Abstract
Background: Greater understanding of the roles of tumor necrosis factor-α, IL-1β, IL-10, and the IL-23/T-helper (Th) 17 and IL-12/Th1 pathways in immune dysregulation in moderate/severe hidradenitis suppurativa (HS) has helped in developing new regimens. We aim to review the use of different immunomodulatory therapies used to manage HS.
Methods: A comprehensive literature search was conducted on the PubMed and Clinicaltrials.gov databases from 1 January 1947 to 31 December 2018. Only clinical trials, case reports, case series and retrospective analyses published in the English language were included.
Results: Our search yielded 107 articles and 35 clinical trials, of which 15 are still ongoing. The tumor necrosis factor-α inhibitors adalimumab and infliximab were the most comprehensively studied agents. Published data from clinical trials support the efficacy of adalimumab, infliximab, anakinra, ustekinumab, bermekimab and apremilast but not etanercept and MEDI8968. Clinical trials for CJM112 have been completed, with results awaiting publication. Trials are underway for secukinumab, IFX-1, INCB054707 and bimekizumab. Biologics used in smaller cohorts include canakinumab, golimumab and rituximab. Most agents are well tolerated and demonstrate a good safety profile, with the most commonly reported adverse event being infections.
Discussion and conclusions: To date, adalimumab is the only biologic which has been approved by the United States Food and Drug Administration for HS. However, other agents also show promise, with further trials underway to evaluate their efficacy, tolerability and safety profiles. Different clinical measurement scores and endpoints used to make direct comparison difficult. Longitudinal surveillance and pooled registry data are paramount to evaluate the long-term safety profile and efficacy of therapy.
Keywords: Hidradenitis suppurativa, biologics, tumor necrosis factor, adalimumab, infliximab, secukinumab
Introduction
Hidradenitis suppurativa (HS), which has an estimated worldwide prevalence of 1%, is a chronic inflammatory follicular occlusive disease predominantly involving the intertriginous areas.1 Clinically, its manifestations vary from inflammatory nodules and abscesses to the formation of sinus tracts and scarring.2 It has a profound adverse impact on patients’ quality of life, and has been closely linked with physical and psychiatric co-morbidities including obesity, hypertension, dyslipidemia, diabetes mellitus, thyroid disorders, polycystic ovarian syndrome, arthropathies and depression.3
Pathophysiology of HS
Histopathological examination of early lesions in HS demonstrates terminal follicular hyperkeratosis, hyperplasia of the follicular epithelium and perifolliculitis. The occlusion of the terminal hair follicle results in dilation and cyst formation, followed by rupture of the hair follicle. The introduction of follicular contents to the surrounding dermis induces an inflammatory response and subsequent formation of abscess, sinus tracts, fibrosis and scars. This is worsened by biofilm formation and secondary infection.4,5
The inflammatory response in HS has in recent years been better characterized, although there are many components that remain to be elucidated. In particular, tumor necrosis factor (TNF)-α, IL-1β, IL-10, and the IL-23/T-helper (Th) 17 and IL-12/Th1 pathways play key roles in immune dysregulation in HS.6,7 In studies of HS skin, significantly increased frequencies of CD4 T cells expressing Th17-associated cytokines and TNF were found infiltrating HS skin.7 Treatment with TNF inhibitors was also related with a significant decrease in IL-17 expressing CD4 T cells in HS skin.7
Staging of HS and implications on therapy
HS has traditionally been staged according to the Hurley staging system, first proposed in 1989 (Table 1).8
Table 1.
Hurley staging of HS
| Stage I (mild) | Abscess formation, single or multiple, without sinus tracts and cauterization. |
| Stage II (moderate) | Recurrent abscesses with tract formation and cicatrization, single or multiple, widely separated lesions. |
| Stage III (severe) | Diffuse or near-diffuse involvement, or multiple interconnected tracts and abscesses across the entire area. |
Note: Data from Hurley.8
Abbreviation: HS, hidradenitis suppurativa.
The Hurley staging system remains useful for determining the severity of disease in individual patients but is limited in monitoring the dynamic characteristics of disease in clinical trials.9 Hence, alternative scoring systems have been developed to better evaluate the efficacy of the intervention, as shown in Table 2.
Table 2.
Scoring systems used in grading HS severity
| HiSCR99 | ≥50% reduction in inflammatory lesion count (abscesses and inflammatory nodules) AND No increase in abscesses or draining fistulas compared to baseline |
|||||
| HS-PGA11 | Clear | No inflammatory or non-inflammatory nodules | ||||
| Minimal | Only the presence of non-inflammatory nodules | |||||
| Mild | 1–4 inflammatory nodules OR 1 abscess or draining fistula AND no inflammatory nodules |
|||||
| Moderate | ≥5 inflammatory nodules OR 1 abscess or draining fistula AND ≥1 inflammatory nodules OR 2–5 abscesses or draining fistulas AND <10 inflammatory nodules |
|||||
| Severe | 2–5 abscesses or draining fistulas AND ≥10 inflammatory nodules | |||||
| Very Severe | >5 abscesses or draining fistulas | |||||
| mSS100 | A |
Number of regions affected (3 points per region): Right axilla Left axilla Right groin Left groin Right gluteal region Left gluteal region Other region |
||||
| B |
For each…: Nodule: 1 point Fistula: 6 points |
|||||
| C |
For each affected region, longest distance between two relevant lesions: <5 cm: 1 point 5–10 cm: 3 points >10 cm: 9 points |
|||||
| D |
For each affected region: If lesions not clearly separated by normal skin: 9 points |
|||||
| The mSS is the total of the subtotals of A to D. | ||||||
| HSSI101 | Points scored | No. of sites | BSA affected (%) | No. of lesions (erythematous, painful) | Drainage (dressing changes per working or leisure hour) | Pain (VAS) |
| 0 | 0 | 0 | 0 | 0 | 0–1 | |
| 1 | 1 | 1 | 1 | |||
| 2 | 2 | 2–3 | 2–3 | 1 | 2–4 | |
| 3 | 3 | 4–5 | 4–5 | >1 | 5–7 | |
| 4 | ≥4 | >5 | >5 | 8–10 | ||
| Sites: Left armpit, right armpit, left side of chest, right side of chest, left groin, right groin, perianal area, sacral area and perineal area. The HSSI is the total of all five assessed domains, with a minimum score of 0 and a maximum score of 19. | ||||||
| IHS4102 | A | Number of nodules | ||||
| B | Number of abscesses | |||||
| C | Number of draining tunnels | |||||
| The IHS4 is derived by the formula: A x 1+ B x 2+ C x 4 | ||||||
Abbreviations: HiSCR, hidradenitis suppurativa clinical response; HS-PGA, HS physician’s global assessment; mSS, modified sartorius score; HSSI, HS severity index; BSA, body surface area; IHS4, International HS Score System.
Conventional medical therapy, involving oral antibiotics and topical treatments, is suitable for treatment of mild to moderate HS. However, there are patients where HS remains resistant to conventional treatment. With the discovery of the key inflammatory mediators in HS, the role of biologics and other immunomodulatory therapies in the targeted treatment of moderate-to-severe HS has been closely studied. Of these, adalimumab remains the only Food and Drug Administration (FDA)-approved biologic for the treatment of HS.10
We present a review of all biologics and immunomodulatory therapies that have been reported in the treatment of HS.
Methods
A review of the literature was conducted by multiple PubMed searches using the keywords “hidradenitis suppurativa“ or ”acne inversa”; with publication date limits from 1 January 1947 to 31 December 2018. Retrieved references were critically appraised. The inclusion criteria were original articles, reports and letters in the English language reporting the treatment of HS with biologic or other immunomodulatory agents, either alone or in combination with conventional drugs or surgery. Articles which were judged to be irrelevant based on the title, abstract or full text, were excluded from the review.
A search of the website Clinicaltrials.gov for planned, in-progress, terminated and completed clinical trials with the terms “hidradenitis suppurativa“ and ”acne inversa” was also performed up to 31 December 2018.
Results
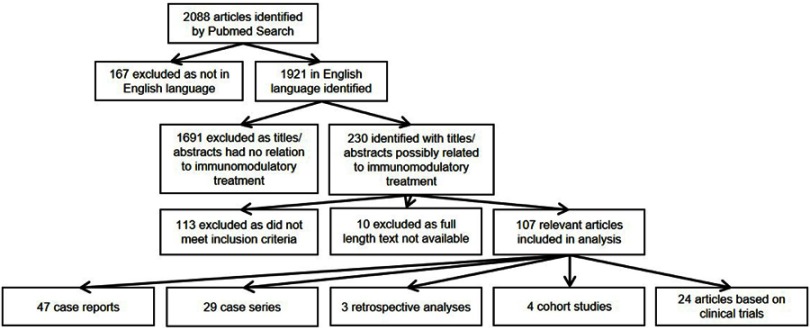
A total of 2,088 articles were retrieved by multiple PubMed searches conducted until 31 December 2018 using the keywords “hidradenitis suppurativa” or “acne inversa”. A total of 107 relevant articles were included in the analysis. A total of 47 case reports, 29 case series, 3 retrospective analyses, 4 cohort studies and 24 articles based on clinical trial data, were selected (Figure 1).
Figure 1.
Selection of articles identified by PubMed search.
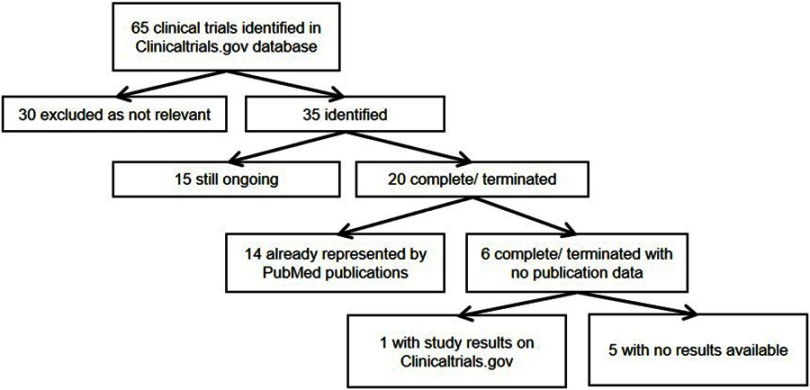
A total of 65 clinical trials were retrieved by a search of the Clinicaltrials.gov database conducted on 31 December 2018, of which 35 were related to immunomodulatory treatment. Twenty of these studies were completed or terminated. To access the results of these trials, the articles retrieved from the PubMed searches were reviewed and matched to their respective clinical trials, using the National Clinical Trial identifier, and the PubMed database was again searched using the terms (“hidradenitis suppurativa“ OR ”acne inversa”) and the medication name. Fourteen of the completed or terminated studies had published articles on PubMed (Figure 2). When trial results were not available in PubMed, results posted on Clinicaltrials.gov were used. In five cases, the trials were listed as “completed” on Clinicaltrials.gov, but neither PubMed indexed journal articles nor posted study results on Clinicaltrials.gov were found. A World Wide Web search was then performed to retrieve any study results available.
Figure 2.
Selection of articles identified by Clinicaltrials.gov search.
Biologic and other immunomodulatory therapies
A total of 19 biologic and other immunomodulatory agents reported in the treatment of HS were identified and categorized according to their mode of action (Table 3). Of these, efalizumab has been withdrawn and was thus excluded from this review. Information from individual published articles included in this review is available in Table 4.
Table 3.
Biologics and other immunomodulatory therapies reported in the treatment of HS
| TNF-α inhibitors | Adalimumab*10,12,14–16,23–27 Infliximab9,31,32 Etanercept51 Golimumab53,54 Certolizumab56 |
| IL-1 inhibitors | Anakinra58,60,103 Canakinumab62,63 Bermekimab66 MEDI896870 |
| IL-12/-23 inhibitors | Ustekinumab72 |
| IL-17 inhibitors | Secukinumab74-76 CJM11279 Bimekizumab82 |
| IL-23 inhibitors | Guselkumab84-86 |
| Selective PDE-4 inhibitors | Apremilast89,90,104 |
| Complement C5a inhibitors | IFX-191–93 |
| CD-11a inhibitors | Efalizumab**105 |
| CD-20 inhibitors | Rituximab95 |
| JAK-1 inhibitors | INCB05470796,97 |
Notes: aFDA-approved for treatment of HS
bNo longer available, and thus excluded from further review.
Abbreviation: HS, hidradenitis suppurativa.
Table 4.
Articles included in systematic review
| Case reports, case series and retrospective studies | ||||||
|---|---|---|---|---|---|---|
| Case reports | ||||||
| Study | Subject characteristics | Therapies | Dose | Treatment duration | Efficacy | Complications |
| Koilakou S et al106 | One 38-year-old male | ADA | 40 mg EOW | NR | No | None |
| Benhadou F et al 201822 | One 55-year-old female | ADA | 160 mg once | 1 week | Yes | Erythroderma |
| Van der Zee HH et al 201353 | One 51-year-old female | ADA, GOL, ANK | ADA 40 mg EOW, GOL 50 mg monthly, ANK 100 mg monthly | ADA for 2 years, GOL for 1 year, ANK for 1 year | All ineffective | None |
| Moul DK et al 2006107 | One 67-year-old male | ADA | 40 mg EOW | 5 months | Yes | None |
| Harde V et al 2008108 | One 32-year-old male | ADA | 80 mg once, then 40 mg weekly | 6 months | Yes | None |
| de Wet J et al 201717 | One 42-year-old male | ADA | 160 mg at week 0, 80 mg at week 2, and 40 mg weekly for 5 weeks | 8 weeks | Yes | None |
| Samycia M et al109 | One 48-year-old male | ETA, INF, ADA | ETA 50 mg weekly, then twice weekly, INF 5 mg/kg, then 10 mg/kg every 6 weeks, ADA 80 mg at week 0 then 40 mg EOW then weekly |
ETA for 4 months, INF for 1 year 6 months, ADA for 1 year |
No Yes, for 1 year Yes |
Fatigue (from INF) |
| Diamantova et al 2014110 | One 50-year-old female | ADA | 80 mg at week 0 then 40 mg EOW | 8 weeks | Yes | None |
| Bosnić et al 2016111 | One 39-year-old male | ADA | 40 mg EOW | 1 year | Yes | None |
| Saraceno et al 201518 | One 50-year-old male | ADA | 40 mg EOW | 36 weeks | Yes | None |
| Bessaleli et al 2018112 | One 33-year-old female | ADA | 40 mg, then increased to 80 mg weekly | 8 months | Yes | Cervical squamous cell carcinoma in situ |
| Crowley EL et al 2018113 | One 32-year-old male | ADA | 80 mg at week 0 and then 40 mg weekly | 3 years | Yes | Oral candidiasis |
| Molina-Leyva et al 2018114 | One 39-year-old female | ADA | NR | 16 weeks | DLQI reduced from 18 to 0 at week 4 and 16 | None |
| Gorovoy et al 2009115 | One 47-year-old female | INF, ADA | INF NR, ADA 40 mg EOW | 15 months for each biologic | Initial response for INF, good response for ADA | Infusion reaction with urticaria (INF) |
| Murphy et al 201519 | One 26-year-old female | INF, ADA | INF 5 mg/kg for 2 doses, ADA 160 mg/80 mg then 40 mg EOW | INF NR, ADA for 2 months |
Response to both | Drug hypersensitivity (INF) |
| Friedman et al 201813 | One 48-year-old female | ANK | 100 mg/day for 9 months, then 200 mg/day for 15 months | 24 months | Moderate response | Drug-induced sarcoidosis |
| Zarchi et al 2013116 | One 37-year-old female | ANK | 200 mg/day | 1 year | Yes | Staphylococcus aureus pustular folliculitis |
| Jaeger T et al 201364 | One 27-year-old male | CAN | 150 mg every 3–6 weeks for 8 injections | NR | Yes | NR |
| Tekin B et al 201765 | One 27-year-old male | INF, CAN | INF 5 mg/kg for 8 sessions, CAN 150 mg every 4 weeks for 3 doses | NR | Slight improvement with INF, worsening with CAN | Worsening of HS with CAN |
| Zangrilli A et al 2008117 | One 32-year-old male | ETA | 50 mg 2x/week for 24 weeks, then 25 mg 2x/week for 24 weeks | 48 weeks | Yes | None |
| Tursi A 201654 | One 42-year-old female | GOL | 200 mg once then 100 mg every 4 weeks | NR | Yes | None |
| Roussomoustakaki M et al 200338 | One 29-year-old female | INF | 5 mg/kg at 0, 2 and 6 weeks | 6 weeks | Yes | None |
| Adams DR et al 2003118 | One 17-year-old male | INF | 5 mg/kg at 0, 2, 6 weeks, and 7 months | 7 months | Yes | None |
| Rosi YL et al 2005119 | One 30-year-old female | INF | 5 mg/kg at 0, 2 and 6 weeks | 6 weeks | Yes | None |
| Husein-ElAhmed H et al 2011120 | One 47-year-old male | INF | 4.6 mg/kg at weeks 0, 2, 6, 10 | 10 weeks | Yes, but recurrence in 2 weeks | None |
| von Preussen AC et al 2012121 | One 42-year-old female | INF | 5 mg/kg at 0, 2 and 6 weeks and then every 8 weeks | NR | Yes | None |
| Montes-Romero JA et al 200837 | One 39-year-old male | INF | 5 mg/kg at weeks 0, 2, 6 and 14 |
NR | Yes | None |
| Goertz RS et al 200841 | One 54-year-old male | INF | 5 mg/kg for 7 infusions | 2 years | Yes, but effect plateaued | New superinfection |
| Blazquez I et al 201339 | One 50-year-old female | INF | INF 5 mg/kg at 0, 2 and 6 weeks and then every 8 weeks | 6 months | Yes | None |
| Groleau PF et al 201540 | One 51-year-old male | INF | I5 mg/kg at 0, 2 and 6 weeks and then 7.5 mg/kg every 8 weeks for 1 year, then 5 mg/kg every 8 weeks twice, then 2.5 mg/kg every 8 weeks twice Stopped for 7 months, then 5 mg/kg every 8 weeks when relapsed |
NR | Yes, relapsed after cessation | None |
| Martínez F et al 200142 | One 30-year-old male | INF | 5 mg/kg for 2 doses | NR | Yes | Generalized erythema and dyspnea |
| Lebwohl B et al 2003122 | One 21-year-old male | INF | NR | NR | Yes, but recurred on prolonged sitting | None |
| Thielen AM et al 2006123 | One 48-year-old male | INF | 5 mg/kg at 0, 2 and 6 weeks then every 8 weeks | 104 weeks | Yes | Limited herpes zoster |
| Gori A et al 201244 | One 19-year-old male | INF | 5 mg/kg at 0, 2 and 6 weeks then every 8 weeks | 14 weeks | Yes | Acne |
| Alecsandru D et al 2010124 | One 47-year-old male | INF | 5 mg/kg at 0, 2 and 6 weeks then every 8 weeks | NR | Yes, flared upon stopping | None |
| Poulin Y et al 2009125 | One 25-year-old female | ETA, INF | ETA 50 mg twice a week, INF 5 mg/kg at 0, 2 and 6 weeks then every 6 weeks | 26 months of ETA, 20 months of INF | Worsened with ETA, improved with INF | ETA caused flare of HS |
| Staub J et al 201536 | One 22-year-old female | ETA, ADA, ANK, INF | ETA, ADA and ANK NR, INF 5 mg/kg at 0 and 2 weeks then every 8 weeks | 10 months of ETA, 5 months of ADA, 20 months of INF Not stated for ANK |
Improved with INF in combination with dapsone, steroids and cyclosporine but relapsed on tailing cyclosporine | None |
| Ozer I et al 201635 | One 43-year-old male | INF | 5 mg/kg at 0, 2 and 6 weeks then every 8 weeks | 2 years | Yes | None |
| Kozub P et al 2012126 | One 53-year-old female | INF | 500 mg at 0, 2 and 6 weeks then every 8 weeks | 43 weeks | Initial improvement but plateaued until addition of dapsone | None |
| Vossen MG et al 201143 | One 21-year-old male | ETA, INF | ETA 50 mg/week, INF 5 mg/kg | NR | NR | Gemella morbillorum bacteremia |
| Takahashi H et al 201795 | One 19-year-old male | RIT | 200 mg for 2 doses 1 year apart | 1 year | Yes | None |
| Thorlacius L et al 201774 | One 47-year-old male | ADA, INF, ANK, SEC | ADA, INF, ANK NR, SEC 300 mg/week for 4 weeks then every 4 weeks after | 7 years | Not improved with ADA, INF, ANK. Responded to SEC | Oral candidiasis (SEC) |
| Schuch A et al 201775 | One 24-year-old male | ADA, INF SEC | ADA, INF NR, SEC 300 mg/week for 4 weeks then every 4 weeks after | NR | Not improved with ADA and INF, responded to SEC | None |
| Giuseppe P et al 2018127 | One 37-year-old male | INF, SEC | INF 5 mg/kg, SEC 300 mg/week for 4 weeks then every 4 weeks after | NR | Partial improvement with INF, improved with SEC | None |
| Jørgensen AR et al 2018128 | One 36-year-old female | INF, ADA, UST, SEC | INF, ADA, UST NR, SEC 300 mg/week for 5 weeks then every month | 1 year | No response to INF, ADA, UST, improved with SEC but small relapse | Throat infections, fever |
| Santos-Pérez MI et al 2014129 | One 50-year-old female | ADA, UST | ADA 80 mg, then 40 mg every 2 weeks, UST 45 mg at weeks 0, 4 and then every 12 weeks | 2 years of ADA, 1.5 years of UST | Stable then worsened on ADA, improved with UST but 2 exacerbations reported | None |
| Sharon VR et al 2012130 | One 55-year-old male | ADA, UST | ADA NR, UST 45 mg at weeks 0, 4 and 12, then 90 mg every 8 weeks | NR | Did not respond to ADA, improved with UST but flares 2 weeks prior to next dose | None |
| Scheinfeld N 201445 | One 47-year-old male | INF | 500 mg for 3 courses | NR | NR | Metastatic cutaneous squamous cell carcinoma |
| Case series | ||||||
| Study | Subject characteristics | Therapies | Dose | Treatment duration | Efficacy | Complications |
| Blanco R et al 2009131 | Six patients, two males and four females | ADA in six, ETA in one | ADA 40 mg EOW, increased to weekly if inadequately controlled, decreased to 3-weekly if in remission | Mean of 21.5 months | ETA ineffective, ADA effective | Pain at injection site, severe facial cellulitis in one patient |
| Chinniah N et al 2014132 | Six patients, four males and two females | ADA in three cases, INF in four cases, ETA in one case** | NR | Mean 25.3 months | Significant response to ADA in two, INF in three, ETA in one | Neurological adverse events in one patient on ADA |
| Houriet C et al 201763 | Two (One male, one female) | CAN | One given 150 mg monthly, one given 150 mg on day 1 and 15 and then monthly | One for 26 months, one for 12 months | Reduction in Sartorius score and VAS for both patients | None |
| Sun NZ et al 201762 | Two females | CAN and ANK in one, ADA, INF and CAN in one | INF 5 mg/kg at week 0, 2 and 6, CAN 150 mg every 8 weeks, ANK 100 mg daily for first patient, ADA 40 mg weekly, INF 6 mg/kg and CAN 150 mg weekly for second patient | NR | INF effective in one patient, ADA partial response in same patient | INF - hypersensitivity reaction in first patient, suspicion of drug-related interstitial nephritis in second patient |
| Sand FL et al 201556 | 29 patients | ADA in 22, ETA in five, INF in six, CER in two** | ADA 40 mg once weekly, ETA 50 mg once weekly, INF 5 mg/kg every 8 weeks, ADA 40 mg once weekly | Mean of 13 months (1–50 months) | 12 out of 22 responded to ADA, two of five to ETA, one of six to INF, none of two for CER | ADA – Meningealia, headache, fever in one patient, pneumonia in two patients, visual disturbances and headache in one patient; ETA – urosepticemia in one patient, sebopsoriasis in one patient; INF –sensory and motor polyneuropathy, myalgia and arthralgia in one patient, recurrent tonsillitis in one patient |
| Zhao CY et al 201821 | Four males | ADA | NR | 10–60 months | 50–100% improvement | One with development of melanoma in situ, one with worsening of Charcot-Marie-Tooth syndrome, one with drug-induced lupus |
| Patil 2018133 | Two males | ADA biosimilar (ZRC-3197) | 40 mg weekly for 3 weeks, then EOW for 3 months | 3 months and 3 weeks | >50% reduction in abscess and inflammatory nodule count in both patients | None |
| Menis et al 2014103 | Two males | ANK | 100 mg daily | 12 weeks | No | One with worsening of HS |
| Cusack et al 2006134 | Six females | ETA | 25 mg twice weekly | 17–40 weeks | All six improved, between 44% and 73% in DLQI reduction | Increased frequency of upper respiratory tract infections in one patient |
| Lasocki et al 2010135 | Four (One male, three female) | INF | 5 mg/kg at week 0, 2 and 6, then 8-weekly maintenance infusions. | 38–54 weeks | Improvement in all patients, but with recurrence in all after cessation of therapy | Headache and vomiting in one patient |
| Lozeron et al 200920 | One male | INF | 5 mg/kg at 0, 2, 6, 12 and 18 weeks | 18 weeks | Not effective | Lewis-Summer Syndrome (demyelinating neuropathy) |
| Elkjaer et al 2008136 | Two males | INF | 5 mg/kg/day at 0, 2 and 6 weeks and then 5 weekly | NR | Effective | Infusion reactions in one patient |
| Antonucci et al 2008137 | Two (one male, one female) | INF | 5 mg/kg on weeks 0, 2 and 6 and then every 8 weeks | 59 weeks | Effective in one, ineffective in one | None |
| Delage et al 201132 | Seven (Three males, Four females) | INF | 5 mg/kg on weeks 0, 2 and 6 and then every 8 weeks | 6–110 weeks | Effective in six | One with eczema-like eruption, one with pretragian abscess |
| Brunasso et al 2008138 | Seven (Three males, four females) | INF | 5 mg/kg on weeks 0, 2 and 6 and then every 8 weeks | Mean 58.6 weeks (4–72) | Improvements in pain, discharge, area reduction and DLQI. 3 with new lesions during therapy | One with adverse drug reaction, not further specified |
| Moschella 2007139 | Three (One male, two females) | INF | 5 mg/kg on weeks 0, 2 and 6 and then varying subsequent dosing | 54–80 weeks | Effective in all | None |
| Sullivan et al 2003140 | Five (One male, four females) | INF | 5 mg/kg at week 0 for all five patients, and again at week 4–6 for three patients | 0– 6 weeks | Effective in all | Presumed Mycobacterium tuberculosis lymph node infection in one patient |
| Torres et al 2010141 | Two patients | INF | 5 mg/kg on weeks 0, 2 and 6 and then every 8 weeks | 7 months | Ineffective | None |
| Usmani et al 2006142 | Four patients (two males, two females) | INF | 5 mg/kg, varying dosing | Up to 11 months | Two with good response, one with mild response, one with poor response | One with INF-induced lupus, one with infusion reaction |
| Fernández-Vozmediano et al 2007143 | Six patients (Two males, four females) | INF | 5–10 mg/kg at weeks 0, 2 and 6, then every 4 weeks | 6 months | Improvement to stage I in four cases, stage II in two cases | One with headache |
| Moriarty et al 201431 | 11 patients (Eight males, three females) | INF | 5–10 mg/kg at weeks 0, 2 and 6, then every 4 to 8 weeks | Median 49.1 months | All with initial improvement, two with secondary failure | Nine cutaneous infections requiring antibiotics, four respiratory tract infections requiring antibiotics, one episode of tonsillitis requiring antibiotics, one case of Hodgkin lymphoma 36 months after cessation of INF |
| Kovacs et al 201884 | Three (Two males, one female) | GUS | 100 mg at weeks 0 and 4, then 8 weekly | At least 12 weeks | Improvements in IHS4, VAS and DLQI at 12 weeks in all patients | None |
| Weber et al 201788 | Nine patients (Six males, three females) | APR | 30 mg twice daily | 2 days to 9 months | Improvement in five of six who persisted with therapy | Two with weight loss, one with loose stool, one with dry cough, one with nausea, one with reflux |
| Zhang et al 2014144 | 22 (nine males, 13 females) | 15 on INF, seven on ADA | NR | 1 month to 3 years | 14 with improvement (11 with INF, three with ADA) | Three with infusion reactions, two with fatigue, one with anaphylaxis, one with heart failure, one with dyspnea, one with recurrent HSV. One death from lung malignancy, one death from metastatic perianal squamous cell carcinoma (both on INF) but no direct causal relationship established. |
| DeFazio et al 2016145 | 11 patients | Eight on INF, three on UST | Mean 10.5 months (6 to 15 months) | NR | Effective in seven, local recurrence in four, of which one was after 4 months of INF | None |
| Monné et al 2014146 | Four patients | Four on ADA, of which one also tried INF and ETA | Varying doses | NR | ADA effective in three of four, ETA and INF ineffective | None |
| Gulliver et al 2011147 | Three (one male, two female) | UST | 45 mg at 0, 1 and 4 months | 6 months | One ineffective, one 25–49% disease clearance, one complete clearance | One patient with S. aureus of right axilla, one patient with cystitis, psoriasiform dermatitis and arthritis |
| Retrospective analyses | ||||||
| Study | Subject characteristics | Therapies | Dose | Treatment duration | Efficacy | Complications |
| van Rappard et al 2012148 | 30 patients (17 males and 13 females) | INF | Weeks 0, 2 and 6 and subsequently every 8 weeks. |
Mean 9.3 months | 10 free of lesions, 13 improved, 4 moderately improved, 3 no response | Noted in 12 patients, not further specified |
| Martin-Ezquerra G et al 2014149 | 19 patients (ten males, nine females) | ADA in 11 cases, INF in ten cases, UST in two cases, ETA in two cases** | NR | Mean of 12 months | Physician-judged at least partial responses for ADA in eight, INF in seven, UST in three, none for ETA | Severe infusion reaction and hypertriglyceridemia in two patients |
| Bettoli, Manfredini et al 2018150 | 34 patients (19 males, 15 females) | ADA | NR | Varying, up to at least 1 year | Among group receiving ADA for >1 year, 60% achieved HiSCR at 3 months and maintained up to 12 months. | NR |
| Kyriakou et al 2018151 | 19 (five males, 14 females) | ADA | 160 mg at week 0, 80 mg at week 2, 40 mg at week 4 and 40 mg weekly after | At least 24 weeks | 63.1% achieved clinical response at 24 weeks (defined by HS-PGA score of clear, minimal or mild with at least two-grade improvement from baseline). | None# |
| Casseres et al 201885 | Eight (five males, three females) | GUS | 100 mg at week 0, 4 and then 8 weekly | Up to 10 months | Five (63%) with improvement | None |
| Cohort studies | ||||||
| Study | Subject characteristics | Therapies | Dose | Treatment duration | Efficacy | Complications |
| van Rappard et al 2011152 | 19 patients (12 males, seven females) | Ten on INF, nine on ADA | INF 5 mg/kg at weeks 0, 2 and 6. ADA 40 mg EOW. | 6 weeks | 46% reduction in Sartorius score in INF group vs 34% in ADA group | One on INF with acute arthritis and myalgia, three on ADA with fatigue, one with injection site pain |
| Sbidian et al 2016153 | 67 patients (30 males, 37 females) | 17 on ADA, 63 on ADA, eight on ETA** | INF 5 mg/kg, ADA 40 mg EOW, ETA 50 mg twice a week | NR | Treatment with ADA associated with at least partial response compared to ETA or INF with hazards ratio of 6.6 (p=0.001) | One each with hepatitis, lupus, repeated urinary tract infection and pulmonary embolism |
| Shanmugam et al 2018112 | 68 patients (23 males, 45 females; 31 ever had biologics, 37 never had biologics) | NR | NR | NR | Ever receiving biologics associated with sharper decline in HS activity, biologic use associated with significant reduction in HSS and Hurley stage, effect of biologics greater in patients who received surgery, combination therapy with surgery and biologics associated with higher probability of achieving 75% reduction in active nodule count. | NR |
| Clinical trials | |||||||
|---|---|---|---|---|---|---|---|
| Study | Trial characteristics | Subject characteristics | Therapies | Dose | Treatment duration | Efficacy | Complications |
| Kimball et al 2016,12 Zouboulis et al 201815 | Randomized double-blind placebo-controlled Phase III trial, with open-label extension to 168 weeks | 307 for PIONEER I, 326 for PIONEER II | ADA | 40 mg weekly vs 40 mg EOW vs placebo | 36 weeks | More patients in the weekly ADA group achieved HiSCR [41.8% versus 26.0% in PIONEER I (p=0.003) and 58.9% vs 27.6% in PIONEER II (p<0.001)]. Mean duration of infection-free HiSCR was significantly longer in ADA compared to placebo group (p< 0.001).154 More patients on ADA achieved a ≥30% in the Patient’s Global Assessment of Skin Pain, both PIONEER I (ADA [40.3%] vs placebo [24.9%]; OR =2.03, p=0.004) and II (ADA [61.2%] vs placebo [24.8%]; OR=4.78, p<0.001).155 Achievement of HiSCR was maintained through week 168 in 52.3% in weekly ADA group and 57.1% of responders plus partial responders.15 |
Among ADA group, new psoriasiform eruptions and psoriasis in ten patients, one case of squamous cell carcinoma of the nose, and one death from cardiorespiratory arrest 42 days after the last dose of ADA in a 35-year-old man with a history of diabetes mellitus, smoking and a family history of ischemic heart disease. Adverse events observed during open-label extension were similar to safety profiles observed in PIONEER studies.15 Safety analysis of every week and EOW dosing showed similar adverse event rates.16 |
| Jiménez-Gallo et al 2018156 | Prospective open label study | 19 (11 males, eight females) | ADA | 40 mg weekly | 36 weeks | HiSCR in 68.42%. | NR |
| Kimball et al 201211 | Phase II parallel randomized, placebo-controlled trial with a blinded 16-week period and an open-label 36-week period | 154 | ADA | 40 mg weekly vs 40 mg EOW vs placebo | 52 weeks | 17.6% in weekly ADA vs 9.6% in EOW group vs 3.9% in placebo group achieved HS-PGA score of clear, minimal, or mild with at least a two-grade improvement relative to baseline score at week 16 (EOW vs placebo difference, 5.6% [95% CI, 4.0% to 15.3%]; p 0.25; weekly vs placebo difference, 13.7% [CI, 1.7% to 25.7%]; p 0.025). Proportion with clinical response decreased after change from weekly to EOW dosing. A shorter time to achieving HiSCR in weekly ADA vs EOW group.99 | 24 listed serious adverse events: rectal fissure, inadequately controlled diabetes mellitus, goiter, hidradenitis, interstitial lung disease, pilonidal cyst, viral infection, anemia, bacteremia, vocal cord neoplasm, bacterial genital infection, Escherichia infection, penile swelling, purulent discharge, pustular rash, scrotal swelling, noncardiac chest pain, and cellulitis. |
| Arenbergerova et al 2010157 | Open label trial | Eight (three males, five females) | ADA | 80 mg at week 0, 40 mg at week 1, then 40 mg EOW | 12 months | Mean reduction in pain and discharge of 86.5% and 72.8% respectively at 12 months, and 56.6% and 50.1% at 24 months | One case of EBV infection |
| Amano et al 2010101 | Open-label Phase II trial | Ten, of which six completed treatment | ADA | 160 mg at week 0, 80 mg at week 1, 40 mg EOW | 12 weeks | None responded, as defined by at least 50% decrease in HSSI score | No serious adverse events |
| Leslie et al 201460 | Open-label trial | 6 (Two males, four females), of which five completed the study | ANK | 100 mg daily | 8 weeks | Significant improvement in modified Sartorius score during treatment and rebound during follow-up post-treatment | None |
| Tzanetakou et al 201658 | Randomized double-blind placebo-controlled trial | 20 patients (ten in anakinraANK arm, ten in placebo arm), of which 19 completed study | ANK | 100 mg daily | 12 weeks | At week 12, 78% in ANK arm vs 20% in placebo arm had reduction in disease activity score, defined as the sum of scores of all affected areas (two largest diameters in each affected areas multiplied by degree of inflammation at each lesion), and 78% vs 30% achieved HiSCR. At week 24, 10% vs 33% achieved HiSCR. | No serious adverse events |
| Vossen et al 201889 | Randomized double-blind placebo-controlled trial | 20 (15 given apremilastAPR and five given placebo) of which 18 completed the study | APR | 30 mg BD | 16 weeks | Eight of 15 achieved HiSCR in APR arm compared to none in placebo arm (p=0.055) at week 16. | 38 adverse events in APR arm vs 11 in placebo arm, none categorized as severe. |
| Cusack et al 2006134 | Open-label trial | Six | ETA | 25 mg twice weekly | 17 to 40 weeks | 44 to 73% reduction in DLQI 12 to 24 weeks after starting treatment | Increased incidence of URTIs in one patient |
| Giamarellos-Bourboulis et al 200747 | Open-label Phase II trial | Ten three males, seven females) | ETA | 50 mg weekly | 12 weeks | >50% decrease in disease activity (defined as sum of lesions with each lesion evaluated with the formula: lesion diameters multiplied by severity) in six patients at week 12 and seven patients at week 24. | No serious adverse events: three patients with self-limited injection site erythema, one patient with right gluteal abscess |
| Sotiriou et al 200850 | Open- label trial | Four (one male and three females) | ETA | 25 mg twice a week | 6 months | 54–73% reduction in DLQI | One case of mild injection site reaction |
| Lee et al 200949 | Open-label trial | 15, of which ten completed treatment | ETA | 50 mg weekly for 12 weeks, then 25 mg weekly for 2 weeks | 14 weeks | Only three (20%) of patients achieved 20% reduction in PGA, based on intention to treat analysis. | No serious adverse events reported |
| Pelekanou et al 201048 | Open-label Phase II trial | Ten | ETA | 50 mg weekly | Initial 12-week duration, then subsequent 0 to 96-week course depending on recurrence | Three who improved in mSS with no recurrence and 7 who improved and then recurred after stopping treatment, of which five had a positive response to repeat treatment | None |
| Adams et al 201051 | Randomized trial with 12-week double-blind placebo-controlled phase followed by 12-week open-label phase | 20 patients (ten in etanerceptETA arm and ten in placebo arm), of which 14 completed entire study | ETA | 50 mg twice weekly | 24 weeks | No statistically significant difference in physician global assessment between treatment and placebo arms | No serious adverse events |
| Paradela et al 201233 | Open-label trial | Ten (four males, six females) | INF | 5 mg/kg at week 0, 2, 6 and then 8-weekly | 29 to 181 weeks | Eight with response (defined as 50% reduction in hidradenitis severity score), of which four relapsed (defined as 40% increase in score of initial response) | One case of mycobacterial folliculitis, one case of scrotal abscess, two cases of psoriasis, five cases of positive anti-nuclear antibodies, two cases of transient hyperlipidemia |
| Grant et al 20109 | Clinical trial with 8-week double-blind placebo-controlled phase followed by 14- to 22-week open label phase | 38 patients (15 in infliximabINF arm and 23 in placebo arm) | INF | 5 mg/kg at week 0, 2 and 6 and then 8 weekly | 22 weeks | At week 8, 60% of patients in INF arm vs 5.6% of patients in placebo arm had 25% to less than 50% reduction in HSSI, and 13.3% of patients in INF arm vs 88.9% in placebo arm had less than 25% decrease in HSSI (p<0.001). | Serious adverse events: one pregnancy, one case of hypertension, one infusion reaction requiring hospitalization. |
| Mekkes et al 200734 | Open-label trial | 11, of which ten (four males, six females) completed the study | INF | 5 mg/kg at week 0, 2 and 6 | 6 weeks | Improvements in all patients within 2–6 weeks, as defined by reduction in mSS. At one year, improvements in 6 patients, but recurrences in 4. | One with numbness and pain in both legs, one with anaphylactic shock, and one with myalgia and fever |
| Kanni et al 201866 | Randomized double-blind placebo-controlled trial | 20 patients (10 in bermekimabBER arm and 10 in placebo arm) | BER | 7.5 mg/kg every 14 days | 12 weeks | 60% in BER arm vs 10% in placebo arm achieved HiSCR at week 12, and 40% vs 0% at week 24. | 19 HS exacerbations in BER group, of which 2 required hospitalization |
| Blok et al 201672 | Open-label trial | 17 (four males, 13 females) | UST | Week 0, 4, 16 and 28–45 mg per dose for participants weighing <100 kg and 90 mg per dose for those >100 kg | 28 weeks | Marked improvement of mSS in six (35%), moderate in eight (47%), mild in one (6%), no change/worsening in two (12%) at 40 weeks. Mean mSS of intention-to-treat population decreased from 112.12 to 60.18 at week 40 (46.33% improvement, p<0.01). Eight (47%) achieved HiSCR at week 40. | Headache, fatigue, URTI |
| Guo et al 201791 | Open-label phase II trial | 12 | IFX-1 | 800 mg on days 1, 4, 8, 15, 22, 29, 36, 43 and 50 | 50 days | 83% achieved HiSCR at Day 134 (95% CI: 0.52–0.98). | NR |
Notes: **Overlapping due to switches in therapy. #Study excluded patients who discontinued treatment due to adverse event or had to receive another therapeutic modality.
Abbreviations: ADA, adalimumab; GOL, golimumab; ANK, anakinra; ETA, etanercept; INF, infliximab; CAN, canakinumab; RIT, rituximab; SEC, secukinumab; UST, ustekinumab; CER, certolizumab; GUS, guselkumab; APR, apremilast; BER, bermekimab; EOW, every other week; NR, not reported; PASH, pyoderma gangrenosum, acne and suppurative hidradenitis; NSAID, non-steroidal anti-inflammatory drug; HPV, human papillomavirus; SAPHO, synovitis, acne, pustulosis, hyperostosis and osteitis; HCV, hepatitis C virus; Nd-YAG, neodymium-doped yttrium aluminum garnet; CO2, carbon dioxide; IHS4, international HS severity score system; HSV, herpes simplex virus; HSS, HS score; HiSCR, HS clinical response; URTI, upper respiratory tract infection; PGA, physician global assessment; mSS, modified sartorius score; EBV, Epstein-Barr virus, HSSI, HS severity index.
TNF-α inhibitors
Adalimumab
Adalimumab is a recombinant human anti-TNF-α IgG1 monoclonal antibody. When used for HS, it is given subcutaneously as an initial dose of 160 mg, followed by a dose of 80 mg 2 weeks later, and a maintenance dose of 40 mg weekly thereafter.10 In 2015, it became the first and, to date, only FDA-approved biologic agent for the treatment of moderate/severe HS.10
A Phase II study by Kimball et al in 2012 first demonstrated that a significantly greater proportion receiving adalimumab weekly (17.6%) compared to placebo (3.9%) achieved the primary clinical endpoint of a HS-PGA score of clear, minimal or mild with at least a two-grade improvement relative to baseline scores at week 16. This effect was not significantly demonstrated in the group of patients receiving adalimumab every other week (9.6%) (weekly vs placebo difference 13.7%, p=0.025; every other week vs placebo difference 5.6%, p=0.25).11 After changing from weekly to every other week dosing, a decreased proportion of patients showed a clinical response.11
The subsequent PIONEER I and II Phase III trials involved a total of 633 patients with moderate-to-severe HS with an inadequate response to oral antibiotics. A significantly higher proportion of patients given adalimumab achieved HiSCR, compared to patients given placebo after 12 weeks of treatment (PIONEER I: 41.8% vs 26.0%, p=0.003; PIONEER II: 58.9% vs 27.6%, p<0.001).12 Most adverse events observed were mild or moderate in severity. Of note, in the group of patients treated with adalimumab, there were new psoriasiform eruptions and psoriasis in ten patients, one case of squamous cell carcinoma of the nose, and one death from cardiorespiratory arrest 42 days after the last dose of adalimumab in a 35-year-old man with a history of diabetes mellitus, smoking and a family history of ischemic heart disease.12 Secondary efficacy data also showed a greater proportion of subjects achieving a ≥30% reduction in the Patient’s Global Assessment of Skin Pain (PGA-SP) in both PIONEER I (adalimumab vs placebo [24.9%]; OR=2.03, p=0.004) and PIONEER II (adalimumab [61.2%] vs placebo [24.8%]; OR=4.78, p<0.001).13,14
An open-label extension trial of the PIONEER I and II trials also confirmed that patients who continued to receive weekly adalimumab maintained a long-term response with a HiSCR rate of 52.3% at week 168 and a decrease in the Dermatology Life Quality Index (DLQI) of 5.1–6.8 points at week 72, with no new safety risks identified.15
In a study by Ryan et al that analyzed the safety data of adalimumab in HS, there were no new safety concerns identified with the weekly dosing of adalimumab compared with every other week dosing.16
In case reports, adalimumab has also shown effectiveness in treating HS associated with pyoderma gangrenosum, acne and psoriatic arthritis.17–19 However, some case reports and series have drawn caution to the use of adalimumab, reporting adverse events such as erythroderma, melanoma, demyelinating disorders and drug-induced lupus.20–22
There are currently post-marketing surveillance trials of adalimumab underway, assessing quality of life, effectiveness of treatment and safety profile.23–27 In addition, the safety, efficacy and cost-effectiveness of adalimumab in conjunction with surgery are currently under investigation by two Phase IV trials.28,29
Infliximab
Infliximab is a chimeric mouse/human anti-TNF-α monoclonal antibody. It is currently FDA-approved for use in inflammatory bowel disease, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis;30 and has been used as an off-label treatment in patients with HS resistant to adalimumab.9,31,32 It is currently dosed as an intravenous infusion 5 mg/kg body weight on week 0, 2, 6 and thereafter every 8 weeks.30 However, reports suggest that the dosing regimen for HS requires further refinement.31
In a descriptive single-center study involving 10 patients, no long-term curative effect was uniformly seen.33 In another study evaluating the efficacy of a single course (three infusions) of infliximab in 10 patients, three patients did not have a recurrence at 2 years, whereas the other seven had an average time of 8.5 months to recurrence of lesions (4.3–13.4 months).34
In a Phase II randomized study comprising a double-blind placebo-controlled treatment phase, an open-label crossover treatment phase and an observational phase, 38 patients with moderate-to-severe HS as defined by a HSSI score >8 were selected. More patients treated with regular infliximab responded with a 25% to <50% decrease in HSSI compared to placebo (60% vs 5.6%), whereas most patients treated with placebo had a <25% decrease in HSSI compared with infliximab (88.9% vs 13.3%, p<0.001).9 After 8 weeks of treatment, there were significant improvements in the infliximab group compared to the placebo group in terms of mean DLQI change (−10 vs −1.6, p=0.003) and mean PGA scores (1.8 vs 4.7, p<0.001).9 Most adverse events were mild and none were considered unexpected.9
In case reports, infliximab demonstrated efficacy in treating HS associated with pyoderma gangrenosum, acne, Crohn’s disease and systemic amyloidosis.35–42
However, infliximab has also in cases been associated with paradoxical worsening of facial acne vulgaris, demyelinating neuropathies, metastatic cutaneous squamous cell carcinoma and a case of Gemella morbillorum bacteremia complicated by brain abscesses.20,43–45
Etanercept
Etanercept is a dimeric TNF-α inhibitor. It is approved for use in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, plaque psoriasis and psoriatic arthritis.46
After showing mixed results in open-label trials,47–50 it was examined under a double-blind, placebo-controlled study in 20 patients with moderate-to-severe HS. In patients given etanercept 50 mg twice weekly for 24 weeks, no significant improvement in HS was found.51
Golimumab
Golimumab is an anti-TNF-α human monoclonal antibody, approved for use in rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and ulcerative colitis.52
To date, it has been used in two case reports in the treatment of HS. In a case of a patient with concomitant Hurley Stage 3 HS and psoriatic arthritis, the use of subcutaneous golimumab 50 mg once weekly did not result in clinical improvement of HS (after adalimumab and anakinra had failed).53 However, in a later case report published in 2016 of a 42-year-old female with Hurley Stage 2 HS and pyostomatitis vegetans on a background of ulcerative colitis, golimumab subcutaneously 200 mg once followed by 100 mg every 4 weeks, together with amoxicillin-clavulanate, resulted in complete and sustained remission of HS, pyostomatitis vegetans and ulcerative colitis.54
There are no clinical trials underway to further assess golimumab in HS.
Certolizumab
Certolizumab is a PEGylated Fab fragment of a humanized TNF-α monoclonal antibody that is FDA-approved for the treatment of Crohn’s disease, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and plaque psoriasis.55 Its use in HS was described briefly in a case series, where it was used in two patients but found to be ineffective.56
IL-1 inhibitors
Anakinra
Anakinra is a recombinant IL-1 receptor inhibitor which is FDA-approved for use in rheumatoid arthritis and neonatal-onset multisystem inflammatory disease.57 It has been given as a 100 mg subcutaneous daily dose in HS.57
It has been studied in a double-blind, randomized, placebo-controlled Phase II clinical trial involving 20 patients. There were significantly more patients with a decreased disease activity score in the anakinra group compared to the placebo group after 12 weeks of treatment (78% vs 20%, p=0.02) and achieving HiSCR at the end of 12 weeks (78% vs 30%, p=0.04).58 However, at 24 weeks, the difference in patients achieving HiSCR was not statistically significant (10% vs 33%, p=0.28).58
In later case reports, there were also experiences of failure of anakinra therapy, or even worsening of HS related to anakinra use, suggesting the need for further clinical trials.59 Painful injection site reactions are also commonly reported with the use of anakinra, limiting its tolerability for some patients.60 It was also linked with drug-induced sarcoidosis in one case report.13
Canakinumab
Canakinumab is a human monoclonal anti-IL-1β antibody which is FDA-approved for use in cryopyrin-associated periodic syndromes and systemic juvenile idiopathic arthritis.61 It has been given up to 150 mg subcutaneous weekly dose in the treatment of HS. To date, it has shown mixed results in case reports and series.62–65
Bermekimab
Bermekimab (MABp1) is an anti-IL 1α human monoclonal antibody. In a recent Phase II trial involving 20 patients with moderate-to-severe HS either randomized to bermekimab or placebo for 12 weeks, 60% of patients on bermekimab achieved HiSCR at week 12 compared to 10% on placebo (P=0.035).66 Twelve weeks after cessation of treatment, 40% of patients on bermekimab had a positive HiSCR compared to 0% of patients on placebo.66 No adverse events related to bermekimab were reported.66
MEDI8968
MEDI8968 is a fully human immunoglobulin monoclonal antibody that selectively binds to the IL-1R1 receptor to inhibit activation by IL-1α and IL-1β. It has been studied for use in osteoarthritis, rheumatoid arthritis and chronic obstructive pulmonary disease.67–69 A Phase IIa study evaluating the safety, tolerability and efficacy of MEDI8968 for the treatment of subjects with moderate-to-severe HS was terminated early due to a lack of efficacy.70
IL-12/-23 inhibitors
Ustekinumab
Ustekinumab is a human monoclonal antibody that acts by binding to and inhibiting the p40 subunit on IL-12 and IL-23. It is FDA-approved for use in plaque psoriasis, psoriatic arthritis and Crohn’s disease.71 Patients weighing 100 kg and below receive 45 mg per dose, and those weighing above 100 kg receive 90 mg per dose.71
In a Phase II open-label study involving 17 patients on ustekinumab, the majority of patients showed moderate to marked improvement, as defined by a significant decrease in the mSS and modified HS Lesional Area Severity Index.72 Forty-seven percent of patients achieved HiSCR.72 Adverse events were mild and temporary, most commonly headache, fatigue and upper respiratory tract infections. The authors of the Phase II study suggested that the dosing regimen in HS may have to be further intensified.72
IL-17 inhibitors
Secukinumab
Secukinumab is a human IgG1k monoclonal antibody that acts as an IL-17A inhibitor. It is FDA-approved for moderate-to-severe plaque psoriasis, psoriatic arthritis and ankylosing spondylitis.73 Given at 300 mg subcutaneously weekly for 1 month followed by 4-weekly maintenance dosing, it has shown dramatic improvement in case reports of patients in whom other biologic therapies failed.74,75 An exploratory pilot study on the safety and feasibility of secukinumab in HS patients is currently underway,76 and there are two randomized double-blind multicenter trials to compare the efficacy, safety and tolerability of 2-weekly and 4-weekly secukinumab 300 mg in patients with moderate-to-severe HS.77,78
CJM112
CJM112 is a human monoclonal anti-IL-17A antibody. A Phase II study involving 66 patients with moderate-to-severe chronic -HS has been completed, but results are not available at present.79
Bimekizumab
Bimekizumab is a humanized anti-IL17A and IL-17F monoclonal antibody which has been studied and found to be effective in patients with psoriasis.80,81 A Phase II trial is currently underway to investigate its use in moderate-to-severe HS, with no results available at the time of writing.82
IL-23 inhibitors
Guselkumab
Guselkumab is an anti-IL-23 monoclonal antibody that has been FDA-approved for use in adults with moderate-to-severe plaque psoriasis. It is given subcutaneously 100 mg at week 0, week 4 and every 8 weeks thereafter.83 A case series involving three patients with severe HS, given guselkumab, found significant reductions in the IHS4, VAS for pain and (DLQI for all three patients.84 Another retrospective chart review of eight patients with moderate-to-severe HS given guselkumab found that 63% of patients reported improvements, with suggestions to further intensify the dosing regimen.85 No adverse events were documented in both articles. A Phase II multicenter randomized double-blind placebo-controlled trial has been initiated to evaluate its efficacy in the treatment of moderate-to-severe HS.86
Selective PDE-4 inhibitors
Apremilast
Apremilast is an orally administered PDE-4 inhibitor which is FDA-approved for use in patients with moderate-to-severe plaque psoriasis and active psoriatic arthritis. It is titrated to a target dose of 30 mg twice daily.87
In a reported case series of nine patients with Hurley stages II–III HS who had responded poorly to other treatments, five of six patients who persisted with treatment showed a good clinical response, with a significant improvement in the Sartorius score (73.17±67.76 to 56.17±44.89, p=0.028), VAS (7.17±0.98 to 2.00±2.10, p=0.026) and DLQI (21.33±8.91 to 9.33±5.85, p=0.027).88
In a double-blind, randomized, placebo-controlled trial involving 20 patients with moderate HS, 8 of 15 patients (53.3%) given apremilast achieved a positive HiSCR at week 16 compared to zero of five in the placebo group (p=0.055). Patients receiving apremilast also showed a significantly lower abscess and nodule count (mean difference −2.6; 95% CI −6.0 to −0.9; p=0.011), numerical rating scales for pain (mean difference −2.7; 95% CI −4.5 to −0.9; p=0.009), itch (mean difference −2.8; 95% CI −5.0 to −0.6; p=0.015) and disease burden (mean difference −1.8; 95% CI −3.7 to −0.01; p=0.049) compared to placebo. There were no major adverse events documented.89
Another Phase II open-label trial involving 20 patients has been completed, with no results available at present.90
Complement 5a inhibitors
IFX-1
IFX-1 is a human C5a-specific monoclonal antibody. Preliminary data from an open-label clinical study involving 12 patients, 75% of patients achieved HiSCR at day 50 (95% CI 0.43–0.95) and 83% at day 134 (95% CI 0.52–0.98).91,92
Another Phase II study is currently underway to determine its efficacy and safety.93
CD-20 inhibitors
Rituximab
Rituximab is a chimeric monoclonal antibody against the CD20 protein. It is FDA-approved for use in non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, microscopic polyangiitis and pemphigus vulgaris.94
In one case report in a kidney transplant recipient with idiopathic carpotarsal osteolysis who suffered chronic active antibody-mediated rejection and also developed HS, low-dose rituximab with two courses of 200 mg each were given with dramatic improvement of HS without remission of rejection.95
There are no further studies underway to evaluate the efficacy of rituximab.
JAK-1 inhibitors
INCB054707
INCB054707 is an orally administered inhibitor of the Janus kinase 1 pathway. There are currently two Phase II trials underway, with no other information available at the time of writing.96,97
Discussion
The increased understanding of the inflammatory pathways in HS provides many exciting therapeutic opportunities for patients with HS resistant to conventional methods of therapy. As more molecular targets are identified, immunomodulatory therapies can be developed, and their dosing regimens further refined. The efficacy, or lack thereof, of individual therapies also provides key insights into disease pathophysiology.
TNF-α inhibition in HS has been demonstrated to be useful. Adalimumab is presently the only FDA-approved biologic for use in HS and should thus be the drug of choice in moderate-to-severe HS where conventional treatment has proven ineffective. It is worth noting from our systematic review that where HS is associated with pyoderma gangrenosum, acne, Crohn’s disease or systemic amyloidosis, infliximab may also be considered as an effective off-label treatment.
Etanercept and MEDI8968 have already proven to be ineffective. Other therapies, involving smaller cohorts, have shown partial or mixed responses, with larger trials underway to further assess their efficacy in HS. The varying clinical measurement scores and endpoints used in these trials to determine treatment responsiveness potentially complicate direct comparison between different agents.
Most biologics and immunomodulatory therapies exhibit a generally well-tolerated safety profile. However, long-term safety concerns, including infection risks, especially latent tuberculosis reactivation, demyelinating disorders, and the development of malignancy from chronic immunosuppression will need to be evaluated through longitudinal surveillance and pooled registry data.98 These issues are especially pertinent in the treatment of HS, where the dosing regimen for biologics is typically more intensive compared to other inflammatory diseases, such as psoriasis. Patient selection remains important, as complete response is not the norm, not all patients with HS tolerate or respond well to immunomodulatory therapy, and may benefit from other modes of treatment, such as surgery.
In patients with severe HS that does not fully respond even to biologic treatment, we may consider biologics as an adjunct to surgery, where biologics are used to debulk disease to minimize the area required for surgical resection. Results from Phase IV trials to assess the combination of adalimumab with surgery will help refine the treatment approach for this category of severe HS.28,29
Ultimately, it is hoped that the use of immunomodulatory therapies will help overcome some of the challenges in treating severe HS, alleviating the impact on sufferers’ quality of life and morbidity associated with the disease. However, more quality data is required on their efficacy, safety and use in specific sub-populations before we can achieve truly targeted treatment of HS.
Disclosure
Hazel H Oon is a clinical investigator for Janssen, Novartis and Pfizer. She has also served as a speaker and advisory board member for AbbVie, Janssen, Novartis and Eli Lilly. Hazel H Oon reports grants and personal fees from AbbVie, Eli Lilly, Janssen, Novartis, and Pfizer outside the submitted work.The authors report no other conflicts of interest in this work.
References
- 1.Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3(1):54–60. doi: 10.1007/s13671-013-0064-8 [DOI] [Google Scholar]
- 2.Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44(7):535–540. doi: 10.1111/j.1365-4632.2004.02536.x [DOI] [PubMed] [Google Scholar]
- 3.Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71(6):1144–1150. doi: 10.1016/j.jaad.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 4.von Laffert M, Helmbold P, Wohlrab J, Fiedler E, Stadie V, Marsch WC. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19(6):533–537. doi: 10.1111/j.1600-0625.2009.00915.x [DOI] [PubMed] [Google Scholar]
- 5.Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105–115. doi: 10.2147/CCID.S111019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173(6):1431–1439. doi: 10.1111/bjd.14075 [DOI] [PubMed] [Google Scholar]
- 7.Moran B, Sweeney CM, Hughes R, et al. Hidradenitis suppurativa is characterized by dysregulation of the Th17: tregCell axis, which is corrected by anti-TNF therapy. J Invest Dermatol. 2017;137(11):2389–2395. doi: 10.1016/j.jid.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 8.Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. Dermatologic Surgery New York: Marcel Dekker. 1989;729–739. [Google Scholar]
- 9.Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62(2):205–217. doi: 10.1016/j.jaad.2009.06.050 [DOI] [PubMed] [Google Scholar]
- 10.AbbVie. Humira; 2017. Available from: http://www.rxabbvie.com/pdf/humira.pdf. Accessed December 31, 2018.
- 11.Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. doi: 10.7326/0003-4819-157-12-201212180-00004 [DOI] [PubMed] [Google Scholar]
- 12.Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370 [DOI] [PubMed] [Google Scholar]
- 13.Friedman BE, English JC 3rd. Drug-induced sarcoidosis in a patient treated with an interleukin-1 receptor antagonist for hidradenitis suppurativa. JAAD Case Rep. 2018;4(6):543–545. doi: 10.1016/j.jdcr.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimball AB, Sundaram M, Shields AL, et al. Adalimumab alleviates skin pain in patients with moderate to severe hidradenitis suppurativa: secondary efficacy results from the PIONEER I and PIONEER II randomized controlled trials. J Am Acad Dermatol. 2018;79:1141–1143. doi: 10.1016/j.jaad.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 15.Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80(1):60–69.e2. doi: 10.1016/j.jaad.2018.05.040 [DOI] [PubMed] [Google Scholar]
- 16.Ryan C, Sobell JM, Leonardi CL, et al. Safety of Adalimumab Dosed Every Week and Every Other Week: focus on Patients with Hidradenitis Suppurativa or Psoriasis. Am J Clin Dermatol. 2018;19(3):437–447. doi: 10.1007/s40257-017-0341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Wet J, Jordaan HF, Kannenberg SM, Tod B, Glanzmann B, Visser WI. Pyoderma gangrenosum, acne, and suppurative hidradenitis syndrome in end-stage renal disease successfully treated with adalimumab. Dermatol Online J. 2017;23:12. [PubMed] [Google Scholar]
- 18.Saraceno R, Babino G, Chiricozzi A, Zangrilli A, Chimenti S. PsAPASH: a new syndrome associated with hidradenitis suppurativa with response to tumor necrosis factor inhibition. J Am Acad Dermatol. 2015;72(1):e42–44. doi: 10.1016/j.jaad.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 19.Murphy B, Morrison G, Podmore P. Successful use of adalimumab to treat pyoderma gangrenosum, acne and suppurative hidradenitis (PASH syndrome) following colectomy in ulcerative colitis. Int J Colorectal Dis. 2015;30(8):1139–1140. doi: 10.1007/s00384-014-2110-9 [DOI] [PubMed] [Google Scholar]
- 20.Lozeron P, Denier C, Lacroix C, Adams D. Long-term course of demyelinating neuropathies occurring during tumor necrosis factor-alpha-blocker therapy. Arch Neurol. 2009;66(4):490–497. doi: 10.1001/archneurol.2009.11 [DOI] [PubMed] [Google Scholar]
- 21.Zhao CY, Fernandez-Penas P. Is it worthy to treat hidradenitis suppurativa with adalimumab in patients with melanoma and other debilitating systemic diseases? A series of clinical dilemmas. Australas J Dermatol. 2018;59(4):e297–e298. doi: 10.1111/ajd.12829 [DOI] [PubMed] [Google Scholar]
- 22.Benhadou F, Hellgren G, Willaert F, Del Marmol V. Acute erythroderma in a patient receiving TNF-alpha-blocking therapy for hidradenitis suppurativa. Case Rep Dermatol. 2018;10(1):7–12. doi: 10.1159/000485911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US National Library of Medicine. Canadian humira post marketing observational epidemiological study: assessing humira real-life effectiveness and impact on HS burden of illness - full text view - ClinicalTrials.gov; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02896920?term=adalimumab&cond=hidradenitis&rank=12. Accessed December 31, 2018.
- 24.US National Library of Medicine. Effectiveness of adalimumab in moderate to severe HidrAdenitis suppurativa patients - a multi country study in Real Life Setting (HARMONY); 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02786576. Accessed December 31, 2018.
- 25.US National Library of Medicine. Post-marketing surveillance of adalimumab in Korean Hidradenitis Suppurativa Subjects (HS rPMS); 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT03001115. Accessed December 31, 2018.
- 26.US National Library of Medicine. Open-label study of adalimumab in Japanese subjects with hidradenitis suppurativa; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02904902. Accessed December 31, 2018.
- 27.US National Library of Medicine. Post Marketing Observational Study (PMOS) to assess quality of life in swedish Hidradenitis Suppurativa (HS) Patients (HOPE); 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02739828. Accessed December 31, 2018.
- 28.US National Library of Medicine. Safety and Efficacy of Humira (Adalimumab) for Hidradenitis Suppurativa (HS) Peri-Surgically (SHARPS Study) (SHARPS); 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02808975. Accessed December 31, 2018.
- 29.US National Library of Medicine. Cost-effectiveness of Adalimumab and Surgery vs Adalimumab in HS (HS-COST); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03221621?cond=%28%22hidradenitis+suppurativa%22+OR+%22acne+inversa%22%29&rank=46. Accessed December 31 2018.
- 30.US Food & Drug Administration. REMICADE (infliximab) label; 2013. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf. Accessed December 31, 2018.
- 31.Moriarty B, Jiyad Z, Creamer D. Four-weekly infliximab in the treatment of severe hidradenitis suppurativa. Br J Dermatol. 2014;170(4):986–987. doi: 10.1111/bjd.12713 [DOI] [PubMed] [Google Scholar]
- 32.Delage M, Samimi M, Atlan M, Machet L, Lorette G, Maruani A. Efficacy of infliximab for hidradenitis suppurativa: assessment of clinical and biological inflammatory markers. Acta Derm Venereol. 2011;91(2):169–171. doi: 10.2340/00015555-1025 [DOI] [PubMed] [Google Scholar]
- 33.Paradela S, Rodriguez-Lojo R, Fernandez-Torres R, Arevalo P, Fonseca E. Long-term efficacy of infliximab in hidradenitis suppurativa. J Dermatolog Treat. 2012;23(4):278–283. doi: 10.3109/09546634.2012.683767 [DOI] [PubMed] [Google Scholar]
- 34.Mekkes JR, Bos JD. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br J Dermatol. 2008;158(2):370–374. doi: 10.1111/j.1365-2133.2007.08332.x [DOI] [PubMed] [Google Scholar]
- 35.Ozer I, Karacin C, Adisen E, Guz G, Ali Gurer M. Two diseases one remedy? Systemic amyloidosis secondary to hidradenitis suppurativa: treatment with infliximab. Dermatol Ther. 2017;30:2. doi: 10.1111/dth.12445 [DOI] [PubMed] [Google Scholar]
- 36.Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29(11):2243–2247. doi: 10.1111/jdv.12765 [DOI] [PubMed] [Google Scholar]
- 37.Montes-Romero JA, Callejas-Rubio JL, Sanchez-Cano D, Gonzalez-Martinez FJ, Navas-Parejo A, Ortego-Centeno N. Amyloidosis secondary to hidradenitis suppurativa. Exceptional response to infliximab. Eur J Intern Med. 2008;19(6):e32–33. doi: 10.1016/j.ejim.2007.11.014 [DOI] [PubMed] [Google Scholar]
- 38.Roussomoustakaki M, Dimoulios P, Chatzicostas C, et al. Hidradenitis suppurativa associated with Crohn‘s disease and spondyloarthropathy: response to anti-TNF therapy. J Gastroenterol. 2003;38(10):1000–1004. doi: 10.1007/s00535-003-1185-9 [DOI] [PubMed] [Google Scholar]
- 39.Blazquez I, Gonzalez-Lama Y, Roustan G. Crohn‘s disease and hidradenitis suppurativa. An uncommon association that responds to infliximab. J Crohns Colitis. 2013;7(12):e717–718. doi: 10.1016/j.crohns.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 40.Groleau PF, Grossberg AL, Gaspari AA. Hidradenitis suppurativa and concomitant pyoderma gangrenosum treated with infliximab. Cutis. 2015;95(6):337–342. [PubMed] [Google Scholar]
- 41.Goertz RS, Konturek PC, Naegel A, et al. Experiences with a long-term treatment of a massive gluteal acne inversa with infliximab in Crohn‘s disease. Med Sci Monit. 2009;15(1):CS14–C18. [PubMed] [Google Scholar]
- 42.Martínez FNP, Benlloch S, Ponce J. Hidradenitis suppurativa and Crohn’s disease: response to treatment with infliximab. Inflamm Bowel Dis. 2001;7(4):323–326. [DOI] [PubMed] [Google Scholar]
- 43.Vossen MG, Gattringer KB, Khalifeh N, et al. Gemella morbillorum bacteremia after anti-tumor necrosis factor alpha as acne inversa therapy. J Clin Microbiol. 2012;50(3):1109–1112. doi: 10.1128/JCM.06161-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gori A, Rossari S, Bruscino N, Tripo L. Paradoxical effect of infliximab in a patient with hidradenitis suppurativa. Dermatol Ther. 2012;25(4):376–378. doi: 10.1111/j.1529-8019.2012.01471.x [DOI] [PubMed] [Google Scholar]
- 45.Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20:3. [PubMed] [Google Scholar]
- 46.US Food & Drug Administration. Enbrel (etanercept) Label; 2012. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf. Accessed December 31, 2018.
- 47.Giamarellos-Bourboulis EJ, Pelekanou E, Antonopoulou A, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158(3):567–572. doi: 10.1111/j.1365-2133.2007.08372.x [DOI] [PubMed] [Google Scholar]
- 48.Pelekanou A, Kanni T, Savva A, et al. Long-term efficacy of etanercept in hidradenitis suppurativa: results from an open-label phase II prospective trial. Exp Dermatol. 2010;19(6):538–540. doi: 10.1111/j.1600-0625.2009.00967.x [DOI] [PubMed] [Google Scholar]
- 49.Lee RA, Dommasch E, Treat J, et al. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2009;60(4):565–573. doi: 10.1016/j.jaad.2008.11.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sotiriou E, Apalla Z, Ioannidos D. Etanercept for the treatment of hidradenitis suppurativa. Acta Derm Venereol. 2009;89(1):82–83. doi: 10.2340/00015555-0545 [DOI] [PubMed] [Google Scholar]
- 51.Adams DR, Yankura JA, Fogelberg AC, Anderson BE. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146(5):501–504. doi: 10.1001/archdermatol.2010.72 [DOI] [PubMed] [Google Scholar]
- 52.US Food & Drug Administration. SIMPONI (golimumab) label; 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125289s024lbl.pdf. Accessed December 31, 2018.
- 53.van der Zee HH, Prens EP. Failure of anti-interleukin-1 therapy in severe hidradenitis suppurativa: a case report. Dermatology. 2013;226(2):97–100. doi: 10.1159/000343221 [DOI] [PubMed] [Google Scholar]
- 54.Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48(12):1511–1512. doi: 10.1016/j.dld.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 55.US Food & Drug Administration. CIMZIA (certolizumab pegol) for injection; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125160s283lbl.pdf. Accessed December 31, 2018.
- 56.Sand FL, Thomsen SF. Off-label use of TNF-alpha inhibitors in a dermatological university department: retrospective evaluation of 118 patients. Dermatol Ther. 2015;28(3):158–165. doi: 10.1111/dth.12222 [DOI] [PubMed] [Google Scholar]
- 57.US Food & Drug Administration. Kineret® (anakinra) for injection, for subcutaneous use; 2012. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf. Accessed December 31, 2018.
- 58.Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of Anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152(1):52–59. doi: 10.1001/jamadermatol.2015.3903 [DOI] [PubMed] [Google Scholar]
- 59.Russo V, Alikhan A. Failure of Anakinra in a case of severe hidradenitis suppurativa. J Drugs Dermatol. 2016;15(6):772–774. [PubMed] [Google Scholar]
- 60.Leslie KS, Tripathi SV, Nguyen TV, Pauli M, Rosenblum MD. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(2):243–251. doi: 10.1016/j.jaad.2013.09.044 [DOI] [PubMed] [Google Scholar]
- 61.US Food & Drug Administration. ILARIS® (canakinumab) for injection, for subcutaneous use; 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/BLA125319_858687lbl.pdf. Accessed December 31, 2018.
- 62.Sun NZ, Ro T, Jolly P, Sayed CJ. Non-response to interleukin-1 antagonist canakinumab in two patients with refractory pyoderma gangrenosum and hidradenitis suppurativa. J Clin Aesthet Dermatol. 2017;10(9):36–38. [PMC free article] [PubMed] [Google Scholar]
- 63.Houriet C, Seyed Jafari SM, Thomi R, et al. Canakinumab for severe hidradenitis suppurativa: preliminary experience in 2 cases. JAMA Dermatol. 2017;153(11):1195–1197. doi: 10.1001/jamadermatol.2017.2392 [DOI] [PubMed] [Google Scholar]
- 64.Jaeger T, Andres C, Grosber M, et al. Pyoderma gangrenosum and concomitant hidradenitis suppurativa–rapid response to canakinumab (anti-IL-1beta). Eur J Dermatol. 2013;23(3):408–410. doi: 10.1684/ejd.2013.2018 [DOI] [PubMed] [Google Scholar]
- 65.Tekin B, Salman A, Ergun T. Hidradenitis suppurativa unresponsive to canakinumab treatment: a case report. Indian J Dermatol Venereol Leprol. 2017;83(5):615–617. doi: 10.4103/ijdvl.IJDVL_147_16 [DOI] [PubMed] [Google Scholar]
- 66.Kanni T, Argyropoulou M, Spyridopoulos T, et al. MABp1 targeting IL-1alpha for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randomized study. J Invest Dermatol. 2018;138(4):795–801. doi: 10.1016/j.jid.2017.10.030 [DOI] [PubMed] [Google Scholar]
- 67.Cohen SB, Proudman S, Kivitz AJ, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther. 2011;13(4):R125. doi: 10.1186/ar3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cardiel MH, Tak PP, Bensen W, et al. A phase 2 randomized, double-blind study of AMG 108, a fully human monoclonal antibody to IL-1R, in patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(5):R192. doi: 10.1186/ar3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calverley PMA, Sethi S, Dawson M, et al. A randomised, placebo-controlled trial of anti-interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir Res. 2017;18(1):153. doi: 10.1186/s12931-017-0633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.US National Library of Medicine. Assessment of the Safety, Tolerability and Efficacy of MEDI8968 in Subjects With Moderate to Severe Hidradenitis Suppurativa - Full Text View - ClinicalTrials.gov.; 2013. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01838499?term=medi8968&cond=Hidradenitis&rank=1. Accessed December 31, 2018.
- 71.US Food & Drug Administration. STELARA® (ustekinumab) injection, for subcutaneous or intravenous use; 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf. Accessed December 31, 2018.
- 72.Blok JL, Li K, Brodmerkel C, Horvatovich P, Jonkman MF, Horvath B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174(4):839–846. doi: 10.1111/bjd.14338 [DOI] [PubMed] [Google Scholar]
- 73.US Food & Drug Administration. COSENTYX® (secukinumab) injection, for subcutaneous use; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125504s013lbl.pdf. Accessed December 31, 2018.
- 74.Thorlacius L, Theut Riis P, Jemec GBE. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 201. 8;179(1):182–185. doi:10.1111/bjd.15769 [DOI] [PubMed] [Google Scholar]
- 75.Schuch A, Fischer T, Boehner A, Biedermann T, Volz T. Successful treatment of severe recalcitrant hidradenitis suppurativa with the interleukin-17A antibody secukinumab. Acta Derm Venereol. 2018;98(1):151–152. doi: 10.2340/00015555-2794 [DOI] [PubMed] [Google Scholar]
- 76.US National Library of Medicine. Exploratory Trial Evaluating Cosentyx (Secukinumab) for Patients With Moderate-to-Severe Hidradenitis Suppurativa - Full Text View - ClinicalTrials.gov.; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03099980?term=secukinumab&cond=hidradenitis&rank=1. Accessed December 31, 2018.
- 77.US National Library of Medicine. Study of Efficacy and Safety of Two Secukinumab Dose Regimens in Subjects With Moderate to Severe Hidradenitis Suppurativa (HS) (SUNRISE); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03713632?cond=%28%22hidradenitis+suppurativa%22+OR+%22acne+inversa%22%29&rank=8. Accessed December 31, 2018.
- 78.US National Library of Medicine. This is a Study of Efficacy and Safety of Two Secukinumab Dose Regimens in Subjects With Moderate to Severe Hidradenitis Suppurativa (HS). (SUNSHINE); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03713619?cond=%28%22hidradenitis+suppurativa%22+OR+%22acne+inversa%22%29&rank=9. Accessed December 31, 2018.
- 79.US National Library of Medicine. Efficacy, Safety, and Pharmacokinetics Study of CJM112 in Hidradenitis Suppurativa Patients - Full Text View - ClinicalTrials.gov.; 2015. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02421172?term=cjm112&cond=Hidradenitis&rank=1. Accessed December 31, 2018.
- 80.Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277 e210–286 e210. doi: 10.1016/j.jaad.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 81.Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001. doi: 10.1111/bcp.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.US National Library of Medicine. A Study to Test the Efficacy, Safety and Pharmacokinetics of Bimekizumab in Subjects With Moderate to Severe Hidradenitis Suppurativa. - Full Text View - ClinicalTrials.gov.; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03248531?term=bimekizumab&cond=Hidradenitis&rank=1. Accessed December 31, 2018.
- 83.US Food & Drug Administration. TREMFYA (guselkumab) injection, for subcutaneous use; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761061Orig1s000lbl.pdf. Accessed December 31, 2018.
- 84.Kovacs M, Podda M. Guselkumab in the treatment of severe Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33(3):e140–e141. doi:10.1111/jdv.15368 [DOI] [PubMed] [Google Scholar]
- 85.Casseres RG, Kahn JS, Her MJ, Rosmarin D. Guselkumab in the treatment of hidradenitis suppurativa: a retrospective chart review. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 86.US National Library of Medicine. A Study to Evaluate the Efficacy, Safety, and Tolerability of Guselkumab for the Treatment of Participants With Moderate to Severe Hidradenitis Suppurativa (HS) (NOVA); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03628924. Accessed December 31, 2018.
- 87.US Food & Drug Administration. OTEZLA® (apremilast) tablets, for oral use label; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed December 31, 2018.
- 88.Weber P, Seyed Jafari SM, Yawalkar N, Hunger RE. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: a case series of 9 patients. J Am Acad Dermatol. 2017;76(6):1189–1191. doi: 10.1016/j.jaad.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 89.Vossen ARJVV, van der Zee HH, Prens EP. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80(1):80–88. doi:10.1016/j.jaad.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 90.US National Library of Medicine. Single Center Study of Apremilast for the Treatment of Hidradenitis Suppurativa - Full Text View - ClinicalTrials.gov.; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02695212?term=apremilast&cond=Hidradenitis&rank=1. Accessed December 31, 2018.
- 91.Guo RHM, Zenker O, Giamarellos-Bourboulis EJ, Riedemann N IFX-1 blocking the anaphylatoxin 1 blocking the anaphylatoxin 1 blocking the anaphylatoxin C5a – an anti an anti-inflammatory effect in patients with hidradenitis suppurativa; 2017. Available from: https://www.inflarx.de/dam/jcr:ec982d1e-f82b-4992-a1b0-ff6e69a04b6b/Guo_2017_poster.pdf. Accessed December 31, 2018.
- 92.US National Library of Medicine. Studying Complement Inhibition in Patients With Moderate to Severe Hidradenitis Suppurativa - Full Text View - ClinicalTrials.gov.; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT03001622?term=ifx+1&cond=Hidradenitis&rank=1. Accessed December 31, 2018.
- 93.US National Library of Medicine. Efficacy and Safety Study of IFX-1 in Patients With Moderate to Severe Hidradenitis Suppurativa (HS) (SHINE); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03487276. Accessed December 31, 2018.
- 94.US Food & Drug Administration. RITUXAN® (rituximab) injection, for intravenous use label; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103705s5450lbl.pdf. Accessed December 31, 2018.
- 95.Takahashi K, Yanagi T, Kitamura S, et al. Successful treatment of hidradenitis suppurativa with rituximab for a patient with idiopathic carpotarsal osteolysis and chronic active antibody-mediated rejection. J Dermatol. 2018;45(5):e116–e117. doi:10.1111/1346-8138.14144 [DOI] [PubMed] [Google Scholar]
- 96.US National Library of Medicine. A Placebo-Controlled Study of the Safety of INCB054707 in Participants With Hidradenitis Suppurativa - Full Text View - Clinicaltrials.gov; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03607487. Accessed December 31, 2018.
- 97.US National Library of Medicine. A Study of the Safety of INCB054707 in Participants With Hidradenitis Suppurativa - Full Text View - Clinicaltrials.gov.; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03569371. Accessed December 31, 2018.
- 98.Lee RA, Eisen DB. Treatment of hidradenitis suppurativa with biologic medications. J Am Acad Dermatol. 2015;73(5 Suppl 1):S82–88. doi: 10.1016/j.jaad.2015.07.053 [DOI] [PubMed] [Google Scholar]
- 99.Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30(6):989–994. doi: 10.1111/jdv.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831–839. doi: 10.1111/j.1365-2133.2009.09198.x [DOI] [PubMed] [Google Scholar]
- 101.Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49(8):950–955. doi: 10.1111/j.1365-4632.2010.04545.x [DOI] [PubMed] [Google Scholar]
- 102.Zouboulis CC, Tzellos T, Kyrgidis A, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. doi: 10.1111/bjd.15748 [DOI] [PubMed] [Google Scholar]
- 103.Menis D, Maronas-Jimenez L, Delgado-Marquez AM, Postigo-Llorente C, Vanaclocha-Sebastian F. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy. Br J Dermatol. 2015;172(3):810–811. doi: 10.1111/bjd.13292 [DOI] [PubMed] [Google Scholar]
- 104.US National Library of Medicine. Short-term Safety, Efficacy and Mode of Action of Apremilast in Moderate Suppurative Hidradenitis - Full Text View - ClinicalTrials.gov.; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03049267?term=apremilast&cond=Hidradenitis&rank=2. Accessed December 31, 2018.
- 105.Strober BE, Kim C, Siu K. Efalizumab for the treatment of refractory hidradenitis suppurativa. J Am Acad Dermatol. 2007;57(6):1090–1091. doi: 10.1016/j.jaad.2007.07.032 [DOI] [PubMed] [Google Scholar]
- 106.Koilakou S, Karapiperis D, Tzathas C. A case of hidradenitis suppurativa refractory to anti-TNFalpha therapy in a patient with Crohn‘s disease. Am J Gastroenterol. 2010;105(1):231–232. doi: 10.1038/ajg.2009.489 [DOI] [PubMed] [Google Scholar]
- 107.Moul DK, Korman NJ. The cutting edge. Severe hidradenitis suppurativa treated with adalimumab. Arch Dermatol. 2006;142(9):1110–1112. doi: 10.1001/archderm.142.9.1110 [DOI] [PubMed] [Google Scholar]
- 108.Harde V, Mrowietz U. Treatment of severe recalcitrant hidradenitis suppurativa with adalimumab. J Dtsch Dermatol Ges. 2009;7(2):139–141. doi: 10.1111/j.1610-0387.2008.06918.x [DOI] [PubMed] [Google Scholar]
- 109.Samycia M, Brassard A. Adalimumab in treatment-resistant hidradenitis suppurativa following recurrence after extensive affected area excision: a review of biologics therapy. J Cutan Med Surg. 2013;17 Suppl 1:S23–S32. doi: 10.2310/7750.2012.11144 [DOI] [PubMed] [Google Scholar]
- 110.Diamantova D, Lomickova I, Cetkovska P. Adalimumab treatment for hidradenitis suppurativa associated with Crohn‘s disease. Acta Dermatovenerol Croat. 2014;22(4):291–293. [PubMed] [Google Scholar]
- 111.Bosnic D, Zarkovic B, Baresic M, Zarkovic M, Anic B. Improvement of overlapping hidradenitis suppurativa and ankylosing spondylitis after the introduction of adalimumab. Reumatologia. 2016;54(6):321–325. doi: 10.5114/reum.2016.64910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shanmugam VK, Mulani S, McNish S, Harris S, Buescher T, Amdur R. Longitudinal observational study of hidradenitis suppurativa: impact of surgical intervention with adjunctive biologic therapy. Int J Dermatol. 2018;57(1):62–69. doi: 10.1111/ijd.13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crowley EL, O‘Toole A, Gooderham MJ. Hidradenitis suppurativa with SAPHO syndrome maintained effectively with adalimumab, methotrexate, and intralesional corticosteroid injections. SAGE Open Med Case Rep. 2018;6:2050313X18778723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molina-Leyva A, Badiola J. Severe refractory hidradenitis suppurativa successfully treated with adalimumab in an HIV-positive/hepatitis C virus-positive patient. Aids. 2018;32(16):2436–2438. doi: 10.1097/QAD.0000000000001998 [DOI] [PubMed] [Google Scholar]
- 115.Gorovoy I, Berghoff A, Ferris L. Successful treatment of recalcitrant hidradenitis suppurativa with adalimumab. Case Rep Dermatol. 2009;1(1):71–77. doi: 10.1159/000251217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149(10):1192–1194. doi: 10.1001/jamadermatol.2013.5377 [DOI] [PubMed] [Google Scholar]
- 117.Zangrilli A, Esposito M, Mio G, Mazzotta A, Chimenti S. Long-term efficacy of etanercept in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2008;22(10):1260–1262. doi: 10.1111/j.1468-3083.2008.02617.x [DOI] [PubMed] [Google Scholar]
- 118.Adams DR, Gordon KB, Devenyi AG, Ioffreda MD. Severe hidradenitis suppurativa treated with infliximab infusion. Arch Dermatol. 2003;139(12):1540–1542. doi: 10.1001/archderm.139.12.1540 [DOI] [PubMed] [Google Scholar]
- 119.Rosi YL, Lowe L, Kang S. Treatment of hidradenitis suppurativa with infliximab in a patient with Crohn‘s disease. J Dermatolog Treat. 2005;16(1):58–61. doi: 10.1080/09546630410024547 [DOI] [PubMed] [Google Scholar]
- 120.Husein-ElAhmed H, Fernandez-Pugnaire MA, Ruiz-Carrascosa JC. Severe hidradenitis suppurative in an HIV-positive male: use of multiple treatment modalities, including tumor necrosis factor blockade. AIDS Patient Care STDS. 2011;25(9):507–508. doi: 10.1089/apc.2011.0158 [DOI] [PubMed] [Google Scholar]
- 121.von Preussen AC, Flux K, Hartschuh W, Hartmann M. Acne inversa successfully treated with infliximab. Int J Dermatol. 2012;51(8):1011–1013. doi: 10.1111/j.1365-4632.2010.04670.x [DOI] [PubMed] [Google Scholar]
- 122.Lebwohl B, Sapadin AN. Infliximab for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2003;49(5 Suppl):S275–276. doi: 10.1016/S0190 [DOI] [PubMed] [Google Scholar]
- 123.Thielen AM, Barde C, Saurat JH. Long-term infliximab for severe hidradenitis suppurativa. Br J Dermatol. 2006;155(5):1105–1107. doi: 10.1111/j.1365-2133.2006.07528.x [DOI] [PubMed] [Google Scholar]
- 124.Alecsandru D, Padilla B, Izquierdo JA, Fernandez-Cruz E, Sanchez-Ramon S. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146(12):1343–1345. doi: 10.1001/archdermatol.2010.372 [DOI] [PubMed] [Google Scholar]
- 125.Poulin Y. Successful treatment of hidradenitis suppurativa with infliximab in a patient who failed to respond to etanercept. J Cutan Med Surg. 2009;13(4):221–225. doi: 10.2310/7750.2008.08034 [DOI] [PubMed] [Google Scholar]
- 126.Kozub P, Simaljakova M. Hidradenitis suppurativa treated with combination of infliximab and dapsone. Bratisl Lek Listy. 2012;113(5):319–323. [DOI] [PubMed] [Google Scholar]
- 127.Giuseppe P, Nicola P, Valentina C, et al. A case of moderate hidradenitis suppurativa and psoriasis treated with secukinumab. Ann Dermatol. 2018;30(4):462–464. doi: 10.5021/ad.2018.30.4.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jorgensen AR, Yao Y, Thomsen SF. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med. 2018;2018:8685136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos-Perez MI, Garcia-Rodicio S, Del Olmo-Revuelto MA, Pozo-Roman T. Ustekinumab for hidradenitis suppurativa: a case report. Actas Dermosifiliogr. 2014;105(7):720–722. doi: 10.1016/j.ad.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 130.Sharon VR, Garcia MS, Bagheri S, et al. Management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92(3):320–321. doi: 10.2340/00015555-1229 [DOI] [PubMed] [Google Scholar]
- 131.Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145(5):580–584. doi: 10.1001/archdermatol.2009.49 [DOI] [PubMed] [Google Scholar]
- 132.Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies. Australas J Dermatol. 2014;55(2):128–131. doi: 10.1111/ajd.12136 [DOI] [PubMed] [Google Scholar]
- 133.Patil S. Low-dose adalimumab biosimilar (ZRC-3197) in the treatment of hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2018;84(6):745–747. doi: 10.4103/ijdvl.IJDVL_232_18 [DOI] [PubMed] [Google Scholar]
- 134.Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis suppurativa. Br J Dermatol. 2006;154(4):726–729. doi: 10.1111/j.1365-2133.2005.07067.x [DOI] [PubMed] [Google Scholar]
- 135.Lasocki A, Sinclair R, Foley P, Saunders H. Hidradenitis suppurativa responding to treatment with infliximab. Australas J Dermatol. 2010;51(3):186–190. doi: 10.1111/j.1440-0960.2010.00623.x [DOI] [PubMed] [Google Scholar]
- 136.Elkjaer M, Dinesen L, Benazzato L, Rodriguez J, Logager V, Munkholm P. Efficacy of infliximab treatment in patients with severe fistulizing hidradenitis suppurativa. J Crohns Colitis. 2008;2(3):241–245. doi: 10.1016/j.crohns.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 137.Antonucci A, Negosanti M, Negosanti L, Iozzo I, Varotti C. Acne inversa treated with infliximab: different outcomes in 2 patients. Acta Derm Venereol. 2008;88(3):274–275. doi: 10.2340/00015555-0397 [DOI] [PubMed] [Google Scholar]
- 138.Brunasso AM, Delfino C, Massone C. Hidradenitis suppurativa: are tumour necrosis factor-alpha blockers the ultimate alternative? Br J Dermatol. 2008;159(3):761–763. doi: 10.1111/j.1365-2133.2008.08735.x [DOI] [PubMed] [Google Scholar]
- 139.Moschella SL. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? A report of three treated cases. Int J Dermatol. 2007;46(12):1287–1291. doi: 10.1111/j.1365-4632.2007.03293.x [DOI] [PubMed] [Google Scholar]
- 140.Sullivan TP, Welsh E, Kerdel FA, Burdick AE, Kirsner RS. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149(5):1046–1049. [DOI] [PubMed] [Google Scholar]
- 141.Torres T, Selores M. Treatment of hidradenitis suppurativa with infliximab. An Bras Dermatol. 2010;85(4):576. doi: 10.1590/S0365-05962010000400028 [DOI] [PubMed] [Google Scholar]
- 142.Usmani N, Clayton TH, Everett S, Goodfield MD. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32(2):204–205. [DOI] [PubMed] [Google Scholar]
- 143.Fernandez-Vozmediano JM, Armario-Hita JC. Infliximab for the treatment of hidradenitis suppurativa. Dermatology. 2007;215(1):41–44. doi: 10.1159/000102032 [DOI] [PubMed] [Google Scholar]
- 144.Zhang J, Reeder VJ, Hamzavi IH. Use of biologics in the treatment of hidradenitis suppurativa: a review of the Henry Ford Hospital experience. Br J Dermatol. 2014;171(6):1600–1602. doi: 10.1111/bjd.13186 [DOI] [PubMed] [Google Scholar]
- 145.DeFazio MV, Economides JM, King KS, et al. Outcomes after combined radical resection and targeted biologic therapy for the management of recalcitrant hidradenitis suppurativa. Ann Plast Surg. 2016;77(2):217–222. doi: 10.1097/SAP.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bahillo Monne C, Honorato Guerra S, Schoendorff Ortega C, Gargallo Quintero AB. Management of hidradenitis suppurativa with biological therapy: report of four cases and review of the literature. Dermatology. 2014;229(4):279–287. doi: 10.1159/000365076 [DOI] [PubMed] [Google Scholar]
- 147.Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26(7):911–914. doi: 10.1111/j.1468-3083.2011.04123.x [DOI] [PubMed] [Google Scholar]
- 148.Van Rappard DC, Mekkes JR. Treatment of severe hidradenitis suppurativa with infliximab in combination with surgical interventions. Br J Dermatol. 2012;167(1):206–208. doi: 10.1111/j.1365-2133.2012.10807.x [DOI] [PubMed] [Google Scholar]
- 149.Martin-Ezquerra G, Masferrer E, Masferrer-Niubo M, et al. Use of biological treatments in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29(1):56–60. doi: 10.1111/jdv.12438 [DOI] [PubMed] [Google Scholar]
- 150.Bettoli V, Manfredini M, Calamo G. et al. Long-term adalimumab treatment of hidradenitis suppurativa: results and practical insights from a real-life experience. Dermatol Ther;2018:e12737. doi: 10.1111/dth.12737 [DOI] [PubMed] [Google Scholar]
- 151.Kyriakou A, Trigoni A, Galanis N, Sotiriadis D, Patsatsi A. Efficacy of adalimumab in moderate to severe hidradenitis suppurativa: real life data. Dermatol Rep. 2018;10(2):7859. doi: 10.4081/dr.2018.7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Rappard DC, Leenarts MF, Meijerink-van ‘T Oost L, Mekkes JR. Comparing treatment outcome of infliximab and adalimumab in patients with severe hidradenitis suppurativa. J Dermatolog Treat. 2012;23(4):284–289. doi: 10.3109/09546634.2011.571657 [DOI] [PubMed] [Google Scholar]
- 153.Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-alpha therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013. Br J Dermatol. 2016;174(3):667–670. doi: 10.1111/bjd.14199 [DOI] [PubMed] [Google Scholar]
- 154.Giamarellos-Bourboulis EJ, Sobell J, Ryan C, Wolkenstein PJ, Geng Z, Mulder GD. Infection-free clinical response among patients with hidradenitis suppurativa who were treated with adalimumab: results from two phase 3 studies. Wounds. 2017;29(11):E98–E102. [PubMed] [Google Scholar]
- 155.Kimball AB, Sundaram M, Shields AL, et al. Adalimumab alleviates skin pain in patients with moderate-to-severe hidradenitis suppurativa: secondary efficacy results from the PIONEER I and PIONEER II randomized controlled trials. J Am Acad Dermatol. 2018;79(6):1141–1143. doi: 10.1016/j.jaad.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 156.Jimenez-Gallo D, de la Varga-Martinez R, Ossorio-Garcia L, Collantes-Rodriguez C, Rodriguez C, Linares-Barrios M. Effects of adalimumab on T-helper-17 lymphocyte- and neutrophil-related inflammatory serum markers in patients with moderate-to-severe hidradenitis suppurativa. Cytokine. 2018;103:20–24. doi: 10.1016/j.cyto.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 157.Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49(12):1445–1449. doi: 10.1111/j.1365-4632.2010.04638.x [DOI] [PubMed] [Google Scholar]