Abstract
Background
Enset (Ensete ventricosum, Musaceae) is an African crop that currently provides the staple food for approx. 20 million Ethiopians. Whilst wild enset grows over much of East and Southern Africa and the genus extends across Asia to China, it has only ever been domesticated in the Ethiopian Highlands. Here, smallholder farmers cultivate hundreds of landraces across diverse climatic and agroecological systems.
Scope
Enset has several important food security traits. It grows over a relatively wide range of conditions, is somewhat drought-tolerant, and can be harvested at any time of the year, over several years. It provides an important dietary starch source, as well as fibres, medicines, animal fodder, roofing and packaging. It stabilizes soils and microclimates and has significant cultural importance. In contrast to the other cultivated species in the family Musaceae (banana), enset has received relatively little research attention. Here, we review and critically evaluate existing research, outline available genomic and germplasm resources, aspects of pathology, and explore avenues for crop development.
Conclusion
Enset is an underexploited starch crop with significant potential in Ethiopia and beyond. Research is lacking in several key areas: empirical studies on the efficacy of current agronomic practices, the genetic diversity of landraces, approaches to systematic breeding, characterization of existing and emerging diseases, adaptability to new ranges and land-use change, the projected impact of climate change, conservation of crop wild relatives, by-products or co-products or non-starch uses, and the enset microbiome. We also highlight the limited availability of enset germplasm in living collections and seedbanks, and the lack of knowledge of reproductive and germination biology needed to underpin future breeding. By reviewing the current state of the art in enset research and identifying gaps and opportunities, we hope to catalyse the development and sustainable exploitation of this neglected starch crop.
Keywords: Biodiversity, biotic and abiotic resistance, climate adaptation, crop wild relatives (CWRs), domestication, Ensete ventricosum, false banana, food security, germplasm collections, pests and pathogens, sustainable agriculture, tropical crop ecology
INTRODUCTION
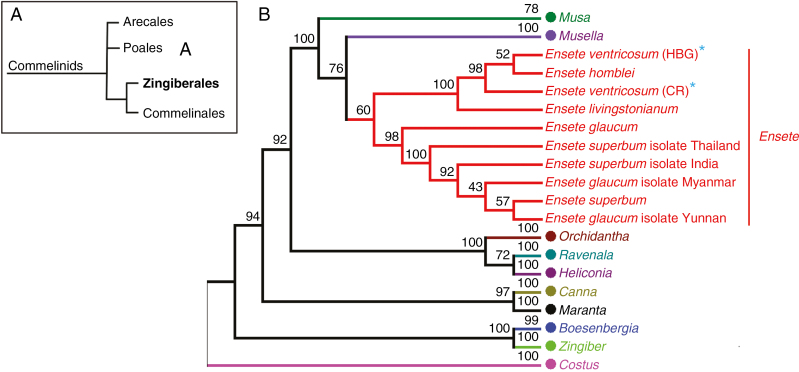
Enset [Ensete ventricosum (Welw.) Cheesman] is a large perennial monocarpic herbaceous plant, similar in form to the related bananas of the genus Musa (Fig. 1). The two genera, together with the monotypic Musella (Franch.) C.Y. Wu ex H.W. Li, form the family Musaceae within the Monocot order Zingiberales (Fig. 2A). Like banana, enset has a pseudostem of overlapping leaf sheaths, large paddle-shaped (oblong-lanceolate) leaves and produces a massive pendulous inflorescence with banana-like fruits. However, unlike sweet and starchy banana (with the latter called plantain in some contexts, although there is no botanical distinction between banana and plantain), which are widely farmed for their fruits, it is instead the swollen pseudostem base, leaf sheaths and underground corm that provide a year-round dietary starch source, typically harvested 4–7 years after planting. Despite a widespread distribution in eastern, central and southern tropical Africa (Baker and Simmonds, 1953; Lock, 1993), enset has only been domesticated in Ethiopia (Brandt et al., 1997). Here, hundreds of landraces are found in diverse climatic and agroecological systems (Birmeta et al., 2002; Tesfaye and Lüdders, 2003; Yemataw et al., 2014a) where they provide the staple food source for approx. 20 million rural people (Supplementary Data, Fig. S1 – see Supplementary data Information for population estimation methods).
Fig. 1.
Domesticated Enset ventricosum in Ethiopia. (A, B) Original plates of ‘Ensete’ from Bruce (1790). (C) Large enset plants (landrace ‘Medasho’) grown by small scale farmers in Teticha (Sidama Zone, SNNPR region). (D) A typical enset home garden near Butajira (Gurage Zone, SNNPR region). (E) An enset germplasm collection at Yerefezy research station, University of Wolkite (Gurage Zone, SNNPR region). Clear differences in morphology can be observed, with substantial differences in growth rate under local environmental conditions.
Fig. 2.
Evolutionary relationships of genus Ensete. (A) The genus Ensete is included in the Musaceae, one of eight families of the monocot order Zingiberales which together with the Commelinales is sister to the Poales that contain the cereal crops including wheat, maize and rice. (B) Evolutionary relationships of the genus Ensete within the Zingiberales based on ITS sequences, including collapsed sister genera within Musaceae and outgroups representing the eight families (see Supplementary Data Fig. S2 for an expanded tree and Supplementary Data Information for method details). Provenance of the two E. ventricosum accessions are Hamburg Botanic Garden (HBG) and Costa Rica (CR; Introduced).
Enset has historically been ascribed as a ‘tree against hunger’ (Brandt et al., 1997), due to the domesticated plant having important attributes that support the food security of communities that cultivate it. These attributes were evident during the devastating famines of the 1980s, where enset-growing communities reported little-to-no food insecurity (Dessalegn, 1995). Most significant is the apparent ability of enset to withstand environmental stress, including periods of drought (Quinlan et al., 2015). Enset can also be harvested at any time of the year and at any stage over several years (including when it is immature), and enset-derived starch can also be stored for long periods (Birmeta, 2004). Enset also provides fibres, medicines, animal fodder and packaging material (Brandt et al., 1997). It stabilizes soils and microclimates (Abate et al., 1996) and is culturally significant (Kanshie, 2002; Negash and Niehof, 2004; Tewodros and Tesfaye, 2014). Enset has a complex management system supported by extensive ethnobotanical knowledge (Borrell et al., unpubl. res.). In a comparison of starch crops, enset has been reported to produce the highest yield per hectare in Ethiopia (Tsegaye and Struik, 2001; Kanshie, 2002) with relatively low inputs and management requirements. Enset therefore has the ability to support a larger population per unit area than regions relying on growing cereals (Yirgu, 2016). As a result of these qualities, enset farming provides a long-term, sustainable food supply capable of buffering not only seasonal and periodic food deficits, with minimum off-farm input, but also demonstrates potential that exceeds its current utilization in South-West Ethiopia.
Despite the current and potential importance of enset, relatively little is known about its biology and ecology. In this review we aim to (1) summarize the existing knowledge and current research effort both nationally in Ethiopia and internationally; (2) identify critical knowledge gaps in the ecology, diversity and distribution of enset to direct future research effort; and (3) catalyse the development of resources needed to enable the sustainable exploitation of enset diversity as a resilient climate-smart crop of the future. Concurrently, we also acknowledge the importance of local ethnobotanic knowledge, management and plant processing; these topics will be reviewed in due course (Borrell et al., unpubl. res.). Finally, we introduce the online resource www.enset-project.org to make various tools and data available to researchers both in Ethiopia and internationally.
THE GENUS ENSETE: EVOLUTION AND SYSTEMATICS
Ensete Bruce ex Horan. is a monophyletic genus (Li et al., 2010) with seven described species in Africa and Asia (Table 1). Although first reported by Bruce (1790) during travels in Ethiopia (Fig. 1A, B), and formally described by Horaninow (1862) it was not until almost a century and a half later that Cheesman (1947) elevated the informal ‘giant bananas’ group within Musa to re-establish the genus Ensete. Of the 20 reported synonyms, 65 % relate to Ensete ventricosum (Welw.) Cheesman. The sister genus Musella (Li, 1978) was originally placed under Musa and Ensete (Cheesman, 1947; Simmonds, 1960). Whilst the sole species of this genus, M. laisocarpa (the golden lotus banana), occupies a unique geographical distribution, drier and cooler than any other member of the family, there is continuing debate as to whether it should be treated as a member of Ensete or as its sister (Liu et al., 2003; Li et al., 2010).
Table 1.
Accepted species of the genus Ensete, with details of conservation and domestication.
| Accepted species* | Common names and synonyms | Conservation status† and distribution | Domestication status | Uses | Notes |
|---|---|---|---|---|---|
| Africa | |||||
| Ensete homblei | None | DD; possibly restricted range | Crop wild relative | Unknown | The majority of locality information for this species, is from historical herbarium collections. Reported to die down to the corm in the dry season (Timberlake and Martins, 2010). |
| Ensete ventricosum | Abyssinian banana, False banana, E. edule | LC; widely distributed | Domesticated & crop wild relative | Human food; animal fodder; fibre; packaging; medicine; ornamental | Due to the practice of harvesting domestic enset before the flowers mature, there is probably limited gene flow between wild and cultivated populations (Birmeta et al., 2004). |
| Ensete livingstonianum | E. gilettii | LC; widely distributed. | Crop wild relative | Unknown | Reported to die down to the corm in the dry season (J.S.B., pers. obs.). |
| Ensete perrieri | Madagascar banana, Musa perrieri | CR; endemic to Madagascar; only three known mature individuals. | Crop wild relative | Unknown | Reported to die down to the corm in the dry season (Schatz and Phillipson, 2011). Possibly present in the ornamental trade, but genetic confirmation of identity required. |
| Asia | |||||
| Ensete superbum | Cliff banana | EN; endemic to India | Crop wild relative | Human food; packaging; medicine; ornamental | Overharvesting from the wild of leaves, seeds and young plants has been reported (Bhise et al., 2015). |
| Ensete glaucum | Snow banana | LC; widely distributed (E. glaucum var. wilsonii listed as DD) | Evidence of utilization | Animal fodder; cultural; ornamental | Denham and Donohue (2009). There is some doubt as to whether E. glaucum var. wilsonii (Tucher) Häkkinen is distinct from E. glaucum. The two species are largely sympatric, with E. glaucum var. wilsonii being a smaller, higher elevation species endemic to Yunnan (Wu and Kress, 2000). Possibly present in the ornamental trade, but could be E. glaucum. |
| Ensete lecongkietii | Orphan banana | NE (Not Evaluated); endemic to Vietnam | Crop wild relative | Unknown | The most recently described Ensete species (Luu et al., 2012). |
| Close relatives | |||||
| Musella lasiocarpa | Golden lotus banana E. lasiocarpa, Musella lasiocarpum | DD; essentially unknown in the wild, but common in agricultural areas. | Semi-cultivated | Medicinal use; human food; animal fodder; fibre; alleviation of soil erosion; ornamental. | There is some degree of conflict in the literature over whether the genus Musella is truly distinct from the genus Ensete (Liu et al., 2003; Li et al., 2010). We follow recent evidence (Janssens et al., 2016). |
*Sources: All species reported here are considered accepted species by POWO (WCSP, 2018).
†IUCN Red List conservation status classifications: NE, Not Evaluated; DD, Data Deficient; LC, Least Concern; EN, Endangered; CR, Critically Endangered.
Currently, Ensete (seven species) and the sister genera Musa (approx. 70 species) and Musella (one species) belong to the Musaceae within the order Zingiberales, together with eight tropical plant families (Fig. 2B, Supplementary Data Table S1), some including genera known for their medicinal properties and ornamental use. APG IV (Chase et al., 2016) has confirmed the position of Zingiberales as a monophyletic order within the monocots, placing it in the commelinoid clade, as the sister group to Commelinales, but has not addressed the interfamilial relationships of the other families belonging within the order (Fig. 2B). Understanding the relationship and genomic organization of Ensete as sister to Musa may provide novel insights into the evolution of the globally important Musaceae family.
Like the other Musaceae genera, Ensete originated in northern Indo-Burma during the early Eocene, probably followed by a single African colonization via gradual overland dispersal during a more mesic climate period (Janssens et al., 2016). The presence of Eocene Ensete fossils in North America (Manchester and Kress, 1993) establishes that the genus also reached the New World. Ensete differs from bananas in being mainly African in distribution, monocarpic, having large seed size (up to 18 mm compared to 10 mm) and an apparent adaptation to cooler and drier environments than most Musa species (Cheesman, 1947; Baker and Simmonds, 1953). Musa and Ensete can be further distinguished by the presence of ‘T’-shaped embryos and granulose papillose pollen grains in Ensete, and their absence in Musa (Bekele and Shigeta, 2011). Ensete does not normally produce suckers, whereas Musa does – although a small number of suckering E. ventricosum landraces are known to occur in Ethiopia (provenance unknown). In the field, Ensete are perhaps best distinguished from Musa by their more rigid and upright leaves (J.S.B., pers. obs.).
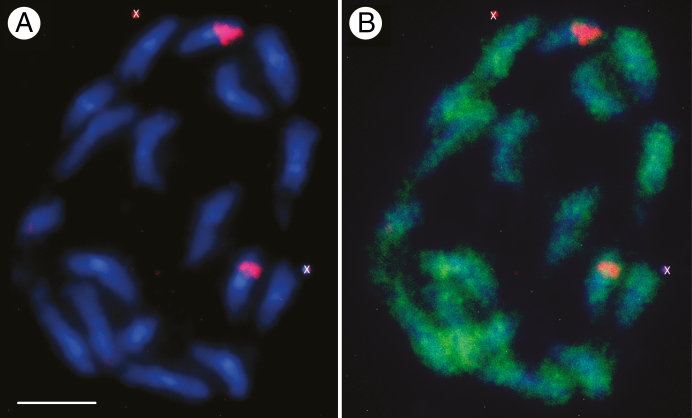
A further distinction is that Ensete is currently only reported to be diploid with 2n = 2x = 18 (Westphal, 1975; Diro et al., 2003) and this is consistent with flow cytometry measurements in ten individuals (J. S. Heslop-Harrison and P. Tomaszewska pers. comm.) and chromosome counts (Fig. 3). By contrast, Musa has species with x = 7, 10 and 11 at various ploidy levels; domesticated varieties are commonly sterile, parthenocarpic triploids (2n = 3x = 33) (Bartoš et al., 2005). Species in the genus Ensete have a relative small DNA content, reported to be about 620 Mb per haploid genome for E. livingstonianum (measured by flow cytometry, Bartoš et al., 2005) and 547 Mb for E. ventricosum (estimated from whole genome sequencing, Harrison et al., 2014). This is similar to the genome size of haploid Musa species, ranging from 580 to 800 Mb measured by flow cytometry (Bartoš et al., 2005), and the lower estimates from whole genome sequencing of 523 Mb for M. acuminata (D’Hont et al., 2012). Ensete species have n = 9 chromosomes (Westphal, 1975) and the karyotype consists of mainly bi-armed chromosomes of similar size, each slightly bigger than those in banana (Fig. 3). Musa species from the section Eumusa, which includes the bananas M. acuminata and M. balbisiana, all have n = 11. Other Musa species outside the section Eumusa (in sections Australimusa and Rhodochlamys) have n = 9 and n = 10. Molecular cytogenetics of E. ventricosum localized 5S rDNA sequences at the short arm of a medium pair of chromosomes (Fig. 3) adjacent to the secondary constriction harbouring 45S rDNA. In Musa species, 5S and 45S rDNA are usually adjacent to each other and this has also been observed in E. livingstonianum (Bartoš et al., 2005). The latter has been reported to have additional minor 5S sites, which are either lost in E. ventricosum, or more probably varietal differences exist. Whilst phylogenetic relationships within the genus Ensete and to other genera within the Zingiberales are poorly known (Fig. 2), there does appear to be support for a distinction between African and Asian Ensete lineages (Li et al., 2010; Janssens et al., 2016).
Fig. 3.
Metaphase chromosomes of Ensete ventricosum ‘Maurelli’ 2n = 18. Chromosomes appear blue with the DNA stain DAPI and show two distinct 5S rDNA loci (red) at the ends of a medium sized chromosome pair (A). The simple sequence repeat AAC (green) is distributed along all chromosome arms (B). Sequences were mapped by fluorescence in situ hybridization (FISH) using the method of Schwarzacher and Heslop-Harrison (2000, for details see Supplementary Data Information). Scale bar = 5 μm; ‘x’ denotes unspecified soil/contamination in image.
In Musa there are over 1000 landraces with high genetic diversity, indicating multiple origins from different wild M. acuminata and its hybrids with M. balbisiana (Heslop-Harrison and Schwarzacher, 2007). The movement and interactions of various human groups have played an important role in generating this diversity (Perrier et al., 2011). Most landraces arise via selection (by farmers) of spontaneously occurring mutants with parthenocarpic fruit production. These are brought under cultivation, multiplied and distributed by vegetative propagation. Extensive hybridization has occurred, including between diploid wild species or genotypes, involving unreduced gametes, and perhaps residual fertility of triploids (Heslop-Harrison and Schwarzacher, 2007). Due to the high levels of domestic diversity indicated in genetic studies (Tobiaw and Bekele, 2011; Olango et al., 2015) and the overlapping spatial distribution of wild and domesticated enset, it seems likely that there were also multiple domestication events in enset, and/or frequent local introgression from wild populations. However, unlike Musa, we hypothesize that all domesticated enset landraces arose from a single species, E. ventricosum, as this is the only member of the genus present in Ethiopia or the surrounding region. More detailed diversity studies of wild and domestic enset in Ethiopia are required to elucidate the number of domestication events and population structure.
Among communities in Ethiopia, E. ventricosum is unusual in that human–enset interactions currently span the entire spectrum of domestication intensity, from wild procurement to full domestication (Hildebrand, 2001). As such, there is limited evidence to elucidate the timeline of domestication, not least because the crop has never moved outside its centre of origin and diversity. Nevertheless, whilst wild enset is considered largely inedible, except during periods of severe food insecurity, smallholders report that domesticated landraces are more palatable (Table 2). There are no data about the presence and genetics of secondary products that may be eliminated during domestication. Several authors have suggested that enset was first cultivated by growing wild plants in the terminal Pleistocene or Early Holocene (Brandt, 1984; Hildebrand, 2001). Although there is little evidence for this, it would compete with, or pre-date, the first evidence of intense Musa cultivation (~6500 years before present) in the New Guinea Highlands (Denham et al., 2003). Evidence from Uganda and Cameroon dates Musa cultivation in Africa to at least 2500 years before the present (Mdiba et al., 2001; Lejju et al., 2006), although these data have not met with universal acceptance (Neumann and Hildebrand, 2009). There is limited evidence that enset, although not used today, may have been historically consumed in northern Uganda (Thomas, 1940; Hamilton et al., 2016). It has also been suggested that Ensete once formed an ‘Ensete belt’ in East Africa from north-east Lake Victoria south-east to the Usambara Mountains, Tanzania (Langhe et al., 1994), and was used in times of food scarcity. This is largely consistent with the data presented in Fig. 4, and we note that genetic characterization of these populations would provide crucial insights into their history. Furthermore, among some communities (outside Ethiopia) enset is reported to maintain a cultural significance (Philippson, 1990). It has been suggested that this ancient care of Ensete in Africa contributed to the rapid and widespread adoption of the bananas arriving from Asia, with the oldest names relating to banana apparently derived from those in use for Ensete (Langhe et al., 1994). Elsewhere outside Africa, Ensete is reported to have been used as an emergency food source in Vietnam during the Second World War, with the growing point used as a vegetable (Oyen and Lemmens, 2002). Similarly, parts of E. glaucum are consumed in New Guinea, particularly the ripe fruits which are eaten raw (Kennedy, 2009), suggesting additional potential among underexploited wild relatives.
Table 2.
Wild and domesticated traits in E. ventricosum
| Character | Wild enset | Domesticated enset |
|---|---|---|
| Morphology | ||
| Leaf colour | Green/glaucous | Green, red, yellow, purple |
| Midrib colour | Green | Green, red, yellow, purple, black |
| Petiole colour | Green | Green, red, yellow, purple, black |
| Pseudostem colour | Green | Green, red, yellow, purple, black |
| Pseudostem shape | Conical | Conical, basal enlargement possible in some varieties |
| Corm size | Small | Enlarged |
| Corm colour | Dark (reported sometimes black) | Cream to white |
| Wax | Not present | Present on ventral leaf blade |
| Discoloration of tissue after cutting | Present | Uncommon |
| Palatability | ||
| Pseudostem edibility | Bitter | Edible |
| Corm edibility | Bitter, largely inedible | Variable, generally sweet. Edible |
| Genetics | ||
| Genetic diversity | High | High |
| Chromosome number | n = 9 | n = 9 |
| Ploidy | Diploid | Diploid |
| Reproduction | ||
| Reproduction method | Sexual | Asexual (sexual also possible) |
| Sucker production | No | In some varieties |
| Seed dormancy | Unknown | Unknown |
| Other uses | ||
| Medicinal use | None reported | Yes |
| Fibre | None reported | Yes |
| Disease susceptibility | ||
| Bacterial wilt | Unknown | Highly susceptible (but some are tolerant) |
| Mealybug | Unknown | Highly susceptible |
| Frost | Unknown, suspected intolerant | Tolerant |
Fig. 4.
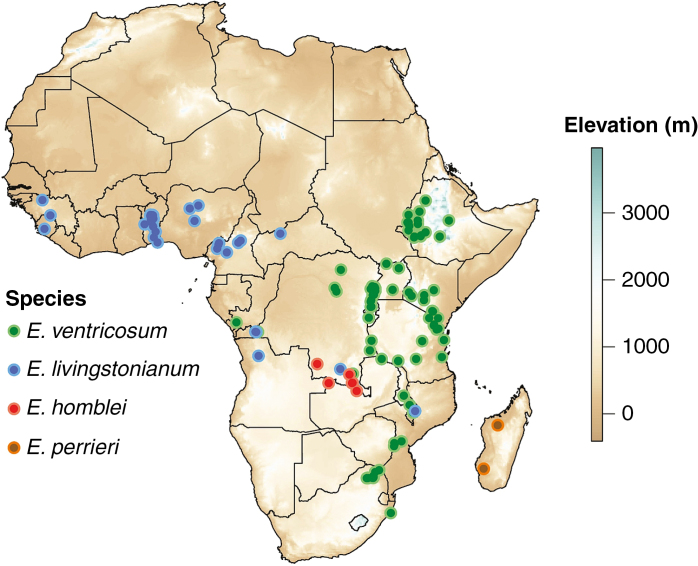
Three species of Ensete occur in mainland Africa, Ensete homblei, E. livingstonianum and E. ventricosum, with a fourth, E. perrieri, restricted to Madagascar. E. ventricosum is likely to be the most widespread species within the Musaceae, occurring over much of central, south-east and east Africa. Whilst the contemporary distribution reaches as far north as the Ethiopian Highlands, it has been suggested that enset was historically known to the Egyptians (Simoons, 1965). By comparison, distribution records for E. homblei, E. livingstonianum and their numerous synonyms are sparse. Ensete livingstonianum appears to be a species of drier habitats and is reported to die back in the dry season. It has a more westerly distribution than E. ventricosum, although they are likely to be sympatric over at least a portion of their range (Baker and Simmonds, 1953). Comparatively, E. homblei is recorded from only a handful of locations in the south-eastern Congo, and neighbouring northern Zambia. This could represent low sampling effort, rarity or both. Finally, E. perrieri is known from only three mature individuals, and is likely to be the most endangered crop wild relative of enset. Due to difficulty in distinguishing species with varying morphology, of different ages, and sometimes only from seed samples, it is possible that some geographically disjunct records represent misidentification, particularly for E. livingstonianum and E. ventricosum. Records presented here are collated from the literature (Cheesman, 1947; Baker and Simmonds, 1953), online databases (GBIF, 2018), herbaria (AAU, K) and personal observations.
THE DISTRIBUTION OF WILD AND DOMESTICATED ENSETE
Ensete consists of three very widespread species (E. ventricosum and E. livingstonianum in Africa; E. glaucum in Asia) and five other localized endemics or near-endemics (Fig. 4). Three species have been formally assessed for the IUCN red list, of which two (E. ventricosum and E. livingstonianum) are ‘Least Concern’ and one (E. perrieri) is ‘Critically Endangered’. Although not assessed, E. superbum would probably meet the criteria for ‘Endangered’, and all other non-cultivated species could be considered ‘Data Deficient’. Musella lasiocarpa may be extinct in the wild (Liu et al., 2003). Ensete ventricosum is the only Ensete species in Ethiopia (Brandt et al., 1997), occurring in the South and South-West (Tsegaye and Struik, 2002) across the Southern Nations, Nationalities and People’s Regional (SNNPR) region, as well as the neighbouring regions of Oromia and parts of Benishangul-Gumuz (Fig. 5). Hereafter, we refer to E. ventricosum as enset and we distinguish wild from domestic landraces. Spelling of regions, zones and other place names follows Davis et al. (2018).
Fig. 5.
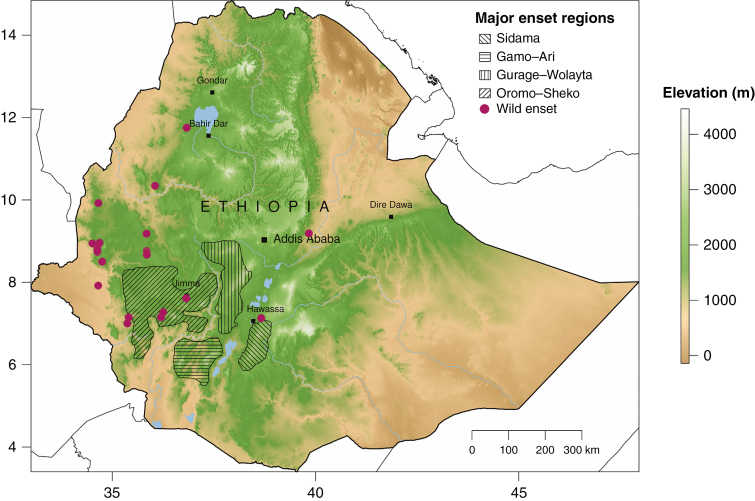
Distribution of major domesticated enset-growing regions (shaded polygons) and wild enset records (red points) in Ethiopia. Whilst domestic enset is occasionally encountered in the wider area, these four enset farming areas represent the major centres of cultivation, where enset is frequently the most important starch staple. The Sidama zone (SNNPR region) is predominantly high elevation, with enset sometimes grown together with crops such as coffee under sparse shade trees. At the highest elevations, enset is subject to frost damage. The Gurage-Wolayta cultivation area encompasses (from north to south) adjacent zones (Gurage, Hadiya, Kambata and Wolayta) in the SNNPR region. The northern part of Gurage is markedly drier than many other areas of enset cultivation. Here enset is predominantly grown in dense stands with few other crops and no shade trees. Gamo (Gamo zone) and Ari (South Omo zone) are relatively poorly known areas of enset cultivation, with high spatial variation in enset importance. Sheka and Dawro (SNNPR region) and adjacent areas in Oromia (Oromia region) are also relatively poorly researched. Here domestic enset occurs in close proximity to wild enset.
Wild enset in Ethiopia is considered by some researchers to be range-restricted and declining (S. Demissew, pers. obs.) although there is a paucity of data to support or refute this. Birmeta et al. (2004) report that wild enset occurs mainly around the city of Bonga (SNNPR region; Kaffa zone) and in a smaller area by the Omo river (SNNPR region; Gamo Gofa zone) whilst Garedew et al. (2017) report wild enset to be widely distributed in Sheka forest (SNNPR region; Skeka zone). Herbarium records indicate historical presence in Metekel (Benishangul-Gumuz region), West Wellega (Oromia region), Kefa and Sidama zones (SNNPR region). Observations of wild enset are further complicated by escaped domestic enset occurring on the periphery of villages or in neighbouring forests (e.g. a cluster of 15 enset plants closely resembling domestic varieties in Harenna forest, several hundred metres from the nearest habitation; J.S.B., pers. obs.).
As a forest species, the wild enset distribution will be affected by regional rates of forest loss. Ethiopia currently has less than 4 % forest cover, down from a potential climax vegetation maximum of 25–35 % (Reusing, 1998; Moat et al., 2018). It is possible that wild enset has become extinct in some areas, such as the Rift Valley area around Hawassa (SNNPR region; Sidama zone), where an estimated 82 % of forest has been lost since 1972 (Dessie and Kleman, 2007). This area has a strong and diverse enset culture, and is considered by some the origin of enset domestication (Simoons, 1965), yet there is no contemporary evidence of wild enset. By comparison, domesticated enset is considerably more widespread in Ethiopia, suggesting substantial niche expansion for the cultivated crop. The distribution of domesticated enset appears to reflect both amenable ecological conditions, population density (Yemataw et al., 2014b) and the presence of ethnic groups for which it is a staple (Tsegaye and Struik, 2002; ethnobotanical aspects also reviewed by Borrell et al., unpubl. res.). Enset is a highland crop cultivated at altitudes ranging from 1200 to more than 3100 m a.s.l. (Simoons, 1965; Brandt et al., 1997; Tsegaye and Struik, 2001, 2002) and is reported to perform best at elevations of 2000–2750 m (Brandt et al., 1997). According to Bezuneh and Feleke (1966) the soil type of enset cultivation areas is moderately acidic to slightly basic (pH 5.6–7.3), with 0.10–0.15 % total nitrogen and 2–3 % organic matter. Similarly, Shank (1994) reported that enset often performs best in acidic, heavy clay soils that retain high levels of organic matter when manured. Preferred climatic conditions are reported to be an average air temperature of 16–20 °C and an annual rainfall of 1100–1500 mm, evenly distributed throughout the year (Brandt et al., 1997).
The suitability of environmental conditions for enset cultivation across the domestic distribution clearly differs, as yield, age to maturity and maximum obtainable size vary considerably (Tsegaye and Struik, 2001; J. Borrell and A. Davis, pers. obs.), although this is probably confounded by agriculture practice and landrace selection (Shumbulo et al., 2012). At the upper elevation limit, low temperatures and frost has been hypothesized as a constraint; at the lower limit, water availability (Brandt et al., 1997). Various authors have defined enset as drought-tolerant (Shumbulo et al., 2012; Harrison et al., 2014) and it is widely regarded as ‘drought-resistant’ in Ethiopia (Birmeta, 2004) although there is a lack of rigorous evidence to demonstrate this.
The geographical range of wild enset (in Ethiopia) is more limited, perhaps due to more specific ecological requirements or alternatively loss of habitat (Fig. 5). According to several authors it is restricted to 1200–1600 m a.s.l. (Brandt et al., 1997). Baker and Simmonds (1953) described enset as a species of swamps, river banks or forest clearings, at middle altitudes, rarely or never in dense shade. Across its regional distribution, they record altitudes ranging from 1300 to 2300 m. Contemporary wild populations have been reported in humid forest, frequently along river banks, often consisting of 10–200 plants (Birmeta et al., 2004). It therefore seems that distribution and environmental tolerance of domesticated enset, relative to its wild progenitor, has been expanded through the domestication process.
COMPARATIVE MORPHOLOGY OF WILD AND DOMESTICATED ENSET
The vegetative morphology of domestic enset is highly variable (Fig. 1). Pseudostem colours include red, green, purple, black and many colour combinations (Yemataw et al., 2014b). Mature height ranges from 2 m in dwarf variants to more than 10 m for enset plants occurring in the Sidama area. According to farmers, corm size, tissue quality for starch, root structure for harvestability, drought, frost and disease tolerance are all variable among clonal genotypes (Tsegaye and Struik, 2001; Bizuayehu, 2002; Tewodros and Tesfaye, 2014). This indicates high phenotypic diversity. By comparison wild enset is predominantly green (also referred to as ‘white’ in Ethiopia). Hildebrand (2001) showed that wild and domesticated enset differ in growth pattern, with the former increasing girth more consistently with age and the latter attaining larger girth earlier in development. This could be evidence of farmer selection for earlier maturing genotypes. Domesticated enset is also characterized by further traits that are not observed in wild enset. Hildebrand (2001) recorded the presence of a wax bloom on the ventral leaf blade and hypothesized that this is a water stress response to hotter conditions and sunlight exposure in farms, as opposed to the conditions in the forests where wild enset is found. A general comparison of wild and domestic traits is given in Table 2.
Floral morphology
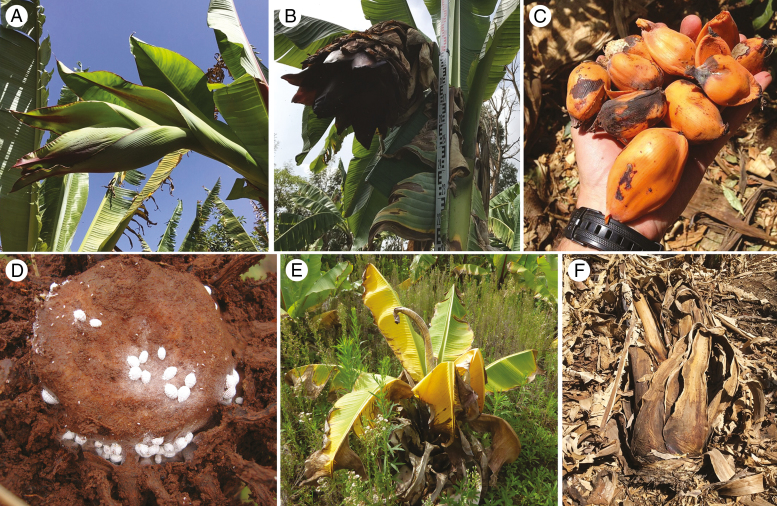
This is poorly known (Fig. 6), largely due to the fact that enset is harvested before flowering to maximize starch yield and is exclusively multiplied using vegetative propagation techniques. Despite this, there appears to be variation in inflorescence length, fruit shape and size and some farmers may use fruit morphology to differentiate landraces (S. Tamrat, pers. comm.). Whether all cultivated varieties produce viable seed, or indeed whether all varieties flower is currently unknown. Similarly, the mode of pollination and seed dispersal has not been studied extensively, with various authors suggesting self-pollination (Tabogie, 1999), nectar-seeking insects (Shigeta, 1990), bats (Fleming et al., 2009) or monkeys (Hildebrand, 2001) as vectors.
Fig. 6.
Floral morphology and diseases of enset. (A) A young inflorescence (landrace: ‘Dima’). (B) A mature inflorescence (landrace: Touzoma). (C) Ripe enset fruits (landrace: ‘Lemat’). (D) A mealybug-infested corm. (E) A young enset plant showing symptoms of bacterial wilt (Xanthomonas wilt of enset). (F) An enset plant recently killed by bacterial wilt.
Seed morphology and germination
As with other Musaceae, seed germination is generally poor and inconsistent, with a thorough understanding of germination requirements not yet achieved. Desiccated seeds have successfully been used in germination tests (Tesfaye, 1992), suggesting that storage behaviour is orthodox (Ellis and Roberts, 1980). According to Priestley (1986), E. ventricosum seeds can be maintained for 1–2 years in commercial storage. The thick testa with a cutinous inner integument (Graven et al., 1996) provides considerable protection, and has led people to consider enset as being physically dormant and requiring scarification (Tesfaye, 1992); however, it seems that enset seeds are able to imbibe water without mechanical intervention to equilibrium in 4 d (Bezuneh, 1971; Karlsson et al., 2013). Furthermore, whilst soaking can improve imbibition, it is not essential and numerous chemical treatments have been applied to enset seeds with little success (Tesfaye, 1992; Karlsson et al., 2013). Enset embryos do not extend within the seed (Karlsson et al., 2013), so they are not therefore morphologically dormant. Eco-physiological germination tests have so far been inconclusive, and an area of exploration could be the role of alternating temperatures, as this is important for Musa seed germination (Stotzky et al., 1962; Ellis et al., 1985; Chin, 1996), but has given inconclusive results for enset (Bezuneh, 1971; Tesfaye, 1992).
Like Musa (Cox et al., 1960; Asif et al., 2001), in vitro germination of excised embryos has been used as an alternative technique to provide access to enset plant regeneration and the development of new genotypes (Negash et al., 2000; Diro et al., 2003, 2004). Progress on this, and other in vitro techniques (shoot tip culture, callus culture and somatic embryogenesis) has been reviewed by Diro et al. (2004).
In a comparison of wild and domesticated enset, it is important to note that wild enset, to the best of our knowledge, engages exclusively in sexual reproduction, whilst in a farm setting domestic enset is exclusively clonally propagated by farmers (J.S. Borrell, pers. obs.). When permitted to flower (enset is normally harvested before flowering) seed production and fertility appears further diminished in domesticated plants (Hildebrand, 2001), which may pose challenges for germplasm conservation. Dissection of a small number (n = 4) of wild, naturalized and domestic enset showed a marked difference in well-formed, viable seeds per fruit and per infructescence. Wild enset tends to have thousands of seeds, whilst domestic enset has few fruits with full-sized seeds, and low numbers of viable seeds in each fruit, possibly due to the absence of suitable pollinators in the domestic environment, or reduced fitness resulting from a domestication bottleneck.
DIVERSITY OF WILD AND DOMESTICATED ENSET
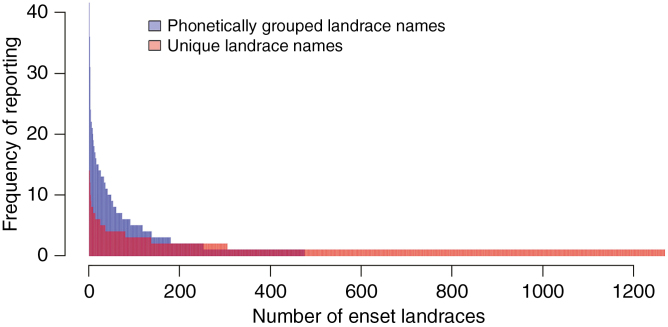
Whilst enset has only been domesticated in a comparatively small region of the species’ wild distribution, the reported phenotypic diversity of cultivated enset landraces is exceptionally high (Shank, 1994; Brandt et al., 1997; Tsegaye and Struik, 2002; Bizuayehu, 2008). Farmers claim to maintain diverse enset varieties for several reasons, including: different qualities that suit different food products, alternative uses such as fibre, fodder or medicine, and different climatic and pest tolerance (Olango et al., 2014; Yemataw et al., 2014a).The Areka Agricultural Research Centre (Wolayta Zone, Ethiopia), for example, reports that it maintains 623 distinct enset landraces from 12 major enset-growing areas of Ethiopia (Yemataw et al., 2017). In our own literature survey, we recorded 1270 unique vernacular names for wild and domestic enset varieties from 28 publications (Supplementary Data Table S2). After clustering similar sounding names, we still recovered 475 phonetic groups (Fig. 7). Furthermore, there was remarkably low commonality between studies with the vast majority of enset landraces being referenced in the literature only once.
Fig. 7.
Frequency of enset landrace vernacular names in the literature. In comparison with the high number of domesticated enset vernacular names, only four names were reported for wild enset (E. ventricosum) in our survey.
Indigenous knowledge
Farmers use vernacular names for identification of enset clones, with up to 26 landrace names being recorded from a single farm (Yemataw et al., 2014a). Whilst vernacular names are known to vary considerably based on region, language and ethnic group, it is difficult to know if this represents distinct diversity or vernacular duplication. Indeed, the true number of landraces may be considerably less or more than we report: as in many other crops, the same genotype may be given multiple names (synonyms), or different genotypes given the same name [homonyms; see for apple (Malus) Liang et al., 2015].
Phenotypes
Whilst the majority of studies rely on indigenous knowledge for identification of differing landraces (Olango et al., 2014; Yemataw et al., 2016), several authors have also attempted to document and analyse enset landraces using phenotypic characters (morphology). Initially, Zippel and Kefale (1995) developed a field survey technique for the rapid identification of enset clones based on morphological characters, principally colour. In subsequent research, Tabogie (1997) reported significant variation among 79 enset accessions collected from different parts of Ethiopia and attempted to associate yield with different traits. Bekele et al. (2013) undertook a similar study and categorized 120 distinct enset landraces into 11 clusters. The most important morphological descriptors included pseudostem circumference, corm weight and fibre yield, with maturity period and number of leaves also contributing useful information. This suggests that there is indeed high diversity in desirable crop traits. Other authors report similar morphological diversity in field surveys (Yemataw et al., 2012, 2014a, 2017). However due to the vast number of landraces and considerable variability between individuals, the degree of precision and consistency in morphological studies is unclear.
Genotypes
In comparison with other important food crops, there are few studies employing molecular markers for germplasm characterization and evaluation of genetic diversity in enset (Table 3). In the first studies of their kind, random amplified polymorphic DNA (RAPD) was used to measure the genetic diversity and relatedness of 111 cultivated enset clones collected from nine enset-growing regions (Birmeta et al., 2002) and 146 cultivated enset clones collected from four regions, in Ethiopia (Negash et al., 2002). The authors reported a high level of genetic variability among the tested germplasm as well as considerable duplication of vernacular names (for landraces) among the collection and suggested that full identity between two clones can only be determined by more extensive genome comparison. In a later study, domesticated enset was then compared to five wild enset populations by Birmeta et al. (2004), with the two groups found to cluster separately in an UPGMA (unweighted pair group method with arithmetic mean) analysis based on RAPD markers.
Table 3.
Previous genetic and genomic studies of wild and domestic E. ventricosum in Ethiopia
| Marker | Aims | No. of markers | No. of genotypes | Origin | Reference |
|---|---|---|---|---|---|
| AFLP | Genetic diversity and identity of cultivated enset clones | 180 loci | 146 domesticated clones | Domesticated | Negash et al. (2002) |
| RAPD | Genetic diversity among Ethiopian enset clones | 97 loci | 111 domesticated clones | Domesticated | Birmeta et al. (2002) |
| RAPD | Comparison of wild and cultivated gene pools in Ethiopia | 72 loci | 5 wild populations (48 plants), 9 domesticated clones | Wild and domesticated | Birmeta et al. (2004) |
| ISSR | Genetic diversity of cultivated enset clones | 26 loci | 71 domesticated clones | Domesticated | Tobiaw and Bekele (2011) |
| SNP | Genome sequence | – | 1 domesticated clone | Domesticated | Harrison et al. (2014) |
| SSR | Cross-taxa transferability of markers, genetic diversity and phylogenetic relationship with Musa spp. | 34 markers | 6 wild and 64 domesticated clones | Wild and domesticated | Olango et al. (2015) |
| SNP | Genome assemblies, phylogenetics and SNP datasets | 20 000 | 17 domesticated clones | Domesticated | Yemataw et al. (2018) |
AFLP, amplified fragment length polymorphism; RAPD, random amplified polymorphic DNA; ISSR, inter simple sequence repeat; SNP, single nucleotide polymorphism.
Subsequently, 71 cultivated enset clones collected from two different areas of south-western Ethiopia (Keffa and Dawro zones, SNNPR region) were evaluated with inter simple sequence repeat (ISSR) markers to estimate genetic variation (Tobiaw and Bekele, 2011). Two ISSR markers produced 26 clear scoreable bands and clustered all the 71 cultivated enset landraces in to two major groups, which aligned with their collection regions. Olango et al. (2015) developed the first set of genomic microsatellite markers from pyrosequencing of an enriched genomic library of E. ventricosum and examined their cross-genus transferability to related taxa, using them to estimate genetic diversity, as well as relationships between wild and domesticated enset accessions. The analysis demonstrated that intra-population allelic variation contributed more to genetic diversity than inter-population variations. Phylogenetic data combined with principal components analysis results revealed that wild enset clustered together and were distinct from domesticated enset landraces sampled across the region (Olango et al., 2015).
More recently there has been an effort to enable enhanced enset research through the publication of the E. ventricosum draft genome sequence (Harrison et al., 2014), with an approximate size of 547 Mb (GenBank accession number AMZH02). Whilst the original ‘JungleSeeds’ assembly has unknown provenance and is only distantly related to Ethiopian plants, the subsequent assemblies of the Ethiopian landraces ‘Onjamo’, ‘Bedadeti’ and ‘Derea’ are likely to be of more use to researchers. A further 17 E. ventricosum accessions have subsequently been re-sequenced using Illumina HiSeq and MiSeq platforms and raw reads aligned against the published E. ventricosum ‘Bedadeti’ reference genome sequence (Yemataw et al., 2018). Available genome sequences are reported in Table 4.
Table 4.
Comparison of available E. ventricosum genome sequences and assemblies, with related Musa species
| Landrace | E. ventricosum ‘Bedadeti’ | E. ventricosum ‘JungleSeeds’ | E. ventricosum ‘Onjamo’ | E. ventricosum ‘Derea’ | M. acuminata subsp. malaccensis | M. itinerans | M. balbisiana |
|---|---|---|---|---|---|---|---|
| BioSample | SAMN02854351 | SAMN01797775 | SAMN05751581 | SAMN05729394 | SAMEA2272344 | SAMN04505257 | SAMN02333823 |
| GenBank assembly | GCA_000818735.2 | GCA_000331365.2 | GCA_001884845.1 | GCA_001884805.1 | GCA_000313855.2 | GCA_001649415.1 | –* |
| Coverage | 30 | 40 | 21 | 18.4 | 20.5 | 92 | 41.4 |
| Scaffold count | 45 745 | 52 692 | 51 525 | – | 7 512 | 28 415 | – |
| Scaffold N-50 | 21 097 | 13 866 | 16 208 | – | 1 311 088 | 195 772 | – |
| Contig count | 46 254 | 71 088 | 54 038 | 60 129 | 29 249 | 55 966 | 180 175 |
| Contig N-50 | 20 943 | 11 721 | 15 546 | 12 314 | 28 326 | 35 438 | 7 884 |
| Size (Mb) | 451.3 | 437.3 | 444.8 | 429.5 | 472.2 | 455.0 | 402.5 |
| Total gap length | 10 455 | 286 734 | 46 732 | 0 | 81 753 624 | 39 952 266 | 61 115 757 |
*Assembly is available at: http://banana-genome-hub.southgreen.fr/organism/Musa/balbisiana [18 October 2018].
Crop wild relatives (CWRs)
Of the studies reporting vernacular, phenotypic and genetic diversity of enset, almost all exclusively address domesticated enset landraces. Only two studies, by Birmeta et al. (2004) and Olango et al. (2015), included formal analysis of wild enset accessions in Ethiopia. Therefore, whilst wild and domesticated enset are distinct, the relationship between domesticated landraces and their wild crop progenitors, as well as their value in breeding programmes, is unclear.
Spatial patterns of diversity
Without a clear understanding of enset diversity (and how it is partitioned across vernacular taxonomies, phenotypic and genetic components, and wild vs. domestic), it is difficult to draw conclusions on the geographical distribution of diversity in Ethiopia. Negash et al. (2002) documented 146 clones from four enset-growing zones: Kefa-Sheka (western SNNPR region), Sidama (eastern SNNPR region), Hadiya and Wolayta (both in central SNNPR region) in Ethiopia. Birmeta (2004) recorded 111 clones from nine enset-growing areas. Emerging from these studies are a first indication of regional patterns of diversity; for example, Yemataw et al. (2014a) found Hadiya (within the Gurage-Wolayta enset area; Fig. 5) to have the highest landrace richness, as well as the greatest number of unique landraces, whilst Sidama had the lowest (Fig. 5). In Sidama, Tesfaye and Lüdders (2003) found enset diversity to be correlated with elevation, whilst in the Gamo Highlands Samberg et al. (2010) found enset diversity peaked at 2500–2800 m with an average of 15 landraces per farm, and only six or seven per farm below 2000 m and above 3000 m, respectively, thus hinting at important biogeographical patterns. Despite the above studies, a national assessment of areas of enset diversity is lacking.
NEAR-TERM THREATS: ENSET PESTS AND PATHOGENS
Pests and diseases affecting enset growth and yield represent the most serious short-term threat to enset production. The most important disease is Xanthomonas wilt of enset (XWE; Xanthomonas campestris pv. musacearum), together with enset root mealy bug (Cataenococcus ensete) infestation (Fig. 6). Additional pests (nematodes, mole rat, porcupine, termites) and diseases (bacterial, fungal and viral) currently cause moderate to limited damage.
Xanthomonas wilt of enset (XWE)
Caused by the pathogen Xanthomonas campestris pv. Musacearum, XWE was first observed on enset in Ethiopia in the 1930s (Castellani, 1939), but only identified as X. campestris pv. musacearum on enset in 1968 (Yirgou and Bradbury, 1968) and subsequently on banana in 1974 (Yirgou and Bradbury, 1974). Various symptoms characterize the disease: leaf yellowing, distortion and wilting/collapse, and pockets of yellow or cream-coloured slimy ooze are visible in cut vascular tissues in leaf sheaths, leaf midribs and real stem (Blomme et al., 2017). Vascular bundles often become discoloured, although this symptom is not as conspicuous as the internal discoloration observed in banana. Total yield loss is expected once the disease takes hold, although plant recovery has been observed in tolerant landraces (e.g. the landraces ‘Mazia’, ‘Badadeti’ and ‘Astara’, J.S. Borrell, pers. obs.; Hunduma et al., 2015).
The main mode of spread of XWE is through cultivation tools and contaminated planting material. However, porcupines, warthogs and mole rats often eat rhizomes and, in the process, can transmit XWE (Brandt et al., 1997). Insect-vector transmission via flowers does not occur in cultivated enset as plants are harvested before or at flower emergence. The incidence of XWE in wild enset is not known. More broadly, the pathogen arrived in Uganda and the Eastern Democratic Republic of Congo in 2001 and has since spread across most of the highland banana production zones of east and central Africa, probably from the disease reservoir in enset (Blomme et al., 2017). Control measures that could prevent, reduce or eliminate the spread of XWE include the disinfection of tools between use on different plants, preventing animals from browsing infected plants, fencing infected sites and the rigorous removal of infected plants (Quimio and Tessera, 1996). We also note concurrent genomic research on X. campestris pv. musacearum which has identified evidence of two distinct sublineages, suggesting more than one introductory event, and candidate virulence factors that may facilitate host infection (Nakato et al., 2018).
Pests affecting enset growth and yield
The enset root mealy bug (Cataenococcus ensete) is a major pest of enset in southern Ethiopia, having been first reported at Wonago (Tsedeke, 1988; Addis et al., 2008). Enset root mealybugs have an elongate-oval body covered with bright white wax secretions on the dorsal and lateral sides. Although the insect has been present in various parts of the enset-growing region, it has only become a serious threat to enset production in recent years (Addis et al., 2008). The insect attacks enset of all ages, but particularly young plants, with symptoms including retarded growth, dried out outer leaves (but with a green central shoot) and eventual plant death, especially under moisture stress. Enset plants attacked by root mealybugs have a significantly lower number of roots as compared to healthy plants. As a result, mealy bug-damaged enset plants are more easily uprooted. Mealy bugs are mainly spread through infested planting materials (Bizuayehu, 2002; Addis et al., 2008), and thus production of mealy bug-free planting materials is a key control measure.
Although symptoms are often not clearly visible, root necrosis due to nematodes poses an increasing constraint to enset production (Addis et al., 2006). Bogalel et al. (2004) carried out a nematode survey at 25 enset cultivation sites, representative of seven agro-ecological zones. The predominant nematode species found was Pratylenchus goodeyi (5640 per 100 g fresh root weight), followed by Aphelenchoides ensete and Meloidogyne spp. The nematode Aphelenchoides ensete was also isolated from leaves that showed severe streak-like symptoms on young enset plants. In a subsequent study, 294 enset plants across the enset-growing region were assessed for root damage and sampled for nematode identification. Twelve plant parasitic nematode taxa were identified: P. goodeyi was the most common species, present in about 90 % of samples, with Ektaphelenchoides spp. and Meloidogyne spp. also observed (Addis et al., 2006).
Minor diseases caused by bacterial, fungal and viral pathogens
The fungal disease Sclerotium root and corm rot of Enset is characterized by a gradual rotting of roots and leaf sheaths at the soil level and stunted plant growth (Quimio and Tessera, 1996). The causal agent was identified as a Sclerotium sp. which can gain entry to enset plants through damaged roots and corms. The pathogen survives in disintegrating root and corm tissue present in the soil (Quimio and Tessera, 1996). A second fungal disease, Cephalosporium inflorescence spot of enset, causes extensive necrosis of flower bracts and necrotic spots on leaf sheaths of mature plants (Tessera and Quimio, 1994). Finally, Enset streak is believed to be caused by a badnavirus (Tessera et al., 1996) and chlorotic and yellow mosaics, streaks and stripes are characteristic leaf symptoms of the disease. Severely affected plants have also narrow distorted leaves and become stunted. Early infection results in a significant reduction in yield. The major means of dissemination of the disease is through infected corms or suckers arising from an infected corm. A complete overview of enset bacterial, fungal and viral diseases is provided in Jones (2000).
LONG-TERM THREATS: CLIMATE CHANGE AND DECLINING DIVERSITY
In the longer term, shifting environmental conditions due to climate change and declining farm diversity of landraces are likely to be increasingly important threats to enset agriculture (Adhikari et al., 2015). Social changes through urbanization, mobility and labour are all threats, too, to traditional farming practices. Threats to germplasm diversity are compounded by a lack of nationally and internationally secure germplasm collections, including both in vivo and long-term storage as seeds or through cryopreservation, with the strong restrictions on germplasm movement and confidentiality considerations limiting opportunities outside Ethiopia and public availability of knowledge. Climate change is projected to substantially impact all agricultural systems in East Africa, resulting in declining and more variable yields, with subsequently adaptation and transitions to new growing areas becoming necessary (Challinor et al., 2014; Adhikari et al., 2015; Rippke et al., 2016). Despite this, the projected impact on enset cultivation has not been assessed. Concurrently, several authors have suggested an overall decline in the diversity of enset landraces on farms in Ethiopia (Negash et al, 2002; Birmeta et al., 2004; Zengele, 2017), although there have been no systematically repeated surveys or clear empirical data to support this.
Enset susceptibility to climate change
Ethiopia’s mean annual temperature increased by 1.3 °C between 1960 and 2006 at an average rate of 0.28 °C per decade (McSweeney et al., 2010). Nationally, mean annual temperature is projected to increase by 1.3–3.1 °C by the 2060s and 1.5–5.1 °C by the 2090s (McSweeney et al., 2010). Historical precipitation patterns are less clear due to strong inter-annual and inter-decadal variation, but appear to have declined slightly overall (Jury and Funk, 2013; Mekasha et al., 2014). Future projections indicate increasing annual precipitation but are highly variable (McSweeney et al., 2010; Mekasha et al., 2014).
Despite these past and future climatic changes, there have been no studies assessing the projected impact on enset. In studies on Coffea arabica, for which there is substantial environmental niche overlap with enset, Moat et al. (2017) showed that 39–59 % of the current growing area could experience climate changes large enough to render them unsuitable for coffee farming, and Davis et al. (2012) report a 38–90 % reduction in climatically suitable areas for wild populations. Coffee requires the correct environmental conditions at specific times of the growing cycle for successful flowering and fruiting (Moat et al., 2017). By contrast, enset is less susceptible to short-term temperature or precipitation variation that can detrimentally impact coffee crops, and is not reliant on a sexual reproduction cycle for food production (DaMatta and Cochicho Ramalho, 2006).
Enset germplasm collections
Whilst empirical evidence of declining enset diversity is lacking, systematic collection and maintenance of diverse crop germplasm is important to maximize use and availability in sustainable agricultural development, and guard against the erosion of genetic diversity. Bioversity International (a CGIAR Research Centre) is currently committed to the long-term preservation of the entire banana genepool. This has been achieved through the collection and maintenance of 4928 Musa germplasm accessions, encompassing numerous crop wild relatives, including a handful of Ensete spp. (Ruas et al., 2017). Collected accessions are preserved as living collections across numerous partner organizations, as well as in vitro under slow growth conditions and using cryopreservation. To facilitate this, the Musa Germplasm Information System (MGIS) was developed (https://www.crop-diversity.org/mgis/) which has served to accelerate Musa research (e.g. MusaNet, 2016). Virus-free Musa germplasm is now freely available for international distribution upon request through the MGIS website; between 1985 and 2014 the Bioversity International Musa Germplasm Transit Centre (ITC) distributed over 17 000 samples among 109 countries worldwide. However, to date only six Ensete accessions are available through the MGIS database (Table 5), of which only two are E. ventricosum. Of these two, one is termed the ‘red mutant’ which is most likely a commonly available horticultural cultivar named ‘Maurelii’, the other – arguably the most important accession – is of unknown provenance, but reported to be wild. Therefore, it is possible that despite 5000 Musaceae accessions, domesticated landraces of this important tropical crop are not conserved internationally.
Table 5.
Internationally available Ensete germplasm accessions
| Species | Landrace | Accession number | Institute code | Field collection | In vitro | Lyophilized leaves | Cryopreserved | Available for distribution | Origin | Origin |
|---|---|---|---|---|---|---|---|---|---|---|
| E. gilletii (livingstonianum) | – | ITC1389 | BEL084 (ITC) | No | Yes | No | Yes | No | Wild | NGA_Jos, Plateau State, Nigeria |
| E. glaucum | Pisang Pidak | IFRI-001 | IDN150 (ICHORD) | Yes | No | No | No | No | Wild | Indonesia |
| E. glaucum subsp. glaucum | Vudu Vudu | ITC0775 | BEL084 (ITC) | No | Yes | Yes | Yes | Yes | Wild | Papua New Guinea |
| E. ventricosum subsp ventricosum | – | ITC1387 | BEL084 (ITC) | No | Yes | Yes | No | Yes | Wild | Unknown |
| E. ventricosum subsp ventricosum | red mutant | ITC1388 | BEL084 (ITC) | No | Yes | Yes | No | Yes | Wild | Unknown |
| E. unknown | Chuoi Nguon | VNIO60 | VNM007 (FAVRI) | Yes | No | No | No | No | Landrace | Vietnam |
Two comparatively very large living collections of domesticated enset exist at field sites in Ethiopia. The first, Areka field station (part of the Southern Agricultural Research Institute), reports to maintain a collection of approx. 600 landraces (Harrison et al., 2014) from several regions of Ethiopia, with four clonal replicates of each. Second is a newer collection at the University of Wolkite (Fig. 1C), which maintains approx. 110 landraces from the Gurage region with up to 15 replicates of each. Information on these collections, such as the landraces they contain, is not publicly available. Finally, Guzzon and Muller (2016) conducted a review of the availability of stored and fresh seeds of E. ventricosum, E. homblei and E. livingstoninum. Of the 27 African genebanks, 42 botanic gardens and four researchers contacted, only one collection was available: one accession of E. ventricosum collected in Tanzania maintained at the Millennium Seed Bank, RBG Kew, UK, stored in orthodox conditions (15 % relative humidity, −20 °C).
FUTURE RESEARCH PRIORITIES
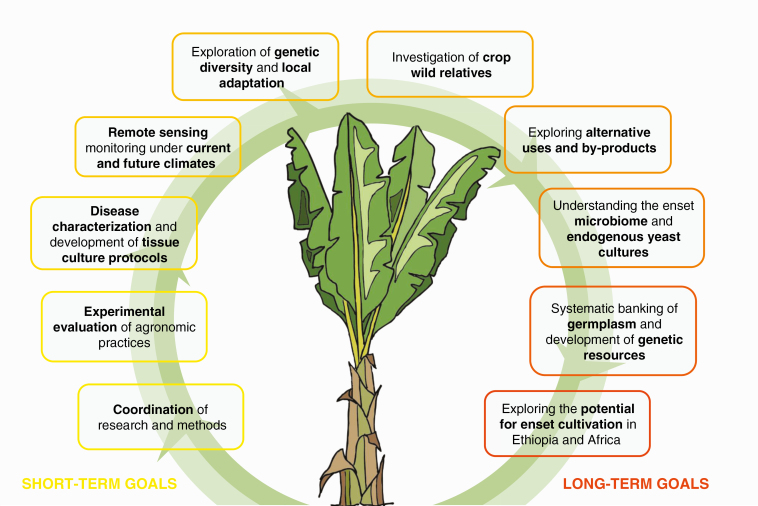
Global food demand is increasing, and is likely to continue to increase into the second half of this century (Godfray et al., 2010). By 2050, a projected 100–110 % increase in global crop demand, relative to 2005 levels, will be required (Tilman et al., 2011). In the latter half of the 20th century this has been largely met, not through substantial growth in cropland, but by improvements in crop productivity often dubbed the ‘Green Revolution’ (Evenson and Gollin, 2003). In this article we have shown that despite unique and valuable crop attributes, as well as the dependency of 20 million Ethiopians, enset has been overlooked by modern crop improvement research. This therefore represents an opportunity for sustainable exploitation to support livelihoods and improve food security in the region. Here, building on the literature examined in this review, we identify our ten priority areas for research (summarized in Fig. 8) and make recommendations for short- and long-term development of enset as a key food security crop.
Fig. 8.
Roadmap for the sustainable development and exploitation of the Ethiopian starch crop enset for food security and to support livelihoods.
1. Coordination of research and methods
Whilst enset has only been domesticated in Ethiopia, enset research encompasses researchers from at least 40 institutions in 11 countries. Currently, despite positive national and international collaborations (e.g. Brandt et al., 1997; Yemataw et al., 2018), enset research is still disconnected with many interesting and important research programmes running in isolation. Partly, the aim of this review is to draw together many disparate aspects of enset research to facilitate discoverability and collaboration by researchers.
In addition, we relate the experience of the Global Musa Genomics Consortium (http://www.musagenomics.org) which sought to bring together expertise and enable close collaboration, the sharing of materials, resources, data and technology to accelerate Musa breeding efforts (Roux et al., 2011). It is our view that enset research and food security in Ethiopia could benefit from such an approach, with equitable and appropriate access and benefit sharing agreements in place. Here, we present and make available the resource www.enset-project.org, which will act as an open repository for data emerging from our current research programme.
2. Experimental evaluation of enset agronomic practices
There are numerous cultural practices employed in enset cultivation that are reported to significantly influence growth and yield, but few of these have been empirically evaluated in robust, replicated and controlled experiments (reviewed by Borrell et al., unpubl. res.). A key practice, for instance, involves systematic transplanting of enset at specific ages, which appears to have a dramatic impact on the resulting pseudostem and corm size (Yemataw et al., 2016), perhaps by delaying maturity. In a study by Tsegaye (2007), transplanting treatments significantly affected height, pseudostem circumference and dry matter yield, and increased partitioning of dry matter to harvestable parts. To our knowledge a similar practice has not been reported in any other crop. Other practices include aeration of the soil, mulching with discarded plant material, companion plants, the use of fertilizer, various rhizome preparation practices for vegetative multiplication, as well as treatments for pests and diseases. However, the efficacy of these practices is largely unknown and they represent an important first step in optimizing enset agriculture.
3. Disease characterization and development of disease-free tissue culture protocols
Whilst several pests and pathogens are known to affect enset cultivation in Ethiopia, their impact on regional yields is yet to be quantified. Similarly, geographical patterns in disease incidence are not yet available. In the medium term, it is likely that different degrees of disease tolerance may be identified across enset landraces and crop wild relatives. This genetic diversity will provide the long-term basis for crop breeding to generate disease-resistant genotypes. The generation of disease-free tissue culture protocols (e.g. Tripathi et al., 2015) is also likely to play an important role. Concurrently, ongoing surveillance to identify newly emerging pathogens, or the potential for transmission from related species (e.g. the widely cultivated Musa), is an important safeguard for enset sustainability.
4. Remote sensing under current and future climates
Estimates of the land area under enset cultivation (e.g. Shank; 1994; Central Statistical Agency, CSA, Government of Ethiopia, 2004), and associated yields (Pijls et al., 1995; Tsegaye and Struik, 2001; Sahle et al., 2018) are highly variable and have been historically hampered by difficult access to remote areas. The long-term nature of enset cultivation, local differences in cultivated landraces, plant growth rates, agronomic practice and dependency on co-staple crop productivity in any given period make estimating enset production difficult (Cochrane and Adam, 2017). Therefore, standardized empirical analyses of the land area under enset cultivation, yield components and inter-annual trends are lacking.
Advances in the resolution and availability of satellite data (e.g. MODIS, Sentinel 2) are increasingly being applied to vegetation and crop surveys (Hütt et al., 2016; Immitzer et al., 2016; Moat et al., 2017). Thus, in the near term there may be the potential to use freely available satellite data to directly monitor annual enset production. Furthermore, this approach could be applied to mapping bacterial wilt outbreaks. Concurrently, improved regional bioclimatic datasets (e.g. Worldclim2) and an enhanced network of climate stations and data loggers across the enset-growing region will allow better characterization of the enset environmental niche and stress conditions. The impact of climate change under a range of future scenarios is yet to be quantified for enset and will form an important part of any future development strategy, as undertaken for coffee in Ethiopia (Moat et al., 2017; Davis et al., 2018).
5. Exploration of genetic diversity and local adaptation
High enset genetic diversity distributed over a wide range of environmental conditions suggests that the domestication process may have facilitated adaptation of landraces to local conditions, and indeed to a wider range of conditions than its wild progenitor. Because enset is a clonally reproducing and distributed plant, this represents a powerful system to investigate the genomic basis of adaptive traits. Key steps to achieve this would be the characterization of existing enset genetic diversity using high-resolution genomic markers, standardized methods to measure fitness and yield as well as robust monitoring of environmental conditions. Concurrently, assessing the risk of erosion to enset genetic diversity through the loss or decline of landraces should be a priority for future enset monitoring strategies. In the medium term this could similarly be extended to monitoring of crop wild relative diversity. In the long term, with the prerequisite knowledge of germination biology, novel sexual breeding utilizing mapping populations and pan-genomic sequencing may enable the development of improved landraces, tolerant of disease, better adapted to current and future climates, or with desirable yield or by-product attributes (Tester and Langridge, 2010).
6. Investigation of crop wild relatives
During the process of domestication, crops typically experience a genetic bottleneck resulting in reduced variation when compared to wild progenitors. CWRs are therefore an important source of genetic diversity for crop improvement (Jarvis et al., 2008), and may possess desirable traits that have been lost in domesticated landraces. The susceptibility of wild enset to pathogens such as XWE, for example, is currently unknown, and they may harbour important genetic diversity for disease tolerance or resistance (see for example in wheat, Ali et al., 2016; Rasheed et al., 2018). Wild enset is also reported to have a higher seed set and germination rate than domesticated enset, so understanding the reasons why this is diminished in the latter will be important in developing seed-based germplasm collections for breeding.
Whilst CWRs have been used extensively for breeding in other species (Tester and Langridge, 2010), such as improving drought tolerance in wheat (Farooq, 2004), their use is anticipated to further increase due to advances in molecular technology (Hajjar and Hodgkin, 2007). Therefore, long-term conservation of wild diversity is a key foundation for the sustainable exploitation of enset. A similar approach has already been undertaken in Ethiopia through the formation of biosphere reserves for wild populations of Arabica coffee (Coffea arabica) (Davis et al., 2012; Aerts et al., 2016).
7. Exploring alternative uses
In addition to being a major dietary starch source, enset also has the potential to produce other valuable by-products. Fibre can be extracted from the pseudostem and leaves, and is comparable with other natural fibres such as flax, sisal and hemp (Teli and Terega, 2017). High value wax is currently extracted from closely related banana species (Yanagida et al., 2003) and several authors have identified the importance of enset as animal fodder (Fekadu and Ledin, 1997; Mohammed et al., 2013). Enset is also widely considered as an important medicinal plant in Ethiopia, and is used in particular to treat individuals with fractured or broken bones, as well as for placental discharge in humans and livestock (Tsehaye and Kebebew, 2006; Assefa and Fitamo, 2016). The chemical basis of these uses has not been explored.
8. Understanding the enset microbiome and endogenous yeast cultures
The plant microbiome is probably in the order of tens of thousands of species, and its relevance to plant health and yield is only beginning to be understood (Berendsen et al., 2012). Importantly, there are indications that the microbiome may have a role in the suppression of plant diseases. Therefore, in the long term, characterization of the enset microbiome across different agroecological environments, combined with whole genome and population genomic studies, may provide novel pathways to crop improvement. Concurrently, a significant component of enset agriculture is fermentation of the pseudostem and corm tissue using endogenous yeasts. This practice is currently performed by farmers and is thus highly variable. Development of improved fermentation cultures may result in rapidly improved product quality, as well as provide an opportunity to improve micro-nutritional content.
9. Systematic germplasm banking and development of genetic resources
Given their economic and food security value, Ensete species, and particularly domesticated enset landraces, are currently severely underrepresented in global ex situ germplasm collections. This chronically limits the potential for plant breeding and crop improvement. In the long term, under scenarios of habitat loss, agricultural intensification, disease spread, climate change and introduction of high-yielding genotypes, both wild and domestic enset are at risk of losing genetic diversity, and with it potentially important adaptive traits.
Whilst a large number of landraces are present in two collections in Ethiopia, germplasm management of vegetatively propagated plants species such as enset is costly, time-consuming, vulnerable to poor documentation and requires a large land surface area for proper maintenance. Therefore, a key research goal should be further exploration of the potential for germplasm banking as seeds, together with a strategy to collect germplasm from a wide range of spatial and ecological conditions. Conventional breeding and ex situ conservation by seed also requires an understanding of seed desiccation tolerance, longevity in storage and, essentially, germination requirements. As with Musa, much of this is not well understood.
Similarly, access to domestic enset germplasm outside of Ethiopia is challenging and historically limited. If (with appropriate access and benefit sharing agreements) additional domesticated germplasm could be admitted in to the ITC genebank (Leuven, Belgium) it will not only be more readily accessible to the scientific community (benefiting research and sustainable exploitation), but would also safeguard a critically important tropical crop.
10. Exploring the potential for enset cultivation in Ethiopia and Africa
Within Ethiopia, domesticated enset appears to occupy a range of conditions distinct and somewhat broader than wild enset. Similarly, the wild distribution outside Ethiopia of E. ventricosum extends as far as South Africa, encompassing a range of environmental conditions not found in Ethiopia. Therefore, it seems likely that the climatic envelope of this already tolerant crop could be further enlarged and it could potentially be introduced to new areas.
In Ethiopia, a current research programme has collected four landraces from Dilla (Gedio zone; SNNPR region) and introduced these to a novel enset cultivation area near Ankober (North Shewa; Amhara region), north of Addis Ababa, to investigate performance. An equally important component is the concurrent introduction of enset harvesting and processing cultural knowledge (see Borrell et al., unpubl. res.). Further afield, a second project is exploring the potential introduction of enset to Zambia in an effort to combat hidden hunger (Cardenas et al., 2018). Future research effort should focus on characterizing the environmental requirements of enset to predict habitat suitability and assess the feasibility of introduction to novel areas.
BOX 1. In memoriam Mark Goodwin
We are deeply saddened to write that our co-author, colleague and friend, Mark Goodwin (Fig. 9), passed away suddenly in his University office on 25 August 2018, aged just 58. During the day, he had held many discussions related to enset, including ways to connect researchers and build collaborations. Mark’s particular expertise was in the delivery of impact from research projects, linking with pedagogy and the importance of advanced training. After finishing his PhD, Mark worked for the UK Government’s Overseas Development Administration and The Open University, before joining the University of Leicester in 2006. Within the Department of Genetics and Genome Biology, he was a leader in the Centre for Excellence in Teaching and Learning (CETL), led the Virtual Genetics Education Centre project, managed the Leicester-Gondar PhD programme and was an Academic Partner for British Council programmes in Afghanistan and Bangladesh. Mark was a co-investigator for the GCRF Foundation project on enset leading to the work presented in this publication. Mark was taken from us much too soon, and we will greatly miss his input to the delivery of impact from research programmes.
Fig. 9.
Mark Anthony John Goodwin: 9 August 1960 to 25 August 2018 (left) and in Hawassa, Ethiopia with enset in May 2012 (right). Images Pat Heslop-Harrison.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Species and associated GenBank accession numbers for ITS sequences included in the phylogenetic analysis (Fig. 2 and Supplementary Data Fig. S2). Table S2: Landrace names for domesticated varieties of Ensete ventricosum, identified in the review of available literature. Fig. S1: Estimated population size of the major enset-growing regions. Fig. S2: Phylogenetic relationships of Ensete species within the Zingerberales. Supplementary Information: Detailed information on methods used to prepare Figs 2, 3 and 7 and Supplementary Data Figs S1 and S2.
ACKNOWLEDGEMENTS
The authors wish to acknowledge collaborators from Wolkite University, Hawassa University, Addis Ababa University, the Ethiopian Biodiversity Institute and the Southern Agricultural Research Institute for enabling and facilitating research on enset in Ethiopia. This work was supported by the GCRF Foundation Awards for Global Agricultural and Food Systems Research, entitled, ‘Modelling and genomics resources to enhance exploitation of the sustainable and diverse Ethiopian starch crop enset and support livelihoods’ [Grant No. BB/P02307X/1].
LITERATURE CITED
- Abate T, Hiebsch C, Brandt SA, Gebremariam S. 1996. Enset-based sustainable agriculture in Ethiopia. Addis Abeba, Ethiopia: Institute of Agricultural Research. [Google Scholar]
- Addis T, Blomme G, Turyagyenda L, Van den Berg E, De Waele D. 2006. Nematodes associated with enset and banana in the highlands of Ethiopia. International Journal of Nematology 16: 118–125. [Google Scholar]
- Addis T, Azerefegne F, Blomme G, Kanaujia K. 2008. Biology of the enset root mealybug Cataenococcus ensete and its geographical distribution in southern Ethiopia. Journal of Applied Biosciences 8: 251–260. [Google Scholar]
- Adhikari U, Nejadhashemi AP, Woznicki SA. 2015. Climate change and eastern Africa: a review of impact on major crops. Food and Energy Security 4: 110–132. [Google Scholar]
- Aerts R, Geeraert L, Berecha G, et al. . 2016. Conserving wild Arabica coffee: emerging threats and opportunities. Agriculture, Ecosystems & Environment 237: 75–79. [Google Scholar]
- Ali N, Heslop-Harrison J, Ahmad H, Graybosch RA, Hein GL, Schwarzacher T. 2016. Introgression of chromosome segments from multiple alien species in wheat breeding lines with wheat streak mosaic virus resistance. Heredity 117: 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif MJ, Mak C, Othman RY. 2001. In vitro zygotic embryo culture of wild Musa acuminata ssp. malaccensis and factors affecting germination and seedling growth. Plant Cell, Tissue and Organ Culture 67: 267–270. [Google Scholar]
- Assefa AS, Fitamo D. 2016. Diversity of enset landraces (Ensete ventricosum (Welw) Cheesman) in Aleta Chuko District, Sidama Zone, South Nation Nationality People and Regional State, Ethiopia. Journal of Plant Sciences 4: 1–7. [Google Scholar]
- Baker RED, Simmonds NW. 1953. The genus Ensete in Africa. Kew Bulletin 8: 405–416. [Google Scholar]
- Bartoš J, Alkhimova O, Doleželová M, De Langhe E, Doleźel J. 2005. Nuclear genome size and genomic distribution of ribosomal DNA in Musa and Ensete (Musaceae): taxonomic implications. Cytogenetic and Genome Research 109: 50–57. [DOI] [PubMed] [Google Scholar]
- Bekele A, Diro M, Yeshitla M. 2013. The diversity and associated yield components of enset (Ensete ventricosum) based on its agro-morphological. Journal of Science 36: 49–54. [Google Scholar]
- Bekele E, Shigeta M. 2011. Phylogenetic relationships between Ensete and Musa species as revealed by the trnT trnF region of cpDNA. Genetic Resources and Crop Evolution 58: 259–269. [Google Scholar]
- Berendsen RL, Pieterse CMJ, Bakker PAHM. 2012. The rhizosphere microbiome and plant health. Trends in Plant Science 17: 478–486. [DOI] [PubMed] [Google Scholar]
- Bezuneh T. 1971. The role of Musacae in Ethiopian agriculture. I. The genus Ensete. Acta Horticulturae 21: 97–100. [Google Scholar]
- Bezuneh T, Feleke A. 1966. The production and utilization of the genus Ensete in Ethiopia. Economic Botany 20: 65–70. [Google Scholar]
- Bhise MR, Rahangdale SS, Rahangdale SR. 2015. Effect of leaf harvesting on reproduction and natural populations of Indian wild banana Ensete superbum (Roxb.) Cheesman (Zingiberales: Musaceae). Journal of Threatened Taxa 7: 7181–7185 [Google Scholar]
- Birmeta G. 2004. Genetic variability and biotechnological studies for the conservation and improvement of Ensete ventricosum. PhD Thesis, University of Agricultural Sciences Alnarp, Sweden: Acta Universitatis Agriculturae Sueciae Agraria 502. [Google Scholar]
- Birmeta G, Nybom H, Bekele E. 2002. RAPD analysis of genetic diversity among clones of the Ethiopian crop plant Ensete ventricosum. Euphytica 124: 315–325. [Google Scholar]
- Birmeta G, Nybom H, Bekele E. 2004. Distinction between wild and cultivated enset (Ensete ventricosum) gene pools in Ethiopia using RAPD markers. Hereditas 140: 139–148. [DOI] [PubMed] [Google Scholar]
- Bizuayehu T. 2002. Studies on landrace diversity, in vivo and in vitro regeneration of Enset (Ensete ventricosum Welw.). PhD Thesis, Awassa College of Agriculture, Awassa, Ethiopia. [Google Scholar]
- Bizuayehu T. 2008. On Sidama folk identification, naming, and classification of cultivated enset (Ensete ventricosum) varieties. Genetic Resources and Crop Evolution 55: 1359–1370. [Google Scholar]
- Blomme G, Dita M, Jacobsen KS, et al. . 2017. Bacterial diseases of bananas and enset: current state of knowledge and integrated approaches toward sustainable management. Frontiers in Plant Science 8:, 1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogalel M, Mekete T, Protection P. 2004. Survey of plant parasitic nematodes and banana weevil. Nematologia Mediterranea 32: 223–227. [Google Scholar]
- Brandt SA. 1984. New perspectives on the origins of food production in Ethiopia. In: Clark JD, Brandt SA, eds. From hunters to farmers: the causes and consequences of food production in Africa. Berkley, CA: University of California Press, 173–190. [Google Scholar]
- Brandt SA, Spring A, Hiebsch C, et al. . 1997. The “Tree Against Hunger”: enset-based agricultural system in Ethiopia. Washington, DC: American Association for the Advancement of Science. [Google Scholar]
- Bruce J. 1790. Travels to discover the source of the Nile, in the years 1768, 1769, 1770, 1771, 1772 and 1773. London: G.J. and J. Robinson. [Google Scholar]
- Cardenas D, Hensel C, Molla E. 2018. Students4Kids: winning project, growing the tree against hunger (Ensete ventricosum) in Zambia. In: Biesalski HK, Birmer R (eds) Hidden hunger: strategies to improve nutrition quality. World Reviews of Nutrition and Dietetics 118: 221–226. [DOI] [PubMed] [Google Scholar]
- Castellani E. 1939. Su un marciume dell’ ensete. L’Agric. Coloniale Firenze 33: 297–300. [Google Scholar]
- Central Statistical Agency (CSA), Government of Ethiopia 2004. Agricultural Sample Survey 2003/2004, Vol. 1: Area and Production of Major Crops. Addis Ababa: Central Statistical Authority. [Google Scholar]
- Challinor A, Watson J, Lobell D, Howden S, Smith D, Chhetri N. 2014. A meta-analysis of crop yield under climate change and adaptation. Nature Climate Change 4: 287–291. [Google Scholar]
- Chase MW, Christenhusz MJM, Fay MF, et al. . 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Cheesman EE. 1947. Classification of the Bananas I: the genus Ensete Horan. Kew Bulletin 2: 97–106. [Google Scholar]
- Chin HF. 1996. Germination and storage of banana seeds. In: Frison AE, Horry JP, De Waele D (eds) New frontiers in resistance breeding for nematodes, Fusarium and Sigatoka. Montpellier: INIBAP, 218–227. [Google Scholar]
- Cochrane L, Adam TA. 2017. Knowledge gaps and opportunities for future research on Ethiopian food security and agriculture. Ethiopian Journal of Applied Scences and Technology 8: 33–41. [Google Scholar]
- Cox EA, Stotzky G, Goos RD. 1960. In vitro culture of Musa balbisiana Colla embryos. Nature 185: 403–404. [Google Scholar]
- D’Hont A, Denoeud F, Aury JM, et al. . 2012. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488: 213–217. [DOI] [PubMed] [Google Scholar]
- DaMatta FM, Cochicho Ramalho JD. 2006. Impacts of drought and temperature stress on coffee physiology and production: a review. Brazilian Journal of Plant Physiology 18: 55–81. [Google Scholar]
- Davis AP, Gole TW, Baena S, Moat J. 2012. The impact of climate change on indigenous Arabica coffee (Coffea arabica): predicting future trends and identifying priorities. PLoS ONE 7: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Wilkinson T, Challa ZK, Williams J, Baena S, Gole TW, Moat J. 2018. Coffee atlas of Ethiopia. Richmond: Kew Publishing. [Google Scholar]
- Denham T, Donohue M. 2009. Pre-Austronesian dispersal of banana cultivars west from New Guinea: linguistic relics from eastern Indonesia. Archaeology in Oceania 44: 18–28. [Google Scholar]
- Denham TP, Haberle SG, Lentfer C, et al. . 2003. Origins of agriculture at Kuk Swamp in the highlands of New Guinea. Science 301: 189–193. [DOI] [PubMed] [Google Scholar]
- Dessalegn R. 1995. Resilience and vulnerability: enset agriculture in southern Ethiopia. Journal of Ethiopian Studies 28: 23–51. [Google Scholar]
- Dessie G, Kleman J. 2007. Pattern and magnitude of deforestation in the South Central Rift Valley Region of Ethiopia. Mountain Research and Development 27: 162–168. [Google Scholar]
- Diro M, van Staden J, Bornman CH. 2003. In vitro regeneration of Ensete ventricosum from zygotic embryos of stored seeds. South African Journal of Botany 69: 364–369. [Google Scholar]
- Diro M, van Staden J, Bornman CH. 2004. Propagation of Ensete in vitro: a review. South African Journal of Botany 70: 497–501. [Google Scholar]
- Ellis RH, Roberts EH. 1980. Improved equations for the prediction of seed longevity. Annals of Botany 45: 13–30. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1985. Handbook of seed technology for genebanks. Volume II. Compendium of specific germination information and test recommendations. Rome: International Board for Plant Genetic Resources. [Google Scholar]
- Evenson R, Gollin D. 2003. Assessing the impact of the Green Revolution, 1960 to 2000. Science 300: 758. [DOI] [PubMed] [Google Scholar]
- Fekadu D, Ledin I. 1997. Weight and chemical composition of the plant parts of enset (Ensete ventricosum) and the intake and degradability of enset by cattle. Livestock Production Science 49: 249–257. [Google Scholar]
- Farooq S. 2004. Production of low input and stress tolerant wheat germplasm through the use of biodiversity residing in the wild relatives. Hereditas 135: 211–215. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Geiselman C, Kress WJ. 2009. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104: 1017–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garedew B, Ayiza A, Haile B, Kasaye H. 2017. Indigenous knowledge of enset (Ensete ventricosum (Welw.) Cheesman): cultivation and management practice by Shekicho People, Southwest Ethiopia. Journal of Plant Sciences 5: 6–18. [Google Scholar]
- GBIF 2018. Global Biodiversity Information Facility. Free and open access to biodiversity data https://www.gbif.org/ (accessed 15 October 2018). [Google Scholar]
- Godfray HCJ, Beddington JR, Crute IR, et al. . 2010. Food security: the challenge of feeding nine billion people. Science 327: 812–818. [DOI] [PubMed] [Google Scholar]
- Graven P, De Koster CG, Boon JJ, Bouman F. 1996. Structure and macromolecular composition of the seed coat of the Musaceae. Annals of Botany 77: 105–122. [Google Scholar]
- Guzzon F, Müller JV. 2016. Current availability of seed material of enset (Ensete ventricosum, Musaceae) and its Sub-Saharan wild relatives. Genetic Resources and Crop Evolution 63: 185–191. [Google Scholar]
- Hajjar R, Hodgkin T. 2007. The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156: 1–13. [Google Scholar]
- Hamilton AC, Karamura D, Kakudidi E. 2016. History and conservation of wild and cultivated plant diversity in Uganda: forest species and banana varieties as case studies. Plant Diversity 38: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J, Moore K, Paszkiewicz K, et al. . 2014. A draft genome sequence for Ensete ventricosum, the drought-tolerant “Tree against hunger.” Agronomy 4: 13–33. [Google Scholar]
- Heslop-Harrison JS, Schwarzacher T. 2007. Domestication, genomics and the future for banana. Annals of Botany 100: 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand E. 2001. Morphological characterization of domestic vs. forest-growing Ensete ventricosum (Welw.) Cheesman, Musaceae, in Sheko district, Bench-Maji Zone, southwest Ethiopia. Bioogise Skrifter 54: 287–309. [Google Scholar]
- Horaninow PF. 1862. Prodromus Monographiae Scitaminarum. St. Petersburg. [Google Scholar]
- Hunduma T, Kassahun S, Hilu E, Oli M. 2015. Evaluation of enset clones resistance against enset bacterial wilt disease (Xanthomonas campestris pv. musacearum). Journal of Veterinary Science & Technology 6: 1–7. [Google Scholar]
- Hütt C, Koppe W, Miao Y, Bareth G. 2016. Best accuracy land use/land cover (LULC) classification to derive crop types using multitemporal, multisensor, and multi-polarization SAR satellite images. Remote Sensing 8: 684. [Google Scholar]
- Immitzer M, Vuolo F, Atzberger C. 2016. First experience with Sentinel-2 data for crop and tree species classifications in central Europe. Remote Sensing 8: 166. [Google Scholar]
- Janssens SB, Vandelook F, De Langhe E, et al. . 2016. Evolutionary dynamics and biogeography of Musaceae reveal a correlation between the diversification of the banana family and the geological and climatic history of Southeast Asia. New Phytologist 210: 1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A, Lane A, Hijmans RJ. 2008. The effect of climate change on crop wild relatives. Agriculture, Ecosystems & Environment 126: 13–23. [Google Scholar]
- Jones DR. 2000. Diseases of banana, abacá and enset. Walingford: CABI Publishing. [Google Scholar]
- Jury MR, Funk C. 2013. Climatic trends over Ethiopia: regional signals and drivers. International Journal of Climatology 33: 1924–1935. [Google Scholar]
- Kanshie KT. 2002. Five thousand years of sustainablity? A Case study on Gedeo Land Use (Southern Ethiopia). Heelsum: Treemail Publishers. [Google Scholar]
- Karlsson LM, Tamado T, Dalbato AL, Mikias Y. 2013. Seed morphology and germination of Ensete ventricosum (Musaceae). Seed Science and Technology 41: 357–370. [Google Scholar]
- Kennedy J. 2009. Bananas and people in the homeland of genus Musa: not just pretty fruit. Ethnobotany Research and Applications 7: 179–198. [Google Scholar]
- De Langhe E, Swennen R, Vuylsteke D. 1994. Plantain in the Eary Bantu World. Azania: Archaeological Research in Africa 29–30: 147–160. [Google Scholar]
- Lejju BJ, Robertshaw P, Taylor D. 2006. Africa’s earliest bananas? Journal of Archaeological Science 33: 102–113. [Google Scholar]
- Li LF, Häkkinen M, Yuan YM, Hao G, Ge XJ. 2010. Molecular phylogeny and systematics of the banana family (Musaceae) inferred from multiple nuclear and chloroplast DNA fragments, with a special reference to the genus Musa. Molecular Phylogenetics and Evolution 57: 1–10. [DOI] [PubMed] [Google Scholar]
- Li XW. 1978. The Musaceae of Yunnan. Acta Phytotaxonomica Sinica 16: 54–64. [Google Scholar]
- Liang W, Dondini L, De Franceschi P, Paris R, Sansavini S, Tartarini S. 2015. Genetic diversity, population structure and construction of a core collection of apple cultivars from Italian germplasm. Plant Molecular Biology Reporter 33: 458–473. [Google Scholar]
- Liu A-Z, Kress WJ, Long C-L. 2003. The ethnobotany of Musella lasiocarpa (Musaceae), an endemic plant of southwest China. Economic Botany 57: 279–281. [Google Scholar]
- Lock JM. 1993. Musaceae. In: Polehill, RM. (ed.) Flora of Tropical East Africa: prepared at the Royal Botanic Gardens/Kew with assistance from the East African Herbarium. Rotterdam: A.A. Balkema on behalf of the The East African Governments. [Google Scholar]
- Luu HT, Nguyen QD, Vu NL, Vo TL. 2012. Ensete lecongkietii (Musaceae) - a new species from Vietnam. Folia Malaysiana 13: 43–50. [Google Scholar]
- Manchester SR, Kress WJ. 1993. Fossil bananas (Musaceae): Ensete oregonense sp. nov. from the Eocene of Western North America and its phytogeographic significance. American Journal of Botany 80: 1264–1272. [Google Scholar]
- McSweeney C, New M, Lizcano G. 2010. UNDP Climate Change Country Profiles: Ethiopia. https://www.geog.ox.ac.uk/research/climate/projects/undp-cp/ (accessed 15 October 2018). [Google Scholar]
- Mdiba C, Doutrelepont H, Vrydaghs L, et al. . 2001. First archaeological evidence of banana cultivation in central Africa during the third millenium before present. Vegetation History and Archaeobotany 10: 1–6. [Google Scholar]
- Mekasha A, Tesfaye K, Duncan AJ. 2014. Trends in daily observed temperature and precipitation extremes over three Ethiopian eco-environments. International Journal of Climatology 34: 1990–1999. [Google Scholar]
- Moat J, Williams J, Baena S, et al. . 2017. Resilience potential of the Ethiopian coffee sector under climate change. Nature Plants 3: 17081. [DOI] [PubMed] [Google Scholar]
- Moat JF, Tadesse W, Davis AP, Gole 2018. Least concern to endangered: applying climate change projections profoundly influences the extinction risk assessment for wild Arabica coffee. Global Change Biology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed B, Martin G, Laila MK. 2013. Nutritive values of the drought tolerant food and fodder crop enset. African Journal of Agricultural Research 8: 2326–2333. [Google Scholar]
- MusaNet 2016. Global strategy for the conservation and use of Musa genetic resources ( B., Laliberté, ed.). Montpellier: Bioversity International. [Google Scholar]
- Nakato V, Mahuku G, Coutinho T. 2018. Pathogen profile Xanthomonas campestris pv. musacearum: a major constraint to banana, plantain and enset production in central and east Africa over the past decade. Molecular Plant Pathology 19: 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash A, Niehof A. 2004. The significance of enset culture and biodiversity for rural household food and livelihood security in southwestern Ethiopia. Agriculture and Human Values 21: 61–71. [Google Scholar]
- Negash A, Puite K, Schaart J, Visser B, Krens F. 2000. In vitro regeneration and micropropagation of enset from Southwestern Ethiopia. Plant Cell, Tissue and Organ Culture 6: 153–158. [Google Scholar]
- Negash A, Tsegaye A, van Treuren R, Visser B. 2002. AFLP analysis of enset clonal diversity in south and southwestern Ethiopia for conservation. Crop Science 42: 1105–1111. [Google Scholar]
- Neumann K, Hildebrand E. 2009. Early bananas in Africa: the state of the art. Ethnobotany Research and Applications 7: 353–362. [Google Scholar]
- Olango T, Tesfaye B, Catellani M, Pè M. 2014. Indigenous knowledge, use and on-farm management of enset (Ensete ventricosum (Welw.) Cheesman) diversity in Wolaita, Southern Ethiopia. Journal of Ethnobiology and Ethnomedicine 10: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olango TM, Tesfaye B, Pagnotta MA, Pè ME, Catellani M. 2015. Development of SSR markers and genetic diversity analysis in enset (Ensete ventricosum (Welw.) Cheesman), an orphan food security crop from Southern Ethiopia. BMC Genetics 16: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyen LPA, Lemmens RHMJ. 2002. Plant resources of tropical Africa. Precursor. Wageningen: Programme PROTA. [Google Scholar]
- Perrier X, De Langhe E, Donohue M, et al. . 2011. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proceedings of the National Academy of Sciences 108: 11311–11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippson G. 1990. Langue et histoire au Kilimanjaro et alentour: diffusion démique et diffusion culturelle. http://www.ddl.cnrs.fr/fulltext/Philippson/Philippson_2007.pdf (accessed 15 October 2018). [Google Scholar]
- Pijls LTJ, Timmer AAM, Wolde-Gebriel Z, West CE. 1995. Cultivation, preparation and consumption of enset in Ethiopia. Journal of Science, Food and Agriculture 67: 1–11. [Google Scholar]
- Priestley D. 1986. Seed aging: implications for seed storage and per- sistence in the soil. Ithaca, NY: Comstock Publishing Associates. [Google Scholar]
- Quimio AJ, Tessera M. 1996. Diseases of enset. In: Tsedeke A, Clifton H, Steven BA, Gebre-Mariam S (eds) Enset-based sustainable agriculture in Ethiopia. Proceedings of the International Workshop on enset. Addis Ababa: Ethiopian Institute of Agricultural Research, 188–203. [Google Scholar]
- Quinlan RJ, Quinlan MB, Dira S, Caudell M, Sooge A, Assoma AA. 2015. Vulnerability and resilience of sidama enset and maize farms in southwestern Ethiopia. Journal of Ethnobiology 35: 314–336. [Google Scholar]
- Rasheed A, Mujeeb-Kazi A, Ogbonnaya FC, He Z, Rajaram S. 2018. Wheat genetic resources in the post-genomics era: promise and challenges. Annals of Botany 121: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusing M. 1998. Monitoring of forest resources in Ethiopia. Addis Ababa: Ministry of Agriculture; http://hdl.handle.net/123456789/1055 (accessed 15 Ocotber 2018). [Google Scholar]
- Rippke U, Ramirez-villegas J, Jarvis A, et al. . 2016. Timescales of transformational climate change adaptation in sub-Saharan African agriculture. Nature Climate Change 6: 605–609. [Google Scholar]
- Roux N, Rouard M, Huang XL, Smith M. 2011. Opportunities for bridging the gap between genomics and genetic improvement in Musa spp. Acta Horticulturae 897: 509–516. [Google Scholar]
- Ruas M, Guignon V, Sempere G, et al. . 2017. MGIS: managing banana (Musa spp.) genetic resources information and high-throughput genotyping data. Database 2017: bax046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahle M, Yeshitela K, Saito O. 2018. Mapping the supply and demand of Enset crop to improve food security in Southern Ethiopia. Agronomy for Sustainable Development 38: 7. [Google Scholar]
- Samberg LH, Shennan C, Zavaleta ES. 2010. Human and environmental factors affect patterns of crop diversity in an ethiopian highland agroecosystem diversity in an Ethiopian highland agroecosystem. The Professional Geographer 62: 395–408. [Google Scholar]
- Schatz G, Phillipson P. 2011. Catalogue of the vascular plants of Madagascar. Monographs in Systematic Botany. St Louis, MO: Missouri Botanical Garden. [Google Scholar]
- Schwarzacher T, Heslop-Harrison P. 2000. Practical in situ hybridization. Oxford: BIOS Scientific Publishers. [Google Scholar]
- Shank R. 1994. The enset culture: a technical report on Enset ventricosum or ‘False Banana’. Addis Ababa: United Nations-Emergencies Unit for Ethiopia. [Google Scholar]
- Shigeta M. 1990. Folk in-situ conservation of ensete [Ensete ventricosum (Welw.) E.E. Cheesman]: toward the interpretation of indigenous agricultural science of the Ari, Sowthwestern Ethiopia. African Study Monographs 10: 93–107. [Google Scholar]
- Shumbulo A, Gecho Y, Tora M. 2012. Diversity, challenges and potentials of enset (Ensete ventricosum) production: in case of Offa Woreda, Wolaita Zone, Southern Ethiopia. Food Science and Quality Management 7: 24–31. [Google Scholar]
- Simmonds NW. 1960. Notes on banana taxonomy. Kew Bulletin 14: 198–212. [Google Scholar]
- Simoons FJ. 1965. Some questions on the economic prehistory of Ethiopia. The Journal of African History 6: 1–13. [Google Scholar]
- Stotzky G, Cox EA, Goos RD. 1962. Seed germination studies in Musa. I. Scarification and aseptic germination of Musa balbisiana. American Journal of Botany 49: 515–520. [Google Scholar]
- Tabogie E. 1997. Morphological characterization of enset clones and the association of yield with different traits. M.Sc. Thesis, School of Graduate Studies, Alemaya, Ethiopia. [Google Scholar]
- Tabogie E. 1999. Floral morphology of enset. AgriTop 14: 3–4. [Google Scholar]
- Teli MD, Terega JM. 2017. Chemical, physical and thermal characterization of Ensete ventricosum plant fibre. International Research Journal of Engineering and Technology 4: 67–75. [Google Scholar]
- Tesfaye B, Lüdders P. 2003. Diversity and distribution patterns of enset landraces in Sidama, Southern Ethiopia. Genetic Resources and Crop Evolution 50: 359–371. [Google Scholar]
- Tesfaye M. 1992. The effect of soaking, temperature and other pretreatments of the germination of ‘enset’ seed. Seed Science and Technology 20: 533–538. [Google Scholar]
- Tessera M, Lohuis D, Peters D. 1996. A badna virus in Ensete. In: Bekele E, Abdulah A, Yemane A (eds) Proceedings of the 3rd annual conference of the crop protection society of Ethiopia. Addis Ababa: Crop Protection Society of Ethiopia, 143–148. [Google Scholar]
- Tessera M, Quimio AJ. 1994. Research on enset pathology. In: Herath E, Desalegn L (eds). Proceedings of the 2nd National Hhorticultural Workshop of Ethiopia. Addis Ababa: Institute of Agricultural Research, 217–225. [Google Scholar]
- Tester M, Langridge P. 2010. Breeding technologies to increase crop production in a changing world. Science 327: 818–822. [DOI] [PubMed] [Google Scholar]
- Tewodros M, Tesfaye W. 2014. Farmers indigenous knowledge and assessment of enset (Ensete ventricosum Welw. Cheesman) cultivars for major insect pests in Ojojia water shade Kembata- tembaro zone, South Ethiopia. Sky Journal of Agricultural Research 3: 112–119. [Google Scholar]
- Thomas AS. 1940. Uganda banana varieties and their uses ( JD, Tothill, ed.). Oxford: Oxford University Press. [Google Scholar]
- Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences, USA 108: 20260–20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake JR, Martins ES. 2010. Flora Zambesiaca Volume 13, Part 4. Richmond: Royal Botanic Gardens Kew. [Google Scholar]
- Tobiaw DC, Bekele E. 2011. Analysis of genetic diversity among cultivated enset (Ensete ventricosum) populations from Essera and Kefficho, southwestern part of Ethiopia using inter simple sequence repeats (ISSRs) marker. African Journal of Biotechnology 10: 15697–15709. [Google Scholar]
- Tripathi JN, Oduor RO, Tripathi L. 2015. A high-throughput regeneration and transformation platform for production of genetically modified banana. Frontiers in Plant Science 6: 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsedeke A. 1988. Insect and mite pests of horticultural and miscellaneous plants in Ethiopia. IAR Handbook 1. Addis Ababa: Institute of Agricultural Research. [Google Scholar]
- Tsegaye A. 2007. Effects of repetitive transplanting and leaf pruning on growth and dry matter partitioning of enset (Ensete ventricosum (Welw.) Cheesman). Journal of Agronomy 6: 45–52. [Google Scholar]
- Tsegaye A, Struik PC. 2001. Enset (Ensete ventricosum (Welw.) Cheesman) kocho yield under different crop establishment methods as compared to yields of other carbohydrate-rich food crops. NJAS - Wageningen Journal of Life Sciences 49: 81–94. [Google Scholar]
- Tsegaye A, Struik PC. 2002. Analysis of enset (Ensete ventricosum) indigenous production methods and farm-based biodiversity in major enset-growing regions of southern Ethiopia. Experimental Agriculture 38: 291–315. [Google Scholar]
- Tsehaye Y, Kebebew F. 2006. Diversity and cultural use of enset (Enset ventricosum (Welw.) Cheesman) in Bonga in situ. Ethonbotany Research & Applications 4: 147–157. [Google Scholar]
- Westphal E. 1975. Agricultural Systems in Ethiopia, Agricultural Research Report 826. Addis Ababa: Centre for Agricultural Publishing and Documentation, College of Agriculture, Haile Sellassie I University, and Wageningen: Wageningen Agricultural University. [Google Scholar]
- Wu T-L, Kress WJ. 2000. ENSETE Horaninow, Prodr. Monogr. Scitam. 8, 40. 1862. Flora of China 24: 297–313. [Google Scholar]
- Yanagida T, Shimizu N, Kimura T. 2003. Properties of wax extracted from banana leaves. In: 2003 ASAE Annual Meeting. St Joseph, Michigan, USA: American Society of Agricultural and Biological Engineers; doi: 10.13031/2013.14121w [DOI] [Google Scholar]
- Yemataw Z, Mohamed H, Diro M, Addis T, Blomme G. 2012. Genetic variability, inter-relationships and path analysis in enset (Ensete ventricosum) clones. The African Journal of Plant Sciences and Biotechnology 6: 21–25. [Google Scholar]
- Yemataw Z, Mohamed H, Diro M, Addis T, Blomme G. 2014a Ethnic-based diversity and distribution of enset (Ensete ventricosum) clones in southern Ethiopia. Journal of Ecology and the Natural Environment 6: 244–251. [Google Scholar]
- Yemataw Z, Mohamed H, Diro M, Addis T, Blomme G. 2014b Enset (Ensete ventricosum) clone selection by farmers and their cultural practices in southern Ethiopia. Genetic Resources and Crop Evolution 61: 1091–1104. [Google Scholar]
- Yemataw Z, Tesfaye K, Zeberga A, Blomme G. 2016. Exploiting indigenous knowledge of subsistence farmers’ for the management and conservation of Enset (Ensete ventricosum (Welw.) Cheesman) (Musaceae family) diversity on-farm. Journal of Ethnobiology and Ethnomedicine 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemataw Z, Chala A, Ambachew D, Studholme D, Grant M, Tesfaye K. 2017. Morphological variation and inter-relationships of quantitative traits in enset (Ensete ventricosum (welw.) Cheesman) germplasm from South and South-Western Ethiopia. Plants 6: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemataw Z, Muzemil S, Ambachew D, et al. . 2018. Genome sequence data from 17 accessions of Ensete ventricosum, a staple food crop for millions in Ethiopia. Data in Brief 18: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirgou D, Bradbury JF. 1968. Bacterial wilt of enset (Ensete ventricosum) incited by Xanthomonas musacearum sp. n. Phytopathology 58: 111–112. [Google Scholar]
- Yirgou D, Bradbury JF. 1974. A note on wilt of banana caused by the enset wilt organism Xanthomonas musacearum. East African Agricultural and Forestry Journal 40: 111–114. [Google Scholar]
- Yirgu T. 2016. Land use dynamics and challenges of enset (Ensete ventricosum) agriculture in the upper reaches of Baso-Deme watershed, Gamo Highland, SW Ethiopia. Global Journal of Interdisciplinary Social Sciences 5: 20–28. [Google Scholar]
- Zengele AG. 2017. Genetic erosion of enset (Ensete ventricosum Welw. Cheesman) in Wolaita Zone, Southern Ethiopia: a review. Advances in Life Science and Technology 61: 7–14. [Google Scholar]
- Zippel K, Kefale A. 1995. Field guide to enset landraces of North Omo, Ethiopia. FRP Technical Pamphlet. Zürich: Eidgenössische Technische Hochschule (ETH). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.