Graphical abstract
Keywords: Hepatocellular carcinoma, Hepatitis C virus, Direct-acting antiviral agents, Occurrence, Recurrence
Highlights
-
•
Conflicting reports are available for the likelihood of HCC recurrence after DAAs.
-
•
Weak evidence is existing for the de novo occurrence of HCC following DAAs.
-
•
Geographical and ethnic differences could explain variable results among studies.
-
•
Observed marked heterogeneity in the design and inclusion criteria of studies.
-
•
Identifying patients at increased risk is very important for the management of HCC.
Abstract
Hepatitis C virus clearance is expected in more than 95% of patients treated with direct-acting antivirals (DAAs). However, an extensive debate about the impact of DAAs on the development of hepatocellular carcinoma (HCC) is currently ongoing. This review aimed to explore currently available evidence about the relationship between DAAs and HCC development. The American studies and some European studies clearly showed no relation, while the Japanese and Egyptian studies and the other European studies showed an increased risk of developing HCC after DAA exposure. These conflicting results may be due to geographical and ethnic variations and differences in the design and inclusion criteria among the studies. After reviewing the data from these different studies, it seems that some patients are at increased risk of developing HCC after DAA exposure. Identifying those at increased risk is very important for the management of HCC in light of the potentially major consequences of HCC for the patients’ quality of life and the subsequent major burden imposed on healthcare resources.
Introduction
Liver cirrhosis is the most common risk factor for hepatocellular carcinoma (HCC). Moreover, hepatitis C virus (HCV) is the leading cause of chronic liver disease in United States, Europe and many other countries like Egypt [1], [2]. Hepatocarcinogenesis risk in HCV infected patients with advanced cirrhosis ranges from 2% to 8% per year [3].One of the lessons learned during the era of interferon (IFN)-based therapy for hepatitis C virus (HCV) was that eradication of HCV reduced the risk of developing hepatocellular carcinoma (HCC), irrespective of the degree of hepatic fibrosis. This finding was demonstrated by multiple studies and meta-analyses [4], [5]. Moreover, patients with previously ablated HCC who achieved a sustained virologic response (SVR) due to IFN-based therapy had better prognoses than those who did not [6]. Additionally, achieving a SVR has been found to be the single most important factor predicting a lower risk of developing HCV-induced HCC [7]. Notably, IFN-based therapy was limited to patients without advanced cirrhosis. The introduction of highly effective direct-acting antivirals (DAAs) was generally expected by practicing hepatologists to lead to the extension of this benefit to all patients, including those who were not candidates for IFN-based therapy [8]. However, the clinical experience of using DAAs has resulted in a major debate regarding the relationship between DAAs and the development of HCC. Many authors have suggested that there is a link between the use of DAAs and the development of HCC, while others have insisted that DAAs are protective against HCC development. In this review, we explored the available evidence to identify possible explanations for these conflicting viewpoints and to develop suggestions for future research needed to solve this ongoing debate.
Studies that suggested a link between HCC development/recurrence and DAA therapy
HCC occurrence is defined as new appearance of HCC in patients with no history of liver tumor. While HCC recurrence is defined as reappearance of HCC in patients who had previous successful radical treatment for HCC [9]. In 2016, Reig and his colleagues [10] published an initial report that showed an unexpectedly high recurrence rate of previously treated HCC. The authors retrospectively analysed a cohort of 58 patients who had previously received treatment for HCV-HCC followed by DAA-based HCV treatment. The study reported a 27.6% recurrence rate after a median follow-up of 5.7 months [10]. The major limitations of this study were using crude recurrence rate, including patients who received TACE therapy, which is a palliative treatment and finally using time of DAA initiation instead of time of HCC treatment as a baseline to calculate recurrence-free period [11].This study was the first to report this risk of increased recurrence, and it sparked global interest in conducting further research. This initial study was soon followed by another retrospective study from Italy by Conti et al. The study included 344 chronic HCV patients with cirrhosis who received different DAA regimens; 91% of the patients achieved a SVR. The patients were followed for 24 weeks. The authors reported a 29% recurrence rate for those with a history of HCC and a 3.16% incidence rate [de novo] in those without a history of prior HCC irrespective of DAA regimen used [12]. As this study had no control group, they compared their data with those of a historic cohort of untreated patients with cirrhosis at their centre. The historic cohort was found to have a 3.2% incidence rate of de novo HCC, which was similar to the rate among the chronic HCV patients without a history of prior HCC who received DAA therapy. The authors concluded that HCV eradication in patients with cirrhosis did not provide protection against the development of HCC. Similar results were reported by Cardoso et al. [13] and Kozbial et al. [14]; those studies reported 7.4% and 6.6% incidence rates of HCC within a-one and 2 years of follow-up, respectively, of patients with cirrhosis who received DAA. Moreover, Nakao and his colleagues [15] reported 1.7% and 7% HCC incidence rates one and two years after DAA therapy, respectively, in 242 Japanese patients.
In another European study from Belgium, Bielen and his colleagues made an important observation. Although they found no difference in the HCC incidence rates between patients who received DAAs with or without pegylated (Peg)-IFN treatment, interestingly, they reported an HCC recurrence rate of 15% in patients treated with DAAs alone compared to 0% in those who received a combination of Peg-IFN (which is an immune-modulator) and DAAs [16]. Although selection bias could not be excluded in that study (patients who are not candidates for IFN-based therapy may have more advanced chronic liver disease and cirrhosis and therefore a higher HCC risk), it highlighted the potential role of immunomodulation in the recurrence of HCC after DAA therapy.
In 2017, reports from non-European countries emerged, and they supported the same hypothesis. Ida et al. [17] reported a 5% incidence rate and a 12% recurrence rate of HCC in a Japanese cohort with cirrhosis who were treated with daclatasvir and asunaprevir and followed for 15 months. Minami et al. [18] and Virlogeux et al. [19] reported the highest HCC recurrence rates (54.4% and 47.8%, respectively). The former followed 163 Japanese patients with a history of HCC for 14.5 months, and the latter included 23 French patients with cirrhosis; both studies included patients treated with TACE or radiotherapy. Kolly et al. [20] included patients from 3 European countries who were treated for HCC with ablation, resection or TACE. They reported a 42.5% recurrence rate after 21 months of follow-up. Similar observations were reported by Shimizu and his colleagues [21]. Additionally, Yang et al., in their small study with a total of 58 patients, noticed a 27.8% recurrence rate in patients who received bridge therapy before liver transplantation (LT) (ablation, TACE) [22], and Nagata et al. reported a 29% recurrence rate after a median follow-up of 27.6 months in patients who were treated with ablation or resection for early-stage HCC [23]. Finally, an Egyptian study reported that HCC recurrence was observed in 42% of 62 patients who were treated by DAAs after successful HCC management using one of the following modalities: 1. percutaneous ethanol injection (PEI), 2. thermal ablation by RFA or microwave ablation (MWA), 3. hepatic resection, and 4. TACE. More than 80% of these patients developed recurrence within 6 months of treatment initiation [24].
Notably, in addition to HCC recurrence, the biology of HCC and the pattern of recurrence after DAA treatment have been investigated. In 2017, Reig and colleagues reported that they found more aggressive HCC recurrence after DAA treatment, as defined by an advanced Barcelona Clinic Liver Cancer (BCLC) stage [25]. Additionally, Renzulli et al. found a more aggressive pattern of HCC recurrence with early vascular invasion after DAA therapy [26]. Abdelaziz and his colleagues noticed that compared with patients with de novo HCC after DAA therapy, patients with recurring HCC after DAA therapy had a lower 1-year survival rate and were less responsive to ablation therapy [27]. The annual incidence of HCC in patients with HCV-related cirrhosis is 2–8%, and after reviewing recent publications, we can conclude that no data showed an increase in the risk of HCC occurrence after DAA exposure.
Studies that found no link between HCC development/recurrence and DAA therapy
Two large studies from the USA published in 2017 did not find any increase in the incidence of HCC after DAA therapy. Ioannou et al. retrospectively studied 62,354 HCV patients and found that achieving a SVR, regardless of the type of therapy (DAAs alone, DAAs in combination with IFN-based therapy or IFN-based therapy alone), reduced the risk of developing de novo HCC by 71% [28]. However, the study indicated that the risk of developing HCC was higher in patients with cirrhosis than in those without cirrhosis. Additionally, Kanwal et al. studied 22,500 patients and reported similar results [29]. None of these studies examined the rate of HCC recurrence. Another important large prospective study conducted by Calvaruso et al. in 2018 observed a 2.9% incidence rate of HCC after following 2249 patients with cirrhosis for one year [30]. They also confirmed the beneficial effects of HCV eradication in patients with different stages of cirrhosis and reported that patients with compensated cirrhosis without portal hypertension experienced a significant reduction in the HCC incidence rate after HCV eradication [30]. This was also confirmed by Romano and colleagues who followed 3917 HCV patients after DAA therapy with mean follow up period of 536 (±192) days. They reported that HCC occurrence rate was 0.46% in F3, 1.49% in CTP-A and 3.61% in CTP-B cirrhotics; in the first year [31]. Similarly, Hasson et al. did not observe any increase in the incidence of HCC in patients co-infected with HCV and HIV who were treated with DAAs [32]. Another large study conducted by Calleja and his colleagues, which included 4000 HCV patients who were treated with DAAs, confirmed a low incidence rate of de novo HCC (0.93%) but reported a 30% recurrence rate of HCC after 18 months of follow-up [33]. The study also indicated that HCC was more common in patients with cirrhosis, irrespective of their SVR status [33].
Furthermore, Nagata et al. studied 1897 Japanese patients and observed a 2.5% incidence rate of de novo HCC in patients who received IFN-based therapy and a 1.1% incidence rate in those who received IFN-free therapy. Surprisingly, the same study found a 53% recurrence rate in the IFN-based group and a 29% recurrence rate in the IFN-free group [23]. Zavaglia et al. reported a 3.2% recurrence rate in their cohort of Italian patients who were treated with DAAs [34], while Torres et al. [35] found no HCC recurrence after a 12-month follow-up period. However, both studies lacked control groups and included small numbers of patients (32 patients in the former and 7 in the latter). Zeng et al. reported the same results in a letter to the editor of the Journal of Hepatology [36]. Notably, a prospective study by Ogawa et al. with152 patients who received treatment for HCC (ablation, resection, TACE and radiotherapy) reported a 16.5% recurrence rate [37]. Another large prospective study conducted in Italy by Cabibbo and his colleagues with143 patients and a 9-month median follow-up period found 12% and 26.6% HCC recurrence rates at 6 months and 1 year after DAA therapy, respectively. They also reported that only a previous history of HCC recurrence and tumour size were independent risk factors for early HCC recurrence [38]. However, they included patients with different HCC stages who were treated with resection, RFA and TACE.
Cheung et al. from the UK found a cumulative incidence rate of 5.4% in patients who achieved a SVR after DAA therapy; they reported a much higher rate (11.3%) in those who did not achieve a SVR, indicating a protective effect of viral clearance. They also reported a 6.8% HCC recurrence rate. Importantly, they studied 406 patients with decompensated cirrhosis who were already at a higher risk of HCC development than other patients, and their finding strongly suggested a beneficial effect of HCV clearance in those patients [39].
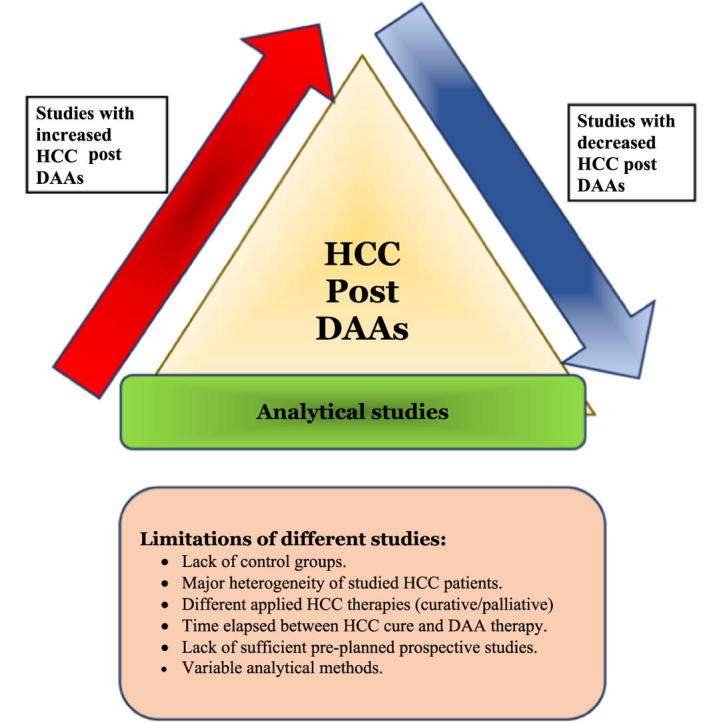
Potential pitfalls of studies and reasons of disparity in results
Collectively, most of the aforementioned studies, whether they supported or refuted the potential link between DAA therapy and HCC development/recurrence, share several common main limitations that can be summarized as follows:
-
•
The studies lacked control groups;
-
•
There was major heterogeneity within the groups of patients with HCC in terms of clinicopathologic features, such as cirrhosis grade, tumour morphology and HCC stage;
-
•
Different HCC therapies were applied in the various studies, ranging from palliative TACE to potentially curative options such as ablation and surgery;
-
•
Time elapsed between the presumed eradication of HCC and treatment with DAAs;
-
•
The studies were retrospective rather than prospective; and
-
•
There was variability in the analytical methods used [8].
Analytical studies
Analytical and comparative time-dependent studies may be more useful in solving this debate than retrospective studies; however, conflicting results of these studies remain a major challenge to drawing a firm conclusion. The first analytical study discussing this issue was conducted by Pol and colleagues, who compared DAA-exposed and non-exposed groups from the French ANRS Hepather cohort. They found no increased risk of recurrence in those exposed to DAAs (HR: 1.21, 95% CI 0.62-2.34). One major strength of this study was that they analysed 3 distinct cohorts, and their results were consistent among the three groups [40]. However, the study included patients who received DAAs at least 23 months after HCC treatment. Thus, they included patients with indolent HCC, and due to this delayed disease-free window, the tumours detected could be considered denovo HCC rather than recurrent disease. In their analysis they also reported that there was no increase in HCC recurrence after DAA exposure in liver transplant recipients, an issue that is under investigation in published articles [40].
A systematic review and meta-analysis performed by Waziry et al. analysed 26 studies and found that there was no increased risk of developing HCC following DAA therapy in patients with cirrhosis. They reported a reduction in individual risk of 63% [41]. Although they found a higher HCC recurrence rate, they attributed this to a shorter duration of follow-up (cohort effect) and older age of the included patients (higher baseline risk). Notably, another important time-dependent analytical study from Egypt performed by our group in cooperation with the Emerging Disease Epidemiology Unit at the Institute Pasteur was recently published [8]. This was the first propensity score-matched comparative time-dependent analysis of DAA-exposed and non-exposed patients who were previously treated for HCC and confirmed to have achieved a complete radiological response. The major strengths of the study were as follows the exclusion of patients who were treated by non-curative options to manage HCC; the exclusion of those treated by IFN-based therapy combined with DAAs; the initiation of follow-up from point of HCC eradication, according to the mRECIST criteria; the inclusion of a matched control group; and the adjustment for baseline factors and the time since complete radiological response of HCC through inverse probability weighting. Importantly, in contrast to the French data, our results showed a 4-fold increase in the rate of HCC recurrence after DAA treatment in patients with a history of successfully treated HCC when compared to the matched control patients who were not treated with DAAs. Remarkably, after a median follow-up period of 16 months, a 37.7% recurrence rate was observed in DAA-exposed patients [8]. In this study there was no difference in recurrence rate in patients who received DAA early after HCC treatment (less than 3 months) and those who received it later (more than 6 months). However another report presented in international liver conference 2018 pointed to the importance of time elapsed since HCC complete response after treatment and HCC recurrence rate after DAA exposure but still waiting full data [42].
What really happened according to available evidence?
Many explanations have been suggested; some authors linked the development of HCC to baseline risk factors such as advanced fibrosis grade, co-infection with hepatitis B virus (HBV) or age. Another hypothesis suggests that DAAs induce the dysregulation of immune surveillance mechanisms, following the very rapid viral clearance; this phenomenon has been confirmed by multiple studies. This dysregulation may result in the reconstitution of innate immunity, with the downregulation of type II and III IFNs, their receptors and IFN-stimulated genes. A reduction in the activation of IFN may, in turn, allow the growth of malignant cells due to the anti-angiogenic and anti-proliferative properties of IFN, which DAAs lack [43]. Additionally, one of the changes in the immune system that has been described after HCV eradication is the reduction in the numbers of cytotoxic activity of natural killer (NK) cells in the liver, which supports a faster progression of HCC foci [43].This observation was emphasized by an interesting study performed by Monto and colleagues who noticed that all 11 patients who developed HCC after second generation DAA had NK cell inhibitory KIR/HLA types suggesting the genetic based impaired immune-surveillance ability in those patients [44]. Another potential mechanism could be related to microRNA (miRNA) 122, which is the most common miRNA in hepatocytes. Previous studies confirmed that it functions as a tumour suppressor gene in HCC [45]. Interestingly, miRNA 122 was found to be downregulated after a SVR was achieved through DAA therapy, which may contribute to an increased risk of HCC recurrence [46]. Finally, another important study by Villani et al. demonstrated that during treatment with DAAs, the level of vascular endothelial growth factor becomes elevated significantly and remains elevated for 3 months after the cessation of DAA treatment, which may eventually lead to the development/recurrence of HCC [47]. Many studies have investigated potential predictors of HCC recurrence after DAA exposure. Table 1 summarizes the studies that found independent risk factors for de novo HCC development or recurrence after DAA exposure.
Table 1.
Summary of studies that found predictors of HCC development after DAA exposure.
| Study | Predictors for occurrence | Predictors for recurrence |
|---|---|---|
| Conti et al. [12] | Liver cirrhosis, Child stage B, Thrombocytopenia | Increased age, liver stiffness score |
| Cabibbo et al. [38] | N/A | Previous HCC recurrence, baseline tumor size |
| Minami et al. [18] | N/A | Alpha-fetoprotein -L3, DCP, number of previous HCC managements, Time elapsed between last HCC treatment and DAA start |
| Kanwal et al. [29] | Liver cirrhosis, alcohol use | |
| Ogawa et al. [37] | N/A | Liver cirrhosis, Time between HCC management and DAA treatment less than one year, Palliative HCC treatment [TACE, radiotherapy |
| Ikeda et al. [49] | N/A | Multiple HCC treatment sessions, Alpha-fetoprotein level, Prothrombin time |
| Mettke et al. [48] | MELD score, Alpha-fetoprotein level | |
| Kolly et al. [20] | N/A | Time between HCC treatment and starting DAA therapy |
| Nagata et al. [23] | N/A | Alpha-fetoprotein, WFA-M2BP level |
| Calvaruso et al. [30] | Hypoalbuminemia, absence of SVR, thrombocytopenia | |
| Shimizu et al. [21] | N/A | HBcAb positivity, TACE |
| Mashiba et al. [50] | N/A | Alpha-fetoprotein level, SVR, baseline BCLC stage of HCC |
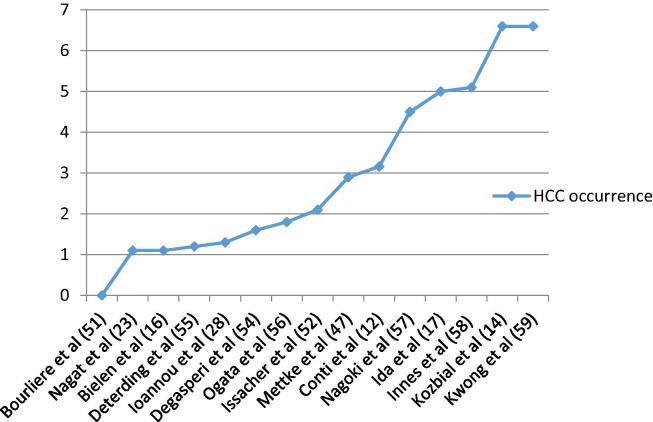
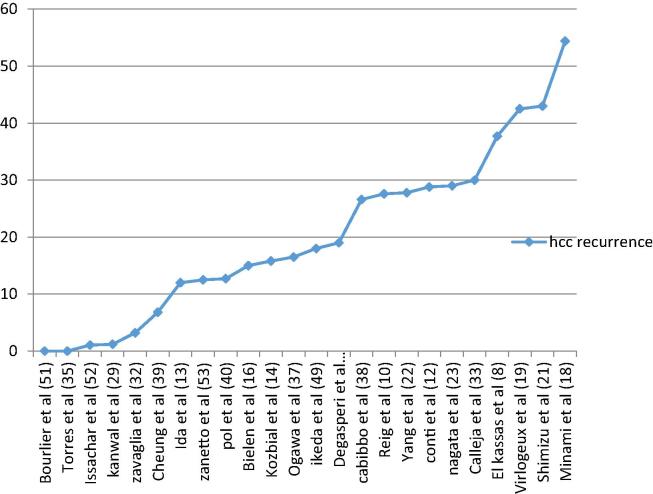
Fig. 1, Fig. 2 illustrate the relevant studies and the risk of HCC reported in each study, arranged in an ascending manner.
Fig. 1.
HCC occurrence rate after DAA observed by various studies. [51], [52], [54], [55], [56], [57], [58], [59].
Fig. 2.
HCC recurrence rate after DAA observed by various studies. [51], [52], [53].
Conclusions and future perspectives
While it appears that there is no increased risk of de novo HCC in cirrhotic patients receiving DAAs, the existence of early protective effects has been questioned; this suggests that continued HCC surveillance in patients with cirrhosis should be mandatory, even after achieving a SVR. Notably, most American studies and some European studies showed no increase in the risk of HCC recurrence after treatment with DAAs. However, other European, Japanese and Egyptian studies showed the opposite effect, with an increased HCC recurrence rate following DAA therapy in HCC patients; these conflicting findings indicate that geographical variations and differences in viral genotypes may play a role in HCC recurrence after DAA therapy. Some reports acknowledged that they were unable to account for geographical variations. It seems that after DAA exposure, some patients with HCV are at increased risk of de novo HCC development, and other patients with a history of treated HCC are at increased risk of HCC recurrence. Identifying those at increased risk is very important for the management of HCC, as HCC has potentially major ramifications for the patients’ quality of life and, consequently, imposes a major burden on healthcare costs, especially in developing countries.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Mohamed El Kassas is an Associate Professor and chief of Endemic Medicine and Hepato-Gastroenterology Department, Faculty of Medicine, Helwan University, Cairo, Egypt, member of Specialized Scientific Council for Medical Research, ASRT, president of Egyptian Association for Research and Training in HepatoGastroenterology (EARTH), and winner of the state encouraging award in medical sciences 2016. Professor El Kassas has a research interest in viral hepatitis, focusing his work on the management of HCV and HBV, and their relation to hepatic carcinogenesis. He has participated in several clinical trials, authoring or co-authoring 50 peer-reviewed articles in the field of hepatology.

Tamer Elbaz is an Assistant Professor of Hepatology and Gastroenterology working at Endemic Medicine and Hepatogastroenterology Department, Faculty of Medicine, Cairo University, Egypt. He certainly focused his studies on the management of viral hepatitis. He has many publications that described the safety and efficacy of HCV lines of treatment in Egypt. Also, major part of his publications focused on diagnosis, prognosis and management of hepatocellular carcinoma. He is an active participant of the multidisciplinary hepatocellular carcinoma clinics at Kasr Al Ainy Hospital and he is a member of several projects for management of HCV using the recently discovered drugs.

Mohamed Salaheldin graduated from Ain Shams University 2004. He worked as gastroenterology and hepatology resident in Tropical Medicine Departement, Ain Shams University from 2006 till 2009 and received master degree in 2009 from Ain Shams University. He joined Ain Shams Hepatoma Group in 2007 and he is active member till now. He worked as assistant lecturer of gastroenterology and hepatology in Tropical Medicine Departement, Ain Shams University from 2010 till 2014. He received MD degree in 2014. He works as a lecturer of gastroenterology and hepatology in Tropical Medicine Departement, Ain Shams University from 2014 till now.

Lobna Abdelsalam is a consultant geneticist, working in the genome unit, Faculty of Medicine, Cairo University. She has a long track of research work with focus on genetic aspects of viral hepatitis and hepatocellular carcinoma. She has participated in several research projects, authoring or co-authoring a large number of peer-reviewed manuscripts in the field of genetics.

Ahmed Omar Kaseb is an Associate Professor and Program Director of Hepatocellular Carcinoma (HCC), at the Department of Gastrointestinal Medical Oncology at the University of Texas MD Anderson Cancer Center, Houston, Texas, USA. His research also examines the differential effects of demographics and hepatitis status on treatment outcome in HCC. Based on his interest and research, he has developed several protocols in the treatment and staging of HCC, including a new HCC staging system. Dr. Kaseb has authored and co-authored over 100 articles, books, abstracts, and editorials, and currently serves as the Editor-in-Chief of the international “Journal of hepatocellular Carcinoma”.

Gamal Esmat is a distinguished Professor at Endemic Medicine and Hepatogastroenterology Department, Cairo University. He was the vice president of Cairo University for graduate studies and research. He published many scientific articles in top journals and was mainly concerned with hepatitis, hepatocellular carcinoma, and liver transplantation. He is a member of numerous scientific societies and organizations and was the president of IASL (International Association for the study of the liver) during 2005-2008 and is recently the WHO consultant for management of HBV. He was awarded State Merit Award in Medical Science 2010 and Egyptian Nile Award in Science 2016.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Messina J.P., Humphreys I., Flaxman A., Brown A., Cooke G.S., Pybus O.G. Global distribution and prevalence of hepatitis C virus genotype. Hepatology. 2015;61(1):77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandeel A., Genedy M., El-Refai S., Funk A.L., Fontanet A., Talaat M. The prevalence of hepatits C infection in Egypt 2015: implication for future policy on prevention and treatment. Liver Int. 2017;37(1):45–53. doi: 10.1111/liv.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioannou G.N., Splan M.F., Weiss N.S., McDonald G.B., Beretta L., Lee S.P. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2007;5:938–945. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Morgan R.L., Baack B., Smith B.D., Yartel A., Pitasi M., Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann. Int. Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K., Saitoh S., Arase Y., Chayama K., Suzuki Y., Kobayashi M. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–1130. doi: 10.1002/hep.510290439. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang L., Zeng X., Yang Z., Meng Z., Hoshida Y. Effect and safety of interferon for hepatocellular carcinoma: a systematic review and meta-analysis. PLoS ONE. 2013;8[9] doi: 10.1371/journal.pone.0061361. e61361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon C., Jung K.S., Kim D.Y., Baatarkhuu O., Park J.Y., Kim B.K. Lower incidence of hepatocellular carcinoma and cirrhosis in hepatitis C patients with sustained virological response by pegylated interferon and ribavirin. Dig. Dis. Sci. 2015;60:573–581. doi: 10.1007/s10620-014-3361-6. [DOI] [PubMed] [Google Scholar]
- 8.El Kassas M., Funk A.L., Salaheldin M., Shimakawa Y., Eltabbakh M., Jean K. Increased recurrence rates of hepatocellular carcinoma after DAA therapy in a hepatitis C-infected Egyptian cohort: a comparative analysis. J. Viral. Hepat. 2018;25:623–630. doi: 10.1111/jvh.12854. [DOI] [PubMed] [Google Scholar]
- 9.Llovet J.M., Villanueva A. Liver cancer: Effect of HCV clearance with direct-acting antiviral agents on HCC. Nat. Rev. Gastroenterol. Hepatol. 2016;13:561–562. doi: 10.1038/nrgastro.2016.140. [DOI] [PubMed] [Google Scholar]
- 10.Reig M., Mariño Z., Perelló C., Iñarrairaegui M., Ribeiro A., Lens S. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Cammà C., Cabibbo G., Craxì A. Direct antiviral agents and risk for HCC early recurrence: much ado about nothing. J. Hepatol. 2016;65:861–862. doi: 10.1016/j.jhep.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Conti F., Buonfiglioli F., Scuteri A., Crespi C., Bolondi L., Caraceni P. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso H., Vale A.M., Rodrigues S., Gonçalves R., Albuquerque A., Pereira P. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J. Hepatol. 2016;65:1070–1071. doi: 10.1016/j.jhep.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Kozbial K., Moser S., Schwarzer R., Laferl H., Al-Zoairy R., Stauber R. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J. Hepatol. 2016;65:856–858. doi: 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Nakao Y., Hashimoto S., Abiru S., Komori A., Yamasaki K., Nagaoka S. Rapidly growing, moderately differentiated HCC: a clinicopathological characteristic of HCC occurrence after IFN-free DAA therapy? J. Hepatol. 2018;68(4):854–855. doi: 10.1016/j.jhep.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Bielen R., Moreno C., Van Vlierberghe H., Bourgeois S., Mulkay J.P., Vanwolleghem T. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C-infected patients treated with direct-acting antivirals with and without pegylated interferon: a Belgian experience. J. Viral. Hepat. 2017;24:976–981. doi: 10.1111/jvh.12726. [DOI] [PubMed] [Google Scholar]
- 17.Ida H., Hagiwara S., Kono M., Minami T., Chishina H., Arizumi T. Hepatocellular carcinoma after achievement of sustained viral response with daclatasvir and asunaprevir in patients with chronic hepatitis C virus infection. Dig. Dis. 2017;35:565–573. doi: 10.1159/000480183. [DOI] [PubMed] [Google Scholar]
- 18.Minami T., Tateishi R., Wake T., Nishibatake M., Nakagomi R., Sato M. Hepatocellular carcinoma recurrence after curative treatments in patients with chronic hepatitis C who underwent direct-acting. Antiviral therapy. Hepatology. 2017;66:760A–761A. [Google Scholar]
- 19.Virlogeux V., Pradat P., Hartig-Lavie K., Bailly F., Maynard M., Ouziel G. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017;37:1122–1127. doi: 10.1111/liv.13456. [DOI] [PubMed] [Google Scholar]
- 20.Kolly P., Waidmann O., Vermehren J., Moreno C., Vögeli I., Berg T. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: a European multicentre study. J. Hepatol. 2017;67:876–878. doi: 10.1016/j.jhep.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu H., Matsui K., Iwabuchi S., Fujikawa T., Nagata M., Takatsuka K. Relationship of hepatitis B virus infection to the recurrence of hepatocellular carcinoma after direct acting antivirals. Indian J. Gastroenterol. 2017;36:235–238. doi: 10.1007/s12664-017-0755-3. [DOI] [PubMed] [Google Scholar]
- 22.Yang J.D., Aqel B.A., Pungpapong S., Gores G.J., Roberts L.R., Leise M.D. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J. Hepatol. 2016;65:859–860. doi: 10.1016/j.jhep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Nagata H., Nakagawa M., Asahina Y., Sato A., Asano Y., Tsunoda T. Liver Conference Study Group. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017;67:933–939. doi: 10.1016/j.jhep.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Hassany M., Elsharkawy A., Maged A., Mehrez M., Asem N., Gomaa A. Hepatitis C virus treatment by direct-acting antivirals in successfully treated hepatocellular carcinoma and possible mutual impact. Eur. J. Gastroenterol. Hepatol. 2018;30(8):876–881. doi: 10.1097/MEG.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 25.Reig M., Mariño Z., Perelló C., Iñarrairaegui M., Lens S., Díaz A. PS-031- Tumour recurrence after Interferon-free treatment for hepatitis C in patients with previously treated hepatocellular carcinoma discloses a more aggressive pattern and faster tumour growth. J. Hepatol. 2017;66:S20. [Google Scholar]
- 26.Renzulli M., Buonfiglioli F., Conti F., Brocchi S., Serio I., Foschi F.G. Imaging features of microvascular invasion in hepatocellular carcinoma developed after direct-acting antiviral therapy in HCV-related cirrhosis. EurRadiol. 2018;28:506–513. doi: 10.1007/s00330-017-5033-3. [DOI] [PubMed] [Google Scholar]
- 27.Abdelaziz A.O., Nabil M.M., Abdelmaksoud A.H., Shousha H.I., Cordie A.A., Hassan E.M. De-novo versus recurrent hepatocellular carcinoma following direct-acting antiviral therapy for hepatitis C virus. Eur. J. Gastroenterol. Hepatol. 2018;30(1):39–43. doi: 10.1097/MEG.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 28.Ioannou G.N., Green P.K. Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. pii: S0168-8278(17)32273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanwal F., Kramer J., Asch S.M., Chayanupatkul M., Cao Y., El- Serag H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Calvaruso V., Cabibbo G., Cacciola I., Petta S., Madonia S., Bellia A. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155(2):411–421.e4. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Romano A., Angeli P., Piovesan S., Noventa F., Anastassopoulos G., Chemello L. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J. Hepatol. 2018;69(2):345–352. doi: 10.1016/j.jhep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Hasson H., Merli M., Messina E., Bhoori S., Salpietro S., Morsica G. Occurrence of hepatocellular carcinoma in HIV/HCV co-infected patients treated with direct-acting antivirals. J. Hepatol. 2017;67:415–417. doi: 10.1016/j.jhep.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Calleja J.L., Crespo J., Rincón D., Ruiz-Antorán B., Fernandez I., Perelló C. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J. Hepatol. 2017;66:1138–1148. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Zavaglia C., Okolicsanyi S., Cesarini L., Mazzarelli C., Pontecorvi V., Ciaccio A. Is the risk of neoplastic recurrence increased after prescribing direct-acting antivirals for HCV patients whose HCC was previously cured? J. Hepatol. 2017;66:236–237. doi: 10.1016/j.jhep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Torres H.A., Vauthey J.N., Economides M.P., Mahale P., Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J. Hepatol. 2016;65:862–864. doi: 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Q.L., Li Z.Q., Liang H.X., Xu G.H., Li C.X., Zhang D.W. Unexpected high incidence of hepatocellular carcinoma in patients with hepatitis C in the era of DAAs: too alarming? J, Hepatol. 2016;65:1068–1069. doi: 10.1016/j.jhep.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa E., Furusyo N., Nomura H., Dohmen K., Higashi N., Takahashi K., Kawano A., Azuma K., Satoh T., Nakamuta M., Koyanagi T., Kato M., Shimoda S., Kajiwara E., Hayashi J. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment. Pharmacol. Ther. 2018;47(1):104–113. doi: 10.1111/apt.14380. [DOI] [PubMed] [Google Scholar]
- 38.Cabibbo G., Petta S., Calvaruso V., Cacciola I., Cannavò M.R., Madonia S. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol. Ther. 2017;46:688–695. doi: 10.1111/apt.14256. [DOI] [PubMed] [Google Scholar]
- 39.Cheung Michelle C.M., Walker Alex J., Hudson Benjamin E., Verma Suman, McLauchlan John, Mutimer David J., Brown Ashley, Gelson William T.H., MacDonald Douglas C., Agarwal Kosh, Foster Graham R., Irving William L. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016;65(4):741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 40.ANRS collaborative study group on hepatocellular carcinoma [ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts]. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J. Hepatol. 2016;65:734–740. doi: 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 41.Waziry R., Hajarizadeh B., Grebely J., Amin J., Law M., Danta M. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Gambato M., Russo F.P., Piovesan S., Romano A., Zanetto G., Anastassopoulos G. HCC recurrence under all-oral DAAs-based antiviral therapy in HCV-infected patients: data from Navigatore web platform. J. Hepatol. 2018;68(suppl 1):S85–S86. [Google Scholar]
- 43.Chu P.S., Nakamoto N., Taniki N., Ojiro K., Amiya T., Makita Y. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS ONE. 2017;12:e0179096. doi: 10.1371/journal.pone.0179096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unexpected Hepatocellular Carcinoma (HCC) following treatment‐induced HCV clearance in a VA cohort. Monto A, Ryan J, Niemi E, segal M, Lanier L. Hepatol. 2017;66:S1. 1412.
- 45.Nakao K., Miyaaki H., Ichikawa T. Antitumor function of microRNA-122 against hepatocellular carcinoma. J Gastroenterol. 2014;49:589–593. doi: 10.1007/s00535-014-0932-4. [DOI] [PubMed] [Google Scholar]
- 46.Waring J.F., Dumas E.O., Abel S., Coakley E., Cohen D.E., Davis J.W. Serum miR-122 may serve as a biomarker for response to direct acting antivirals: effect of paritaprevir/R with dasabuvir or ombitasvir on miR-122 in HCV-infected subjects. J Viral Hepat. 2016;23:96–104. doi: 10.1111/jvh.12470. [DOI] [PubMed] [Google Scholar]
- 47.Villani R., Facciorusso A., Bellanti F., Tamborra R., Piscazzi A., Landriscina M. DAAs rapidly reduce inflammation but increase serum VEGF level: a rationale for tumor risk during anti-HCV treatment. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mettke F, Schlevogt B, Deterding K, Wranke A, Smith A, Port K, et al. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does notchange the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther 2018;47:516–525. [DOI] [PubMed]
- 49.Ikeda K., Kawamura Y., Kobayashi M., Kominami Y., Fujiyama S., Sezaki H. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci. 2017;62:2932–2942. doi: 10.1007/s10620-017-4739-z. [DOI] [PubMed] [Google Scholar]
- 50.Mashiba T., Joko K., Kurosaki M., Ochi H., Osaki Y., Kojima Y. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourliere M., Gane E.J., Jacobson I., Gordon S.C., Sulkowski M.S., McNabb B.L. Long-term follow up of patients with chronic HCV and no or minimal fibrosis shows low risk for liver- related morbidity and mortality after achieving SVR with DAA based therapy: results from the Gilead SVR Registry. Hepatology. 2017;66:518A–519A. [Google Scholar]
- 52.Issachar A., Sneh-Arbib O., Braun M., Shlomai A., Oxtrud E., Harif Y. LBP-509-Occurrence and recurrence of malignancies post DAA Treatment in 5.1% of patients-single center experience. J. Hepatol. 2017;66:S97. [Google Scholar]
- 53.Zanetto A., Shalaby S., Vitale A., Mescoli C., Ferrarese A., Gambato M. Dropout rate from the liver transplant waiting list because of hepatocellular carcinoma progression in hepatitis C virus-infected patients treated with direct-acting antivirals. Liver Transpl. 2017;23:1103–1112. doi: 10.1002/lt.24790. [DOI] [PubMed] [Google Scholar]
- 54.Degasperi E., D’Ambrosio R., Sangiovanni A., Aghemo A., Soffredini R., De Nicola S. Low rates of de novo or recurrent hepatocellular carcinoma in HCV cirrhotic patients treated with direct-acting antivirals (DAAs): a singlecenter experience. Hepatology. 2017;66:46A. [Google Scholar]
- 55.Deterding K., Mauss S., Pathil A., Buggisch P., Schott E., Cornberg M. PS-096-Long-term follow-up after IFN-free therapy of advanced HCV-associated liver cirrhosis: continued improvement of liver function parameters – results from the German Hepatitis C-Registry (DHC-R) J. Hepatol. 2017;66:S55. [Google Scholar]
- 56.Ogata F., Kobayashi M., Akuta N., Osawa M., Fujiyama S., Kawamura Y. Outcome of all-oral direct-acting antiviral regimens on the rate of development of hepatocellular carcinoma in patients with Hepatitis C virus genotype 1-related chronic liver disease. Oncology. 2017;93:92–98. doi: 10.1159/000470910. [DOI] [PubMed] [Google Scholar]
- 57.Nagaoki Y., Imamura M., Aikata H., Daijo K., Teraoka Y., Honda F. The risks of hepatocellular carcinoma development after HCV eradication are similar between patients treated with peg-interferon plus ribavirin and direct-acting antiviral therapy. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0182710. e0182710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Innes H., Barclay S.T., Hayes P.C., Fraser A., Dillon J.F., Stanley A. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J. Hepatol. 2018;68(4):646–654. doi: 10.1016/j.jhep.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Kwong A.J., Kim W., Flemming J.A. Continued increase in incidence of de novo hepatocellular carcinoma among liver transplant registrants with hepatitis C virus infection. Hepatology. 2017;66:71A. doi: 10.1002/hep.30045. [DOI] [PMC free article] [PubMed] [Google Scholar]