Abstract
Approximately 200 cases of leprosy are reported each year in the United States, and about 175 of the cases are diagnosed for the first time. Florida contributes a large number of reported cases each year and is showing an increasing incidence. Studies from other southern U.S. states demonstrate infection with the same strain of Mycobacterium leprae, confirming human armadillo exposure as the main risk factor. In contrast, cases from Florida show no clear risk factor. We present three cases (two foreign born and one autochthonous case) from Hillsborough county Florida, that were reported from this non-endemic area during the past five years. The first case was a 35-year-old male from Mexico, with history of exposure from a Haitian inmate, who presented with multiple erythematous non-tender cutaneous lesions and numbness in both hands. Biopsy confirmed borderline lepromatous leprosy. The second case was a 34-year-old male, from Florida who presented with sparse annular non-supporative lesions on left cheek with one-month duration and denied leprosy or armadillo exposure. Biopsy confirmed the diagnosis of borderline tuberculoid leprosy. The third case was a 38-year-old female, from Puerto Rico who presented with disseminated painless plaques, edema of the hands with numbness and paresthesia. She denied leprosy or armadillo exposure. The biopsy confirmed borderline lepromatous leprosy with erythema nodosum leprosum. Our case series demonstrates that a history of armadillo exposure is not always present. Other risk factors need to be considered when leprosy is a possible diagnosis in a patient.
Keywords: Leprosy, Armadillo exposure, Mycobacterium, Leprae
Introduction
Leprosy, or Hansen’s disease (HD), is a chronic granulomatous disease caused by Mycobacterium leprae, an acid-alcohol-fast, obligate intracellular bacillus, that primarily affects the skin, peripheral nervous system, the mucosa of upper respiratory tract and eyes [1,2]. Leprosy is a non-highly contagious disease, and the majority of the world population has natural immunity [3]. The prolonged human exposure to nasal secretions of an infected person is the principal known mechanism of transmission [1]. However, zoonotic transmission of the disease has been described from contact with nine-banded armadillos, found only in the US (Dasypus novemcinctus) [4], which are the only known non-human reservoirs of M. leprae. After a whole-genome sequencing analysis, Truman et al. found that wild armadillos from southern states and U.S. born patients with leprosy are infected with the same strain of M. leprae, demonstrating that zoonotic transmission from nine-banded armadillos is the principal source of infection in this region [5]. Furthermore, Levis et al. reported in 2000, the first autochthonous case of leprosy in Toronto, (where there are no wild armadillos) and suggested immigration from endemic countries as the likely scenario for autochthonous transmission of HD [6,7].
Although it is considered a rare infection, the Health Resources and Services Administration reported around 178 new cases in the United States in 2015. The six states (California, Texas, Louisiana, Hawaii, Florida and New York) reported the highest number (72%–129/178) of registered cases across the country. Out of the 129 registered cases, 29 cases were from the State of Florida, the highest incidence from any other previous years [8,9]. The majority of these cases were diagnosed in native-born U.S. citizens suggesting indigenous transmission within the population [3].
Although leprosy can present subtly as a skin lesion, which requires skin biopsy for diagnosis, physicians also need to be aware of other risk factors for leprosy infection. Besides wild armadillo exposure, a history of close and prolonged contact with immigrant individuals or with non-immigrant individuals that frequently travel to endemic regions should be a consideration [10].
We report three cases of leprosy with no previous armadillo exposure. These cases were diagnosed in Hillsborough County in Florida. The demographics of all three cases are reported in Table 1.
Table 1.
Demographics of Leprosy Cases.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Sex | Male | Male | Female |
| Age (years) | 35 | 34 | 38 |
| Place of Birth | Mexico | Alabama, U.S. | Puerto Rico |
| Exposure to infected humans | + | − | − |
| Exposure to armadillos | − | − | − |
| Type of Leprosy | Borderline Lepromatous | Tuberculoid Leprosy | Borderline Lepromatous |
| Time between arrival to U.S. and presentation | Not known | Not known | 6 months |
Case reports
Case 1
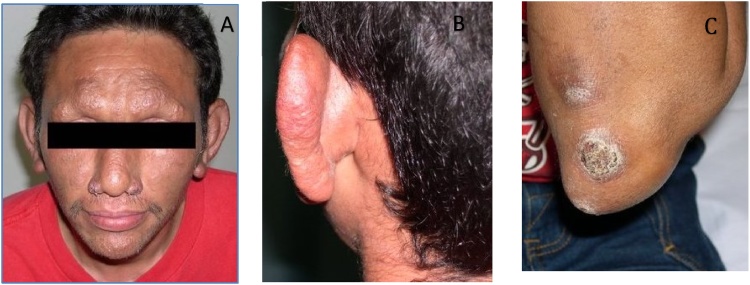
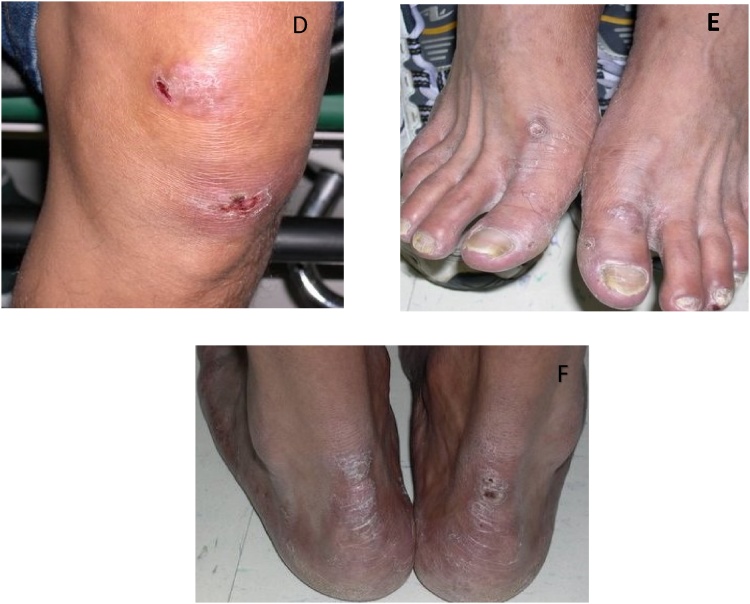
A previously healthy 35-year-old male originally from Mexico presented in May, 2012, with an eight-month history of disseminated circular non-tender skin lesions on the face, trunk, buttocks, and thighs with associated numbness in both hands for four months. The patient reported having lived in New York, Kentucky, and Georgia for periods of few months after he migrated from Mexico. He worked in construction and had a history of incarceration in Tampa and Miami facilities. During that time, he recalled exposure to an inmate of Haitian descent who had similar exudative skin lesions but didn’t have the diagnosis of leprosy. Also, the patient stated childhood exposure to leprosy-like skin lesions from his grandfather. On physical examination, circular, erythematous, non-tender cutaneous lesions with raised borders and variable sizes were located on the face, trunk, upper extremities, and thighs. (Figs. A–F) After biopsy confirmation of borderline lepromatous leprosy, he started treatment with dapsone, rifampin and minocycline. Patient successfully completed 18 months of therapy with full resolution of symptoms. Two years after completion of the therapy, patient presented with fever and new circular, erythematous, non-tender cutaneous lesions with raised borders (Fig. 1, Fig. 2). Treatment was started for reactional leprosy: erythema nodosum leprosum. Patient completed 5 months of therapy with prednisone and thalidomide. At his 5 year follow-up visit the patient remained asymptomatic.
Fig. 1.
Skin manifestations of Mycobacterium leprae infection. A) Leonine facies with madarosis (complete loss of eyebrows) and bilateral nodular lesions of nasal ala. B) Left ear nodularity. C) Crusted ulcerative lesions of the left elbow.
Fig. 2.
Skin manifestations of Mycobacterium leprae infection. D) Annular ulcerated plaques over the left knee. E) Hyper-pigmented nodular lesions of the dorsal aspect of the feet. F) Hyper-pigmented plaques of the posterior heel bilaterally with crusted ulcerative lesion on the right side.
Case 2
A 34 -year-old male visited the county clinic in August, 2012 complaining of an annular cutaneous lesion on his left cheek of one-month duration that increased in size progressively. The patient was born in Alabama but lived in Cocoa Beach Florida since childhood and moved to Tampa Florida, a few months before presentation. His other travel history involved Minnesota and The Bahamas. He denied being previously in contact with individuals with leprosy, and denied any contact with armadillos. Physical exam showed sparse annular, non-suppurative lesions with raised borders on face and trunk and smaller lesions located on lumbosacral and left antecubital areas. The biopsy showed Borderline Tuberculoid Leprosy. The patient was started on rifampin and dapsone and successfully completed 13 months of therapy. All lesions resolved and patient remained symptom-free at his 6 month follow up visit. Attempts to locate him for subsequent visits were unsuccessful.
Case 3
A 38-year-old female from Puerto Rico who recently moved to Florida reported a five-year history of recurrent rash and skin lesions. She presented in February, 2017 with a three-month history of non- painful flat lesion located between the 3rd and 4th finger of the right hand, associated with edema and numbness. Additional lesions disseminated to scalp, arms, chest, back and lower extremities. These increased in size, became erythematous, raised and painful. She had associated fever, fatigue, and weakness. Initially, she was treated with topical and oral antifungals for presumed dermatophytosis, but the lesions became larger with associated burning and pinprick sensation in lower limbs. The patient worked in a geriatric facility as a nurse aid but denied known leprosy and armadillo exposure. Initial examination revealed non-pitting edema on hands and feet with full range of motion (ROM), more than ten disseminated plaques with erythematous borders on upper and lower extremities and decreased light touch sensation with negative Monofilament test (10 g) on both hands. A biopsy confirmed borderline lepromatous leprosy. The patient was hospitalized, and triple-drug therapy regimen was started with minocycline, dapsone, and rifampin; Prednisone was tapered later in the treatment. In the follow-up visit the patient continued to have neuropathy in hands and lower extremities. The pain improved and scalp lesions resolved however, the skin lesions on the extremities persisted. Table 1
Discussion
In this report, we describe two cases of leprosy among foreign-born patients and one autochthonous case of leprosy
Case 1, had multiple risk factors that need to be considered when suspecting leprosy infection, including the patient’s Mexican origin, exposure to an inmate with similar skin lesions during incarceration, and history of exposure to an infected relative. Prior studies considered lepromatous leprosy as a travel-related hazard for travelers to Mexico [10]. Sotiriou et al. described two cases of HD in siblings from Mexico caused by M. lepromatosis suggesting family clustering of leprosy [11].
Case 2 is a U.S. born patient with no known history of exposure to leprosy or armadillos, who might had been infected during his travel to the Caribbean. Due to the prolonged incubation period that characterizes leprosy, he developed the manifestations years later. Another possibility is unnoticed exposure to an individual with HD while living in Florida, given the increasing foreign population in this state [12,13].
This data supports that international migration of patients with HD is a likely scenario for human transmission as stated before by Rendini et al. [7] The incidence of immigration in Florida was consistently increased between 2010–2014, and about two-thirds of all immigrants to Florida from abroad came from the Americas with a specific increment in those from the Caribbean, and South America [13]. Although notably fewer cases have been recently registered among people migrating from Cuba and Puerto Rico (1.69% of total cases for each country in 2015) [3], immigration from these areas still represents the most likely risk factor for leprosy infection in our Case 3.
The increased migratory flow of people from areas where leprosy is still endemic will undoubtedly have an impact on the incidence of leprosy in the U.S. where HD has been considered controlled for decades [12]. Moreover, the risk of transmission inside U.S. territory will be expected to increase in areas with higher affluence of immigrants from endemic countries.
Interestingly, among the cases of leprosy reported in the U.S. in 2015, two-thirds were U.S. born citizens without a history of leprosy exposure or residency in endemic countries. Moreover, multiple autochthonous cases of leprosy have been reported in Florida [4,[14], [15], [16], [17]] and several of them have been attributed to zoonotic transmission from armadillos [5,[15], [16], [17]]. A Canadian patient with leprosy who had only traveled to Florida, was found to have M. leprae strain 3I-2-v1, which is found in most infected armadillos [5,15]. Analyses of nuclear microsatellite DNA demonstrated that western population of armadillos from Texas and Louisiana, historically infected by M.leprae [5], have merged with the non-infected eastern population of armadillos [18]. More recently, five patients with biopsy proven HD were interviewed by Domozych et al, in Central Florida, and were genotyped of their M. leprae isolates. There was no history of contact with infected humans and armadillos. One patient who had direct contact with armadillo, was found to be infected with M. leprae SNP 3 K strain, not identified before in armadillos [4]. For the control of infection, it is important to clarify the mode of transmission of this disease. It is unclear that the patients in our study and a more recent study in Central Florida, how our subjects who did not recall any direct contact with armadillos contracted HD and the studies warrant further investigation.
Truman et al conducted genome sequencing studies of Mycobacterium. Leprea that included patients and wild armadillos from wide range of areas in the Southern US including Arkansas, Alabama, Louisiana, Mississippi and Texas. [5] With the increasing incidence of leprosy cases in the state of Florida, perhaps further genome sequencing specifically in the wild armadillos from Florida might highlight association between armadillo exposure and human infection with leprosy.
These data highlight the importance of considering the country of origin, the travel and residency history and contact with immigrants from endemic areas, as part of the standard evaluation of patients with skin and neurological lesions, suggestive of leprosy. The treatment with the multidrug regimen should be effective although careful decision making on the choice of therapy and experience is required. Clinicians should consider that in spite of the fact that leprosy is a treatable disease; delays in the diagnosis and adequate treatment might increase the risk of progression to leprosy-associated disabilities.
Authorship statement
The following roles were assigned in the preparation of this manuscript.
Sadaf Aslam, MD, MS: First Author, manuscript preparation, writing and submission, cases study, literature searches.
Jellyana Peraza, MD: Manuscript/abstract preparation and writing, case study, literature searches.
Andrew Mekaiel MD: Abstract preparation and poster presentation, literature searches.
Manual Castro, MD: Abstract preparation and poster presentation, literature searches.
Beata Casanas DO: Manuscript preparation and review, cases study, literature searches.
Contributor Information
Sadaf Aslam, Email: saslam@health.usf.edu.
Jellyana Peraza, Email: jellyanaperaza@gmail.com.
Andrew Mekaiel, Email: andrewmekaiel@gmail.com.
Manuel Castro, Email: manuel_cb@hotmail.com.
Beata Casanas, Email: bcasanas@health.usf.edu.
References
- 1.Eichelmann K. Leprosy. An update: definition, pathogenesis, classification, diagnosis, and treatment. Actas Dermosifiliogr. 2013;104(7):554–563. doi: 10.1016/j.adengl.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Organization, W.H . 2018. Leprosy factsheet.http://www.who.int/mediacentre/factsheets/fs101/en/ [cited 2018 March]; Available from: [Google Scholar]
- 3.Program . U.S. Department of Health Resources and Services Adminstration; 2015. A summary of Hansen’s disease in the United States. [Google Scholar]
- 4.Domozych R. Increasing incidence of leprosy and transmission from armadillos in Central Florida: a case series. JAAD Case Rep. 2016;2(3):189–192. doi: 10.1016/j.jdcr.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truman R.W. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364(17):1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boggild A.K. Leprosy in Toronto: an analysis of 184 imported cases. CMAJ. 2004;170(1):55–59. [PMC free article] [PubMed] [Google Scholar]
- 7.Rendini T., Levis W. Autochthonous leprosy without Armadillo exposure, Eastern United States. Emerg Infect Dis. 2017;23(11):1928. doi: 10.3201/eid2311.171145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2017. Health, Florida department of health reportable and infectious diseases (Hansens disease) [Google Scholar]
- 9.WHO; 2018. Repository, global health observatory data, number of new leprosy cases. [Google Scholar]
- 10.Virk A. Mycobacterium lepromatosis lepromatous leprosy in US citizen who traveled to disease-endemic areas. Emerg Infect Dis. 2017;23(11):1864–1866. doi: 10.3201/eid2311.171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotiriou M.C., Stryjewska B.M., Hill C. Two cases of leprosy in siblings caused by Mycobacterium lepromatosis and review of the literature. Am J Trop Med Hyg. 2016;95(3):522–527. doi: 10.4269/ajtmh.16-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos J.M., Romero D., Belinchon I. Epidemiology of leprosy in Spain: the role of the international migration. PLoS Negl Trop Dis. 2016;10(3) doi: 10.1371/journal.pntd.0004321. p. e0004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Rayer Stefan. 2016. Foreign in-migration to Florida, 2005 - 2014 bureau of economic and business research.https://www.bebr.ufl.edu/population/website-article/foreign-migration-florida-2005 [cited 2018; Available from: –2014. [Google Scholar]
- 14.Anderson K.L. A case of leprosy in central Florida. Cutis. 2017;100(5):327–329. [PubMed] [Google Scholar]
- 15.Bonnar P.E. Leprosy in Nonimmigrant Canadian man without travel outside North America, 2014. Emerg Infect Dis. 2018;24(1):165–166. doi: 10.3201/eid2401.170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harb J. Two cases of lepromatous leprosy from exposure to armadillos in Florida. Skinmed. 2017;15(5):391–393. [PubMed] [Google Scholar]
- 17.Villada G. Autochthonous borderline tuberculoid leprosy in a man from Florida. Lepr Rev. 2016;87(1):101–103. [PubMed] [Google Scholar]
- 18.Loughry W.J. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45(1):144–152. doi: 10.7589/0090-3558-45.1.144. [DOI] [PubMed] [Google Scholar]