Abstract
Background
The intraoperative lung protective effect of mechanical ventilation of different positive end-expiratory pressure (PEEP) levels on patients undergoing abdominal laparoscopic surgery with the steep Trendelenburg position remains undefined. The purpose of the study was to explore the optimal PEEP.
Methods
Sixty patients scheduled for abdominal laparoscopic surgery were randomized to four groups including: PEEP 0, 4, 8 and 12 cmH2O. The pulmonary dynamic compliance (Cdyn), dead space to tidal volume ratio (VD/VT), and intrapulmonary shunt ratio (QS/QT) were measured after anesthesia induction (T0), 5 min after pneumoperitoneum (PNP) with position change (T1), 30 (T2) and 60 min (T3) after PEEP, and end of surgery (T4).
Results
Cdyn increased when different levels of PEEP (including the 4, 8, and 12 cmH2O) were used vs. no PEEP (P<0.05). The VD/VT in PEEP 8 and 12 cmH2O were significantly improved than no PEEP (P<0.05). Meanwhile, the QS/QT in PEEP 12 cmH2O was higher than others during the procedures.
Conclusions
A moderate PEEP level (8 cmH2O) with low tidal volume was sufficient to improve Cdyn and to decrease VD/VT without increasing QS/QT, which was suggested to be a good choice of intraoperative lung protective ventilation during abdominal laparoscopic surgery with Trendelenburg position.
Keywords: Positive end-expiratory pressure (PEEP), pulmonary dynamic compliance (pulmonary Cdyn), dead space to tidal volume ratio (VD/VT), intrapulmonary shunt
Introduction
Laparoscopic surgery offers various postoperative benefits including quicker recovery and shorter hospital stay (1,2). But, the pneumoperitoneum (PNP) of 11–14 mmHg and extreme steep Trendelenburg position (T-position) of 25–40 used during the abdominal laparoscopic procedures can cause the splinting of the diaphragm, decrease of respiratory compliance (3), increase of intrathoracic pressure and the reduction of functional residual volume, which all contribute to the formation of atelectasis and the mismatching of the ventilation/perfusion ratio ending up with the postoperative pulmonary complications and prolonged hospital stay (4,5). Positive end-expiratory pressure (PEEP) prevents atelectasis (6,7) by consistently reopening the collapsed lung tissue (5). Also, it was proved to preserve homogeneous regional ventilation during laparoscopic surgery (8), and improve the postoperative pulmonary functions (9). However, given that individual opening and closing pressure cannot be determined in the operation room, many anaesthetists currently compromise on a rather low standard PEEP targeting the lower plateau pressure to reduce driving pressure and achieve seemingly adequate ventilation during laparoscopic surgery (10), which shows no specific evidence in protecting the lung. The clinicians are eager to know which level of PEEP might be truly beneficial during laparoscopic surgery, might provide compensatory alveolar pressure against the collapsing alveolar pressure and improve the pulmonary gas exchange and respiratory mechanics.
To address this question, this study investigated the effects of different PEEP levels on both respiratory mechanics and pulmonary gas exchange, and explored which level of PEEP should be used intraoperatively to benefit the patients undergoing laparoscopic surgery with steep T-position.
Methods
Study design and patients
This was a prospective study performed at the Department of Anesthesiology of Shanghai General Hospital from March 2016 to March 2017. This study was approved by the Medical Ethics Committee of Shanghai General Hospital and registered at the Chinese Clinical Trial Registry (ChiCTR-IOR-16008184). All patients were provided a written informed consent before participation.
Sixty consecutive patients were enrolled. The inclusion criteria: (I) American Society of Anesthesiologists (ASA) physical status I–II; (II) scheduled to undergo abdominal laparoscopic surgery (radical rectectomy or colectomy); (III) surgery was planned to be performed in the 30° T-position. The exclusion criteria: (I) <20 or >60 years of age; (II) obesity (body mass index, BMI >28 kg/m2); (III) any cardiovascular or pulmonary disorders; (IV) abnormal lung-function test results; or (V) abnormal blood test results for renal or hepatic function.
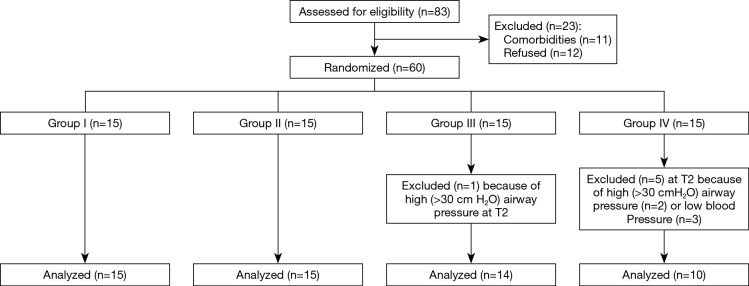
The enrolled patients were divided into four groups (n=15/group) (Figure 1). Group I was the control group and did not receive PEEP. After induction of anesthesia and the creation of the PNP, patients in groups II, III, and IV received PEEP at 4, 8, and 12 cmH2O.
Figure 1.
Study flowchart. Group I: control group (no PEEP); Group II: received PEEP at 4 cmH2O; Group III: received PEEP at 8 cmH2O; Group IV: received PEEP at 12 cmH2O. PEEP, positive end-expiratory pressure.
Outcomes
The primary outcomes were the changes of pulmonary dynamic compliance (Cdyn), dead space to tidal volume ratio (VD/VT), and intrapulmonary shunt ratio (QS/QT) in different PEEP levels at different time points.
Randomization
The randomization schedule was concealed from the investigators and generated by an independent statistician (Supplement I). The same person prepared sequentially numbered envelopes that were sealed and opaque to maintain allocation concealment until the time of randomization. The corresponding author enrolled the study subjects after evaluating eligibility. Patients were assigned to study groups by opening the randomization envelopes just before the start of anesthesia. Cdyn, PetCO2 (end tidal carbon dioxide partial pressure), PaCO2 (arterial partial pressure of carbon dioxide), PaO2 (arterial partial pressure of oxygen), SaO2 (arterial oxygen saturation), PvO2 (central venous partial pressure of oxygen), SvO2 (central venous oxygen saturation), and HB (hemoglobin levels) were measured at five time points (T0: after anesthesia induction before PNP and position change; T1: 5 min after PNP and T-position; T2: 30 min after PEEP; T3: 60 min after PEEP; and T4: end of the surgery before extubation).
Anesthesia management
All patients had fasted for 8–12 hours before surgery, and 500 mL of crystalloid solution was given before anesthesia induction. In the operating room, routine monitoring was established, including electrocardiogram (ECG), heart rate (HR), peripheral arterial oxygen saturation via pulse oximetry (SpO2), and PetCO2, using a S/5 monitor (Datex Ohmeda, Helsinki, Finland). A 20-G catheter (Angiocath, 8608376, H4774-2, BD Medical, Franklin Lake, NJ, USA) was inserted in the radial artery to monitor arterial blood pressure continuously. A 14-G catheter (Beihe Medical Co. LTD, Foshan, China) was placed in the right internal jugular vein under local anesthesia with lidocaine for hemodynamic measurements and blood sampling. Blood samples were taken to assess PaCO2, PaO2, SaO2, PvO2, SvO2, and HB.
Anesthesia was induced with midazolam (1–2 mg), sufentanyl (0.2–0.4 µg/kg), propofol (1.5–2.5 mg/kg), and rocuronium (0.6–1 mg/kg). These drugs were administered by intravenous injection during surgery. After tracheal intubation, airway pressure was maintained between 30 and 40 cmH2O for 30 s. Volume-controlled ventilation with a low tidal volume of 6–8 mL/kg (ideal body weight), a respiratory rate (RR) of 10–16 breaths/min, and an I/E ratio of 1:1.5 was maintained to keep PetCO2 within 35–45 mmHg and peak airway pressure (Ppeak) ≤30 cmH2O. Anesthesia was maintained with sevoflurane (1–1.2 minimum alveolar concentration) inhalation in 100% oxygen at a 1 L/min fresh air flow. Additional rocuronium (10 mg each time) and sufentanyl (5–10 µg each time) were administered to maintain constant muscle paralysis and a sufficient level of analgesia. Patients were placed in a 30° steep T-position after PNP of 12 mmHg intra-abdominal pressure under continuous monitoring (UHI-4, Olympus Medical Systems Corp, Tokyo, Japan). PEEP was applied until the end of the operation. Measurements were recorded at the five time points mentioned above.
Pulmonary mechanics and gas exchange parameters
The arterial and venous blood samples were taken at each time point for blood gas analysis (Radiometer, Copenhagen, Denmark). The central venous catheter was placed to collect blood samples instead of mixed venous blood samples (11). The pulmonary parameters including Cdyn were obtained directly from continuous airway monitoring technique (S/5, Datex Ohmeda).
The VD/VT was calculated with the following Eq. [1] (12):
| VD/VT = (PaCO2 − PetCO2)/PaCO2 | [1] |
| PaCO2 and PetCO2 were obtained directly from the arterial blood gas analysis and end-respiratory carbon dioxide monitoring. The QS/QT was calculated with the following Eq. [2] (13): |
| QS/QT = (CcO2 − CaO2)/(CcO2 − CvO2) | [2] |
CcO2 is the pulmonary capillary oxygen content. When the patient inhaled 100% oxygen, the CcO2 can be estimated from the Eq. [3] (13):
| CcO2 = 1.34× HB × SaO2 +0.003×(713− PaO2/0.8) | [3] |
CaO2 is the arterial oxygen content, and can be estimated from the Eq. [4] (13):
| CaO2 = 1.34× HB × SaO2 + 0.003× PaO2 | [4] |
CvO2 is the mixed venous oxygen content, and can be estimated from the Eq. [5] (13):
| CvO2 = 1.34× HB × SvO2 + 0.003× PvO2 | [5] |
SaO2, PaO2, SvO2, PvO2 and HB were all obtained from the result of blood gas analysis.
Statistical analysis
Power analysis was performed with G*Power 3.1. A total of 56 (14 patients per group) were required with a power of 80% and a P value of 0.05. Statistical analyses were performed using SPSS 19 (IBM Inc., Armonk, NY, USA). Continuous data were tested using the Kolmogorov-Smirnov test. Normally distributed continuous data were presented as means ± standard deviation (SD). Non-normally distributed continuous variables were presented using medians (range). Categorical data were presented as frequencies and analyzed using the Chi-square test. The hemodynamic parameters, Cdyn, VD/VT, and QS/QT at each time point were analyzed with two-way ANOVA (Tukey’s post hoc test) and the differences between time points in each group were analyzed with one-way ANOVA (Tukey’s post hoc test). P values <0.05 were considered statistically significant.
Results
Characteristics of the patients
Figure 1 presents the study flowchart. The present study included 60 patients, divided into four groups: PEEP 0 group (Group I), PEEP 4 group (Group II), PEEP 8 group (Group III), and PEEP 12 group (Group IV). Baseline characteristics did not differ between the groups (Table 1). All procedures were performed without complications and there was no conversion to open surgery. One patient in Group III and two in Group IV were excluded from the analyses because of high peak airway pressures (Ppeak >30 cmH2O). In addition, three patients in Group IV were excluded because of the hypotension (mean arterial pressure <65 mmHg) that could not be corrected by vasoactive agents (e.g., ephedrine, phenylephrine, perdipine). The hemodynamic measurements (HR, mean arterial pressure, and SpO2) did not differ among the four groups at any time (Table 2).
Table 1. Baseline characteristics of the patients.
| Variable | I | II | III | IV | P |
|---|---|---|---|---|---|
| Age (years) | 55±10 | 56±12 | 52±13 | 53±9 | 0.778 |
| BMI (kg/m2) | 24.3±3.0 | 22.8±2.0 | 23.8±2.1 | 24.0±2.8 | 0.398 |
| Types of surgery | |||||
| LAP rectectomy (n) | 6 (40.0) | 8 (53.3) | 7 (46.7) | 7 (46.7) | 0.911 |
| LAP colectomy (n) | 9 (60.0) | 7 (46.7) | 8 (53.3) | 8 (53.3) | |
| Pneumoperitoneum duration (min) | 126±30 | 116±26 | 113±28 | 117±32 | 0.629 |
| Operative time (min) | 154±19 | 146±27 | 143±28 | 153±22 | 0.539 |
Group I: control group (no PEEP); Group II: received PEEP at 4 cmH2O; Group III: received PEEP at 8 cmH2O; Group IV: received PEEP at 12 cmH2O. n=15/group. Data shown as are mean ± SD or n (%). BMI: body mass index, weight (kg)/height2 (m); PEEP, positive end-expiratory pressure.
Table 2. Hemodynamic parameters of the patients.
| Group | Factor | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| I | n | 15 | 15 | 15 | 15 | 15 |
| MAP (mmHg) | 87.0±11.9 | 88.0±10.4 | 84.2±11.4 | 82.4±9.4 | 85.6±10.0 | |
| HR (bpm) | 63.7±10.9 | 67.5±8.7 | 65.4±9.0 | 68.8±9.6 | 71.3±8.2 | |
| II | n | 15 | 15 | 15 | 15 | 15 |
| MAP (mmHg) | 91.2±8.5 | 89.7±6.6 | 88.4±5.4 | 86.3±4.3 | 92.4±7.2 | |
| HR (bpm) | 64.7±10.1 | 67.5±8.7 | 66.5±7.4 | 66.0±7.4 | 72.5±8.1 | |
| III | n | 15 | 15 | 14 | 14 | 14 |
| MAP (mmHg) | 86.0±12.0 | 85.7±10.6 | 84.5±9.1 | 83.0±9.1 | 83.8±12.2 | |
| HR (bpm) | 67.6±7.0 | 69.9±8.1 | 68.5±8.4 | 67.0±6.2 | 70.1±6.8 | |
| IV | n | 15 | 15 | 10 | 10 | 10 |
| MAP (mmHg) | 86.3±12.9 | 102.3±13.5 | 90.5±9.7 | 90.4±10.5 | 89.4±11.1 | |
| HR (bpm) | 61.0±8.0 | 70.8±13.5 | 67.9±6.5 | 66.0±5.6 | 62.7±5.4 |
Data are shown as means ± SD. Group I: control group (no PEEP); Group II: received PEEP at 4 cmH2O; Group III: received PEEP at 8 cmH2O; Group IV: received PEEP at 12 cmH2O. T0: baseline (after anesthesia induction but before PNP and position change); T1: 5 min after PNP and position change; T2: 30 min and T3: 60 min after PEEP; T4: end of surgery but before extubation. No significant difference at all time points among the groups (all P>0.05). PNP, pneumoperitoneum; MAP, mean arterial pressure; HR, heart rate.
Pulmonary parameter
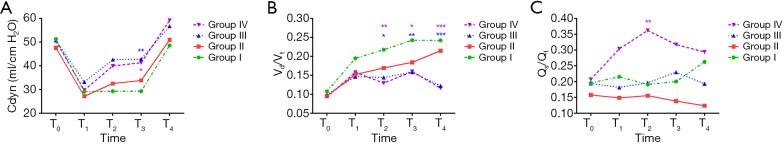
The Cdyn decreased significantly from T0 to T1, but did not differ between groups (Table 3). There were improvement of Cdyn in Groups II, III and IV at T2 compared with T1, and at T4 compared with T0. At T3, the Cdyn of Groups III and IV were higher than in Group I (Figure 2A).
Table 3. Pulmonary and pulmonary gas exchange parameters of the patients.
| Group | Factor | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| I | Cdyn (mL/cmH2O) | 51.33±10.96 | 29.07±8.66# | 29.27±8.02 | 29.27±8.60 | 48.60±10.72 |
| VD/VT (%) | 10.73±5.24 | 19.47±4.88# | 21.73±4.70 | 24.27±4.91 | 24.20±6.75# | |
| QS/QT (%) | 19.56±12.24 | 21.52±15.15 | 19.07±10.30 | 20.01±13.58 | 26.20±20.78 | |
| II | Cdyn (mL/cmH2O) | 47.60±10.61 | 27.13±5.78# | 32.53±5.99^ | 33.80±5.47 | 50.93±9.79# |
| VD/VT (%) | 9.47±5.80 | 15.20±7.04# | 16.93±6.91 | 18.40±7.76 | 21.47±7.58# | |
| QS/QT (%) | 15.75±4.47 | 14.83±6.58 | 15.53±7.60 | 13.82±7.85 | 12.33±6.29 | |
| III | Cdyn (mL/cmH2O) | 50.71±10.97 | 33.14±8.96# | 42.57±10.26^ | 42.79±10.72 | 56.71±11.06# |
| VD/VT (%) | 9.86±7.78 | 14.64±6.77# | 14.43±7.96 | 15.79±7.53 | 12.21±6.84 | |
| QS/QT (%) | 19.41±9.79 | 18.17±11.66 | 19.60±10.24 | 22.96±14.79 | 19.28±9.26 | |
| IV | Cdyn (mL/cmH2O) | 50.40±23.50 | 29.70±7.63# | 39.90±13.99^ | 41.30±13.09 | 59.20±19.33# |
| VD/VT (%) | 9.30±9.07 | 15.80±6.84# | 12.90±8.37 | 16.00±7.53 | 11.60±7.38 | |
| QS/QT (%) | 20.71±10.28 | 30.35±17.54 | 36.19±14.67# | 31.71±16.12# | 29.36±15.48 |
Data are shown as means ± SD. Group I: control group (no PEEP); Group II: received PEEP at 4 cmH2O; Group III: received PEEP at 8 cmH2O; Group IV: received PEEP at 12 cmH2O. T0: baseline (after anesthesia induction but before PNP and position change); T1: 5 min after PNP and position change; T2: 30 min and T3: 60 min after PEEP; T4: end of surgery but before extubation. #, P<0.05, compared to the baseline in the same group; ^, P<0.05, compared to the previous time point in the same group. PNP, pneumoperitoneum.
Figure 2.
Cdyn (A), VD/VT (B), and QS/QT (C) in the four groups (without the excluded patients). T0: baseline (after anesthesia induction but before PNP and position change); T1: 5 min after PNP and position change; T2: 30 min and T3: 60 min after PEEP; T4: end of surgery but before extubation (supine position without PNP). Group I: control group (no PEEP); Group II: received PEEP at 4 cmH2O; Group III: received PEEP at 8 cmH2O; Group IV: received PEEP at 12 cmH2O. *, P<0.05; **, P<0.01; ***, P<0.001. PEEP, positive end-expiratory pressure; PNP, pneumoperitoneum.
Pulmonary gas exchange parameters
The VD/VT increased significantly from T0 to T1 in all patients (Table 3). There was an increase of VD/VT in Groups I and II at T4 compared with T0, while no significant differences were found in Groups III and IV (Table 3). From T2 to T4. The VD/VT in Groups III and IV was significantly lower than Group I at the same time points (Figure 2B).
Compared with T0, there were significant increases of QS/QT in Group IV at T2 and T3, while no changes were found in the other groups (Table 3). At T2, the QS/QT in Group IV was higher than in the other groups (Figure 2C).
Discussion
The main finding of the present study was that setting the ventilation of a moderate PEEP level (8 cmH2O) with low tidal volume was sufficient to improve pulmonary Cdyn and VD/VT without increasing the intrapulmonary shunt in patients undergoing laparoscopic surgery with steep T-position. The results showed that a low level of PEEP may not be effective to compensate for the effects of PNP and T-position, and a high level of PEEP was associated with increased intrapulmonary shunt and haemodynamic depression.
PNP along with the steep T-position used in the laparoscopic surgery can cause a reduction of lung volume (14) resulting in impaired lung function during and after the surgery. Usually, anaesthetists can conquer the negative effects of PNP and steep T-position by using lung-protective ventilation strategies, mainly including PEEP, low-tidal volume and recruitment maneuvers. However, the correct value of PEEP remains a matter of debate. Haliloglu et al. (9) found that the postoperative pulmonary functions were less impaired in patients ventilated with a tidal volume of 6 mL/kg and 8 cmH2O PEEP than the patients ventilated with a tidal volume of 10 mL/kg and no PEEP. Similarly, Karsten et al. (8) also validated that PEEP (10 cmH2O) can preserve homogeneous regional ventilation and improve oxygenation and respiratory compliance in patients undergoing laparoscopic surgery. Our results suggested that using a moderate level of PEEP combined with low tidal volume improved both the respiratory mechanics and pulmonary gas exchange. The main effect of PEEP is to maintain the recruitment of alveolar units that were previously collapsed (15). Moderate level of PEEP in our study might have provided appropriate pressure to keep the alveolar units open without reducing venous reflux, which may lead to increased intrapulmonary shunt and haemodynamic depression.
Increased level of PEEP (12 cmH2O) combined with low tidal volume improved the Cdyn and reduced the VD/VT. However, the PEEP level of 12 cmH2O in our research was associated with higher QS/QT and haemodynamic depression. The most common complications of a high level of PEEP are hemodynamic effects and barotraumas. A high level of PEEP along with PNP and the steep T-position can lead to the increasing pressure of peak pressure, plateau (16) and intrapulmonary venous, which eventually resulted in the rise of QS/QT caused by the increasing intrapulmonary shunt and the mismatching of the ventilation/perfusion ratio. A previous trail (17) published on Lancet 2014 also showed that a high level of PEEP (12 cmH2O) does not protect against postoperative pulmonary complications, and much more likely to cause haemodynamic depression. Our outcome showed that the high level of PEEP was not only associated with hemodynamic trouble, but also performed poorly in improving the respiratory mechanics and pulmonary gas exchange intraoperatively. Increased level of PEEP (12 cmH2O) combined with low tidal volume may not be an ideal selection for laparoscopic surgery.
On the contrary, we found no advantages of using low level of PEEP (4 cmH2O) combined with low tidal volume. PEEP of 4 cmH2O during laparoscopic surgery is not high enough to act against a superimposed pressure (the hydrostatic pressure at the dependent portion of the lung resulting from the weight of the tissue above, which is the main reason for lung collapse) (18) and resulted in repeated opening and closing of small airways which might cause postoperative pulmonary complications (6,19). In 2015, Bender et al. (20) reported that the use of low PEEP (less than 5 cmH2O) intraoperatively has decreased significantly. However, there is still some voice of supporting low PEEP level ventilation. Park et al. (21) found that the low tidal volume with PEEP (5 cmH2O) during PNP with 30° reverse T-position and 20° left lateral tilt was associated with less incidences of pulmonary complications. Another study (22) suggested that low tidal volume with PEEP (5 cmH2O) application may be a good alternative for preventing high CO2 levels and yielding better oxygenation and lower extubation time in patients undergoing prolonged laparoscopic urology with T-position. Several differences might explain the opposite results: (I) these trials above enrolled the laparoscopic surgery with different surgical position; (II) they compared the different results on respiratory function caused by protective lung ventilation and conventional ventilation, not the different levels of PEEP.
There are several limitations to our study. The patients inhaled 100% oxygen instead of air-oxygen mixture due to the limitation of our central air supply department and the devices equipped in the operation room. Inhaling 100% oxygen may limit the efficacy of recruitment maneuvers and PEEP. One hundred percent oxygen can cause the collapse of partial alveolar, which eventually leads to the mismatch of ratio of ventilation and blood flow. This may explain the higher VD/VT and QS/QT of our results than theoretical data. Another limitation concerns not including standardization of the administration of fluid during the study. The two limitations mentioned above could be trivial since the situation was similar in each group. The hemodynamics of all the patients were monitored by invasive arterial blood pressure monitoring, which allows to reverse the changes rapidly. Vasoactive agents (e.g., ephedrine, phenylephrine, perdipine) were also used to stabilize the blood pressure and the HR, which may have some possible effects on vasodilation and contraction of pulmonary blood vessels. However, these vasoactive agents are all short-acting medication without possibility to affect the final results.
In conclusion, in this study we explored the intraoperative lung protective ventilation of different PEEP levels in patients with good functional status and without cardiopulmonary co-morbidities undergoing radical rectectomy or colectomy laparoscopic procedures with steep T-position. The moderate PEEP level (8 cmH2O) combined with low tidal volume could lead to better Cdyn and lower VD/VT without increasing QS/QT. Meanwhile, the high level of PEEP (12 cmH2O) was associated with increased QS/QT and haemodynamic depression. Our results suggest that a moderate PEEP level combined with low tidal volume could be a good choice of intraoperative lung protective ventilation for the patients undergoing abdominal laparoscopic surgery in the steep T-position. Further study regarding the clinical outcomes of different levels of PEEP is needed.
Acknowledgements
Acknowledgements to the Department of Anesthesiology, Shanghai General Hospital.
Supplementary
Supplement I CONSORT 2010 checklist of information to include when reporting a randomised trial*
Ethical Statement: This study was approved by the Medical Ethics Committee of Shanghai General Hospital and registered at the Chinese Clinical Trial Registry (ChiCTR-IOR-16008184). All patients were provided a written informed consent before participation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
we strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement.org.
| Section/topic | Item No. | Checklist item | Reported on page No. |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | P 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | P 2 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | P 4 |
| 2b | Specific objectives or hypotheses | P 4-5 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | P 5, 24 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | P 5 | |
| Participants | 4a | Eligibility criteria for participants | P 5 |
| 4b | Settings and locations where the data were collected | P 5 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | P 6-8 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | P 5-8 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | P 5-6 | |
| Sample size | 7a | How sample size was determined | P 8 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | P 9 | |
| Randomisation | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | P 6 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | P 6 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | P 6 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | P 6 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | – |
| 11b | If relevant, description of the similarity of interventions | – | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | P 8-9 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | P 8-9 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | P 9,24 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | P 9,24 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | |
| 14b | Why the trial ended or was stopped | P 9,24 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | P 20 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | P 9 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | P 9-10, P 21-22, 25 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | – | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | – |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | – |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | P 13 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | P 10, 13-14 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | – |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | P 5 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | P 6-7 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | P 16 |
References
- 1.Murray A, Lourenco T, de Verteuil R, et al. Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess 2006;10:1-141, iii-iv. 10.3310/hta10450 [DOI] [PubMed] [Google Scholar]
- 2.Noblett SE, Horgan AF. A prospective case-matched comparison of clinical and financial outcomes of open versus laparoscopic colorectal resection. Surg Endosc 2007;21:404-8. 10.1007/s00464-006-9016-8 [DOI] [PubMed] [Google Scholar]
- 3.Suh MK, Seong KW, Jung SH, et al. The effect of pneumoperitoneum and Trendelenburg position on respiratory mechanics during pelviscopic surgery. Korean J Anesthesiol 2010;59:329-34. 10.4097/kjae.2010.59.5.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahata O, Kunisawa T, Nagashima M, et al. Effect of age on pulmonary gas exchange during laparoscopy in the Trendelenburg lithotomy position. Acta Anaesthesiol Scand 2007;51:687-92. 10.1111/j.1399-6576.2007.01311.x [DOI] [PubMed] [Google Scholar]
- 5.Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med 2005;31:1327-35. 10.1007/s00134-005-2761-7 [DOI] [PubMed] [Google Scholar]
- 6.Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 2005;102:838-54. 10.1097/00000542-200504000-00021 [DOI] [PubMed] [Google Scholar]
- 7.Cinnella G, Grasso S, Spadaro S, et al. Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology 2013;118:114-22. 10.1097/ALN.0b013e3182746a10 [DOI] [PubMed] [Google Scholar]
- 8.Karsten J, Luepschen H, Grossherr M, et al. Effect of PEEP on regional ventilation during laparoscopic surgery monitored by electrical impedance tomography. Acta Anaesthesiol Scand 2011;55:878-86. 10.1111/j.1399-6576.2011.02467.x [DOI] [PubMed] [Google Scholar]
- 9.Haliloglu M, Bilgili B, Ozdemir M, et al. Low Tidal Volume-Positive End-expiratory Pressure versus High Tidal Volume-Zero Positive End-expiratory Pressure and Postoperative Pulmonary Functions in Robot-assisted Laparoscopic Radical Prostatectomy. Med Princ Pract 2017;26:573-8. 10.1159/000484693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladha K, Melo MF, Mclean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015;351:h3646. 10.1136/bmj.h3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahvanainen J, Meretoja O, Nikki P. Can central venous blood replace mixed venous blood samples? Crit Care Med 1982;10:758-61. 10.1097/00003246-198211000-00012 [DOI] [PubMed] [Google Scholar]
- 12.Bhalla AK, Rubin S, Newth CJ, et al. Monitoring Dead Space in Mechanically Ventilated Children: Volumetric Capnography Versus Time-Based Capnography. Respir Care 2015;60:1548-55. 10.4187/respcare.03892 [DOI] [PubMed] [Google Scholar]
- 13.Zeng YM. Critical Care Medicine. 1st ed. Beijing: People’s Health Publishing House, 2000:53-6. [Google Scholar]
- 14.Loring SH, Behazin N, Novero A, et al. Respiratory mechanical effects of surgical pneumoperitoneum in humans. J Appl Physiol 2014;117:1074-9. 10.1152/japplphysiol.00552.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pintado MC, De Pablo R, Trascasa M, et al. Individualized PEEP Setting in Subjects With ARDS: A Randomized Controlled Pilot Study. Respir Care 2013;58:1416-23. 10.4187/respcare.02068 [DOI] [PubMed] [Google Scholar]
- 16.Oba Y, Salzman GA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med 2000;343:813; author reply 813-4. [PubMed] [Google Scholar]
- 17.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology , Hemmes SN, Gama de Abreu M, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. 10.1016/S0140-6736(14)60416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cressoni M, Chiumello D, Carlesso E, et al. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology 2014;121:572-81. 10.1097/ALN.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 19.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. a concept of atelectasis. N Engl J Med 1963;269:991-6. 10.1056/NEJM196311072691901 [DOI] [PubMed] [Google Scholar]
- 20.Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative Lung-Protective Ventilation Trends and Practice Patterns: A Report from the Multicenter Perioperative Outcomes Group. Anesth Analg 2015;121:1231-9. 10.1213/ANE.0000000000000940 [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Kim BG, Oh AH, et al. Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: prospective, randomized and controlled trial. Surg Endosc 2016;30:4598-606. 10.1007/s00464-016-4797-x [DOI] [PubMed] [Google Scholar]
- 22.Ela Y, Bakı ED, Ateş M, et al. Exploring for the safer ventilation method in laparoscopic urologic patients? Conventional or low tidal? J Laparoendosc Adv Surg Tech A 2014;24:786-90. 10.1089/lap.2014.0004 [DOI] [PubMed] [Google Scholar]