Abstract
Background and Aims
The positive effects of species diversity on the functioning and production of ecosystems have been discussed widely in the literature. In agriculture, these effects are increasingly being applied to mixed-species crops and particularly to temporary grasslands. However, the effects of increases in genetic diversity (i.e. within-species diversity) on productivity in multispecies crops have not been much studied. Nevertheless, genetic diversity may have strong positive effects on agricultural ecosystems and positively influence production and species abundances in multispecies covers. We examine here the effects of genetic diversity on temporary multispecies grasslands.
Methods
From a real situation, a breeder’s field trial, we describe a study with five seed mixtures, each containing seven species (three grasses and four legumes) but with three different levels of genetic diversity (low, medium and high) created by using different numbers of cultivars per species. From the perspective of a plant breeder, we analyse measurements of biomass production over a 5-year period.
Key Results
We show a positive effect of genetic diversity on production, on production stability and on the equilibrium of species abundances in the mixtures over the 5-year period of the experiment. The legume/grass proportions were best balanced, having the highest within-species diversity.
Conclusions
For the first time in a field-plot study, we demonstrate the major role played by within-species genetic diversity on the production, stability and species composition of temporary grasslands. Our key results seem to find their explanation in terms of shifts in the peaks of species biomass production during the season, these shifts likely leading to temporal species complementarity. Our study suggests major benefits will arise with increases in the genetic diversity of multispecies crops. Genetic diversity may be useful in helping to meet new crop-diversification challenges, particularly with multispecies grasslands. Genetic and species diversity will likely provide additional levers for improving crops in diversified systems.
Keywords: Cultivar and species mixtures, genetic diversity, production stability, species equilibrium, grass species, legume species
INTRODUCTION
Many ecological studies have demonstrated the positive effects of species diversity on ecosystem functioning (Loreau, 1998; Gamfeldt et al., 2008). It is now widely accepted that species diversity affects biomass production functions (Hector et al., 1999; Tilman et al., 1996, 2001), ecosystem services (Hooper et al., 2005; Isbell et al., 2011; Finn et al., 2013) and the stability of ecosystem processes (Tilman et al., 2006; Isbell et al., 2009; Jiang and Pu, 2009; Gross et al., 2014). In community ecology, the principal mechanism used to explain the positive effects of species diversity is complementarity in resource use. This results from a diminution of competitive intensity between species, promoted by their niche difference, and thus favours the abundance of each and so their coexistence. Complementarity may operate at a temporal level, for instance if the growths of two species are not in synchrony. Although widely described at species level, the effects of diversity at genetic level (i.e. at the within-species diversity level) have scarcely been investigated in multispecies crops. Nevertheless, a number of studies have shown the importance of genetic diversity on the functioning of multispecies plant covers, i.e. plant communities (Whitlock et al., 2007; Prieto et al., 2015). Despite the high potential importance of genetic diversity in multispecies crops (Prieto et al., 2015) and its significance in the breeding of plant species for use in mixtures (Litrico and Violle, 2015) in diversified agro-ecosystems studies of the effects of genetic diversity in multispecies crops are few. As noted by Barot et al. (2017), this contrasts with studies of the effects of genetic diversity in monospecies crops.
In multispecies temporary grasslands, farmers use grassland grasses and legume species. However, there has as yet been little work that serves to guide the choice of genetic diversity within the individual species in a species mixture. Temporary multispecies grasslands offer a good model through which to study this question. Some plant breeders seek to improve their seeds for use in temporary multispecies grasslands by utilizing the available genetic diversity to increase production, to engender production stability and to maintain species balance. The aims of this study are, using a case study, (1) to generate information about the effects of genetic diversity on community functioning, particularly on biomass production and species abundance, and (2) to link the effects of genetic diversity on community functioning with values for species growth synchrony.
The contribution of plant ecology to crop science has expanded in recent years. One of the challenges for agriculture in future work on agro-ecosystems will be to lay more equal weight on aspects of plant production and those of environmental sustainability (Dooley, 2005). Hopefully, this perspective will also help minimize conflict between these two objectives (Nemecek and Erzinger, 2005). The use of multispecies crops, in particular those including legumes, is increasingly seen as a way of making plant productive systems more sustainable. An increasing number of studies have emphasized the positive effects of species diversity in agro-ecosystems (Tilman et al., 2001; Hooper et al., 2005; Cardinale et al., 2012) and these favour diversification in agriculture. Sown multispecies grasslands are a major component of crop diversification, to increase the environmental sustainability of agriculture (O’Mara, 2012). These crops can play a number of positive roles in promoting ecosystem services in cropping systems by affecting the nitrogen economy (Weigelt et al., 2009; Gross et al., 2010), limiting weed populations (Hector et al., 2001; Frankow-Lindberg, 2012) and increasing soil fertility (Garbeva et al., 2006). By these means they can also reduce the need for pesticide application. An increasing proportion of temporary grasslands in cash-crop rotations can also help secure forage production and hence livestock farming autonomy, especially in the face of projected changes in climate, which include increases in the incidence and severity of water deficit. Lastly, temporary grasslands can also have positive environmental outcomes (Soussana and Lemaire, 2014; Kunrath et al., 2015), their multispecies composition being important in ensuring this multifunctional role (Weigelt et al., 2009). Temporary grasslands are frequently sown as species mixtures, and the inclusion of high within-species diversity is indeed possible. This genetic diversity could influence the functioning of these crops (Prieto et al., 2015) to extents, and in ways, that have yet to be assessed. A better understanding of the effects of genetic diversity on community functioning is also likely to be useful in dealing with new challenges faced by agronomy associated with diversification. In particular, breeders need to provide farmers with forage plant cultivars designed for mixtures, and this involves taking plant–plant interactions into account.
Some plant breeders have undertaken trials to test the effects of the genetic variability of the individual species they use in sown grassland mixtures on mixture production over significant periods of time. Here, we take advantage of such a situation occurring in a breeder’s experiment, to examine the effects of genetic diversity on temporary multispecies grasslands in the particular case of an experiment set up by a plant breeder to identify optimal seed mixtures for farmers. Monitoring over a 5-year period, we first analyse the effects of genetic diversity on mixture biomass production and on species abundances in a grassland community, and second we examine whether there has been any effect of genetic diversity on species growth synchrony and/or any link between genetic diversity and biomass production and species abundances.
MATERIALS AND METHODS
Experimental design
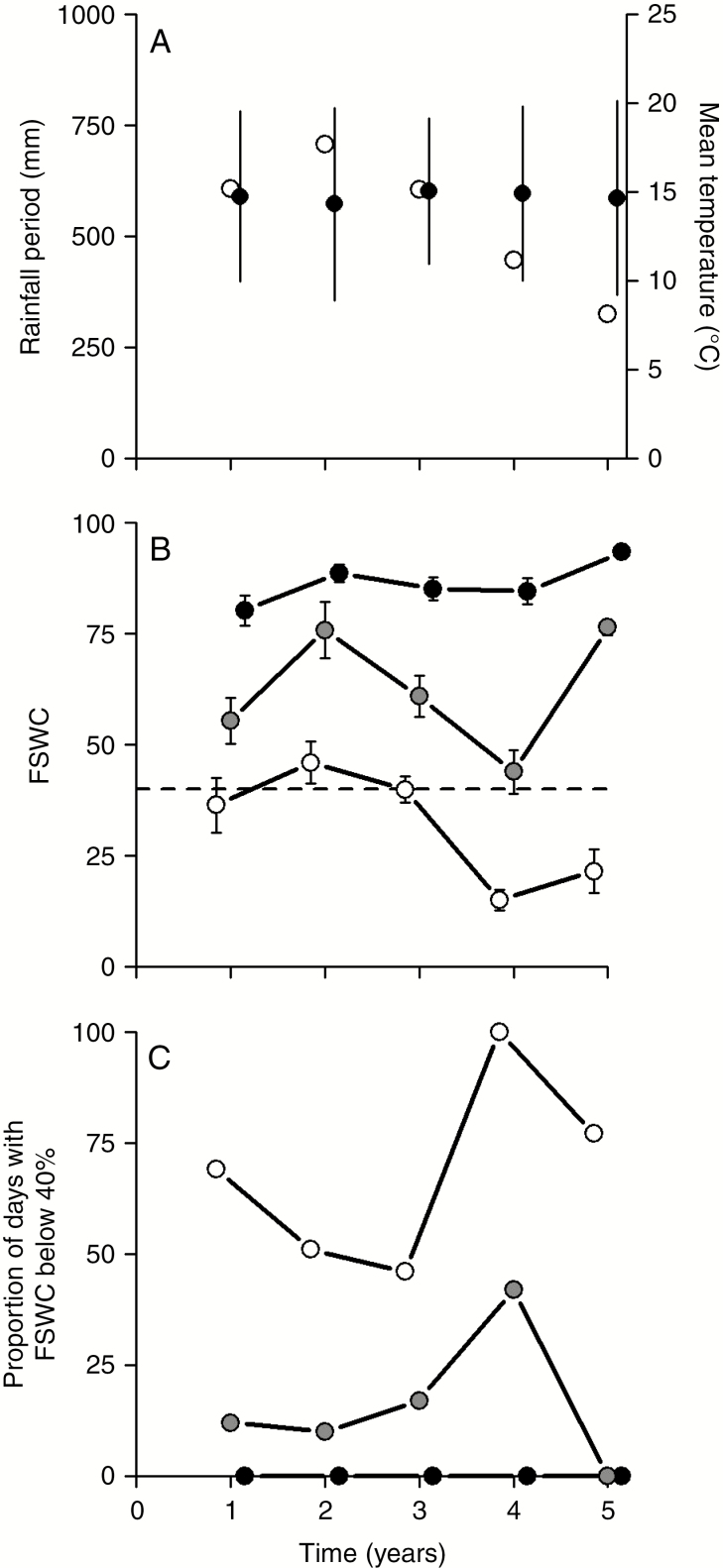
In September 2011, the plant breeder at Jouffray Drillaud sowed a total of 15 multispecies grassland mixtures in micro-plots (5 × 1.3 m) at the Jouffray Drillaud Station, Saint Sauvant, France (46°21′37″ N, 0°03′25″ E). The climate of this region is oceanic temperate with wet winters and medium-dry summers. During the 5-year experimental period, the average annual rainfall was 852 mm and the average annual temperature was 12.1°C. The micro-plots were fully exposed to the weather, with no supplementary irrigation applied during the period. The soil is a Cambisol with a silty–loamy texture in the surface horizon and clay in the subsoil horizon (Hubert, 2008) (Supplementary Data Fig. S1). The organic matter concentration varied from ~1.9 % in the 0–30 cm soil layer, down to ~0.4 % at 135 cm depth, with only traces of free CaCO3. The field capacity at the site was close to 150 mm (Kunrath et al., 2015). To quantify variations in water deficit intensity during regrowth, between seasons and also between years, two indices were computed. A simple daily soil-water balance considered a single soil reservoir of 150 mm depth at maximum soil-water availability (SMWA) (simplified from Kunrath et al., 2015). All mixtures were considered to be exposed to the same SMWA and to the same daily potential evapotranspiration rate. The first index was the fraction of soil water content (FSWC) averaged over the whole regrowth period. The second index was computed following the FAO estimate of threshold values for grassland species and enumerating the proportion of days between two cuts, when the FSWC was <40 %. Rainfall, global solar irradiance, air temperature and humidity and wind-run at 2 m height were recorded daily by an automatic weather station belonging to the INRA network located 2 km from the experimental site. The related database CLIMATIK provided the certified data and the potential evapotranspiration was calculated using the Penman equation calibrated for an irrigated tall fescue turf.
Each micro-plot contained a single seed mixture sown in eight rows, each 5 m long. Each seed mixture contained seven species (all species common in temporary grasslands). The mixtures all contained three grass species (Dactylis glomerata, Festuca arundinacea and Lolium perenne) and four legume species (Trifolium repens, Trifolium pratense, Lotus corniculatus and Medicago sativa). The seed mixture in each micro-plot had the same total weight of seed and the same species proportions (Table 1). The genetic diversity of each species (controlled by number of cultivars) varied between the seed mixtures. The cultivars of each species were chosen by the breeder from those commonly in use in agriculture, that are available in the French catalogue but are of contrasting phenology, aerial architecture and biomass production (Table 1). This situation would seem to provide an excellent opportunity to explore mechanisms of complementarity existing between species, and also between cultivars. For each species, up to six cultivars were used, except for T. pratense, T. repens and L. corniculatus, for which fewer cultivars were available; three of the seed mixtures contained only one cultivar per species (M-1, M-2 and M-3), one mixture contained two or three cultivars per species (M-4) and one mixture contained two to six cultivars per species (M-5) (Table 1). The five seed mixtures were replicated three times (from the same seed lots) and were randomly distributed in three blocks. There was a total of 15 plots (3 × 5). No nitrogen (N) fertilizer was applied during the experimental period. This limited the soil N resources to soil mineralization of organic N and to fixation of atmospheric N by the legumes.
Table 1.
Proportions of species and cultivars sown for five mixtures (M-1, M-2, M-3, M-4, M-5). The proportions are those of seed mass. Each cultivar used is described according to four agronomic traits. Each trait is described according to three levels: low (+), medium (++), high (+++). A cultivar code was assigned to cultivars not yet registered and named (D. glomerata, F. arundinacea and L. perenne)
| Proportions (seed mass) | Description of cultivars | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Cultivar | M-1 | M-2 | M-3 | M-4 | M-5 | Yield | Height | Leaf area | Phenology |
| D. glomerata | 0.115 | 0.115 | 0.115 | 0.115 | 0.115 | |||||
| Vaillant | 1.000 | 0.000 | 0.000 | 0.333 | 0.166 | ++ | +++ | ++ | + | |
| Lucullus | 0.000 | 0.000 | 0.000 | 0.333 | 0.166 | ++ | ++ | ++ | ++ | |
| Accord | 0.000 | 0.000 | 0.000 | 0.333 | 0.166 | ++ | ++ | + | + | |
| Otop | 0.000 | 0.000 | 1.000 | 0.000 | 0.166 | ++ | ++ | +++ | ++ | |
| E1V5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.166 | + | + | + | +++ | |
| President | 0.000 | 1.000 | 0.000 | 0.000 | 0.166 | +++ | +++ | +++ | ++ | |
| F. arundinacea | 0.231 | 0.231 | 0.231 | 0.231 | 0.231 | |||||
| Soni | 1.000 | 0.000 | 0.000 | 0.500 | 0.125 | ++ | ++ | ++ | +++ | |
| Elodie | 0.000 | 0.000 | 0.000 | 0.250 | 0.125 | +++ | ++ | ++ | +++ | |
| Noria | 0.000 | 0.000 | 0.000 | 0.250 | 0.125 | + | ++ | ++ | ++ | |
| Gardian | 0.000 | 1.000 | 0.000 | 0.000 | 0.250 | +++ | +++ | +++ | + | |
| E3V5 | 0.000 | 0.000 | 1.000 | 0.000 | 0.250 | + | + | + | ++ | |
| Mariellendo | 0.000 | 0.000 | 0.000 | 0.000 | 0.125 | ++ | ++ | ++ | +++ | |
| L. perenne | 0.115 | 0.115 | 0.115 | 0.115 | 0.115 | |||||
| Gagny | 0.000 | 0.000 | 0.000 | 0.333 | 0.166 | ++ | ++ | + | ++ | |
| Aberstar | 1.000 | 0.000 | 0.000 | 0.333 | 0.166 | +++ | ++ | + | ++ | |
| Juras | 0.000 | 0.000 | 0.000 | 0.333 | 0.166 | ++ | + | ++ | +++ | |
| Tonnus | 0.000 | 1.000 | 0.000 | 0.000 | 0.166 | +++ | +++ | +++ | + | |
| E6V5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.166 | ++ | ++ | ++ | ++ | |
| Rgmaroc | 0.000 | 0.000 | 1.000 | 0.000 | 0.166 | ++ | +++ | + | + | |
| T. repens | 0.115 | 0.115 | 0.115 | 0.115 | 0.115 | |||||
| Abervantage | 1.000 | 0.000 | 0.000 | 0.333 | 0.333 | ++ | + | + | ++ | |
| Aran | 0.000 | 0.000 | 0.000 | 0.333 | 0.333 | ++ | ++ | ++ | ++ | |
| Giga | 0.000 | 1.000 | 1.000 | 0.333 | 0.333 | ++ | +++ | +++ | +++ | |
| T. pratense | 0.115 | 0.115 | 0.115 | 0.115 | 0.115 | |||||
| Diplo | 1.000 | 1.000 | 0.000 | 0.666 | 0.666 | ++ | ++ | ++ | ++ | |
| Formica | 0.000 | 0.000 | 1.000 | 0.333 | 0.333 | ++ | + | + | ++ | |
| L. corniculatus | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 | |||||
| Leo | 1.000 | 0.000 | 0.000 | 0.500 | 0.500 | ++ | + | + | ++ | |
| Altus | 0.000 | 1.000 | 0.000 | 0.500 | 0.250 | ++ | +++ | +++ | + | |
| PX-ete | 0.000 | 0.000 | 1.000 | 0.000 | 0.250 | ++ | + | +++ | ++ | |
| M. sativa | 0.231 | 0.231 | 0.231 | 0.231 | 0.231 | |||||
| timbale | 1.000 | 0.000 | 0.000 | 0.333 | 0.166 | +++ | +++ | +++ | ++ | |
| Galaxie | 0.000 | 1.000 | 0.000 | 0.333 | 0.166 | +++ | +++ | ++ | ++ | |
| Kali | 0.000 | 0.000 | 0.000 | 0.333 | 0.166 | ++ | +++ | ++ | ++ | |
| Meldor | 0.000 | 0.000 | 0.000 | 0.000 | 0.166 | ++ | +++ | ++ | ++ | |
| Rafia | 0.000 | 0.000 | 1.000 | 0.000 | 0.166 | ++ | +++ | ++ | ++ | |
| Luzelle | 0.000 | 0.000 | 0.000 | 0.000 | 0.166 | ++ | + | + | ++ |
Total and species biomass
For the 5 years of the experiment the whole canopy of each plot was harvested three times each year (spring, summer and autumn, 2012–16). All plots were cut at 5 cm above ground level and at the same time. Harvest date was decided based on a visual assessment of the above-ground standing biomass. Fresh biomass was weighed for each harvest and each plot. A sample was taken from each harvest and each plot, and weighed (fresh biomass), dried to constant weight at 60 °C for 72 h and weighed again (dry biomass). The dry biomass of each harvest and each plot was estimated from the ratio between the fresh and dry masses for the samples. At each harvest, four quadrats (0.33 × 0.15 m) were placed randomly in each plot and the species dry biomasses were separated. These samples were dried and weighed to measure the dry mass proportion of each species in the total dry biomass. The estimation of species dry biomass was arduous, so was carried out on only two of the three blocks for years 1, 2 and 3 and on all blocks for years 4 and 5. It was in these latter years that the difference between species abundances began to be large. For each plot and each year, the annual total biomass (for all species) and annual species biomass (for each species) were calculated by summation of the three biomass measurements (spring, summer and autumn). The inter-annual coefficients of variation (CVs) for annual total biomass were calculated as:
where σʹ is the standard deviation of annual dry biomass between years and µʹ is the mean annual dry biomass over 5 years. The CVs were calculated for each plot.
Evenness index
To define the species abundance equilibrium for each mixture, we adapted the Pielou evenness index (Pielou, 1966), which is based on the Shannon index (Shannon and Weaver, 1963). Unlike the Pielou and Shannon–Weaver indices, which are based on the numbers of individuals of each species, the proportion of species in our evenness index (E) is based on the dry biomass of each species in the plot. First, the diversity index (H) was computed as in Pielou (1966) but with species dry biomass proportions replacing Pielou’s species numerical abundance proportions;
where bi represents the dry biomass proportion of species i. In this equation, the maximum value taken by H occurs when the proportions between species dry biomass are equal. Under these conditions, Hmax = log S, where S represents the number of species. The value 0 was assigned to extinct species. The evenness index is then defined as:
with values of E varying from 0 to 1. For this study, S was always the total number of species involved and this was fixed in our experiment at seven. The inter-annual CV of the evenness index was calculated for each plot as:
where σj is the standard deviation of E between years in plot j and µj is the index mean over 5 years in plot j.
Species synchrony
The species synchrony index (Loreau and de Mazancourt, 2008) was calculated for each year and for each plot. The species synchrony index measures the level of synchrony in the above-ground growth (dry biomass) peak of each species. It is frequently used in ecology to explain plant community stability. The synchrony index ( of plot j was calculated as:
where is the variance of total dry biomass in plot j over subsequent seasonal measurements (three measures per year: spring, summer, autumn) and is the standard deviation of the dry biomass of species i in mixture j over subsequent seasonal measurements. The value ψ = 1 indicates that the species in the community are perfectly synchronous in their growth in the plot. For the value ψ = 0 the growths of the species in the plot are totally asynchronous.
Statistical analyses
Index calculation and statistical analyses were carried out each year using R software (v. 3.2.4; R Development Core Team, 2016). The Shannon–Weaver index was calculated with the Vegan package (Oksanen et al., 2016).
The mixture effect was tested on means of annual total biomass, annual species biomass, evenness index, synchrony index, inter-annual CVs of total biomass and inter-annual CVs of the evenness index. The normality and the homoscedasticity of residuals were checked using the Shapiro and Bartlett tests. We conducted ANOVA with the aov function in R. Our design was balanced, with mixture and block as fixed factors according to the following model (model 1):
where Yklm is the variable explained, is the fixed mixture effect for level k of the mixture factor, is the fixed block effect for level l of the block factor and is the model error for the explained variable Y corresponding to observation m. Tukey tests (multiple comparisons) were carried out when the mixture effect was significant, for 2 × 2 comparison of mixtures (TuKeyHSD function). Correlation was assessed between the evenness index and the synchrony index, between the evenness index and total annual biomass and between the synchrony index and total annual biomass with a regression test (lm function).
From the climate data, we observed (Fig. 1) a stronger drought in the two last years of the 5-year trial. To disentangle the effects on total annual biomass of grassland age (time since sowing) and that of drought, we analysed data with a repeated measures analysis of variance (aov function in R with error term). Our design was balanced, with mixture, block, drought and age as fixed factors and individuals as random factor using the following model (model 2):
Fig. 1.
Climatic data. (A) Rainfall during the measurement periods (March to October) and mean annual temperature (values are mean ± standard deviation) from the ‘climatik’ database (Lusignan INRA station). (B) Fraction of soil water content (FSWC) averaged during regrowth each year. Black points represent the values of useful reserve for the first cut, grey points the second cut and white points the third cut. The dashed line represents a threshold of 40 %, below which grassland species are considered to be under water stress (FAO estimate of threshold values for grasslands). Values are mean ± confidence interval. (C) Proportion of days during regrowth when the FSWC index was <40 % for each year. Black points represent the value of FSWC for the first cut, grey points the second cut and white points the third cut.
where j, k, l and m have the same meaning as in the previous equations, Yjklm is the variable explained, αk is the fixed mixture effect, βl is the fixed block effect, [corresponding to number of days (Julian day)per year that FSWC was <40 %]Γm, γm (the number of years since sowing) is the covariable introduced in the model, uj is the within-subject variability (error term in the aov function) linked to plot j (plot identity) and ɛjklm is the model error; (ατ)km is the interaction between mixture effect and the drought covariable, (αγ)km is the interaction between mixture effect and the age covariable, and (τΓ)m is the interaction between drought and age. The percentage of total variance explained by each factor and covariable was computed as:
where is the percentage explained by factor h, is the sum of squares of factor h and is the total sum of squares of the model.
RESULTS
Total and species annual biomass
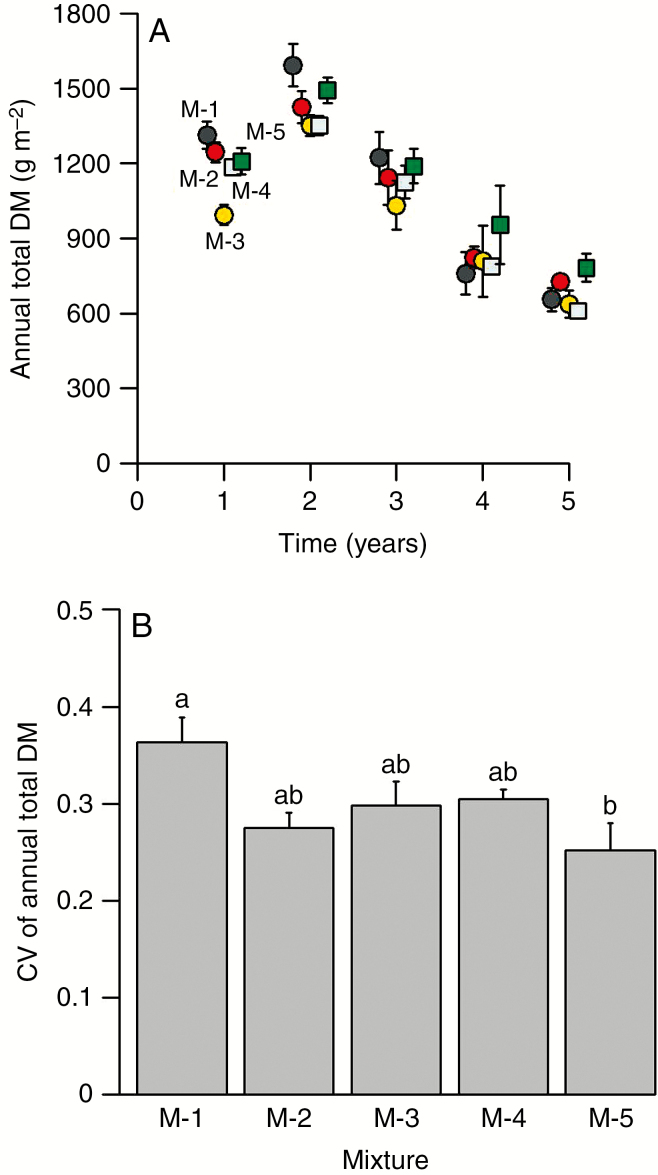
Total annual biomass production (all species) changed over the 5-year period of the experiment (Fig. 2A). Values lay in the range 600–1600 g m−2, with an increase between year 1 and year 2, and then a decline to year 5. This trend was much the same for all mixtures. The rainfall events during the growth period and the FSWC followed much the same pattern during the 5 years of the experiment (Fig. 1A, B). Total biomass was affected by both drought intensity (number of days per year when FSWC was <40 %) and by mixture age (Table 2). Drought significantly affected total biomass change from year to year (representing 24 % of variation) with cumulative effects of mixture age (representing 40 % of variation). In the first, second and fifth years, annual total biomass (all species) showed significant differences between mixtures (Table 3, Fig. 2A). Mixture M-5, with the highest genetic diversity, was among the most productive (1200–1600 g m−2) in the first 2 years and also exhibited the highest productivity in the final year (around 800 g m−2; Fig. 2A). Furthermore, for M-5, the inter-annual CV of total biomass (F4,2 = 4.26, P < 0.05) (Fig. 2B) was lower than that of the most productive of low-diversity mixtures (M-1) and tended to be the lowest measured amongst all mixtures.
Fig. 2.
Production and variation of total biomass. (A) Annual total biomass of five mixtures: M-1 (black points), M-2 (red points), M-3 (yellow points), M-4 (blue squares) and M-5 (green squares). Values are mean ± standard error. (B) Mean inter-annual CVs of annual total biomass per mixture. Values are mean ± standard error. Different letters indicate significant differences (P < 0.05). DM, dry matter.
Table 2.
Analysis of variance from model M-2 to explain total annual biomass
| Effect | D.f | F value | P value | Percentage of variance explained* |
| Mixture | 4 | 6.14 | 0.01 | 4 |
| Block | 2 | 16.86 | <0.01 | 5.5 |
| Drought | 1 | 210.54 | <0.001 | 23.9 |
| Age | 1 | 355.104 | <0.001 | 40.4 |
| Mixture × drought | 4 | 2.87 | 0.03 | 1.3 |
| Mixture × age | 4 | 2.87 | 0.03 | 1.3 |
| Drought × age | 1 | 147.05 | <0.001 | 16.7 |
*Percentage of variance explained by each factor.
Table 3.
Analysis of variance from model M-1 to explain annual total biomass, annual biomass for each species, and indices of evenness and synchrony
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | D.f | F value | P value | D.f | F value | P value | D.f | F value | P value | D.f | F value | P value | D.f | F value | P value | |
| Total annual biomass | ||||||||||||||||
| Mixture | 4 | 7.01 | 0.01 | 4 | 14.11 | <0.01 | 4 | 1.28 | 0.36 | 4 | 1.08 | 0.43 | 4 | 4.16 | 0.04 | |
| Block | 2 | 0.95 | 0.43 | 2 | 19.02 | <0.001 | 2 | 6.15 | 0.02 | 2 | 6.52 | 0.02 | 2 | 3.64 | 0.08 | |
| D. glomerata biomass | ||||||||||||||||
| Mixture | 4 | 5.53 | 0.06 | 4 | 0.08 | 0.98 | 4 | 2.05 | 0.25 | 4 | 1.71 | 0.24 | 4 | 3.14 | 0.08 | |
| Block | 1 | 0.35 | 0.59 | 1 | 1.34 | 0.31 | 1 | 1.04 | 0.37 | 2 | 8.05 | 0.01 | 2 | 13.25 | <0.01 | |
| F. arundinacea biomass | ||||||||||||||||
| Mixture | 4 | 6.80 | <0.05 | 4 | 47.46 | <0.01 | 4 | 1.95 | 0.27 | 4 | 5.58 | 0.02 | 4 | 23.87 | <0.01 | |
| Block | 1 | 2.79 | 0.17 | 1 | 0.30 | 0.61 | 1 | 0.50 | 0.52 | 2 | 0.60 | 0.57 | 2 | 0.17 | 0.85 | |
| L. perenne biomass | ||||||||||||||||
| Mixture | 4 | 10.81 | 0.02 | 4 | 4.44 | 0.09 | 4 | 6.93 | 0.04 | 4 | 1.59 | 0.27 | 4 | 2.74 | 0.10 | |
| Block | 1 | 4.48 | 0.10 | 1 | 6.09 | 0.07 | 1 | 0.69 | 0.45 | 2 | 1.33 | 0.32 | 2 | 0.62 | 0.56 | |
| T. repens biomass | ||||||||||||||||
| Mixture | 4 | 81.77 | <0.01 | 4 | 4.26 | 0.09 | 4 | 0.69 | 0.63 | 4 | 0.67 | 0.63 | 4 | 2.02 | 0.18 | |
| Block | 1 | 21.88 | 0.01 | 1 | 1.80 | 0.25 | 1 | 3.49 | 0.14 | 2 | 4.68 | 0.05 | 2 | 16.26 | <0.01 | |
| T. pratense biomass | ||||||||||||||||
| Mixture | 4 | 8.12 | 0.03 | 4 | 2.25 | 0.23 | 4 | 1.08 | 0.47 | 4 | 0.90 | 0.51 | 4 | 0.35 | 0.84 | |
| Block | 1 | 0.78 | 0.43 | 1 | 0.94 | 0.39 | 1 | 1.74 | 0.26 | 2 | 1.37 | 0.31 | 2 | 0.49 | 0.63 | |
| L. corniculatus biomass | ||||||||||||||||
| Mixture | 4 | 4.69 | 0.08 | 4 | 3.23 | 0.14 | 4 | 1.07 | 0.48 | 4 | 4.99 | 0.03 | 4 | 0.98 | 0.47 | |
| Block | 1 | 1.92 | 0.24 | 1 | 3.62 | 0.13 | 1 | 0.72 | 0.44 | 2 | 2.54 | 0.14 | 2 | 0.16 | 0.86 | |
| M. sativa biomass | ||||||||||||||||
| Mixture | 4 | 14.90 | 0.01 | 4 | 1.06 | 0.48 | 4 | 1.33 | 0.40 | 4 | 5.31 | 0.02 | 4 | 2.27 | 0.15 | |
| Block | 1 | 7.98 | 0.05 | 1 | 3.70 | 0.13 | 1 | 1.97 | 0.23 | 2 | 1.36 | 0.31 | 2 | 1.49 | 0.28 | |
| Evenness index | ||||||||||||||||
| Mixture | 4 | 4.61 | 0.08 | 4 | 0.74 | 0.61 | 4 | 3.81 | 0.11 | 4 | 2.43 | 0.13 | 4 | 22.97 | <0.001 | |
| Block | 1 | 0.01 | 0.93 | 1 | 2.45 | 0.19 | 1 | 1.83 | 0.25 | 2 | 6.11 | 0.03 | 2 | 5.92 | 0.03 | |
| Synchrony index | ||||||||||||||||
| Mixture | 4 | 1.43 | 0.36 | 4 | 0.66 | 0.65 | 4 | 2.35 | 0.21 | 4 | 4.08 | 0.04 | 4 | 1.59 | 0.27 | |
| Block | 1 | 0.42 | 0.55 | 1 | 0.77 | 0.43 | 1 | 0.01 | 0.92 | 2 | 3.42 | 0.08 | 2 | 0.38 | 0.70 |
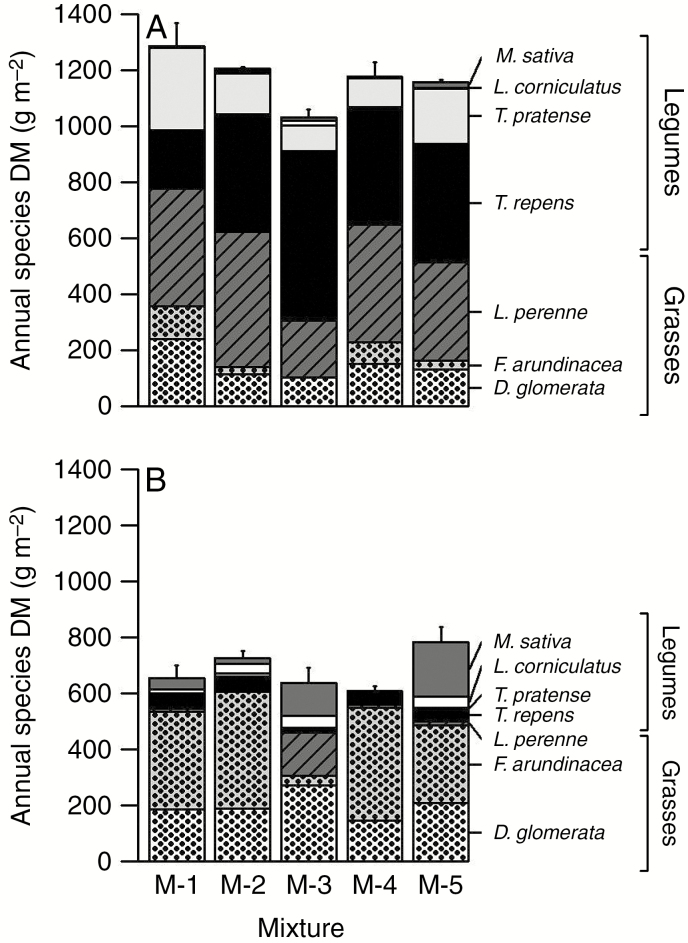
The species composition of the mixtures showed significant change for several species and over several years (Table 3). Within all mixtures, the initial (year 1) abundances of F. arundinacea and D. glomerata accounted for lower proportions of total biomass than L. perenne and T. repens, but in year 5 the situation was fully reversed (Fig. 3A, B). Until year 4, the differences between mixtures were not explained by their genetic diversities. However, the mixture with greatest genetic diversity (M-5) had significantly higher species abundance representation (Fig. 3B, Table 3). Our results show that genetic diversity of species can influence the contribution of species to total mixture biomass. This was also significant for the grass–legume biomass proportions (Fig. 3A, B) and was confirmed by the species evenness result (see next section).
Fig. 3.
Annual species biomass. (A) Mean of annual species biomass of each mixture for the first year of the experiment with D. glomerata, F. arundinacea, L. perenne, T. repens, T. pratense, L. corniculatus and M. sativa. Values are mean ± standard error. (B) Mean of annual species biomass of each mixture for the fifth year of the experiment with D. glomerata, F. arundinacea, L. perenne, T. repens, T. pratense, L. corniculatus and M. sativa. Values are mean ± standard error. DM, dry matter.
Species evenness and species synchrony indices
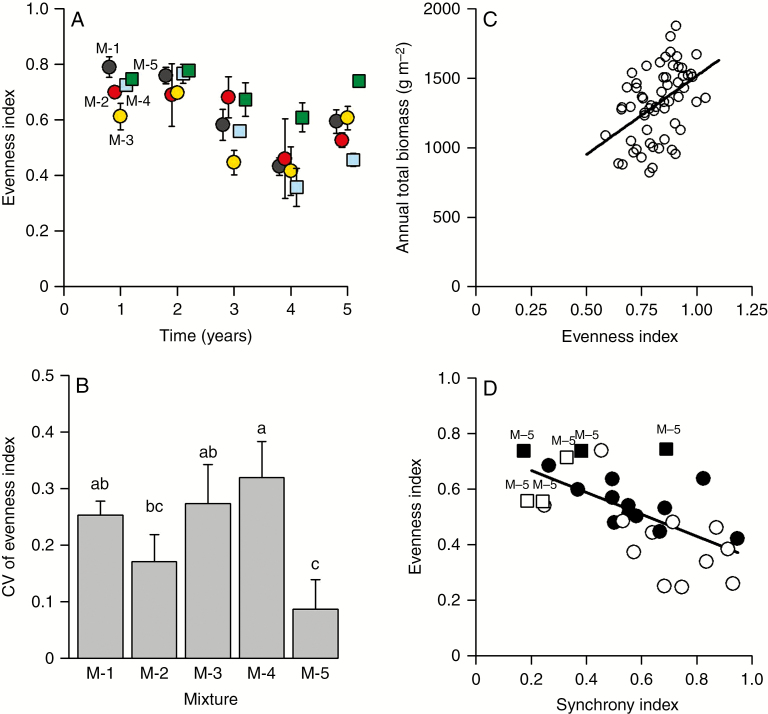
Among the more productive mixtures, only in M-5 did the evenness index remain almost unchanged, exhibiting the highest values throughout the 5-year experiment (Fig. 4A). Hence, the inter-annual CV of evenness index for mixture M-5 was among the lowest (Fig. 4B). The species evenness in M-5 was significantly higher (near 0.8) than in any other mixture in year 5, the final year of measurement (Fig. 4A, Table 3). These results emphasize the positive influence of species genetic diversity on the equilibrium of species abundances in a mixture in the field over a period of time. Lastly, when the drought effect was removed (analysis of residuals of regression model with the number of days when the FSWC index was <40 % as variable), a correlation was maintained (R2 = 0.20, P < 0.001) between evenness index and total annual biomass (Fig. 4C).
Fig. 4.
Equilibrium and synchrony level of species. (A) Equilibrium species abundances estimated from yearly evenness index for the five mixtures: M-1 (black points), M-2 (red points), M-3 (yellow points), M-4 (blue squares) and M-5 (green squares). (B) Inter-annual CV of the evenness index. Values are mean ± standard error. Different letters indicate significant differences (P < 0.05). (C) Annual total biomass as a function of evenness index for the 5 years of the experiment with the drought effect removed; residuals of models with the number of days when the fraction of soil water content (FSWC) index was <40 % as fixed factor. The regression through both sets of points is significant (P < 0.001), with R2 = 0.20. (D) Synchrony index as a function of evenness index for the fourth year (white points) and fifth year (black points). Square points correspond to mixture M-5 and round points correspond to the other mixtures. The regression through both sets of points is significant (P < 0.001), with R2 = 0.38.
The synchrony index varied between ~0.2 and 0.75 in year 4 and between 0.35 and 0.75 in year 5. The mixture effect on the synchrony index was significant in year 4 (Table 3), while the synchrony value for M-5 was the weakest. A similar but non-significant trend was found in year 5. Finally, there was a negative correlation (R2 = 0.38, P < 0.001) between the evenness index and the synchrony index in year 4 and year 5 (Fig. 4D).
DISCUSSION
As expected, the dynamics of biomass production over the years changed with increasing age of the grassland (Hopkins et al., 1995). Drought intensity also affected total biomass, with a cumulative effect of mixture age. Although the water deficit intensity may have differed slightly between mixtures, the mixture with the highest species genetic diversity showed the highest productivities during the last few years of the trial, after the cumulative effects of grassland age and drought. It also showed the lowest CVs. Our results show not only an effect of genetic diversity on the biomass production of mixtures but also demonstrate that the stability of biomass production tends to increase with high genetic diversity. This finding is consistent with that from a recent study using grassland micro-plots in boxes of artificial soil exposed to drought (Prieto et al., 2015). It also supports the ecological hypothesis that diversity is a source of community stability (Gross et al., 2014). One novel result of our study is the positive effect of genetic diversity on the balance of species abundance over a period of time. That is, the genetic diversity of the various species constituting a community affects the species proportions. The results for both biomass production stability and species evenness for the mixture M-4, which had intermediate genetic diversity, were not intermediate, as might have been expected. Two possible explanations are that (1) the species genetic diversity of M-4 was too low to obtain a significant genetic diversity benefit – in other words, a minimum level of genetic diversity may be required for the complementarity mechanism to be activated; and (2) M-4 did not contain particularly competitive cultivars. The effects of the identity of the cultivars may have become entangled with the effects of increasing genetic diversity. The results for the medium-diversity mixture may be explained by the absence of some cultivars. This hypothesis, leading to selection of competitive cultivars, could be studied with molecular tools to shed light on the dynamics of multi-cultivar swards over time. However, our key result is the negative relationship found between the evenness index and synchronicity. This suggests a temporal complementarity between species driven by high genetic diversity. This result fits with the study of Prieto et al. (2015).
For more sustainable, productive and persistent sown grasslands in crop rotations, it is important that we are able to determine the most effective level of genetic diversity of species. It is also important to identify the genetic traits that should be most diverse; some traits may not be useful at all and others quite critical. In particular we need to identify those traits most involved in species complementarity (Litrico and Violle, 2015; Wagg et al., 2017). In our experiment, the trait differences were focused on the cultivars’ phenologies and leaf traits (Table 1). In further studies, it would be interesting to test the effects of diversity in a number of other traits and with other species. In a plant community, differentiation of species ecological niches (Macarthur and Levins, 1967) through differentiation of traits should allow species to reduce the negative effects of plant interaction (i.e. competition) by promoting species complementarity. The variations in evenness and species abundance suggest that traits linked to growth seasonality (e.g. the timing of biomass peaks during the year), and therefore phenology, could be important. Meanwhile, at least a certain level of diversification within species would seem to be desirable. Asynchrony of peak biomass between species leads to temporal niche differentiation of growth (Prieto et al., 2015). This contributes to the species diversity effect on production stability and on ecosystem functioning. The relation between genetic diversity and asynchrony of peak biomass between species under artificial conditions was highlighted recently by Prieto et al. (2015). Our results support the potential effect of genetic diversity and, for the first time, demonstrate this support in the field. Selected genotypes within species could be complementary for growth relative to genotypes within other species and thus reduce competition and increase complementarity between species by shifting the timing of biomass peaks. The benefits of high genetic diversity could have occurred simultaneously with the increase in competitive pressures (after the early years). Species genetic diversity may increase the likelihood of particular genotypes being present that are better adapted to the conditions generated by the presence of other species and their associated selection pressures. In this way, genotype-level selection could lead to species-level complementarity effects. Our study displays a negative correlation between peak biomass synchrony among species and equilibrium of species abundance. This fosters the growth of asynchronous species, decreases competition between species and favours the growth of each species. The equilibrium of species abundance seems to improve total biomass production through the year, probably through species competition equilibrium.
Many studies to date have focused on species diversity effects within crops. However, the genetic diversity effect on the production, stability and species composition of multispecies crops has been largely overlooked. Along with species diversity, genetic diversity can have a nested effect on the performance of a species mixture (Litrico and Huyghe, 2018). The results presented here are based on a particular set of species, of cultivars and of environmental conditions. It remains for our findings to be confirmed for other plant materials and environments through similarly structured experiments. Nevertheless, our results are in broad agreement with the few studies that have been made into the effects of genetic diversity in plant mixtures (Whitlock et al., 2007; Prieto et al., 2015). Here, we provide insights that may present avenues along which to explore the mechanisms underlying the observed ecological effects. We suggest the hypothesis that ecological mechanisms, such as complementarity, may depend on selection at the genetic level. The genetic diversity of a species in a mixture should, over time, ensure the presence of genotypes adapted to selection pressures imposed by the other species in the mixture. In this way, genetic diversity should lead to essential complementarity between species, improved biomass production and species equilibrium stability in multispecies crops.
Conclusions
This study has significance for areas of agriculture and agro-ecology where crop diversification is required. The high genetic diversity of individual species in a multispecies mixture should raise the biomass production and stability of the plant community. Such stabilities are important in agriculture to ensure enduring production and quality (Sturludottir et al., 2014). The nutritional quality of forage crops is influenced by species composition, especially when legumes are included (Deak et al., 2007). This study shows, for the first time, the importance of genetic diversity obtained through the mixing of cultivars in improving the effects of species mixtures in temporary grasslands. Our results suggest temporal complementarity may be a very good way to optimize grassland functioning, as well as suggesting a target for the composition of seed mixtures for breeders. This study also contributes to the discussion of the need to include genetic diversity in breeding programmes of cultivars intended for multispecies crops and suggests ways in which this may help meet the challenges faced by modern agriculture (Litrico and Violle, 2015).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the URP3F technical team and particularly Dominique Dénoue and Brigitte Bonneau, who provided important experimental assistance. We also thank the Jouffray Drillaud technical team that conducted the experiment. Finally, we thank two anonymous referees for their relevant comments on our work. The ANR project (PRAISE, ANR-13- ADAP-0015) co-funded this work. The PhD grant of Julien Meilhac was supported by the Region Poitou-Charente and INRA (BAP division and EcoServ Metaprogram).
LITERATURE CITED
- Barot S, Allard V, Cantarel A, et al. 2017. Designing mixtures of varieties for multifunctional agriculture with the help of ecology. A review. Agronomy for Sustainable Development 37: 13. [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486: 59–67. [DOI] [PubMed] [Google Scholar]
- Deak A, Hall MH, Sanderson MA, Archibald DD. 2007. Production and nutritive value of grazed simple and complex forage mixtures. Agronomy Journal 99: 814–821. [Google Scholar]
- Dooley EE. 2005. Millennium ecosystem assessment. Environmental Health Perspectives 113: A591. [Google Scholar]
- Finn JA, Kirwan L, Connolly J, et al. 2013. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: a 3-year continental-scale field experiment. Journal of Applied Ecology 50: 365–375. [Google Scholar]
- Frankow-Lindberg BE. 2012. Grassland plant species diversity decreases invasion by increasing resource use. Oecologia 169: 793–802. [DOI] [PubMed] [Google Scholar]
- Gamfeldt L, Hillebrand H, Jonsson PR. 2008. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 89: 1223–1231. [DOI] [PubMed] [Google Scholar]
- Garbeva P, Postma J, van Veen JA, van Elsas JD. 2006. Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environmental Microbiology 8: 233–246. [DOI] [PubMed] [Google Scholar]
- Gross K, Cardinale BJ, Fox JW, et al. 2014. Species richness and the temporal stability of biomass production: a new analysis of recent biodiversity experiments. American Naturalist 183: 1–12. [DOI] [PubMed] [Google Scholar]
- Gross N, Liancourt P, Choler P, Suding KN, Lavorel S. 2010. Strain and vegetation effects on local limiting resources explain the outcomes of biotic interactions. Perspectives in Plant Ecology, Evolution and Systematics 12: 9–19. [Google Scholar]
- Hector A, Schmid B, Beierkuhnlein C, et al. 1999. Plant diversity and productivity experiments in European grasslands. Science 286: 1123–1127. [DOI] [PubMed] [Google Scholar]
- Hector A, Dobson K, Minns A, Bazeley-White E, Lawton JH. 2001. Community diversity and invasion resistance: an experimental test in a grassland ecosystem and a review of comparable studies. Ecological Research 16: 819–831. [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs 75: 3–35. [Google Scholar]
- Hopkins A, Murray PJ, Bowling PJ, Rook AJ, Johnson J. 1995. Productivity and nitrogen uptake of aging and newly sown swards of perennial ryegrass (Lolium perenne L.) at different sites and with different nitrogen-fertilizer treatments. European Journal of Agronomy 4: 65–75. [Google Scholar]
- Hubert F. 2008. Clay mineral assemblage characterisation of two temperate soils using direct profile fitting of diffractograms. Mineralogical and pedological outputs. PhD Thesis, Université de Poitiers. [Google Scholar]
- Isbell F, Calcagno V, Hector A, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477: 199–202. [DOI] [PubMed] [Google Scholar]
- Isbell FI, Polley HW, Wilsey BJ. 2009. Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecology Letters 12: 443–451. [DOI] [PubMed] [Google Scholar]
- Jiang L, Pu ZC. 2009. Different effects of species diversity on temporal stability in single-trophic and multitrophic communities. American Naturalist 174: 651–659. [DOI] [PubMed] [Google Scholar]
- Kunrath TR, de Berranger C, Charrier X, et al. 2015. How much do sod-based rotations reduce nitrate leaching in a cereal cropping system? Agricultural Water Management 150: 46–56. [Google Scholar]
- Litrico I, Violle C. 2015. Diversity in plant breeding: a new conceptual framework. Trends in Plant Science 20: 604–613. [DOI] [PubMed] [Google Scholar]
- Litrico I, Huyghe C. 2018. Can increased within-field diversity boost ecosystem services and crop adaptability to climatic uncertainty? In: Lemaire G, Carvalho PC, Kronberg S, Recous S, eds. Agro-ecosystem diversity: reconciling contemporary agriculture and environmental quality. Academic Press, 191–197. doi:10.1016/B978-0-12-811050-8.00011-X. [Google Scholar]
- Loreau M. 1998. Biodiversity and ecosystem functioning: a mechanistic model. Proceedings of the National Academy of Sciences of the USA 95: 5632–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. 2008. Species synchrony and its drivers: neutral and nonneutral community dynamics in fluctuating environments. American Naturalist 172: E48–E66. [DOI] [PubMed] [Google Scholar]
- Macarthur R, Levins R. 1967. The limiting similarity convergence and divergence of coexisting species. American Naturalist 101: 377–385. [Google Scholar]
- Nemecek T, Erzinger S. 2005. Modelling representative life cycle inventories for Swiss arable crops. International Journal of Life Cycle Assessment 10: 68–76. [Google Scholar]
- O’Mara FP. 2012. The role of grasslands in food security and climate change. Annals of Botany 110: 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet G, Friendly M, et al. 2016. Community ecology package. R package Version 2.4-1. https://cran.r-project.org, https://github.com/vegandevs/vegan [Google Scholar]
- R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN: 3-900051-07-0. http://www.R-project.org [Google Scholar]
- Pielou EC. 1966. Species-diversity and pattern-diversity in the study of ecological succession. Journal of Theoretical Biology 10: 370–383. [DOI] [PubMed] [Google Scholar]
- Prieto I, Violle C, Barre P, Durand JL, Ghesquiere M, Litrico I. 2015. Complementary effects of species and genetic diversity on productivity and stability of sown grasslands. Nature Plants 1: 5. [DOI] [PubMed] [Google Scholar]
- Shannon CE, Weaver W. 1963. The mathematical theory of communication. Urbana: University Illinois Press. [Google Scholar]
- Soussana JF, Lemaire G. 2014. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agriculture Ecosystems & Environment 190: 9–17. [Google Scholar]
- Sturludottir E, Brophy C, Belanger G, et al. 2014. Benefits of mixing grasses and legumes for herbage yield and nutritive value in Northern Europe and Canada. Grass and Forage Science 69: 229–240. [Google Scholar]
- Tilman D, Wedin D, Knops J. 1996. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379: 718–720. [Google Scholar]
- Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. 2001. Diversity and productivity in a long-term grassland experiment. Science 294: 843–845. [DOI] [PubMed] [Google Scholar]
- Tilman D, Reich PB, Knops JMH. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441: 629–632. [DOI] [PubMed] [Google Scholar]
- Wagg C, Ebeling A, Roscher C, et al. 2017. Functional trait dissimilarity drives both species complementarity and competitive disparity. Functional Ecology 31: 2320–2329. [Google Scholar]
- Weigelt A, Weisser WW, Buchmann N, Scherer-Lorenzen M. 2009. Biodiversity for multifunctional grasslands: equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 6: 1695–1706. [Google Scholar]
- Whitlock R, Grime JP, Booth R, Burke T. 2007. The role of genotypic diversity in determining grassland community structure under constant environmental conditions. Journal of Ecology 95: 895–907. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.