Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is considered as the most frequent cause of chronic hepatic disease in adults. It is strictly correlated with insulin resistance, frequently associated with components of metabolic syndrome, and, similarly to the latter, it has been correlated with high risk of developing type 2 diabetes and cardiovascular diseases. Systemic arterial hypertension has been suggested to be associated with NAFLD in approximately 40% of the cases, and NAFLD has been independently associated with an increased risk of arterial hypertension in observational studies. Therefore, we can infer that treating arterial hypertension in NAFLD carriers will be often necessary and that the potential beneficial effects of the antihypertensive might, in this context, influence the choice of the respective drug. The renin-angiotensin system has been correlated to the whole basic physiopathogenic mechanism of NAFLD in experimental models. Based on these findings, we conducted this study to evaluate the effects of the ACE-inhibitor ramipril, used preventively, in NAFLD induced in rabbits fed hyperlipidaemic diet. Methods: Twenty-nine rabbits were divided into three groups (normal, placebo, and ramipril). The placebo and ramipril groups were fed a ration containing 0.925% cholesterol. The groups were orally administered 0.35 mg/kg/day of ramipril, and an equivalent volume of vehicle was administered to the placebo group. At the end of the 8th week, all rabbits underwent segmental hepatic resection and were euthanized. Blood samples were collected to determine glucose, insulin, creatinine, total cholesterol, triglycerides, HDL-C, and aminotransferase levels at baseline and euthanasia. Haematoxylin and eosin and Gomori trichrome-stained slides were analysed based on the histological scoring system for NAFLD. Sudan III-stained slides were analysed by morphometry and immunostained based on the Allred scoring system. Results: When compared with placebo, ramipril significantly diminished the development of steatosis (P=0.032), lobular inflammation (P=0.006), hepatocellular ballooning (P=0.023), and fibrosis (P=0.02). Based on the NAFLD activity score (NAS), ramipril significantly reduced the development of non-alcoholic steatohepatitis (NASH) (P=0.003). Conclusions: The preventive use of ramipril in rabbits fed hyperlipidaemic diet, attenuates the development of the whole NAFLD histopathological spectrum and based on NAS, ramipril significantly reduced the development of NASH.
Keywords: Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, renin-angiotensin system, hypertension, atherosclerosis, insulin resistance
Introduction
Non-alcoholic fatty liver disease (NAFLD) is considered as the most frequent cause of chronic hepatic disease in adults [1,2]. It is strictly correlated with insulin resistance (IR) [3-5], frequently associated with components of metabolic syndrome (MS) [5-7], and, similarly to the latter, it has been correlated with high risk of developing type 2 diabetes (T2DM) [5] and cardiovascular diseases (CVD) [8-13]. IR has been considered the basic cause of lipid accumulation in hepatocytes (first hit) which generates oxidative stress, lipid peroxidation, and inflammatory response (second hit) thus resulting in hepatocellular lesion and fibrotic substitution.
The renin-angiotensin system (RAS) has been correlated to the whole basic physiopathogenic mechanism of NAFLD in experimental models. Systemic infusion of angiotensin 2 (AT2) in rats induced IR [14] and hepatic oxidative stress, increased the serum levels and hepatic concentration of tumor necrosis factor-alpha (TNF-α), promoted the infiltration of inflammatory cells in the liver, increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and the activation of hepatic stellate cells (HSC) [15]. In human HSC culture, AT2 increased the production of reactive oxygen species and the expression of genes involved in hepatic fibrogenesis [16]. In rat HSC culture, AT2 stimulated the release of type I collagen and transforming growth factor beta 1 (TGF-β1) by activating its type 1 receptor [16]. In transgenic rats with high AT2 tissue levels, a significant increase in hepatic oxidative stress associated with an enhanced NADPH oxidase activity was observed [17].
In NAFLD animal models, namely rats, significant results were obtained with the use of angiotensin-converting enzyme (ACE) inhibitor perindopril [18] and angiotensin 2 type 1 receptor (AT1) antagonists irbesartan [18], telmisartan [19,20], and olmesartan [21,22]. A significant improvement was observed in steatosis, inflammatory response, and hepatic fibrosis with the aforementioned drugs [18-22]. IR, as assessed by the homeostasis model assessment index, improved with perindopril, irbesartan [18], and olmesartan [21]. Oxidative stress improved with telmisartan [19] and olmesartan [21,22]. In hypercholesterolaemic rabbits, olmesartan prevented lobular inflammation and fibrosis, while reducing significantly steatosis and steatohepatitis (NASH), as assessed by the NAFLD activity score (NAS) [23].
A significant prevalence of MS in NAFLD carriers (> 40%) [1], an increased risk to develop NAFLD in MS carriers [6], and the correlation of this syndrome with NAFLD progression and severity have been reported [3]. Systemic arterial hypertension has been suggested to be associated with NAFLD in approximately 40% of the cases [1], and NAFLD has been independently associated with an increased risk of arterial hypertension in observational studies [24,25]. Therefore, we can infer that treating arterial hypertension in NAFLD carriers will be often necessary and that the potential beneficial effects of the antihypertensive might, in this context, influence the choice of the respective drug.
Based on these findings, we conducted this study to evaluate the effects of the ACE-inhibitor ramipril, used preventively, in NAFLD induced in rabbits fed hyperlipidaemic diet.
Methods
Animals, and biochemical assays
Twenty-nine white adult male rabbits (New Zealand), with a mean age of 4 months, were selected for this study. The animals were handled in compliance with the Guiding Principles in the Care and Use of Animals, and protocol approval was obtained from the Pontifical Catholic University Animal Research Committee. The animals were divided into three groups: placebo group, 10 rabbits; ramipril group, 10 rabbits; and control group, 9 rabbits. During the 56 days of the study, the animals from control group were fed a specific diet (Nuvilab; Nuvital, Colombo, Brazil) that does not cause metabolic abnormalities. The animals from placebo group and ramipril group were fed a specific diet plus 0.925% cholesterol (cholesterol ≥ 92.5%; Sigma-Aldrich, St. Louis, USA). All groups were fed the respective diet ad libitum. With regard to the Ramipril group, 0.35 mg/kg/day of ramipril (commercially available from Sanofi Aventis) was administered orally from day 1 to day 56. The placebo (vehicle) was administered orally to placebo group throughout the study period at the same dosage. On day 56, the animals from the three groups underwent liver resection. Anaesthesia was induced with ketamine (30 mg/kg) and intramuscular xylazine (6 mg/kg). After the procedure, the rabbits were euthanized with a lethal dose of barbiturate. Blood samples were obtained on the first day of the experiment and immediately before euthanasia by cardiac puncture. Clinical laboratory assessment included fasting serum glucose, insulin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Measurements were obtained using two automated systems (Dimension RxL Max; Siemens, Munich, Germany. Abbott Architect i1000SR; Abbott, Chicago, USA).
Histological, morphometric, and immunohistochemistry analyses
The liver segments of the right lateral and left medial lobes were fixed with 10% formaldehyde buffered with phosphate and then paraffin embedded for haematoxylin and eosin staining and Gomori trichrome staining. Slides with the two histological sections (4 µm thick) were prepared and analysed by blinded observers for steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis based on the histological scoring system for NAFLD [26] using an Olympus BX 50 microscope (Tokyo, Japan).
For Sudan III staining, cryofixation and frozen sections of the liver segments was performed. The Sudan III stained slides were photographed with a digitizer (Axio Scan.Z1; ZEISS, Oberkochen, Germany), and images were analysed to estimate hepatic steatosis with an image analyser (Image-Pro Plus 4.5.0.29; Media Cybernetics, Rockville, USA). For each slide, approximately 600 images were obtained using a 20× objective. Approximately 500 images were excluded, resulting in approximately 100 satisfactory images per animal. The positive control was used as a “mask” displaying sufficient levels of tissue stained. The mask was then superimposed on images of the slides and an image analysis was performed to identify the positive areas in the slides based on the ideal pattern staining indicated by the mask, and these results were transformed to measures of positive areas in square micrometres (μm2).
Immunohistochemistry was performed to estimate the expression of inducible nitric oxide synthase (iNOS) in liver tissue as an indirect parameter of nitrative/oxidative stress. Histological sections were stained with mouse monoclonal anti-iNOS antibody (1/200 dilution; ab129372, Abcam), and immunostained slides were analysed blindly based on the Allred score [27], using an Olympus BX 50 microscope (Tokyo, Japan). The Allred score is defined as the sum of the proportion and intensity scores. The proportion score is determined by the overall proportion of stained cells (0= none; 1= < 1/100; 2=1/100 to < 1/10; 3=1/10 to < 1/3; 4=1/3 to 2/3; 5= > 2/3) and the intensity score by the intensity of staining in the positive cells (0= none; 1= weak; 2= intermediate; 3= strong).
Statistical analysis
Data were analysed with software SPSS, version 20 (IBM, New York, USA). Results are described by means and standard deviations or by frequencies and percentages. The interaction between group (placebo, ramipril, and control) and time (baseline and euthanasia) was analysed using ANOVA (mixed model) with one between-subjects factor (group) and one within-subjects factor (time). Considering that most of the variables presented a significant interaction between group and time, the analysis was performed using the one-way ANOVA model (group comparisons at baseline and euthanasia) and paired Student t-test (time comparisons for each group). Effect size was reported by eta-squared measures, r-value, and Cohen’s d. Categorical variables were analysed by Fisher’s exact test. Normality distribution was evaluated using Shapiro-Wilk test, and data from variables that did not meet this condition were submitted to a logarithmic transformation. A p-value < 0.05 was indicative of statistical significance.
Results
Metabolic and lipid profiles
The weight of the animals at baseline was similar in the three groups. The average body weight of rabbits in the control group was higher than that in the other groups, and no difference was observed between the ramipril and placebo groups at euthanasia (Table 1).
Table 1.
Weight and serum biochemical parameters at baseline and eutanásia
| PHASE | GROUP | n | WT | GLC | INS | CREAT |
|---|---|---|---|---|---|---|
| Baseline | Placebo | 10 | 2996±250 | 141.0±32.6 | 5.00±1.58 | 1.16±0.20 |
| Ramipril | 10 | 3080±314 | 123.7±48.0 | 4.59±1.09 | 1.23±0.22 | |
| Control | 9 | 3255±329 | 163.7±37.6 | 5.1±2.27 | 1.28±0.15 | |
| p-value (η2)* | 0.179 (η2=0.12) | 0.055 (η2=0.20) | 0.910 (η2=0.01) | 0.394 (η2=0.07) | ||
| Euthanasia | Placebo | 10 | 2989±255a | 150.8±22.3 | 3.09±2.24a | 1.44±0.29 |
| Ramipril | 10 | 3182±197a | 125.3±43.1 | 2.08±2.01a | 1.47±0.26 | |
| Control | 9 | 3541±374 | 142.0±25.9 | 6.7±3.43 | 1.23±0.20 | |
| p-value (η2)* | 0.001 (η2=0.42) | 0.160 (η2=0.13) | 0.002 (η2=0.39) | 0.099 (η2=0.16) | ||
| Baseline x Euthanasia | Placebo | 10 | 0.904 (d=0.04) | 0.234 (d=0.40) | 0.045 (d=0.74) | 0.007 (d=1.11) |
| p-value (Cohen’s d)** | Ramipril | 10 | 0.346 (d=0.31) | 0.938 (d=0.03) | 0.004 (d=1.19) | 0.049 (d=0.72) |
| Control | 9 | 0.001 (d=1.79) | 0.240 (d=0.42) | 0.280 (d=0.39) | 0.544 (d=0.21) |
Data represent means ± standard deviations. WT, weight (grams); GLC, glucose (mg/dl); INS, insulin (µUI/ml); CREAT, creatinine (mg/dl).
oneway ANOVA and LSD post hoc test; η2, eta-squared effect size measure;
paired Student’s t test; d, Cohen’s d effect size measure;
significant difference compared to control group (WT): placebo (P < 0.001, r=0.68), ramipril (P=0.010, r=0.54); INS: placebo (P=0.016, r=0.57), ramipril (P < 0.001, r=0.70).
Glucose, insulin, creatinine, aminotransferase, and lipid serum levels were also similar in the three groups at baseline, and these parameters were similar between the placebo and ramipril groups at euthanasia (Tables 1 and 2). Compared to the control group, there was a significant increase in lipid and AST levels, and a significant decrease in insulin levels in the placebo and ramipril groups at euthanasia (Tables 1 and 2). Insulin levels in the control group were similar at baseline and euthanasia (Table 1).
Table 2.
Serum aminotransferase and lipids at baseline and euthanasia
| PHASE | GROUP | AST | ALT | CHOL | HDL | TGC |
|---|---|---|---|---|---|---|
| Baseline | Placebo | 52.8±14.8 | 85.9±29.7 | 31.9±16.2 | 22.4±11.0 | 49.5±16.3 |
| Ramipril | 51.2±18.1 | 68.7±29.2 | 38.4±28.0 | 22.5±9.2 | 45.7±19.4 | |
| Control | 70.0±43.7 | 92.2±47.8 | 18.4±10.7 | 14.7±6.6 | 70.7±34.6 | |
| p-value* | 0.463 | 0.332 | 0.107 | 0.128 | 0.099 | |
| Euthanasia | Placebo | 86.1±32.8a | 98.5±47.9a | 970.7±156.6a | 306.2±72.1a | 384.9±307.7a |
| Ramipril | 86.5±39.6a | 104.3±54.1a | 989.3±102.7a | 234.2±80.6a,b | 282.7±175.6a | |
| Control | 50.6±23.0 | 92.7±37.7 | 18.1±6.19 | 13.0±6.2 | 76.2±48.6 | |
| p-value* | 0.019 | 0.876 | < 0.001 | < 0.001 | 0.001 | |
| Baseline x Euthanasia | Placebo | 0.023 (d=0.87) | 0.383 (d=0.29) | < 0.001 (d=6.29) | < 0.001 (d=4.06) | 0.007 (d=1.09) |
| p-value (Cohen’s d)** | Ramipril | 0.027 (d=0.84) | 0.120 (d=0.54) | < 0.001 (d=10.3) | < 0.001 (d=2.67) | 0.002 (d=1.34) |
| Control | 0.218 (d=0.45) | 0.976 (d=0.01) | 0.919 (d=0.03) | 0.479 (d=0.25) | 0.769 (d=0.10) |
Data represent means ± standard deviations. AST, aspartate aminotransferase (U/L); ALT, alanine aminotransferase (U/L); CHOL, total cholesterol (mg/dl); HDL, high-density lipoprotein cholesterol (mg/dl); TGC, triglyceride (mg/dl).
Oneway ANOVA and LSD post hoc test;
Paired Student’s t test.
Significant difference compared to control group (AST): placebo (P=0.013, r=0.55), ramipril (P=0.013, r=0.50); CHOL: placebo (P < 0.001, r=0.98), ramipril (P < 0.001, r=0.99); HDL: placebo (P < 0.001, r=0.95), ramipril (P < 0.001, r=0.89); TGC: placebo (P=0.004, r=0.58), ramipril (P=0.042, r=0.64).
Significant difference compared to placebo group (HDL): P=0.018, r=0.44.
Histological, morphometric, and immunohistochemistry analysis
Steatosis was observed in all animals of the placebo group and in 90% of the ramipril group. The lowest steatosis score was observed in 40% of the ramipril group, which was not observed in the placebo group. The highest steatosis score was noted in 10% and 50% of the ramipril and placebo groups, respectively (Table 3). The comparison between the two groups showed a significant difference (P=0.032), pointing to a decrease in steatosis with ramipril (Figure 1). All animals of both placebo and ramipril groups developed lobular inflammation. However, 90% of the ramipril group developed slight inflammation (score 1), and the highest inflammation score was not observed in any animal of this group (Table 3). A significant difference was found between the two groups (P=0.006), showing a decreased inflammatory response with ramipril (Figure 1).
Table 3.
Results of histological analysis according to the histological scoring system for NAFLD
| ITEM | DEFINITION | SCORE | PERCENTAGE FOR GROUP | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo | Ramipril | Control | ||||
| Steatosis | < 5% | 0 | 0% | 10% | 100% | 0.032 |
| Grade | 5 a 33% | 1 | 0% | 40% | ||
| > 33 a 66% | 2 | 50% | 40% | |||
| > 66% | 3 | 50% | 10% | |||
| Lobular | No foci | 0 | 0% | 0% | 100% | 0.006 |
| Inflammation | < 2 foci per 200X field | 1 | 20% | 90% | ||
| 2-4 foci per 200X field | 2 | 40% | 10% | |||
| > 4 foci per 200X field | 3 | 40% | 0% | |||
| Hepatocellular | None | 0 | 0% | 10% | 100% | 0.023 |
| Balooning | Few balloon cells | 1 | 20% | 70% | ||
| Many cells/prominent ballooning | 2 | 80% | 20% | |||
| Fibrosis Stage | None | 0 | 0% | 0% | 100% | 0.020 |
| Perisinusoidal or periportal | 1 | 0% | 0% | |||
| Mild, zone 3, perisinusoidal | 1A | 20% | 60% | |||
| Moderate, zone 3, perisinusoidal | 1B | 10% | 30% | |||
| Portal/periportal | 1C | 50% | 10% | |||
| Perisinusoidal and portal/periportal | 2 | 20% | 0% | |||
| NAS | Not NASH | 0-2 | 0% | 20% | 100% | 0.003 |
| Borderline | 3-4 | 0% | 50% | |||
| NASH | ≥ 5 | 100% | 30% | |||
NAS, nonalcoholic fatty liver disease activity score; NASH, nonalcoholic steatohepatitis. p, p value: placebo vs ramipril (Fisher’s exact test, after grouping the scores into two categories).
Figure 1.
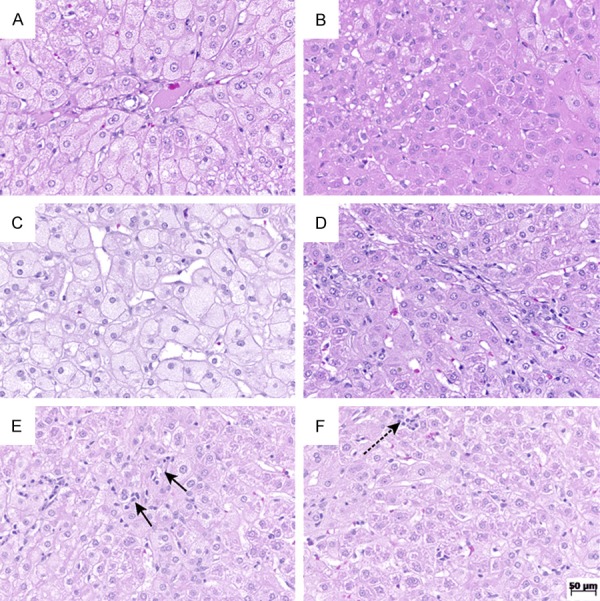
Hematoxylin and eosin stain at original magnification, 200×. A. Rabbit from placebo group with high score of ballooning; B. Liver from the ramipril group with preserved lobular architecture, showing no ballooning; C. Rabbit from placebo group with high score of steatosis; D. Rabbit from the ramipril group with minor degree of steatosis; E. Rabbit from the placebo group with high score of lobular inflammation; F. Rabbit from the ramipril group with score 1 of lobular inflammation.
Hepatocellular ballooning occurred in all animals of the placebo group and in 90% of the ramipril group. However, the lowest ballooning score occurred in 70% and 20% of the ramipril and placebo groups, respectively (Table 3). The comparison between the two groups showed a significant reduction (P=0.023) of ballooning degeneration with ramipril (Figure 1). Fibrosis scores between 1A and 1C and between 1A and 2 were observed in the placebo and ramipril groups, respectively. Fibrosis scores 1C and 2 were observed in 90% of the placebo group, and lower scores (1A e 1B) in 70% of the animals belonging to the ramipril group (Table 3). A significant difference was found between the two groups (P=0.02), pointing to a reduction of hepatic fibrosis with ramipril (Figure 2). NAS correlated with the presence of NASH (score ≥ 5) in the entire placebo group and in 30% of the ramipril group. Scores ≤ 2 correlated with the absence of NASH, which occurred in 20% of the ramipril group (Table 3). Comparison between the two groups showed a significant difference, suggesting an attenuating effect of ramipril in the development of NASH (P=0.003). None of the histological alterations that compose the histological scoring system for NAFLD were observed in the control group. Therefore, the control group was characterized as normal (Table 3; Figure 3).
Figure 2.
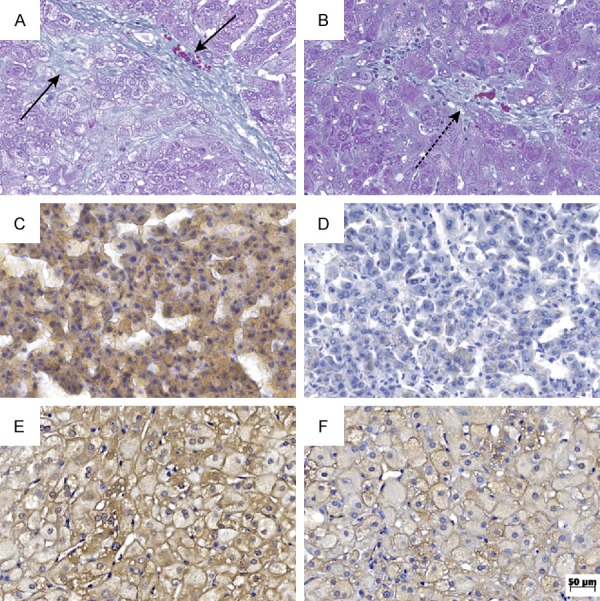
Photomicrographs at original magnification, 200×. A. Liver from the placebo group with score 2 of fibrosis (Gomori staining); B. Fibrosis score 1A observed in the ramipril group (Gomori staining); C. Rabbit from the placebo group with high score of steatosis (Sudan III stain); D. Rabbit from the ramipril group with minor degree of steatosis (Sudan III stain); E. Rabbit from the placebo group with high Allred score (iNOS immunostaining); F. Rabbit from the ramipril group with Allred score 5 (iNOS immunostaining).
Figure 3.
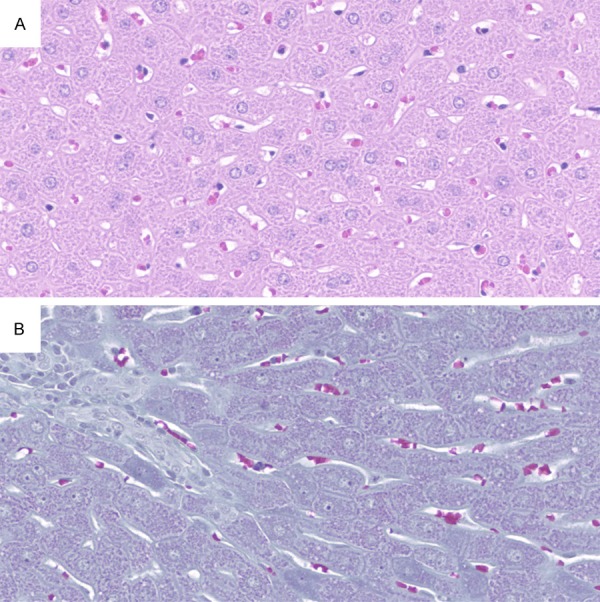
Photomicrographs from the control group at original magnification, 200×. Liver with preserved lobular architecture, showing no histopathological changes. A. Hematoxylin and eosin stain; B. Gomori staining.
The mean area of positivity was estimated by morphometry of Sudan III-stained slides, in the placebo (19.9±10.6 µm2) and ramipril (11±8.2 µm2) groups. Comparison between the two groups showed a significant difference (P=0.049), in agreement with the histological analysis of haematoxylin and eosin-stained slides (Figure 2).
The expression of iNOS, estimated by Allred score, in the placebo group was significantly higher compared with ramipril (P < 0.001), suggesting a reduction of iNOS expression with ramipril (Figure 2).
Discussion
Approximately four decades after the description of NAFLD, despite growing interest and efforts of the scientific community, its pharmacological therapy remains undefined. No drugs have been approved by the regulatory organs for NAFLD or NASH treatment [28-30]. The present recommendation is based on the approach used for associated diseases such as obesity, insulin resistance, diabetes, and dyslipidaemia [28,31]. Hypertension is significantly prevalent in adults and is frequently associated with NAFLD [1,24,25]. Therefore, we can infer that criteria for the choice of antihypertensive drugs in this condition are needed.
In the present study, ramipril was used in a dosage calculated by allometric extrapolation, equivalent to approximately 5 mg per day for humans [32]. Compared with placebo, ramipril significantly decreased steatosis, lobular inflammation, and hepatic fibrosis (Table 3). Similar results were observed in other rat models of NAFLD with the ACE-inhibitor perindopril and the AT1 blockers irbesartan, telmisartan, and olmesartan [18-22]. Nevertheless, in these studies, NASH was not adequately characterised according to established histological criteria [31]. In the present study, placebo group rabbits developed histological alterations resembling human NASH, and in the ramipril group all these histological alterations, including steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis were significantly attenuated. Furthermore, histological analysis was performed based on a validated [26] and recognized [31] histological scoring system for NAFLD.
NAS is defined as an unpondered sum of steatosis, lobular inflammation, and ballooning scores. In the validation study, scores ≤ 2 and ≥ 5 were correlated with the absence and presence of NASH, respectively. Scores 3 and 4 were distributed nearly evenly among the three defined diagnostic categories (not NASH, borderline, and NASH) [26]. In our study, the presence of NASH, estimated by NAS, was observed in all rabbits of the placebo group and in 30% of the ramipril group. NAS correlated with the absence of NASH in 20% of the ramipril group. A comparison between both groups showed a significant difference, suggesting an attenuating effect of ramipril in the development of steatohepatitis (Table 3). A similar result was observed in an experiment with olmesartan in the same animal model using the same histological analysis criteria [23]. Although these studies evaluated different forms of renin-angiotensin system blockade, these results may be considered consonant with our results.
The weight of the animals in the ramipril and placebo groups, as well as the total cholesterol and triglyceride levels, was similar at baseline and euthanasia (Tables 1 and 2). Therefore, these variables did not influence the results. Glucose, insulin, aminotransferases and creatinine levels were also similar in both groups (Tables 1 and 2), suggesting that these parameters had no influence in the histological results. The behaviour of the glucose and aminotransferases serum levels was similar in a study using olmesartan in the same animal model [23], suggesting an inherent pattern to the model used. The lack of difference between the placebo and ramipril groups in the analysis of glucose, insulin, and aminotransferases variables might characterise a limitation of the model used in the present study. However, the whole histopathological spectrum of NAFLD, as well as the difference between the groups was well characterised. The disease was induced by hyperlipidaemia. This was associated with NAFLD, found in 69.16% of the cases in a recently published meta-analysis [1]. Moreover, no standard animal model for NAFLD exists currently. In our study, peripheral IR was not assessed by euglycaemic hyperinsulinaemic clamp, considered the gold standard for this evaluation. However, the glucose levels were similar in the three groups, and the levels of insulin were lower in the placebo and ramipril groups compared with the control group at euthanasia. Furthermore, insulin levels in the control group were similar at baseline and at euthanasia (Table 1). Therefore, we can infer that peripheral IR was not characterised in our study, raising the possibility that histological alterations compatible with NAFLD developed in the model used were related with hepatic insulin resistance. In other animal models, the increase of iNOS hepatic expression has been associated with hepatic IR [33-35] and nitro-oxidative stress [33,34]. In the present study, iNOS expression, estimated by the analysis of immunostained slides, was significantly higher in the placebo group compared with the ramipril group (P < 0.001), suggesting that a reduction of hepatic iNOS expression was observed with ramipril.
The widely described correlation between NAFLD and IR [3-5], frequent association of this hepatopathy with MS components [5-7] in 39.34% of hypertension cases [1], increased risk of NAFLD-carriers to develop T2DM [10] and CVD [8-13], increased mortality rate due to CVD in NAFLD carriers [36,37], and the wide association of RAS with the pathophysiology of this hepatic disease [14-17] might influence a possible definition of criteria for the choice of drugs to treat patients with NAFLD and hypertension.
ACE inhibitors (captopril, enalapril, lisinopril, ramipril, and trandolapril) and AT1 blockers (losartan, valsartan, and candesartan) significantly decreased T2DM incidence in randomised and controlled clinical trials, selected in two meta-analyses [38,39]. Ramipril, in randomised clinical trial, controlled placebo, reduced CVD mortality, the incidence of acute myocardial infarction, and stroke in high-risk patients [40].
In the present study, ramipril significantly reduced the development of steatosis, lobular inflammation, and hepatic fibrosis. Based on the criteria of NAS, ramipril significantly diminished the development of NASH. Therefore, we can conclude that in rabbits fed hyperlipidaemic diet, the preventive use of ramipril attenuates the development of the whole NAFLD histopathological spectrum. Further studies are required to confirm these findings, increase the knowledge on the association of NAFLD and hypertension, and consolidate the criteria to choose the most appropriate drugs or therapeutic classes for the treatment of arterial hypertension in NAFLD carriers.
Disclosure of conflict of interest
None.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the united states from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 5.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB Jr, Kempf J, Zinman B, Haffner SM. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 6.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 8.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, Lee HS, Larson J, Therneau TM, Kim WR. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325–1330. doi: 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- 14.Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002;40:872–879. doi: 10.1161/01.hyp.0000040262.48405.a8. [DOI] [PubMed] [Google Scholar]
- 15.Bataller R, Gabele E, Morris T, Lehnert M, Yang L, Brenner DA. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G642–651. doi: 10.1152/ajpgi.00037.2003. [DOI] [PubMed] [Google Scholar]
- 16.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG (mRen2) 27 (Ren2) rats. J Hepatol. 2008;49:417–428. doi: 10.1016/j.jhep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toblli JE, Muñoz MC, Cao G, Mella J, Pereyra L, Mastai R. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese zucker rats. Obesity. 2008;16:770–776. doi: 10.1038/oby.2007.114. [DOI] [PubMed] [Google Scholar]
- 19.Fujita K, Yoneda M, Wada K, Mawatari H, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Maeyama S, Saito S, Iwasaki T, Terauchi Y, Nakajima A. Telmisartan, an angiotensin II type 1 receptor blocker, controls progress of nonalcoholic steatohepatitis in rats. Dig Dis Sci. 2007;52:3455–3464. doi: 10.1007/s10620-007-9741-4. [DOI] [PubMed] [Google Scholar]
- 20.Kudo H, Yata Y, Takahara T, Kawai K, Nakayama Y, Kanayama M, Oya T, Morita S, Sasahara M, Mann DA, Sugiyama T. Telmisartan attenuates progression of steatohepatitis in mice: role of hepatic macrophage infiltration and effects on adipose tissue. Liver Int. 2009;29:988–996. doi: 10.1111/j.1478-3231.2009.02006.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto E, Dong YF, Kataoka K, Yamashita T, Tokutomi Y, Matsuba S, Ichijo H, Ogawa H, Kim-Mitsuyama S. Olmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase-1 inhibition. Hypertension. 2008;52:573–580. doi: 10.1161/HYPERTENSIONAHA.108.112292. [DOI] [PubMed] [Google Scholar]
- 22.Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, Takahashi M, Akisawa N, Iwasaki S, Oben JA, Onishi S. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375–1381. doi: 10.1002/hep.21638. [DOI] [PubMed] [Google Scholar]
- 23.Sturzeneker MC, Ioshii SO, Villela Baroncini LA, Précoma DB. Olmesartan severely weakened the development of NASH in an animal model of hypercholesterolemia. Atherosclerosis. 2011;216:97–102. doi: 10.1016/j.atherosclerosis.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 24.López-Suárez A, Guerrero JM, Elvira-González J, Beltrán-Robles M, Cañas-Hormigo F, Bascuñana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol. 2011;23:1011–1017. doi: 10.1097/MEG.0b013e32834b8d52. [DOI] [PubMed] [Google Scholar]
- 25.Ryoo JH, Suh YJ, Shin HC, Cho YK, Choi JM, Park SK. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J Gastroenterol Hepatol. 2014;29:1926–1931. doi: 10.1111/jgh.12643. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response toadjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 28.Filozof C, Goldstein BJ, Williams RN, Sanyal A. Non-alcoholic steatohepatitis: limited available treatment options but promising drugs in development and recent progress towards a regulatory approval pathway. Drugs. 2015;75:1373–1392. doi: 10.1007/s40265-015-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American association for the study of liver diseases-U.S. food and drug administration joint workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 32.Pachaly JR. Terapêutica por Extrapolação Alométrica. In: Cubas ZS, Silva JCR, Catão-Dias JL, editors. Tratado de animais selvagens. São Paulo: Roca; 2006. pp. 1215–1223. [Google Scholar]
- 33.Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54:1340–1348. doi: 10.2337/diabetes.54.5.1340. [DOI] [PubMed] [Google Scholar]
- 34.Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 2010;59:861–871. doi: 10.2337/db09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinozaki S, Choi CS, Shimizu N, Yamada M, Kim M, Zhang T, Shiota G, Dong HH, Kim YB, Kaneki M. Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice. J Biol Chem. 2011;286:34959–34975. doi: 10.1074/jbc.M110.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 37.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 38.Tocci G, Paneni F, Palano F, Sciarretta S, Ferrucci A, Kurtz T, Mancia G, Volpe M. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and diabetes: a meta-analysis of placebo-controlled clinical trials. Am J Hypertens. 2011;24:582–590. doi: 10.1038/ajh.2011.8. [DOI] [PubMed] [Google Scholar]
- 39.Abuissa H, Jones PG, Marso SP, O’Keefe JH Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]


