Abstract
The incorporation of C-glycosides in drug design has become a routine practice for medicinal chemists. These naturally occurring building blocks exhibit attractive pharmaceutical profiles, becoming an extensive topic of synthetic efforts in recent decades.[1] Described herein is a practical, scalable, and versatile route for the synthesis of non-anomeric and unexploited C-acyl glycosides via a Ni/photoredox dual catalytic system. Utilizing an organic photocatalyst, an arsenal of glycosyl-based radicals is generated and efficiently coupled with highly functionalized carboxylic acids at room temperature. Distinctive features of this transformation include its mild conditions, impressive compatibility with a wide array of functional groups, and most significantly, preservation of the anomeric carbon: a handle for further, late-stage derivatization.
Keywords: Glycosides; 1,4-Dihydropyridine; Acylation; Carboxylic Acids; Single Electron Transfer
Graphical Abstract
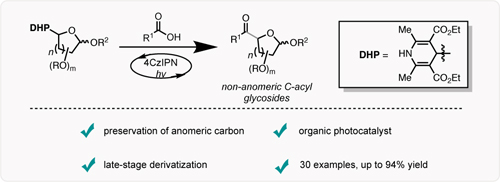
C-acyl glycosides, naturally occurring building blocks, have recently been the focus of extensive research efforts due to their enhanced biological activities and unique chemical structure. We describe a practical and versatile route toward non anomeric C-acyl glycosides via Ni/Photoredox dual catalysis. Key to this transformation is the preservation of the anomeric carbon as a handle for further late-stage derivatization. This process is operationally simple and widely applicable to various functional groups. An organic photocatalyst is utilized to generate an array of glycosyl-based radicals that engage in cross-coupling with in-situ activated carboxylic acids to access medicinally relevant compounds.
Glycodiversification of drug scaffolds is a powerful strategy used to invoke unique chemical diversity and enhance pharmacokinetic and/or pharmacodynamic properties of medicinal targets.[1,2] Glycosidic attachment in natural products renders increased solubility, membrane transport, and specificity in cellular tissues.[3] A privileged class of saccharides containing a C—C bond linking a carbohydrate unit to an aglycone or another sugar moiety are known as C-glycosides.[1c,4] These compounds are ubiquitous in nature and serve as key motifs in numerous antitumor, antibiotic, and type II antidiabetic agents.[1c] Given their in vivo resistance toward basic, acidic, and enzymatic hydrolysis, C-glycosides have proven to be successful mimetic forms of the more labile O-glycosides.[4] In particular, C-acyl glycosides have demonstrated important biological activities such as inhibition against reactive oxygen species, playing a major role in cell signaling (oxidative stress), and glutamate-induced cell death.[5] It is worth highlighting that C-acyl-glycosylation has proven efficient in providing unique synthetic disconnections toward complex, bioactive molecules. [6] A prominent example is the synthesis of zaragozic acid C, a potent squalene synthase inhibitor, based on a photochemical Csp3-H acylation strategy of a glycosidic moiety.[6a]
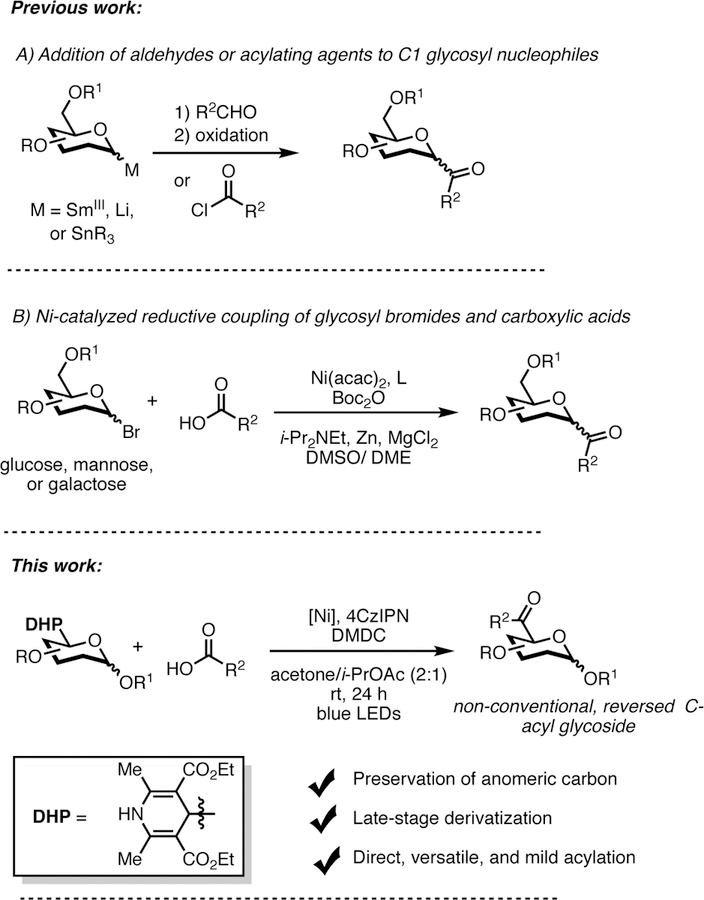
Consequently, various strategies have been devised to introduce acyl groups at the anomeric carbon in glycosides. Most common methods include: i) nucleophilic additions of organometallic reagents to C-glycosyl aldehydes followed by an oxidation step,[7] ii) addition of aldehydes[8] or electrophilic acylating agents[9] to glycosyl-based lithium, tin or samarium reagents (Scheme 1A), and iii) addition of Grignard reagents to glyconitriles[10] or masked aldehydes such as glycosyl benzothiazoles.[11] These strategies rely on harsh conditions and reactive organometallic reagents, thus limiting their widespread applicability in pharmaceutical settings.
Scheme 1.
Recent developments in the synthesis of C-acyl glycosides.
More recently, Gong et al. described a nickel-catalyzed reductive coupling of aliphatic carboxylic acids with glycosyl bromides with complete α-selectivity in the mannose series (Scheme 1B).[12] This report documents the most straightforward route toward C-acyl glycosides to date.
Traditional transition metal-catalyzed cross-coupling reactions remain in large part inapplicable in the context of Cacyl-glycosylation because of the Csp3-hybridized nature of the anomeric position. As a result, the development of efficient and catalytic transformations toward C-acyl glycosides is highly desired. Specifically, non-anomeric acylation approaches that preserve the anomeric carbon for further functionalization are highly limited and challenging.
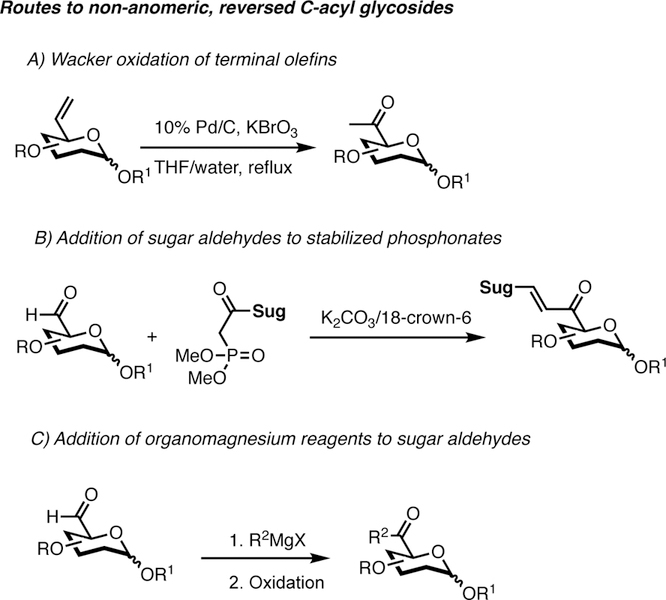
Routes based on Wacker oxidation of terminal olefins have been reported for the synthesis of non-anomeric C-acyl glycosides (Scheme 2A).[13] However, such transformations are limited to methyl ketones and require elevated temperatures. Other strategies to assemble complex sugar units take advantage of the addition of sugar aldehydes to stabilized sugar phosphonates (Scheme 2B).[14] Conversion of glycosyl carboxylic acids to more reactive acyl chlorides has been utilized in the synthesis of α-diazocarbonyl saccharides.[15] Finally, the addition of organomagnesium or organolithium reagents to sugar aldehydes at C5 has been described (Scheme 2C).[16] These transformation are generally not applicable to molecules possessing delicate functional groups.
Scheme 2.
Acylation strategies of non-anomeric glycoside.
Recent efforts have demonstrated that Ni/photoredox cross-coupling reactions are valuable tools for the construction of new C—C bonds.[17] These processes are governed by a single-electron transmetalation pathway, with an inherent low activation energy barrier favoring Csp3-hybridized nucleophiles.[18] In this context, we explored 1,4-dihydropyridines (1,4-DHPs)[19] as glycosyl-based radical precursors. These coupling partners are bench stable and can be prepared from inexpensive starting materials.[19c] Although 1,4-DHPs stem from the corresponding C-formyl glycosides, they are of immense synthetic value as they have low oxidation potentials and thus are amenable for fragmentation using inexpensive organic photocatalysts in lieu of stoichiometric oxidants.[19c] To address the demands associated with the synthesis of non-anomeric Cacyl glycosides, we investigated the feasibility of a crosscoupling reaction of in situ activated carboxylic acids[20] with glycosyl based DHPs in an attempt to access such challenging structural motifs.
Studies were initiated by evaluating suitable activators of hydrocinnamic acid using high-throughput experimentation techniques (see Supporting Information)20 Dimethyl dicarbonate (DMDC)21 served as an ideal activator, and 4CzIPN, an inexpensive organic dye, performed more efficiently than Ir-and Ru-based photocatalysts.
Subsequently, the generality of this transformation with respect to the DHP coupling partner was examined (Table 1). Various functionalized glycosidic scaffolds are well-tolerated under the reaction conditions, affording the desired ketones in high yields and acceptable diastereoselectivities. Sterically hindered pyranose and furanose moieties (2a, 2b, and 2e) performed equally well when compared to those with less steric constraints (2c and 2d). It is worth noting that synthetically challenging glycoside 2f, exhibiting a free hydroxyl group, can be obtained in good yield in one-step starting from the corresponding DHP 1e. Non-glycosyl based DHPs successfully furnished the desired ketones (2g, 2h, and 2i) in high yields.
Table 1.
Scope of 1,4-DHPs in the Cross-Coupling with Carboxylic Acids.[a]
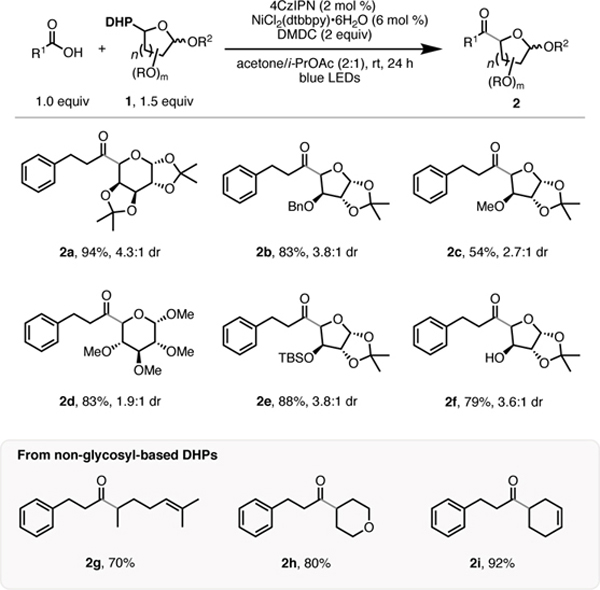 |
All cited yields are isolated. See Supporting Information for experimental details.
Next, we focused our attention on the compatibility of abundant carboxylic acids as cross-coupling partners (Table 2). Nucleophilic free hydroxyl groups are tolerated despite their potential reactivity with DMDC (2l). Glycosides bearing small, strained rings were incorporated with no loss in reactivity (2m, 2o, 2p, 2r, and 2s). An N-Boc-protected amino acid and an Fmoc-protected amine afforded the desired ketones in high yields and excellent diasteroselectivities (2k and 2n, respectively). Glycoside 2w incorporating indomethacin, and 3b and 3c derived from naproxen, were successfully obtained. Glycosides containing steroidal moieties, abundant motifs in synthetic drugs and natural products, are amenable to this cross-coupling (2x). The feasibility of using non-glycosyl based DHPs was successfully examined in the synthesis of dialkyl ketones 3b-3f.
Table 2.
Carboxylic Acid Scope under Optimized Reaction Conditions.[a]
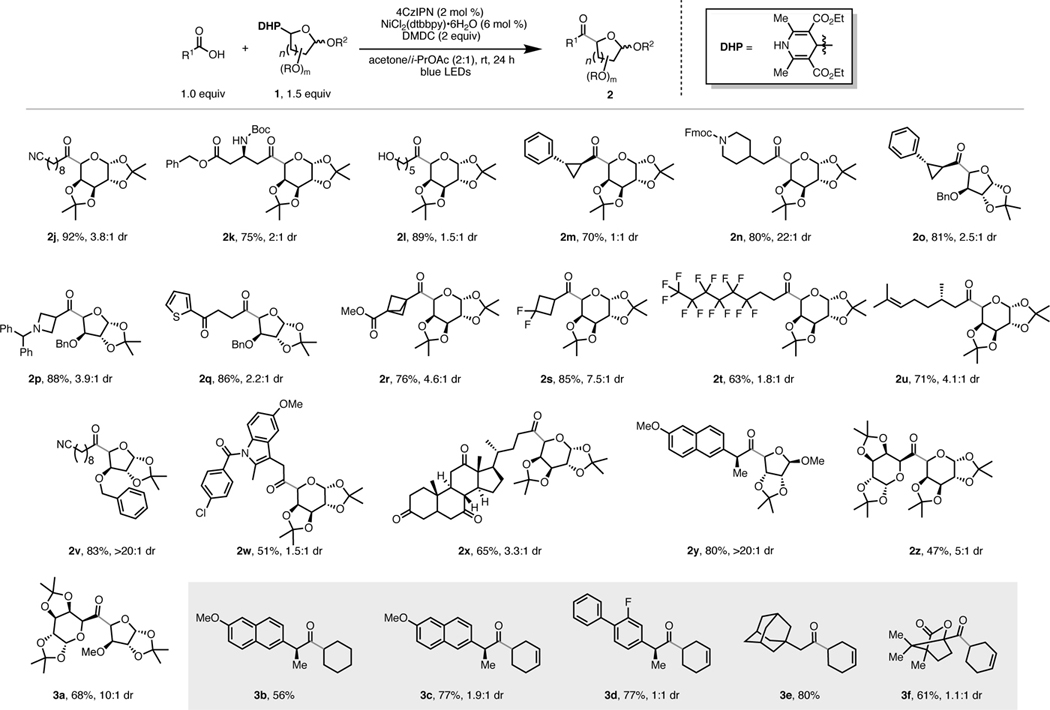 |
All cited yields are isolated. See Supporting Information for experimental details.
Most notably, employing glycosyl-based carboxylic acids allowed the generation of disaccharide alkyl ketones (2z and 3a) with complete retention of configuration in the saccharide moiety derived from the carboxylic acid, despite the challenging steric demands of both cross-coupling partners. To the best of our knowledge, this structural motif is currently unreported in the literature. Therefore, this acylation strategy provides a straightforward route to new chemical space in carbohydrate chemistry.
To assess the scalability of this protocol, we conducted the reaction to access 2x on 2.5 mmol scale (Scheme 3). Because of its economical profile [~$5/mmol], 4CzIPN is an attractive photocatalyst for large-scale applications. The resulting yield was comparable to the reaction on 0.3 mmol scale. This demonstrates the usefulness of glycosyl-based DHPs in the synthesis of C-acyl glycosides, especially within the context of rapid, late-stage functionalization of desired targets bearing a carboxylic acid handle.
Scheme 3.
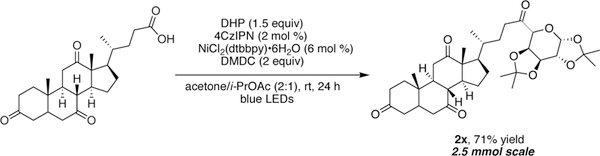
Scale-up of C-acyl-glycosylation under Ni/photoredox conditions.
In conclusion, this report details a practical and versatile route toward the acylation of highly functionalized, non-anomeric, C-acyl glycosides in high yields and acceptable diastereoselectivities. Utilizing a dual-catalytic Ni/photoredox system, we are able to employ a vast array of glycosyl-based radicals to preserve the anomeric carbon; a useful handle for further structural elaboration. This mild acylation protocol can be utilized in industrial settings to access medicinally relevant C-acyl glycosides, as well as provide unique and alternative disconnections in the synthetic design of complex, bioactive molecules.
Supplementary Material
Acknowledgements
The authors are grateful for the financial support provided by the National Institutes of Health (NIGMS 1 R01 GM113878 to G. A. M.). J.K.M. was supported by the Bristol-Myers Squibb (BMS) Graduate Fellowship in Synthetic Organic Chemistry. We sincerely thank Dr. Álvaro Gutierrez-Bonet of the University of Pennsylvania (UPenn) for useful discussions. We thank Dr. Charles W. Ross, III (UPenn) for assistance in obtaining HRMS data, and Dr. Jun Gu for NMR assistance.
Footnotes
Supporting information for this article is given via a link at the end of the document
References
- [1].a) For selected examples, see: Bililign T, Griffith BR, Thorson JS, Nat. Prod. Rep 2005, 22, 742; [DOI] [PubMed] [Google Scholar]; b) Hultin PG, Curr. Top. Med. Chem 2005, 5, 1. [DOI] [PubMed] [Google Scholar]; c) Stambasky J, Hocek M, Kocovsky P, Chem. Rev 2009, 109, 6729. [DOI] [PubMed] [Google Scholar]
- [2].Holemann A, Seeberger PH, Curr. Opin. Biotechnol 2004, 15, 615. [DOI] [PubMed] [Google Scholar]
- [3].Gantt RW, Peltier-Pain P, Thorson JS, Nat. Prod. Rep 2011, 28, 1811. [DOI] [PubMed] [Google Scholar]
- [4].Koester DC, Holkenbrink A, Werz DB, Synthesis 2010, 19, 3217. [Google Scholar]
- [5].a) Reports on the biological activities of C-acyl glycosides: Wu Q, Cho J-G, Lee D-S, Lee D-Y, Song N-Y, Kim Y-C, Lee K-T, Chung H-G, Choi M-S, Jeong T-S, Ahn E-M, Kim G-S, Baek N-I, Carbohydr. Res 2013, 372, 9; [DOI] [PubMed] [Google Scholar]; b) Disadee W, Mahidol C, Sahakitpichan P, Sitthimonchai S, Ruchirawat S, Kanchanapoom T, Phytochemistry 2012, 74, 115. [DOI] [PubMed] [Google Scholar]
- [6].a) For selected examples, see: Kawamata T, Nagatomo M, Inoue M, J. Am. Chem. Soc 2017, 139, 1814; [DOI] [PubMed] [Google Scholar]; b) Inuki S, Aiba T, Kawakami S, Akiyama T, Inoue J-I, Fujimoto Y, Org. Lett 2017, 19, 3079; [DOI] [PubMed] [Google Scholar]; c) Dhar S, La Clair JJ, Leon B, Hammons JC, Yu Z, Kashyap MK, Castro JE, Burkart MD, J. Am. Chem. Soc 2016, 138, 5063. [DOI] [PubMed] [Google Scholar]
- [7].a) For examples highlighting nucleophilic additions to C-glycosyl aldehydes, see: Picard J, Lubin-Germain N, Uziel J, Augé J, Synthesis 2006, 6, 979; [Google Scholar]; b) Kolympadi M, Fontanella M, Venturi C, André S, Gabius H-J, Jiménez-Barbero J, Vogel P, Chem.-Eur. J 2009, 15, 2861; [DOI] [PubMed] [Google Scholar]; c) Geng Y, Kumar A, Faidallah HM, Albar HA, Mhkalid IA, Schmidt RR, Bioorg. Med. Chem 2013, 21, 4793. [DOI] [PubMed] [Google Scholar]
- [8].a) For examples highlighting glycosyl-based nucleophiles, see: Lesimple P, Beau J-M, Bioorg. Med. Chem 1994, 2, 1319; [DOI] [PubMed] [Google Scholar]; b) Mazéas D, Skrydstrup T; Beau J-M, Angew. Chem., Int. Ed. Engl 1995, 34, 909. [Google Scholar]
- [9].Belosludtev YY, Bhatt RK, Falck JR, Tetrahedron Lett 1995, 36, 5881. [Google Scholar]
- [10].Guisot NES, Ella Obame I, Ireddy P, Nourry A, Saluzzo C, Dujardin G, Dubreuil D, Pipelier M, Guillarme S, J. Org. Chem 2016, 81, 2364. [DOI] [PubMed] [Google Scholar]
- [11].Dondoni A, Catozzi N, Marra A, J. Org. Chem 2005, 70, 9257. [DOI] [PubMed] [Google Scholar]
- [12].Zhao C, Jia X, Wang X, Gong H, J. Am. Chem. Soc 2014, 136, 17645. [DOI] [PubMed] [Google Scholar]
- [13].Kulkarni MG, Shaikh YB, Borhade AS, Chavhan SW, Dhondge AP, Gaikwad DD, Desai MP, Birhade DR, Dhatrak NR, Tetrahedron Lett 2013, 54, 2293. [Google Scholar]
- [14].Potopnyk MA, Cmoch P, Cieplak M, Gajewska A, Jarosz S, Tetrahedron: Asymmetry 2011, 22, 780. [Google Scholar]
- [15].Guan Z, Zhang LH, Sinay P, Zhang Y, J Org Chem 2012, 77, 8888. [DOI] [PubMed] [Google Scholar]
- [16].Kolympadi M, Fontanella M, Venturi C, Andre S, Gabius HJ, Jimenez-Barbero J, Vogel P, Chem.-Eur. J 2009, 15, 2861. [DOI] [PubMed] [Google Scholar]
- [17].a) For reviews on Ni/photoredox dual catalysis, see: Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, Molander GA, Acc. Chem. Res 2016, 49, 1429; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Prier CK, Rankic DA, MacMillan DWC Chem. Rev 2013, 49, 1429; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Matsui JK, Lang SB, Heitz DR, Molander GA, ACS. Catal 2017, 7, 2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tellis JC, Primer DN, Molander GA, Science 2014, 345, 433; [DOI] [PMC free article] [PubMed] [Google Scholar]; Primer DN, Karakaya I, Tellis JC, Molander GA, J. Am. Chem. Soc 2015, 137, 2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) For selected examples, see: Chen W, Liu Z, Tian J, Li J, Ma J, Cheng X, Li G, J. Am. Chem. Soc 2016, 138, 12312; [DOI] [PubMed] [Google Scholar]; b) Gutiérrez-Bonet Á, Remeur C, Matsui JK, Molander GA, J. Am. Chem. Soc 2017, 139, 12251; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gutiérrez-Bonet Á, Tellis JC, Matsui JK, Vara BA, Molander GA, ACS. Catal 2016, 6, 8004; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Buzzetti L, Prieto A, Roy SR, Melchiorre P, Angew. Chem., Int. Ed. Engl 2017, 56, 15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schmink JR, Bellomo A, Berritt S, Aldrichimica Acta 2013, 46, 71. [Google Scholar]
- [21].Amani J, Molander GA, Org. Lett 2017, 19, 3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


