Abstract
Background and Aims
Micronutrient deficiency in cereals is a problem of global significance, severely reducing grain yield and quality in marginal soils. Ancient landraces represent, through hundreds of years of local adaptation to adverse soil conditions, a unique reservoir of genes and unexplored traits for enhancing yield and abiotic stress tolerance. Here we explored and compared the genetic variation in a population of Northern European barley landraces and modern elite varieties, and their tolerance to manganese (Mn) limitation.
Methods
A total of 135 barley accessions were genotyped and the genetic diversity was explored using Neighbor–Joining clustering. Based on this analysis, a sub-population of genetically diverse landraces and modern elite control lines were evaluated phenotypically for their ability to cope with Mn-deficient conditions, across three different environments increasing in complexity from hydroponics through pot experiments to regional field trials.
Key Results
Genetically a group of Scottish barley landraces (Bere barley) were found to cluster according to their island of origin, and accessions adapted to distinct biogeographical zones with reduced soil fertility had particularly larger Mn, but also zinc (Zn) and copper (Cu) concentrations in the shoot. Strikingly, when grown in an alkaline sandy soil in the field, the locally adapted landraces demonstrated an exceptional ability to acquire and translocate Mn to developing leaves, maintain photosynthesis and generate robust grain yields, whereas modern elite varieties totally failed to complete their life cycle.
Conclusions
Our results highlight the importance of gene pools of local adaptation and the value of ancient landrace material to identify and characterize genes that control nutrient use efficiency traits in adverse environments to raise future crop production and improve agricultural sustainability in marginal soils. We propose and discuss a model summarizing the physiological mechanisms involved in the complex trait of tolerance to Mn limitation.
Keywords: Barley landraces, Hordeum vulgare, evolutionary biology, genetic diversity, adaptation, marginal soils, micronutrients, nutrient use efficiency, sustainable agriculture
INTRODUCTION
It is a key global challenge to increase crop productivity while preserving natural resources, and at the same time facing climate change and degradation of arable lands. Hence, it is time to re-think how we grow plants to meet the United Nations sustainable development goals of zero hunger and responsible crop production (United Nations, 2015). However, this goal is currently quite distant considering that it is contingent on the need to produce on poorly fertile marginal soils with inherent nutrient limitations.
To grow and yield efficiently, plants require 17 essential elements. Of these, several micronutrients are necessary for photosynthesis, where they have key functions in electron transport processes. Light-induced water splitting is catalysed by the manganese (Mn) cluster in the oxygen-evolving complex (OEC) of photosystem II (PSII), and its function is a prerequisite to extract electrons for driving photosynthesis. Iron (Fe) is present as haem and sulphur-Fe proteins and Fe-enzymes functioning in electron transfer and redox reactions within the various photosynthetic complexes. Iron, copper (Cu) and zinc (Zn) are co-factors of the superoxide dismutases (Fe-SOD and Cu-Zn-SOD), which scavenge reactive oxidative species in the chloroplast, and plastocyanin is a mobile Cu-containing protein involved in electron transport from cytochrome b6f to photosystem I (PSI) during photosynthesis.
Manganese deficiency is a widespread problem, particularly in dry, calcareous and sandy soils, which favour the oxidation of Mn into plant-unavailable Mn oxides (Schmidt et al., 2016a). Barley is particularly sensitive to Mn deficiency, which significantly reduces crop yields and may even cause complete crop loss in severe winters (Schmidt et al., 2013). Novel genotypes which are able to acquire and utilize Mn and other micronutrients more efficiently are needed to achieve global food security. To achieve this, one strategy is to explore the variation contained in ancient landraces, where barley is an excellent genetic model, because it has been adapted to a wide range of adverse environments during its domestication and subsequent cultivation and expansion (Dawson et al., 2015; Pankin and von Korff, 2017). For millennia, farmers have selected for specific agricultural traits to maximize yields, including adaptations providing crop robustness and stability under nutrient stress conditions (Dwivedi et al., 2016). The advantage of utilizing ancient landraces for plant genetic improvement has been demonstrated in previous studies investigating the molecular basis of adaptation to, for example, high soil boron in wheat landraces (Pallotta et al., 2014), aluminium tolerance in landraces of barley (Fujii et al., 2012) and phosphorus efficiency in landraces of rice (Gamuyao et al., 2012). These clearly emphasize the impact and power of genetic variation hidden in ancient landraces originating from diverse agronomic zones. In recent research, an unprecedented variation in the ability of Northern European barley landraces and commercial varieties to tolerate Mn deficiency has been observed (George et al., 2014). Apparently these landraces had a greater capacity to acquire Mn, suggesting a wider genetic diversity in tolerance to Mn deficiency compared with modern elite varieties.
Here we explore and compare the genetic variation in a population of Northern European ancient landraces and modern elite varieties grown across three different environments, i.e. hydroponics, pot experiments and field trials under natural conditions. Using chlorophyll a (Chl a) fluorescence as a well-established proxy for tolerance towards Mn deficiency (Schmidt et al., 2013), we begin to reveal the complex key adaptive traits conserved in the landraces, but lost from elite modern varieties.
MATERIALS AND METHODS
Barley accessions
The accessions used in this study are part of a ‘heritage’ barley collection which includes landraces and locally adapted cultivars from the UK mainland and Scottish islands, comprising two- and six-row types (Supplementary Data Table S1). This was supplemented with landraces from Denmark, Norway and Sweden, as well as two accessions from the Faeroe Islands. Accessions were sourced from the Germplasm Resources Unit (GRU) at the John Innes Centre in Norwich, Science and Advice for Scottish Agriculture (SASA) in Edinburgh, Nordic Genetic Resource Center previously known as the Nordic Genebank (NGB) in Sweden, the USDA Germplasm Resources Information Network (GRIN) or the N.I. Vavilov All Russian Institute of Plant Genetic Resources (VIR).
Genetic analysis
DNA extraction and 9K select genotyping
Genomic DNA for genotyping was extracted from 7-day-old germinated seedling leaf tissue using Qiagen DNeasy Plant Mini kits (Qiagen, Hilden, Germany). Genotyping using the 9K Illumina SNP chip (Comadran et al., 2012) was performed at TraitGenetics, GmbH in Germany, and single nucleotide polymorphism (SNP) alleles were called using GenomeStudio Genotyping Module v2.0.2 (Illumina, San Diego, CA, USA). The data were filtered to remove >5 % missing SNP data, and any heterozygous calls were scored as missing data. The genetic relationship between these accessions was analysed using Neighbor–Joining clustering by means of a simple matching distance matrix executed in PAST, version 1.91 (Hammer et al., 2001). STRUCTURE analysis of individuals was undertaken with the STRUCTURE 2.2 software package (Falush et al., 2007), based on 25 000 ‘burn-in’ replications and a further 25 000 Markov chain Monte Carlo (MCMC) steps; other parameters were kept at default settings.
Plant growth conditions and experimental set-up
Hydroponics experiment 1
Barley seeds of 24 selected barley genotypes (Bere and UK landraces, Fig. 1A) were germinated in vermiculite for 6 d in a glasshouse with minimum temperatures of 18 °C:15 °C, light:dark, and a photoperiod of 16 h:8 h, light:dark (minimum 250 μmol photons m−2 s−1 light intensity). One seedling of each of the 24 different landraces was transferred into each of six 28 L hydroponic containers. Plants were supplied with an aerated nutrient solution (pH 6–6.5) according to Schmidt et al. (2013) except for Mn additions. Control plants received increasing concentrations of MnSO4·H2O [100 nm week 1 (split application), 500 nm weeks 2 and 3, 715 nm week 4). The Mn-deficient plants received only 100 nm MnSO4·H2O in week 1 (split application) and then no MnSO4·H2O for the rest of the growth. At 31 d after transplanting, the youngest fully developed leaf (YFDL) from each plant was harvested and freeze-dried for inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis.
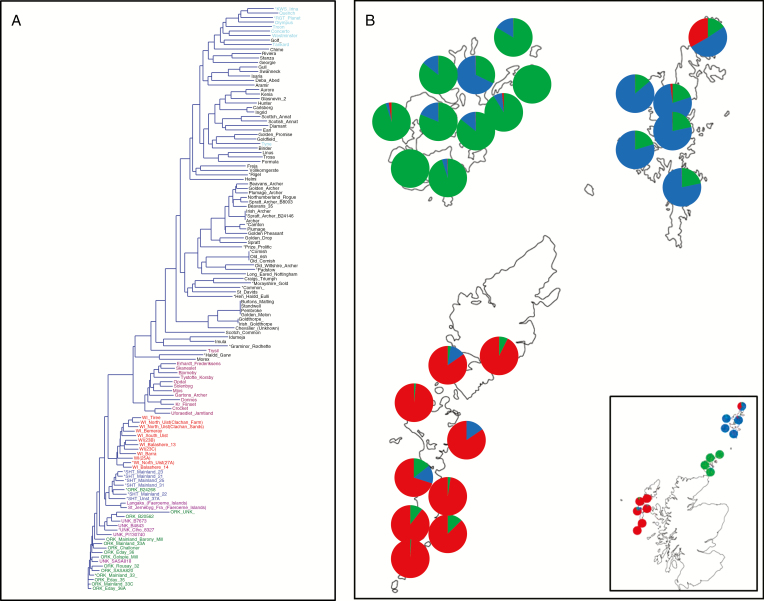
Fig. 1.
Biogeographical map: genotypic variation of barley with distribution across geographical zones. (A) Hierarchical clustering using Neighbor–Joining and an association matrix based on simple matching for 130 barley (Hordeum vulgare) accessions using 9K iSelect SNP chip (Comadran et al., 2012) (Supplementary Data Table 1). To simplify, two-row landraces UK are coloured black, two-row modern cultivars are light blue, six-row Scandinavian landraces are purple, Bere landraces from Orkney are green, those from the Shetlands are blue, those from the Western Isles are red and those of unknown origin are pink (most group close to Orkney accessions). Two accessions from the Faeroe Islands group closely to Shetlands and Bere North Ronaldsay; the furthermost of the Orkney Isles is close to most of the Shetland accessions. Accessions with asterisks are used in pot, hydroponics and field experiments. (B) STRUCTURE results (K = 3) for Bere accessions only from Scottish Islands (Western Isles, islands off the West coast of Scotland latitude of 58.217 and longitude –6.37; Orkney Isles, North of Scotland with latitude of 58.985 and longitude of –2.960; Shetland Isles are 80 km from Orkney at a latitude of 60.347 and the longitude is –1.236); the inset map shows the true position of Western and Orkney Isles in relation to the mainland of Scotland (Shetlands are positioned closer to Orkney for illustration). Q profiles (membership of each of the three groups) are shown as pie charts and located close to collection sites. For example, most accessions from Orkney have similar Q values for membership of that particular K group (green) and range from 0.322 to 0.999. Similar membership profiles were identified for the Western Isles (red) and Shetland Isles (blue).
Hydroponics experiment 2
In a smaller hydroponic set-up, plants of KWS Irina, Bere Unst Shetland and Bere Clho 8327 were cultivated in 4 L containers under control conditions (as described for experiment 1) or under Mn-deficient conditions, with plants receiving 200 nm MnSO4·H2O (split in three) during the first 10 d after transplanting. At 25 d after transplanting, fluorescence was measured and recorded using Imaging PAM, one YFDL per rep was harvested for ICP-OES analysis and the remaining YFDLs were collected and immediately frozen in liquid nitrogen for subsequent thylakoid isolation and analysis.
Pot experiment
Seedlings of three modern elite varieties (KWS Irina, RGT Planet and Graminor Rødhette), and four Bere landraces (Bere North Ronaldsay, Bere West Orkney, Bere Shetland and Bere North Uist) (Table 1) were transplanted to 1 L pots with either Orkney soil (pH 7.8; loamy sand, see field trials for further information) supplemented with fertilizer [Yara Mila 14–3–15–10 (NPKS) + Mg, Cu and B] at a rate of 2 g kg–1 soil or into 1 L pots with compost (15 mm dark peat, light peat and sod pea 3:6:1 including 50 kg of clay, 1 kg of aquasorb, 4 kg of lime/dolodust and 0.4 L wetting agent per m3) supplemented with fertilizers [1.3 kg of base 15–10–20 (NPK) + trace elements and Nutricote 70 day (slow release) 16–10–10 (NPK) per m3]. Four plants per genotype were planted separately in each of the two soil types, giving a total number of 56 individual plants. The plants were grown in a glasshouse at a photoperiod of 17 h:7 h, light:dark, a minimum light intensity of 200 μmol photons m–2 s–1 and temperatures between 25 and 15 °C. Soil moisture was maintained by regularly watering the pots with tap water and pots were rotated twice a week. From 14 d of growth, fluorescence measurements were recorded every 3–4 d (the dynamic data are presented in Fig. 4A) until flag leaf stage (BBCH 39). At maturity, plants were left to dry before collection of ears for threshing to determine grain yield.
Table 1.
List of barley genotypes characterized for Mn deficiency tolerance in pot and field trial experiments
| Barley genotype | Row type | Origin | Soil type of adaptation | Soil parent material | Soil pH | |
|---|---|---|---|---|---|---|
| Modern elites |
KWS Irina | 2 | ||||
| RGT Planet | 2 | |||||
| Graminor Rødhette | 6 | |||||
| Bere landraces | Bere Unst Shetland | 6 | Shetland | Magnesian gleys rich in organic matter | Drifts derived from ultra- basic igneous rocks | 6.2 |
| Bere North Uist | 6 | Uist | Brown calcareous soils | Shelly sands | 7.2 | |
| Bere North Ronaldsay | 6 | Orkney | Calcareous gleys | Shelly sands | 7.3 | |
| Bere West Orkney | 6 | Orkney | Saline gleys | Drifts derived from sandstones | 4.8 |
Row type and genotype origin (Supplementary Data Table S1) including the corresponding soil characteristics of the geographical region are given.
Soil data are retrieved from United Kingdom Soil Observatory (UKSO) and Scotland’s Soils websites.
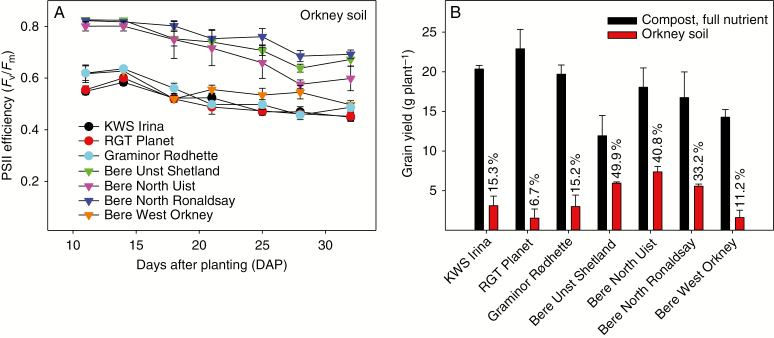
Fig. 4.
Pot experiments performed under semi-controlled glasshouse conditions. (A) Three modern elite barley genotypes (circles) and four Bere barley landraces (triangles, originating from the Western Isles, Shetland and Orkney Islands) were cultivated in Orkney soil (high pH sandy soil) or in nutrient-replete compost. PSII efficiency (Fv/Fm) was recorded until the flag leaf stage. (B) Grain yield (g per plant). The values are means ± s.e. (n = 4).
Field trials
Field experiments were undertaken during the growing season 2017 at Dundee, Scotland (latitude, longitude: 56.484303, –3.118515) and at Burray, Orkney Islands, Scotland (latitude, longitude: 58.848128, –2.915237). In Orkney, the soil was a brown calcareous soil with a textural class of sandy loam, composed of 77 % sand, 11 % silt, 12 % clay, 7.2 % organic matter and a pH of 7.8 (measured in water). Soil-available Mn and Fe, and Cu and Zn (EDTA) were 1.2, 71.1, 10.1 and 1.8 mg L–1, respectively. At the Dundee site, the soil was a Brown Earth with a sandy silt loam texture and a slightly acidic pH of 5.9.
The same seven genotypes as explored in the pot experiment (Table 1) were planted in a randomized complete block design (Alpha design) with four replicates per genotype (28 plots in total). Plot size was 2.87 m2 for the Dundee site and 2.31 m2 for the Orkney site, with a target plant population of 300 plants m–2. Raxil Star was applied as a seed dressing (5 mL kg–1 seed). Trial maintenance included fertilization corresponding to 66 kg N ha–1 total (split 2:1 at planting and later top dressing) for the Dundee site and 47 kg N ha–1 (at planting) for the Orkney site. Chemical pest, fungicide and weed control was applied according to normal practice. The number of days until ear emergence was recorded and, in Orkney, the number of tillers that carried spikes was counted in the middle 25 cm length of the middle two rows of each plot. At maturity, grains were harvested using a small plot combine. At the Orkney site, plants were initially hand cut from each plot and gathered into sheaves, and subsequently combined for the Bere landraces, but for the modern elite genotypes the ears were few and mostly empty, and were therefore threshed using a stationary laboratory thresher (Haldrup LT-15, Haldrup GmbH, Ilshofen, Germany). Grains were dried at 30 °C for 24 h. Thousand grain weight (TGW) was determined using a Marvin digital seed analyser (GTA sensorik GmbH, Germany), and the nitrogen and moisture contents were recorded using a grain analyser (Infratec 1241 grain analyser, Foss Analytics, Denmark).
Leaf and thylakoid analyses
Chlorophyll a fluorescence measurements
Chlorophyll a fluorescence was recorded with a portable Plant Efficiency Analyzer (HANDY-PEA, Hansatech Instruments Limited, King’s Lynn, UK) or Imaging PAM (MAXI measuring head, Walz, Germany). The YFDLs were dark adapted for 25 min before measurements, and PSII photochemical efficiency was calculated as the ratio of Fv to Fm using the PEA Plus software (version 1.10) or visualized using the Imaging-Win software (version 2.41a). Measurement of Chl a fluorescence (Fv/Fm values) has been demonstrated to be a powerful tool to reveal the Mn status of the plant and consequently the progression of Mn deficiency (Schmidt et al., 2013; Leplat et al., 2016).
Leaf digestion and analysis of total element concentrations
The YFDLs harvested from each plant (hydroponics and pot experiment) or from 3–5 plants per plot (field experiments) were dried in paper bags in an oven at 60–70 °C for approx. 72 h. The dried leaves were digested in ultrapure acids (70 % HNO3 and 30 % H2O2) at 240 °C and 200 bars for 15 min using a pressurized microwave digestion system (Ultrawave, Milestone Srl, Sorisole, Italy) according to Hansen et al. (2013). Certified reference material [apple (Malus domestica Borkh.) leaf, NIST 1515, National Institute of Standards and Technology] was included in each digestion batch to evaluate data quality (the accuracy and precision of the measurements). Following digestion, leaf element concentrations were determined using ICP-OES (Agilent 5100, Agilent Technologies, USA).
Thylakoid isolation and analysis of Mn binding in PSII complexes by SEC-ICP-MS
The YFDLs were used for thylakoid isolation as described previously (Teicher and Scheller, 1998). Leaves were disrupted in homogenization buffer [0.4 m sucrose, 10 mm NaCl, 5 mm MgCl2, 20 mm Tricine (pH 7.9), 10 mm l-ascorbate and 10 mm NaF] in a laboratory blender (Waring Laboratory LB20E, Connecticut, USA). The homogenate was filtered through a double layer of Miracloth (pore size 22–25 mm), and centrifuged for 10 min at 6000 g. The supernatant was discarded, and the pellet was resuspended in washing buffer [5 mm Tricine (pH 7.9), 10 mm NaF]. Subsequently, the washed thylakoids were pelleted by centrifugation for 10 min at 11 200 g, and finally re-suspended in a storage buffer [0.4 m sucrose, 10 mm NaCl, 5 mm MgCl2, 20 mm Tricine (pH 7.9), 10 mm NaF and 20 % glycerol]. The samples were immediately snap-frozen in liquid nitrogen and stored at –80 °C. The protein concentration in each sample was determined using the Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA) according to the protocol of the manufacturer. For size-exclusion chromatography (SEC), the thylakoids were solubilized using n-dodecyl α-d-maltoside (α-DM) as described in Schmidt et al. (2015), and subsequently passed through a 0.45 μm nylon membrane filter (Q-max RR syringe filters; Frisenette, Denmark) and 50 μg of total protein were applied to a size-exclusion column (BioBasic SEC 1000; Thermo Fisher Scientific) using an inert HPLC system (Ultimate® 3000; Dionex, Thermo Fisher Scientific). The metal binding in fractionated photosynthetic complexes was analysed by ICP-mass spectrometry (MS) (Agilent 8800 ICP-QQQ-MS) as previously described (Schmidt et al., 2015).
Data analysis
All data, the mean of three to four replicates and genotypic differences were analysed with one-way or two-way analysis of variance (ANOVA) followed by least significant difference (l.s.d.) test, and P-values <0.05 were considered significant. Data were tested for normality and skewness. Mean values (X) are listed with their associated s.e values (X ± s.d./√n). All statistical analyses were performed using SAS (SAS Institute; version 9.4).
RESULTS
Very little is known about the genetic variation within Northern European landraces, and much of the published research on adaptation has focused on landraces and wild relatives from the Fertile Crescent (Morrell and Clegg, 2007; Dawson et al., 2015; Russell et al., 2016; Pankin and von Korff, 2017). Although these studies have enhanced our understanding of the dynamics of genetic diversity, the impact on contemporary breeding has still to be realized. In this study, a small collection of locally adapted ‘heritage’ accessions from the UK and Scandinavia, most of which have been grown for hundreds of years, surviving both changes in climate and agricultural practice, have been sourced and genotyped with >6000 mapped SNPs (Comadran et al., 2012) (Supplementary Data Table S1).
Genotypic variation in ancient barley landraces according to geographical zones
A simple matching Neighbor–Joining tree (Fig. 1A; for an enlarged version, see Supplementary Data Fig. S1) clearly distinguishes two-row and six-row cultivars and landraces, but more importantly highlights the distinctiveness and highly geographical clustering of the Scottish Island six-row types, known collectively as Bere barley. Each individual is colour coded based on similarity to one another, clearly showing genotypic geographically distinct island patterns (Fig. 1B). Three distinct groups can be identified based on the origin of the individuals evolved on the major island groups of Scotland, being the Western Isles, Orkney Islands and Shetland Islands (Fig. 1B). The colour groupings illustrate the differences and uniqueness amongst island groups and at the same time indicate similarities of individuals within an island (Fig. 1B). Interestingly, the diversity within the Bere six-row collection is less than the small number of six-row types surveyed from Scandinavian countries and the two-row accessions, with 53.6 % of SNPs polymorphic compared with 67.4 and 87.4 %, respectively.
Bere landraces retain superior micronutrient efficiency traits
To exploit the adaptive traits to micronutrient limitation, resulting from a long history of cultivation in adverse environments, a sub-population of 24 contrasting landraces was further characterized in hydroponics under Mn-replete or Mn-deficient conditions. The successful induction of Mn deficiency was verified by leaf Mn concentrations below 11 μg Mn g–1 d. wt (Fig. 2A), while plants cultivated under Mn-replete conditions had leaf Mn concentrations well above the critical Mn concentration level, ranging between 39 and 161 μg Mn g–1 d. wt (Reuter et al., 1997). The plants did not suffer from any other nutrient deficiencies (Supplementary Data Table S2).
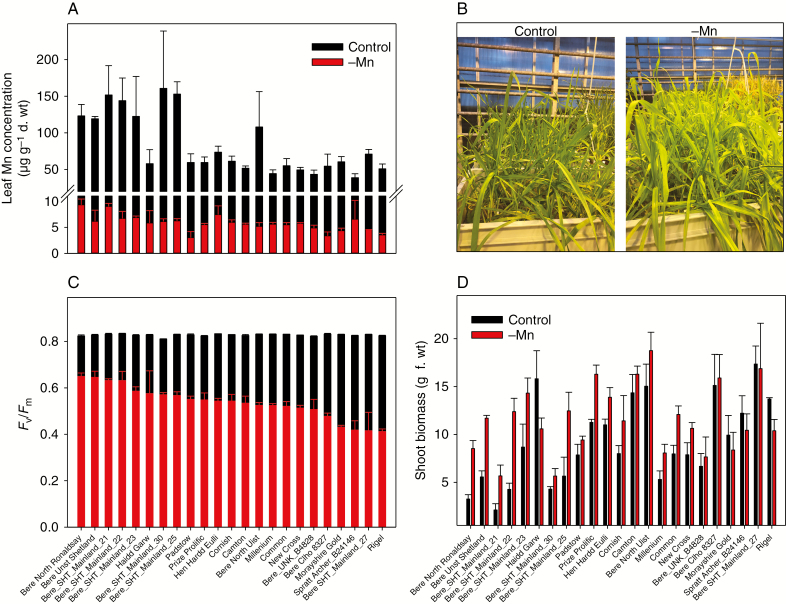
Fig. 2.
Tolerance to Mn deficiency in barley landraces in hydroponic growth conditions. Barley landraces were grown in hydroponics under Mn-replete (Control) or Mn-deficient conditions (–Mn). (A) Leaf Mn concentration (μg g–1 d. wt) in the youngest fully developed leaf (YFDL). (B) Appearance and vigour of control plants (left photo; full nutrient) and Mn-deficient plants (right photo) at 30 d after transplantation (DAT) to hydroponics. (C) PSII efficiency (Fv/Fm) in the YFDL of plants exposed to control or Mn deficiency conditions. (D) Shoot biomass (g f. wt). In (A), (C) and (D), the values are means± s.e. (n = 3).
At harvest, 30 days after planting (DAP), clear visual differences between leaves from the two treatments were observed (Fig. 2B). When cultivated under Mn-replete conditions, all plants appeared healthy with dark green leaves and Fv/Fm values above the theoretical maximum of 0.8, indicating optimal PSII efficiency and photosynthesis (Fig. 2C) (Stirbet and Govindjee, 2011). In contrast, the Mn-deficient plants revealed floppy and pale green leaves, in agreement with the well-known characteristics of interveinal chlorosis symptoms in Mn-deficient plants (Hannam and Ohki, 1988; Schmidt et al., 2016a). However, comparing the shoot growth of Mn-deficient plants with that of control plants (Fig. 2D), it was rather surprising that both leaf size and shoot biomass were increased for Mn-deficient plants as compared with plants grown under Mn-replete conditions; in fact, 75 % of the landraces had greater root and shoot biomass under Mn-limiting conditions as compared with the control.
The barley landraces revealed great diversity in Mn efficiency, as indicated by the large span in Fv/Fm values, ranging from 0.41 to 0.65 (Fig. 2C) under Mn-deficient conditions. This is, to our knowledge, the first documentation showing such large variation in Mn efficiency within genotypes adapted to local environments (Ceccarelli, 1994; Hebbern et al., 2005; Dwivedi et al., 2016). In addition to this, our data show that the most Mn-efficient Bere landraces (landraces displaying the greatest Fv/Fm values in Fig. 2C) were also found to accumulate up to 4.1 times more Mn in the leaf tissue when grown under Mn-replete conditions compared with the Mn-inefficient two-row landraces (Fig. 2A). Furthermore, the Mn-efficient Bere landraces revealed 3.7 and 2.8 times greater Zn and Cu concentrations in the leaf tissue, respectively, compared with other UK landraces (Supplementary Data Table S2). This is remarkable considering that all 24 landraces were co-cultivated under the same conditions, thereby having access to the same amount and composition of nutrients under Mn-deficient and Mn-replete conditions.
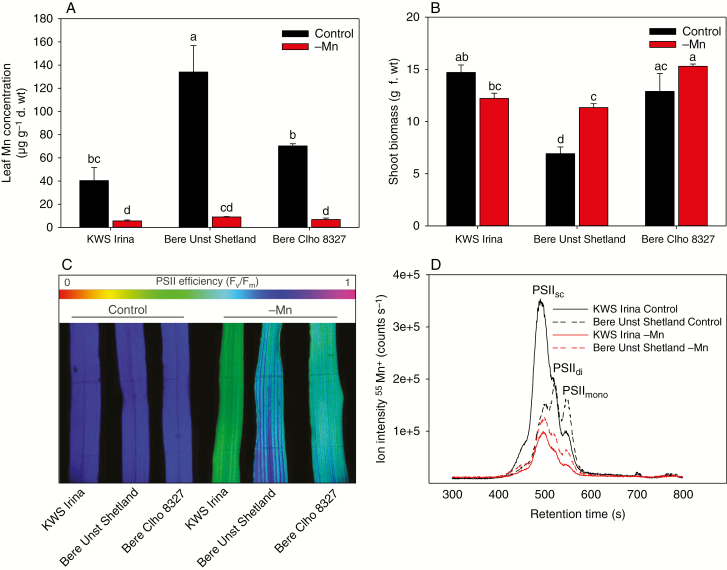
In a subsequent hydroponics experiment, two Bere landraces, which contrasted in terms of Mn efficiency (Bere Unst Shetland and Bere Clho 8327, cf. Fig. 2C), were compared against the modern elite genotype KWS Irina (Fig. 3). Under control (Mn-replete) conditions, Bere Unst Shetland had elevated leaf Mn concentrations of >100 μg Mn g–1 d. wt (Fig. 3A) and further allocated up to 2.5 times more Zn and Cu in the leaf tissue compared with the modern elite KWS Irina (Supplementary Data Table S3). The recorded biomass data were consistent with the observations described above, with the Bere landraces having larger shoots under Mn deficiency (Fig. 3B in accordance with Fig. 2D) compared with the control. In contrast, a more typical decrease in biomass under Mn deficiency was observed for the modern elite KWS Irina (Fig. 3B). Intact leaves were investigated using imaging Chl a fluorescence analysis (Fig. 3C). Under control conditions, the Fv/Fm values were >0.8 for all genotypes. However, under Mn-deficient conditions, distinct differences in PSII functionality appeared, demonstrating much better Mn efficiency in Bere Unst Shetland compared with Bere Clho 8327, but especially compared with KWS Irina (Fig. 3C). Furthermore, a highly specialized allocation of Mn was observed for the Mn-efficient genotype Bere Unst Shetland, as revealed by a prioritized clustering of functional PSII complexes closest to the veins and mid-rib vein of the leaf (Fig. 3C). This is in close agreement with the observable interveinal chlorosis in leaves of plants experiencing severe Mn deficiency. This observation was much more prominent in the Bere landraces compared with the elite KWS Irina. To examine differences in PSII functionality further, Mn loading in isolated and fractionated PSII super- and subcomplexes was investigated according to Schmidt et al. (2015) (Fig. 3D). Under control conditions, more Mn was bound in PSII supercomplexes of the modern elite KWS Irina, compared with Bere Unst Shetland which had a more even distribution of Mn binding between PSII supercomplexes, PSII dimers and PSII momomers (Fig. 3D). Under Mn-deficient conditions, Mn incorporation into PSII was severely reduced for both genotypes, but was less pronounced for Bere Unst Shetland compared with the control. Under Mn-deficient conditions, the macro-organization of PSII complexes was similar for both genotypes (Fig. 3D).
Fig. 3.
Comparison of landrace and modern elite genotypes. A modern elite barley genotype (cv. KWS Irina) and two barley landraces contrasting in terms of Mn efficiency (Bere Unst Shetland and Bere Clho 8327; Fig. 2A, C) were cultivated under Mn-replete or Mn-deficient conditions in hydroponics. (A) Leaf Mn concentration (μg g–1 d. wt) in the youngest fully developed leaf. (B) Shoot biomass (g f. wt). (C) PSII efficiency (Fv/Fm) for the colour scale indicates the Fv/Fm values. (D) Manganese size-exclusion chromatograms of thylakoids isolated from KWS Irina (solid lines) and Bere Unst Shetland (dashed lines) grown under control (black lines) or Mn-deficient (red lines) conditions (representative chromatograms are shown out of three independent replicates per genotype/treatment). SC, supercomplexes; di, dimers; mono, monomers. In (A) and (B), the values are means ± s.e. (n = 3). Bars with the same letter are not significantly different (P ≥ 0.05).
Mn efficiency traits have been lost from modern elite varieties
Based on the data obtained from hydroponics (Fig. 2), four Bere landraces (ranking from being Mn efficient to Mn inefficient, all six-row types) were selected together with two modern elite genotypes commonly grown in Northern Europe (KWS Irina and RGT Planet, both two-row types) and a six-row modern elite variety Graminor Rødhette (Table 1). The seven accessions were genotyped, demonstrating that the modern elite genotypes were genetically very distant from the Bere landraces (Fig. 1A).
Plants of the seven genotypes were grown to maturity under semi-controlled glasshouse conditions either in nutrient-replete compost (control conditions) or in soil from Orkney, which has a high pH and limited plant availability of Mn. Already at 11 DAP, for plants grown in Orkney soil, Chl a fluorescence measurements enabled separation of the genotypes into two distinct groups (Fig. 4A). Bere Unst Shetland, Bere North Ronaldsay and Bere North Uist were able to retain maximal PSII efficiency (Fv/Fm values ≥0.80) up to 14 DAP, then started to decline, reaching Fv/Fm values of 0.60–0.69 at the flag leaf stage (Fig. 4A), but without displaying any pronounced differences in biomass growth compared with control plants. By comparison, the Fv/Fm values of the elite genotypes were reduced to between 0.45 and 0.49 (Fig. 4A), corresponding to severe Mn deficiency (Schmidt et al., 2016b), and plant biomass development was clearly compromised.
Leaf Mn concentrations were <6.2 μg Mn g–1 d. wt for the modern elite varieties and the Bere West Orkney, but >13 μg Mn g–1 d. wt for the three Mn-efficient Bere landraces (Supplementary Data Table S4). The Fv/Fm values of plants grown in compost (Mn sufficient) were >0.82 for all genotypes throughout the experimental period (data not shown), and leaf Mn concentrations ranged between 45 and 170 μg Mn g–1 d. wt. The Mn-efficient Bere barley landraces accumulated up to 2.8 and 2.4 times more Zn and Cu, respectively, in the leaf tissue, compared with the modern elite genotypes (Supplementary Data Table S4). Grain yields were severely reduced in the elite genotypes by 85–93 %, whereas the yield of landraces adapted to high pH soils was limited by 50–67 % (Fig. 4B) when grown in low Mn-available soil compared with compost soil, and TGWs were unaffected (Supplementary Data Table S5).
The Mn-efficient Bere landraces are superior to elite varieties in alkaline marginal agricultural systems
To test the robustness and stability of the pronounced Mn efficiency trait further, the seven genotypes evaluated in the pot experiment were tested under naturally occurring field conditions in Orkney (soil with limited Mn availability) and Dundee (nutrient-replete soil). The observed existence of differential Mn efficiency among the genotypes was found to be even more dramatic in the field (Fig. 5) than was observed in hydroponics (Figs 2 and 3) and pot experiments (Fig. 4).
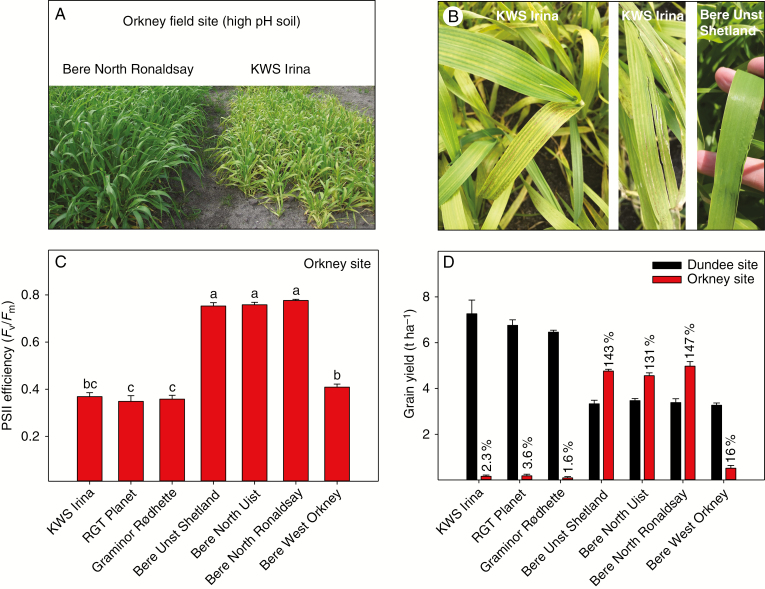
Fig. 5.
Field trials conducted in Orkney (calcareous sandy soil, pH 7.8) or mainland Dundee (Mn-replete site). (A) Visual appearance of the Bere barley landrace (Bere North Ronaldsay, left plot) and the modern elite genotype (KWS Irina, right plot) growing in Orkney. (B) Severe Mn deficiency symptoms of interveinal chlorosis (younger leaves) and necrotic aligned interveinal spots (older leaves) in the modern elite genotype KWS Irina (left photo), together with wind-damaged leaves (centre photo). An unaffected leaf of Bere Unst Shetland is shown to the right. (C) PSII efficiency (Fv/Fm) of the youngest fully developed leaves of Orkney-grown plants. Values are means ± s.e. (n = 4). Bars with the same letter are not significantly different (P ≥ 0.05). (D) Grain yields obtained in the Dundee (black bars) and Orkney (red bars) field sites. The values are means ± s.e (n = 3–4).
In Orkney, at early growth stages (BBCH 20 and onwards), remarkable visual differences in growth, biomass and overall plant vigour became apparent when comparing the Mn-efficient Bere landraces and the modern elite genotypes in the field (Fig. 5A). The Mn-efficient genotypes were thriving, showing no signs of Mn deficiency symptoms (Fig. 5B, right photo), whereas the modern elite genotypes displayed severe Mn deficiency symptoms (Fig. 5B, left and centre photos). These symptoms included floppy and soft leaves easily damaged by high winds (Fig. 5B, centre photo), severe chlorosis in the youngest leaves and multiple necrotic interveinal spots in the older leaves (Fig. 5B, left photo), along with impeded plant growth resulting in delayed growth stages (Fig. 5A) and poor competition against weeds. The dramatic differences between the varieties were unchanged as the season progressed. This was demonstrated by Chl a fluorescence measurements at growth stage BBCH 33–37, showing Fv/Fm values <0.37 in the three modern elite varieties, while the three Mn-efficient Beres maintained Fv/Fm values above 0.75 (Fig. 5C).
In the modern elite genotypes, leaf Mn concentrations reached only 4 μg Mn g–1 d. wt [far below the critical Mn threshold (Reuter et al., 1997)], whereas the Mn-efficient Bere landraces translocated 5- to 6-fold more Mn to the shoot, with sufficient concentrations ranging between 19 and 23 μg Mn g–1 d. wt (Supplementary Data Table S4). The Mn-efficient Bere landraces further revealed up to 1.7 and 2.0 times greater leaf Zn and Cu concentrations, respectively, compared with the modern elite genotypes (Supplementary Data Table S4). The plants did not suffer from other nutrient deficiencies (Supplementary Data Table S4). The ripening of the modern elite varieties was considerably delayed, so that they were harvested 1 month later than the Bere landraces. Even though the elite genotypes utilized photosynthetic energy to set spikes (Supplementary Data Table S5), the majority of the harvestable ears were empty, comprising mostly husk, as demonstrated by the small TGW (Supplementary Data Table S5), indicating unsuccessful grain filling and resulting in an extremely poor grain yield of <0.2 t ha–1 (Fig. 5D). It is important to note that these grains were largely empty, without embryo and endosperm. In contrast, the Mn-efficient Bere landraces produced impressive grain yields ranging between 4.6 and 5.0 t ha–1, achieved without Mn fertilization (Fig. 5D).
In the corresponding Mn-replete site in Dundee, growth and plant development were successful for all genotypes, having leaf Mn concentrations ranging between 15 and 25 μg Mn g–1 d. wt (Supplementary Data Table S4), and optimal concentrations of all other analysed essential elements (Supplementary Data Table S4). Notably, the Mn-efficient Bere landraces accumulated up to 2.6 and 2.8 times more Zn and Cu in the leaf tissue, respectively, compared with the modern elite genotypes. The modern elite genotypes revealed superior brackling and lodging resistance, whereas lodging was a serious issue for the taller Bere landraces. Even more importantly, the modern elite genotypes generated excellent grain yields (Fig. 5D) up to 7.2 t ha–1, proving their superior growth and high-yielding properties when cultivated under optimal nutrient regimes. In contrast, the Bere landraces achieved grain yields from 3.3 to 3.5 t ha–1 (Fig. 5D), which was less than the corresponding yields obtained at the Orkney field site.
Genotypic variation is associated with wider micronutrient efficiency
The Mn-efficient Bere landraces demonstrated exceptional Mn accumulation in the leaf tissue, across all environmental conditions, from hydroponics, through controlled soil pot experiments to the field. Simultaneously, the Mn-efficient Bere landraces accumulated 2.4 to 2.8 times greater Zn and Cu leaf concentrations compared with the modern elite genotypes (Supplementary Data Tables S2–S4). This observation suggests that the landraces may retain traits associated with wider micronutrient efficiencies. However, Fe seems to constitute an important exception as there are no indications that the Mn efficiency trait is associated with increased Fe uptake. In fact, when grown under Mn-deficient conditions in Orkney, leaf Fe concentrations ranged between 49 and 60 μg g–1 d. wt and between 70 and 80 μg g–1 d. wt for the Mn-efficient Bere landraces and the modern elite genotypes, respectively.
DISCUSSION
Adaptive traits to Mn deficiency have developed independently from biogeographical zones
During its domestication and subsequent cultivation, barley has adapted to a wide range of environments, and here we have demonstrated the unique ability of Bere barley landraces to generate robust grain yields under severe Mn-deficient conditions, while modern elite varieties completely failed to set seeds. Although it is well established that varietal differences exist, this is a unique example of the local adaptation of landraces to marginal soils, and is an exemplar of how we may be able to take advantage of these landraces to target genes for specific environmentally limiting conditions. It was particularly striking that the sub-group of Bere barley landraces demonstrating superior tolerance to Mn deficiency were those that have become adapted to alkaline, sandy and high organic matter soils (Table 1), which inherently reduce plant Mn availability (Schmidt et al., 2016a). The Bere West Orkney genotype, which showed no superior Mn efficiency compared with the elite genotypes, has adapted to more acidic soils where Mn deficiency is not likely to be critical. Although the Bere barley landraces were found to cluster genetically according to their islands of origin (Fig. 1B), it appears that Bere barley landraces from distinct biogeographical zones have developed different but equivalent strategies to overcome Mn-deficient conditions (Figs 4 and 5). Under such strong and geographically varied selection pressure, it seems likely that the adaptation to Mn deficiency has developed independently.
Mn deficiency tolerance is a complex trait
The Mn-efficient Bere landraces had grain yields ranging between 4.6 and 5.0 t ha–1 in the field which was achieved without any supplementation of Mn. This leaves the plants with the only option to mobilize Mn in the root rhizosphere and to increase Mn uptake, Mn translocation and/or internal Mn use efficiency in PSII. Either way, the large effects of Mn deficiency observed for the non-adapted modern elite genotypes, but absent in the adapted Bere landraces, are truly exceptional compared with previous genotypic studies in barley (Hebbern et al., 2005; Husted et al., 2009; Schmidt et al., 2015, 2016b; Leplat et al., 2016). However, although the Bere landraces demonstrated an impressive adaptation in the ability to maintain photosynthesis under Mn-deficient conditions, there was a yield penalty when grown under optimal conditions where a range of other undesirable traits come into play. The Bere barley landraces accumulated Mn under controlled Mn-replete conditions (Figs 2A and 3A; Supplementary Data Table S4), which together with their decreased biomass (Figs 2D and 3B) implies an impaired ability to regulate Mn uptake and homeostasis under these conditions.
The physiological mechanisms underlying Mn efficiency in plants are still not fully understood. Unique traits in the landraces may involve mechanisms yet to be elucidated or polymorphisms in single genes controlling processes that are already known. Even so, our results suggest that the landrace phenotype is a complex and polygenic trait, and may include a combination of mechanisms ranging from Mn mobilization in the rhizosphere, to uptake, translocation as well as Mn delivery to dependent processes in photosynthesis. These physiological processes are discussed in the following section based on the results obtained in this study.
Responsive root traits confer tolerance to environmental changes in Mn levels
Changing the chemistry of the rhizosphere to mobilize plant-available Mn2+ via root exudates, proton release (Rengel and Marschner, 2005; George et al., 2014; Rengel, 2015; Liu et al., 2017) or promoting soil microflora activity (Marschner et al., 2003) may contribute to enhance Mn acquisition under field conditions where Mn availability is limited (Fig. 5). Previous studies with traditional landraces have demonstrated upregulation of root phytase activity under Mn deficiency (George et al., 2014), enabling mobilization of Mn from the phytate-bound pool in soils. However, considering the enormous genotypic variations in tolerance to Mn deficiency observed in the hydroponic experiments (Figs 2 and 3), root traits are unlikely to be the singular responsive mechanism to Mn deficiency. More likely to be involved is the primary uptake of Mn, mediated by high and low affinity root transport proteins. So far, the only known Mn transporters in barley roots are the Natural Resistance Associated Macrophage Protein NRAMP5 (Sasaki et al., 2012; Wu et al., 2016) and the Iron-Regulated Transporter IRT1 (Pedas et al., 2008; Long et al., 2018). The expression of Nramp5 is unaffected by Mn deficiency, but is slightly upregulated under Fe deficiency (Wu et al., 2016); however, its regulation at the protein level remains unknown. Iron-Regulated Transporter 1 is a broad-spectrum metal transporter that also transports Fe, Zn, Co and Cd, and has previously been associated with genotypic differences in Mn uptake (Pedas et al., 2005, 2008). In this context, both Mn and Fe deficiency have been shown to induce an upregulation of HvIRT1 in genotypes with contrasting Mn efficiency, with the greatest expression levels found in the Mn-efficient genotype (Pedas et al., 2008). In this context, recent research has demonstrated that IRT1 in Arabidopsis acts like a sensor for soil concentration of metals (Dubeaux et al., 2018), by a sophisticated iron-dependent transcriptional network of regulation, controlling IRT1 to ensure optimal metal (including Mn and Zn) absorption by roots. It could be speculated that an adapted (IRT1) transport system exists in Bere barley landraces, where a reduced Fe sensing and/or xylem loading of Fe will increase Mn uptake. This hypothesis is further supported by the homeostatic interplay between Mn and Fe uptake observed in the Mn-efficient Bere landraces when grown in an alkaline calcareous soil (Supplementary Data Table S4). Studies by Eroglu et al. (2016) have demonstrated Fe deficiency-induced chlorosis phenotypes related to a disturbed Mn homeostasis in arabidopsis lacking the vacuolar Mn transporter MTP8, indicating that high Mn stress induces Fe deficiency. Given the distinct biogeographical adaptive traits of the Bere barley landraces to high pH soil conditions, where both Mn and Fe availabilities are limited due to their redox sensitivity, such an Fe sensing and uptake strategy for micronutrient in general would be advantageous.
Regulation of Mn translocation and delivery to photosystem II
Manganese uptake by the roots and the translocation of Mn to demanding leaf tissues solely relies on xylem transport, while remobilization is virtually absent due to the phloem immobility of Mn in plants. In graminaceous plant species, upon nutrient uptake by the roots and the subsequent translocation to the shoots, nutrients are preferentially distributed via a well-organized system in the nodes (Shao et al., 2017). Here a junction of vasculatures connects the leaf and the stem, and within nodes, the intervascular transfer of nutrients is mediated by nutrient-specific transporters (Yamaji and Ma, 2017). In rice, a node-switch transport protein, NRAMP3, functions in preferential Mn distribution to active developing tissues under Mn-limiting conditions (Yamaji et al., 2013). However, the mechanisms controlling the intervascular transport and allocation of Mn in leaves, including variations at the genotypic level, remain to be identified. Notably, our data show that the Mn-efficient Bere barley landrace has a distinct Mn allocation pattern along the veins of the leaf (Fig. 3C), which could represent an optimized strategy to ensure immediate access of Mn for PSII repair under Mn limitation. Furthermore, marked differences in Mn loading to PSII of chloroplasts was observed, not only under Mn-deficient conditions but also under control conditions. We have previously revealed differential Mn loading to PSII as a process contributing to Mn efficiency in barley (Schmidt et al., 2015). This increased internal utilization of Mn was further associated with increased photosynthetic protective non-photochemical quenching mechanisms and PSII stability, the latter mediated by the extrinsic proteins PsbP and PsbQ of PSII (Schmidt et al., 2016b). At the mechanistic level, the genotypic differences in Mn delivery to PSII supercomplexes observed between the Mn-efficient landrace and the modern elite genotype under control conditions may reflect adjustments to Mn toxicity given the leaf Mn accumulation of 140 μg Mn g–1 d. wt in the Bere Unst Shetland landrace compared with 40 μg g–1 d. wt in the elite genotype KWS Irina. The greater Mn loading to all PSII complexes observed for Bere Unst Shetland under Mn-deficient conditions compared with KWS Irina, may involve barley homologues of the recently identified arabidopsis chloroplast high affinity Mn import proteins CMT1 (Eisenhut et al., 2018) and PAM71 (Schneider et al., 2016), which could be present in the Mn-efficient Bere landraces, but lost from the elites. Indeed, an efficient and timely incorporation of Mn in PSII is crucial under low Mn regimes, but could also involve unidentified Mn chaperones to ensure adequate Mn supply to the thylakoid lumen (Schmidt et al., 2016a; Krieger-Liszkay and Thomine, 2018).
In conclusion, the presented results provide an illustrative case study for the use of traditional landraces as sources of genes and traits to improve agricultural sustainability, and comprise a strong argument to save and maintain extant populations of landraces. We identified a unique Mn-efficient phenotype in Bere landraces that can be used to cope with a timely issue of global significance. The Mn efficiency trait is likely to be polygenic and highly complex, and might include a range of key processes along the entire pathway from Mn mobilization in soil via root exudates, to ion transporter activity and regulation as well as homeostatic responses at the leaf level to regulate photosynthesis and other processes of basic metabolism. In future studies, phenotyping crosses between Mn-efficient landraces and Mn-inefficient modern elite lines could provide more insight into the genetic basis and help resolve the complexity of the trait. The potential areas of research to unravel the trait are summarized in Fig. 6. Studies into these avenues of research will provide additional information on the molecular basis for micronutrient efficiency and the underlying genetic control to develop novel genotypes matching site-specific soils and climatic conditions, and thereby unlock the production potential of marginal soils.
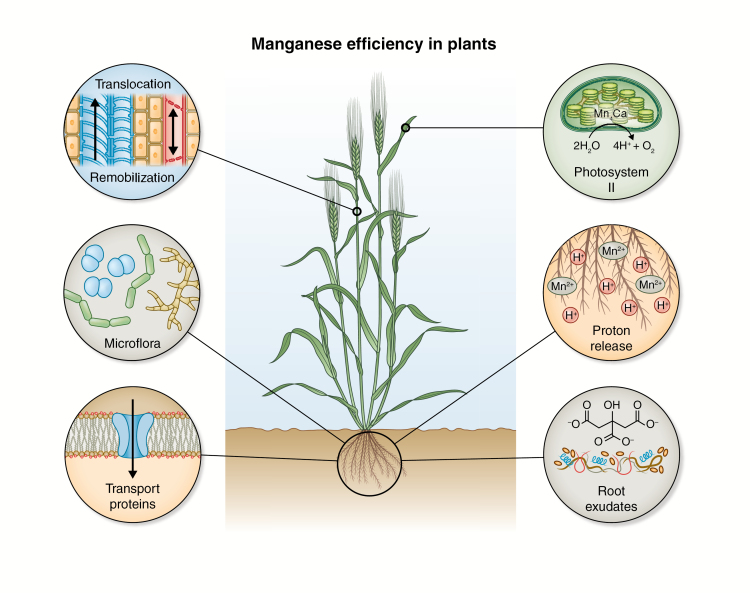
Fig. 6.
Conceptual model summarizing selected key processes controlling manganese (Mn) efficiency in plants. Proton release: increased proton-pumping activity releases H+ into the rhizosphere and promotes lowering of pH, which dissolves Mn(IV) oxides into plant-available Mn2+ (Mizuno et al., 2006). Root exudates: exudation of organic acids such as citrate, malate, oxalate and oxaloactetate to the rhizosphere changes soil Mn solubility through their ability to reduce Mn(IV) oxides by ligand exchange and complexing the released Mn2+ to increase plant availability (Gherardi and Rengel, 2004; Rengel and Marschner, 2005; Chen et al., 2015; Rengel, 2015; Liu et al., 2017). Under Mn deficiency, root phytase exudation can be upregulated in plants, to solubilize Mn2+ complexed with inositol phosphates (George et al., 2014). Microflora: rhizosphere micro-organisms play important roles in plant tolerance towards Mn deficiency. The rhizosphere is rich in bacteria and fungi, with the ability to change the redox state of Mn and thereby influence the plant availability of Mn (Kothari et al., 1991; Posta et al., 1994; Marschner et al., 2003). Transport proteins: a range of plasma membrane-bound proteins localized at the root surface (IRT1 and NRAMP1;5) (Pedas et al., 2008; Cailliatte et al., 2010; Sasaki et al., 2012; Wu et al., 2016) and endogenous transport proteins (VIT1, CAX2, ECA1;3, ZIP1, NRAMP2;3;4 and MTP8, 11) (Pedas et al., 2014; Alejandro et al., 2017; Shao et al., 2017) are regulated by the Mn status of plants and are involved in primary uptake of Mn2+. Translocation and remobilization: upon Mn uptake by the roots, Mn loading and unloading of xylem and phloem is mediated by Mn transport proteins (Yamaji et al., 2013). Furthermore, the chemical environment of the vascular tissue fluid (Álvarez-Fernández et al., 2014), and leaf transpiration (Hebbern et al., 2009) influence Mn mobility within plants. Photosystem II: at the leaf level, the chloroplast Mn transporters, CMT1 (Eisenhut et al., 2018) and PAM71 (Schneider et al., 2016), are involved in Mn2+ homeostasis and required for Mn delivery to PSII super- and subcomplexes to fulfil the catalytic function of Mn in water splitting in photosynthesis. The peripheral proteins PsbP and PsbQ of PSII protect the Mn cluster of PSII and are important for PSII stability and functionality (Schmidt et al., 2016a, b).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: details for accessions presented in Fig. 1. Table S2: leaf element concentrations of UK landraces grown in hydroponics. Table S3: leaf element concentrations of an elite genotype and Bere barley landraces grown in hydroponics. Table S4: leaf element concentration for plants grown in pot and field experiments. Table S5: phenotypic scores recorded for Orkney and mainland Dundee field trials. Figure S1: enlarged version of Fig. 1A.
ACKNOWLEDGEMENTS
This work was supported by Independent Research Fund Denmark – Technology and Production Sciences [grant no. DFF-5054-00042 to S.B.S.] and by the Scottish Government Rural and Environment Science and Analytical Services (RESAS). S.B.S, J.R., T.S.G. and S.H. designed the experiments. J.R. conducted the genetic analysis of Fig. 1 (with assistance from A.B. and P.E.H.). S.B.S carried out the hydroponic experiments in Figs 2 and 3, and pot experiments in Fig. 4 (with assistance from L.K.B.). S.B.S and P.M planned and performed the field trials in Fig. 5 (with assistance from J.W.). S.B.S and S.H. constructed the model in Fig. 6. S.B.S and J.R. (with significant input from T.S.G., and S.H.) analysed the data. S.B.S, T.S.G, J.R. and S.H. wrote the manuscript. All authors commented on the manuscript.
LITERATURE CITED
- Alejandro S, Cailliatte R, Alcon C, et al. 2017. Intracellular distribution of manganese by the trans-Golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. The Plant Cell 29: 3068–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Fernández A, Díaz-Benito P, Abadía A, López-Millán A-F, Abadía J. 2014. Metal species involved in long distance metal transport in plants. Frontiers in Plant Science 5: 105. doi: 10.3389/fpls.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. 2010. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. The Plant Cell 22: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli S. 1994. Specific adaptation and breeding for marginal conditions. Euphytica 77: 205–219. [Google Scholar]
- Chen Z, Sun L, Liu P, Liu G, Tian J, Liao H. 2015. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiology 167: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comadran J, Kilian B, Russell J, et al. 2012. Natural variation in a homolog of Antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nature Genetics 44: 1388–1392. [DOI] [PubMed] [Google Scholar]
- Dawson IK, Russell J, Powell W, Steffenson B, Thomas WTB, Waugh R. 2015. Barley: a translational model for adaptation to climate change. New Phytologist 206: 913–931. [DOI] [PubMed] [Google Scholar]
- Dubeaux G, Neveu J, Zelazny E, Vert G. 2018. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Molecular Cell 69: 953–964. [DOI] [PubMed] [Google Scholar]
- Dwivedi SL, Ceccarelli S, Blair MW, Upadhyaya HD, Are AK, Ortiz R. 2016. Landrace germplasm for improving yield and abiotic stress adaptation. Trends in Plant Science 21: 31–42. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Hoecker N, Schmidt SB, et al. 2018. The plastid envelope CHLOROPLAST MANGANESE TRANSPORTER1 is essential for manganese homeostasis in Arabidopsis. Molecular Plant 11: 955–969. [DOI] [PubMed] [Google Scholar]
- Eroglu S, Meier B, von Wirén N, Peiter E. 2016. The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiology 170: 1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. 2007. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Molecular Ecology Notes 7: 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Yokosho K, Yamaji N, et al. 2012. Acquisition of aluminium tolerance by modification of a single gene in barley. Nature Communications 3: 713. doi: 10.1038/ncomms1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, et al. 2012. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488: 535–539. [DOI] [PubMed] [Google Scholar]
- George TS, French AS, Brown LK, et al. 2014. Genotypic variation in the ability of landraces and commercial cereal varieties to avoid manganese deficiency in soils with limited manganese availability: is there a role for root-exuded phytases? Physiologia Plantarum 151: 243–256. [DOI] [PubMed] [Google Scholar]
- Gherardi MJ, Rengel Z. 2004. The effect of manganese supply on exudation of carboxylates by roots of lucerne (Medicago sativa). Plant and Soil 260: 271–282. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: http://palaeo-electronica.org/2001_2001/past/issue2001 _2001.htm. [Google Scholar]
- Hannam RJ, Ohki K. 1988. Detection of manganese deficiency and toxicity in plants. In: Graham RD, Hannam RJ, Uren NC, eds. Manganese in soils and plants. Dordrecht: Kluwer Academic Publishers, 243–259. [Google Scholar]
- Hansen TH, de Bang TC, Laursen KH, Pedas P, Husted S, Schjoerring JK. 2013. Multielement plant tissue analysis using ICP spectrometry. Methods in Molecular Biology 953: 121–141. [DOI] [PubMed] [Google Scholar]
- Hebbern CA, Pedas P, Schjoerring JK, Knudsen L, Husted S. 2005. Genotypic differences in manganese efficiency: field experiments with winter barley (Hordeum vulgare L.). Plant and Soil 272: 233–244. [Google Scholar]
- Hebbern CA, Laursen KH, Ladegaard AH, et al. 2009. Latent manganese deficiency increases transpiration in barley (Hordeum vulgare). Physiologia Plantarum 135: 307–316. [DOI] [PubMed] [Google Scholar]
- Husted S, Laursen KH, Hebbern CA, et al. 2009. Manganese deficiency leads to genotype-specific changes in fluorescence induction kinetics and state transitions. Plant Physiology 150: 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari SK, Marschner H, Römheld V. 1991. Effect of a vesicular–arbuscular mycorrhizal fungus and rhizosphere micro‐organisms on manganese reduction in the rhizosphere and manganese concentrations in maize (Zea mays L.). New Phytologist 117: 649–655. [Google Scholar]
- Krieger-Liszkay A, Thomine S. 2018. Importing manganese into the chloroplast: many membranes to cross. Molecular Plant 11: 1109–1111. [DOI] [PubMed] [Google Scholar]
- Leplat F, Pedas PR, Rasmussen SK, Husted S. 2016. Identification of manganese efficiency candidate genes in winter barley (Hordeum vulgare) using genome wide association mapping. BMC Genomics 17: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhou M, Delhaize E, Ryan PR. 2017. Altered expression of a malate-permeable anion channel, OsALMT4, disrupts mineral nutrition. Plant Physiology 175: 1745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LZ, Persson DP, Duan FY, et al. 2018. The iron-regulated transporter 1 plays an essential role in uptake, translocation and grain-loading of manganese, but not iron, in barley. New Phytologist 217: 1640–1653. [DOI] [PubMed] [Google Scholar]
- Marschner P, Fu Q, Rengel Z. 2003. Manganese availability and microbial populations in the rhizosphere of wheat genotypes differing in tolerance to Mn deficiency. Journal of Plant Nutrition and Soil Science 166: 712–718. [Google Scholar]
- Mizuno T, Hirano K, Hosono A, Kato S, Obata H. 2006. Continual pH lowering and manganese dioxide solubilization in the rhizosphere of the Mn‐ hyperaccumulator plant Chengiopanax sciadophylloides. Soil Science & Plant Nutrition 52: 726–733. [Google Scholar]
- Morrell PL, Clegg MT. 2007. Genetic evidence for a second domestication of barley (Hordeum vulgare) east of the Fertile Crescent. Proceedings of the National Academy of Sciences, USA 104: 3289–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M, Schnurbusch T, Hayes J, et al. 2014. Molecular basis of adaptation to high soil boron in wheat landraces and elite cultivars. Nature 514: 88–91. [DOI] [PubMed] [Google Scholar]
- Pankin A, von Korff M. 2017. Co-evolution of methods and thoughts in cereal domestication studies: a tale of barley (Hordeum vulgare). Current Opinion in Plant Biology 36: 15–21. [DOI] [PubMed] [Google Scholar]
- Pedas P, Hebbern CA, Schjoerring JK, Holm PE, Husted S. 2005. Differential capacity for high-affinity manganese uptake contributes to differences between barley genotypes in tolerance to low manganese availability. Plant Physiology 139: 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S. 2008. Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiology 148: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P, Schiller Stokholm M, Hegelund JN, Ladegard AH, Schjoerring JK, Husted S. 2014. Golgi localized barley MTP8 proteins facilitate Mn transport. PLoS One 9: e113759. doi: 10.1371/journal.pone.0113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posta K, Marschner H, Römheld V. 1994. Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. Mycorrhiza 5: 119–124. [Google Scholar]
- Rengel Z. 2015. Availability of Mn, Zn and Fe in the rhizosphere. Journal of Soil Science and Plant Nutrition 15: 397–409. [Google Scholar]
- Rengel Z, Marschner P. 2005. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytologist 168: 305–312. [DOI] [PubMed] [Google Scholar]
- Reuter DJ, Edwards DG, Wilhelm NS. 1997. Temperate and tropical crops. In: Reuter DJ, Robinson JB, eds, Plant analysis: an interpretation manual, 2nd edn Clayton, Australia: CSIRO Publishing, 81–279. [Google Scholar]
- Russell J, Mascher M, Dawson IK, et al. 2016. Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nature Genetics 48: 1024–1030. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Yokosho K, Ma JF. 2012. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. The Plant Cell 24: 2155–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SB, Pedas P, Laursen KH, Schjoerring JK, Husted S. 2013. Latent manganese deficiency in barley can be diagnosed and remediated on the basis of chlorophyll a fluorescence measurements. Plant and Soil 372: 417–429. [Google Scholar]
- Schmidt SB, Persson DP, Powikrowska M, et al. 2015. Metal binding in photosystem II super- and subcomplexes from barley thylakoids. Plant Physiology 168: 1490–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SB, Jensen PE, Husted S. 2016a Manganese deficiency in plants: the impact on photosystem II. Trends in Plant Science 21: 622–632. [DOI] [PubMed] [Google Scholar]
- Schmidt SB, Powikrowska M, Krogholm KS, et al. 2016. b Photosystem II functionality in barley responds dynamically to changes in leaf manganese status. Frontiers in Plant Science 7: 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Steinberger I, Herdean A, et al. 2016. The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. The Plant Cell 28: 892–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JF, Yamaji N, Shen RF, Ma JF. 2017. The key to Mn homeostasis in plants: regulation of Mn transporters. Trends in Plant Science 22: 215–224. [DOI] [PubMed] [Google Scholar]
- Stirbet A, Govindjee. 2011. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B 104: 236–257. [DOI] [PubMed] [Google Scholar]
- Teicher HB, Scheller HV. 1998. The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH. Plant Physiology 117: 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations 2015. Sustainable development goals – 17 goals to transform our world http://www.un.org/sustainabledevelopment/sustainable-development-goals/ (18 January 2018).
- Wu D, Yamaji N, Yamane M, Kashino-Fujii M, Sato K, Feng Ma J. 2016. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiology 172: 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF. 2017. Node-controlled allocation of mineral elements in Poaceae. Current Opinion in Plant Biology 39: 18–24. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF. 2013. A node-based switch for preferential distribution of manganese in rice. Nature Communications 4: 2442. doi: 10.1038/ncomms3442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.