Abstract
Background
Persistent depressive disorder (PDD) is defined as a depressive disorder with a minimum illness duration of two years, including four diagnostic subgroups (dysthymia, chronic major depression, recurrent major depression with incomplete remission between episodes, and double depression). Persistent forms of depression represent a substantial proportion of depressive disorders, with a lifetime prevalence ranging from 3% to 6% in the Western world. Growing evidence indicates that PDD responds well to several acute interventions, such as combined psychological and pharmacological treatments. Yet, given the high rates of relapse and recurrences of depression following response to acute treatment, long‐term continuation and maintenance therapy are of great importance. To date, there has been no evidence synthesis available on continuation and maintenance treatments of PDDs.
Objectives
To assess the effects of pharmacological and psychological (either alone or combined) continuation and maintenance treatments for persistent depressive disorder, in comparison with each other, placebo (drug/attention placebo/non‐specific treatment control), and treatment as usual (TAU). Continuation treatments are defined as treatments given to currently remitted people (remission is defined as depressive symptoms dropping below case level) or to people who previously responded to an antidepressant treatment. Maintenance therapy is given during recovery (which is defined as remission lasting longer than six months).
Search methods
We searched Ovid MEDLINE (1950‐ ), Embase (1974‐ ), PsycINFO (1967‐ ) and the Cochrane Central Register of Controlled Trials (CENTRAL) to 28 September 2018. An earlier search of these databases was also conducted for RCTs via the Cochrane Common Mental Disorders Controlled Trial Register (CCMD‐CTR) (all years to 11 Dec 2015). In addition we searched grey literature resources as well as the international trial registers ClinicalTrials.gov and ICTRP to 28 September 2018. We screened reference lists of included studies and contacted the first author of all included studies.
Selection criteria
We included randomized (RCTs) and non‐randomized controlled trials (NRCTs) in adults with formally diagnosed PDD, receiving pharmacological, psychological, or combined continuation and maintenance interventions.
Data collection and analysis
Two review authors independently selected studies and extracted and analyzed data. The primary efficacy outcome was relapse/recurrence rate of depression. The primary acceptance outcome was dropout due to any reason other than relapse/recurrence. We performed random‐effects meta‐analyses using risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CI).
Main results
We included 10 studies (seven RCTs, three NRCTs) involving 840 participants in this review, from which five studies investigated continuation treatments and five studies investigated maintenance treatments. Overall, the included studies were at low‐to‐moderate risk of bias. For the three NRCTs, the most common source of risk of bias was selection of reported results. For the seven RCTs, the most common sources of risk of bias was non‐blinding of outcome assessment and other bias (especially conflict of interest due to pharmaceutical sponsoring).
Pharmacological continuation and maintenance therapies
The most common comparison was antidepressant medication versus tablet placebo (five studies). Participants taking antidepressant medication were probably less likely to relapse or to experience a recurrent episode compared to participants in the placebo group at the end of the intervention (13.9% versus 33.8%, RR 0.41, 95% CI 0.21 to 0.79; participants = 383; studies = 4; I² = 54%, moderate quality evidence). Overall dropout rates may be similar between participants in the medication and placebo group (23.0% versus 25.5%, RR 0.90, 95% CI 0.39 to 2.11; RCTs = 4; participants = 386; I² = 64%, low quality evidence). However, sensitivity analyses showed that the primary outcome (rate of relapse/recurrence) showed no evidence of a difference between groups when only including studies with low risk of bias.
None of the studies compared pharmacological or psychological treatments versus TAU.
Psychological continuation and maintenance therapies
One study compared psychological therapies versus attention placebo/non‐specific control. One study compared psychotherapy with medication. The results of the studies including psychotherapy might indicate that continued or maintained psychotherapy could be a useful intervention compared to no treatment or antidepressant medication. However, the body of evidence for these comparisons was too small and uncertain to draw any high quality conclusions.
Combined psychological and pharmacological continuation and maintenance therapies
Three studies compared combined psychological and pharmacological therapies with pharmacological therapies alone. One study compared combined psychological and pharmacological therapies with psychotherapeutic therapies alone. However, the body of evidence for these comparisons was too small and uncertain to draw any high quality conclusions
Comparison of different antidepressant medications
Two studies reported data on the direct comparison of two antidepressants. However, the body of evidence for this comparison was too small and uncertain to draw any high quality conclusions.
Authors' conclusions
Currently, it is uncertain whether continued or maintained pharmacotherapy (or both) with the reviewed antidepressant agents is a robust treatment for preventing relapse and recurrence in people with PDD, due to moderate or high risk of bias as well as clinical heterogeneity in the analyzed studies.
For all other comparisons, the body of evidence was too small to draw any final conclusions, although continued or maintained psychotherapy might be effective compared to no treatment. There is need for more high quality trials of psychological interventions. Further studies should address health‐related quality of life and adverse events more precisely, as well as assessing follow‐up data.
Plain language summary
Long‐term treatment for people with persistent depression
Why is this review important?
Depressive disorders that persist for at least two years cause considerable problems. Even after successful treatment, they frequently recur. Common treatments are antidepressant medicines and psychological treatments (talking therapies), or a combination of both. Long‐term treatments should prevent the recurrence of depressive symptoms.
Who will be interested in this review?
‐ People with persisting depression (longer than two years), friends, families, and carers.
‐ General practitioners, psychiatrists, clinical psychologists, psychological therapists, and pharmacists.
What questions does this review aim to answer?
In adults with persistent depression who improved with acute (short‐term) treatment:
‐ Is receiving continued antidepressant medicine, psychological treatment, or a combination of both more effective in preventing recurrence of depression compared to placebo (a pretended treatment) or care as usual?
‐ Is receiving continued antidepressant medicine, psychological treatment, or a combination of both equally accepted as receiving placebo or usual care?
‐ Is one treatment more effective or more accepted than another?
Which studies does the review include?
We searched medical databases and other sources to find all relevant studies completed up to September 2018. The studies had to compare antidepressant treatment, psychological treatment, or a combination of both, with each other, with placebo, or with care as usual for preventing recurrence of depression in adults diagnosed with persistent depression. We included 10 studies involving 840 participants. Five studies compared antidepressant medicine with placebo.
One study compared psychological therapies versus attention placebo/non‐specific control. One study compared psychotherapy with medication. Three studies compared combined psychological and pharmacological therapies with pharmacological therapies alone. One study compared combined psychological and pharmacological therapies with psychotherapeutic therapies alone.
Two studies compared two different antidepressants with each other.
Overall, the included studies were at low‐to‐moderate risk of bias.
What does the evidence from the review tell us?
According to GRADE, there was moderate quality evidence that participants taking medication treatment probably had less relapses/recurrences and may have lower dropouts than those taking placebo. The risk of depression returning in participants receiving a placebo (instead of antidepressant medicine) was 34%. In comparison, participants who remained on antidepressant medicines had a lower risk for recurrence of 13%. The continued treatment lasted between four months and two years. Antidepressant were as well accepted as placebo. However, as most of the included studies showed risk of bias and there were some inconsistent results between the different studies, it cannot be concluded with certainty whether continued or maintained pharmacotherapy (or both) is a convincing treatment for people with PDD. Additionally, as studies on the long‐term effects of medication are lacking, recommendations on the necessary duration of medication treatment cannot be drawn.
The benefits of psychological therapies or combined treatment remained unclear, due to the small number of studies.
What should happen next?
This review cannot provide clear, certain evidence regarding whether continued antidepressant medication (compared to placebo tablet) reduces the risk of depression recurring in adults with persistent depression. However, only a few studies have been done. Further studies should especially address psychological and combined long‐term treatments.
Summary of findings
for the main comparison.
| Pharmacological continuation and maintenance treatment compared with placebo for persistent depressive disorder | ||||||
|
Patient or population: people with persistent depressive disorder Settings: outpatient treatment Intervention: pharmacological continuation or maintenance treatment (sertraline, phenelzine, nefazodone, desipramine) Comparison: tablet placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pharmacotherapy | |||||
|
Relapse/recurrence (end of intervention) |
338 per 1000 | 139 per 1000a (71 to 267) | RR 0.41 (0.21 to 0.79) | 383 (4 studies) | ⊕⊕⊕⊝b Moderate | See Characteristics of included studies table for the criteria of relapse/recurrence. |
|
Dropout due to any reason (end of intervention) |
255 per 1000 | 230 per 1000a (99 to 538) | RR 0.90 (0.39 to 2.11) | 386 (4 studies) | ⊕⊕⊝⊝c, d Low | "Dropout due to any reason" was all reported dropouts due to other reasons than relapse/recurrence. 1 study only reported dropouts in the first month of the maintenance treatment phase (Kocsis 1996). As the maintenance treatment lasted 24 months, the dropout rate in this study was very likely to be underestimated. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aAssumed risk calculated as the proportion of participants on placebo with the outcome (relapse/recurrence or dropout any) in the four included studies, multiplied by 1000.
bDowngraded due to limitations in the design and implementation of available studies suggesting high likelihood of bias (there were studies with high or unclear risk of bias in almost all RoB‐Domains (except detection bias)).
cDowngraded due to unexplained heterogeneity between studies (I² = 64%). Due to the small number of included studies, subgroup or meta‐regression analyses were not performed. In two studies, dropout rates were higher in the intervention group, in two studies they were lower.
dDowngraded due to imprecision of results (the overall confidence interval was wide and the confidence intervals of two included studies are also very wide).
Background
Description of the condition
Persistent forms of depression that last for two years or longer represent a substantial proportion of depressive disorders (Boland 2002; Gilmer 2005; Keller 1992; Spijker 2002). Within the literature, four subtypes can be distinguished: dysthymia, chronic major depression, recurrent major depression with incomplete remission between episodes, and double depression (Dunner 2005). Dysthymic disorder is defined as a condition with mild depressive symptoms persisting for at least two years. Major depressive episode (MDD), chronic type, refers to a more severe condition that meets full criteria for major depression continuously for a minimum of two years. People who have recovered to the point at which they no longer meet full criteria for an MDD but continue to experience significant symptoms for at least two years are referred to as having recurrent major depression with incomplete remission between episodes. The superimposition of an MDD on antecedent dysthymia is referred to as double depression (Klein 2010). In the Diagnostic and Statistical Manual of Mental Disorders – 5th Edition (DSM‐5), the new diagnostic category of persistent depressive disorder was introduced subsuming dysthymic as well as chronic major depressive disorders (APA 2013).
The mean length of persistent depression is between 17 and 30 years (Gilmer 2005; Kocsis 2008), and the lifetime prevalence for persistent depressive disorders is estimated to range from 3% to 6% in the Western world (Kessler 2005; Klein 2010; Murphy 2012). In comparison to acute forms of depression, persistent depressive disorders are associated with longer treatment duration; increased loss of physical wellbeing; increased comorbidity; more severe impairments in social, psychological, and emotional functioning; increased healthcare utilization; more frequent suicide attempts; and more frequent hospitalizations (Arnow 2003; Gilmer 2005). Thus, persistent depression is likely to make a large contribution to the high burden of disease that is associated with unipolar depression according to disability‐adjusted life years (WHO 2008).
Description of the intervention
Overall, a large number of different interventions exist for the treatment of unipolar depression, including psychological, pharmacological, and combined psychological and pharmacological therapies. Evidence from randomized controlled trials (RCTs) as well as meta‐analyses suggests that these interventions are effective in the acute treatment of depression, including persistent forms of depression (Cuijpers 2010; Cuijpers 2013; Imel 2008; Keller 2000; Kriston 2014; Spijker 2013; von Wolff 2012; von Wolff 2013). Still, there is also evidence that relevant numbers of people do not respond to treatment, do not reach complete remission, and develop persisting residual symptoms long term (Epstein 2014). It is estimated that probably half of people with depressive disorders develop a chronic course (Klein 2011).
Moreover, acute‐phase treatments often fail to prevent relapse (which is defined as the return of symptoms of depression before a full remission has been achieved) and recurrence (which is defined as the appearance of another new episode of depression after full remission of a previous episode has been achieved) in major depression. For example, after scheduled termination of acute‐phase cognitive therapy (CT), relapse/recurrence rates were 29% in the first year and 54% in the second year (Vittengl 2007). In the same study, even when other depression‐specific psychological therapies and even higher doses of pharmacotherapy were used after the acute‐phase treatment, relapse and recurrence rates were still high (Vittengl 2007). Further, there are studies showing that 30% to 50% of people considered to be remitted still have to deal with residual depressive symptoms (Nutt 2007).
Thus, following response to acute treatment, long‐term continuation and maintenance therapy might be required to prevent relapse or recurrence of symptoms. Continuation treatments are defined as treatments given to currently remitted people (remission is defined as depressive symptoms dropping below case level) or to people who previously responded to an antidepressant treatment. Maintenance therapy is given during recovery (which is defined as remission lasting longer than six months; Frank 1991; NICE 2010). The German National Clinical Practice Guideline for Unipolar Depression recommends a combination of pharmacotherapy and psychological therapy as acute‐phase treatment for people with persistent forms of depression (DGPPN 2015). Additionally, a continued psychological therapy or pharmacotherapy (or both) is recommended to prevent relapse and recurrence. Specifically, the type of treatment that was successful in the acute phase is recommended to be continued (APA 2010; DGPPN 2015; NICE 2010). However, the recommendations concerning the continuation of therapy are based on people with unipolar depression in general, specific recommendations regarding people with persistent depressive disorders are lacking.
Hence, a systematic review of evidence regarding the effectiveness of pharmacological, psychological, and combined pharmacological and psychological therapies as continuation and maintenance treatments for people with persistent forms of depression is needed.
How the intervention might work
Acute treatments aim to reduce depressive symptoms and re‐establish psychosocial functioning. In comparison, continuation and maintenance treatments aim to maintain (or improve) the psychofunctional status reached by acute treatment, and to reduce the likelihood of relapse and recurrence in the long‐term (DGPPN 2015). Therefore, continuation and maintenance treatments are considered to be more than a pure extension of acute treatments, because continuation/maintenance treatments differ in frequency and content over the course of the illness in comparison to acute treatments.
Psychological continuation and maintenance interventions are usually offered less frequently than acute psychological therapy, aiming to monitor symptoms and to integrate techniques and strategies into daily life in the long‐term (DGPPN 2015). Different programmes targeting the prevention of relapse and recurrence focus on a range of effect mechanisms. CT approaches focus on the generalization of skills achieved during acute therapy (Jarrett 1998), or the cognitive content of negative thinking (Bockting 2005). Mindfulness‐based cognitive therapy (MBCT) was especially developed to reduce relapse and recurrence in depression (Piet 2011; Segal 2002), and teaches people to deal with negative feelings and thoughts as a part of their lives through becoming aware of negative cognitive patterns. Maintenance interpersonal psychotherapy (IPT) aims to complement skills gained in the acute‐phase therapy and teaches people to take responsibility in the prevention of future episodes by recognizing and preventing stressful environmental and social circumstances (Beshai 2011). Still, it remains challenging to completely understand the mechanisms of preventing relapse and recurrence (Beshai 2011).
The exact therapeutic mechanisms of antidepressants are not yet clear (Pringle 2011). Most antidepressants seem to increase the concentrations of monoamine neurotransmitters (e.g. serotonin or noradrenaline) in the synaptic cleft (Berton 2006). However, the effect of most antidepressants fully develops after some weeks, indicating that neurophysiological changes of brain tissue (e.g. changes in sensitivity and frequency of receptors), occurring in the presence of a constant level of active ingredients, are necessary for permanent improvement. Depending on the type of active ingredient, antidepressants can have mood‐enhancing, anxiolytic, or sedative effects and are able to increase or decrease inner drive. Moreover, the placebo effect is of particular importance in the treatment of depression. There are studies assuming that the more severe the depressive symptoms are, the greater the benefit of antidepressants seem to be compared to placebo (Anderson 2008; Kirsch 2008). However, one meta‐analysis performed on patient‐level data regarding the response to antidepressant medication showed that initial depression severity and outcomes were similarly related in treatment and placebo groups (Rabinowitz 2016).
A number of studies have shown that the risk of relapse or recurrence of depression is associated with residual symptoms following acute treatment phases (APA 2010; NICE 2010). These findings lead to the therapeutic goal of sustained remission and recommendations of international treatment guidelines to continue antidepressant medication after acute‐phase treatment (APA 2010; NICE 2010).
Why it is important to do this review
Research that focuses on the prevention of recurrence of depression was identified as a top priority in the project "Depression: asking the right questions" (MQ 2016). The high prevalence and the severe personal, societal, and economic consequences of persistent depressive disorder underline the need for adequate treatment strategies (Gilmer 2005). Growing evidence indicates that persistent depressive disorder responds well to several acute interventions, such as combined psychological and pharmacological treatments, although the number of RCTs is still limited (Spijker 2013). Yet, given the high rates of relapse and recurrences of depression following response to acute treatment, long‐term continuation and maintenance therapy are of great importance (Beshai 2011).
Several RCTs have supported the effectiveness of continuation and maintenance therapies for depression (Browne 2002; Jarrett 2001; Jarrett 2013; Keller 2007; Klein 2004; Petersen 2010; Vittengl 2009). One meta‐analysis on relapse prevention with antidepressant drug treatment of depressive disorders showed that continued antidepressant medication produced a robust reduction in relapse (Glue 2010). Another meta‐analysis summarized the findings of long‐term effects of cognitive behavioural therapy (CBT) (Vittengl 2007). Participants who responded to acute treatment and continued to receive CBT showed a significant reduction in relapse and recurrence rates in comparison to inactive as well as active controls.
Although most evidence addresses acute treatments for persistent depressive disorder or long‐term treatments for acute depressive episodes, some studies have addressed the effectiveness of long‐term treatments of persistent depressive disorder (Gelenberg 2003; Harrison 1986; Keller 1998a; Klein 2004; Kocsis 1996; Kocsis 2003; Koran 2001; Rouillon 1989; Stangier 2013).
We found no systematic review on the comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder.
In summary, this systematic review may be highly relevant as:
persistent depressive disorders have a high prevalence and serious personal, societal, and economic consequences;
no evidence synthesis is available on continuation and maintenance treatments of persistent depressive disorders;
high quality evidence synthesis is needed for clinical guideline recommendations.
Objectives
To assess the effects of pharmacological and psychological continuation and maintenance treatments for persistent depressive disorder, in comparison with each other; placebo (drug/attention placebo/non‐specific treatment control); and treatment as usual (TAU). In addition, to assess the effects of combined psychological and pharmacological continuation and maintenance treatments, in comparison with either of these treatments alone.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), and non‐randomized controlled trials (NRCTs). We considered NRCTs in this review as we expected a limited number of RCTs. There were no restrictions regarding other design characteristics. There were no cross‐over or cluster RCTs eligible for inclusion in this review; however, future versions of this review could consider including these trials.
Types of participants
Characteristics
We included participants aged 18 years or older of any gender and ethnicity.
Diagnosis
We included participants who had a diagnosis of persistent depressive disorder or had had this diagnosis before their last previous acute treatment. The diagnosis of depression needed to rely on a formal classification system, such as the International Classification of Diseases (ICD) (WHO 1992), or the Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA 2013). Participants needed to be either currently remitted from persistent depressive disorder or needed to have at least partially responded to an acute intervention (at least 25% symptom reduction from baseline) at the beginning of the continuation or maintenance treatment. We included participants described as 'treatment resistant' if they fulfilled the formerly mentioned criteria. As the distinction between subtypes of persistent depressive disorder (chronic major depression, dysthymia, double depression, or recurrent depression without a complete remission between episodes) is controversial, inclusion was primarily driven by the duration of the existing depressive disorder. Consequently, we included studies investigating participants with chronic major depression, dysthymia, double depression, or recurrent depression without a complete remission between episodes if the target disorders were or had been of at least two years' duration. We excluded studies reporting to investigate 'chronically depressed' participants without fulfilling these criteria (e.g. less than two years' duration).
Comorbidities
We included studies that did not define specific concurrent mental or somatic conditions as inclusion criteria but reported on comorbidities in addition to the persistent depressive disorder. We excluded studies focusing exclusively on persistently depressed participants with a specific concurrent mental or somatic disorder as we assumed that the interventions in these types of studies (primarily) addressed the comorbid condition and were not focused exclusively on persistent depression.
Setting
There were no restrictions based on settings.
Subset data
We only considered studies in which both participants with persistent and acute forms of depression were included if they reported data separately for the persistent subgroup (or if 80% or more of the total sample had a diagnosis of persistent depression). If randomization was based on the total sample, we included studies and categorized them as NRCTs.
Types of interventions
Experimental intervention
We considered pharmacological, psychological, and combined continuation and maintenance interventions. We defined continuation treatments as treatments given to currently remitted people or to people who previously responded to an antidepressant treatment, whereas we defined maintenance treatments as treatments given to people who were currently recovered. Continuation/maintenance treatments needed to be started within one year after termination of an acute treatment. We considered all interventions that satisfied these definitions. Additionally, we considered studies that did not report all the above mentioned criteria but reported data on interventions that were clearly labelled as 'continuation' or 'maintenance' treatments. We considered pharmacological interventions including the oral administration of classified antidepressants:
tricyclic antidepressant (TCA);
selective serotonin reuptake inhibitor (SSRI);
monoamine oxidase inhibitor (MAOI);
alpha2‐receptor antagonist;
selective noradrenaline‐dopamine reuptake inhibitor (SNDRI);
melatonin receptor agonist;
serotonin 5HT2C receptor antagonist
noradrenergic and specific serotonergic antidepressants (NaSSA);
selective serotonin noradrenaline reuptake inhibitor (SSNRI).
We also considered the following as they can be used (alone or in combination) in treating different forms of depression (DGPPN 2015):
non‐classified antidepressants (Trazodone);
lithium;
Hypericum perforatum;
antipsychotic drugs.
Psychological therapies had to fulfil the following criteria.
The intervention must have been based on a scientific theory (described in detail or manualized or referenced, or a combination of these).
At least one contact between therapist and participant either face‐to‐face or via telecommunication technologies (e.g. online therapy) must have taken place. Thus, for example, the general dissemination of information material in form of leaflets in waiting rooms was not considered as a psychological therapy.
The intervention must have considered the personal needs of the participant or a group of participants and must have been individually tailored in an interpersonal process. Thus, we included group therapies.
Concerning psychological therapies, we considered behaviour therapy/behaviour modification, CBT, third‐wave CBTs, psychodynamic therapies, humanistic therapies, integrative therapies, systemic therapies, and other psychologically oriented interventions (based on the definition of the Cochrane Common Mental Disorders Group) for inclusion.
Combined interventions included the administration of one or more pharmacological agents combined with one or more psychological therapy.
Somatic (e.g. electroconvulsive therapy, vagus nerve stimulation, acupuncture), non‐pharmacological (e.g. physical exercise, bright light therapy), and organizational (e.g. case management) interventions were not considered as including too many different interventions was likely to result in large clinical and methodological heterogeneity.
Comparator intervention
We included both controlled and comparative effectiveness studies. The comparators were:
pharmacological placebo (participants received placebo tablets);
attention‐placebo/non‐specific control (participants received a treatment that involved non‐specific psychosocial factors or assessment only);
treatment as usual (TAU);
(other) psychological therapy;
(other) pharmacological treatment;
(other) combined psychological/pharmacological therapy.
Types of outcome measures
Primary outcomes
-
Relapse/recurrence rate of depression, preferentially defined as:
fulfilment of formal diagnostic criteria for depression (DSM, ICD), or as
-
exceeding a cut‐off on a depression symptom rating scale used by the authors, specifically:
Hamilton Depression Rating Scale (HAM‐D; Hamilton 1960);
Montgomery‐Åsberg Depression Rating Scale (MADRS; Montgomery 1979);
Beck‐Depression‐Inventory (BDI; Beck 1996);
Inventory of Depressive Symptomatology (IDS; Rush 2000);
Patient Health Questionnaire (PHQ; Spitzer 1999); or
any other depression symptom scale.
Due to the long tradition of depression research, most instruments used in clinical trials are usually psychometrically sound. Such measures were preferred throughout the review (referenced or sufficient psychometric quality (or both) reported).
Dropout due to any reason.
Secondary outcomes
Symptom severity of depression at the end of treatment (metric outcome of depression scale as defined above).
Health‐related quality of life (e.g. World Health Organization Quality of Life (WHOQOL) (Skevington 2004).
Dropout due to any type of adverse event (for the definition of adverse events see below).
Any type of adverse event (defined as any potentially negative event occurring during or after treatment in relation to a patient including symptoms of all body parts (e.g. headache, dizziness, dry mouth); psychological symptoms (e.g. depressed mood, suicidal thoughts); and psychosocial, legal, and economic consequences (e.g. conflicts with the partner, stigmatization) (Ladwig 2014; Nebeker 2004; Rief 2011).
Serious adverse events (defined as adverse events leading to serious consequences such as death, mortal danger, hospitalization, or disability; FDA 2016). Note that adverse events need to be differentiated from side effects that are defined as any adverse event that can be attributed to a lege artis intervention.
Timing of outcome assessment
The primary outcome time point was the 'end of the intervention' (regardless of the duration of the intervention). Additionally, we planned to analyze data at 'one year after the end of the intervention' providing that enough data were available. If one‐year‐data were not available, we planned to use data that ranged between six and 18 months after the end of the intervention with a preference for the time that was closest to one year after the end of the intervention. However, only one study provided follow‐up data 12 weeks after the end of the intervention.
Hierarchy of outcome measures
If more than one diagnostic definition or depression symptom rating scale (or both) was available (concerning relapse or recurrence rate of depression), we used the presented hierarchy to select measures (priority starting with: fulfilment of formal diagnostic criteria, continuing with HAM‐D, MADRS, etc.).
Search methods for identification of studies
Electronic searches
Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR)
The Cochrane Common Mental Disorders Group maintains a specialized register of RCTs, the CCMD‐CTR (description in Appendix 1). The Group's Information Specialist ran an initial search of the CCMD‐CTR (11 December 2015) for study records using the following controlled search terms (condition only): ("chronic depression" or "dysthymia"or "dysthymic disorder" or "persistent depressive disorder" or "recurrent depression")
The Information Specialist also searched the CCMD‐CTR‐references register (11 December 2015) using a more sensitive set of terms (condition only): ("chronic* depress*" or "double depress*" or dysthymi* or (depress* NEAR2 recurr*) or "persistent depressive disorder"):ti,ab,kw,ky,mh,mc,emt
[Key: ti = title; ab = abstract; kw = keywords; ky = additional keywords; mh = MeSH terms; mc = MeSH checkwords; emt = EMTREE terms]
As the scope of this review covers RCTs and NRCTs the information specialist also ran a scoping search of Ovid PsycINFO (11 December 2015) (Appendix 2). We screened the records retrieved from the CCMD‐CTR and PsycINFO for continuation and maintenance trials, prior to running all other database searches. The information specialist used these as a test set to develop the search strategy further, to prevent the retrieval of too many irrelevant references.
The Information Specialist ran complementary searches on the following bibliographic databases (September 2016 and 2018) using relevant subject headings and search syntax, appropriate to each resource (Appendix 2):
Cochrane Central Register of Controlled Trials (CENTRAL to Issue 9, 2018);
Ovid MEDLINE (1946 to 28 September 2018);
Ovid Embase (1974 to 28 September 2018);
Ovid PsycINFO (all years to 28 September 2018).
We also searched the international trial registers ClinicalTrials.gov and ICTRP to 28‐09‐2018, using the following terms for ClinicalTrials.gov: (“chronic depression” OR “double depression” OR dysthymia OR dysthymic OR “recurrent depression” OR “recurrent depressive disorder” OR “persistent depressive disorder”) AND (continuation OR maintenance) and the following terms for ICTRP: chronic depression AND continuation OR double depression AND continuation OR dysthymia AND continuation OR dysthymic AND continuation OR recurrent depression AND continuation OR recurrent depressive disorder AND continuation OR persistent depressive disorder AND continuation OR chronic depression AND maintenance OR double depression AND maintenance OR dysthymia AND maintenance OR dysthymic AND maintenance OR recurrent depression AND maintenance OR recurrent depressive disorder AND maintenance OR persistent depressive disorder AND maintenance.
There were no restrictions on date, language, or publication status applied to the searches. The search of the CCMD‐CTR was not repeated in 2018 as it was out of date at the time.
Searching other resources
Grey literature
We searched the following sources of grey literature.
ProQuest Dissertations and Theses Database (www.proquest.com/; searched
11 August 2015).
Depression. The Treatment and Management of Depression in Adults (NICE 2010).
S3 Guideline/National Disease Management Guideline. Unipolar Depression (DGPPN 2015).
Canadian Network for Mood and Anxiety Treatments (CANMAT). Clinical guidelines for the management of major depressive disorder in adults (Kennedy 2009).
Open Grey (www.opengrey.eu/; retrieved 11 August 2015).
As the first search on grey literature revealed no additional results, we did not repeat it in September 2018, only the main searches (see above) were updated.
Handsearching
As all relevant journals are included in the bibliographic databases being searched, we conducted no further handsearches in journals.
Reference lists
We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies missed from the original electronic searches (e.g. unpublished or in‐press citations). We also conducted a cited reference search on the Web of Science.
Correspondence
We contacted the first author of all included studies to request information on unpublished or ongoing studies or additional trial data.
Data collection and analysis
Selection of studies
Two review authors (KM, SL, RM, or AJ) independently screened titles and abstracts for inclusion of all the potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve' (ineligible). We retrieved the full‐text reports/publications and two review authors (KM, SL, or RM) independently screened the full‐texts and selected studies for inclusion. We recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a fourth review author (AJ). We identified and excluded duplicate records and we collated multiple reports that related to the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of included studies and Characteristics of excluded studies tables.
Data extraction and management
We used a data collection form, which had been piloted on one study in the review (Klein 2004), to extract study characteristics and outcome data. Three review authors (KM, SL, RM) extracted data from this study for piloting the data collection form. Extraction of the data of the remaining included studies was undertaken by two review authors (KM, SL, or RM), who independently extracted study characteristics and data. We extracted the following.
Methods: study design, time of randomization, total duration of study, location, study setting, and date of study (year).
Participants: number of participants (n), mean age, age range, % women, diagnostic subgroup, mean age of onset, length of current/last episode, number of previous episodes.
Interventions: intervention, comparison, type of acute treatment previous to continuation/maintenance treatment.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding of the trial.
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (KM, SL, or RM). One review author (SL) transferred data into Review Manager 5 (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (KM) spot‐checked study characteristics for accuracy against the trial report.
Main comparisons
We chose seven main comparisons from the list of possible comparisons based on clinical importance and expected frequency of the comparisons in clinical trials:
pharmacological continuation and maintenance therapies versus placebo;
pharmacological continuation and maintenance therapies versus TAU;
psychological continuation and maintenance therapies versus attention placebo/non‐specific control;
psychological continuation and maintenance therapies versus TAU;
psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies;
combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone;
combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone.
Assessment of risk of bias in included studies
Two review authors (KM, SL, or RM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (KM, SL, or RM). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We judged each potential source of bias as high, low, or unclear and provided a supporting quotation from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarized the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
We used the ROBINS‐I tool for assessing the quality of non‐randomized studies in meta‐analyses to assess the quality of NRCTs (Sterne 2016). This tool shows substantial overlap with the risk of bias ratings in RCTs, but additionally includes two domains at the preintervention level (bias due to confounding, bias in selection of participants into the study) and one domain at the intervention level (bias in classification of interventions).
We included no cluster‐randomized trials; however, in updates of this review we will consider recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomized trials in cluster‐randomized trials (Higgins 2011).
We used sensitivity analyses to consider the risk of bias. Moreover, we took the risk of bias into account when interpreting the treatment effects.
Measures of treatment effect
Dichotomous data
To increase clinical applicability of the findings, we calculated the risk ratio (RR) and 95% confidence intervals (CI) of the primary outcomes relapse/recurrence and dropout due to any reason, as they are more likely to help clinicians to make informed decisions in specific clinical situations. For rare outcomes (adverse events) or endpoints with highly varying baseline rates, we estimated odds ratios (OR) and 95% CIs. When the overall results were significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB). None of the included studies in this review used time‐to‐event data, however in future versions of this review primary studies should consider pooled hazard ratios for calculations.
Continuous data
We analyzed continuous data as mean differences (MD) and 95% CIs when studies used the same rating scale. When studies used different scales, we calculated standardized mean differences (SMD) and 95% CIs. We entered data presented as a scale with a consistent direction of effect. We undertook meta‐analyses only where this was meaningful (i.e. if the treatments, participants, and underlying clinical question were similar enough for useful pooling). We planned to narratively describe skewed data reported as medians and interquartile ranges if effect size calculation was not possible.
Time‐to‐event data
We planned to consider pooled hazard ratios for calculation of time‐to‐event data.
Unit of analysis issues
Cross‐over and cluster‐randomized trials
As we expected a small number of overall available studies, data from cross‐over trials and cluster‐randomized trials were planned for inclusion in the analysis, regardless of the level of randomization. None of the studies in this review was either a cross‐over or a cluster‐randomized trial. However, in updates of this review, cluster‐randomized trials should include direct effect estimates of the primary studies, only if they were obtained from analyses that accounted for the clustering in the data (e.g. using a multilevel model). Otherwise, the effect estimates should be approximated using an inflated standard error that incorporates the design effect (Higgins 2011). For cross‐over trials, only the first comparison (precross‐over) meeting our inclusion criteria should be used.
Studies with multiple treatment groups
For studies with multiple treatment groups, for each of the main objectives addressed in our review, we considered only data from the comparison of interest. If the study provided more than one comparison of interest for one of the main objectives, we planned to divide the number of participants in the arm used several times by the number of arms for all analyses to avoid including participants more than once in the analysis. However, this procedure was not necessary in our analyses.
Dealing with missing data
In case of missing or unclear data, we contacted corresponding authors or study sponsors to obtain key study characteristics and missing numerical outcome data when possible (e.g. when a study was identified as abstract only). We documented all requests and correspondence.
For all studies, we planned to calculate effect sizes using the intention‐to‐treat (ITT) principle (i.e. analyzing all participants allocated to the respective study arm). For the primary outcomes, all randomized participants were included in the analyses (when possible) irrespective of how the authors of the primary studies defined their ITT sample. For all other outcomes, we followed the definition of the ITT sample provided by the authors. Where authors reported no ITT data, we used the data provided.
Assessment of heterogeneity
We tested statistical heterogeneity between studies for significance using Cochrane's Q‐test and quantified it using the I² statistic (Higgins 2003).
Results were visually displayed as forest plots. We expected considerable clinical heterogeneity between studies. I² values in the range of 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity. Based on this classification, we considered I² values in the range of 50% to 100% as relevant statistical heterogeneity that was to be further explored. As "thresholds for the interpretation of I² can be misleading, since the importance of inconsistency depends on several factors" (Higgins 2011), this was only a rough orientation. Therefore, we decided on a case‐by‐case‐basis if the determined heterogeneity needed to be further explored.
Assessment of reporting biases
We tested for possible reporting biases and small‐study effects using visual examination of funnel plots (when useful). We planned to use Egger's test for test of publication bias, requiring a minimum of 10 studies per comparison (Sterne 2001).
Data synthesis
All analyses used a random‐effects model (DerSimonian 1986). We used a random‐effects rather than fixed‐effect model because we assumed that the included studies would not be functionally equivalent and would show considerable clinical (concerning population, intervention) and methodological (concerning quality) heterogeneity. Results are visually displayed as forest plots.
If it was not possible to combine studies via meta‐analysis, we provided a narrative summary.
Subgroup analysis and investigation of heterogeneity
To identify possible treatment effect moderators, we planned a priori defined subgroup analyses (in case of categorical predictors) or meta‐regression analyses (in case of metric predictors) for the primary outcomes.
We considered the following variables in subgroup analyses:
subtype of persistent depressive disorder (dysthymia versus other): a possibly moderating effect of subtype would suggest that a distinction between these subtypes might be used for allocation of people to treatments (differential indication). In contrast, a possible homogeneity of effects across subtypes may suggest that a distinction is of little relevance in the day‐to‐day practice. We planned to test dysthymia against other subtypes as dysthymia is assumed to be the most frequently mentioned subtype;
mean age of onset: the age of onset is known as a relevant predictor, it should be assessed if people with early onset need different treatments;
applied intervention (CBTs versus other, SSRIs versus other): experience shows that CBT approaches/SSRIs are the most frequent forms of psychological therapies/antidepressants to be studied. Therefore, we decided to test these approaches versus other approaches. Evidence on the best available treatments (in case of considerable differences) is indispensable for guideline recommendations;
duration of continuation/maintenance treatment (weeks): for guideline recommendations and clinical practice, it is indispensable to know if different treatment durations result in different outcomes, e.g. if longer treatments lead to better outcomes.
In case of considerable heterogeneity between study results that could not be explained by the a priori defined subgroup and meta‐regression analyses, we planned to perform a series of a posteriori (explorative) meta‐regression analyses to identify sources of heterogeneity. A priori and a posteriori analyses should be clearly labelled as such.
Sensitivity analysis
We performed sensitivity analyses excluding studies with a high or unclear risk of bias (separately for each of the seven domains according to Cochrane's 'Risk of bias' tool, when possible) or outlying findings (or both). Results were contrasted to those acquired with data from all studies to control for possible effects of study quality on pooled effects.
We planned additional sensitivity analysis: excluding trials without a randomization on person level (second phases of cross‐over trials, NRCTs, and cluster‐randomized trials) and excluding trials without (re)randomization immediately before the continuation/maintenance phase to control for possible design effects.
'Summary of findings' table
We included the comparison of effectiveness of pharmacotherapy versus placebo for persistent depressive disorder in Table 1. 'Summary of findings' tables include a summary of the quality of evidence, the magnitude of effects of the according intervention and a summary of available data on the primary outcomes (relapse/recurrence and dropout due to any reason). We expressed findings as measures of RR and absolute risk, with 95% CIs and used the GRADE approach to assess the quality of the body of evidence (Guyatt 2011).
Results
Description of studies
Results of the search
The CCMD Information Specialist conducted initial searches in December 2015 of the CCMD‐CTR (studies and references registers) and Ovid PsycINFO, retrieving a total of 4489 records. In September 2016, after we had screened the initial search results the Information Specialist ran further searches of CENTRAL and a cross‐search of Ovid MEDLINE, Embase and PsycINFO, retrieving an additional 929 records (after exclusion of duplicates). In October 2016, we searched and screened several sources of grey literature, reference lists of relevant systematic reviews and studies, and correspondended with authors of included studies to date, which yielded an additional 834 records. After removal of duplicates, two review authors (SL, KM, RM, or AJ) independently screened all 5520 records by title and abstract and excluded 5000 records as they did not meet our inclusion criteria. Two out of three review authors (SL, KM, or RM) independently checked each of the remaining 520 full‐text reports for eligibility. We included 17 publications (representing 10 studies) for the qualitative synthesis, and out of this pool we used 10 publications for the quantitative synthesis. One additional study is classified as 'awaiting classification', as the recruiting process has not finished yet (NCT03219879). The PRISMA flow diagram displays the details of the selection process (see Figure 1).
1.
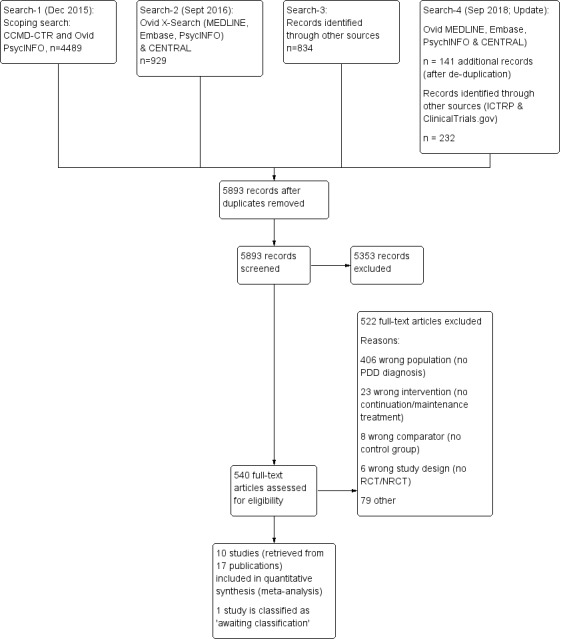
Study flow diagram. NRCT: non‐randomized controlled trial; PDD: persistent depressive disorder; RCT: randomized controlled trial.
In September 2018, the Information Specialist repeated the searches of Ovid MEDLINE, Embase, PsycINFO together with a search of the Cochrane Library trials database (CENTRAL). The update search retrieved 141 additional records (after de‐duplication). We screened these 141 records and identified 20 records for which we did a full‐text screening. Only one study fulfilled the inclusion criteria, but this study was already included in the systematic review (duplicate).
We also repeated the searches in the WHO International ClinicalTrials Registry Platform (ICTRP) and ClinicalTrials.gov (to September 2018) and identified 232 new records. Of these 232 records, 231 were excluded and one is a duplicate (classified as 'awaiting classification', see above).
Included studies
We included 17 publications, describing 10 studies: five continuation and five maintenance studies. In Table 2, column 'Study ID', we mentioned the 10 main publications. Three further publications described the acute phases of the included continuation and maintenance studies (Keller 1998a; Keller 2000; Marin 1994; see Table 2, column 'Related acute‐phase study'), one publication was a study protocol (Rush 1998), and three publications (Berndt 2000; Kocsis 1997; Kocsis 2002) provided additional analyses on the studies of Keller 1998b and Koran 2001. Thus, these seven publications provided information missing in the 10 main publications (e.g. location of the study, comorbidities, detailed information on the interventions).
1. Overview of included studies.
| Related acute‐phase study | Study ID | Treatment arms | Continuation/maintenance (treatment duration) | Study design | Diagnosis |
| Keller 1998b | Koran 2001 | Sertraline Imipramine |
Continuation (16 weeks) | NRCT | Chronic major depressive disorder, double depression |
| Keller 1998b | Sertraline Placebo |
Maintenance (76 weeks) | RCT | Chronic major depressive disorder, double depression | |
| Harrison 1986 | Harrison 1986 | Phenelzine Placebo |
Continuation (26 weeks) | RCT | Dysthymia, double depression |
| Keller 2000 | Kocsis 2003 | Nefazodone CBASP Combination |
Continuation (16 weeks) | NRCT | Chronic major depressive disorder, double depression, recurrent depressive disorder with incomplete interepisode remission |
| Gelenberg 2003 | Nefazodone Placebo |
Maintenance (52 weeks) | RCT | Chronic major depressive disorder, double depression, recurrent depressive disorder with incomplete interepisode remission | |
| Klein 2004 | CBASP Assessment only |
Maintenance (52 weeks) | RCT | Chronic major depressive disorder, double depression, recurrent depressive disorder with incomplete interepisode remission | |
| Hellerstein 2001 | Hellerstein 2001 | Fluoxetine Fluoxetine + group psychotherapy |
Continuation (16 weeks) | RCT | Dysthymia |
| Marin 1994 | Kocsis 1995 | Imipramine Desipramine |
Continuation (16–20 weeks) | NRCT | Dysthymia, double depression |
| Kocsis 1996* | Desipramine Placebo |
Maintenance (104 weeks) | RCT | Chronic major depressive disorder, dysthymia, double depression | |
| Miller 2001* | Desipramine Placebo |
Maintenance (104 weeks) | RCT | Dysthymia |
*These groups are partially overlapping (see above).
CBASP: Cognitive Behavioral Analysis System of Psychotherapy; NRCT: non‐randomized controlled trial; RCT: randomized controlled trial.
There were partially overlapping participant groups between the different continuation/maintenance treatment studies that followed one acute treatment study. However, these studies focused on different comparisons and were not included in the same analyses. Two exceptions were the studies of Kocsis 1996 and Miller 2001. These two studies focused on the same comparison (desipramine versus placebo) during the maintenance phase, but analyzed different diagnostic subgroups. While Miller 2001 analyzed solely participants with dysthymia, Kocsis 1996 included participants with a chronic MDD and double depression. Both studies shared the group of participants with dysthymia, although just partially as Miller 2001 also included participants with dysthymia not involved in Kocsis 1996.
Two studies investigated solely continuation treatments (Harrison 1986; Hellerstein 2001). The other eight studies followed three acute treatment studies, and investigated both continuation and maintenance treatments (Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 1995; Kocsis 2003; Koran 2001; Miller 2001).
Comparisons
We predefined seven relevant comparisons.
1. Pharmacological continuation and maintenance therapies versus placebo
Five of 10 studies included comparisons of an antidepressant medication with a pharmacological placebo (Gelenberg 2003; Harrison 1986; Keller 1998b; Kocsis 1996; Miller 2001). Two of these five studies compared desipramine versus placebo in the maintenance phase, but used different subgroups for analyses (see Table 2) (Kocsis 1996; Miller 2001).
2. Pharmacological continuation and maintenance therapies versus treatment as usual
There were no studies comparing pharmacological therapies versus TAU.
3. Psychological continuation and maintenance therapies versus attention placebo/non‐specific control
One study compared psychotherapy versus assessment only (Klein 2004).
4. Psychological continuation and maintenance therapies versus treatment as usual
There were no studies comparing psychological therapies versus TAU.
5. Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies
One study with three treatment arms compared pharmacological, psychological, and combined continuation therapy (Kocsis 2003).
6. Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone
Two studies compared combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone (Hellerstein 2001; Kocsis 2003 with three treatment arms (pharmacological, psychological, and combined continuation therapy)).
7. Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone
One study compared combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone (Kocsis 2003 with three treatment arms (pharmacological, psychological, and combined continuation therapy)).
We included one a posteriori comparison.
8. Pharmacological continuation and maintenance therapies versus other pharmacological continuation and maintenance therapies (post hoc)
Two studies compared pharmacological continuation and maintenance therapies versus other pharmacological continuation and maintenance therapies (post hoc) (Kocsis 1995; Koran 2001).
Design
Two studies used a randomized, controlled, parallel‐group design to investigate the continuation treatment phase (Harrison 1986; Hellerstein 2001). Three studies investigated a continuation treatment within an NRCT, that is, the participants continued to receive the same treatment that was effective during acute treatment (Kocsis 1995; Kocsis 2003; Koran 2001). Each of these three studies was followed by maintenance treatments applying a randomized, controlled, parallel‐group design (Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 1996; Miller 2001). Continuation treatments lasted between 16 and 26 weeks, maintenance treatments between 52 and 104 weeks. All 10 studies involved preceded acute treatments in their study design (see Table 2).
Sample size
Study size varied widely. The two studies investigating solely a continuation treatment randomized 12 participants (Harrison 1986) and 40 participants (Hellerstein 2001). Two studies randomized 329 participants (Kocsis 2003) and 386 participants (Koran 2001) for the continuation phase and rerandomized 82 participants (Klein 2004) and 161 participants (Keller 1998b) for the subsequent maintenance phase. Another study (Kocsis 1995) randomized 73 participants to the continuation phase and rerandomized between 27 participants (Miller 2001) and 53 participants (Kocsis 1996) to the subsequent maintenance phase.
Setting
Two studies were multicentre (Harrison 1986; Hellerstein 2001), and five were single centre (Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 2003; Koran 2001). One study was conducted multicentre during the continuation treatment (Kocsis 1995), and continued single centre during the maintenance phase (Kocsis 1996; Miller 2001). All studies were conducted in the US and used an outpatient setting for treatment.
Inclusion criteria
All studies required the participants to meet DSM criteria for persistent depressive disorder by the time of entering the study (i.e. start of acute treatment). Two continuation treatment studies included people with dysthymia (Harrison 1986; Hellerstein 2001), whereby the latter one focused on people with early‐onset dysthymia. While Koran 2001 and Keller 1998b included participants with either a chronic depressive episode or double depression, Gelenberg 2003, Kocsis 2003, and Klein 2004 additionally included participants with recurrent depression with incomplete interepisode remission. Kocsis 1995 analyzed participants with either dysthymia or double depression in the continuation treatment phase. The subsequent maintenance treatment phase included participants with either chronic major depressive disorder, dysthymia, or double depression, whereby Miller 2001 analyzed only people with dysthymia.
All studies used explicit response or remission criteria for entry into continuation or maintenance phases. Participants were required to show at least clinical response or partial remission, scoring below 15 on the HAM‐D (Gelenberg 2003; Klein 2004; Kocsis 2003), or to range between a score of 11 and 12 on the HAM‐D (Kocsis 1996; Miller 2001). Harrison 1986 required the participants to reach a score of 1 or 2 ("very much improved" or "much improved") on the Clinical Global Impression (CGI). Keller 1998b and Koran 2001 required participants to fulfil both a HAM‐D score of 15 or less and a CGI score of less than 3 (i.e. no more than mild depression). One study additionally defined specific remission criteria based on the Longitudinal Interval Follow‐up Evaluation (LIFE) for participants with double depression (Koran 2001), scoring 1 (no symptoms) or 2 (some symptoms) during four weeks. Compared to acute‐phase baseline scores, six studies determined response or remission with at least 50% decrease of symptoms (Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 2003; Koran 2001; Miller 2001), and one study with at least 40% reduction of symptoms (Hellerstein 2001). For the studies investigating continuation treatments, participants had to achieve the defined response or remission criteria directly at the end of acute treatment (Hellerstein 2001; Kocsis 2003), or had to maintain the specific score for the last four weeks before entering the continuation phase (Koran 2001). For the studies investigating maintenance treatments, participants had to continue their response or remission throughout the end of continuation treatment for being eligible to enter the maintenance phase (Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 1996; Miller 2001). Participants included in the studies had to be aged between 21 and 65 years (Hellerstein 2001; Keller 1998b; Koran 2001) or between 18 and 75 years (Gelenberg 2003; Klein 2004; Kocsis 2003).
Exclusion criteria
Five studies described criteria for excluding participants prior to study entry (i.e. before starting the acute treatment of the study programme). Six studies excluded participants who failed to respond to either at least one adequate trial of antidepressant medication (Harrison 1986; Keller 1998b; Koran 2001) or who failed to respond to three or more previous trials of antidepressant medication or at least two trials of empirical supported psychotherapy, or both (Gelenberg 2003; Klein 2004; Kocsis 2003). Nine studies (except Harrison 1986) excluded participants with serious medical illness, DSM diagnosed axis I disorders (if principal), personality disorders, present psychotic symptoms, or immediate suicidal risk. Five studies excluded participants who took concomitant (psychoactive) medication or who had received electroconvulsive therapy either within three months (Keller 1998b; Koran 2001) or three years prior to study entry (Gelenberg 2003; Klein 2004; Kocsis 2003). Hellerstein 2001 excluded participants who underwent another parallel psychotherapy, and Koran 2001 and Keller 1998b excluded participants who started another psychotherapy within the previous three months before entering study.
Participant characteristics
While one study reported that the majority of included participants were aged in their 30s or 40s (Harrison 1986), all other studies provided mean age scores of participants varying between 36 and 45 years. Harrison 1986 included predominantly women (83%), while the proportion of women varied between 50% and 66% in all other studies. Distribution of diagnostic subgroups differed among the included studies, whereby eight studies treated participants of several diagnostic subgroups, and two studies analyzed solely people with dysthymia (Hellerstein 2001; Miller 2001). The number of participants with double depression varied between 23% and 63% (Gelenberg 2003; Harrison 1986; Keller 1998b; Klein 2004; Kocsis 1995; Kocsis 1996; Kocsis 2003; Koran 2001). Six studies treated participants with a chronic depressive episode (Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 1996; Kocsis 2003; Koran 2001), of which the amount varied between 11% and 55%. Three studies also treated participants diagnosed with a recurrent depressive episode with incomplete interepisode remission (Gelenberg 2003; Klein 2004; Kocsis 2003); the amount varied between 22% and 29%. Three studies also treated participants with dysthymia, of which the amount varied between 37% and 40% (Harrison 1986; Kocsis 1995; Kocsis 1996).
Although other axis I disorders (if principal) and personality disorders were an exclusion criterion in almost all studies, some studies described percentages of single comorbid mental conditions with the following percentages:
Keller 1998b: 26% anxiety disorders, 37% substance abuse, 30% alcohol abuse, 48% axis II disorders;
Klein 2004: 27% anxiety disorders, 26% substance abuse;
Kocsis 2003: 31% anxiety disorders, 29% alcohol abuse;
Kocsis 1996: 34% anxiety disorders, 48% axis II disorders.
As only serious medical illnesses were an exclusion criterion, participants could have more harmless illnesses. However, none of the included studies reported data on comorbid somatic conditions.
Six studies provided data on the mean age of onset, which ranged from 12.3 to 29.5 years. The mean length of the current/previous episode was 73.2 to 105.6 months (data provided in five studies: Gelenberg 2003; Keller 1998b; Klein 2004; Kocsis 2003; Koran 2001). Four studies provided data on the number of previous episodes and reported a mean number of 1.3 to 3.0 episodes (Hellerstein 2001; Keller 1998b; Klein 2004; Koran 2001). Three studies reported the mean lifetime duration of the depressive disorder (Harrison 1986: 15.0 years; Koran 2001: 16.6 years; Miller 2001: 24.0 years).
Four studies reported data on previous medications (Keller 1998b; Kocsis 1996; Kocsis 2003; Miller 2001), the percentages of participants previously received antidepressants ranged from 22% (Keller 1998b) to 57% (Kocsis 2003).
Five studies reported data on previously received psychotherapy (Hellerstein 2001; Keller 1998b; Kocsis 2003; Miller 2001); this applied to 63% (Keller 1998b) to 85% (Miller 2001) of the included participants.
Types of intervention
Antidepressant drugs and drug placebo interventions
Continuation treatment: one continuation treatment study compared an active antidepressant drug with a tablet placebo (Harrison 1986). This study used the MAOIs phenelzine as active treatment for 26 weeks. Participants in the active group received on average phenelzine 51 mg daily. Participants in the placebo group discontinued phenelzine treatment over a period of 14 days by reducing the daily dose by 15 mg every two to three days. Two continuation treatment studies included a direct comparison of two antidepressant medications. Koran 2001 compared sertraline (SSRI) 50 mg to 200 mg per day to imipramine (TCA) 50 mg to 300 mg per day for 16 weeks. The dose could be adapted by 50 mg per day each week depending on the participant's symptoms and adverse effects. The second study compared two TCAs during 16 to 20 weeks of treatment (Kocsis 1995). Participants received the same final dose achieved during acute treatment (imipramine 300 mg per day or desipramine 200 mg per day). Two continuation treatment studies included comparisons of antidepressant medication alone versus medication plus psychotherapy. Kocsis 2003 investigated three active treatment arms, including nefazodone (SNDRI) alone, psychotherapy alone (Cognitive Behavioral Analysis System of Psychotherapy), and nefazodone plus psychotherapy over 16 weeks. In both medication arms, participants received nefazodone 300 mg per day to 600 mg per day. Hellerstein 2001 compared fluoxetine (SSRI) alone with fluoxetine plus group psychotherapy over 16 weeks. Participants in both arms received fluoxetine 20 mg per day to 80 mg per day.
Maintenance treatment: four maintenance treatment studies included the comparison of an active antidepressant drug with a tablet placebo (Gelenberg 2003; Keller 1998b; Kocsis 1996; Miller 2001). Of these four, two studies analyzed the same comparison (desipramine versus placebo) but with focus on different diagnostic subgroups (Kocsis 1996; Miller 2001). In Keller 1998b, the participants in the active treatment group received a flexible daily dose of sertraline hydrochloride 50 mg to 200 mg (SSRI) for 76 weeks. Participants in the placebo arm reduced the sertraline dose by 50 mg every week and received placebo substitution. Gelenberg 2003 used nefazodone SNDRI at the same dose being effective during the previous continuation phase (300 mg per day and 600 mg per day) over 52 weeks. Participants in the placebo arm received identical (but inactive) tablets without any stepwise reduction between continuation and maintenance phase. Kocsis 1996 and Miller 2001 used desipramine (TCA) over 104 weeks as maintenance treatment. Participants in the active group maintained the dose (75mg per day to 350 mg per day) of the previous continuation phase. Participants in the placebo arm reduced their dose by 25% per week during the first month of maintenance treatment and subsequently started a treatment with identical placebo tablets.
Types of psychological therapies
Three studies investigated psychotherapeutic treatments, two continuation treatment studies and one maintenance treatment study.
Kocsis 2003 examined the Cognitive Behavioral Analysis System of Psychotherapy (CBASP; McCullough 2000) during the 16‐week continuation phase. Participants received six sessions of manualized CBASP, both in the CBASP and the combined treatment arm. The continuation treatment study of Hellerstein 2001 compared fluoxetine (SSRI) alone with a group receiving fluoxetine plus manualized group psychotherapy in a 16‐week continuation phase. The combined group received treatment according to an unpublished manual of Cognitive‐Interpersonal Group Psychotherapy for Chronic Depression (CIGP‐CD), combining cognitive and interpersonal approaches. Up to 10 participants formed a group with weekly meetings of 90 minutes.
Klein 2004 investigated CBASP versus assessment only in a 52‐week maintenance phase which followed the study of Kocsis 2003 (see above). Participants in the CBASP group received one session every four weeks for up to 13 sessions, and were evaluated by an independent evaluator every four weeks. Participants in the assessment only group met the project co‐ordinator and the independent evaluator also every four weeks, hence received some attention but no active treatment.
Process evaluation of psychological treatments
One continuation treatment study (Hellerstein 2001) and one maintenance treatment study (Klein 2004) provided information on process evaluation.
Hellerstein 2001 involved two clinical psychology PhD students with extensive psychotherapy training for conducting the group therapy in the continuation treatment phase. On a weekly basis, these two students met a senior psychiatrist supervisor for two months for reviewing how to conduct the treatment with the CIGP‐CD treatment manual. By the start of the study treatment, sessions with the participants were audiotaped and supervised weekly for adherence to the manual.
Information on the CBASP sessions in the maintenance treatment study was inferable from the main publication (Klein 2004). There was additional information from the publication of the acute treatment phase (Keller 2000). The CBASP sessions were all videotaped and conducted by psychotherapists with at least two or five years of experience (dependent on last degree achieved). The therapists underwent a two‐day training workshop with James P McCullough (founder of CBASP) including an evaluation of two videotaped pilot cases before starting treatment with study participants. Throughout the maintenance phase, the designated supervisors at each site assessed therapist's adherence to treatment procedures biweekly by reviewing videotapes. These supervisors were directly supervised by James P McCullough. Treatment adherence was measured using a CBASP‐specific rating scale developed by McCullough (McCullough 2000). In case of non‐adherence, an immediate meeting with the respective therapist was scheduled and opportunities for improvement were discussed.
Types of outcome measures
Primary outcomes
The primary efficacy outcome was rates of relapse or recurrence of depression, defined by exceeding a specific score on the HAM‐D or on the severity of the CGI, by fulfilling DSM criteria for an MDD, by clinical judgement of the research team during a predefined range of time, or a combination of these.
Two continuation studies applied criteria for relapse, either scoring below a satisfactory response during four weeks (Koran 2001), or scoring three or more on the CGI during two weeks (Harrison 1986).
In the maintenance treatment study of Keller 1998b, participants had to fulfil DSM criteria for an MDD during three consecutive weeks, a CGI rating of three or more, and an increase of at least four points on the HAM‐D (compared to maintenance baseline) to be diagnosed as having a recurrence. One week later, a senior investigator determined the diagnosis within a clinical interview to confirm relapse. Two other maintenance treatment studies defined a participant's recurrence by a score of 12 or more on the HAM‐D and a score below 60 on the GAS on three consecutive ratings within four weeks (Kocsis 1996; Miller 2001). Also, if a participant fulfilled these criteria on just one rating but was considered to need urgently alternative treatment, the participant was rated as being recurred.
One continuation treatment study (Kocsis 2003), and two maintenance treatment studies (Gelenberg 2003; Klein 2004), applied the same criteria for recurrence, as they followed the same acute treatment study (Keller 2000). These three studies required the participants to score 16 or higher on the HAM‐D, to fulfil DSM criteria for an MDD on two consecutive visits, and to undergo a clinical interview with a senior investigator confirming recurrence. They also applied another definition in case a participant scored 16 or more on the HAM‐D but did not fulfil MDD criteria or discontinued before a second visit for clarification. Then, senior investigators reviewed the data of such participants at the end of the study, discussed and decided if and at what time an MDD had occurred, and if the participant could be considered to have recurred.
Two studies did not address relapse or recurrence (Hellerstein 2001; Kocsis 1995).
The primary safety/acceptability outcome was dropout due to any reason other than recurrence. Nine studies reported overall dropout rates. Most of the studies also reported reasons for dropout (see 'Secondary outcomes' below). One study did not report any dropouts (Miller 2001).
Secondary outcomes
Metric outcomes of depression severity scales and quality of life measures were reviewed as secondary efficacy outcomes. Six studies reported changes in severity of depressive symptoms from pre‐ to post‐treatment on the HAM‐D (Gelenberg 2003; Harrison 1986; Hellerstein 2001; Keller 1998b; Klein 2004; Koran 2001). Three studies included quality of life measures (Hellerstein 2001; Keller 1998b; Koran 2001). The continuation treatment study of Koran 2001 reported data on the Quality of Life Enjoyment and Satisfaction Questionnaire (Q‐LES‐Q), a self‐report measure obtaining the degree of enjoyment and satisfaction in different areas of daily functioning. In the subsequent maintenance treatment study, Keller 1998b used the 36‐item Short‐Form Health Survey (SF‐36), and reported data on three subscales (social functioning, role limitations owing to emotional problems, role limitations owing to physical health problems) in a further publication (Kocsis 2002). Hellerstein 2001 used the Satisfaction With Life Scale (SWLS), a self‐report measure of subjective life satisfaction. Only one study reported follow‐up outcome data (both for the HAM‐D and the SWLS scores) 12 weeks after the end of the intervention.
Measures of safety (i.e. dropout due to any type of adverse event and the occurrence of any or severe adverse events) were also reviewed. Five of 10 studies reported dropout due to adverse events other than recurrence, and all of these studies compared antidepressant medication with placebo or another medication (Gelenberg 2003; Harrison 1986; Keller 1998b; Kocsis 1995; Koran 2001). Reasons for such dropout were adverse effects, insufficient response, intercurrent illness, or dispute with staff. Two studies reported the occurrence of any adverse events, including adverse effects (e.g. headache, insomnia, sexual problems) for the majority of participants (Keller 1998b), and adverse effects (especially sleep disturbances and sexual problems) for all participants in the medication arm (Harrison 1986), although Harrison 1986 reported no data for the placebo group.
Excluded studies
The major reason for exclusion of studies was the non‐fulfilment of the diagnosis 'persistent depressive disorder' (see Figure 1). Some studies involved participants with recurrent depressive disorder with complete interepisode remission (e.g. Jarrett 2013), other studies also involved chronic forms of depression, but the percentage of chronic forms was less than 80% (e.g. Thase 2001) or provided no separate analyses of diagnostic subgroups of persistent depression (e.g. Petersen 2010). Other studies did not apply clear response or remission criteria for participants to be eligible for entering continuation/maintenance treatment (i.e. all participants from the acute phase could take part in the following treatment phases) (e.g. Schramm 2017).
Risk of bias in included studies
Of the 10 included studies, seven were RCTs and three were NRCTs. These three NRCTs were continuation treatment studies (Kocsis 1995; Kocsis 2003; Koran 2001), and were labelled as NRCTs for this review as the acute‐phase responders were not rerandomized for the continuation treatment. We used the 'Risk of bias' tool on a three‐point scale (low/high/unclear risk) for the RCTs (Figure 2; Figure 3). We used the ROBINS‐I tool (Sterne 2016), using a five‐point scale (low/moderate/serious/critical/unclear risk) for the NRCTs (Figure 4; Figure 5; Table 3; Table 4; Table 5).
2.
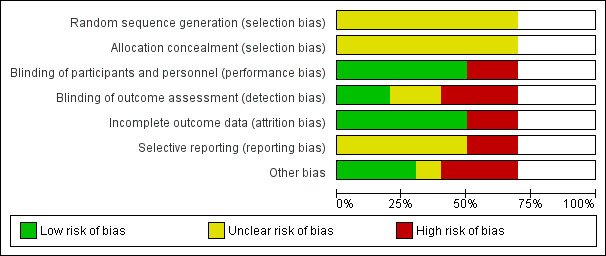
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included seven randomized controlled trials. Blank space in rows containing no information indicate missing information on the 'Risk of bias' scale for the three non‐randomized controlled trials.
3.
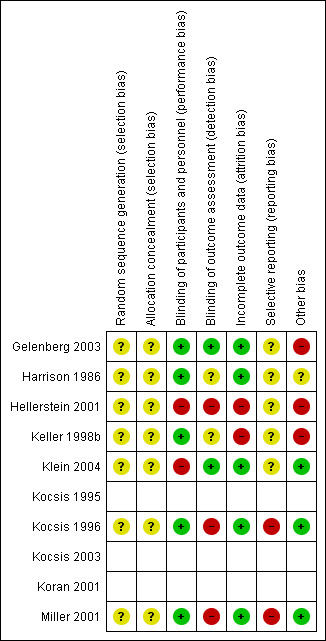
Risk of bias summary: review authors' judgements about each risk of bias item for each included randomized controlled trial (seven studies). Blank space in rows containing no information indicate missing information on the 'Risk of bias' scale for the three non‐randomized controlled trials.
4.
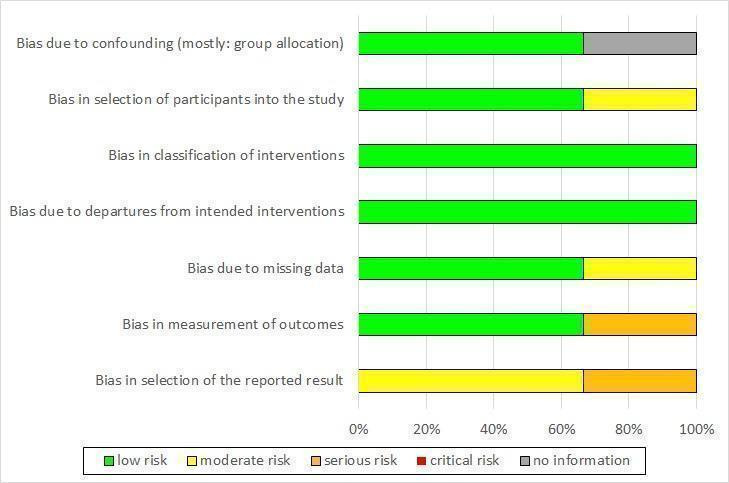
Risk of bias graph non‐randomized controlled trials (NRCT): review authors' judgement about each risk of bias item presented as percentages across all included NRCTs (three studies).
5.
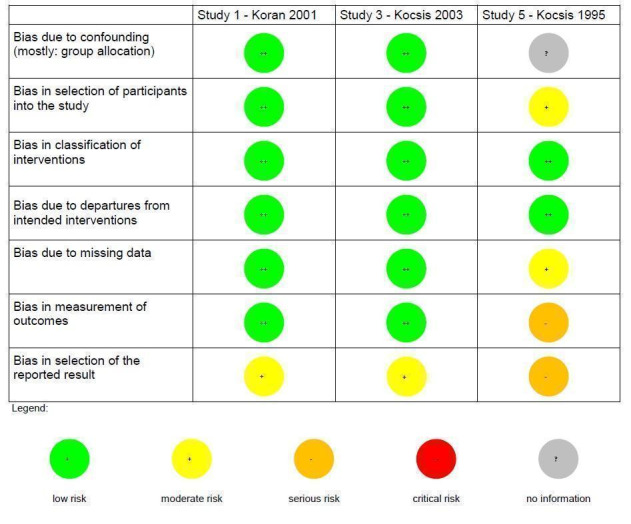
Risk of bias summary non‐randomized controlled trials (NRCT): review authors' judgements about each risk of bias item for each included NRCT (three studies).
2. Risk of bias (non‐randomized trials) – Kocsis 1995.
| Risk of bias (ROBINS‐I tool) | Rating | Explanation of judgement | Possible ratings |
| Bias due to confounding | 5 | No information how participants were allocated to groups in the acute treatment. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of participants into the study | 2 | Different length of drugs and procedures during acute treatment (participants of 3 protocols were included for analyses of continuation treatment). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in classification of interventions | 1 | Intervention was well defined: IMI and DMI were continued on an open basis at the same final dose achieved during the acute phase. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to departures from intended interventions |
1 | No indication for departures from intended interventions, check of plasma levels was performed. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to missing data | 2 | Proportions of missing participants differed substantially across interventions: 26% in the IMI group and 6% in the DMI group, in the IMI group 4 participants did not comply with the follow‐up assessment, reasons for dropout were reported; but proportion of dropout due to dissatisfaction with treatment was similar for IMI and DMI (7% and 6%). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in measurement of outcomes | 3 | Participants in the IMI protocols were seen and rated once at week 26 of treatment. Participants on the DMI protocol were seen and rated every 2 weeks through week 26. Lack of blinding: participants and raters were aware of the treatment. (see p. 214) | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of the reported result | 3 | Not all predefined outcomes were reported separately for both groups. Some data were assessed every 2 weeks, these data were not reported. In general, data were not reported for HAM‐D and GAS for the DMI vs IMI. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
DMI: desipramine; GAS: Global Assessment Scale; HAM‐D: Hamilton Depression Rating Scale; IMI: imipramine.
3. Risk of bias (non‐randomized trials) – Kocsis 2003.
| Risk of bias (ROBINS‐I tool) | Rating | Explanation of judgement | Possible ratings |
| Bias due to confounding | 1 | Randomization before acute phase, exclusion of cross‐over participants. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of participants into the study | 1 | All eligible participants were included, cross‐over participants were excluded; acute phase treatment had the same length and measurement times for all groups. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in classification of interventions | 1 | Intervention status was well described (planned and actual dose of medication as well as number of CBASP sessions). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to departures from intended interventions | 1 | Medication doses as well as number of CBASP sessions were within the planned range. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to missing data | 1 | Number of missing data was low and comparable in all groups (2–3%). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in measurement of outcomes | 1 | Methods of outcome assessment were comparable across intervention groups. Quote: "Trained independent evaluators unaware of treatment assignment completed the HAM‐D‐24 at each assessment visit." (p. 77), no means and standard deviations reported, unclear if other subscales were evaluated. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of the reported result | 2 | No study protocol existed, but all measures mentioned in the methods section were reported in the outcome section. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
CBASP: Cognitive Behavioral Analysis System of Psychotherapy; HAM‐D: Hamilton Depression Rating Scale.
4. Risk of bias (non‐randomized trials) – Koran 2001.
| Risk of bias (ROBINS‐I tool) | Rating | Explanation of judgement | Possible ratings |
| Bias due to confounding | 1 | Randomization before acute phase. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of participants into the study | 1 | All possible participants were included (direct and cross‐over). All measures existing from the beginning of the intervention. The study flow was clearly described since acute treatment. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in classification of interventions | 1 | Intervention status is well defined (dose ranges are described in section 2.3 and 3.4). | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to departures from intended interventions |
1 | Assignment to intervention. Quote: "For both treatment groups, 10% of patients had dose increases aimed at improving outcome" (p. 31); same for both groups with regard to the main outcome; adapting dose is usual practice; no deviation from intended treatment, they counted the tablets. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias due to missing data | 1 | Quote: "For all patients, including drop outs, pill counts indicated compliance rates of 88.7% for imipramine and 84.7% for sertraline. No differences were found between diagnostic groups or between acute and crossover patients." (p. 31); Proportions of and reasons for missing participants were similar across intervention groups; less than 5% dropout for the main outcome; proportions of missing data were comparable and are addressed in the analyses with LOCF. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in measurement of outcomes | 1 | Information from Rush et al., 1998 (study protocol): reliable ratings (p. 593); quote: "continued on the same double‐blind medication dose for an additional 16 weeks" (p. 593); assessment methods comparable across groups. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
| Bias in selection of the reported result | 2 | Outcomes correspond to the ones named in the protocol, but protocol just for acute phase; not all measures used during the acute phase were used in continuation phase. | Code 1 = low risk, 2 = moderate risk, 3 = serious risk, 4 = critical risk, 5 = no information |
LOCF: last observation carried forward.
Risk of bias in randomized controlled trials (seven studies)
Random sequence generation (selection bias)
None of the included RCTs described random sequence generation (unclear risk of bias in all seven RCTs).
Allocation (selection bias)
All seven RCTs had an unclear risk of bias concerning allocation as there was no information on the allocation process.
Blinding (performance bias and detection bias)
Blinding of participants and personnel (performance bias)
Five RCTs reported that participants and personnel were blinded (low risk) (Gelenberg 2003; Harrison 1986; Keller 1998b; Kocsis 1996; Miller 2001). Two RCTs were psychotherapy studies (Hellerstein 2001; Klein 2004). Therefore, participants and personnel were aware of the treatment condition (high risk).
Blinding of outcome assessment (detection bias)
In two RCTs, the outcome assessors were independent and blind to the treatment condition (low risk; Gelenberg 2003; Klein 2004). In two RCTs, there was no information on this domain (unclear risk; Harrison 1986; Keller 1998b). In three RCTs, the assessors were study clinicians not blinded to the treatment condition (high risk; Hellerstein 2001; Kocsis 1996; Miller 2001).
Incomplete outcome data (attrition bias)
In five RCTs, the risk concerning incomplete outcome data was low (Gelenberg 2003; Harrison 1986; Klein 2004; Kocsis 1996; Miller 2001). In these studies, the number of participants with missing data was low (below 5%), there were no missing data, or the (main) outcome was reported for all included participants. Two RCTs had a high risk of bias as the number of participants with missing data was very high, and we considered the used imputation methods (last observation carried forward (LOCF)) as rather inadequate for this context (see Discussion) (Hellerstein 2001; Keller 1998b).
Selective reporting (reporting bias)
Five RCTs had an unclear risk concerning reporting bias (Gelenberg 2003; Harrison 1986; Hellerstein 2001; Keller 1998b; Klein 2004). There were no study protocols for the continuation or maintenance treatments available. Nevertheless, we did not rate the risk of bias as high as outcomes in relevant domains were reported and there was no specific indication for selective reporting. Two RCTs were at high risk as results were not reported for all applied measures (Kocsis 1996; Miller 2001).
Other potential sources of bias
To operationalize the domain of other relevant sources of bias, we assessed three subdomains separately: insufficient treatment adherence, allegiance bias/conflict of interest, and attention bias. Ratings from these three subdomains were then summarized to one overall rating of 'other potential sources of bias' for each RCT (see Figure 3 and Characteristics of included studies table). If one of these three subdomains was at high risk of bias, we assessed the overall rating as high. In the case that two (or three) domains indicated an unclear risk and one (or no) domain indicated a low risk, the overall rating was unclear. The overall rating was low risk if two or three domains indicated a low risk and no or one domain indicated an unclear risk.
Three studies were at high risk, especially because conflict of interest was considered very likely (pharmaceutical sponsoring) (Gelenberg 2003; Hellerstein 2001; Keller 1998b). One study had an unclear risk as information on the three subdomains was mostly lacking (Harrison 1986). Three studies had a low risk as there was – for example – serum level control to ensure the treatment adherence (Klein 2004; Kocsis 1996; Miller 2001). Moreover, the investigators ensured that all groups received the same amount of attention and there were – in case of sponsoring from a pharmaceutical company – also other, independent authors involved in the publication.
Risk of bias in non‐randomized controlled trials (three studies)
Bias due to confounding (mostly: group allocation)
Two NRCTs were at low risk of bias due to confounding as participants were randomized before the acute treatment (Kocsis 2003; Koran 2001). In one NRCT, there was no information on how participants were allocated to the groups in the acute treatment phase (unclear risk; Kocsis 1995).
Bias in selection of participants into the study
Two NRCTs included all eligible participants and described the process of inclusion and the study flow clearly (Kocsis 2003; Koran 2001). Kocsis 1995 included participants from three different acute‐phase treatment protocols with different treatment durations and medication, thus the risk was moderate.
Bias in classification of interventions
All three NRCTs defined the intervention status well, for example, the planned and actual dose of the pharmacological intervention and the number of psychotherapy sessions was described, indicating a low risk (Kocsis 1995; Kocsis 2003; Koran 2001).
Bias due to departures from intended interventions
In all three NRCTs, there was no indication for departures from intended interventions (e.g. plasma level checks were performed and the dose range of medication or the number of psychotherapy sessions were within the planned range (Kocsis 1995; Kocsis 2003; Koran 2001). Therefore, the risk was low.
Bias due to missing data
In the studies of Koran 2001 and Kocsis 2003, the number of participants with missing data was low (less than 5% for the main outcome) and comparable across the intervention groups. The risk was moderate for Kocsis 1995, as the proportions of participants with missing data differed substantially across the groups, but reasons for dropout were reported.
Bias in measurement of outcomes
The assessment methods were reliable, comparable across treatment groups and performed by trained independent raters in the studies of Koran 2001 and Kocsis 2003. In the study of Kocsis 1995, the frequency of ratings differed across treatment groups and participants and raters were aware of the treatment (serious risk).
Bias in selection of the reported result
In the study of Koran 2001, the outcomes correspond to the ones named in the methods section. The study protocol focused solely on the acute phase of the study, and not all measures described in the protocol were reported in the publication of the continuation treatment study (moderate risk). For the study reported by Kocsis 2003, no study protocol was available, but all measures reported in the methods section were also reported in the results section (moderate risk). In the study reported by Kocsis 1995, the risk of bias was rated as serious as not all predefined outcomes were reported.
Effects of interventions
See: Table 1
For all comparisons, we analyzed ITT data when possible. Six studies reported ITT data for all outcome measures included in this review. In the other four studies, data for 2% to 10% (Hellerstein 2001; Kocsis 1996; Kocsis 2003; Koran 2001) of the ITT sample were missing for single outcome measures at the end of the intervention. Missing data were not replaced, calculations were based on the data provided in the publications. Data on the dropout rate (overall dropout and dropout due to adverse events) were consistently based on the complete ITT sample. The only study with follow‐up data provided data on depression severity at follow‐up for the complete ITT sample and data on quality of life at follow‐up for 85% of the participants of the ITT sample (Hellerstein 2001).
1. Pharmacological continuation and maintenance therapies versus placebo
Five studies compared pharmacological continuation and maintenance therapies with placebo. Keller 1998b compared sertraline (participants = 77) with placebo (participants = 84), Harrison 1986 compared phenelzine (participants = 5) with placebo (participants = 7), and Gelenberg 2003 compared nefazodone (participants = 76) with placebo (participants = 84). Both Kocsis 1996 and Miller 2001 compared desipramine with placebo, analyzing different diagnostic subgroups: Kocsis 1996 (desipramine: participants = 28; placebo: participants = 25), Miller 2001 (desipramine: participants = 14; placebo: participants = 13). As Kocsis 1996 and Miller 2001 evaluated partially overlapping groups (see above), we considered only the data of the larger group (Kocsis 1996) here. The sample of Kocsis 1996 was replaced by the sample of Miller 2001 in the sensitivity analyses.
Primary outcomes
1.1. Relapse/recurrence rates of depression
The four included studies were all RCTs. Three of them were maintenance treatment studies (Gelenberg 2003; Keller 1998b; Kocsis 1996), while Harrison 1986 was a continuation treatment study. All studies used different antidepressants from varying classes. Participants taking antidepressant medication had significant fewer relapses or recurrences compared to the placebo group at end of the intervention, with moderate heterogeneity (RR 0.41, 95% CI 0.21 to 0.79; participants = 383; studies = 4; I² = 54%; Analysis 1.1; Figure 6). This translated to an NNTB of six.
1.1. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 1 Relapse/recurrence.
6.
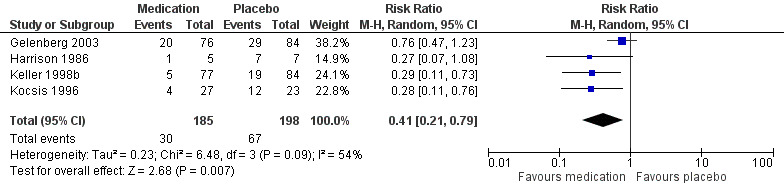
Forest plot of comparison: 1 Medication versus placebo, outcome: 1.1 Relapse/recurrence.
1.2. Dropout due to any reason
Four RCTs provided overall dropout rates at the end of the intervention. Three were maintenance treatment studies (Gelenberg 2003; Keller 1998b; Kocsis 1996), while Harrison 1986 was a continuation treatment study. We found no significant differences between medication and placebo, with substantial heterogeneity (RR 0.90, 95% CI 0.39 to 2.11; participants = 386; I² = 64%; Analysis 1.2). Two studies reported fewer dropouts in the medication arm (Gelenberg 2003; Kocsis 1996), while the other two studies reported fewer dropouts in the placebo group (Harrison 1986; Keller 1998b).
1.2. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 2 Dropout due to any reason.
Secondary outcomes
1.3. Symptom severity of depression
Three RCTs provided means and standard deviations (SDs). Two were maintenance treatment studies (Gelenberg 2003; Keller 1998b), while Harrison 1986 was a continuation treatment study. Participants in the medication groups showed a significantly lower symptom severity on the HAM‐D at the end of the intervention compared to the placebo groups, with moderate to substantial heterogeneity (MD –4.79, 95% CI –8.49 to –1.09; participants = 333; I² = 60%; Analysis 1.3).
1.3. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 3 Depression severity.
1.4. Health‐related quality of life
One RCT provided quality of life measures at the end of the intervention and reported three subscales of the SF‐36 (Keller 1998b). In this maintenance treatment study, participants in the medication group reported both higher social functioning (MD 10.80, 95% CI 3.04 to 18.56; participants = 161; Analysis 1.4) and fewer limitations owing to emotional problems (MD 20.70, 95% CI 7.43 to 33.97; participants = 161; Analysis 1.5). There was no significant difference between groups for the subscale of role limitations owing to physical health problems (Analysis 1.6).
1.4. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 4 SF‐36 Social Functioning score.
1.5. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 5 SF‐36 Emotional Role score.
1.6. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 6 SF‐36 Role Physical score.
1.5. Dropout due to any type of adverse events
Three RCTs provided data on dropouts due to adverse events at the end of the intervention. Two were maintenance treatment studies (Gelenberg 2003; Keller 1998b), while Harrison 1986 was a continuation treatment study. There was no significant difference between medication and placebo groups (OR 3.53, 95% CI 0.67 to 18.70; participants = 333; I² = 46%; Analysis 1.7).
1.7. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 7 Dropout due to any type of adverse event.
1.6. Any type of adverse event
One RCT provided data on experiencing any adverse event (physical problems such as headache or insomnia) at the end of the intervention (Keller 1998b). In this maintenance treatment study, there was no significant difference between medication and placebo (OR 1.47, 95% CI 0.70 to 3.09; participants = 161; Analysis 1.8). The continuation treatment study of Harrison 1986 (RCT) also provided data on adverse events, but solely for the medication group. In the medication group all participants had adverse events.
1.8. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 8 Any type of adverse event.
1.7. Serious adverse events
We found no studies reporting participants experiencing serious adverse events.
2. Pharmacological continuation and maintenance therapies versus treatment as usual
None of the included studies compared pharmacological continuation and maintenance therapies versus TAU.
3. Psychological continuation and maintenance therapies versus attention placebo/non‐specific control
One maintenance treatment study (RCT) provided data on the comparison psychotherapy versus assessment only (Klein 2004).
Primary outcomes
3.1. Relapse/recurrence rates of depression
Rates of relapse/recurrence were significantly lower in the psychotherapy group (RR 0.37, 95% CI 0.14 to 0.93; participants = 82; Analysis 2.1). This translated to an NNTB of five.
2.1. Analysis.
Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 1 Relapse/recurrence.
3.2. Dropout due to any reason
There were no significant differences between the overall dropout rates (RR 0.87, 95% CI 0.41 to 1.81; participants = 82; Analysis 2.2).
2.2. Analysis.
Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 2 Dropout due to any reason.
Secondary outcomes
3.3. Symptom severity of depression
Depression severity was significantly lower in the psychotherapy group at the end of the intervention (MD –4.00, 95% CI –7.05 to –0.95; participants = 82; Analysis 2.3).
2.3. Analysis.
Comparison 2 Psychological continuation and maintenance therapies versus attention placebo/non‐specific control, Outcome 3 Depression severity.
3.4. Health‐related quality of life
The study did not report health‐related quality of life.
3.5. Dropout due to any type of adverse events
The study did not report dropout due to any type of adverse events.
3.6. Any type of adverse event
The study did not report participants experiencing any type of adverse event.
3.7. Serious adverse events
The study did not report participants experiencing serious adverse events.
4. Psychological continuation and maintenance therapies versus treatment as usual
None of the included studies compared psychological continuation and maintenance therapies versus TAU.
5. Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies
One continuation treatment study (NRCT) provided data on the comparison psychotherapy versus medication (Kocsis 2003).
Primary outcomes
5.1. Relapse/recurrence rates of depression
There were no significant differences in relapse/recurrence rates between groups (RR 1.22, 95% CI 0.43 to 3.49; participants = 176; Analysis 3.1).
3.1. Analysis.
Comparison 3 Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies, Outcome 1 Relapse/recurrence.
5.2. Dropout due to any reason
There were no significant differences in dropout rates between groups, although there was a tendency favouring psychotherapeutic treatment (RR 0.56, 95% CI 0.30 to 1.03; participants = 179; Analysis 3.2).
3.2. Analysis.
Comparison 3 Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies, Outcome 2 Dropout due to any reason.
Secondary outcomes
5.3. Symptom severity of depression
The study did not report depression severity scales.
5.4. Health‐related quality of life
The study did not report health‐related quality of life.
5.5. Dropout due to any type of adverse events
The study did not report dropout due to any type of adverse events.
5.6. Any type of adverse event
The study did not report participants experiencing any type of adverse event.
5.7. Serious adverse events
The study did not report participants experiencing serious adverse events.
6. Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone
Two continuation treatment studies compared combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone: Kocsis 2003 (NRCT) compared nefazodone (participants = 91) with nefazodone plus CBASP (participants = 150), while Hellerstein 2001 (RCT) compared fluoxetine only (participants = 19) with fluoxetine plus group psychotherapy (participants = 20).
Primary outcomes
6.1. Relapse/recurrence rates of depression
Kocsis 2003 (NRCT) provided data on relapse/recurrence rates, showing no significant difference between participants taking medication only (nefazodone) or medication combined with psychotherapy (CBASP) (RR 1.23, 95% CI 0.44 to 3.44; participants = 238; Analysis 4.1).
4.1. Analysis.
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 1 Relapse/recurrence.
6.2. Dropout due to any reason
Both studies provided overall dropout rates at the end of the intervention and found no significant differences between medication only and the combined treatment, with no heterogeneity (RR 1.43, 95% CI 0.90 to 2.29; participants = 280; I² = 0%; Analysis 4.2).
4.2. Analysis.
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 2 Dropout due to any reason.
Secondary outcomes
6.3. Symptom severity of depression
One RCT provided continuous data on depression severity scale (Hellerstein 2001). Participants in the combined treatment group showed significantly lower symptom severity on the HAM‐D at the end of the intervention compared to the medication only group (MD 2.80, 95% CI 0.38 to 5.22; participants = 39; Analysis 4.3). This study also provided follow‐up data 12 weeks after the end of the intervention. There was no significant differences between both groups (MD 0.90, 95% CI –3.26 to 5.06; participants = 39; Analysis 4.4).
4.3. Analysis.
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 3 Depression severity.
4.4. Analysis.
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 4 Depression severity – follow‐up.
6.4. Health‐related quality of life
Hellerstein 2001 (RCT) provided health‐related quality of life measures at the end of the intervention and at follow‐up 12 weeks after the end of the intervention. There were no significant differences between groups at either time point, using the SWLS (end: MD –0.50, 95% CI –1.63 to 0.63; participants = 35; Analysis 4.5; follow‐up: MD 0.60, 95% CI –0.56 to 1.76; participants = 33; Analysis 4.6).
4.5. Analysis.
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 5 Health‐related quality of life.
4.6. Analysis.
Comparison 4 Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone, Outcome 6 Health‐related quality of life – follow‐up.
6.5. Dropout due to any type of adverse events
Neither study reported dropout due to any type of adverse events.
6.6. Any type of adverse event
Neither study reported participants experiencing any type of adverse event.
6.7. Serious adverse events
Neither study reported participants experiencing serious adverse events.
7. Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone
One continuation treatment study (NRCT) provided data on the comparison psychotherapy (CBASP, participants = 88) versus combined treatment (nefazodone plus CBASP, participants = 150) (Kocsis 2003).
Primary outcomes
7.1. Relapse/recurrence rates of depression
There was no difference in rates of relapse/recurrence between groups (RR 1.51, 95% CI 0.57 to 4.01; participants = 234; Analysis 5.1).
5.1. Analysis.
Comparison 5 Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone, Outcome 1 Relapse/recurrence.
7.2. Dropout due to any reason
There was no difference in overall dropout rates at the end of the intervention between groups (RR 0.82, 95% CI 0.45 to 1.51; participants = 238; Analysis 5.2).
5.2. Analysis.
Comparison 5 Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone, Outcome 2 Dropout due to any reason.
Secondary outcomes
7.3. Symptom severity of depression
The study did not report depression severity scales.
7.4. Health‐related quality of life
The study did not report health‐related quality of life.
7.5. Dropout due to any type of adverse events
The study did not report dropout due to any type of adverse event.
7.6. Any type of adverse event
The study did not report participants experiencing any type of adverse event.
7.7. Serious adverse events
The study did not report participants experiencing serious adverse events.
8. Comparison of different antidepressant medications (post hoc analyses)
Two continuation treatment studies (NRCTs) compared two different antidepressant medications (Kocsis 1995; Koran 2001). Although we did not predefine this comparison as a main comparison of interest in the study protocol, we opted to report these additional data.
8.1. Imipramine versus desipramine
One study provided data on the comparison of two different TCAs (imipramine and desipramine) (Kocsis 1995).
Primary outcomes
8.1.1. Relapse/recurrence rates of depression
The study did not report relapse/recurrence rates of depression.
8.1.2. Dropout due to any reason
Significantly more participants dropped out in the imipramine group (RR 4.35, 95% CI 1.19 to 15.87; participants = 73; Analysis 6.1). This translated to an NNTB of five, that is, five participants must have been treated with desipramine to maintain one additional participant in treatment.
6.1. Analysis.
Comparison 6 Imipramine (TCA) versus desipramine (TCA), Outcome 1 Dropout due to any reason.
Secondary outcomes
8.1.3. Symptom severity of depression
The study did not report depression severity scales.
8.1.4. Health‐related quality of life
The study did not report health‐related quality of life.
8.1.5. Dropout due to any type of adverse events
There were no significant differences in dropout rates due to adverse events between groups (OR 1.49, 95% CI 0.23 to 9.60; participants = 73; Analysis 6.2).
6.2. Analysis.
Comparison 6 Imipramine (TCA) versus desipramine (TCA), Outcome 2 Dropout due to any type of adverse event.
8.1.6. Any type of adverse event
The study did not report participants experiencing any type of adverse event.
8.1.7. Serious adverse events
The study did not report participants experiencing serious adverse events.
8.2. Imipramine versus sertraline
One study compared a TCA (imipramine) with an SSRI (sertraline) (Koran 2001).
Primary outcomes
8.2.1. Relapse/recurrence rates of depression
There were no significant differences in relapse/recurrence rates between groups (RR 1.27, 95% CI 0.84 to 1.91; participants = 376; Analysis 7.1).
7.1. Analysis.
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 1 Relapse/recurrence.
8.2.2. Dropout due to any reason
There were no significant differences in overall dropout rates between groups (RR 0.81, 95% CI 0.48 to 1.38; participants = 386; Analysis 7.2).
7.2. Analysis.
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 2 Dropout due to any reason.
Secondary outcomes
8.2.3. Symptom severity of depression
There was no significant difference in depression severity (measured with the HAM‐D) at the end of the intervention between groups (MD 0.40, 95% CI –0.97 to 1.77; participants = 377; Analysis 7.3).
7.3. Analysis.
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 3 Depression severity.
8.2.4. Health‐related quality of life
The degree of enjoyment and satisfaction in different areas of daily functioning (Q‐LES‐Q) at the end of the intervention was significantly higher in the sertraline group (MD –4.30, 95% CI –7.31 to –1.29; participants = 347; Analysis 7.4).
7.4. Analysis.
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 4 Health‐related quality of life.
8.2.5. Dropout due to any type of adverse events
There were no significant differences in dropout rates due to adverse events between groups (OR 1.99, 95% CI 0.60 to 6.65; participants = 386) (Analysis 7.5).
7.5. Analysis.
Comparison 7 Imipramine (TCA) versus sertraline (SSRI), Outcome 5 Dropout due to any type of adverse event.
8.2.6. Any type of adverse event
The study did not report participants experiencing any type of adverse event.
8.2.7. Serious adverse events
The study did not report participants experiencing serious adverse events.
Subgroup analyses
We were unable to perform any of the a priori defined subgroup or meta‐regression analyses due to limited number of studies.
Sensitivity analyses
We could perform sensitivity analyses only for one comparison (pharmacological continuation and maintenance therapies versus placebo) as all other comparisons did not provide enough data.
Excluding studies with a high or unclear risk of bias
For each risk of bias domain, we planned to exclude the studies with high or unclear risk to compare these results with the results of the analysis including all studies.
For the domains 'random sequence generation', 'allocation concealment', and 'selective reporting', none of the studies had a low risk of bias, thus these sensitivity analyses could not be performed.
For the domain 'blinding of participants and personnel', all of these studies had a low risk of bias.
For the domain 'blinding of outcome assessment', only Gelenberg 2003 had a low risk of bias. When including only Gelenberg 2003, the difference between medication and placebo for relapse or recurrence rate of depression did not reach significance (RR 0.76, 95% CI 0.47 to 1.23; P = 0.27), whereas for dropout due to any reason, there was a significant difference favouring medication (RR 0.48, 95% CI 0.24 to 0.94; P = 0.03). For depression severity, the difference between medication and placebo was not significant when including Gelenberg 2003 only (MD –2.10, 95% CI –5.08 to 0.88; P = 0.17). For dropout due to any type of adverse event, there was no significant difference between medication and placebo, which was consistent with the original results.
For the domain 'incomplete outcome data', we excluded Keller 1998b as it had a high risk of bias. There was no change for relapse or recurrence rate of depression or dropout due to any reason (except a lower rate of heterogeneity for dropout due to any reason). For severity of depression, the heterogeneity increased when excluding Keller 1998b. Medication was still superior to placebo but the differences between the two groups was not significant (MD –5.63, 95% CI –14.17 to 2.90; P = 0.20). For dropout due to any type of adverse event, the results did not change substantially when excluding Keller 1998b.
For the domain 'other potential sources of bias', the studies of Kocsis 1996 and Miller 2001 (comparing desipramine with placebo) with the partially overlapping subgroup had a low risk of bias. Including only Kocsis 1996, the difference between medication and placebo for relapse or recurrence rate of depression remained significant. For dropout due to any reason, the difference between medication and placebo was not significant, corresponding to the original results. For severity of depression and dropout due to any type of adverse event there were no data available for Kocsis 1996.
In summary, the sensitivity analyses could only focus on three of the seven risk of bias domains and on the two primary outcomes (relapse or recurrence rate of depression, dropout due to any reason) and on two secondary outcomes (severity of depression, dropout due to any type of adverse event).
Excluding trials without a randomization on a personal level or without (re)randomization before the continuation phase
We could not perform these analyses as all studies providing data on the comparison pharmacological continuation and maintenance therapies versus placebo were RCTs with randomization on a personal level.
Post hoc sensitivity analyses
As there were two partially overlapping groups included in our review regarding the comparison pharmacological continuation and maintenance therapies versus placebo (Kocsis 1996; Miller 2001), we decided to perform an a posteriori defined sensitivity analysis (including Miller 2001 instead of Kocsis 1996). Miller 2001 investigated the dysthymic subsample of Kocsis 1996 but also included additional people with dysthymia. Miller 2001 only provided data on relapse or recurrence rate of depression. The RR was slightly lower with a broader CI and a slightly higher heterogeneity when including Miller 2001 instead of Kocsis 1996 (RR 0.38, 95% CI 0.16 to 0.89; I² = 58%; Analysis 1.9). We considered these differences as clinically not meaningful.
1.9. Analysis.
Comparison 1 Pharmacological continuation and maintenance therapies versus placebo, Outcome 9 Relapse/recurrence sensitivity analysis.
Discussion
Summary of main results
This review was based on data from 10 studies, from which five studies investigated continuation treatments and five studies investigated maintenance treatments. All maintenance treatment studies and two continuation treatment studies applied an RCT design. The remaining three continuation treatment studies used an NRCT design. Five studies included comparisons of antidepressant medication versus tablet placebo. Only three studies involved psychological treatment. Two of these three studies investigated the effect of the Cognitive Behavioral Analysis System of Psychotherapy (CBASP) compared to antidepressant medication or combined treatment, or against assessment only, while the other study compared antidepressant medication to medication plus group therapy. We also analyzed data for a posteriori defined comparison, the direct comparison of two antidepressants. All 10 studies reported data at the end of the intervention, while only one study also reported follow‐up data 12 weeks after the end of the intervention.
Pharmacological continuation and maintenance therapies versus placebo
Five studies compared continuation or maintenance antidepressant medication with tablet placebo. The class of antidepressant medication varied between the included studies, SSRIs, MOIs, SNDRIs, and TCAs were used. For the analyses, we excluded one study (Miller 2001), due to an overlapping group of participants with the study of Kocsis 1996 and included four studies involving 383 participants in our analyses. There was moderate quality of evidence that participants taking antidepressant medication had significantly fewer relapses or recurrences and a lower depressive symptom severity score compared to participants taking placebo at the end of the intervention. The results did not change significantly when replacing Kocsis 1996 by Miller 2001. However, the sensitivity analysis showed that the difference between medication and placebo for relapse or recurrence rate of depression and depression severity was not significant when the only study (Gelenberg 2003) with low risk of bias for assessor outcome blinding was included.
Continuation/maintenance antidepressant medication reduced risk of relapse or recurrence rate of depression with an NNTB of six. Heterogeneity between studies was moderate. For the outcome relapse or recurrence rate of depression, three studies showed similar between‐group effect sizes, although these studies varied largely regarding treatment duration and sample size (Harrison 1986; Keller 1998b; Kocsis 1996). In comparison, in Gelenberg 2003, the effect favouring medication was smaller with a rather narrow CI, and as explained above, sensitivity analyses revealed that this effect was not significant when only including Gelenberg 2003. Miller 2001 showed a very strong effect favouring medication with a very large CI (due to small sample size). In Miller 2001, solely participants with dysthymia were treated over a period of 104 weeks, therefore the design and setting of this study is different from the other four studies. Besides, all studies used different antidepressants from varying classes, which could have contributed to the moderate degree of heterogeneity.
We found no significant differences between medication and placebo concerning the overall dropout rate. However, the quality of evidence for this result was rated as low. Heterogeneity was substantial (I² = 64%). In Harrison 1986, no participant in the placebo group dropped out, but they reported that all participants in the placebo group relapsed (therefore a dropout due to other reasons than relapse/recurrence was not possible by definition). In addition, two studies investigated rather small sample sizes, resulting in large CIs (Harrison 1986; Kocsis 1996).
Participants taking antidepressant medication had a significantly lower depressive symptom severity score compared to participants taking placebo at the end of the intervention (based on three RCTs). Two of these studies, both maintenance treatment studies, showed very similar results, both in means and SDs pre‐ and post‐treatment and generally, in design of the study (maintenance phase, lasting 52 weeks (Gelenberg 2003) or 76 weeks (Keller 1998b)). Treatment duration in the continuation treatment study of Harrison 1986 was 26 weeks, and participants in the medication arm reported lower and participants in the placebo group higher symptom severity scores compared to the corresponding groups in the other two studies. These factors might have contributed to considerable heterogeneity between the three studies.
Regarding relapse or recurrence rate of depression and severity of depression, the results are unconvincing whether or not continued or maintained (or both) pharmacotherapy was superior to tablet placebo in persistent depressed participants.
Three RCTs provided data on dropout due to any type of adverse event at the end of the intervention and low quality of evidence indicated no significant difference between medication and placebo (Gelenberg 2003; Harrison 1986; Keller 1998b). These three studies varied in sample size, dropout rates due to adverse events in general and between treatment arms, potentially contributing to the moderate degree of heterogeneity. In the continuation treatment study of Harrison 1986, no participant in the placebo group dropped out due to adverse events, but all of these participants relapsed (before), and this study had a small sample size and rather short treatment duration compared to the other two studies. The maintenance treatment study of Keller 1998b showed considerable differences in dropout rates between the groups, favouring placebo, and treatment lasted 76 weeks. In comparison to the other two studies, Gelenberg 2003 showed the smallest difference in dropout rates due to an adverse event between the medication and placebo group during the maintenance treatment phase. It was unclear if participants actually took the medication throughout the entire maintenance phase as no laboratory tests were reported (see Characteristics of included studies table). This could possibly lead to less reporting of adverse events.
One study provided quality of life measures, in which participants in the medication group benefited compared to placebo (Keller 1998b).
Psychological continuation and maintenance therapies versus attention placebo/non‐specific control
One maintenance treatment study with 52 weeks' duration, involving 82 participants, compared psychological continuation and maintenance therapies versus attention placebo/non‐specific control (Klein 2004). The study showed fewer relapse or recurrence rate of depression and a lower depression severity score in the CBASP group compared to the assessment‐only group at the end of the intervention. Maintained CBASP treatment reduced the risk of relapse or recurrence with an NNTB of five. Overall dropout rates were similar in both treatment arms. It might be assumed that maintained active psychotherapy has a positive effect on depression outcomes compared to assessment only.
Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies
Only one study, involving 179 participants, provided data on the active comparison of psychotherapy with medication during the continuation treatment phase (Kocsis 2003). Although participants receiving CBASP and participants taking nefazodone did not differ regarding relapse or recurrence of depression and overall dropout rate at the end of the intervention, there was a tendency favouring psychotherapeutic treatment observable regarding dropout.
Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone
Three studies compared combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone. One continuation treatment study, involving 238 participants, provided relapse or recurrence rates of depression and showed no statistically significant differences between the group taking nefazodone and the group receiving CBASP plus nefazodone (Kocsis 2003). Two studies, involving 280 participants, provided overall dropout rates, showing no statistically significant differences between medication alone and the combined treatment (Hellerstein 2001; Kocsis 2003). One study, involving 39 participants, reported a significant lower depression severity score for the combined group compared to medication alone at the end of the intervention (Hellerstein 2001). However, this effect did not remain at 12 weeks' follow‐up. This same study provided quality of life measures, and found no differences between groups at the end of the intervention and follow‐up.
Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone
One continuation treatment study, involving 238 participants, compared combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone. It found no significant differences for relapse or recurrence of depression and overall dropout rates between the CBASP group and the CBASP plus nefazodone group (Kocsis 2003).
Comparison of different antidepressant medications (post hoc analyses)
Two studies reported data on the direct comparison of two antidepressants. One continuation treatment study, involving 73 participants, compared two TCAs, where overall dropout rates were higher in the imipramine group compared to the desipramine group (Kocsis 1995). Compared to imipramine, desipramine reduced the dropout risk with a NNTB of five. Dropout rates due to any type of adverse events did not differ significantly between the two groups. The imipramine sample was relatively small (participants = 23) and only half the size of the desipramine sample. The three dropouts in the desipramine group dropped out due to dissatisfaction with treatment response or due to adverse effects. In the imipramine group, one participant discontinued because of adverse effects, one participant had a dispute with the staff, and four participants did not comply with the follow‐up assessment. Data on relapse or recurrence rates of depression were not provided in this comparison.
The second study, a continuation treatment study involving 386 participants, compared a TCA with an SSRI, showing no significant differences between groups regarding relapse or recurrence of depression, overall dropout rate, dropout rate due to any type of adverse events, and severity of depression severity at the end of the intervention (Koran 2001). This study provided quality of life measures, in which participants of the SSRI group reported a significantly higher quality of life at the end of the intervention.
Overall completeness and applicability of evidence
Only 10 studies were identified for inclusion in this review. Most of the studies retrieved in the search could not be included in the review as: they treated solely participants with recurrent depression (with clear interepisode remission and an episode duration shorter than two years); they did not clearly assess the percentage of diagnostic subgroups of persistent depressive disorder; or they reported no subgroup analyses. Thus, it can be assumed that there are more studies available involving people with persistent depression, but as no specific percentages were available from the publications or following contact with the authors, these data could not be considered for this review. Moreover, some long‐term studies had to be excluded because they did not define clear response or remission criteria for entering the next treatment phase which we required for inclusion in the review as response or remission are considered as accurate criteria of continuation or maintenance treatments (Frank 1991). Overall, this resulted in a rather small body of evidence available for addressing the objectives of this review.
Regarding the 10 included studies, for most comparisons only one or two studies provided data for the analyses, limiting the informative value of the presented results. For the comparison of antidepressant medication and placebo, five studies provided consistent data although they used different classes of medication. Only three of the included studies involved psychological treatments, two of them applied to individual participants (CBASP), the other applied as a group therapy, both conducted in an outpatient setting in the US. More studies are required to evaluate the different forms of psychotherapy (e.g. IPT, CBT, CBASP, MBCT, psychodynamic therapy) in varying treatment settings (individual, group), cultures, and healthcare systems. Moreover, just two studies investigated the combination of psychotherapy and antidepressant medication although guidelines already recommend combined treatment for persistent depressive disorders (DGPPN 2015). Also, we expected to include comparisons with TAU, investigating if long‐lasting continuation and maintenance treatments are implemented in healthcare systems and evaluating these treatments under natural conditions. Unfortunately, we identified no studies using this comparator.
From the 10 included studies, five were continuation studies and five were maintenance studies. Maintenance treatment studies varied largely regarding the duration of treatment (12 to 24 months), while the preceding continuation treatment studies were more similar in duration (16 to 20 weeks). Per definition, participants remitted or at least partially responded during acute treatment should start continuation treatment within one year after terminating acute treatment. Then, maintenance treatments should be given during recovery, which is defined as remission lasting longer than six months (Frank 1991; NICE 2010). Three studies providing a continuation treatment phase defined a duration of 16 to 20 weeks' treatment before participants entered a subsequent maintenance phase. This does not correspond to the recommended criterion of six months' recovery before entering maintenance treatment. Still, we decided to include these studies in the review and followed the definition of the authors as they described reasonable criteria for participants being eligible to enter the maintenance phase. We kept the term 'maintenance treatment' for these treatments as they were longer than the examined continuation treatments, consistently. Due to the small amount of included studies, we did not differentiate between effects of continuation versus maintenance treatments during the analyses, which would be valuable considering the distinct criteria of remission/recovery for both treatment phases. Particularly in persistent depressive disorder with participants showing severe levels and duration of symptoms, clear criteria for receiving both treatments following acute therapy are required. Therefore, a consistent application of the terms continuation and maintenance treatment and corresponding implementation into research and health care is needed before definite conclusions about the effectiveness of such treatments can be drawn.
For this review, the primary efficacy outcome was relapse or recurrence. Most of the studies applied rather strict criteria for participants to fulfil this outcome criterion at the end of the intervention (e.g. exceeding cut‐offs during two or three consecutive sessions followed by a personal interview with a study investigator clarifying a potential diagnosis). Such procedures contribute to keeping participants longer in the study programme and possibly underestimate absolute relapse and recurrence rates. Different definitions of this outcome between the included studies also prevent a comparison of absolute relapse/recurrence rates between the examined treatments. Additionally, it must be kept in mind that two different target figures were mixed here: relapse defined the return of symptoms before full remission was reached, while recurrence indicated a new episode after a full remission had been reached.
The primary acceptance outcome was dropout due to any reason (other than relapse/recurrence), of which nine of the 10 studies reported data. Specific reasons for dropout and specific adverse events were rarely described. Instead, general statements about adverse events were reported (e.g. that adverse effects evolved in at least 10% of participants). But, next to adverse effects, other negative events such as interpersonal problems (e.g. conflicts with others) might occur during or after treatment. Such adverse events were reported very rarely in the included studies of this review, but should be addressed clearly in future research (Meister 2016). Especially in long‐term treatments such as continuation and maintenance treatments, dropout is considered likely and should be assessed in more detail (e.g. if participants dropped out due to aspects of the intervention itself or due to other reasons).
Another secondary outcome of interest of this review were quality of life measures. Only three of the 10 studies addressed this outcome although psychotherapeutic and pharmacological treatments are considered to improve quality of life in depressive disorders (Kamenov 2017). As persistent depressive disorders are characterized by a chronic course, an exclusive focus on improvement of depressive symptomatology over a long time might be too narrow to describe health status of participants completely. Including quality of life measures more frequently into studies is recommended.
Moreover, we intended to analyse data at the time point 'one year after the end of the intervention'. Surprisingly, just one of the 10 studies provided follow‐up data, and even this study addressed a short follow‐up duration (12 weeks). Particularly in persistent depressive disorder, we consider the evaluation of long‐term effects beyond termination of treatment as highly relevant and valuable information to provide recommendations on when therapy should be extended, changed, or terminated.
PDD is a rather severe form of depression with people probably experiencing comorbid disorders including personality disorders, suicidality or psychotic symptoms, and usually undergoing several other treatments before entering a study, which in turn was an exclusion criterion in some of the included studies for this review. This raises the question about what type of participants joined the studies we included in this review – severely impaired or less impaired people? Although these previously mentioned exclusion criteria were defined (e.g. other mental disorders as main diagnoses), a high percentage of included participants experienced comorbid conditions (e.g. in Keller 1998b and Kocsis 1996 half of the sample had an axis‐II‐diagnosis). Additionally, anxiety disorders and substance abuse was highly prevalent in samples reporting comorbid conditions. Further, as lifetime duration of depression ranged from 15 to 24 years, we can assume that – despite the exclusion criteria – severely impaired participants were included in our analyses with illness duration and comorbidity rates comparable to those known from previous research (Gilmer 2005; Kocsis 2008). However, not all studies reported on comorbidity and chronicity, so this information was not available for the whole sample.
Six studies had failing previous trials of antidepressant medication as an exclusion criterion (i.e. studies excluded so‐called treatment‐resistant participants). But, as response is a requirement for continuation and maintenance treatment, results of those studies are not applicable to treatment‐resistance anyway.
Experience with prior treatment is common in this severely impaired group and was not an exclusion criterion in the analyzed studies – especially the experience with psychotherapy was high (63% to 85%) in the five studies providing data on this issue.
Another aspect regarding the applicability of evidence is date of publication. Studies included in this review were published between 1986 and 2004. The current practice including available medication and psychotherapy for treating people with persistent forms of depression might have been different at that time. For example, nefazodone, which was used in two of the included studies (Gelenberg 2003; Kocsis 2003), was withdrawn from the market in 2003 (Kocsis 2003) and 2004 (Gelenberg 2003) in some countries due to the rare incidence of hepatoxicity (Cosgrove‐Mather 2004).
Quality of the evidence
For most of the planned comparisons only one or two studies provided data. Thus, we reported a 'Summary of findings' table only for the comparison of pharmacological continuation and maintenance therapies versus placebo, referring to the quality of evidence of the primary outcomes relapse/recurrence and overall dropout.
Limitations in study design or execution (risk of bias)
We included seven RCTs and three NRCTs involving 840 participants with persistent depressive disorder. Two of the three NRCTs were evaluated as having almost no risk of bias in the seven domains, while the other study was classified between moderate and serious risk for more than half of the domains. The seven included RCTs varied regarding risk of bias domains, and were rated mostly as low or unclear risk of bias, with one exception. One study was at high risk of bias in four domains and unclear risk of bias in three domains (Hellerstein 2001). In general, none of the studies addressed allocation concealment and random sequence generation and, therefore, were at unclear risk, and selective reporting at unclear risk in five of these seven studies. One study with high risk of bias in the domain selective reporting analyzed data on dropout during the 104‐week maintenance phase, but only the rates during the first month of treatment, probably underestimating the actual dropout rate over time (Kocsis 1996).
To separate the two primary outcomes (relapse/recurrence and overall dropout), we did not include the participants with relapse/recurrence in the overall dropout rates. This must be kept in mind when interpreting the absolute dropout rates. Additionally, as in one study, all participants in the placebo group relapsed and there was no information on the time of relapse, the dropout rate in this group was zero, possibly overestimating the acceptance of this treatment. If the participants relapsed early, there was 'no chance' for accepting or not accepting the treatment. Two studies reported incomplete outcome data at high risk (Hellerstein 2001; Keller 1998b), as they applied the LOCF method for imputing missing values. In Keller 1998b, 70% of the participants in the placebo group dropped out during the 76‐week maintenance treatment, thus the missing data were replaced by the last available measure of the participant. Although this method is commonly used for analyzing longitudinal data, we consider this procedure inappropriate within the context of continuation/maintenance treatments as it potentially underestimates relapse and recurrence rates. As LOCF assumes that the missing data after the participant's dropout stay the same as the last value observed for that participant (Shih 2002), we assumed that LOCF provided rather optimistic estimates instead of conservative estimates. This assumption of stability is rather unlikely for persistent depressive disorder over long periods of time considering the high likelihood of recurrences reported in previous research.
Analyses regarding dropout rates for the comparison of antidepressant medication versus placebo resulted in serious heterogeneity between studies. Due to the small number of studies, we were unable to analyse these differences statistically. These circumstances in combination with the mentioned limitations of the studies contributed to downgrading the body of evidence for this comparison regarding the outcome dropout (other than relapse/recurrence) to low (see Table 1).
Inconsistency of results
Data were inconsistent in regard to overall dropout rates. Dropout rates varied between studies from 4% (Kocsis 1996) and 100% (Harrison 1986). This unexplained heterogeneity contributed to downgrading the quality of evidence to low regarding overall dropout rates in the comparison of antidepressant medication versus placebo.
Indirectness of evidence
All included studies directly addressed the objective of this review, namely the comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder.
Imprecision of results
Two of four studies addressing overall dropout rates in the comparison of antidepressant medication versus placebo showed wide CIs (Harrison 1986; Kocsis 1996). This contributed to downgrading the quality of evidence to low.
Publication bias
Due to the small number of included studies, we applied funnel plot only for the comparison antidepressant medication versus tablet placebo. Apparently, the funnel plot on relapse/recurrence was asymmetrical, with an overhang of small studies showing a large difference in favour of medication), while the funnel plot on dropout was symmetrical. The application of statistical tests (e.g. Egger's test) for funnel plot asymmetry was not conducted, as it is not advisable due to the small number of studies. The Cochrane Handbook for Systematic Reviews of Interventions recommends these tests when there are at least 10 studies, otherwise the power is too low (Higgins 2011).
Potential biases in the review process
We used a broad search strategy for identifying all relevant studies regarding continuation and maintenance treatments in PDD. The main search was conducted in the specialized register of RCTs of the Cochrane Common Mental Disorders Group (CCMD‐CTR) searching for study and reference records. Moreover, we searched other databases, grey literature, clinical trials registers, and contacted relevant authors in this field. We had to exclude a considerable number of studies as most either did not include specifically participants with PDD or did not report the percentage of treated PDD participants. Although we were in email contact with the first authors of the included papers regarding this missing information, they either did not reply to our queries or simply had specific data no longer available due to conducting these studies several years ago. Therefore, we assumed that more studies than those included in this review were conducted with participants with PDD.
At least two review authors independently screened records, and extracted and analyzed data to prevent any severe bias in the used methods.
There was marked clinical heterogeneity between the included studies, especially regarding sample size, subtype of persistent depression, type of treatment (e.g. type of antidepressant medication), treatment duration, and definition of relevant outcomes. Therefore, we are not reporting on a homogeneous group of participants, manifesting in both studies investigating a specific subgroup (e.g. solely people with dysthymia) and studies investigating all four diagnostic subgroups of PDD, making it difficult to generalize the results to PDD in general. This also applied to the type of treatment: studies included continuation and maintenance treatment with lengths between 16 weeks and two years, which allows no conclusion on continuation or maintenance treatment in general.
We required the included studies to report on ICD or DSM diagnosis of PDD, neglecting total duration of illness or other potential indicators of impairment. But, as we required clear response or remission criteria for considering continuation or maintenance treatment studies for this review, symptom level of included participants was considered rather homogeneous. The pharmaceutical industry funded some of the included studies with grants. Conducting long‐term continuation and maintenance studies requires considerable financial support, potentially leading to bias of the found results if covered by third parties. However, we considered risk of publication bias as low but the application of a statistical test was not advisable due to the small number of studies. Additionally, the required length of treatment – especially of maintenance studies – is possibly one reason for the lack of studies in this field.
Another aspect of potential bias was the allowance or prescribing of concomitant treatments in addition to the treatment provided by the study. Two of the included studies investigating antidepressant medication reported that participants received ongoing psychotherapy. One study reported that 60% of the participants received ongoing psychotherapy while treated with sertraline or imipramine, yet there were no group differences (Koran 2001). In two studies, about 40% of the participants of both treatment groups (desipramine, placebo) were on stable long‐term psychotherapy regimens (Kocsis 1996; Miller 2001). Although parallel treatment is not necessarily considered to bias the results, especially when proportions are similar between treatment groups, the observed individual change is not solely based on study treatment but also on parallel treatment in the respective two studies. Additionally, interaction effects may occur: 1. the effect of antidepressant medication could be underestimated because the received psychotherapy makes an additional treatment (antidepressant medication) less meaningful or 2. receiving parallel psychotherapy intensifies the effect of medication or keeps the participant motivated to stay on medication. However, the other studies did not mention any information about parallel treatments or explicitly stated that no concomitant treatment was allowed.
Despite the discussed conceptual and methodological concerns, we considered conducting a meta‐analysis appropriate, especially for the comparison of antidepressant medication versus tablet placebo. However, sensitivity analyses addressed differences in risk of bias between studies, and found no significant differences between medication and placebo regarding the primary outcome rate of relapse/recurrence when only studies with low risk of bias were included. Heterogeneity was planned to be addressed through subgroup and meta‐regression analyses but, due to the low number of studies included, we were only able to discuss differences between studies on a descriptive level. In general, for the majority of comparisons we had to describe the results based on one single study, not being able to report pooled results.
This review used two primary outcomes, rate of relapse/recurrence and rate of overall dropout due to other reasons than relapse/recurrence. Most of the studies reported on both outcomes separately. Still, there is open discussion if both outcomes overlap conceptually in continuation and maintenance treatment studies. For example, in Harrison 1986, all participants of the placebo group relapsed, resulting in zero dropouts reported during treatment as participants simply had "no chance" for dropping out due to relapsing before, possibly overestimating the acceptance of placebo treatment.
Due to the low number of included studies, we were unable to undertake all planned subgroup and sensitivity analyses. However, in future versions of this review, differences between subgroups should be tested formally (Bucher 1997; Deeks 2008; Song 2003), and all meta‐regression analyses should be performed using the restricted maximum likelihood estimate method, a recommended random‐effects approach that accounts for residual between‐trial heterogeneity (Thompson 1999).
Continuation and maintenance treatment studies are usually performed as complex studies, that is, studies with different treatment phases (acute, continuation, and maintenance treatment). Criteria and procedures of transition from one into the next phase differed between studies: some studies defined response, some remission as an eligibility criterion for the next phase, including different definitions of response and remission (see above). Some studies did not rerandomize participants when entering the new phase (especially concerning the transition from acute to continuation treatment). Other studies rerandomized responders from one treatment arm to a different treatment arm in the next phase, for example Klein 2004 randomized continuation phase responders of CBASP to CBASP or assessment only in the maintenance phase. These different procedures complicate the comparability between different studies. Moreover, studies mostly described different treatment phases, resulting in partly overlapping patient groups included in different analyses.
As persistent depression is a chronic condition, relapses or recurrences are common in this population. Longer treatment durations (i.e. longer observation periods) probably increase the chance to observe relapses or recurrences, this must be kept in mind when comparing the results of different studies with varying treatment durations.
Concerning data analysis, there is no widely accepted consensus on how to deal with missing data in meta‐analysis when primary data are not available. In acute‐phase studies, researchers can replace missing values with methods such as LOCF, if study authors do not report adequate ITT analyses. However, in the case of continuation and maintenance studies, we assume that LOCF would produce rather optimistic instead of conservative estimates (see above) due to its concept of stability of measures over time (Shih 2002). Thus, we had to deal with the available data sets. However, percentages of missing data were low: data on overall dropout rates were complete, the percentage of missing data concerning other outcomes ranged between 0% and 10%.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first review evaluating the effectiveness of continuation and maintenance treatments in people with PDD.
A previous review investigated the efficacy and acceptability of acute treatments in PDD by applying network meta‐analytic methods, showing that several antidepressant medications were superior to placebo, and that several evidence‐based treatments exist (Kriston 2014). Due to the small number of included studies in our review, comparisons between different antidepressants could not systematically be investigated.
In line with the review of von Wolff 2012 on acute treatment in persistent depression, our review could also not provide a clear superiority of combined treatments compared to pharmacological monotherapy.
Regarding continuation and maintenance studies, Wilkinson 2016 evaluated treatments for older people with depressive disorders. This updated Cochrane Review identified seven studies of which six compared continued or maintained antidepressant medication with placebo, favouring antidepressants regarding relapse/recurrence at 12 months, but showing no significant differences between treatment arms at six or 24 months' follow‐up. Although this result is in line with our result for this comparison and outcome, Wilkinson downgraded the level of evidence to low (GRADE) compared to the GRADE rating of moderate level evidence in our review. Like in our review, Wilkinson 2016 included just two studies involving psychological treatment, and data reported on this as well as combined treatments were too few to draw conclusions.
Regarding long‐term effects of psychological treatments, Vittengl 2007 conducted a meta‐analysis on effects of acute and continued cognitive therapies CTs in depression. They found high relapse and recurrence rates (29% within one year and 54% within two years) for participants discontinuing after acute CT. Those participants continuing CT had significantly fewer relapses and recurrences compared to active controls (e.g. receiving pharmacotherapy) at follow‐up (10 to 255 weeks after end of continuation‐phase treatment), with relapse/recurrence rates of 42% (continued CT) and 61% (active controls) over 114 weeks on average. In comparison, rates of relapse/recurrence were similar at end of (20 to 52 weeks') continuation phase for both CT and other active treatments, with relapse/recurrence rates of 10% (continued CT) and 22% (active controls) over 27 weeks on average. Although the results of Vittengl 2007 are encouraging to consider continued pharmacotherapy or psychotherapy (or both) in depression treatment, the analyses were based on only five to eight studies.
Guidi 2016 conducted a meta‐analysis on the sequential integration of pharmacotherapy and psychotherapy in major depressive disorder, that is participants received pharmacotherapy in the acute phase and psychotherapy in the residual phase. Receiving cognitive‐behavioural therapy (CBT) during continuation of antidepressant drugs was superior to antidepressants alone or TAU. Further, participants receiving CBT who had medication tapered and discontinued were significantly less likely to relapse compared to clinical management or continued pharmacotherapy. These analyses were based on 13 studies.
Authors' conclusions
Implications for practice.
The comparison of antidepressant medication versus placebo showed coherent results based on five studies favouring pharmacotherapy as an effective continuation and maintenance treatment for participants with persistent depressive disorder (PDD) compared to tablet placebo regarding relapse/recurrence. However, the quality of evidence was rated moderate and sensitivity analyses showed that the primary outcome (rate of relapse/recurrence) did not reach significance when only including studies with low risk of bias.
On this basis, it cannot be concluded with certainty that continued or maintained pharmacotherapy (or both) with the reviewed antidepressant agents is a robust treatment for preventing relapse and recurrence in people with PDD. As long‐term follow‐up data were not available in most of the studies, this review cannot draw any conclusions about an appropriate duration of antidepressant medication intake, or when to taper off or stop medication. Studies in this review predominantly used tapering down of medication following an a priori‐defined scheme, and this is the likely approach in clinical practice.
Moreover, in two of the five studies reporting a benefit from antidepressant medication compared to placebo, about half of the sample size had ongoing psychotherapy in addition to study treatment. Thus, it is unclear if the individual course of the analyzed participants is only attributable to the medication provided by the study, or also to the parallel psychotherapy treatment. This might be an indication that people with PDD prefer a combined treatment in clinical practice anyway.
For all other planned comparisons the body of evidence with mostly just one or two studies providing too little data to draw final conclusions about recommendations for other types of treatment.
For the type of antidepressant medication as well as distinct treatment options for specific patient populations (e.g. subtype of persistent depression), the reported data of the included studies were too few to draw final conclusions or recommendations. Even concerning the comparison of antidepressants versus placebo, meta‐regression or subgroup analyses were not possible due to the small number of eligible studies. Also, all included studies were conducted in the US and were published between 1986 and 2004. Thus, differences between cultures and healthcare systems, as well as current developments regarding recommendations of clinical guidelines are not covered by the studies selected for this review. Conclusions and recommendations of this review should be interpreted on this background.
Implications for research.
The lack of studies on continuation and maintenance treatments in people with persistent depression emphasizes the need for further primary studies – especially on psychological and combined treatments. The results of Vittengl 2007 suggest that long‐term psychotherapy is effective in depression in general, emphasizing the need for verification the transferability of these results for the population of people with persistent depression. Moreover, studies should also focus on treating older adults who also experience persistent forms of depression, and who might respond differently (Wilkinson 2016).
Further studies should assess health‐related quality of life as well as adverse events. Lack of reporting (consistently) on adverse events is also a common problem in studies on acute treatment of PDD, especially in psychotherapeutic studies (Meister 2016). Generally, psychotherapeutic studies fail to report on adverse events (Lilienfeld 2007; Nutt 2008). Additionally, further studies should address follow‐up evaluations. Comparing continuing treatment to stopping treatment in the long run is necessary to draw conclusions on the recommendable duration of continuation and maintenance treatments. Moreover, cost‐effectiveness analyses are important to compare the relative costs and effects of different treatments, which we consider especially relevant in the context of long‐term treatments.
People with persistent forms of depression are likely to have a long duration of illness including a complex treatment history. Although it might be difficult to assess those aspects in detail in retrospect, it seems highly relevant to take previous treatments and their effects into account when organizing further treatments. In this regard, there might be a conceptual overlap between participants who are treatment resistant and participants showing persistent forms of depression. Future research should try to differentiate what type of treatments are effective dependent on previous treatments, especially in participants with rather long treatment histories. This also includes investigating the impact of comorbidities and their treatments as persistent forms of depression are associated with increased comorbidity (Arnow 2003; Gilmer 2005).
For this review, we decided to only include studies reporting on clear response criteria between treatment phases, that is, only responders were allowed to continue treatment as this reflects the current definition of continuation and maintenance treatments (Frank 1991; NICE 2010). As a consequence, we had to exclude some well‐performed studies evaluating (acute) long‐term treatments not following these strict definitions. However, those long‐term studies investigated treatments that changed regarding frequency and length over time, similar to studies we defined as continuation and maintenance treatments. This probably reflects clinical practice as it is likely that there is often no clear distinction between long‐term acute treatment on the one side and continuation and maintenance treatment on the other side. Nevertheless, for conducting research and ensuring the comparability of study results, we recommend standardized procedures. First, assessing and reporting response, remission, and recovery data at the end of the intervention (or the intervention phase). Second, defining whether or not participants receive further treatment and the reasons for it. Third, defining the frequency, duration, and contents of each treatment phase, and whether or not those aspects change between treatment phases.
As there is broad evidence on depression treatments on the one hand and a lack of studies fulfilling the inclusion criteria of this review on the other hand, clear diagnostic procedures as well as clear reporting concerning the persistence of depressive symptoms is necessary. It is reasonable that several excluded studies also examined participants with PDD, which could have been analyzed here if data on this subgroup were reported. The lack of reporting on this specific diagnosis reflects the fact that chronic major depression and recurrent depression without full interepisode remission may be designated as "(recurrent) major depression" in Diagnostic and Statistical Manual of Mental Disorders – 4th Edition (DSM‐IV) and International Classification of Diseases 10th Revision (ICD‐10), ignoring the persistence of depressive symptoms. However, the new category "persistent depressive disorder" implemented in Diagnostic and Statistical Manual of Mental Disorders – 5th Edition (DSM‐5) (duration of at least two years) increases the likelihood of a precise diagnosis concerning persistent symptoms.
History
Protocol first published: Issue 11, 2017 Review first published: Issue 5, 2019
| Date | Event | Description |
|---|---|---|
| 8 February 2019 | Feedback has been incorporated | feedback of sign‐off editor had been incorporated |
Acknowledgements
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service, or the Department of Health and Social Care.
Appendices
Appendix 1. CCMD‐CTR
Description of the Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR)
The Cochrane Common Mental Disorders Group maintains a specialized register of randomized controlled trials (RCT) the CCMD‐CTR. This register contains over 39,000 reference records (reports of RCTs) for depression, anxiety, and other common mental disorders. A percentage of the reference records have been tagged to 12,500 individual, PICO coded study records (with coding based on the EU‐Psi coding manual). Reports of trials for inclusion in the register are collated from (weekly), generic searches of MEDLINE, Embase, and PsycINFO, quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies can be found on the Group's website, with an example of the core MEDLINE search displayed in below.
In 2016 the Group's Specialized Register (CCMD‐CTR) became out of date with the Editorial Group's move from Bristol to York.
The search strategy listed below is the weekly Ovid MEDLINE search which was used to inform the Group's specialized register (to June 2016). It is based on a list of terms for all conditions within the scope of the Cochrane Common Mental Disorders Group plus a sensitive RCT filter.
1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record.
Appendix 2. Other database searches
Ovid PsycINFO (all years to 11 December 2015).
Initial PsycINFO search used to scope the literature and balance the sensitivity/specificity of the other database searches.
[Condition] 1. (chronic* depress*).ti,ab,id. 2. (double depress*).ti,ab,id. 3. DYSTHYMIC DISORDER/ 4. MAJOR DEPRESSION/ and ("CHRONICITY (Disorders)"/ or CHRONIC ILLNESS/) 5. (dysthymi*).ti,ab,id. 6. RECURRENT DEPRESSION/ 7. (depress* adj2 recurr*).ti,ab,id. 8. persistent depressive disorder.ti,ab,id. 9. or/1‐8 [Maintenance] 10. MAINTENANCE THERAPY/ 11. (maintenance or maintained).ti,ab,id. 12. continuation.ti,ab,id. 13. (stable or stabilise*1).ab. 14. RELAPSE PREVENTION/ 15. "RELAPSE (Disorders)"/ 16. or/10‐15 [Controlled Trials Filter] 17. exp EXPERIMENTAL DESIGN/ 18. TREATMENT EFFECTIVENESS EVALUATION/ 19. MENTAL HEALTH PROGRAM EVALUATION/ 20. (empirical study or longitudinal study or prospective study or quantitative study).md. 21. "2000".md. [treatment outcome/clinical study] 22. RETROSPECTIVE STUDIES/ 23. EVIDENCE BASED PRACTICE/ 24. (study or trial or treatment* or intervention or therap* or psychotherap*).ti. 25. (control* adj3 (group*1 or study or trial)).ti,ab,id. 26. (waitlist* or wait list* or treatment* as usual or TAU or care as usual or standard care or standard treatment*).ti,ab,id. 27. placebo.ti,ab,id. 28. PLACEBO/ 29. (RCT or random*).ti,ab,id. 30. (crossover* or cross over*).ti,ab,id. 31. (quasi experimental).ti,ab,id. 32. (longitudinal or cohort).ti,ab,id. 33. (case adj (control or report or series)).ti,ab,id. 34. (cross‐sectional).ti,ab,id. 35. (experimental or quantitative or pilot).ti,ab,id. 36. or/17‐35 37. (9 and 16 and 36) [Psychotherapies] 38. exp PSYCHOTHERAPY/ 39. exp PSYCHOTHERAPEUTIC TECHNIQUES/ 40. exp COGNITIVE TECHNIQUES/ 41. exp COUNSELING/ 42. 3300.cc. [Classification Code: Health & Mental Health Treatment & Prevention] 43. 3310.cc. [Classification Code: Psychotherapy & Psychotherapeutic Counseling] 44. 3311.cc. [Classification Code: Cognitive Therapies] 45. 3312.cc. [Classification Code: Behavior Therapy & Behavior Modification] 46. 3313.cc. [Classification Code: Group & Family Therapy] 47. 3314.cc. [Classification Code: Interpersonal & Client Centered & Humanistic Therapy] 48. 3315.cc. [Classification Code: Psychoanalytic Therapy] 49. (CBT or c‐CBT or iCBT or coping skills or counsel?ing or mindfulness or psychoanal* or psychotherap* or rehabilitat*).ti,ab,id. 50. ((psychologic* or psychodynamic or behavio?r or cognitive) adj3 (intervent* or therap* or treat* or manag*)).ti,ab,id. 51. (Abreaction or Acting Out or Adlerian or Adolescent Psychotherap* or Age Regression or Analytical Psychotherap* or Anger Control or Anger Management or Art Therap* or Assertive* Training or Autogenic Training or Autosuggestion or Aversion Therap* or Balint Group or Behavio?r Contracting or Behavio?r Modification or Behavio?r Therap* or Bibliotherap* or Biofeedback or Body Psychotherap* or Brief Psychotherap* or Caregiver Support or Child Psychotherap* or Client Cent* Therapy or Cognitive Behavio?r Therap* or Cognitive Behavio?ral Stress Management or Cognitive Rehabilitation or Cognitive Restructuring or Cognitive Therap* or Colo?r Therap* or Conjoint Therap* or Contingency Management or Conversion Therap* or Conversational Therap* or Countertransference or Couples Therap* or Covert Sensitization or Crisis Intervention).ti,ab,id,de. 52. (Dance Therap* or Dialectical Behavio?r Therap* or (Dream* adj3 Analys*) or Eclectic Psychotherap* or Eclectic Therap* or Emotion* Focus* Therap* or Emotional Freedom Technique or Encounter Group Therap* or Existential Therap* or Experiential Psychotherap* or Exposure Therap* or Expressive Psychotherap* or Eye Movement Desensiti#ation or Family Therap* or Free Association or Geriatric Psychotherap* or Gestalt Therap* or Griefwork or Group Psychotherap* or Group Therap* or Guided Image* or Holistic Psychotherap* or Humanistic Psychotherap* or Hypnosis or Hypnotherapy or Hypnoti#zability or Implosive Therap* or Individual Psychotherap* or Insight Therap* or Integrative Psychotherap* or Integrative Therap* or Interpersonal Psychotherap*).ti,ab,id,de. 53. (Logotherap* or Marathon Group Therap* or Marital Therap* or Meditation or Mental Healing or Metacognitive Therap* or Milieu Therap* or Mind train* or Morita Therap* or Music Therap* or Narrative Therap* or Nondirective Therap* or Personal Construct Therap* or Person Cent* Therap* or Persuasion Therap* or Pet Therap* or Play Therap* or Primal Therap* or Problem Solving Therap* or Psychoanalysis or Psychoanalytic Therap* or Psychodrama or Psychodynamic Psychotherapy or Psychotherapeutic Counsel* or Psychotherapeutic Processes or Psychotherapeutic Training or (Psychotherap* adj3 Rational‐Emotive)) .ti,ab,id,de. 54. (Rational Emotive Behavio?r Therap* or Reality Therap* or Reciprocal Inhibition Therap* or Relationship Therap* or Relaxation Stress Management or Relaxation Technique* or Relaxation Therap* or Relaxation Training or Reminiscence Therap* or Role Playing or Self Analys* or Self Esteem Building or Sensitivity Training Group* or Sex Therap* or Sleep Phase Chronotherap* or Socioenvironmental Therap* or Sociotherap* or Solution Focused Therap* or Support Group* or (Support adj3 Psycho*) or Systematic Desensiti#ation or Therapeutic Communit* or Transactional Analysis or Validation Therap*).ti,ab,id,de. 55. or/38‐54 [Antidepressants] 56. PSYCHOPHARMACOLOGY/ or NEUROPSYCHOPHARMACOLOGY/ 57. 3340.cc. [Classification Code: Clinical Psychopharmacology] 58. exp ANTIDEPRESSANT DRUGS/ 59. NEUROTRANSMITTER UPTAKE INHIBITORS/ or exp SEROTONIN NOREPINEPHERINE REUPTAKE INHIBITORS/ or exp SEROTONIN REUPTAKE INHIBITORS/ 60. exp MONOAMINE OXIDASE INHIBITORS/ 61. exp TRICYCLIC ANTIDEPRESSANT DRUGS/ 62. (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic*).ti,ab,id,de. 63. (Agomelatine or Alnespirone or Amoxapine or Amfebutamone or Amiflamine or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or (Atomoxetine or Tomoxetine) or Benactyzine or Brofaromine or Bupropion or Butriptylin* or Cianopramine or Cilobamine or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clorimipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Dapoxetine or Deanol or Dibenzepin* or Demexiptilin* or Deprenyl or Desipramine or Desvenlafaxine or Dibenzepin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Duloxetine or DVS‐233 or Enilospirone or Eptapirone or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluparoxan or Furazolidone or Fluvoxamine or Harmaline or Harmine or Hyperforin or Hypericum or John* Wort or Idazoxan or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Imipraminoxide or Isocarboxazid* or Lesopitron or Levomilnacipran or Lithium or Lofepramin* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or LY2216684 or Maprotiline or Medifoxamine or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptyline or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezin* or Pirandamine or Piribedil or Pirlindole or Pivagabine or Pizotyline or Propizepine or (Protriptylin* or Pertofrane) or Quinupramine or Quipazine or Reboxetine or Ritanserin or Rolipram or Scopolamine or Selegiline or Sertraline or (Setiptiline or Teciptiline) or Tandospirone or Tetrindole or Thiazesim or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromine or Trazodone or Trimipramine or 5‐Hydroxytryptophan or 5‐HT or Tryptophan or Hydroxytryptophan or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone or Zimeldine or (Alaproclate or Caroxazone or Diclofensine or Fenfluramine)) .ti,ab,id,de. 64. or/56‐63 [Mood Stabilisers or Antipsychotics] 65. MOOD STABILIZERS/ 66. exp ANTICONVULSIVE DRUGS/ 67. exp NEUROLEPTIC DRUGS/ 68. ((mood stabili?er*1 or lithium or eslicarbazepine or licarbazepine or valnoctamide or carbamazepine or valproate or valproic acid or divalpro* or ziprasidone or gabapentin or lamotrigine or topiramate) or (antipsychotic*1 or amisulpride or aripiprazole or asenapine or cariprazine or clozapine or haloperidol or iloperidone or lurasidone or olanzapine or quetiapin* or paliperidone or prosulpride or risperidone)).ti,ab,id,de. 69. or/65‐68 70. (9 and (55 or 64 or 69) and 36) 71. 37 or 68
Validated Ovid cross‐search‐1 (all years to 4 October 2016 and updated 28 Sepetmber 2018) PsycINFO 1987 ‐, Embase 1974 ‐, MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 ‐
1. MAINTENANCE THERAPY/ 2. (continuation or maintenance).ti. 3. ((continuation or maintenance) adj2 (efficacy or effectiveness or medicat* or pharmacotherap* or phase or study or therap* or psychotherap* or treatment*)).ti,ab,id,kf,kw. 4. ((continu* or maint*) adj (medicat* or pharmacotherap* or therap* or psychotherap* or treatment*)).ti,ab,id,kf,kw. 5. or/1‐4 6. chronic* depress*.ti,ab,id,kf,kw. 7. doubl* depress*.ti,ab,id,kf,kw. 8. DYSTHYMIC DISORDER/ or DYSTHYMIA/ 9. dysthymi*.ti,ab,id,kf,kw. 10. MAJOR DEPRESSION/ or DEPRESSIVE DISORDER/ or DEPRESSIVE DISORDER, MAJOR/ 11. "CHRONICITY (DISORDERS)"/ or CHRONIC ILLNESS/ or CHRONIC DISEASE/ or RECURRENCE/ or RECURRENT DISEASE/ or RECURRENCE RISK/ or REMISSION/ 12. (10 and 11) 13. RECURRENT DEPRESSION/ 14. (depress* adj2 recurr*).ti,ab,id,kf,kw. 15. persistent depressive disorder.ti,ab,id,kf,kw. 16. (6 or 7 or 8 or 9 or 12 or 13 or 14 or 15) 17. (5 and 16)
[Key: ti = title; ab = abstract; kf = author keyword MEDLINE; kw = author keyword Embase; id = key concepts PsycINFO]
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 9, 2016 and Issue 9, 2018 #1 (continuation or maintenance):ti #2 ((continuation or maintenance) near/3 (efficacy or effectiveness or medicat* or pharmacotherap* or phase or study or therap* or psychotherap* or treatment*)) #3 ((continu* or maint*) next (medicat* or pharmacotherap* or therap* or psychotherap* or treatment*)) #4 (#1 or #2 or #3) #5 "chronic* depress*" #6 "doubl* depress*" #7 MeSH descriptor: [DYSTHYMIC DISORDER] this term only #8 dysthymi* #9 MeSH descriptor: [DEPRESSIVE DISORDER] this term only #10 MeSH descriptor: [DEPRESSIVE DISORDER, MAJOR] this term only #11 MeSH descriptor: [CHRONIC DISEASE] explode all trees #12 MeSH descriptor: [RECURRENCER] this term only #13 (#9 or #10) and (#11 or #12) #14 (depress* near/3 recurr*) #15 (persistent next depress*) #16 (#5 or #6 or #7 or #8 or #13 or #14 or #15) #17 (#4 and #16)
The 2016 search identified 929 new references.
Update search (28 September 2018)
The following search string was appended to the update search of both Ovid and the Cochrane Library Trials database (all years to date) (with appropriate syntax amendments for CENTRAL): ((longterm or long term or continu* or maintain*) and ((prevent* or probability or reduc* or time to or decreas* or risk?) adj2 recurrence)).ti,ab,id,kf,kw. AND ((dysthymi*.ti. or (depress*.ti. and (chronic or persist*).mp.) or double depression.ti,ab,id,kf,kw.))
The 2018 search identified 141 new references.
Data and analyses
Comparison 1. Pharmacological continuation and maintenance therapies versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse/recurrence | 4 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 2 Dropout due to any reason | 4 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.39, 2.11] |
| 3 Depression severity | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐8.49, ‐1.09] |
| 4 SF‐36 Social Functioning score | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 10.80 [3.04, 18.56] |
| 5 SF‐36 Emotional Role score | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 20.70 [7.43, 33.97] |
| 6 SF‐36 Role Physical score | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐9.76, 13.96] |
| 7 Dropout due to any type of adverse event | 3 | 333 | Odds Ratio (M‐H, Random, 95% CI) | 3.53 [0.67, 18.70] |
| 8 Any type of adverse event | 1 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.70, 3.09] |
| 9 Relapse/recurrence sensitivity analysis | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.89] |
Comparison 2. Psychological continuation and maintenance therapies versus attention placebo/non‐specific control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse/recurrence | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.14, 0.93] |
| 2 Dropout due to any reason | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.81] |
| 3 Depression severity | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐7.05, ‐0.95] |
Comparison 3. Psychological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse/recurrence | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.43, 3.49] |
| 2 Dropout due to any reason | 1 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.03] |
Comparison 4. Combined psychological and pharmacological continuation and maintenance therapies versus pharmacological continuation and maintenance therapies alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse/recurrence | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.44, 3.44] |
| 2 Dropout due to any reason | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.90, 2.29] |
| 3 Depression severity | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 2.8 [0.38, 5.22] |
| 4 Depression severity – follow‐up | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐3.26, 5.06] |
| 5 Health‐related quality of life | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.63, 0.63] |
| 6 Health‐related quality of life – follow‐up | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.56, 1.76] |
Comparison 5. Combined psychological and pharmacological continuation and maintenance therapies versus psychotherapeutic continuation and maintenance therapies alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse/recurrence | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.57, 4.01] |
| 2 Dropout due to any reason | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.45, 1.51] |
Comparison 6. Imipramine (TCA) versus desipramine (TCA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout due to any reason | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [1.19, 15.87] |
| 2 Dropout due to any type of adverse event | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.23, 9.60] |
Comparison 7. Imipramine (TCA) versus sertraline (SSRI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse/recurrence | 1 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.84, 1.91] |
| 2 Dropout due to any reason | 1 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.48, 1.38] |
| 3 Depression severity | 1 | 377 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.97, 1.77] |
| 4 Health‐related quality of life | 1 | 347 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐7.31, ‐1.29] |
| 5 Dropout due to any type of adverse event | 1 | 386 | Odds Ratio (M‐H, Random, 95% CI) | 1.99 [0.60, 6.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gelenberg 2003.
| Methods | Design: RCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (52 weeks) Comparison groups: nefazodone vs placebo Funded by: Bristol‐Myers Squibb |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 160 Criteria for relapse/recurrence: "If depressive symptoms began to emerge, as evidenced by a HAM‐D‐24 score of 16 or greater, another evaluation was scheduled within 2 weeks. Evaluations continued every 2 weeks until either the symptoms subsided or recurrence criteria were met. Recurrence was defined as a HAM‐D‐24 score of 16 or greater, together with a diagnosis of MDD as determined from a DSM‐IV MDD checklist administered by the independent evaluator, on two consecutive visits. At the second of these visits, the recurrence also needed confirmation by each site's senior investigator based on a clinical interview. In addition, because some patients had elevated HAM‐D‐24 scores but did not meet MDD criteria, or discontinued before the confirmatory visit, a committee of senior investigators conducted a blinded review of all patient data at the end of the study. Recurrence was declared if there was consensus among the committee that an episode of MDD had occurred. The committee also indicated the date of onset of the recurrence. The final definition of time‐to‐recurrence was based on the first recurrence declared by either one of the two methods to define recurrence." (p. 809) Age distribution in sample (mean): nefazodone: 44.4 (SD 11.1), placebo: 44.1 (SD 8.4) Sex distribution in sample (% women): nefazodone 69.7; placebo 65.5 Diagnoses in sample: nefazodone: 34.2% chronic major depressive disorder, 36.8% double depression, 29.0% recurrent depressive disorder without complete remission between episodes; placebo: 28.6% chronic major depressive disorder, 42.9% double depression, 28.6% recurrent depressive disorder without complete remission between episodes Depression severity at continuation/maintenance baseline (mean): HAM‐D‐24 nefazodone: 5.9 (SD 4.4); placebo: 5.6 (SD 4.0) Age of onset (mean): nefazodone: 24.1 (SD 13.3) years; placebo: 27.7 (SD 12.7) years Length current/last major episode (mean): nefazodone: 100.8 (SD 129.6) months; placebo: 87.6 (SD 90.0) months |
|
| Interventions | Maintenance treatment (52 weeks) Nefazodone (participants = 76) Name (class and type): nefazodone (SNDRI) Planned dosage of drug: 300–600 mg/day Dosage of drug (mean): 485.9 (SD 115.6) mg/day Placebo (participants = 84) Name (class and type): placebo Planned dosage of placebo: NR Dosage of placebo (mean): 504.0 (SD 115.9) mg/day Notes: for all medication visits, any formal psychotherapeutic interventions were proscribed. Participants in the placebo arm received identical (but inactive) tablets without any tapering down between continuation and maintenance phase. |
|
| Outcomes | Relapse/recurrence HAMD‐24 mean Dropout any Dropout due to adverse events |
|
| Notes | Probably conflict of interest because of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind comparison: participants assigned to placebo received apparently identical, inactive tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary dependent measure was the 24‐item HAM‐D, which was rated by trained, independent evaluators blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of missing data, main outcomes seem to be reported for all participants, see statistical methods. (p. 809) |
| Selective reporting (reporting bias) | Unclear risk | Unclear, no information on the measures in the maintenance phase, but relevant outcomes (HAM‐D, recurrence, adverse events) were reported, no study protocol. |
| Other bias | High risk | Insufficient treatment adherence: the medication doses were within the planned range, no laboratory tests are reported. Allegiance bias/conflict of interest: supported by grants from Bristol‐Myers Squibb. Attention bias: medication visits were equal in both groups. |
Harrison 1986.
| Methods | Design: RCT Phases: continuation treatment (26.1 weeks) after response to phenelzine treatment Comparison groups: phenelzine vs placebo Funded by: probably internal funding by the authors' institution, no information given |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 12 Criteria for relapse/recurrence: "Patients were considered to have relapsed and were withdrawn from the protocol if they scored 3 or more on the CGI for 2 consecutive weeks. Patients received a score of 3 on the CGI only if they had a clear recurrence of depressive symptoms." (p. 347) Age distribution in sample: unclear Sex distribution in sample (% women): 83.3 Diagnoses in sample: phenelzine: 20.0% dysthymia, 80.0% double depression; placebo: 58.0% dysthymia, 42.0% double depression Depression severity at continuation/maintenance baseline (mean): HAM‐D phenelzine: 1.8 (SD 1.3); placebo: 4.4 (SD 3.9) Age of onset: unclear Length current/last major episode in months: unclear |
|
| Interventions | Continuation treatment (26.1 weeks) Phenelzine (participants = 5) Name (class and type): phenelzine (MAOI) Planned dosage of drug: unclear Dosage of drug (mean): 51.0 (SD 7.4) mg/day Placebo (participants = 7) Name (class and type): tablet placebo Planned dosage of placebo: NR Dosage of placebo: NR Notes: the placebo group discontinued phenelzine treatment over 14 days by tapering the daily dose by 15 mg every 2–3 days according to a predetermined schedule. No information about concomitant treatments. |
|
| Outcomes | Relapse/recurrence HAM‐D mean Dropout any Dropout due to adverse event Experiencing any adverse event (no data available for the placebo group) Serious adverse events (no data available for the placebo group) |
|
| Notes | After relapse, participants were treated openly as clinically indicated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The double blind condition was maintained by providing individual daily medication packets in which the number of tablets was kept constant by substituting matching placebo." (pp. 346–7) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Unclear risk | Insufficient treatment adherence: no information on treatment adherence. Allegiance bias/conflict of interest: no information about funding/possible conflict of interest. Attention bias: no indication for attention bias, all participants in the placebo group also saw the physician. |
Hellerstein 2001.
| Methods | Design: RCT Phases: acute (8 weeks), continuation (16 weeks) Comparison groups: fluoxetine vs fluoxetine + group psychotherapy Funded by: grant from Eli Lilly Company. |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 40 Criteria for relapse/recurrence: not available Age distribution in sample (mean): 45.1 (SD 9.8) years Sex distribution in sample (% women): 50 Diagnoses in sample: 100% dysthymia Depression severity at continuation/maintenance baseline (mean): HAM‐D 21: fluoxetine: 7.8 (SD 4.7); combination: 6.2 (SD 4.9) Age of onset: unclear Length current/last major episode in months: unclear |
|
| Interventions | Continuation treatment (16 weeks) Fluoxetine (participants = 18) Name (class and type): fluoxetine (SSRI) Planned dosage of drug: 20–80 mg/day Dosage of drug (mean): 38.8 (SD 18.9) mg/day Combination (participants = 19) Name (class and type): fluoxetine (SSRI) + group psychotherapy (CT/IPT) Planned number of sessions + dosage of drug: 16 sessions + 20–80 mg/day Dosage of drug (mean): 37.4 (SD 17.3) mg/day Notes: participants were not allowed to currently undergo another psychotherapy. In the medication group, psychiatrists were instructed not to engage in psychotherapy, counselling, or supportive interventions. |
|
| Outcomes | HAM‐D‐21 mean (end of intervention and follow‐up) Dropout any SWLS (end of intervention and follow‐up) |
|
| Notes | Possibly conflict of interest (funded by Eli Lilly); discrepant information given in text vs tables; sometimes also unclear/discrepant: information given in text itself; treatment/group therapy = CIGP‐CD manual, which is not classified by Cochrane. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Psychotherapy trial, no blinding possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Unblinded raters" (p. 101) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF method for physician rated scales: 7/35 (20%) dropout at follow‐up (36 weeks), these scales are main outcomes; no comment why participants dropped out. |
| Selective reporting (reporting bias) | Unclear risk | No existing study protocol. |
| Other bias | High risk | Quote: "Insufficient treatment adherence: Sessions were audiotaped and reviewed in weekly supervision meetings for adherence to the manual." (pp. 96–7) Allegiance bias/conflict of interest: financed by pharmaceutical company, but unclear/no further information. Attention bias: more attention in the combination group as this group also received psychotherapy. Other: very likely that randomization was before acute treatment, but it was not described clearly. Discrepant information in the text. |
Keller 1998b.
| Methods | Design: RCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (76 weeks) Comparison groups: sertraline vs placebo Funded by: grant from Pfizer (NY) |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 161 Criteria for relapse/recurrence: recurrence: DSM‐III‐R criteria for major depression for ≥ 3 weeks; CGI severity score of ≥ 4 (at least moderate severity); CGI improvement score ≥ 3 (minimally improved or less); and an increase in HAM‐D score ≥ 4 points higher than the maintenance baseline; next visit 1 week later in total ≥ 4 weeks of clinical worsening; additionally: senior investigator supporting diagnosis/recurrence. (pp. 1666–7) Age distribution in sample (mean): sertraline: 40.8 (SD 9.0) years; placebo: 42.4 (SD 9.7) years Sex distribution in sample (% women): sertraline: 62.3; placebo: 69.0 Diagnoses in sample: sertraline: 52.0% chronic major depressive disorder, 48.0% double depression; placebo: 43.0% chronic major depressive disorder, 57.0% double depression Depression severity at continuation/maintenance baseline (mean): sertraline: 5.5 (SD 4.2); placebo: 6.3 (SD 3.7) Age of onset (mean): sertraline: 24.9 (SD 11.2) years; placebo: 25.7 (SD 12.5) years Length current/last major episode (mean): sertraline: 88.2 (SD 121.7) months; placebo: 54.9 (SD 80.8) months |
|
| Interventions | Maintenance treatment (76 weeks) Sertraline (participants = 77) Name (class and type): sertraline (SSRI) Planned dosage of drug: 50–200 mg/day Dosage of drug (mean): 146.1 mg/day Placebo (participants = 84) Name (class and type): placebo tablets Planned dosage of placebo: unclear Dosage of placebo (mean): 3.4 tablets/day Notes: participants in the placebo arm tapered sertraline by 50 mg reduction per week as placebo substitution. No information about concomitant treatments. |
|
| Outcomes | Relapse/recurrence HAM‐D‐24 mean Dropout any SF‐36 Dropout due to adverse event Experiencing any adverse event |
|
| Notes | Probably conflict of interest because of funding. They used 2 different criteria for relapse/recurrence, we extracted the stricter one; therefore, maybe less relapse observed than actual happened, in combination with numerous of dropouts with possible bias of results. "Patients meeting recurrence criteria could continue in the study if both patient and study physician agreed that no change in the study medication was indicated at that time. Instead, an increase in daily dose was undertaken at a rate of 50mg/week up to the maximum daily dose of 200mg of sertraline hydrochloride. A similar double‐blind titration was also used for patients receiving placebo treatment." (further details see p. 1667) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Quote: "To maintain blinding, this group of patients continued (as a parallel but non‐randomised group) receiving imipramine during subsequent continuation and maintenance phases… The integrity of the study's double‐blind component was not compromised." (p. 1666) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 70% dropout in the placebo group. The data were replaced by the LOCF ‐method (i.e. 70% of data replaced by last observation point, the participant's condition was probably better at this earlier time). |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Insufficient treatment adherence: no information. Allegiance bias/conflict of interest: whole study financed by Pfizer. Attention bias: most likely, each treatment group gained same attention (as both groups received tablets). |
Klein 2004.
| Methods | Design: RCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (52 weeks) Comparison groups: CBASP vs assessment only Funded by: Bristol‐Myers Squibb |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 82 Criteria for relapse/recurrence: "Recurrence was defined in the protocol as a HRSD‐24 [HAM‐D] score of 16 or greater on two consecutive visits and a diagnosis of MDD as determined from a DSM–IV MDD checklist administered by the independent evaluator. At the second of these visits, the recurrence also needed confirmation by the site's senior investigator on the basis of a clinical interview." (p. 683) Age distribution in sample (mean): CBASP: 44.2 (SD 11.7) years; assessment only: 46.0 (SD 11.1) years Sex distribution in sample (% women): CBASP: 81.0; assessment only: 52.5 Diagnoses in sample: CBASP: 50.0% chronic major depressive disorder, 26.2% double depression, 23.8% recurrent depressive disorder with incomplete remission between episodes; assessment only: 60.0% chronic major depressive disorder, 20.0% double depression, 20.0% recurrent depressive disorder with incomplete remission between episodes Depression severity at continuation/maintenance baseline (mean): HAM‐D‐24: CBASP: 6.6 (SD 3.8); assessment only: 6.2 (SD 4.4) Age of onset (mean): CBASP: 27.0 (SD 12.4) years; assessment only: 29.5 (SD 13.5) years Length current/last major episode in months (mean): CBASP: 92.4 (SD 115.2); assessment only: 85.2 (SD 122.4) |
|
| Interventions | Maintenance treatment (52 weeks) Name (class and type): CBASP Planned number of sessions: 13 Number of sessions (mean): 11.1 (SD 3.8) Name (class and type): assessment only Planned number of sessions: 13 Number of sessions: unclear Notes: "In both conditions, all psychotropic medication and non‐protocol psychotherapy were prohibited." (p. 683) |
|
| Outcomes | Relapse/recurrence HRSD‐24 (HAM‐D) mean Dropout any |
|
| Notes | Probably conflict of interest because of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As CBASP was compared to assessment only, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The primary outcome measure throughout all phases of the study was the HRSD‐24, which was administered by certified rates who were unaware of patient's treatment condition. Patients in the CBASP condition were also seen by the independent evaluator every 4 weeks but did not receive an honorarium. All patients were reminded at each visit not to mention anything that might reveal their treatment condition to the independent evaluator. If patients had questions or concerns about the study, they were instructed to raise them with the project coordinator rather than the independent evaluator. In the rare instances that the blind was broken, the patient was seen by a different independent evaluator at subsequent visits. In both conditions, all psychotropic medication and non‐protocol psychotherapy were prohibited." (p. 683) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We compared time to recurrence between the CBASP and assessment only groups using survival analysis. Patients who failed to complete the maintenance phase were included in these analyses using all available data up to the time of exiting the study." (p. 684) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol only described outcome measurements for the acute phase. |
| Other bias | Low risk | Insufficient treatment adherence. Quote: "Sessions were videotaped and reviewed weekly–biweekly by the site supervisor or James P. McCullough to assess adherence to the treatment procedures. Adherence was assessed using a rating scale described in McCullough (2000). When non‐adherence was identified, it was immediately discussed with the therapist and efforts at remediation were provided." (p. 683) Allegiance bias/conflict of interest: some authors were well‐known CBASP therapists (e.g. J McCullough), but there were also other authors; interests were balanced across authors. Attention bias: in both conditions, participants saw the therapist or project co‐ordinator every 4 weeks. The project co‐ordinator provided them with some attention but no active treatment. |
Kocsis 1995.
| Methods | Design: NRCT Phases: acute (6–10 weeks), continuation (16–20 weeks), maintenance (104.4 weeks) Comparison groups: imipramine vs desipramine Funded by: no information |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 73 Criteria for relapse/recurrence: no information; this outcome was not addressed. Age distribution in sample (mean): 36.0 (SD 10.0) years Sex distribution in sample (% women): 64.1 Diagnoses in sample: 37.0% dysthymia, 63.0% double depression Depression severity at continuation/maintenance baseline: unclear Age of onset: unclear Length current/last major episode in months: unclear |
|
| Interventions | Continuation treatment (16–20 weeks) Imipramine (participants = 23) Name (class and type): imipramine (TCA) Planned dosage of drug: 300 mg/day Dosage of drug: unclear Sertraline (participants = 50) Name (class and type): desipramine (TCA) Planned dosage of drug: 200 mg/day Dosage of drug (mean): 232 (SD 72) mg/day Notes: "Patients were allowed to remain in stable long‐term psychotherapy during the study but were not allowed to enter into new psychotherapy arrangements." (p. 214) No data provided about the percentage of participants receiving parallel psychotherapy. "Concomitant psychotropic medications were proscribed." (p. 214) |
|
| Outcomes | Dropout any Dropout due to adverse event |
|
| Notes | There were 3 different treatment arms in the acute treatment, but it was unclear how participants were allocated to the different treatment arms, e.g. if there were randomized. Additionally, the rationale of the acute treatment was unclear (e.g. some participants received medication on a double blind and some on an open basis). | |
Kocsis 1996.
| Methods | Design: RCT Phases: acute (10 weeks), continuation (16 weeks), maintenance (104.4 weeks) Comparison groups: desipramine vs placebo Funded by: grant from the National Institute of Mental Health |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 53 Criteria for relapse/recurrence: "Suspected relapse occurred when a HAM‐D score rose above 12 during the maintenance phase. Clinicians discussed and encouraged compliance and obtained a plasma drug level, which was reviewed by a non blind observer who was not involved in the treatment. The non‐blind observer gave instructions or dummy instructions for dosage adjustments. Relapse was defined as HAM‐D scores greater than 12 and GAS scores below 60 on three successive ratings over a period of 4 weeks or at least one rating meeting these criteria and an urgent need for alternative treatment for a depressive syndrome." (p. 771) Age distribution in sample (mean): 36.9 (SD 9.6) years Sex distribution in sample (% women): 57.4 Diagnoses in sample: 10.9% chronic major depressive disorder, 39.5% dysthymia, 49.6% double depression Depression severity at continuation/maintenance baseline: unclear Age of onset (mean): 12.6 (SD 6.9) years Length current/last major episode in months: unclear |
|
| Interventions | Maintenance treatment (104.4 weeks) Desipramine (participants = 28) Name (class and type): desipramine (TCA) Planned dosage of drug: 75–350 mg/day Dosage of drug: unclear Placebo (participants = 25) Name (class and type): placebo Planned dosage of drug: participants in the placebo group were tapered by approximately 25% per week over the month and then received identical placebo at the same dose equivalent for the next 23 months or until relapse. Dosage of drug: unclear Notes: participants in the placebo arm were tapered down by 25% per week during the first month of maintenance treatment followed by receiving identical placebo tablets. Stable psychotherapeutic treatment was allowed during the study, 39% of participants from the desipramine group and 40% of participants from the placebo group were in stable psychotherapeutic treatment during the study. |
|
| Outcomes | Relapse/recurrence Dropout any |
|
| Notes | Desipramine (norpramine) and matching placebo were provided by Marion Merrill Dow Inc., Kansas City, MO. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A third possible limitation in the present study was the absence of independent raters. Ratings were done by study clinicians who may have been able to guess the maintenance treatment based on side effects." (p. 773). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50/53 (5.7% dropout during maintenance treatment) participants completed ≥ 1 month of maintenance treatment, outcome data were provided for this sample. |
| Selective reporting (reporting bias) | High risk | No study protocol available. Quote: "A self‐rated measure of social impairment and function, the Social Adjustment Scale‐Self‐rated was completed at the beginning and end of each phase of the study." (p. 771) AND: "Subjects were seen and rated each month during the maintenance phase." (p. 771). Very incomplete data in the text/tables (just full vs partial remission and relapse, but no presentation of clear data about HAM‐D, GAS, and SAS‐SR). |
| Other bias | Low risk | Insufficient treatment adherence: control of plasma drug concentrations. Allegiance bias/conflict of interest: no indication for conflict of interest. Attention bias: no differences between the groups. Participants were seen and rated each month during the maintenance phase. |
Kocsis 2003.
| Methods | Design: NRCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (52 weeks) Comparison groups: nefazodone vs CBASP vs combination Funded by: Bristol‐Myers Squibb |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 329 Criteria for relapse/recurrence: "Two definitions of relapse were utilized. Any patient who scored higher than 15 on the HAM‐D was considered at risk for a relapse of MDD. In all such cases, an independent evaluator completed the DSM‐IV criteria checklist for MDD, and if the patient met DSM‐IV symptom criteria, the treating clinician was notified. A confirmatory visit was scheduled within 14 days and the HAM‐D and MDD criteria checklist assessment were repeated. Patients meeting MDD criteria were evaluated by an independent senior investigator to confirm relapse. In addition, an investigator could declare a relapse on de facto grounds in the case of an exacerbation of depressive symptomatology with marked incapacity and clinically significant suicidal ideation, including psychiatric hospitalizations resulting from such exacerbations. Patients not meeting relapse criteria but continuing to score higher than 15 on the HAM‐D were followed every other week until their outcome was clarified." (p. 77) Age distribution in sample (mean): nefazodone: 43.1 (SD 9.7) years; CBASP: 44.0 (SD 10.8) years; combination: 44.6 (SD 9.4) years Sex distribution in sample (% women): nefazodone: 58.7; CBASP: 66.3; combination: 67.8 Diagnoses in sample: nefazodone: 32.6% chronic major depressive disorder, 41.3% double depression, 26.1% recurrent depressive disorder with incomplete remission between episodes; CBASP: 33.7% chronic major depressive disorder, 46.1% double depression, 20.2% recurrent depressive disorder with incomplete remission between episodes; combination: 32.2% chronic major depressive disorder, 42.1% double depression, 26.6% recurrent depressive disorder with incomplete remission between episodes Depression severity at continuation/maintenance baseline: unclear Age of onset (mean): nefazodone: 26.3 (SD 13.1) years; CBASP: 28.1 (SD 13.5) years; combination: 27.0 (SD 12.9) years Length current/last major episode in months (mean): nefazodone: 92.4 (SD 114.0); CBASP: 105.6 (SD 144.0); combination: 99.6 (SD 120.0) |
|
| Interventions | Continuation treatment (16 weeks) Nefazodone (participants = 91) Name (class and type): nefazodone (SNDRI) Planned dosage of drug: 300–600 mg/day Dosage of drug (mean): 499 (SD 115) mg/day CBASP (participants = 88) Name (class and type): CBASP Planned number of sessions: 6 Number of sessions (mean): 6 (SD 1) Combination (participants = 150) Name (class and type): combination (SNDRI + CBASP) Planned number of sessions + dosage of drug: 6 sessions + 300–600 mg/day Number of sessions + dosage of drug (mean): 5.9 (SD 1.1) sessions + 479 (SD 108) mg/day Notes: "Pharmacotherapists were directed not to provide any psychotherapeutic interventions." (p. 76) |
|
| Outcomes | Relapse/recurrence Dropout any |
|
| Notes | Probably conflict of interest because of funding and connection of the authors to pharmaceutical industry. | |
Koran 2001.
| Methods | Design: NRCT Phases: acute (12 weeks), continuation (16 weeks), maintenance (76 weeks) Comparison groups: sertraline vs imipramine Funded by: grant from Pfizer (NY) |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 386 Criteria for relapse/recurrence: "A full remission of depression was defined as a CGI improvement score (CGI‐I) (Guy, 1976) of 1 or 2 (very much or much improved) and a Hamilton Depression Rating Scale score (HRSD [HAM‐D]) (Hamilton, 1960) ≤ 7. A satisfactory therapeutic response (partial remission) was defined as a CGI‐I ≥2, a HRSD ≤ 15 with a ≥ 50% decrease from baseline, and a CGI severity score (CGI‐S) ≤ 3 (i.e. no more than mild depression). A patient whose scores dropped below a 'satisfactory therapeutic response' for a 4‐week period was considered relapsed." (p. 29) Age distribution in sample (mean): sertraline: 40.2 (SD 9.7) years; imipramine: 43.1 (SD 9.6) years Sex distribution in sample (% women): sertraline: 68.2; imipramine: 57.1 Diagnoses in sample: sertraline: 49.0% chronic major depressive disorder, 51.0% double depression; imipramine: 45.0% chronic major depressive disorder, 55.0% double depression Depression severity at continuation/maintenance baseline (mean): sertraline: 6.7 (SD 3.7); imipramine: 6.9 (SD 3.5) Mean age of onset: unclear Length current/last major episode (mean): sertraline: 73.2 (SD 98.4) months; imipramine: 76.8 (SD 114.0) months |
|
| Interventions | Continuation treatment (16 weeks) Sertraline (participants = 239) Name (class and type): sertraline (SSRI) Planned dosage of drug: 50–200 mg/day Dosage of drug (mean): 149 (SD 55) mg/day Imipramine (participants = 147) Name (class and type): imipramine (TCA) Planned dosage of drug: 50–300 mg/day Dosage of drug: 227 (SD 73) mg/day Notes: "Psychotherapy was not allowed during the study unless it had started at least 3 months before acute phase randomisation and continued throughout all stages of the study without change." (p. 28) 60% of the participants received ongoing psychotherapy during the continuation phase. |
|
| Outcomes | Relapse/recurrence HAM‐D‐24 mean Dropout any Q‐LES‐Q score Dropout due to adverse event |
|
| Notes | Probably conflict of interest because of funding (authors = members of industry who financed study). Further randomized comparison on maintenance treatment of the sertraline group with placebo in the publication of Keller (1998). |
|
Miller 2001.
| Methods | Design: RCT Phases: acute (10–12 weeks), continuation (16 weeks), maintenance (104.4 weeks) Comparison groups: desipramine vs placebo Funded by: supported by grant R01‐MH37103 from the National Institute of Mental Health and from a fund established in the New York Community Trust by DeWitt‐Wallace. |
|
| Participants | Number of participants randomized (NRCT: number of participants included): 27 Criteria for relapse/recurrence: "Recurrence was defined as HAM‐D scores > 12 and GAS scores < 60 on three successive ratings over a period of 4 weeks or at least one rating meeting these criteria and an urgent need for alternative treatment for recurrence of depressive symptoms." (p. 233) Age distribution in sample (mean): desipramine: 34.4 (SD 9.6) years; placebo: 39.0 (SD 11.2) years Sex distribution in sample (% women): desipramine: 43.0; placebo: 46.0 Diagnoses in sample: 100% dysthymia Depression severity at continuation/maintenance baseline (mean): desipramine: 3.1 (SD 2.5); placebo: 3.9 (SD 5.2) Age of onset (mean): desipramine: 14.5 (SD 10.4) years; placebo: 12.3 (SD 8.0) years Length current/last major episode: unclear |
|
| Interventions | Maintenance treatment (104.4 weeks) Desipramine (participants = 14) Name (class and type): desipramine (TCA) Dosage of drug: unclear Dosage of drug (mean): 223 (SD 90) mg/day Placebo (participants = 13) Name (class and type): placebo Planned dosage of placebo: unclear Dosage of placebo (mean): 240 (SD 60) mg/day (dummy dosage) Notes: participants in the placebo arm were tapered down by 25% per week during the first month of maintenance treatment followed by receiving identical placebo tablets. 43% of participants from the desipramine group and 38% of participants from the placebo group were in stable long‐term psychotherapy during the study, a non‐significant difference. |
|
| Outcomes | Relapse/recurrence | |
| Notes | Analysis of the dysthymic subgroup of Kocsis et al. 1996 and some additional participants with dysthymia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind maintenance phase |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Ratings were done by study clinicians who were blinded to treatment assignment, but may have guessed the maintenance treatment based on side effects, potentially biasing ratings of outcome." (p. 235) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for the available outcome. |
| Selective reporting (reporting bias) | High risk | No study protocol available, only recurrence rates were reported, HAM‐D, GAS, and SASR was also measured. |
| Other bias | Low risk | Insufficient treatment adherence: serum level control. Allegiance bias/conflict of interest: no indication for a conflict of interest. Attention bias: same approach in both conditions (quote: "monthly 20–30 minute appointments to monitor clinical status and manage side effects. Therapists provided support and encouragement, and medication compliance was discussed throughout." (p. 232) |
CBASP: Cognitive Behavioral Analysis System of Psychotherapy; CGI: Clinical Global Impression; CIGP‐CD: Cognitive‐Interpersonal Group Psychotherapy for Chronic Depression; CT: cognitive therapy; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders 3rd Edition – Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders 4th Edition; GAS: Global Assessment Scale; HAM‐D: Hamilton Depression Rating Scale; HRSD: Hamilton Rating Scale for Depression (also known as HAM‐D); IPT: interpersonal psychotherapy; LOCF: last observation carried forward; MAOI: monoamine oxidase inhibitor; MDD: major depressive episode; NR: not reported; NRCT: non‐randomized controlled trial; Q‐LES‐Q: Quality of Life Enjoyment and Satisfaction Questionnaire; RCT: randomized controlled trial; SAS‐SR: Social Adjustment Scale – Self‐Report; SD: standard deviation; SF‐36: 36‐item Short‐Form Health Survey; SNDRI: selective noradrenaline‐dopamine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; SWLS: Satisfaction With Life Scale; TCA: tricyclic antidepressant.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bockting 2005 | Included participants were remitted, some with and some without treatment. The interval between acute and continuation treatment was too long in some cases (> 1 year). |
| Fava 2004 | Participants did not meet the criteria of persistent depression (duration < 2 years). |
| Franchini 1997 | Participants did not meet the criteria of persistent depression (duration < 2 years). |
| Frank 2007 | Participants did not meet the criteria of persistent depression (duration < 2 years). |
| Hamidian 2013 | No response during acute treatment required for entering MBCT. |
| Hellerstein 1994 | Acute treatment with long‐term follow‐up |
| Hellerstein 2015 | No comparator (pilot study) |
| Hellerstein 2017 | No response during acute treatment required to enter continuation trial. |
| Holländare 2013 | Participants did not meet the criteria of persistent depression (duration < 2 years). |
| Huijbers 2015 | Participants did not meet the criteria of persistent depression (duration < 2 years). |
| Jarrett 2013 | Participants did not meet the criteria of persistent depression (duration < 2 years); just 5% participants with double depression. |
| Kok 2015 | Participants did not meet the criteria of persistent depression (duration < 2 years); duration of episode maximum 2 years for being eligible. |
| Michalak 2015 | No response during acute treatment required for entering MBCT. |
| Murray 2010 | No response during acute treatment required to enter intervention. |
| Petersen 2010 | Partly meeting criteria for a PDD diagnosis; exact amount of persistent depressed participants unclear. |
| Schramm 2017 | No response during acute treatment required to enter continuation trial. |
| Thase 2001 | < 80% of participants with a PDD diagnosis. |
| van Aalderen 2015 | Participants did not meet the criteria of persistent depression (duration < 2 years). |
| Wiersma 2008 | No response during acute treatment required to enter continuation trial. |
MBCT: mindfulness‐based cognitive therapy; PDD: persistent depressive disorder.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT03219879.
| Methods | Design: RCT Phases: continuation (approximately 26 weeks) Comparison groups: T‐CT (telephone‐delivered cognitive‐behavioral continuation therapy) vs usual care Funded by: University of Zurich |
| Participants | Estimated enrolment: 218 Ages: 18 years to 75 years Sexes Eligible for Study: All Diagnoses: Recurrent major depressive disorder or chronic/persistent depressive disorder based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) |
| Interventions | Continuation treatment (approximately 26 weeks) Behavioral: telephone‐administered continuation therapy The intervention includes eight therapy sessions of approximately 50 minutes duration delivered over the telephone by trained psychotherapists over a time period of six months. The intervention is grounded in the principles of psychological continuation therapy and relapse prevention, and includes strategies such as transferring helpful elements of acute‐phase cognitive‐behavioral therapy for depression to daily life. T‐CT is offered in addition to usual care. Other: Usual care Usual care without any study‐related intervention Other Name: Treatment as usual |
| Outcomes | Primary outcome: Relapse of a major depressive episode (time frame: 6 months, 12 months, and 18 months after baseline) Secondary outcomes: Well‐weeks (time frame: 6 months, 12 months, and 18 months after baseline) Depressive symptoms (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Health‐related quality of life (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Anxiety symptoms (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Psychosocial functioning (time frame: 6 months and 12 months after baseline) Cost of health care utilization (time frame: baseline, 6 months, and 12 months after baseline) Cost‐effectiveness (time frame: baseline, 6 months, and 12 months after baseline) Other Pre‐specified Outcome Measures: T‐CT acceptability (time frame: 6 months after baseline) Treatment satisfaction (time frame: baseline, and 6 months after baseline) Self‐confidence (time frame: baseline, 6 months, and 12 months after baseline) Physical activity (time frame: baseline, 6 months, and 12 months after baseline) Self‐efficacy for depression self‐management (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Self‐management behaviours (time frame: baseline, 3 months, 6 months, and 12 months after baseline) Interpersonal emotion regulation skills (time frame: baseline, 6 months, and 12 months after baseline) Therapeutic alliance (time frame: baseline, 3 months, and 6 months after baseline) |
| Notes |
DSM‐5: Diagnostic and Statistical Manual of Mental Disorders 5th Edition; RCT: randomized controlled trial; T‐CT: telephone‐delivered cognitive‐behavioral continuation therapy
Differences between protocol and review
We replaced the term CCT (clinical controlled trial) by the term NRCT (non‐randomized controlled trial), and updated the name of the tool for analyzing NRCTs to "ROBINS‐I tool" (previously called ACROBAT‐NRSI).
From the seven planned comparisons, we were able to analyse five comparisons. None of the studies provided data for the following two comparisons: pharmacological continuation and maintenance therapies versus treatment as usual (TAU); and psychological continuation and maintenance therapies versus treatment as usual (TAU). We included an additional comparison as two studies provided data: pharmacological continuation and maintenance therapies versus other pharmacological continuation and maintenance therapies (post hoc) medications.
We provided 'Summary of findings' tables for only one comparison (pharmacotherapy versus placebo) as there were few data for the other comparisons.
In the original protocol, we planned analysis of follow‐up data that ranged between six and 18 months after the end of the intervention with a preference for the time that was closest to one year after the end of the intervention. In this review, only one study provided follow‐up data, with time point at 12 weeks after the end of the intervention, which is not in the predefined range of six to 18 months after the end of the intervention. As just one study provided follow‐up data, we still included these data into this review.
Due to the small number of included studies, subgroup and meta‐regression analyses were not performed. Not all predefined sensitivity analyses could be performed due to lack of variation concerning risk of bias in some domains.
To test for publication bias, Eggers' test could not be applied, as it requires a minimum 10 studies per comparison.
We planned to include all participants allocated to the respective study arm in the primary outcome analyses. However, when data on relapse/recurrence were missing for some participants, we used the data provided instead of calculating relapse/recurrence rates ourselves since a classification of all participants without available data as 'relapsed/recurred' probably results in a biased estimate. Continuous data suitable for a calculation of relapse/recurrence rates were not available throughout. Certainly, the percentage of missing data concerning this outcome was low: in five studies relapse/recurrence rates were provided for the whole sample, in two studies this outcome was not addressed and in three studies the amount of missing data ranged between 2% and 10%. Data on the primary acceptance outcome (dropout any) were provided for the whole intention‐to‐treat sample.
Contributions of authors
AJ, LK and MH applied for funding.
SL, KM, AJ, RM, BW, MH, and LK developed the protocol.
KM, SL, AJ and RM screened the literature and extracted the data.
KM, SL, RM and LK conducted the analyses.
KM, SL and RM wrote the first version of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
German Ministry of Education and Research, Germany.
Grant 01KG1403
Declarations of interest
AJ, LK, and MH co‐ordinated the update of the S3 Guideline/National Clinical Practice Guideline "Unipolar Depression" (DGPPN 2015). The expert association DGPPN (editor of the S3 Guideline) provided financial support for the preparation of the Guideline Update to the co‐ordinators' institution (Department of Medical Psychology, University Medical Center Hamburg‐Eppendorf).
KM, BW, AJ, LK, RM, and MH report participating in publicly funded investigator‐initiated primary studies and systematic reviews of interventions for people with depression.
KM, BW, AJ, RM, MH, and SL have had formal training in behavioural psychotherapy.
New
References
References to studies included in this review
Gelenberg 2003 {published data only}
- Gelenberg AJ, Trivedi MH, Rush AJ, Thase ME, Howland R, Klein DN, et al. Randomized, placebo‐controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biological Psychiatry 2003;54(8):806‐17. [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral‐analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine 2000;342(20):1462‐70. [DOI] [PubMed] [Google Scholar]
- Klein DN, Santiago NJ, Vivian D, Blalock JA, Kocsis JH, Markowitz JC, et al. Cognitive‐behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. Journal of Consulting and Clinical Psychology 2004;72(4):681‐8. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Rush AJ, Markowitz JC, Borian FE, Dunner DL, Koran LM, et al. Continuation treatment of chronic depression: a comparison of nefazodone, cognitive behavioral analysis system of psychotherapy, and their combination. Psychopharmacology Bulletin 2003;37(4):73‐87. [PubMed] [Google Scholar]
Harrison 1986 {published data only}
- Harrison W, Rabkin J, Stewart JW, McGrath PJ, Tricamo E, Quitkin F. Phenelzine for chronic depression: a study of continuation treatment. Journal of Clinical Psychiatry 1986;47(7):346‐9. [PubMed] [Google Scholar]
Hellerstein 2001 {published data only}
- Hellerstein DJ, Little SA, Samstag LW, Batchelder S, Muran JC, Fedak M, et al. Adding group psychotherapy to medication treatment in dysthymia: a randomized prospective pilot study. Journal of Psychotherapy Practice and Research 2001;10(2):93‐103. [PMC free article] [PubMed] [Google Scholar]
Keller 1998b {published data only}
- Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, et al. Lost human capital from early‐onset chronic depression. American Journal of Psychiatry 2000;157(6):940‐7. [DOI] [PubMed] [Google Scholar]
- Keller MB, Gelenberg AJ, Hirschfeld RM, Rush AJ, Thase ME, Kocsis JH, et al. The treatment of chronic depression, part 2: a double‐blind, randomized trial of sertraline and imipramine. Journal of Clinical Psychiatry 1998;59(11):598‐607. [DOI] [PubMed] [Google Scholar]
- Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, et al. Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA 1998;280(19):1665‐72. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, A Schatzberg, A Rush, et al. Psychosocial outcomes following long‐term, double‐blind treatment of chronic depression with sertraline vs placebo. Archives of General Psychiatry 2002;59(8):723‐8. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Schatzberg AF, Rush AJ, Korstein S, Klein D, Hirschfeld RM. Maintenance therapy of chronic depression: a placebo‐controlled trial of sertraline. Biological Psychiatry 1997;42(1):240S. [Google Scholar]
- Koran LM, Gelenberg AJ, Kornstein SG, Howland RH, Friedman RA, DeBattista C, et al. Sertraline versus imipramine to prevent relapse in chronic depression. Journal of Affective Disorders 2001;65(1):27‐36. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Koran LM, Keller MB, Markowitz JC, Harrison WM, Miceli RJ, et al. The treatment of chronic depression, part 1: study design and rationale for evaluating the comparative efficacy of sertraline and imipramine as acute, crossover, continuation, and maintenance phase therapies. Journal of Clinical Psychiatry 1998;59(11):589‐97. [PubMed] [Google Scholar]
Klein 2004 {published data only}
- Gelenberg AJ, Trivedi MH, Rush AJ, Thase ME, Howland R, Klein DN, et al. Randomized, placebo‐controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biological Psychiatry 2003;54(8):806‐17. [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral‐analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine 2000;342(20):1462‐70. [DOI] [PubMed] [Google Scholar]
- Klein DN, Santiago NJ, Vivian D, Blalock JA, Kocsis JH, Markowitz JC, et al. Cognitive‐behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. Journal of Consulting and Clinical Psychology 2004;72(4):681‐8. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Rush AJ, Markowitz JC, Borian FE, Dunner DL, Koran LM, et al. Continuation treatment of chronic depression: a comparison of nefazodone, cognitive behavioral analysis system of psychotherapy, and their combination. Psychopharmacology Bulletin 2003;37(4):73‐87. [PubMed] [Google Scholar]
Kocsis 1995 {published data only}
- Kocsis JH, Friedman RA, Markowitz JC, Leon AC, Miller NL, Gniwesch L, et al. Maintenance therapy for chronic depression. A controlled clinical trial of desipramine. Archives of General Psychiatry 1996;53(9):769‐74. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Friedman RA, Markowitz JC, Miller N, Gniwesch L, Bram J. Stability of remission during tricyclic antidepressant continuation therapy for dysthymia. Psychopharmacology Bulletin 1995;31(2):213‐6. [PubMed] [Google Scholar]
- Marin DB, Kocsis JH, Frances AJ, Parides M. Desipramine for the treatment of "pure" dysthymia versus "double" depression. American Journal of Psychiatry 1994;151(7):1079‐80. [DOI] [PubMed] [Google Scholar]
- Miller NL, Kocsis JH, Leon AC, Portera L, Dauber S, Markowitz JC. Maintenance desipramine for dysthymia: a placebo‐controlled study. Journal of Affective Disorders 2001;64(2‐3):231‐7. [DOI] [PubMed] [Google Scholar]
Kocsis 1996 {published data only}
- Kocsis JH, Friedman RA, Markowitz JC, Leon AC, Miller NL, Gniwesch L, et al. Maintenance therapy for chronic depression. A controlled clinical trial of desipramine. Archives of General Psychiatry 1996;53(9):769‐74. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Friedman RA, Markowitz JC, Miller N, Gniwesch L, Bram J. Stability of remission during tricyclic antidepressant continuation therapy for dysthymia. Psychopharmacology Bulletin 1995;31(2):213‐6. [PubMed] [Google Scholar]
- Marin DB, Kocsis JH, Frances AJ, Parides M. Desipramine for the treatment of "pure" dysthymia versus "double" depression. American Journal of Psychiatry 1994;151(7):1079‐80. [DOI] [PubMed] [Google Scholar]
- Miller NL, Kocsis JH, Leon AC, Portera L, Dauber S, Markowitz JC. Maintenance desipramine for dysthymia: a placebo‐controlled study. Journal of Affective Disorders 2001;64(2‐3):231‐7. [DOI] [PubMed] [Google Scholar]
Kocsis 2003 {published data only}
- Gelenberg AJ, Trivedi MH, Rush AJ, Thase ME, Howland R, Klein DN, et al. Randomized, placebo‐controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biological Psychiatry 2003;54(8):806‐17. [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral‐analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine 2000;342(20):1462‐70. [DOI] [PubMed] [Google Scholar]
- Klein DN, Santiago NJ, Vivian D, Blalock JA, Kocsis JH, Markowitz JC, et al. Cognitive‐behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. Journal of Consulting and Clinical Psychology 2004;72(4):681‐8. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Rush AJ, Markowitz JC, Borian FE, Dunner DL, Koran LM, et al. Continuation treatment of chronic depression: a comparison of nefazodone, cognitive behavioral analysis system of psychotherapy, and their combination. Psychopharmacology Bulletin 2003;37(4):73‐87. [PubMed] [Google Scholar]
Koran 2001 {published data only}
- Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, et al. Lost human capital from early‐onset chronic depression. American Journal of Psychiatry 2000;157(6):940‐7. [DOI] [PubMed] [Google Scholar]
- Keller MB, Gelenberg AJ, Hirschfeld RM, Rush AJ, Thase ME, Kocsis JH, et al. The treatment of chronic depression, part 2: a double‐blind, randomized trial of sertraline and imipramine. Journal of Clinical Psychiatry 1998b;59(11):598‐607. [DOI] [PubMed] [Google Scholar]
- Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, et al. Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA 1998;280(19):1665‐72. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, A Schatzberg, A Rush, Klein DN, Howland R, Gniwesch L, et al. Psychosocial outcomes following long‐term, double‐blind treatment of chronic depression with sertraline vs placebo. Archives of General Psychiatry 2002;59(8):723‐8. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Schatzberg AF, Rush AJ, Korstein S, Klein D, Hirschfeld RM. Maintenance therapy of chronic depression: a placebo‐controlled trial of sertraline. Biological Psychiatry 1997;42(1):240S. [Google Scholar]
- Koran LM, Gelenberg AJ, Kornstein SG, Howland RH, Friedman RA, DeBattista C, et al. Sertraline versus imipramine to prevent relapse in chronic depression. Journal of Affective Disorders 2001;65(1):27‐36. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Koran LM, Keller MB, Markowitz JC, Harrison WM, Miceli RJ, et al. The treatment of chronic depression, part 1: study design and rationale for evaluating the comparative efficacy of sertraline and imipramine as acute, crossover, continuation, and maintenance phase therapies. Journal of Clinical Psychiatry 1998;59(11):589‐97. [PubMed] [Google Scholar]
Miller 2001 {published data only}
- Kocsis JH, Friedman RA, Markowitz JC, Leon AC, Miller NL, Gniwesch L, et al. Maintenance therapy for chronic depression. A controlled clinical trial of desipramine. Archives of General Psychiatry 1996;53(9):769‐74. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Friedman RA, Markowitz JC, Miller N, Gniwesch L, Bram J. Stability of remission during tricyclic antidepressant continuation therapy for dysthymia. Psychopharmacology Bulletin 1995;31(2):213‐6. [PubMed] [Google Scholar]
- Marin DB, Kocsis JH, Frances AJ, Parides M. Desipramine for the treatment of "pure" dysthymia versus "double" depression. American Journal of Psychiatry 1994;151(7):1079‐80. [DOI] [PubMed] [Google Scholar]
- Miller NL, Kocsis JH, Leon AC, Portera L, Dauber S, Markowitz JC. Maintenance desipramine for dysthymia: a placebo‐controlled study. Journal of Affective Disorders 2001;64(2‐3):231‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bockting 2005 {published data only}
- Bockting C, Schene AH, Spinhoven P, Koeter MW, Wouters LF, Huyser J, et al. Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2005;73(4):647‐57. [DOI] [PubMed] [Google Scholar]
Fava 2004 {published data only}
- Fava GA, Ruini C, Rafanelli C, Finos L, Conti S, Grandi S. Six‐year outcome of cognitive behavior therapy for prevention of recurrent depression. American Journal of Psychiatry 2004;161:1872‐6. [DOI] [PubMed] [Google Scholar]
Franchini 1997 {published data only}
- Franchini L, Gasperini M, Perez J, Smeraldi E, Zanardi R. A double‐blind study of long‐term treatment with sertraline or fluvoxamine for prevention of highly recurrent unipolar depression. Journal of Clinical Psychiatry 1997;58(3):104‐7. [DOI] [PubMed] [Google Scholar]
Frank 2007 {published data only}
- Frank E, Kupfer DJ, Buysse DJ, Swartz HA, Pilkonis PA, Houck PR, et al. Randomized trial of weekly, twice‐monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. American Journal of Psychiatry 2007;164(5):761‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamidian 2013 {published data only}
- Hamidian S, Omidi A, Mousavinasab SM, Naziri G. Comparison of the effect of mindfulness‐based cognitive therapy accompanied by pharmacotherapy with pharmacotherapy alone in treating dysthymic patients. Iranian Red Crescent Medical Journal 2013;15(3):239‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hellerstein 1994 {published data only}
- Hellerstein DJ, Yanowitch P, Rosenthal J, Hemlock C, Kasch K, Samstag L W, et al. Long‐term treatment of double depression: a preliminary study with serotonergic antidepressants. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1994;18(1):139‐47. [DOI] [PubMed] [Google Scholar]
Hellerstein 2015 {published data only}
- Hellerstein DJ, Erickson G, Stewart JW, McGrath PJ, Hunnicutt‐Ferguson K, Reynolds SK, et al. Behavioral activation therapy for return to work in medication‐responsive chronic depression with persistent psychosocial dysfunction. Comprehensive Psychiatry 2015;57:140‐7. [DOI] [PubMed] [Google Scholar]
Hellerstein 2017 {published data only}
- Hellerstein DJ, Hunnicutt‐Ferguson K, Stewart JW, McGrath PJ, Keller S, Peterson BS, et al. Do social functioning and symptoms improve with continuation antidepressant treatment of persistent depressive disorder? An observational study. Journal of Affective Disorders 2017;210:258‐64. [DOI] [PubMed] [Google Scholar]
Holländare 2013 {published data only}
- Holländare F, Anthony SA, Randestad M, Tillfors M, Carlbring P, Andersson G, et al. Two‐year outcome of Internet‐based relapse prevention for partially remitted depression. Behaviour Research and Therapy 2013;51(11):719‐22. [DOI] [PubMed] [Google Scholar]
Huijbers 2015 {published data only}
- Huijbers MJ, Spinhoven P, Spijker J, Ruhe HG, Schaik DJ, Oppen P, et al. Adding mindfulness‐based cognitive therapy to maintenance antidepressant medication for prevention of relapse/recurrence in major depressive disorder: randomised controlled trial. Journal of Affective Disorders 2015;187:54‐61. [DOI] [PubMed] [Google Scholar]
Jarrett 2013 {published data only}
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher‐risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry 2013;70(11):1152‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kok 2015 {published data only}
- Kok G, Burger H, Riper H, Cuijpers P, Dekker J, Marwijk H, et al. The three‐month effect of mobile Internet‐based cognitive therapy on the course of depressive symptoms in remitted recurrently depressed patients: results of a randomized controlled trial. Psychotherapy and Psychosomatics 2015;84(2):90‐9. [DOI] [PubMed] [Google Scholar]
Michalak 2015 {published data only}
- Michalak J, Schultze M, Heidenreich T, Schramm E. A randomized controlled trial on the efficacy of mindfulness‐based cognitive therapy and a group version of cognitive behavioral analysis system of psychotherapy for chronically depressed patients. Journal of Consulting and Clinical Psychology 2015;83(5):951‐63. [DOI] [PubMed] [Google Scholar]
Murray 2010 {published data only}
- Murray G, Michalak EE, Axler A, Yaxley D, Hayashi B, Westrin A, et al. Relief of Chronic Or Resistant Depression (Re‐ChORD): a pragmatic, randomized, open‐treatment trial of an integrative program intervention for chronic depression. Journal of Affective Disorders 2010;123(1‐3):243‐8. [DOI] [PubMed] [Google Scholar]
Petersen 2010 {published data only}
- Petersen TJ, Pava JA, Buchin J, Matthews JD, Papakostas GI, Nierenberg AA, et al. The role of cognitive‐behavioral therapy and fluoxetine in prevention of recurrence of major depressive disorder. Cognitive Therapy and Research 2010;34(1):13‐23. [Google Scholar]
Schramm 2017 {published data only}
- Schramm E, Kriston L, Zobel I, Bailer J, Wambach K, Backenstrass M, et al. Effect of disorder‐specific vs nonspecific psychotherapy for chronic depression: a randomized clinical trial. JAMA Psychiatry 2017;74(3):233‐42. [DOI] [PubMed] [Google Scholar]
Thase 2001 {published data only}
- Thase ME, Nierenberg AA, Keller MB, Panagides J. Efficacy of mirtazapine for prevention of depressive relapse: a placebo‐controlled double‐blind trial of recently remitted high‐risk patients. Journal of Clinical Psychiatry 2001;62(10):782‐8. [DOI] [PubMed] [Google Scholar]
van Aalderen 2015 {published data only}
- Aalderen JR, Donders AR, Peffer K, Speckens AE. Long‐term outcome of mindfulness‐based cognitive therapy in recurrently depressed patients with and without a depressive episode at baseline. Depression and Anxiety 2015;32(8):563‐9. [DOI] [PubMed] [Google Scholar]
Wiersma 2008 {published data only}
- Wiersma J, Schaik D, Oppen P, McCullough J, Schoevers R, Dekker J, et al. Treatment of chronically depressed patients: a multisite randomized controlled trial testing the effectiveness of 'Cognitive Behavioral Analysis System of Psychotherapy' (CBASP) for chronic depressions versus usual secondary care. BMC Psychiatry 2008;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT03219879 {published data only}
- NCT03219879. Telephone‐administered Relapse Prevention for Depression (NaTel) [Telephone‐administered Cognitive‐behavioral Relapse Prevention for Patients With Chronic and Recurrent Depression: A Multi‐center Randomized Clinical Trial]. clinicaltrials.gov/ct2/show/NCT03219879 (first received 18 July 2017).
Additional references
Anderson 2008
- Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology (Oxford, England) 2008;22(4):343‐96. [DOI] [PubMed] [Google Scholar]
APA 2010
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder: Third Edition. Washington (DC): American Psychiatric Association, 2010. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders – 5th Edition (DSM‐5). Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Arnow 2003
- Arnow BA, Constantino MJ. Effectiveness of psychotherapy and combination treatment for chronic depression. Journal of Clinical Psychology 2003;59(8):893‐905. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory‐II. San Antonio (TX): The Psychological Corporation, 1996. [Google Scholar]
Berndt 2000
- Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, et al. Lost human capital from early‐onset chronic depression. American Journal of Psychiatry 2000;157(6):940‐7. [DOI] [PubMed] [Google Scholar]
Berton 2006
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Reviews. Neuroscience 2006;7(2):137‐51. [DOI] [PubMed] [Google Scholar]
Beshai 2011
- Beshai S, Dobson KS, Bockting CL, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clinical Psychology Review 2011;31(8):1349‐60. [DOI] [PubMed] [Google Scholar]
Boland 2002
- Boland RJ, Keller MB. Course and outcome of depression. In: Gotlib IH, Hammen CL editor(s). Handbook of Depression. New York (NY): Guilford Press, 2002:43‐60. [Google Scholar]
Browne 2002
- Browne G, Steiner M, Roberts J, Gafni A, Byrne C, Dunn E, et al. Sertraline and/or interpersonal psychotherapy for patients with dysthymic disorder in primary care: 6‐month comparison with longitudinal 2‐year follow‐up of effectiveness and costs. Journal of Affective Disorders 2002;68(2‐3):317‐30. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Cosgrove‐Mather 2004
- Cosgrove‐Mather B. Anti‐depressant taken off market, 2014. www.cbsnews.com/news/anti‐depressant‐taken‐off‐market/ (accessed June 6, 2017).
Cuijpers 2010
- Cuijpers P, Straten A, Schuurmans J, Oppen P, Hollon Steven D, Andersson G. Psychotherapy for chronic major depression and dysthymia: a meta‐analysis. Clinical Psychology Review 2010;30(1):51‐62. [DOI] [PubMed] [Google Scholar]
Cuijpers 2013
- Cuijpers P, Sijbrandij M, Koole Sander L, Andersson G, Beekman Aartjan T, Reynolds Charles F. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta‐analysis of direct comparisons. World Psychiatry 2013;12(2):137‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Systematic Reviews in Health Care. BMJ Publishing Group, 2008:285‐312. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
DGPPN 2015
- DGPPN, BÄK, KBV, AWMF, AkdÄ, BPtK, BApK, DAGSHG, DEGAM, DGPM, DGPs, DGRW, editor(s), for the Guideline Group Unipolar Depression. S3‐guideline/national disease management guideline unipolar depression – long version, 1st edition: version 5, 2009. www.leitlinien.de/mdb/downloads/nvl/depression/depression‐1aufl‐vers5‐lang.pdf (accessed 12 August 2015).
Dunner 2005
- Dunner DL. Dysthymia and double depression. International Review of Psychiatry 2005;17(1):3‐8. [DOI] [PubMed] [Google Scholar]
Epstein 2014
- Epstein I, Szpindel I, Katzman MA. Pharmacological approaches to manage persistent symptoms of major depressive disorder: rationale and therapeutic strategies. Psychiatry Research 2014;220 Suppl 1:S15‐33. [DOI] [PubMed] [Google Scholar]
FDA 2016
- US Food, Drug Administration. What is a serious adverse event? 2016. www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm (accessed retrieved 6 June 2017).
Frank 1991
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry 1991;48(9):851‐5. [DOI] [PubMed] [Google Scholar]
Gilmer 2005
- Gilmer WS, Trivedi MH, Rush AJ, Wisniewski SR, Luther J, Howland RH, et al. Factors associated with chronic depressive episodes: a preliminary report from the STAR‐D project. Acta Psychiatrica Scandinavica 2005;112(6):425‐33. [DOI] [PubMed] [Google Scholar]
Glue 2010
- Glue P, Donovan MR, Kolluri S, Emir B. Meta‐analysis of relapse prevention antidepressant trials in depressive disorders. Australian and New Zealand Journal of Psychiatry 2010;44(8):697‐705. [DOI] [PubMed] [Google Scholar]
Guidi 2016
- Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta‐analysis of the sequential model and a critical review of the literature. American Journal of Psychiatry 2016;173(2):128‐37. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton A. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Imel 2008
- Imel ZE, Malterer MB, McKay KM, Wampold BE. A meta‐analysis of psychotherapy and medication in unipolar depression and dysthymia. Journal of Affective Disorders 2008;110(3):197‐206. [DOI] [PubMed] [Google Scholar]
Jarrett 1998
- Jarrett RB, Basco MR, Risser R, Ramanan J, Marwill M, Kraft D, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients?. Journal of Consulting and Clinical Psychology 1998;66(6):1036‐40. [DOI] [PubMed] [Google Scholar]
Jarrett 2001
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Archives of General Psychiatry 2001;58(4):381‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamenov 2017
- Kamenov K, Twomey C, Cabello M, Prina AM, Ayuso‐Mateos JL. The efficacy of psychotherapy, pharmacotherapy and their combination on functioning and quality of life in depression: a meta‐analysis. Psychological Medicine 2017;47(3):414‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keller 1992
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, et al. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5‐year prospective follow‐up of 431 subjects. Archives of General Psychiatry 1992;49(10):809‐16. [DOI] [PubMed] [Google Scholar]
Keller 1998a
- Keller MB, Gelenberg AJ, Hirschfeld RM, Rush AJ, Thase ME, Kocsis JH, et al. The treatment of chronic depression, part 2: a double‐blind, randomized trial of sertraline and imipramine. Journal of Clinical Psychiatry 1998;59(11):598‐607. [DOI] [PubMed] [Google Scholar]
Keller 2000
- Keller MB, McCullough JP, Klein DN, Arnow BA, Rush AJ, Nemeroff CB, et al. A comparison of nefazadone, the Cognitive Behavioral Analysis System of Psychotherapy and their combination for the treatment of chronic depression. New England Journal of Medicine 2000;322:1462‐70. [DOI] [PubMed] [Google Scholar]
Keller 2007
- Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, Nemeroff CB, et al. The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study: outcomes from the 2‐year and combined maintenance phases. Journal of Clinical Psychiatry 2007;68(8):1246‐56. [DOI] [PubMed] [Google Scholar]
Kennedy 2009
- Kennedy SH, Lam RW, Parikh SV, Patten SB, Ravindran AV. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. Journal of Affective Disorders 2009;117:S1‐2. [DOI] [PubMed] [Google Scholar]
Kessler 2005
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 2005;62(6):593‐602. [DOI] [PubMed] [Google Scholar]
Kirsch 2008
- Kirsch I, Deacon BJ, Huedo‐Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta‐analysis of data submitted to the Food and Drug Administration. PLoS Medicine 2008;5(2):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2010
- Klein DN. Chronic depression: diagnosis and classification. Current Directions in Psychological Science 2010;19(2):96‐100. [Google Scholar]
Klein 2011
- Klein JP, Steinlechner S, Sipos V, Schweiger U. Psychotherapie chronischer Depression nach dem Cognitive Behavioral Analysis System of Psychotherapy (CBASP) – Konzept. Psychotherapie, Psychosomatik, Medizinische Psychologie 2011;61(12):526‐35. [DOI] [PubMed] [Google Scholar]
Kocsis 1997
- Kocsis JH, Schatzberg AF, Rush AJ, Korstein S, Klein D, Hirschfeld RM. Maintenance therapy of chronic depression: a placebo‐controlled trial of sertraline. Biological Psychiatry 1997;42(1):240S. [Google Scholar]
Kocsis 2002
- Kocsis JH, Schatzberg A, Rush A, Klein DN, Howland R, Gniwesch L, et al. Psychosocial outcomes following long‐term, double‐blind treatment of chronic depression with sertraline vs placebo. Archives of General Psychiatry 2002;59(8):723‐8. [DOI] [PubMed] [Google Scholar]
Kocsis 2008
- Kocsis JH, Gelenberg AJ, Rothbaum B, Klein DN, Trivedi MH, Manber R, et al. Chronic forms of major depression are still undertreated in the 21st century: systematic assessment of 801 patients presenting for treatment. Journal of Affective Disorders 2008;110(1‐2):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kriston 2014
- Kriston L, Wolff A, Westphal A, Holzel LP, Harter M. Efficacy and acceptability of acute treatments for persistent depressive disorder: a network meta‐analysis. Depression and Anxiety 2014;31(8):621‐30. [DOI] [PubMed] [Google Scholar]
Ladwig 2014
- Ladwig I, Rief W, Nestoriuc Y. Welche risiken und nebenwirkungen hat psychotherapie? – entwicklung des Inventars zur Erfassung Negativer Effekte von Psychotherapie (INEP). Verhaltenstherapie 2014;24(4):252‐63. [Google Scholar]
Lilienfeld 2007
- Lilienfeld SO. Psychological treatments that cause harm. Perspectives on Psychological Science 2007;2(1):53‐70. [DOI] [PubMed] [Google Scholar]
Marin 1994
- Marin DB, Kocsis JH, Frances AJ, Parides M. Desipramine for the treatment of "pure" dysthymia versus "double" depression. American Journal of Psychiatry 1994;151(7):1079‐80. [DOI] [PubMed] [Google Scholar]
McCullough 2000
- McCullough JP. Treatment for Chronic Depression: Cognitive Behavioral Analysis System of Psychotherapy. New York (NY): Guilford Press, 2000. [DOI] [PubMed] [Google Scholar]
Meister 2016
- Meister R, Wolff A, Mohr H, Nestoriuc Y, Harter M, Holzel L, et al. Adverse event methods were heterogeneous and insufficiently reported in randomized trials on persistent depressive disorder. Journal of Clinical Epidemiology 2016;71:97‐108. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
MQ 2016
- MQ Transforming Mental Health Through Research. Depression: asking the right questions. Project report. Identifying priorities for depression research, 2016. www.depressionarq.org (accessed prior to 19 October 2018).
Murphy 2012
- Murphy JA, Byrne GJ. Prevalence and correlates of the proposed DSM‐5 diagnosis of chronic depressive disorder. Journal of Affective Disorders 2012;139(2):172‐80. [DOI] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795‐801. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Collaborating Centre for Mental Health. Depression. The Treatment and Management of Depression in Adults (Updated Edition): National Clinical Practice Guideline 90. Leicester and London: The British Psychological Society and the Royal College of Psychiatrists, 2010. [PubMed] [Google Scholar]
Nutt 2007
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. Journal of Psychopharmacology (Oxford, England) 2007;21(5):461‐71. [DOI] [PubMed] [Google Scholar]
Nutt 2008
- Nutt DJ, Sharpe M. Uncritical positive regard? Issues in the efficacy and safety of psychotherapy. Journal of Psychopharmacology (Oxford, England) 2008;22(1):3‐6. [DOI] [PubMed] [Google Scholar]
Piet 2011
- Piet J, Hougaard E. The effect of mindfulness‐based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta‐analysis. Clinical Psychology Review 2011;31(6):1032‐40. [DOI] [PubMed] [Google Scholar]
Pringle 2011
- Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2011;35(7):1586‐92. [DOI] [PubMed] [Google Scholar]
Rabinowitz 2016
- Rabinowitz J, Werbeloff N, Mandel FS, Menard F, Marangell L, Kapur S. Initial depression severity and response to antidepressants v. placebo: patient‐level data analysis from 34 randomised controlled trials. British Journal of Psychiatry 2016;209(5):427‐8. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rief 2011
- Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E. Assessing general side effects in clinical trials: reference data from the general population. Pharmacoepidemiology and Drug Safety 2011;20(4):405‐15. [PUBMED: 21442687] [DOI] [PubMed] [Google Scholar]
Rouillon 1989
- Rouillon F, Phillips R, Serrurier D, Ansart E, et al. Rechutes de dépression unipolaire et efficacité de la maprotiline [Prophylactic efficacy of maprotiline on relapses of unipolar depression]. L'Encéphale: Revue de Psychiatrie Clinique Biologique et Thérapeutique 1989;15(6):527‐34. [PubMed] [Google Scholar]
Rush 1998
- Rush AJ, Koran LM, Keller MB, Markowitz JC, Harrison WM, Miceli RJ, et al. The treatment of chronic depression, part 1: study design and rationale for evaluating the comparative efficacy of sertraline and imipramine as acute, crossover, continuation, and maintenance phase therapies. Journal of Clinical Psychiatry 1998;59(11):589‐97. [PubMed] [Google Scholar]
Rush 2000
- Rush AJ, Carmody T, Reimitz P‐E. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS‐C) and Self‐Report (IDS‐SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research 2000;9(2):45‐59. [Google Scholar]
Segal 2002
- Segal ZV Williams JM, Teasdale JD. Mindfulness‐based Cognitive Therapy for Depression: a New Approach to Preventing Relapse. New York (NY): Guilford, 2002. [Google Scholar]
Shih 2002
- Shih WJ. Problems in dealing with missing data and informative censoring in clinical trials. Current Controlled Trials in Cardiovascular Medicine 2002;3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skevington 2004
- Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL‐BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research 2004;13(2):299‐310. [DOI] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny A‐M, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ (Clinical Research Ed.) 2003;326(7387):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spijker 2002
- Spijker J, Graaf Rde, Bijl RV, Beekman AT, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). British Journal of Psychiatry 2002;181:208‐13. [DOI] [PubMed] [Google Scholar]
Spijker 2013
- Spijker J, Straten A, Bockting CL, Meeuwissen JA, Balkom A. Psychotherapy, antidepressants, and their combination for chronic major depressive disorder: a systematic review. Canadian Journal of Psychiatry 2013;58(7):386‐92. [DOI] [PubMed] [Google Scholar]
Spitzer 1999
- Spitzer RL, Kroenke K, Williams J B. Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282(18):1737‐44. [DOI] [PubMed] [Google Scholar]
Stangier 2013
- Stangier U, Hilling C, Heidenreich T, Risch AK, Barocka A, Schlosser R, et al. Maintenance cognitive‐behavioral therapy and manualized psychoeducation in the treatment of recurrent depression: a multicenter prospective randomized controlled trial. American Journal of Psychiatry 2013;170(6):624‐32. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001;323(7304):101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; Vol. 355:i4919. [DOI] [PMC free article] [PubMed]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Vittengl 2007
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta‐analysis of cognitive‐behavioral therapy's effects. Journal of Consulting and Clinical Psychology 2007;75(3):475‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vittengl 2009
- Vittengl JR, Clark LA, Jarrett RB. Continuation‐phase cognitive therapy's effects on remission and recovery from depression. Journal of Consulting and Clinical Psychology 2009;77(2):367‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Wolff 2012
- Wolff A, Hölzel L, Westphal A, Harter M, Kriston L. Combination of pharmacotherapy and psychotherapy in the treatment of chronic depression: a systematic review and meta‐analysis. BMC Psychiatry 2012;12(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Wolff 2013
- Wolff A, Holzel LP, Westphal A, Harter M, Kriston L. Selective serotonin reuptake inhibitors and tricyclic antidepressants in the acute treatment of chronic depression and dysthymia: a systematic review and meta‐analysis. Journal of Affective Disorders 2013;144(1‐2):7‐15. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International Classification of Mental and Behavioural Disorders, 10th Revision (ICD‐10). Geneva: WHO, 1992. [Google Scholar]
WHO 2008
- World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: WHO Press, 2008. [Google Scholar]
Wilkinson 2016
- Wilkinson P, Izmeth Z. Continuation and maintenance treatments for depression in older people. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD006727.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
von Wolff 2014
- Wolff A, Liebherz S. Comparative effectiveness of Continuation and Maintenance Treatments for Chronic Depression (COMACHRON): a systematic review. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014009928 (accessed prior to 18 October 2018).


