Abstract
Measles virus causes a highly infectious disease in NHP. Clinical signs range from asymptomatic to fatal, although measles virus is most well-known for its characteristic generalized maculopapular rash. Along with appropriate quarantine practices, restricted human access, and appropriate personal protective equipment, vaccines are used to combat the risk of infection. The canine distemper–measles vaccine (CDMV), administered at the manufacturer's standard dose (1.0 mL IM), has been shown to be effective against clinical measles disease in rhesus macaques (Macaca mulatta). The goal of the current study was to test whether doses smaller than the manufacturer's recommended dose stimulated adequate antibody production to protect against infection. We hypothesized that either 0.25 or 0.5 mL IM of CDMV would stimulate antibody production comparable to the manufacturer's recommended dose. We found that the 0.25-mL dose was less effective at inducing antibodies than either the standard (1.0 mL) or 0.5-mL dose, which both yielded similar titers. The primary implication of this study informs balancing resource allocation and providing efficacious immunity. By using half the manufacturer-recommended dose, the 50% cost reduction may provide sufficient monetary incentive to implement, maintain, or modify measles vaccination programs at NHP facilities.
Abbreviations: CDMV, canine distemper–measles vaccine; MV, measles virus
Measles virus (MV) is one of the most infectious diseases in humans,22,50,55 and NHP in contact with humans are at constant risk.6,43 MV is the prototypic member of the genus Morbillivirus and family Paramyxoviridae.24 All Morbilliviruses are highly infectious, closely related, and most likely have evolved from a common ancestor.24 These viruses generally spread through the respiratory route, initially infecting and replicating in the immune system and inducing profound immune suppression; consequently, morbilliviruses have the potential to cause acute large-scale outbreaks with high morbidity and mortality in naive populations.25,54 Morbilliviruses include canine distemper virus (infects dogs, coyotes, wolves, and seals), rinderpest (cattle), peste des petits ruminants (goats and sheep), phocine distemper virus (seals and otters), dolphin morbillivirus, pilot whale morbillivirus, Longman beaked whale morbillivirus, feline morbillivirus, and unclassified morbillivirus-related viruses (rodents, moles, shrews, and bats).24,25,60,61,74,80,82
MV is spread by direct contact, aerosols, and fomites.6,26 Measles is typically characterized by a generalized maculopapular rash, although clinical signs can range from asymptomatic to fatal. Clinical illness demonstrates a prodromal period of 2 to 3 d consisting of a fever, malaise, and anorexia, followed by coryza, keratoconjunctivitis, and a dry cough; generalized lymphadenopathy and splenomegaly are commonly noted also. Pathognomonic ‘Koplik spots’ on the buccal mucosa are rare. The measles rash usually appears from 3 to 5 d after the onset of clinical signs. The rash often is first noticed on the head, especially the face, and rapidly spreads down the neck, trunk, and extremities over the next several days. In the late stages, the rash darkens, the fever decreases, and systemic manifestations resolve. The rash fades in the same top-down sequence as it appeared and may be associated with desquamation.12-14,47,50 In addition, MV induces a transient yet profound immunosuppression that can last for weeks to months,23,24,39,40,53 causing dysfunction of both the humoral (antibody) and cell-mediated immune systems for as long as 6 mo, resulting in increased susceptibility to pneumonia, which is the most common cause of death associated with measles infection,22 as well as enteropathy, abortion, encephalitis, and even as a direct cause of death.23,43,49,53 Stressed or immunosuppressed animals are even more likely to experience severe opportunistic sequelae, likely from disruption of the mucosal barrier,39,40,43 and transient immunosuppression can interfere with delayed-type hypersensitivity reactions, such as skin testing for Mycobacterium tuberculosis,15,32,75,79,83 further complicating preventative health measures.
Due to the rapid exhaustion of susceptible hosts, MV cannot be maintained below a minimum density threshold.48 Wild NHP typically do not live in populations that are sufficiently large and dense to maintain a transmission cycle.40,59 Herd immunity exceeding 90% to 94% is essential to prevent transmission.30,34,55,85 MV is endemic only in high-density human populations, and humans are the only natural reservoir host.18,22,59 Therefore, exposure to humans remains the major risk factor for measles infection in NHP.43 Human-to-NHP transmission of MV has been reported to produce marked morbidity and mortality in NHP populations.43 Historically, measles was a common infectious disease in NHP, and numerous outbreaks, involving several species, have been reported in the past.43,67,85 New World NHP species are especially susceptible to MV; they may present with respiratory or gastrointestinal symptoms and may develop a more severe form of disease with increased mortality.2,27,45,46
Prior to the introduction of the measles vaccine in humans, measles was nearly a universal disease,58 and as many as 50% of all childhood deaths from infectious disease were associated with MV.53 Safe and effective attenuated modified live measles vaccines, first introduced in 1963, have led to a substantial reduction in morbidity and mortality. In humans, these vaccines are 90% to 95% effective and provide protection for more than 20 y.22 However, research shows that declines in measles vaccination rates are associated with resurgences of clinical cases.30,34,53,63 These outbreaks pose a renewed health risk for NHP having contact with humans.
Effective occupational health and vaccination programs for animal care staff, donning personal protective equipment (for example, clothing, gloves, masks, and face shields), and restricting human access have limited and reduced MV exposure at most NHP facilities. However, immunity to measles is not all or nothing, but rather a continuum of clinical conditions. Humans with subclinical infections and suboptimal immunity may serve as a source of virus introduction.16,84 In addition, focusing only on occupational and vaccination programs for the animal care staff may result in large, susceptible NHP populations.19,71 The potential consequences of MV infection and disease in NHP can be devastating and far-reaching and include health, research, and occupational effects, as well as financial implications. Therefore, prevention of MV NHP infection and transmission is contingent on concerted quarantine practices and animal vaccination protocols.75 MV is a vaccine-preventable disease in both humans and NHP.
We and others have evaluated various measles vaccines for use in NHP.18,85 Although formulations previously investigated have been proven to provide adequate immunity to MV, they are impractical to implement in large-scale NHP settings for various reasons, including feasibility, availability, and financial considerations. In addition, for achieving the best immunity while still providing optimal individual and herd immunity, a 2-dose regime is recommended, which would be more economical if half or a quarter of the manufacturer's recommended dose were used. Moreover, a reduced dose volume could have the additional advantage of reducing potential local muscle damage and pain,10,37 furthering the animal welfare benefits.
After previously investigating all of the commercially available measles vaccines currently on the market, we have found the canine distemper–measles vaccine (CDMV) formulation to be a safe, efficacious, and economical measles vaccine for use in NHP, as of the time when the current study was conducted.18,85 In addition, the renewed availability of CDMV, as well as the unavailability of alternatives, were factors in initiating the current dose study. Therefore, we hypothesized that although the full dose of CDMV recommended by the manufacturer (1.0 mL IM) will be the most effective and yield the highest average measles antibody titer, smaller doses (0.25 or 0.5 mL IM) will also have high average titers and prove to provide adequate immunity for clinical and research use. The objective of the current study was to determine the minimal appropriate dose of CDMV that can be administered to NHP to promote and enhance animal welfare as well as to potentially save resources for reallocation to other aspects of the research and facility. Therefore, we performed a modified dose volume trial of CDMV in juvenile rhesus macaques.
Materials and Methods
Animals.
The 65 animals (full dose group, n = 22; half dose, n = 21; quarter dose, n = 22) used in this study were previously unvaccinated, juvenile rhesus macaques (Macaca mulatta) of both sexes (age, 11 to 17 mo) that were housed outdoors in half-acre breeding corrals composed of large multimale, multifemale social groups of all ages at the California National Primate Research Center (University of California, Davis, Davis, CA). Animals were fed chow twice daily (LabDiet Monkey Diet 5047, Purina Laboratory, St Louis, MO), offered water free-choice through automatic watering devices, supplemented with fruits and vegetables biweekly, and provided with species-appropriate environmental enrichment, manipulanda, and foraging opportunities. Daily health checks were performed by trained personnel according to standard operating procedures. This study was approved by the IACUC of the University of California, Davis. Animals were maintained in accordance with the USDA Animal Welfare Act and Regulations and the Guide for the Care and Use of Laboratory Animals.4,5,42 The animal care and use program of the University of California, Davis, is fully AAALAC-accredited, is USDA-registered, and maintains a Public Health Services Assurance.57
Study design and sample collection.
For this study, all unvaccinated juveniles older than 6 mo undergoing routine biannual health screening in 3 separate corrals were selected and assigned to individual groups to receive 1 of 3 CDMV vaccine regimens as follows: the juveniles from the first corral received the full-dose (1.0 mL IM) vaccination routinely used at the facility, those from the second corral received the half dose (0.5 mL IM), and those from the third corral received the quarter dose (0.25 mL IM). Each corral was vaccinated with a separate dose to reduce any potential, although unlikely, for viral shedding that might affect the results and to decrease variability. All subjects received a physical examination at the time of vaccination, and 1 mL whole blood was collected from all subjects for assessments of measles and neutralizing antibody titers at 0, 6, and 12 mo after vaccination. The blood was allowed to clot at ambient temperature to prevent hemolysis, which can potentially interfere with test results. The clotted blood was centrifuged at 800 × g for 15 min within 6 h of collection and the serum was separated from the clot. Serum samples were stored at –70 °C prior to analysis.
Vaccine.
The measles vaccine used in this study was Vanguard DM (Zoetis, Parsippany, NJ), as previously investigated.18 It is an attenuated modified live bivalent vaccine in which the MV derives from the Enders attenuated Edmonston strain; this vaccine is propagated in chick embryo cell culture, has a 10-fold higher MV titer per dose than Attenuvax (Merck, Kenilworth, NJ),52 and is administered to healthy puppies 6 to 12 wk in age to aid in the prevention of canine distemper disease. The MV component is designed to provide puppies with temporary cross-protection from canine distemper virus regardless of maternal antibody levels.77 The safety and efficacy of this vaccine was previously validated in rhesus macaques at the manufacturer's recommended dose of 1.0 mL IM18 and is currently in use at the facility. The freeze-dried vaccine was reconstituted according to the manufacturer's recommendations by using the sterile diluent provided and aseptic technique and was stored at 2 to 7 °C until administered.88
Measles antibody.
Total IgG antibody, reported as the highest dilution of test serum showing antibodies against measles, was determined by using a multiplex microbead immunoassay (Luminex 100/200 system), with microbead-coupled recombinant measles nucleocapsid antigen and controls (Charles River Labs, Wilmington, MA). The instrument is an analyzer that uses the principles of flow cytometry to simultaneously measure multiple antibody–antigen analytes in a single well of a microtiter plate. For this protocol, 2-fold dilutions (starting at 1:50) of individual samples were made to determine the titer. The dilutions were added to antigen-coated beads diluted in Prionex (Millipore Sigma, Burlington, MA)–PBS–BSA; 50 µL each of the bead solution and diluted sample were added to a filter plate and left to mix on a shaker for 1 h at room temperature. After 1 h, the wells were washed and subsequently incubated on the shaker for 30 min with 100 μL of biotinylated goat antihuman antibodies diluted in BSA–PBS buffer solution. Next, the wells were washed with buffer before the addition of 100 μL of streptavidin–R-phycoerythrin in BSA–PBS and incubation on the shaker for 30 min. Finally, the wells were washed once more to remove unbound material, after which the beads were analyzed spectrophotometrically by using 2 lasers which identify the antibody–antigen complexes bound to the uniquely dyed xMAP microspheres. Digital signal processors and the xPONENT software (Millipore Sigma) were used to acquire and analyze the data. By measuring the spectral properties of the beads, the median fluorescence index for each antigen was determined. The median fluorescence index of the sample was compared with a positive–negative cutoff value of 3000 median fluorescent units to determine the presence of antibody.
Neutralization titers.
The samples were tested for neutralizing antibodies in a plaque reduction–microneutralization assay as previously described.85 Briefly, 50 TCID units of MVvac2GPF, a molecular clone of the Moraten vaccine strain expressing green fluorescent protein,38,85 were incubated with 4-fold dilutions of the test serum, starting at 1:20. The neutralization media was DME with 2% FCS and antibiotics, and the cell line was low-passage (less than 6 mo) Vero cells.3 Rhesus measles immune globulin was used as the positive control, and media in place of test serum was used as the negative control. After an incubation period of 3 d, the plates were examined for green plaques under a UV microscope at 10× magnification, and each well was marked as either positive (green fluorescence) or negative for virus. Any fluorescence at all was considered positive. Data were analyzed by using the Reed Muench calculation template, which measured a reciprocal titer for each sample.65
Statistical analysis.
Statistical analysis was performed in JMP version 14 (SAS Institute, Cary, NC). Comparisons between the geometric mean antibody titers of the various doses at each time point for each assay were evaluated by ANOVA followed by Tukey–Kramer post hoc analysis.18,41 Results were considered statistically significant when they had a P value less than 0.05.
Results
Measles antibody.
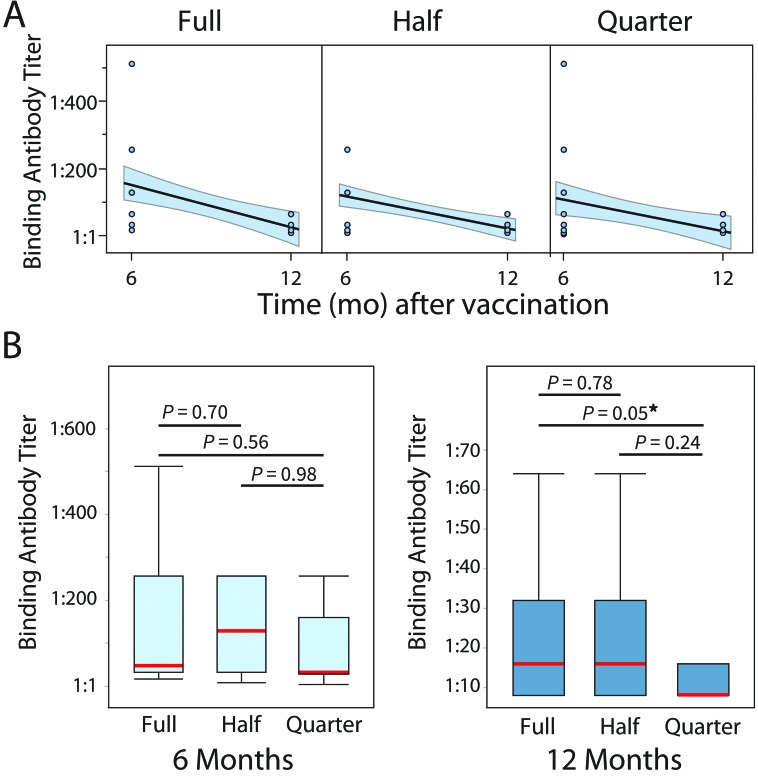
The median, mode, and range of the IgG binding antibody titers for rhesus macaques comprising each CDMV dose group (full, half, quarter) at 6 and 12 mo after vaccination are shown in Tables 1 and 2. From the 6-mo stage to the 12-mo stage, the average titer for the full, half, and quarter doses decreased by 2.06 (84%), 2.45 (82%), and 3.03, respectively (88%; Tables 1 and 2). Our data suggest that total IgG binding antibody titers against measles at 6 and 12 mo after vaccination were measurable and decreased longitudinally in all 3 vaccine regimens (Figure 1 A). In addition, we found no statistically significant differences in the measured binding antibody titer between doses at 6 mo. However, at 12 mo after vaccination, there was a marginally significant (P = 0.05) difference between the titers for the half and quarter doses but not between the half and full doses of CDMV (Figure 1 B).
Table 1.
Binding antibody titers, reported as the highest dilution of test serum showing antibodies against measles, for all 3 dose groups at 6 mo after vaccination
| Median titer | Mode titer | Titer range | % negative (no antibody) | |
| Full dose | 48 | 32 | 16–512 | 0% |
| Half dose | 128 | 32 | 8–256 | 0% |
| Quarter dose | 32 | 32 | 4–512 | 0% |
Data regarding titers are given as reciprocal values.
Table 2.
Binding antibody titers, reported as the highest dilution of test serum showing antibodies against measles, for all 3 dose groups at 12 mo after vaccination
| Median titer | Mode titer | Titer range | % negative (no antibody) | |
| Full dose | 16 | 8 | 8–64 | 0% |
| Half dose | 16 | 8 | 8–64 | 0% |
| Quarter dose | 8 | 8 | 8–64 | 0% |
Data regarding titers are given as reciprocal values.
Figure 1.
(A) Line of fit for measles binding antibody titers, reported as the highest dilution of test serum showing antibodies against measles, with 95% CI (shaded region). A longitudinal downward trend is observed for all doses. (B) Box plots of post-vaccination antibody dilution measurements (6 and 12 mo after vaccination) suggest no difference between groups in the binding antibody titers at 6 mo after vaccination. However, animals in the quarter-dose group showed slightly lower binding antibody titer (marginally significant, P = 0.05) at 12 mo after vaccination. Red lines illustrate the medians, whiskers depict the minimal and maximal values, and the bands are the 1st and 2nd quartiles. *, P = 0.05.
Neutralizing antibody.
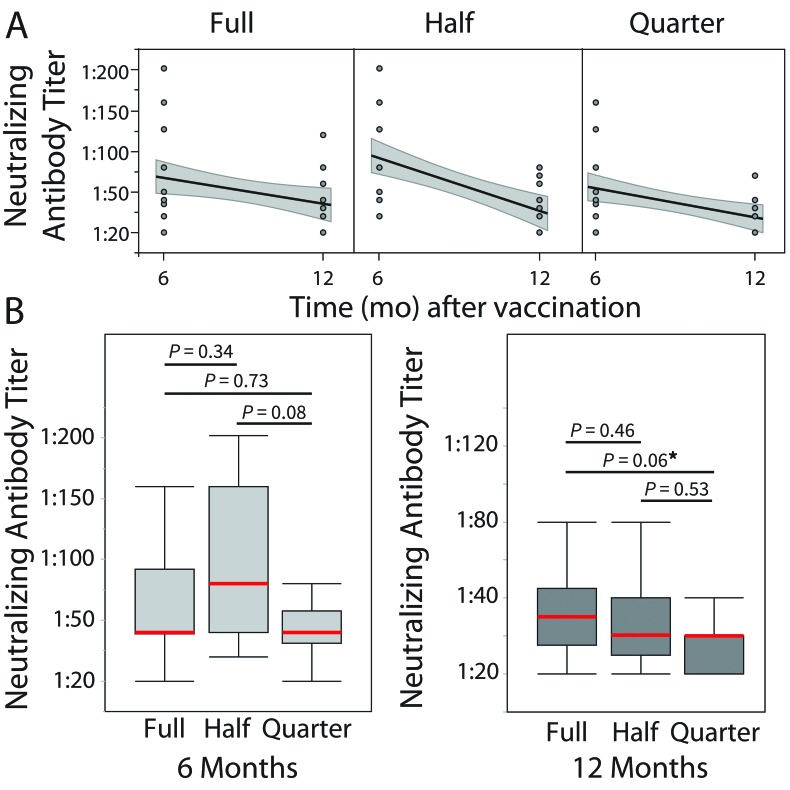
Tables 3 and 4 summarize the 6- and 12-mo post-vaccination neutralizing antibody titers for the full-, half-, and quarter-dose groups. From the 6-mo stage to the 12-mo stage, the average titer for the full, half, and quarter doses decreased by 0.69 (38%), 1.39 (62%), and 1.38 (61%), respectively (Tables 2 A and B). Our data suggest that although neutralizing titers decreased over time after vaccination (Figure 2 A), similarly to the IgG antibody levels, no statistically significant differences between the 3 vaccine regimens were found, except for a marginally lower (P = 0.06) titer in the half-dose group compared with the quarter-dose group only at 12 mo after vaccination (Figure 2 B). These results indicate that, at both 6 and 12 mo, a full dose of CDMV has a similar effect to the half dose but not the quarter dose in rhesus macaques.
Table 3.
Neutralizing antibody titers, reported as the highest dilution of test serum showing antibodies against measles, for all 3 dose groups at 6 mo after vaccination
| Median titer | Mode titer | Titer range | % negative (no antibody) | |
| Full dose | 40 | 40 | <20–202 | 9.0% |
| Half dose | 80 | 40 | 20–202 | 0% |
| Quarter dose | 40 | 40 | <20–160 | 18.2% |
Data regarding titers are given as reciprocal values.
Table 4.
Neutralizing antibody titers, reported as the highest dilution of test serum showing antibodies against measles, for all 3 dose groups at 12 mo after vaccination
| Median titer | Mode titer | Titer range | % negative (no antibody) | |
| Full dose | 30 | 40 | <20–120 | 22.7% |
| Half dose | 20 | 20 | <20–120 | 23.8% |
| Quarter dose | 20 | 20 | <20–70 | 27.2% |
Data regarding titers are given as reciprocal values.
Figure 2.
(A) Line of fit for neutralizing titers, reported as the highest dilution of test serum showing antibodies against measles, with 95% CI (shaded region). Similar to results from binding antibody measurement, a longitudinal downward trend is observed for all doses. (B) Box plots of post-vaccination neutralizing dilution titers (6 and 12 mo after vaccination) suggests no difference between groups in the neutralizing antibody titers at 6 mo after vaccination. However, animals in the quarter-dose group showed slightly lower albeit nonsignificant (P = 0.06) neutralizing titers at 12 mo after vaccination. Red lines illustrate the medians, whiskers depict the minimal and maximal values, and the bands are the 1st and 2nd quartiles. *, P = 0.05.
Discussion
NHP have contributed a vast amount to biomedical research by serving as an important preclinical and translational research model.28,29,35,36,72 In 2010, approximately 70,000 NHP were housed in United States research facilities for biomedical research.44 In particular, NHP are a valuable animal model for the study of MV from pathogenesis to long-term immunologic memory and for the development of new vaccines.23,87 MV continues to kill more than 89,000 human infants and children each year because currently licensed vaccines cannot be administered in infants younger than 9 mo, despite the vaccine-preventable nature of the disease in adult populations.20
MV is highly infectious for many species of NHP. Despite restricted visitation policies, use of personal protective equipment for staff handling animals, and maintenance of closed breeding colonies, the potential for humans with suboptimal immunity and subclinical infections to serve as sources of virus introduction into susceptible NHP colonies remains a possibility, especially with current MV vaccination in most human populations below the threshold level required for herd immunity. In addition, there are potential zoonotic concerns regarding NHP-to-human transmission. This underscores the importance of measles vaccination programs in biomedical research facilities housing NHP, as previously recommended.18,85
Vaccination is the most effective strategy against morbilliviruses.24 The latest attenuated measles vaccines are among the safest in use worldwide today.31 Local reactions at the site of injection are negligible. The main reported reaction is a mild, measles-like syndrome, which occurs in 2% to 30% of humans at approximately 1 wk after vaccination.31
In addition, morbilliviruses have recently been shown to induce cross-protection within genera.11 Cross-protection against canine distemper virus from MV vaccination has been demonstrated previously in dogs, macaques, and mice.7,9,25,77 However, some concerns exist regarding potential attenuated replication of canine distemper virus due to CDMV in NHP, especially given that NHP have been shown to be susceptible to infection by canine distemper virus through both experimental vaccination as well as natural occurrence.17,21,24,25,56,64,70,73,78,86 Although no evidence supporting this concern exists in the literature, the principal danger with an attenuated vaccine is that the organism, because it is still alive, can sometimes recover its virulence and cause disease in vaccinees.62 However, MV vaccination has been shown to induce partial protection against canine distemper virus in macaques,25 which should further reduce this concern. In addition, MV vaccination may provide general protection against all infectious diseases and protect polymicrobial herd immunity for as long as 3 y,1,53 which may be particularly helpful in high-density, outdoor NHP colonies, where diseases can spread readily. Moreover, due to their pleomorphic nature and long-lasting stimulation of the immune system, morbilliviruses are now being considered as vaccine vectors for protection against other infectious disease agents, including oncolytic viruses.11
The most commonly used, commercially available measles vaccines investigated for use in NHP include Attenuvax (Merck), M-Vac Vaccine (Serum Institute of India, Pune, India) and CDMV.18,85 In addition, a human measles–mumps–rubella vaccine (Merck) is available and includes an Attenuvax component, but its polyvalent formulation results in increased costs and vaccination against additional agents, making it less desirable for use in large research NHP colonies; consequently this vaccine was not investigated in previous studies. Since the completion of the CDMV safety and efficacy study in 1996, the facility has adopted the use of CDMV with the only hiatus being from 2007 to 2013 resulting from unavailability of the vaccine. Thus far, more than 9100 rhesus macaques, 450 long-tailed macaques (Macaca fasicularis), 60 titi monkeys (Callicebus moloch), and 6 squirrel monkeys (Saimiri spp.) have been vaccinated at our center. As in the original study, animals continue to be observed closely both clinically and pathologically by board-certified laboratory animal veterinarians and pathologists, respectively, for any adverse effects that could possibly be linked to morbillivirus infection, and none have been noted. Local injection site reactions have been nonexistent. In addition, we have received anecdotal reports that many other NHP facilities have been and still are using CDMV in macaques and other NHP species without any reported adverse effects.
In the current study, the mean, median, and mode titers for all 3 doses were within 1 dilution of each other. A single-dilution difference is within the typical normal range of testing variation for these and other immunoassays; thus, at least a 4-fold (2-dilution) difference is typically required to report seroconversion or a change in titer.33,85 As previously shown, the production of neutralizing antibodies against MV is considered protective in rhesus macaques,18 such that it formed the basis of comparison. Although there were no statistically significant differences between the neutralizing titers for all 3 groups at 6 mo after vaccination, there was a marginally significant difference between the full and quarter doses (Figures 1 B and 2 B), and there was a higher percentage of animals with negative antibody tests in the quarter-dose group. The full- and half-dose groups had similar titers at 12 mo, but the half-dose group had fewer subjects with no detectable antibody at 6 mo. In addition, we noted an increased range of titers within the quarter-dose group at 6 mo, perhaps suggesting that interindividual variation in immune response becomes a factor with the quarter dose of CDMV, making it potentially less reliable.
In humans, especially in developing countries, the optimal MV vaccine strategy is a 2-dose approach, with the first dose as early as 6 mo and the second ranging from 12 mo to school entry, depending on the national immunization program standards, because booster vaccination has been shown to provide increased immunity in subjects with low to no detectable antibody responses.8,68,69,76 In light of these considerations, we selected as subjects previously unvaccinated, juvenile (age, 11 to 17 mo) rhesus macaques of both sexes housed outdoors in half-acre breeding corrals; it was beyond the scope of the current study to evaluate the optimal timing of booster measles vaccination. At our facility, we elect not to vaccinate juveniles until they are at least 6 mo of age, to sufficiently reduce documented interference and inhibition of the immune response by maternal antibody.1,51
The implications of our current results are substantial. The potential cost associated with the implementation and maintenance of a measles vaccination program can be a deterrent to vaccination. As compared with the manufacturer's recommended 1.0 mL dose, using a half dose results in a 50% cost reduction yet provides comparable immunity. With rising management costs, NHP facilities must strive to decrease costs without diminishing animal welfare. This 50% cost reduction may provide sufficient incentive to implement and maintain a new NHP MV vaccination program or to modify an existing one, for example, by ensuring a 2-dose approach as is standard in humans. Alternately, the monetary savings from half-dose vaccination with CDMV could be used to improve another aspect of animal welfare. Moreover, the 50% dose volume reduction has the additional advantage of reducing potential local muscle damage when administered to young and small NHP species, given that the degree of muscle trauma and subsequent pain and inevitable rise in creatinine protein kinase are related to the volume of injectate.10,37 Lastly, live-attenuated virus vaccines have been shown to undergo a dose-sparing effect due to the subsequent replication of the vaccine vector in vivo, which is illustrated by our results and offers a powerful practical advantage when considering a measles vaccination protocol in large NHP colonies.66,81 Therefore, the results of the current study indicate that using a reduced dose of measles vaccine is safe, efficacious, and more affordable than previously thought, allowing facilities to support the protection of these valuable animal model resources via a measles vaccination protocol.
In conclusion, vaccination remains the most effective intervention strategy to combat morbillivirus infections, and we can now provide adequate protection against measles by using half of the manufacturer-recommended dose of CDMV yet still achieve full-dose immunity to MV, because the production of neutralizing antibodies against MV in rhesus has previously been shown to be protective.18 In addition, using this decreased dose may lead to a 50% vaccine cost reduction, and savings might be an economic incentive to implement or augment a measles booster program to prevent disease outbreak or, with the financial savings, to enhance animal welfare in other ways.
Acknowledgments
The project described was partially supported by the Office of the Director of NIH (P51 OD011107-54). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the Director or the NIH. We thank the members of the Pathogen Detection Laboratory and animal care staff at the CNPRC for their dedicated project support.
References
- 1.Aaby P, Clements CJ. 1989. Measles immunization research: a review. Bull World Health Organ 67:443–448. [PMC free article] [PubMed] [Google Scholar]
- 2.Abee CR, Mansfield K, Tardif SD, Morris T. 2012. Nonhuman primates in biomedical research: diseases. San Francisco (CA): Elsevier Academic Press. [Google Scholar]
- 3.Ammerman NC, Beier-Sexton M, Azad AF. 2008. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol 4 Appendix:A.4E.1–A.4E.7. 10.1002/9780471729259.mca04es11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 5.Animal Welfare Regulations. 2013. 9 CFR §3.129.
- 6.Bailey C, Mansfield K. 2010. Emerging and reemerging infectious diseases of nonhuman primates in the laboratory setting. Vet Pathol 47:462–481. 10.1177/0300985810363719. [DOI] [PubMed] [Google Scholar]
- 7.Barrett T. 1999. Morbillivirus infections, with special emphasis on morbilliviruses of carnivores. Vet Microbiol 69:3–13. 10.1016/S0378-1135(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 8.Bass JW, Halstead SB, Fischer GW, Podgore JK, Pearl WR, Schydlower M, Wiebe RA, Ching FM. 1976. Booster vaccination with further live attenuated measles vaccine. JAMA 235:31–34. 10.1001/jama.1976.03260270017018. [DOI] [PubMed] [Google Scholar]
- 9.Beauverger P, Buckland R, Wild TF. 1993. Measles virus antigens induce both type-specific and canine distemper virus cross-reactive cytotoxic T lymphocytes in mice: localization of a common Ld-restricted nucleoprotein epitope. J Gen Virol 74:2357–2363. 10.1099/0022-1317-74-11-2357. [DOI] [PubMed] [Google Scholar]
- 10.Bergeson PS, Singer SA, Kaplan AM. 1982. Intramuscular injections in children. Pediatrics 70:944–948. [PubMed] [Google Scholar]
- 11.Billeter MA, Naim HY, Udem SA. 2009. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr Top Microbiol Immunol 329:129–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blake FG, Trask JD. 1921. Studies on measles. I. Susceptibility of monkeys to the virus of measles. J Exp Med 33:385–412. 10.1084/jem.33.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake FG, Trask JD. 1921. Studies on measles. II. Symptomatology and pathology in monkeys experimentally infected. J Exp Med 33:413–422. 10.1084/jem.33.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake FG, Trask JD. 1921. Studies on measles: III. Acquired immunity following experimental measles. J Exp Med 33:621–626. 10.1084/jem.33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brody JA, Overfield T, Hammes LM. 1964. Depression of the tuberculin reaction by viral vaccines. N Engl J Med 271:1294–1296. 10.1056/NEJM196412172712505. [DOI] [PubMed] [Google Scholar]
- 16.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. 1990. Measles antibody: reevaluation of protective titers. J Infect Dis 162:1036–1042. 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 17.Choi YK, Simon MA, Kim DY, Yoon BI, Kwon SW, Lee KW, Seo IB, Kim DY. 1999. Fatal measles virus infection in Japanese macaques (Macaca fuscata). Vet Pathol 36:594–600. 10.1354/vp.36-6-594. [DOI] [PubMed] [Google Scholar]
- 18.Christe KL, McChesney MB, Spinner A, Rosenthal AN, Allen PC, Valverde CR, Roberts JA, Lerche NW. 2002. Comparative efficacy of a canine distemper–measles and a standard measles vaccine for immunization of rhesus macaques (Macaca mulatta). Comp Med 52:467–472. [PubMed] [Google Scholar]
- 19.Clements CJ, Cutts FT. 1995. The epidemiology of measles: 30 years of vaccination. Curr Top Microbiol Immunol 191:13–33. [DOI] [PubMed] [Google Scholar]
- 20.Dabbagh A, Patel MK, Dumolard L, Gacic-Dobo M, Mulders MN, Okwo-Bele J-M, Kretsinger K, Papania MJ, Rota PA, Goodson JL. 2017. Progress toward regional measles elimination—worldwide, 2000–2016. MMWR Morb Mortal Wkly Rep 66:1148–1153. 10.15585/mmwr.mm6642a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalldorf G, Douglass M, Robinson HE. 1938. The sparing effect of canine distemper on poliomyelitis in Macaca mulatta. J Exp Med 67:333–343. 10.1084/jem.67.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Quadros CA. 2004. Can measles be eradicated globally? Bull World Health Organ 82:134–138. [PMC free article] [PubMed] [Google Scholar]
- 23.de Swart RL. 2008. The pathogenesis of measles revisited. Pediatr Infect Dis J 27 10 Suppl:S84–S88. 10.1097/INF.0b013e31816857fe. [DOI] [PubMed] [Google Scholar]
- 24.de Vries RD, Duprex WP, de Swart RL. 2015. Morbillivirus infections: an introduction. Viruses 7:699–706. 10.3390/v7020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries RD, Ludlow M, Verburgh RJ, van Amerongen G, Yüksel S, Nguyen DT, McQuaid S, Osterhaus AD, Duprex WP, de Swart RL. 2014. Measles vaccination of nonhuman primates provides partial protection against infection with canine distemper virus. J Virol 88:4423–4433. 10.1128/JVI.03676-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries RD, Mesman AW, Geijtenbeek TB, Duprex WP, de Swart RL. 2012. The pathogenesis of measles. Curr Opin Virol 2:248–255. 10.1016/j.coviro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Delpeut S, Sawatsky B, Wong XX, Frenzke M, Cattaneo R, von Messling V. 2017. Nectin-4 interactions govern measles virus virulence in a new model of pathogenesis, the squirrel monkey (Saimiri sciureus). J Virol 91:1–11. 10.1128/JVI.02490-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebeling M, Küng E, See A, Broger C, Steiner G, Berrera M, Heckel T, Iniguez L, Albert T, Schmucki R, Biller H, Singer T, Certa U. 2011. Genome-based analysis of the nonhuman primate Macaca fascicularis as a model for drug safety assessment. Genome Res 21:1746–1756. 10.1101/gr.123117.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson B, Street SL, Wright H, Pearson C, Jia Y, Thompson SL, Allibone P, Dubay CJ, Spindel E, Norgren RB., Jr 2007. Single nucleotide polymorphisms (SNPs) distinguish Indian-origin and Chinese-origin rhesus macaques (Macaca mulatta). BMC Genomics 8:43 10.1186/1471-2164-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine PE. 1993. Herd immunity: history, theory, practice. Epidemiol Rev 15:265–302. 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 31.Fine PEM. 1993. Safety of measles vaccines. In: Kurstak E, editor. Measles and poliomyelitis: vaccines, immunization, and control. New York (NY): Springer Vienna; 10.1007/978-3-7091-9278-8_7 [DOI] [Google Scholar]
- 32.Fireman P, Friday G, Kumate J. 1969. Effect of measles vaccine on immunologic responsiveness. Pediatrics 43:264–272. [PubMed] [Google Scholar]
- 33.Ford RB, Mazzaferro EM. 2012. Section 5 - Laboratory diagnosis and test protocols, p 551–634. In: Ford RB, Mazzaferro E, editors. Kirk and Bistner's handbook of veterinary procedures and emergency treatment, 9th ed Saint Louis (MO): Elsevier Saunders. [Google Scholar]
- 34.Fox JP. 1983. Herd immunity and measles. Rev Infect Dis 5:463–466. 10.1093/clinids/5.3.463. [DOI] [PubMed] [Google Scholar]
- 35.Friedman H, Ator N, Haigwood N, Newsome W, Allan JS, Golos TG, Kordower JH, Shade RE, Goldberg ME, Bailey MR, Bianchi P. 2017. The critical role of nonhuman primates in medical research. Pathog Immun 2:352–365. 10.20411/pai.v2i3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csürös M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O'brien WE, Prüfer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234. 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 37.Greenblatt DJ. 1976. Intramuscular injection of drugs. N Engl J Med 295:542–546. 10.1056/NEJM197609022951006. [DOI] [PubMed] [Google Scholar]
- 38.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. 2008. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clin Vaccine Immunol 15:1054–1059. 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilleman MR. 2001. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine 20:651–665. 10.1016/S0264-410X(01)00384-X. [DOI] [PubMed] [Google Scholar]
- 40.Hilleman MR. 2002. Overview of the needs and realities for developing new and improved vaccines in the 21st century. Intervirology 45:199–211. 10.1159/000067911. [DOI] [PubMed] [Google Scholar]
- 41.Horne AD, Lachenbruch PA, Getson PR, Hsu HS. 2001. Analysis of studies to evaluate immune response to combination vaccines. Clin Infect Dis 33 Suppl 4:S306–S311. 10.1086/322566. [DOI] [PubMed] [Google Scholar]
- 42.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 43.Jones-Engel L, Engel GA, Schillaci MA, Lee B, Heidrich J, Chalise M, Kyes RC. 2006. Considering human-primate transmission of measles virus through the prism of risk analysis. Am J Primatol 68:868–879. 10.1002/ajp.20294. [DOI] [PubMed] [Google Scholar]
- 44.Lankau EW, Turner PV, Mullan RJ, Galland GG. 2014. Use of nonhuman primates in research in North America. J Am Assoc Lab Anim Sci 53:278–282. [PMC free article] [PubMed] [Google Scholar]
- 45.Levy BM, Mirkovic RR. 1971. An epizootic of measles in a marmoset colony. Lab Anim Sci 21:33–39. [PubMed] [Google Scholar]
- 46.Lorenz D, Albrecht P. 1980. Susceptibility of tamarins (Saguinus) to measles virus. Lab Anim Sci 30:661–665. [PubMed] [Google Scholar]
- 47.Lowenstine LJ. 1993. Measles virus infection, nonhuman primates, p 108–118. In: Jones TC, Mohr U, Hunt RD, editors. Nonhuman primates I. Monographs on pathology of laboratory animals. Berlin (Germany): Springer. [Google Scholar]
- 48.MacArthur JA, Mann PG, Oreffo V, Scott GB. 1979. Measles in monkeys: an epidemiological study. J Hyg (Lond) 83:207–212. 10.1017/S0022172400025985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McChesney MB, Fujinami RS, Lerche NW, Marx PA, Oldstone MB. 1989. Virus-induced immunosuppression: infection of peripheral blood mononuclear cells and suppression of immunoglobulin synthesis during natural measles virus infection of rhesus monkeys. J Infect Dis 159:757–760. 10.1093/infdis/159.4.757. [DOI] [PubMed] [Google Scholar]
- 50.McChesney MB, Miller CJ, Rota PA, Zhu YD, Antipa L, Lerche NW, Ahmed R, Bellini WJ. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 233:74–84. 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- 51.McKee A, Ferrari MJ, Shea K. 2015. The effects of maternal immunity and age structure on population immunity to measles. Theor Ecol 8:261–271. 10.1007/s12080-014-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merck. 2017. MMR II (measles, mumps, and rubella virus vaccine, live) [technical detailer]. Whitehouse Station (NJ): Merck [Google Scholar]
- 53.Mina MJ, Metcalf CJ, de Swart RL, Osterhaus AD, Grenfell BT. 2015. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 348:694–699. 10.1126/science.aaa3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moss WJ. 2007. Measles still has a devastating impact in unvaccinated populations. PLoS Med 4:1–10. 10.1371/journal.pmed.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moss WJ, Griffin DE. 2006. Global measles elimination. Nat Rev Microbiol 4:900–908. 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagata T, Ochikubo F, Yoshikawa Y, Yamanouchi K. 1990. Encephalitis induced by a canine distemper virus in squirrel monkeys. J Med Primatol 19:137–149. [PubMed] [Google Scholar]
- 57.National Institutes of Health. 2002. Public health service policy on humane care and use of laboratory animals. Bethesda (MD): Office of Laboratory Animal Welfare. [Google Scholar]
- 58.Orenstein WA, Papania MJ, Wharton ME. 2004. Measles elimination in the United States. J Infect Dis 189 Suppl 1:S1–S3. 10.1086/377693. [DOI] [PubMed] [Google Scholar]
- 59.Orenstein WA, Strebel PM, Papania M, Sutter RW, Bellini WJ, Cochi SL. 2000. Measles eradication: is it in our future? Am J Public Health 90:1521–1525. 10.2105/AJPH.90.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osterhaus AD, de Vries P, van Binnendijk RS. 1994. Measles vaccines: novel generations and new strategies. J Infect Dis 170 Suppl 1:S42–S55. 10.1093/infdis/170.Supplement_1.S42. [DOI] [PubMed] [Google Scholar]
- 61.Park ES, Suzuki M, Kimura M, Maruyama K, Mizutani H, Saito R, Kubota N, Furuya T, Mizutani T, Imaoka K, Morikawa S. 2014. Identification of a natural recombination in the F and H genes of feline morbillivirus. Virology 468-470:524–531. 10.1016/j.virol.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Pastoret P-P, Brochier B. 1998. Rhabdovirus, infection and immunity, p 2098–2102. In: Delves PJ, Roitt IM, editors. Encyclopedia of immunology. San Diego (CA): Academic Press. [Google Scholar]
- 63.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. 2016. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA 315:1149–1158. 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu W, Zheng Y, Zhang S, Fan Q, Liu H, Zhang F, Wang W, Liao G, Hu R. 2011. Canine distemper outbreak in rhesus monkeys, China. Emerg Infect Dis 17:1541–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed LJ, Muench H. 1938. A simple method of estimating 50% endpoints. Am J Epidemiol 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 66.Robert-Guroff M. 2007. Replicating and nonreplicating viral vectors for vaccine development. Curr Opin Biotechnol 18:546–556. 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts J, Lerche N, Anderson J, Markovits J, Maul D. 1988. Epizootic measles at the California Primate Research Center. Abstracts of scientific papers. Thirty-ninth Annual Meeting of the American Association for Laboratory Animal Science, Detroit, Michigan, 9–13 October 1988. Lab Anim Sci 38:492–492 [Google Scholar]
- 68.Rosenthal SR, Clements CJ. 1993. Two-dose measles vaccination schedules. Bull World Health Organ 71:421–428. [PMC free article] [PubMed] [Google Scholar]
- 69.Rouderfer V, Becker NG, Hethcote HW. 1994. Waning immunity and its effects on vaccination schedules. Math Biosci 124:59–82. 10.1016/0025-5564(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 70.Sakai K, Yoshikawa T, Seki F, Fukushi S, Tahara M, Nagata N, Ami Y, Mizutani T, Kurane I, Yamaguchi R, Hasegawa H, Saijo M, Komase K, Morikawa S, Takeda M. 2013. Canine distemper virus associated with a lethal outbreak in monkeys can readily adapt to use human receptors. J Virol 87:7170–7175. 10.1128/JVI.03479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salmi AA. 1995. Measles virus, p 956–962. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. editors. Manual of clinical microbiology, 6th ed Washington (DC): ASM Press. [Google Scholar]
- 72.Salyards GW, Lemoy MJ, Knych HK, Hill AE, Christe KL. 2017. Pharmacokinetics of a novel, transdermal fentanyl solution in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 56:443–451. [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarz AJ, Boyer PA, Zirbel LW, York CJ. 1960. Experimental vaccination against measles. I. Tests of live measles and distemper vaccine in monkeys and 2 human volunteers under laboratory conditions. J Am Med Assoc 173:861–867. 10.1001/jama.1960.03020260001001. [DOI] [PubMed] [Google Scholar]
- 74.Sharp CR, Nambulli S, Acciardo AS, Rennick LJ, Drexler JF, Rima BK, Williams T, Duprex WP. 2016. Chronic infection of domestic cats with feline morbillivirus, United States. Emerg Infect Dis 22:760–762. 10.3201/eid2204.151921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staley EC, Southers JL, Thoen CO, Easley SP. 1995. Evaluation of tuberculin testing and measles prophylaxis procedures used in rhesus macaque quarantine conditioning protocols. Lab Anim Sci 45:125–130. [PubMed] [Google Scholar]
- 76.Stetler HC, Orenstein WA, Bernier RH, Herrmann KL, Sirotkin B, Hopfensperger D, Schuh R, Albrecht P, Lievens AW, Brunell PA. 1986. Impact of revaccinating children who initially received measles vaccine before 10 months of age. Pediatrics 77:471–476. [PubMed] [Google Scholar]
- 77.Strating A. 1975. Measles vaccine in dogs: efficacy against aerosol challenge with virulent canine distemper virus. J Am Vet Med Assoc 167:59–62. [PubMed] [Google Scholar]
- 78.Sun Z, Li A, Ye H, Shi Y, Hu Z, Zeng L. 2010. Natural infection with canine distemper virus in hand-feeding rhesus monkeys in China. Vet Microbiol 141:374–378. 10.1016/j.vetmic.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Tamashiro VG, Perez HH, Griffin DE. 1987. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J 6:451–454. 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 80.US Department of Commerce. National Oceanic and Atmospheric Administration. [Internet]. 2013. Morbillivirus infection in dolphins, porpoises, and whales. [Cited 29 August 2017]. Available at: http://www.nmfs.noaa.gov/pr/health/mmume/faqs_morbillivirus_ume.html.
- 81.Virnik K, Hockenbury M, Ni Y, Beren J, Pavlakis GN, Felber BK, Berkower I. 2013. Live attenuated rubella vectors expressing SIV and HIV vaccine antigens replicate and elicit durable immune responses in rhesus macaques. Retrovirology 10:1–15. 10.1186/1742-4690-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilkinson DA, Melade J, Dietrich M, Ramasindrazana B, Soarimalala V, Lagadec E, le Minter G, Tortosa P, Heraud JM, de Lamballerie X, Goodman SM, Dellagi K, Pascalis H. 2014. Highly diverse morbillivirus-related paramyxoviruses in wild fauna of the southwestern Indian Ocean Islands: evidence of exchange between introduced and endemic small mammals. J Virol 88:8268–8277. 10.1128/JVI.01211-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willy ME, Woodward RA, Thornton VB, Wolff AV, Flynn BM, Heath JL, Villamarzo YS, Smith S, Bellini WJ, Rota PA. 1999. Management of a measles outbreak among Old World nonhuman primates. Lab Anim Sci 49:42–48. [PubMed] [Google Scholar]
- 84.Wintermeyer L, Myers MG. 1979. Measles in a partially immunized community. Am J Public Health 69:923–927. 10.2105/AJPH.69.9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yee JL, NPRC Breeding Colony Management Constortium. McChesney MB, Christe KL. 2015. Multicenter safety and immunogenicity trial of an attenuated measles vaccine for NHP. Comp Med 65:448–454. [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshikawa Y, Ochikubo F, Matsubara Y, Tsuruoka H, Ishii M, Shirota K, Nomura Y, Sugiyama M, Yamanouchi K. 1989. Natural infection with canine distemper virus in a Japanese monkey (Macaca fuscata). Vet Microbiol 20:193–205. 10.1016/0378-1135(89)90043-6. [DOI] [PubMed] [Google Scholar]
- 87.Zhu YD, Heath J, Collins J, Greene T, Antipa L, Rota P, Bellini W, McChesney M. 1997. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology 233:85–92. 10.1006/viro.1997.8575. [DOI] [PubMed] [Google Scholar]
- 88.Zoetis Inc. 2018. Vanguard DM canine distemper–measles vaccine modified live virus [technical detailer]. New York (NY): Zoetis. [Google Scholar]