Abstract
How individuals respond to chronic stress varies. Susceptible individuals ultimately develop depression; whereas resilient individuals live normally. In this study, our objective was to examine the effect of branched-chain amino acids (BCAA), commonly used by athletes, on susceptibility to stress. Male C57BL/6 mice were subjected to daily defeat sessions by a CD1 aggressor, for 10 days. On day11, the behavior of mice was assessed using the social interaction test, elevated plus maze and open field. Mice received the BCAA leucine, isoleucine or valine before each defeat session. Furthermore, we examined whether BCAA regulate brain derived neurotrophic factor (BDNF) signaling by using a brain-permeable tropomyosin receptor kinase B (TRKB) inhibitor, ANA-12. We also tested the effect of voluntary exercise and high protein diets on susceptibility to stress. Mice exposed to chronic stress displayed increased susceptibility and social avoidance. BCAA promoted resilience to chronic stress, rescued social avoidance behaviors and increased hippocampal BDNF levels and TRKB activation. Inhibition of TRKB signaling abolished the ability of BCAA to promote resilience to stress and to rescue social avoidance. Interestingly, we found that BCAA activate the exercise-regulated PGC1a/FNDC5 pathway known to induce hippocampal BDNF signaling. Although both voluntary exercise and BCAA promoted resilience to stress, combining them did not yield synergistic effects confirming that they affect similar pathways. We also discovered that high protein diets mimic the effect of BCAA by rescuing social deficits induced by chronic stress and increase Bdnf expression in the hippocampus. Our data indicate that BCAA, exercise and high protein diets rescue susceptibility to stress by activating the hippocampal BDNF/TRKB signaling.
Keywords: Branched chain amino acids, Chronic social defeat stress, BDNF, FNDC5, Exercise, High protein diet
Highlights
-
•
BCAA promote resilience to stress and rescue social avoidance via activation of hippocampal BDNF/TRKB signaling.
-
•
BCAA induce hippocampal BDNF/TRKB signaling by activating the exercise-regulated PGC1a/FNDC5 pathway.
-
•
BCAA and voluntary exercise affect similar pathways.
-
•
HPD promote resilience to stress, rescue social avoidance and induce hippocampal Bdnf expression.
1. Introduction
Depression is one of the leading causes of disability worldwide (Smith, 2014). Although multiple antidepressants are available, less than half of depressed patients achieve complete remission (Block and Nemeroff, 2014). To develop novel therapies, recent work has focused on identifying cellular pathways that regulate susceptibility and resilience to stress. Some individuals appear to be more susceptible to stress, a major cause of depression. The gene expression networks that promote susceptibility to stress and depression have been analyzed (Bagot et al., 2016, 2017; Benatti et al., 2012; Krishnan et al., 2007). Interestingly, brain derived neurotrophic factor (Bdnf) expression is reduced in the hippocampi of rats that are susceptible to stress as compared to resilient rats (Duclot and Kabbaj, 2013). Indeed, chronic stress decreases hippocampal Bdnf expression (Jiang et al., 2014; Tsankova et al., 2006) and antidepressant treatment reverses this downregulation (Tsankova et al., 2006). The therapeutic effect of antidepressants is lost in the hippocampal Bdnf knockouts (Adachi et al., 2008; Bjorkholm and Monteggia, 2016; Monteggia et al., 2004, 2007) and chronic stress-induced memory deficits are reversed by regular exercise via induction of BDNF signaling (Kim and Leem, 2016). BDNF levels are also reduced in patients with depression and antidepressant treatment restores normal levels. A human BDNF polymorphism that changes valine (Val) to methionine (Met) at codon 66 (Val66Met), and that causes a 30% reduction in BDNF levels is associated with increased risk of depression and anxiety disorders (Notaras et al., 2015; Soliman et al., 2010; Yu et al., 2012).
In addition to altered gene expression profiles in the brain of depressed patients, fluctuations in the levels of a wide range of metabolites have been detected. For example, the levels of branched-chain amino acids (BCAA), leucine (Leu), isoleucine (Ile) and Val are decreased in patients suffering from psychiatric disorders such as major depressive disorder (Baranyi et al., 2016), immune-related major depression (Baranyi et al., 2018) and bipolar disorder (Fellendorf et al., 2018). BCAA were identified among twenty-four metabolites whose hippocampal levels were increased after antidepressant treatment and Leu was proposed to serve as a biomarker that links the responses to antidepressant treatment in the serum to the hippocampus (Webhofer et al., 2011). Even though, Leu exhibits antidepressant properties in an inflammation-induced depression model (Walker et al., 2018), the relevance of BCAA in the etiology of depression remains unclear.
In this work, we decided to determine whether BCAA protect against stress. We tested the hypothesis that BCAA promote resilience to stress in a chronic social defeat stress (CSDS) model, a validated model of depression (Berton et al., 2006; Krishnan et al., 2007). Our results suggest that BCAA promote resilience to stress and rescue social avoidance behavior by increasing hippocampal BDNF levels through the activation of the exercise-regulated PGC1a/FNDC5 pathway. Indeed, BCAA induce the activation of the BDNF receptor, tropomyosin receptor kinase B (TRKB), and inhibition of TRKB signaling abolishes the ability of BCAA to promote resilience to stress. Although both voluntary exercise and BCAA promote resilience to stress, combining them does not yield synergistic effects confirming that they affect similar pathways. We also show that a high protein diet (HPD) can mimic the effects of BCAA on social behavior potentially through activation of hippocampal Bdnf expression. Our results suggest that BCAA, HPD and exercise promote resilience to stress by activating hippocampal BDNF signaling.
2. Methods
2.1. Animal housing and BCAA injections
Adult male C57BL/6 mice received daily intraperitoneal injections of saline, Leu (32.5 mg/kg), Ile (32.5 mg/kg), Val (28.5 mg/kg), and ANA-12 (0.5 mg/kg). The doses of the BCAA were selected based on the observation that the used concentration of Leu activates the mammalian target of rapamycin (mTOR) and protein synthesis (Poncet et al., 2014). Animal care and use was in accordance with the guidelines and as approved by the ACUC.
Exercise paradigm: Male C57BL/6 mice were individually housed. They were divided into 2 groups: sedentary animals or exercising animals. The exercising animals were housed with free access to a running wheel.
Diets: Male C57BL/6 mice were divided into 2 groups: a group that received a standard diet (STD) and a group that received a high protein diet (HPD). The composition of the diet is included in Table 1. The diets were designed to be isocaloric.
Table 1.
Composition of the STD and HPD.
| Ingredients | Standard Diet | High Protein Diet |
|---|---|---|
| Casein (in g) | 85 | 262 |
| Corn Starch (in g) | 374 | 134 |
| Sucrose (in g) | 60 | 99 |
| Cellulose (in g) | 30 | 31 |
| Oil (in g) | 24 | 31 |
| Fat (in g) | – | – |
| Vitamin mix (in g) | 6 | 12 |
| Mineral mix (in g) | 21 | 31 |
| Total weight (in g) | 600 | 600 |
2.2. RNA extraction and Real Time PCR
Total RNA was prepared from hippocampi using the Rneasy Plus Mini RNA extraction kit (Qiagen) and reverse transcription was performed using QuantiTect reverse transcription kit (Qiagen) according to the manufacturer's protocol. Real-time PCR was performed using a standard PCR protocol, with Sybr green dye (BioRad).
The primer sequences used are BdnfI: (CAGGACAGCAAAGCCACAAT and GCCTTCATGCAACCGAAGTA) and Gapdh: (CTCTCTGCTCCTCCCTGTTC and CCGACCTTCACCATTTTGTC).
2.3. Chronic social defeat stress (CSDS) model
We used the CSDS model in mice to mimic the symptoms of depression. The CSDS paradigm was performed as previously described (Golden et al., 2011). Cages were divided into two compartments separated by a perforated plexiglass. First, we screened and selected the aggressive CD-1 mice. Subsequently, experimental mice were subjected to ten days of CSDS sessions. During these ten days, experimental mice were introduced into the compartment of the aggressor mouse for 7 min. After this timed direct physical contact, mice were placed in the adjacent compartment allowing only sensory interaction with the aggressor. On the eleventh day, social behavioral testing was performed followed by animal sacrifice and brain tissue harvesting.
2.4. Social interaction test
The social interaction test was performed one day after the last defeat session as previously described (Kaidanovich-Beilin et al., 2011). Mice were habituated for 5 min in a cage containing three compartments with two compartments having a circular wire enclosure. After the habituation phase, a social stimulus C57BL/6J mouse (ten weeks) was confined to one of the circular enclosures and the experimental mouse was reintroduced to the cage in the central chamber. The movement of the experimental mouse was monitored using a camera for 10 min. The time spent in each compartment (central, social and non-social) was measured by the ANY-maze program. The total time spent by the mouse in the social compartment was divided by the total time spent in the non-social compartment and the mouse was considered susceptible if the ratio is < 1 and resilient if the ratio is > 1 (Henriques-Alves and Queiroz, 2015).
2.5. Elevated plus maze
The elevated plus maze (EPM) is a validated test for anxiety (File, 2001). Mice were allowed to navigate the maze for 5 min. The time spent in the closed arms was recorded with a camera and measured by the ANY-maze program.
2.6. Open field
Each mouse was allowed to freely explore the open field for 5 min. The average distance travelled was recorded with a camera and measured by the ANY-maze program.
2.7. Immunoblot analyses
To determine TRKB, pTRKB, PGC1a, FNDC5, GAPDH and ACTIN protein levels, total cellular proteins were extracted by lysing the hippocampi in RIPA-B (1% Triton X-100, 1% SDS, 50 mm Tris-Cl, pH 7.4, 500 mm NaCl and 1 mm EDTA) in the presence of the protease inhibitors cocktail (SIGMA) and MG132 (SIGMA), followed by Benzonase nuclease (SIGMA) digestion for 15 min. Antibodies against TrkB and pTRKB were a kind gift from Dr. Moses V. Chao. These antibodies were used at 1:1000 and 1:500 dilutions respectively. Antibodies against PGC1a (ab54881), FNDC5 (ab1748333), GAPDH (ab8245, Abcam) and b-ACTIN (AC-74; Sigma Aldrich) were used at dilutions of 1:1000, 1:1000, 1:1000 and1:1000 respectively. Proteins were visualized using the ChemiDoc Imaging System (BioRad). Band quantification was analyzed with ImageJ software (Daaboul et al., 2017). The Western blot images shown in the different figures are representative images.
2.8. Statistical analysis
Unpaired t-test, 1way or 2way ANOVA followed by the Dunnett, Tukey or Bonferroni post tests were used to measure statistical significance. p < 0.05 was considered to be statistically significant. Data are represented as average ± standard error of the mean (SEM).
3. Results
3.1. BCAA promote resilience to chronic social defeat stress
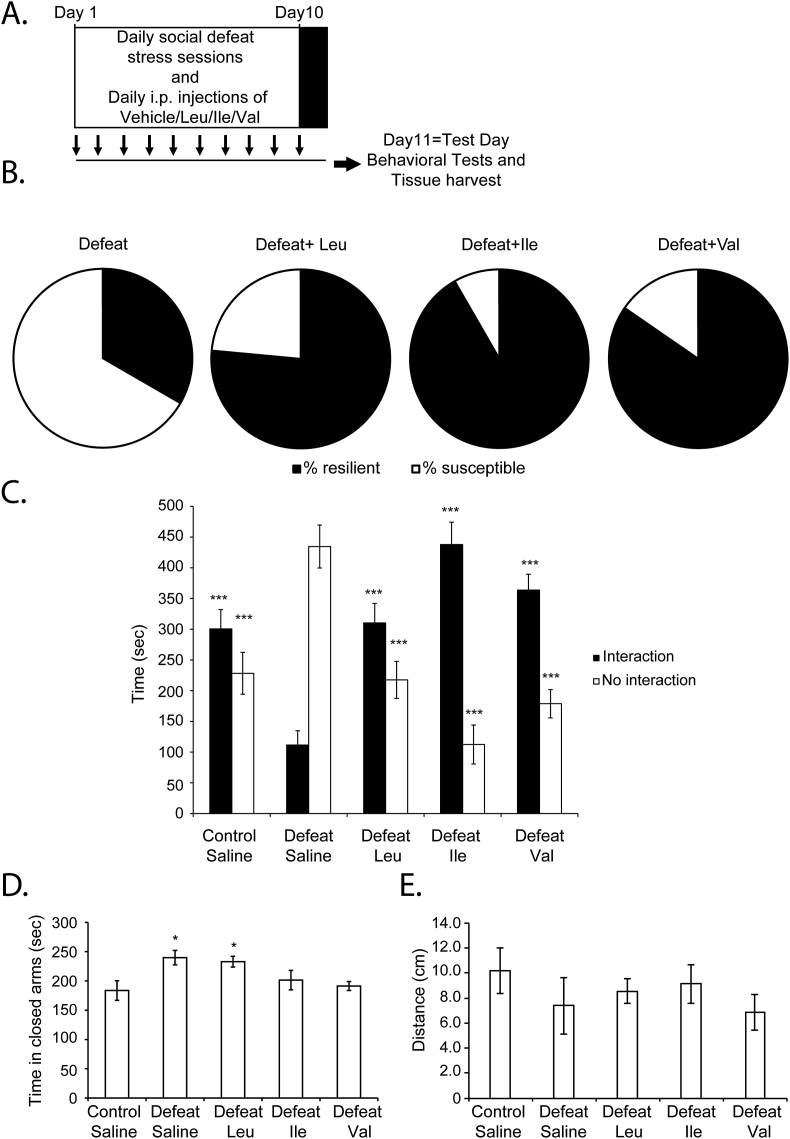
To determine whether BCAA promote resilience to stress, we subjected C57BL/6J male mice (6–7weeks) to a CSDS paradigm (Berton et al., 2006; Krishnan et al., 2007). For ten days, mice received intraperitoneal injections of either saline, Leu, Ile or Val. We used a Leu concentration (32.5 mg/kg) that was previously reported to activate mTOR and protein synthesis (Poncet et al., 2014) and injected equivalent concentrations of Ile and Val. Fifteen minutes after each injection, mice were subjected to defeat sessions (Fig. 1A). Twenty-four hours after the final defeat session, mice underwent social interaction testing to screen for susceptibility versus resilience to stress. Mice exposed to CSDS and that exhibit social avoidance behaviors are classified as susceptible, whereas mice exposed to CSDS and that exhibit normal behavior are classified as resilient (Krishnan et al., 2007). We divided the mice into susceptible or resilient by calculating the social interaction ratio. We found that all BCAA promote resilience to stress. The percentage of resilient mice within the defeat group increased from 33.3% (8/24) in the defeat group receiving saline to 76.5% (13/17) in the defeat group receiving Leu, 91.7% (11/12) in the defeat group receiving Ile and 84.6% (11/13) in the defeat group receiving Val (Fig. 1B).
Fig. 1.
BCAA mediate resilience to chronic social defeat stress and rescue social avoidance behavior. (A) The CSDS paradigm consists of ten days of daily defeat sessions. Each day, the experimental mouse is subjected to direct physical contact with an aggressor mouse for 7 min. On the eleventh day, behavioral tests and brain tissue collection are conducted. Mice receive daily intraperitoneal injections of either saline, Leu, Ile, or Val 15 min before each defeat session. (B) Leu, Ile and Val increase resilience to stress. In the group of mice (n = 24) receiving saline and subjected to CSDS, 33.3% are resilient to stress, whereas 66.7% are susceptible to stress. In the group of mice (n = 17) receiving Leu (32.5 mg/kg, daily for ten days) and subjected to CSDS, 76.5% are resilient to stress, whereas 23.5% are susceptible to stress. In the group of mice (n = 12) receiving Ile (32.5 mg/kg, daily for ten days) and subjected to CSDS, 91.7% are resilient to stress, whereas 8.3% are susceptible to stress. In the group of mice (n = 13) receiving Val (28.5 mg/kg, daily for ten days) and subjected to CSDS, 84.6% are resilient to stress, whereas 15.4% are susceptible to stress. (C) Intraperitoneal injections of Leu, Ile and Val reverse the social avoidance phenotype induced by CSDS as shown by the increase in the time spent in interaction zone of the social interaction test. Statistical significance was measured by 2way Anova followed by Bonferroni posttests. Significance was measured versus the defeat groups. ***p < 0.001. The n numbers for the control, defeat, defeat + Leu, defeat + Ile and defeat + Val are 16, 16, 17,12 and 13 respectively. (D) Intraperitoneal injections of Ile and Val, but not Leu decrease anxiety. defeat + saline and defeat + Leu animals exhibit anxiety-like behavior as measured by the significant increase in the time spent in the closed arm of the elevated plus maze (EPM), whereas defeat + Ile and defeat + Val behave similar to Control. Statistical significance was measured by one-way anova followed by Dunnett's multiple comparison test. Significance was measured versus the control groups *p < 0.05. The n numbers for control + saline, defeat + saline, defeat + Leu, defeat + Ile and defeat + Val are 16, 16, 17, 12 and 13 respectively. (E) There was no significant difference in the distance travelled in the open field between the different mice groups.
3.2. BCAA enhance social interaction
In addition to promoting resilience to stress, BCAA also rescue social avoidance behavior characteristic of CSDS. Defeat mice receiving saline spent significantly less time interacting with the social stimulus as compared to the control mice (Fig. 1C). This social avoidance behavior was reversed by all three BCAA. The average time spent interacting with the social stimulus was significantly higher in the defeat mice receiving Leu, Ile and Val as compared to the defeat mice receiving saline (Fig. 1C). These results confirm that BCAA rescue the social behavior deficits induced by the CSDS paradigm.
3.3. BCAA partially decrease anxiety
We also assessed anxiety-like behaviors using the elevated plus maze (EPM). In the EPM, we measured the time spent exploring the closed arms of the maze to assess the anxiety levels of the different mice groups. A significant increase in the time spent exploring the closed arms of the maze is associated with anxious behavior. We found that mice subjected to CSDS (defeat + saline group) spent significantly more time in the closed arms of the maze as compared to the control mice (Fig. 1D). This effect was reversed only by Ile and Val, but not Leu administration. The defeat sessions and the different BCAA treatments did not affect locomotive behavior, as the average distance travelled by all mice groups in the open field was comparable (Fig. 1E). Our results demonstrate that BCAA promote resilience to stress and rescue social avoidance behavior. Their effects on anxiety were not as robust, but rather were divergent, suggesting as previously reported that the pathways regulating social avoidance behavior and anxiety maybe different (Karnib et al., 2019). In addition, our results are consistent with Ile and Val engaging pathways that protect against anxiety and Leu failing to activate these pathways. We next decided to understand how BCAA mediate resilience to stress.
3.4. BCAA increase hippocampal Bdnf expression and BDNF/TRKB signaling in mice exposed to CSDS
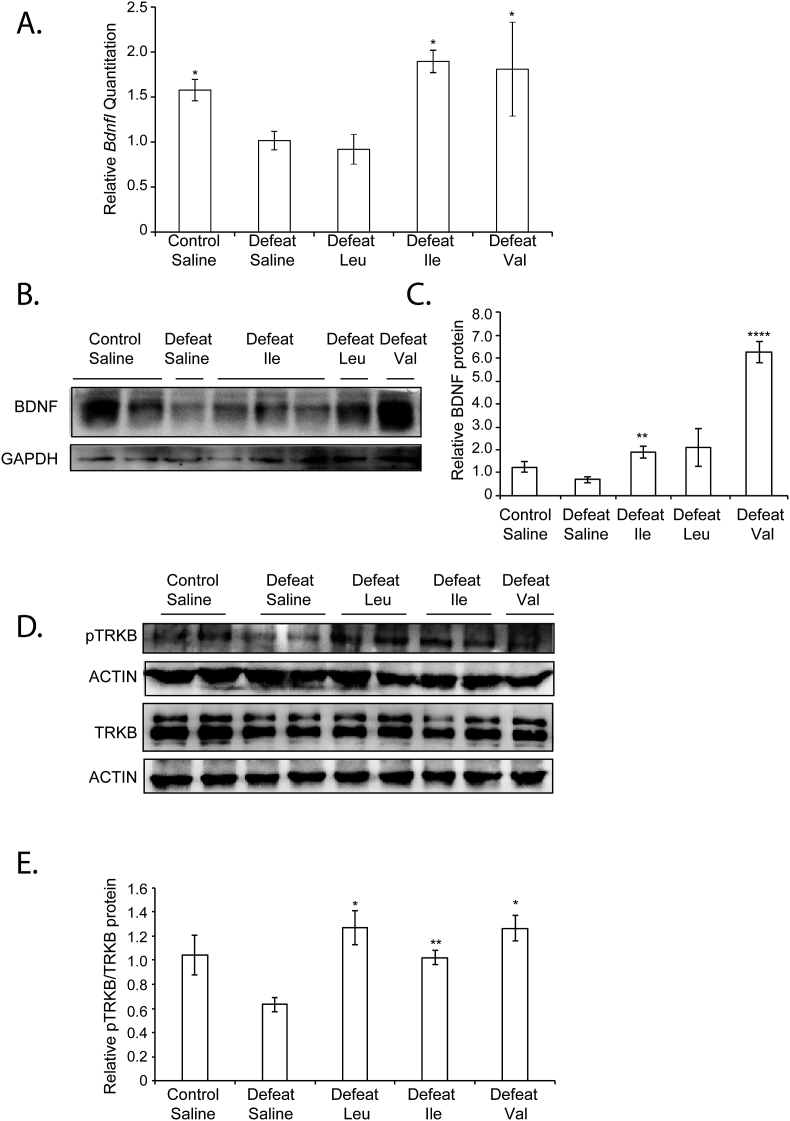
Because hippocampal Bdnf expression is necessary for the ability of antidepressants to promote resilience to CSDS and to rescue social avoidance behavior (reviewed in: Bjorkholm and Monteggia, 2016), we studied how BCAA affect Bdnf expression in the hippocampus. We measured Bdnf exon 1 (BdnfI) mRNA expression using RT Real Time PCR. The rodent Bdnf gene consists of eight non-coding exons and a single coding exon. Many transcripts are generated through alternative splicing of the different non-coding exons with the common coding exon (Pruunsild et al., 2011). We focused on BdnfI since it is both a neuronal activity-dependent (Tabuchi et al., 2002) and an exercise-dependent transcript (Sleiman et al., 2016; Tong et al., 2001). We found that defeat mice that received only Ile or Val, but not Leu had significantly increased hippocampal BdnfI mRNA when compared to defeat mice that received saline (Fig. 2A). We also assessed hippocampal BDNF protein levels using western blots. We found that defeat mice that received Ile and Val had significantly increased hippocampal BDNF protein levels as compared to defeat mice receiving saline (Fig. 2B and C). Even though we observed some increases in hippocampal BDNF protein levels in defeat mice receiving Leu, these increases were not statistically significant. To understand whether BCAA activate BDNF signaling, we analyzed the phosphorylation of the BDNF receptor TRKB using western blots. Interestingly, we found that all three BCAA significantly increased TRKB phosphorylation (Fig. 2D and E). Taken together, our results suggest that Leu, Ile and Val activate BDNF signaling in the hippocampi of defeat mice. In addition, our data suggest that both Ile and Val activate the BDNF/TRKB activity by inducing BDNF gene expression, whereas Leu activates the pathway at the level of the TRKB receptor potentially independent of significant effects on BDNF levels.
Fig. 2.
BCAA restore control levels of BDNF protein and BDNF signaling in the hippocampus. (A) CSDS significantly decrease BDNFI expression in the hippocampus as measured by real-time RTPCR. Intraperitoneal injections of Ile and Val reverse this effect and restore control hippocampal BDNFI expression in animals subjected to CSDS. The number of animal used for control + saline, defeat = saline, defeat + Leu, defeat + Ile and defeat + Val are 13, 13 4, 3 and 3 respectively. Statistical significance was measured by one-way anova followed by Dunnett's multiple comparison test. *p < 0.05. Defeat vs control: p = 0.0228, defeat vs defeat + Leu: p = 0.9700, defeat vs defeat + Ile: p = 0.0264 and defeat vs defeat + Val: p = 0.0362. (B) Representative Western blot images depicting BDNF levels in hippocampus of control + saline, defeat + saline, defeat + Leu, defeat + Ile and defeat + Val animals. (C) Quantification of the BDNF western blots. Significance was measured versus defeat * p < 0.05. The n of hippocampi analyzed are 7,5,4,3, and 2 for control + saline, defeat + saline, defeat + Leu, defeat + Ile and defeat + Val groups respectively. Defeat vs control: p = 0.0940 and df = 10, defeat vs defeat + Leu: p = 0.1068 and df = 7, defeat vs defeat + Ile: p = 0.0082 and df = 6 and defeat vs defeat + Val: p < 0.0001 and df = 5. (D) Representative Western blot images depicting phosphorylated TRKB levels in hippocampus of control, defeat, defeat + Leu, defeat + Ile and defeat + Val animals. (E) Quantification of the phosphorylated TRKB western blots. Significance was measured versus defeat * p < 0.05. The n of hippocampi analyzed are 6,4,5,6, and 2 for control + saline, defeat + saline, defeat + Leu, defeat + Ile and defeat + Val groups respectively. Defeat vs control: p = 0.5853 and df = 9, defeat vs defeat + Leu: p = 0.0191 and df = 7, defeat vs defeat + Ile: p = 0.0022 and df = 8 and defeat vs defeat + Val: p = 0.0331 and df = 4.
3.5. BCAA mediate resilience to stress and rescue social avoidance behavior by activating BDNF/TRKB signaling
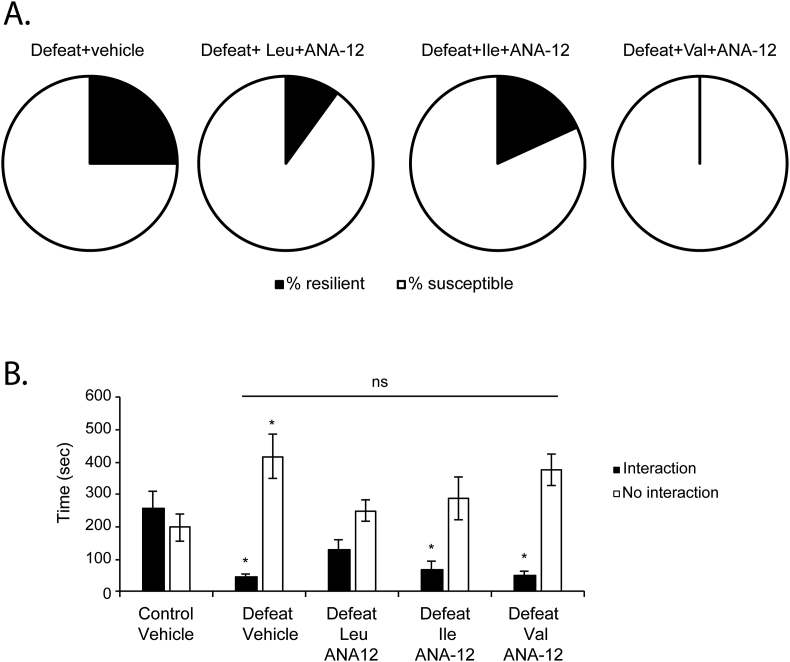
In order to test whether BCAA promote resilience to stress by modulating hippocampal BDNF/TRKB signaling, we studied the effect of a combinatorial treatment of BCAA and a selective TRKB inhibitor, ANA-12, on social behavior in response to CSDS. ANA-12 is a potent and TRKB-selective ligand that prevents activation of this receptor by BDNF without altering TRKA and TRKC functions (Cazorla et al., 2011). ANA-12 crosses the blood-brain barrier and can be detected in the brain (Cazorla et al., 2011). In order to understand whether BCAA promote resilience to stress by modulating BDNF signaling, mice received daily injections of vehicle, Leu + ANA-12, Ile + ANA-12 or Val + ANA-12. After the injections, the mice were exposed to CSDS. We found that all of the combined treatments failed to promote resilience to stress (Fig. 3A). Taken together, these experiments suggest that BCAA mediate resilience to stress by activating BDNF/TRKB signaling. We also studied the social interaction behaviors of the mice receiving the combined treatment. The combined treatment of all BCAA with ANA-12 did not significantly rescue social avoidance phenotype (Fig. 3B). Indeed, no significant increase in the time spent interacting with the social stimulus was observed in mice exposed to CSDS and receiving either treatment as compared to defeat mice receiving vehicle (Fig. 3B). These results support the hypothesis that BCAA rescue social interaction deficits by restoring hippocampal BDNF signaling. We were next interested in understanding the molecular pathways activated by the BCAA to induce BDNF signaling.
Fig. 3.
BCAA mediate resilience to stress and rescue social avoidance behavior by activating BDNF/TRKB signaling. (A) The combined treatment of Leu, Ile and Val with the TRKB inhibitor ANA-12 failed to increase resilience to stress. In the group of mice receiving saline and subjected to CSDS, 25% are resilient to stress, whereas 75% are susceptible to stress. In the group of mice receiving Leu + ANA-12 and subjected to CSDS, 10% are resilient to stress, whereas 90% are susceptible to stress. In the group of mice receiving Ile + ANA-12 and subjected to CSDS, 18% are resilient to stress, whereas 82% are susceptible to stress. In the group of mice receiving Val + ANA-12 and subjected to CSDS, 0% are resilient to stress, whereas 100% are susceptible to stress. The n numbers for the control + vehicle, defeat + vehicle, defeat + Leu + ANA-12, defeat + Ile + ANA-12 and defeat + Val + ANA- 32, 10, 11, 11. (B) Intraperitoneal injections of Leu, Ile and Val in combination with ANA-12 do not rescue the social avoidance phenotype associated with CSDS as shown by the lack of increase in the time spent in interaction zone of the social interaction test. Statistical significance was measured by 2way Anova followed by Tukey's multiple comparison test. Significance was measured versus both control + vehicle or defeat + vehicle groups. *p < 0.05. The n numbers for the control + vehicle, defeat + vehicle, defeat + Leu + ANA-12, defeat + Ile + ANA-12 and defeat + Val + ANA-12 are 6, 8, 10, 11 and 11. Interaction: (defeat + vehicle vs control + vehicle: p = 0.0268, defeat + vehicle vs defeat + Leu: p = 0.6559, defeat + vehicle vs defeat + Ile: p = 0.9930 and defeat + vehicle vs defeat + Val: p > 0.9999) and No Interaction: (defeat + vehicle vs control + vehicle: p = 0.0227, defeat + vehicle vs defeat + Leu: p = 0.0630 defeat + vehicle vs defeat + Ile: p = 0.2149 and defeat + vehicle vs defeat + Val: p = 0.9659).
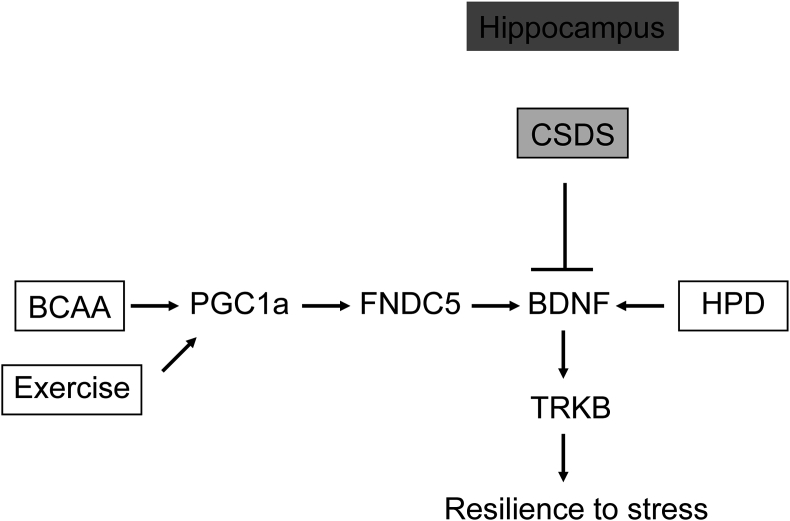
3.6. BCAA activate the exercise-regulated PGC1a/FNDC5 pathway known to induce Bdnf expression
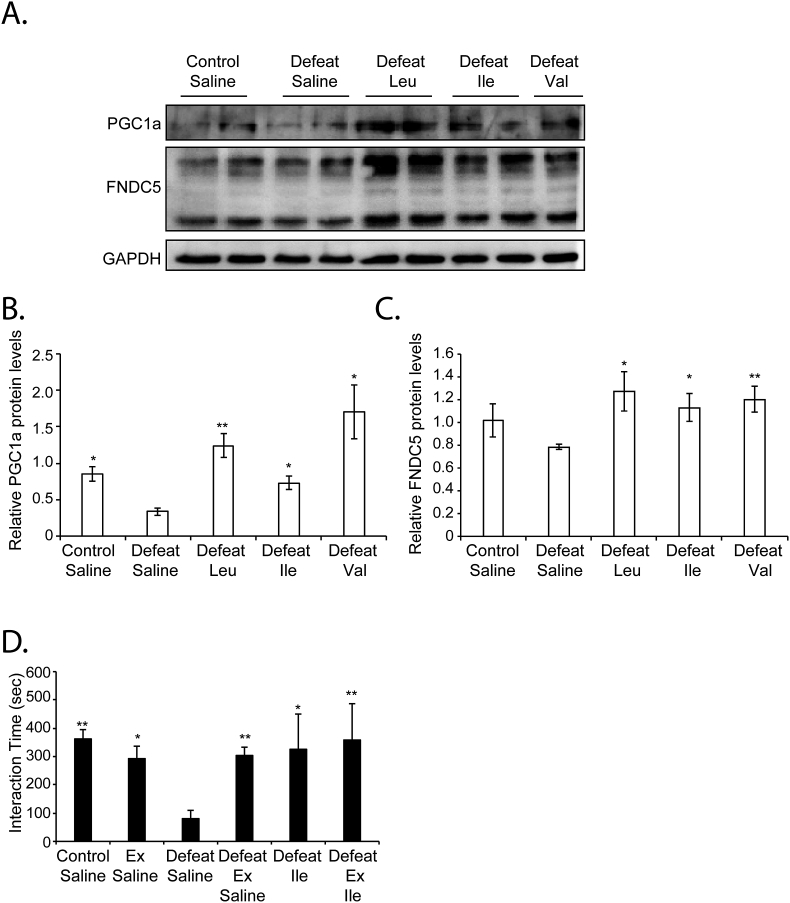
Like BCAA, exercise is a potent inducer of BDNF signaling in the hippocampus (El Hayek et al., 2019; Lourenco et al., 2019; Oliff et al., 1998; Sleiman and Chao, 2015; Sleiman et al., 2016; Wrann et al., 2013). Indeed, the exercise-activated pathway that leads to hippocampal BDNF induction has been deciphered (El Hayek et al., 2019; Wrann et al., 2013). Exercise increases the levels of the transcriptional coactivator PGC1a, which in turn activates the expression of Fndc5. FNDC5, is a protein that is processed and secreted, and that induces BDNF/TRKB activation through an unknown mechanism (El Hayek et al., 2019; Lourenco et al., 2019; Wrann et al., 2013). Since both exercise and diet are environmental factors that lead to BDNF signaling in the hippocampus, we tested whether BCAA also activate the PGC1a/FNDC5 pathway. Indeed, we found that the administration of BCAA increases the protein levels of PGC1a (Fig. 4A and B) and FNDC5 (Fig. 4A and C) in the hippocampus. Considering the already well-established causal relationship between the PGC1a/FNDC5 pathway and hippocampal BDNF signaling, our data is consistent with BCAA activating this pathway to promote resilience to stress and rescue social avoidance behavior. Interestingly, we found that even though both voluntary wheel running (VWR) and Ile can rescue social avoidance behavior as measured by the increase in the time spent interacting with a social stimulus, we do not observe synergistic nor additive effects when we combine VWR with Ile treatment (Fig. 4D). These results are consistent with the hypothesis that both exercise and BCAA mediate resilience to stress by engaging the BDNF/TRKB signaling pathway. Indeed, previous work has already established that exercise reverses chronic stress-induced memory deficits via BDNF induction (Kim and Leem, 2016).
Fig. 4.
BCAA activate the PGC1A/FNDC5 pathway known to activate BDNF signaling in the hippocampus (A) BCAA restore control hippocampal levels of PGC1a and FNDC5 in animals subjected to CSDS. Representative Western blot image depicting hippocampal PGC1a and FNDC5 in control, defeat, defeat + Leu, defeat + Ile and defeat + Val mice. (B) Quantification of the PGC1A western blots. Statistical significance was measured by the unpaired t-test. Significance was measured versus defeat * p < 0.05. The n of hippocampi analyzed are 3 for control + saline, defeat + saline, defeat + Leu, defeat + Ile and defeat + Val groups respectively. Defeat + saline vs control = saline: p = 0.0102 and df = 4, defeat + saline vs defeat + Leu: p = 0.0063 and df = 4, defeat + saline vs defeat + Ile: p = 0.0175 and df = 4 and defeat + saline vs defeat + Val: p = 0.0213 and df = 4 (C) Quantification of the FNDC5 western blots. Statistical significance was measured by the unpaired t-test. Significance was measured versus defeat * p < 0.05. The n of hippocampi analyzed are 4,4,3,3, and 3 for control + saline, defeat + saline, defeat + Leu, defeat + Ile and defeat + Val groups respectively. Defeat vs control: p = 0.1572 and df = 6, defeat vs defeat + Leu: p = 0.0226 and df = 5, defeat vs defeat + Ile: p = 0.0226 and df = 5 and defeat vs defeat + Val: p = 0.0090 and df = 5. (D) VWR and intraperitoneal injections of Ile alone or combined reverse the chronic social defeat phenotype as shown by the increase in the time spent in interaction zone of the social interaction test. Statistical significance was measured by 1way Anova followed by Dunnett's multiple comparison test. Significance was measured versus the defeat groups. *p < 0.05 and **p < 0.01. The n numbers for the control + saline, exercise + saline, defeat + saline, defeat + Exercise (Ex), defeat + Ile and defeat + Ex + Ile are 7, 6, 7, 8, 3 and 4 respectively. Defeat + saline vs control + saline: p = 0.0013, defeat + saline vs Ex + saline: p = 0.0244, defeat + saline vs defeat + Ex + saline: p = 0.0090, defeat + saline vs defeat + Ile: p = 0.0375 and defeat + saline vs defeat + Ex + Ile: p = 0.0071.
3.7. High protein diet (HPD) promotes resilience to stress and rescues social deficits
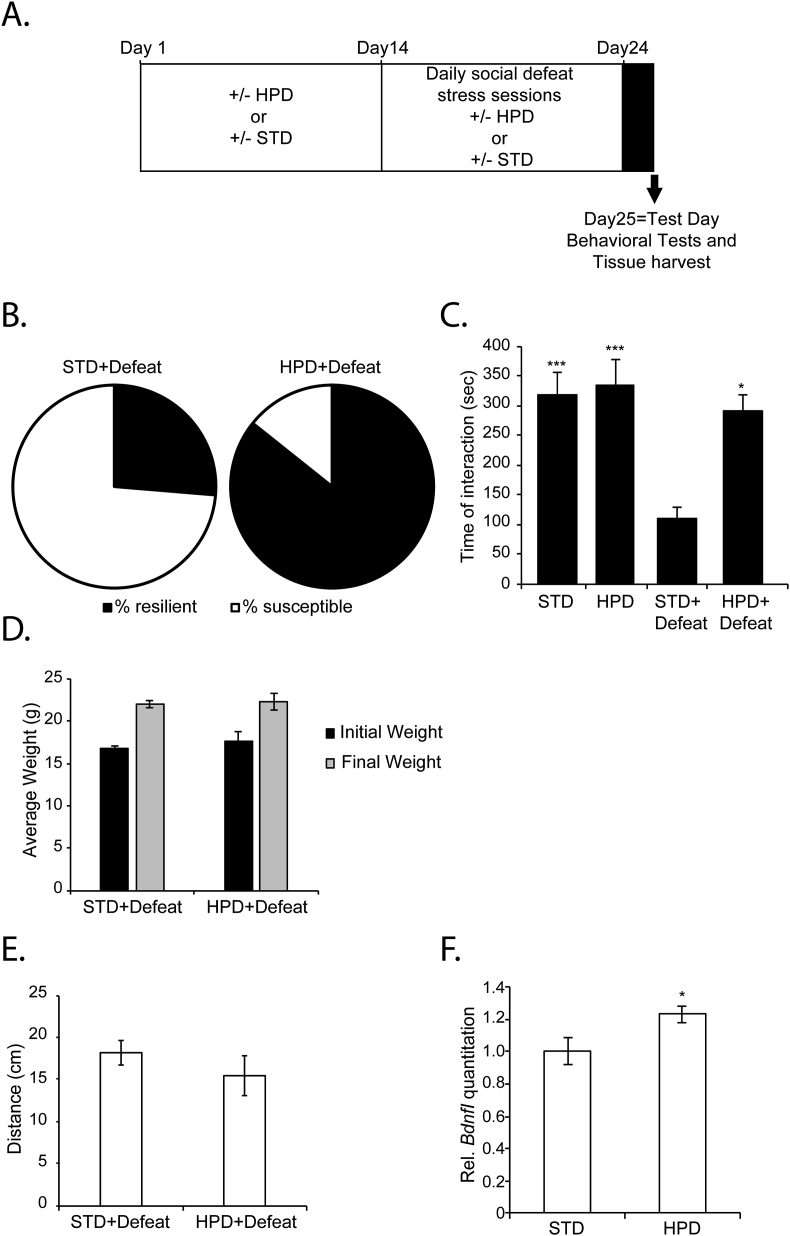
We next decided to test whether like BCAA, a high protein diet (HPD) can also promote resilience to stress and rescue social avoidance behavior. Several studies have associated carbohydrate-enriched and high fat diets with social behavior, yet little is known about the effect of HPDs. For example, carbohydrate-enriched diets increase anxiety and depression after exposure to stress (Santos et al., 2018). High-fat diets, on the other hand, are initially anxiolytic, but prolonged use promotes anxiety and depression (Del Rio et al., 2016; Hassan et al., 2018; Xu et al., 2018). To test whether a HPD mediates resilience to CSDS, mice were either fed a standard diet (STD) or a HPD for two weeks then were subjected to CSDS (Fig. 5A). 26% of the mice that received the STD and that were subjected to CSDS were resilient to stress, whereas 85.7% of the mice that received the HPD and that were subjected to CSDS were resilient to stress (Fig. 5B). Indeed, the mice that received the HPD and that were subjected to CSDS spent significantly more time interacting with a social stimulus as compared to mice that were subjected to CSDS and that received the STD (Fig. 5C). The effects of the HPD on resilience to stress and social behavior were independent of mice weight (Fig. 5D) or changes in locomotor behavior (Fig. 5E). Interestingly, mice that received the HPD had significantly higher hippocampal BdnfI mRNA expression as compared to mice that received the STD (Fig. 5F). Taken together, our data are consistent with HPD, BCAA and exercise protecting against depression by inducing hippocampal BDNF/TrkB signaling (Fig. 6).
Fig. 5.
HPD promotes resilience to CSDS, rescues social avoidance behaviors and induces hippocampal Bdnf expression. (A) Animals were fed either STD or HPD for two weeks before being subjected to CSDS. Animals continued to receive the different diets during the CSDS. On day 24, behavioral tests and brain tissue collection were conducted. (B) HPD increase resilience to stress. In the group of mice (n = 19) receiving a STD and subjected to CSDS, 26% are resilient to stress. In the group of mice (n = 7) receiving a HPD and subjected to CSDS, 85.7% are resilient to stress. (C) HPD reverses the social avoidance phenotype as shown by the increase in the time spent in interaction zone of the social interaction test. Statistical significance was measured by 2way Anova followed by Tukey's multiple comparison test. Interaction: F(1,43) = 4.886, p = 0.0324, Row Factor F(1,43) = 6.864, p = 0.0121 and column factor F(1,43) = 10.84, p = 0.02. STD + defeat vs HPD + Defeat: p = 0.0153, STD + defeat vs STD: p = 0.001 and STD + defeat vs HPD: p = 0.0002. (D) No significant changes in the weight were observed between the mice receiving a STD or HPD at the end of the experiment. (E) There was no significant difference in the distance travelled in the open field between the different mice groups. (F) Mice receiving a HPD for 4 weeks had significantly increased BdnfI expression levels as compared to mice receiving a STD. Statistical significance was measured by unpaired t-test groups. *p < 0.05. The n number for mice receiving STD and HPD is 10 and 12 respectively.
Fig. 6.
BCAA mediate resilience to stress by inducing the hippocampal BNDF/TRKB signaling through the PGC1a/FNDC5 pathway.
4. Discussion
Changes in the levels of BCAA have been detected in depressed patients. However, our knowledge of their specific roles in the development of depression is limited. Leu acts as an antidepressant in an inflammation-induced depression model (Walker et al., 2018). It produces its antidepressant effects by preventing the transport of the inflammation-induced neurotoxic metabolite kynurenine to the brain (Walker et al., 2018). In this study, we showed that all BCAA promote resilience to CSDS and prevent social avoidance behavior. These observations suggest that the decreased BCAA levels in depressed patients are not circumstantial, but rather relevant to the prevention of the disease. Interestingly, the anxiolytic effects of BCAA were less robust and variable. Both Ile and Val prevented anxious behavior, whereas Leu was not anxiolytic suggesting that BCAA activate different pathways to regulate anxiety. Our results are consistent with previous studies suggesting that BCAA have important functions in the brain (Sperringer et al., 2017). Interestingly BCAA supplementation improves cognitive function in mice subjected to traumatic brain injury (Cole et al., 2010; Paterno et al., 2018) and extends life span (D'Antona et al., 2010; Mansfeld et al., 2015). One critical aspect in considering BCAA supplementation to enhance cognitive function and rescue depression is dosing. Elevated levels of BCAA are associated with a high risk of metabolic disorder such as insulin resistance as well as maple syrup urine disorder (Al-Haddad et al., 2016; Lynch and Adams, 2014).
We also discovered that BCAA promote resilience to stress by activating hippocampal BDNF/TRKB signaling. BDNF signaling regulates neuronal survival, synaptic plasticity, neurotransmitter release, long-term potentiation and memory formation. Disruption of BDNF signaling is associated with the pathophysiology of many psychiatric disorders such as depression and anxiety (Mitre et al., 2017). Specifically, decreases in BDNF levels and TRKB signaling in the hippocampus have been associated with depression and increased susceptibility to CSDS (Bjorkholm and Monteggia, 2016). Indeed, antidepressants can mediate their effects by restoring BDNF signaling in the hippocampus (Bjorkholm and Monteggia, 2016; Monteggia et al., 2004, 2007; Tsankova et al., 2006). Even though all three BCAA induced TRKB signaling, our data suggest that Ile and Val specifically affect BdnfI gene expression.
In addition to antidepressants, voluntary exercise as well as lactate, a metabolite produced during exercise, promote resilience to CSDS and anxiety disorders (Karnib et al., 2019; Mattar et al., 2017; Mul, 2018; Mul et al., 2018; Rizk et al., 2018) and increase BDNF signaling. Multiple reports have deciphered the exercise-regulated molecular pathways responsible for hippocampal BDNF activation and identified the PGC1a/FNDC5 as a major BDNF inducer (El Hayek et al., 2019; Sleiman et al., 2016; Wrann et al., 2013). PGC1a induces the expression of the transmembrane protein FNDC5 which is cleaved and secreted as irisin. Irisin is thought to activate BDNF signaling and this exercise-induced pathway is critical to rescue cognitive impairment associated with Alzheimer's disease mouse models (Lourenco et al., 2019; Wrann et al., 2013). In this work, we discovered that like exercise, BCAA can engage this hippocampal pathway known to induce BDNF activity to promote resilience to CSDS. Indeed, we found that combining exercise with Ile did not induce synergistic effects on resilience to stress suggesting that both paradigms mediate their effects by engaging similar pathways. These data are consistent with previous reports suggesting that exercise reverses chronic-stress induced cognitive functions by inducing hippocampal BDNF signaling (Kim and Leem, 2016).
Finally, we were also able to expand our results to show that HPD rich in BCAA also promote resilience to stress and rescue social avoidance. Associations between life-style choices including diets and psychiatric disorders have been established (Doumit et al., 2016, 2018; Mattar et al., 2019; Quirk et al., 2013; Sanchez-Ruiz et al., 2019; Zeeni et al., 2018). Previous work has implicated prolonged use of both carbohydrate-enriched diets and high fat diets in promoting depression and anxiety, but no information was available about how HPD affect social behavior (Del Rio et al., 2016; Eudave et al., 2018; Hassan et al., 2018; Santos et al., 2018; Xu et al., 2018). Our work suggests that short-term use of HPD and BCAA can be useful. In future, further investigation may be required to distinguish between effects of short-term use of BCAA and long-term use, a phenomenon that is prevalent with athletes, on resilience to stress and depression. Even though our results are consistent with BCAA mediating the positive effects of the HPD, we have evidence that suggests that these positive effects are not exclusively mediated by BCAA. Indeed, preliminary results suggest that the amino acid methionine (Met) promotes resilience to CSDS and rescues social avoidance behavior (SFS, unpublished results). This is of great interest considering that Met is the precursor of S-Adenosyl Methionine (SAM), which is a cofactor for DNA and histone methyltransferases. Alternatively, how Tryptophan (Trp) affects CSDS may also be relevant since Trp is a precursor of serotonin and since recent studies have revealed that histones can be serotonylated (Farrelly et al., 2019). Ultimately, the effects of Met and Trp on CSDS should be addressed because these amino acids affect epigenetic modifications which can lead to changes in brain gene expression patterns, a hallmark of depression (Nestler et al., 2016).
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the Lebanese American University School of Arts and Sciences SPIII fund, Lebanese American University Graduate Research fund to SFS and the National Council for Scientific Research, Lebanon, grant #699. This work is also supported by Lebanese American University grants to JSS. SFS conceived the study, performed experiments, analyzed the data and wrote the manuscript. PN, EAH performed the experiments with the help of LEH, NK, MK, NB, VJ, AG and JN. JSS analyzed the results, provided valuable reagents and helped write the manuscript. NZ and MB designed the diets, provided valuable advise on the use of these diets and edited the manuscript.
References
- Adachi M. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Haddad R. Epigenetic changes in diabetes. Neurosci. Lett. 2016;625:64–69. doi: 10.1016/j.neulet.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Bagot R.C. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron. 2016;90:969–983. doi: 10.1016/j.neuron.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot R.C. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol. Psychiatry. 2017;81:285–295. doi: 10.1016/j.biopsych.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi A. Branched-chain amino acids as new biomarkers of major depression - a novel neurobiology of mood disorder. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi A. Metabolomics approach in the investigation of depression biomarkers in pharmacologically induced immune-related depression. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti C. Transcriptional profiles underlying vulnerability and resilience in rats exposed to an acute unavoidable stress. J. Neurosci. Res. 2012;90:2103–2115. doi: 10.1002/jnr.23100. [DOI] [PubMed] [Google Scholar]
- Berton O. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bjorkholm C., Monteggia L.M. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S.G., Nemeroff C.B. Emerging antidepressants to treat major depressive disorder. Asian J Psychiatr. 2014;12:7–16. doi: 10.1016/j.ajp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Cazorla M. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Investig. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.T. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc. Natl. Acad. Sci. U. S. A. 2010;107:366–371. doi: 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metabol. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Daaboul H.E. Antitumor activity of beta-2-himachalen-6-ol in colon cancer is mediated through its inhibition of the PI3K and MAPK pathways. Chem. Biol. Interact. 2017;275:162–170. doi: 10.1016/j.cbi.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Del Rio D. Effect of high-fat diets on mood and learning performance in adolescent mice. Behav. Brain Res. 2016;311:167–172. doi: 10.1016/j.bbr.2016.04.052. [DOI] [PubMed] [Google Scholar]
- Doumit R. Predictors of disordered eating in young males. Community Ment. Health J. 2018;54:236–244. doi: 10.1007/s10597-017-0163-2. [DOI] [PubMed] [Google Scholar]
- Doumit R. Anxiety as a moderator of the relationship between body image and restrained eating. Psychiatr. Care. 2016;52:254–264. doi: 10.1111/ppc.12126. [DOI] [PubMed] [Google Scholar]
- Duclot F., Kabbaj M. Individual differences in novelty seeking predict subsequent vulnerability to social defeat through a differential epigenetic regulation of brain-derived neurotrophic factor expression. J. Neurosci. 2013;33:11048–11060. doi: 10.1523/JNEUROSCI.0199-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hayek L. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J. Neurosci. 2019 Mar 27;39(13):2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudave D.M. Effects of high fat or high sucrose diet on behavioral-response to social defeat stress in mice. Neurobiol Stress. 2018;9:1–8. doi: 10.1016/j.ynstr.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly L.A. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535–539. doi: 10.1038/s41586-019-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellendorf F.T. Branched-chain amino acids are associated with metabolic parameters in bipolar disorder. World J. Biol. Psychiatr. 2018:1–6. doi: 10.1080/15622975.2018.1487077. [DOI] [PubMed] [Google Scholar]
- File S.E. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Golden S.A. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.M. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2018:1–17. doi: 10.1080/1028415X.2018.1465713. [DOI] [PubMed] [Google Scholar]
- Henriques-Alves A.M., Queiroz C.M. Ethological evaluation of the effects of social defeat stress in mice: beyond the social interaction ratio. Front. Behav. Neurosci. 2015;9:364. doi: 10.3389/fnbeh.2015.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B. SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway. Int. J. Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O. Assessment of social interaction behaviors. J. Vis. Exp. 2011 Feb 25;48 doi: 10.3791/2473. pii: 2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnib N. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology. 2019 May;44(6):1152–1162. doi: 10.1038/s41386-019-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.M., Leem Y.H. Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience. 2016;324:271–285. doi: 10.1016/j.neuroscience.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Krishnan V. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lourenco M.V. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat. Med. 2019;25:165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C.J., Adams S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun. 2015;6:10043. doi: 10.1038/ncomms10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar L. Movie violence acutely affects food choices in young adults. Eat. Behav. 2019;33:7–12. doi: 10.1016/j.eatbeh.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Mattar L. Effect of 7-minute workout on weight and body composition. J Sports Med Phys Fitness. 2017;57:1299–1304. doi: 10.23736/S0022-4707.16.06788-8. [DOI] [PubMed] [Google Scholar]
- Mitre M. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 2017;131:13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia L.M. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia L.M. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Mul J.D. Voluntary exercise and depression-like behavior in rodents: are we running in the right direction? J. Mol. Endocrinol. 2018;60:R77–R95. doi: 10.1530/JME-17-0165. [DOI] [PubMed] [Google Scholar]
- Mul J.D. Voluntary wheel running promotes resilience to chronic social defeat stress in mice: a role for nucleus accumbens DeltaFosB. Neuropsychopharmacology. 2018 Aug;43(9):1934–1942. doi: 10.1038/s41386-018-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J. Epigenetic basis of mental illness. Neuroscientist. 2016;22:447–463. doi: 10.1177/1073858415608147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol. Psychiatry. 2015;20:916–930. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- Oliff H.S. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Paterno R. Memory deficit in an object location task after mild traumatic brain injury is associated with impaired early object exploration and both are restored by branched chain amino acid dietary therapy. J. Neurotrauma. 2018;35:2117–2124. doi: 10.1089/neu.2017.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet N. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J. Neurosci. 2011;31:3295–3308. doi: 10.1523/JNEUROSCI.4540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk S.E. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175. doi: 10.1186/1471-244X-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk M. High-intensity exercise is associated with a better nutritional status in anorexia nervosa. Eur. Eat. Disord. Rev. 2018 Dec 26:1–10. doi: 10.1002/erv.2661. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruiz M.J. Personality, emotion-related variables, and media pressure predict eating disorders via disordered eating in Lebanese university students. Eat. Weight Disord. 2019;24:313–322. doi: 10.1007/s40519-017-0387-8. [DOI] [PubMed] [Google Scholar]
- Santos C.J. Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr. Neurosci. 2018;21:33–39. doi: 10.1080/1028415X.2016.1213529. [DOI] [PubMed] [Google Scholar]
- Sleiman S.F., Chao M.V. Downstream consequences of exercise through the action of BDNF. Brain Plast. 2015;1:143–148. doi: 10.3233/BPL-150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman S.F. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 2016;5 doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. Mental health: a world of depression. Nature. 2014;515:181. doi: 10.1038/515180a. [DOI] [PubMed] [Google Scholar]
- Soliman F. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperringer J.E. Branched-chain amino acids and brain metabolism. Neurochem. Res. 2017;42:1697–1709. doi: 10.1007/s11064-017-2261-5. [DOI] [PubMed] [Google Scholar]
- Tabuchi A. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J. Biol. Chem. 2002;277:35920–35931. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- Tong L. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol. Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Tsankova N.M. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Walker A.K. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol. Psychiatry. 2018 Jul 9 doi: 10.1038/s41380-018-0076-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webhofer C. Metabolite profiling of antidepressant drug action reveals novel drug targets beyond monoamine elevation. Transl. Psychiatry. 2011;1 doi: 10.1038/tp.2011.56. e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann C.D. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metabol. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. High-fat diet mediates anxiolytic-like behaviors in a time-dependent manner through the regulation of SIRT1 in the brain. Neuroscience. 2018;372:237–245. doi: 10.1016/j.neuroscience.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Yu H. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J. Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeni N. Media, technology use, and attitudes: associations with physical and mental well-being in youth with implications for evidence-based practice. Worldviews Evidence-Based Nurs. 2018;15:304–312. doi: 10.1111/wvn.12298. [DOI] [PubMed] [Google Scholar]