Abstract
Inbred strain 13/N guinea pigs are frequently used as animal models in studies of emerging and high-pathogenicity viruses. To date, clinical reference intervals have not been established for hematology and clinical chemistry parameters in this strain. We obtained whole-blood samples from the cranial vena cava of healthy strain 13/N colony animals for inhouse CBC and clinical chemistry analyses. Analyte values were investigated to determine subpopulation differences according to age and sex. Glucose, albumin, ALP, lymphocyte percentage, Hgb, and MCHC decreased with age, whereas neutrophil and monocyte percentages, BUN, creatinine, calcium, and amylase increased with age. Total protein and WBC counts increased over the first 300 d of life before stabilizing. Across all age categories, female guinea pigs consistently had lower RBC, Hct, Hgb, ALT, ALP, and amylase levels and higher MCV values than males. These trends were strongest in adults (age, 151 through 900 d). Most parameters stabilized by 300 d; previous studies used 60 d or 120 d as adult age and 90 to 120 d as sexual maturity. We recommend age group definitions of 0 through 150 d for juveniles, 151 through 900 d for adults, and older than 900 d for geriatric adult strain 13/N guinea pigs.
Origins of strain 13/N guinea pigs date back to their generation in 1906 by the USDA.7 This tricolored strain is notably valuable in the development of animal models for highly pathogenic viruses, such as arenaviruses that cause hemorrhagic fevers in humans.15,22,23 Strain 13/N guinea pigs are more susceptible to infection with Lassa and Lujo viruses than are mice and outbred guinea pig strains.4,12 Uniquely, strain 13/N animals typically uniformly succumb to infection by these agents and mirror many characteristics of human disease without the need for rodent-adapted virus variants. Therefore, these animals have been used to investigate pathogenesis, screen therapeutics, and develop vaccine candidates for arenaviruses and other high-containment, high-consequence agents.4,5,11,13,21,26 In addition, recent attempts have been made to establish this strain as a new model for Zika virus infection, albeit thus far unsuccessfully.16
Due to the limited number of strain 13/N guinea pig colonies maintained and their specialized use in infectious disease research, few studies report the normal health parameters of these animals. Importantly, to date, no comprehensive reports of clinical reference intervals for this strain have been published. Blood from both healthy control and infected animals is routinely analyzed during novel viral pathogenesis studies, but determining significant hallmarks of the disease can be challenging without a larger database for comparisons.3,26 Benchmarking normal baselines will provide better insight into the overall health of strain 13/N guinea pigs and validate deviations from expected ranges as clinically relevant when they are used as a human disease model.
Here we establish reference intervals for hematology and clinical chemistry analytes in a healthy strain 13/N population. Published reference intervals for other strains of guinea pigs have often been limited in the number, age, and sex of animals included. Studies that included a wider age range saw values for WBC, neutrophils, monocytes, total protein, BUN, and creatinine increase over time, whereas lymphocyte percentages and ALP decrease with age.14,22,28 We sought to determine similar age correlations in CBC and clinical chemistry analytes by collecting samples from strain 13/N guinea pigs representing a wide age range. In particular, we aimed to determine the age at which hematology and clinical chemistry values in juvenile strain 13/N guinea pigs normalize and are comparable to those of a stable adult population.
Previous studies have shown that levels of the liver enzymes ALP and ALT differed between females and males in other strains of guinea pigs, and CBC parameters including RBC, Hgb, and Hct were lower in females than males.22,28 Because discerning differences between males and females is important when interpreting clinical data, we investigated sex-associated differences in both hematology and clinical chemistry analytes in strain 13/N guinea pigs. We hypothesized that, as in other lab animal species and guinea pig strains, WBC in strain 13/N animals would increase with age, whereas the ratio of leukocytes would invert from lymphocyte-dominant to neutrophil-dominant. In addition, we predicted that the chemistry analytes BUN and creatinine would increase with age. We anticipated that multiple analytes would differ depending on the animal's sex, particularly those related to erythrocytes (RBC, Hgb, Hct), as seen in other strains.
Materials and Methods
Animals.
Healthy strain 13/N guinea pigs were selected from the Centers for Disease Control and Prevention breeding colony, originating from 22 breeding age animals obtained from Iowa State University in October 2012, for establishing hematology (age, 1 through 44 mo; 33 male and 27 female) and clinical chemistry (age, 1 through 46 mo, 38 male and 39 female) reference intervals. Among these animals was a cohort of 16 pups (10 male and 6 female), from which blood samples were collected monthly from 1 to 6 mo for analysis of baseline changes in growing animals.
Animal work was approved by the Centers for Disease Control and Prevention IACUC and conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, at an AAALAC-accredited facility.10 Active breeding groups and sows with pups younger than 6 wk were housed in conventional rack-style caging (Techniplast USA, West Chester, PA). All other animals were socially housed in sex-segregated floor pens with paper (Techboard and Poly Pads, Shepherd Specialty Papers, Watertown, NY) and paper nesting material (EnviroDri, FiberCore LLC, Cleveland, OH). Guinea pigs were provided unrestricted bottled spring water (Niagara Bottling, Ontario, CA) and pelleted diet (LabDiet 5025, Land O'Lakes, St Louis, MO) with daily timothy hay and supplemental vitamin C-rich fresh vegetables 3 times weekly. Environmental enrichment was provided in the form of plastic tunnels (Guinea Pig Hut, Bio-Serv, Flemington, NJ) and wood gnawing materials (manzanita sticks and wood blocks, Bio-Serv). Environmental parameters were maintained within a temperature range of 68 to 79 °F (20.0 to 26.1 °C) and 30% to 70% relative humidity on a 12:12-h light:dark cycle. Annual health monitoring of the colony includes serology (Guinea Pig Basic Opti-Spot, IDEXX BioResearch, Columbia, MO), fecal examinations, and baseline CBC and clinical chemistry analyses. All animals were free of Clostridium piliforme, guinea pig parainfluenza virus 3, lymphocytic choriomeningitis virus, murine pneumonia virus, Sendai virus, and Encephalitozoon cuniculi, as determined by using serologic screening tests.
Blood collection.
Blood was collected from the cranial vena cava of each anesthetized guinea pig (3% to 5% isoflurane anesthesia) by using a 25-gauge needle and 1-mL syringe (Becton–Dickinson, Franklin Lakes, NJ) and immediately deposited into EDTA microtainer tubes (Becton-Dickinson) and lithium–heparin microtubes (catalog no. 41.1393.105, Sarstedt, Nümbrecht, Germany). Automated blood smears were made in duplicate at the time of venipuncture from whole blood (HemaPrep Automated Blood Smearing Instrument, JP Gilbert Company, Boyertown, PA). Each animal was sampled once for this study, except for the young pup cohort, where serial sampling was performed monthly. Blood sample amounts ranged from 0.5 to 1 mL and were limited to a maximum of 7.5% of total blood volume by weight. Samples were obtained between 1000 and 1400 to limit the influence of natural daily fluctuations. For samples exhibiting visible clots or platelet histogram abnormalities, additional blood for reevaluation was taken 5 to 7 d later than the initial sample.
Automated and manual sample analysis.
Blood was examined within 4 h of collection at room temperature. CBC were performed on a VetScan HM5 hematology analyzer (Abaxis, Union City, CA) by using EDTA-treated whole blood. Several individual samples were run in duplicate or triplicate as a secondary quality-control method and were averaged in the dataset. The hematology analyzer was maintained in accordance with manufacturer guidelines, including an instrument performance study. All CBC values, other than WBC differentials (which were assessed manually), were analyzed by using the automated platform. Blood smears were processed with Wright–Giemsa stain (Shandon Wright–Giemsa Kit, Thermo Fisher Scientific, Waltham, MA) in a flooded slide preparation. Slides were randomized and masked for a 7-way WBC differential count (neutrophils, lymphocytes, monocytes, eosinophils, Foa–Kurloff cells, basophils, and nucleated RBC) performed in duplicate (by SG and TH; Figure 1). Results of the 7-way differentials were combined and averaged for each animal. Nucleated RBC did not exceed 3 per 100 WBC counted and were not used to adjust the overall automated WBC count. When high-quality blood smears were unavailable, the automated WBC differential data for neutrophils, lymphocytes, and monocytes were used at these time points. These animals were not included in calculations of the eosinophil, basophil, and Foa–Kurloff percentages due to lack of data and have been notated appropriately in the accompanying tables. Chemistry panels were analyzed by using whole blood in lithium–heparin tubes and the General Chemistry 13 panel on the Piccolo Xpress Analyzer (Abaxis).
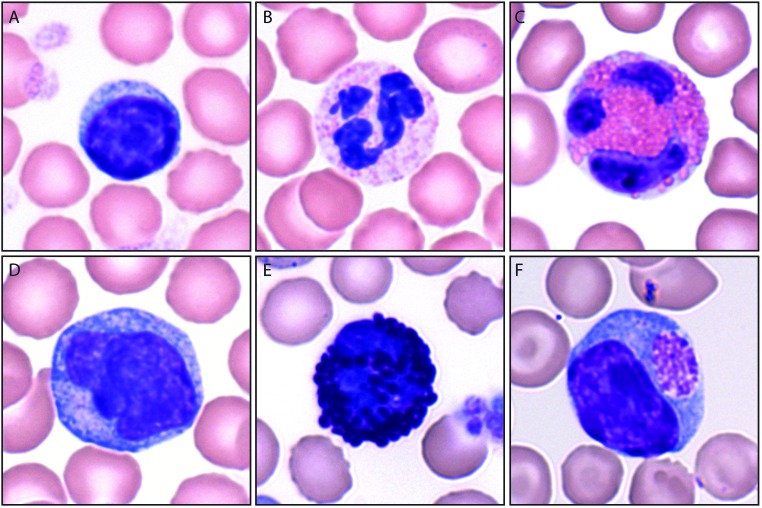
Figure 1.
WBC commonly found in guinea pig blood smears. (A) Lymphocytes have a higher nuclear:cytoplasmic ratio than (D) monocytes. Eosinophils (C) are easily differentiated from (B) neutrophils, which also are known as heterophils in rabbits and guinea pigs, by the larger, more prominent pink granules in eosinophils. (E) Basophils contain large blue-purple granules. (F) Foa-Kurloff cells (F) contain pink intracytoplasmic inclusion bodies of varying size and have shown natural killer activity in vitro. Wright-Giemsa staining; magnification, ×100 (oil immersion).
Statistical methods.
Data were analyzed by using Prism (version 7.01, GraphPad Software, La Jolla, CA). Results were screened for outliers by using the Grubbs method and evaluated for normality with the D'Agostino–Pearson test. A single time point for each animal was selected at random for data analysis, to limit any potential bias of including multiple time points for individual animals. One male pup was removed from analysis due to underlying clinical disease. Correlation with age was evaluated using the Spearman coefficient. For the chemistry panel parameters GGT and creatinine, for which zero values are unavailable with this analyzer, the midway point of the lower range was used (2.5 U/L and 0.1 mg/dL, respectively).
To isolate the effects of sex from those of age, guinea pigs were separated into 3 age groups: juvenile (age, 0 through 150 d; weight, 285 to 915 g; CBC: males, n = 9; females, n = 7 female; chemistry: males, n = 11; females, n = 8); adult, (age, 151 through 900 d; weight, 400 to 1200 g; CBC: males, n = 18; females, n = 16; chemistry: males, n = 16; females, n = 22); and geriatric adult (age, older than 900 d; weight, 735 to 1100 g; CBC: males, n = 5; females, n = 4; chemistry: males, n = 10; females, n = 9). Cut-offs for age bracket were determined in light of the established age of sexual maturity, onset of decreased fecundity, and development of age-related diseases in animals of the facility's strain 13/N colony.8,20 When generating reference intervals according to sex, a one-time point for each pup at 150 d or older was integrated with adult cohort data. An unpaired t test or nonparametric Mann–Whitney test was used to evaluate sex-related differences. In the serially sampled pup cohort, one-way ANOVA or Kruskal–Wallis nonparametric tests were used to compare monthly averages with the adult cohort (age 151 through 900 d of age). Results were corrected for multiple comparisons by using the false discovery rate method with an α value of 0.05 and were considered statistically significant at a P value less than 0.05.
Results
Hematology.
Common hematology parameters (WBC; neutrophil, lymphocyte, monocyte, eosinophil, Foa–Kurloff cell, and basophil percentages; RBC; Hgb; Hct; MCV; MCH; MCHC; platelet count; and MPV) for strain 13/N guinea pigs were evaluated (Figures 2 and 3). On the basis of these values, reference intervals were established for animals 151 to 900 d of age (Table 1). Age was correlated with various CBC parameters in both the colony population overall (neutrophil, lymphocyte, and monocyte percentages; Hgb; MCHC; and MPV) and when the population was separated according to sex (neutrophil and lymphocyte percentages; RBC; Hgb; MCH; MCHC; and MPV; Table 2). In the instrument performance study, all analytes had an interassay CV of less than 7%, except monocytes (CV, 9.72%). The monocyte variation had limited effects on WBC count ranges due to the use of manual blood-smear differentials at most time points. Manual differential data for eosinophils, Foa–Kurloff cells, and basophils were unavailable from 12 of 59 animals for correlation calculations, given that, on average, each of these 3 cell types accounted for less than 3% of the overall WBC manual differential counts, the absence of these data points is unlikely to distort the data. Total WBC counts peaked between 260 to 310 d and slowly decreased thereafter (Figure 3 A). Percentages of neutrophils (r = 0.771) and monocytes (r = 0.263) increased with age, whereas lymphocyte percentage (r = –0.797) decreased with age (Figure 3 A). Hgb (r = –0.307) and subsequently MCHC (r = –0.334) decreased with age, but no statistically significant differences were seen for erythrocyte counts (Table 2, Figure 2). Platelet levels decreased from birth to 800 d before abruptly increasing in the geriatric adult cohort, thus negating any overall age correlation effects (Figure 3 A). Platelet size (measured by mean volume) increased with age (r = 0.469).
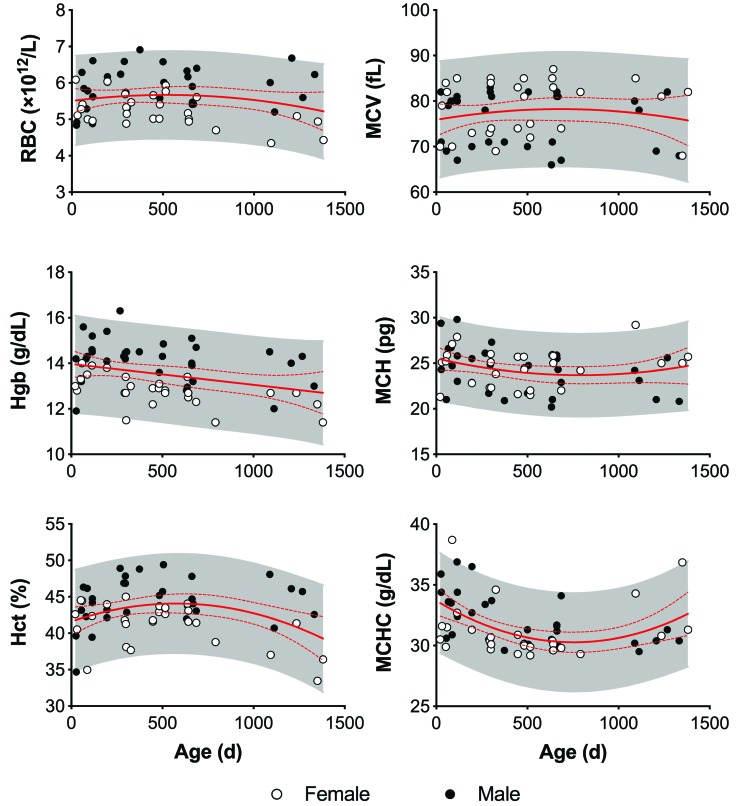
Figure 2.
Erythrocyte analysis by sex and age in strain 13/N guinea pigs. Erythrocyte number (RBC), Hgb, and Hct are consistently higher in males than females. These parameters peak by 450 d and then decrease over time. Erythrocyte volume (MCV) has a bimodal distribution that is undifferentiated by age or sex. The solid and dashed red lines represent the mean and 95% CI, respectively, of each parameter. The shaded area represents the 95% predictive interval of each parameter.
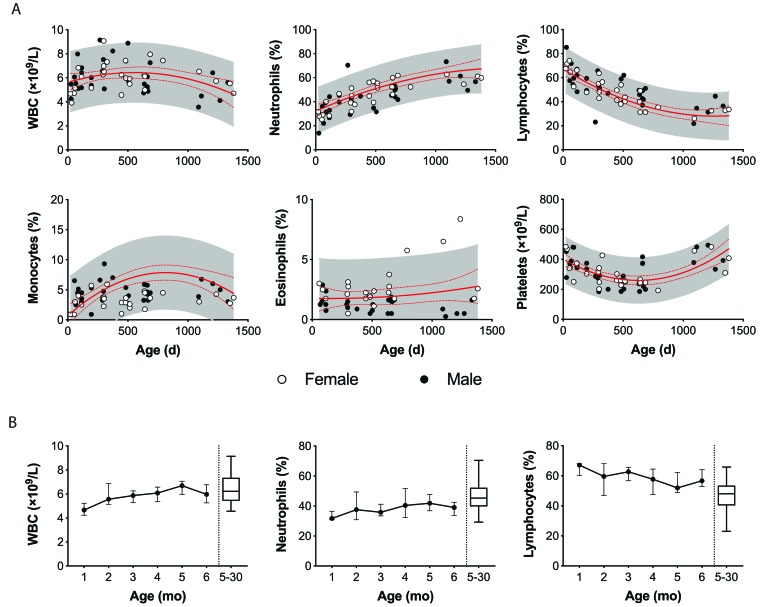
Figure 3.
Lymphocyte analysis by sex and age in strain 13/N guinea pigs. (A) Changes with age in WBC, neutrophils, lymphocytes, monocytes, eosinophils, and platelets in female and male strain 13/N guinea pigs. (B) Longitudinal sampling of 15 strain 13/N guinea pig pups revealed a steady increase in total WBC and neutrophils and decreasing percentage of lymphocytes over the first 6 mo of life compared with the adult cohort (age, 5–30 mo). The solid and dashed red lines represent the mean and 95% CI, respectively, of each parameter. The shaded area represents the 95% predictive interval of each parameter.
Table 1.
Hematology reference intervals for strain 13/N guinea pigs (age, 150–900 d)
| Female (n = 28) | Male (n = 27) | |||||||||
| Mean | 1 SD | Median | 2.5–97.5 Percentile | Interquartile range | Mean | 1 SD | Median | 2.5–97.5 Percentile | Interquartile range | |
| WBC (×109/L) | 6.3 | 1.1 | 6.1 | 4.6–9.1 | 5.5–7.1 | 6.4 | 1.4 | 6.3 | 4.7–10.1 | 5.3–7.3 |
| Neutrophils (%) | 45 | 10.5 | 45 | 24–62 | 38–52 | 43 | 9 | 42 | 30–70 | 39–49 |
| Lymphocytes (%) | 49 | 11 | 49 | 31–70 | 40–57 | 50 | 9 | 51 | 23–66 | 43–58 |
| Monocytes (%)b | 3.5 | 1.2 | 3.5 | 1–6 | 2.75–4.5 | 4.5 | 1.7 | 4.4 | 2.5–9.3 | 3.3–5.5 |
| Eosinophils (%)a,c | 2.2 | 1.1 | 2 | 0.5–5.75 | 1.4–2.75 | 1 | 0.6 | 1.3 | 0.25–2.5 | 0.5–1.5 |
| Foa-Kurloff cells (%)a,c | 0.4 | 0.3 | 0.25 | 0–1 | 0.125–0.75 | 1 | 0.6 | 0.9 | 0.125–2.75 | 0.75–1.25 |
| Basophils (%)a | 0.2 | 0.2 | 0.25 | 0–0.625 | 0–0.4 | 0.2 | 0.2 | 0.25 | 0–0.5 | 0–0.5 |
| RBC (×1012/L)c | 5.3 | 0.4 | 5.3 | 4.7–6.3 | 5.0–5.7 | 6.0 | 0.5 | 6.0 | 5.3–6.9 | 5.7–6.4 |
| Hgb (g/dL)c | 13 | 0.9 | 12.9 | 11.4–15.6 | 12.7–13.4 | 14.4 | 0.8 | 14.3 | 12.8–16.3 | 13.9–15.1 |
| Hct (%)c | 42 | 3 | 42 | 35–47 | 39–44 | 46 | 2.2 | 47 | 42–49.4 | 44.0–47.8 |
| MCV (fL)b | 79 | 6 | 82 | 69–87 | 74–85 | 76 | 6 | 80 | 66–83 | 71–82 |
| MCH (pg) | 24.5 | 2.2 | 25 | 21.5–29 | 22.3–25.9 | 24 | 2 | 24.5 | 20.2–27.5 | 22.5–25.5 |
| MCHC (g/dL) | 30.9 | 1.9 | 30.1 | 29.2–36.9 | 29.8–30.9 | 31.4 | 1.8 | 30.8 | 29.2–36.5 | 30.1–32.7 |
| Platelets (×109/L) | 278 | 56 | 259 | 193–427 | 248–304 | 260 | 71 | 237 | 164–417 | 201–293 |
| Mean platelet volume (fL) | 7.6 | 0.2 | 7.6 | 7.1–8.2 | 7.4–7.7 | 7.5 | 0.1 | 7.5 | 7.2–7.8 | 7.4–7.6 |
Analytes with statistically significant differences according to sex in mean (Gaussian) or median (non-Gaussian) values are reported.
Due to the lack of data from manual differential counts, analysis for these parameters was performed by using data from 21 females and 23 males.
P < 0.05 between sex-specific values.
P < 0.01 between sex-specific values.
Table 2.
Correlation (r) of hematology parameter values with age in female and male strain 13/N guinea pigs
| Female (n = 27) | Male (n = 32) | |
| WBC (×109/L) | 0.1799 | –0.1934 |
| Neutrophils (%) | 0.8424d | 0.7396d |
| Lymphocytes (%) | –0.8690d | –0.7541d |
| Monocytes (%) | 0.3039 | 0.2552 |
| Eosinophils (%)a | 0.2442 | –0.2442 |
| Foa-Kurloff cells (%)a | 0.0812 | 0.2213 |
| Basophils (%)a | 0.2814 | –0.2329 |
| RBC (×1012/L) | –0.4274b | 0.1893 |
| Hct (%) | –0.3506 | 0.1839 |
| Hgb (g/dL) | –0.6496d | –0.2217 |
| MCV (fL) | 0.1346 | 0.0031 |
| MCH (pg) | –0.0159 | –0.3694b |
| MCHC (g/dL) | –0.1817 | –0.5468c |
| Platelets (×109/L) | –0.2327 | –0.0624 |
| Mean platelet volume (fL) | 0.4517b | 0.4961c |
Due to lack of data from manual differential counts, analysis was performed by using data from 22 females and 25 males for these parameters.
Values that differ according to age are indicated (bP < 0.05, cP < 0.01, dP < 0.001).
Among the cohort of serially sampled pups, WBC, neutrophil percentage, RBC, Hgb, Hct, and mean platelet volume increased with age over the 6-mo period (Figure 3 B). By 60 d, total WBC, RBC, Hgb, and Hct reached values similar to those of the adult (that is, 151 to 900 d old) population, but MCH, MCHC, and platelet values were higher in juveniles than adults at all time points.
Sex-related differences were observed in several hematologic analytes, both in the relative strength of age correlations for various analytes (Table 2) and expected value range (Table 1). Male guinea pigs had higher RBC (P < 0.0001), Hct (P < 0.001), and Hgb (P < 0.0001) and percentages of Foa–Kurloff cells (P < 0.001) and monocytes (P < 0.01) than females, whereas EOS percentages (P < 0.0001) and MCV (P < 0.05) were higher in females. Male sex had a stronger correlation with MCHC and mean platelet volume than female (r = –0.547 and 0.496, respectively), whereas females showed stronger association with decreasing Hgb (r = –0.650). Total RBC counts decreased with age in female guinea pigs (r = –0.427), and MCH decreased with age in males (r = -0.369), but these correlations were not apparent in the overall population when both sexes were combined.
Clinical chemistry.
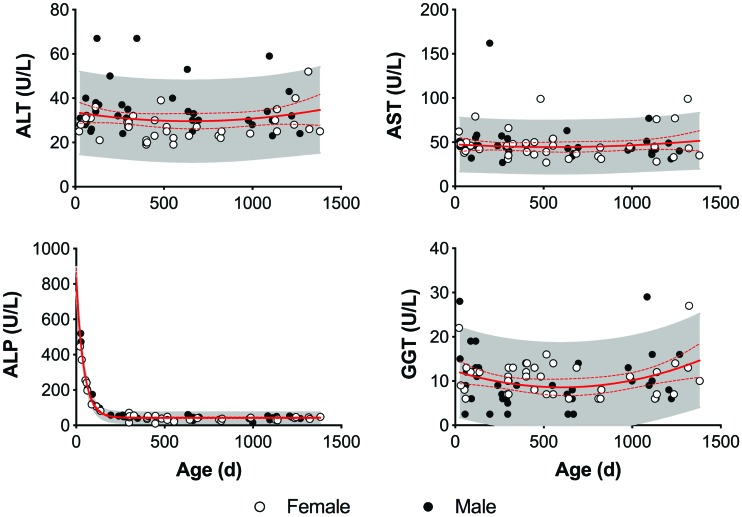
Eleven blood analytes—glucose, BUN, creatinine, calcium, total protein, albumin, AST, ALT, ALP, GGT, total bilirubin, and amylase—were evaluated to establish clinical chemistry reference intervals for strain 13/N guinea pigs 151 to 900 d old (Table 3) and to determine any notable differences based on sex or age. Several chemistry analytes varied with age, both visually (Figures 4 and 5) and statistically (Table 4). Glucose (r = –0.282), albumin (r = –0.386), and ALP (r = –0.728) decreased with age, whereas BUN (r = 0.488), creatinine (r = 0.590), calcium (r = 0.246), and amylase (r = 0.337) increased with age. Total protein peaked at 400 to 450 d and then slowly decreased with age (Figure 4).
Table 3.
Clinical chemistry reference intervals for strain 13/N guinea pigs (age, 150 to 900 d)
| Female (n = 28) | Male (n = 27) | |||||||||
| Mean | 1 SD | Median | 2.5–97.5 Percentile | Interquartile range | Mean | 1 SD | Median | 2.5–97.5 Percentile | Interquartile range | |
| Glucose (mg/dL) | 143 | 16 | 145 | 115–179 | 127–154 | 149 | 29 | 150 | 80–218 | 138–167 |
| BUN (mg/dL) | 20.5 | 3.3 | 21 | 14–26 | 18–23 | 20.6 | 2.8 | 20 | 17–29 | 19–22 |
| Creatinine (mg/dL)c | 0.32 | 0.2 | 0.3 | 0.1–0.8 | 0.1–0.5 | 0.5 | 0.2 | 0.5 | 0.1–0.9 | 0.4–0.6 |
| Total protein (g/dL) | 5.4 | 0.3 | 5.5 | 4.7–5.9 | 5.1–5.7 | 5.2 | 0.2 | 5.2 | 4.8–5.6 | 5.1–5.4 |
| Albumin (g/dL) | 2.9 | 0.2 | 2.9 | 2.7–3.3 | 2.7–3.0 | 2.9 | 0.2 | 2.9 | 2.2–3.2 | 2.7–3 |
| Calcium (mg/dL) | 11.4 | 0.5 | 11.5 | 10.4–12.2 | 11–11.8 | 11.5 | 0.4 | 11.5 | 10.5–12.2 | 11.2–11.9 |
| ALP (U/L)b | 43 | 15 | 44 | 10–74 | 32–53 | 53 | 16 | 54 | 16–83 | 45–67 |
| ALT (U/L)c | 27 | 6 | 27 | 19–39 | 22–30 | 36 | 9 | 34 | 24–67 | 30–39 |
| AST (U/L)a | 46 | 15 | 45 | 27–99 | 35–50 | 50 | 25 | 44 | 27–162 | 40–54 |
| GGT (U/L)b | 10 | 3 | 11 | 6–16 | 7–13 | 8 | 4 | 8 | 2.5–21 | 6–10 |
| Total bilirubin (mg/dL)a | 0.3 | 0.04 | 0.3 | 0.3–0.4 | 0.3–0.3 | 0.32 | 0.04 | 0.3 | 0.3–0.4 | 0.3–0.3 |
| Amylase (U/L)c | 1149 | 131 | 1190 | 850–1380 | 1031–1253 | 1297 | 120 | 1305 | 964–1519 | 1243–1371 |
Analytes with statistically significant differences by sex in mean (Gaussian) or median (non-Gaussian) are reported.
Due to machine error, analysis of these parameters was performed by using data from 26 females and 25 males.
P < 0.05 between sex-specific values.
P < 0.01 between sex-specific values.
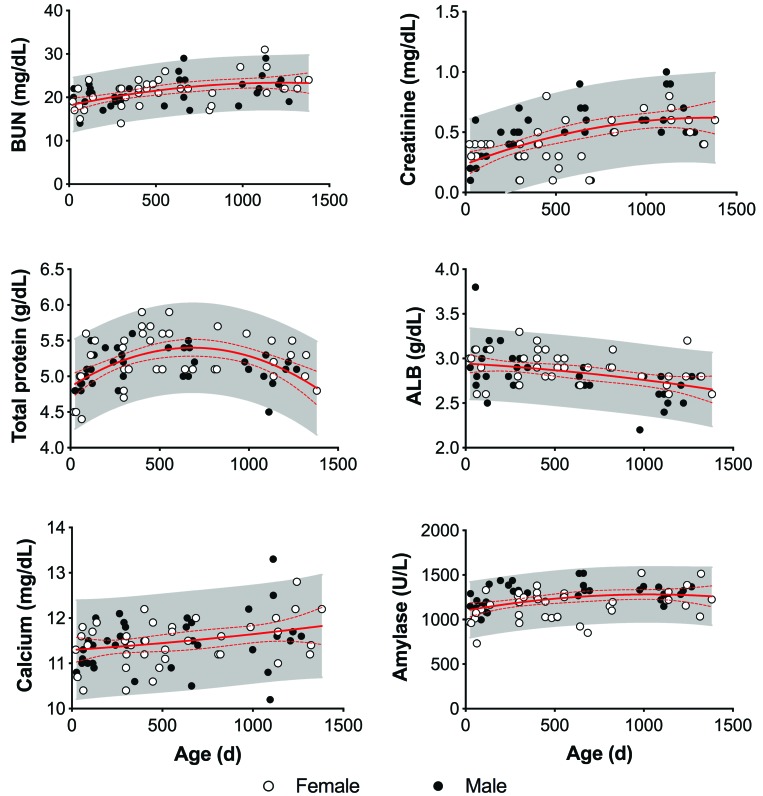
Figure 4.
Effect of age and sex on clinical chemistry in strain 13/N guinea pigs. BUN and creatinine increase with age in strain 13/N guinea pigs. Total protein sharply increases for the first 400 d of life, followed by a steady drop along with albumin concentration, as a secondary sign of protein loss. In addition, calcium and amylase increased with age. The solid and dashed red lines represent the mean and 95% CI, respectively, of each parameter. The shaded area represents the 95% predictive interval of each parameter.
Figure 5.
Effect of age and sex on liver enzymes in strain 13/N guinea pigs. ALP sharply decreases in the first 200 d. Males have a consistently higher ALT than females, but this trend is not seen with AST or GGT. The solid and dashed red lines represent the mean and 95% CI, respectively, of each parameter. The shaded area represents the 95% predictive interval of each parameter.
Table 4.
Correlation (r) of clinical chemistry parameter values with age in female and male strain 13/N guinea pigs
| Female (n = 39) | Male (n = 37) | |
| Glucose (mg/dL) | 0.0737 | –0.4438d |
| BUN (mg/dL)a | 0.5097e | 0.4462d |
| Creatinine (mg/dL) | 0.5049e | 0.6588e |
| Calcium (mg/dL) | 0.2998 | 0.3455c |
| Total protein (g/dL) | 0.1904 | 0.1461 |
| Albumin (g/dL) | –0.2255 | –0.5747e |
| AST (U/L)b | –0.1822 | –0.2513 |
| ALT (U/L) | –0.9258 | –0.0006 |
| ALP (U/L) | –0.6220e | –0.8036e |
| GGT (U/L) | –0.0638 | –0.073 |
| Total bilirubin (mg/dL)b | 0.06772 | 0.08633 |
| Amylase (U/L) | 0.3121 | 0.3978d |
Due to machine error, these parameters were calculated by using data from 38 females and a36 males or b35 males.
Values that differ significantly between age groups are indicated (cP < 0.05, dP < 0.01, and eP < 0.001).
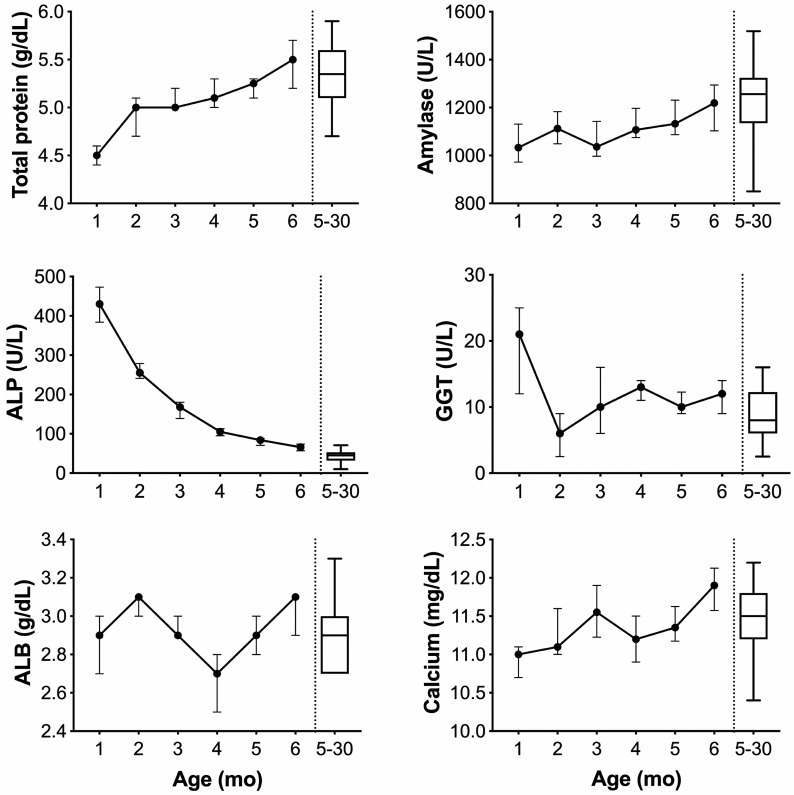
Longitudinal analysis of the juvenile cohort's clinical chemistry results revealed increases in BUN, calcium, total protein, ALT, and amylase as the pups grew (Figure 6). Conversely, ALP (0 through 184 d) and GGT (0 through 66 d) dropped precipitously before a gradual decrease to adult levels. Stabilization of parameters typical of the adult population was achieved at 3 mo (calcium), 4 mo (BUN), 5 mo (total protein), and 6 mo (amylase; Figure 6). The liver enzymes ALT and ALP remained elevated, whereas creatinine remained below adult levels at 6 mo.
Figure 6.
Longitudinal sampling of clinical chemistry analytes in 15 strain 13/N guinea pig pups. Total protein (TP) and amylase (AMY) increase with age in strain 13/N guinea pig pups. Albumin (ALB) did not significantly rise in this same period, indicating that most of the protein increase is related to immunoglobulin production in the developing immune system. High ALP levels are often seen at high levels in young, growing animals. Here, ALP appears to reach levels similar to those of adults by 6 mo of age. GGT drops suddenly after 1 mo before steadily increasing within the adult range. Calcium (CA) reaches adult 5-30 mo values by 3 mo and continues to rise through 6 mo.
BUN was correlated with age more strongly in female guinea pigs, whereas creatinine and ALP had a stronger relationship to age in males (Table 4). Age correlations existed for albumin (r = –0.575) and amylase (r = 0.398) in male but not female guinea pigs. Amylase (P < 0.01), ALP (P < 0.05), and ALT (P < 0.0001) were higher in males overall (Table 3). When further stratified by age, creatinine (P < 0.05), ALT (P < 0.0001), ALP (P < 0.05), and amylase (P < 0.01) were higher in adult (that is, 151 through 900 d old) males, whereas GGT was higher (P < 0.0001) in adult females. Female geriatric adults (that is, older than 900 d) had higher (P < 0.05) albumin concentrations than age-matched males.
Discussion
The strain 13/N guinea pig reference intervals (Tables 1 and 3) established here show notable sex- and age-associated differences that are important for use in clinical decision-making and research data interpretation. Importantly, these data reflect the full spectrum of age cohorts maintained in breeding colonies and encompass animals that may be retained for use as aged subjects in research studies.
Hematology parameters in strain 13/N guinea pigs follow similar trends seen in established reference intervals for Weiser–Maples, hairless Dunkin–Hartley, and Hartley strains.14,24,25 Our hypothesis of a lymphocyte to neutrophil inversion with age was consistent with previous literature.25 In contrast to previous studies, WBC did not continue to increase but instead peaked around 300 d before slowly decreasing (Figure 3 A).14 Platelets in strain 13/N guinea pigs were lower than reported in studies on other strains.28 Erythrocyte volume, measured as MCV, was bimodal, with peaks centered at 70 and 82 fL (Figure 2), but this phenotypic variation in erythrocyte volume was not separated by age, sex, or presence of anisocytosis on blood smears. Distinct MCV subpopulations have not been reported in previous guinea pig studies.
We used manual evaluation of WBC in this study to complement the automated CBC point-of-care analyzer. Although subject to some variability, manual WBC differential counts are considered the standard of care in small mammal medicine and provide additional details regarding RBC, WBC, and platelet morphology, including the opportunity to assess percentages of circulating Foa–Kurloff cells. Unique to guinea pigs, Foa–Kurloff cells display a large, granular intracytoplasmic vacuole containing periodic acid–Schiff positive material and act as cytotoxic natural killer cells with potential antitumor activity.1,6,18,19,22 These vacuoles can exceed the size of the nucleus and therefore may interfere with obtaining accurate automated WBC differential counts. Although Foa–Kurloff cells are typically associated with pregnancy in older females, several studies have noted no significant difference in peripheral blood Foa–Kurloff cell numbers between males and females older than 2 to 3 mo.1,17,28 In contrast, we measured higher Foa–Kurloff percentages in males (Table 1). This difference may be due to a manual assessment of these cells (compared with automated analysis) and categorization of any mononuclear cell containing an eosinophilic intracytoplasmic inclusion body as Foa–Kurloff cells in this study.
By using the manual counting method, as many as 400 cells in each sample were blindly evaluated between 2 readers, thus increasing the sensitivity of hematologic data acquisition. Slides in this study were created from fresh whole blood and without dilution, to minimize artifacts from EDTA or incomplete mixing. Despite our efforts to limit variance, blood smears evaluations can be discrepant from the true circulating blood components due to smear quality and the accumulation of large leukocytes at the feathered edge. Trends in age correlation were consistent between manual and automated data collection, with the manual cell counting method providing a more normalized distribution of monocyte percentages compared with the point-of-care analyzer.
In addition, clinical chemistry values in strain 13/N guinea pigs were largely within established reference values for Hartley, normal and euthymic Dunkin–Hartley, and Weiser–Maples strains, with the exception of glucose, ALP, and creatinine.14,22,24 Glucose was elevated in strain 13/N compared with other strains, likely due to lack of fasting prior to sample collection. Previous studies in other strains provided a wider range for ALP but included animals as young as 3 wk, when bone-associated isoforms of ALP are typically elevated due to skeletal growth.22,25 ALP in strain 13/N animals decreased until 9 mo, similar to trends seen in inbred Weiser–Maples guinea pigs, indicating continued bone development well past the onset of sexual maturity at 3 to 4 mo.8,14,20 Strain 13/N control animals in a dietary hypervitaminosis D study had similar ALP and creatinine values to those in our study, but lower overall total calcium levels.9 Calcium in our current study increased over time (Figure 4) and may correspond with the development of renal disease in this strain, but we are unable to draw definitive conclusions without measuring other electrolyte levels, including magnesium and phosphorus.1 Total protein rose rapidly through 150 d without a concurrent increase in albumin, indicating increasing immunoglobulin production in the developing immune system. Unlike in Weiser–Maples guinea pigs, in which total protein peaks at 200 d, we saw total protein levels continue to rise from 150 to 400 d in strain 13/N animals before slowly decreasing thereafter (Figure 4).14 Our study is the first to correlate serum amylase with age in guinea pigs (Figure 4). Increasing amylase levels in other species commonly indicate kidney, liver, pancreas, or salivary gland tissue damage, and the elevated levels seen here may indicate subclinical inflammation in aging animals.25 Nephrosclerosis is commonly seen in aged strain 13/N guinea pigs from our colony and is the most likely source of this progressive rise in amylase concentration.
Limitations of this study include a smaller sample size at the extreme ends of the age range. Expanded analyses of month-to-month changes in the youngest animals, especially in females, may efficiently isolate interactions between growth and sex. In this colony, young female guinea pigs are bred by 8 mo to avoid increased dystocia risk from pelvic symphysis fusion.8,20 Consequently, data collected from females 150 to 240 d old was limited by pregnancy status and decreased statistical power in this age range. Likewise, data expansion in the senior adult (that is, older than 900 d) animal cohort could help categorize clinicopathologic changes associated with renal disease and other age-related changes and disease processes. Several animals older than 900 d were removed from analysis due to markedly elevated BUN and creatinine; microcytic, hypochromic anemia; and concurrent clinical symptoms of illness, such as progressive weight loss and generalized muscle wasting. These findings are consistent with chronic renal disease and was confirmed as nephrosclerosis at necropsy.1 Given that most animals in this colony show some degree of renal insufficiency after 3 y of age, characterization of the temporal progression and severity of disease could prove beneficial.
Other sources of potential alterations in determined reference intervals may include the type of anesthesia administered, use of point-of-care analyzers, and sample type. Collecting a large volume of blood in guinea pigs typically requires deep sedation or anesthesia to safely access the cranial vena cava and jugular vein. The isoflurane used in this study is known to influence hematology and chemistry values in guinea pigs, including WBC, Hct, TP, GLU, and ALP values. However, this gas anesthetic produces milder changes than other approaches, such as ketamine-xylazine anesthesia.27
We specifically selected point-of-care instruments for use in these studies, given that these devices are used almost exclusively in BSL3 and BSL4 laboratories, where research involving strain 13/N animals is often performed. Although the type of analyzer used may influence analyte outcome, the previous analysis showed only minor differences between hematology analyzer systems when the instruments are maintained by using correct quality-control measures.2 Small differences in clinical chemistry values may be expected due to the use of whole blood rather than serum, both of which are valid testing methods for the chemistry analyzer used. Consulting with the manufacturer representative indicated potential analyte variance in albumin, calcium, and total bilirubin due to the use of a human blood analyte system rather than a veterinary system. However, the manufacturer's veterinary clinical chemistry rotor was not validated for use with guinea pigs at the time of submission. Consistency in our intervals with those of other strains, excluding age as a factor, indicates that the difference in rotors may have minimal effects.
In summary, strain 13/N guinea pigs follow age-related trends in hematology and clinical chemistry analytes similar to those seen in other strains. The age and sex of the animal should be taken into strong consideration when interpreting clinical diagnostics, especially for erythrocyte-focused analytes such as RBC, Hgb, and Hct. Many parameters were stable by 150 to 200 d of age, but skeletal maturity, peak immunoglobulin production, and transformation of the WBC ratio from predominantly lymphocytes to neutrophils were not achieved until close to 400 d of age. Given these data, we propose general age cutoffs of 0 to 150 d for juvenile animals, 150 to 900 d for adult animals, and older than 900 d for senior adult strain 13/N guinea pigs. Further data collection will help to refine these age cutoffs or identify new biologically relevant subpopulations. In addition, future establishment of reference values for urinalysis and clotting factors such as prothrombin time and activated prothrombin time could be valuable in this specialized guinea pig strain, especially given their use as models for studying many hemorrhagic fever viruses.
Acknowledgments
We thank Dr Cassandra Tansey for technical assistance with animal work, Timothy Flietstra for assistance in statistical analysis, and the Comparative Medicine Branch at CDC for their mentorship and support through the Laboratory Animal Medicine Residency Program.
References
- 1.Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits, 4th ed Ames (IA): Wiley–Blackwell. [Google Scholar]
- 2.Becker M, Moritz A, Giger U. 2008. Comparative clinical study of canine and feline total blood cell count results with 7 in-clinic and 2 commercial laboratory hematology analyzers. Vet Clin Pathol 37:373–384. 10.1111/j.1939-165X.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell TM, Shaia CI, Bearss JJ, Mattix ME, Koistinen KA, Honnold SP, Zeng X, Blancett CD, Donnelly GC, Shamblin JD, Wilkinson ER, Cashman KA. 2017. Temporal progression of lesions in guinea pigs infected with lassa virus. Vet Pathol 54:549–562. 10.1177/0300985816677153. Erratum in: Corrigendum. 2018. Vet Pathol 55:355 [DOI] [PubMed] [Google Scholar]
- 4.Bird BH, Dodd KA, Erickson BR, Albariño CG, Chakrabarti AK, McMullan LK, Bergeron E, Ströeher U, Cannon D, Martin B, Coleman-McCray JD, Nichol ST, Spiropoulou CF. 2012. Severe hemorrhagic fever in strain 13/N guinea pigs infected with Lujo virus. PLoS Negl Trop Dis 6:1–13. 10.1371/journal.pntd.0001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashman KA, Smith MA, Twenhafel NA, Larson RA, Jones KF, Allen RD, 3rd, Dai D, Chinsangaram J, Bolken TC, Hruby DE, Amberg SM, Hensley LE, Guttieri MC. 2011. Evaluation of Lassa antiviral compound ST193 in a guinea pig model. Antiviral Res 90:70–79. 10.1016/j.antiviral.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debout C, Taouji S, Izard J. 1995. Increase of a guinea pig natural killer cell (Kurloff cell) during leukemogenesis. Cancer Lett 97:117–122. 10.1016/0304-3835(95)03960-5. [DOI] [PubMed] [Google Scholar]
- 7.Festing MFW. 1979. Inbred strains in biomedical research. London (United Kingdom): Macmillan; 10.1007/978-1-349-03816-9 [DOI] [Google Scholar]
- 8.Hargaden M, Singer L. 2012. Anatomy, physiology, and behavior, p 575–602. Chapter 20. In: Suckow MA, Stevens KA, Wilson RP, editors. The laboratory rabbit, guinea pig, hamster, and other rodents. New York (NY): Academic Press. [Google Scholar]
- 9.Holcombe H, Parry NM, Rick M, Brown DE, Albers TM, Refsal KR, Morris J, Kelly R, Marko ST. 2014. Hypervitaminosis D and metastatic calcification in a colony of inbred strain 13 guinea pigs, Cavia porcellus. Vet Pathol 52:741–751. 10.1177/0300985814551423. [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 11.Jahrling PB. 1983. Protection of Lassa virus-infected guinea pigs with Lassa-immune plasma of guinea pig, primate, and human origin. J Med Virol 12:93–102. 10.1002/jmv.1890120203. [DOI] [PubMed] [Google Scholar]
- 12.Jahrling PB, Smith S, Hesse RA, Rhoderick JB. 1982. Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun 37:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kainulainen MH, Spengler JR, Welch SR, Coleman-McCray JD, Harmon JR, Klena JD, Nichol ST, Albariño CG, Spiropoulou CF. 2018. Use of a scalable replicon-particle vaccine to protect against lethal Lassa virus infection in the guinea pig model. J Infect Dis 217:1957–1966. 10.1093/infdis/jiy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagaki M, Yamaguchi M, Nakamura M, Sakurada K, Suwa T, Sasa H. 2005. Age-related changes in haematology and serum chemistry of Weiser–Maples guineapigs (Cavia porcellus). Lab Anim 39:321–330. 10.1258/0023677054307042. [DOI] [PubMed] [Google Scholar]
- 15.Lukashevich IS. 2013. The search for animal models for Lassa fever vaccine development. Expert Rev Vaccines 12:71–86. 10.1586/erv.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller LJ, Nasar F, Schellhase CW, Norris SL, Kimmel AE, Valdez SM, Wollen-Roberts SE, Shamblin JD, Sprague TR, Lugo-Roman LA, Jarman RG, Yoon IK, Alera MT, Bavari S, Pitt MLM, Haddow AD. 2018. Zika virus infection in Syrian golden hamsters and strain 13 guinea pigs. Am J Trop Med Hyg 98:864–867. 10.4269/ajtmh.17-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilny AA. 2008. Clinical hematology of rodent species. Vet Clin North Am Exot Anim Pract 11:523–533. 10.1016/j.cvex.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Pouliot N, Maghni K, Blanchette F, Cironi L, Sirois P, Stankova J, Rola-Pleszczynski M. 1996. Natural killer and lectin-dependent cytotoxic activities of Kurloff cells: target cell selectivity, conjugate formation, and Ca2+ dependency. Inflammation 20:647–671. 10.1007/BF01488802. [DOI] [PubMed] [Google Scholar]
- 19.Pouliot N, Maghni K, Sirois P, Rola-Pleszczynski M. 1996. Guinea pig Kurloff (NK-like) cells mediate TNF-dependent cytotoxic activity: analogy with NC effector cells. Inflammation 20:263–280. 10.1007/BF01488203. [DOI] [PubMed] [Google Scholar]
- 20.Quesenberry KE, Donnelly TM, Mans C. 2012. Biology, husbandry, and clinical techniques of guinea pigs and chinchillas, p 279–294. In: Quesenberry KE, Carpenter JW, editors. Ferrets, rabbits, and rodents, 3rd ed St Louis (MO): Saunders. [Google Scholar]
- 21.Safronetz D, Mire C, Rosenke K, Feldmann F, Haddock E, Geisbert T, Feldmann H. 2015. A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl Trop Dis 9:1–14. 10.1371/journal.pntd.0003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharp P. 2018. The laboratory guinea pig, p 305–329. Chapter 8. In: Kurtz DM, Travlos GS, editors. The clinical chemistry of laboratory animals, 3rd ed Boca Raton (FL): CRC Press. [Google Scholar]
- 23.Vela E. 2012. Animal models, prophylaxis, and therapeutics for arenavirus infections. Viruses 4:1802–1829. 10.3390/v4091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waner T, Avidar Y, Peh HC, Zass R, Bogin E. 1996. Hematology and clinical chemistry values of normal and euthymic hairless adult male Dunkin–Hartley guinea pigs (Cavia porcellus). Vet Clin Pathol 25:61–64. 10.1111/j.1939-165X.1996.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 25.Washington IM, Van Hoosier G. 2012. Clinical biochemistry and hematology, p 57–116. In: Suckow M, Stevens K, Wilson R, editors. The laboratory rabbit, guinea pig, hamster, and other rodents. New York (NY): Academic Press. [Google Scholar]
- 26.Welch SR, Scholte FEM, Albariño CG, Kainulainen MH, Coleman-McCray JD, Guerrero LW, Chakrabarti AK, Klena JD, Nichol ST, Spengler JR, Spiropoulou CF. 2018. The S genome segment is sufficient to maintain pathogenicity in intraclade Lassa virus reassortants in a guinea pig model. Front Cell Infect Microbiol 8:1–13. 10.3389/fcimb.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams WR, Johnston MS, Higgins S, Izzo AA, Kendall LV. 2016. Blood profiles in unanesthetized and anesthetized guinea pigs (Cavia porcellus). Lab Anim (NY) 45:35–41. 10.1038/laban.911. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman KL, Moore DM, Smith SA. 2010. Hematology of guinea pigs, p 893–898. Chapter 112.In: Weiss DJ, Wardrop KJ, editors. Schalm's veterinary hematology, 6th ed Ames (IA): Wiley–Blackwell. [Google Scholar]