Abstract
Background: Sodium Benzoate (SB) significantly improved positive, negative, and cognitive symptoms as add on treatment in schizophrenia. Olanzapine (Ola), the most effective atypical antipsychotic drug, has been linked to hepatic steatosis, acute kidney injury, reproductive side effects and poor effect on negative symptoms in some patients. Goals: is to compare the efficacy and check the safety of long-term monotherapy with SB 0.01 mg/Kg versus Ola on male cognitive, memory, hepatic, renal and testicular functions in rat model of schizophrenia. Methods: 48 young adult male rats were divided into 6 groups; C: control; O: received Ola; SB: received SB; K: received single IP ketamine (Ket) injection; K+O: received Ola and Ket and K+SB: received SB and Ket. Ola and SB given orally for 3 or 10 weeks for behavioral or serological studies respectively. We measured activities of daily life (ADL), spatial learning and memory in radial arm water maze (RAWM), serum parameters of hepatic, renal and testicular functions. Results: Both Ola and SB significantly improved hoarding and burrowing, caused significant decrease in time to reach target (TRT), working memory errors (WME) in K+O and K+SB groups compared to K group. Ola caused significant increase in ALT, AST and creatinine and decrease in serum LH, testosterone compared to controls. SB caused significant rise in serum LH, ALT, AST and decrease in protein and albumin compared to both C and O groups. Conclusion: Both Ola and SB improved ADL, cognitive and memory functions. Although SB saved testicular and renal functions, it worsened liver function compared to Ola.
Keywords: Schizophrenia, olanzapine (ola), sodium benzoate (sb), radial arm water maze (RAWM), activities of daily life (ADL), testis, liver, kidney
Introduction
Schizophrenia is considered as one of the most disabling diseases socially and economically that affect about 1% of the population [1]. The most accepted two theories that explain the pathogenesis of schizophrenia are the dopamine and glutamate hypotheses [2]. Olanzapine (Ola) has been proved to be one of the most effective second-generation antipsychotics that block serotonin 5HT2A and dopamine receptors [3]. However, the use of Ola has been linked to several side effects as weight gain, hepatic steatosis, metabolic syndrome and gonadal dysfunction [4,5]. Moreover, it has poor effect on management of negative and cognitive symptoms of some schizophrenia patients [6]. Glutamatergic antipsychotics that modulate NMDARs have sparked a new hope in treatment of schizophrenia [1].
Sodium benzoate is D-amino acid oxidase (DAO) inhibitor and considered as relatively safe natural food and drug preservative by FDA. The role of DAO, an enzyme that catabolizes D-amino acids, has been investigated in many psychiatric diseases particularly schizophrenia [7]. D-amino acids act as allosteric modulator of N-methyl-D-aspartate receptors (NMDARs) whose hypofunction is implicated in the pathophysiology of schizophrenia [8]. The disturbed genetic expression of NMDAR in patients as well as the ability of NMDAR antagonists to cause psychotic manifestation in previously stabilized patients or healthy volunteers reinforced the hypothesis of NMDAR malfunction in schizophrenia [9]. Elevation of D-amino acids either by exogenous administration [10] or by increasing their endogenous levels using reuptake inhibitors or inhibiting their catabolism could modulate NMDAR function [11]. Randomized double blind placebo-controlled trials showed that SB was effective as an adjuvant therapy in controlling the negative symptoms of refractory schizophrenia [12,13]. The central goal of the current study is to compare the efficacy and check the safety of systemic administration of very small dose SB (0.01 mg/Kg) as monotherapy versus Ola on male daily life activities, cognitive, spatial learning and memory functions in ketamine-induced rat model schizophrenia. In the chronic study we investigated the possible toxicity of both drugs on hepatic, renal and testicular function.
Methods
Drugs and chemicals
Sodium benzoate was manufactured by Bayer (AG Kaiser-Wilhelm-Allee 51373 Leverkusen Germany, 500 mg dissolved in 500 ml distilled water) and given at concentration of 0.01 mg/Kg/day for 10 weeks. Olanzapine was manufactured by Multi-Apex for Pharmaceutical Industries (S.A.E.-Badr City-Egypt). Each tab of Ola 10 mg was mashed and dissolved in 10 ml DW and given at dose (1 mg/kg/day) by gavage for 10 weeks [14]. Ketamine hydrochloride injection (USP ROTEX MEDICA, TRITTAU, Germany; ampoule 10 ml at 50 mg/ml) was given as single IP injection of Ket (25 mg/Kg) half an hour before the behavioral test [15].
Animal model
Forty-eight young adult male Albino Wistar rats (80-150 gm) were obtained from the Animal Core Facility, Assiut University, Assiut, Egypt. Rats were transported to Physiology lab 1 week before start of treatment for habituation. They were kept under 12 hr light/dark cycle and 25°C with free access to food and water (Ad Libitum). All experiments were carried out in strict accordance with the applicable national and international guidelines. The protocol was approved by the Local Experimental Ethical Committee at Deanship of Scientific Research of Assiut University, Assiut, Egypt. All surgery was done under ketamine anesthesia, and all efforts were made to minimize suffering.
Experimental design of research
Rats were used for two regimen protocols; behavioral subacute study starting from the third week of treatment including 30 rats and chronic ten weeks study including 18 rats intended for serological examination. In the serological study, animals were divided into three groups; (C) group served as control received DW; (O) group received Ola 1 mg/Kg; (SB) group: received SB 0.01 mg/Kg; treatment given orally daily by gavage for 10 weeks. In the behavioral study rats were divided into three groups 10 animals each and subjected to behavioral tests starting from the third week of treatment; K: received ketamine 25 mg/Kg as single IP injection 30 min before the test; K+O: received Ola and single Ket injection; K+SB group: received SB and single Ket injection. The behavior group was tested twice before and after administration of Ket.
Behavioral tests
Radial arm water maze (RAWM)
The RAWM is composed of six arms, made of stainless steel with an open central area, diameter 50-55 cm, the length of the arm was 30-35 cm and the width was 13-15 cm. The depth of the water maze was about 70 cm and the height of the pedestal 62.5 cm. Water level was 1 cm above the pedestal and the inside of the water maze as well as the pedestal were painted black. The rats were tested over three days (15 trials each); the goal arm is held constant for all trials of the same day, with a different start arm on successive trials. Ketamine was given as single dose 30 min before testing on the third day. Three parameters were calculated time to reach target (TRT), working memory error (WME) and reference memory error (RME) for each trial. Entering an incorrect arm for the first time was considered as RME and entering incorrect arm twice was calculated as WME. The first day was for training and not included in the scoring. TRT and memory errors were averaged across three successive trials and presented in 5 blocks on the second and third day and named as (C, O and SB on the second day and as K, K+O and K+SB on the third day) [16].
Activities of daily life
Rat were subjected to one week of habituation at which the rats were tested in groups to enhance learning. Testing was done for each rat separately for hoarding and burrowing [17].
Burrowing
A tube with elevated front end and closed back end filled with 800 gm of sand was put in the home cage 3 hours before the dark phase. The amount of the sand burrowed was measured the next morning by weighing the tube at the beginning of the test and the next morning.
Hoarding
Rats were fasted during the day time and allowed an access to 100 gm of food pellet during night only through wire meshed tubes that were connected to their home cages through doors. We measured the amount of the collected food the next morning.
Biochemical parameters
After 12 hours fasting at the end of 10 weeks, rats in all groups were anaesthetized with ketamine (50 mg/Kg). Blood samples were collected, and serum was separated by centrifugation at 3000 rpm for 10 minutes and stored at -20°C for further biochemical analysis. Serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) were estimated using ELISA (LH Kit Cat. No PT-LH_96 and FSH Kit Cat. No.PT-FSH-96, Pishtaz Teb Diagnostics, JTC Diagnosemittel UG Schulweg 8 D-34516 Voehl/Germany). Serum alanine transaminase (ALT) and aspartate transaminase (AST) were estimated using ELISA kits (GPT/ALT FL IFCC and GOT/AST FL IFCC; Chema Diagnostica, Via Campania 2/4 60030 Monsano (AN)-ITALY-EU). Serum free testosterone was estimated using ELIZA (DBC testosterone Kit Cat. No. CAN-TE-250; version 5.1; Diagnostic Biochem Canada Inc., 41 Byron Ave, Dorchester, Ontario, Canada, N0L 1G2) and following manufacture instructions. Absorbance was read using Automated ELIZA plate reader (Robonite-Readwell-India).
Albumin was estimated using Spectrum Diagnostics albumin reagent (Cat. No 211 001). Total protein was estimated using Spectrum Diagnostics total protein reagent (Cat. No 310001), both from Egyptian Company of Biotechnology (S.A.E., Obour city industrial area. Block 20008, piece 19A. Cairo. Egypt). Serum urea was estimated using Berthelot. Enzymatic colorimetric (Cat. No 2 × 100 ml); serum creatinine was estimated using Jaffe. Colorimetric-kinetic (Cat. No 2 × 100 ml), both from Diamond Diagnostics, D-P International Head Office: 23 El-Montzh St., Heliopplis, Cairo, Egypt. All biochemical analysis was done following the manufacture instructions and using Auto Biochemistry analyzer (Robonite Prietest-touch-India).
Statistical analysis
GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) was used for data analysis. Data were presented as mean ± SEM. Data were compared among groups using One Way ANOVA or Two-Way ANOVA with Bonferroni Multiple Comparison as posthoc test as appropriate. A (P) value of less than 0.05 was considered to represent a statistically significant difference.
Results
Effect of olanzapine (Ola) vs sodium benzoate (SB) on burrowing and hoarding behavior in rat model of ketamine (Ket)-induced schizophrenia
We found that Ket caused significant reduction in the amount of sand burrowed (P<0.001) and the amount of food hoarded in K group compared to C group (P<0.001) (Figure 1A, 1B). Ola caused significant rise in the amount of sand burrowed (P<0.001) (Figure 1A) and the amount of food hoarded (P<0.01) (Figure 1B) in K+O group compared to K group. SB caused significant rise in the amount of sand burrowed (P<0.001) (Figure 1A) and the amount of food hoarded (P<0.05) (Figure 1B) in K+SB groups compared to K group. Insignificant difference between C, O, SB, K+O and K+SB groups was found (P>0.05) (Figure 1A, 1B).
Figure 1.
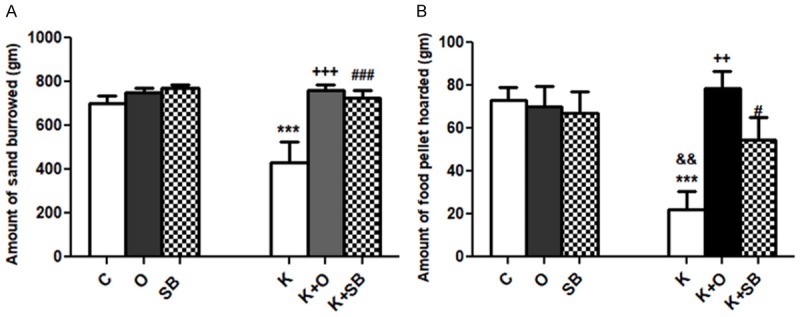
Effect of olanzapine (Ola) vs sodium benzoate (SB) on activities of daily life in rat model of ketamine (Ket)-induced schizophrenia. A: Burrowing; B: Hoarding behavior; data represent mean ± standard error (M ± SEM); C: control group; O: olanzapine group; SB: sodium benzoate group; K: Ket group; K+O: Ket+Ola; K+SB: Ket+SB; One Way ANOVA with Bonferroni Multiple Comparison posthoc test ***P<0.001; +++P<0.001; ++P<0.01; ###P<0.001; #P<0.05; &&P<0.01; (*) P value of K vs C, (&) P value of K vs O or SB groups; (+) P value of K vs K+O group; (#) P value of K vs K+SB; P value of <.05 is considered significant; N = 10 in each group.
Effect of olanzapine vs sodium benzoate on ketamine (Ket)-induced impaired performance in radial arm water maze (RAWM)
The present study showed significant decrease in time to reach target (TRT) in K+O and K+SB compared to K group (P<0.05) (Figure 2A). Significant decrease in working memory errors (WME) in K+O and K+SB compared to K group found (P<0.05) (Figure 2B). Moreover, comparison within each group demonstrated significant decrease in TRT and WME with successive trials in all the studied groups except K group (Figure 2A, 2B). Significant improvement in reference memory errors (RME) with increasing number of trials in all the studied groups including ketamine group shown (Figure 2C).
Figure 2.
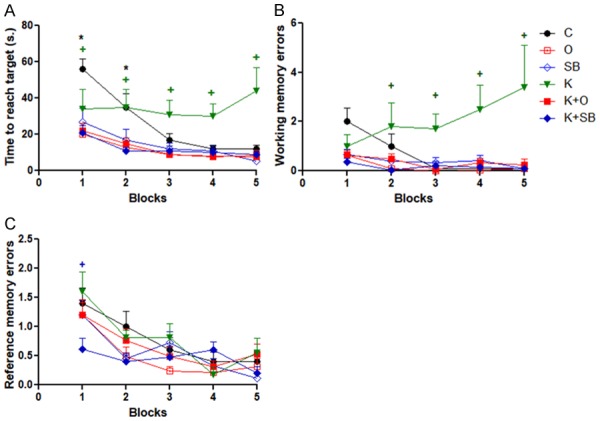
Effect of olanzapine (Ola) vs sodium benzoate (SB) on rat performance in radial arm water maze (RAWM). A: Time to reach target (TRT); B: Working memory errors (WME); C: Reference memory errors (RME); data represent mean ± standard error (M ± SEM); C: control group; O: Ola group; SB: SB group; K: Ket group; K+O: Ket+Ola; K+SB: Ket+SB; Two-way ANOVA with Bonferroni Multiple Comparison posthoc test; [TRT: (+) P value of K vs C (B1, B5), K vs O, K+O or K+SB (B2-5) & K vs SB (B3-5); (*) P value of C vs O, K+O or K+SB (B1,B2)]; WME: [(+) P value of K vs C, SB, K+SB or K+O (B4, B5) & K vs O (B2-B5)]; RME: [(+) P value of K+SB vs C or K (B1)]; *P<0.05; +P<0.05; P value of <.05 is considered significant; N = 10 in each group.
Effect of chronic treatment of olanzapine and sodium benzoate on weight gain and relative organ weight
Our results showed significant increase in TBW within each group (P<0.001) with time and insignificant difference in TBW in between groups (P>0.05) (Figure 3A). We found significant decrease in mean relative testicular weight of O group compared to C group (P<0.01), significant difference in testicular weight between O group and SB (P<0.05) and insignificant difference in testicular weight between C and SB groups (Figure 3B). Insignificant difference in mean relative liver weight between the three groups demonstrated (Figure 3B). Significant decrease in relative brain weight of O group compared to C group (P<0.01), significant difference in brain weight between O group and SB (P<0.05) and insignificant difference in brain weight between C and SB groups found (Figure 3B). Significant decrease in mean relative kidney weight of O group compared to C group and insignificant difference in kidney weight between of SB group compared to C or O groups (P>0.05) (Figure 3B).
Figure 3.
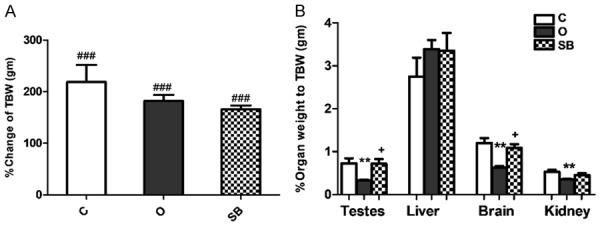
Effect of olanzapine versus sodium benzoate on total body weight and relative organ weight of male albino rat. Data represent mean ± standard error (M ± SEM); C: control group; O: olanzapine group; SB: sodium benzoate group; A: % change in total body weight (%TBW = final weight/original weight*100); B: % relative organ weight = organ weight/TBW*100); One Way ANOVA with Bonferroni’s Multiple Comparison posthoc test ###P<0.001; **P<0.01; +P<0.05; (#) P value of final TBW vs original TBW; (*) P value of Ola group vs C group; (+) P value of O group vs SB group; P value of <.05 is considered significant; N = 6 in each group.
Effect of chronic treatment of olanzapine and sodium benzoate on liver and kidney functions
The current work showed significant rise of serum level of liver enzymes ALT (P<0.05) and AST (P<0.05) in O group compared to C group, significant rise of serum level of ALT (P<0.001) and AST (P<0.001) in SB group compared to C group and significant difference in ALT (P<0.01) and AST (P<0.001) between SB and O groups (Figure 4A). Significant decrease in serum total protein level in SB group compared to both C (P<0.001) and O (P<0.01) groups and significant decrease in serum albumin level in SB group compared to both C (P<0.01) and O (P<0.05) groups (Figure 4B). Insignificant difference in serum urea level between the three studied groups (C, O and SB groups) found (Figure 4C). Significant rise in serum creatinine level (P<0.001) in O group compared to both C and SB groups and insignificant difference in serum creatinine level between C and SB groups shown (Figure 4D).
Figure 4.
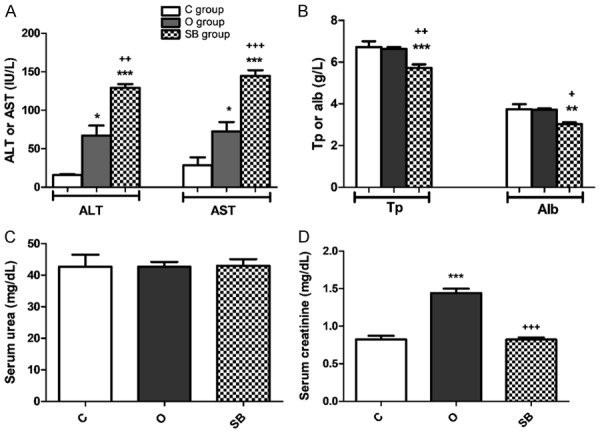
Olanzapine versus sodium benzoate effect on serum level of; (A) Alanine transaminase (ALT) & aspartate transaminase (AST); (B) Total protein (Tp) & albumin (alb); (C) Serum urea and (D) serum creatinine of male albino rat. Data represent mean ± standard error (M ± SEM); C: control group; O: olanzapine group; SB: sodium benzoate group; ANOVA with Bonferroni’s Multiple Comparison posthoc test ***P<0.001; **P<0.01; *P<0.05; +++P<0.001; ++P<0.01; +P<0.05; (*) P value of Ola or SB groups vs C group; (+) P value of Ola group vs SB group; P value of <.05 is considered significant; N = 6 in each group.
Effect of chronic treatment of olanzapine and sodium benzoate on testicular function
We found insignificant difference in serum FSH between the three studied groups C, O and SB (Figure 5). Significant decrease in serum LH level in O group (P<0.001) compared to both C and SB groups and significant rise in serum LH in SB group (P<0.01) compared C group demonstrated (Figure 5). Significant decrease in serum free testosterone level in O group compared to both C group (P<0.01) and SB group (P<0.05) shown (Figure 5).
Figure 5.
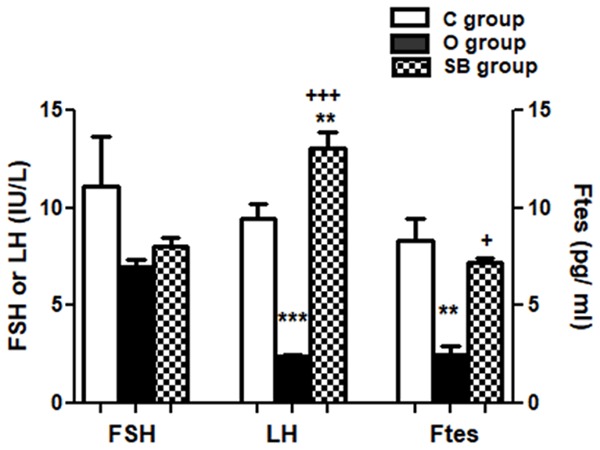
Olanzapine versus sodium benzoate effect on serum level of luteinizing hormone (LH), follicle stimulating hormone (FSH) and free testosterone (Ftes) of male albino rats. Data represent mean ± standard error (M ± SEM); C: control group; O: olanzapine group; SB: sodium benzoate group; One Way ANOVA with Bonferroni’s Multiple Comparison posthoc test ***P<0.001; **P<0.01; +++P<0.001; +P<0.05; (*) P value of O or SB groups vs C group; (+) P value of Ola group vs SB group; P value of <.05 is considered significant; N = 6 in each group.
Discussion
Several previous studies reported impaired community interaction, ability to get employed and lead independent life in patients with schizophrenia [18]. Loss of ability to do daily usual tasks is as important as cognitive disability that imposes socioeconomic burden in psychotic patients. Activity of daily life tests in rodents such as burrowing, and hoarding are considered as good indicator of development of animal model as well as testing the efficacy of pharmacological agents [19]. Up to our knowledge, no previous study showed the effect of SB or Ola on ADL and/or Ket-induced deficit in those tests in rodents. The current work showed that Ket caused significant reduction in the amount of sand burrowed and food hoarded in K group compared to C group. Both Ola and SB caused significant rise in the amount of sand burrowed as well as the amount of food hoarded compared to K group. Insignificant difference between C, O, SB, K+O and K+SB groups was found.
Most previous studies used dry radial arm maze (RAM) or Morris water maze (MWM) tasks to evaluate spatial learning and memory function of the brain. Previous study demonstrated impaired working memory in delayed non-match to sample (DNMS) task and normal spatial memory in MWM with Ola treatment at a dose of 7.5 mg/Kg daily from postnatal day 28 to day 49 in rats [20]. Another study indicated that oral daily treatment with Ola 5-10 mg/Kg for 2-8 weeks impaired acquisition concerning earlier sessions that improved with repetition and reduced swim speeds in MWM, delayed non-match-to-position trials in the RAM, and decreased activity in open field assessment [21]. Up to our knowledge, no previous study tested effect of SB or Ola on Ket-induced changes of rat performance in RAWM. The present work demonstrated that both Ola and SB caused significant improvement in spatial learning, memory and cognitive function in RAWM. The results of the present study showed that co administration of both Ola+Ket and SB+Ket significantly reduced the latency to reach the pedestal (TRT) and working memory errors (WME) compared to administration of Ket alone. Moreover, comparison within each group demonstrated significant decrease in TRT and WME with more trials in all the studied groups except Ket group. Significant improvement in reference memory errors (RME) with trials in all the studied groups including ketamine group was found.
In line with our results, previous study reported that Ola at dose range of 0.4-1.25 mg/kg improved the cognitive and memory functions and reduced the MK-801-induced working memory errors in RAM task [14]. Another study reported improvement of learning and memory in novel object recognition task, Barnez maze and T maze in mice model of Alzheimer’s disease treated with 100 mg/Kg cinnamon verum for 2 months [22]. They reported that cinnamon verum induced sharp peak of its metabolite SB in hippocampus after 7 days of treatment. It was reported that DAO knockout mice showed better performance in Morris water maze (MWM) with peripherally located platform and worse performance with centrally located one [23]. On the other hand, recent study in mice reported impaired memory performance and increased oxidative stress with oral administration of SB at (0.56, 1.125, and 2.25 mg/ml) in the drinking water for 4 weeks using rotarod test, shuttle box and step-down test [24]. Those conflicting results may be explained on the base of dose difference as well as different test protocol. We may speculate that the effect of SB and Ola was dose sensitive since using Ola in small dose around 1 mg/Kg improved cognitive and memory function in the previous studies.
Previous studies showed conflicting results on changes in body weight with Ola and SB administration in rodents. We found that administration of Ola at a dose of 1 mg/Kg for 10 weeks caused insignificant change in weight gain and significant decrease in mean relative testicular, kidney and brain weights compared to control. Administration of SB at a dose of 0.01 mg/Kg for 10 weeks caused insignificant change in weight gain and relative organ weight compared to control. We found significant difference in testicular, and brain weights between O group and SB group. In line with us, recent study showed insignificant change in percent weight gain in male rats compared to controls with 5 mg/Kg Ola treatment for 8 weeks [25]. However, the same study reported significant decrease in percent weight gain with higher doses 10-15 mg/Kg on the next 4 to 6 weeks of continued Ola treatment. A more recent study on female rats demonstrated significant increase in body weight compared to controls with 4 mg/Kg Ola administered for 28 days [26]. Supporting the current study, recent study showed insignificant change in weight gain with 100 mg/Kg SB administration for 28 days in male rats [27]. However, most of the previous toxicology studies of SB in rodents reported significant reduction in body weight gain compared to controls with dose range from 25 to 400 mg/Kg [28,29]. These conflicting results may be explained by sex, dose and time difference of SB or Ola treatment.
Several clinical studies and case reports estimated elevation of serum transaminases with Ola treatment [30,31]. The current study showed that administration of Ola at a dose of 1 mg/Kg for 10 weeks caused significant increase in serum alanine transaminase (ALT), aspartate transaminase (AST) and insignificant change in serum total protein (Tp) and albumin (Alb) levels compared to controls. Previous study of us showed that Ola administration caused significant rise of serum transaminases in female rats [32]. In contrast to us, another study reported insignificant change in serum transaminases with the administration of 5, 10 and 15 mg/Kg Ola for 14 weeks duration in rats [25].
Administration of SB at a dose of 0.01 mg/Kg for 10 weeks caused significant further rise in serum ALT and AST levels compared to C or O groups and significant decrease in serum total protein and albumin. Supporting us, previous study reported significant rise in ALT and AST and decrease in serum Alb level with the use of SB (25, 100 and 400 mg/Kg) for 28 days in rats [29]. Another study demonstrated significant rise of serum transaminases with SB 30, 60 and 120 mg/kg that was given every other day for 14 days [33].
This study showed that Ola at 1 mg/Kg for 10 weeks treatment caused deleterious effects on the kidney as it decreased significantly the kidney weight and increased serum creatinine level compared to C and SB groups. In line with this result, previous study of about 200,000 older adults reported 2-fold increased risk for acute kidney injury within 90 days of Ola administration [34]. Several case reports showed that the acute use of atypical antipsychotic drugs (AADs) such as Ola induced chronic interstitial nephritis or renal affection [35,36].
Our results showed insignificant difference in kidney weight and serum creatinine with SB administration. In agreement with us, previous old study in rats reported that SB given at doses ranging between 0.016-1.09 g/kg for 30 days with diet didn’t cause any side effects [37]. However, most previous toxicity studies that used higher doses of SB (100-1350 mg/Kg) reported rise in serum urea and/or creatinine that was dose dependent [29,38].
Electronic searching study using data from 1291 patients with schizophrenia showed that Ola may cause more rise of prolactin and thence, impaired testicular function if compared to other AADs except risperidone [4]. In contrast, another study reported that the mean serum FSH, LH and testosterone levels does not significantly change after 8 weeks of treatment with Ola if compared to their initial evaluation done after 3 weeks of the start of treatment [5]. The current study showed that Ola caused significant decrease in serum LH, Ftes compared to C and SB groups. In line with us, previous study in female rats reported significant rise of serum prolactin, disruption of estrous cycle, increased food intake, weight gain and insulin resistance after 21 days of oral administration of Ola 10 mg/Kg [39]. Another study reported trend toward low reproductive hormones with prolactin elevation in both males and females treated with antipsychotic drugs (APDs) [40]. Taken together, the rise in serum prolactin causes disturbance in gonadotropin secretion resulting in impaired gonadal function.
We found that SB caused significant rise in serum LH and insignificant change in FSH and Ftes compared to both control and Ola groups. In agreement with us, recent study showed insignificant change in circulating total testosterone with 100 mg/Kg SB for 28 days in rats, however, it significantly decreased serum FSH and LH levels [27]. A recent clinical review reported several benefits of sodium benzoate in treatment of neuropsychiatric diseases including schizophrenia and recommended low dose as high dose may have negative effect on brain neurochemistry as well as other organ functions [41]. Therefore, we may speculate that the apparently conflicting results may be explained on the base of dose difference between the current study and others. Finally, further long-term studies using small doses in animals and humans are needed to prove the efficacy and safety of those drugs in treatment of psychotic disorders.
Conclusion
This study provides evidence for the efficacy of sodium benzoate as monotherapy in very small dose on cognitive, spatial learning and memory functions of the brain as well as its safety except on liver function when compared with the standard AAD (olanzapine). In contrast to most of previous studies, the current work provides an evidence for the efficacy of small dose of Ola on cognitive, spatial learning and memory functions of the brain. Although more studies are needed to validate our findings, the current work not only reinforces the chronic side effects of Ola on hepatic, renal and testicular functions but also encourages investigating safety and efficacy of smaller dose of Ola in the microgram range and searching for prophylactic preventive measures or using novel DAO inhibitors that have less hepatic toxicity.
Disclosure of conflict of interest
None.
Abbreviations
- Ola
Olanzapine
- SB
Sodium benzoate
- APDs
Antipsychotic drugs
- Ket
Ketamine
- DAO
D-amino acid oxidase inhibitor
- LH
Luteinizing hormone
- FSH
Follicle stimulating hormone
- Ftes
Free testosterone
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- Tp
Total protein
- alb
Albumin
- RAWM
Radial arm water maze
- TRT
Time to reach target
- WME
Working memory error
- RME
Reference memory error
References
- 1.Stępnicki P, Kondej M, Kaczor AA. Current concepts and treatments of schizophrenia. Molecules. 2018;23 doi: 10.3390/molecules23082087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. 2014;28:287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondej M, Stępnicki P, Kaczor AA. Multi-target approach for drug discovery against schizophrenia. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, Kissling W, Leucht S. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010:CD006654. doi: 10.1002/14651858.CD006654.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konarzewska B, Wołczyński S, Szulc A, Galińska B, Popławska R, Waszkiewicz N. Effect of risperidone and olanzapine on reproductive hormones, psychopathology and sexual functioning in male patients with schizophrenia. Psychoneuroendocrinology. 2009;34:129–39. doi: 10.1016/j.psyneuen.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19(Suppl 1):38–52. doi: 10.1017/S1092852914000601. [DOI] [PubMed] [Google Scholar]
- 7.Coyle JT. NMDA receptors and schizophrenia: a brief history. Schizophr Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marek GJ, Behl B, Bespalov AY, Gross G, Lee Y, Schoemaker H. Glutamatergic (N-methyl-D-aspartate receptor) hypofrontality in schizophrenia: too little juice or a mis wired brain? Mol Pharmacol. 2010;77:317–326. doi: 10.1124/mol.109.059865. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho SE, Na KS, Cho SJ, Kang SG. Low d-serine levels in schizophrenia: a systematic review and meta-analysis. Neurosci Lett. 2016;634:42–51. doi: 10.1016/j.neulet.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Betts JF, Schweimer JV, Burnham KE, Burnet PW, Sharp T, Harrison PJ. D-amino acid oxidase is expressed in the ventral tegmental area and modulates cortical dopamine. Front Synaptic Neurosci. 2014;6:11. doi: 10.3389/fnsyn.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, Lane HY. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2018;84:422–432. doi: 10.1016/j.biopsych.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Lane HY, Lin C, Green MF, Hellemann G, Huang CC, Chen PW, Tun R, Chang YC, Tsai GE. Add-on treatment of benzoate for schizophrenia a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry. 2013;70:1267–1275. doi: 10.1001/jamapsychiatry.2013.2159. [DOI] [PubMed] [Google Scholar]
- 14.Mutlu O, Celikyurt IK, Ulak G, Tanyeri P, Akar FY, Erden F. Effects of olanzapine and clozapine on radial maze performance in naive and MK-801-treated mice. Arzneimittelforschung. 2012;62:4–8. doi: 10.1055/s-0031-1291360. [DOI] [PubMed] [Google Scholar]
- 15.Razoux F, Garcia R, Isabelle Léna I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32:719–727. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- 16.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–9. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 17.Deacon RMJ. A novel approach to discovering treatment for Alzheimer’s disease. J Alzheimers Dis Parkinsonism. 2014;4:142. [Google Scholar]
- 18.Fervahaa G, Agida O, McDonalda K, Foussiasa G, Remingtona G. Daily activity patterns in remitted first-episode schizophrenia. Compr Psychiatry. 2014;55:1182–1187. doi: 10.1016/j.comppsych.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Weston-Green K, Babic I, de Santis M, Pan B, Montgomery MK, Mitchell T, Huang XF, Nealon J. Disrupted sphingolipid metabolism following acute clozapine and olanzapine administration. J Biomed Sci. 2018;25:40. doi: 10.1186/s12929-018-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milstein JA, Elnabawi A, Vinish M, Swanson T, Enos JK, Bailey AM, Kolb B, Frost DO. Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS One. 2013;8:e57308. doi: 10.1371/journal.pone.0057308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry AV Jr, Warner SE, Vandenhuerk L, Pillai A, Mahadik SP, Zhang G, Michael G, Bartlett MG. Negative effects of chronic oral chlorpromazine and olanzapine treatment on the performance of tasks designed to assess spatial learning and working memory in rats. Neuroscience. 2008;156:1005–1016. doi: 10.1016/j.neuroscience.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modi KK, Roy A, Brahmachari S, Rangasamy SB, Pahan K. Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of Alzheimer’s disease. PLoS One. 2015;10:e0130398. doi: 10.1371/journal.pone.0130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchett D, Taylor AM, Barkus C, Engle SJ, Brandon NJ, Sharp T, Foster RG, Harrison PJ, Peirson SN, Bannerman DM. Searching for cognitive enhancement in the morris water maze: better and worse performance in D-amino acid oxidase knockout (Dao-/-) mice. Eur J Neurosci. 2016;43:979–989. doi: 10.1111/ejn.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoshnoud MJ, Siavashpour A, Bakhshizadeh M, Rashedinia M. Effects of sodium benzoate, a commonly used food preservative, on learning, memory, and oxidative stress in brain of mice. J Biochem Mol Toxicol. 2018;32:e22022. doi: 10.1002/jbt.22022. [DOI] [PubMed] [Google Scholar]
- 25.Shah R, Subhan F, Ali G, Ullah I, Ullah S, Shahid M, Ahmad N, Fawad K. Olanzapine induced biochemical and histopathological changes after its chronic administration in rats. Saudi Pharm J. 2016;24:698–704. doi: 10.1016/j.jsps.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parasuraman S, Zhen KM, Banik U, Christapher PV. Ameliorative effect of curcumin on olanzapine-induced obesity in sprague-dawley rats. Phcog Res. 2017;9:247–252. doi: 10.4103/pr.pr_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kehinde OS, Christianah OI, Oyetunji OA. Ascorbic acid and sodium benzoate synergistically aggravate testicular dysfunction in adult Wistar rats. Int J Physiol Pathophysiol Pharmacol. 2018;10:39–46. [PMC free article] [PubMed] [Google Scholar]
- 28.Kehinde OS, Christianah OI, Oyetunji OA. Effect of sodium benzoate treatment on body weight of wistar rats. Indian Vet J. 2010;87:303–304. [Google Scholar]
- 29.Dewangan D. M.V. Sc. Thesis (2009), Department of veterinary pathology, College of veterinary science and animal husbandry. Studies on toxicopathology of sodium benzoate in rats. Submitted to the INDIRA GANDHI KRISHI VISHWAVIDYALAYA, RAIPUR, ROLL NO.-9949 I.D. NO.-UG/VETY/DURG/2002/18. [Google Scholar]
- 30.Telles-Correia D, Barbosa A, Cortez-Pinto H, Campos C, Rocha NB, Machado S. Psychotropic drugs and liver disease: a critical review of pharmacokinetics and liver toxicity. Gastrointest Pharmacol Ther. 2017;8:26–38. doi: 10.4292/wjgpt.v8.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slim M, Medina-Caliz I, Gonzalez-Jimenez A, Cabello MR, Mayoral-Cleries F, Lucena MI, Andrade RJ. Hepatic safety of atypical antipsychotics: current evidence and future directions. Drug Saf. 2016;39:925–43. doi: 10.1007/s40264-016-0436-7. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoud GS, Mostafa DG. Vitamin D aggravates the metabolic side effects of olanzapine in female rats. IOSR-JDMS 2016; 15 (6) Ver. I: 12-20. ISSN: 2279-0853, p-ISSN: 2279-0861. DOI: 10.9790/0853-1506021220. [Google Scholar]
- 33.Ibekwe SE, Uwakwe AA, Monanu MO. In vivo effects of sodium benzoate on plasma amino transferase and alkaline phosphatase of wistar albino rats. Scient Res Essay. 2007;2:006–009. [Google Scholar]
- 34.Hwang YJ, Dixon SN, Reiss JP, Wald R, Parikh CR, Gandhi S, Shariff SZ, Pannu N, Nash DM, Rehman F, Garg AX. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med. 2014;161:242–248. doi: 10.7326/M13-2796. [DOI] [PubMed] [Google Scholar]
- 35.He L, Peng Y, Fu X, Chen X, Liu H. Dibenzodiazepine derivative quetiapine- and olanzapine-induced chronic interstitial nephritis. Renal Failure. 2013;35:657–659. doi: 10.3109/0886022X.2013.780615. [DOI] [PubMed] [Google Scholar]
- 36.Kanofsky JD, Woesner ME, Harris AZ, Kelleher JP, Gittens K, Jerschow E. A case of acute renal failure in a patient recently treated with clozapine and a review of previously reported cases. Prim Care Companion CNS Disord. 2011;13:3. doi: 10.4088/PCC.10br01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth HF Jr, Carpenter CP. Further experience with the range finding test in the industrial toxicology laboratory. J Ind Hyg Toxicol. 1948;30:63–68. [PubMed] [Google Scholar]
- 38.Abd-AlGadir MI, Ihaimer MM, Sabah Elkheir MK, Idris OF. Effect of benzoic acid and combination of benzoic with citric acid as food additives on the renal function of experimental rats. Asian J Clin Nutri. 2009;1:83–87. [Google Scholar]
- 39.Evers S. The a-typical effects of olanzapine on body weight regulation: and the possible counter effects of topiramate. Uni of Groningen. 2015 [S.l.]: [S.n.]. 273 p. [Google Scholar]
- 40.Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology. 2003;28(Suppl 2):55–68. doi: 10.1016/s0306-4530(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 41.Piper JD, Piper PW. Benzoate and sorbate salts: a systematic review of the potential hazards of these invaluable preservatives and the expanding spectrum of clinical uses for sodium benzoate. Compr Rev Food Sci Food Saf. 2017;16 doi: 10.1111/1541-4337.12284. [DOI] [PubMed] [Google Scholar]


