carcinoma | Obs. | OPG levels (biomarker) | Assessment of bone biomarkers for TKI response in RCC with bone metastases, HRQoL and comparison of imaging techniques | na | T | NCT02747173 |
| Renal transplantation, immunosuppression | Int. | OPG levels (biomarker) | Everolimus | IV | Unk | NCT01239472 |
| Renal cancer | Int. | TRAIL (therapeutic) | T-cells transduced with T-cell receptor recognizing TRAIL bound to the DR4 | I/II | T | NCT00923390 |
| Diabetic patients with proteinuria | Int. | TWEAK levels (biomarker) | Calcium channel blocker amlodipine | IV | C | NCT01738945 |
| Diabetes, hypertension proteinuria | Int. | TWEAK levels (biomarker) | Renin angiotensin system blockage, calcium channel blocker amlodipine; valsartan | IV | C | NCT00921570 |
| Lupus nephritis | Int. | TWEAK (therapeutic) | BIIB023 anti TWEAK monoclonal antibody; mycophenolate mofetil; oral corticosteroids | II | T | NCT01930890 |
| Lupus nephritis | Int. | TWEAK (therapeutic) | BIIB023 anti TWEAK monoclonal antibody; placebo; mycophenolate mofetil; oral corticosteroids | II | T | NCT01499355 |
| Advanced solid tumours | Int. | Anti Fn14 (therapeutic) | PDL192 anti-TWEAKR monoclonal antibody | I | C | NCT00738764 |
A: active; C: completed; T: terminated; Int: interventional; Obs: observational; With: withdrawn; R: recruiting; Unk: unknown; *: not recruiting yet; na.: not available (accessed November 2018).
Apart from circulating OPG, also urinary OPG has raised interest as a potential biomarker of renal disease. Several studies have reported a correlation between urinary OPG and both disease status and prognosis of patients with lupus nephritis [134,135]. In particular, Gupta and Kiani showed an association between urinary OPG and renal disease activity. In addition, urinary OPG was elevated in patients with poor response to therapy, disease relapse or CKD development [134]. Overall, all these studies suggest that OPG could serve as a biomarker of CKD development and progression [136,137]. For this reason, several ongoing clinical studies have included OPG measurement to monitor CKD progression and its complications (Table 1).
Besides CKD development and progression, it has been argued that measuring OPG could help differentiate between low-turnover and high-turnover osteodystrophy in hemodialysis patients. Nevertheless, the studies focusing on this topic have shown contradictory results. In a study on 39 chronic hemodialysis patients who underwent bone biopsies, OPG levels were lower in the patients with low-turnover bone disease than in those with predominant hyperparathyroidism or mixed osteodystrophy, for intact PTH levels lower than 300 pg/ml [138]. By contrast, Haas and colleagues found that OPG levels were lower in patients with high-turnover bone disease as compared with those with low or normal-turnover bone disease [139].
TWEAK in non-diabetic kidney disease
As compared with TRAIL and OPG, there is much more information on the actions of TWEAK and Fn14 on the kidney. Table 2 summarizes the effects that TWEAK has on different kidney cells in vitro [140], where it promotes fibrosis, inflammation, proliferation, as well as cell death [81,85,94,96,109,110,114,115,141–159]. In line with these effects, in vivo studies suggest that TWEAK/Fn14 blockade might protect against renal injury/disease (Table 2). In models of AKI, such as folic acid nephropathy, which is characterized by tubular cell death, proliferation, and inflammation, TWEAK deficiency or Ab anti-TWEAK decreased kidney apoptosis and inflammation [114,145,146] and improved kidney function [114,160]. Similar effects were found in a model of AKI induced by ischemia-reperfusion, where Fn14 blockade reduced renal apoptosis, fibrosis, and inflammation [161]. Consistent with these observations, TWEAK administration exacerbated proinflammatory changes in the kidney of high-fat diet-fed ApoE-knockout mice, while anti-TWEAK neutralizing antibodies significantly reduced renal, as well as vascular inflammation [162]. Also in a model of chronic graft-versus-host lupus nephritis, Fn14 deficiency and/or anti-TWEAK neutralizing antibodies were able to significantly decrease proinflammatory cytokine expression, macrophage infiltration, as well as proteinuria [163]. In addition, TWEAK deficiency significantly reduced apoptosis, inflammation, and fibrosis after ureter ligation [143], as well as the cell proliferation in the remnant kidney after unilateral nephrectomy [114], suggesting an involvement of TWEAK/Fn14 axis in the compensatory tubular cell proliferation that follows uninephrectomy [114] (Table 2).
Table 2. Renal actions of TWEAK/Fn14: key findings from in vitro and in vivo models.
| In vitro key effect | Cell type | Mechanisms | Ref. |
|---|---|---|---|
| Fibrosis | Mesangial cells | TGFbeta1 and fibronectin increase through PKG-I down-regulation | [141] |
| Tubular cells | Endothelial–mesenchymal transition via NF-κB | [142] | |
| Phenotypic changes via NF-κB and ERK activation: F-actin redistribution, loss of epithelial and tight junction proteins, vimentin expression | |||
| Renal fibroblasts | Decrease in collagen I and fibronectin protein levels | [143] | |
| Inflammation | Tubular cells | MCP-1, RANTES increase via NF-κB and JAK2 kinase activation | [144] |
| MCP-1, RANTES, and IL-6 increase | [145] | ||
| CCL21 increase via non-canonical NF-κB activation | [96] | ||
| CXCL16 increase via NF-κB | [146] | ||
| CD74 and DDT increase | [147] | ||
| IL-6 and other chemokines via EGFR activation, ERK activation | [148] | ||
| CXCL10 increase via MAP3K14 and non-canonical NF-κB pathway | [149] | ||
| Modulation of NF-κB components Bcl3 overexpression – which decreases NF-κB transcriptional activity | [150] | ||
| Modulation of NF-κB components: NF-kBiz overexpression – which has anti-inflammatory anti-apoptotic effects | [151] | ||
| Podocytes | MCP-1 increase via NF-κB | [152] | |
| CCL19, RANTES increase via NF-κB | [153] | ||
| CCL21 increase via non-canonical NF-κB pathway | |||
| Induction of multiple inflammatory cytokines/chemokines and adhesion molecules | [110] | ||
| Renal fibroblasts | MCP-1 and RANTES increase via NF-κB | [143] | |
| Mesangial cells | IL-6, IL-8, MCP-1, and CCL5 increase via NF-κB | [85] | |
| Induction of multiple inflammatory cytokines/chemokines and adhesion molecules | [110] | ||
| MCP-1, RANTES, CXCL10, and CXCL1 increase | [109] | ||
| Proliferation | Tubular cells | Cell number increase, cyclin D1 expression via MAPK (ERK/p38), PI3K/Akt, NF-κB | [114] |
| Mesangial cells | Promotion of cell proliferation and cell cycle activity | [85] | |
| Renal fibroblasts | Increase in mitosis number, cyclin D1 expression via Ras/ERK pathway | [143] | |
| Cell death | Tubular cells | (Late) Necroptosis via RIPK1, RIPK3, MLKL | [154] |
| Apoptosis and increased inflammatory gene expression | [155] | ||
| In an inflammatory milieu, induction of apoptosis, activation of caspase-8, -9 -3, Bid cleavage and mitochondrial injury | [115] | ||
| Others | Tubular cells | Klotho down-regulation via NF-κB | [156] |
| MAGED2 up-regulation – modulation of electrolyte transport | [157] | ||
| PGC-1alpha and mitochondrial function down-regulation | [158] | ||
| Endothelial cells | Endothelin-1 increase and ECE1 up-regulation via AP-1 and NF-kB | [159] | |
| Cell growth and migration, enhanced FGF-2 and VEGF-A mitogenic activity | [81] | ||
| Vascular smooth muscle cells | Enhanced inorganic phosphate-induced calcification via both canonical and non-canonical NF-κB pathways | [94] |
| In vivo eperimental model | Intervention | Effect | Ref. |
|---|---|---|---|
| Healthy state | TWEAK administration | Increase in inflammation (chemokines and IL-6) via NF-κB activation | [145] |
| Increase in inflammation (CCL21) via non-canonical NF-κB | [96] | ||
| Increase in inflammation (CXCL16 and CD3 infiltration) via NF-κB | [146] | ||
| Klotho reduction | [156] | ||
| PGC-1alpha reduction | [158] | ||
| Folic acid nephropathy | Anti-TWEAK AB | Reduction in inflammation (chemokines and IL-6) | [145] |
| TWEAK-KO mice | Reduction in apoptosis and proliferation and renal function improvement | [114] | |
| Anti-TWEAK AB TWEAK-KO mice | Reduction in inflammation (CCL21) | [96] | |
| Anti-TWEAK AB | Reduction in inflammation (CXCL16 and CD3 infiltration) | [146] | |
| Anti-TWEAK AB TWEAK-KO mice | Reversal in klotho down-regulation | [156] | |
| Anti-TWEAK AB | Reversal PGC-1alpha | [158] | |
| I/R injury | Fn14 blockade | Reduction in fibrosis and increased survival | [161] |
| HFD-fed ApoE-KO mice | TWEAK administration | Increase in inflammation (RANTES, MCP-1, macrophage infiltration) via NF-κB | [162] |
| Anti-TWEAK AB | Reduction in inflammation (RANTES, MCP-1, macrophage infiltration) | ||
| cGVH-induced lupus | Fn14 blockade | Reduction in IgG deposition, IL-6, MCP-1, RANTES, IP-10, macrophage infiltration Reduction in proteinuria |
[163] |
| Anti-TWEAK AB | Reduction in inflammation Reduction in proteinuria |
||
| Unilateral nephrectomy | TWEAK-KO mice | Reduction in tubular cell proliferation in the remnant kidney | [114] |
| Ureter ligation | TWEAK-KO mice | Reduction in apoptosis, inflammation, and fibrosis | [143] |
| Adeno-TWEAK | Increase in apoptosis, inflammation, and fibrosis | [143] |
Abbreviations: AB: antibody; AP-1: activator protein 1; Bcl3: B-cell lymphoma 3-encoded protein; CCL19: chemokine (C–C motif) ligand 19; CCL21: chemokine (C–C motif) ligand 21; CD74: cluster of differentiation 74; CXCL1: chemokine (C–X–C motif) ligand 1; CXCL10: C–X–C motif chemokine 10 (also known as IP-10); CXCL16: chemokine (C–X–C motif) ligand 16; DDT: d-dopachrome tautomerase cytokine (also known as MIF-2); ECE-1: endothelin-converting enzyme-1; EGFR: epidermal growth factor receptor; ERK: extracellular signal-regulated kinase; FGF-2: fibroblast growth factor 2; IL: interleukin; I/R: ischemia/reperfusion; JAK2: Janus Kinase 2; KO: knockout; MAGED2: melanoma antigen-encoding gene D2; MAPK: mitogen-activated protein kinase; MAP3K14: mitogen-activated protein kinase kinase kinase 14 (also known as NIK); MCP-1: monocyte chemoattractant protein 1; MLKL: mixed lineage domain-like protein; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NF-kBiz: NF-κBInhibitor Zeta; PGC-1 alpha: peroxisome proliferator-activated receptor-γ coactivador-1 alpha; PI3K: phosphoinositide 3-kinase; PKG-I: protein kinase G-I; RANTES: regulated on activation, normal T cell expressed and secreted (also known as CCL5); RIPK: receptor interacting protein kinase; TGFbeta1: transforming growth factor beta 1; VEGF-A: vascular endothelial growth factor A.
Overall, these studies have provided sound evidence for the development and use of anti-TWEAK therapies against renal injury. Consistent with this, a humanized anti-TWEAK monoclonal IgG antibody BII023 is currently under investigation. The first study on the effects of BII023 showed that a single dose had a favorable safety and tolerability profile in patients with rheumatoid arthritis [164], where it reduced serum biomarkers of inflammation. However, in the ATLAS study (NCT01499355), the same treatment did not demonstrate sufficient efficacy when added to mycophenolate and corticosteroids, in patients with lupus nephritis, such that the best modality to use anti-TWEAK therapies is still under investigation [164,165].
Apart from the potential of anti-TWEAK therapies, accumulating evidence suggests that TWEAK could serve also as a CKD biomarker [166] (Table 1). Rather surprisingly, circulating TWEAK seems to be lower in patients undergoing hemodialysis than healthy controls [167,168], and to increase in patients who have undergone renal transplant [168]. Nevertheless, within the hemodialysis range, higher TWEAK levels have been found associated with a shorter time to death, and hemodyialysis patients with high TWEAK have been found at increased CV and all-cause mortality risk [167]. Based on these results, Carrerro and colleagues have put forward that TWEAK might be an additive, but not a primary marker, of the high mortality rate seen in hemodialysis patients [167]. With respect to lupus nephritis, while circulating TWEAK does not discriminate between patients with and without lupus nephritis [169,170], urinary (uTWEAK) has been found selectively higher in patients with lupus nephritis as compared with other autoimmune disease patients and healthy subjects [169], such that uTWEAK measurement has been proposed as a non-invasive tool to monitor this disease [171,172].
TRAIL, OPG, and TWEAK in diabetes and diabetic kidney disease
OPG and diabetic nephropathy
Diabetes mellitus is now globally the single leading cause of end-stage renal disease (ESRD) and renal replacement therapy worldwide [173]. So far, OPG is probably one of the TNF superfamily members that have been studied the most in the setting of diabetic nephropathy. First of all, experimental studies have shown that the onset of diabetes is associated with an increase in circulating OPG [174], and that diabetes significantly increases OPG expression in peripheral tissues, such as the vessels [16,175]. In addition, microarray studies have confirmed that OPG is up-regulated in the kidneys of diabetic patients [100]. Moreover, it has been shown that OPG levels, which are elevated in patients with type 1 diabetes mellitus (T1DM) [176], correlate with glycemic status [177]. Similar results have been found in patients with type 2 diabetes mellitus (T2DM), who displayed significantly higher levels of OPG as compared with healthy controls, and the patients whose glucose levels were poorly controlled had the highest levels of OPG. All these studies are summarized in a recent review by Perez de Ciriza and colleagues [178].
With respect to diabetic nephropathy, OPG levels have been found significantly higher in patients with T1DM and diabetic nephropathy as compared with those with normal kidney function [177,179]. In these patients, OPG levels have been associated not only with the presence but also with the severity of diabetic kidney disease [180]. Likewise, OPG levels have been found significantly higher in patients with T2DM and kidney damage as compared with those with normal kidney function, where OPG was found to be independently associated with the severity of diabetic nephropathy [181]. Interestingly, several experimental studies suggest that OPG is not only a risk marker but also a risk factor of disease, meaning that it has a causal role on disease development and progression. This is supported by the observations that OPG administration was associated not only with pancreatic [182] and adipose tissue damage [73], and metabolic abnormalities, but also with kidney damage [132].
TRAIL and diabetic nephropathy
In keeping with the view that TRAIL and OPG have opposite effects [32], the literature suggests that TRAIL might have a protective role against diabetes. The first evidence of a link between TRAIL and diabetes was provided by the observation that TRAIL-knockout mice developed heightened autoimmune responses and diseases, such as autoimmune diabetes [183]. Further experimental studies strengthened this relationship, as TRAIL blockade exacerbated T1DM [184] and TRAIL delivery attenuated disease severity [26,185]. In line with these observations, we have demonstrated that TRAIL treatment significantly ameliorated glucose abnormalities in high-fat diet-fed mice [22,25]. Other Authors have reported that TRAIL deficiency worsened glucose control in T2DM animal models [186,187]. In addition, looking at the kidney, the same authors found that TRAIL deficiency contributed to diabetic nephropathy in a mouse model of T2DM, as TRAIL-knockout mice displayed increased urinary protein loss and greater glomerular damage as compared with wild-type mice [187].
Clinical studies have shown that TRAIL levels are significantly lower in patients with T1DM [17] and T2DM [188,189] at onset as compared with healthy subjects, and that this reduction becomes less pronounced a few months after the start of the therapy [17,188]. A decrease in circulating TRAIL has been found also in patients with diabetic nephropathy as compared with healthy controls [190–192]. By contrast, studies on the tissue expression of TRAIL have shown that in T1DM patients there is an increase in TRAIL positive T-cells [193,194]. As for the kidney, TRAIL expression was found increased in renal biopsies of diabetic patients, where it became detectable also in the glomeruli. Based on in vitro studies showing that TRAIL had apoptotic effects on tubular cells exposed to high glucose and pro-inflammatory conditions, Lorz and colleagues have hypothesized that TRAIL could contribute to diabetic nephropathy [100]. Nevertheless, given the limited number of papers and their partly contradictory results, at this stage, it is difficult to draw any conclusion on the exact role of TRAIL in the development and progression of diabetic nephropathy. Further studies are needed to clarify TRAIL significance and potential applications in diabetic kidney disease.
TWEAK and diabetic nephropathy
Despite being largely studied in acute kidney injury, TWEAK actions/significance in the setting of diabetic nephropathy remain/s to be clarified. Nevertheless, cross-sectional studies have shown a link between the soluble circulating TWEAK and diabetes. In particular, T1DM patients exhibited lower levels of TWEAK as compared with healthy subjects [195]. Likewise, also T2DM patients had lower levels of TWEAK and, in this setting, kidney disease had an additive and independent effect on it. In other words, as compared with healthy subjects, whose TWEAK levels were 669 ± 201 ug/l, in patients with diabetes TWEAK levels were 516 ± 187 ug/l, and in those with diabetes and chronic hemodialysis TWEAK levels were 317 ± 132 ug/l [196]. In addition, a large prospective nested case–control study has shown that TWEAK concentrations were significantly lower in patients developing diabetes, highlighting that decreased circulating TWEAK could be used to predict T2DM [197].
Given that TWEAK-transgenic mice exhibited greater body weight gain and fat mass as well as insulin resistance and glucose intolerance, and that TWEAK inhibited insulin-stimulated glucose uptake in vitro, it has been argued that TWEAK might promote metabolic abnormalities and diabetes development [198]. Therefore, TWEAK reduction in patients with diabetes is counterintuitive and the mechanisms underlying such findings are poorly understood. The same could be said for nephropathy/diabetic nephropathy, as TWEAK administration worsened renal damage in ApoE-knockout mice [162], and yet diabetic patients undergoing hemodialysis exhibit low levels of TWEAK [196]. To explain this apparent paradox the hypotheses that have been postulated include a possible uptake of TWEAK by Fn14, which increases in disease states/inflammatory conditions [199], or the increase in CD163, which is a DcR for TWEAK [199]. CD163 is a monocyte-macrophage surface receptor, which acts as a DcR for TWEAK [78], and it increases in inflammatory conditions, as well as in CKD, leading to a reduction in the TWEAK/CD163 ratio [200].
Role of TRAIL, OPG, and TWEAK in cardiovascular disease of CKD patients
CKD and CVD are often associated with co-existing pathologies that can begin, intensify and sustain each other [201,202]. CKD patients are at risk of developing CVD proportionally to the severity of kidney disease, decrease in glomerular filtration rate and increase in proteinuria [203]. As a consequence, CKD patients have an early onset and a higher risk of CVD, including stroke, coronary artery disease, peripheral vascular disease, and congestive heart failure, as compared with the general population [202]. Epidemiological data underline that patients with CKD die at an accelerated rate, which is 10-30 times higher than that of subjects without CKD [204,205]. Of note, in ESDR patients, CVD is the leading cause of morbidity and mortality with the ability of reducing the 5-year survival rate of patients on dialysis to only 40–50% [206,207]. Given that the traditional risk factors for CVD (including diabetes, dyslipidemia, hypertension, smoking, age and gender, as well as medical and family history) do not fully explain the accelerated development and progression of CVD in patients with CKD, it is current scientific opinion that other non-traditional risk factors must be involved. These non-traditional risk factors include hemodialysis, disorders of phospho-calcium metabolism, such as vitamin D deficiency, hyperparathyroidism, hyperphosphatemia [208,209], as well as the modification of the plasma levels of soluble proteins involved in vascular homeostasis, such as fibroblast growth factor-23 (FGF-23) and its co-receptor klotho, which are examples of recognized systems that contribute to CVD in course of CKD [210].
Also OPG, TRAIL, and TWEAK seem to be involved in CVD development and progression in patients with CKD. OPG has been recently identified as a potential risk marker of CVD in CKD patients [127]. Endothelial cells are a source of OPG when exposed to an inflammatory milieu, suggesting a role for OPG – and possibly the entire OPG/RANK/RANKL axis – in vascular injury and atherosclerosis. Increased OPG levels are associated with an increased CVD risk, although the increase in OPG production can be the attempt to protect the vessel walls from vascular damage/calcification [31]. Increased OPG levels have been reported in pre-dialysis patients and those undergoing dialysis, as well as in transplant recipients, where it may predict vascular calcification progression and patient survival. In this regard, Montanez-Barragan and colleagues have argued that, even in the context of uremia, the increase in OPG might be a protective response against kidney and vascular injury [137]. Consistent with this concept, recombinant OPG has been evaluated in phase I clinical trials, although additional studies are needed to confirm its potential [137]. Overall, so far, OPG levels appear a promising independent biomarker of CVD in patients with acute/chronic cardio-metabolic disease [31].
By contrast to OPG, circulating TRAIL levels are significantly decreased in patients with myocardial infarction and/or acute coronary syndrome [32,58] and they are inversely associated with an increased risk of CVD and cardiac mortality [58,60]. Similarly, an inverse association between TRAIL levels and mortality risk was observed in patients with advanced heart failure and CKD [32]. In particular, Kuznieeski and colleagues reported that CKD patient exhibited high OPG levels and OPG/TRAIL ratio and that OPG levels were associated with a higher risk of CVD and mortality among patients with renal failure [211]. The same Authors observed that OPG levels and OPG/TRAIL ratio predicted long-term mortality (all-cause and CVD mortality) and that they correlated with aortic pulse wave velocity and with N-terminal pro b-type natriuretic peptide, that are two independent predictors of CVD morbidity and mortality, supporting the role of OPG and OPG/TRAIL ratio as biomarkers of CV dysfunction and predictor of mortality in late stage of CKD. In the context of the NEFRONA study [212], our group has recently investigated the role of circulating TRAIL in predicting the progression of subclinical atheromatosis in CKD patients [213]. We reported that the baseline levels of soluble TRAIL were independently and inversely associated with novel plaque formation in 3–5-stage patients, establishing the proof of concept for the use of recombinant TRAIL to prevent/attenuate atheromatous progression in CKD patients not undergoing dialysis [213].
Recent studies have highlighted also the importance of TWEAK in the evaluation of the CV risk in kidney disease. It is known that TWEAK contributes to endothelial cell dysfunction. As a matter of fact, both TWEAK and Fn14 have been detected in human atherosclerotic plaques, suggesting that they can both play a role in the atherogenic process, probably also via reactive oxygen species (ROS) and mtROS modulation [214]. In the setting of kidney disease, it has been demonstrated that circulating TWEAK decreases across CKD stages and that this decrease is associated with an increased risk of CVD. For these reasons, TWEAK has been indicated as predictor of mortality in dialysis and non-dialysis CKD patients, as well as a biomarker of CVD outcomes [215]. Moreover, it has been observed that atherosclerotic burden and atheromatosis progression in CKD patients free of CVD are accompanied by low TWEAK, suggesting that TWEAK could serve as biomarker to predict CVD risk even before clinical manifestations [216,217].
Mineral and bone disorders are common in CKD patients and contribute to the burden of CV and bone complications in CKD. Vascular calcification of the arterial wall and cardiac valves is a hallmark of atherosclerosis. Bozic and colleagues analyzed the serum levels OPG, Osteopontin (OPN), and TWEAK as predictors of CVD outcomes in CKD patients (with or without CVD risk factors) without any history of CVD [218]. In detail, they observed that higher levels of OPG (or OPN) alongside lower levels of TWEAK were associated with significantly greater risk of non-fatal and fatal CV events [218]. Although other inflammatory markers are associated with plaque progression and instability in the general population [219,220], the combination of OPG, OPN, and TWEAK showed good discriminative power suggesting that marker combinations integrating different biological mechanisms might better stratify CVD risk in CKD [218].
In this scenario, CKD can be considered as an atypical disease, whose CVD outcomes depend on both traditional and new risk factors [210]. Although the underlying mechanisms linking CKD to accelerated CVD remain to be fully elucidated, the level fluctuations of OPG, TRAIL, and TWEAK influence the predictability of CVD outcomes in CKD patients. Overall, a multi-marker analysis strategy integrating these biomarkers with other known CVD risk factors might improve clinical risk prediction of CVD in kidney disease.
Conclusion perspectives
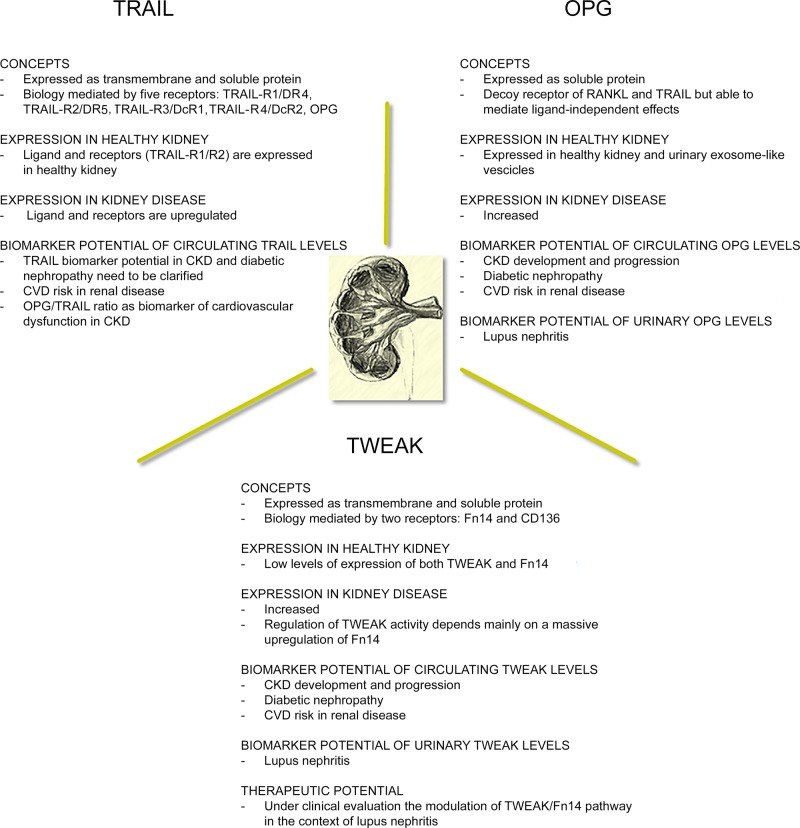
Several lines of evidence suggest a specific involvement of TRAIL, OPG, and TWEAK in the pathogenesis of kidney disease, such that they are regarded as new potential biomarkers of kidney disease and its complications, as well as potential therapeutic targets (Figure 3). In particular, ongoing clinical studies on renal diseases have already included OPG and TWEAK as biomarkers to monitor disease-related progression and complications. Besides circulating OPG levels, also urinary OPG has been proposed as a biomarker of disease status and prognosis in this setting. Likewise, due to a large body of evidence that has highlighted the harmful role of TWEAK in kidney injury, the TWEAK–Fn14 system is emerging not only as a potential biomarker of renal disease but also as a promising therapeutic target, and strategies blocking TWEAK/Fn14, which appear to be safe and well tolerated, are under clinical investigation. By contrast, the significance of TRAIL in the setting of kidney disease remains to be fully elucidated.
Figure 3. Snapshot of key suggestions on the involvement of the TRAIL/OPG/TWEAK triad in kidney disease.
The figure summarizes evidence and suggestions about the role of the TNF-superfamily triad in kidney disease in the light of potential clinical translations as disease biomarkers and/or therapeutic strategies.
Further studies are needed to clarify if OPG has a causative role in kidney disease, to understand the exact role of TRAIL in renal disease development/progression, and to optimize TWEAK-Fn14-targeting therapeutics. Overall, due to their belonging to the same family, a better comprehension of their changes and specific effects, alone and in combination, might facilitate the identification of new diagnostic/therapeutic strategies, in line with the concept that it is the integration of biological, molecular, and genetic data that will allow the identification and validation of new clinically-relevant biomarkers for kidney disease [221].
Abbreviations
- AKI
acute kidney injury
- CKD
chronic kidney disease
- CNS
central nervous system
- CV
cardiovascular
- CVD
cardiovascular disease
- DcR
decoy receptor
- DD
death domain
- DR
death receptors
- ERK
extracellular signal regulated kinase
- ESRD
end-stage renal disease
- FGF-23
fibroblast growth factor-23
- Fn14
fibroblast growth factor-inducible molecule 14
- MAPK
mitogen-activated protein kinase
- MCNS
minimal-change nephrotic syndrome
- NF-kB
nuclear factor kB
- OPG
osteoprotegerin
- OPN
osteopontin
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- RANK
receptor activator of nuclear factor kappa-Β
- RANKL
RANK ligand
- ROS
reactive oxygen species
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TNF
tumor necrosis factor
- TRAF
TNF receptor-associated factors
- TRAIL
TNF-related apoptosis inducing ligand
- TWEAK
TNF-like weaker inducer of apoptosis
- uTWEAK
urinary TWEAK
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Locksley R.M., Killeen N. and Lenardo M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B.B. (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 10.1038/nri1184 [DOI] [PubMed] [Google Scholar]
- 3.Bodmer J.L., Schneider P. and Tschopp J. (2002) The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 10.1016/S0968-0004(01)01995-8 [DOI] [PubMed] [Google Scholar]
- 4.Vanamee E.S. and Faustman D.L. (2018) Structural principles of tumor necrosis factor superfamily signaling. Sci. Signaling 11, 10.1126/scisignal.aao4910 [DOI] [PubMed] [Google Scholar]
- 5.Croft M., Benedict C.A. and Ware C.F. (2013) Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discovery 12, 147–168 10.1038/nrd3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegmund D., Lang I. and Wajant H. (2017) Cell death-independent activities of the death receptors CD95, TRAILR1, and TRAILR2. FEBS J. 284, 1131–1159 10.1111/febs.13968 [DOI] [PubMed] [Google Scholar]
- 7.Voltan R., Secchiero P., Casciano F., Milani D., Zauli G. and Tisato V. (2016) Redox signaling and oxidative stress: cross talk with TNF-related apoptosis inducing ligand activity. Int. J. Biochem. Cell Biol. 81, 364–374 10.1016/j.biocel.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 8.Mahalingam D., Szegezdi E., Keane M., de Jong S. and Samali A. (2009) TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat. Rev. 35, 280–288 10.1016/j.ctrv.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Falschlehner C., Emmerich C.H., Gerlach B. and Walczak H. (2007) TRAIL signalling: decisions between life and death. Int. J. Biochem. Cell Biol. 39, 1462–1475 10.1016/j.biocel.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 10.MacFarlane M. (2003) TRAIL-induced signalling and apoptosis. Toxicol. Lett. 139, 89–97 10.1016/S0378-4274(02)00422-8 [DOI] [PubMed] [Google Scholar]
- 11.Agostinis C., Bulla R., Tisato V., De Seta F., Alberico S., Secchiero P.. et al. (2012) Soluble TRAIL is elevated in recurrent miscarriage and inhibits the in vitro adhesion and migration of HTR8 trophoblastic cells. Hum. Reprod. 27, 2941–2947 10.1093/humrep/des289 [DOI] [PubMed] [Google Scholar]
- 12.Biolo G., Secchiero P., De Giorgi S., Tisato V. and Zauli G. (2012) The energy balance positively regulates the levels of circulating TNF-related apoptosis inducing ligand in humans. Clin. Nutr. 31, 1018–1021 10.1016/j.clnu.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 13.Morano D., Rolfo A., Tisato V., Farina A., Rimondi E., Scutiero G.. et al. (2018) Lower maternal serum tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) levels in early preeclampsia. A retrospective study. Pregnancy Hypertension. 12, 1–5 10.1016/j.preghy.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 14.Tisato V., Gonelli A., Voltan R., Secchiero P. and Zauli G. (2016) Clinical perspectives of TRAIL: insights into central nervous system disorders. Cell. Mol. Life Sci. 73, 2017–2027 10.1007/s00018-016-2164-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tisato V., Secchiero P., Bonaccorsi G., Bergamini C., Greco P., Zauli G.. et al. (2017) Low circulating TRAIL levels are associated with increase of resistin and lipocalin-2/ngal adipokines in postmenopausal women. Mediators Inflamm. 2017, 5356020 10.1155/2017/5356020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toffoli B., Fabris B., Bartelloni G., Bossi F. and Bernardi S. (2016) Dyslipidemia and diabetes increase the OPG/TRAIL ratio in the cardiovascular system. Mediators Inflamm. 2016, 6529728 10.1155/2016/6529728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tornese G., Iafusco D., Monasta L., Agnoletto C., Tisato V., Ventura A.. et al. (2014) The levels of circulating TRAIL at the onset of type 1 diabetes are markedly decreased in patients with ketoacidosis and with the highest insulin requirement. Acta Diabetol. 51, 239–246 10.1007/s00592-013-0507-5 [DOI] [PubMed] [Google Scholar]
- 18.Singh A.V., Subhashree L., Milani P., Gemmati D. and Zamboni P. (2010) Interplay of iron metallobiology, metalloproteinases, and FXIII, and role of their gene variants in venous leg ulcer. Int. J. Lower Extremity Wounds 9, 166–179 10.1177/1534734610384653 [DOI] [PubMed] [Google Scholar]
- 19.Tornese G., Tisato V., Monasta L., Vecchi Brumatti L., Zauli G. and Secchiero P. (2015) Serum TRAIL levels increase shortly after insulin therapy and metabolic stabilization in children with type 1 diabetes mellitus. Acta Diabetol. 52, 1003–1006 10.1007/s00592-015-0731-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zauli G., Tisato V., Melloni E., Volpato S., Cervellati C., Bonaccorsi G.. et al. (2014) Inverse correlation between circulating levels of TNF-related apoptosis-inducing ligand and 17beta-estradiol. J. Clin. Endocrinol. Metab. 99, E659–64 10.1210/jc.2013-4193 [DOI] [PubMed] [Google Scholar]
- 21.Tisato V., Rimondi E., Brombo G., Volpato S., Zurlo A., Zauli G.. et al. (2016) Serum soluble tumor necrosis factor-related apoptosis-inducing ligand levels in older subjects with dementia and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 41, 273–280 10.1159/000446275 [DOI] [PubMed] [Google Scholar]
- 22.Bernardi S., Toffoli B., Tisato V., Bossi F., Biffi S., Lorenzon A.. et al. (2018) TRAIL reduces impaired glucose tolerance and NAFLD in the high-fat diet fed mouse. Clin. Sci. 132, 69–83 10.1042/CS20171221 [DOI] [PubMed] [Google Scholar]
- 23.Tisato V., Garrovo C., Biffi S., Petrera F., Voltan R., Casciano F.. et al. (2014) Intranasal administration of recombinant TRAIL down-regulates CXCL-1/KC in an ovalbumin-induced airway inflammation murine model. PLoS One 9, e115387 10.1371/journal.pone.0115387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bossi F., Bernardi S., Zauli G., Secchiero P. and Fabris B. (2015) TRAIL modulates the immune system and protects against the development of diabetes. J. Immunol. Res. 2015, 680749 10.1155/2015/680749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardi S., Zauli G., Tikellis C., Candido R., Fabris B., Secchiero P.. et al. (2012) TNF-related apoptosis-inducing ligand significantly attenuates metabolic abnormalities in high-fat-fed mice reducing adiposity and systemic inflammation. Clin. Sci. 123, 547–555 10.1042/CS20120176 [DOI] [PubMed] [Google Scholar]
- 26.Zauli G., Toffoli B., di Iasio M.G., Celeghini C., Fabris B. and Secchiero P. (2010) Treatment with recombinant tumor necrosis factor-related apoptosis-inducing ligand alleviates the severity of streptozotocin-induced diabetes. Diabetes 59, 1261–1265 10.2337/db09-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Luthy R.. et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 10.1016/S0092-8674(00)80209-3 [DOI] [PubMed] [Google Scholar]
- 28.Tan K.B., Harrop J., Reddy M., Young P., Terrett J., Emery J.. et al. (1997) Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene 204, 35–46 10.1016/S0378-1119(97)00509-X [DOI] [PubMed] [Google Scholar]
- 29.Tsuda E., Goto M., Mochizuki S., Yano K., Kobayashi F., Morinaga T.. et al. (1997) Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem. Biophys. Res. Commun. 234, 137–142 10.1006/bbrc.1997.6603 [DOI] [PubMed] [Google Scholar]
- 30.Emery J.G., McDonnell P., Burke M.B., Deen K.C., Lyn S., Silverman C.. et al. (1998) Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 273, 14363–14367 10.1074/jbc.273.23.14363 [DOI] [PubMed] [Google Scholar]
- 31.Rochette L., Meloux A., Rigal E., Zeller M., Cottin Y. and Vergely C. (2018) The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Therapeutics 182, 115–132 10.1016/j.pharmthera.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 32.Bernardi S., Bossi F., Toffoli B. and Fabris B. (2016) Roles and clinical applications of OPG and TRAIL as biomarkers in cardiovascular disease. Biomed. Res. Int. 2016, 1752854 10.1155/2016/1752854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chicheportiche Y., Bourdon P.R., Xu H., Hsu Y.M., Scott H., Hession C.. et al. (1997) TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 272, 32401–32410 10.1074/jbc.272.51.32401 [DOI] [PubMed] [Google Scholar]
- 34.Trebing J., Arana J.A., Salzmann S. and Wajant H. (2014) Analyzing the signaling capabilities of soluble and membrane TWEAK. Methods Mol. Biol. 1155, 31–45 10.1007/978-1-4939-0669-7_4 [DOI] [PubMed] [Google Scholar]
- 35.Levin A., Tonelli M., Bonventre J., Coresh J., Donner J.A., Fogo A.B.. et al. (2017) Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390, 1888–1917 10.1016/S0140-6736(17)30788-2 [DOI] [PubMed] [Google Scholar]
- 36.Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K.. et al. (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3, 673–682 10.1016/1074-7613(95)90057-8 [DOI] [PubMed] [Google Scholar]
- 37.Pitti R.M., Marsters S.A., Ruppert S., Donahue C.J., Moore A. and Ashkenazi A. (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 271, 12687–12690 10.1074/jbc.271.22.12687 [DOI] [PubMed] [Google Scholar]
- 38.Ashkenazi A. and Herbst R.S. (2008) To kill a tumor cell: the potential of proapoptotic receptor agonists. J. Clin. Invest. 118, 1979–1990 10.1172/JCI34359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J.H., Kirkiles-Smith N.C., McNiff J.M. and Pober J.S. (2003) TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J. Immunol. 171, 1526–1533 10.4049/jimmunol.171.3.1526 [DOI] [PubMed] [Google Scholar]
- 40.Li X., Han W.Q., Boini K.M., Xia M., Zhang Y. and Li P.L. (2013) TRAIL death receptor 4 signaling via lysosome fusion and membrane raft clustering in coronary arterial endothelial cells: evidence from ASM knockout mice. J. Mol. Med. 91, 25–36 10.1007/s00109-012-0968-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delmas D., Rebe C., Micheau O., Athias A., Gambert P., Grazide S.. et al. (2004) Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene 23, 8979–8986 10.1038/sj.onc.1208086 [DOI] [PubMed] [Google Scholar]
- 42.Tisato V., Gallo S., Melloni E., Celeghini C., Passaro A., Zauli G.. et al. (2018) TRAIL and ceruloplasmin inverse correlation as a representative crosstalk between inflammation and oxidative stress. Mediators Inflamm. 2018, 9629537 10.1155/2018/9629537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan G., O’Rourke K., Chinnaiyan A.M., Gentz R., Ebner R., Ni J.. et al. (1997) The receptor for the cytotoxic ligand TRAIL. Science 276, 111–113 10.1126/science.276.5309.111 [DOI] [PubMed] [Google Scholar]
- 44.Bernardi S., Secchiero P. and Zauli G. (2012) State of art and recent developments of anti-cancer strategies based on TRAIL. Recent Pat. Anticancer Drug Discov. 7, 207–217 10.2174/157489212799972927 [DOI] [PubMed] [Google Scholar]
- 45.Lim B., Allen J.E., Prabhu V.V., Talekar M.K., Finnberg N.K. and El-Deiry W.S. (2015) Targeting TRAIL in the treatment of cancer: new developments. Expert Opin. Ther. Targets 19, 1171–1185 10.1517/14728222.2015.1049838 [DOI] [PubMed] [Google Scholar]
- 46.Sheridan J.P., Marsters S.A., Pitti R.M., Gurney A., Skubatch M., Baldwin D.. et al. (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277, 818–821 10.1126/science.277.5327.818 [DOI] [PubMed] [Google Scholar]
- 47.Goodall M.L., Fitzwalter B.E., Zahedi S., Wu M., Rodriguez D., Mulcahy-Levy J.M.. et al. (2016) The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev. Cell 37, 337–349 10.1016/j.devcel.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jouan-Lanhouet S., Arshad M.I., Piquet-Pellorce C., Martin-Chouly C., Le Moigne-Muller G., Van Herreweghe F.. et al. (2012) TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 19, 2003–2014 10.1038/cdd.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills K.R., Reginato M., Debnath J., Queenan B. and Brugge J.S. (2004) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. PNAS 101, 3438–3443 10.1073/pnas.0400443101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He W., Wang Q., Xu J., Xu X., Padilla M.T., Ren G.. et al. (2012) Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2- and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy 8, 1811–1821 10.4161/auto.22145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degli-Esposti M.A., Dougall W.C., Smolak P.J., Waugh J.Y., Smith C.A. and Goodwin R.G. (1997) The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7, 813–820 10.1016/S1074-7613(00)80399-4 [DOI] [PubMed] [Google Scholar]
- 52.Chaudhary P.M., Eby M., Jasmin A., Bookwalter A., Murray J. and Hood L. (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7, 821–830 10.1016/S1074-7613(00)80400-8 [DOI] [PubMed] [Google Scholar]
- 53.Secchiero P., Gonelli A., Carnevale E., Milani D., Pandolfi A., Zella D.. et al. (2003) TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation 107, 2250–2256 10.1161/01.CIR.0000062702.60708.C4 [DOI] [PubMed] [Google Scholar]
- 54.Zauli G., Sancilio S., Cataldi A., Sabatini N., Bosco D. and Di Pietro R. (2005) PI-3K/Akt and NF-kappaB/IkappaBalpha pathways are activated in Jurkat T cells in response to TRAIL treatment. J. Cell. Physiol. 202, 900–911 10.1002/jcp.20202 [DOI] [PubMed] [Google Scholar]
- 55.Chen D., Yu J. and Zhang L. (2016) Necroptosis: an alternative cell death program defending against cancer. Biochim. Biophys. Acta 1865, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Secchiero P., Gonelli A., Corallini F., Ceconi C., Ferrari R. and Zauli G. (2010) Metalloproteinase 2 cleaves in vitro recombinant TRAIL: potential implications for the decreased serum levels of TRAIL after acute myocardial infarction. Atherosclerosis 211, 333–336 10.1016/j.atherosclerosis.2010.02.024 [DOI] [PubMed] [Google Scholar]
- 57.Bernardi S., Bossi F., Toffoli B., Giudici F., Bramante A., Furlanis G.. et al. (2017) Association between thyroid hormones and TRAIL. Clin. Biochem. 50, 972–976 10.1016/j.clinbiochem.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 58.Secchiero P., Corallini F., Ceconi C., Parrinello G., Volpato S., Ferrari R.. et al. (2009) Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS One 4, e4442 10.1371/journal.pone.0004442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michowitz Y., Goldstein E., Roth A., Afek A., Abashidze A., Ben Gal Y.. et al. (2005) The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J. Am. Coll. Cardiol. 45, 1018–1024 10.1016/j.jacc.2004.12.065 [DOI] [PubMed] [Google Scholar]
- 60.Volpato S., Ferrucci L., Secchiero P., Corallini F., Zuliani G., Fellin R.. et al. (2011) Association of tumor necrosis factor-related apoptosis-inducing ligand with total and cardiovascular mortality in older adults. Atherosclerosis 215, 452–458 10.1016/j.atherosclerosis.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niessner A., Hohensinner P.J., Rychli K., Neuhold S., Zorn G., Richter B.. et al. (2009) Prognostic value of apoptosis markers in advanced heart failure patients. Eur. Heart J. 30, 789–796 10.1093/eurheartj/ehp004 [DOI] [PubMed] [Google Scholar]
- 62.Bernardi S., Norcio A., Toffoli B., Zauli G. and Secchiero P. (2012) Potential role of TRAIL in the management of autoimmune diabetes mellitus. Curr. Pharm. Des. 18, 5759–5765 10.2174/138161212803530925 [DOI] [PubMed] [Google Scholar]
- 63.Secchiero P., Candido R., Corallini F., Zacchigna S., Toffoli B., Rimondi E.. et al. (2006) Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation 114, 1522–1530 10.1161/CIRCULATIONAHA.106.643841 [DOI] [PubMed] [Google Scholar]
- 64.Schoppet M., Henser S., Ruppert V., Stubig T., Al-Fakhri N., Maisch B.. et al. (2007) Osteoprotegerin expression in dendritic cells increases with maturation and is NF-kappaB-dependent. J. Cell. Biochem. 100, 1430–1439 10.1002/jcb.21129 [DOI] [PubMed] [Google Scholar]
- 65.Yasuda H., Shima N., Nakagawa N., Mochizuki S.I., Yano K., Fujise N.. et al. (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139, 1329–1337 10.1210/endo.139.3.5837 [DOI] [PubMed] [Google Scholar]
- 66.Boyce B.F. and Xing L. (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Therapy 9, S1 10.1186/ar2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu H., Lacey D.L., Dunstan C.R., Solovyev I., Colombero A., Timms E.. et al. (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. PNAS 96, 3540–3545 10.1073/pnas.96.7.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaguchi K., Kinosaki M., Goto M., Kobayashi F., Tsuda E., Morinaga T.. et al. (1998) Characterization of structural domains of human osteoclastogenesis inhibitory factor. J. Biol. Chem. 273, 5117–5123 10.1074/jbc.273.9.5117 [DOI] [PubMed] [Google Scholar]
- 69.Mosheimer B.A., Kaneider N.C., Feistritzer C., Djanani A.M., Sturn D.H., Patsch J.R.. et al. (2005) Syndecan-1 is involved in osteoprotegerin-induced chemotaxis in human peripheral blood monocytes. J. Clin. Endocrinol. Metab. 90, 2964–2971 10.1210/jc.2004-1895 [DOI] [PubMed] [Google Scholar]
- 70.Nagy E.E., Varga-Fekete T., Puskas A., Kelemen P., Brassai Z., Szekeres-Csiki K.. et al. (2016) High circulating osteoprotegerin levels are associated with non-zero blood groups. BMC Cardiovasc. Disord. 16, 106 10.1186/s12872-016-0287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiechl S., Schett G., Wenning G., Redlich K., Oberhollenzer M., Mayr A.. et al. (2004) Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109, 2175–2180 10.1161/01.CIR.0000127957.43874.BB [DOI] [PubMed] [Google Scholar]
- 72.Ueland T., Yndestad A., Oie E., Florholmen G., Halvorsen B., Froland S.S.. et al. (2005) Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation 111, 2461–2468 10.1161/01.CIR.0000165119.62099.14 [DOI] [PubMed] [Google Scholar]
- 73.Bernardi S., Fabris B., Thomas M., Toffoli B., Tikellis C., Candido R.. et al. (2014) Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol. Cell. Endocrinol. 394, 13–20 10.1016/j.mce.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 74.Jono S., Otsuki S., Higashikuni Y., Shioi A., Mori K., Hara K.. et al. (2010) Serum osteoprotegerin levels and long-term prognosis in subjects with stable coronary artery disease. J. Thromb. Haemost. 8, 1170–1175 10.1111/j.1538-7836.2010.03833.x [DOI] [PubMed] [Google Scholar]
- 75.Vik A., Mathiesen E.B., Brox J., Wilsgaard T., Njolstad I., Jorgensen L.. et al. (2011) Serum osteoprotegerin is a predictor for incident cardiovascular disease and mortality in a general population: the Tromso Study. J. Thromb. Haemost. 9, 638–644 10.1111/j.1538-7836.2011.04222.x [DOI] [PubMed] [Google Scholar]
- 76.Venuraju S.M., Yerramasu A., Corder R. and Lahiri A. (2010) Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J. Am. Coll. Cardiol. 55, 2049–2061 10.1016/j.jacc.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 77.Wiley S.R., Cassiano L., Lofton T., Davis-Smith T., Winkles J.A., Lindner V.. et al. (2001) A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 15, 837–846 10.1016/S1074-7613(01)00232-1 [DOI] [PubMed] [Google Scholar]
- 78.Bover L.C., Cardo-Vila M., Kuniyasu A., Sun J., Rangel R., Takeya M.. et al. (2007) A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J. Immunol. 178, 8183–8194 10.4049/jimmunol.178.12.8183 [DOI] [PubMed] [Google Scholar]
- 79.Polek T.C., Talpaz M., Darnay B.G. and Spivak-Kroizman T. (2003) TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J. Biol. Chem. 278, 32317–32323 10.1074/jbc.M302518200 [DOI] [PubMed] [Google Scholar]
- 80.Maecker H., Varfolomeev E., Kischkel F., Lawrence D., LeBlanc H., Lee W.. et al. (2005) TWEAK attenuates the transition from innate to adaptive immunity. Cell 123, 931–944 10.1016/j.cell.2005.09.022 [DOI] [PubMed] [Google Scholar]
- 81.Donohue P.J., Richards C.M., Brown S.A., Hanscom H.N., Buschman J., Thangada S.. et al. (2003) TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler. Thromb. Vasc. Biol. 23, 594–600 10.1161/01.ATV.0000062883.93715.37 [DOI] [PubMed] [Google Scholar]
- 82.Frejo L., Requena T., Okawa S., Gallego-Martinez A., Martinez-Bueno M., Aran I.. et al. (2017) Regulation of Fn14 Receptor and NF-kappaB Underlies Inflammation in Meniere’s Disease. Front. Immunol. 8, 1739 10.3389/fimmu.2017.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boulamery A. and Desplat-Jego S. (2017) Regulation of neuroinflammation: what role for the tumor necrosis factor-like weak inducer of apoptosis/Fn14 pathway? Front. Immunol. 8, 1534 10.3389/fimmu.2017.01534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Q., Xiao S. and Xia Y. (2017) TWEAK/Fn14 activation participates in skin inflammation. Mediators Inflamm. 2017, 6746870 10.1155/2017/6746870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun F., Teng J., Yu P., Li W., Chang J. and Xu H. (2018) Involvement of TWEAK and the NF-kappaB signaling pathway in lupus nephritis. Exp. Therapeutic Med. 15, 2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Escote X., Gomez-Zorita S., Lopez-Yoldi M., Milton-Laskibar I., Fernandez-Quintela A., Martinez J.A.. et al. (2017) Role of omentin, vaspin, cardiotrophin-1, TWEAK and NOV/CCN3 in obesity and diabetes development. Int. J. Mol. Sci. 18, 10.3390/ijms18081770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J., Wei L. and Xia Y. (2017) Roles of tumour necrosis factor-related weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway in lupus nephritis. Nephrology 22, 101–106 10.1111/nep.12957 [DOI] [PubMed] [Google Scholar]
- 88.Perez J.G., Tran N.L., Rosenblum M.G., Schneider C.S., Connolly N.P., Kim A.J.. et al. (2016) The TWEAK receptor Fn14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics. Oncogene 35, 2145–2155 10.1038/onc.2015.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lassen U.N., Meulendijks D., Siu L.L., Karanikas V., Mau-Sorensen M., Schellens J.H.. et al. (2015) A phase I monotherapy study of RG7212, a first-in-class monoclonal antibody targeting TWEAK signaling in patients with advanced cancers. Clin. Cancer Res.: An Off. J. Am. Assoc. Cancer Res. 21, 258–266 10.1158/1078-0432.CCR-14-1334 [DOI] [PubMed] [Google Scholar]
- 90.Ye S., Fox M.I., Belmar N.A., Sho M., Chao D.T., Choi D.. et al. (2017) Enavatuzumab, a humanized anti-TWEAK receptor monoclonal antibody, exerts antitumor activity through attracting and activating innate immune effector cells. J. Immunol. Res. 2017, 5737159 10.1155/2017/5737159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam E.T., Eckhardt S.G., Messersmith W., Jimeno A., O’Bryant C.L., Ramanathan R.K.. et al. (2018) Phase I study of enavatuzumab, a first-in-class humanized monoclonal antibody targeting the TWEAK receptor, in patients with advanced solid tumors. Mol. Cancer Ther. 17, 215–221 10.1158/1535-7163.MCT-17-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Culp P.A., Choi D., Zhang Y., Yin J., Seto P., Ybarra S.E.. et al. (2010) Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clin. Cancer Res.: An Off. J. Am. Assoc. Cancer Res. 16, 497–508 10.1158/1078-0432.CCR-09-1929 [DOI] [PubMed] [Google Scholar]
- 93.Hu G., Zeng W. and Xia Y. (2017) TWEAK/Fn14 signaling in tumors. Tumour Biol. 39, 1010428317714624 10.1177/1010428317714624 [DOI] [PubMed] [Google Scholar]
- 94.Henaut L., Sanz A.B., Martin-Sanchez D., Carrasco S., Villa-Bellosta R., Aldamiz-Echevarria G.. et al. (2016) TWEAK favors phosphate-induced calcification of vascular smooth muscle cells through canonical and non-canonical activation of NFkappaB. Cell Death Dis. 7, e2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burkly L.C. (2015) Regulation of tissue responses: the TWEAK/Fn14 pathway and other TNF/TNFR superfamily members that activate non-canonical NFkappaB signaling. Front. Immunol. 6, 92 10.3389/fimmu.2015.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanz A.B., Sanchez-Nino M.D., Izquierdo M.C., Jakubowski A., Justo P., Blanco-Colio L.M.. et al. (2010) TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One 5, e8955 10.1371/journal.pone.0008955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saitoh T., Nakayama M., Nakano H., Yagita H., Yamamoto N. and Yamaoka S. (2003) TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J. Biol. Chem. 278, 36005–36012 10.1074/jbc.M304266200 [DOI] [PubMed] [Google Scholar]
- 98.Lambert M., Pepin G., Peralta-Zaragoza O., Matusiak R., Ly S., Landry P.. et al. (2016) TWEAK Negatively Regulates Human Dicer. Non-Coding RNA 2, 10.3390/ncrna2040012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burkly L.C. (2014) TWEAK/Fn14 axis: the current paradigm of tissue injury-inducible function in the midst of complexities. Semin. Immunol. 26, 229–236 10.1016/j.smim.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 100.Lorz C., Benito-Martin A., Boucherot A., Ucero A.C., Rastaldi M.P., Henger A.. et al. (2008) The death ligand TRAIL in diabetic nephropathy. J. Am. Soc. Nephrol. 19, 904–914 10.1681/ASN.2007050581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yun T.J., Chaudhary P.M., Shu G.L., Frazer J.K., Ewings M.K., Schwartz S.M.. et al. (1998) OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J. Immunol. 161, 6113–6121 [PubMed] [Google Scholar]
- 102.Devarapu S.K., Grill J.F., Xie J., Weidenbusch M., Honarpisheh M., Vielhauer V.. et al. (2017) Tumor necrosis factor superfamily ligand mRNA expression profiles differ between humans and mice during homeostasis and between various murine kidney injuries. J. Biomed. Sci. 24, 77 10.1186/s12929-017-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toffoli B., Pickering R.J., Tsorotes D., Wang B., Bernardi S., Kantharidis P.. et al. (2011) Osteoprotegerin promotes vascular fibrosis via a TGF-beta1 autocrine loop. Atherosclerosis 218, 61–68 10.1016/j.atherosclerosis.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 104.Cretney E., Takeda K., Yagita H., Glaccum M., Peschon J.J. and Smyth M.J. (2002) Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 168, 1356–1361 10.4049/jimmunol.168.3.1356 [DOI] [PubMed] [Google Scholar]
- 105.Bucay N., Sarosi I., Dunstan C.R., Morony S., Tarpley J., Capparelli C.. et al. (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 12, 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spierings D.C., de Vries E.G., Vellenga E., van den Heuvel F.A., Koornstra J.J., Wesseling J.. et al. (2004) Tissue distribution of the death ligand TRAIL and its receptors. J. Histochem. Cytochem.: Off. J. Histochemistry Soc. 52, 821–831 10.1369/jhc.3A6112.2004 [DOI] [PubMed] [Google Scholar]
- 107.Nguyen V., Cudrici C., Zernetkina V., Niculescu F., Rus H., Drachenberg C.. et al. (2009) TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects. Clin. Immunol. 132, 32–42 10.1016/j.clim.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benito-Martin A., Ucero A.C., Zubiri I., Posada-Ayala M., Fernandez-Fernandez B., Cannata-Ortiz P.. et al. (2013) Osteoprotegerin in exosome-like vesicles from human cultured tubular cells and urine. PLoS One 8, e72387 10.1371/journal.pone.0072387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campbell S., Burkly L.C., Gao H.X., Berman J.W., Su L., Browning B.. et al. (2006) Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J. Immunol. 176, 1889–1898 10.4049/jimmunol.176.3.1889 [DOI] [PubMed] [Google Scholar]
- 110.Gao H.X., Campbell S.R., Burkly L.C., Jakubowski A., Jarchum I., Banas B.. et al. (2009) TNF-like weak inducer of apoptosis (TWEAK) induces inflammatory and proliferative effects in human kidney cells. Cytokine 46, 24–35 10.1016/j.cyto.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 111.Burkly L.C., Michaelson J.S., Hahm K., Jakubowski A. and Zheng T.S. (2007) TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine 40, 1–16 10.1016/j.cyto.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 112.Sanz A.B., Izquierdo M.C., Sanchez-Nino M.D., Ucero A.C., Egido J., Ruiz-Ortega M.. et al. (2014) TWEAK and the progression of renal disease: clinical translation. Nephrol. Dial. Transplant. 29, i54–i62 10.1093/ndt/gft342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanz A.B., Ruiz-Andres O., Sanchez-Nino M.D., Ruiz-Ortega M., Ramos A.M. and Ortiz A. (2016) Out of the TWEAKlight: elucidating the role of Fn14 and TWEAK in acute kidney injury. Semin. Nephrol. 36, 189–198 10.1016/j.semnephrol.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 114.Sanz A.B., Sanchez-Nino M.D., Izquierdo M.C., Jakubowski A., Justo P., Blanco-Colio L.M.. et al. (2009) Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J. Cell. Mol. Med. 13, 3329–3342 10.1111/j.1582-4934.2009.00766.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Justo P., Sanz A.B., Sanchez-Nino M.D., Winkles J.A., Lorz C., Egido J.. et al. (2006) Cytokine cooperation in renal tubular cell injury: the role of TWEAK. Kidney Int. 70, 1750–1758 10.1038/sj.ki.5001866 [DOI] [PubMed] [Google Scholar]
- 116.Hoste E.A., Kellum J.A., Selby N.M., Zarbock A., Palevsky P.M., Bagshaw S.M.. et al. (2018) Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625 10.1038/s41581-018-0052-0 [DOI] [PubMed] [Google Scholar]
- 117.Romagnani P., Remuzzi G., Glassock R., Levin A., Jager K.J., Tonelli M.. et al. (2017) Chronic kidney disease. Nat. Rev. Dis. Primers 3, 17088 10.1038/nrdp.2017.88 [DOI] [PubMed] [Google Scholar]
- 118.He L., Wei Q., Liu J., Yi M., Liu Y., Liu H.. et al. (2017) AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92, 1071–1083 10.1016/j.kint.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Susantitaphong P., Cruz D.N., Cerda J., Abulfaraj M., Alqahtani F., Koulouridis I.. et al. (2013) World incidence of AKI: a meta-analysis. Clin. J. Am. Soc. Nephrol. 8, 1482–1493 10.2215/CJN.00710113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomas M.C., Cooper M.E. and Zimmet P. (2016) Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 12, 73–81 10.1038/nrneph.2015.173 [DOI] [PubMed] [Google Scholar]
- 121.Okuyama S., Komatsuda A., Wakui H., Aiba N., Fujishima N., Iwamoto K.. et al. (2005) Up-regulation of TRAIL mRNA expression in peripheral blood mononuclear cells from patients with minimal-change nephrotic syndrome. Nephrol. Dial. Transplant. 20, 539–544 10.1093/ndt/gfh673 [DOI] [PubMed] [Google Scholar]
- 122.Leng X., Zhang Q., Chen Z. and Wang D. (2014) Blocking TRAIL-DR5 signaling with soluble DR5 alleviates acute kidney injury in a severely burned mouse model. Int. J. Clin. Exp. Pathol. 7, 3460–3468 [PMC free article] [PubMed] [Google Scholar]
- 123.Adachi T., Sugiyama N., Gondai T., Yagita H. and Yokoyama T. (2013) Blockade of death ligand TRAIL inhibits renal ischemia reperfusion injury. Acta Histochem. Cytochem. 46, 161–170 10.1267/ahc.13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sari F., Yalcin A.D., Genc G.E., Sarikaya M., Bisgin A., Cetinkaya R.. et al. (2016) Autosomal dominant polycystic disease is associated with depressed levels of soluble tumor necrosis factor-related apoptosis-inducing ligand. Balkan Med. J. 33, 512–516 10.5152/balkanmedj.2016.150685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rudnicki M., Perco P., B D.H., Leierer J., Heinzel A., Muhlberger I.. et al. (2016) Renal microRNA- and RNA-profiles in progressive chronic kidney disease. Eur. J. Clin. Invest. 46, 213–226 10.1111/eci.12585 [DOI] [PubMed] [Google Scholar]
- 126.Carlsson A.C., Ingelsson E., Sundstrom J., Carrero J.J., Gustafsson S., Feldreich T.. et al. (2017) Use of proteomics to investigate kidney function decline over 5 years. Clin. J. Am. Soc. Nephrol. 12, 1226–1235 10.2215/CJN.08780816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liabeuf S., Barreto D.V., Barreto F.C., Chasseraud M., Brazier M., Choukroun G.. et al. (2010) The circulating soluble TRAIL is a negative marker for inflammation inversely associated with the mortality risk in chronic kidney disease patients. Nephrol. Dial. Transplant. 25, 2596–2602 10.1093/ndt/gfq042 [DOI] [PubMed] [Google Scholar]
- 128.Kazama J.J., Shigematsu T., Yano K., Tsuda E., Miura M., Iwasaki Y.. et al. (2002) Increased circulating levels of osteoclastogenesis inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am. J. Kidney Dis. 39, 525–532 10.1053/ajkd.2002.31402 [DOI] [PubMed] [Google Scholar]
- 129.Upadhyay A., Larson M.G., Guo C.Y., Vasan R.S., Lipinska I., O’Donnell C.J.. et al. (2011) Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol. Dial. Transplant. 26, 920–926 10.1093/ndt/gfq471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rashtchizadeh N., Ghorbanihaghjo A., Argani H., Mahmoudi Meimand S., Safa J., Vatankhahan H.. et al. (2012) Serum receptor activator of nuclear factor-kappa B ligand, osteoprotegrin, and intact parathyroid hormone in hemodialysis and renal transplant patients. Ther. Apher. Dial. 16, 600–604 10.1111/j.1744-9987.2012.01097.x [DOI] [PubMed] [Google Scholar]
- 131.Mohamed G.B. and Abdel-Latif E.A. (2011) Serum osteoprotegerin (OPG) in children with primary nephrotic syndrome. Saudi J. Kidney Dis. Transpl. 22, 955–962 [PubMed] [Google Scholar]
- 132.Bernardi S., Toffoli B., Bossi F., Candido R., Stenner E., Carretta R.. et al. (2017) Circulating osteoprotegerin is associated with chronic kidney disease in hypertensive patients. BMC Nephrol. 18, 219 10.1186/s12882-017-0625-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lewis J.R., Lim W.H., Zhu K., Wong G., Dhaliwal S.S., Lim E.M.. et al. (2014) Elevated osteoprotegerin predicts declining renal function in elderly women: a 10-year prospective cohort study. Am. J. Nephrol. 39, 66–74 10.1159/000357787 [DOI] [PubMed] [Google Scholar]
- 134.Kiani A.N., Johnson K., Chen C., Diehl E., Hu H., Vasudevan G.. et al. (2009) Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J. Rheumatol. 36, 2224–2230 10.3899/jrheum.081112 [DOI] [PubMed] [Google Scholar]
- 135.Gupta R., Aggarwal A., Sinha S., Rajasekhar L., Yadav A., Gaur P.. et al. (2016) Urinary osteoprotegerin: a potential biomarker of lupus nephritis disease activity. Lupus 25, 1230–1236 10.1177/0961203316636470 [DOI] [PubMed] [Google Scholar]
- 136.Candido R. (2014) The osteoprotegerin/tumor necrosis factor related apoptosis-inducing ligand axis in the kidney. Curr. Opin. Nephrol. Hypertens. 23, 69–74 10.1097/01.mnh.0000437611.42417.7a [DOI] [PubMed] [Google Scholar]
- 137.Montanez-Barragan A., Gomez-Barrera I., Sanchez-Nino M.D., Ucero A.C., Gonzalez-Espinoza L. and Ortiz A. (2014) Osteoprotegerin and kidney disease. J. Nephrol. 27, 607–617 10.1007/s40620-014-0092-x [DOI] [PubMed] [Google Scholar]
- 138.Coen G., Ballanti P., Balducci A., Calabria S., Fischer M.S., Jankovic L.. et al. (2002) Serum osteoprotegerin and renal osteodystrophy. Nephrol. Dial. Transplant. 17, 233–238 10.1093/ndt/17.2.233 [DOI] [PubMed] [Google Scholar]
- 139.Haas M., Leko-Mohr Z., Roschger P., Kletzmayr J., Schwarz C., Domenig C.. et al. (2002) Osteoprotegerin and parathyroid hormone as markers of high-turnover osteodystrophy and decreased bone mineralization in hemodialysis patients. Am. J. Kidney Dis. 39, 580–586 10.1053/ajkd.2002.31409 [DOI] [PubMed] [Google Scholar]
- 140.Sanz A.B., Sanchez-Nino M.D. and Ortiz A. (2011) TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 80, 708–718 10.1038/ki.2011.180 [DOI] [PubMed] [Google Scholar]
- 141.Martin P., Mora I., Cortes M.A., Calleros L., Garcia-Jerez A., Ortiz A.. et al. (2014) Relevant role of PKG in the progression of fibrosis induced by TNF-like weak inducer of apoptosis. Am. J. Physiol. Ren. Physiol. 307, F75–F85 10.1152/ajprenal.00398.2013 [DOI] [PubMed] [Google Scholar]
- 142.Berzal S., Gonzalez-Guerrero C., Rayego-Mateos S., Ucero A., Ocana-Salceda C., Egido J.. et al. (2015) TNF-related weak inducer of apoptosis (TWEAK) regulates junctional proteins in tubular epithelial cells via canonical NF-kappaB pathway and ERK activation. J. Cell. Physiol. 230, 1580–1593 10.1002/jcp.24905 [DOI] [PubMed] [Google Scholar]
- 143.Ucero A.C., Benito-Martin A., Fuentes-Calvo I., Santamaria B., Blanco J., Lopez-Novoa J.M.. et al. (2013) TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim. Biophys. Acta 1832, 1744–1755 10.1016/j.bbadis.2013.05.032 [DOI] [PubMed] [Google Scholar]
- 144.Ucero A.C., Berzal S., Ocana-Salceda C., Sancho M., Orzaez M., Messeguer A.. et al. (2013) A polymeric nanomedicine diminishes inflammatory events in renal tubular cells. PLoS One 8, e51992 10.1371/journal.pone.0051992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanz A.B., Justo P., Sanchez-Nino M.D., Blanco-Colio L.M., Winkles J.A., Kreztler M.. et al. (2008) The cytokine TWEAK modulates renal tubulointerstitial inflammation. J. Am. Soc. Nephrol. 19, 695–703 10.1681/ASN.2007050577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Izquierdo M.C., Sanz A.B., Mezzano S., Blanco J., Carrasco S., Sanchez-Nino M.D.. et al. (2012) TWEAK (tumor necrosis factor-like weak inducer of apoptosis) activates CXCL16 expression during renal tubulointerstitial inflammation. Kidney Int. 81, 1098–1107 10.1038/ki.2011.475 [DOI] [PubMed] [Google Scholar]
- 147.Valino-Rivas L., Cuarental L., Grana O., Bucala R., Leng L., Sanz A.. et al. (2018) TWEAK increases CD74 expression and sensitizes to DDT proinflammatory actions in tubular cells. PLoS One 13, e0199391 10.1371/journal.pone.0199391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rayego-Mateos S., Morgado-Pascual J.L., Sanz A.B., Ramos A.M., Eguchi S., Batlle D.. et al. (2013) TWEAK transactivation of the epidermal growth factor receptor mediates renal inflammation. J. Pathol. 231, 480–494 10.1002/path.4250 [DOI] [PubMed] [Google Scholar]
- 149.Ortiz A., Husi H., Gonzalez-Lafuente L., Valino-Rivas L., Fresno M., Sanz A.B.. et al. (2017) Mitogen-activated protein kinase 14 promotes AKI. J. Am. Soc. Nephrol. 28, 823–836 10.1681/ASN.2015080898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Poveda J., Sanz A.B., Carrasco S., Ruiz-Ortega M., Cannata-Ortiz P., Sanchez-Nino M.D.. et al. (2017) Bcl3: a regulator of NF-kappaB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells. Exp. Mol. Med. 49, e352 10.1038/emm.2017.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Poveda J., Sanz A.B., Rayego-Mateos S., Ruiz-Ortega M., Carrasco S., Ortiz A.. et al. (2016) NFkappaBiz protein downregulation in acute kidney injury: modulation of inflammation and survival in tubular cells. Biochim. Biophys. Acta 1862, 635–646 10.1016/j.bbadis.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 152.Sanchez-Nino M.D., Poveda J., Sanz A.B., Mezzano S., Carrasco S., Fernandez-Fernandez B.. et al. (2013) Fn14 in podocytes and proteinuric kidney disease. Biochim. Biophys. Acta 1832, 2232–2243 10.1016/j.bbadis.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 153.Valino-Rivas L., Gonzalez-Lafuente L., Sanz A.B., Ruiz-Ortega M., Ortiz A. and Sanchez-Nino M.D. (2016) Non-canonical NFkappaB activation promotes chemokine expression in podocytes. Sci. Rep. 6, 28857 10.1038/srep28857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martin-Sanchez D., Fontecha-Barriuso M., Carrasco S., Sanchez-Nino M.D., Massenhausen A.V., Linkermann A.. et al. (2018) TWEAK and RIPK1 mediate a second wave of cell death during AKI. PNAS 115, 4182–4187 10.1073/pnas.1716578115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Poveda J., Sanchez-Nino M.D., Glorieux G., Sanz A.B., Egido J., Vanholder R.. et al. (2014) p-cresyl sulphate has pro-inflammatory and cytotoxic actions on human proximal tubular epithelial cells. Nephrol. Dial. Transplant. 29, 56–64 10.1093/ndt/gft367 [DOI] [PubMed] [Google Scholar]
- 156.Moreno J.A., Izquierdo M.C., Sanchez-Nino M.D., Suarez-Alvarez B., Lopez-Larrea C., Jakubowski A.. et al. (2011) The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J. Am. Soc. Nephrol. 22, 1315–1325 10.1681/ASN.2010101073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Valino-Rivas L., Cuarental L., Agustin M., Husi H., Cannata-Ortiz P., Sanz A.B.. et al. (2018) MAGE genes in the kidney: identification of MAGED2 as upregulated during kidney injury and in stressed tubular cells. Nephrol. Dial. Transplant., 10.1093/ndt/gfy367 Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 158.Ruiz-Andres O., Suarez-Alvarez B., Sanchez-Ramos C., Monsalve M., Sanchez-Nino M.D., Ruiz-Ortega M.. et al. (2016) The inflammatory cytokine TWEAK decreases PGC-1alpha expression and mitochondrial function in acute kidney injury. Kidney Int. 89, 399–410 10.1038/ki.2015.332 [DOI] [PubMed] [Google Scholar]
- 159.Martinez-Miguel P., Medrano-Andres D., Griera-Merino M., Ortiz A., Rodriguez-Puyol M., Rodriguez-Puyol D.. et al. (2017) Tweak up-regulates endothelin-1 system in mouse and human endothelial cells. Cardiovasc. Res. 113, 207–221 10.1093/cvr/cvw239 [DOI] [PubMed] [Google Scholar]
- 160.Sanz A.B., Santamaria B., Ruiz-Ortega M., Egido J. and Ortiz A. (2008) Mechanisms of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 19, 1634–1642 10.1681/ASN.2007121336 [DOI] [PubMed] [Google Scholar]
- 161.Hotta K., Sho M., Yamato I., Shimada K., Harada H., Akahori T.. et al. (2011) Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int. 79, 179–188 10.1038/ki.2010.379 [DOI] [PubMed] [Google Scholar]
- 162.Munoz-Garcia B., Moreno J.A., Lopez-Franco O., Sanz A.B., Martin-Ventura J.L., Blanco J.. et al. (2009) Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol. 29, 2061–2068 10.1161/ATVBAHA.109.194852 [DOI] [PubMed] [Google Scholar]
- 163.Zhao Z., Burkly L.C., Campbell S., Schwartz N., Molano A., Choudhury A.. et al. (2007) TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J. Immunol. 179, 7949–7958 10.4049/jimmunol.179.11.7949 [DOI] [PubMed] [Google Scholar]
- 164.Galluppi G.R., Wisniacki N. and Stebbins C. (2016) Population pharmacokinetic and pharmacodynamic analysis of BIIB023, an anti-TNF-like weak inducer of apoptosis (anti-TWEAK) monoclonal antibody. Br. J. Clin. Pharmacol. 82, 118–128 10.1111/bcp.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Michaelson J.S., Wisniacki N., Burkly L.C. and Putterman C. (2012) Role of TWEAK in lupus nephritis: a bench-to-bedside review. J. Autoimmun. 39, 130–142 10.1016/j.jaut.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yilmaz M.I., Carrero J.J., Ortiz A., Martin-Ventura J.L., Sonmez A., Saglam M.. et al. (2009) Soluble TWEAK plasma levels as a novel biomarker of endothelial function in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 4, 1716–1723 10.2215/CJN.02760409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carrero J.J., Ortiz A., Qureshi A.R., Martin-Ventura J.L., Barany P., Heimburger O.. et al. (2009) Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 4, 110–118 10.2215/CJN.02790608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eskandari Naji H., Ghorbanihaghjo A., Argani H., Raeisi S., Safa J., Alirezaei A.H.. et al. (2017) Serum sTWEAK and FGF-23 Levels in Hemodialysis and Renal Transplant Patients. Int. J. Organ Transplantation Med. 8, 110–116 [PMC free article] [PubMed] [Google Scholar]
- 169.Schwartz N., Rubinstein T., Burkly L.C., Collins C.E., Blanco I., Su L.. et al. (2009) Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res. Therapy 11, R143 10.1186/ar2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang C., Chen L.L., Pan H.F., Leng R.X., Qin W.Z. and Ye D.Q. (2012) Expression of human tumor necrosis factor-like weak inducer of apoptosis in patients with systemic lupus erythematosus. Clin. Rheumatol. 31, 335–339 10.1007/s10067-011-1865-4 [DOI] [PubMed] [Google Scholar]
- 171.El-Shehaby A., Darweesh H., El-Khatib M., Momtaz M., Marzouk S., El-Shaarawy N.. et al. (2011) Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J. Clin. Immunol. 31, 848–856 10.1007/s10875-011-9555-1 [DOI] [PubMed] [Google Scholar]
- 172.Reyes-Thomas J., Blanco I. and Putterman C. (2011) Urinary biomarkers in lupus nephritis. Clin. Rev. Allergy Immunol. 40, 138–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Atkins R.C. and Zimmet P. (2010) Diabetic kidney disease: act now or pay later. Kidney Int. 77, 375–377 10.1038/ki.2009.509 [DOI] [PubMed] [Google Scholar]
- 174.Secchiero P., Corallini F., Pandolfi A., Consoli A., Candido R., Fabris B.. et al. (2006) An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am. J. Pathol. 169, 2236–2244 10.2353/ajpath.2006.060398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vaccarezza M., Bortul R., Fadda R. and Zweyer M. (2007) Increased OPG expression and impaired OPG/TRAIL ratio in the aorta of diabetic rats. Med. Chem. 3, 387–391 10.2174/157340607781024456 [DOI] [PubMed] [Google Scholar]
- 176.Xiang G.D., Sun H.L. and Zhao L.S. (2007) Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients. Diabetes Res. Clin. Pract. 76, 199–206 10.1016/j.diabres.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 177.Rasmussen L.M., Tarnow L., Hansen T.K., Parving H.H. and Flyvbjerg A. (2006) Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur. J. Endocrinol. 154, 75–81 10.1530/eje.1.02049 [DOI] [PubMed] [Google Scholar]
- 178.Perez de Ciriza C., Lawrie A. and Varo N. (2015) Osteoprotegerin in cardiometabolic disorders. Int. J. Endocrinol. 2015, 564934 10.1155/2015/564934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gordin D., Soro-Paavonen A., Thomas M.C., Harjutsalo V., Saraheimo M., Bjerre M.. et al. (2013) Osteoprotegerin is an independent predictor of vascular events in Finnish adults with type 1 diabetes. Diabetes Care 36, 1827–1833 10.2337/dc12-2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang S.T., Xu J.M., Wang M., Chen F.L. and Ding G. (2013) Increased plasma osteoprotegerin concentrations in Type 1 diabetes with albuminuria. Clin. Nephrol. 79, 192–198 10.5414/CN107536 [DOI] [PubMed] [Google Scholar]
- 181.Chang Y.H., Lin K.D., He S.R., Hsieh M.C., Hsiao J.Y. and Shin S.J. (2011) Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism 60, 1064–1069 10.1016/j.metabol.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 182.Toffoli B., Bernardi S., Candido R., Sabato N., Carretta R., Corallini F.. et al. (2011) Osteoprotegerin induces morphological and functional alterations in mouse pancreatic islets. Mol. Cell. Endocrinol. 331, 136–142 10.1016/j.mce.2010.08.019 [DOI] [PubMed] [Google Scholar]
- 183.Lamhamedi-Cherradi S.E., Zheng S.J., Maguschak K.A., Peschon J. and Chen Y.H. (2003) Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL-/- mice. Nat. Immunol. 4, 255–260 10.1038/ni894 [DOI] [PubMed] [Google Scholar]
- 184.Mi Q.S., Ly D., Lamhamedi-Cherradi S.E., Salojin K.V., Zhou L., Grattan M.. et al. (2003) Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes 52, 1967–1975 10.2337/diabetes.52.8.1967 [DOI] [PubMed] [Google Scholar]
- 185.Dirice E., Sanlioglu A.D., Kahraman S., Ozturk S., Balci M.K., Omer A.. et al. (2009) Adenovirus-mediated TRAIL gene (Ad5hTRAIL) delivery into pancreatic islets prolongs normoglycemia in streptozotocin-induced diabetic rats. Hum. Gene Ther. 20, 1177–1189 10.1089/hum.2009.039 [DOI] [PubMed] [Google Scholar]
- 186.Di Bartolo B.A., Chan J., Bennett M.R., Cartland S., Bao S., Tuch B.E.. et al. (2011) TNF-related apoptosis-inducing ligand (TRAIL) protects against diabetes and atherosclerosis in Apoe (-)/(-) mice. Diabetologia 54, 3157–3167 10.1007/s00125-011-2308-0 [DOI] [PubMed] [Google Scholar]
- 187.Cartland S.P., Erlich J.H. and Kavurma M.M. (2014) TRAIL deficiency contributes to diabetic nephropathy in fat-fed ApoE-/- mice. PLoS One 9, e92952 10.1371/journal.pone.0092952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiang G., Zhang J., Ling Y. and Zhao L. (2014) Circulating level of TRAIL concentration is positively associated with endothelial function and increased by diabetic therapy in the newly diagnosed type 2 diabetic patients. Clin. Endocrinol. (Oxf.) 80, 228–234 10.1111/cen.12312 [DOI] [PubMed] [Google Scholar]
- 189.Bisgin A., Yalcin A.D. and Gorczynski R.M. (2012) Circulating soluble tumor necrosis factor related apoptosis inducing-ligand (TRAIL) is decreased in type-2 newly diagnosed, non-drug using diabetic patients. Diabetes Res. Clin. Pract. 96, e84–e86 10.1016/j.diabres.2012.02.028 [DOI] [PubMed] [Google Scholar]
- 190.Choi J.W., Fujii T. and Fujii N. (2018) Fas and tumor necrosis factor-related apoptosis-inducing ligand are closely linked to the levels of glycated and fetal hemoglobin in patients with diabetes mellitus. Clin. Lab. 64, 767–775 10.7754/Clin.Lab.2018.171130 [DOI] [PubMed] [Google Scholar]
- 191.Arik H.O., Yalcin A.D., Gumuslu S., Genc G.E., Turan A. and Sanlioglu A.D. (2013) Association of circulating sTRAIL and high-sensitivity CRP with type 2 diabetic nephropathy and foot ulcers. Med. Sci. Monit. 19, 712–715 10.12659/MSM.889514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chang W.W., Liang W., Yao X.M., Zhang L., Zhu L.J., Yan C.. et al. (2018) Tumour necrosis factor-related apoptosis-inducing ligand expression in patients with diabetic nephropathy. J. Renin Angiotensin Aldosterone Syst. 19, 1470320318785744 10.1177/1470320318785744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cheung S.S., Metzger D.L., Wang X., Huang J., Tai J., Tingle A.J.. et al. (2005) Tumor necrosis factor-related apoptosis-inducing ligand and CD56 expression in patients with type 1 diabetes mellitus. Pancreas 30, 105–114 10.1097/01.mpa.0000148515.77497.4b [DOI] [PubMed] [Google Scholar]
- 194.Salehi E., Vodjgani M., Massoud A., Keyhani A., Rajab A., Shafaghi B.. et al. (2007) Increased expression of TRAIL and its receptors on peripheral T-cells in type 1 diabetic patients. Iran J. Immunol. 4, 197–205 [PubMed] [Google Scholar]
- 195.Llaurado G., Gonzalez-Clemente J.M., Maymo-Masip E., Subias D., Vendrell J. and Chacon M.R. (2012) Serum levels of TWEAK and scavenger receptor CD163 in type 1 diabetes mellitus: relationship with cardiovascular risk factors. a case-control study. PLoS One 7, e43919 10.1371/journal.pone.0043919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kralisch S., Ziegelmeier M., Bachmann A., Seeger J., Lossner U., Bluher M.. et al. (2008) Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 199, 440–444 10.1016/j.atherosclerosis.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 197.Diaz-Lopez A., Chacon M.R., Bullo M., Maymo-Masip E., Martinez-Gonzalez M.A., Estruch R.. et al. (2013) Serum sTWEAK concentrations and risk of developing type 2 diabetes in a high cardiovascular risk population: a nested case-control study. J. Clin. Endocrinol. Metab. 98, 3482–3490 10.1210/jc.2013-1848 [DOI] [PubMed] [Google Scholar]
- 198.Sato S., Ogura Y., Tajrishi M.M. and Kumar A. (2015) Elevated levels of TWEAK in skeletal muscle promote visceral obesity, insulin resistance, and metabolic dysfunction. FASEB J. 29, 988–1002 10.1096/fj.14-260703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vendrell J. and Chacon M.R. (2013) TWEAK: a new player in obesity and diabetes. Front. Immunol. 4, 488 10.3389/fimmu.2013.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Valdivielso J.M., Coll B., Martin-Ventura J.L., Moreno J.A., Egido J., Fernandez E.. et al. (2013) Soluble TWEAK is associated with atherosclerotic burden in patients with chronic kidney disease. J. Nephrol. 26, 1105–1113 10.5301/jn.5000245 [DOI] [PubMed] [Google Scholar]
- 201.Di Lullo L., House A., Gorini A., Santoboni A., Russo D. and Ronco C. (2015) Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 20, 259–272 10.1007/s10741-014-9460-9 [DOI] [PubMed] [Google Scholar]
- 202.Lainscak M. (2009) 10. Cardiovascular risk in chronic kidney disease. EJIFCC 20, 73–76 [PMC free article] [PubMed] [Google Scholar]
- 203.Major R.W., Cheng M.R., Grant R.A., Shantikumar S., Xu G., Oozeerally I.. et al. (2018) Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS One 13, e0192895 10.1371/journal.pone.0192895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.de Jager D.J., Grootendorst D.C., Jager K.J., van Dijk P.C., Tomas L.M., Ansell D.. et al. (2009) Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302, 1782–1789 10.1001/jama.2009.1488 [DOI] [PubMed] [Google Scholar]
- 205.Albertus P., Morgenstern H., Robinson B. and Saran R. (2016) Risk of ESRD in the United States. Am. J. Kidney Dis. 68, 862–872 10.1053/j.ajkd.2016.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Go A.S., Chertow G.M., Fan D., McCulloch C.E. and Hsu C.Y. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 207.Saran R., Li Y., Robinson B., Ayanian J., Balkrishnan R., Bragg-Gresham J.. et al. (2015) US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 66, Svii, S1-305 10.1053/j.ajkd.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shlipak M.G., Fried L.F., Cushman M., Manolio T.A., Peterson D., Stehman-Breen C.. et al. (2005) Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 293, 1737–1745 10.1001/jama.293.14.1737 [DOI] [PubMed] [Google Scholar]
- 209.Buchanan C., Mohammed A., Cox E., Kohler K., Canaud B., Taal M.W.. et al. (2017) Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J. Am. Soc. Nephrol. 28, 1269–1277 10.1681/ASN.2016060686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Covic A., Vervloet M., Massy Z.A., Torres P.U., Goldsmith D., Brandenburg V.. et al. (2018) Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population. Lancet Diab. Endocrinol. 6, 319–331 10.1016/S2213-8587(17)30310-8 [DOI] [PubMed] [Google Scholar]
- 211.Kuzniewski M., Fedak D., Dumnicka P., Stepien E., Kusnierz-Cabala B., Cwynar M.. et al. (2016) Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv. Med. Sci. 61, 269–275 10.1016/j.advms.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 212.Fernandez E. and Martinez Castelao A. (2011) NEFRONA project: open-access database. Nefrologia 31, 5–8 [DOI] [PubMed] [Google Scholar]
- 213.Arcidiacono M.V., Rimondi E., Maietti E., Melloni E., Tisato V., Gallo S.. et al. (2018) Relationship between low levels of circulating TRAIL and atheromatosis progression in patients with chronic kidney disease. PLoS One 13, e0203716 10.1371/journal.pone.0203716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu H., Peng H., Xiang H., Guo L., Chen R., Zhao S.. et al. (2018) TWEAK/Fn14 promotes oxidative stress through AMPK/PGC1alpha/MnSOD signaling pathway in endothelial cells. Mol. Med. Rep. 17, 1998–2004 [DOI] [PubMed] [Google Scholar]
- 215.Yilmaz M.I., Sonmez A., Qureshi A.R., Saglam M., Stenvinkel P., Yaman H.. et al. (2011) Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 1617–1625 10.2215/CJN.10681210 [DOI] [PubMed] [Google Scholar]
- 216.Fernandez-Laso V., Sastre C., Valdivielso J.M., Betriu A., Fernandez E., Egido J.. et al. (2016) Soluble TWEAK and Major Adverse Cardiovascular Events in Patients with CKD. Clin. J. Am. Soc. Nephrol. 11, 413–422 10.2215/CJN.07900715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fernandez-Laso V., Mendez-Barbero N., Valdivielso J.M., Betriu A., Fernandez E., Egido J.. et al. (2017) Soluble TWEAK and atheromatosis progression in patients with chronic kidney disease. Atherosclerosis 260, 130–137 10.1016/j.atherosclerosis.2017.03.043 [DOI] [PubMed] [Google Scholar]
- 218.Bozic M., Mendez-Barbero N., Gutierrez-Munoz C., Betriu A., Egido J., Fernandez E.. et al. (2018) Combination of biomarkers of vascular calcification and sTWEAK to predict cardiovascular events in chronic kidney disease. Atherosclerosis 270, 13–20 10.1016/j.atherosclerosis.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 219.Ammirati E., Moroni F., Norata G.D., Magnoni M. and Camici P.G. (2015) Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. 2015, 718329 10.1155/2015/718329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ammirati E. and Fogacci F. (2017) Clinical relevance of biomarkers for the identification of patients with carotid atherosclerotic plaque: Potential role and limitations of cysteine protease legumain. Atherosclerosis 257, 248–249 10.1016/j.atherosclerosis.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 221.Mulder S., Hamidi H., Kretzler M. and Ju W. (2018) An integrative systems biology approach for precision medicine in diabetic kidney disease. Diabetes Obes. Metab. 20, 6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]