Abstract
Background
Thoracic paravertebral block (TPVB) technique for thoracotomy has seen increased application. The erector spinae plane block (ESPB) technique is simpler to perform than TPVB. However, whether it can be employed as a safe alternative analgesic technique has not been verified by a head-to-head clinical study.
Methods
Ninety-four patients scheduled for thoracotomy lung surgeries were randomly allocated to an ESPB or TPVB group. Patients in both groups were provided with an intravenous patient-controlled analgesia (PCA) device containing sufentanil. Visual analogue scale (VAS) pain scores under the status of rest and cough were recorded at 1, 6, 12, and 24 h postoperatively. In addition, total press times of PCA were read from the PCA memory. The adverse effects, puncture time and success rate of one puncture were also recorded.
Results
There were no significant differences in pain scores at rest and cough between the ESPB and TPVB groups in each of the first two days after surgery, and no difference between the two groups was identified regarding postoperative sufentanil usage (P>0.05). There was no statistical difference in post-operative nausea and vomiting. There was significantly less hypotension (6.7% vs. 21.7%, P=0.04), bradycardia (0 vs. 8.7%, P=0.04), hematoma (0 vs. 10.9%, P=0.02) and a higher success rate of one puncture (82.2% vs. 54.3%, P<0.001) in the ESPB group.
Conclusions
Preoperative single-injection ESPB plus postoperative sufentanil PCA provided similar effects of pain relief for patients undergoing thoracotomy when comparing to TPVB. Yet, ESPB had the advantages of a lower adverse effect incidence.
Keywords: Analgesia, erector spinae plane block (ESPB), thoracic paravertebral block (TPVB), thoracotomy, adverse effects
Introduction
It is common to see thoracic surgical patients, including those undergoing video-assisted thoracoscopic surgery, suffering from severe postoperative pain. The impaired pulmonary functions after surgery may be worsened by this postoperative pain, while effective analgesia can prevent respiratory complications (1). Thoracic epidural block (TEB) and thoracic paravertebral block (TPVB) have been recommended as primary analgesic approaches for pain management after thoracotomy (2). TPVB offers comparable analgesia to TEB with fewer adverse effects, such as postoperative nausea and vomiting (PONV), hypotension and urinary retention, and fewer pulmonary complications (3). In recent years, there have been increased applications of TPVB with ultrasound guidance for thoracotomy (4). However, this is still a particularly challenging technique, as the needle is placed in immediate proximity to the pleura which makes the ultrasound-guided TPVB technique a risk for pleural puncture.
Emerging research has shown that the novel erector spinae plane block (ESPB) can be employed as a simple and safe alternative analgesic technique for acute post-surgical, post-traumatic, and chronic neuropathic thoracic pain in adults (5). However, to date, ESPB has only been described in case reports. Hence, a prospective cohort study was designed to compare the postoperative analgesia, adverse effects, and complications between TPVB and ESPB. As ESPB is simpler to operate than TPVB, we hypothesized that ESPB would provide similar pain relief but reduce adverse effect incidence. In this study, our primary outcomes measured were the postoperative visual analog scales (VAS) for pain under the status of rest and cough at 1, 6, 24, and 48 h after surgery. The secondary endpoints were comparisons of the incidence of adverse effects, puncture time, and success of one-time puncture.
Methods
The trial was designed as a single center, randomized, double-blind, parallel two-arm controlled study. It was approved by the Shanghai General Hospital Ethics Committee (Document No.: Decision Letter of Shanghai General Hospital Institutional Review Board 2018-14, Shanghai General Hospital Ethics Committee, China). The trial was registered at Chinese clinical trial registry (Chictr, http://www.chictr.org.cn/enindex.aspx), registration number: ChiCTR1800016361. Ninety-four adult patients scheduled for the wedge or segmental resection of the lung, or pulmonary lobectomy by posterolateral thoracotomy at Shanghai General Hospital in China, between May 2018 and October 2018 were included. Eligible patients were informed about the study and their written informed consents were received. All patients had an American Society of Anesthesiologists physical status of I-II. Exclusion criteria for patients were the following: ≤18 or ≥81 years old, body mass index (BMI) ≥30 or ≤18 kg/m2, infection of the skin at the site of the needle puncture, known allergies to any of the study drugs, pre-existing pain syndromes, pregnancy, severe hepatic disorders (phosphatase alkaline, aspartate aminotransferase, alanine aminotransferase greater than 2 times normal reference range), renal disorders (serum creatinine greater than 2 mg/dL, oliguria, anuria, or hemodialysis), or cardiovascular disorders (New York Heart Association functional class greater than II).
At the preoperative visit, all patients were instructed on how to evaluate their own pain by using a 10-cm visual analog pain scale (0= no pain, 10= maximum pain imaginable) and how to use the patient-controlled analgesia (PCA) device. Patients were randomized to receive either single-shot ESPB (ESPB group) or TPVB (TPVB group) in addition to patient-controlled analgesia (PCA) according to a computer-generated random number table.
The nerve block was performed in the preoperative block area following standardized monitoring, including noninvasive blood pressure (BP), electrocardiogram (EKG), and pulse oximetry (PO). Oxygen 2–3 L/min was applied through the nasal cannula, and midazolam 0.025 mg/kg iv. was given. All blocks were performed by the same three experienced senior attending doctors in ultrasonic-guided nerve blocks. The patient was placed in the lateral position. A Navi Ultrasound Scanner (Wisonic Medical Technology, Shenzhen, China) was used for block performance. The blocks were performed at the T5 level of the spine using an in-plane approach. Sensory block of the 5th intercostal space in the midaxillary line was assessed by bilaterally using cold perception until 30 minutes after nerve block. If sensory blockade did not occur, the patient was excluded from the study.
Patients underwent general anesthesia with standardized monitoring. General anesthetic induction was conducted with intravenous 0.3 µg/kg sufentanil, 1.5–2.0 mg/kg propofol, and 0.6 mg/kg rocuronium. Endobronchial intubation was performed with a double-lumen tube. Bispectral index monitoring was used to monitor the depth of anesthesia. Desflurane was used for anesthesia maintenance, with a BIS target range of 40–60. In the process of anesthesia, sufentanil 0.1 µg/kg and rocuronium 0.15 mg/kg was administered at one-hour intervals. Before closing the incision, flurbiprofen axetil sodium 0.1 was given intravenously. Three milligrams granisetron was also administered to prevent postoperative nausea and vomiting. Ringer’s solution was infused at a rate of 6 mL/kg/h during surgery.
All patients were extubated at the end of surgery and then transferred to the post-anesthesia care unit (PACU). In PACU, all subjects were given sufentanil 0.02 µg/kg/mL by PCA. The device was programmed to deliver sufentanil 0.03 µg/kg with a 15-min lockout period, and 0.03 µg/kg/h background infusion. After observation for one hour, patients were transferred to the intensive care unit (ICU) of the thoracic department for close monitoring.
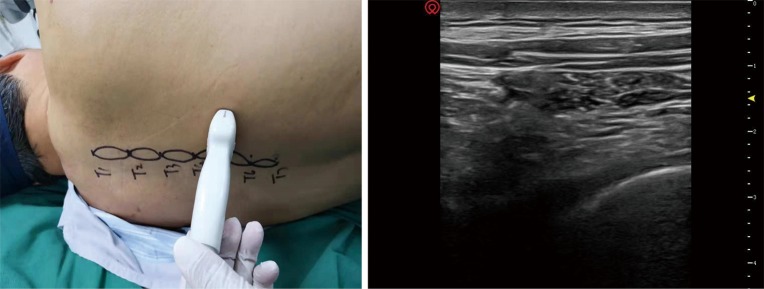
In the ESPB group, a high-frequency linear ultrasound probe was placed in a longitudinal orientation 3 cm from the midline. Once the erector spinae muscle and the transverse processes had been identified, a 22G 90-mm spinal needle (TuoRen Medical Instrument Finty Company, Zhengzhou, China) was inserted after standard skin disinfection in a caudad-to-cephalad direction using a sterile probe cover until the tip lay in the interfacial plane deep to the erector spinae muscle (Figure 1). After hydrolocalization with normal saline, this plane was opened. Twenty milliliters of 0.25% bupivacaine (Harvest Pharmaceutical Co. Ltd., Shanghai, China) was administered for block performance.
Figure 1.
Transducer position (left) and corresponding ultrasound image (right) of the thoracic 330 paravertebral block (TPVB).
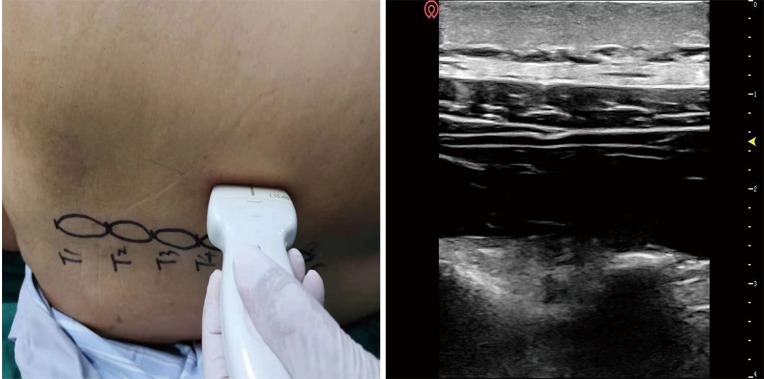
In the TPVB group, a high-frequency linear ultrasound probe was placed in a vertical orientation 2–3 cm lateral to the midline. Once the transverse process, internal intercostal membrane and parietal pleura had been identified, a 22G 90-mm spinal needle (TuoRen Medical Instrument Finty (Company, Zhengzhou, China) was inserted after standard skin disinfection laterally to medically Use a sterile probe cover until the tip lay in the thoracic paravertebral space beyond the internal intercostal membrane (Figure 2). After injection with normal saline, to confirm ventral pressing of the parietal pleura, 20 mL of 0.25% bupivacaine (Harvest Pharmaceutical Co. Ltd., Shanghai, China) was administered for block performance.
Figure 2.
Transducer position (left) and corresponding ultrasound image (right) of the erector spinae 334 plane block (ESPB).
During the first 48 h after surgery, all patients received flurbiprofen axetil 50 mg every 6 h intravenously and dezocine 10 mg intramuscularly as rescue analgesia if VAS score at rest was greater than 5, or the patient demanded additional analgesia. In the presence of nausea, with or without vomiting, granisetron 3 mg was given intravenously and repeated once if nausea persisted (maximum dose of 6 mg per day). Complications after puncturing of the blockade (hematoma in the puncture sites, pneumothorax) were evaluated by the same senior thoracic surgeon. Bradycardia, defined as heart rate (HR) <50 beats/min, was treated with atropine 0.5 mg intravenously. Hypotension, defined as a decrease in mean arterial pressure (MAP) of >20% from the baseline value was treated with mephedrone 6 mg intravenously. Tachycardia, defined as HR >110 beats/min was treated with esmolol 10 mg intravenously. Hypertension, defined as an increase in MAP of >20% from the baseline value, was treated with nicardipine 0.5 mg intravenously.
The age, weight, height, and The American Society of Anesthesiologists (ASA) class of each patient was recorded, as were the type and duration of surgery. The success rate of one-time puncture and operating time were documented. Patient satisfaction with a puncture was rated by the following score: 1= very dissatisfied, 2= dissatisfied, 3= dissatisfied, 4= very dissatisfied. Using the VAS, patients were asked to rate their pain under the status of rest and cough at 1, 6, 24, and 48 h after surgery. The total press times of PCA were read from the PCA memory. Forty-eight hours after the operation, patients were asked to rate their overall satisfaction with pain management using the following score: 1= very dissatisfied, 2= dissatisfied, 3= dissatisfied, 4= very dissatisfied. The adverse effects and unwanted effects were also recorded. Patients were not told which anesthetic procedure they had undergone to ensure they were masked. In addition, the masked assessment was done in all groups.
The differences of VAS pain scores under the status of rest and cough recorded at 1, 6, 12, and 24 h postoperatively were the primary outcomes. The secondary outcomes including total press times of PCA were read from the PCA memory. The adverse effects, puncture time, and success rate of one-time puncture was also recorded. The sample size was calculated based on the primary outcome measurement, the VAS scores at rest at 6 h after surgery. The results of a previous pilot study showed that the means of the TPVB and ESPB groups were 2.0 and 1.6 respectively, with a standard deviation (SD) of 0.6 in both groups. Using a two-sided t-test, 72 patients were required to achieve a power of 80% with an α value of 0.05 for detecting the differences between them. Taking into consideration possible dropouts, 94 patients (47 in each group) were enrolled in the study.
All statistical analyses were performed using IBM SPSS for Windows® version 22.0 software (SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to determine the normality of data distribution. Continuous variables were expressed as mean ± standard deviation, and median (25th–75th percentiles), and categorical variables as counts (percentages). Comparisons of normally distributed continuous variables between the groups were performed using Student’s t-test, while non-normally distributed continuous variables between the groups were compared using the Mann-Whitney U test. Comparisons of categorical variables between the groups were performed using Fisher’s Exact Chi-Square test, the Yates Chi-Square test, and the Monte Carlo Chi Square test. A two-sided P value <0.05 was considered statistically significant.
Results
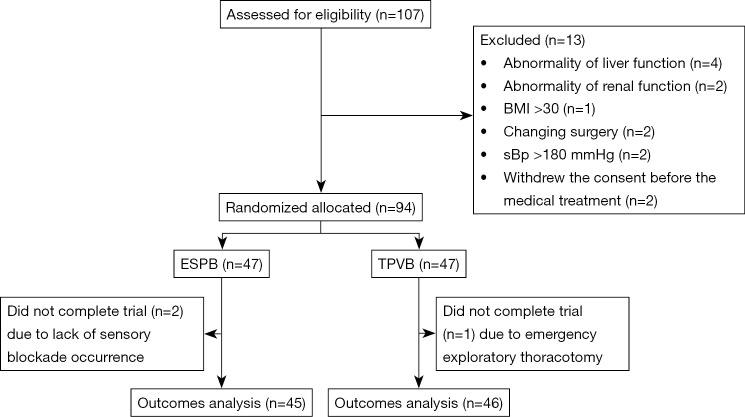
Of the 107 patients that were screened, 13 (12%) were excluded, and 94 (88%) underwent randomization between May 1, 2018, and October 21, 2018. Two patients in the ESPB group were excluded because sensory blockade did not occur; one patient in TPVB group was unable to assess the pain at 48 h after the operation because of an emergency exploratory thoracotomy. Thus, 91 participants (46 patients in the TPVB group and 45 patients in the ESPB group) ultimately completed the study (Figure 3). The patients’ characteristics are presented in Table 1. There was no statistical difference in terms of sex ratio, age, height, weight, ASA class, hypertension, diabetes mellitus, coronary disease, duration of surgery, and types of surgery.
Figure 3.
The participant flow chart.
Table 1. Patients’ characteristics.
| Characteristics | ESPB group, n=45 | TPVB group, n=46 | P value |
|---|---|---|---|
| Sex (male/female) | 22/23 | 19/27 | 0.47 |
| Age (years)a | 61.73±9.32 | 59.39±9.95 | 0.25 |
| Weight (kg)a | 63.27±12.15 | 63.09±9.37 | 0.94 |
| Height (cm)a | 163.38±8.03 | 163.74±7.84 | 0.83 |
| ASA | I/II: 10/35 | I/II: 8/38 | 0.56 |
| Hypertension | 23 (51.1%) | 21 (45.7%) | 0.92 |
| Diabetes mellitus | 7 (15.6%) | 9 (19.6%) | 0.62 |
| Coronary disease | 3 (6.7%) | 2 (4.3%) | 0.63 |
| Duration of surgery (min)a | 78.33±29.62 | 72.61±24.47 | 0.32 |
| Types of surgery | |||
| Wedge resection | 17 (37.8%) | 14 (30.4%) | 0.46 |
| Segmentectomy | 6 (13.3%) | 4 (8.7%) | 0.48 |
| Lobectomy | 22 (48.9%) | 28 (60.9%) | 0.91 |
a, Mean ± SD. ESPB, erector spinae plane block; TPVB, thoracic paravertebral block.
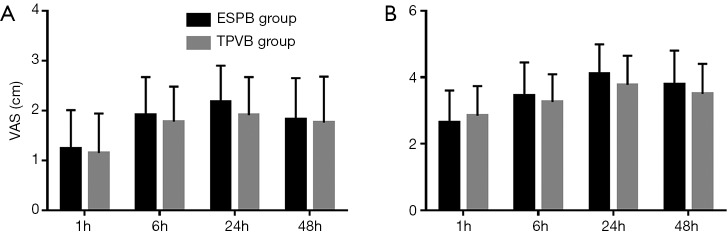
The pain scores (VAS) after surgery are shown in Figure 4. There was no difference in VAS at rest and during coughing between the TPVB group and ESPB group at 1, 6, 24, and 48 h after surgery. The difference of total press times of PCA between the groups was not statistically significant (Table 2). There was no difference in satisfaction with pain management between the ESPB group and the TPVB group (P=0.73; Mann-Whitney rank-sum test). One patient in the TPVB Group experienced excruciating pain of 6/10 VAS intensity approximately 11 hours after surgery, which was controlled by 10 mg dezocine intramuscularly.
Figure 4.
VAS at 1, 6, 24, and 48 h after surgery. (A) At rest; (B) during coughing post-surgery.
Table 2. Incidence of adverse effects; puncture time; success of puncture by once; press times of PCA.
| Variable | ESPB (n, %) | TPVB (n, %) | P value |
|---|---|---|---|
| Hypotension | 3, 6.7 | 10, 21.7 | 0.04 |
| Bradycardia | 0, 0 | 4, 8.7 | 0.04 |
| Hypertension | 3, 6.7 | 1, 2.2 | 0.29 |
| Tachycardia | 1, 2.2 | 0, 0 | 0.31 |
| PONV | 8, 17.8 | 11, 23.9 | 0.47 |
| Hematoma | 0, 0 | 5, 10.9 | 0.02 |
| Puncture time (min)a | 6.82±1.47 | 10.67±1.94 | 0.00 |
| Success of puncture by once | 37, 82.2 | 25, 54.3 | 0.00 |
| Press times of PCAa | 3.644±1.28 | 3.54±1.46 | 0.52 |
| Satisfaction with punctureb | 3.53±0.05 | 3.24±0.48 | 0.01 |
a, Mean ± SD. b, Mann-Whitney rank sum test. PCA, patient-controlled analgesia; ESPB, erector spinae plane block; TPVB, thoracic paravertebral block; PONV, postoperative nausea and vomiting.
The incidence of adverse effects, puncture time, success of one-time puncture, and press times of PCA is shown in Table 2. Hypotension occurred significantly more frequently in the TPVB group than in the ESPB group (21.7% vs. 6.7% respectively), as did bradycardia (4 patients in the TPVB group versus 0 patients in the ESPB group) (Table 2). Statistically, the frequency of hematoma was significantly higher in the TPVB group as compared to the ESPB group (P=0.02). Patients in the ESPB group felt more satisfied with puncture than those in the TPVB group (P=0.01; Mann-Whitney rank-sum test). The time to complete the single-shot erector spinae plane block under the guide of ultrasound was shorter than in TPVB. More cases in the ESPB group were punctured successfully once than those in the TPVB group. There were no differences in the presence of hypertension and tachycardia between the ESPB group and the TPVB group. No statistically significant differences were noted with respect to PONV.
Discussion
Over the past decade, the use of paravertebral block in patients undergoing thoracic surgery has increased, and some study results have even suggested that TPVB should replace TEB for thoracotomy patients (6,7). ESPB is a newly defined regional anesthesia technique which is a useful intervention in thoracic neuropathic pain and acute pain after thoracic surgery or trauma (5). To the best of our knowledge, this is the first controlled trial to compare postoperative analgesia between TPVB and ESPB after thoracic surgery. In the present study, the efficacy of thoracic paravertebral and erector spinae plane block was investigated by VAS scores and total consumption of sufentanil after the operation. We found that TPVB and ESPB provided a comparable amount of pain relief during the first 48 hours.
A continuous peripheral nerve block providing a prolonged block that may be titrated to the desired effect provides adequate analgesia which may even decrease the incidence and/or severity of chronic, persistent postsurgical pain (8). In our study, the patients undergoing thoracotomy were treated with single-injection peripheral nerve blocks which had no technical challenges in terms of infusion or catheter usage when combining with intravenous infusion of sufentanil and flurbiprofen axetil. The conclusion of our study was that both the TPVB and ESPB groups were provided with satisfactory postoperative pain control at rest in the first 48 hours.
Forero et al. (5) and Gurkan et al. (9) demonstrated that single-shot ultrasound-guided ESPB with 20 mL 0.25% bupivacaine at the T4 vertebral level before breast surgery exhibited a significant analgesic effect and reduced opioid consumption. Adhikary et al. (10) reported that an ESPB provided better analgesia in a pediatric case of video-assisted thoracic surgery (VATS) when compared with a thoracic epidural which failed to cover the upper thoracic dermatomes that had previously been employed on the same patient for a similar procedure on the opposite side. Nath et al. (11). utilized the ultrasound-guided continuous ESPB in two cases of open posterolateral thoracotomy and received excellent results. The ESPB and catheter placement performance after thoracotomy in in a lung transplant recipient provides effective postoperative analgesia (12). A single-injection ESPB was performed on 3 fresh cadavers at the T5 vertebral level by Adhikary et al. They found that 20 mL of a radiocontrast dye mixture produces epidural and neural foraminal spread across 2–5 levels which may have clinical effects similar to thoracic paravertebral blockade, and intercostal spread over 5 to 9 levels (13).
Ten patients in the TPVB group were treated with intravenous injection of ephedrine because of hypotension during the operation, while this occurred with only 3 patients in the ESPB group. There were significantly more patients (4 patients) in the TPVB group who suffered bradycardia intraoperatively than in the ESPB group (0 patients). We hypothesize that the incidence of hypotension and bradycardia increase may be a result of unilateral sympathetic blockage of TPVB.
The paravertebral block may replace epidural analgesia for thoracic surgery as it offers equivalent analgesic effect with fewer adverse effects like hypotension, urinary retention, and pulmonary complications (14). However, as the paravertebral space is often small, even with the guidance of ultrasound, the risk for pleural puncture, especially with a less skilled anesthesiologist, is still present (15). ESPB is a relatively safer method in which the transverse process acts as an anatomical barrier and avoids needle insertion into pleura (9). The relatively superficial nature of the ESPB, with the needle tip distant from the pleura and no structures at risk of needle injury in the immediate vicinity provides advantages of technical simplicity, direct ultrasound visualization, and less concern in the case of a hematoma (5,12). In our study, 5 patients in the TPVB group experienced hematoma occurring at the puncture site, and ESPB showed advantages of shorter puncture time, a higher success rate of single puncture, and higher satisfaction with the puncture. There was no difference between the two groups in terms of PONV (ESPB: 17.8%; TPVB: 21.7%), which may be explained by the similar cumulative sufentanil consumptions postoperatively.
In our setting, a single-injection nerve block was combined with sufentanil PCA. However, our results show that at 1 hour after surgery patients had VAS scores ≤3 when coughing, while at 6, 24, and 48 h, patients had VAS scores >3 when coughing, which implies inadequate analgesia. Also, in our study, there was one patient in the TPVB group who suffered excruciating pain at 11 hours after operation. This may be explained by the limited time in single-injection in block anesthesia.
This study design has three main limitations. First, the primary outcome, VAS, is not an objective indicator, so it may affect the efficacy evaluation. In addition, the sample size of this study is small. Therefore, the results may need to be further validated by a larger sample size test. Another limitation is that there was an intravenous catheter rather than an indwelling one for postoperative analgesia, and this could have had an impact on the analgesic effect of the two methods.
In summary, both preoperative single-dose thoracic paravertebral block plus postoperative sufentanil PCA and erector spinal plane block provide enough pain relief for patients undergoing thoracotomy. Erector spinal plane block has advantages in terms of greater technical simplicity, lower incidence of hypotension, and the prevention of hematoma. It may be thus considered as a viable alternative to thoracic paravertebral block in establishing postoperative analgesia.
Acknowledgements
None.
Ethical Statement: The trial was approved by the Shanghai General Hospital Ethics Committee (Document No.: Decision Letter of Shanghai General Hospital Institutional Review Board 2018-14, Shanghai General Hospital Ethics Committee, China). This trial was registered at the Chinese clinical trial registry (Chictr) (http://www.chictr.org.cn/enindex.aspx). The registration number is ChiCTR1800016361.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Xue FS, Li BW, Zhang GS, et al. The influence of surgical sites on early postoperative hypoxemia in adults undergoing elective surgery. Anesth Analg 1999;88:213-9. [DOI] [PubMed] [Google Scholar]
- 2.Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. 10.1093/bja/ael020 [DOI] [PubMed] [Google Scholar]
- 4.Hara K, Sakura S, Nomura T, et al. Ultrasound guided thoracic paravertebral block in breast surgery. Anaesthesia 2009;64:223-5. 10.1111/j.1365-2044.2008.05843.x [DOI] [PubMed] [Google Scholar]
- 5.Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 2016;41:621-7. 10.1097/AAP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 6.Daly DJ, Myles PS. Update on the role of paravertebral blocks for thoracic surgery: Are they worth it? Curr Opin Anaesthesiol 2009;22:38-43. 10.1097/ACO.0b013e32831a4074 [DOI] [PubMed] [Google Scholar]
- 7.Conlon NP, Shaw AD, Grichnik KP. Postthoracotomy paravertebral analgesia: Will it replace epidural analgesia? Anesthesiol Clin 2008;26:369-80, viii. 10.1016/j.anclin.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 8.Ilfeld BM. Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg 2017;124:308-35. 10.1213/ANE.0000000000001581 [DOI] [PubMed] [Google Scholar]
- 9.Gurkan Y, Aksu C, Kus A, et al. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: A randomized controlled study. J Clin Anesth 2018;50:65-8. 10.1016/j.jclinane.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 10.Adhikary SD, Pruett A, Forero M, et al. Erector spinae plane block as an alternative to epidural analgesia for post-operative analgesia following video-assisted thoracoscopic surgery: A case study and a literature review on the spread of local anaesthetic in the erector spinae plane. Indian J Anaesth 2018;62:75-8. 10.4103/ija.IJA_693_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath S, Bhoi D, Mohan VK, et al. Usg-guided continuous erector spinae block as a primary mode of perioperative analgesia in open posterolateral thoracotomy: A report of two cases. Saudi J Anaesth 2018;12:471-4. 10.4103/sja.SJA_755_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelava M, Anthony D, Elsharkawy H. Continuous erector spinae block for postoperative analgesia after thoracotomy in a lung transplant recipient. J Cardiothorac Vasc Anesth 2018;32:e9-11. 10.1053/j.jvca.2018.04.041 [DOI] [PubMed] [Google Scholar]
- 13.Adhikary SD, Bernard S, Lopez H, et al. Erector spinae plane block versus retrolaminar block: A magnetic resonance imaging and anatomical study. Reg Anesth Pain Med 2018;43:756-62. [DOI] [PubMed] [Google Scholar]
- 14.Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. 10.1213/01.ane.0000333274.63501.ff [DOI] [PubMed] [Google Scholar]
- 15.Voscopoulos C, Palaniappan D, Zeballos J, et al. The ultrasound-guided retrolaminar block. Can J Anaesth 2013;60:888-95. 10.1007/s12630-013-9983-x [DOI] [PubMed] [Google Scholar]