Abstract
Background: Seminoma accounts for the most part of cases of testicular germ cell tumor, which is the most common malignancy among males between ages 15 and 44 years. Understanding the molecular mechanism of tumorigenesis is important for better clinical diagnosis and treatment.
Purpose: We performed bioinformatics analysis to better understand seminoma at the genetic level and to explore potential candidate genes or molecules for diagnosis, treatment, and prognosis.
Methods: A gene expression profile (GSE8607), containing 40 seminoma samples and three healthy testes samples, was analyzed to identify differentially expressed genes (DEGs) associated with the occurrence of seminoma. Functional annotation was then performed using gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses. Cytoscape with Search Tool for the Retrieval of Interacting Genes was used to construct a protein–protein interaction (PPI) network of the DEGs and select hub genes. Moreover, validation of expression level and Kaplan–Meier analysis for overall survival were conducted to those hub genes.
Results: A total of 1,636 DEGs were identified between seminoma and healthy samples, including 701 up-regulated in seminoma that were enriched in the regulation of immune responses, defense responses, receptor activity, and signal transducer activity; 935 were down-regulated in seminoma and were associated with reproductive processes, kinase activity, and carbohydrate derivative binding. Five hub genes were selected from the PPI network according to the degree of connectivity: IL6, VEGFA, IL10, CCR5, and CXCR4. Among them, high expression levels of CCR5 and CXCR4 were associated with poor prognosis for seminoma patients. Four modules selected from the PPI network revealed that seminoma was connected with the Janus kinase-signal transducers and activators of transcription signaling pathway, chemokine signaling pathway, endocytosis, and cytokine–cytokine receptor interaction.
Conclusion: These identified DEGs and hub genes facilitate our knowledge of the underlying molecular mechanism of seminoma and have the potential to be used as diagnostic biomarkers or therapeutic targets for seminoma.
Keywords: seminoma, bioinformatics analysis, DEGs, key genes, pathways
Introduction
Testicular germ cell tumors (TGCT) are rare tumors in the general population but are the most commonly occurring malignancy among males between ages 15 and 44 years.1 Radical orchidectomy is the standard treatment method.2,3 Recently, immunotherapy and gene therapy are suggested to be potential therapeutic strategies.4–6 Understanding the molecular mechanism of tumorigenesis is important for a better clinical application of the novel treatments, as well as early diagnosis of disease and prognostic prediction. In the present study, we aim to identify key genes associated with the tumorigenesis of seminoma, based on the data from the Gene Expression Omnibus (GEO) database, for a better knowledge about the mechanism at the genetic level and exploring potential candidate genes or molecules for diagnosis, treatment, and prognosis.
Materials and methods
Microarray data
The gene expression profile (GSE8607), containing 40 seminoma samples and three healthy testes samples, was downloaded from the GEO database. GSE8607, which was submitted by Gashaw, was based on the Affymetrix GPL8300 platform ([HG_U95Av2Affymetrix] Human Genome U95 Version 2 Array). The average age of the 40 patients with seminoma was 37.6 (23–56) years old, while 53 years old in the control group. The American Joint Committee on Cancer (AJCC) stage distribution of these seminoma samples is as follows: 35 with stage I, 3 with stage II, and 2 with stage III.
Identification of DEGs
GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) is a free online tool for comparing several groups of gene expression data in a GEO Series.7 We applied it to detect the differentially expressed genes (DEGs) between seminoma samples and normal testes samples. Adjust P-value<0.05 was thought significant statistically and |logFC| ≥2 was set as the cut-off criterion for a better accuracy and significance as described earlier.8,9
GO and KEGG pathway analysis
Gene ontology (GO) analysis is a useful method for functional studies of high-throughput genomes or transcriptome data,10,11 and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis is employed for the systematic analysis of gene functions by linking genomic information with higher-order functional information.12 To visualize core biological processes (BP), molecular functions (MF), and cellular component (CC) pathways among the DEGs, we used Functional Annotation Tool of Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/).13 Adjust P-value<0.05 was considered statistically significant.
Hub genes selection and analysis of modules from PPI networks
Search Tool for the Retrieval of Interacting Genes (STRING) is a biological database for building protein–protein interaction (PPI) networks, providing a system-wide view of interactions between each member.14 DEGs were mapped to STRING to analyze their relationships with each other, and a combined score of >0.4 was set as the cut-off criterion described before. PPI networks were then established using Cytoscape software,15 which visually explores biomolecular interaction networks composed of proteins, genes, and other molecules. The plug-in Centiscape was used to search for the most important nodes in a network by calculating centrality parameters for each node.15 Hub genes were selected from the PPI network according to the degree of connectivity. The plug-in Molecular Complex Detection (MCODE) was used to select modules from the PPI network with the criteria as described before: node score cutoff=0.2, degree cutoff=2, max depth=100, and k-core=2.9 Pathway analyses of genes in each module were performed using DAVID, with adjust P-value<0.05 considered statistically significant.
Comparison of the hub genes expression level
GEPIA (http://gepia.cancer-pku.cn/index.html) is a web server for cancer and normal gene expression profiling and interactive analyses, based on the data from TCGA and the GTEx project.16 We used it to validate the different expression levels of the hub genes between seminoma tissues and normal testes tissues. Then, the box plot was performed to visualize the relationship.
Survival analyses of hub genes
The expression profiles of 134 seminoma samples, along with the clinical data were downloaded from the TCGA database (http://tcga-data.nci.nih.gov) for survival analyses of hub genes. The hazard ratio (HR) with 95% confidence intervals and log-rank P-value were calculated and displayed. Log-rank P-value<0.05 was considered statistically significant.
Results
Identification of DEGs
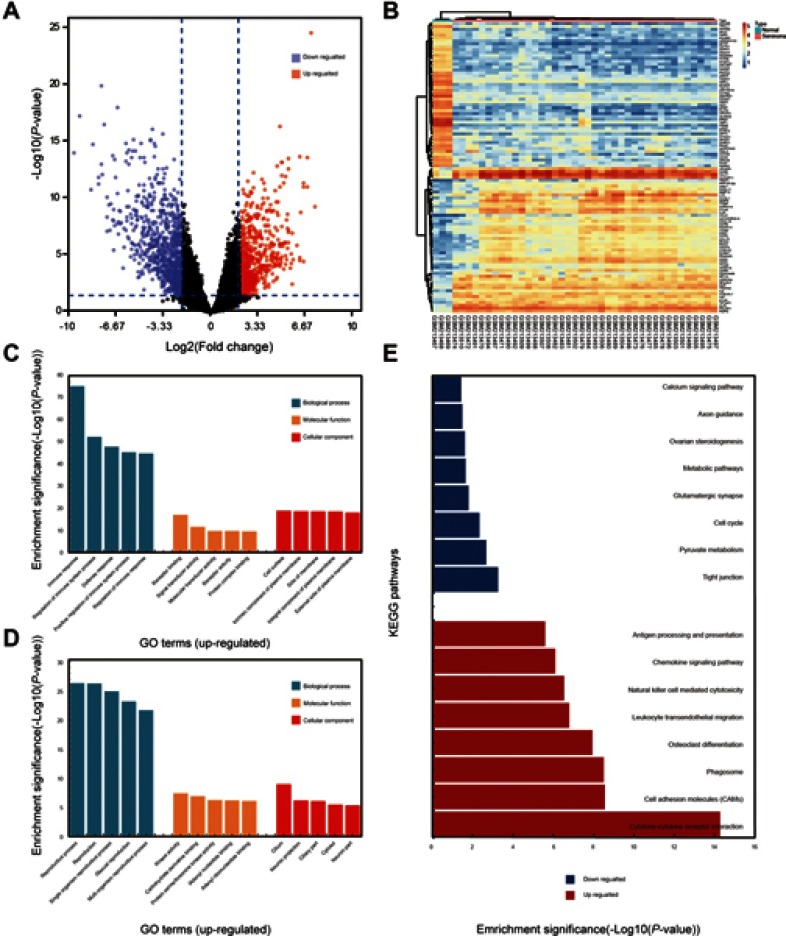
The study included 40 seminoma samples and three healthy testes samples. A total of 1,636 DEGs were selected after the analysis of GSE8607 by GEO2R. Of these, 701 were up-regulated and 935 were down-regulated in seminoma samples compared with healthy controls. A volcano plot and heat map of DEGs are shown in Figure 1A and B, respectively.
Figure 1.
Selection of DEGs and function annotation. (A) Volcano plot of the DEGs (adjust P-value <0.05 and |logFC| ≥2 were set as the cut-off criteria). (B) Heat map of the top 100 DEGs (top 50 up-regulated and down-regulated genes). (C) Genes ontology analysis of up-regulated DEGs. (D) Genes ontology analysis of down-regulated DEGs. (E) KEGG pathway analysis of DEGs.
GO term and KEGG pathway analyses
For a deeper insight into the DEGs, we performed GO and KEGG pathway enrichment analyses. GO BP analysis revealed that up-regulated DEGs were mainly involved in the regulation of immune system processes and defense responses; while down-regulated DEGs were mainly associated with reproductive processes. GO MF analysis showed that the up-regulated DEGs were mainly enriched in receptor binding, signal transducer activity, receptor activity, molecular transducer activity, and protein complex binding; while down-regulated DEGs were significantly enriched in kinase activity, carbohydrate derivative binding, protein serine/threonine kinase activity, adenyl nucleotide binding, and adenyl ribonucleotide binding. GO CC analysis showed that up-regulated DEGs were involved with intrinsic components of plasma membranes, cell surface, side of membranes, integral components of plasma membranes, and external side of plasma membranes; while down-regulated DEGs were mainly associated with cilium and neuron projections. GO analysis findings are shown in Figure 1C and D, and Table 1.
Table 1.
Gene ontology analysis of DEGs associated with seminoma
| Expression | Category | Term | Count | % | P-value | FDR |
|---|---|---|---|---|---|---|
| Up-regulated | GOTERM_BP_FAT | Immune response | 206 | 36.20 | 6.12E−76 | 1.19E−72 |
| GOTERM_BP_FAT | Regulation of immune system process | 166 | 29.17 | 4.71E−53 | 9.19E−50 | |
| GOTERM_BP_FAT | Defense response | 168 | 29.53 | 1.39E−48 | 2.71E−45 | |
| GOTERM_BP_FAT | Positive regulation of immune system process | 130 | 22.85 | 4.61E−46 | 9.00E−43 | |
| GOTERM_BP_FAT | Regulation of immune response | 126 | 22.14 | 1.75E−45 | 3.41E−42 | |
| GOTERM_MF_FAT | Receptor binding | 112 | 19.68 | 1.01E−17 | 1.62E−14 | |
| GOTERM_MF_FAT | Signal transducer activity | 113 | 19.86 | 2.74E−12 | 4.42E−09 | |
| GOTERM_MF_FAT | Receptor activity | 104 | 18.28 | 2.08E−10 | 3.36E−07 | |
| GOTERM_MF_FAT | Molecular transducer activity | 104 | 18.28 | 2.08E−10 | 3.36E−07 | |
| GOTERM_MF_FAT | Protein complex binding | 61 | 10.72 | 3.30E−10 | 5.33E−07 | |
| GOTERM_CC_FAT | Intrinsic component of plasma membrane | 132 | 23.20 | 1.36E−19 | 2.02E−16 | |
| GOTERM_CC_FAT | Cell surface | 81 | 14.24 | 2.44E−19 | 3.60E−16 | |
| GOTERM_CC_FAT | Side of membrane | 60 | 10.54 | 2.75E−19 | 4.06E−16 | |
| GOTERM_CC_FAT | Integral component of plasma membrane | 128 | 22.50 | 2.89E−19 | 4.27E−16 | |
| GOTERM_CC_FAT | External side of plasma membrane | 44 | 7.73 | 8.94E−19 | 1.32E−15 | |
| Down-regulated | GOTERM_BP_FAT | Reproductive process | 145 | 19.03 | 9.16E−27 | 1.79E−23 |
| GOTERM_BP_FAT | Reproduction | 145 | 19.03 | 1.06E−26 | 2.08E−23 | |
| GOTERM_BP_FAT | Single organism reproductive process | 133 | 17.45 | 2.37E−25 | 4.63E−22 | |
| GOTERM_BP_FAT | Sexual reproduction | 98 | 12.86 | 1.32E−23 | 2.58E−20 | |
| GOTERM_BP_FAT | Multi-organism reproductive process | 107 | 14.04 | 4.11E−22 | 8.04E−19 | |
| GOTERM_MF_FAT | Kinase activity | 74 | 9.71 | 6.64E−08 | 1.08E−04 | |
| GOTERM_MF_FAT | Carbohydrate derivative binding | 141 | 18.50 | 2.01E−07 | 3.27E−04 | |
| GOTERM_MF_FAT | Protein serine/threonine kinase activity | 43 | 5.64 | 9.12E−07 | 1.49E−03 | |
| GOTERM_MF_FAT | Adenyl nucleotide binding | 103 | 13.52 | 1.06E−06 | 1.72E−03 | |
| GOTERM_MF_FAT | Adenyl ribonucleotide binding | 102 | 13.39 | 1.35E−06 | 2.20E−03 | |
| GOTERM_CC_FAT | Cilium | 55 | 7.22 | 1.78E−09 | 2.70E−06 | |
| GOTERM_CC_FAT | Neuron projection | 75 | 9.84 | 1.04E−06 | 1.58E−03 | |
| GOTERM_CC_FAT | Ciliary part | 37 | 4.86 | 1.40E−06 | 2.12E−03 | |
| GOTERM_CC_FAT | Cytosol | 196 | 25.72 | 5.46E−06 | 8.26E−03 | |
| GOTERM_CC_FAT | Neuron part | 92 | 12.07 | 7.43E−06 | 1.12E−02 |
Table 2 and Figure 1E show the results of KEGG analysis. Up-regulated DEGs were mainly enriched in cytokine–cytokine receptor interactions, cell adhesion molecules (CAMs), phagosomes, osteoclast differentiation, leukocyte transendothelial migration, natural killer cell-mediated cytotoxicity, chemokine signaling pathways, and antigen processing and presentation; down-regulated DEGs were significantly enriched in tight junctions, pyruvate metabolism, the cell cycle, glutamatergic synapses, metabolic pathways, ovarian steroidogenesis, axon guidance, and calcium signaling pathways.
Table 2.
KEGG pathway analysis of DEGs associated with seminoma
| Expression | Pathway ID | Term | Count | % | P-value | FDR | Genes |
|---|---|---|---|---|---|---|---|
| Up-regulated | hsa04060 | Cytokine−cytokine receptor interaction | 44 | 7.73 | 5.35E−15 | 6.77E-12 | IL1R2, CCL3, CCL2, PDGFB, TNFRSF25, CCR1, CXCL9, FASLG, CCL8, KIT, CCL5, CXCL11, CCL4, TNFRSF4, IL10, FLT3LG, CXCL10, TNFRSF1B, TNFRSF11B, IL23A, CXCR5, CXCR4, IFNA5, IL10RA, CXCR6, IFNG, CSF2RB, IL1B, IL2RG, LTB, CSF2RA, CD27, IL6, IL2RB, CCL19, IL6R, IFNAR2, CCR8, TNFSF10, CCR5, CXCL13, CCR2, VEGFA, ACVR1 |
| hsa04514 | Cell adhesion molecules (CAMs) | 27 | 4.75 | 2.78E−09 | 3.54E−06 | ITGAL, CLDN7, CD8A, CDH1, L1CAM, ITGB2, HLA-DMB, CDH3, HLA-DMA, ITGAM, NRCAM, CD2, HLA-DRB4, CD4, HLA-DPB1, HLA-DOA, CD28, ICAM1, PTPRC, SELL, ICAM2, ITGA4, HLA-E, HLA-F, PECAM1, HLA-DPA1, HLA-DRA | |
| hsa04145 | Phagosome | 28 | 4.92 | 3.17E−09 | 4.03E−06 | TUBB2B, TLR2, ITGB2, HLA-DMB, ATP6V1B1, HLA-DMA, ITGAM, TUBB, TAP2, HLA-DRB4, HLA-DPB1, FCGR3A, THBS1, HLA-DOA, FCGR3B, NCF2, NCF4, CTSS, HLA-E, HLA-F, CYBA, CORO1A, CYBB, ITGA5, HLA-DPA1, FCGR2A, CD14, HLA-DRA | |
| hsa04380 | Osteoclast differentiation | 25 | 4.39 | 1.15E−08 | 1.46E−05 | NCF2, NCF4, ACP5, NFKB2, STAT1, JUNB, BTK, STAT2, LILRB1, IRF9, IFNAR2, LILRB2, CYBA, TNFRSF11B, CYBB, LILRB3, LCK, IFNG, LILRB4, IL1B, FCGR2A, FCGR3A, FCGR3B, TYROBP, LCP2 | |
| hsa04670 | Leukocyte transendothelial migration | 22 | 3.87 | 1.63E−07 | 2.07E−04 | ICAM1, ITGAL, CLDN7, MYL7, ITK, NCF2, MMP9, NCF4, SIPA1, ACTN1, ITGB2, ITGA4, ITGAM, VASP, THY1, CYBA, CYBB, RAC2, CXCR4, PECAM1, MSN, RHOH | |
| hsa04650 | Natural killer cell mediated cytotoxicity | 22 | 3.87 | 2.94E−07 | 3.73E−04 | ICAM1, ITGAL, PTPN6, ICAM2, CD247, FASLG, ITGB2, GZMB, NCR1, CD48, IFNAR2, TNFSF10, SH2D1A, RAC2, IFNA5, LCK, IFNG, FCER1G, FCGR3A, FCGR3B, TYROBP, LCP2 | |
| hsa04062 | Chemokine signaling pathway | 27 | 4.75 | 8.07E−07 | 1.03E−03 | CCL3, CCL2, CCR1, CXCL9, CCL8, CXCL11, CCL5, CCL4, CXCL10, RAC2, CXCR5, CXCR4, CXCR6, ITK, LYN, HCK, CCL19, STAT1, CCL18, STAT2, CCR8, CCR5, ARRB2, CXCL13, CCR2, GRK5, XCL2 | |
| hsa04612 | Antigen processing and presentation | 16 | 2.81 | 2.59E−06 | 3.29E−03 | CD8A, CTSS, HLA-DMB, HLA-E, HLA-DMA, CD74, HLA-F, B2M, TAP2, IFNG, HLA-DRB4, CD4, HLA-DPA1, HLA-DPB1, HLA-DOA, HLA-DRA | |
| Down-regulated | hsa04530 | Tight junction | 17 | 2.23 | 5.54E−04 | 7.16E−01 | PPP2R1B, PRKCZ, CLDN18, MYL5, MAGI1, GNAI1, MYH3, MYLPF, MYH7, ACTN3, CTNNA2, CSNK2A2, EPB41L3, MYH11, EXOC3, TJP3, TJP2 |
| hsa00620 | Pyruvate metabolism | 8 | 1.05 | 2.13E−03 | 2.73E+00 | HAGH, LDHC, ME1, ME3, ACYP1, PDHA2, ACACB, PC | |
| hsa04110 | Cell cycle | 14 | 1.84 | 4.68E−03 | 5.90E+00 | CCNE2, CDKN1C, E2F2, FZR1, CDKN1B, RBL2, CDKN2B, CCNH, DBF4, CDKN2D, CDK6, PTTG1, CDC25C, CCNA1 | |
| hsa04724 | Glutamatergic synapse | 12 | 1.57 | 1.61E−02 | 1.90E+01 | PRKACG, SLC1A2, DLGAP1, GNAO1, GRIA2, ADCY9, GNAI1, GRM8, SLC1A6, PPP3CC, CACNA1C, GRK3 | |
| hsa01100 | Metabolic pathways | 72 | 9.45 | 2.27E−02 | 2.58E+01 | LDHC, KYNU, CYP2J2, PGAM2, ITPKA, CKB, AKR1C3, TYR, AKR1C4, AGPS, PTGES, RGN, PDHA2, PCYT2, NT5E, NMNAT2, DDC, ALDH6A1, HYAL3, PDXK, GATM, CYP11A1, PFKP, CDS1, GMPS, NME5, ALOX15, ADO, PTGDS, CHPF, RFK, ABAT, PLA2G6, INPP4B, INPP1, GALNT3, ME1, CHKA, ME3, MVD, GK2, HMGCS1, HPRT1, ALDH1A1, TYMS, CKMT2, PLCH1, HSD17B3, PLCD1, PCYT1B, PRPS1L1, UGT8, MTMR6, SPAM1, GAD1, ODC1, PLA2G16, UAP1, SPHK2, MAOA, BCKDHB, MGAT4C, SI, ACACB, GAPDHS, CYP17A1, ACSM1, PYGM, HMGCS2, GAMT, PC, LIPF | |
| hsa04913 | Ovarian steroidogenesis | 7 | 0.92 | 2.46E−02 | 2.76E+01 | AKR1C3, PRKACG, CYP17A1, CYP2J2, ADCY9, CYP11A1, STAR | |
| hsa04360 | Axon guidance | 12 | 1.57 | 3.29E−02 | 3.52E+01 | EPHA5, DCC, EPHA4, PAK3, GNAI1, ROBO1, EFNB2, SEMA3D, SRGAP3, PPP3CC, SEMA3C, EPHB1 | |
| hsa04020 | Calcium signaling pathway | 15 | 1.97 | 3.76E−02 | 3.92E+01 | SPHK2EPHB1 ERBB4, SLC25A4, PHKG2, ITPKA, PRKACG, CAMK4, ADCY9, PLN, PDE1A, PPP3CC, PLCD1, CACNA1C, MYLK, HTR2A |
Identification of hub genes and analysis of modules from PPI networks
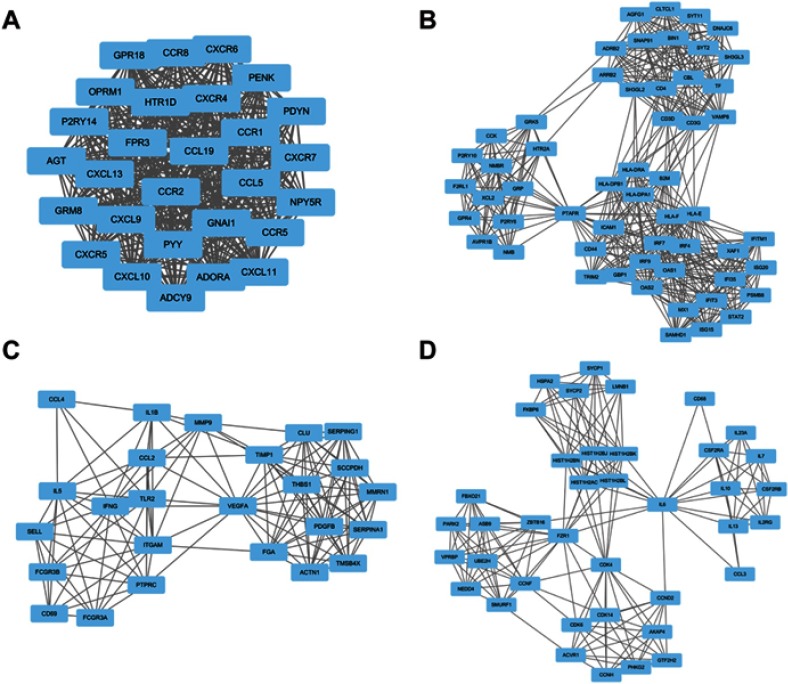
According to the information from STRING, the top five hub nodes were selected, including interleukin 6 (IL6), vascular endothelial growth factor A (VEGFA), including interleukin 10 (IL10), C-C motif chemokine receptor 5 (CCR5), and C-X-C motif chemokine receptor 4 (CXCR4). Analysis of the relationship between 1,074 nodes and 4,633 edges by plug-in MCODE enabled four modules to be selected, and then functional annotation of the genes from these modules was conducted by DAVID (Figure 2, Tables 3–6). The genes in these modules were mainly involved in the Janus kinase-signal transducers and activators of transcription (Jak–STAT) signaling pathway, chemokine signaling pathway, endocytosis, and cytokine–cytokine receptor interactions.
Table 4.
The enriched pathways of module 2
| Term | P-value | FDR | Genes |
|---|---|---|---|
| Endocytosis | 8.30E−07 | 8.76E−04 | SH3GL3, ADRB2, ARRB2, CBL, DNAJC6, GRK5, HLA-E, BIN1, CLTCL1, SH3GL2, HLA-F |
| Antigen processing and presentation | 3.03E−06 | 3.19E−03 | CD4, HLA-DPA1, HLA-DPB1, HLA-E, HLA-DRA, HLA-F, B2M |
| Cell adhesion molecules (CAMs) | 1.09E−04 | 1.15E−01 | ICAM1, CD4, HLA-DPA1, HLA-DPB1, HLA-E, HLA-DRA, HLA-F |
| Neuroactive ligand-receptor interaction | 6.68E−04 | 7.03E−01 | P2RY6, P2RY10, ADRB2, AVPR1B, F2RL1, NMBR, PTAFR, HTR2A |
| Phagosome | 9.50E−03 | 9.58E+00 | HLA-DPA1, HLA-DPB1, HLA-E, HLA-DRA, HLA-F |
Table 5.
The enriched pathways of module 3
| Term | P-value | FDR | Genes |
|---|---|---|---|
| Cytokine−cytokine receptor interaction | 3.34E−05 | 3.56E−02 | IL5, CCL2, PDGFB, VEGFA, IFNG, IL1B, CCL4 |
| Phagosome | 8.49E−04 | 9.01E−01 | TLR2, THBS1, FCGR3A, FCGR3B, ITGAM |
| Osteoclast differentiation | 6.00E−03 | 6.21E+00 | IFNG, IL1B, FCGR3A, FCGR3B |
| Complement and coagulation cascades | 1.66E−02 | 1.64E+01 | FGA, SERPING1, SERPINA1 |
| Rap1 signaling pathway | 2.15E−02 | 2.07E+01 | PDGFB, VEGFA, THBS1, ITGAM |
Figure 2.
Top four modules from the PPI network. (A) modules 1; (B) modules 2; (C) modules 3; (D) modules 4.
Table 3.
The enriched pathways of module 1
| Term | P-value | FDR | Genes |
|---|---|---|---|
| Chemokine signaling pathway | 4.19E−17 | 4.35E−14 | GNAI1, CCR1, CXCL9, CCL19, CXCL11, CCL5, CXCL10, CCR8, CXCR5, ADCY9, CCR5, CXCR4, CXCL13, CCR2, CXCR6 |
| Cytokine−cytokine receptor interaction | 1.36E−12 | 1.41E−09 | CCR1, CXCL9, CCL19, CXCL11, CCL5, CXCL10, CCR8, CCR5, CXCR5, CXCL13, CXCR4, CCR2, CXCR6 |
| Neuroactive ligand-receptor interaction | 2.23E−04 | 2.31E−01 | OPRM1, GRM8, P2RY14, FPR3, HTR1D, ADORA1, NPY5R |
| Toll-like receptor signaling pathway | 4.97E−03 | 5.04E+00 | CXCL9, CCL5, CXCL11, CXCL10 |
| Regulation of lipolysis in adipocytes | 1.46E−02 | 1.42E+01 | ADCY9, GNAI1, ADORA1 |
Table 6.
The enriched pathways of module 4
| Term | P-value | FDR | Genes |
|---|---|---|---|
| Jak-STAT signaling pathway | 9.16E−08 | 9.76E−05 | IL6, IL23A, CCND2, IL7, CSF2RB, IL13, IL2RG, IL10, CSF2RA |
| Cytokine-cytokine receptor interaction | 2.40E−07 | 2.56E−04 | IL6, CCL3, IL23A, IL7, CSF2RB, IL13, IL2RG, IL10, CSF2RA, ACVR1 |
| Cell cycle | 1.66E−03 | 1.75E+00 | FZR1, CCND2, CCNH, CDK6, CDK4 |
| Ubiquitin mediated proteolysis | 2.39E−03 | 2.52E+00 | FZR1, NEDD4, SMURF1, PARK2, UBE2H |
| PI3K-Akt signaling pathway | 1.33E−02 | 1.33E+01 | IL6, CCND2, IL7, IL2RG, CDK6, CDK4 |
Expression level and survival analysis of hub genes
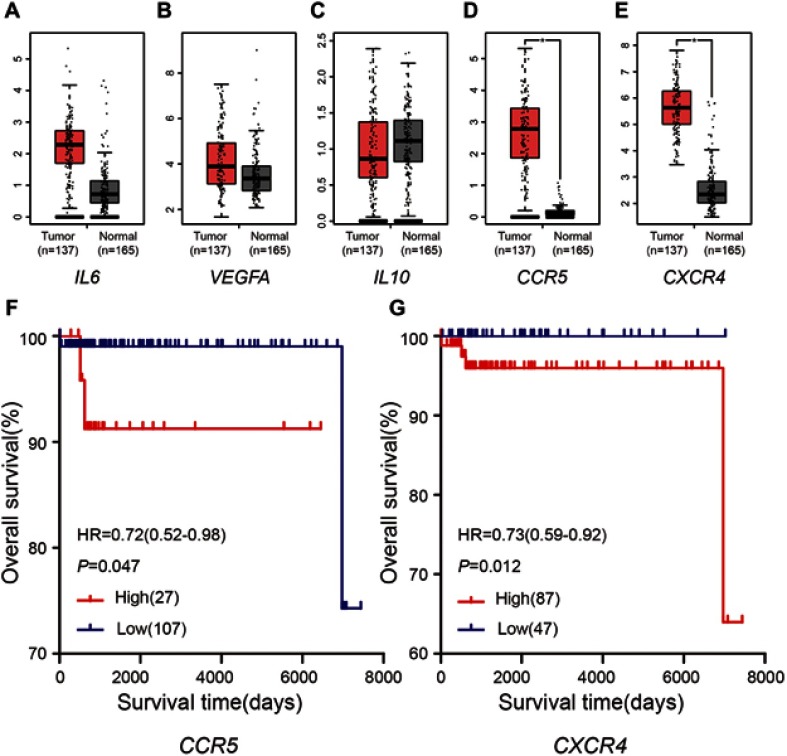
Compared with the normal tissues, CCR5 and CXCR4 revealed higher expression levels in seminoma tissues (P<0.05), but IL6, VEGFA and IL10 not (Figure 3A–E). Besides, 134 seminoma samples from TCGA database, grouped by the different expression of CCR5 and CXCR4, were used to conduct survival analyses. Table 7 shows the clinicopathological characteristics in patients with seminoma from TCGA cohort. It was found that increased expression level of CCR5 (HR 0.72 [0.52–0.98], P=0.047) was associated with poor overall survival for seminoma patients, as well as CXCR4 (HR 0.73 [0.59–0.92], P=0.012) (Figure 3F and G).
Figure 3.
Expression levels and survival analyses of DEGs. (A–E) Expression levels of the five hub genes. *P-value <0.05. (F–G) Kaplan-Meier plot of overall survival for CCR5 and CXCR4 in seminoma patients.
Abbreviation: HR, hazard ratio.
Table 7.
The clinicopathological characteristics in patients with seminoma from TCGA cohort
| Expression of CCR5 | P-value | Expression of CXCR4 | P-value | ||||
|---|---|---|---|---|---|---|---|
| Low (n=107) | High (n=27) | Low (n=47) | High (n=87) | ||||
| Age | 0.305 | 0.239 | |||||
| <40 y | 88 | 25 | 42 | 71 | |||
| ≥40 y | 19 | 2 | 5 | 16 | |||
| Race | 0.442 | 0.133 | |||||
| White | 95 | 23 | 45 | 74 | |||
| Yellow | 7 | 1 | 1 | 7 | |||
| Black | 5 | 3 | 1 | 6 | |||
| AJCC stage | 0.288 | 0.588 | |||||
| I | 76 | 23 | 33 | 68 | |||
| II | 14 | 2 | 7 | 9 | |||
| III | 17 | 2 | 7 | 10 | |||
| Survival | 0.380 | 0.297 | |||||
| Yes | 105 | 25 | 47 | 83 | |||
| No | 2 | 2 | 0 | 4 | |||
Note: *p<0.05.
Discussion
Seminoma accounts for the most part of cases of TGCT, which is the most common malignancy among males between ages 15 and 44 years.1 Understanding its molecular mechanism in genetic aspects is important for diagnosis and treatment. In the present study, we analyzed the gene expression profile GSE8607, containing 40 seminoma samples and three healthy testes samples, by bioinformatics methods, to explore the hub genes which may play crucial roles in tumorigenesis. We identified 1,636 DEGs, of which 701 were up-regulated and 935 were down-regulated in seminomas compared with control testes.
GO analysis showed that up-regulated DEGs were mainly enriched in defense responses, receptor activity, regulation of immune response processes, and signal transducer activity, while down-regulated DEGs were mainly enriched in reproductive processes, kinase activity, and carbohydrate derivative binding. Regarding KEGG pathway enrichment analysis, up-regulated DEGs were enriched in CAMs, natural killer cell-mediated cytotoxicity, cytokine–cytokine receptor interactions, and chemokine signaling pathways. Previous studies have suggested that cell adhesion plays an important part in the growth, progression, and metastasis of tumors. Moreover, high expression level of CAMs was reported to be associated with poor prognosis in lung and breast cancer, and many other tumor types.17–19 In recent years, natural killer T cells were found to be an effective treatment for several cancers, but their efficacy in seminoma remains unknown.20
We showed that down-regulated DEGs were mainly associated with tight junctions, metabolic pathways, axon guidance, the cell cycle, and calcium signaling pathways. Tight junctions of healthy testes separate the internal and external environment of the testis and protect it from harmful substances. However, it is difficult to maintain a normal structure and function in cancer tissue. Moreover, studies have suggested that the loss of cell cycle regulation leads to genomic instability, and the cell cycle is thought to play an important role in the etiology of spontaneous cancers.21 Recent evidence indicated that physiological calcium signaling regulated aerobic metabolism, but that pathological calcium overload contributed to cell death.22 Therefore, monitoring these processes and pathways may aid the diagnosis or treatment of seminoma.
IL6, VEGFA, IL10, CCR5, and CXCR4 were selected as hub genes because of their high degree of connectivity. Existing evidence suggests that immunologic factors may affect the development of seminoma, with the inflammatory cytokines IL6 and IL10 thought to promote tumor immune evasion through local immunosuppression. Parker et al reported that the extent of lymphocyte infiltration in seminomas was associated with a reduced risk of disease recurrence,23 while Klein et al documented major roles for IL6 in shaping the surrounding tumor microenvironment by influencing local immune responses.24 IL6 may, therefore, have the potential to become a novel diagnostic and immunotherapeutic factor for seminoma.25 However, details about signaling and intercellular interaction require further investigation. Mohamed reported that IL10 secreted by tumor-infiltrating monocytes/macrophages (CD14+/CD16+) separated from inflammatory breast cancer patients positively correlated with the expression level of CPB2, which was crucial to lymphovascular invasion in inflammatory breast cancer.26 However, further studies are required to clarify the role of IL10 in seminoma.
Angiogenesis is crucial to the progression of many kinds of tumors because of the need for nutrition from blood vessels.27 VEGFA is a subtype of VEGF that functions as a potent angiogenic factor in blood vessel formation and regulates the progression of tumors.28,29 VEGFA promotes tumor proliferation and angiogenesis by activating phosphoinositide 3-kinase (PI3K)/AKT and extracellular-signal-regulated kinase (ERK) signaling pathways.30–32 Although anti-angiogenesis therapies targeting VEGFA inhibit the progression of many tumor types, their efficacy in seminoma needs to be investigated. The gene CCR5, encoding one of the receptors of C-C chemokine ligand 5 (CCL5), promotes carcinogenesis, stroma genesis, and tumor progression.33 CCL5/CCR5 operates via PI3K/AKT, mitogen-activated protein kinase kinase, and ERK, which in turn activate nuclear factor-κB, leading to activation of αvβ3 integrin and contributing to cell migration.34–36 CCL5/CCR5 was found to be a biomarker of poor prognosis in various cancer types such as pancreas, prostate, breast, ovarian, and renal cancers.37–41 CXCR4 is one of the most commonly overexpressed cytokine receptors in malignant tumors, and it accepts the signal of its ligand CXCL12 to mediate cell adhesion, angiogenesis, proliferation, metastasis, and survival.42 Both CXCR4 and CXCL12 are important components of the signaling mechanisms that facilitate the normal migration of primordial germ cells from the hindgut to the genital ridges in fish and mammals.43 Although they were reported to play an important role in the metastasis of seminoma by activating MAP kinase and PI3K pathways, they have a limited role in tumor survival and proliferation.44 In the present study, we found that high expression levels of CCR5 and CXCR4 were associated with poor prognosis for seminoma patients. Maybe they are potential diagnostic biomaker or predictor for prognosis when more studies confirm their values.
Analysis of the four modules selected from the PPI network in the present study suggested that the Jak–STAT signaling pathway, chemokine signaling pathway, endocytosis, and cytokine–cytokine receptor interactions may be associated with the occurrence of seminoma. The Jak–STAT pathway is an evolutionarily conserved signaling cascade which mediates the response to cytokines and growth factors. Cellular responses to the activation of this pathway include differentiation, proliferation, migration, apoptosis, and cell survival. Moreover, Jak–STAT signaling is integral to homeostatic and developmental processes such as hematopoiesis, immune development, stem cell maintenance, and organismal growth,45 while its activation by dysregulated chemokines induces the occurrence and growth of cancer. Palagani et al reported that inhibiting the Jak–STAT pathway might prevent pancreatic cancer progression,46 and Li et al showed that its inhibition would promote the antiproliferative effect of methotrexate in small cell lung cancer.47 However, the relationship between seminoma and the Jak–STAT pathway requires further investigation. Maybe the Jak–STAT pathway is a promising candidate signaling pathway which affects the tumorigenesis of seminoma.
In conclusion, we used bioinformatics analysis of DEGs to identify key genes and pathways that are closely associated with the occurrence of seminoma. Our work identified several potential targets for biomarkers and for understanding underlying molecular mechanisms. However, further study is required to determine the functions of these candidates.
Acknowledgments
We thank Sarah Williams, PhD, from Liwen Bianji, Edanz Group China, for editing the English text of a draft of this manuscript. This study was supported by Foundation of Fujian Provincial Department of Finance (Grant number: 2018B011).
Abbreviation list
TCGT, testicular germ cell tumors; AJCC, American Joint Committee on Cancer; DEGs, differentially expressed genes; GO, Gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; STRING, Search Tool for the Retrieval of Interacting Genes; PPI, protein–protein interaction; MCODE, Molecular Complex Detection; GEO, Gene Expression Omnibus; BP, biological processes; MF, molecular functions; CC, cellular component; DAVID, Database for Annotation, Visualization and Integrated Discovery; IL 6, interleukin 6; VEGFA, vascular endothelial growth factor A; CCR5, C-C motif chemokine receptor 5; CXCR4, C-X-C motif chemokine receptor 4; Jak–STAT, the Janus kinase-signal transducers and activators of transcription signaling pathway.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ghazarian AA, Kelly SP, Altekruse SF, Rosenberg PS, McGlynn KA. Future of testicular germ cell tumor incidence in the United States: forecast through 2026. Cancer. 2017;123:2320–2328. doi: 10.1002/cncr.30597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith ZL, Werntz RP, Eggener SE. Testicular cancer: epidemiology, diagnosis, and management. Med Clin North Am. 2018;102:251–264. doi: 10.1016/j.mcna.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 4.Fankhauser CD, Curioni-Fontecedro A, Allmann V, et al. Frequent PD-L1 expression in testicular germ cell tumors. Br J Cancer. 2015;113:411–413. doi: 10.1038/bjc.2015.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada Y, Takayama K-I, Fujimura T, et al. A novel prognostic factor TRIM44 promotes cell proliferation and migration, and inhibits apoptosis in testicular germ cell tumor. Cancer Sci. 2017;108:32–41. doi: 10.1111/cas.13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Duan H, Zhou S, et al. microRNA-199a-3p functions as tumor suppressor by regulating glucose metabolism in testicular germ cell tumors. Mol Med Rep. 2016;14:2311–2320. doi: 10.3892/mmr.2016.5472 [DOI] [PubMed] [Google Scholar]
- 7.Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254 [DOI] [PubMed] [Google Scholar]
- 8.Liang B, Li C, Zhao J. Identification of key pathways and genes in colorectal cancer using bioinformatics analysis. Med Oncol. 2016;33:111. doi: 10.1007/s12032-016-0829-6 [DOI] [PubMed] [Google Scholar]
- 9.Sun C, Yuan Q, Wu D, Meng X, Wang B. Identification of core genes and outcome in gastric cancer using bioinformatics analysis. Oncotarget. 2017;8:70271–70280. doi: 10.18632/oncotarget.20082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006;34:D322–D326. doi: 10.1093/nar/gkj021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashburner M, Ball CA, Blake JA, et al; The Gene Ontology Consortium. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 14.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Jiang Y, Xie D, et al. Inhibition of cell-adhesion protein DPYSL3 promotes metastasis of lung cancer. Respir Res. 2018;19:41. doi: 10.1186/s12931-018-0740-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J-D, Hong C-Q, Huang W-H, et al. L1 cell adhesion molecule and its soluble form sL1 exhibit poor prognosis in primary breast cancer patients. Clin Breast Cancer. 2018;18:e851–e861. doi: 10.1016/j.clbc.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Gao M, Ma Y, Wang L, Shen Y, Liu X. Inhibition of cell migration by focal adhesion kinase: time-dependent difference in integrin-induced signaling between endothelial and hepatoblastoma cells. Int J Mol Med. 2018;41:2573–2588. doi: 10.3892/ijmm.2018.3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldowska M, Bojarska-Junak A, Rolinski J. A brief review of clinical trials involving manipulation of invariant NKT cells as a promising approach in future cancer therapies. Cent-Eur J Immunol. 2017;42:181–195. doi: 10.5114/ceji.2017.69361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. [DOI] [PubMed] [Google Scholar]
- 22.Logan CV, Szabadkai G, Sharpe JA, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46:188–193. doi: 10.1038/ng.2851 [DOI] [PubMed] [Google Scholar]
- 23.Parker C, Milosevic M, Panzarella T, et al. The prognostic significance of the tumour infiltrating lymphocyte count in stage I testicular seminoma managed by surveillance. Eur J Cancer. 2002;38:2014–2019. [DOI] [PubMed] [Google Scholar]
- 24.Klein B, Schuppe H-C, Bergmann M, Hedger MP, Loveland BE, Loveland KL. An in vitro model demonstrates the potential of neoplastic human germ cells to influence the tumour microenvironment. Andrology. 2017;5:763–770. doi: 10.1111/andr.12365 [DOI] [PubMed] [Google Scholar]
- 25.Klein B, Haggeney T, Fietz D, et al. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum Reprod. 2016;31:2192–2202. doi: 10.1093/humrep/dew211 [DOI] [PubMed] [Google Scholar]
- 26.Mohamed HT, El-Husseiny N, El-Ghonaimy EA, et al. IL-10 correlates with the expression of carboxypeptidase B2 and lymphovascular invasion in inflammatory breast cancer: the potential role of tumor infiltrated macrophages. Curr Probl Cancer. 2018;42:215–230. doi: 10.1016/j.currproblcancer.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 27.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/jco.2005.06.081 [DOI] [PubMed] [Google Scholar]
- 28.London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768. [PubMed] [Google Scholar]
- 29.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 30.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046 [DOI] [PubMed] [Google Scholar]
- 31.Lee T-H, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent L, Jin DK, Karajannis MA, et al. Fetal stromal-dependent paracrine and intracrine vascular endothelial growth factor-a/vascular endothelial growth factor receptor-1 signaling promotes proliferation and motility of human primary myeloma cells. Cancer Res. 2005;65:3185–3192. doi: 10.1158/0008-5472.can-04-3598 [DOI] [PubMed] [Google Scholar]
- 33.Cambien B, Richard-Fiardo P, Karimdjee BF, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRbeta in colorectal carcinoma. PLoS One. 2011;6:e28842. doi: 10.1371/journal.pone.0028842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C-Y, Fong Y-C, Lee C-Y, et al. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 2009;77:794–803. doi: 10.1016/j.bcp.2008.11.014 [DOI] [PubMed] [Google Scholar]
- 35.Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells. 2012;30:2309–2319. doi: 10.1002/stem.1194 [DOI] [PubMed] [Google Scholar]
- 36.Kato T, Fujita Y, Nakane K, et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine. 2013;64:251–257. doi: 10.1016/j.cyto.2013.06.313 [DOI] [PubMed] [Google Scholar]
- 37.Singh SK, Mishra MK, Eltoum I-EA, Bae S, Lillard JW, Singh R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci Rep. 2018;8:1323. doi: 10.1038/s41598-018-19643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo T, Ito F, Nakazawa H, Horita S, Osaka Y, Toma H. High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. J Urol. 2004;171:2171–2175. [DOI] [PubMed] [Google Scholar]
- 39.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–134. doi: 10.1002/pros.20306 [DOI] [PubMed] [Google Scholar]
- 40.Khalid A, Wolfram J, Ferrari I, et al. Recent advances in discovering the role of CCL5 in metastatic breast cancer. Mini Rev Med Chem. 2015;15:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukishiro S, Suzumori N, Nishikawa H, Arakawa A, Suzumori K. Elevated serum RANTES levels in patients with ovarian cancer correlate with the extent of the disorder. Gynecol Oncol. 2006;102:542–545. doi: 10.1016/j.ygyno.2006.01.029 [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Cheng G, Hao M, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doitsidou M, Reichman-Fried M, Stebler J, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. [DOI] [PubMed] [Google Scholar]
- 44.McIver SC, Loveland KL, Roman SD, Nixon B, Kitazawa R, McLaughlin EA. The chemokine CXCL12 and its receptor CXCR4 are implicated in human seminoma metastasis. Andrology. 2013;1:517–529. doi: 10.1111/j.2047-2927.2013.00081.x [DOI] [PubMed] [Google Scholar]
- 45.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palagani V, Bozko P, El Khatib M, et al. Combined inhibition of Notch and JAK/STAT is superior to monotherapies and impairs pancreatic cancer progression. Carcinogenesis. 2014;35:859–866. doi: 10.1093/carcin/bgt394 [DOI] [PubMed] [Google Scholar]
- 47.Li HX, Zhao W, Shi Y, et al. Retinoic acid amide inhibits JAK/STAT pathway in lung cancer which leads to apoptosis. Tumour Biol. 2015;36:8671–8678. doi: 10.1007/s13277-015-3534-8 [DOI] [PubMed] [Google Scholar]