Graphical abstract
Keywords: Hepatitis delta virus, Virus life cycle, Chronic hepatitis, Epidemiology, Treatment, Hepatitis delta management
Highlights
-
•
Hepatitis D virus is a defective virus, dependent on hepatitis B virus for its assembly.
-
•
Hepatitis D virus infection affects 62–72 million people worldwide.
-
•
Chronic hepatitis D is the most severe chronic viral hepatitis.
-
•
Current interferon-based antiviral treatments have dismal efficiency and are poorly tolerated.
-
•
Host-targeting molecules inhibiting the viral life cycle are currently in clinical development.
Abstract
Hepatitis delta virus (HDV) is a defective virus that requires the hepatitis B virus (HBV) to complete its life cycle in human hepatocytes. HDV virions contain an envelope incorporating HBV surface antigen protein and a ribonucleoprotein containing the viral circular single-stranded RNA genome associated with both forms of hepatitis delta antigen, the only viral encoded protein. Replication is mediated by the host cell DNA-dependent RNA polymerases. HDV infects up to72 million people worldwide and is associated with an increased risk of severe and rapidly progressive liver disease. Pegylated interferon-alpha is still the only available treatment for chronic hepatitis D, with poor tolerance and dismal success rate. Although the development of antivirals inhibiting the viral replication is challenging, as HDV does not possess its own polymerase, several antiviral molecules targeting other steps of the viral life cycle are currently under clinical development: Myrcludex B, which blocks HDV entry into hepatocytes, lonafarnib, a prenylation inhibitor that prevents virion assembly, and finally REP 2139, which is thought to inhibit HBsAg release from hepatocytes and interact with hepatitis delta antigen. This review updates the epidemiology, virology and management of HDV infection.
Introduction
Hepatitis D or delta is caused by the hepatitis delta virus (HDV), a human pathogen first identified in 1977 [1]. HDV is a defective RNA virus that does not encode its own envelope proteins and depends on the expression of the hepatitis B virus (HBV) surface antigen (HBsAg) in the same cell to complete its life cycle. HDV can enter hepatocytes not expressing HBsAg and efficiently replicate its genome and express the hepatis delta antigen (HDAg); however, no secretion of infectious particles occurs. Hepatitis D is hence the result of either an acute coinfection by HBV and HDV or a HDV superinfection of patients chronically infected with HBV.
Chronic hepatitis D (CHD) is arguably the most aggressive type of viral hepatitis and is associated with an increased risk of cirrhosis, liver decompensation and hepatocellular carcinoma (HCC) [2], but the management of HDV has evolved little during the past years. The main treatment remains pegylated interferon-alpha (IFN-alpha), with unsatisfactory results. Nucleos(t)ide analogues specific for HBV have no effect on HDV replication. However, several host-targeting molecules with a specific impact on HDV life cycle are currently under development.
Epidemiology
Worldwide, ∼248 to 292 million people are chronically infected with HBV [3], [4]. Based on these estimations, ∼15 to 20 million of these patients were initially thought to be also affected by HDV [5]. These figures were challenged by a recent meta-analysis, proposing that a staggering 62–72 million people may live with HDV worldwide [6], a prevalence almost two-times greater than that of human immunodeficiency virus (HIV) infection (estimated to infect 36.9 million persons in 2017, according to the World Health Organization). These estimates imply a disease burden much higher than previously considered and one that is still debated [7]. Indeed, the exact global prevalence of HDV infection remains unknown because of heterogeneous and non-standardised screening practices and the inaccessibility to testing in many endemic areas.
In Mongolia, HDV infects ∼60% of the HBsAg-positive individuals, corresponding to the highest reported prevalence worldwide [8]. Other highly affected areas include the Amazon basin [9], West Africa [10], [11], the Mediterranean basin [12] and Eastern Europe [13].
In Western Europe, although high prevalence rates were described in Italy early after HDV identification, a subsequent decrease was documented as consequence of improved socio-economic conditions and mass vaccination campaigns against HBV [14], [15]. HDV prevalence now seems to be very low in some European countries, and in close association with intravenous drug use (IVDU) [16]. However, no decrease has been observed in other areas, likely because of migration from endemic regions [17], [18].
In the United States, HDV infection has for long been considered rare and screening recommendations are limited to high-risk populations [19]. Unfortunately, several recent studies highlight the presence of suboptimal testing rates and suggest that the prevalence may be much higher that previously considered [20], [21], [22].
As HBV, HDV can be transmitted by blood and blood-derived products and sexual contact. Vertical transmission is however rare. In highly endemic populations, transmission occurs mainly through intrafamilial and iatrogenic spread [23] in association with poor hygiene conditions [24]. In low endemicity regions in the northern hemisphere, iatrogenic and intrafamilial transmission, while accounting for infections occurred in the past, are no longer common and IVDU is now the main transmission route [6]. Sexual transmission, although less frequent than for HBV or HIV, seems to be important in regions where HBV infection is endemic, such as Taiwan [25], [26].
Virology
Classification
HDV is the smallest known virus infecting mammals, for which humans are the only natural reservoir. Other susceptible mammalian hosts have been identified as well as used for research purposes; these include chimpanzees, tree shrews (both with HBV as a helper virus) and woodchucks (in the presence of the woodchuck hepatitis virus, WHV). Although HBV orthologues have been found in a variety of non-human mammals and have been shown to have potential cross-species infectivity [27], no HDV orthologue had been described until the very recent identification of HDV-like agents in birds and snakes [28], [29].
Due to its distinct characteristics, HDV has been postulated to originate from plant viroids or cellular circular RNAs and is currently the sole member of the Deltavirus genus [30], [31], [32].
There are eight HDV genotypes, highly heterogeneous, with up to ∼40% of sequence divergence [33]. Genotype 1 is present worldwide and is the predominant virus in Europe and North America. Genotype 3 is identified in South America, while genotypes 2 and 4 are common in East Asia and genotypes 5–8 are mainly found in Africa (refer to [34] for a review of genotype distribution). Although good quality studies are limited, different genotypes seem to be associated with distinct liver disease severity. In comparison with genotype 1, genotypes 2 and 4 seem to cause milder liver disease. Genotype 2 in particular, has been associated to a lower incidence of cirrhosis, HCC and decreased mortality than genotype 1 in a prospective study conducted in Asia [35]. Genotype 3, on the other hand, is associated with a more severe course of acute infections and a higher risk of acute liver failure [36].
A co-evolution of HBV and HDV genotypes can be suggested by the frequency of specific genotype pairs, the most commonly reported being the combination of HDV genotype 3 with HBV genotype F [37]. However, these associations have been argued to merely result from geographic distribution, given these are not strict combinations [36] and HDV virion assembly has been shown to be possible with several HBV genotypes [38].
Viral structure
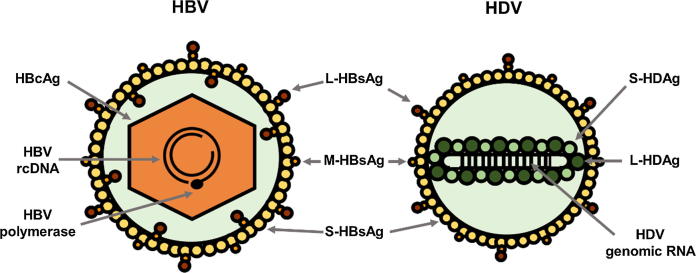
HDV circulating virions were firstly characterised in chimpanzees infected with serum from an Italian chronic carrier [39]. As represented in Fig. 1. These 35–37 nm particles are composed of an envelope and a ribonucleoprotein (RNP).
Fig. 1.
Structure of HBV and HDV virions. Both viruses use HBV surface proteins (S-, M- and L-HBsAg) for their assembly. HBV icosahedral capsid is formed by multimerisation of its core protein (HBcAg) and contains one copy of the viral partially double-stranded DNA genome (or relaxed circular DNA, rcDNA) and the viral polymerase. HDV virions contain one copy of the viral circular, single-stranded RNA genome (that has 70% of sequence complementarity, allowing its folding into a rod-like structure), associated with both forms of its only protein (large and small delta antigen or S- and L-HDAg), forming the viral ribonucleoprotein (RNP).
Since HDV is a defective virus and does not code for its own surface proteins, it uses the three forms of HBV surface proteins (small or S-HBsAg, medium or M-HBsAg and large or L-HBsAg) on which it depends to form its own envelope and egress and re-entry into hepatocytes. These proteins share a common C-terminus (S domain, the only constituent of S-HBsAg). M-HBsAg contains an N-terminal hydrophilic domain named PreS2 and, relative to M-HBsAg, L-HBsAg N-terminus consists of an additional domain named PreS1 [40].
The RNP, present both in viral particles and infected cells, contains the viral genome associated with both isoforms of the hepatitis delta antigen (HDAg) — small, S-HDAg, and large, L-HDAg — forming a structure that is essential for the nuclear trafficking of HDV RNA and for viral assembly [41]. Although debate surrounds its complete characterisation, its assembly depends on the oligomerisation of HDAg molecules and the secondary structure of the HDV genome [42], [43], [44], [45].
The HDV genome, whose complete structure was first reported in 1986, is a circular, covalently closed, single-stranded RNA of ∼1680 nucleotides with 74% internal base pairing, allowing the folding into a partially double-stranded rod-like structure [46].
During HDV replication in the infected cells, two other main forms of viral RNAs can be found: the antigenome, which is a replication intermediate and the exact complement of the genome sequence [47], [48], and the HDV mRNA coding for the two isoforms of the HDAg [49]. Ribozymes (small, self-cleaving RNA sequences) have been described in both the HDV genome and antigenome and are responsible for the cleavage of the multimeric linear RNA molecules that arise during replication [50].
Viral life cycle
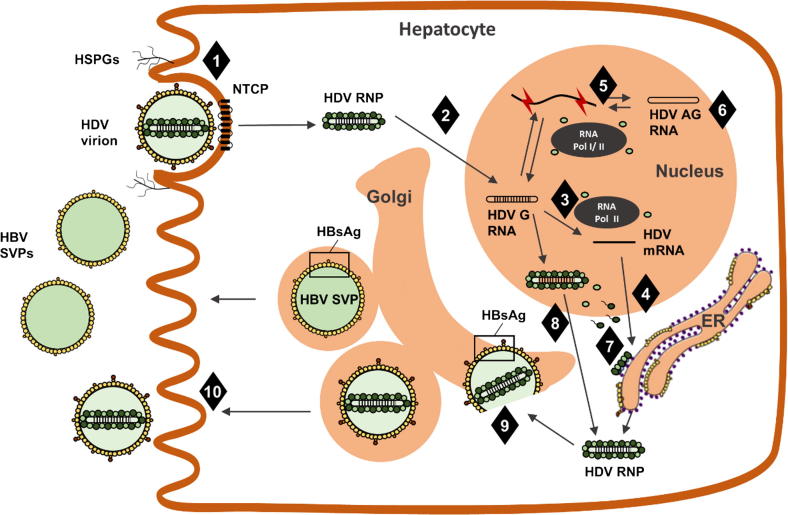
The following paragraphs describe the main steps of the HDV life cycle and Fig. 2. depicts a simplified, schematic version.
Fig. 2.
HDV life cycle. HDV entry (step 1) is mediated by a first attachment step, resulting from viral interaction with HSPGs, and later specific interaction of L-HBsAg with the viral receptor, NTCP. This step is inhibited by Myrcludex B. The viral RNP is then transported to the nucleus (step 2) where it releases the viral genome that serves as template to transcription of HDV mRNA (step 3), from which HDAg is translated (step 4). Replication of viral RNA (step 5) is mediated by cellular DNA-dependent RNA polymerases in the presence of S-HDAg, through a rolling-circle mechanism, with formation of multimeric and antigenomic RNA intermediates. During replication, antigenomic RNA can be edited by ADAR1 (step 6), leading to the expression of L-HDAg molecules (as detailed in Fig. 3). Farnesylation of L-HDAg (step 7), a step inhibited by lonafarnib, is necessary for regulation of replication and viral assembly. The newly formed HDV RNPs are assembled in the nucleus (step 8), exported and then enveloped by HBV surface glycoproteins (step 9) through the interaction of farnesylated L-HDAg with HBsAg. HDV virions are thought to be secreted through the Golgi (step 10) in parallel with HBV SVPs. Although the precise mechanism of action of REP 2139 is not fully characterized, it has been shown not to interfere with viral entry of HBV or HDV but appears to affect HDV secretion by inhibiting secretion of HBsAg and also potentially by interacting with HDAg. The exact mechanism of action of interferons (both alpha and lambda) is not represented, as it is still not fully known (although it is believed to involve an inhibition of viral RNA replication). ADAR1, adenosine deaminase acting on RNA 1; AG, antigenome; ER, endoplasmic reticulum; G, genome; HBV, hepatitis B virus; HDV, hepatitis B virus; HSPGs, heparan sulfate proteoglycans; NTCP, sodium taurocholate co-receptor peptide; RNP, ribonucleoprotein; SVPs, subviral particles.
Viral entry
HDV is considered to target primarily hepatocytes. Although the possibility of extra-hepatic replication in a natural infection has been hypothesised for HBV, namely in lymphocytes [51], [52], no such evidence exists for HDV [53]. However, HDV replication can take place in a wide range of mammalian cells, if the genome is experimentally delivered, suggesting that its hepatotropism depends exclusively on the presence of the receptor [54].
HDV is considered to enter hepatocytes through the same mechanisms as HBV, given that both viruses share a similar envelope [2]. Infectivity of both viruses depends on the presence of L-HBsAg, in particular on the 75 amino acids located at its N-terminal (in the PreS1 domain), where an essential myristoylation site is located [40], as well as specific amino acid residues of S-HBsAg. However, M-HBsAg does not seem necessary [55], [56].
A first, non-specific step consists in the viral attachment to the heparin sulfate proteoglycans (HSPGs) exposed on the outer face of the host cell membrane [57], [58], [59]. One particular HSPG, glypican-5, has been identified as an entry factor that only partially justifies HDV and HBV dependence on HSPGs, as its abrogation was not sufficient to completely prevent infection [60].
Attachment, although necessary, is not sufficient to allow viral entry and further interaction of the virus with its specific receptor is needed. For the first three decades following the identification of both HBV and HDV, this receptor was unknown. In 2012, the human sodium taurocholate cotransporting peptide (hNTCP, encoded by SLC10A1) was convincingly shown to be a functional receptor to both HBV and HDV in hepatocytes [61]. This molecule is located at the basolateral membrane of hepatocytes and is involved in the uptake of bile acids. The interaction of the bile acid binding domain of NTCP with the myristoylated N-terminal sequence of the PreS1 region of L-HBsAg was shown to be both necessary and sufficient for HBV and HDV infection [61], [62]. The post-entry steps involved in the release of the HDV RNP in the cytoplasm and its transport into the nucleus, where transcription and replication subsequently occur, are not fully characterised.
Replication
HDV replication occurs in the nucleus and is completely independent of HBV. Since HDV does not possess its own RNA-dependent RNA polymerase or use the polymerase of its helper virus, the host cell DNA-dependent RNA polymerases likely mediate its replication. Several lines of evidence support the involvement of RNA polymerase II, but a debate is still ongoing concerning the role of the other cell RNA polymerases in HDV replication [54], [63]. Both RNA polymerases I and III have been shown to bind HDV RNA. While RNA polymerase I seems implicated in the antigenomic transcription, no precise function has been reported so far for RNA polymerase III [64].
The mechanisms through which the virus is able to hijack the cell DNA-dependent RNA polymerase(s) for its RNA replication are still largely unknown and, although similar mechanisms have been shown to play a role in plant viroid replication [65], HDV constitutes a unique case in human virology. The secondary structure of HDV RNA might play a role in the capacity of the host RNA polymerase II to use it as template, as the enzyme recognises sites located at the two poles of the rod-like structure of the genome [66]. Arguably, S-HDAg would also play an important role in this process [67]. Indeed, the regulation of viral replication involves both forms of HDAg: while L-HDAg, which is essential for viral assembly, has an inhibitory effect on replication [68], [69], the transcription of the viral RNAs cannot occur in the absence of S-HDAg [70], [71]. S-HDAg has been shown to interact with several subunits of both RNA polymerases I and II and the interaction with the latter may involve the recruitment of chromatin remodelling complexes onto HDV RNA [67], [72]. To account for the need of S-HDAg, the transcription of the HDV mRNA is believed to precede the HDV RNA replication. The cellular RNA polymerase II mediates the transcription of this 800 nucleotide-long mRNA, which has the same characteristics as cellular mRNAs (a 5′ cap and a poly-A tail) [54]. This step ensures the availability of S-HDAg, which then favours the initiation of replication.
The replication of HDV RNA follows a rolling-circle mechanism starting with the synthesis of multimeric linear transcripts complementary to the genome. HDV RNA antigenomic ribozyme self-cleavage separates the different monomers from the multimeric transcripts. Monomers are then ligated into circular antigenomic molecules, serving as the template for genomic-strand progeny molecules [66]. The mechanism underlying the circularisation of the HDV genome, either by ribozyme mediated self-ligation or a cellular ligase, is still under debate [73].
RNA editing and L-HDAg synthesis
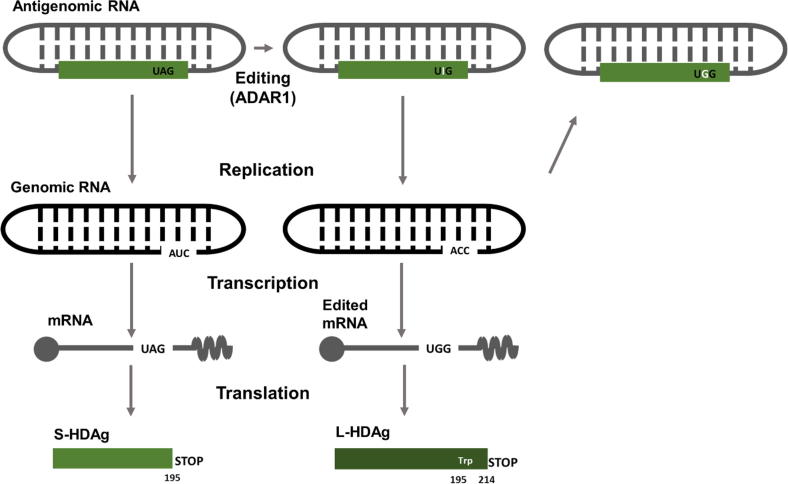
A single open reading frame in the HDV antigenome directs the synthesis of both isoforms of HDAg (S-HDAg and L-HDAg). These two isoforms differ by an additional C-terminal stretch of 19 amino acids in L-HDAg. As described before, the replication of HDV RNA requires S-HDAg, whereas L-HDAg is essential for virion assembly. The C-terminal domain of L-HDAg enables the interaction with HBsAg [74]. The relative ratio between these two HDAg isoforms regulates the equilibrium between replication and virion assembly. The editing of the antigenomic RNA by adenosine deaminase acting on RNA 1 (ADAR1) drives the transition from S-HDAg mRNA to L-HDAg mRNA transcription [75]. This cellular enzyme has two isoforms: the small, which is expressed constitutively and the large, whose expression is stimulated by type I IFN. Contradictory results still exist regarding the role of each ADAR-1 isoform in the HDV life cycle [76], [77]. ADAR1 acts on a particular site of the HDV antigenome, called the amber/W site. This site is a UAG amber stop codon, leading to translation termination and consequent S-HDAg production. ADAR1 deaminates an adenosine (UAG → UIG) and the resulting inosine is recognised as a guanosine in the next replication cycle, leading to a ACC triplet on the genome (instead of the original AUC). Transcription of this triplet generates a tryptophan-encoding UGG codon in the mRNA that no longer works as a stop codon. Consequently, translation proceeds for an additional 19 amino acids, resulting in L-HDAg synthesis [75], as depicted in Fig. 3.
Fig. 3.
Differential expression of S- and L-HDAg as a consequence of antigenome RNA editing by ADAR-1. The HDV antigenomic RNA has one single open reading frame from which the two isoforms of HDAg are expressed. Adenosine deaminase acting on RNA-1 (ADAR1) catalyses editing of the amber/W site on the antigenomic HDV RNA and adenosine 1012 is converted to inosine. After replication and mRNA transcription, the original stop codon (AUG, terminating the synthesis of S-HDAg) is converted into UGG, coding for a tryptophan (Trp) residue and allowing translation to proceed until the next stop codon, which results in the addition of 19 amino acids (L-HDAg).
Post-translational modifications of HDAg proteins
Both HDAg proteins undergo post-translational modifications critical for their respective functions. Phosphorylation of two serine residues of S-HDAg allows interactions with the cellular RNA polymerase II, enabling the replication of HDV RNA [78], [79]. A farnesylation signal (C211XXQ box) in the additional 19 amino acids of L-HDAg enables a farnesyl lipid group to be added covalently to the cysteine at position 211 by a cellular farnesyltransferase. This farnesylated form of L-HDAg inhibits the replication of HDV RNA and is essential for virion assembly [74]. Indeed, agents that inhibit the addition of the farnesyl lipid group to the C-terminus of L-HDAg prevent its interaction with HBsAg, consequently inhibiting HDV virion secretion both in vitro and in vivo [80], [81] and constitute a novel therapeutic approach for HDV infection (see Drugs in Clinical Development below).
Assembly and release
As mentioned previously, although HDV can replicate and synthesise new RNPs independently of HBV, its release from hepatocytes depends on the presence of HBsAg in the same cell. HDV assembly depends on the specific interaction between the farnesylated N-terminus of L-HDAg and the S region of HBsAg [82], and it has been shown that, unlike HBV, HDV RNP can efficiently be assembled with the small form of HBsAg (S-HBsAg) [83]. However, the large form of HBsAg (L-HBsAg) is needed to form infectious virions (as mentioned earlier, it mediates the interaction between the virus and its hepatocyte receptor, NTCP). The relevance of the non-infectious, S-HBsAg enveloped, HDV particles in a natural infection is still to be demonstrated.
In the context of a HBV infection, the three forms of HBsAg, which are produced in much higher amounts than required for HBV virion production, can self-assemble and be secreted as “empty” subviral particles (SVPs). These non-infectious SVPs (constituted of an envelope devoid of HBV capsid or nucleic acids) are secreted in large excess relative to the infectious virions and are thought to play a role in HBV escape to the immune response. HBV SVPs are secreted through the Golgi, while infectious virions follow the multivesicular body pathway [84]. Given that the composition of the HDV envelope is close to that of SVPs and that titers of circulating HDV virions are higher than those of HBV virions, approaching those of HBV SVPs [2], [85], it is likely that HDV uses the SVP secretion pathway for its assembly and release.
Molecular interactions with HBV
Studies conducted in experimental models have shown a decrease in HBV replication in the context of HDV infection, with minimal impact on the expression of HBsAg, as demonstrated by an increased HBsAg/HBV DNA ratio in the HBV-HDV co-infected cells, in comparison with HBV monoinfected cells [86], [87], [88]. This observation has been confirmed in patients, although the viral dominance patterns seem to fluctuate over time [89], [90].
Several mechanisms may be used by HDV to inhibit its helper virus replication, while ensuring a constant pool of HBsAg for its own assembly. Firstly, the possibility of an epigenetic regulation of cccDNA transcriptional activity by HDAg has been suggested from both in vitro results and patients samples [91], raising the possibility of a differential transcription of PreS/S mRNA vs pregenomic mRNA. Secondly, both isoforms of HDAg have been shown to interact with and strongly repress both HBV enhancer sequences, with a direct impact on HBV replication [92]. Thirdly, HDAg, being an RNA-binding protein that has recently been shown to interact with specific cellular RNAs [93], [94], [95], may bind to HBV mRNAs and selectively affect their stability. Finally, accumulating evidence suggests that, in HBV-infected patients, integrated HBV DNA is an abundant source of HBsAg, even in the absence of HBV replication [96], [97], [98]. Furthermore, HBsAg derived from integrated HBV DNA has been shown to support assembly and release of infectious HDV particles [99]. While the impact of this mechanism in vivo is still to be demonstrated, it is tempting to hypothesise that HDV can complete its cycle using HBsAg produced from integrated HBV DNA, devoid of HBV replication in the same hepatocyte.
Indirect mechanisms of interference through a deregulation of the hepatocyte innate immune response are also possible. HBV has classically been considered not to be recognised by the innate immune system [100]. While this notion has been challenged by evidence suggesting that the virus may in fact actively counteract the interferon response [101], [102], two recent studies support the concept of HBV as a “stealth virus” [103], [104]. HDV, on the other hand, has been shown to induce a strong type I IFN response [86], [105] as a result of the recognition of viral RNAs by melanoma differentiation antigen 5 (MDA5) [106]. The consequent increased expression of antiviral IFN-stimulated genes (ISGs), such as MxA, may contribute to the inhibition of HBV replication [92].
The interplay between HDV and the host cell IFN response is however far from being clarified. HDV replication is itself inhibited by the administration of exogenous IFN-alpha [86], [107]. The mechanisms could involve, among others, the increased synthesis of L-HDAg as a consequence of the stimulation of ADAR1 expression [108]. Furthermore, IFN-alpha has been shown to inhibit HDV propagation during cell division, suggesting yet another antiviral mechanism [109] [Zeng Z et al, 2018 International HBV Meeting]. It is tempting to hypothesise that the virus may have developed mechanisms to resist the strong IFN response induced by its own replication, and HDV has indeed been shown to interfere with the JAK/STAT signalling pathway, a mechanism that might play a role in viral persistence [110].
Clinical presentation and natural history of the disease
Two modalities of HDV infection exist: simultaneous coinfection with HBV and HDV superinfection of a person chronically carrying HBV. Coinfection translates into acute hepatitis, during which aminotransferase levels can follow a typical biphasic course, corresponding to an initial HBV spread followed by HDV propagation. As for HBV monoinfection, in most immunocompetent adult patients (90–95%), it progresses to resolution of both HBV and HDV infections. The risk of acute liver failure is however much higher than that during acute HBV monoinfection [111], [112]. Acute HDV superinfection of a patient chronically infected with HBV is associated with an episode of acute hepatitis that can be mistaken for a HBV flare. In this setting, the risk of acute liver failure is particularly high [111]. More than 90% of HBV carriers superinfected with HDV progress to chronic dual infection [111], [112].
CHD is considered the most severe form of chronic viral hepatitis, with a faster progression towards cirrhosis and a higher risk of decompensation and mortality [113]. Indeed, 10–15% of chronically infected patients might develop cirrhosis within 5 years from infection and up to 80% after 30 years [114].
The association between HDV and HCC is still debated. On the one hand, decompensation of chronic liver disease, and not HCC, has been shown to be the most common complication of CHD [115], [116]. On the other hand, despite the long-standing belief that HDV-infected patients do not present an increased risk of HCC, several cohort studies have recently found that this risk may indeed be as much as nine times higher than in HBV monoinfected patients [20], [113], [117], [118]. Furthermore, persisting HDV replication was shown to be a risk factor for liver disease progression and HCC [119]. Other factors of disease progression are male sex, cirrhosis at presentation and lack of antiviral therapy [116].
Diagnosis of HDV infection
Recommendations of the major societies currently differ in the screening strategy for HDV diagnosis. European guidelines advise for HDV screening of all HBV-infected patients [120]. In the United States, and despite increasing evidence pointing to a suboptimal diagnosis of HDV infection [20], [21], screening is only advised in patients with specific risk factors (including migrants from regions with high HDV endemicity, a history of IVDU or high-risk sexual behaviour, individuals infected with HCV or HIV and patients with elevated aminotransferases with low or undetectable HBV DNA) [19]. Given the recent evidence suggesting that the global burden of disease may be higher than previously estimated, screening of all HBsAg-positive patients may be considered. Such a strategy would not only allow a more accurate determination of the prevalence of HDV infection, but it would also lead to wider and earlier therapeutic interventions, decreasing the burden of disease complications. Furthermore, access to care would be significantly strengthened by the implementation of point of care diagnosis.
Several markers can be used for the diagnosis of HDV infection. Anti-HDV antibodies can be detected by enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA). Anti-HDV total antibody is currently used as a first screening approach for the detection of HDV infection. However, two main limitations should be borne in mind, justifying the need for complementary approaches for diagnosis confirmation. Firstly, total anti-HDV antibody can be undetectable in the early weeks of acute infection. Secondly, anti-HDV IgG may persist after HDV infection, not allowing the distinction between active and resolved infection. A quantitative microarray antibody capture (QMAC) assay for the quantification of anti-HDV IgG has been recently validated for HDV diagnosis in Mongolia and the United States and was shown to correlate with detection of HDV RNA [8], [121]. Anti-HDV IgM appears earlier during the acute infection and has been shown to correlate with disease activity during chronic infection [122]; however, it is frequently undetectable in this setting and hence does not allow diagnosis confirmation nor distinction between acute and chronic infection. Serum HDAg is only transiently detected in the acute phase of HDV infection and its measurement is of limited utility.
Infection confirmation relies on the detection of HDV RNA by quantitative RT-PCR, which, together with a positive anti-HDV antibody, allows to distinguish between chronic and past infections and to follow response to treatment [123]. However, unlike for other viruses, lack of standardisation is still problematic for HDV and PCR results are often not comparable between laboratories [124]. Recent steps have been taken towards standardisation, with the availability of a World Health Organization standard, allowing result reporting in international units (IU) and new pangenotypic commercial assays [125]. In cases where a liver biopsy sample is obtained, intrahepatic HDAg may be detected by immunohistochemistry and HDV RNA by in situ hybridisation. Although determination of HDV genotype is possible by PCR, its use is limited to research settings.
Management of HDV-infected patients
The management of acute hepatitis D relies on general support measures or referral for liver transplantation if acute liver failure develops. No antiviral treatment has proven useful [126]. There are currently no specific direct-acting antiviral treatments for HDV and, although several host-targeting molecules are under development, current recommendations for CHD treatment are limited to a prolonged course of pegylated IFN-alpha.
Patient follow-up and treatment outcomes
The ideal endpoint for CHD treatment would be the clearance of both HBV and HDV infections from the liver, translating into anti-HBs seroconversion, to prevent liver disease progression. Although on-treatment and post-treatment kinetics of serum HDV RNA have been used to monitor treatment response, cumulative evidence exists that, unlike HCV infection, they fail to predict long-term virological outcome. Indeed, although undetectable HDV viremia is expected to be a marker of on-treatment response, a classical definition of sustained virological response (persistently undetectable viral RNA for 24 weeks after treatment) should be used with caution in CHD, as later relapses have been shown to occur in more than 50% of the patients treated with pegylated IFN-alpha [127].
Currently available antiviral treatments
Pegylated IFN-alpha
Although, as described above, its precise mechanism of action still needs to be clarified, IFN-alpha remains the only recommended treatment for CHD [19], [120]. Pegylated IFN-alpha, having a prolonged plasma half-life, allows a once-a-week administration, with better efficiency and compliance than standard IFN-alpha [123], [128]. Indeed, in a meta-analysis performed in 2011, standard IFN-alpha treatment was associated with a 17% sustained suppression of HDV RNA at six months follow-up (compared to 25% in pegylated IFN-alpha) and with more frequent and severe adverse events (e.g. anorexia, nausea, weight loss, alopecia, leukopenia and thrombocytopenia) [129]. Results are comparable for pegylated IFN-alpha 2a and 2b [130].
Data from clinical trials do not allow an accurate prediction of response and no robust stopping rules exist. However, HDV RNA negativity at 24 weeks of treatment has been identified as a predictor of sustained HDV RNA suppression during follow-up [131]. Nevertheless, optimal treatment duration has not been established. In most studies, pegylated IFN-alpha was used for 48 weeks and this is now the recommended treatment duration. As shown in Table 1, results diverge among studies and considering the variability of PCR performances recently demonstrated [124], [132], the reported sustained responses at 24 weeks of post-treatment follow-up may have been overestimated in earlier studies. In one large randomised clinical trial, a 48-week course of pegylated IFN-alpha led to a persistently undetectable HDV RNA 24 weeks after treatment in 25%–30% of patients [133]. Shorter treatment durations (three to six months) have been evaluated in preliminary studies, resulting in suppression of HDV replication and improvement of liver disease in some patients but universal relapse after treatment discontinuation [134], [135]. Prolonging the duration beyond 48 weeks has not shown any additional benefit in a large cohort [136], although particular patients have been suggested to benefit from prolonged courses of treatment [131].
Table 1.
Summary of studies evaluating IFN-alpha treatment of chronic hepatitis D.
| Treatment | Treatment modalities | Number of patients | Sustained suppression of HDV RNA at 24-weeks of follow-up | References |
|---|---|---|---|---|
| IFN-alpha: 3–18 Mio units 3x/week | 3–12 months | 201 | 17% | [129] |
| Pegylated IFN-alpha 2b: 1.5 µg/kg qw | 18 months | 16 | 25% | [137] |
| 18 months + Ribavirin (1–1.2 g qd for 12 months) | 22 | 18% | [137] | |
| 12 months | 14 | 43% | [123] | |
| 12 months | 12 | 17% | [138] | |
| 12 months | 48 | 25% | [139] | |
| Pegylated IFN-alpha 2a: 180 µg/kg qw | 12 months | 29 | 26% | [133] |
| 12 months + adefovir (10 mg qd for 12 months) | 31 | 31% | [133] | |
| Pegylated IFN-alpha 2b: 1.5 µg/kg qw or Pegylated IFN-alpha 2a: 180 µg/kg qw |
12 months | 104 | 23% | [140] |
Abbreviations: qw, weekly; qd, daily.
A summary of the main studies evaluating the efficiency of IFN-based regimens in HDV infection is provided in Table 1.
Nucleoside/nucleotide analogues
Nucleoside/nucleotide analogues (NUCs) act on the HBV reverse transcriptase, and efficiently inhibit HBV replication, with little effect on HBsAg expression. Although in theory inhibiting the helper virus is expected to affect HDV life cycle, in reality, NUCs are not effective against HDV. Molecules tested in HDV infection include famciclovir [141], ribavirin (in combination with pegylated IFN-alpha [142]), lamivudine [143] and entecavir [144], but none demonstrated effectiveness.
A clinical trial tested adefovir as monotherapy or in combination with pegylated IFN-alpha, but none of these treatments showed a better efficacy than pegylated IFN-alpha alone, and adefovir treatment alone had no effect on HDV viremia [133]. The same result was later observed with tenofovir [136]. However, a prospective South American study reported encouraging results with the combination of entecavir and pegylated IFN-alpha for 48 weeks in patients infected with HDV genotype 3, as 21 of the 22 patients included had an undetectable HDV RNA level at the end of treatment and at the six months follow-up, and 20 of 22 had undetectable level of HBV DNA at the six months follow-up, suggesting that genotype 3 might react differently to these molecules, potentially being easier to treat [145]. Finally, a study conducted with HIV co-infected patients treated with tenofovir for 58 weeks showed a good response with no detectable levels of HBV DNA in all patients and with no detectable levels of HDV RNA in 53% of them. Furthermore, an improvement in liver fibrosis severity was observed in 60% of patients who achieved undetectable HDV RNA levels [146]. Although this improvement may be a mere consequence of the immune reconstitution resulting from antiretroviral treatment, a benefit of prolonged therapy with NUCs in CHD cannot be excluded.
Drugs in clinical development
As HDV depends on the host cell RNA polymerases for its replication, and even though alternative viral targets as the ribozyme could eventually be inhibited [147], the development of antiviral molecules that directly and specifically target this step has not been successful. The alternative strategies currently being developed are based either on the indirect stimulation of the innate immune system (as is the case of IFN-lambda) or of cell targets involved in other steps of the viral life cycle as entry (Myrcludex B) and viral assembly and release (lonafarnib and REP 2139). A summary of the most relevant results obtained in recent clinical trial results is presented in Table 2.
Table 2.
Summary of the studies evaluating molecules in clinical development.
| Treatment | Treatment duration | Number of patients | Virological outcome | Development stage and References |
|---|---|---|---|---|
| Pegylated IFN-lambda 120 or 180 µg qw sc |
48 weeks | 33 | At week 24 of treatment: 4/10 patients are HDV PCR-negative |
Phase 2 [148] |
| Myrcludex B 2 mg/Kg qd sc, 24 weeks followed by pegylated IFN-alpha monotherapy, 48 weeks | 72 weeks | 24 | Decline in HDV RNA at week 24 of treatment: 1.67 log10 decrease in HDV RNA |
Phase 2 [149] |
| Myrcludex B 2 mg/Kg qd sc + pegylated IFN 24 weeks followed by Pegylated IFN-alpha monotherapy, 24 weeks | 48 weeks | Decline in HDV RNA at week 24 of treatment: 2.59 log10 decrease in HDV RNA |
||
| Pegylated IFN-alpha monotherapy | 48 weeks | Decline in HDV RNA at week 24 of treatment: 2.17 log10 |
||
| Myrcludex B 2, 5 or 10 mg qd sc | 24 weeks | 120 | Decline in HDV RNA at week 24 of treatment: 2 mg: 1.75 log10 5 mg: 1.6 log10 10 mg: 2.7 log10 |
Phase 2b [150] |
| Tenofovir 245 mg qd po | 24 weeks | Decline in HDV RNA at week 24 of treatment: 0.18 log10 |
||
| Myrcludex B 2 or 5 mg qd sc + pegylated IFN-alpha sc | 48 weeks | 30 | Decline in HDV RNA at week 48 of treatment: 2 mg: 3.62 log10 5 mg: 4.48 log10 |
Phase 2 [151] |
| Myrcludex B 2 mg qd sc | 48 weeks | 15 | Decline in HDV RNA at week 48 of treatment: 2.84 log10 |
|
| Pegylated IFN-alpha sc | 48 weeks | 15 | Decline in HDV RNA at week 48 of treatment: 1.14 log10 |
|
| Lonafarnib 100 or 200 mg bid iv | 4 weeks | 14 | Decline in HDV RNA at day 28 of treatment: 100 mg: 0.73 log10 200 mg: 1.54 log10 |
Phase 2A [152] |
| Lonafarnib 200 mg bid po | 12 weeks | 3 | Variation in HDV RNA at week 12 of treatment: 0.03 log10 |
Phase 2 [153] |
| Lonafarnib 300 mg bid po | 12 weeks | 3 | Decrease in HDV RNA at week 12 of treatment: 1.78 log10 |
|
| Lonafarnib 100 mg tid po | 5 weeks | 3 | Decrease in HDV RNA at week 4 of treatment: 1.31 log10 |
|
| Lonafarnib 100 mg bid po + pegylated IFN-alpha) qw sc | 8 weeks | 3 | Decrease in HDV RNA at week 8 of treatment: 2.19 log10 |
|
| LNF 100 mg po bid + ritonavir 100 mg qd po | 8 weeks | 3 | Decrease in HDV RNA at week 8 of treatment: 2.97 log10 |
|
| Lonafarnib 50 mg bid po (increased at 4 week intervals to 75 mg and then 100 mg) + ritonavir 100 mg bid po | 24 weeks | 15 | Dose escalation possible in 10 patients At the end of treatment, mean HDV RNA decline was 1.58 ± 1.38 log10 IU/mL |
Phase 2 [154] |
| Lonafarnib 50, 75 or 100 mg qd + ritonavir 100 mg qd po | 12 or 24 weeks | 21 | Decrease in HDV RNA at week 12 of treatment: 50 mg: 1.6 log10 75 mg: 1.3 log10 100 mg: 0.83 log10 Decrease in HDV RNA up to 3.7 log10 at week 24 of treatment |
Phase 2 [155] |
| Lonafarnib 50 mg bid po + ritonavir 100 mg bid po or Lonafarnib 25 mg bid po + Ritonavir 100 mg bid po or Lonafarnib 50 mg bid po + ritonavir 100 mg bid po + pegylated IFN-alpha qw sc or Lonafarnib 25 mg bid + Ritonavir 100 mg bid + pegylated IFN-alpha qw sc or Lonafarnib 50 mg bid po + ritonavir 100 mg bid po + addition of pegylated IFN-alpha qw for weeks 12–24 |
24 weeks | 33 |
Decrease in HDV RNA at week 24 of treatment: 21 of 33 patients had a > 2 log10 decrease in HDV RNA |
Phase 2 [156] |
| REP 2139-Ca 500 mg qw iv 15 weeks followed by REP 2139-Ca qw + pegylated IFN-alpha 15 weeks followed by pegylated IFN-alpha 33 weeks | 63 weeks | 12 | - At week 30 of treatment: >5log decline in HDV RNA in 11 patients Undetectable HDV RNA in 10 patients - At the end of treatment: HBs seroconversion in 5 patients; Undetectable HDV RNA in 9 patients; −18 months after treatment: 4 patients HBsAg negative 7 patients maintain undetectable HDV RNA |
Phase 2 [157], [158] |
Abbreviations: bid, twice a day; iv, intravenous; po, per os; qw, weekly; qd, daily; sc, subcutaneous, tid, three times per day.
Pegylated IFN-lambda
IFN-lambda is a type III IFN with structural features, receptor characteristics and biological activities that are distinct from IFN-alpha, while sharing common ISG induction pathways associated with its antiviral activity. It has been shown to have an antiviral effect against HDV comparable with IFN-alpha in humanised mice [107]. In patients with chronic hepatitis B, the administration of IFN-lambda in a pegylated formulation led to virological outcomes equivalent to those of pegylated IFN-alpha, but with a better tolerability, which makes it a potentially attractive option for the treatment of CHD [159]. It is currently being evaluated in phase II clinical trials both in monotherapy (NCT02765802) and in combination with lonafarnib and ritonavir (NCT03600714).
Myrcludex B
Myrcludex B, a myristoylated lipopeptide, inhibits the entry of HBV and HDV in hepatocytes. Its sequence corresponds to the N-terminal amino acids (2–48) of L-HBsAg and inhibits viral entry by binding to its natural receptor, NTCP, at the basolateral membrane of hepatocytes (Fig. 2). Data from preclinical studies indicate that the antiviral effect can occur without interference with the bile acid transport function of NTCP. Indeed, while bile acid transport can be affected by high doses of Myrcludex B (IC50 47 nmol/L), effective viral entry inhibition can be achieved at much lower doses (IC50 80 pmol/L) [40].
In a phase Ib/IIa trial, 24 patients with CHD received standard pegylated IFN-alpha monotherapy or 24 weeks of Myrcludex B as monotherapy or in combination with pegylated IFN-alpha. Although no changes in HBsAg levels (the primary endpoint) were observed, the serum HDV RNA levels were significantly reduced (1.67 log10 in the Myrcludex B group, 2.6 log10 in the Myrcludex B plus pegylated IFN-alpha and 2.2 log10 in the pegylated IFN-alpha monotherapy arm) [149]. While some patients achieved undetectable HDV RNA levels at the end of treatment, viral rebound was universal after treatment cessation.
A more recent phase II study on a larger group of 120 subjects aimed to determine the optimal dose and potential serious adverse effects of Myrcludex B. Randomised into four arms, patients first received tenofovir for 12 weeks, followed by tenofovir alone or combined with different doss of Myrcludex B for 24 weeks, and finally tenofovir alone again for 24 weeks [150]. The primary endpoint was a 2 log10 reduction in HDV RNA from baseline and was achieved by 77% of the patients in the arm receiving the highest dose of Myrcludex B (10 mg). During treatment with Myrcludex B, the serum HDV RNA levels decreased in a dose-dependent manner (1.75 log, 1.6 log and 2.7 log decrease in the 2, 5 and 10 mg arms, respectively) and ALT improved in 50% of the patients. However, at the end of 12 weeks of follow-up, only ∼10% of patients in each of the three arms treated with Myrcludex B had maintained a virological response (i.e. 2 log10 reduction in HDV RNA) and no response was reported in the arm receiving tenofovir alone, supporting the need for longer-term treatment with Myrcludex B. The treatment seems to be well tolerated with no serious adverse events, despite a slight, asymptomatic increase of bile acids [150]. Interestingly, an encouraging linear decline in intrahepatic HDV RNA levels was demonstrated overtime, suggesting that Myrcludex B monotherapy is associated with a decrease in the number of HDV-infected hepatocytes [160].
End of treatment results of a subsequent multicentre trial evaluating a 48-week course of Myrcludex B (2 or 5 mg) in combination with pegylated IFN-alpha, compared with each therapy alone were presented at AASLD 2018. Fifty percent of the patients in the combination arms had undetectable HDV RNA at the end of treatment (compared to 13% in the monotherapy groups), with median declines compared to baseline of 4.48 log in the high-dose combination group (compared to 1.14 log in the pegylated IFN-alpha monotherapy arm) [131], [151]. Daily subcutaneous injections are currently needed, although an oral formulation is under development. A phase III clinical trial is expected to start early 2019.
Lonafarnib
As mentioned before, farnesylation of L-HDAg is an important post-translational modification, as it enables the interaction of HDV RNP with the HBV envelope. Lonafarnib is a farnesyl-transferase inhibitor preventing the farnesylation of L-HDAg and consequently its interaction with HBsAg (Fig. 2) and has been shown to abrogate the secretion of HDV viral particles both in vitro and in vivo [80], [81]. A 2015 phase IIa clinical study showed that HDV RNA levels were significantly reduced in patients treated with lonafarnib for 28 days, in comparison to placebo (0.73 log10 IU/ml and 1.54 log10 IU/ml in the 100 mg and 200 mg group, respectively) and these reductions were proportional to the circulating drug levels [152]. However, lonafarnib has significant adverse effects, such as nausea, diarrhoea, abdominal bloating and weight loss.
A more recent study combined a low dosage of lonafarnib with ritonavir, a cytochrome P450 3A4 inhibitor [153]. Ritonavir allows the administration of smaller doses of lonafarnib to achieve sufficient serum levels, leading to a better tolerability than the equivalent dose without ritonavir. Four weeks of treatment with lonafarnib 100 mg thrice daily led to a 1.2 log decrease in HDV RNA, whereas a 2.4 log decline was observed with a treatment of 100 mg of lonafarnib twice daily combined with ritonavir. Moreover, lonafarnib added to pegylated IFN-alpha showed a decrease of 1.8 log of HDV RNA after four weeks. However, after 8 weeks of treatment with either lonafarnib + ritonavir or lonafarnib + pegylated IFN-alpha, almost all patients returned to the pre-treatment HDV RNA levels within 4–24 weeks post-treatment [153]. A new phase III study has recently been announced.
REP 2139
Nucleic acid polymers (NAPs) are amphipathic molecules with a broad antiviral spectrum. Although their precise mechanisms of action are still debated, their anti-HBV action seems to result from an inhibition HBsAg release from hepatocytes [161]. As for HDV, an additional interaction with HDAg has been described and may account for the observed antiviral effect (current evidence on the mechanisms of action of NAPs has been recently reviewed in [162]). Furthermore, the drastic reduction of circulating HBAg levels shown in patients is thought to promote a normalisation of the humoral immune response [157].
A recent uncontrolled trial included 12 patients that were treated with REP 2139-Ca in combination with pegylated IFN-alpha (patients received REP 2139-Ca only for 15 weeks, followed by a combination of REP 2139-Ca and pegylated IFN-alpha for 15 weeks and finally pegylated IFN-alpha only for 33 weeks) [157]. At the end of combination therapy (week 30), 10/12 patients had undetectable HDV RNA and 9/12 patients had HBsAg declines > 2log10 from baseline, 6 of whom had HBsAg seroconversion. At the end of treatment 9 patients remained HDV RNA negative with 6 still having HBsAg seroconversion. Eighteen months after removal of treatment, HDV RNA was still negative in 7 patients and HBs seroconversion was still present in 4 patients [158].
Elevations of aminotransferases were documented in nearly 50% of patients [157]. However, aminotransferases normalized in these patients during follow-up and no other alterations in liver function were documented (with the exception of one patient with bilirubin elevation). Although the results are overall promising, larger phase III trials are required before establishing the efficacy and safety of this treatment.
A long-term follow-up study in CHB is currently under way (with encouraging results presented in 2018 [158]) and a clinical trial is planned to evaluate REP 2139-Mg (once-a-week subcutaneous administration) in combination with tenofovir and pegylated IFN-alpha.
Vaccination/ prevention
HBV vaccination protects effectively against both HBV and HDV infection. Vaccination campaigns have indeed reduced the reservoir of HBV patients that can be potentially infected by HDV. A study published in 2007 [163] showed a clear correlation between the introduction of vaccination for HBV and the decrease in HDV incidence particularly among those 15–24 years old, probably also because of reduced iatrogenic transmission. Countries with high HDV endemicity, such as Brazil and Mongolia, have adopted universal HBV vaccination programmes, with an expected impact on the absolute number of new infections. No perspectives for a vaccination strategy to prevent HDV infection in HBV-infected patients currently exist, as results in animal models have been discouraging [164].
Conclusions and future perspectives
Hepatitis D is considered the most severe form of chronic viral hepatitis. It currently has no satisfactory treatment and a better understanding of its pathogenesis is warranted. HDV infection is highly endemic in resource-limited countries, where clinical trials are difficult to conduct and, while it is considered infrequent in developed countries, its real prevalence may be underestimated. Thanks to significant advances in the characterisation of the viral life cycle, several host-targeting molecules are currently in clinical evaluation with promising results.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
DA was funded by the Nuovo Soldati Foundation.
Biographies

Nathalie Mentha is a medical student at the Faculty of Medicine of the University of Geneva. She completed her master thesis focusing on the hepatitis delta virus and particularly on the new treatment development with the Department of Pathology and Immunology of the University of Geneva in 2018. She will graduate as a physician in September 2019.

Sophie Clément is a senior scientist at the Viropathology Unit, headed by Professor Negro (University Hospitals of Geneva, Switzerland) since 2005. She had obtained her PhD in Human Sciences from Lyon I University in 1995. After a post-doctoral training at Northwestern University (Chicago), she joined the laboratory directed by Professor Gabbiani at the Faculty of Medicine (Geneva), mainly focusing her interest on fibrosis. She is now involved in projects focusing on the metabolic disorders associated with viral hepatitis, and more specifically on insulin resistance and steatosis. She has published ∼30 peer-reviewed journal articles in the hepatology field.

Francesco Negro is a Professor at the Departments of Medicine and of Pathology and Immunology of the University Hospital of Geneva, Switzerland. His research focuses on metabolic alterations induced by HCV and has participated into several collaborative works on treatment, epidemiology and public health issues related to viral hepatitis. He has (co)authored ∼300 peer-reviewed manuscripts in the field of hepatology (h-index 61). He is member of the Governing Board of the European Association for the Study of the Liver (EASL) and of the HCV clinical practice guidelines panels of EASL and WHO, and Chairman of the Swiss Hepatitis C Cohort Study.

Dulce Alfaiate is a post-doctoral researcher in the Department of Pathology and Immunology of the Faculty of Medicine of the University of Geneva, in the laboratory of Professor Francesco Negro. Dr. Alfaiate earned her Medical Degree at the University of Lisbon in 2003 and her specialist title in Infectious Diseases in 2011. She was awarded her PhD by the University of Lyon in 2015, for her work on the interactions between hepatitis B and hepatitis delta viruses. Her current research interests focus on the pathogenesis of liver disease in patients chronically infected by hepatitis viruses.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Rizzetto M., Canese M.G., Aricò S., Crivelli O., Trepo C., Bonino F. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sureau C., Negro F. The hepatitis delta virus: replication and pathogenesis. J Hepatol. 2016;64:S102–S116. doi: 10.1016/j.jhep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet Lond Engl. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 5.Wedemeyer H., Negro F. Devil hepatitis D: an orphan disease or largely underdiagnosed? Gut. 2018 doi: 10.1136/gutjnl-2018-317403. [DOI] [PubMed] [Google Scholar]
- 6.Chen H.-Y., Shen D.-T., Ji D.-Z., Han P.-C., Zhang W.-M., Ma J.-F. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2018 doi: 10.1136/gutjnl-2018-316601. [DOI] [PubMed] [Google Scholar]
- 7.Stockdale A.J., Kreuels B., Henrion M.R.Y., Giorgi E., Kyomuhangi I., Geretti A.M. Hepatitis D prevalence: problems with extrapolation to global population estimates. Gut. 2018 doi: 10.1136/gutjnl-2018-317874. [DOI] [PubMed] [Google Scholar]
- 8.Chen X., Oidovsambuu O., Liu P., Grosely R., Elazar M., Winn V.D. A novel quantitative microarray antibody capture (Q-MAC) assay identifies an extremely high HDV prevalence amongst HBV infected Mongolians. Hepatol Baltim Md. 2016 doi: 10.1002/hep.28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarponi C.F. de O., da Silva R.D.N., de Souza Filho J.A., Guerra M.R.L., Pedrosa M.A.F., Mol M.P.G. Hepatitis delta prevalence in South America: a systematic review and meta-analysis. Rev Soc Bras Med Trop. 2019;52:e20180289. doi: 10.1590/0037-8682-0289-2018. [DOI] [PubMed] [Google Scholar]
- 10.Groc S., Abbate J.L., Le Gal F., Gerber A., Tuaillon E., Albert J.-L. High prevalence and diversity of hepatitis B and hepatitis delta virus in Gabon. J Viral Hepat. 2019;26:170–182. doi: 10.1111/jvh.12991. [DOI] [PubMed] [Google Scholar]
- 11.Butler E.K., Rodgers M.A., Coller K.E., Barnaby D., Krilich E., Olivo A. High prevalence of hepatitis delta virus in Cameroon. Sci Rep. 2018;8:11617. doi: 10.1038/s41598-018-30078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amini N., Alavian S.M., Kabir A., Aalaei-Andabili S.H., Saiedi Hosseini S.Y., Rizzetto M. Prevalence of hepatitis d in the eastern mediterranean region: systematic review and meta analysis. Hepat Mon. 2013;13 doi: 10.5812/hepatmon.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gheorghe L., Csiki I.E., Iacob S., Gheorghe C., Trifan A., Grigorescu M. Hepatitis delta virus infection in Romania: prevalence and risk factors. J Gastrointest Liver Dis JGLD. 2015;24:413–421. doi: 10.15403/jgld.2014.1121.244.dtv. [DOI] [PubMed] [Google Scholar]
- 14.Sagnelli E., Sagnelli C., Pisaturo M., Macera M., Coppola N. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol WJG. 2014;20:7635–7643. doi: 10.3748/wjg.v20.i24.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroffolini T., Sagnelli E., Sagnelli C., Russello M., De Luca M., Rosina F. Hepatitis delta infection in Italian patients: towards the end of the story? Infection. 2017;45:277–281. doi: 10.1007/s15010-016-0956-1. [DOI] [PubMed] [Google Scholar]
- 16.Aguilera A., Trastoy R., Rodríguez-Calviño J., Manso T., de Mendoza C., Soriano V. Prevalence and incidence of hepatitis delta in patients with chronic hepatitis B in Spain. Eur J Gastroenterol Hepatol. 2018;30:1060–1062. doi: 10.1097/MEG.0000000000001163. [DOI] [PubMed] [Google Scholar]
- 17.Servant-Delmas A., Le Gal F., Gallian P., Gordien E., Laperche S. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2014;59:126–128. doi: 10.1016/j.jcv.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Wranke A., Pinheiro Borzacov L.M., Parana R., Lobato C., Hamid S., Ceausu E. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the Hepatitis Delta International Network (HDIN) Liver Int Off J Int Assoc Study Liver. 2017 doi: 10.1111/liv.13604. [DOI] [PubMed] [Google Scholar]
- 19.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.-M., Hwang J.P., Jonas M.M. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatol Baltim Md. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushner T., Serper M., Kaplan D.E. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63:586–592. doi: 10.1016/j.jhep.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel E.U., Thio C.L., Boon D., Thomas D.L., Tobian A.A.R. Prevalence of Hepatitis B and Hepatitis D virus infections in the United States, 2011–2016. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019 doi: 10.1093/cid/ciz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins E.B., Glenn J. Prevalence of hepatitis delta virus (HDV) infection in the United States: results from an ICD-10 review. Gastroenterology. 2017;152:S1085. [Google Scholar]
- 23.Niro G.A., Casey J.L., Gravinese E., Garrubba M., Conoscitore P., Sagnelli E. Intrafamilial transmission of hepatitis delta virus: molecular evidence. J Hepatol. 1999;30:564–569. doi: 10.1016/s0168-8278(99)80185-8. [DOI] [PubMed] [Google Scholar]
- 24.Noureddin M., Gish R. Hepatitis delta: epidemiology, diagnosis and management 36 years after discovery. Curr Gastroenterol Rep. 2014;16:365. doi: 10.1007/s11894-013-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascarella S., Negro F. Hepatitis D virus: an update. Liver Int Off J Int Assoc Study Liver. 2011;31:7–21. doi: 10.1111/j.1478-3231.2010.02320.x. [DOI] [PubMed] [Google Scholar]
- 26.Liaw Y.F., Chiu K.W., Chu C.M., Sheen I.S., Huang M.J. Heterosexual transmission of hepatitis delta virus in the general population of an area endemic for hepatitis B virus infection: a prospective study. J Infect Dis. 1990;162:1170–1172. doi: 10.1093/infdis/162.5.1170. [DOI] [PubMed] [Google Scholar]
- 27.Bonvicino C.R., Moreira M.A., Soares M.A. Hepatitis B virus lineages in mammalian hosts: potential for bidirectional cross-species transmission. World J Gastroenterol. 2014;20:7665–7674. doi: 10.3748/wjg.v20.i24.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wille M., Netter H.J., Littlejohn M., Yuen L., Shi M., Eden J.-S. A Divergent hepatitis D-Like agent in birds. Viruses. 2018;10 doi: 10.3390/v10120720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetzel U., Szirovicza L., Smura T., Prahauser B., Vapalahti O., Kipar A. Identification of a novel deltavirus in boa constrictor. BioRxiv. 2018 doi: 10.1128/mBio.00014-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flores R., Owens R.A., Taylor J. Pathogenesis by subviral agents: viroids and hepatitis delta virus. Curr Opin Virol. 2016;17:87–94. doi: 10.1016/j.coviro.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Taylor J.M. Host RNA circles and the origin of hepatitis delta virus. World J Gastroenterol WJG. 2014;20:2971–2978. doi: 10.3748/wjg.v20.i11.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnius L., Taylor J., Mason W.S., Sureau C., Dény P., Norder H. ICTV virus taxonomy profile: deltavirus. J Gen Virol. 2018;99:1565–1566. doi: 10.1099/jgv.0.001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dény P. Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol. 2006;307:151–171. doi: 10.1007/3-540-29802-9_8. [DOI] [PubMed] [Google Scholar]
- 34.Alfaiate D., Dény P., Durantel D. Hepatitis delta virus: From biological and medical aspects to current and investigational therapeutic options. Antiviral Res. 2015;122:112–129. doi: 10.1016/j.antiviral.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Su C.-W., Huang Y.-H., Huo T.-I., Shih H.H., Sheen I.-J., Chen S.-W. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. 2006;130:1625–1635. doi: 10.1053/j.gastro.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Gomes-Gouvêa M.S., Soares M.C.P., Bensabath G., de Carvalho-Mello I.M.V.G., Brito E.M.F., Souza O.S.C. Hepatitis B virus and hepatitis delta virus genotypes in outbreaks of fulminant hepatitis (Labrea black fever) in the western Brazilian Amazon region. J Gen Virol. 2009;90:2638–2643. doi: 10.1099/vir.0.013615-0. [DOI] [PubMed] [Google Scholar]
- 37.Casey J.L., Niro G.A., Engle R.E., Vega A., Gomez H., McCarthy M. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J Infect Dis. 1996;174:920–926. doi: 10.1093/infdis/174.5.920. [DOI] [PubMed] [Google Scholar]
- 38.Freitas N., Abe K., Cunha C., Menne S., Gudima S.O. Support of the infectivity of hepatitis delta virus particles by the envelope proteins of different genotypes of hepatitis B virus. J Virol. 2014;88:6255–6267. doi: 10.1128/JVI.00346-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzetto M., Hoyer B., Canese M.G., Shih J.W., Purcell R.H., Gerin J.L. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci USA. 1980;77:6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban S., Bartenschlager R., Kubitz R., Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Tavanez J.P., Cunha C., Silva M.C.A., David E., Monjardino J., Carmo-Fonseca M. Hepatitis delta virus ribonucleoproteins shuttle between the nucleus and the cytoplasm. RNA N Y N. 2002;8:637–646. doi: 10.1017/s1355838202026432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornillez-Ty C.T., Lazinski D.W. Determination of the multimerization state of the hepatitis delta virus antigens in vivo. J Virol. 2003;77:10314–10326. doi: 10.1128/JVI.77.19.10314-10326.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin B.L., Chasovskikh S., Dritschilo A., Casey J.L. Hepatitis delta antigen requires a flexible quasi-double-stranded RNA structure to bind and condense hepatitis delta virus RNA in a ribonucleoprotein complex. J Virol. 2014;88:7402–7411. doi: 10.1128/JVI.00443-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gudima S., Chang J., Moraleda G., Azvolinsky A., Taylor J. Parameters of human hepatitis delta virus genome replication: the quantity, quality, and intracellular distribution of viral proteins and RNA. J Virol. 2002;76:3709–3719. doi: 10.1128/JVI.76.8.3709-3719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuccola H.J., Rozzelle J.E., Lemon S.M., Erickson B.W., Hogle J.M. Structural basis of the oligomerization of hepatitis delta antigen. Struct Lond Engl. 1993;1998(6):821–830. doi: 10.1016/s0969-2126(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 46.Wang K.S., Choo Q.L., Weiner A.J., Ou J.H., Najarian R.C., Thayer R.M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986;323:508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- 47.Chen P.J., Kalpana G., Goldberg J., Mason W., Werner B., Gerin J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci USA. 1986;83:8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macnaughton T.B., Lai M.M.C. Genomic but not antigenomic hepatitis delta virus RNA is preferentially exported from the nucleus immediately after synthesis and processing. J Virol. 2002;76:3928–3935. doi: 10.1128/JVI.76.8.3928-3935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gudima S., Wu S.Y., Chiang C.M., Moraleda G., Taylor J. Origin of hepatitis delta virus mRNA. J Virol. 2000;74:7204–7210. doi: 10.1128/jvi.74.16.7204-7210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo M.Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988;62:4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coffin C.S., Mulrooney-Cousins P.M., Peters M.G., van Marle G., Roberts J.P., Michalak T.I. Molecular characterization of intrahepatic and extrahepatic hepatitis B virus (HBV) reservoirs in patients on suppressive antiviral therapy. J Viral Hepat. 2011;18:415–423. doi: 10.1111/j.1365-2893.2010.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi S.S., Coffin C.S. Hepatitis B virus lymphotropism: emerging details and challenges. Biotechnol Genet Eng Rev. 2018;34:139–151. doi: 10.1080/02648725.2018.1474324. [DOI] [PubMed] [Google Scholar]
- 53.Negro F., Korba B.E., Forzani B., Baroudy B.M., Brown T.L., Gerin J.L. Hepatitis delta virus (HDV) and woodchuck hepatitis virus (WHV) nucleic acids in tissues of HDV-infected chronic WHV carrier woodchucks. J Virol. 1989;63:1612–1618. doi: 10.1128/jvi.63.4.1612-1618.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor J.M. Replication of the hepatitis delta virus RNA genome. Adv Virus Res. 2009;74:103–121. doi: 10.1016/S0065-3527(09)74003-5. [DOI] [PubMed] [Google Scholar]
- 55.Sureau C., Guerra B., Lee H. The middle hepatitis B virus envelope protein is not necessary for infectivity of hepatitis delta virus. J Virol. 1994;68:4063–4066. doi: 10.1128/jvi.68.6.4063-4066.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abou-Jaoudé G., Sureau C. Entry of hepatitis delta virus requires the conserved cysteine residues of the hepatitis B virus envelope protein antigenic loop and is blocked by inhibitors of thiol-disulfide exchange. J Virol. 2007;81:13057–13066. doi: 10.1128/JVI.01495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamas Longarela O., Schmidt T.T., Schöneweis K., Romeo R., Wedemeyer H., Urban S. Proteoglycans act as cellular hepatitis delta virus attachment receptors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leistner C.M., Gruen-Bernhard S., Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol. 2008;10:122–133. doi: 10.1111/j.1462-5822.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 59.Schulze A., Gripon P., Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatol Baltim Md. 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- 60.Verrier E.R., Colpitts C.C., Bach C., Heydmann L., Weiss A., Renaud M. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatol Baltim Md. 2016;63:35–48. doi: 10.1002/hep.28013. [DOI] [PubMed] [Google Scholar]
- 61.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. ELife. 2012;3:00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Modahl L.E., Macnaughton T.B., Zhu N., Johnson D.L., Lai M.M. RNA-Dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol Cell Biol. 2000;20:6030–6039. doi: 10.1128/mcb.20.16.6030-6039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greco-Stewart V.S., Schissel E., Pelchat M. The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III. Virology. 2009;386:12–15. doi: 10.1016/j.virol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Rackwitz H.R., Rohde W., Sänger H.L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981;291:297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- 66.Greco-Stewart V.S., Miron P., Abrahem A., Pelchat M. The human RNA polymerase II interacts with the terminal stem-loop regions of the hepatitis delta virus RNA genome. Virology. 2007;357:68–78. doi: 10.1016/j.virol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Abeywickrama-Samarakoon N., Cortay J.-C., Sureau C., Alfaiate D., Levrero M., Dény P. [Hepatitis delta virus replication and the role of the small hepatitis delta protein S-HDAg] Med Sci MS. 2018;34:833–841. doi: 10.1051/medsci/2018209. [DOI] [PubMed] [Google Scholar]
- 68.Chang F.L., Chen P.J., Tu S.J., Wang C.J., Chen D.S. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci USA. 1991;88:8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modahl L.E., Lai M.M. The large delta antigen of hepatitis delta virus potently inhibits genomic but not antigenomic RNA synthesis: a mechanism enabling initiation of viral replication. J Virol. 2000;74:7375–7380. doi: 10.1128/jvi.74.16.7375-7380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuo M.Y., Chao M., Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamaguchi Y., Filipovska J., Yano K., Furuya A., Inukai N., Narita T. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science. 2001;293:124–127. doi: 10.1126/science.1057925. [DOI] [PubMed] [Google Scholar]
- 72.Cao D., Haussecker D., Huang Y., Kay M.A. Combined proteomic-RNAi screen for host factors involved in human hepatitis delta virus replication. RNA N Y N. 2009;15:1971–1979. doi: 10.1261/rna.1782209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reid C.E., Lazinski D.W. A host-specific function is required for ligation of a wide variety of ribozyme-processed RNAs. Proc Natl Acad Sci USA. 2000;97:424–429. doi: 10.1073/pnas.97.1.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glenn J.S., Watson J.A., Havel C.M., White J.M. Identification of a prenylation site in delta virus large antigen. Science. 1992;256:1331–1333. doi: 10.1126/science.1598578. [DOI] [PubMed] [Google Scholar]
- 75.Casey J.L. Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol. 2012;353:123–143. doi: 10.1007/82_2011_146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong S.K., Lazinski D.W. Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc Natl Acad Sci USA. 2002;99:15118–15123. doi: 10.1073/pnas.232416799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartwig D., Schütte C., Warnecke J., Dorn I., Hennig H., Kirchner H. The large form of ADAR 1 is responsible for enhanced hepatitis delta virus RNA editing in interferon-alpha-stimulated host cells. J Viral Hepat. 2006;13:150–157. doi: 10.1111/j.1365-2893.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 78.Abbas Z., Afzal R. Life cycle and pathogenesis of hepatitis D virus: a review. World J Hepatol. 2013;5:666–675. doi: 10.4254/wjh.v5.i12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong S.-Y., Chen P.-J. Phosphorylation of serine 177 of the small hepatitis delta antigen regulates viral antigenomic RNA replication by interacting with the processive RNA polymerase II. J Virol. 2010;84:1430–1438. doi: 10.1128/JVI.02083-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bordier B.B., Marion P.L., Ohashi K., Kay M.A., Greenberg H.B., Casey J.L. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol. 2002;76:10465–10472. doi: 10.1128/JVI.76.20.10465-10472.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bordier B.B., Ohkanda J., Liu P., Lee S.-Y., Salazar F.H., Marion P.L. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J Clin Invest. 2003;112:407–414. doi: 10.1172/JCI17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang S.B., Lai M.M. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993;67:7659–7662. doi: 10.1128/jvi.67.12.7659-7662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sureau C., Guerra B., Lanford R.E. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993;67:366–372. doi: 10.1128/jvi.67.1.366-372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe T., Sorensen E.M., Naito A., Schott M., Kim S., Ahlquist P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci USA. 2007;104:10205–10210. doi: 10.1073/pnas.0704000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bonino F., Heermann K.H., Rizzetto M., Gerlich W.H. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986;58:945–950. doi: 10.1128/jvi.58.3.945-950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alfaiate D., Lucifora J., Abeywickrama-Samarakoon N., Michelet M., Testoni B., Cortay J.-C. HDV RNA replication is associated with HBV repression and interferon-stimulated genes induction in super-infected hepatocytes. Antiviral Res. 2016;136:19–31. doi: 10.1016/j.antiviral.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Lütgehetmann M., Mancke L.V., Volz T., Helbig M., Allweiss L., Bornscheuer T. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatol Baltim Md. 2012;55:685–694. doi: 10.1002/hep.24758. [DOI] [PubMed] [Google Scholar]
- 88.Wu J.C., Chen P.J., Kuo M.Y., Lee S.D., Chen D.S., Ting L.P. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J Virol. 1991;65:1099–1104. doi: 10.1128/jvi.65.3.1099-1104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pollicino T., Raffa G., Santantonio T., Gaeta G.B., Iannello G., Alibrandi A. Replicative and transcriptional activities of hepatitis B virus in patients coinfected with hepatitis B and hepatitis delta viruses. J Virol. 2011;85:432–439. doi: 10.1128/JVI.01609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schaper M., Rodriguez-Frias F., Jardi R., Tabernero D., Homs M., Ruiz G. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol. 2010;52:658–664. doi: 10.1016/j.jhep.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 91.Raffa G, Niro GA, Raimondo G, Pollicino T. Hepatitis delta virus exerts an epigenetic control over hepatitis B virus transcription/replication activity. Hepatology, vol. 54, p. 1083A–1083A.
- 92.Williams V., Brichler S., Radjef N., Lebon P., Goffard A., Hober D. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol. 2009;90:2759–2767. doi: 10.1099/vir.0.011239-0. [DOI] [PubMed] [Google Scholar]
- 93.Alves C., Cheng H., Tavanez J.P., Casaca A., Gudima S., Roder H. Structural and nucleic acid binding properties of hepatitis delta virus small antigen. World J Virol. 2017;6:26–35. doi: 10.5501/wjv.v6.i2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen M., Du D., Zheng W., Liao M., Zhang L., Liang G. Small hepatitis delta antigen selectively binds to target mRNA in hepatic cells: a potential mechanism by which hepatitis D virus downregulates glutathione S-transferase P1 and induces liver injury and hepatocarcinogenesis. Biochem Cell Biol Biochim Biol Cell. 2018:1–10. doi: 10.1139/bcb-2017-0321. [DOI] [PubMed] [Google Scholar]
- 95.Wang C.-C., Chang T.-C., Lin C.-W., Tsui H.-L., Chu P.B.C., Chen B.-S. Nucleic acid binding properties of the nucleic acid chaperone domain of hepatitis delta antigen. Nucleic Acids Res. 2003;31:6481–6492. doi: 10.1093/nar/gkg857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freitas N., Lukash T., Gunewardena S., Chappell B., Slagle B.L., Gudima S.O. Relative abundance of integrant-derived viral RNAs in infected tissues harvested from chronic hepatitis B virus carriers. J Virol. 2018;92 doi: 10.1128/JVI.02221-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu B., Wang R., Fu J., Su M., Du M., Liu Y. Integration of hepatitis B virus S gene impacts on hepatitis B surface antigen levels in patients with antiviral therapy. J Gastroenterol Hepatol. 2018;33:1389–1396. doi: 10.1111/jgh.14075. [DOI] [PubMed] [Google Scholar]
- 98.Tu T., Budzinska M.A., Shackel N.A., Urban S. HBV DNA integration: molecular mechanisms and clinical implications. Viruses. 2017;9 doi: 10.3390/v9040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freitas N., Cunha C., Menne S., Gudima S.O. Envelope proteins derived from naturally integrated hepatitis B virus DNA support assembly and release of infectious hepatitis delta virus particles. J Virol. 2014;88:5742–5754. doi: 10.1128/JVI.00430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wieland S., Thimme R., Purcell R.H., Chisari F.V. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lebossé F., Testoni B., Fresquet J., Facchetti F., Galmozzi E., Fournier M. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol. 2017;66:897–909. doi: 10.1016/j.jhep.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 102.Luangsay S., Gruffaz M., Isorce N., Testoni B., Michelet M., Faure-Dupuy S. Early inhibition of hepatocyte innate responses by hepatitis B virus. J Hepatol. 2015;63:1314–1322. doi: 10.1016/j.jhep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 103.Suslov A., Boldanova T., Wang X., Wieland S., Heim M.H. Hepatitis B virus does not interfere with innate immune responses in the human liver. Gastroenterology. 2018;154:1778–1790. doi: 10.1053/j.gastro.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 104.Mutz P., Metz P., Lempp F.A., Bender S., Qu B., Schöneweis K. HBV bypasses the innate immune response and does not protect HCV from antiviral activity of interferon. Gastroenterology. 2018;154(1791–1804) doi: 10.1053/j.gastro.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 105.Giersch K., Allweiss L., Volz T., Helbig M., Bierwolf J., Lohse A.W. Hepatitis Delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Z., Filzmayer C., Ni Y., Sültmann H., Mutz P., Hiet M.-S. Hepatitis D virus replication is sensed by MDA5 and induces IFN-β/λ responses in hepatocytes. J Hepatol. 2018;69:25–35. doi: 10.1016/j.jhep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 107.Giersch K., Homs M., Volz T., Helbig M., Allweiss L., Lohse A.W. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDV infected humanized mice. Sci Rep. 2017;7:3757. doi: 10.1038/s41598-017-03946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hartwig D., Schoeneich L., Greeve J., Schütte C., Dorn I., Kirchner H. Interferon-alpha stimulation of liver cells enhances hepatitis delta virus RNA editing in early infection. J Hepatol. 2004;41:667–672. doi: 10.1016/j.jhep.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 109.Giersch K., Bhadra O.D., Volz T., Allweiss L., Riecken K., Fehse B. Hepatitis delta virus persists during liver regeneration and is amplified through cell division both in vitro and in vivo. Gut. 2017 doi: 10.1136/gutjnl-2017-314713. [DOI] [PubMed] [Google Scholar]
- 110.Pugnale P., Pazienza V., Guilloux K., Negro F. Hepatitis delta virus inhibits alpha interferon signaling. Hepatol Baltim Md. 2009;49:398–406. doi: 10.1002/hep.22654. [DOI] [PubMed] [Google Scholar]
- 111.Farci P., Niro G.A. Clinical features of hepatitis D. Semin Liver Dis. 2012;32:228–236. doi: 10.1055/s-0032-1323628. [DOI] [PubMed] [Google Scholar]
- 112.Yurdaydın C., Idilman R., Bozkaya H., Bozdayi A.M. Natural history and treatment of chronic delta hepatitis. J Viral Hepat. 2010;17:749–756. doi: 10.1111/j.1365-2893.2010.01353.x. [DOI] [PubMed] [Google Scholar]
- 113.Fattovich G., Giustina G., Christensen E., Pantalena M., Zagni I., Realdi G. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep) Gut. 2000;46:420–426. doi: 10.1136/gut.46.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fattovich G., Boscaro S., Noventa F., Pornaro E., Stenico D., Alberti A. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis. 1987;155:931–935. doi: 10.1093/infdis/155.5.931. [DOI] [PubMed] [Google Scholar]
- 115.Romeo R., Del Ninno E., Rumi M., Russo A., Sangiovanni A., de Franchis R. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136:1629–1638. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 116.Niro G.A., Smedile A., Ippolito A.M., Ciancio A., Fontana R., Olivero A. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol. 2010;53:834–840. doi: 10.1016/j.jhep.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 117.Ji J., Sundquist K., Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:790–792. doi: 10.1093/jnci/djs168. [DOI] [PubMed] [Google Scholar]
- 118.Béguelin C., Moradpour D., Sahli R., Suter-Riniker F., Lüthi A., Cavassini M. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol. 2017;66:297–303. doi: 10.1016/j.jhep.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 119.Romeo R., Foglieni B., Casazza G., Spreafico M., Colombo M., Prati D. High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 121.Mahale P., Aka P.V., Chen X., Liu P., Fram B.J., Wang A.S. Hepatitis D viremia among injection drug users in San Francisco. J Infect Dis. 2018;217:1902–1906. doi: 10.1093/infdis/jiy157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wranke A., Heidrich B., Ernst S., Calle Serrano B., Caruntu F.A., Curescu M.G. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Castelnau C., Le Gal F., Ripault M.-P., Gordien E., Martinot-Peignoux M., Boyer N. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatol Baltim Md. 2006;44:728–735. doi: 10.1002/hep.21325. [DOI] [PubMed] [Google Scholar]
- 124.Le Gal F., Brichler S., Sahli R., Chevret S., Gordien E. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatol Baltim Md. 2016;64:1483–1494. doi: 10.1002/hep.28772. [DOI] [PubMed] [Google Scholar]
- 125.Le Gal F., Dziri S., Gerber A., Alloui C., Ben Abdesselam Z., Roulot D. Performance characteristics of a new consensus commercial kit for Hepatitis D virus RNA viral load quantification. J Clin Microbiol. 2017;55:431–441. doi: 10.1128/JCM.02027-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niro G.A., Rosina F., Rizzetto M. Treatment of hepatitis D. J Viral Hepat. 2005;12:2–9. doi: 10.1111/j.1365-2893.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 127.Heidrich B., Yurdaydın C., Kabaçam G., Ratsch B.A., Zachou K., Bremer B. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatol Baltim Md. 2014;60:87–97. doi: 10.1002/hep.27102. [DOI] [PubMed] [Google Scholar]
- 128.Farci P., Roskams T., Chessa L., Peddis G., Mazzoleni A.P., Scioscia R. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740–1749. doi: 10.1053/j.gastro.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 129.Abbas Z., Khan M.A., Salih M. Jafri W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev. 2011:CD006002. doi: 10.1002/14651858.CD006002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abbas Z., Abbas M. Management of hepatitis delta: need for novel therapeutic options. World J Gastroenterol. 2015;21:9461–9465. doi: 10.3748/wjg.v21.i32.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yurdaydin C. New treatment option for delta virus: is a cure in sight? J Viral Hepat. 2019 doi: 10.1111/jvh.13081. [DOI] [PubMed] [Google Scholar]
- 132.Brichler S., Le Gal F., Butt A., Chevret S., Gordien E. Commercial real-time reverse transcriptase PCR assays can underestimate or fail to quantify hepatitis delta virus viremia. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013;11:734–740. doi: 10.1016/j.cgh.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 133.Wedemeyer H., Yurdaydìn C., Dalekos G.N., Erhardt A., Çakaloğlu Y., Değertekin H. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322–331. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 134.Di Bisceglie A.M., Martin P., Lisker-Melman M., Kassianides C., Korenman J., Bergasa N.V. Therapy of chronic delta hepatitis with interferon alfa-2b. J Hepatol. 1990;11(Suppl 1):S151–S154. doi: 10.1016/0168-8278(90)90185-t. [DOI] [PubMed] [Google Scholar]
- 135.Porres J.C., Carreño V., Bartolomé J., Moreno A., Galiana F., Quiroga J.A. Treatment of chronic delta infection with recombinant human interferon alpha 2c at high doses. J Hepatol. 1989;9:338–344. doi: 10.1016/0168-8278(89)90143-8. [DOI] [PubMed] [Google Scholar]
- 136.Wedemeyer H., Yurdaydin C., Hardtke S., Caruntu F.A., Curescu M.G., Yalcin K. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19:275–286. doi: 10.1016/S1473-3099(18)30663-7. [DOI] [PubMed] [Google Scholar]
- 137.Niro G.A., Ciancio A., Gaeta G.B., Smedile A., Marrone A., Olivero A. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatol Baltim Md. 2006;44:713–720. doi: 10.1002/hep.21296. [DOI] [PubMed] [Google Scholar]
- 138.Erhardt A., Gerlich W., Starke C., Wend U., Donner A., Sagir A. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int Off J Int Assoc Study Liver. 2006;26:805–810. doi: 10.1111/j.1478-3231.2006.01279.x. [DOI] [PubMed] [Google Scholar]
- 139.Gheorghe L., Iacob S., Simionov I., Vadan R., Constantinescu I., Caruntu F. Weight-based dosing regimen of peg-interferon α-2b for chronic hepatitis delta: a multicenter Romanian trial. J Gastrointest Liver Dis JGLD. 2011;20:377–382. [PubMed] [Google Scholar]
- 140.Abbas Z., Memon M.S., Mithani H., Jafri W., Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther. 2014;19:463–468. doi: 10.3851/IMP2728. [DOI] [PubMed] [Google Scholar]
- 141.Yurdaydin C., Bozkaya H., Gürel S., Tillmann H.L., Aslan N., Okçu-Heper A. Famciclovir treatment of chronic delta hepatitis. J Hepatol. 2002;37:266–271. doi: 10.1016/s0168-8278(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 142.Gunsar F., Akarca U.S., Ersoz G., Kobak A.C., Karasu Z., Yuce G. Two-year interferon therapy with or without ribavirin in chronic delta hepatitis. Antivir Ther. 2005;10:721–726. [PubMed] [Google Scholar]
- 143.Yurdaydin C., Bozkaya H., Onder F.O., Sentürk H., Karaaslan H., Akdoğan M. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat. 2008;15:314–321. doi: 10.1111/j.1365-2893.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 144.Abbas Z., Memon M.S., Umer M.A., Abbas M., Shazi L. Co-treatment with pegylated interferon alfa-2a and entecavir for hepatitis D: A randomized trial. World J Hepatol. 2016;8:625–631. doi: 10.4254/wjh.v8.i14.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borzacov L.M.P., de Figueiredo Nicolete L.D., Souza L.F.B., Dos Santos A.O., Vieira D.S., Salcedo J.M.V. Treatment of hepatitis delta virus genotype 3 infection with peg-interferon and entecavir. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2016;46:82–88. doi: 10.1016/j.ijid.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 146.Soriano V., Vispo E., Sierra-Enguita R., de Mendoza C., Fernández-Montero J.V., Labarga P. Efficacy of prolonged tenofovir therapy on hepatitis delta in HIV-infected patients. AIDS Lond Engl. 2014;28:2389–2394. doi: 10.1097/QAD.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 147.Chia J.-S., Wu H.-L., Wang H.-W., Chen D.-S., Chen P.-J. Inhibition of hepatitis delta virus genomic ribozyme self-cleavage by aminoglycosides. J Biomed Sci. 1997;4:208–216. doi: 10.1007/BF02253420. [DOI] [PubMed] [Google Scholar]
- 148.Hamid S.S., Etzion O., Lurie Y., Bader N., Yardeni D., Channa S.M. A phase 2 randomized clinical trial to evaluate the safety and efficacy of pegylated interferon lambda monotherapy in patients with chronic hepatitis delta virus infection. Interim results from the LIMT HDV Study (abstr.) Hepatology. 2017;66:496A. [Google Scholar]
- 149.Bogomolov P., Alexandrov A., Voronkova N., Macievich M., Kokina K., Petrachenkova M. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490–498. doi: 10.1016/j.jhep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 150.Wedemeyer H., Bogomolov P., Blank A., Allweiss L., Dandri-Petersen M., Bremer B. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol. 2018;68:S3. [Google Scholar]
- 151.Wedemeyer H., Schoeneweis K., Bogomolov P.O., Voronkova N.V., Chulanov V., Stepanova T. Interim results of a multicentre, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of Myrcludex B in combination with peg-interferon alpha 2a in patients with chronic HBV/Hdv co-infection. Hepatology. 2018;68 11A-11A. [Google Scholar]
- 152.Koh C., Canini L., Dahari H., Zhao X., Uprichard S.L., Haynes-Williams V. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167–1174. doi: 10.1016/S1473-3099(15)00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yurdaydin C., Keskin O., Kalkan Ç., Karakaya F., Çalişkan A., Karatayli E. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatol Baltim Md. 2018;67:1224–1236. doi: 10.1002/hep.29658. [DOI] [PubMed] [Google Scholar]
- 154.Wedemeyer H., Port K., Deterding K., Wranke A., Kirschner J., Bruno B. A phase 2 dose-escalation study of lonafarnib plus ritonavir in patients with chronic hepatitis D: final results from the Lonafarnib with ritonavir in HDV-4 (LOWR HDV-4) study. J Hepatol. 2017;66:S24. [Google Scholar]
- 155.Koh C., Surana P., Han T., Fryzek N., Kapuria D., Etzion O. A phase 2 study exploring once daily dosing of ritonavir boosted lonafarnib for the treatment of chronic delta hepatitis–end of study results from the LOWR HDV-3 study. J Hepatol. 2017;66:S101–S102. [Google Scholar]
- 156.Yurdaydin C., Kalkan C., Karakaya F., Caliskan A., Karatayli S., Keskin O. Subanalysis of the LOWR HDV-2 study reveals high response rates to Lonafarnib in patients with low viral loads. J Hepatol. 2018;68:S89. [Google Scholar]
- 157.Bazinet M., Pântea V., Cebotarescu V., Cojuhari L., Jimbei P., Albrecht J. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877–889. doi: 10.1016/S2468-1253(17)30288-1. [DOI] [PubMed] [Google Scholar]
- 158.Bazinet M., Pantea V., Cebotarescu V., Cojuhari L., Jimbei P., Vaillant A. Establishment of persistent functional remission of HBV and HDV infection following REP 2139 and pegylated interferon alpha 2a therapy in patients with chronic HBV/HDV co-infection: 18 month follow-up results from the REP 301-LTF study. J Hepatol. 2018;68:S509. [Google Scholar]
- 159.Chan H.L.Y., Ahn S.H., Chang T.-T., Peng C.-Y., Wong D., Coffin C.S. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B) J Hepatol. 2016;64:1011–1019. doi: 10.1016/j.jhep.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 160.Allweiss L., Dettmer C., Volz T., Giersch K., Alexandrov A., Wedemeyer H. Strong intrahepatic decline of hepatitis D virus RNA and antigen after 24 weeks of treatment with Myrcludex B in combinationwith Tenofovir in chronic HBV/HDV infected patients: Interim results from a multicenter, open-label phase 2b clinical trial. J Hepatol. 2018;68:S90. [Google Scholar]
- 161.Blanchet M., Sinnathamby V., Vaillant A., Labonté P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15 cells. Antiviral Res. 2019;164:97–105. doi: 10.1016/j.antiviral.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 162.Vaillant A. REP 2139: antiviral mechanisms and applications in achieving functional control of HBV and HDV infection. ACS Infect Dis. 2018 doi: 10.1021/acsinfecdis.8b00156. [DOI] [PubMed] [Google Scholar]
- 163.Mele A., Mariano A., Tosti M.E., Stroffolini T., Pizzuti R., Gallo G. Acute hepatitis delta virus infection in Italy: incidence and risk factors after the introduction of the universal anti-hepatitis B vaccination campaign. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007;44:e17–e24. doi: 10.1086/510433. [DOI] [PubMed] [Google Scholar]
- 164.Roggendorf M. Perspectives for a vaccine against hepatitis delta virus. Semin Liver Dis. 2012;32:256–261. doi: 10.1055/s-0032-1323631. [DOI] [PubMed] [Google Scholar]