Abstract
Background
Nasal nitric oxide (nNO) could be a biomarker for nasal passage inflammation and sinus ostial patency. We have aimed to investigate the nNO concentration and the effect of antibiotic therapy in children with perennial allergic rhinitis (PAR) children with/without acute bacterial sinusitis.
Methods
We enrolled a cohort of 90 and 31 children with PAR, without and with acute unilateral maxillary sinusitis, and 79 normal children. Acute bacterial maxillary sinusitis was diagnosed based on clinical signs and symptoms, radiographic examination and nasal fibroendoscopy. Rhinitis control assessment test (RCAT), rhinomanometry, nNO and fractional exhaled NO (FENO) measurements were performed before and 2 weeks after antibiotic therapy.
Results
We found significantly higher mean nNO levels, FENO values, and total nasal resistance in children with PAR than in normal children (p < 0.05). Acute unilateral maxillary sinusitis was associated with lower lesion-side nNO levels, higher FENO values, total nasal resistance, and poor RCAT scores (p < 0.05). In multivariate analysis, age, IgE, and acute maxillary sinusitis were significant factors influencing nNO levels in children with PAR. The lesion-side nNO levels, FENO values, total nasal resistance, and RCAT scores were reversed after antibiotic therapy (p < 0.05). The lesion-side nNO levels were significantly correlated to nasal obstructive scores (r = 0.59, p < 0.05) and expiratory nasal resistance (r = −0.54, p < 0.05) in the acute maxillary sinusitis. A cut-off nNO value of 538 ppb showed 100% sensitivity and 94.9% specificity, to predict PAR from normal children. An nNO value of 462 ppb showed 100% sensitivity and 100% specificity to discriminate between the lesion-side and the unaffected sinus-side in PAR children with acute unilateral maxillary sinusitis.
Conclusions
We conclude that the obstruction of NO from the sinus into the nasal passage is the likely explanation for the decreased lesion-side nNO levels in acute unilateral maxillary sinusitis. nNO is a non-invasive biomarker with high sensitivity to diagnose and monitor treatment responses of PAR patients with acute rhinosinusitis. Both nNO and FENO levels return to baseline following antibiotic therapy, supporting the “one airway one disease” concept.
Abbreviations: ARIA, Allergic rhinitis and its impact on asthma; Der p, Dermatophagoides pteronyssinus; Der f, Dermatophagoides farinae; FENO, Fractional Exhaled NO; NO, Nitric oxide; nNO, Nasal nitric oxide; RCAT, Rhinitis control assessment test
Introduction
Nitric oxide (NO), which is synthesized from l-arginine by inducible NO synthase (iNOS), is mainly produced by the epithelial and inflammatory cells in inflammatory airway disease.1, 2, 3 Bronchial fractional exhaled NO (FENO) measurement is used as a non-invasive approach to evaluate the degree of eosinophilic airway inflammation and to monitor treatment responses in asthmatic patients.4, 5, 6 Nasal NO (nNO) refers to the NO produced from ciliary epithelial cells in the paranasal sinus mucosa and seems to increase ciliary motility, thereby maintaining mucociliary clearance.7, 8 High nNO levels play an important role in mucosal immunity against airway pathogens, making the sinuses sterile.9 In patients with primary ciliary dyskinesia and cystic fibrosis, extremely low nNO levels have been demonstrated along with high levels of sensitivity and specificity.3, 10
Measurements of nNO are completely noninvasive and can easily be performed in children.11 nNO measurements may be a useful marker for evaluating nasal inflammation in allergic rhinitis (AR) patients,12. nNO production is triggered mainly by airborne allergens in combination with iNOS induction, and AR patients tend to show significant nNO levels.13, 14 However, there are some limitations in the measurement of nNO to monitor nasal inflammation in AR patients. Firstly, there is no consensus regarding the reference values for nNO levels obtained with different measurement techniques.15, 16, 17, 18 Secondly, detection of nNO concentrations could be influenced by medications, such as vasoconstrictors and intranasal steroid treatments,19 and finally, nNO levels may be low in AR patients with nasal polyps. These limitations make interpretations based on a single measure difficult in clinical practice.15, 16, 17, 18, 19, 20, 21
Rhinosinusitis is common in children with AR. Many studies have reported that nNO levels are affected by chronic rhinosinusitis with nasal polyps.22, 23 In chronic rhinosinusitis, nNO concentrations are thought to be reduced because of damage to ciliary beating and sinus ostial occlusion, with the gaseous NO in the sinuses failing to reach the nasal cavities.24 Some authors have proposed that nNO could be a post-operative biomarker to monitor the success of treatment for chronic rhinosinusitis, since nNO levels seem to correlate with radiographic staging and symptom improvement.22, 23, 24, 25
Acute rhinosinusitis is a frequent complication of upper respiratory infections that involve inflammation of the paranasal sinus mucosa and leads to mucosal swelling with mechanical obstruction of the paranasal sinus ostia.26 Acute rhinosinusitis is an important predisposing factor in difficult-to-control cases of AR with persistent nasal symptoms and chronic cough.27 However, there are few studies evaluating the value of nNO levels in the diagnosis of acute bacterial maxillary sinusitis in AR children. Our study aims to investigate the relationship between nNO levels and clinical characteristics, rhinitis control assessment test (RCAT) results, anterior rhinomanometry findings, and FENO levels in perennial AR patients with/without acute unilateral maxillary sinusitis and to determine the effect of antibiotic therapy for the sinusitis. Validation of nNO as a biomarker to predict and monitor treatment response in PAR patients with acute sinusitis will be of great value in the field of rhinology.
Methods
Subjects & clinical examination
This study prospectively recruited 200 children aged 6–13 years, including 121 PAR patients without (n = 91) and with (n = 31) acute unilateral maxillary sinusitis, from the Changhua Christian Hospital, Taiwan. PAR was defined according to the allergic rhinitis and its impact on asthma (ARIA) guidelines. At the initial visit, assessment of allergic rhinitis history, rhinitis control assessment test (RCAT), anterior rhinomanometry, nNO and FENO measurement using NIOX (Aerocrine, Sweden), and allergen-specific IgE testing for house dust mites (Dermatophagoides pteronyssinus [Der p], Dermatophagoides farina [Der f]) using the CAP system (Pharmacia Diagnostics, Uppsala, Sweden) were performed. Allergen-specific IgE testing for house dust mites (Der p, Der f) was performed as sensitization with house dust mites represents 90% of the allergy sensitization cases in Taiwan.28 Acute unilateral maxillary sinusitis was defined by signs and symptoms of acute sinusitis, such as purulent nasal discharge, purulent pharyngeal drainage, nocturnal and diurnal cough, and nasal congestion for at least 10 days prior to investigation.26 Acute unilateral maxillary sinusitis was confirmed by an otorhinolaryngologist using clinical otolaryngological assessments, video nasal fiberoptic endoscopy, and radiographic examinations (Water's view). The endoscopic signs of acute bacterial maxillary sinusitis included mucopurulent discharge, primarily from the middle meatus, and/or edema and/or mucosal obstruction, primarily in the middle meatus. Study subjects meeting one of the following criteria were excluded from the study: abnormal nasal and palatal anatomical structure, nasal polyps, prior nasal or sinus surgery, chronic cardiorespiratory diseases, history of asthma or nasal allergy requiring leukotriene receptor antagonist in the last seven days, and oral or nasal corticosteroids in the last one month. Seventy-nine age- and sex-matched children with normal serum IgE levels (<45 kU/L), who showed negative findings in skin prick tests, absence of specific allergen IgE (<0.35 kU/L) in the CAP assessments, and normal nasal fibroendoscopy and anterior rhinometry findings, were selected as healthy control subjects.
The study subjects were classified into normal control, PAR, and PAR with acute unilateral bacterial maxillary sinusitis groups according to the inclusion and exclusion criteria. All the enrolled children underwent clinical otolaryngological assessments and video nasal fiberoptic endoscopy using a Pentax endoscope diameter: 2.7 mm). For treatment of their acute bacterial sinusitis, patients received, depending on their age and drug allergy history, oral amoxicillin/clavulanate 50 mg/kg/day (GlaxoSmithKline, UK) for 10 days, according to their response to therapy. All enrolled patients were followed up for 48–72 h to observe the response of initial antibiotic treatment. Indications for hospitalization and parenteral antibiotic administration included toxic-appearance, complications, and treatment failure in the outpatient clinic. The uncomplicated patients with penicillin allergy received oral cephalosporin treatment for a total of 10 days.26 Patients with acute unilateral bacterial maxillary sinusitis were evaluated using RCAT, anterior rhinomanometry, and nNO and FENO measurements during the acute phase of the disease and 2 weeks after the start of antibiotic therapy. All sinusitis patients had not received antibiotic therapy for at least 2 months before the study, and none had recurrent sinusitis. Patients were examined by a physician, who was blinded to the nNO and FENO data, before and after treatment to confirm the diagnosis and response to therapy. This study was approved by the institutional review board (Changhua Christian Hospital), and written informed consent was obtained from each participant and the patient's legal guardians (CCH IRB No. 140906 and No. 160207).
Rhinitis control assessment test
The RCAT29 was performed in PAR patients with acute unilateral bacterial maxillary sinusitis before and after antibiotic treatment, and the RCAT scores were assessed by patients and their guardians. Score assessments were based on the intensity of the following symptoms: nasal congestion, nasal sneezing, watery eyes, interference of nasal or other allergy symptoms with sleep, avoidance of daily activity because of nasal or other allergy symptoms, and control of nasal or other allergy symptoms over the past week on a 5-point scale (1 = extremely often, 2 = often, 3 = sometimes, 4 = rarely, 5 = never experienced nasal congestion, nasal sneezing, watery eyes, and avoidance of daily activity because of nasal or other allergy symptoms; 1 = all the time, 2 = a lot, 3 = somewhat, 4 = a little, 5 = not at all in the “nasal or other allergy symptoms interfere with sleep” group; and 1 = not at all, 2 = a little, 3 = somewhat, 4 = very, 5 = completely in the “how well were the nasal or other allergy symptoms controlled” item) (Table 3).
Table 3.
Rhinitis control assessment test (RCAT) results among perennial allergic rhinitis (PAR) patients, and PAR with acute unilateral bacterial maxillary sinusitis patients before and after antibiotic therapy.
| RCAT items (Frequency) | PAR group (n = 90) | PAR with acute maxillary sinusitis group (n = 31) |
|
|---|---|---|---|
| Baseline | Follow-up (2 weeks) | ||
| Nasal congestion | 2.8 ± 1.0 | 1.7 ± 0.7* | 3.2 ± 0.9† |
| Nasal sneeze | 2.6 ± 1.1 | 2.6 ± 1.0 | 3.5 ± 1.2*† |
| Watery eyes | 3.6 ± 1.2 | 3.6 ± 1.1 | 3.9 ± 1.1 |
| Interfere with sleep | 4.1 ± 1.0 | 3.8 ± 1.2 | 4.5 ± 0.8† |
| Avoid daily activity | 4.0 ± 1.0 | 3.1 ± 1.1* | 4.1 ± 0.9† |
| Nasal symptoms controlled | 3.1 ± 1.2 | 2.4 ± 1.0* | 3.2 ± 0.9† |
| RCAT sum scores | 20.3 ± 4.5 | 17.3 ± 4.3* | 22.4 ± 4.5*† |
Rhinitis control assessment test (RCAT) rate nasal congestion, nasal sneezing, watery eyes, nasal or other allergy symptoms interfere with sleep, avoid daily activity because of nasal or other allergy symptoms and how well with nasal or other allergy symptoms controlled on a 1- to 5-point scale on the past week. (1 = extremely often, 2 = often, 3 = sometimes, 4 = rarely, 5 = never in “nasal congestion, nasal sneeze, watery eyes and avoid daily activity” items; 1 = all the time, 2 = a lot, 3 = somewhat, 4 = a little, 5 = not at all in “interfere with sleep” item; 1 = not at all, 2 = a little, 3 = somewhat, 4 = very, 5 = completely in “how well were the nasal or other allergy symptoms controlled” item).
Data presented with mean ± SD; *mean P < 0.05 when compared to perennial allergic rhinitis patients group; † mean P < 0.05 when compared to baseline after antibiotic therapy.
Nasal and fractional exhaled NO
nNO and FENO levels were measured using an online electrochemical analyzer equipped with nNO software (NIOX MINO; Aerocrine AB, Sweden) in compliance with the American Thoracic Society/European Respiratory Society recommendations.18 Briefly, nNO levels were measured from the nostril while holding the breath with an aspiration flow of 5 mL/s. The NO levels derived from the nose were recorded by introducing a nasal olive connected to the analyzer into one nostril. The nNO concentration was automatically calculated by the NIOX MINO system. The nasal olive was then placed in the other nostril and the test was repeated. Measurements were made in triplicate for both nostrils and the mean nNO level was used for the analysis. The subjects rested for 15 min after nNO measurement before undergoing the FENO examination. FENO measurements were made according to standard guidelines.5 All children avoided physical exercise and consumption of foods rich in nitrogen such as sausages, various animal offal, lettuce, and spinach within two hours of nNO measurement. Finally, all enrolled children satisfied all the requirements and successfully completed the nNO and FENO measurements.
Rhinomanometry
On the same day, study participants underwent anterior active rhinomanometry (Masterscreen rhino; Carefusion, Germany). The children wore a tight-fitting facemask and breathed through one nostril with the mouth closed. A sensor was placed in the contralateral nostril and pre- and postnasal pressures were recorded via airflow and pressure transducers. The rhinomanometry results were considered to be related to nasal flows at 150 Pa. Three or more nasal resistance measurements were performed for each patient and the mean was recorded when reproducible values were achieved.
Statistical analysis
Categorical variables were expressed as numbers. A one-Sample Kolmogorov–Smirnov test was performed to check the normality assumption for the distribution of continuous variables, which were described as mean ± standard deviation (SD). Comparisons of the nNO concentrations before and after antibiotic treatment were performed using a paired t-test. The means of groups of datasets were compared using one-way ANOVA, followed by Bonferroni multiple comparisons at a type I error level of 0.05. The case-control study enrolled PAR patients with/without sinusitis with ratio of 1:3 based on the proportion of acute sinusitis and PAR in the general population distribution. Independent t-tests were used to assess whether baseline data and intra-group data were significantly different between the two treatment groups. A post-hoc power analysis using G*Power (3.1.9, Dusseldorf, Germany) revealed a power of 1.0 using the baseline nasal nitric oxide level as the main variable of interest, an alpha level of 0.05, and an effect size f-value of 4.94. Correlation analyses were performed to study the relationship between the nNO levels and expiratory nasal resistance and nasal obstruction scores at the acute phase of unilateral bacterial maxillary sinusitis. Univariate and multiple linear regression analyses were used to measure the correlations between nasal NO and clinical parameters. The Youden index with area under the curve (AUC) was used to determine the cutoff lesion-side nNO to predict acute unilateral bacterial maxillary sinusitis in PAR patients and normal healthy subjects. All data were analyzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). A p value less than 0.05 was considered to be statistically significant.
Results
Clinical characteristics of the subjects
This study enrolled a total of 200 children who successfully underwent the nNO measurements, including 79 normal children and 121 PAR patients without (n = 90) and with (n = 31) unilateral maxillary sinusitis. The demographic data are presented in Table 1. There were no significant intergroup differences in mean age, height, weight, male-to-female ratio, or body mass index. There were no significant differences in the total IgE, Der p-specific IgE, and Der f-specific IgE levels between PAR patients without and with acute unilateral maxillary sinusitis (Table 1). None of the patients required inpatient antibiotic therapy during the study.
Table 1.
Demographic characteristics and clinical parameters in perennial allergic rhinitis (PAR) patients, PAR with acute unilateral maxillary s inusitis patients and normal healthy children.
| Characteristic | PAR group |
PAR with acute |
Healthy controls |
|---|---|---|---|
| sinusitis group | |||
| Number of patients (n) | 90 | 31 | 79 |
| Mean age (y) | 9.7 ± 2.4 | 8.6 ± 2.0 | 10.1 ± 1.9 |
| Sex (M:F) | 61 : 29 | 17: 14 | 55 : 24 |
| Height, cm | 139.4 ± 16.3 | 126.9 ± 24.4 | 142.4 ± 14.4 |
| Weight, kg | 38.2 ± 16.2 | 32.2 ± 11.2 | 40.8 ± 12.2 |
| Body mass index | 18.9 ± 5.1 | 18.6 ± 4.7 | 19.6 ± 3.4 |
| Total IgE (IU/mL) | 482.0 ± 525.3 | 344.6 ± 346.9 | - - |
| Der p (kU/L) | 43.9 ± 38.6 | 44.6 ± 35.3 | - - |
| Der f (kU/L) | 50.3 ± 38.5 | 37.2 ± 34.7 | - - |
Data presented as mean ± SD.
Der p, Dermatophagoides pteronyssinus; Der f, Dermatophagoides farinae.
Acute unilateral maxillary sinusitis in PAR patients was associated with lower nNO levels, high expiratory nasal resistance, and poor RCAT scores
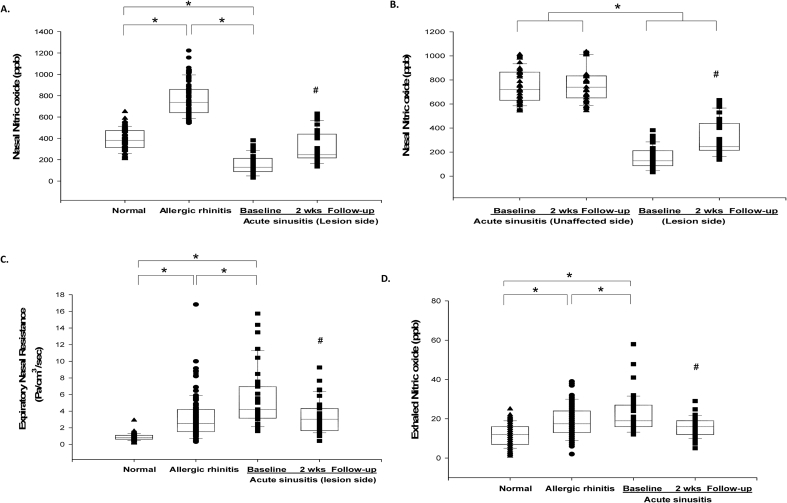
The nNO levels in normal children showed a normal distribution (389.9 ± 97.2 ppb; Fig. 1A). nNO values in PAR patients (765.4 ± 152.1 ppb) were significantly higher than those in normal children (P < 0.05; Fig. 1A). In patients with acute unilateral maxillary sinusitis, lesion-side nNO levels (151.2 ± 87.5 ppb) were significantly lower than those in PAR patients and normal subjects (P < 0.05; Fig. 1A), and nNO levels in the unaffected sinus side (748.1 ± 130.9 ppb) were significantly higher than those on the lesion side (P < 0.05; Fig. 1B) but not different from those in PAR patients without sinusitis. High lesion-side expiratory nasal resistance (Fig. 1C) and poor RCAT scores (Table 3) were observed in patients with acute unilateral maxillary sinusitis (P < 0.05). Bronchial FENO levels were significantly higher in acute unilateral maxillary sinusitis patients (22.5 ± 9.3 ppb) than in PAR patients without sinusitis (18.7 ± 8.6 ppb) and normal subjects (11.8 ± 5.4 ppb) (P < 0.05) (Fig. 1D).
Fig. 1.
The differences in nasal nitric oxide (nNO) levels in acute unilateral bacterial maxillary sinusitis patients, with comparisons between (A) allergic rhinitis patients and control subjects and (B) the non-affected side in these patients. Expiratory nasal resistance (C) and bronchial exhaled nitric oxide (FENO) levels (D) in the study groups. *Mean P < 0.05 for comparisons among study groups. #mean P < 0.05 when compared to baseline after antibiotic therapy.
Antibiotic therapy improved lesion-side nNO, FENO, nasal resistance, and RCAT scores in PAR children with acute unilateral maxillary sinusitis
The lesion-side nNO concentration increased significantly to a mean value of 312.5 ± 143.7 ppb in PAR subjects with unilateral sinusitis after antibiotic therapy (p < 0.05) (Fig. 1A). In these patients, the nNO levels in the unaffected sinus side did not show significant differences after antibiotic therapy (Fig. 1B). The changed nNO levels in the lesion-side increased significantly when compared to the unaffected lesion-side (161.4 ± 164.6 ppb vs 9.6 ± 64.2 ppb; p < 0.05) among PAR subjects with unilateral sinusitis after antibiotic therapy and PAR controls (161.4 ± 164.6 ppb vs −26.3 ± 177.8 ppb; p < 0.05). In addition, the lesion-side expiratory nasal resistance (Fig. 1C) and clinical characteristics and RCAT scores (Table 3) significantly improved in the recovery phase after antibiotic therapy (p < 0.05). The bronchial FENO concentration also significantly decreased after recovery in acute unilateral maxillary sinusitis patients (15.7 ± 4.7 ppb vs 22.5 ± 9.3 ppb; p < 0.05, Fig. 1D).
Correlation between nNO levels, clinical characteristics, and FENO values in PAR patients
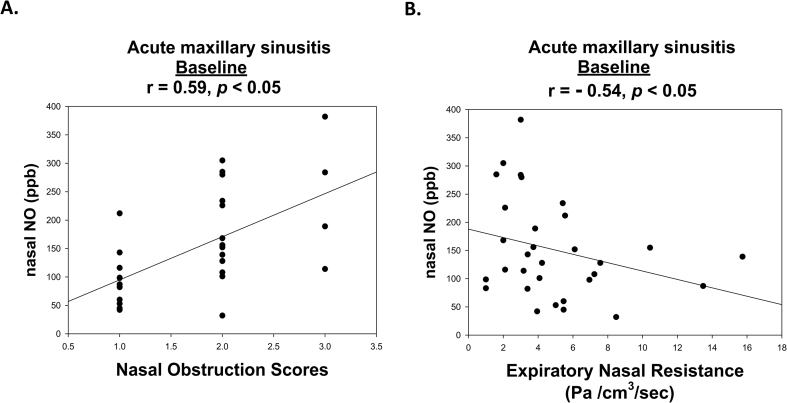
Multiple linear regression analysis demonstrated that nNO levels were significantly associated with age, total IgE levels, and acute maxillary sinusitis diagnosis (P < 0.05) (Table 2). The nNO levels significantly correlated with bronchial FENO levels in PAR patients without acute maxillary sinusitis (r = 0.62, P < 0.05, data not shown). There were moderate negative correlations between nNO levels and expiratory nasal resistance (r = −0.54, P < 0.05) and moderate positive correlations between nNO levels and nasal obstruction improvement scores (r = 0.59, P < 0.05) in the acute phase of unilateral maxillary sinusitis (Fig. 2).
Table 2.
Regression analysis for the nasal nitric oxide level in perennial allergic rhinitis patients `
| Univariate analysis |
Multiple linear regression |
|||
|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | |
| Age | 31.5 [7.9–55.0] | 0.009 | 11.4 [1.2–21.6] | 0.029 |
| Gender | −85.3 [−196.4–25.9] | 0.133 | ||
| Height | 5.3 [2.1–8.6] | 0.001 | ||
| Weight | 3.6 [0.2–7.1] | 0.039 | ||
| BMI | 2.2 [−8.5 – 12.9] | 0.691 | ||
| Total nasal resistance | −25.4 [−39.2 to −11.7] | <0.001 | ||
| Total IgE | 0.2 [0.1–0.3] | 0.001 | 0.1 [0.1–0.2] | <0.001 |
| Der p | 1.1 [−0.3 – 2.5] | 0.121 | ||
| Der f | 1.9 [0.3–3.4] | 0.022 | ||
| Nasal congestion | 82.0 [34.3–129.8] | 0.001 | ||
| Nasal sneeze | −45.6 [−94.4–3.3] | 0.067 | ||
| Watery eyes | −34.6 [−80.9–11.7] | 0.143 | ||
| Interfere with sleep | 15.4 [−33.7–64.6] | 0.001 | ||
| Avoid daily activity | 66.5 [19.5–113.4] | 0.005 | ||
| Nasal symptoms controlled | 17.3 [−28.4–62.9] | 0.459 | ||
| RCAT sum scores | 5.5 [−5.9–16.9] | 0.348 | ||
| Acute sinusitis diagnosis | 614.2 [−670.3 to −558.1] | <0.001 | 588.1 [−643.9–−532.3] | 0.001 |
RCAT, Rhinitis control assessment test; Der p, Dermatophagoides pteronyssinus; Der f, Dermatophagoides farinae.
Adjusted for covariate factors, including age and IgE and acute sinusitis diagnosis group.
Fig. 2.
Pearson's correlation test for nasal nitric oxide (nNO) values, severity of nasal obstruction, and expiratory nasal resistance in perennial allergic rhinitis patients with acute unilateral maxillary sinusitis.
Cutoff nNO value for diagnosis of PAR in children with/without acute maxillary sinusitis
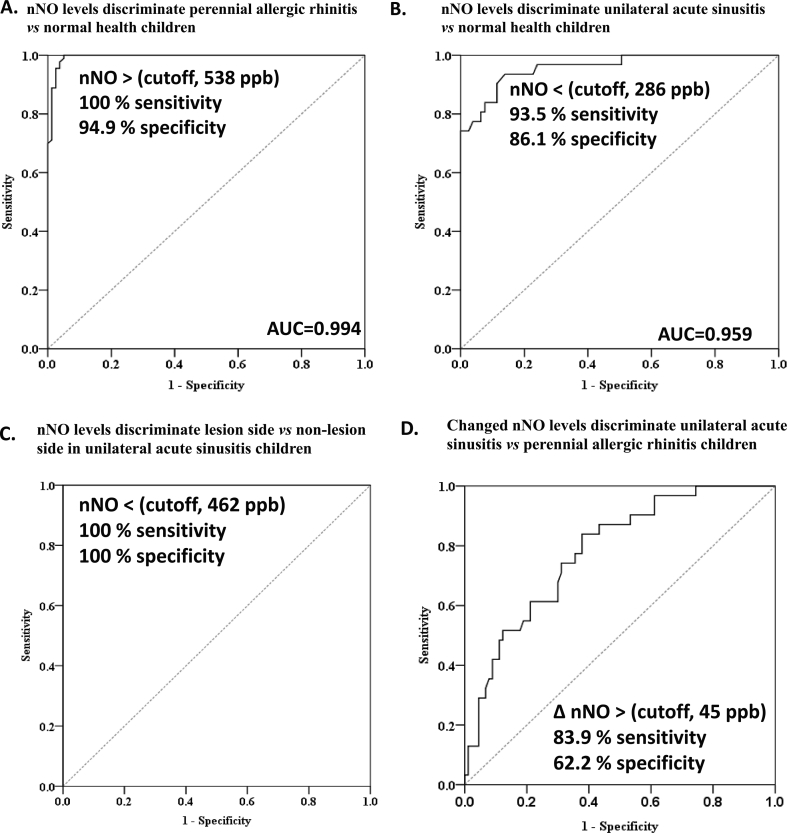
Because nNO measurements can be useful in the diagnosis of acute maxillary sinusitis, we used the receiver operating characteristic curve to determine the degree of significance for the differential diagnosis. The diagnostic AUC values for nNO levels were significantly high in PAR patients (AUC = 0.994) and PAR patients with acute unilateral maxillary sinusitis (0.959) in comparison with normal healthy children (Fig. 3A, B). The cut-off lesion-side nNO value for discrimination of PAR with acute unilateral maxillary sinusitis from normal healthy subjects with high sensitivity (93.5%) and high specificity (86.2%; Fig. 3B) was 286 ppb. The cut-off- lesion-side nNO value with 462 ppb was high sensitivity (100%) and specificity (100%) for discrimination of non-lesion side nNO value in PAR with acute unilateral maxillary sinusitis (Fig. 3C). In addition, we found cut-off-value of increased nNO levels (over 45 ppb) with 83.9% sensitivity and 62.2% specificity to diagnose acute unilateral maxillary sinusitis in PAR children (Fig. 3D).
Fig. 3.
Cutoff values of nasal nitric oxide (nNO) for differentiating (A) perennial allergic rhinitis and (B) acute unilateral maxillary sinusitis in comparison with normal healthy children and (C) lesion-side vs non-lesion side in unilateral acute sinusitis children by receiver operating characteristic curves. (D) Cutoff-values of increased nNO levels (over 45 ppb) after antibiotic treatment with 83.9% sensitivity and 62.2% specificity values to diagnose acute unilateral maxillary sinusitis in PAR children.
Discussion
nNO is not routinely measured in clinical practice, mainly because of the few studies of a large series with emphasis on individual parameters and the lack of consensus concerning the most suitable measurement technique. The normal reference values of nNO in young healthy children may serve as a starting point for noninvasive monitoring of allergic airway inflammation. The previously published mean nNO concentrations in healthy children ranged from 200 to 450 ppb.15, 16, 17 We assessed the feasibility of nNO measurements by means of an online constant-flow standardized method, and all children were able to perform the test.11, 24 We determined the normal nNO levels in 6–13 years non-atopy health children, with the average nNO levels ranging from 300 to 500 ppb (389.9 ± 97.2 ppb) after careful nasal fibroendoscopy and anterior rhinometry assessment.
Multivariate analysis showed that age, total IgE levels, and acute maxillary sinusitis diagnosis were significant factors influencing the nNO levels. The positive relationship between nNO levels and age in PAR patients may be due to the accelerated pneumatization of developing paranasal sinuses and mucosal surface area during childhood.30 The elevated IgE levels and eosinophilia in the inflamed nasal mucosa may contribute to the characteristic nNO increases in PAR patients.13, 31 We observed that increased nNO levels were related to RCAT with nasal obstruction, suggesting that nNO may be a potential clinical biomarker of upper airway inflammation and sinus mucosal health in PAR patients.
Several studies have reported an nNO reduction in sinusitis patients, principally due to sinus ostial obstruction and failure of sinus gaseous NO to reach the nasal cavities.22, 23, 24, 25 Another explanation for the reduced NO nasal concentrations during sinusitis could be an impairment of NOS-2 expression in the ciliary epithelial cells of sinus mucosa.32, 33 We found that acute sinusitis patients associated with low nNO levels, which was confirmed due to ostiomeatal occlusion, and the lesion-side nNO levels were negatively correlated with expiratory nasal resistance. When the atopic status of the patients was considered, we could easily distinguish acute maxillary sinusitis based on lower nNO concentrations.
Among sinusitis patients, nNO levels recovered significantly with medical or surgical treatments after resolution of the sinus ostial obstruction and clearance of the infectious material within the sinus cavity.34, 35, 36 We found that the lesion-side nNO level was largely decreased in acute unilateral maxillary sinusitis patients and that recovery of nNO levels was associated with amelioration of nasal obstructive symptoms and expiratory nasal resistance after antibiotic therapy. Following up changes in nNO levels over time could help assessment of the patency of the sinus ostium and determine the effectiveness of therapeutic interventions.
Our data also showed that treatment of acute unilateral bacterial maxillary sinusitis with antibiotics improves symptoms, increases nNO levels, and decreases bronchial FENO, which is a marker for bronchial inflammation. Using standard velum closure techniques, the cross-contamination risk between nNO and FENO was minimized during NO measurement. Therefore, the increase in nNO production in the nasal membrane is related to the inflammatory status rather than contamination from the lower airway. Williamson et al. demonstrated that nNO and FENO levels were correlated in healthy volunteers.37 Monitoring FENO concentrations is a useful tool to evaluate inflammation of the lower airways in PAR patients.38, 39 The results of our study indicated that high FENO levels had a positive association (r = 0.62) with nNO levels in PAR patients without asthma. Interestingly, we found that bronchial FENO levels were significantly higher in PAR patients with acute rhinosinusitis, and the pathogenic mechanisms may be caused by postnasal drip of inflammatory material. Furthermore, we found that both abnormal FENO and lesion-side nNO levels recovered to baseline after antibiotic therapy, supporting the “one airway, one disease” concept, which posits a link between inflammation in the united upper and lower airways.40
This study had some limitations. We did not perform sinus aspiration to verify bacterial etiology or computed tomography (CT) scanning for evaluation of sinus abnormalities among children with acute unilateral bacterial maxillary sinusitis. Although imaging studies with CT scanning are the gold standard for sinusitis diagnosis, they are not usually necessary in the evaluation of children with uncomplicated acute bacterial rhinosinusitis. Sinus aspiration is an invasive procedure that is not routinely performed in children.
Most clinicians agree that the diagnosis of acute bacterial sinusitis in children can be made after viral respiratory infections when children show persistent symptoms over 10 days with two or more of the following findings: discolored nasal discharge, nasal obstruction, and cough.26 At least 7 days observation was always suggested in the literature or guidelines for diagnosis with acute bacterial sinusitis. Roentgenography may cause radiation exposure in young children. Intranasal endoscopy is a reliable and minimal invasive tool but may require anesthesia and cause uncomfortable to pediatric patients with potential mucosal trauma and bleeding. Measurements of nNO can be used as a noninvasive, nonradioactive diagnostic tool for early diagnosis in PAR children with acute rhinosinusitis. PAR children with acute sinusitis presented with a marked reduction in lesion-side nNO levels, higher total nasal resistance, and poorer RCAT scores. The study clearly demonstrates the relationship between expiratory nasal resistance and nNO, which supports the hypothesis that the decrease in nNO can be attributed to sinus ostial obstruction rather than changes in inflammation. nNO also can be a sensitivity biomarker to monitor the success of antibiotic treatment for acute rhinosinusitis, especially in the obscured treatment outcome, since nNO levels seem to correlate with symptom and total nasal resistance improvement.
We conclude that nNO certainly represents a useful biomarker for diagnosis and treatment-response monitoring in cases of acute maxillary sinusitis. Differential diagnosis in PAR patients with and without complications such as nasal polyps and sinusitis can be performed by checking for a decrease in nNO levels and increase in nasal resistance. We suggest that measurement of nNO levels and nasal resistance in individual sides may add understanding to the pathophysiologic role of NO in the regulation of upper airway inflammation and better differential diagnosis and treatment of PAR children with and without other co-morbidities.
Conflicts of interest
The authors have no conflicts of interest relevant to this article to disclose. The authors also have no financial relationships relevant to instruments used in this study to disclose.
Ethics approval and consent to participate
This study was approved by the institutional review board (Changhua Christian Hospital), and written informed consent was obtained from each participant and the patient's legal guardians (CCH IRB No. 140906 and No. 160207). Privacy and confidentiality of medical information was ensured.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported in part by grants from the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-371-008 and MOST 107-2314-B-371 -011 -MY2), Changhua Christian Hospital (Y_103_ 0188 and Y_104_0252 and Y_104_0127 and Y_105_0023 and Y_105_0050 and Y_105_0246).
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Acknowledgement
We thanks the Mr. Vikas Narang, Senior Vice President, Editage for language editing in our manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100027.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Antosova M., Mokra D., Pepucha L. Physiology of nitric oxide in the respiratory system. Physiol Res. 2017;66:S159–S172. doi: 10.33549/physiolres.933673. [DOI] [PubMed] [Google Scholar]; Antosova M, Mokra D, Pepucha L, Plevkova J, Buday T, Sterusky M, et al. Physiology of nitric oxide in the respiratory system. Physiol Res. 2017;66:S159-S172. [DOI] [PubMed]
- 2.Voynow J.A., Montpetit A.J. Nasal NO as a biomarker: don't say NO to the many challenges of translational medicine. Pediatr Pulmonol. 2015;50:100–102. doi: 10.1002/ppul.23111. [DOI] [PubMed] [Google Scholar]; Voynow JA, Montpetit AJ. Nasal NO as a biomarker: don't say NO to the many challenges of translational medicine. Pediatr Pulmonol. 2015;50:100-102. [DOI] [PubMed]
- 3.Manna A., Montella S., Maniscalco M., Maglione M., Santamaria F. Clinical application of nasal nitric oxide measurement in pediatric airway diseases. Pediatr Pulmonol. 2015;50:85–99. doi: 10.1002/ppul.23094. [DOI] [PubMed] [Google Scholar]; Manna A, Montella S, Maniscalco M, Maglione M, Santamaria F. Clinical application of nasal nitric oxide measurement in pediatric airway diseases. Pediatr Pulmonol. 2015;50:85-99. [DOI] [PubMed]
- 4.Petsky H.L., Kew K.M., Chang A.B. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev. 2016;11:CD011439. doi: 10.1002/14651858.CD011439.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev. 2016;11:CD011439. [DOI] [PMC free article] [PubMed]
- 5.Tsai Y.G., Sun H.L., Chien J.W., Chen C.Y., Lin C.H., Lin C.Y. High exhaled nitric oxide levels correlate with nonadherence in acute asthmatic children. Ann Allergy Asthma Immunol. 2017;118:521–523. doi: 10.1016/j.anai.2017.01.031. [DOI] [PubMed] [Google Scholar]; Tsai YG, Sun HL, Chien JW, Chen CY, Lin CH, Lin CY. High exhaled nitric oxide levels correlate with nonadherence in acute asthmatic children. Ann Allergy Asthma Immunol. 2017;118:521-523. [DOI] [PubMed]
- 6.Chien J.W., Lin C.Y., Yang K.D., Lin C.H., Kao J.K., Tsai Y.G. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43:1018–1026. doi: 10.1111/cea.12119. [DOI] [PubMed] [Google Scholar]; Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43:1018-1026. [DOI] [PubMed]
- 7.Antosova M., Mokra D., Tonhajzerova I. Nasal nitric oxide in healthy adults - reference values and affecting factors. Physiol Res. 2017;66:S247–S255. doi: 10.33549/physiolres.933680. [DOI] [PubMed] [Google Scholar]; Antosova M, Mokra D, Tonhajzerova I, Mikolka P, Kosutova P, Mestanik M, et al. Nasal nitric oxide in healthy adults - reference values and affecting factors. Physiol Res. 2017;66:S247-S255. [DOI] [PubMed]
- 8.Yao Y., Xie S., Yang C., Zhang J., Wu X., Sun H. Biomarkers in the evaluation and management of chronic rhinosinusitis with nasal polyposis. Eur Arch Oto-Rhino-Laryngol. 2017;274:3559–3566. doi: 10.1007/s00405-017-4547-2. [DOI] [PubMed] [Google Scholar]; Yao Y, Xie S, Yang C, Zhang J, Wu X, Sun H. Biomarkers in the evaluation and management of chronic rhinosinusitis with nasal polyposis. Eur Arch Otorhinolaryngol. 2017;274:3559-3566. [DOI] [PubMed]
- 9.Jankowski R., Nguyen D.T., Poussel M., Chenuel B., Gallet P., Rumeau C. Sinusology. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:263–268. doi: 10.1016/j.anorl.2016.05.011. [DOI] [PubMed] [Google Scholar]; Jankowski R, Nguyen DT, Poussel M, Chenuel B, Gallet P, Rumeau C. Sinusology. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:263-268. [DOI] [PubMed]
- 10.Shapiro A.J., Josephson M., Rosenfeld M. Accuracy of nasal nitric oxide measurement as a diagnostic test for primary ciliary dyskinesia. a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:1184–1196. doi: 10.1513/AnnalsATS.201701-062SR. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shapiro AJ, Josephson M, Rosenfeld M, Yilmaz O, Davis SD, Polineni D, et al. Accuracy of nasal nitric oxide measurement as a diagnostic test for primary ciliary dyskinesia. a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:1184-1196. [DOI] [PMC free article] [PubMed]
- 11.Weschta M., Deutschle T., Riechelmann H. Nasal fractional exhaled nitric oxide analysis with a novel hand-held device. Rhinology. 2008;46:23–27. [PubMed] [Google Scholar]; Weschta M, Deutschle T, Riechelmann H. Nasal fractional exhaled nitric oxide analysis with a novel hand-held device. Rhinology. 2008;46:23-27. [PubMed]
- 12.Wang P.P., Wang G.X., Ge W.T., Tang L.X., Zhang J., Ni X. Nasal nitric oxide in allergic rhinitis in children and its relationship to severity and treatment. Allergy Asthma Clin Immunol. 2017;13:20. doi: 10.1186/s13223-017-0191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang PP, Wang GX, Ge WT, Tang LX, Zhang J, Ni X. Nasal nitric oxide in allergic rhinitis in children and its relationship to severity and treatment. Allergy Asthma Clin Immunol 2017;13:20. [DOI] [PMC free article] [PubMed]
- 13.Yuksel H., Kirmaz C., Yilmaz O. Nasal mucosal expression of nitric oxide synthases in patients with allergic rhinitis and its relation to asthma. Ann Allergy Asthma Immunol. 2008;100:12–16. doi: 10.1016/S1081-1206(10)60398-5. [DOI] [PubMed] [Google Scholar]; Yuksel H, Kirmaz C, Yilmaz O, et al. Nasal mucosal expression of nitric oxide synthases in patients with allergic rhinitis and its relation to asthma. Ann Allergy Asthma Immunol. 2008;100:12-16. [DOI] [PubMed]
- 14.Duong-Quy S., Vu-Minh T., Hua-Huy T. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J Asthma Allergy. 2017;10:75–82. doi: 10.2147/JAA.S129047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Duong-Quy S, Vu-Minh T, Hua-Huy T, Tang-Thi-Thao T, Le-Quang K, Tran-Thanh D, et al. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J Asthma Allergy. 2017;10:75-82. [DOI] [PMC free article] [PubMed]
- 15.Menou A., Babeanu D., Paruit H.N., Ordureau A., Guillard S., Chambellan A. Normal values of offline exhaled and nasal nitric oxide in healthy children and teens using chemiluminescence. J Breath Res. 2017;11:036008. doi: 10.1088/1752-7163/aa76ef. [DOI] [PubMed] [Google Scholar]; Menou A, Babeanu D, Paruit HN, Ordureau A, Guillard S, Chambellan A. Normal values of offline exhaled and nasal nitric oxide in healthy children and teens using chemiluminescence. J Breath Res. 2017;11:036008. [DOI] [PubMed]
- 16.You S., Zhang J., Bai Y., Ji L., Wang H. Normal values of nasal NO and exhaled NO in young Chinese people aged 9 - 22 years. World J Otorhinolaryngol Head Neck Surg. 2016;2:22–27. doi: 10.1016/j.wjorl.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; You S, Zhang J, Bai Y, Ji L, Wang H. Normal values of nasal NO and exhaled NO in young Chinese people aged 9 - 22 years. World J Otorhinolaryngol Head Neck Surg. 2016;2:22-27. [DOI] [PMC free article] [PubMed]
- 17.Piacentini G.L., Bodini A., Peroni D.G. Nasal nitric oxide levels in healthy pre-school children. Pediatr Allergy Immunol. 2010;21:1139e1145. doi: 10.1111/j.1399-3038.2010.00989.x. [DOI] [PubMed] [Google Scholar]; Piacentini GL, Bodini A, Peroni DG, et al. Nasal nitric oxide levels in healthy pre-school children. Pediatr Allergy Immunol. 2010;21:1139e1145. [DOI] [PubMed]
- 18.American Thoracic Society; European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]; American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912-930. [DOI] [PubMed]
- 19.Alobid I., Benitez P., Valero A., Munoz R., Langdon C., Mullol J. Oral and intranasal steroid treatments improve nasal patency and paradoxically increase nasal nitric oxide in patients with severe nasal polyposis. Rhinology. 2012;50:171–177. doi: 10.4193/Rhin10.140. [DOI] [PubMed] [Google Scholar]; Alobid I, Benitez P, Valero A, Munoz R, Langdon C, Mullol J. Oral and intranasal steroid treatments improve nasal patency and paradoxically increase nasal nitric oxide in patients with severe nasal polyposis. Rhinology. 2012;50:171-177. [DOI] [PubMed]
- 20.Delclaux C., Malinvaud D., Chevalier-Bidaud B., Callens E., Mahut B., Bonfils P. Nitric oxide evaluation in upper and lower respiratory tracts in nasal polyposis. Clin Exp Allergy. 2008;38:1140–1147. doi: 10.1111/j.1365-2222.2008.03006.x. [DOI] [PubMed] [Google Scholar]; Delclaux C, Malinvaud D, Chevalier-Bidaud B, Callens E, Mahut B, Bonfils P. Nitric oxide evaluation in upper and lower respiratory tracts in nasal polyposis. Clin Exp Allergy. 2008;38:1140-1147. [DOI] [PubMed]
- 21.Torretta S., Bossi A., Capaccio P. Nasal nitric oxide in children with adenoidal hypertrophy: a preliminary study. Int J Pediatr Otorhinolaryngol. 2010;74(6):689–693. doi: 10.1016/j.ijporl.2010.03.025. [DOI] [PubMed] [Google Scholar]; Torretta S, Bossi A, Capaccio P, Marchisio P, Esposito S, Brevi A, et al. Nasal nitric oxide in children with adenoidal hypertrophy: a preliminary study. Int J Pediatr Otorhinolaryngol. 2010;74(6):689-693. [DOI] [PubMed]
- 22.Liu C., Zheng M., He F., Wang X., Zhang L. Role of exhaled nasal nitric oxide in distinguishing between chronic rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy. 2017;31:389–394. doi: 10.2500/ajra.2017.31.4480. [DOI] [PubMed] [Google Scholar]; Liu C, Zheng M, He F, Wang X, Zhang L. Role of exhaled nasal nitric oxide in distinguishing between chronic rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy. 2017;31:389-394. [DOI] [PubMed]
- 23.Maniscalco M. Nasal nitric oxide as biomarker in the evaluation and management of chronic rhino-sinusitis with nasal polyposis. Eur Arch Oto-Rhino-Laryngol. 2017;274:3817–3818. doi: 10.1007/s00405-017-4591-y. [DOI] [PubMed] [Google Scholar]; Maniscalco M. Nasal nitric oxide as biomarker in the evaluation and management of chronic rhino-sinusitis with nasal polyposis. Eur Arch Otorhinolaryngol. 2017;274:3817-3818. [DOI] [PubMed]
- 24.Fu C.H., Tseng H.J., Huang C.C., Chang P.H., Chen Y.W., Lee T.J. Nasal nitric oxide in unilateral sinus disease. PLoS One. 2017;12:e0171965. doi: 10.1371/journal.pone.0171965. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fu CH, Tseng HJ, Huang CC, Chang PH, Chen YW, Lee TJ. Nasal nitric oxide in unilateral sinus disease. PLoS One. 2017;12:e0171965. [DOI] [PMC free article] [PubMed]
- 25.Frendø M., Håkansson K., Schwer S. Exhaled and nasal nitric oxide in chronic rhinosinusitis patients with nasal polyps in primary care. Rhinology. 2018;56:59–64. doi: 10.4193/Rhino17.111. [DOI] [PubMed] [Google Scholar]; Frendo M, Hakansson K, Schwer S, Ravn AT, Meteran H, Porsbjerg C, et al. Exhaled and nasal nitric oxide in chronic rhinosinusitis patients with nasal polyps in primary care. Rhinology. 2018;56:59-64. [DOI] [PubMed]
- 26.Wald E.R., Applegate K.E., Bordley C. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262–e280. doi: 10.1542/peds.2013-1071. [DOI] [PubMed] [Google Scholar]; Wald ER, Applegate KE, Bordley C, Darrow DH, Glode MP, Marcy SM, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280. [DOI] [PubMed]
- 27.Wei J.L. Chronic nasal dysfunction in children: allergic rhinitis? Infectious? What to do if neither? Curr Opin Otolaryngol Head Neck Surg. 2015;23:491–498. doi: 10.1097/MOO.0000000000000207. [DOI] [PubMed] [Google Scholar]; Wei JL. Chronic nasal dysfunction in children: Allergic rhinitis? Infectious? What to do if neither? Curr Opin Otolaryngol Head Neck Surg. 2015;23:491-498. [DOI] [PubMed]
- 28.Wan K.S., Yang W., Wu W.F. A survey of serum specific-lgE to common allergens in primary school children of Taipei City. Asian Pac J Allergy Immunol. 2010;28:1–6. [PubMed] [Google Scholar]; Wan KS, Yang W, Wu WF. A survey of serum specific-lgE to common allergens in primary school children of Taipei City. Asian Pac J Allergy Immunol. 2010;28:1-6. [PubMed]
- 29.Meltzer E.O., Schatz M., Nathan R., Garris C., Stanford R.H., Kosinski M. Reliability, validity, and responsiveness of the rhinitis control assessment test in patients with rhinitis. J Allergy Clin Immunol. 2013;131:379–386. doi: 10.1016/j.jaci.2012.10.022. [DOI] [PubMed] [Google Scholar]; Meltzer EO, Schatz M, Nathan R, Garris C, Stanford RH, Kosinski M. Reliability, validity, and responsiveness of the Rhinitis Control Assessment Test in patients with rhinitis. J Allergy Clin Immunol. 2013;131:379-386. [DOI] [PubMed]
- 30.Baraldi E., Azzolin N., Biban P., Zacchello F. Effect of antibiotic therapy on nasal nitric oxide concentration in children with acute sinusitis. Am J Respir Crit Care Med. 1997;155:1680–1683. doi: 10.1164/ajrccm.155.5.9154876. [DOI] [PubMed] [Google Scholar]; Baraldi E, Azzolin N, Biban P, Zacchello F. Effect of antibiotic therapy on nasal nitric oxide concentration in children with acute sinusitis. Am J Respir Crit Care Med. 1997:155:1680-1683. [DOI] [PubMed]
- 31.Nesic V.S., Djordjevic V.Z., Tomic-Spiric V., Dudvarski Z.R., Soldatovic I.A., Arsovic N.A. Measuring nasal nitric oxide in allergic rhinitis patients. J Laryngol Otol. 2016;130:1064–1071. doi: 10.1017/S0022215116009087. [DOI] [PubMed] [Google Scholar]; Nesic VS, Djordjevic VZ, Tomic-Spiric V, Dudvarski ZR, Soldatovic IA, Arsovic NA. Measuring nasal nitric oxide in allergic rhinitis patients. J Laryngol Otol. 2016;130:1064-1071. [DOI] [PubMed]
- 32.Asano T., Takemura M., Kanemitsu Y. Combined measurements of fractional exhaled nitric oxide and nasal nitric oxide levels for assessing upper airway diseases in asthmatic patients. J Asthma. 2018;55:300–309. doi: 10.1080/02770903.2017.1332203. [DOI] [PubMed] [Google Scholar]; Asano T, Takemura M, Kanemitsu Y, Yokota M, Fukumitsu K, Takeda N, et al. Combined measurements of fractional exhaled nitric oxide and nasal nitric oxide levels for assessing upper airway diseases in asthmatic patients. J Asthma. 2018;55:300-309. [DOI] [PubMed]
- 33.Autio T.J., Koskenkorva T., Leino T.K., Koivunen P., Alho O.P. Longitudinal analysis of inflammatory biomarkers during acute rhinosinusitis. Laryngoscope. 2017;127:E55–E61. doi: 10.1002/lary.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]; Autio TJ, Koskenkorva T, Leino TK, Koivunen P, Alho OP. Longitudinal analysis of inflammatory biomarkers during acute rhinosinusitis. Laryngoscope. 2017;127:E55-61. [DOI] [PMC free article] [PubMed]
- 34.Fu C.H., Huang C.C., Chen Y.W., Chang P.H., Lee T.J. Nasal nitric oxide in relation to quality-of-life improvements after endoscopic sinus surgery. Am J Rhinol Allergy. 2015;29:e187–e191. doi: 10.2500/ajra.2015.29.4249. [DOI] [PubMed] [Google Scholar]; Fu CH, Huang CC, Chen YW, Chang PH, Lee TJ. Nasal nitric oxide in relation to quality-of-life improvements after endoscopic sinus surgery. Am J Rhinol Allergy. 2015;29:e187-e191. [DOI] [PubMed]
- 35.Ragab S.M., Lund V.J., Saleh H.A., Scadding G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy. 2006;61:717–724. doi: 10.1111/j.1398-9995.2006.01044.x. [DOI] [PubMed] [Google Scholar]; Ragab SM, Lund VJ, Saleh HA, Scadding G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy. 2006;61:717-724. [DOI] [PubMed]
- 36.Lanz M.J., Prendes S., Peyrou N., Toledo G., Ferrer C.M. Nasal nitric oxide as a noninvasive marker in the antibiotic treatment of acute bacterial sinusitis. J Allergy Clin Immunol. 2008;121:530–531. doi: 10.1016/j.jaci.2007.09.034. [DOI] [PubMed] [Google Scholar]; Lanz MJ, Prendes S, Peyrou N, Toledo G, Ferrer CM. Nasal nitric oxide as a noninvasive marker in the antibiotic treatment of acute bacterial sinusitis. J Allergy Clin Immunol. 2008;121:530-531. [DOI] [PubMed]
- 37.Williamson P.A., Vaidyanathan S., Clearie K., Stewart M., Lipworth B.J. Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann Allergy Asthma Immunol. 2010;105:162–167. doi: 10.1016/j.anai.2010.05.014. [DOI] [PubMed] [Google Scholar]; Williamson PA, Vaidyanathan S, Clearie K, Stewart M, Lipworth BJ. Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann Allergy Asthma Immunol. 2010;105:162-167. [DOI] [PubMed]
- 38.Yoon J., Choi Y.J., Lee E. Allergic rhinitis in preschool children and the clinical utility of FeNO. Allergy Asthma Immunol Res. 2017;9:314–321. doi: 10.4168/aair.2017.9.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yoon J, Choi YJ, Lee E, Cho HJ, Yang SI, Kim YH, et al. Allergic Rhinitis in Preschool Children and the Clinical Utility of FeNO. Allergy Asthma Immunol Res. 2017;9:314-321. [DOI] [PMC free article] [PubMed]
- 39.Krantz C., Janson C., Borres M.P., Nordvall L., Alving K., Malinovschi A. Nasal nitric oxide is associated with exhaled NO, bronchial responsiveness and poor asthma control. J Breath Res. 2014;8:026002. doi: 10.1088/1752-7155/8/2/026002. [DOI] [PubMed] [Google Scholar]; Krantz C, Janson C, Borres MP, Nordvall L, Alving K, Malinovschi A. Nasal nitric oxide is associated with exhaled NO, bronchial responsiveness and poor asthma control. J Breath Res. 2014;8:026002. [DOI] [PubMed]
- 40.Heffler E., Pizzimenti S., Badiu I. Nasal nitric oxide is a marker of poor asthma control. J Breath Res. 2013;7:026009. doi: 10.1088/1752-7155/7/2/026009. [DOI] [PubMed] [Google Scholar]; Heffler E, Pizzimenti S, Badiu I, Guida G, Ricciardolo FL, Bucca C, et al. Nasal nitric oxide is a marker of poor asthma control. J Breath Res. 2013;7:026009. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.