Abstract
Avian communities from South America harbor an extraordinary diversity of Leucocytozoon species (Haemosporida, Leucocytozoidae). Here, of 890 birds sampled, 10 (1.2%) were infected with Leucocytozoon parasites. Among them, two new species were discovered and described. Leucocytozoon grallariae sp. nov. and Leucocytozoon neotropicalis sp. nov. were found in non-migratory highland passeriforms belonging to the Grallaridae and Cotingidae, respectively. They both possess gametocytes in fusiform host cells. However, due to combining microscopic examination and molecular detection, it was revealed that these parasites were present in co-infections with other Leucocytozoon species, which gametocytes develop in roundish host cells, therefore exhibiting two highly distant parasite lineages isolated from the same samples. Remarkably, the lineages obtained by cloning the mtDNA genomes were not captured by the classic nested PCR, which amplifies a short fragment of cytochrome b gene. Phylogenetic analyses revealed that the lineages obtained by the classic nested PCR clustered with parasites possessing gametocytes in roundish host cells, while the lineages obtained by the mtDNA genome PCR protocol were closely related to Leucocytozoon parasites possessing gametocytes in fusiform host cells. These findings suggest problems with the sensitivity of the molecular protocols commonly used to detect Leucocytozoon species. A detailed analysis of the primers used in the classic nested PCR revealed a match with DNA sequences from those parasites that possess gametocytes in roundish host cells (i.e., Leucocytozoon fringillinarum), while they differ with the orthologous regions in the mtDNA genomes isolated from the samples containing the two new species. Since these are mixed infections, none of the lineages detected in this study can be assigned accurately to the new Leucocytozoon morphospecies that develops in fusiform host cells. However, phylogenetic analyses allowed us to hypothesize their most probable associations. This study highlights the need for developing detection methods to assess the diversity of Leucocytozoon parasites accurately.
Keywords: Andean mountains, Birds, Co-infection, New species, Cotingidae, Grallaridae, Passeriforms
Graphical abstract
Highlights
-
•
Molecular diversity of Leucocytozoon is underestimated.
-
•
We described two new Leucocytozoon species infecting passerines endemic of Neotropics.
-
•
Commonly PCR protocols failed to detect Leucocytozoon lineages from Neotropical region.
1. Introduction
The Andes in South America are recognized as hotspots for avian endemism. This region includes approximately 133 different ecosystems (Morrone, 2001; Josse et al., 2009) with more than 2000 avian species reported, including nearly 600 endemic species (Myers et al., 2000; Herzog and Kattan, 2011). Such host species richness seems to drive a high diversity of avian haemosporidian parasites that is just starting to be characterized using microscopy and Polymerase Chain Reaction (PCR)-based detection methods (i.e. Merino et al., 2008; Rodríguez et al., 2009; Jones et al., 2013; Mantilla et al., 2013, 2016; Matta et al., 2014; Galen and Witt, 2014; Harrigan et al., 2014; González et al., 2014, 2015; Marzal et al., 2015; Lotta et al., 2016; Moens et al., 2016; Moens and Pérez-Tris, 2016; Cadena-Ortiz et al., 2018; de Aguilar et al., 2018; Gil-Vargas and Sedano-Cruz, 2019).
Molecular techniques present significant advantages for parasite detection, particularly in samples with very low parasitemia (sub-microscopic infections). Many molecular protocols targeting to different molecular markers such as the rRNA (Richard et al., 2002), nuclear sequences (Bensch et al., 2004), the apicoplast (Caseinolytic protease C-Clpc) (Martinsen et al., 2008), and mitochondrial genes (Cytochrome b -cytb, Cytochrome oxidase subunit I and III – cox1, cox3) (Escalante et al., 1998; Bensch et al., 2000; Perkins and Schall, 2002; Hellgren et al., 2004; Pacheco et al., 2018b) have been used with diagnosis purpose. Currently, most of the avian haemosporidian inventories rely mainly on molecular detection. Unfortunately, most of the sequences of haemosporidian, including Leucocytozoon, reported in the Neotropics remain without being associated with a morphospecies because few studies applied both microscopic and molecular diagnosis in parallel. That is an obstacle for a reliable estimate of parasite species diversity.
PCR-based methods commonly overlook the presence of co-infections (Bernotienė et al., 2016; Pacheco et al., 2018a). The two main reasons are 1) the primers may have a higher affinity for one of the parasites in the sample, and 2) there may be an uneven amount of the template for each of the species or lineages in the co-infected samples (Perez-Tris and Bensch, 2005; Bernotienė et al., 2016; Pacheco et al., 2018a). Nevertheless, there are many reported cases where the amplification fails to detect haemosporidian parasite DNA in samples with an evident high intensity of parasitemia (Zehtindjiev et al., 2012; Schaer et al., 2015; Bernotienė et al., 2016), indicating that other variables are also involved in the PCR performance in co-infections (Pacheco et al., 2018a). Indeed, co-infections of avian haemoporasites are common in natural populations (Perez-Tris and Bensch, 2005; Van Rooyen et al., 2013; Bernotienė et al., 2016; Pacheco et al., 2018a), making this problem far more complicated (Bernotienė et al., 2016; Ciloglu et al., 2018; Pacheco et al., 2018a). Although recent publications have provided alternatives to overcome the issue of mixed infections (Perez-Tris and Bensch, 2005; Bernotienė et al., 2016; Pacheco et al., 2018a), the molecular detection of lineages belonging to the same genus in the same sample and their linkage to certain morphospecies remains challenging. For example, in the case of birds sampled in Colombian mountain ranges, co-infections of Leucocytozoon species with parasites of other genera have been detected in 25.4% of samples. Furthermore, in 18.2% of the co-infections two or more species of Leucocytozoon were observed in the same blood film (Lotta et al., 2016).
In this study, new leucocytozoid species were found in two non-migratory species of passerines (Undulated Antpitta, Grallaria squamigera and Green and black Fruiteater, Pipreola riefferii) from highland ecosystems of Colombia. These parasites were described using both microscopic and molecular diagnosis. Importantly, both bird species were co-infected with different lineages of Leucocytozoon species. Using morphological and phylogenetic analyses, a possible linkage was proposed between the new morphospecies under description and other lineages amplified from co-infections. Furthermore, we also discussed current problems in molecular diagnosis of Leucocytozoon parasites.
2. Materials and methods
2.1. Study sites
During this study, 840 birds belonging to 139 species were caught using mist nets in the central and eastern Andean mountain ranges of Colombia (Table 1). In the central mountain chain (Cordillera Central), 686 birds belonging to 118 species were captured during April, July, August, and December of 2015 and January of 2016 (Table 1). Sample sites included three distinct life zones (Cuatrecasas, 1958): (i) Subandean Forest (SF) (1800–2600 m above sea level (masl)); represented by the Fauna and Flora Sanctuary Otún Quimbaya (FFS), El Cedral station and Ucumarí Natural Regional Park (NRP); (ii) the Andean Forest (AF) (2900–3500 masl) as found in the locality of El Bosque, and (iii) the Paramo ecosystem (P) (3900–4100 masl) as represented by the Otún Lake. In the eastern mountain range (Cordillera Oriental) 154 birds, belonging to 42 species were captured during January of 2015 and December 2016 (Table 1) at the Bosque Palacio de Chingaza (NNP), which is an Andean Forest (2900–3500 masl) life zone.
Table 1.
Birds captured in Los Nevados National Natural Park, and Palacio forest at Chingaza NNP in this study.
| Life zone | Altitudinal range | N° cap (N° infected) per chain of mountains |
||
|---|---|---|---|---|
| Eastern | Central | |||
| Anseriformes | ||||
| Anatidae | ||||
| Anas flavirostris | AF | 2900–3500 | 1(0) | |
| Apodiformes | ||||
| Trochilidae | ||||
| Adelomyia melanogenys | SAF | 1800–2600 | 5(0) | |
| Aglaiocercus kingii | SAF | 1800–2600 | 1(0) | |
| Amazilia franciae | SAF | 1800–2600 | 4(0) | |
| Boissonneaua flavescens | AF | 2900–3500 | 1(0) | |
| Boissonneaua flavescens | SAF | 1800–2600 | 2(0) | |
| Coeligena | SAF | 1800–2600 | 7(0) | |
| Coeligena helianthea | AF | 2900–3500 | 1(0) | |
| Coeligena torquata | SAF | 1800–2600 | 3(0) | |
| Coeligena torquata | AF | 2900–3500 | 1(0) | |
| Colibri coruscans | AF | 2900–3500 | 3(0) | |
| Colibri thalassinus | SAF | 1800–2600 | 3(0) | |
| Doryfera ludovicae | SAF | 1800–2600 | 2(0) | |
| Ensifera | AF | 2900–3500 | 1(0) | 3(0) |
| Eriocnemis cupreoventris | AF | 2900–3500 | 2(0) | |
| Eriocnemis derbyi | AF | 2900–3500 | 14(0) | |
| Eriocnemis derbyi | SAF | 1800–2600 | 1(0) | |
| Heliangelus amethysticollis | AF | 2900–3500 | 1(0) | |
| Heliangelus exortis | AF | 2900–3500 | 1(0) | 7(0) |
| Heliangelus exortis | SAF | 1800–2600 | 5(1) | |
| Lafresnaya lafresnayi | AF | 2900–3500 | 6(0) | 4(0) |
| Metallura tyrianthina | AF | 2900–3500 | 3(0) | |
| Metallura tyrianthina | P | 3900–4100 | 2(0) | |
| Phaethornis guy | SAF | 1800–2600 | 4(0) | |
| Phaethornis syrmatophorus | SAF | 1800–2600 | 3(0) | |
| Schistes geoffroyi | SAF | 1800–2600 | 1(0) | |
| Columbiformes | ||||
| Columbidae | ||||
| Zenaida auriculata | SAF | 1800–2600 | 2(0) | |
| Charadriiformes | ||||
| Charadiidae | ||||
| Vanellus chilensis | SAF | 1800–2600 | 2(0) | |
| Falconiformes | ||||
| Accipitridae | ||||
| Rupornis magnirostris | SAF | 1800–2600 | 1(0) | |
| Passeriformes | ||||
| Cinclidae | ||||
| Cinclus leucocephalus | AF | 2900–3500 | 3(0) | |
| Corvidae | ||||
| Cyanolyca viridicyanus | SAF | 1800–2600 | 2(0) | |
| Cyanocorax yncas | SAF | 1800–2600 | 3(0) | |
| Cotingidae | ||||
| Pipreola riefferii§ | AF | 2900–3500 | 1(1) | |
| Dendrocolaptidae | ||||
| Pseudocolaptes boissonneautii | AF | 2900–3500 | 1(0) | |
| Emberizidae | ||||
| Arremon brunneinucha | SAF | 1800–2600 | 4(0) | |
| Arremon brunneinucha | AF | 2900–3500 | 2(1) | |
| Arremon torquatus | AF | 2900–3500 | 4(0) | |
| Atlapetes albinucha | SAF | 1800–2600 | 6(0) | |
| Atlapetes albinucha | AF | 2900–3500 | 2(0) | |
| Atlapetes pallidinucha | AF | 2900–3500 | 7(1) | |
| Atlapetes schistaceus | AF | 2900–3500 | 6(1) | |
| Zonotrichia capensis | SAF | 1800–2600 | 93(0) | |
| Zonotrichia capensis | P | 3900–4100 | 6(0) | |
| Zonotrichia capensis | AF | 2900–3500 | 17(0) | 30(0) |
| Fringillidae | ||||
| Astragalinus psaltria | AF | 2900–3500 | 2(0) | |
| Astragalinus psaltria | SAF | 1800–2600 | 4(0) | |
| Euphonia laniirostris | SAF | 1800–2600 | 3(0) | |
| Euphonia xanthogaster | SAF | 1800–2600 | 4(0) | |
| Saltator atripennis | SAF | 1800–2600 | 1(0) | |
| Furnariidae | ||||
| Cinclodes excelsior | P | 3900–4100 | 6(0) | |
| Dendrocincla tyrannina | AF | 2900–3500 | 1(0) | |
| Dendrocincla tyrannina | SAF | 1800–2600 | 2(0) | |
| Hellmayrea gularis | AF | 2900–3500 | 1(0) | |
| Leptasthenura andicola | P | 3900–4100 | 6(0) | |
| Lepidocolaptes lacrymiger | SAF | 1800–2600 | 1(0) | |
| Margarornis squamiger | AF | 2900–3500 | 12(0) | |
| Premnoplex brunnescens | AF | 2900–3500 | 1(0) | |
| Premnoplex brunnescens | SAF | 1800–2600 | 2(0) | |
| Synallaxis azarae | SAF | 1800–2600 | 10(0) | |
| Syndactyla subalaris | SAF | 1800–2600 | 1(0) | |
| Xiphocolaptes promeropirhynchus | SAF | 1800–2600 | 3(0) | |
| Grallariidae | ||||
| Grallaria squamigera* | AF | 2900–3500 | 1(1) | |
| Hirundinidae | ||||
| Orochelidon murina | AF | 2900–3500 | 2(0) | 7(0) |
| Orochelidon murina | P | 3900–4100 | 5(0) | |
| Pygochelidon cyanoleuca | SAF | 1800–2600 | 14(0) | |
| Pyroderus scutatus | SAF | 1800–2600 | 4(0) | |
| Stelgidopteryx ruficollis | SAF | 1800–2600 | 2(0) | |
| Icteridae | ||||
| Molothrus bonariensis | SAF | 1800–2600 | 2(0) | |
| Momotidae | ||||
| Momotus momota | SAF | 1800–2600 | 1(0) | |
| Parulidae | ||||
| Basileuterus tristriatus | SAF | 1800–2600 | 2(0) | |
| Cardellina canadiensis | SAF | 1800–2600 | 8(1) | |
| Myioborus sp. | AF | 2900–3500 | 1(0) | |
| Myioborus miniatus | SAF | 1800–2600 | 6(0) | |
| Myioborus ornatus | AF | 2900–3500 | 4(0) | 6(0) |
| Myiothlypis coronata | AF | 2900–3500 | 1(0) | |
| Myiothlypis coronata | SAF | 1800–2600 | 5(0) | |
| Myiothlypis nigrocristatus | AF | 2900–3500 | 13(0) | 1(0) |
| Setophaga fusca | SAF | 1800–2600 | 1(0) | |
| Rhinocryptidae | ||||
| Scytalopus infasciatus | AF | 2900–3500 | 1(0) | |
| Scytalopus micropterus | AF | 2900–3500 | 2(0) | |
| Thraupidae | ||||
| Anisognathus igniventris | AF | 2900–3500 | 3(0) | |
| Anisognathus lacrymosus | AF | 2900–3500 | 5(0) | |
| Buthraupis montana | AF | 2900–3500 | 1(1) | |
| Catamblyrhynchus diadema | AF | 2900–3500 | 3(0) | |
| Catamenia homochroa | P | 3900–4100 | 12(0) | |
| Catamenia inornata | P | 3900–4100 | 18(0) | |
| Conirostrum rufum | AF | 2900–3500 | 1(0) | |
| Diglossa albilatera | AF | 2900–3500 | 2(0) | 3(0) |
| Diglossa albilatera | SAF | 1800–2600 | 1(0) | |
| Diglossa cyanea | AF | 2900–3500 | 7(0) | 18(0) |
| Diglossa cyanea | SAF | 1800–2600 | 3(0) | |
| Diglossa humeralis | P | 3900–4100 | 9(0) | |
| Diglossa humeralis | AF | 2900–3500 | 11(0) | |
| Diglossa sittoides | SAF | 1800–2600 | 6(0) | |
| Euphonia laniirostris | SAF | 1800–2600 | 3(0) | |
| Euphonia xanthogaster | SAF | 1800–2600 | 4(0) | |
| Hemispingus atropileus | AF | 2900–3500 | 4(0) | 2(0) |
| Hemispingus frontalis | AF | 2900–3500 | 1(0) | |
| Hemispingus sp. | AF | 2900–3500 | 1(0) | |
| Hemispingus superciliaris | SAF | 1800–2600 | 1(1) | |
| Hemispingus superciliaris | AF | 2900–3500 | 3(0) | 1(0) |
| Hemispingus verticalis | AF | 2900–3500 | 1(0) | |
| Phrygilus unicolor | P | 3900–4100 | 64(0) | |
| Pipraeidea melanonota | SAF | 1800–2600 | 5(0) | |
| Saltator atripennis | SAF | 1800–2600 | 3(0) | |
| Saltator maximus | SAF | 1800–2600 | 1(0) | |
| Sericossypha albocristata | SAF | 1800–2600 | 5(0) | |
| Sporophila nigricollis | SAF | 1800–2600 | 14(0) | |
| Tangara arthus | SAF | 1800–2600 | 1(0) | |
| Tangara cyanicollis | SAF | 1800–2600 | 1(0) | |
| Tangara gyrola | SAF | 1800–2600 | 2(0) | |
| Tangara heinei | SAF | 1800–2600 | 1(0) | |
| Tangara labradorides | SAF | 1800–2600 | 1(0) | |
| Tangara nigroviridis | SAF | 1800–2600 | 2(0) | |
| Tangara vassorii | AF | 2900–3500 | 6(1) | |
| Tangara vitriolina | SAF | 1800–2600 | 6(0) | |
| Thraupis episcopus | SAF | 1800–2600 | 4(0) | |
| Thraupis palmarum | SAF | 1800–2600 | 2(0) | |
| Tiaris olivaceus | SAF | 1800–2600 | 2(0) | |
| Troglodytidae | ||||
| Henicorhina leucophrys | SAF | 1800–2600 | 7(0) | |
| Henicorhina leucophrys | AF | 2900–3500 | 3(0) | |
| Pheugopedius genibarbis | SAF | 1800–2600 | 3(0) | |
| Pheugopedius mystacalis | SAF | 1800–2600 | 3(0) | |
| Troglodytes aedon | P | 3900–4100 | 2(0) | |
| Troglodytes aedon | AF | 2900–3500 | 2(0) | |
| Troglodytes aedon | SAF | 1800–2600 | 8(0) | |
| Troglodytes solstitialis | SAF | 1800–2600 | 1(0) | |
| Turdidae | ||||
| Catharus aurantiirostris | SAF | 1800–2600 | 1(0) | |
| Catharus ustulatus | SAF | 1800–2600 | 1(0) | |
| Myadestes ralloides | SAF | 1800–2600 | 1(0) | |
| Turdus fuscater | P | 3900–4100 | 2(0) | 2(0) |
| Turdus fuscater | AF | 2900–3500 | 5(0) | |
| Turdus fuscater | SAF | 1800–2600 | 1(0) | |
| Turdus ignobilis | SAF | 1800–2600 | 14(0) | |
| Tyrannidae | ||||
| Contopus fumigatus | SAF | 1800–2600 | 1(0) | |
| Elaenia albiceps | SAF | 1800–2600 | 1(0) | |
| Elaenia frantzii | SAF | 1800–2600 | 1(0) | |
| Empidonax alnorum | SAF | 1800–2600 | 1(0) | |
| Empidonax sp. | SAF | 1800–2600 | 1(0) | |
| Mecocerculus leucophrys | AF | 2900–3500 | 3(0) | 7(0) |
| Mecocerculus leucophrys | SAF | 1800–2600 | 1(0) | |
| Mecocerculus stictopterus | AF | 2900–3500 | 12(0) | |
| Mionectes olivaceus | SAF | 1800–2600 | 1(0) | |
| Mionectes striaticolis | SAF | 1800–2600 | 11(0) | |
| Mionectes srtiaticolis | AF | 2900–3500 | 2(0) | |
| Myiarchus tuberculifer | SAF | 1800–2600 | 1(0) | |
| Myiophobus sp. | SAF | 1800–2600 | 1(0) | |
| Myiotheretes striaticollis | AF | 2900–3500 | 1(0) | |
| Nephelomyias pulcher | SAF | 1800–2600 | 1(0) | |
| Ochthoeca cinnamomeiventris | AF | 2900–3500 | 3(0) | 3(0) |
| Ochthoeca diadema | SAF | 1800–2600 | 2(0) | |
| Ochthoeca diadema | AF | 2900–3500 | 4(0) | |
| Ochthoeca fumicolor | P | 3900–4100 | 5(0) | |
| Ochthoeca rufipectoralis | AF | 2900–3500 | 1(0) | |
| Phyllomyias nigrocapillus | SAF | 1800–2600 | 1(0) | |
| Phyllomyias nigrocapillus | AF | 2900–3500 | 1(0) | |
| Phylloscartes poecilotis | SAF | 1800–2600 | 2(0) | |
| Pyrrhomyias cinnamomeus | SAF | 1800–2600 | 4(0) | |
| Sayornis nigricans | SAF | 1800–2600 | 9(0) | |
| Serpophaga cinerea | SAF | 1800–2600 | 4(0) | |
| Sicalis flaveola | SAF | 1800–2600 | 3(0) | |
| Tyrannus melancholicus | SAF | 1800–2600 | 2(0) | |
| Uromyias agilis | AF | 2900–3500 | 6(0) | |
| Uromyias agilis | SAF | 1800–2600 | 1(0) | |
| Zimmerius viridiflavus | SAF | 1800–2600 | 12(0) | |
| Vireonidae | ||||
| Cyclarhis nigrirostris | SAF | 1800–2600 | 1(0) | |
| Piciformes | ||||
| Ramphastidae | ||||
| Aulacorhynchus haematopygus | SAF | 1800–2600 | 1(0) | |
| Picidae | ||||
| Picumnus olivaceus | SAF | 1800–2600 | 1(0) | |
| Trogoniformes | ||||
| Trogonidae | ||||
| Trogon collaris | SAF | 1800–2600 | 1(0) | |
| Trogon personatus | AF | 2900–3500 | 1(0) | |
| Total per locality | 154(4) | 686(6) | ||
| Leucocytozoon sp. prevalence | 2.6% | 0.9% | ||
| Leucocytozoon sp. nov. prevalence | 0.6% | 0.14% | ||
| Leucocytozoon sp. overall prevalence | 1.19% | |||
| Total | 840(10) | |||
The number of birds capture per species followed of the occurrence of Leucocytozoon sp. (in parenthesis), is given. §Hosts infected with L. neotropicalis sp. nov, * hosts infected with L. grallariae sp. nov. Life zone as follow Sub-Andean Forest (SAF); Andenan Forest (AF); Paramo (P); and altitudinal range data is also provided.
2.2. Sampling and blood film examination
Birds were identified according to the taxonomic lists of the South American Classification Committee (SACC) (Remsen et al., 2012). Blood samples were collected by brachial or tarsal vein puncture or toenail clipping (last method for the tiny hummingbirds). For each bird, three thin smears were made, and 50 μl of blood were stored in an EDTA-anticoagulant solution or SET buffer (0.05 M Tris, 0.15 M NaCl, 0.5 M EDTA, pH 8.0). Blood films were air dried immediately in the field, and then fixed with absolute methanol and stained with 30% Giemsa solution in the laboratory according to Valkiūnas (2005). The smears were double-blind scanned by microscopic examination using an Olympus BX43 microscope, and digital images were captured with an Olympus DP27 digital camera, processed with cellSens software standard 1.13 (Olympus, Tokyo, Japan). For the morphological characterization of parasites, more than 100 images were taken, and the best images for morphometrical measurements were selected using ImageJ (Schneider et al., 2012), following the recommendations of Valkiūnas et al. (2010). The intensity of parasitemia was determined by an actual counting of the number of infected cells per 10,000 erythrocytes (Muñoz et al., 1999). A Student's t-test implemented in XLSTAT (Addinsoft, 2017) was used to determine statistical significance between the mean values of parasite morphometric measurements. A P-value of 0.05 or less was considered significant.
2.3. DNA extraction, PCR amplification and sequencing of cytochrome b gene and DNA mitochondrial genome
DNA extractions were carried out using a standard phenol–chloroform protocol (Sambrook et al., 1989). Cytochrome b gene (cytb) amplifications were done by using a nested PCR protocol (Hellgren et al., 2004). Purifications of PCR products were performed with ethanol and ammonium acetate protocol according to Bensch et al. (2000) and then visualized on a 1.5% agarose gel. All purified PCR products were subsequently sequenced in both senses using a 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Five independent amplifications were carried out for all available samples infected with the new Leucocytozoon species and made careful visual inspection of the electropherograms to confirm that the lineage sequences obtained were not chimeric products as a result of mixed infections. The cytb sequences obtained in this study were submitted to GenBank under accession numbers MH909275 (L_GRSQU_02) and MH909276 (L_PIRIE_02).
In addition to the cytb gene, two Leucocytozoon mitochondrial genomes (mtDNA) from one Undulated Antpitta (Grallaria squamigera, Grallaridae) and one Green-and-black Fruiteater (Pipreola riefferii, Cotingidae) were amplified, cloned and sequenced. PCR products were amplified with TaKaRa LA TaqTM Polymerase (TaKaRa Mirus Bio Inc., Shiga, Japan) as described by (Pacheco et al., 2011, 2018b) using primers forward 5′ GA GGA TTC TCT CCA CAC TTC AAT TCG TAC TTC and reverse 5′ CAG GAA AAT WAT AGA CCG AAC CTT GGA CTC. Then, a nested PCR was performed using the internal oligos forward 5′ TTTCATCCTTAAATCTCGTAAC 3'/reverse 5′ GACCGAACCTTGGACTCTT 3'. PCR amplifications for both PCR (outer and inner) were carried out in a 50 μl volume using 20 ng of total genomic DNA. The PCR conditions were as follow: a partial denaturation at 94 °C for 1 min and 30 cycles of 30 s at 94 °C and 7 min at 68 °C, followed by a final extension of 10 min at 72 °C. Following the manufacturer's directions, six independent PCR products (bands of approximately 6 kb) were excised from the gel, purified using QIAquick® Gel extraction kit (Qiagen, GmbH, Hilden, Germany), and four of them were cloned in the pGEM®-T Easy Vector systems (Promega, Madison, WI, USA) and two were directly sequenced. For at least three clones from each independent PCR and two PCR products, we sequenced both strands using an Applied Biosystems 3730 capillary sequencer. There were no inconsistencies among the clones and between the direct sequencing of the PCR products and clones. We submitted the mtDNA genome sequences to GenBank under accession numbers MK103894 and MK103895. In addition, following the above-mentioned methodologies, new DNA extractions and amplifications of the parasite cytb gene were performed using samples infected with Leucocytozoon pterotenuis (Blood film: GERPH07966- Blood sample: UNAL:GERPH:PA262), and a Leucocytozoon sp. (Blood film: GERPH-07737- Blood sample:UNAL:GERPH:AN18). These two parasites were previously detected and described by us in other species of the Grallariidae (Grallaria ruficapilla and Grallaria quitensis respectively; as Leucocytozoon pteroteunis (Lotta et al., 2015).
2.3.1. Phylogenetic analysis
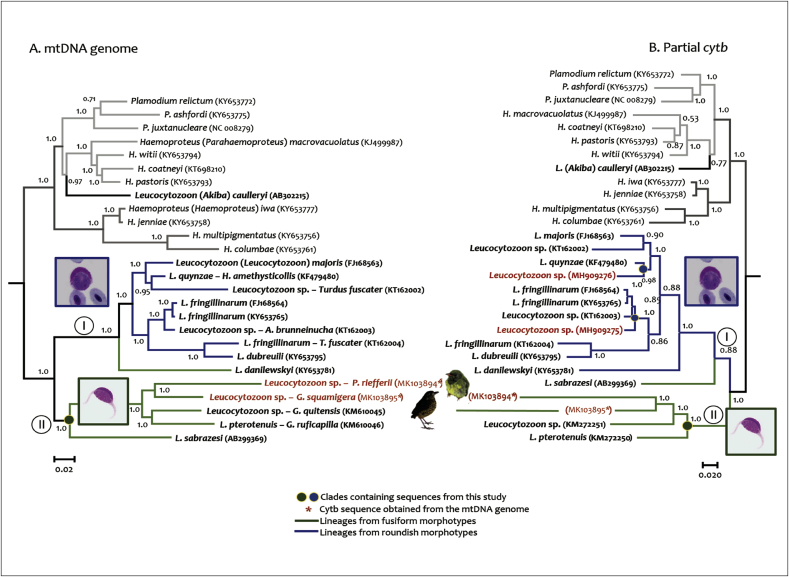
First, phylogenetic relationships of the new Leucocytozoon species were estimated from an alignment using partial cytb gene sequences (476 base pairs (bp)). This alignment, constructed in MEGA 7 (Kumar et al., 2016) and aligned with Clustal Omega tool (McWilliam et al., 2013), included 88 lineages of parasites from passerine and non-passerine of South American birds and lineages belonging to unidentified morphospecies that had been deposited in the GenBank (Benson et al., 2015) and MalAvi database (Bensch et al., 2009), as well as the new sequences reported in this study (Supplementary Table S1). A second alignment was done with only 28 cytb partial sequences (476 bp) including the partial cytb sequences available and the cytb from the mtDNA genomes obtained in this study. Finally, an alignment was done using 26 mtDNA genomes (5487 bp excluding gaps) in order to show the phylogenetic relationship between the new parasites that we found, and the ones reported previously. It is important to note that for both birds infected with the new Leucocytozoon sp., no inconsistencies between the mtDNA genomes clones and mtDNA genomes obtained by direct sequencing of PCR products were found.
In all the cases we performed phylogenetic reconstructions using Bayesian methods implemented on MrBayes v3.1.2 (Ronquist and Huelsenbeck, 2003), through the CIPRES portal (Miller et al., 2010). For partial cytb sequences and mtDNA genomes, phylogenetic relationships were estimated under the General Time-Reversible model (GTR+Γ+I), which was the best model that fit these data, according to the corrected Akaike information criterion implemented on jModelTest 2.1.1 (Darriba et al., 2012). Two independent Markov Chain Monte Carlo (MCMC) simulations were conducted simultaneously for 5 × 106 generations, sampled every 100 generations. After discarding 25% of the trees as a burn-in period, a majority rule consensus phylogeny was obtained from 75,000 trees. Then, the phylogeny was visualized and edited using FigTree v1.3.1 (Rambaut, 2006). We estimated genetic distances between lineages using a Kimura two-parameter model of substitution, implemented in MEGA 7.0 (Kumar et al., 2016).
2.4. Analysis of primers
Affinity of the primers proposed by Hellgren et al. (2004) with the cytb gene sequences obtained from Leucocytozoon parasites was evaluated by aligning the oligonucleotides with lineages of parasite species with gametocytes developing in roundish host cells, like Leucocytozoon fringillinarum and L. dubreuili (Perkins, 2008; Pacheco et al., 2018b) and lineages obtained from those parasites, which gametocytes develop in fusiform host cells, such as L. pterotenuis (Lotta et al., 2015) and the two species described in this study.
2.5. Ethical statement
Samples were collected using a non-invasive methodology, approved by the “Comité de Bioetica of Departamento de Ciencias para la Salud Animal,” Facultad de Medicina Veterinaria y de Zootecnia, Universidad Nacional de Colombia (Permit number CBE-FMVZ-016). Bird capture and manipulation were done in a way that reduced stress caused by these activities. Once the blood samples were taken, the birds were released. Fieldwork were conducted under authorization of “Unidad Administrativa Especial del Sistema de Parques Nacionales Naturales de Colombia UAESPNN - Subdirección técnica” and “Autoridad Nacional de Licencias Ambientales, ANLA” (file 4120E183893 of 2011, resolution 0787 of 2013, and resolution 255 of 2014).
3. Results
3.1. Prevalence of infection and description of parasites
Leucocytozoon infections were detected in 10 birds of the 840 sampled (1.2%) by microscopic examination of blood smears. Molecular characterization was made only on the positive samples by microscopic examinations. Whilst gametocytes of Leucocytozoon quynzae, L fringillinarum and Leucocytozoon sp. were observed in eight individuals belonging to Emberizidae, Parulidae, Thraupidae and Trochilidae (Table 1), two new morphologically readily distinct species of Leucocytozoon were found in widely distributed South American native non-migrating passerine bird species (McMullan et al., 2011; BirdLife International, 2017a, 2017b): one Undulated Antpitta (Grallaria squamigera) and one Green and black Fruiteater (Pipreola riefferii) (Table 1).
Undulated Antpitta (Grallaridae) is distributed in the Andean mountain ranges above 2000 masl in Neotropical humid forests and scrublands of Bolivia, Peru, Ecuador, Colombia and Venezuela (BirdLife International, 2017a). In general, Antpitta birds are elusive, rarely seen and demanding in their capture and sampling. Green and black Fruit-eaters (Cotingidae) are arboreal birds that inhabit tropical moist montane forests above 1000 to 3300 masl from Peru to Venezuela (BirdLife International, 2017b).
3.2. Description of parasites
3.2.1. Leucocytozoon (Leucocytozoon) grallariae sp. nov
Young gametocytes (Fig. 1) markedly influence the shape of host cells from earliest stages of their development. Growing parasites were of oval or ellipsoid shapes; they closely adhered to the host cell nuclei, which were markedly enlarged, deformed and assumed crescent shapes (Fig. 1A–C). The host cell cytoplasm was present around growing gametocytes, and it was very evident. Advanced young gametocytes often possessed invaginations on their sides, which were opposite to the host cell nuclei, and that gave the growing gametocytes the shapes of giant beans with approximately equally rounded ends (Fig. 1 B). Host cells assumed ellipsoid shapes from early stages of gametocyte development (Fig. 1 A).
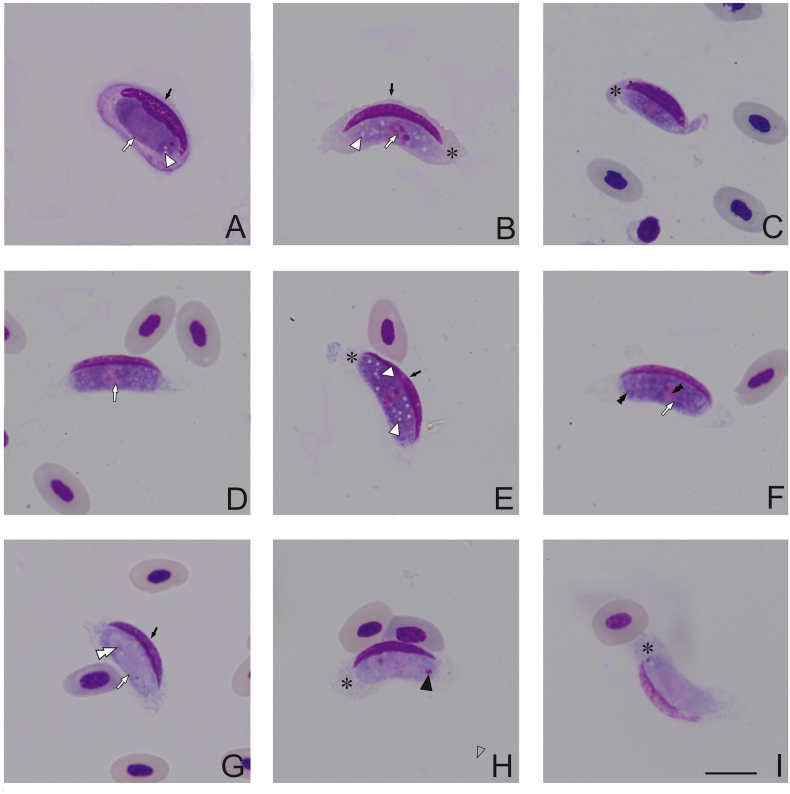
Fig. 1.
Leucocytozoon grallariae sp. nov. from Undulated Antpitta (Grallaria squamigera) captured at Palacio forest in the Chingaza National Natural Park (NNP), Colombia. Immature gametocytes (A–C), macrogametocytes (D–F) and microgametocytes (G–I) in fusiform host cells. Nucleus of host cells (black arrows ) possessing mature gametocytes assumes a slender waning moon shape (D–I). Parasite nuclei are indicated by white arrows (
) possessing mature gametocytes assumes a slender waning moon shape (D–I). Parasite nuclei are indicated by white arrows ( ) and parasite nucleolus – by double white arrow tips (
) and parasite nucleolus – by double white arrow tips ( ). Host cell cytoplasm is distorted by developing parasites forming a thin rim that develops into the cytoplasmic processes (asterisk *). In mature gametocytes, vacuoles are indicated by white arrow tips (
). Host cell cytoplasm is distorted by developing parasites forming a thin rim that develops into the cytoplasmic processes (asterisk *). In mature gametocytes, vacuoles are indicated by white arrow tips ( ). Volutin granules are indicated by double black arrow tips (
). Volutin granules are indicated by double black arrow tips ( ) and azurophilic granule by black arrow tips (
) and azurophilic granule by black arrow tips ( ). Giemsa-stained thin blood films. Scale bar = 10 μm.
). Giemsa-stained thin blood films. Scale bar = 10 μm.
Macrogametocytes developed in fusiform host cells (Fig. 1). In Leucocytozoon species, as the gametocytes develop, they cause considerable distortion of the host cells, producing two distinct host cell-parasite complex forms: roundish and fusiform. In this Leucocytozoon species, the gametocytes only in fusiform host cells were observed. Gametocytes induced marked hypertrophy and deformation of the host cells and displacement of their nuclei, which lay on the periphery of gametocytes. The host cell nuclei acquired slender waning moon shapes; usually extending up to ½ of the circumference of gametocytes, and they could reach the fusiform processers, but they never extended into the processes (Fig. 1C–E). In both types of host cells, the nuclei looked homogenous.
The host-cell cytoplasm forms two short cytoplasmic processes located close to ends of gametocytes. Fusiform processes were variable in form (Fig.1 D, F–I) nevertheless, those processes, which length was similar to their largest width were common (Table 2), and that was a characteristic feature of the development of this species. It is worth mentioning that the cytoplasmic processes were occasionally observed as being unequally long at both sides of the same parasite (Fig. 1H and I), probably as a consequence of the host cell deformation during blood film preparation. Some remnants of host cell cytoplasm were seen covering around half of the perimeter of the gametocyte-host cell complex as a thin rim (Fig. 1G–I), which is uncommon in avian leucocytozoids. Additionally, host cells with fully grown gametocytes could possess irregular shapes and blunt processes, while they were often pointed in host cells with immature growing gametocytes (Fig. 1G–I).
Table 2.
Morphometric parameters of gametocytes and host cells of Leucocytozoon grallariae sp. nov.
| Feature |
Leucocytozoon grallariae sp. nov |
|
|---|---|---|
| Macrogametocyte n = 15 | Microgametocyte n = 10 | |
| Parasite | ||
| Length | 14.7–23.6 (20.2 ± 2.5) | 15.8–21.3 (18.5 ± 2.1) |
| Width | 4,5-5,5 (5,4 ± 0,5) | 4.1–6.1 (5.1 ± 0.6) |
| Area | 79.7–109.0 (97.4 ± 9.6) | 71.3–93.4 (81.7 ± 6.0) |
| Perimeter | 39.9–54.4 (47.1 ± 4.3) | 39.6–49.3 (43.5 ± 3.4) |
| Parasite nucleus | ||
| Length | 2.7–4.9 (3.6 ± 0.6) | 7.2–12.9 (10.0 ± 1.9) |
| Width | 3.1–5.4 (4.4 ± 0.6) | 3.0–4.5 (3,7 ± 0.4) |
| Area | 9.9–18.0 (13.1 ± 2.2) | 23.4–49.4 (32.8 ± 9.0) |
| Host-cell parasite complex | ||
| Length | 24.8–32.1 (29.6 ± 2.5) | 24.7–33.9 (29.4 ± 2.7) |
| Width | 7.3–10.3 (8.5 ± 1.0) | 7.0–10.3 (8.4 ± 0.9) |
| Area | 162.6–196.4 (185.1 ± 12.8) | 152.2–188.1 (168.8 ± 11.0) |
| Host-cell nucleus | ||
| Length | 15.8–22.9 (21.1 ± 1.8) | 17.5–21.6 (18.9 ± 1.2) |
| Width | 1.5–3.6 (2.2 ± 0.5) | 2.0–2.6 (2.3 ± 0.2) |
| Area | 27.0–49.1 (32.6 ± 5.5) | 25.7–34.8 (31.3 ± 2.3) |
| Perimeter of parasite covered | 15.3–22.1 (21.1 ± 1.8) | 17.2–20.5 (18.2 ± 1.1) |
| Cytoplasmic processesa | ||
| Length | 3.3–7.6 (5.0 ± 1.0) | 3.8–7.8 (5.5 ± 1.3) |
| Width | 3.7–8.0 (5.7 ± 1.0) | 4.4–8.0 (5.8 ± 1.1) |
| Area | 15.4–35.3 (24.1 ± 3.7) | 20.0–35.3 (26.4 ± 4.5) |
a Measurements are given in μm or μm2 (for area). Minimum and maximum values as well as mean ± SD are provided.
Only one of 2 cytoplasmic processes was measured for each parasite.
The gametocyte cytoplasm was granular in appearance; it often possessed small vacuoles and tiny volutin granules (Fig. 1E and F). Vacuoles were of different sizes, but not greater than 3.0 μm in their maximum diameter; they were observed in 97 gametocytes. Vacuoles were seen in growing (immature) gametocytes (Fig. 1 B). Parasite nuclei were compact; they were seen mainly in the central position (67% of reported cases) in gametocytes and were of roundish (Fig. 1D–F) or various oval (Fig. 1 E) shapes. Nucleoli were visible in 50% of the gametocytes.
The general configuration of the Microgametocytes and other features (Fig. 1G–I) were as for macrogametocytes with the usual haemosporidian sexual dimorphic characters that were the pale stained cytoplasm and large diffuse nuclei (Table 2). The proportion of microgametocytes and macrogametocytes in the type material was approximately 3:5. A prominent azurophilic granule was seen only in the cytoplasm of 90% of microgametocytes (Fig. 1H and I) located close to the parasite nuclei.
3.2.2. Taxonomic summary
Type host: Undulated Antpitta Grallaria squamigera (Grallaridae, Passeriformes).
Additional hosts: unknown.
Type locality: Palacio Forest at the transition zone of Chingaza NNP (4° 41′ N, 73° 50′ W, 2950 masl), Cundinamarca, Colombia.
Distribution: This parasite has been recorded only at the type locality.
Type specimens: Hapantotype (accession No. UNAL:GERPH:PA340 -XI, intensity of parasitemia 0.25%, collected by Nubia E. Matta, 18 January 2015) and deposited in the biological collection GERPH (Grupo de Estudio Relación Parásito Hospedero) at the Universidad Nacional de Colombia, Bogotá, Colombia. Parahapantotypes (accession Nos., UNAL:GERPH:PA340 – I to UNAL:GERPH:PA340 –X and UNAL:GERPH:PA340 –XII to UNAL:GERPH:PA340 -XIX) are deposited in the same collection. Digital images of blood stages of the parasite in the type preparations are available on request from GERPH.
DNA sequences: The partial mitochondrial DNA genome (5891 bp) that includes cox1, cox3 and cytb genes (GenBank accession number MK103895) was obtained from the type host Grallaria squamigera.
Site of infection: Blood cells, of which the origin is unclear due to marked deformation caused by gametocytes.
Prevalence: Only one individual of the host species was collected and found to be infected, so the sample size does not allow to estimate of prevalence. The parasite was morphologically detected in 1 of out of 684 examined birds (0.12%). This or similar parasites were not found in 840 sampled birds of different species at Palacio Forest and Los Nevados NNP (Table 1).
Etymology: The species name refers to the host genus name Grallaria, which is the type host of the parasite belongs.
3.2.3. Remarks
Seventeen species of Leucocytozoon, which gametocytes develop in fusiform host cells are reported to date (Supplentary Table S2), but only four of them, Leucocytozoon maccluri (Greiner, 1976), Leucocytozoon balmorali (Peirce, 1984), Leucocytozoon hamiltoni (Valkiūnas et al., 2002), and Leucocytozoon pterotenuis (Lotta et al., 2015) are described in birds of the.
Passeriformes. The new species described here, Leucocytozoon grallariae, found in a passerine bird, can be readily distinguished from the four Leucocytozoon species mentioned above due to two distinctive morphological characters. First, the fusiform processes are short (Table 2) in the host cells with fully grown gametocytes of L. grallariae (Fig. 1D–I). The length of these processes often does not exceed their largest width (Table 2, Fig. 1 G, H). This is not the case in L. maccluri, L. balmorali, L. hamiltoni and L. pterotenuis; their gametocytes develop fusiform host cells, which fusiform processes are greater in length than in largest width. Second, the host cell nuclei assume the slender waning moon shapes, and the nuclei could reach the cytoplasmic processes, but never extended into them. None of these two characters are features of L. maccluri, L. balmorali, L. hamiltoni, L. pterotenuis (see Valkiūnas, 2005; Lotta et al., 2015).
Gametocytes in many Leucocytozoon species described in non-passerine birds develop in fusiform host cells (Table S2). During L. grallariae infection, the host cell nuclei extend up to ½ of the circumference of the gametocytes, and the host cell nuclei might reach the beginning of cytoplasmic processes. This feature is not characteristic of other leucocytozoids where gametocytes developed fusiform host cells, and this feature can be used during identification of L. grallariae.
During microscopic examination of the type material, a co-infection with Leucocytozoon species with gametocytes developing in roundish host cells were detected (Fig. 3, Table S3). Gametocytes developing in fusiform host cells were ten times more often observed (parasitemia intensity is 0.25%) than gametocytes developing in roundish host cells (0.01%). In addition to the lineage associated with the GenBank No. MK103895 for the partial mtDNA genome reported above, one cytb lineage of 476 bp (L_GRSQU 02 GenBank No. MH909275) was amplified from the same blood sample of this type host. Genetic distance between both lineages was 0.23 (Table 5), which suggest that they belong to a different species. However, from these two lineages obtained in the same sample, we propose that lineage MK103895 obtained by cloning corresponds to the parasite, which gametocytes develop in fusiform host cells in the sample (see discussion below).
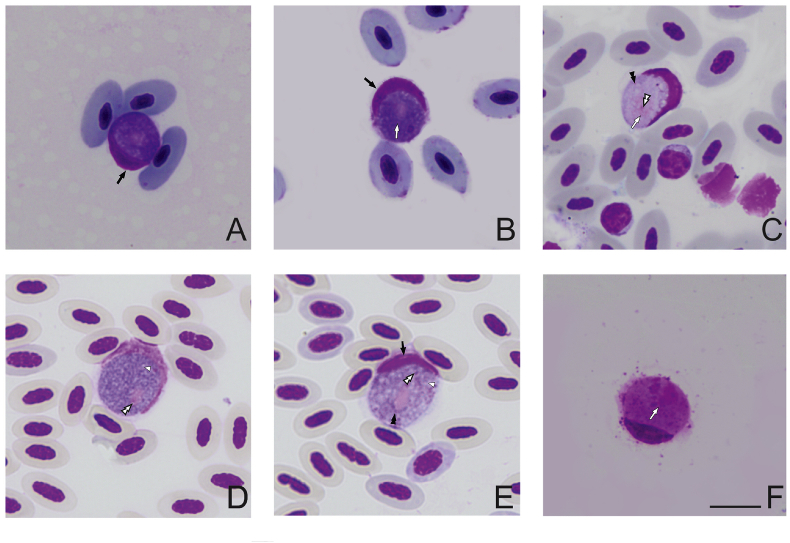
Fig. 3.
Gametocytes developing roundish host cells observed in type material of Leucocytozoon grallariae (A–C) and Leucocytozoon neotropicalis (D–F). Host cell nucleus (black arrows ) deformed as a cap resembles the parasites of the Leucocytozoon fringillinarum group. The white arrow indicates the parasite nuclei (
) deformed as a cap resembles the parasites of the Leucocytozoon fringillinarum group. The white arrow indicates the parasite nuclei ( ), double white arrow tips shows the nucleolus (
), double white arrow tips shows the nucleolus (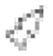 ). Vacuoles (white arrow tips
). Vacuoles (white arrow tips ) are present, while volutin granules (double black arrow tips
) are present, while volutin granules (double black arrow tips ) are few or absent in some gametocytes. Giemsa-stained thin blood films. Although gametocytes in roundish host cells were observed in both samples, those co-existing with Leucocytozoon grallariae (A–C), are significative smaller than Leucocytozoon sp. gametocytes found in type host of L. neotropicalis (D–F) (see Table S3). Scale bar = 10 μm.
) are few or absent in some gametocytes. Giemsa-stained thin blood films. Although gametocytes in roundish host cells were observed in both samples, those co-existing with Leucocytozoon grallariae (A–C), are significative smaller than Leucocytozoon sp. gametocytes found in type host of L. neotropicalis (D–F) (see Table S3). Scale bar = 10 μm.
Table 5.
Genetic distance between cytochrome b lineages of Leucocytozoon spp. shown in FigS1. Calculations were made using Kimura two-parameter model of substitutions.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AB299369 | |||||||||||||||||||||||||
| 2 | JQ988502PERU | 0,19 | ||||||||||||||||||||||||
| 3 | JQ988749PERU | 0,18 | 0,23 | |||||||||||||||||||||||
| 4 | JQ988751PERU | 0,18 | 0,03 | 0,22 | ||||||||||||||||||||||
| 5 | KF479480 | 0,17 | 0,09 | 0,19 | 0,08 | |||||||||||||||||||||
| 6 | KF874701PERU | 0,18 | 0,05 | 0,22 | 0,04 | 0,09 | ||||||||||||||||||||
| 7 | KF874740PERU | 0,18 | 0,05 | 0,23 | 0,03 | 0,11 | 0,02 | |||||||||||||||||||
| 8 | KF874751PERU | 0,18 | 0,10 | 0,21 | 0,09 | 0,07 | 0,09 | 0,10 | ||||||||||||||||||
| 9 | KF874796PERU | 0,18 | 0,04 | 0,22 | 0,04 | 0,10 | 0,02 | 0,03 | 0,09 | |||||||||||||||||
| 10 | KF874797PERU | 0,16 | 0,05 | 0,21 | 0,04 | 0,08 | 0,02 | 0,02 | 0,07 | 0,02 | ||||||||||||||||
| 11 | KF874801PERU | 0,17 | 0,07 | 0,22 | 0,06 | 0,09 | 0,07 | 0,06 | 0,10 | 0,06 | 0,06 | |||||||||||||||
| 12 | KF874814PERU | 0,17 | 0,05 | 0,21 | 0,04 | 0,08 | 0,03 | 0,03 | 0,07 | 0,03 | 0,01 | 0,06 | ||||||||||||||
| 13 | KM272250COL | 0,18 | 0,24 | 0,05 | 0,23 | 0,21 | 0,22 | 0,23 | 0,23 | 0,23 | 0,21 | 0,22 | 0,21 | |||||||||||||
| 14 | KM272251COL | 0,23 | 0,21 | 0,15 | 0,21 | 0,22 | 0,20 | 0,21 | 0,22 | 0,21 | 0,20 | 0,24 | 0,20 | 0,17 | ||||||||||||
| 15 | KT247892COL | 0,17 | 0,09 | 0,22 | 0,08 | 0,12 | 0,08 | 0,09 | 0,14 | 0,09 | 0,08 | 0,10 | 0,09 | 0,23 | 0,22 | |||||||||||
| 16 | KY646032COL | 0,18 | 0,10 | 0,19 | 0,10 | 0,05 | 0,10 | 0,11 | 0,08 | 0,10 | 0,08 | 0,10 | 0,08 | 0,20 | 0,24 | 0,12 | ||||||||||
| 17 | KY646033COL | 0,18 | 0,09 | 0,19 | 0,09 | 0,04 | 0,10 | 0,10 | 0,07 | 0,10 | 0,08 | 0,10 | 0,08 | 0,21 | 0,23 | 0,12 | 0,04 | |||||||||
| 18 | MG714923BRAZIL | 0,18 | 0,12 | 0,23 | 0,11 | 0,08 | 0,10 | 0,11 | 0,07 | 0,11 | 0,08 | 0,10 | 0,09 | 0,23 | 0,26 | 0,13 | 0,10 | 0,08 | ||||||||
| 19 | NC_012450 | 0,19 | 0,09 | 0,23 | 0,09 | 0,07 | 0,09 | 0,10 | 0,08 | 0,09 | 0,08 | 0,10 | 0,08 | 0,23 | 0,23 | 0,11 | 0,08 | 0,08 | 0,08 | |||||||
| 20 | NC_012451 | 0,18 | 0,04 | 0,23 | 0,03 | 0,09 | 0,04 | 0,03 | 0,09 | 0,03 | 0,03 | 0,06 | 0,04 | 0,23 | 0,21 | 0,08 | 0,10 | 0,09 | 0,11 | 0,09 | ||||||
| 21 | MK103895 | 0,21 | 0,24 | 0,15 | 0,24 | 0,22 | 0,26 | 0,27 | 0,24 | 0,25 | 0,24 | 0,26 | 0,24 | 0,17 | 0,18 | 0,23 | 0,22 | 0,22 | 0,25 | 0,24 | 0,24 | |||||
| 22 | MK103894 | 0,31 | 0,30 | 0,24 | 0,29 | 0,30 | 0,30 | 0,32 | 0,28 | 0,30 | 0,29 | 0,30 | 0,30 | 0,26 | 0,25 | 0,27 | 0,30 | 0,29 | 0,29 | 0,30 | 0,30 | 0,25 | ||||
| 23 | MH909276 | 0,19 | 0,04 | 0,22 | 0,02 | 0,10 | 0,02 | 0,01 | 0,10 | 0,02 | 0,03 | 0,06 | 0,03 | 0,23 | 0,21 | 0,08 | 0,11 | 0,09 | 0,11 | 0,09 | 0,03 | 0,25 | 0,30 | |||
| 24 | MH909275 | 0,18 | 0,10 | 0,19 | 0,09 | 0,06 | 0,10 | 0,10 | 0,07 | 0,10 | 0,09 | 0,10 | 0,09 | 0,22 | 0,24 | 0,14 | 0,06 | 0,05 | 0,08 | 0,08 | 0,10 | 0,23 | 0,28 | 0,10 | ||
| 25 | FJ168562 | 0,21 | 0,22 | 0,27 | 0,22 | 0,21 | 0,22 | 0,22 | 0,23 | 0,24 | 0,22 | 0,24 | 0,22 | 0,24 | 0,29 | 0,21 | 0,23 | 0,21 | 0,24 | 0,22 | 0,22 | 0,28 | 0,32 | 0,22 | 0,22 |
Lineages obtained from the type samples of L. gralariae sp. nov. and L. neotropicalis sp. nov. are.indicated in bold. Lineage of Haemoproteus columbae was used as outgroup, and it is indicated in italics.
3.2.4. Leucocytozoon (Leucocytozoon) neotropicalis sp. nov
Macrogametocytes (Fig. 2A–E) develop in fusiform host cells; the shape of gametocytes is oval-elongate (their length is greater than width, Table 3). However, the morphology of host-parasite complexes with fusiform processes is readily distinguishable from L. grallariae (compare Fig. 1 F, H and Fig. 2 A, F, I). The host cell nuclei are pushed aside, deformed like a homogeneous band of variable width that extends close to half of the circumference of gametocyte (Fig. 2 A, I), but never extends into the cytoplasmic processes. This is similar in both L. grallariae and L. neotropicalis cells, so these parasites cannot be distinguished by this character (see the description of L. grallariae and compare Fig. 2 D, F, H and Fig. 2 A, G, I.). These species can be readily distinguished due to the length of the cytoplasmic processes. Mainly, the latter is significantly longer (Student's t-test, α = 0.05 P < 0.0001) and narrower in their maximum width (P < 0.0001) than in L. grallariae (see Table 2, compare Fig. 2. F, H and Fig. 2 A, F, I).
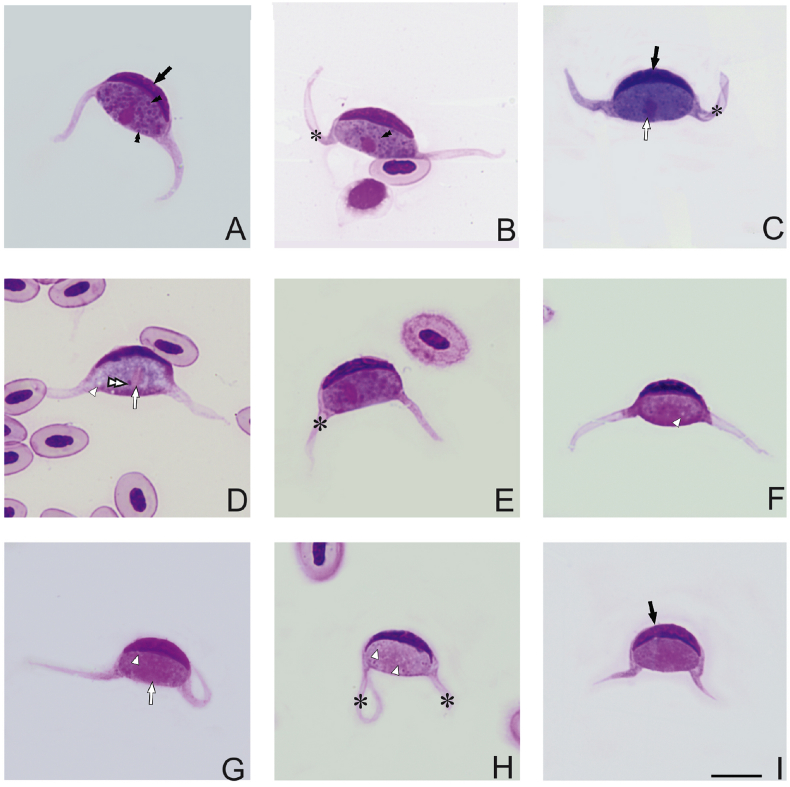
Fig. 2.
Leucocytozoon neotropicalis sp. nov. from the peripheral blood of its type vertebrate host Green-and-black Fruiteater (Pipreola riefferii) captured at Los Nevados NNP, Colombia. Macrogametocytes (A–E) and microgametocytes (F–I). Black arrows (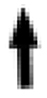 ) indicate the deformed host cell nuclei. Parasite nuclei are indicated by white arrow (
) indicate the deformed host cell nuclei. Parasite nuclei are indicated by white arrow (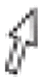 ) and nucleoli are shown by double white arrowtips (
) and nucleoli are shown by double white arrowtips (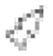 ). Volutin granules are indicated by double black arrowtips (
). Volutin granules are indicated by double black arrowtips ( ) and vacuoles – by white arrowtips (
) and vacuoles – by white arrowtips ( ). Uneven cytoplasmic processes may acquire a ribbon-like appearance (asterisk *). Giemsa-stained thin blood films. Scale bar = 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
). Uneven cytoplasmic processes may acquire a ribbon-like appearance (asterisk *). Giemsa-stained thin blood films. Scale bar = 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Morphometric parameters of gametocytes and host cells of Leucocytozoon neotropicalis sp. nov. Measurements of Leucocytozoon eurystomi and Leucocytozoon lovati are provided for comparison. Measurements are given in μm or μm2 (for area). Minimum and maximum values as well as mean ± SD are provided.
| Feature |
Leucocytozoon neotropicalis sp. nov |
Leucocytozoon eurystomia,b | Leucocytozoon lovatib | |
|---|---|---|---|---|
| Macrogametocyte n = 6 | Microgametocyte n = 1 | |||
| Parasite | ||||
| Length | 14.4–16.0 (15.3 ± 0.6) | 11.4–17.9 (13.6 ± 1.5) | 22.6–29.6 (25.2 ± 1.3) | 14.1–22.0 (17.5 ± 1.4) |
| Width | 7.0–8.2 (7.8 ± 1.0) | 5.7–10.0 (7.8 ± 1.4) | 7.0–12.2 (8.1 ± 1.0) | 6.7–13.1 (11.0 ± 0.7) |
| Area | 86.1–101.2 (95.2 ± 4.9) | 64.9–114.9 (83.7 ± 17.4) | (182 ± 18.5) | |
| Perimeter | 34.1–40.9 (38.8 ± 1.6) | 31.4–48.9 (37.4 ± 4.8) | (63.0 ± 5.9) | |
| Parasite nucleus | ||||
| Length | 2.0–3.1 (2.8 ± 0.5) | 5.3–10.5 (8.0 ± 1.5) | 2.8–6.4 (4.1 ± 0.4) | 3.2–6.8 (4.2 ± 0.3) |
| Width | 3.3–4.1 (4.0 ± 0.4) | 4.4–7.0 (5.6 ± 0.8) | 1.4–5.7 (3.5 ± 0.3) | 1.8–4.4 (3.6 ± 0.3) |
| Area | 7.0–9.6 (8.6 ± 1.0) | 32.5–48.4 (41.4 ± 4.7) | (11.8 ± 2.6) | |
| Host-cell parasite complex | ||||
| Length | 35.5–56.6 (44.7 ± 5.3) | 29.8–55.8 (41.4 ± 8.6) | (39.2 ± 6.3) | |
| Width | 9.5–10,8 (10.2 ± 0.5) | 9.2–10.8 (9.9 ± 0.5) | (9.1 ± 0.7) | |
| Area | 161.0–200.1 (181.8 ± 10.4) | 127.4–181.7 (151.2 ± 13.0) | (202.2 ± 21.1) | |
| Host-cell nucleus | ||||
| Length | 14.8–19.7 (17.7 ± 1.8) | 14.2–18.3 (16.3 ± 1.3) | 18.3–26.0 (21.7 ± 2.3) | 9.8–16.3 (13.5 ± 1.5) |
| Width | 2.4–3.7 (2.9 ± 3.0) | 2.4–3.7 (3.0 ± 0.4) | ||
| Area | 28.2–39.6 (35.7 ± 4.3) | 23.6–42.0 (33.6 ± 5.3) | (39.0 ± 7.2) | |
| Perimeter of parasite covered | 14.1–18.2 (16.5 ± 1.8) | 14.2–18.3 (16.3 ± 1.3) | (16.9 ± 2.3) | |
| Cytoplasmic processesc | ||||
| Length | 11.3–22.0 (15.7 ± 2.8) | 7.6–24.4 (14.8 ± 4.5) | ||
| Width | 2.6–5.4 (3.8 ± 0.8) | 1.9–5.3 (3.7 ± 0.9) | ||
| Area | 18.2–29.0 (25.0 ± 5.3) | 14.4–26.1 (21.2 ± 3.2) | ||
According to Bennet et al., (1993).
According to Valkiūnas (2005).
Only one of 2 cytoplasmic processes was measured for each parasite.
Two long thin fusiform spindle-shaped processes of the host cells' cytoplasm reach up to 14 μm (Table 3), and that never is observed in L. grallariae. The length of the cytoplasmic processes can be different in the same host-parasite complex (Fig. 2J and K), and that probably is a result of deformation during the preparation of blood films. Cytoplasmic processes are thin and often flattened appearing like a ribbon (Fig. 3F–H). It is important to note that mature gametocytes often have a flattened form on the side, located on the opposite side of the host cell nuclei (Fig. 2 H, J-K). This character is not observed in L. grallariae (Fig. 1I–K).
In the type material, we observed tiny volutin granules and small vacuoles (up to 0.47 μm in diameter) in 47.9% of fully grown gametocytes (Fig. 2A–E). The parasite nucleus was roundish in 49.3% of 103 observed gametocytes (Fig. 2A–C) or elongated (Fig. 2 E); its position was mainly more or less central, but sometimes was off-centre. The nucleolus was variable both in shape and position, being visible in 53.2% of 103 observed parasites (Fig. 2 B, D).
Microgametocytes (Fig. 2F–I): General configuration and other features were similar to the macrogametocytes with the usual haemosporidian sexual dimorphic characters. The proportion of microgametocytes and macrogametocytes in the type material was approximately 1:2.
Taxonomic summary.
Type host: Green-and-black Fruiteater Pipreola riefferii (Cotingidae, Passeriformes).
Additional hosts: unknown.
Type locality: El Bosque, Los Nevados National Natural Park (NNP) (4° 43 N; 75° 27 W, 3150 masl), Risaralda, Colombia.
Type specimens: Hapantotype (accession Nos. UNAL:GERPH:OT1354-II). The intensity of the infection of the lineage MK103894 is 0.33%, it was collected by Melisa Galarza (27 December 2015) and deposited in the biological collection GERPH (Grupo de Estudio Relación Parásito Hospedero) at the Universidad Nacional de Colombia, Bogotá, Colombia. Parahapantotypes (accession Nos. UNAL:GERPH:OT1354-I, UNAL:GERPH:OT1354-III, other data as for the hapantotype) are deposited in the same collection. Digital images of the blood stages of the parasite in the type preparations are available on request from GERPH.
Partial mitochondrial DNA genome (5811 bp) that includes cox1, cox3 and cytb genes (GenBank accession number MK103895) was obtained from the type host Pipreola riefferii.
Site of infection: Blood cells, the specific cell is unknown due to the marked deformation by developing gametocytes.
Prevalence: Only one individual of the host species was collected and found infected, so the sample size does not allow to estimate the prevalence. Parasite was detected by microscopy in 1 of out of 684 examined birds (0.12%). In the type locality, 1 of 686 birds captured at Los Nevados NNP (0.14%) was infected, as determined by microscopic examination.
Etymology: The species name (neotropicalis) was derived from the name of the zoogeographical region where this parasite was found.
3.2.5. Remarks
Leucocytozoon neotropicalis is one of the six Leucocytozoon species that parasitize passerine birds and possess gametocytes developing in fusiform host cells. The main differences between L. neotropicalis and L. grallariae are specified in the description of the former parasite. Both of these new leucocytozoids have gametocytes developing fusiform host cells, which can be distinguished from other leucocytozoids due to the unique shape of their host cell nuclei (see Remarks on L. grallariae).
Due to the presence of long and narrow cytoplasmic processes in host cells, L. neotropicalis is similar to L. lovati (Valkiūnas, 2005) and L. eurystomi (Bennett et al., 1993; Valkiūnas, 2005) (Table 3). However, the nuclei of host cells never reach the cytoplasmic processes in the last two parasites. Because of this character, these species can be readily distinguished.
Microscopic examination of blood smears from type series revealed the presence of co-infection of a parasite with gametocytes developing in roundish host cells. Overall, the configuration of the nuclei in roundish host cells resembles the same characters observed in the L. fringillinarum group (Fig. 3.). The reported gametocytes in roundish host cells are bigger than those observed in the sample with coinfection with L. grallariae (Student's t-test for parasite area: p < 0.0001, α = 0.05, Table S3). In contrast to the macrogametocytes of L. neotropicalis, the volutin granules are not pronounced or absent in roundish gametocytes of this parasite (Fig. 3D–F). It worth mentioning that, for both new species as well as for L. pterotenuis (Lotta et al., 2015), the gametocytes developing in fusiform host cells were the most common, and their parasitemia was on average ten to seventeen times higher than the species with gametocytes developing roundish host cells. Indeed, in the type material of L. neotropicalis parasitemia of gametocytes developing fusiform host cells was 0.21%, while it did not exceed 0.01% for gametocytes developing roundish host cells.
Two distantly related lineages with a genetic distance of 0.29 between them (L_PIRIEF_01, cytb gene GenBank No. MH909276 and partial mtDNA genome that included cytb, cox1, cox3, GenBank No. MK103894), were amplified from the same sample, which makes it difficult to link the lineages with their morphotypes. Based on phylogenetic analysis, we suggest that the last one lineage (GenBank No. MK103894) corresponds to L neotropicalis n. sp. (see discussion below).
3.3. Sequencing of the cytochrome b gene and the DNA mitochondrial genome
Two lineages were isolated from each of the samples containing L. grallariae or L. neotropicalis. The partial cytb fragments obtained using the primers suggested by Hellgren et al. (2004) were very distant from the cytb lineages obtained by the mtDNA genome amplification protocol (Pacheco et al., 2011, Pacheco et al., 2018b, Pacheco et al., 2018a). In other words, different Leucocytozoon parasite sequences were obtained in the same sample using different protocols and that corresponded to the microscopic observation of possible co-infections in these samples.
Even though both protocols for the amplification of cytb fragments and the mtDNA genome were run at least two times independently, each protocol amplified different lineages (lineages isolated from G. squamigera: GenBank cytb accession No. MH909275 vs GenBank mtDNA accession No. MK103895; lineages isolated from P. riefferii: GenBank cytb accession No. MH909276 vs GenBank mtDNA accession No. MK103894). Similar results were obtained when new molecular studies were performed with the Grallaridae bird samples reported by Lotta et al. (2015), where parasites were described as Leucocytozoon pterotenuis. With the new analysis, we realized that the cytb fragment obtained along with the partial mitochondrial genomes (mtDNA) identified with GenBank accession No. KM272250 and the short cytb fragment amplified with Hellgren's primers identified with GenBank accession No. KY646032 were different. Thereby, we will be referring to the description of Leucocytozoon pteroteunuis (Lotta et al., 2015) as a partial description of the parasite (according to the (International Commission on Zoological Nomenclature, 1999) the description “in part”), because according to the molecular analyses performed, gametocytes in roundish host cells observed in the sample likely do not belong to L. pterotenuis, but to other Leucocytozoon species (see discussion). Thus, the species name L. pterotenuis is valid only in part, mainly for gametocytes developing in fusiform host cells, but not to gametocytes in roundish host cells.
3.4. Analysis of primer affinities
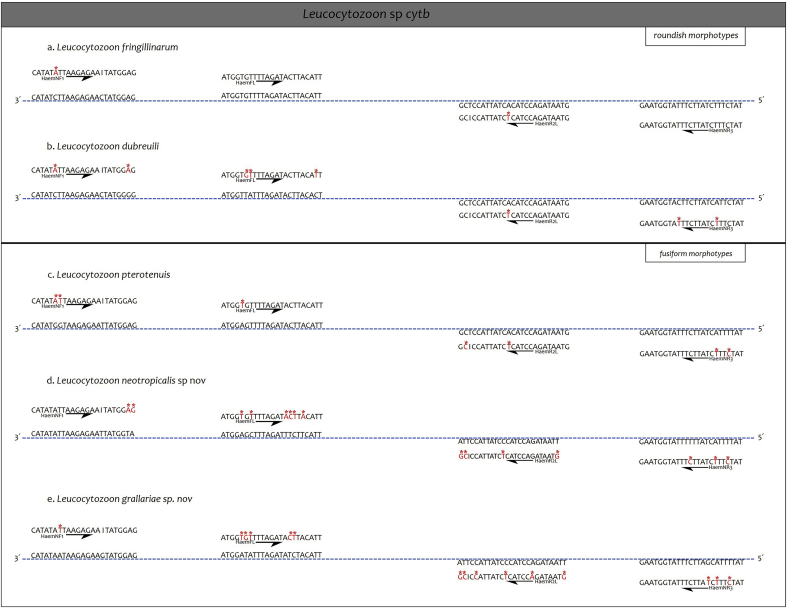
The success of amplification is highly dependent on the primer's affinity for the target sequence and the parasitemia that determines the amount of parasite present DNA (Perez-Tris and Bensch, 2005; Pacheco et al., 2018a). After verification of affinities of the primers proposed by Hellgren et al. (2004) with the all complete cytb gene sequences of Leucocytozoon parasites available for passerine birds, it was noticed that oligo-sequences matched the cytb sequences of L. fringillinarum (Genbank accession No. KY653765) and L. dubreuili (Genbank accession No. KY653795), which both have gametocytes developing roundish host cells (Fig. 4 A, B). In contrast, we noticed that the primer HaemR2L did not completely match with the mtDNA sequences obtained using the mtDNA genome amplification protocol used for L. pterotenuis (in part) (Genbank No.KM610046). Indeed, the base pairs at the 3′ end of the primer, as well as the two last base pair of the 5’, did not match with the cytb gene sequences obtained from the mtDNA genome of L. grallariae (Genbank No. MK103895) nor L. neotropicalis (Genbank No. MK103894) (Fig. 4).
Fig. 4.
Primer affinity analyses of the primers suggested by Hellgren et al. (2004). Parasites with gametocytes developing roundish host cells are: (a) L. fringillinarum, and (b) L. dubreuili; and parasites with fusiform host cell are (c) L. pterotenuis (in part), (d) L. neotropicalis sp. nov, and (e) L. grallariae sp. nov. An asterisk over the base pair highlights mismatches between the sequences and the primers. Note that primers HaemNR3 and HaemR2L are presented in 3′-5′ sense to fit the parasite sequences.
3.5. Phylogenetic analysis
In the phylogenetic reconstructions based on partial mitochondrial genomes and 476 cytb fragments (Fig. 5 and S1), two main clades that resemble the classification of parasites according to morphological features were observed. Thus, parasite lineages of leucocytozoids with gametocytes developing round host cells were part of a separate clade (Fig. 5 and S1 clade I). An exception is L. danilewskyi, in which the gametocytes develop both roundish and fusiform host cells. Meanwhile, parasites that produce gametocytes in fusiform cells are part of a separate monophyletic group (identified as clade II). Within this, lineages Genbank No. MK103894 of L. neotroplcalis and Genbank No. MK103895 of L. grallariae samples form a clade that is the sister lineage to L. pterotenuis (in part) (KM272250) and Leucocytozoon sp. (KM272251). These parasites are closely related to L. sabrazesi (a morphological synonym of Leucocytozoon macleani, AB299369), a parasite infecting Galliformes birds, in whose gametocytes develop in fusiform host cells (Fig. 5A, Table 4). Thus, the phylogenetic analyses suggested a link between the parasite morphotypes and their sequences for both samples with co-infection.
Fig. 5.
(A) A Bayesian phylogenetic hypothesis of Leucocytozoon species constructed only with partial mitochondrial genomes (5485 bp excluding gaps) and (B) partial cytb gene sequences of leucocytozoids. Branch colors indicate the parasite morphology, with green branches representing parasites in fusiform host cells, and blue branches correspond to a species that develops in roundish host cells. Notice that, since parasite mitochondrial genomes (mtDNA) corresponding to the partial cytb fragments of the MH909275 and MH909276 sequences could not be amplified, they were not included in the phylogenetic hypothesis constructed with mtDNA (Fig. 5A). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 4.
Genetic distance (d) and standard errors (SE) between pairs of species using cox1,cox3, cytb and mitochondrial genomes (mtDNA) sequences of Leucocytozoon lineages (Fig. 5).
| Genetic distance (d ± SE) |
||||
|---|---|---|---|---|
| cox1 | cox3 | cytb | mitochondrial genome | |
| L. neotropicalisa vs. L. grallariaeb | 0.090 ± 0.007 | 0.105 ± 0.011 | 0.129 ± 0.007 | 0.095 ± 0.004 |
| L. neotropicalis vs. L. pterotenuisc | 0.103 ± 0.008 | 0.116 ± 0.011 | 0.141 ± 0.007 | 0.108 ± 0.004 |
| L. grallariae vs. L. pterotenuis | 0.095 ± 0.007 | 0.119 ± 0.011 | 0.080 ± 0.006 | 0.082 ± 0.004 |
| L. neotropicalis vs. L. sabrazesi | 0.156 ± 0.010 | 0.169 ± 0.013 | 0.176 ± 0.006 | 0.149 ± 0.005 |
| L. grallariae vs. L. sabrazesi | 0.095 ± 0.009 | 0.188 ± 0.014 | 0.136 ± 0.006 | 0.131 ± 0.005 |
| L. quynzae vs. L. neotropicalis | 0.160 ± 0.009 | 0.161 ± 0.012 | 0.141 ± 0.008 | 0.146 ± 0.005 |
| L. quynzae vs. L. grallariae | 0.167 ± 0.010 | 0.181 ± 0.014 | 0.080 ± 0.007 | 0.133 ± 0.005 |
| L. fringillinarum vs. L. neotropicalis | 0.171 ± 0.009 | 0.178 ± 0.013 | 0.175 ± 0.007 | 0.150 ± 0.004 |
| L. fringillinarum vs. L. grallariae | 0.176 ± 0.010 | 0.192 ± 0.014 | 0.140 ± 0.007 | 0.139 ± 0.005 |
| L. majoris vs. L. fringillinarum | 0.089 ± 0.007 | 0.099 ± 0.011 | 0.048 ± 0.004 | 0.058 ± 0.004 |
Lineages amplified and cloned from.
P. riefferii infected with L. neotropicalis, (GenBank MK103894).
G. squamigera infected with L. grallariae (GenBank MK103895), and.
G. ruficapilla infected with L. pterotenuis (Lotta et al., 2015 (partim)) (GenBank KM610046).
It is worth noting that, since parasite mitochondrial genomes (mtDNA) corresponding to the partial cytb fragments of the MH909275 and MH909276 sequences could not be amplified, they were not included in the phylogenetic hypothesis constructed with mtDNA (Fig. 5A).
Interestingly, in clade I, the partial cytb lineages MH909275, MH909276 and KY646032 obtained from samples of L. neotropicalis, L. grallariae and L. pterotenuis (in part) respectively using the primers proposed by Hellgren et al. (2004) share a recent common ancestor with L. fringillinarum and L. quynzae (Fig. 5B and S1). This suggests that these lineages likely correspond to the roundish host cell morphotype coexisting with the fusiform host cell morphospecies present in the samples infected with L. neotropicalis, L. grallariae and L. pterotenuis (in part). On the other hand, cytb fragments obtained from mtDNA genome lineages KM272250, MK103894 and MK103895 form a monophyletic group (Fig. 5B clade II) that is a sister clade of parasites developing roundish host cells plus L. sabrazesi (synonym of Leucocytozoon macleani) (AB299369) and L. danilewskyi (KY653781) (Fig. 5B clade II). Both Leucocytozoon sabrazesi and L. danilewskyi are parasites with gametocytes that develop both roundish and fusiform host cells.
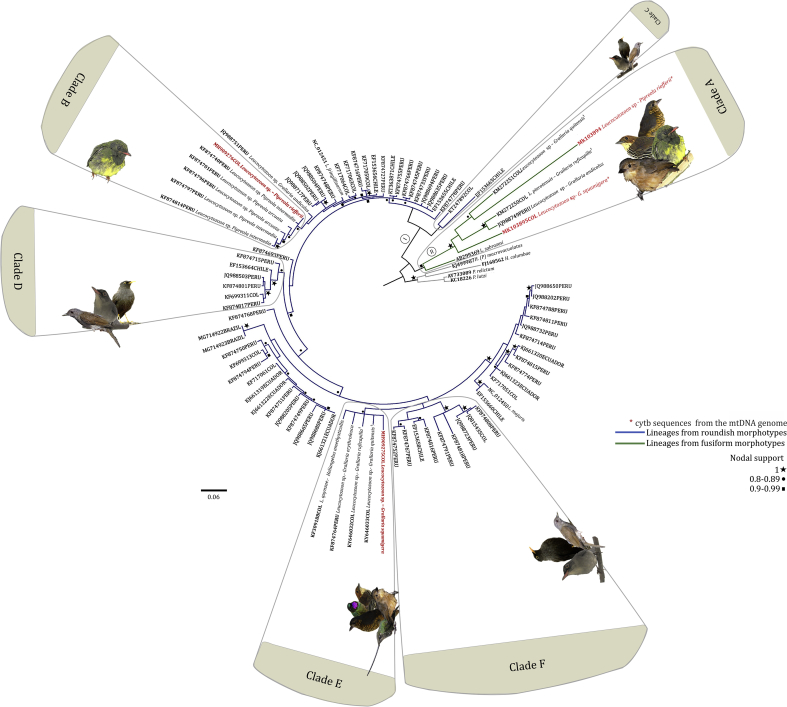
Phylogenetic relationships of parasite lineages with sequences isolated from South American birds are depicted in Supplementary Figure S1 (see also Supplementary Table S1). It is noteworthy that the cytb lineage L_GRSQU 02 (Genbank No. MH909275) obtained by PCR from the sample infected with L. grallariae was placed in a well-supported clade along with the lineage KY646032 isolated from the type material of L. pterotenuis (in part) (Fig. S1, clade E). Within the clade E, a partial cytb sequence of L. quynzae and the lineage KF874769 obtained from a Peruvian Grallaria erythroleuca specimen were included. Genetic distances between the isolate L_GRSQU_02 (Genbank No. MH909275) and the lineages of parasites previously reported in other species of Grallaridae (KY646032, KY646033, and KF874764) were 0.05, and 0.06 respectively; while it was 0.05 for L. quynzae (Fig.S1, Table 5). Furthermore, the linage L_PIRIE 02 (Genbank No. MH909276) fell into a clade composed by parasites infecting other species of Pipreola (Fig. S1, Clade IB). Genetic distances between this lineage and the Peruvian lineages isolated from Pipreola (P.) intermedia (Genbank accession No. KF874740, KF874814) and Pipreola arcuata (Genbank accession Nos. KF874701, KF874796) ranged between 0,01 and 0.03 (clade B).
Consistently with phylogenetic reconstructions performed with mtDNA genome, lineages MK103895 and MK103894 fell into a clade that included L. sabrazesi and L. pterotenuis (in part) (Fig. 5A, clade II). The genetic distance between the mtDNA genome lineage MK103894 of L. neotropicalis and its sister taxa (KM272250) was 0.25, while the genetic distance of lineage MK103895 L. sabrazesi, L. pterotenuis (in part) and MK103894 were 0.21, 0.17, and 0.25 respectively. The large genetic distances estimated using both the partial mtDNA and the cytb sequences obtained from the new species as well as L. pterotenuis (in part) using the methodologies above mentioned are reported in Table 5.
4. Discussion
The combined use of information obtained from morphological and molecular characterizations provided opportunities to distinguish and describe two new parasite species L. neotropicalis and L. grallariae. Gametocytes of both species develop fusiform host cells that are quite scarce in the Neotropic leucocytozoids (Lotta et al., 2016, 2015) and are rare in leucocytozoids parasitizing Passeriformes birds (Valkiūnas, 2005). Nevertheless, the standard PCR protocols used to diagnose and characterize the leucocytozooids (i.e. Bensch et al., 2000; Hellgren et al., 2004; Perkins and Schall, 2002) likely underestimate the Leucocytozoon diversity. The mispriming found in this study explain how such protocols failed to detect parasite lineages that, in this case, seem to be endemic from the Neotropical region. Besides other factors that cause a sub-estimate of Leucocytozoon parasite prevalence and diversity are the sampling bias of avian hosts (most of them belong to orders Passeriformes or Apodiformes), as well as that some studies rely solely on molecular methods and did not use microscopic examination for the parasite detection (i.e., Galen and Witt, 2014; Harrigan et al., 2014).
4.1. Prevalence of Leucocytozoon parasites
In the Neotropics, the overall percentage of naturally infected birds with Leucocytozoon parasite is generally low (e.g., Harrigan et al., 2014; Lotta et al., 2016; Fecchio et al., 2018). Indeed, the above-cited studies report 0.06%, 1.16%, 4.6% respectively. The percentage found in this study used microscopy solely so chronical non-patent infections could be overlooked by this methodology. However, the percentage obtained (1.2%) was similar to the ones reported in the two studies mentioned above, where molecular detection of all samples was performed. However, this value is lower when it was compared with other prevalence data obtained in other zoogeographical regions (e.g. 16.2% in the Holarctic region, see Valkiūnas, 2005). New molecular protocols together with microscopy may change our perspective of how frequently these parasites are found in the neotropics. In the avian species studied in Colombia, there is marked variation in the frequency of Leucocytozoon parasites’ found across different families. For example, all Grallariidae species studied so far are infected (100%), followed by Thraupidae (29.2%), Emberizidae (19.4%) and Turdidae (7.9%) (Lotta et al., 2016).
In this study, 4 out of 4 examined grallariids were infected. Although the sample size is small, we can speculate that these birds are highly susceptible to Leucocytozoon infection. Indeed, different Leucocytozoon species were found in these birds even in sympatric transmission, as is the case of L. pterotenuis (in part) in Grallaria ruficapilla described by (Lotta et al., 2015) and L. grallariae (Grallaria squamigera) which showed high genetic diversity (Table 5) and distinct morphological differences (Fig. 1, Fig. 2; see also Fig. 1 in Lotta et al., 2015). An ecological aspect that can drive this feature is the preference of these birds to inhabit areas and build their nest near streams and small brooks (Stiles and López, 1995; Londoño et al., 2004), being them a readily available blood source for simuliids, their parasite vectors. Simuliids also have preferences for running and clean waters (Coscarón and Coscarón-Arias, 2007).
4.2. Analysis of primer affinities
With the affinity analysis of the primers proposed by Hellgren et al. (2004) (Fig. 4), we determine that those primers can amplify Leucocytozoon parasites with gametocytes that developed round host cells, like L. fringillinarum and L. dubreuili. Also, different studies have proved their efficiency in the detection of other leucoytozoids with gametocytes in fusiform host cells, such as L. buteonis (Krone et al., 2008), L. danilewskyi (Ortego and Cordero, 2009), and L simondi (Smith and Ramey, 2015). However, these primers did not match properly with the cytb sequences of L. pterotenuis, L. grallariae, and L. neotropicalis; which gametocytes develop in fusiform host cells recently described in the Neotropics. One possible explanation for these results may be due to the design of the Hellgren et al. (2004) primers, particularly the primer HaemR2L, since only the cytb sequences of L. dubreuili and L. simondi were available at that time (Perkins and Schall, 2002).
It is worth mentioning that despite of microscopic report of two different morphologies of Leucocytozoon parasites in blood films of type samples of L. neotropicalis, L. grallariae and L. pterotenuis (in part, previously described by Lotta et al., 2015), the presence of co-infections were confirmed only when molecular analyses (partial cytb and mitochondrial DNA genome amplification) were performed in parallel.
None of the partial cytb lineages obtained by mtDNA amplification could be amplified using the primers and protocols suggested by Hellgren et al. (2004). That calls for the development of a new set of primer for nested PCR-based methods diagnosis of avian leucocytozoids. The new mtDNA genomes sequences obtained in this and previous studies conducted in the Neotropics (i.e., Matta et al., 2014; Lotta et al., 2016; Pacheco et al., 2018b) and now available in public databases can be helpful for the design of a new set of primer and PCR protocols.
4.3. Diversity of Leucocytozoon lineages and primers used
Two different parasite lineages were amplified in each sample where a new parasite species was described, and that was consistent with microscopic examination. The genetic distances between the cytb fragments obtained by direct sequencing of the PCR product and the cloned mitochondrial lineages ranged from 0.20 to 0.29 (Table 5), indicating that two different species were co-infecting each sample. Similar to this, after the publication of L. pterotenuis (in part, Lotta et al., 2015), the authors found that cytb lineages isolated from Grallaria ruficapilla and Grallaria quitensis (obtained using the Hellgren's protocol, Hellgren et al., 2004) and used for the reconstruction of phylogenetic relationships were different from those obtained using the protocol for the parasite mtDNA genome amplification. Nevertheless, both sets of lineages - partial mtDNA genomes (GenBank accession numbers KM610045 and KM610046) and partial cytb genes (GenBank accession numbers KY646032 and KY646033) are true lineages. The last one we presume belongs to a morphotype also present in co-infection with L. pterotenuis (in part, Lotta et al., 2015).
The presence of parasites with gametocytes developing fusiform and roundish host cells in the smears does not always implicate a co-infection. Indeed, Desser (1967) proved that Leucocytozoon simondi has gametocytes that develop both roundish and fusiform host cells depending on the stage of exo-erythrocytic development. Mainly, merozoites from the hepatic meronts and megalomeronts produced gametocytes that develop in roundish and fusiform host cells, respectively. Nevertheless, in all our phylogenetic reconstructions, partial cytb fragments fell into a different clade to those cytb sequences derived from the mtDNA sequences. Furthermore, the microscopic examination revealed that fusiform host cells were more often seen than roundish ones in all the three samples where we have found new Leucocytozoon species. For example, in G. ruficapilla, parasitemia of L. pteroteunuis gametocytes in fusiform host cells and roundish host cells was 0.06% and 0.01%, respectively (Lotta et al., 2015). The same pattern was found in the birds infected with L. grallariae and L. neotropicalis (see above in the description remarks). The parasitemia and aforementioned analyses of primers suggest that DNA fragments of parasites from gametocytes in roundish host cells more likely were amplified by the Hellgren et al. primers (Hellgren et al., 2004).
4.4. Phylogenetic relationships of parasite lineages
The association of molecular lineages and morphospecies described in this study cannot be definitively proved. However, the close relationship between the 476 bp cytb lineage obtained in the L. neotropicalis (GenBank accession No. MH909276) sample with L. fringillinarum (Fig. 5B, genetic distance 2.8% Table 5) suggests that the L. neotropicalis lineage analyzed correspond to the parasite developing in roundish host cells, which resemble L. fringillinarum morphological species group. On the other hand, Leucocytozoon parasites with gametocytes in fusiform host cells are rare in passerine birds (Lotta et al., 2015; Valkiūnas, 2005). Although L. grallariae lineage is part of the same clade as L. neotropicalis and is closely related to L. pterotenuis (in part), patterns of host specificity of these infections deserve more in-depth studies. It is important to mention that the cytb lineages obtained both for L. grallariae and L. neotropicalis, grouped with other lineages previously reported in Peru and Colombia (Lotta et al., 2016) (Fig.S1. clade B and E).
In the past decade, leucocytozoids have been the subject of intense study in the Neotropical countries (Merino et al., 2008; Rodríguez et al., 2009; Matta et al., 2014; Galen and Witt, 2014; González et al., 2014; Harrigan et al., 2014; Lotta et al., 2015, 2016). New parasite species have been described in birds with distribution limited to the Andean mountains (Matta et al., 2014; Lotta et al., 2015). Due to the bias of the bird's capture method, mainly birds of Passeriformes and Apodiformes out of the 25 bird orders present in these mountain ranges (Herzog and Kattan, 2011) have been extensively sampled. Besides, due to the high endemism and richness of host species in the Neotropics, it is possible that new host-parasite relationships and coevolution have contributed to the generation of new lineages, which are significantly different to previous ones reported in other geographical regions. We encourage researchers to perform more in-depth sampling of birds belonging to other bird orders as well as to go further in genomic studies and life cycle characterization that are helpful for estimates parasite diversity and evolutionary history of avian malaria and relative haemosporidian parasites.
Acknowledgements
We thank the students of the Host-Parasite Relationship Research Group: Avian Haemoparasites Model; especially to Paola González, for field and laboratory assistance. Also, we would like to thank the staff of Unidad Administrativa Especial del Sistema de Parques Nacionales Naturales for supporting field logistics. This study was supported by El Departamento Administrativo de Ciencias, Tecnología e Innovación COLCIENCIAS (contract No. 556–2014, project numbers 110152128340 and 110165944139), Vicerectoria de Investigación - Universidad Nacional de Colombia, project N° 38238 and Division de Investigación Bogotá-Universidad Nacional de Colombia, project N° 18714. The funding sources had no role in the study design, data collection and analysis, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.05.002.
Contributor Information
Ingrid A. Lotta, Email: ialottaa@unal.edu.co.
Nubia E. Matta, Email: nemattac@unal.edu.co.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- Addinsoft . Addinsoft; Paris, France: 2017. XLSTAT 2017: Data Analysis and Statistical Solution for Microsoft Excel. [Google Scholar]; Addinsoft, 2017. XLSTAT 2017: Data Analysis and Statistical Solution for Microsoft Excel. Addinsoft, Paris, France.
- Bennett G., Earlé R., Peirce M., Nandi N. The Leucocytozoidae of South African birds. The coliiformes and coraciiformes. Afr. Zool. 1993;28:74–80. doi: 10.1080/02541858.1993.11448296. [DOI] [Google Scholar]; Bennett, G., Earle, R., Peirce, M., Nandi, N., 1993. The Leucocytozoidae of South African birds. The coliiformes and coraciiformes. Afr. Zool. 28, 74-80. https://doi.org/10.1080/02541858.1993.11448296
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]; Bensch, S., Hellgren, O., Perez-Tris, J., 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 9, 1353-1358. [DOI] [PubMed]
- Bensch S., Péarez-Tris J., Waldenströum J., Hellgren O. Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution. 2004;58:1617–1621. doi: 10.1111/j.0014-3820.2004.tb01742.x. [DOI] [PubMed] [Google Scholar]; Bensch, S., Pearez-Tris, J., Waldenstroum, J., Hellgren, O., 2004. Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution 58, 1617-1621. https://doi.org/10.1111/j.0014-3820.2004.tb01742.x [DOI] [PubMed]
- Bensch S., Stjernman M., Hasselquist D., Örjan Ö., Hannson B., Westerdahl H., Pinheiro R.T. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1583–1589. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bensch, S., Stjernman, M., Hasselquist, D., Orjan, O., Hannson, B., Westerdahl, H., Pinheiro, R.T., 2000. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. Lond. B Biol. Sci. 267, 1583-1589. [DOI] [PMC free article] [PubMed]
- Benson D.A., Clark K., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2015;43:D30–D35. doi: 10.1093/nar/gku1216. [DOI] [PMC free article] [PubMed] [Google Scholar]; Benson, D.A., Clark, K., Karsch-Mizrachi, I., Lipman, D.J., Ostell, J., Sayers, E.W., 2015. GenBank. Nucleic Acids Res. 43, D30-D35. [DOI] [PMC free article] [PubMed]
- Bernotienė R., Palinauskas V., Iezhova T., Murauskaitė D., Valkiūnas G. Avian haemosporidian parasites (Haemosporida): a comparative analysis of different polymerase chain reaction assays in detection of mixed infections. Exp. Parasitol. 2016;163:31–37. doi: 10.1016/j.exppara.2016.01.009. [DOI] [PubMed] [Google Scholar]; Bernotienė, R., Palinauskas, V., Iezhova, T., Murauskaitė, D., Valkiūnas, G., 2016. Avian haemosporidian parasites (Haemosporida): a comparative analysis of different polymerase chain reaction assays in detection of mixed infections. Exp. Parasitol. 163, 31-37. [DOI] [PubMed]
- BirdLife International . 2017. Species Factsheet: Grallaria Squamigera.http://www.birdlife.org [WWW Document]. URL. [Google Scholar]; BirdLife International, 2017a. Species factsheet: Grallaria squamigera. [WWW Document]. URL http://www.birdlife.org (accessed 9.17.2017).
- BirdLife International . 2017. Species Factsheet: Pipreola Riefferii.http://www.birdlife.org [WWW Document]. URL. [Google Scholar]; BirdLife International, 2017b. Species factsheet: Pipreola riefferii. [WWW Document]. URL http://www.birdlife.org (accessed 9.17.2017).
- Cadena-Ortiz H., Mantilla J.S., de Aguilar J.R., Flores D., Bahamonde D., Matta N.E., Bonaccorso E. Avian haemosporidian infections in rufous-collared sparrows in an Andean dry forest: diversity and factors related to prevalence and parasitaemia. Parasitology. 2018:1–9. doi: 10.1017/S0031182018002081. [DOI] [PubMed] [Google Scholar]; Cadena-Ortiz, H., Mantilla, J.S., de Aguilar, J.R., Flores, D., Bahamonde, D., Matta, N.E., Bonaccorso, E., 2018. Avian haemosporidian infections in rufous-collared sparrows in an Andean dry forest: diversity and factors related to prevalence and parasitaemia. Parasitology 1-9. [DOI] [PubMed]
- Ciloglu A., Ellis V.A., Bernotienė R., Valkiūnas G., Bensch S. A new one-step multiplex PCR assay for simultaneous detection and identification of avian haemosporidian parasites. Parasitol. Res. 2018:1–11. doi: 10.1007/s00436-018-6153-7. https://doi.org/doi: 10.1007/s00436-018-6153-7 [DOI] [PubMed] [Google Scholar]; Ciloglu, A., Ellis, V.A., Bernotienė, R., Valkiūnas, G., Bensch, S., 2018. A new one-step multiplex PCR assay for simultaneous detection and identification of avian haemosporidian parasites. Parasitol. Res. 1-11. https://doi.org/doi: 10.1007/s00436-018-6153-7 [DOI] [PubMed]
- Coscarón S., Coscarón-Arias C. Neotropical Simuliidae (Diptera: Simuliidae) Aquat. Biodivers. Lat. Am. 2007 [Google Scholar]; Coscaron, S., Coscaron-Arias, C., 2007. Neotropical Simuliidae (Diptera: Simuliidae). Aquat. Biodivers. Lat. Am.
- Cuatrecasas J. Aspectos de la vegetación natural de Colombia. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 1958;10:221–268. [Google Scholar]; Cuatrecasas, J., 1958. Aspectos de la vegetacion natural de Colombia. Rev. Acad. Colomb. Cienc. Exactas Fisicas Nat. 10, 221-268.
- Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9 doi: 10.1038/nmeth.2109. 772–772. [DOI] [PMC free article] [PubMed] [Google Scholar]; Darriba, D., Taboada, G.L., Doallo, R., Posada, D., 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772-772. [DOI] [PMC free article] [PubMed]
- de Aguilar J.R., Castillo F., Moreno A., Peñafiel N., Browne L., Walter S.T., Karubian J., Bonaccorso E. Patterns of avian haemosporidian infections vary with time, but not habitat, in a fragmented Neotropical landscape. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206493. [DOI] [PMC free article] [PubMed] [Google Scholar]; de Aguilar, J.R., Castillo, F., Moreno, A., Peñafiel, N., Browne, L., Walter, S.T., Karubian, J., Bonaccorso, E., 2018. Patterns of avian haemosporidian infections vary with time, but not habitat, in a fragmented Neotropical landscape. PloS One 13, e0206493. [DOI] [PMC free article] [PubMed]
- Desser S. Schizogony and gametogony of Leucocytozoon simondi and associated reactions in the avian host. J. Eukaryot. Microbiol. 1967;14:244–254. doi: 10.1111/j.1550-7408.1967.tb01992.x. [DOI] [PubMed] [Google Scholar]; Desser, S., 1967. Schizogony and gametogony of Leucocytozoon simondi and associated reactions in the avian host. J. Eukaryot. Microbiol. 14, 244-254. [DOI] [PubMed]
- Escalante A.A., Freeland D.E., Collins W.E., Lal A.A. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc. Natl. Acad. Sci. Unit. States Am. 1998;95:8124–8129. doi: 10.1073/pnas.95.14.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]; Escalante, A.A., Freeland, D.E., Collins, W.E., Lal, A.A., 1998. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc. Natl. Acad. Sci. Unit. States Am. 95, 8124-8129. https://doi.org/10.1073/pnas.95.14.8124 [DOI] [PMC free article] [PubMed]
- Fecchio A., Silveira P., Weckstein J., Dispoto J., Anciães M., Bosholn M., Tkach V., Bell J. First record of Leucocytozoon (Haemosporida: Leucocytozoidae) in Amazonia: evidence for rarity in Neotropical lowlands or lack of sampling for this parasite genus? J. Parasitol. 2018;104:168–172. doi: 10.1645/17-182. [DOI] [PubMed] [Google Scholar]; Fecchio, A., Silveira, P., Weckstein, J., Dispoto, J., Anciaes, M., Bosholn, M., Tkach, V., Bell, J., 2018. First record of Leucocytozoon (Haemosporida: Leucocytozoidae) in Amazonia: evidence for rarity in Neotropical lowlands or lack of sampling for this parasite genus? J. Parasitol. 104, 168-172. [DOI] [PubMed]
- Galen S.C., Witt C.C. Diverse avian malaria and other haemosporidian parasites in Andean house wrens: evidence for regional co‐diversification by host‐switching. J. Avian Biol. 2014;45:374–386. [Google Scholar]; Galen, S.C., Witt, C.C., 2014. Diverse avian malaria and other haemosporidian parasites in Andean house wrens: evidence for regional co-diversification by host-switching. J. Avian Biol. 45, 374-386.
- Gil-Vargas D.L., Sedano-Cruz R.E. Genetic variation of avian malaria in the tropical Andes: a relationship with the spatial distribution of hosts. Malar. J. 2019;18:129. doi: 10.1186/s12936-019-2699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gil-Vargas, D.L., Sedano-Cruz, R.E., 2019. Genetic variation of avian malaria in the tropical Andes: a relationship with the spatial distribution of hosts. Malar. J. 18, 129. [DOI] [PMC free article] [PubMed]
- González A.D., Lotta I.A., García L.F., Moncada L.I., Matta N.E. Avian haemosporidians from Neotropical highlands: evidence from morphological and molecular data. Parasitol. Int. 2015;64:48–59. doi: 10.1016/j.parint.2015.01.007. [DOI] [PubMed] [Google Scholar]; Gonzalez, A.D., Lotta, I.A., Garcia, L.F., Moncada, L.I., Matta, N.E., 2015. Avian haemosporidians from Neotropical highlands: evidence from morphological and molecular data. Parasitol. Int. 64, 48-59. [DOI] [PubMed]
- González A.D., Matta N.E., Ellis V.A., Miller E.T., Ricklefs R.E., Gutiérrez H.R. Mixed species flock, nest height, and elevation partially explain avian haemoparasite prevalence in Colombia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100695. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gonzalez, A.D., Matta, N.E., Ellis, V.A., Miller, E.T., Ricklefs, R.E., Gutierrez, H.R., 2014. Mixed species flock, nest height, and elevation partially explain avian haemoparasite prevalence in Colombia. PloS One 9, e100695. [DOI] [PMC free article] [PubMed]
- Greiner E.C. Leucocytozoon maccluri sp. n. (Haemosporida: Leucocytozoidae) from a Thailand thrush, Zoothera marginata Blyth. J. Parasitol. 1976;62:545–547. doi: 10.2307/3279409. [DOI] [PubMed] [Google Scholar]; Greiner, E.C., 1976. Leucocytozoon maccluri sp. n. (Haemosporida: Leucocytozoidae) from a Thailand thrush, Zoothera marginata Blyth. J. Parasitol. 62, 545-547. https://doi.org/10.2307/3279409 [PubMed]
- Harrigan R.J., Sedano R., Chasar A.C., Chaves J.A., Nguyen J.T., Whitaker A., Smith T.B. New host and lineage diversity of avian haemosporidia in the northern Andes. Evol. Appl. 2014;7:799–811. doi: 10.1111/eva.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]; Harrigan, R.J., Sedano, R., Chasar, A.C., Chaves, J.A., Nguyen, J.T., Whitaker, A., Smith, T.B., 2014. New host and lineage diversity of avian haemosporidia in the northern Andes. Evol. Appl. 7, 799-811. [DOI] [PMC free article] [PubMed]
- Hellgren O., Waldenström J., Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]; Hellgren, O., Waldenstrom, J., Bensch, S., 2004. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 90, 797-802. [DOI] [PubMed]
- Herzog S.K., Kattan G.H. Patterns of diversity and endemism in the birds of the tropical Andes. Clim. Change Biodivers. Trop. Andes McArthur Found. Inter-Am. Inst. Glob. Change Res. IAI Sci. Comm. Probl. Environ. SCOPE Paris. 2011:245–259. [Google Scholar]; Herzog, S.K., Kattan, G.H., 2011. Patterns of diversity and endemism in the birds of the tropical Andes. Clim. Change Biodivers. Trop. Andes McArthur Found. Inter-Am. Inst. Glob. Change Res. IAI Sci. Comm. Probl. Environ. SCOPE Paris 245-259.
- International Commission on Zoological Nomenclature . fourth ed. International Trust for Zoological Nomenclature; London: 1999. International Code of Zoological Nomenclature. [Google Scholar]; International Commission on Zoological Nomenclature, 1999. International code of zoological nomenclature, fourth edition. ed. International Trust for Zoological Nomenclature, London.
- Jones M., Cheviron Z., Carling M. Spatial patterns of avian malaria prevalence in Zonotrichia capensis on the western slope of the Peruvian Andes. J. Parasitol. 2013;99:903–906. doi: 10.1645/12-147.1. [DOI] [PubMed] [Google Scholar]; Jones, M., Cheviron, Z., Carling, M., 2013. Spatial patterns of avian malaria prevalence in Zonotrichia capensis on the western slope of the Peruvian Andes. J. Parasitol. 99, 903-906. [DOI] [PubMed]
- Josse C., Cuesta F., Navarro G., Barrena V., Cabrera E., Chacón-Moreno E., Ferreira W., Peralvo M., Saito J., Tovar A. Secr. Gen. Comunidad Andina Lima; 2009. Ecosistemas de los Andes del norte y centro. Bolivia, Colombia, Ecuador, Perú y Venezuela. [Google Scholar]; Josse, C., Cuesta, F., Navarro, G., Barrena, V., Cabrera, E., Chacon-Moreno, E., Ferreira, W., Peralvo, M., Saito, J., Tovar, A., 2009. Ecosistemas de los Andes del norte y centro. Bolivia, Colombia, Ecuador, Peru y Venezuela. Secr. Gen. Comunidad Andina Lima.
- Krone O., Waldenström J., Valkiūnas G., Lessow O., Müller K., Iezhova T.A., Fickel J., Bensch S. Haemosporidian blood parasites in European birds of prey and owls. J. Parasitol. 2008;94:709. doi: 10.1645/GE-1357R1.1. [DOI] [PubMed] [Google Scholar]; Krone, O., Waldenstrom, J., Valkiūnas, G., Lessow, O., Muller, K., Iezhova, T.A., Fickel, J., Bensch, S., 2008. Haemosporidian blood parasites in European birds of prey and owls. J. Parasitol. 94, 709. https://doi.org/10.1645/GE-1357R1.1 [DOI] [PubMed]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kumar, S., Stecher, G., Tamura, K., 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870-1874. [DOI] [PMC free article] [PubMed]
- Londoño G.A., Saavedra-R C.A., Osorio D., Martínez J. Notas sobre la anidación del Tororoi Bigotudo (Grallaria alleni) en la Cordillera Central de Colombia. Ornitol. Colomb. 2004;2:19–24. [Google Scholar]; Londoño, G.A., Saavedra-R, C.A., Osorio, D., Martinez, J., 2004. Notas sobre la anidacion del Tororoi Bigotudo (Grallaria alleni) en la Cordillera Central de Colombia. Ornitol Colomb 2, 19-24.
- Lotta I.A., Gonzalez A.D., Pacheco M.A., Escalante A.A., Valkiūnas G., Moncada L.I., Matta N.E. Leucocytozoon pterotenuis sp. nov.(Haemosporida, Leucocytozoidae): description of the morphologically unique species from the Grallariidae birds, with remarks on the distribution of Leucocytozoon parasites in the Neotropics. Parasitol. Res. 2015;114:1031–1044. doi: 10.1007/s00436-014-4269-y. [DOI] [PubMed] [Google Scholar]; Lotta, I.A., Gonzalez, A.D., Pacheco, M.A., Escalante, A.A., Valkiūnas, G., Moncada, L.I., Matta, N.E., 2015. Leucocytozoon pterotenuis sp. nov.(Haemosporida, Leucocytozoidae): description of the morphologically unique species from the Grallariidae birds, with remarks on the distribution of Leucocytozoon parasites in the Neotropics. Parasitol. Res. 114, 1031-1044. [DOI] [PubMed]
- Lotta I.A., Pacheco M.A., Escalante A.A., González A.D., Mantilla J.S., Moncada L.I., Adler P.H., Matta N.E. Leucocytozoon diversity and possible vectors in the neotropical highlands of Colombia. Protist. 2016;167:185–204. doi: 10.1016/j.protis.2016.02.002. [DOI] [PubMed] [Google Scholar]; Lotta, I.A., Pacheco, M.A., Escalante, A.A., Gonzalez, A.D., Mantilla, J.S., Moncada, L.I., Adler, P.H., Matta, N.E., 2016. Leucocytozoon Diversity and Possible Vectors in the Neotropical highlands of Colombia. Protist 167, 185-204. [DOI] [PubMed]
- Mantilla J.S., González A.D., Lotta I.A., Moens M., Pacheco M.A., Escalante A.A., Valkiūnas G., Moncada L.I., Pérez-Tris J., Matta N.E. Haemoproteus erythrogravidus n. sp.(Haemosporida, Haemoproteidae): description and molecular characterization of a widespread blood parasite of birds in South America. Acta Trop. 2016;159:83–94. doi: 10.1016/j.actatropica.2016.02.025. [DOI] [PubMed] [Google Scholar]; Mantilla, J.S., Gonzalez, A.D., Lotta, I.A., Moens, M., Pacheco, M.A., Escalante, A.A., Valkiūnas, G., Moncada, L.I., Perez-Tris, J., Matta, N.E., 2016. Haemoproteus erythrogravidus n. sp.(Haemosporida, Haemoproteidae): Description and molecular characterization of a widespread blood parasite of birds in South America. Acta Trop. 159, 83-94. [DOI] [PubMed]
- Mantilla J.S., González A.D., Valkiūnas G., Moncada L.I., Matta N.E. Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol. Res. 2013;112:4193–4204. doi: 10.1007/s00436-013-3611-0. [DOI] [PubMed] [Google Scholar]; Mantilla, J.S., Gonzalez, A.D., Valkiūnas, G., Moncada, L.I., Matta, N.E., 2013. Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol. Res. 112, 4193-4204. [DOI] [PubMed]
- Martinsen E.S., Perkins S.L., Schall J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol. Phylogenetics Evol. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]; Martinsen, E.S., Perkins, S.L., Schall, J.J., 2008. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 47, 261-273. [DOI] [PubMed]
- Marzal A., García-Longoria L., Cárdenas Callirgos J., Sehgal R. Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol. Invasions. 2015;17:39–45. doi: 10.1007/s10530-014-0718-x. [DOI] [Google Scholar]; Marzal, A., Garcia-Longoria, L., Cardenas Callirgos, J., Sehgal, R., 2015. Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol. Invasions 17, 39-45. https://doi.org/10.1007/s10530-014-0718-x
- Matta N.E., Lotta I.A., Valkiūnas G., González A.D., Pacheco M.A., Escalante A.A., Moncada L.I., Rodríguez-Fandiño O.A. Description of Leucocytozoon quynzae sp. nov.(Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol. Res. 2014;113:457–468. doi: 10.1007/s00436-013-3675-x. [DOI] [PubMed] [Google Scholar]; Matta, N.E., Lotta, I.A., Valkiūnas, G., Gonzalez, A.D., Pacheco, M.A., Escalante, A.A., Moncada, L.I., Rodriguez-Fandiño, O.A., 2013. Description of Leucocytozoon quynzae sp. nov.(Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol. Res. 113, 457-468. [DOI] [PubMed]
- McMullan M., Quevedo A., Donegan T.M. Guía de campo de las aves de Colombia. ProAves. 2011 [Google Scholar]; McMullan, M., Quevedo, A., Donegan, T.M., 2011. Guia de campo de las aves de Colombia. ProAves.
- McWilliam H., Li W., Uludag M., Squizzato S., Park Y.M., Buso N., Cowley A.P., Lopez R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41:W597–W600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]; McWilliam, H., Li, W., Uludag, M., Squizzato, S., Park, Y.M., Buso, N., Cowley, A.P., Lopez, R., 2013. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 41, W597-W600. [DOI] [PMC free article] [PubMed]
- Merino S., Moreno J., Vásquez R.A., Martinez J., Sanchez‐Monsalvez I., Estades C.F., Ippi S., Sabat P., Rozzi R., Mcgehee S. Haematozoa in forest birds from southern Chile: latitudinal gradients in prevalence and parasite lineage richness. Austral Ecol. 2008;33:329–340. [Google Scholar]; Merino, S., Moreno, J., Vasquez, R.A., Martinez, J., Sanchez-Monsalvez, I., Estades, C.F., Ippi, S., Sabat, P., Rozzi, R., Mcgehee, S., 2008. Haematozoa in forest birds from southern Chile: latitudinal gradients in prevalence and parasite lineage richness. Austral Ecol. 33, 329-340.
- Miller M.A., Pfeiffer W., Schwartz T. Gateway Computing Environments Workshop. GCE); Ieee: 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; pp. 1–8. 2010. [Google Scholar]; Miller, M.A., Pfeiffer, W., Schwartz, T., 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees, in: Gateway Computing Environments Workshop (GCE), 2010. Ieee, pp. 1-8.
- Moens M.A., Pérez-Tris J. Discovering potential sources of emerging pathogens: South America is a reservoir of generalist avian blood parasites. Int. J. Parasitol. 2016;46:41–49. doi: 10.1016/j.ijpara.2015.08.001. [DOI] [PubMed] [Google Scholar]; Moens, M.A., Perez-Tris, J., 2016. Discovering potential sources of emerging pathogens: South America is a reservoir of generalist avian blood parasites. Int. J. Parasitol. 46, 41-49. [DOI] [PubMed]
- Moens M.A., Valkiūnas G., Paca A., Bonaccorso E., Aguirre N., Pérez‐Tris J. Parasite specialization in a unique habitat: hummingbirds as reservoirs of generalist blood parasites of Andean birds. J. Anim. Ecol. 2016;85:1234–1245. doi: 10.1111/1365-2656.12550. [DOI] [PubMed] [Google Scholar]; Moens, M.A., Valkiūnas, G., Paca, A., Bonaccorso, E., Aguirre, N., Perez-Tris, J., 2016. Parasite specialization in a unique habitat: hummingbirds as reservoirs of generalist blood parasites of Andean birds. J. Anim. Ecol. 85, 1234-1245. [DOI] [PubMed]
- Morrone J.J. first ed. M&T – Manuales y Tesis SEA; Zaragoza, España: 2001. Biogeografía de América Latina y el Caribe. [Google Scholar]; Morrone, J.J., 2001. Biogeografia de America Latina y el Caribe., 1st ed. M&T - Manuales y Tesis SEA, Zaragoza, España.
- Muñoz E., Ferrer D., Molina R., Adlard R. Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Vet. Rec. 1999;144:632–636. doi: 10.1136/vr.144.23.632. [DOI] [PubMed] [Google Scholar]; Muñoz, E., Ferrer, D., Molina, R., Adlard, R., 1999. Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Vet. Rec. 144, 632-636. [DOI] [PubMed]
- Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]; Myers, N., Mittermeier, R.A., Mittermeier, C.G., Da Fonseca, G.A., Kent, J., 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853. [DOI] [PubMed]
- Ortego J., Cordero P.J. PCR-based detection and genotyping of haematozoa (Protozoa) parasitizing eagle owls, Bubo bubo. Parasitol. Res. 2009;104:467–470. doi: 10.1007/s00436-008-1207-x. [DOI] [PubMed] [Google Scholar]; Ortego, J., Cordero, P.J., 2009. PCR-based detection and genotyping of haematozoa (Protozoa) parasitizing eagle owls, Bubo bubo. Parasitol. Res. 104, 467-470. https://doi.org/10.1007/s00436-008-1207-x [DOI] [PubMed]
- Pacheco M.A., Battistuzzi F.U., Junge R.E., Cornejo O.E., Williams C.V., Landau I., Rabetafika L., Snounou G., Jones-Engel L., Escalante A.A. Timing the origin of human malarias: the lemur puzzle. BMC Evol. Biol. 2011;11:299. doi: 10.1186/1471-2148-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pacheco, M.A., Battistuzzi, F.U., Junge, R.E., Cornejo, O.E., Williams, C.V., Landau, I., Rabetafika, L., Snounou, G., Jones-Engel, L., Escalante, A.A., 2011. Timing the origin of human malarias: the lemur puzzle. BMC Evol. Biol. 11, 299. [DOI] [PMC free article] [PubMed]
- Pacheco M.A., Cepeda A.S., Bernotienė R., Lotta I.A., Matta N.E., Valkiūnas G., Escalante A.A. Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. Int. J. Parasitol. 2018 doi: 10.1016/j.ijpara.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pacheco, M.A., Cepeda, A.S., Bernotienė, R., Lotta, I.A., Matta, N.E., Valkiūnas, G., Escalante, A.A., 2018a. Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. Int. J. Parasitol. [DOI] [PMC free article] [PubMed]
- Pacheco M.A., Matta N.E., Valkiūnas G., Parker P.G., Mello B., Stanley C.E., Lentino M., Garcia-Amado M.A., Cranfield M., Kosakovsky Pond S.L. Mode and rate of evolution of haemosporidian mitochondrial genomes: timing the radiation of avian parasites. Mol. Biol. Evol. 2018;35:383–403. doi: 10.1093/molbev/msx285. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pacheco, M.A., Matta, N.E., Valkiūnas, G., Parker, P.G., Mello, B., Stanley, C.E., Lentino, M., Garcia-Amado, M.A., Cranfield, M., Kosakovsky Pond, S.L., 2018b. Mode and rate of evolution of haemosporidian mitochondrial genomes: timing the radiation of avian parasites. Mol. Biol. Evol. 35, 383-403. https://doi.org/10.1093/molbev/msx285 [DOI] [PMC free article] [PubMed]
- Peirce M.A. Haematozoa of Zambian birds. IV. Description of Leucocytozoon balmorali sp. nov. from Malaconotidae. J. Nat. Hist. 1984;18:223–226. doi: 10.1080/00222938400770181. [DOI] [Google Scholar]; Peirce, M.A., 1984. Haematozoa of Zambian birds. IV. Description of Leucocytozoon balmorali sp. nov. from Malaconotidae. J. Nat. Hist. 18, 223-226. https://doi.org/10.1080/00222938400770181
- Perez-Tris J., Bensch S. Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitology. 2005;131:15–23. doi: 10.1017/s003118200500733x. [DOI] [PubMed] [Google Scholar]; Perez-Tris, J., Bensch, S., 2005. Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitology 131, 15-23. [DOI] [PubMed]
- Perkins S.L. Molecular systematics of the three mitochondrial protein-coding genes of malaria parasites: corroborative and new evidence for the origins of human malaria. DNA Seq. 2008;19:471–478. doi: 10.1080/19401730802570926. [DOI] [PubMed] [Google Scholar]; Perkins, S.L., 2008. Molecular systematics of the three mitochondrial protein-coding genes of malaria parasites: Corroborative and new evidence for the origins of human malaria. DNA Seq. 19, 471-478. [DOI] [PubMed]
- Perkins S.L., Schall J. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitol. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]; Perkins, S.L., Schall, J., 2002. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitol. 88, 972-978. [DOI] [PubMed]
- Rambaut A. Institute of Evolutionary biology, University of Edinburgh; 2006. FigTree. Tree Figure Drawing Tool Version 1.3. 1. [Google Scholar]; Rambaut, A., 2006. FigTree. Tree figure drawing tool version 1.3. 1. Institute of Evolutionary biology, University of Edinburgh.
- Remsen J.V., Cadena C., Jaramillo A., Nores M., Areta J., Claramunt S., Pacheco J., Pérez-Emán J., Robbins M., Stiles F., Stotz D., Zimmer K. 2012. The South American Classification Committee of the American Ornithologists' Union: a New Classification of the Birds of South America.http://www.museum.lsu.edu/∼Remsen/2007-NeotropicalBirding.pdf [WWW Document]. URL. [Google Scholar]; Remsen, J.V., Cadena, C., Jaramillo, A., Nores, M., Areta, J., Claramunt, S., Pacheco, J., Perez-Eman, J., Robbins, M., Stiles, F., Stotz, D., Zimmer, K., 2012. The South American Classification Committee of the American Ornithologists’ Union: a new classification of the birds of South America [WWW Document]. URL http://www.museum.lsu.edu/∼Remsen/2007-NeotropicalBirding.pdf (accessed 9.19.2017).
- Richard F.A., Sehgal R.N., Jones H.I., Smith T.B. A comparative analysis of PCR-based detection methods for avian malaria. J. Parasitol. 2002;88:819–822. doi: 10.1645/0022-3395(2002)088[0819:ACAOPB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]; Richard, F.A., Sehgal, R.N., Jones, H.I., Smith, T.B., 2002. A comparative analysis of PCR-based detection methods for avian malaria. J. Parasitol. 88, 819-822. [DOI] [PubMed]
- Rodríguez O.A., Moya H., Matta N.E. Avian blood parasites in the National natural Park Chingaza: high Andes of Colombia. Hornero. 2009;24:1–6. [Google Scholar]; Rodriguez, O.A., Moya, H., Matta, N.E., 2009. Avian blood parasites in the National Natural Park Chingaza: high Andes of Colombia. Hornero 24, 1-6.
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]; Ronquist, F., Huelsenbeck, J.P., 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572-1574. [DOI] [PubMed]
- Sambrook J., Fritsch E.F., Maniathis T. Lab Press; NY USA: 1989. Molecular Cloning, 2nd Cold Spring Harbor. [Google Scholar]; Sambrook, J., Fritsch, E.F., Maniathis, T., 1989. Molecular Cloning, 2nd Cold Spring Harbor. Lab Press NY USA.
- Schaer J., Reeder D.M., Vodzak M.E., Olival K.J., Weber N., Mayer F., Matuschewski K., Perkins S.L. Nycteria parasites of Afrotropical insectivorous bats. Int. J. Parasitol. 2015;45:375–384. doi: 10.1016/j.ijpara.2015.01.008. [DOI] [PubMed] [Google Scholar]; Schaer, J., Reeder, D.M., Vodzak, M.E., Olival, K.J., Weber, N., Mayer, F., Matuschewski, K., Perkins, S.L., 2015. Nycteria parasites of Afrotropical insectivorous bats. Int. J. Parasitol. 45, 375-384. [DOI] [PubMed]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schneider, C.A., Rasband, W.S., Eliceiri, K.W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. [DOI] [PMC free article] [PubMed]
- Smith M.J., Ramey A. Prevalence and genetic diversity of haematozoa in South American waterfowl and evidence for intercontinental redistribution of parasites by migratory birds. Int. J. Parasitol. Parasites Wildl. 2015;4:22–28. doi: 10.1016/j.ijppaw.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith, M.J., Ramey, A., 2015. Prevalence and genetic diversity of haematozoa in South American waterfowl and evidence for intercontinental redistribution of parasites by migratory birds. Int. J. Parasitol. Parasites Wildl. 4, 22-28. https://doi.org/10.1016/j.ijppaw.2014.12.007 [DOI] [PMC free article] [PubMed]
- Stiles F., López H.A. La situación del Tororoi pechicanela (Grallaria haplonota, Formicariidae) en Colombia. Caldasia. 1995;17:607–610. [Google Scholar]; Stiles, F., Lopez, H.A., 1995. La situacion del Tororoi pechicanela (Grallaria haplonota, Formicariidae) en colombia. Caldasia 17, 607-610.
- Valkiūnas G. CRC Press; Boca Raton: 2005. Avian Malaria Parasites and Other Haemosporidia. [Google Scholar]; Valkiūnas, G., 2005. Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton.
- Valkiunas G., Iezhova T.A., Mironov S.V. Leucocytozoon hamiltoni n. sp. (Haemosporida, Leucocytozoidae) from the Bukharan great tit Parus bokharensis. J. Parasitol. 2002;88:577. doi: 10.2307/3285453. [DOI] [PubMed] [Google Scholar]; Valkiunas, G., Iezhova, T.A., Mironov, S.V., 2002. Leucocytozoon hamiltoni n. sp. (Haemosporida, Leucocytozoidae) from the Bukharan great tit Parus bokharensis. J. Parasitol. 88, 577. https://doi.org/10.2307/3285453 [DOI] [PubMed]
- Valkiūnas G., Sehgal R.N., Iezhova T.A., Hull A.C. Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids. J. Parasitol. 2010;96:170–177. doi: 10.1645/GE-2109.1. [DOI] [PubMed] [Google Scholar]; Valkiūnas, G., Sehgal, R.N., Iezhova, T.A., Hull, A.C., 2010. Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids. J. Parasitol. 96, 170-177. [DOI] [PubMed]
- Van Rooyen J., Lalubin F., Glaizot O., Christe P. Avian haemosporidian persistence and co-infection in great tits at the individual level. Malar. J. 2013;12:40. doi: 10.1186/1475-2875-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Van Rooyen, J., Lalubin, F., Glaizot, O., Christe, P., 2013. Avian haemosporidian persistence and co-infection in great tits at the individual level. Malar. J. 12, 40. [DOI] [PMC free article] [PubMed]
- Zehtindjiev P., Križanauskienė A., Bensch S., Palinauskas V., Asghar M., Dimitrov D., Scebba S., Valkiūnas G. A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction–based protocols for amplification of the cytochrome b gene. J. Parasitol. 2012;98:657–665. doi: 10.1645/GE-3006.1. [DOI] [PubMed] [Google Scholar]; Zehtindjiev, P., Križanauskienė, A., Bensch, S., Palinauskas, V., Asghar, M., Dimitrov, D., Scebba, S., Valkiūnas, G., 2012. A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction-based protocols for amplification of the cytochrome b gene. J. Parasitol. 98, 657-665. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.