Abstract
Two-component regulatory systems (TCSs) are a major mechanism by which bacteria sense and respond to changes in their environment. TCSs typically consist of two proteins that bring about major regulation of the cell genome through coordinated action mediated by phosphorylation. Environmental conditions that activate TCSs are numerous and diverse and include exposure to antibiotics as well as conditions inside a host. The resulting regulatory action often involves activation of antibiotic defenses and changes to cell physiology that increase antibiotic resistance. Examples of resistance mechanisms enacted by TCSs contained in this review span those found in both Gram-negative and Gram-positive species and include cell surface modifications, changes in cell permeability, increased biofilm formation, and upregulation of antibiotic-degrading enzymes.
Keywords: : antibiotic resistance, response regulator, sensor histidine kinase, signal transduction, two-component regulatory system
The escalating issue of antibiotic resistance
Since the introduction of penicillin in 1942, antibiotics have been nothing short of miracle drugs, prolonging life through treatment of previously lethal infections and permitting advances in healthcare through surgery. However, within 2 years of clinical implementation of penicillin, healthcare workers were already beginning to see a loss of drug efficacy due to bacterial resistance. Now, over 70 years later, a post-antibiotic era is either here or uncomfortably close. Measures must be taken to institute better stewardship of current drugs to dedicate funding to research of new drugs as well as to research the mechanisms of antibiotic resistance. This last point may seem comparatively trivial or too little, too late, but an understanding of how bacteria evade antibiotics is crucial to the success of both the former measures.
The intent of this review is to introduce the primary mechanisms by which bacteria activate antibiotic resistance via two-component regulatory systems (TCSs). TCSs are ubiquitous in bacteria and crucial to the maintenance of homeostasis, allowing bacteria to sense changes in their environment and respond accordingly. While the primary purpose of many TCSs is to initiate responses to simple criteria such as environmental changes in nutritional availability and osmolarity, others directly respond to the presence of antibiotics or conditions induced by them. In many cases the identity of the signal is not known, and even TCSs that do not directly sense antibiotics may respond to conditions that overlap with those found inside hosts or may indirectly lead to an increased antibiotic-resistant state. Consequently, TCSs are major players in the realm of infectious disease caused by pathogenic bacteria, and so much so that recent years have seen an increase in research of identifying drugs that target TCSs [1–21].
Fundamentals of bacterial two-component signal transduction
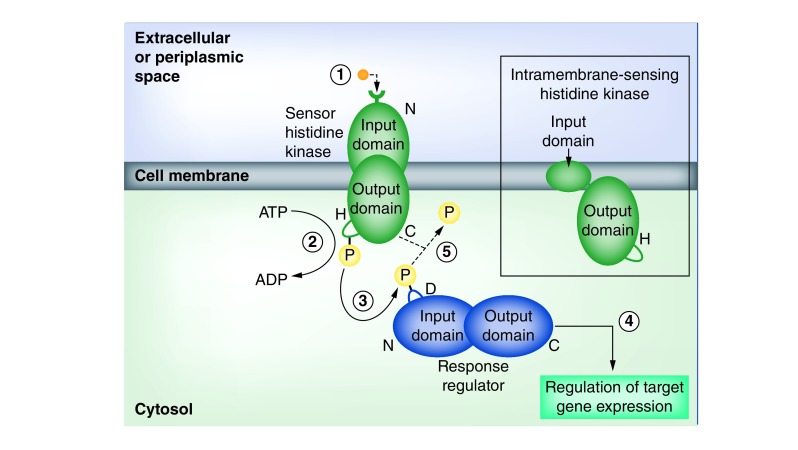
Prototypical bacterial TCSs consist of a pair of proteins: a sensor histidine kinase (HK) and a response regulator (RR) (Figure 1). The HK constitutes a homodimeric integral membrane protein with an N-terminal domain containing a receptor that faces the extracellular or periplasmic space and a C-terminal kinase domain located in the cytosol. These domains are connected by a series of transmembrane helices, the number of which vary from system to system. The variable HK N-terminal domain senses environmental changes via binding of an extracellular ligand or through other conformational changes, which triggers autophosphorylation of a conserved C-terminal histidine residue through adenosine triphosphate hydrolysis. The histidine-bound phosphate is transferred to an aspartate residue in the conserved N-terminal domain of the RR, a homodimeric protein located in the cytosol, which activates the RR’s variable C-terminal output domain, allowing it to regulate genomic expression via targeting of DNA. Through this flow of information through phosphorylation, bacteria are able to effectively sense changes in their surroundings (nutrition, pH, osmotic pressure, antibiotics, etc.) and orchestrate their genetic expression in a way that permits a rapid, cohesive reaction to a dynamic environment [22–26]. Furthermore, the variable natures of the input domain of the HK and the output domain of the RR can allow adaptation to recognize new signaling molecules and/or regulate different genetic foci.
Figure 1. The basic process of two-component signal transduction.
(1) An extracellular ligand (orange) binds to the N-terminal receptor of the sensor histidine kinase (green), embedded in the cell membrane. Binding of the ligand causes (2) the C-terminal kinase domain to hydrolyze adenosine triphosphate and phosphorylate a histidine residue. (3) The phosphate (yellow) is then transferred to an aspartate residue on the N-terminal domain of the cytosolic response regulator (blue). (4) This phosphorylation event activates the response regulator’s C-terminal output domain, which leads to global transcriptional changes. (5) In some cases, the sensor histidine kinase also functions as a phosphatase, and terminates the response regulator’s activation by removal of the phosphate. Inset to right: intramembrane-sensing histidine kinases lack an extracytoplasmic sensory domain and have recently been shown to recruit other sensory proteins in order to function.
The number and organization of proteins comprising a TCS can vary from the archetypal arrangement described above. For example, certain systems utilize an intramembrane-sensing HK (IM-HK), which is significantly smaller than an average HK and does not extend into the extracellular or periplasmic space (Figure 1). The IM-HKs were originally predicted to detect stimuli within or at the surface of the membrane [27,28], but more recent studies indicated that these minimalistic HKs do not carry out sensory functions independently and instead recruit additional proteins that detect membrane stimuli [29–31]. Additionally, despite the name of ‘two-component system’, the domains that compose the HK and RR may exist on multiple separate proteins, forming a phosphorelay. In particular, it is not uncommon for an independent protein to provide the histidine-containing phosphotransfer (HPt) domain that transfers a phosphate to the RR aspartate [32,33]. Finally, an additional gene product may be required for proper functioning of the HK [34] or act as a second RR [35], forming a ‘three-component system’.
A subset of sensor kinases autophosphorylate on a serine or threonine residue, which is similar to signal transduction in eukaryotic cells and has resulted in their being termed eukaryotic-like Ser/Thr kinases (eSTKs). The eSTKs bring about cellular changes by different mechanisms than TCSs, and therefore are not considered to be substitutes of HKs in TCSs. However, interactions in signaling between eSTKs and TCSs can occur [36]. As the purpose of this review is to consider antibiotic resistance controlled specifically by TCSs, eSTKs will not be discussed further.
Mechanisms of TCS-induced antibiotic resistance
In many cases, TCSs respond directly to the presence of antibiotics to bring about a resistant phenotype. In other cases, TCS-enacted global regulation in response to general environmental stressors or changes results in physiological changes that indirectly render the bacteria more resistant to antibiotics. While the types of environmental signals are highly variable, the resulting mechanisms of antibiotic resistance brought about by TCSs will be divided into four categories for the purposes of this review: modification of the cell surface, decreased drug influx or increased drug efflux, upregulation of antibiotic-degrading enzymes and alternative forms of antibiotic resistance, including biofilm production and stress response-induced antibiotic resistance. The following sections will highlight the best characterized TCSs per category of resistance mechanism. Additional TCSs and those that affect antibiotic resistance through an undetermined mechanism can be found in Table 1.
Table 1. Summary of two-component regulatory systems that increase antibiotic resistance.
| Two-component system | Regulation increasing antibiotic resistance | Antibiotic resistance(s) | Example species | Ref. |
|---|---|---|---|---|
| AarG | Increased expression of an aminoglycoside acetyltransferase Global transcriptional changes leading to increased intrinsic resistances |
Aminoglycosides Tetracycline Chloramphenicol Ciprofloxacin |
Providencia stuartii | [37] |
| AdeRS | Upregulation of AdeAB(C) efflux pump | Intrinsic resistance | Acinetobacter baumannii | [38–44] |
| AmgRS | Upregulation of MDR efflux pumps Activation of stress response protects membrane Mutations can give rise to SCVs |
Aminoglycosides Intrinsic resistance |
Pseudomonas aeruginosa | [45–48] |
| BaeSR | Upregulation of MDR efflux pumps | Ceftriaxone Intrinsic resistance |
A. baumannii Escherichia coli Salmonella enterica |
[38–40,42,49–52] |
| BfmRS | Increases formation of biofilm | Chloramphenicol Intrinsic resistance |
A. baumannii | [1,38,39,53–56] |
| BlrAB | Activation of three β-lactamase genes | β-lactams | Aeromonas species | [57–59] |
| BraRS (BceRS, NsaRS) | Upregulation of MDR efflux pumps | Nisin Bacitracin |
Staphylococcus aureus Bacillus subtilis |
[60–66] |
| CesRK | Upregulation of cell-envelope related genes | β-lactams | Listeria monocytogenes | [67–69] |
| CesSR | Upregulation of cell-envelope related genes | Bacitracin Bacteriocins |
Lactococcus lactis | [70] |
| CiaRH | Upregulation of cell-envelope related genes | β-lactams Cefotaxime Polymyxin B |
Streptococcus pneumoniae Group B Streptococcus |
[71–78] |
| ComDE | Increases formation of biofilm | Intrinsic resistance | Streptococcus mutans | [79,80] |
| CopRS | Decreased porin expression | β-lactams Carbapenems |
P. aeruginosa | [81,82] |
| CprRS | Modification of lipid A by 4-aminoarabinose (via arn operon) | Polymyxins Aminoglycosides |
P. aeruginosa | [83–88] |
| CpxAR | Decreased porin expression Upregulation of MDR efflux pumps |
Chloramphenicol Amikacin Nalidixic acid Tetracycline |
Klebsiella pneumoniae E. coli |
[8,89–95] |
| CreBC | Activation of β-lactamase gene Increases formation of biofilm |
β-lactams Intrinsic resistance |
P. aeruginosa | [96–99] |
| CroRS | Upregulation PBP5 | β-lactams | Enterococcus species | [100–106] |
| CzcRS | Decreased porin expression | β-lactams Carbapenems |
P. aeruginosa | [82,107] |
| EnvZ/OmpR | Decreased porin expression Upregulation of MDR efflux pumps |
β-lactams Intrinsic resistance |
E. coli A. baumannii Yersinia pseudotuberculosis S. enterica |
[89,90,108–122] |
| EvgAS | Upregulation of MDR efflux pumps | Intrinsic resistance |
E. coli Klebsiella pneumoniae Vibrio cholera P. aeruginosa |
[95,123–126] |
| fsr | Increases formation of biofilm via production of gelatinase | Intrinsic resistance | Enterococcus faecalis | [127] |
| GacSA | Increases formation of biofilm | Intrinsic resistance | A. baumannii | [128] |
| GraRS (aps) | Modification of teichoic acids via D-alanylation which reverses bacterial surface charge Upregulation of VraFG ABC transporter |
Daptomycin Vancomycin Cationic antibiotics |
S. aureus | [61,129–131] |
| hk11/rr11 | Increases formation of biofilm | Intrinsic resistance | S. mutans | [132] |
| LiaSR | Regulation of cell wall stress responses Increases formation of biofilm |
Vancomycin Bacitracin β-lactams Polymyxin B Nisin Intrinsic resistance |
B. subtilis Streptococcus species |
[34,60,71,133–137] |
| LisRK | Mechanism unknown | Nisin Cephalosporins |
L. monocytogenes | [60,138,139] |
| LsrRS | Activation of ABC transporter LctFEG | Nukacin ISK-1 | S. mutans | [60,61] |
| MisSR | Mechanism unknown; hypothesized upregulation of cell-envelope related proteins to protect the cell | Polymyxins Aminoglycosides |
Neisseria gonorrhoeae Neisseria meningitidis |
[140–142] |
| MtrAB | Upregulation of efflux pumps Regulation of a genetic switch to an MDR colony morphotype |
Isoniazid Rifampicin Ethambutol Vancomycin |
Mycobacterium tuberculosis Mycobacterium smegmatis Mycobacterium avium |
[143–146] |
| NsrRS | Activation of NsrX, a gene hypothesized to interfere with nisin binding to lipid II | Nisin | S. mutans | [60,61] |
| ParRS | Modification of lipid A by 4-aminoarabinose (via arn operon) | Polymyxins Aminoglycosides |
P. aeruginosa | [86–88,147] |
| PdtaRS | Mechanism unknown; hypothesized modification of 30S subunit of ribosome | Tetracycline Penimepicycline Blastocidin S |
M. smegmatis | [148,149] |
| PhoBR | Decreased porin expression | Chloramphenicol Amikacin Nalidixic acid Tetracycline |
K. pneumoniae | [150] |
| PhoPQ | Modification of lipid A by 4-aminoarabinose, phosphoethanolamine (via PmrAB), or palmitate (via PagP) Upregulation of efflux pumps (some species) |
Polymyxins Aminoglycosides |
Enterobacter cloacae P. aeruginosa S. enterica K. pneumoniae Yersinia pestis |
[151–172] |
| PmrAB | Modification of lipid A by 4-aminoarabinose or phosphoethanolamine Mutations can give rise to SCVs in P. aeruginosa |
Polymyxins Aminoglycosides |
A. baumannii S. enterica P. aeruginosa K. pneumoniae |
[39,153,157,161–164,167,168,171,173] |
| RcsBCD | Increases biofilm formation Regulates a gene required for 4-aminoarabinose modification of lipid A |
Daptomycin Cationic antibiotics Intrinsic resistance |
E. coli S. enterica Proteus mirabilis |
[158,174–186] |
| RetS-GacSA | Increases formation of biofilm | Intrinsic resistance | P. aeruginosa | [187–189] |
| SagS | Upregulation of MDR efflux pumps Increases formation of biofilm via BfiSR TCS |
Tobramycin Intrinsic resistance |
P. aeruginosa | [190–197] |
| SmeRySy | Upregulation of SmeZ efflux pump | Aminoglycosides | Stenotrophomonas maltophilia | [198] |
| VanSR | Alters vancomycin target through several actions | Vancomycin | S. aureus | [199–204] |
| VbrKR | Activation of β-lactamase genes | β-lactams | Vibrio parahaemolyticus | [6,205] |
| VicKR | Increases formation of biofilm | Intrinsic resistance | Streptococcus species | [137,206–208] |
| VirRS | Upregulation of ABC transporter AnrAB | Nisin | L. monocytogenes | [60,209,210] |
| VraSR | Increases peptidoglycan synthesis and expression of PBP2 | Methicillin Vancomycin Daptomycin Oxacillin β-lactams |
S. aureus | [60,211–217] |
| WalKR (YycFG) | Mechanism unknown; hypothesized increased permeability or decreased efflux Increases formation of biofilm |
Macrolides Lincosamides Intrinsic resistance |
S. aureus | [218–220] |
| YvcPQ | Upregulation of MDR efflux pumps | Lantibiotics Bacitracin |
Bacillus thuringiensis | [221] |
The purpose of this table is to provide the reader with a list of two-component regulatory systems that can increase antibiotic resistance, the mechanisms by which resistance occurs, examples of antibiotics that can be affected, and examples of bacterial species in which one or more of these resistances can occur. Table entries that occupy the same row are not intended to be exclusively linked.
MDR: Multidrug resistance; SCV: Small colony variant; TCS: Two-component system.
Modification of cell surfaces
Different classes of antibiotics work through a variety of mechanisms that cause bacterial lysis or stasis. However, all antibiotics must first interact with the outermost portion of the cell. In Gram-negative bacteria, this is the outer membrane (OM), and in Gram-positive bacteria, it is the cell wall composed of peptidoglycan. Many antibiotics directly target the OM, peptidoglycan or their biogenesis as their destabilization is lethal to cells [222].
Gram-negative bacteria
Strongly positively charged antibiotics such as polymyxin B, colistin, aminoglycosides, as well as host cationic antimicrobial peptides (CAMPs) take advantage of the net negative charge of the OM in Gram-negative cells. Polymyxin B, colistin and host CAMPs utilize a self-promoted uptake system in which they interact with the OM to form neutral patches that lead to membrane breakage, thereby permitting drug or peptide passage to the periplasm. Here, the amphipathic portion of the cationic molecules insert into the cytoplasmic membrane to form pores, leading to the breakdown of the membrane and cell death. It is also possible for this insertion to occur at the OM [223,224]. Aminoglycosides use the difference in charge to cross the membrane and reach their target – the bacterial ribosome [225–227]. The anionic nature of the OM is due to the prevalence of lipopolysaccharide, which contains a negatively charged lipid A moiety. Bacteria can reverse this state by covalent modification of the lipid A through which the OM becomes positively charged, resulting in decreased or abolished antibiotic action. The three most common modifications to lipid A are the addition of 4-aminoarabinose (4AA), phosphoethanolamine (PEtN) or palmitic acid, the latter of which reduces membrane fluidity but does not affect charge [151,152].
TCSs play a major role in OM lipid A modification, and the two of the best known and most well-characterized TCSs, PhoPQ and PmrAB are found in many Gram-negative bacterial species including but not limited to: Salmonella enterica, Enterobacter cloacae, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Yersinia pestis [151–170]. PhoQ is a HK located in the inner membrane (IM) which activates in response to low concentrations of Mg2+ or to the presence of polycationic peptides. The SK PhoQ then activates the RR PhoP, which can bring about modifications to the OM via genetic regulation. The path leading to lipid A modification differs from species to species. In S. enterica, PhoP can further activate pmrAB, which can lead to PEtN and 4AA modifications to lipid A by activation of the pmrCAB and arnBCADTEF-pmrE operons, respectively [38,164,173,228–232]. In P. aeruginosa, the arnBCADTEF-pmrE operon can be directly induced by PhoP or else via the PmrAB TCS [154]. Furthermore, PmrAB is capable of activating independently of PhoPQ through sensing of low pH or high Fe3+ levels [39,163,171,231], and a recent study of clinical isolates of Acinetobacter baumannii, a multidrug resistant Gram-negative nosocomial pathogen, found mutations in PmrAB could invoke up to a 30-fold increase in transcription of pmrC, which encodes the lipid A phosphoethanolamine transferase [233]. Finally, PhoP can also activate pagB, a gene encoding a palmitoyltransferase which modifies lipid A by addition of a palmitic acid [158].
Two additional TCSs in P. aeruginosa, ParRS and CprRS can activate the arnBCADTEF operon, which regulates 4-AA modification of lipid A in response to the detection of subinhibitory concentrations of polymyxins and colistin [83–87,147]. A recent study further demonstrated the critical need to understand these systems, as the researchers found that 4-AA lipid A modifications are a prerequisite to the evolution of colistin resistance in P. aeruginosa [234].
Finally, in several Gram-negative organisms, including E. coli and Salmonella species, the Rcs TCS, also known as the Rcs phosphorelay, regulates expression of ugd, which is essential to the production and incorporation of 4-AA into the lipopolysaccharide [158,174–184]. Further, in E. coli the Rcs TCS has been shown to increase PagP expression in mature biofilms, leading to increased lipid A palmitoylation and consequently enhanced resistance to treatment with positively charged antibiotics [184,235].
Gram-positive bacteria
The peptidoglycan of bacterial cell walls is formed by alternating sugar residues N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), polymers of which are linked together by cross-linked peptide side chains bound to NAM. During cell wall biosynthesis, the peptide side chains begin as pentapeptides bound to a Lipid II precursor molecule, and in many species the pentapeptide contains a terminal D-Ala D-Ala moiety. The cross-linkage of peptide side chains on opposite strands of the NAM-NAG polymer is catalyzed by a transpeptidase enzyme that recognizes the D-Ala D-Ala signature. The transpeptidase cleaves the distal D-Ala from each side chain being cross-linked and forms a bond between the two side chains.
Both the glycopeptide and β-lactam classes of antibiotics attempt to shut down cell wall synthesis by preventing the final cross-linking step of peptidoglycan synthesis. In the case of glycopeptides, such as vancomycin, inhibition is accomplished by binding of the drug to the D-Ala peptides, which simultaneously blocks polymerization of Lipid II through transglycosylation as well as the transpeptidase action [236]. β-lactams, such as penicillin, mimic the D-Ala-D-Ala moiety and bind to the active site of the transpeptidase enzyme, impeding it from binding its true substrate. For this reason, transpeptidase enzymes that cross-link peptidoglycan are called penicillin binding proteins (PBPs) [237].
The TCSs in many bacterial species can sense antimicrobials or antimicrobial-induced cell wall damage and initiate a response. Vancomycin-resistant Enterococcus and vancomycin-resistant Staphylococcus aureus and several Streptomyces species utilize a chromosomal or plasmid encoded VanSR TCS to counteract vancomycin. The VanS, the HK, is located in the plasma membrane and autophosphorylates on exposure to vancomycin. Subsequent activation of the RR VanR results in the activation of several genes – vanA/B, vanH, vanX and vanY – whose products collectively negate vancomycin’s mechanism of action. The VanA/B ligates D-Lac to D-Ala, lowering the concentration of D-Ala pentapeptides targetable by vancomycin. The VanH reduces pyruvate to D-Lac, further supporting the action of VanA/B. The VanX and VanY work together to deplete the cell of the normal peptidoglycan precursors displaying the D-Ala moiety required for vancomycin recognition [199–204].
In addition to VanSR defenses, S. aureus encodes VraSR and GraRS, both of which prevent antibiotic targeting of cell surfaces. The VraSR acts to mitigate antibiotic degradation of the cell wall specifically during the early and late stages of peptidoglycan synthesis. The system is induced by the action of β-lactams or glycopeptides, increasing resistance to vancomycin, daptomycin and oxacillin [60,211–216]. The VraSR has also been shown to increase expression of PBP2, which plays a role in methicillin and vancomycin resistance [217]. The GraRS, also known as the APS system (antimicrobial peptide sensing), reverses the surface bacterial charge via D-alanylation of teichoic acids to repel positively charged antibiotics and host cAMPs [129,130,238,239].
Enterococcus faecalis and Enterococcus faecium utilize the CroRS TCS to resist β-lactam antibiotics including ampicillin and cephalosporins. In this case the CroS HK activates in response to antimicrobial-induced cell wall damage, and in response, CroR upregulates PBP5, an alternative PBP which can still carry out transpeptidase activity while having a low-binding affinity for β-lactams [100–106].
Several Streptococcus species including S. pneumoniae, S. mutans, S. agalactiae (group B Streptococcus) utilize LiaSR to respond to cell-envelope stressors such as vancomycin, bacitracin and polymyxin B, resulting in activation of genes involved in cell wall maintenance [34,71,133–136].
TCS regulation of drug influx & efflux
Bacteria regulate entry and exit of many molecules via the expression of porins and efflux pumps. Porins, also called outer membrane proteins (OMPs), are β-barrel proteins embedded in the OM of Gram-negative bacteria that allow passive diffusion of molecules. Hydrophilic antibiotics such as β-lactams, aminoglycosides and fluoroquinolones can enter cells via porins, and therefore, downregulation of porins can reduce permeation and decrease susceptibility to a wide variety of antibiotics [240–244].
Efflux pumps are active transport proteins essential to bacterial homeostasis through expulsion of toxic substances and are found in all types of bacteria. Naturally, bacteria also use them to expel antibiotics, and many drug resistances arise from increased expression or activity of efflux pumps [242,243,245]. Some types of pumps transport a wide variety of structurally dissimilar compounds, and it is these that give rise to multiple drug resistances (MDR) and are therefore termed MDR efflux pumps. Increased expression of efflux pumps is often a first step to high levels of resistance as it allows the bacteria to cope with low to intermediate levels of an antibiotic, thereby giving it a chance to gain additional mutations [246]. It is therefore unsurprising that there are many instances of TCS-mediated upregulation of efflux pumps found across many species of MDR bacteria. Here, we will consider a few examples to appreciate the prevalence and diversity of these resistance mechanisms.
Gram-negative bacteria
One of the best known TCSs in Gram-negatives is the EnvZ/OmpR, which is found in a wide variety of species including E. coli, A. baumannii, Yersinia species and S. enterica among others [89,90,108–121]. EnvZ is a HK that primarily senses osmolarity changes and modulates the RR OmpR to regulate expression levels of the OM porins OmpC and OmpF according to levels of certain chemicals in the environment. Reduced expression levels of these OMPs has been seen to upregulate resistance to β-lactams in E. coli and S. enterica Typhimurium [38,89,90,108–110,112], and furthermore, OmpR has been seen to act in additional regulatory pathways in Yersinia, activating the AcrAB-TolC MDR efflux pump [122].
In P. aeruginosa, the TCSs CzcRS and CopRS respond to the presence of metals in the environment. The HK CzcS senses zinc, cobalt, cadmium and copper, while the CopS HK senses copper. Both RRs, CzcR and CopR, repress expression of the OrpD porin. As a result, carbapenem antibiotics, a subclass of β-lactams used to treat highly problematic MDR infections, can no longer access their target PBPs due to the lack of an entry route [81,82,107]. Another P. aeruginosa sensor kinase SagS, a TCS hybrid, activates the RR BlrR via cyclic-di-GMP to turn on MDR ABC transporters [190–197]. Additionally, the AmgRS TCS responds to membrane stress and damage induced by aminoglycosides by upregulating the mexAB-oprM MDR efflux pump [45].
Similarly, K. pneumoniae encodes two TCSs, CpxAR and PhoBR, that repress the KpnO porin, decreasing susceptibility to several antibiotics including chloramphenicol, amikacin, nalidixic acid, and tetracycline. Both TCSs activate due to OM stress [91,150]. Furthermore, CpxAR simultaneously upregulates three MDR RND-family efflux pumps in response to OM stress in K. pneumoniae [91]. A CpxAR homolog in E. coli acts identically, repressing expression of the porins OmpF and OmpC [92,93] and simultaneously activating the mar operon which increases expression of the MDR pump AcrAB-TolC [122].
A. baumannii has at least two TCSs that upregulate MDR efflux pumps. The first, AdeRS, activates the AdeABC MDR pump, and the second, BaeSR, also activates AdeABC as well as two other pumps: AdeIJK and MacAB-TolC [38–44,49]. In E. coli, BaeSR acts similarly, activating the mdtABCD gene cluster encoding a multidrug transporter [50,51]. Finally, BaeSR acts in S. enterica Typhimurium to upregulate two OMPs, resulting in increased resistance to ceftriaxone [52].
In Stenotrophomonas maltophilia, a nosocomial pathogen, the TCS PhoPQ not only contributes to polymyxin resistance through lipid A modification, but also causes resistance to gentamicin, kanamycin, and streptomycin (aminoglycosides) through activation of SmeZ, an efflux protein [172], which is also regulated by an additional S. maltophilia TCS called SmeRySy [198].
Gram-positive bacteria
S. aureus encodes two TCSs that increase resistance by upregulation of various ABC transporters in response to low concentrations of antibiotics: GraRS, which has been implicated in vancomycin resistance through upregulation of the VraFG ABC transporter [61,130,131] and BraRS, also called BceRS or NsaRS, which activates the BraDE and VraDE ABC transporters, increasing resistance to nisin and bacitracin [60–64].
Bacillus subtilis, a soil-dwelling model organism, increases expression of BceAB, an ABC-family efflux pump, via its TCS BceRS in response to exposure to the antibiotic bacitracin [65,66]. Similarly, the YvcPQ TCS in Bacillus thuringiensis, a member of the Bacillus cereus group, modulates efflux via two ABC transporters in response to lantibiotics and bacitracin [221].
Mycobacteria
Finally, Mycobacterium tuberculosis encodes an essential TCS called MtrAB that primarily functions in replication and cell wall biosynthesis. However, MtrA also binds and activates the iniBAC promoter, an operon encoding an efflux pump that increases resistance to isoniazid and ethambutol, two first-line antituberculosis drugs [143–145,247–253]. Additionally, mutations to mtrA in M. smegmatis resulted in decreased resistance to rifampicin and vancomycin, albeit with increased resistance to isoniazid [254]. In the opportunistic pathogen M. avium, MtrAB is required for conversion to a virulent, MDR colony morphotype, presumably by decreasing cell permeability [146].
TCS regulation of antibiotic-modifying enzymes
A discussion of antibiotic resistance would not be complete without mention of the first resistance mechanism clinicians encountered after the introduction of penicillin: the production of enzymes that target the antibiotic itself. As their name suggests, β-lactamases are enzymes that degrade β-lactam antibiotics, which they accomplish through hydrolyzation of the β-lactam ring. They are comprised of many subclasses including but not limited to carbapenemases, cephalosporinases and penicillinases. Other classes of enzymes inactivate antibiotics by direct modification, such as aminoglycoside and chloramphenicol acetyltransferases [255].
The CreBC TCS in P. aeruginosa not only affects biofilm formation, but also activates the chromosomal ampC gene encoding a β-lactamase. The HK CreB directly senses β-lactam activity, making this system particularly effective: it detects a specific threat and responds with a specific countermeasure [96–99].
Another example is the BlrAB TCS found in bacteria of the Aeromonas genus (Gram-negative). Analogous to the CreBC TCS, the BlrB HK autophosphorylates in response to accumulation of pentapeptides in the periplasm, the result of β-lactam inhibition of PBP4. Subsequent phosphorylation of BlrA results in activation of three β-lactamases: a cephalosporinase, a penicillinase and a carbapenemase [57–59].
A final example in the MDR nosocomial pathogen Providencia stuartii, the AarG sensor kinase has been implicated in aminoglycoside resistance through regulation of the aac(2’)-Ia gene encoding an aminoglycoside acetyltransferase. Furthermore, a mutation in aarG gave rise to several additional intrinsic resistances to tetracycline, chloramphenicol and ciprofloxacin, a phenotype that was hypothesized to be mediated via derepression of aarP, encoding a global transcriptional regulator that targets various MDR-associated genes [37].
Alternative forms of resistance
Upregulation of biofilm formation
Biofilms are structurally complex bacterial communities that present a major obstacle to the treatment of many infections. While a thorough discussion of the issues of antibiotic efficacy in eradication of bacteria contained in biofilms is beyond the scope of this review, it is important to convey that biofilm formation generally results in increased tolerance or resistance to antibiotics in several ways including differential gene expression in biofilms, decreased drug penetration through the matrix and the presence of dormant populations of cells or persisters [256–264].
Several Streptococcus species including S. pyogenes, S. mutans and S. pneumoniae utilize the TCS VicKR, which upregulates biofilm formation in response to an as-yet-undetermined environmental signal [137,206–208]. In S. mutans, LiaSR also increases biofilm formation in response to cell envelope stress and can directly react to the antibiotic bacitracin [135,137]. At least two other TCSs, ComDE and hk11/rr11 in S. mutans, also appear to function in the formation of biofilm [79,80,132].
P. aeruginosa is well known for its role in cystic fibrosis-associated lung infections and the tremendous difficulties encountered in treatment due to the impenetrable nature of its biofilm. Therefore, it is not surprising that P. aeruginosa encodes several TCSs that facilitate biofilm formation [187]. The two-component hybrid SagS directs the transition from a planktonic to surface-associated way of life, activating a second TCS, BfiSR, which is essential to the formation of biofilm. As previously noted, SagS simultaneously activates the BrlR RR to upregulate MDR efflux pumps within the biofilm, greatly increasing the biofilm drug tolerance [190–197]. CreBC directly senses β-lactam inhibition of PBP4 and responds by increasing biofilm formation [97]. The GacSA TCS in P. aeruginosa is also essential for biofilm formation and is unique among other GacSA systems in that the GacS sensor kinase cannot autophosphorylate. Instead GacS must be activated by the orphan sensor kinase RetS, which functions as both a kinase and phosphatase to GacS [187–189]. Several more TCSs, including the Roc1 system, Rcs/Pvr, PprAB and PilRS, regulate the expression of cup fimbriae and/or type IV pili required for surface adhesion and prerequisite to biofilm formation in P. aeruginosa [35,185,187,265–268]. Furthermore and finally, small colony variants [269] in this species are known to have increased biofilm production and multidrug resistance [270], and interestingly, a recent study on clinical isolates of P. aeruginosa revealed that mutations in amgRS as well as pmrAB culminated in a highly drug resistant SCV phenotype [46].
BfmRS, a TCS in A. baumannii, also upregulates biofilm production, possibly in response to sublethal concentrations of chloramphenicol, and in doing so, becomes increasingly recalcitrant to treatment [1,38,39,53–56]. Finally, the Rcs TCS is also essential to biofilm formation in E. coli, Proteus mirabilis and S. enterica Typhimurium as well as other species [174–182,186,187].
Stress response-associated antibiotic resistance
A number of TCSs induce cellular stress responses in reaction to environmental changes including poor nutrition, fluctuations in temperature, membrane integrity and oxidative stress. Stress responses often bring about global transcriptional changes, some of which alter the efficacy of antibiotic action. Many of the previously discussed TCSs are considered to function in this manner, including PhoPQ, CpxAR, LiaSR, BaeSR, BceRS, BraRS, VraSR, CroRS and ParRS [34,133,271–273]. Other examples include the AmgRS TCS in P. aeruginosa, which is believed to respond to membrane stress caused by aminoglycoside-induced accumulation of mistranslated peptides by upregulating certain proteases and otherwise implementing measures that protect the membrane [47,48].
Future perspective
TCSs are incredibly versatile and provide bacteria with an indispensable tool for sensing and reacting to their surroundings. Given the essential role of TCSs in bacterial homeostasis as well as the diverse antibiotic resistance mechanisms that can be activated through them, TCSs are an intriguing target for the development of new antimicrobial therapeutics [1–21]. The ability to use predictive software to identify putative compounds that bind one or more TCSs to render them ineffective would be an essential tool in the pharmaceutical targeting of TCSs [2]. Further, the conserved nature of certain domains of the various HKs and RRs both within and across bacterial species could be exploited to develop new broad-spectrum antimicrobials, although portions of these domains share similarity to eukaryotic proteins and thereby could lead to inhibition of host cell processes. Tiwari et al. recently published a review on this topic that covers the benefits and feasibility of medicinal targeting of TCSs and lists several predicted and validated TCS inhibitors [21].
While this review cannot hope to detail the myriad and often pleiotropic effects of TCSs, one can certainly begin to grasp the scope of their importance in antibiotic resistance from the research compiled herein. The numerous instances of TCS-induced resistance mechanisms across both Gram-negative and Gram-positive species highlights the need for further and continued investigation into targeting TCSs in the future development of antimicrobial therapeutics.
Executive summary.
Bacteria utilize coupled sensory-response proteins known as two-component systems (TCSs) to sense environmental changes, including exposure to antibiotics and conditions inside a host, and to respond with activation of genes that increase antibiotic resistance.
TCSs are ubiquitous in bacteria and can initiate a variety of antibiotic resistance mechanisms, including modification of the cell surface, increased efflux, decreased influx, biofilm formation and upregulation of antibiotic-degrading enzymes.
The importance of TCSs to antibiotic resistance, in addition to their role in regulating virulence, has led to the current focus in drug discovery for TCS inhibitors.
Acknowledgments
Authors would like to thank W Shafer of Emory University for critical reading of this manuscript.
Footnotes
Author contributions
PN Rather and ARP Tierney meet the following criteria: substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial & competing interests disclosure
This work is supported by the following awards to PN Rather, VA Merit Award I01 BX001725 and Research Career Scientist Award IK6BX004470; both from the Department of Veterans Affairs and R01AI072219 from the National Institutes of Health. ARP Tierney is supported by the T32 training grant AI106699 from the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Russo TA, Manohar A, Beanan JM. et al. The response regulator BfmR is a potential drug target for acinetobacter baumannii. mSphere 1(3), pii:e00082–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bem AE, Velikova N, Pellicer MT, Baarlen P, Marina A, Wells JM. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 10(1), 213–224 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Worthington RJ, Blackledge MS, Melander C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Future Med. Chem. 5(11), 1265–1284 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Barrett JF, Goldschmidt RM, Lawrence LE. et al. Antibacterial agents that inhibit two-component signal transduction systems. Proc. Natl Acad. Sci. USA 95(9), 5317–5322 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett JF, Hoch JA. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42(7), 1529–1536 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardona ST, Choy M, Hogan AM. Essential two-component systems regulating cell envelope functions: opportunities for novel antibiotic therapies. J. Membr. Biol. 251(1), 75–89 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Cai XH, Zhang Q, Shi SY, Ding DF. Searching for potential drug targets in two-component and phosphorelay signal-transduction systems using three-dimensional cluster analysis. Acta Biochim. Biophys. Sin. (Shanghai) 37(5), 293–302 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Dbeibo L, Van Rensburg JJ, Smith SN. et al. Evaluation of CpxRA as a therapeutic target for uropathogenic Escherichia coli infections. Infect. Immun. 86(3), e00798–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilliard JJ, Goldschmidt RM, Licata L, Baum EZ, Bush K. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 43(7), 1693–1699 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hlasta DJ, Demers JP, Foleno BD. et al. Novel inhibitors of bacterial two-component systems with Gram-positive antibacterial activity: pharmacophore identification based on the screening hit closantel. Bioorg. Med. Chem. Lett. 8(14), 1923–1928 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Milton ME, Minrovic BM, Harris DL. et al. Re-sensitizing multidrug resistant bacteria to antibiotics by targeting bacterial response regulators: characterization and comparison of interactions between 2-aminoimidazoles and the response regulators BfmR from acinetobacter baumannii and QseB from Francisella spp. Front. Mol. Biosci 5, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson K, Yamaguchi Y, Hoch JA. The mechanism of action of inhibitors of bacterial two-component signal transduction systems. J. Biol. Chem. 275(49), 38900–38904 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Stephenson K, Hoch JA. Two-component and phosphorelay signal-transduction systems as therapeutic targets. Curr. Opin. Pharmacol. 2(5), 507–512 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Stephenson K, Hoch JA. Virulence- and antibiotic resistance-associated two-component signal transduction systems of Gram-positive pathogenic bacteria as targets for antimicrobial therapy. Pharmacol. Ther. 93(2–3), 293–305 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Stephenson K, Hoch JA. Histidine kinase-mediated signal transduction systems of pathogenic microorganisms as targets for therapeutic intervention. Curr. Drug Targets Infect. Disord. 2(3), 235–246 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Stephenson K, Hoch JA. Developing inhibitors to selectively target two-component and phosphorelay signal transduction systems of pathogenic microorganisms. Curr. Med. Chem. 11(6), 765–773 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Tang YT, Gao R, Havranek JJ, Groisman EA, Stock AM, Marshall GR. Inhibition of bacterial virulence: drug-like molecules targeting the Salmonella enterica PhoP response regulator. Chem. Biol. Drug Des. 79(6), 1007–1017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utsumi R, Igarashi M. [Two-component signal transduction as attractive drug targets in pathogenic bacteria]. Yakugaku Zasshi 132(1), 51–58 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Velikova N, Fulle S, Manso AS. et al. Putative histidine kinase inhibitors with antibacterial effect against multi-drug resistant clinical isolates identified by in vitro and in silico screens. Sci. Rep. 6, 26085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilke KE, Francis S, Carlson EE. Inactivation of multiple bacterial histidine kinases by targeting the ATP-binding domain. ACS Chem. Biol. 10(1), 328–335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari S, Jamal SB, Hassan SS. et al. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: an overview. Front. Microbiol. 8, 1878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Review of the benefits and feasibility of medicinal targeting of two-component regulatory systems (TCSs) and lists several predicted and validated TCS inhibitors.
- 22.Bourret RB, Silversmith RE. Two-component signal transduction. Curr. Opin. Microbiol. 13(2), 113–115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung J, Hendrickson WA. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 13(2), 116–123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groisman EA. Feedback control of two-component regulatory systems. Annu. Rev. Microbiol. 70, 103–124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Detailed review of TCS functionality, regulation and the significance of these systems to bacterial physiology.
- 25.Nixon BT, Ronson CW, Ausubel FM. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc. Natl Acad. Sci. USA 83(20), 7850–7854 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoch JA. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3(2), 165–170 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50(5), 1591–1604 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Mascher T. Intramembrane-sensing histidine kinases: a new family of cell envelope stress sensors in Firmicutes bacteria. FEMS Microbiol. Lett. 264(2), 133–144 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Mascher T. Bacterial (intramembrane-sensing) histidine kinases: signal transfer rather than stimulus perception. Trends Microbiol. 22(10), 559–565 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Revilla-Guarinos A, Gebhard S, Mascher T, Zuniga M. Defence against antimicrobial peptides: different strategies in Firmicutes. Environ. Microbiol. 16(5), 1225–1237 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Gebhard S, Mascher T. Antimicrobial peptide sensing and detoxification modules: unravelling the regulatory circuitry of Staphylococcus aureus. Mol. Microbiol. 81(3), 581–587 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Gao R, Stock AM. Biological insights from structures of two-componentrproteins. Ann. Rev. Microbiol. 63, 133–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvado B, Vilaprinyo E, Sorribas A, Alves R. A survey of HK, HPt, and RR domains and their organization in two-component systems and phosphorelay proteins of organisms with fully sequenced genomes. PeerJ 3, e1183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan S, Junker A, Helmann JD, Mascher T. Regulation of LiaRS-dependent gene expression in Bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system. J. Bacteriol. 188(14), 5153–5166 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuchma SL, Connolly JP, O'toole GA. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 187(4), 1441–1454 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dworkin J. Ser/Thr phosphorylation as a regulatory mechanism in bacteria. Curr. Opin. Microbiol. 24(Suppl. C), 47–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rather PN, Paradise MR, Parojcic MM, Patel S. A regulatory cascade involving AarG, a putative sensor kinase, controls the expression of the 2′-N-acetyltransferase and an intrinsic multiple antibiotic resistance (Mar) response in Providencia stuartii. Mol. Microbiol. 28(6), 1345–1353 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Lee CR, Lee JH, Park M. et al. Biology of acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infection Microbiol. 7, 55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroger C, Kary SC, Schauer K, Cameron AD. Genetic regulation of virulence and antibiotic resistance in acinetobacter baumannii. Genes (Basel) 8(1), pii:E12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richmond GE, Evans LP, Anderson MJ. et al. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. mBio 7(2), e00430–e00416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams FG, Stroeher UH, Hassan KA, Marri S, Brown MH. Resistance to pentamidine is mediated by AdeAB, regulated by AdeRS, and influenced by growth conditions in Acinetobacter baumannii ATCC 17978. PLoS ONE 13(5), e0197412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coyne S, Courvalin P, Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 55(3), 947–953 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48(9), 3298–3304 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon EJ, Courvalin P, Grillot-Courvalin C. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob. Agents Chemother. 57(7), 2989–2995 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fruci M, Poole K. Aminoglycoside-inducible expression of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: Involvement of the envelope stress-responsive AmgRS two-component system. PLoS ONE 13(10), e0205036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schniederjans M, Koska M, Haussler S. Transcriptional and mutational profiling of an aminoglycoside-resistant Pseudomonas aeruginosa small-colony variant. Antimicrob. Agents Chemother. 61(11), pii:e01178–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Hinz A, Bauerle E. et al. Targeting a bacterial stress response to enhance antibiotic action. Proc. Natl Acad. Sci. USA 106(34), 14570–14575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau CH, Fraud S, Jones M, Peterson SN, Poole K. Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57(5), 2243–2251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin MF, Lin YY, Lan CY. The role of the two-component system BaeSR in disposing chemicals through regulating transporter systems in acinetobacter baumannii. PLoS ONE 10(7), e0132843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baranova N, Nikaido H. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 184(15), 4168–4176 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 184(15), 4161–4167 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu WS, Li PC, Cheng CY. Correlation between ceftriaxone resistance of Salmonella enterica serovar Typhimurium and expression of outer membrane proteins OmpW and Ail/OmpX-like protein, which are regulated by BaeR of a two-component system. Antimicrob. Agents Chemother. 49(9), 3955–3958 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 11(2), e1004691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 4(3), 273–278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi L, Li H, Zhang C. et al. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front. Microbiol. 7, 483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 154(Pt 11), 3398–3409 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Alksne LE, Rasmussen BA. Expression of the AsbA1, OXA-12, and AsbM1 beta-lactamases in Aeromonas jandaei AER 14 is coordinated by a two-component regulon. J. Bacteriol. 179(6), 2006–2013 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niumsup P, Simm AM, Nurmahomed K, Walsh TR, Bennett PM, Avison MB. Genetic linkage of the penicillinase gene, amp, and blrAB, encoding the regulator of beta-lactamase expression in Aeromonas spp. J. Antimicrob. Chemother. 51(6), 1351–1358 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Tayler AE, Ayala JA, Niumsup P. et al. Induction of beta-lactamase production in Aeromonas hydrophila is responsive to beta-lactam-mediated changes in peptidoglycan composition. Microbiology 156(Pt 8), 2327–2335 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Draper LA, Cotter PD, Hill C, Ross RP. Lantibiotic resistance. Microbiol. Mol. Biol. Rev. 79(2), 171–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawada-Matsuo M, Yoshida Y, Zendo T. et al. Three distinct two-component systems are involved in resistance to the class I bacteriocins, Nukacin ISK-1 and nisin A, in Staphylococcus aureus. PLoS ONE 8(7), e69455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blake KL, Randall CP, O'neill AJ. In vitro studies indicate a high resistance potential for the lantibiotic nisin in Staphylococcus aureus and define a genetic basis for nisin resistance. Antimicrob. Agents Chemother. 55(5), 2362–2368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolar SL, Nagarajan V, Oszmiana A. et al. NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 157(Pt 8), 2206–2219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hiron A, Falord M, Valle J, Debarbouille M, Msadek T. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 81(3), 602–622 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Rietkötter E, Hoyer D, Mascher T. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 68(3), 768–785 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Ohki R, Giyanto, Tateno K. et al. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 49(4), 1135–1144 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Gottschalk S, Bygebjerg-Hove I, Bonde M. et al. The two-component system CesRK controls the transcriptional induction of cell envelope-related genes in Listeria monocytogenes in response to cell wall-acting antibiotics. J. Bacteriol. 190(13), 4772–4776 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nielsen PK, Andersen AZ, Mols M, Van Der Veen S, Abee T, Kallipolitis BH. Genome-wide transcriptional profiling of the cell envelope stress response and the role of LisRK and CesRK in Listeria monocytogenes. Microbiology 158(Pt 4), 963–974 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Kallipolitis BH, Ingmer H, Gahan CG, Hill C, Sogaard-Andersen L. CesRK, a two-component signal transduction system in Listeria monocytogenes, responds to the presence of cell wall-acting antibiotics and affects beta-lactam resistance. Antimicrob. Agents Chemother. 47(11), 3421–3429 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez B, Zomer AL, Rodriguez A, Kok J, Kuipers OP. Cell envelope stress induced by the bacteriocin Lcn972 is sensed by the Lactococcal two-component system CesSR. Mol. Microbiol. 64(2), 473–486 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Gomez-Mejia A, Gamez G, Hammerschmidt S. Streptococcus pneumoniae two-component regulatory systems: the interplay of the pneumococcus with its environment. Int. J. Med. Microbiol. 308(6), 722–737 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Mascher T, Heintz M, Zahner D, Merai M, Hakenbeck R. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta-lactam resistance. J. Bacteriol. 188(5), 1959–1968 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lévesque C, Mair R, Perry J, Lau P, Li Y, Cvitkovitch D. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett. Appl. Microbiol. 45(4), 398–404 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zahner D, Kaminski K, Van Der Linden M, Mascher T, Meral M, Hakenbeck R. The ciaR/ciaH regulatory network of Streptococcus pneumoniae. J. Mol. Microbiol. Biotechnol. 4(3), 211–216 (2002). [PubMed] [Google Scholar]
- 75.Quach D, Van Sorge NM, Kristian SA, Bryan JD, Shelver DW, Doran KS. The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses. J. Bacteriol. 191(7), 2023–2032 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mascher T, Zahner D, Merai M, Balmelle N, De Saizieu AB, Hakenbeck R. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J. Bacteriol. 185(1), 60–70 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guenzi E, Gasc AM, Sicard MA, Hakenbeck R. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12(3), 505–515 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Giammarinaro P, Sicard M, Gasc AM. Genetic and physiological studies of the CiaH–CiaR two-component signal-transducing system involved in cefotaxime resistance and competence of Streptococcus pneumoniae. Microbiology 145(Pt 8), 1859–1869 (1999). [DOI] [PubMed] [Google Scholar]
- 79.Li YH, Tang N, Aspiras MB. et al. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184(10), 2699–2708 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183(3), 897–908 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caille O, Rossier C, Perron K. A copper-activated two-component system interacts with zinc and imipenem resistance in Pseudomonas aeruginosa. J. Bacteriol. 189(13), 4561–4568 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raavi, Mishra S, Singh S. Prevention of OprD regulated antibiotic resistance in Pseudomonas aeruginosa biofilm. Microb. Pathog. 112, 221–229 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Breidenstein EB, De La Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19(8), 419–426 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Fernandez L, Breidenstein EB, Song D, Hancock RE. Role of intracellular proteases in the antibiotic resistance, motility, and biofilm formation of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56(2), 1128–1132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gutu AD, Sgambati N, Strasbourger P. et al. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob. Agents Chemother. 57(5), 2204–2215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muller C, Plesiat P, Jeannot K. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and beta-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 55(3), 1211–1221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JY, Chung ES, Na IY, Kim H, Shin D, Ko KS. Development of colistin resistance in pmrA-, phoP-, parR- and cprR-inactivated mutants of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 69(11), 2966–2971 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Fernandez L, Jenssen H, Bains M, Wiegand I, Gooderham WJ, Hancock RE. The two-component system CprRS senses cationic peptides and triggers adaptive resistance in Pseudomonas aeruginosa independently of ParRS. Antimicrob. Agents Chemother. 56(12), 6212–6222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun S, Berg OG, Roth JR, Andersson DI. Contribution of gene amplification to evolution of increased antibiotic resistance in Salmonella typhimurium . . Genetics 182(4), 1183–1195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirakawa H, Nishino K, Yamada J, Hirata T, Yamaguchi A. Beta-lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Antimicrob. Chemother. 52(4), 576–582 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Srinivasan VB, Vaidyanathan V, Mondal A, Rajamohan G. Role of the two component signal transduction system CpxAR in conferring cefepime and chloramphenicol resistance in Klebsiella pneumoniae NTUH-K2044. PLoS ONE 7(4), e33777 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batchelor E, Walthers D, Kenney LJ, Goulian M. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins OmpF and OmpC. J. Bacteriol. 187(16), 5723–5731 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raivio TL, Leblanc SK, Price NL. The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J. Bacteriol. 195(12), 2755–2767 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weatherspoon-Griffin N, Yang D, Kong W, Hua Z, Shi Y. The CpxR/CpxA two-component regulatory system up-regulates the multidrug resistance cascade to facilitate Escherichia coli resistance to a model antimicrobial peptide. J. Biol. Chem. 289(47), 32571–32582 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirakawa H, Nishino K, Hirata T, Yamaguchi A. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 185(6), 1851–1856 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moya B, Dotsch A, Juan C. et al. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5(3), e1000353 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zamorano L, Moya B, Juan C, Mulet X, Blazquez J, Oliver A. The Pseudomonas aeruginosa CreBC two-component system plays a major role in the response to beta-lactams, fitness, biofilm growth, and global regulation. Antimicrob. Agents Chemother. 58(9), 5084–5095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsutsumi Y, Tomita H, Tanimoto K. Identification of novel genes responsible for overexpression of ampC in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 57(12), 5987–5993 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Avison MB, Horton RE, Walsh TR, Bennett PM. Escherichia coli CreBC is a global regulator of gene expression that responds to growth in minimal media. J. Biol. Chem. 276(29), 26955–26961 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Arbeloa A, Segal H, Hugonnet JE. et al. Role of class A penicillin-binding proteins in PBP5-mediated beta-lactam resistance in Enterococcus faecalis . . J. Bacteriol. 186(5), 1221–1228 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Comenge Y, Quintiliani R, Jr., Li L. et al. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J. Bacteriol. 185(24), 7184–7192 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kellogg SL, Kristich CJ. Functional dissection of the CroRS two-component system required for resistance to cell wall stressors in Enterococcus faecalis. J. Bacteriol. 198(8), 1326–1336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kellogg SL, Little JL, Hoff JS, Kristich CJ. Requirement of the CroRS two-component system for resistance to cell wall-targeting antimicrobials in Enterococcus faecium. Antimicrob. Agents Chemother. 61(5), pii:e02461–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-infect. Ther 12(10), 1221–1236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hancock LE, Perego M. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J. Bacteriol. 186(23), 7951–7958 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snyder H, Kellogg SL, Skarda LM, Little JL, Kristich CJ. Nutritional control of antibiotic resistance via an interface between the phosphotransferase system and a two-component signaling system. Antimicrob. Agents Chemother. 58(2), 957–965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perron K, Caille O, Rossier C, Van Delden C, Dumas JL, Kohler T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J. Biol. Chem. 279(10), 8761–8768 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Jaffe A, Chabbert YA, Derlot E. Selection and characterization of beta-lactam-resistant Escherichia coli K-12 mutants. Antimicrob. Agents Chemother. 23(4), 622–625 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mizuno T, Mizushima S. Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes. Mol. Microbiol. 4(7), 1077–1082 (1990). [DOI] [PubMed] [Google Scholar]
- 110.Cai SJ, Inouye M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J. Biol. Chem. 277(27), 24155–24161 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Tipton KA, Rather PN. An ompR/envZ two-component system ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii strain AB5075. J. Bacteriol. (2016) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viveiros M, Dupont M, Rodrigues L. et al. Antibiotic stress, genetic response and altered permeability of E. coli. PLoS ONE 2(4), e365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Russo FD, Silhavy TJ. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J. Mol. Biol. 222(3), 567–580 (1991). [DOI] [PubMed] [Google Scholar]
- 114.Raczkowska A, Skorek K, Brzostkowska M, Lasinska A, Brzostek K. Pleiotropic effects of a Yersinia enterocolitica ompR mutation on adherent-invasive abilities and biofilm formation. FEMS Microbiol. Lett. 321(1), 43–49 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Brzostkowska M, Raczkowska A, Brzostek K. OmpR, a response regulator of the two-component signal transduction pathway, influences inv gene expression in Yersinia enterocolitica O9. Front. Cell. Infect. Microbiol. 2, 153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao H, Zhang Y, Han Y. et al. Phenotypic and transcriptional analysis of the osmotic regulator OmpR in Yersinia pestis. BMC Microbiol. 11, 39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dorman CJ, Chatfield S, Higgins CF, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect. Immun. 57(7), 2136–2140 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alphen WV, Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J. Bacteriol. 131(2), 623–630 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liljestrom P, Maattanen PL, Palva ET. Cloning of the regulatory locus ompB of Salmonella typhimurium LT-2. II. Identification of the envZ gene product, a protein involved in the expression of the porin proteins. Mol. Gen. Genet. 188(2), 190–194 (1982). [DOI] [PubMed] [Google Scholar]
- 120.Liljestrom P, Laamanen I, Palva ET. Structure and expression of the ompB operon, the regulatory locus for the outer membrane porin regulon in Salmonella typhimurium LT-2. J. Mol. Biol. 201(4), 663–673 (1988). [DOI] [PubMed] [Google Scholar]
- 121.Forst SA, Roberts DL. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res. Microbiol. 145(5–6), 363–373 (1994). [DOI] [PubMed] [Google Scholar]
- 122.Raczkowska A, Trzos J, Lewandowska O, Nieckarz M, Brzostek K. Expression of the AcrAB components of the AcrAB-TolC multidrug efflux pump of yersinia enterocolitica is subject to dual regulation by OmpR. PLoS ONE 10(4), e0124248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eguchi Y, Oshima T, Mori H. et al. Transcriptional regulation of drug efflux genes by EvgAS, a two-component system in Escherichia coli. Microbiology 149(Pt 10), 2819–2828 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Nishino K, Yamaguchi A. EvgA of the two-component signal transduction system modulates production of the yhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184(8), 2319–2323 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nishino K, Yamaguchi A. Overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J. Bacteriol. 183(4), 1455–1458 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kato A, Ohnishi H, Yamamoto K, Furuta E, Tanabe H, Utsumi R. Transcription of emrKY is regulated by the EvgA–EvgS two-component system in Escherichia coli K-12. Biosci. Biotechnol. Biochem. 64(6), 1203–1209 (2000). [DOI] [PubMed] [Google Scholar]
- 127.Hancock LE, Perego M. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 186(17), 5629–5639 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cerqueira GM, Kostoulias X, Khoo C. et al. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J. Infect. Dis. 210(1), 46–55 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Herbert S, Bera A, Nerz C. et al. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 3(7), e102 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang SJ, Bayer AS, Mishra NN. et al. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 80(1), 74–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meehl M, Herbert S, Gotz F, Cheung A. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 51(8), 2679–2689 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li YH, Lau PC, Tang N, Svensater G, Ellen RP, Cvitkovitch DG. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184(22), 6333–6342 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mascher T, Zimmer SL, Smith TA, Helmann JD. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis. Antimicrob. Agents Chemother. 48(8), 2888–2896 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pietiainen M, Gardemeister M, Mecklin M, Leskela S, Sarvas M, Kontinen VP. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151(Pt 5), 1577–1592 (2005). [DOI] [PubMed] [Google Scholar]
- 135.Suntharalingam P, Senadheera MD, Mair RW, Levesque CM, Cvitkovitch DG. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J. Bacteriol. 191(9), 2973–2984 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klinzing DC, Ishmael N, Dunning Hotopp JC. et al. The two-component response regulator LiaR regulates cell wall stress responses, pili expression and virulence in group B Streptococcus. Microbiology 159(Pt 7), 1521–1534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kawada-Matsuo M, Komatsuzawa H. Role of Streptococcus mutans two-component systems in antimicrobial peptide resistance in the oral cavity. Jpn. Dent. Sci. Rev. 53(3), 86–94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kallipolitis BH, Ingmer H. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204(1), 111–115 (2001). [DOI] [PubMed] [Google Scholar]
- 139.Cotter PD, Guinane CM, Hill C. The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob. Agents Chemother. 46(9), 2784–2790 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tzeng YL, Datta A, Ambrose K. et al. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. J. Biol. Chem. 279(33), 35053–35062 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Kandler JL, Holley CL, Reimche JL. et al. The MisR response regulator is necessary for intrinsic cationic antimicrobial peptide and aminoglycoside resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 60(8), 4690–4700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johnson CR, Newcombe J, Thorne S. et al. Generation and characterization of a PhoP homologue mutant of Neisseria meningitidis. Mol. Microbiol. 39(5), 1345–1355 (2001). [DOI] [PubMed] [Google Scholar]
- 143.Li Y, Zeng J, Zhang H, He ZG. The characterization of conserved binding motifs and potential target genes for M. tuberculosis MtrAB reveals a link between the two-component system and the drug resistance of M. smegmatis. BMC Microbiol. 10, 242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moker N, Brocker M, Schaffer S, Kramer R, Morbach S, Bott M. Deletion of the genes encoding the MtrA–MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoprotection. Mol. Microbiol. 54(2), 420–438 (2004). [DOI] [PubMed] [Google Scholar]
- 145.Nguyen HT, Wolff KA, Cartabuke RH, Ogwang S, Nguyen L. A lipoprotein modulates activity of the MtrAB two-component system to provide intrinsic multidrug resistance, cytokinetic control and cell wall homeostasis in Mycobacterium. Mol. Microbiol. 76(2), 348–364 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Cangelosi GA, Do JS, Freeman R, Bennett JG, Semret M, Behr MA. The two-component regulatory system mtrAB is required for morphotypic multidrug resistance in Mycobacterium avium. Antimicrob. Agents Chemother. 50(2), 461–468 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fernandez L, Gooderham WJ, Bains M, Mcphee JB, Wiegand I, Hancock RE. Adaptive resistance to the ‘last hope’ antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR–ParS. Antimicrob. Agents Chemother. 54(8), 3372–3382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dadura K, Plocinska R, Rumijowska-Galewicz A. et al. PdtaS deficiency affects resistance of mycobacteria to ribosome targeting antibiotics. Front. Microbiol. 8, 2145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morth JP, Gosmann S, Nowak E, Tucker PA. A novel two-component system found in Mycobacterium tuberculosis. FEBS Lett. 579(19), 4145–4148 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Srinivasan VB, Venkataramaiah M, Mondal A, Vaidyanathan V, Govil T, Rajamohan G. Functional characterization of a novel outer membrane porin KpnO, regulated by PhoBR two-component system in Klebsiella pneumoniae NTUH-K2044. PLoS ONE 7(7), e41505 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 5, 643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in Gram-negative bacteria. Ann. Rev. Bio. 76, 295–329 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng HY, Chen YF, Peng HL. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 17, 60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gooderham WJ, Hancock RE. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 33(2), 279–294 (2009). [DOI] [PubMed] [Google Scholar]
- 155.Guo L, Lim KB, Gunn JS. et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP–phoQ. Science 276(5310), 250–253 (1997). [DOI] [PubMed] [Google Scholar]
- 156.Macfarlane EL, Kwasnicka A, Hancock RE. Role of Pseudomonas aeruginosa PhoP–phoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 146(Pt 10), 2543–2554 (2000). [DOI] [PubMed] [Google Scholar]
- 157.Mcphee JB, Bains M, Winsor G. et al. Contribution of the PhoP–PhoQ and PmrA–PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 188(11), 3995–4006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richards SM, Strandberg KL, Gunn JS. Salmonella-regulated lipopolysaccharide modifications. Subcell. Biochem. 53, 101–122 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Zhou D, Han Y, Qin L. et al. Transcriptome analysis of the Mg2+-responsive PhoP regulator in Yersinia pestis. FEMS Microbiol. Lett. 250(1), 85–95 (2005). [DOI] [PubMed] [Google Scholar]
- 160.Band VI, Crispell EK, Napier BA. et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat. Microbiol. 1(6), 16053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Adams MD, Nickel GC, Bajaksouzian S. et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 53(9), 3628–3634 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beceiro A, Llobet E, Aranda J. et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55(7), 3370–3379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gunn JS. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 16(6), 284–290 (2008). [DOI] [PubMed] [Google Scholar]
- 164.Moskowitz SM, Ernst RK, Miller SI. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186(2), 575–579 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cannatelli A, Di Pilato V, Giani T. et al. In vivo evolution to colistin resistance by PmrB sensor kinase mutation in KPC-producing Klebsiella pneumoniae is associated with low-dosage colistin treatment. Antimicrob. Agents Chemother. 58(8), 4399–4403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ah YM, Kim AJ, Lee JY. Colistin resistance in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 44(1), 8–15 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Miller AK, Brannon MK, Stevens L. et al. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob. Agents Chemother. 55(12), 5761–5769 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moskowitz SM, Brannon MK, Dasgupta N. et al. PmrB mutations promote polymyxin resistance of Pseudomonas aeruginosa isolated from colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 56(2), 1019–1030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jayol A, Nordmann P, Brink A, Poirel L. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob. Agents Chemother. 59(5), 2780–2784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cabot G, Zamorano L, Moya B. et al. Evolution of Pseudomonas aeruginosa Antimicrobial Resistance and Fitness under Low and High Mutation Rates. Antimicrob. Agents Chemother. 60(3), 1767–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 55(8), 3743–3751 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu MC, Tsai YL, Huang YW. et al. Stenotrophomonas maltophilia PhoP, a two-component response regulator, involved in antimicrobial susceptibilities. PLoS ONE 11(5), e0153753 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gunn JS. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 7(1), 57–62 (2001). [PubMed] [Google Scholar]
- 174.Clarke DJ. The Rcs phosphorelay: more than just a two-component pathway. Future Microbiol. 5(8), 1173–1184 (2010). [DOI] [PubMed] [Google Scholar]
- 175.Erickson KD, Detweiler CS. The Rcs phosphorelay system is specific to enteric pathogens/commensals and activates ydeI, a gene important for persistent Salmonella infection of mice. Mol. Microbiol. 62(3), 883–894 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farizano JV, Torres MA, Pescaretti Mde L, Delgado MA. The RcsCDB regulatory system plays a crucial role in the protection of Salmonella enterica serovar Typhimurium against oxidative stress. Microbiology 160(Pt 10), 2190–2199 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Ferrieres L, Clarke DJ. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 50(5), 1665–1682 (2003). [DOI] [PubMed] [Google Scholar]
- 178.Guo XP, Sun YC. New insights into the non-orthodox two component rcs phosphorelay system. Front. Microbiol. 8, 2014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Howery KE, Clemmer KM, Rather PN. The Rcs regulon in Proteus mirabilis: implications for motility, biofilm formation, and virulence. Curr. Genet. 62(4), 775–789 (2016). [DOI] [PubMed] [Google Scholar]
- 180.Howery KE, Clemmer KM, Simsek E, Kim M, Rather PN. Regulation of the min cell division inhibition complex by the Rcs phosphorelay in Proteus mirabilis. J. Bacteriol. 197(15), 2499–2507 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Laubacher ME, Ades SE. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 190(6), 2065–2074 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Ann. Rev. Microbiol. 59, 379–405 (2005). [DOI] [PubMed] [Google Scholar]
- 183.Mouslim C, Groisman EA. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 47(2), 335–344 (2003). [DOI] [PubMed] [Google Scholar]
- 184.Szczesny M, Beloin C, Ghigo JM. Increased osmolarity in biofilm triggers RcsB-dependent lipid A palmitoylation in Escherichia coli. mBio 9(4), pii:e01415–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mikkelsen H, Ball G, Giraud C, Filloux A. Expression of Pseudomonas aeruginosa CupD fimbrial genes is antagonistically controlled by RcsB and the EAL-containing PvrR response regulators. PLoS ONE 4(6), e6018 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pruss BM. Evaluation of CpxRA as a therapeutic target for uropathogenic Escherichia coli infections. Evaluation of CpxRA as a therapeutic target for uropathogenic Escherichia coli infections. J. Bacteriol. 199(18), pii:e00259–17 (2017). [Google Scholar]
- 187.Mikkelsen H, Sivaneson M, Filloux A. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 13(7), 1666–1681 (2011). [DOI] [PubMed] [Google Scholar]
- 188.Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 23(2), 249–259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Parkins MD, Ceri H, Storey DG. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 40(5), 1215–1226 (2001). [DOI] [PubMed] [Google Scholar]
- 190.Dingemans J, Poudyal B, Sondermann H, Sauer K. The Yin and Yang of SagS: distinct residues in the HmsP domain of SagS independently regulate biofilm formation and biofilm drug tolerance. mSphere 3(3), pii:e00192–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Petrova OE, Gupta K, Liao J, Goodwine JS, Sauer K. Divide and conquer: the Pseudomonas aeruginosa two-component hybrid SagS enables biofilm formation and recalcitrance of biofilm cells to antimicrobial agents via distinct regulatory circuits. Environ. Microbiol. 19(5), 2005–2024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gupta K, Marques CN, Petrova OE, Sauer K. Antimicrobial tolerance of Pseudomonas aeruginosa biofilms is activated during an early developmental stage and requires the two-component hybrid SagS. J. Bacteriol. 195(21), 4975–4987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Petrova OE, Sauer K. SagS contributes to the motile-sessile switch and acts in concert with BfiSR to enable Pseudomonas aeruginosa biofilm formation. J. Bacteriol. 193(23), 6614–6628 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Poudyal B, Sauer K. The ABC of biofilm drug tolerance: the MerR-Like Regulator BrlR Is an Activator of ABC Transport Systems, with PA1874-77 Contributing to the Tolerance of Pseudomonas aeruginosa Biofilms to Tobramycin. Antimicrob. Agents Chemother. 62(2), pii:e01981-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chambers JR, Liao J, Schurr MJ, Sauer K. BrlR from Pseudomonas aeruginosa is a c-di-GMP-responsive transcription factor. Mol. Microbiol. 92(3), 471–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liao J, Schurr MJ, Sauer K. The MerR-like regulator BrlR confers biofilm tolerance by activating multidrug efflux pumps in Pseudomonas aeruginosa biofilms. J. Bacteriol. 195(15), 3352–3363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liao J, Sauer K. The MerR-like transcriptional regulator BrlR contributes to Pseudomonas aeruginosa biofilm tolerance. J. Bacteriol. 194(18), 4823–4836 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu CJ, Huang YW, Lin YT, Ning HC, Yang TC. Inactivation of SmeSyRy two-component regulatory system inversely regulates the expression of SmeYZ and SmeDEF efflux pumps in Stenotrophomonas maltophilia. PLoS ONE 11(8), e0160943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Faron ML, Ledeboer NA, Buchan BW. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant enterococcus in the health care setting. J. Clin. Microbiol. 54(10), 2436–2447 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mcguinness WA, Malachowa N, Deleo FR. Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 90(2), 269–281 (2017). [PMC free article] [PubMed] [Google Scholar]
- 201.Novotna GB, Kwun MJ, Hong HJ. In vivo characterization of the activation and interaction of the VanR-VanS two-component regulatory system controlling glycopeptide antibiotic resistance in two related streptomyces species. Antimicrob. Agents Chemother. 60(3), 1627–1637 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Evers S, Courvalin P. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR (B) two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 178(5), 1302–1309 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hong HJ, Hutchings MI, Buttner MJ. Vancomycin resistance VanS/VanR two-component systems. Adv. Exp. Med. Biol. 631, 200–213 (2008). [DOI] [PubMed] [Google Scholar]
- 204.Hutchings MI, Hong HJ, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol. Microbiol. 59(3), 923–935 (2006). [DOI] [PubMed] [Google Scholar]
- 205.Li L, Wang Q, Zhang H, Yang M, Khan MI, Zhou X. Sensor histidine kinase is a beta-lactam receptor and induces resistance to beta-lactam antibiotics. Proc. Natl Acad. Sci. USA 113(6), 1648–1653 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ahn SJ, Burne RA. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 189(17), 6293–6302 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Senadheera MD, Guggenheim B, Spatafora GA. et al. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 187(12), 4064–4076 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stipp RN, Boisvert H, Smith DJ, Höfling JF, Duncan MJ, Mattos-Graner RO. CovR and VicRK Regulate Cell Surface Biogenesis Genes Required for Biofilm Formation in Streptococcus mutans. PLoS ONE 8(3), e58271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Collins B, Curtis N, Cotter PD, Hill C, Ross RP. The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various beta-lactam antibiotics. Antimicrob. Agents Chemother. 54(10), 4416–4423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mandin P, Fsihi H, Dussurget O. et al. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol. Microbiol. 57(5), 1367–1380 (2005). [DOI] [PubMed] [Google Scholar]
- 211.Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, Hiramatsu K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49(3), 807–821 (2003). [DOI] [PubMed] [Google Scholar]
- 212.Chen H, Xiong Z, Liu K. et al. Transcriptional profiling of the two-component regulatory system VraSR in Staphylococcus aureus with low-level vancomycin resistance. Int. J. Antimicrob. Agents 47(5), 362–367 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Boyle-Vavra S, Yin S, Daum RS. The VraS/VraR two-component regulatory system required for oxacillin resistance in community-acquired methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 262(2), 163–171 (2006). [DOI] [PubMed] [Google Scholar]
- 214.Gardete S, Wu SW, Gill S, Tomasz A. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob. Agents Chemother. 50(10), 3424–3434 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Belcheva A, Golemi-Kotra D. A close-up view of the VraSR two-component system. A mediator of Staphylococcus aureus response to cell wall damage. J. Biol. Chem. 283(18), 12354–12364 (2008). [DOI] [PubMed] [Google Scholar]
- 216.Mehta S, Cuirolo AX, Plata KB. et al. VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56(1), 92–102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yin S, Daum RS, Boyle-Vavra S. VraSR two-component regulatory system and its role in induction of pbp2 and vraSR expression by cell wall antimicrobials in Staphylococcus aureus. Antimicrob. Agents Chemother. 50(1), 336–343 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Martin PK, Bao Y, Boyer E. et al. Novel locus required for expression of high-level macrolide-lincosamide-streptogramin B resistance in Staphylococcus aureus. J. Bacteriol. 184(20), 5810–5813 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dubrac S, Boneca IG, Poupel O, Msadek T. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J. Bacteriol. 189(22), 8257–8269 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Martin PK, Li T, Sun D, Biek DP, Schmid MB. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J. Bacteriol. 181(12), 3666–3673 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang S, Li X, Wang X, Li Z, He J. The two-component signal transduction system YvcPQ regulates the bacterial resistance to bacitracin in Bacillus thuringiensis. Arch. Microbiol. 198(8), 773–784 (2016). [DOI] [PubMed] [Google Scholar]
- 222.Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: a guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 33(3), 300–305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A concise and effective introduction to the mechanisms of antibiotic resistance.
- 223.Hancock RE. Peptide antibiotics. Lancet 349(9049), 418–422 (1997). [DOI] [PubMed] [Google Scholar]
- 224.Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob. Agents Chemother. 43(6), 1317–1323 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals 6(12), 1543–1575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jana S, Deb JK. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 70(2), 140–150 (2006). [DOI] [PubMed] [Google Scholar]
- 227.Tran TB, Velkov T, Nation RL. et al. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int. J. Antimicrob. Agents 48(6), 592–597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kox LFF, Wösten M, Groisman EA. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19(8), 1861–1872 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen HD, Groisman EA. The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Ann. Rev. Microbiol. 67, 83–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yuan J, Jin F, Glatter T, Sourjik V. Osmosensing by the bacterial PhoQ/PhoP two-component system. Proc. Natl Acad. Sci. USA 114(50), E10792–E10798 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Groisman EA. The pleiotropic two-component regulatory system PhoP–PhoQ. J. Bacteriol. 183(6), 1835–1842 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ly NS, Yang J, Bulitta JB, Tsuji BT. Impact of two-component regulatory systems PhoP–PhoQ and PmrA-PmrB on colistin pharmacodynamics in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56(6), 3453–3456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wright MS, Jacobs MR, Bonomo RA, Adams MD. Transcriptome remodeling of Acinetobacter baumannii during infection and treatment. mBio 8(2), pii:e02193–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lo Sciuto A, Imperi F. Aminoarabinosylation of lipid A is critical for the development of colistin resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 62(3), pii:e01820–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chalabaev S, Chauhan A, Novikov A. et al. Biofilms formed by Gram-negative bacteria undergo increased lipid a palmitoylation, enhancing in vivo survival. mBio 5(4), pii:e01116–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Qiao Y, Srisuknimit V, Rubino F. et al. Lipid II overproduction allows direct assay of transpeptidase inhibition by beta-lactams. Nat. Chem. Biol. 13(7), 793–798 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 8(6), 423–435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Falord M, Karimova G, Hiron A, Msadek T. GraXSR proteins interact with the VraFG ABC transporter to form a five-component system required for cationic antimicrobial peptide sensing and resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 56(2), 1047–1058 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66(5), 1136–1147 (2007). [DOI] [PubMed] [Google Scholar]
- 240.Achouak W, Heulin T, Pages JM. Multiple facets of bacterial porins. FEMS Microbiol. Lett. 199(1), 1–7 (2001). [DOI] [PubMed] [Google Scholar]
- 241.Pages JM, James CE, Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 6(12), 893–903 (2008). [DOI] [PubMed] [Google Scholar]
- 242.Li XZ, Plesiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 28(2), 337–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fernandez L, Hancock RE. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 25(4), 661–681 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 1794(5), 808–816 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Blair JM, Richmond GE, Piddock LJ. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 9(10), 1165–1177 (2014). [DOI] [PubMed] [Google Scholar]
- 246.Webber MA, Piddock LJV. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 51(1), 9–11 (2003). [DOI] [PubMed] [Google Scholar]
- 247.Colangeli R, Helb D, Sridharan S. et al. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol. Microbiol. 55(6), 1829–1840 (2005). [DOI] [PubMed] [Google Scholar]
- 248.Alland D, Steyn AJ, Weisbrod T, Aldrich K, Jacobs WR., Jr Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition. J. Bacteriol. 182(7), 1802–1811 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zahrt TC, Deretic V. An essential two-component signal transduction system in Mycobacterium tuberculosis. J. Bacteriol. 182(13), 3832–3838 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bretl DJ, Demetriadou C, Zahrt TC. Adaptation to environmental stimuli within the host: two-component signal transduction systems of Mycobacterium tuberculosis. Microbiol. Mol. Biol. Rev. 75(4), 566–582 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Curcic R, Dhandayuthapani S, Deretic V. Gene expression in mycobacteria: transcriptional fusions based on xylE and analysis of the promoter region of the response regulator mtrA from Mycobacterium tuberculosis. Mol. Microbiol. 13(6), 1057–1064 (1994). [DOI] [PubMed] [Google Scholar]
- 252.Via LE, Curcic R, Mudd MH, Dhandayuthapani S, Ulmer RJ, Deretic V. Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J. Bacteriol. 178(11), 3314–3321 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kundu M. The role of two-component systems in the physiology of Mycobacterium tuberculosis. IUBMB Life 70(8), 710–717 (2018). [DOI] [PubMed] [Google Scholar]
- 254.Gorla P, Plocinska R, Sarva K. et al. MtrA response regulator controls cell division and cell wall metabolism and affects susceptibility of mycobacteria to the first line antituberculosis drugs. Front. Microbiol. 9, 2839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 3314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Van Acker H, Van Dijck P, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 22(6), 326–333 (2014). [DOI] [PubMed] [Google Scholar]
- 257.Singh S, Singh SK, Chowdhury I, Singh R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 11, 53–62 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35(4), 322–332 (2010). [DOI] [PubMed] [Google Scholar]
- 259.Stewart PS, William Costerton J. Antibiotic resistance of bacteria in biofilms. Lancet 358(9276), 135–138 (2001). [DOI] [PubMed] [Google Scholar]
- 260.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292(2), 107–113 (2002). [DOI] [PubMed] [Google Scholar]
- 261.Ridenhour BJ, Metzger GA, France M. et al. Persistence of antibiotic resistance plasmids in bacterial biofilms. Evol. Appl. 10(6), 640–647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mah TF. Biofilm-specific antibiotic resistance. Future Microbiol. 7(9), 1061–1072 (2012). [DOI] [PubMed] [Google Scholar]
- 263.Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 34(5), 877–886 (2015). [DOI] [PubMed] [Google Scholar]
- 264.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 4(11), e1000213 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol. Microbiol. 55(2), 368–380 (2005). [DOI] [PubMed] [Google Scholar]
- 266.Hobbs M, Collie ES, Free PD, Livingston SP, Mattick JS. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 7(5), 669–682 (1993). [DOI] [PubMed] [Google Scholar]
- 267.Giraud C, Bernard CS, Calderon V. et al. The PprA–PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 13(3), 666–683 (2011). [DOI] [PubMed] [Google Scholar]
- 268.Bernard CS, Bordi C, Termine E, Filloux A, De Bentzmann S. Organization and PprB-dependent control of the Pseudomonas aeruginosa tad Locus, involved in Flp pilus biology. J. Bacteriol. 191(6), 1961–1973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Proctor RA, Von Eiff C, Kahl BC. et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4(4), 295–305 (2006). [DOI] [PubMed] [Google Scholar]
- 270.Evans TJ. Small colony variants of Pseudomonas aeruginosa in chronic bacterial infection of the lung in cystic fibrosis. Future Microbiol. 10(2), 231–239 (2015). [DOI] [PubMed] [Google Scholar]
- 271.Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J. Antimicrob. Chemother. 67(9), 2069–2089 (2012). [DOI] [PubMed] [Google Scholar]
- 272.Poole K. Stress responses as determinants of antimicrobial resistance in Gram-negative bacteria. Trends Microbiol. 20(5), 227–234 (2012). [DOI] [PubMed] [Google Scholar]
- 273.Poole K. Stress responses as determinants of antimicrobial resistance in Pseudomonas aeruginosa: multidrug efflux and more. Can. J. Microbiol. 60(12), 783–791 (2014). [DOI] [PubMed] [Google Scholar]