Abstract
The AVMA Guidelines for the Euthanasia of Animals considers injection of barbiturates to be an acceptable method of euthanasia in rodents but states there is a potential for pain when administered intraperitoneally. This study examined the potential for pain in mice by assessing visceral pain after intraperitoneal administration and acute pain by using a paw-lick test. Male and female mice (n = 160) intraperitoneally received a euthanizing dose of sodium pentobarbital at a concentration of 5, 50, or 390 mg/mL and were observed for writhing, peritoneum-directed behaviors (PDB), loss of righting reflex, and time to death. Writhing was not observed in any animal. There was no significant difference in the number of mice exhibiting PDB or in the rate of PDB for responders receiving either saline or the 390-mg/mL solution. There was a significant treatment effect on time, with greater concentration and dose resulting in more rapid loss of righting reflex and death. In the second set of experiments, the same solutions were injected subcutaneously into the plantar hindpaw of male and female mice (n = 84). The number of responders, latency until the first lick, and the number of licks per responder were recorded. The number of responders was increased in the 50-mg/mL group; however, there was no difference in latency or the number of licks per responder. These results show that intraperitoneal injection of sodium pentobarbital for euthanasia in mice did not result in increased behavioral signs of pain, and animals lose consciousness more rapidly than the onset of pain seen in the paw-lick test. Therefore, although sodium pentobarbital is capable of inducing inflammation, euthanasia through intraperitoneal administration is rapid and does not result in overt signs of pain when compared with injection of saline.
Abbreviations: LORR, loss of righting reflex; PDB, peritoneum-directed behavior
The AVMA Guidelines on the Euthanasia of Animals defines euthanasia as “ending the life of an individual animal in a way that minimizes or eliminates pain and distress.”3 When viewed in this light, the critical aspects of euthanasia are rapid loss of consciousness and control of pain to minimize distress. One method that the AVMA accepts as providing a gentle and easy death is overdose of barbiturates, including sodium pentobarbital.3 The primary advantages of using sodium pentobarbital as a euthanasia agent are its reliability to induce rapid unconsciousness followed by medullary depression, cardiac and respiratory arrest, and subsequent death.3
In addition, the AVMA Guidelines categorizes methods of euthanasia as acceptable, acceptable with conditions, or unacceptable. Administration of sodium pentobarbital is one of the few methods of euthanasia that is listed as acceptable in laboratory rodents. However, the Laboratory Animals Working Group of the AVMA Panel on Euthanasia identified intraperitoneal injection of sodium pentobarbital as an area in need of further investigation. Specifically, the AVMA Guidelines state that “the degree of pain and methods for controlling pain associated with intraperitoneal administration of sodium pentobarbital have yet to be defined.”3
Concerns regarding the degree of pain associated with intraperitoneal injection of sodium pentobarbital in laboratory rodents arise primarily from 2 studies in rats. One assessed the behavior of rats during euthanasia through intraperitoneal administration of a 150-mg/kg sodium pentobarbital solution with and without lidocaine. Writhing, a hallmark of visceral pain, was reported in all 9 rats euthanized by using sodium pentobarbital alone, whereas only 1 of the 9 rats exhibited writhing when euthanized by using a pentobarbital solution containing lidocaine.2 The second study investigated neuronal activation in the dorsal horn of the spinal cord, by using c-Fos-like immunoreactivity to imply nociception. However, the study involved using intraperitoneal injection of an anesthetic dose of sodium pentobarbital (40 mg/kg), rather than a euthanizing dose.25 The investigators found more activated sensory neurons in the dorsal horn of the spinal cords of rats that received sodium pentobarbital as compared with saline. The addition of lidocaine to the sodium pentobarbital solution resulted in activation of fewer neurons compared with those after sodium pentobarbital alone but at a level that was still greater than in rats injected with saline.25 These studies suggest that sodium pentobarbital is painful to rats when administered intraperitoneally and that this pain can be ameliorated by including a local anesthetic to the solution.
In contrast to these published reports in rats, no studies have been conducted to determine whether euthanasia by intraperitoneal sodium pentobarbital is painful in mice. Here we sought to define the degree of pain associated with intraperitoneal injection of sodium pentobarbital for the euthanasia of mice. Specifically, we hypothesized that mice euthanized by intraperitoneal administration of sodium pentobarbital do not exhibit signs of pain prior to the onset of sedation, as determined by loss of righting reflex (LORR). The potential pain associated with the intraperitoneal administration of a euthanizing dose of sodium pentobarbital was evaluated by: 1) assessing mice for behavioral signs of visceral pain; 2) measuring the onset and extent of acute pain in mice by using the paw-lick test; and 3) assessing local inflammation at the injection site through gross and histologic examination. The time until a pain response occurred and the results of tissue examination were used to infer whether the potential for pain in mice is due to immediate chemical irritancy or inflammation and whether the onset of pain occurs prior to LORR.
Materials and Methods
Animals.
Mice were born as part of an IACUC-approved institutional cross-foster rederivation program or as part of a breeding performance study and were subsequently transferred to an IACUC-approved experimental protocol for these studies. Mice were derived from either C57BL/6NCrl (n = 120; 60 male, 60 female) or Crl:CD1(ICR) (n = 122; 58 male, 64 female) parents. In rare cases when mice could not be obtained from existing studies, they were ordered directly from vendors. Inclusion criteria were sex (male or female), strain or stock (C57BL/6NCrl or Crl:CD1[ICR]), and age (6 to 12 wk), with no prior experimental use. A randomized block design was followed, in which mice were grouped by sex and strain and then randomized according to body weight on each experimental day. Experiments were conducted between 1200 and 1700, after mice were allowed to acclimate to the procedure room for at least 15 min.
All mice used during this study were maintained at the University of Illinois at Chicago (Chicago, IL), an AAALAC-accredited institution, in accordance with the Guide for the Care and Use of Laboratory Animals.16 All procedures were reviewed and approved by the University of Illinois at Chicago Animal Care Committee. Mice were housed in static microisolation cages (Ancare, Bellmore, NY) with autoclaved bedding (Teklad Laboratory Grade Sani-Chips, Envigo, Indianapolis, IN) in same-sex groups under a 14:10-h light:dark cycle, with 2 to 5 mice per cage. Autoclaved municipal water and irradiated chow (Rodent Diet 7912, Envigo) were provided without restriction, cages were changed weekly, and cotton nesting squares (Nestlets, Ancare, Bellmore, NY) were placed in all cages for enrichment.
Mice were maintained in facilities using dirty bedding sentinels with an excluded pathogen list of Mycoplasma spp., Helicobacter spp., murine norovirus, mouse rotavirus, mouse hepatitis virus, mouse parvoviruses, minute virus of mice, pneumonia virus of mice, reovirus 3, Sendai virus, ectromelia, lymphocytic choriomeningitis virus, murine adenovirus 1 and 2, polyomavirus, and Theiler murine encephalomyelitis virus, as well as external and internal parasites.
Intraperitoneal injection.
Male and female C57BL/6NCrl and CD1 mice (n = 160; 10 mice per sex, strain or stock, and treatment) were allowed to acclimate to the test room for at least 15 min prior to injection. Mice were manually restrained and injected in the lower right quadrant of the abdomen by using a 28- or 30-gauge, 1/2-in. needle (Becton Dickinson, Franklin Lakes, NJ). Solutions for injection included 0.9% saline and sodium pentobarbital at 5, 50, or 390 mg/mL. The negative control group received USP sterile 0.9% saline for injection (Baxter, Deerfield, IL). The 5-mg/mL solution was made by diluting a commercially available 50-mg/mL solution for anesthesia (Nembutal, Oak Pharmaceuticals, Lake Forest, IL) to the desired concentration by using USP sterile water for injection (Hospira, Lake Forrest, IL). The 390-mg/mL solution was a commercially available euthanasia solution that does not contain phenytoin (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI). Mice receiving saline were injected with a volume equal to that administered to the 5-mg/mL group. All mice euthanized with the 5- or 50-mg/mL solutions were given a dose of 250 mg/kg, whereas the 390-mg/mL solution was administered to all mice at a uniform volume of 0.1 mL, which corresponds to a dose of approximately 1680 mg/kg in female mice and 1300 mg/kg in males. After injection, mice were immediately placed in an open field (2.5-gal. Glass aquarium, Marineland, Blacksburg, VA) for assessment of writhing, peritoneal-directed behaviors (PDB), LORR, and time to death. PDB was defined as episodes when the mouse attended its abdomen outside of normal grooming, as described previously.5 LORR was defined as the time at which the mouse ceased spontaneous movement and did not regain sternal recumbency when placed in lateral recumbency. In addition, locomotion, rearing, urination, and defecation were recorded. All observations were analyzed for the number of animals that responded in each dose group, average number of events per animal for responders, and number of events per unit time. For mice injected with sodium pentobarbital solutions, behaviors were recorded until LORR, whereas the behaviors of mice injected with saline were scored for 98 s to reflect the longest mean time to LORR, which was seen in the 5-mg/mL group. After LORR, mice were monitored for cessation of heartbeat (recorded as time to death) by digital palpation of the thorax. Three mice that had bleeding or leakage from the injection site were excluded from behavioral analysis.
Assessment of visceral color.
After euthanasia with intraperitoneal sodium pentobarbital, mice underwent necropsy. For mice that received intraperitoneal saline, necropsy was performed after euthanasia by CO2 inhalation, and cervical dislocation was performed on all mice. Four mice were excluded from the study when gross examination determined that the injection entered an organ. Color was assigned a score from 0 to 3 according to degree of redness, with 0 being unaffected tissue. The score was assigned for the region of greatest redness. Tissues were collected from the saline- and 390 mg/mL-injected mice only and fixed in 10% buffered formalin, after which paraffin-embedded sections were prepared and stained with hematoxylin and eosin. Sections were examined by a board-certified veterinary pathologist. Collected tissues included stomach, pancreas, duodenum, jejunum, ileum, cecum, colon, and full-thickness abdominal wall including the area of injection.
Paw-lick assessment.
Studies of male and female C57BL/6NCrl and CD1 mice (n = 84, 5 or 6 mice per sex, strain or stock, and treatment) were completed in 2 cohorts. Mice were randomized by body weight on each day of experiments and allowed to acclimate for at least 15 min after being moved into the testing room. Solutions for injection included 0.9% saline or sodium pentobarbital at 5, 50, or 390 mg/mL. Mice were injected subcutaneously with 15 µL of the appropriate solution in the plantar surface of the left hindpaw, taking care to avoid the foot pads. A 28-gauge, 1/2-in. insulin syringe (Becton Dickinson) was used for each animal. Continuous observations of paw licks were recorded for 10 min and analyzed for the number of mice per treatment that expressed the paw-lick behavior, latency until the first paw lick in responders, and the average number of licks per responder. A cutoff of 10 min was chosen because phase 2 (that is, inflammatory pain) begins at that time.17 Mice were excluded when blood was present at the site of injection. All sodium pentobarbital groups were compared with the group that received saline.
Assessment of hindpaw color and diameter.
During and after the paw-lick experiment, mice were assessed for hindpaw color and diameter. Experiments were conducted in 2 cohorts. The first cohort included mice injected with sterile water for injection or the 5- or 50-mg/mL sodium pentobarbital solution. Sterile water was chosen as the negative control in this cohort because it was the diluent for the 5-mg/mL solution and was a large proportion of this solution. The second cohort included mice injected with 0.9% saline or the 390-mg/mL sodium pentobarbital solution. The color of the injected hindpaw was assessed at 10 min, and an erythema score was assigned according to similarity to preselected color samples (PPG Industries, Pittsburgh, PA). Reference colors were assigned a value from 1 to 5 on the basis of intensity of redness. Mice were subsequently euthanized by CO2 inhalation and cervical dislocation, and the mid-metatarsal dorsopedal diameter of both hindpaws was measured by using digital calipers (Fisher Scientific, Hampton, NH).
Statistical analysis.
All mice were randomized according to body weight; randomization was confirmed by ANOVA. Statistical inference was performed by using multiple linear regression, ANOVA, and the Tukey method for multiple comparisons of continuous response variables; negative binomial regression model for count variables; and the χ2 independence test and multinomial logistic regression for categorical variables. For linear regressions with nonnormal residuals, Box–Cox transformation on the response variables was used, and the regression model was run again. Response variables included time to LORR, time to death, locomotion, rearing, defecation, and urination. The significance level was set at a P value of 0.05. All statistical analyses were performed by using SAS 9.4 (SAS Institute, Cary, NC), and plots were made by using R 3.1.2 (www.r-project.org).
Results
Assessment of behaviors after intraperitoneal injection of mice with sodium pentobarbital.
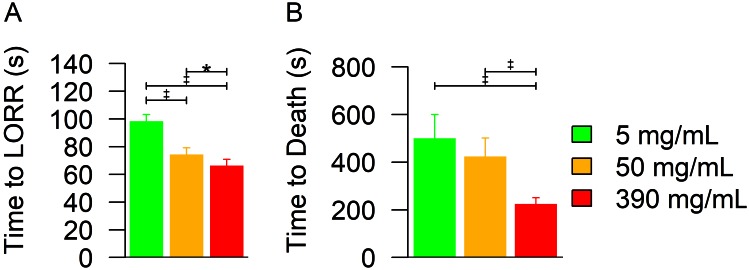
Frequencies of observed behaviors were analyzed by ANOVA with Box–Cox transformation to normalize data. No mouse in any group exhibited writhing after injection (Table 1). The number of mice exhibiting PDB did not differ between groups (P = 0.2374, logistic regression). The PDB rate (mean ± 1 SD) was 0.08 ± 0.21, 0.12 ± 0.45, 0.09 ± 0.26, and 0.43 ± 1.08 PDB per second for 0.9% saline and 5, 50, and 390 mg/mL sodium pentobarbital, respectively. These rates did not differ significantly between mice receiving either saline or sodium pentobarbital (P = 0.0557). All pentobarbital solutions resulted in reliable and rapid unconsciousness according to LORR (Figure 1 A). ANOVA revealed significant (P < 0.0001) treatment and dose effects, with LORR occurring at 98.5 ± 4.7, 74.3 ± 4.9, and 66.4 ± 4.5 s after injection of the 5-, 50-, and 390-mg/mL sodium pentobarbital solutions, respectively. ANOVA after Box–Cox transformation to normalize data demonstrated significant (P < 0.0001) treatment and dose effects on the time to death (Figure 1 B), with lack of heartbeat occurring at 619.6 ± 359.6, 508.3 ± 303.4, and 253.8 ± 118.1 s after injection of the 5-, 50-, and 390-mg/mL solutions, respectively. However, Tukey–Kramer adjustment for multiple comparisons showed that there was no difference in time to death between the 5- and 50-mg/mL groups (P = 0.348). There was a significant increase in locomotion associated with injection of the 390-mg/mL solution compared with saline (P = 0.0002, data not shown) and a significant increase in the frequency of rearing associated with injection of all sodium pentobarbital solutions compared with saline (P = 0.0052, data not shown). There was a significant (P = 0.0013, data not shown) reduction in the frequency of urination associated with the 390 mg/mL solution compared with all other groups. There was no difference in the frequency of defecation between groups (P = 0.0525, data not shown).
Table 1.
Summary of observations after intraperitoneal injection of sodium pentobarbital for euthanasia of mice
| Sodium pentobarbital (mg/mL) | PDB | Peritoneal colora | |
| No. (%) affected | No. (range) events per min | ||
| 0 (n = 31) | 4 (12.9) | 0.08 ± 0.21 (0.00–0.61) | 0 ± 0 |
| 5 (n = 36) | 4 (11.1) | 0.12 ± 0.45 (0.00–2.58) | 0.77 ± 0.42 |
| 50 (n = 36) | 4 (11.1) | 0.09 ± 0.26 (0.00–0.95) | 0.97 ± 0.71 |
| 390 (n = 40) | 9 (22.5) | 0.43 ± 1.08 (0.00–5.33) | 2.15 ± 0.36 |
Data are given as mean ± 1 SD where appropriate.
Writhing did not occur in any group.
Significant (P < 0.0001; Fisher exact test for independence) treatment effect
Figure 1.
(A) Time to LORR and (B) time to death after a euthanizing dose of sodium pentobarbital given intraperitoneally to mice. The 5- and 50-mg/mL solutions were given at 250 mg/kg, whereas the 390-mg/mL solution was given at a uniform volume of 0.1 mL per mouse (that is, approximately 1680 mg/kg in female mice and 1300 mg/kg in male). (A) Time to LORR showed a significant (*, P = 0.0489; ‡, P < 0.0001; ANOVA with Box–Cox transformation to normalize data) treatment effect. (B) Time to death showed a significant (‡, P < 0.0001; ANOVA with Box–Cox transformation to normalize data) dose effect but no significant difference between the 5-and 50-mg/mL solutions. Bars indicate 95% CI.
Gross necropsy and histopathology.
There was positive correlation (P < 0.0001) between the erythema score of peritoneal organs based on visual inspection during gross necropsy (Table 1) and the injected sodium pentobarbital concentration. Scores were 0 ± 0, 0.77 ± 0.42, 0.97 ± 0.71, and 2.15 ± 0.36 for the 0.9% saline and 5-, 50-, and 390-mg/mL sodium pentobarbital solutions, respectively. Representative gastrointestinal and abdominal wall tissues from the saline and 390-mg/mL groups were examined histologically. No histologic evidence suggestive of inflammation was seen in any of the observed sections.
Hindpaw injection of sodium pentobarbital.
During the first 10 min after subcutaneous plantar injection of test solutions, the number of mice that exhibited the paw lick behavior was 8 of 20, 14 of 22, 16 of 22, and 13 of 20 for the saline and 5-, 50-, and 390-mg/mL sodium pentobarbital solution injections, respectively (Table 2). As compared with saline, only the group injected with 50 mg/mL sodium pentobarbital was more likely to exhibit the paw-lick behavior (P = 0.0170, multinomial logistic regression). The latency until the first lick among mice that responded in each group was 271.9 ± 280.7, 291.9 ± 176.9, 254.9 ± 117.2, and 326.3 ± 292.6 s for saline and the 5-, 50-, and 390-mg/mL sodium pentobarbital solutions, respectively. There was no significant difference in latency to the first paw lick between these groups (P = 0.9127). Among mice that expressed the paw-lick behavior, the average number of licks per responder was 3.0 ± 3.0, 1.8 ± 1.1, 1.4 ± 0.8, and 1.5 ± 1.0 licks per mouse for saline and the 5-, 50-, and 390-mg/mL sodium pentobarbital solutions, respectively. There was no significant difference in the average number of licks between these groups (P = 0.8231).
Table 2.
Summary of observations after subcutaneous injection of sodium pentobarbital into hindpaw
| Sodium pentobarbital (mg/mL) | No. of responders | Time (s) until first lick | No. of licks per responder | Paw colorb |
| 0 (n = 20) | 8 | 271.9 ± 280.7 | 3.0 ± 3.0 | 2.2 ± 0.4 |
| 5 (n = 22) | 14 | 291.9 ± 176.9 | 1.8 ± 1.1 | 2.4 ± 0.8 |
| 50 (n = 22) | 16a | 254.9 ± 117.2 | 1.4 ± 0.8 | 4.0 ± 0.0c |
| 390 (n = 20) | 13 | 326.3 ± 292.6 | 1.5 ± 1.0 | 5.0 ± 0.2d |
Data are given as mean ± 1 SD where appropriate.
Significant (P = 0.0170) difference in the number of responders within 10 min between 50-mg/mL and saline groups
Significant (P < 0.0001) effect of treatment on paw color at 10 min after injection
Significant (P < 0.0001) difference in paw color at 10 min after injection between 50-mg/mL and saline groups
Significant (P < 0.0001) difference paw color at 10 min after injection between 390-mg/mL and saline groups
Paw color and diameter.
There was positive correlation (P < 0.0001) between the erythema score at 10 min and the concentration of sodium pentobarbital administered (Table 2). The erythema scores (mean ± 1 SD) were 2.2 ± 0.4, 2.4 ± 0.8, 4.0 ± 0.0, and 5.0 ± 0.2, for saline and the 5-, 50-, and 390-mg/mL sodium pentobarbital solutions, respectively. Similarly, each group showed positive correlation (P < 0.0001) between the average mid-metatarsal dorsopedal paw diameter of the injected paw compared with that of the control paw (Table 3). In addition, differences were noted between sex (P < 0.0001) and strain (P < 0.0001) across all treatment groups.
Table 3.
Mean mid-metatarsal dorsopedal paw diameter (cm) of control compared with injected paws
| Randomized group 1 | Randomized group 2 | ||||||||||||||||
| Sterile water | 5 mg/mL | 50 mg/mL | Saline | 390 mg/mL | |||||||||||||
| Straina | Sexb | Cont | Treat | % diff | Cont | Treat | % diff | Cont | Treata–e | % diff | Cont | Treat | % diff | Cont | Treatf,g | % diff | |
| C57BL/6 | Male | 0.218 | 0.232 | 6.3 | 0.202 | 0.217 | 7.3 | 0.205 | 0.282 | 31.6 | 0.228 | 0.232 | 2.0 | 0.248 | 0.300 | 18.7 | |
| Female | 0.195 | 0.206 | 5.4 | 0.187 | 0.196 | 4.2 | 0.187 | 0.264 | 33.8 | 0.232 | 0.231 | −0.7 | 0.233 | 0.330 | 34.7 | ||
| 0.316 | 0.323 | 2.1 | 0.319 | 0.400 | 22.6 | ||||||||||||
| CD1 | Male | 0.313 | 0.324 | 3.4 | 0.302 | 0.324 | 6.8 | 0.311 | 0.327 | 4.9 | 0.291 | 0.298 | 2.2 | 0.288 | 0.366 | 24.0 | |
| Female | 0.208 | 0.229 | 9.3 | 0.213 | 0.229 | 7.5 | 0.220 | 0.298 | 30.2 | 0.228 | 0.232 | 2.0 | 0.248 | 0.300 | 18.7 | ||
Cont, control; Treat, treated
Percentage difference (% diff) was calculated as [(mean diametertreated – mean diametercontrol) / (average mean of treated and control)] x 100%.
Significant (P < 0.0001) difference in paw diameter between strains
Significant (P < 0.0001) difference in paw diameter between sexes
Significant (P < 0.0001) difference in mean paw diameter between 50-mg/mL and sterile water groups
Significant (P < 0.0001) difference in mean paw diameter between 5- and 50-mg/mL groups
Significant (P < 0.0001) difference in mean paw diameter between control and treated paws for 50-mg/mL groups
Significant (P < 0.0001) difference in mean paw diameter between 390 mg/mL-treated paw and 0.9% saline-treated paws
Significant (P < 0.0001) difference in mean paw diameter between control and treated paws for the 390-mg/mL group
Discussion
The AVMA Guidelines for the Euthanasia of Animals describes an ideal method of euthanasia as one that leads to rapid unconsciousness with minimal pain and distress.3 The AVMA Guidelines consider injection of sodium pentobarbital solutions as acceptable without conditions, but the Laboratory Animals Working Group identified the potential for pain associated with intraperitoneal administration of these solutions as an area in need of further study. In the current study, we assessed the potential for pain associated with sodium pentobarbital injection first by observing mice for behavioral evidence of pain during euthanasia. The extent of pain was then assessed by using the paw-lick test. Finally, pathologic assessment for gross and histologic evidence of local inflammation after injection was performed.
Commercially available 50- and 390-mg/mL formulations of sodium pentobarbital and a single dilution of the 50-mg/mL solution with sterile water to achieve a 5-mg/mL concentration were used for all experiments. The saturated solution was selected because this product is marketed specifically for euthanasia, and it lacks the compound phenytoin, which is a central nervous center depressant and class 1b antiarrhythmic. It has been suggested that barbiturate combination solutions containing phenytoin should not be injected intraperitoneally due to concerns that these formulations may affect cardiac function prior to onset of sedation when given through this route;8,11,22,26 however, this effect has not been demonstrated empirically and is an area in need of further investigation.
Because an ideal method of euthanasia should minimize pain and distress, we first assessed the behavior of mice after the injection of a euthanizing dose of sodium pentobarbital. Prior to our study, it had been noted anecdotally that mice do not exhibit writhing after injection of sodium pentobarbital, in contrast to published reports of writhing in laboratory rats.2,25 This difference in response to sodium pentobarbital administration between rats and mice may be attributed to differences in species-specific behavior, body size, or the volume of solution needed for euthanasia and the resulting rate of absorption. In addition, the studies that reported writhing in rats were performed in Europe, using formulations not commercially available in the United States. Although the composition of the solutions used in European studies is similar to those used in the current study, differences in both the formulation of the solutions and the concentration of sodium pentobarbital are present. Furthermore, recent research demonstrated writhing in rats euthanized by intraperitoneal injection of sodium pentobarbital at concentrations of 80 and 240 mg/mL.28 It is possible that the threshold for writhing occurs at a concentration higher than the 50-mg/mL solution we used and that the 390-mg/mL solution that we used resulted in LORR prior to the development of pain. Perhaps the 80- to 240-mg/mL concentrations used in the other studies28 could result in the development of pain prior to the loss of consciousness. Alternatively the difference might be attributable to one of the other components in the solutions. One previous study2 used a commercially available product that is a solution of sodium pentobarbital in propylene glycol, ethanol, blue dye, and water; this product was diluted in either water or a commercially available lidocaine solution. Another study24 was conducted with a solution formulated in the lab, containing 96% ethanol with propylene glycol, and at a dose that resulted in anesthesia, not euthanasia; the duration of anesthesia or the high concentration of ethanol may have contributed to the study findings. Still another study28 used a solution that is commercially available in Canada and that contains sodium pentobarbital in propylene glycol, ethanol, rhodamine B, benzyl alcohol, and water. The solutions used in the European and Canadian studies were not directly evaluated in our current study due to the lack of availability in the United States; therefore, no definitive conclusions regarding writhing can be made between our study in mice and the studies in rats.
In addition to writhing, our mice were assessed for other behavioral signs of pain after intraperitoneal injection. One behavior that is a possible indication of discomfort after intraperitoneal injection is PDB, which we defined as a behavior in which a mouse inspected its abdomen outside of normal grooming.5 Of note, there was no significant difference in the number of mice that expressed PDB after intraperitoneal injection of pentobarbital compared with saline (P = 0.2374), and the rate at which PDB was expressed did not vary by treatment (P = 0.0557). Although the rate data appear to approach significance, there was no positive correlation between sodium pentobarbital concentration and the rate of PDB. There are several possible explanations for the occurrence of this behavior which make it nonspecific to pain associated with sodium pentobarbital injection in the present study. Possible causes of PDB are pain associated with the needle stick and irritancy of the injected solution. Another possible cause for the behavior could be an unpleasant sensation due to volume expansion in the peritoneal cavity. This effect was more likely a factor in mice that received either saline or the 5-mg/mL sodium pentobarbital solution, given that a volume of 50 mL/kg (0.5 mL per 10 g of body weight) was administered to these mice. This volume, which exceeds the suggested maximal limit of 10 mL/kg set forth by some authors for routine intraperitoneal injection of substances in mice, was necessary to achieve a euthanizing dose of 250 mg/kg of sodium pentobarbital.21 However, both the greatest number of mice to exhibit PDB (9 of 40) and the highest mean rate (0.43 ± 1.08) were seen in the group treated with the 390-mg/mL solution. Although none of the PDB data differed significantly, this may be an area of further study regarding the use of full-strength, saturated sodium pentobarbital solutions for euthanasia in mice and the usefulness of PDB as a measure of abdominal pain or discomfort.
The other behaviors scored after intraperitoneal injection of either sodium pentobarbital or saline (locomotion, rearing, urination, and defecation) have been used to assess distress in rodents.2 In our experiment, mice were administered sodium pentobarbital intraperitoneally for euthanasia and then placed in an open field. Prior to the onset of the pharmacologic effects of sodium pentobarbital, mice are likely to take part in some level of exploratory behavior as well as demonstrate some level of anxiety. These events can be seen as an increase in locomotion and rearing as they explore a novel environment; whereas the increase in defecation might reflect anxiety. For mice that received sodium pentobarbital, increases in locomotion and rearing can also be attributed to the excitatory stage of anesthetic induction. Due to the potential influence of placing a mouse in a novel environment and the anesthetic effect of the sodium pentobarbital, whether the changes in these other behavioral parameters can be attributed to distress associated directly with the injection of sodium pentobarbital is difficult to determine.
Additional aspects of an ideal form of euthanasia are the reliability and rapidity of the method. These features of the various treatments were assessed as the time to LORR and time to death. There was an inverse relationship between concentration and both time to LORR and time to death. The longest times to LORR and death (98.5 ± 4.7 and 619.6 ± 359.6 s, respectively) were observed in mice euthanized by using the 5-mg/mL solution, which was the lowest concentration examined. By comparison, the times to LORR and death in the group euthanized with the 390-mg/mL solution were 66.4 ± 4.5 and 253.8 ± 118.1 s, respectively. When assessing methods of euthanasia according to the reliability of achieving rapid unconsciousness, these data support the use of saturated sodium pentobarbital solutions for the euthanasia of laboratory mice.
The next set of experiments set out to address the AVMA's concern that the extent of pain associated with the injection of sodium pentobarbital has not been assessed. A common method of objectively quantifying pain in animals is the formalin test,10,20 which was expanded from assessing analgesic efficacy to an assessment of pain associated with parenteral injection of various compounds.6,12 This method is commonly used in mice.1,10,15,19,20,23,24 The main benefits of assessing nociceptive effects of injectable agents by using modifications of the formalin test are that the method is quantitative, depends on spontaneous behaviors produced by the animal, elicits responses in a dose-dependent manner, requires little restraint, and differentiates between acute (that is, phase I) chemical irritancy and chronic (that is, phase II) inflammatory pain.4,17,27 In the case of assessing the potential for an injectable drug to cause pain, this model has the added benefit of being clinically applicable.12,17,18
The hindpaw injection evaluation showed that more mice demonstrated paw-licking after treatment with the 50-mg/mL solution compared with saline (P = 0.0170). Although more mice expressed the behavior in this group, they did not do so at a greater frequency than those receiving a saline injection (P = 0.8729). The 390-mg/mL solution did not increase the number of mice that expressed the behavior (P = 0.3394) or the frequency that they expressed it (P = 0.3196). This outcome may have been due to the degree of sedation associated with the 15-µL volume of these solutions. This small amount equates to approximately 25 and 200 mg/kg for the 50- and 390-mg/mL solutions, respectively. Of note, the 200-mg/kg dose of pentobarbital did not result in LORR or euthanasia during the course of observation, and mice were able to express spontaneous behaviors for the full 10 min of observation even though they showed signs of moderate sedation. This result may be due to a slower rate of absorption by the subcutaneous route.
The assessment of paw color at 10 min after injection in the paw-lick studies showed a positive correlation between sodium pentobarbital concentration and intensity of color (P < 0.0001). In particular, 2 individual groups varied significantly from control (50 mg/mL, P < 0.0001; 390 mg/mL, P < 0.0001). Comparing the mid-metatarsal dorsopedal paw diameter revealed a significant increase in diameter for injected compared with control paw in each group (P < 0.0001); however, the difference between the treated and control paw was comparable between the 2 randomized groups. As in the color assessment, only the 50- and 390-mg/mL groups varied significantly from control treatment (P < 0.0001 for both comparisons). Although edema and erythema were seen in the mice that received the 50- and 390-mg/mL solutions, these mice did not express the paw-lick behavior that would be expected due to chemical irritancy, tissue damage, or inflammation.
Importantly, the longest time to unconsciousness based on LORR in the euthanasia observations occurred well in advance of even the earliest onset of the paw-lick behavior (98.5 ± 4.7 s compared with 254.9 ± 117.2 s for the 5- and 50 mg/mL solutions, respectively). Given the rapid onset of sedation and slow development of a pain response, it is distinctly possible that mice are unconscious prior to the onset of pain in the context of intraperitoneal injection for euthanasia. Any pain associated with the injection of sodium pentobarbital is likely due to inflammation and tissue damage, which require some time to develop.
A further difference between the present study and previous studies describing pain during the euthanasia of rats with sodium pentobarbital is the dose. One study2 used a dose of 150 mg/kg for euthanasia, whereas another25 used a nonlethal anesthetic dose of 40-mg/kg for study design purposes.2,25 Our experience is that a dose of 150 mg/kg does not produce reliable euthanasia in CD1 mice, and for this reason, we used a euthanizing dose of 250 mg/kg of sodium pentobarbital; this dose is sufficiently high to produce rapid unconsciousness and death. The time to LORR in the study using 150 mg/kg was 212 ± 10.5 to 213 ± 18.1 s, whereas the time to LORR using 250 mg/kg in our study ranged from 74.3 ± 4.9 to 98.5 ± 4.7 s, and the use of 1300 to 1600 mg/kg in resulted in a time to LORR of 66.4 ± 4.5 s. Similarly, other colleagues found that a higher dose of sodium pentobarbital results in shorter time to unconsciousness in rats, and writhing was reported in some animals of each treatment group, including the saline-injected mice.28 In our current study, by simply using an appropriately high dose to produce rapid and reliable unconsciousness in mice, we avoided producing a prolonged death that allowed for the development of pain. This achievement directly answers another of the questions in the AVMA Guidelines, specifically, identifying means to ameliorate the pain seen in the earlier studies.3
The reason previous investigators used an anesthetic dose of pentobarbital rather than a euthanizing dose was to observe activation of c-Fos,25 and the solution that they injected had a large concentration of ethanol, which also can lead to nociception. Activation of c-Fos occurs with neuronal activation, which in this case was interpreted as a correlate of nociception. Such correlations are nonspecific to chemical irritancy and can be difficult to interpret.7,9,13,14 Due to the time it takes c-Fos to be upregulated during neuronal activation, the mice had to be assessed 3 h after the initial stimulus provided by the intraperitoneal injection of the sodium pentobarbital solution. This prolonged time after injection may more accurately reflect the onset of inflammation rather than of acute pain, which would be relevant during euthanasia.
The rapid loss of consciousness followed by a reliable progression to death currently place barbiturates among the preferred agents available for euthanasia in all species. Among the barbiturates, sodium pentobarbital is the most commonly used. Concern regarding the possibility of pain and the extent of any such pain are important considerations when proposing this agent for the euthanasia of animals. This concern is more pronounced when these compounds are given extravascularly, as is the case in laboratory mice. In our present study, intraperitoneal injection of sodium pentobarbital solutions at various concentrations provided for rapid, reliable euthanasia when given at a dose of 250 mg/kg in laboratory mice and did not cause more than the slight pain or distress associated with the injection of saline.
Acknowledgments
JWD was supported by NIH grant 5 R25 OD010914-04. Additional funding was provided by the Office of the Vice Chancellor for Research at the University of Illinois at Chicago. We thank Dr Herbert Whiteley for histopathologic review of samples; Drs Lisa Halliday, Jeanette Purcell, Cynthia Adams, and Kelly Garcia for their critical review of this project; and Drs Jeanette Purcell and Julia Goldman and Ms Heather Charles for identifying mice to be used on this study.
References
- 1.Adedapo AA, Falayi OO, Oyagbemi AA. 2015. Evaluation of the analgesic, anti-inflammatory, anti-oxidant, phytochemical and toxicological properties of the methanolic leaf extract of commercially processed Moring oleifera in some laboratory animals. J Basic Clin Physiol Pharmacol 26:491–499. 10.1515/jbcpp-2014-0105. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose N, Wadham J, Morton D. 2000. Refinement of euthanasia, p 1159–1170. In: Balls M, van Zeller AM, Halder ME, editors. Progress in the reduction, refinement and replacement of animal experimentation. Oxford: Elsevier. [Google Scholar]
- 3.American Veterinary Medical Association. 2013. AVMA guidelines for the euthanasia of animals, 8th ed Schaumburg (IL): American Veterinary Medical Association. [Google Scholar]
- 4.Bannon AW. 2001. Models of pain: hot-plate and formalin tests in rodents. Curr Protoc Pharmacol. Chapter 5: Unit 5.7. 10.1002/0471141755.ph0507s00 [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC. 1990. Comparative fine structure of action: rules of form and sequence in the grooming patterns of 6 rodent species. Behaviour 113:21–56. 10.1163/156853990X00428. [DOI] [Google Scholar]
- 6.Celozzi E, Lotti VJ, Stapley EO, Miller AK. 1980. An animal model for assessing pain-on-injection of antibiotics. J Pharmacol Methods 4:285–289. 10.1016/0160-5402(80)90048-0. [DOI] [PubMed] [Google Scholar]
- 7.Coggeshall RE. 2005. Fos, nociception and the dorsal horn. Prog Neurobiol 77:299–352. [DOI] [PubMed] [Google Scholar]
- 8.Cooney K, Chappell J, Callan R, Connally B. 2012. Veterinary euthanasia techniques: a practical guide. Hoboken (NJ): Wiley–Blackwell; 10.1002/9781118704585 [DOI] [Google Scholar]
- 9.DeLeo JA, Coombs DW, McCarthy LE. 1991. Differential c-fos-like protein expression in mechanically versus chemically induced visceral nociception. Brain Res Mol Brain Res 11:167–170. 10.1016/0169-328X(91)90118-H. [DOI] [PubMed] [Google Scholar]
- 10.Dubuisson D, Dennis SG. 1977. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4 Supp C:161–174. 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 11.Fakkema DK. 2010. Operational guide for animal care and control agencies: euthanasia by injection. Washington (DC): American Humane Association. [Google Scholar]
- 12.Gupta PK, Patel JP, Hahn KR. 1994. Evaluation of pain and irritation following local administration of parenteral formulations using the rat paw lick model. J Pharm Sci Technol 48:159–166. [PubMed] [Google Scholar]
- 13.Harris JA. 1998. Using c-fos as a neural marker of pain. Brain Res Bull 45:1–8. 10.1016/S0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman GE, Lyo D. 2002. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? J Neuroendocrinol 14:259–268. 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 15.Hunskaar S, Hole K. 1987. The formalin test in mice: dissociation between inflammatory and noninflammatory pain. Pain 30:103–114. 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 17.Langford DJ, Mogil JS. 2008. Pain testing in the laboratory mouse, p 549–560. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals, 2nd ed. Cambridge (MA): Academic Press. [Google Scholar]
- 18.Le Bars D, Gozariu M, Cadden SW. 2001. Animal models of nociception. Pharmacol Rev 53:597–652. [PubMed] [Google Scholar]
- 19.Liu X, Sun W, Zhang B, Tian B, Tang X, Qi N, Haibing H, Huifang L, Xiangqun J. 2013. Clarithromycin-loaded liposomes offering high drug loading and less irritation. Int J Pharm 443:318–327. 10.1016/j.ijpharm.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Melzack R, Melinkoff DF. 1974. Analgesia produced by brain stimulation: evidence of a prolonged onset period. Exp Neurol 43:369–374. 10.1016/0014-4886(74)90178-2. [DOI] [PubMed] [Google Scholar]
- 21.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, Verschoyle R, Westall L, Wilson AB. 2001. Refining procedures for the administration of substances. Lab Anim 35:1–41. 10.1258/0023677011911345. [DOI] [PubMed] [Google Scholar]
- 22.Newberry S, Blinn MK, Bushby PA, Cox CB, Dinnage JD, Griffin KF, Hurley KF, Isaza N, Jones W, Miller L, O'Quin J, Patronek GJ, Smith-Blackmore M, Spindel M. 2010. Guidelines for standards of care in animal shelters. Washington (DC): The Association of Shelter Veterinarians. [Google Scholar]
- 23.Nikfar S, Abdollahi M, Etemad F, Sharifzadeh M. 1997. Effects of sweetening agents on morpine-induced analgesia in mice by formalin test. Gen Pharmacol 29:583–586. 10.1016/S0306-3623(96)00575-7. [DOI] [PubMed] [Google Scholar]
- 24.Paula-Freire LIG, Andersen ML, Molska GR, Köhn DO, Carlini ELA. 2012. Evaluation of the antinociceptive activity of Ocimum gratissiumum L. (Lamiaceae) essential oil and its isolated active principles in mice. Phytother Res 27:1220–1224. 10.1002/ptr.4845. [DOI] [PubMed] [Google Scholar]
- 25.Svendsen O, Kok L, Lauritzen B. 2007. Nociception after intraperitoneal injection of a sodium pentobarbitone formulation with and without lidocaine in rats quantified by expression of neuronal c-fos in the spinal cord—a preliminary study. Lab Anim 41:197–203. 10.1258/002367707780378140. [DOI] [PubMed] [Google Scholar]
- 26.The Humane Society of the United States. 2013. Euthanasia reference manual. Washington (DC): The Humane Society of the United States. [Google Scholar]
- 27.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. 1992. The formalin test: an evaluation of the method. Pain 51:5–17. 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 28.Zatroch KK, Knight CG, Reimer JN, Pang DSJ. 2017. Refinement of intraperitoneal injection of sodium pentobarbital for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res 13:1–7. 10.1186/s12917-017-0982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]