Abstract
Resources detailing the scope, details, and duration for teaching and learning surgical model development in research are poorly described. Situated learning and instructional scaffolding are useful skill-building tools. Herein, we discuss educational theory in the context of a training paradigm for surgical researchers, using our experience with a nonunion femoral fracture model as an example. Stages of learning include cognitive, associative, and autonomous stages. In surgical training, the cognitive stage involves the acquisition of basic knowledge, including anatomy, surgical approach, instrumentation, and suturing, which can be taught by using books, videos, skeletons, and cadavers. To these basic skills, the associative stage adds advanced techniques—including anesthesia, asepsis, hemostasis, and the full surgical procedure—through mentored nonsurvival surgical experiences. After a mentor has assured competence, trainees perform supervised and then independent survival surgeries to complete the autonomous stage. Through these stages, instructional scaffolding is applied in the context of a situated learning environment in which trainees learn in a layered approach through their own experiences. Thus, the proposed training paradigm is structured to teach trainees how to think and act as surgeons so they can adapt and grow, rather than only to ensure technical competency in a specific model. Development and mastery of complex surgical models may require as long as 6 mo to achieve optimal outcomes, depending on the preexisting skill of the research surgeons, technical difficulty, and the stage of model evolution.
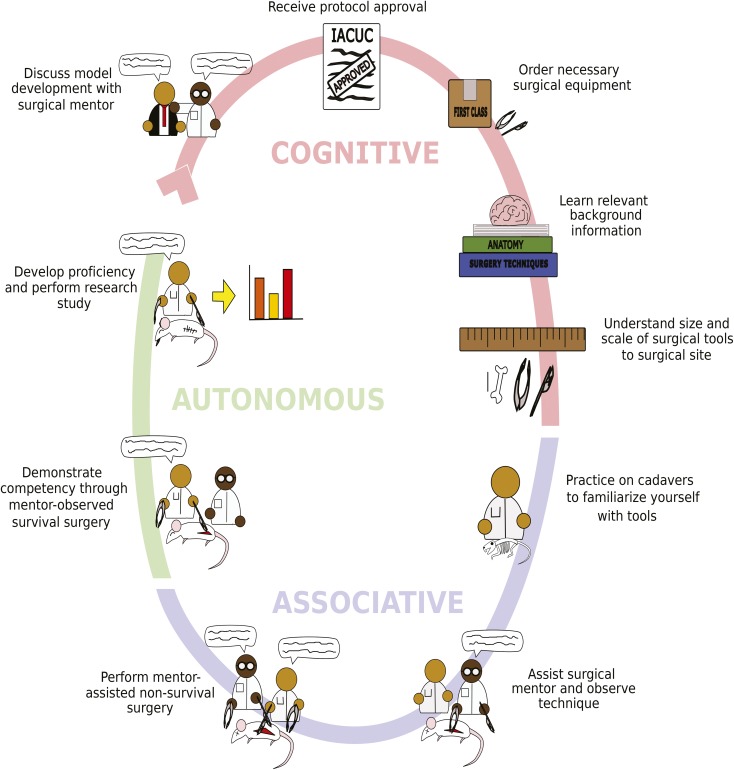
Animal surgical models are important in the advancement of biomedical science and applied research. Both the development and refinement of these models, particularly complex ones, hold multiple challenges, including design, translatability, and technical training. Design and translatability are optimized by the use of team science and the assembly of a diverse, expert clinical and research team. Multidisciplinary teams comprising scientists, engineers, physicians, and veterinarians are common in the refinement of medical device prototypes, for instance. Technical training varies on a case-by-case basis, depending on the knowledge and experience of the surgeon. Even a person who has earned a degree in health sciences (for example, DVM or VMD, MD, DDS or DMD) cannot automatically be considered competent in experimental surgery or research surgical model development and may require additional training.1 However, the steps of the learning and training process are the same regardless of surgeon experience. For expert surgeons, most steps in the process will be short, second-nature, and independently completed, whereas for novices, these same steps should be longer, deliberate, and closely mentored. Herein, we describe an educational paradigm that we have used for surgical model planning and mastery, incorporating the use of instructional scaffolding, situated learning, and mentoring, as needed, for either novice or experienced surgeons (Figure 1).
Figure 1.
Visual guide for training novice surgeons in surgical model development. Artist's rendering of the suggested steps to train novice surgeons. These steps encompass the 3 major stages of learning: cognitive, associative, and autonomous. The cognitive stage should begin with a conversation between the novice surgeon and a surgical mentor, such as a consulting veterinarian. This consultation is followed by gaining IACUC approval for the desired surgical protocol. Next, the appropriate surgical tools should be ordered. The novice surgeon then should begin learning about the surgical model, acquiring relevant background information, and understanding the application of specific methods. The novice surgeon can then use appropriate simulations or anatomic specimens, such as skeletons, to understand spatial relationships between any implants, surgical tools, and the tissues that will be operated on. The novice surgeon begins the associative stage of learning by practicing on cadavers to train hand dexterity and instrument use. Next, the novice surgeon can assist the surgical mentor in performing the desired surgical model to understand the intricacies of the model and to observe mentor expertise in aseptic technique and instrument handling. During this stage, the veterinarian is instructing the novice surgeon. Afterward, the novice surgeon can practice the surgical model with assistance from the mentoring surgeon in a nonsurvival surgery setting. After gaining competence and experience, the autonomous stage begins, in which the novice surgeon can demonstrate mastery of the surgical model to the observing surgical mentor. Finally, the novice surgeon has completed the stages of training and can begin to independently perform surgeries, develop proficiency, and generate study data.
One of the primary risks in surgical model development is that of animal pain and discomfort, particularly during the early stages of experimentation. Therefore, it is paramount to follow the guidance of the institutional oversight body (for example, IACUC)24 tasked with ethical review or regulatory compliance of proposed procedures. The IACUC review process ensures that standards, such as Russell and Burch's ‘three Rs’ (that is, replacement, reduction, and refinement) from the Public Health Service's Policy on Humane Care and Use of Lab Animals and the Guide for the Care and Use of Laboratory Animals are incorporated.12,19,21 Implementation of principles of humane animal use through stepwise surgical training—from skeletons and cadavers to nonsurvival training procedures to survival surgery—minimizes animal use and discomfort as surgeon abilities are improved. Skill refinement results in reduced animal pain and distress and decreases the need for repeat studies resulting from poor technique.
In addition to input during the IACUC review process, a veterinarian board-certified by the American College of Laboratory Animal Medicine may be involved in surgical model planning and development. The board's position statement on rodent survival surgery recommends seeking the guidance of additional veterinary specialists, such as veterinary anesthesiologists and surgeons, when warranted.2 Development of complex surgical models will benefit from input not only from veterinary specialists but also from physicians practicing in the relevant clinical specialty, to ensure that the resulting model is maximally translatable and practical. In the examples we describe, all complex model development profited from the input of both board-certified human surgeons and a board-certified veterinary surgeon collaborating with multidisciplinary scientific teams. This level of expertise may not be needed for less-complicated models.
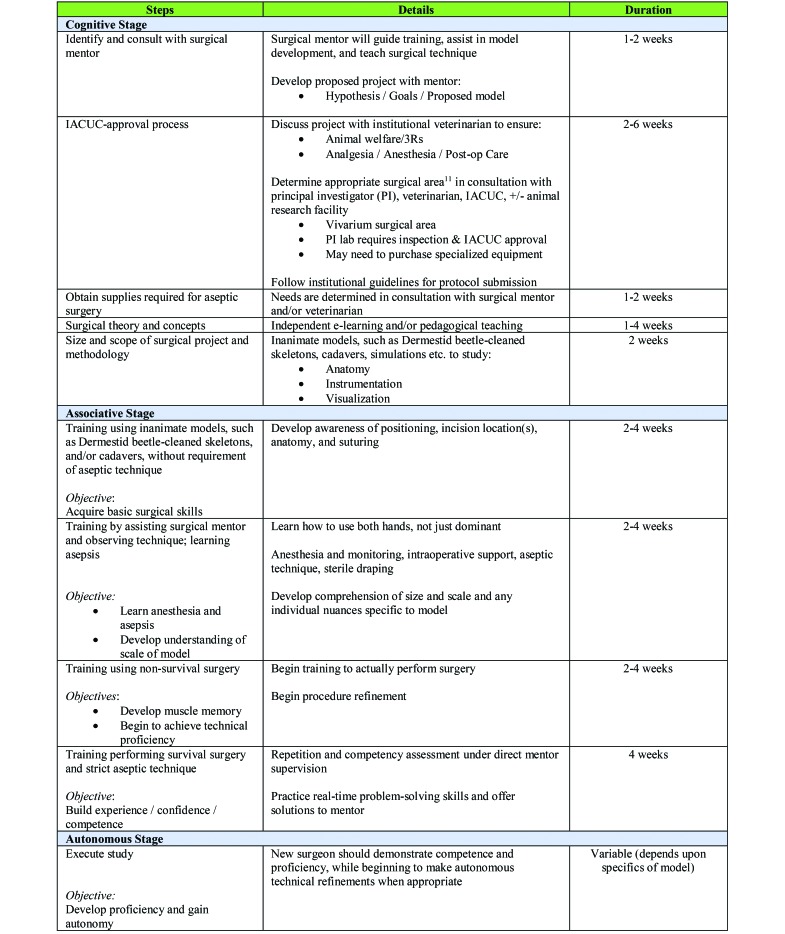
The goal of this overview is to detail a schema for training research surgeons to skillfully develop, master, and execute surgical models, thereby improving scientific practice, data quality, reproducibility, and animal welfare. We describe the utility of adapting standardized teaching approaches to surgical training (for example, situated learning and instructional scaffolding), define the 3 stages of intellectual engagement (cognitive, associative, autonomous), and detail suggestions for initial training of new surgeons with hands-on mentoring. As such, this training guide provides a working training plan and workflow for either experienced surgeons or novices in collaboration with their surgical mentors (Figure 2). Ideally, training will result in not only development and mastery of a specific model but also improved surgical knowledge, problem-solving skills, and confidence. The principles discussed can be incorporated in most research facilities—not only academic biomedical surgical facilities like ours—and likely are translatable to many surgical training scenarios.
Figure 2.
Plan for training new surgeons in surgical model development. Description of training plan involves preparation and the training components incorporated within cognitive, associative, and autonomous stages, as well as the estimated duration for each component. Note that durations are dependent on the complexity of surgery, experience of trainee, and length of study.
Situated Learning and Instructional Scaffolding
In the early 1990s, Jean Lave and Etienne Wagner developed a theoretical framework around the concept of situated learning, which suggests that active participation in a learning experience will lead to better material absorption and improved learning outcomes. By using situated learning, teachers structure a learning environment around real, daily activities, such as field trips for students. Students become ‘situated’ within the learning event, leading to the construction of a knowledge base developed from their own experiences. This model is in contrast to traditional pedagogical learning, which develops abstract learning experiences read from a textbook or delivered in lecture format by teachers and that students then attempt to apply later in real-world scenarios. Pedagogical learning can lead, for example, to a situation where a student is able to pass exams but unable to apply the learned information at a job site.4 Situated learning is directly applicable to the teaching of surgical models and is the long-time foundation of both veterinary and human clinical education.23
Surgical skills are acquired through 3 consecutive learning stages: cognitive, associative, and autonomous.20 Teaching surgical skills through consecutive learning stages utilizes the concept of instructional scaffolding, in which learning objectives are broken down into smaller packets of learning that, when combined, result in the ability to identify and solve a complex problem. Instructional scaffolding strategies include mentor demonstration of a technique, followed immediately by surgical trainees attempting to replicate the practice, which requires the trainees to use their own experiences and prior knowledge as a platform to complete the desired learning objective. Instructional scaffolding will engage learners in complex, problem-centered activities that can be scaled to a trainee's knowledge base and level of expertise.
Cognitive stage.
During the cognitive stage of learning, surgical trainees must learn basic surgical theory, vocabulary, and concepts. Textbooks, lectures, and electronic (e-) learning should be used as tools to develop procedural knowledge. In fact, if the availability of surgical training courses at the trainee's institution is limited, they may have to rely on e-learning.3 E-learning allows for incorporation of video, audio, images, and animations and is a flexible and accessible route for training. In addition, e-learning can diminish professional boundaries and hierarchies (for example, professor and student), which may enable trainees to feel more relaxed and amenable in describing their knowledge gaps and insecurities if they are receiving remote guidance from experts at other institutions. However, e-learning should not be viewed as a sole method for training. Hands-on mentoring is still considered the best approach for skills acquisition and basic training, with studies showing that hands-on mentoring is superior to e-learning for teaching laboratory rodent research techniques.6,25
Associative stage.
The associative stage of learning involves the novice surgeon practicing their surgical skills. This stage is particularly critical for having a mentor present and not relying on e-learning. In a 1-on-1 setting, mentors are able to customize their teaching approaches to specifically address any gaps in learning or motor skills unique to individual trainees. A common issue for novice surgeons is an overreliance on using their dominant hand during surgical procedures. Being able to see how a mentor uses both hands to perform a surgical task in real-time can accelerate this area of training. As such, the use of instructional scaffolding is particularly robust during this stage. Surgical mentors should view themselves not as a content transmitter but rather as a facilitator of learning by tracking progress, aiding the learner in context cues, and offering encouragement.
Autonomous stage.
Alongside instructional scaffolding, mentors can use the concept of entrustment, whereby mentors gradually decrease personal involvement and verbal instruction, leading to trainee autonomy, the third stage of surgical training. The combination of instructional scaffolding with entrustment will lead to genuine skill acquisition and confidence by surgeons to ensure skillful execution of a surgical procedure. Surgical training is composed of many iterations of these interactions that combine intellectual acuity with physical coordination and cooperation between mentors and trainees.23 Furthermore, surgical training is an ongoing and continuous process, given that surgeons must stay abreast of new and refined methods, analgesics, and anesthetics. This constant evolution of the field is why it is critical for a training plan to teach trainees how to think and act as surgeons so that they can adapt and grow rather than only to ensure technical competency in a specific model. An important aspect of professional surgical conduct is self-recognition of the limitations of one's abilities. Intensive training of novice surgeons in research is unable—and not intended—to make them experts of all surgical principles and procedures. Well-trained surgeons should be able to determine when they are reaching the extent of their skills and when additional input or expertise is needed.
After trainees have progressed through these learning stages, they should be both competent in the process of model development and proficient in surgical methods specific to their studied model. Competency has been defined as the ability to apply knowledge and skills to adequately perform surgical procedures, according to standards accepted by the laboratory animal science community.8 Mentor can assess competency by observing surgical trainees perform a given task within the surgical environment and determining whether the task was performed correctly. Proficiency is defined as advancement in knowledge or skill. Therefore, once trainees are competent in a surgical procedure, they can demonstrate proficiency by consistently and accurately performing the procedure. Proficiency is commonly assessed by someone other than the original mentor, if possible, to mitigate bias and ensure objectivity. Extensive methods for developing competency and proficiency-based assessments have been described in detail and commonly include score sheets and checklists.5,17 These assessments can be specifically tailored by mentors to meet trainees’ needs, given that our proposed paradigm focuses on individual trainees rather than institutions.
Training to Perform Aseptic Surgery
In our laboratory, we have established a murine model of atrophic nonunion fracture of the femur to continue studying the osteogenic potential of hematopoietic stem cells.15,16 This protocol was adapted from previously described methods.9 During generation of this model, we applied the plan we describe herein to train a graduate student with no prior surgical experience to perform this surgery and developed the model to be repeatable, precise, and able to maintain rigorous asepsis standards. The mentor was a veterinarian who was board-certified by the American College of Veterinary Surgeons and who had extensive experience in surgical model development. In addition, our intention was to maintain the best possible environment to maximize animal welfare, which, in turn, positively affects data reproducibility and minimizes procedural complications. Throughout the following sections, we describe each step of the training plan, as outlined in Figure 1 and described in detail in Figure 2, and use examples from how we implemented training of the novice surgeon to become competent and proficient. These examples illustrate how the incorporation of learning principles and practices can improve surgical training, expertise, and aseptic standards.
Consult with surgical mentor.
First, each trainee should identify a surgical mentor, preferably at their local institution, who has the availability, resources, and experience to successfully implement the training plan. In our situation, a board-certified veterinary surgeon served as the surgical mentor, with additional procedural advice from a human orthopedic surgeon. The consultation should include a discussion on the primary desired outcomes of the model, the animal species used, any previous relevant experience of the novice surgeon, and the generation of a timeline to estimate the duration, depth, and breadth of training.
Most laboratory animal veterinarians can serve as surgical mentors, especially for well-established procedures (for example, subcutaneous implantation of mini-osmotic pumps, ovariectomy). For more complex and novel models, it can be helpful to consult a veterinary or human surgeon during the design and refinement stages of the model development process. These surgeons can make critical translational contributions due to their experience with clinical applications and complications and because they are aware of cutting-edge methodology and understand possible pitfalls.1 We have found that promoting team science by assembling an appropriate interdisciplinary research group is key to achieving best outcomes.
A full schema of the surgical model should be created and reviewed by all relevant parties before any surgeries are performed on live animals. By laying this groundwork early and by using an instructional scaffolding approach, trainees will be more prepared to handle live surgical situations, thus leading to a lowered risk of avoidable mistakes and waste of resources. In the same manner, just as one would not learn calculus without first learning how to add and subtract, the ability to perform intricate surgical procedures requires learning simple steps and then layering complexity. Using this approach will vastly improve the surgeon's skills prior to performing surgeries planned for long-term experimental endpoints.
Receive approval for surgical protocol.
Before attempting any surgeries, the trainee and mentor need to meet with the attending veterinarian (or designee) at their institution to discuss the protocol before its submission to the institutional oversight body.2 Discussions will focus on anesthetic and analgesic plans; animal care before, during, and after surgery; location for surgery; recordkeeping; and humane endpoints. Protocols can take multiple weeks to process, depending on the institution, and the submitted protocol should be thoroughly detailed and consider animal welfare in the context of the 3Rs,24 as described earlier.
Order surgical equipment.
In addition to having the training to visualize the technique that will be used, having a mentor or a collaborator with surgical experience aid in choosing surgical instruments can be beneficial. This advantage is due to the wide array of available surgical instruments, each of which is manufactured for a specific purpose that may not be immediately obvious to novices. Similarly, the choice of suture material, if required, is dependent on the desired outcome. Surgical equipment should be ordered immediately after the specifics of the proposed surgical model have been established and the protocol approved by the appropriate institutional regulatory body.
Learn background information.
As described earlier, before beginning surgical procedures, trainees should learn relevant background information—including basic surgical theory, vocabulary, concepts, and anatomy—that is pertinent to the model of interest. For example, in the case of our murine nonunion fracture example, the trainee learned that, compared with companion species, such as dogs and cats, mice have a large angular third trochanter on the proximolateral femur, thus presenting a flaring of the diaphysis rather than a flat surface. This feature was likely to cause technical complications during surgery, particularly because of where specific pins had to be placed. Another example is the various similarities and differences between sheep and human cardiovascular systems that make sheep an ideal model for cardiovascular surgical device testing.7 Textbooks, lectures, and e-learning should be used as tools to develop procedural knowledge. Although mentors can assist in providing resources and testing comprehension, much of this step during training can be performed independently by trainees.
Understand scale and size of instruments.
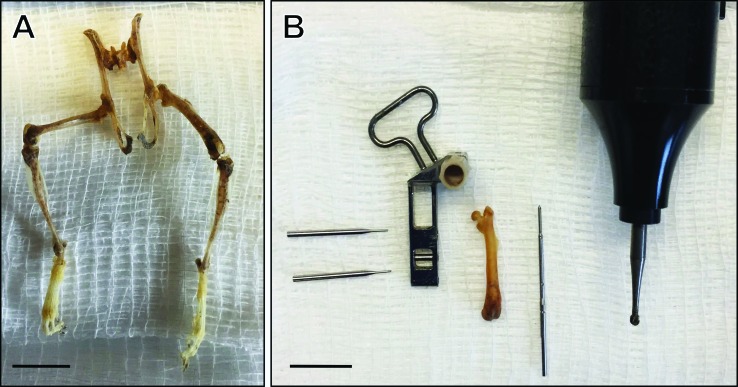
We have found it useful to have trainees first understand the size, scope, and relative ratio of instruments and implants to the surgical site before beginning any surgical practice. Other authors provide a similar recommendation for initial training in basic surgical techniques, for which they have developed cost-effective simulation models that allow for practice and skill refinement of maintaining asepsis, minimal dissection, proper tissue handling, correct use of suture material, and appropriate instrument use that preclude the need for live animals.22 In our fracture model example, an ideal facilitator for this training in scale and size of instruments came about because of the availability of a dermestid beetle colony available within the institution's animal facility. Dermestid beetles (also known as skin beetles) eat dead flesh from cadavers, thus leaving ‘cleaned’ skeletons that allow for visualizing the anatomy and unique anatomic bony landmarks in mice, such as the large flared third trochanter on the proximolateral mouse femur.13 Using these skeletons is a valuable cognitive tool that mitigates the need for live animals during initial training, leads to a foundational understanding of the 3D anatomy, and facilitates planning surgical refinements. In our laboratory's atrophic nonunion femur fracture model, the trainee was able to use dermestid-beetle–cleaned mouse femurs to practice the angle of entry of the locking nail into the medullary cavity and for visualizing the size of surgical implants relative to the femur (Figure 3). These practices led to increased efficiency when performing these surgeries in live animals. Experienced surgeons who are developing new models may also benefit from progression through preparation, practice, and execution.
Figure 3.
Dermestid beetle–cleaned skeletons for training surgical techniques. (A) Dermestid beetle–cleaned skeleton allows visualization of the size and scale of the surgical area and skeletal anatomy. (B) Equipment for LockingMouseNail (RISystem, Davos, Switzerland) surgery alongside an isolated left femur gives trainee an idea of the size ratio between surgical implants and femur. From left to right: locking pins, locking nail guide arm, mouse femur, locking nail, and microdrill with 1.6-mm burr. Scale bar, 1 cm.
Practice hand dexterity with cadavers.
This stage can be combined with those previously mentioned, but the purpose here is to begin progressing from the cognitive stage to the associative stage of learning. Depending on the model being developed, different training specimens, such as cadavers, can be used. Trainees should begin familiarizing themselves with the various surgical tools and equipment and actively practice incision placements, suturing, and various aspects of the procedure. In our nonunion fracture example, the trainee first used cleaned skeletons to practice implant placement and then progressed to training on cadavers to practice incision placement, fracture generation, and suturing. This stage should be purely motor-skill–driven, without emphasis on correct animal site preparation, asepsis, or anesthesia maintenance.
At our institution, investigators have used cadaveric tissues, full cadavers, and nonsurvival training surgeries in the early developmental phases of complex porcine surgical models, including kyphosis,10 radiation retinopathy,14 and temporomandibular joint dysfunction. These large-animal models have taken 3 y, 4 mo, and 2 mo, respectively, for development and refinement by highly experienced orthopedic, ophthalmologic, and oral–maxillofacial surgeons who had the assistance of a full veterinary anesthetic and surgical support team. In 2010, colleagues at our institution published their experience in training experienced surgeons in liver transplantation in rodents, demonstrating a reduction in training duration from 6 to 12 mo to 3 to 6 mo when balanced injectable was used rather than isoflurane anesthesia.11 Experienced surgeons are able to master complex procedures more quickly when they are focusing on a single task rather than managing anesthesia and surgery simultaneously. We expect that this limitation is magnified for novice surgeons.
Assist surgical mentor and observe surgical and aseptic techniques.
The next stage in the training paradigm is to assist the surgical mentor and observe the mentor's surgical and aseptic techniques during the procedure. In this stage of training, the importance of situated learning cannot be overstated. The situated learning approach allows the mentor to give real-time feedback to the trainee and supports the ‘see one, do one’ approach. Situated learning accelerates the trainee's learning, knowledge acquisition, skill refinement, and muscle memory. The trainee can observe the mentor's subtle maneuvers and muscle movements, use of both hands without overreliance on dominant hand, understand the relationship between musculoskeletal anatomy and incision placement, and learn how to manipulate surgical instruments and suture. In addition, the mentor can demonstrate correct aseptic technique, including animal preparation and maintenance of the sterile field during surgery.
By using aseptic technique, the surgeon will greatly reduce the risk for development of postsurgical wound infections and associated complications. Wound infections can cause pain, and distress and may lead to premature euthanasia. The consequences of infection can be particularly catastrophic in orthopedic models, like our atrophic nonunion fracture model, because implants are often used and complicate elimination of infection. Likewise, even minor infections can change animal physiology and behavior, consequently affecting study results. Aseptic technique is essential for sound scientific practice, data integrity, reproducibility, and animal welfare.5 Aseptic technique requires careful preparation and attentiveness by both the surgeon and any associated assistants. Although ample preparation and planning are required, they are well worth the effort, for the reasons just enumerated. Therefore, it is critical that the mentor has a strong foundational understanding of aseptic technique and is able to communicate and demonstrate this understanding to the trainee.
Perform mentor-assisted nonsurvival surgery.
After assisting the surgical mentor and understanding how to handle surgical instruments correctly, trainees perform mentor-assisted nonsurvival surgery. The purpose of this stage of training is to focus on procedural skill and workflow rather than on flawless aseptic technique. Anesthesia maintenance can be emphasized during this stage, but doing so is not critical, given that the surgeries are nonsurvival procedures. The mentor should be actively involved during this stage and provide real-time, positive, and constructive feedback. This step can be the most difficult for which to secure mentor cooperation, because patience and extended attention are needed without extensive direct involvement in conducting the procedure. A skilled mentor will identify when the trainee has reached his or her limit of duration for the training session, keeping the learning environment positive and minimizing trainee frustration. This stage is completed once the trainee has demonstrated procedural competence.
Demonstrate competency by performing survival surgery.
After trainees gain competence and experience, the autonomous stage begins, in which novice surgeons can demonstrate mastery of the surgical model to the observing surgical mentor. During this stage of training, trainees begin performing survival surgeries, ensuring appropriate animal site preparation, anesthetic maintenance, instrument handling, aseptic technique, procedural competence, and correct suturing. In addition to the surgical procedure itself, surgeon trainees learn appropriate postoperative care during this time. We have found that using a mesh handling device (that is, spatula) to move mice with surgically created fractures is the best approach to minimize pain, distress, and trauma postoperatively. We typically use this device to move mice from cage to induction chamber when performing microCT analysis on operated mice.23 Once a mentor is confident in a trainee's ability to perform the surgery successfully with minimal instruction and to provide postoperative care, trainees can progress to the next stage.
Gain autonomy and execute research study.
At this point, trainees should be competent in the procedure and ready to establish autonomy. A mentor's presence during surgery should no longer be required. Any planned research studies using the model can be performed and data can be generated. Proficiency will be developed over time. A marker of proficiency is the demonstrated ability of now-autonomous surgeons becoming mentors themselves and training new surgeons in the model.
Because the mentor no longer needs to be present, a surgical assistant can be highly beneficial for ensuring that appropriate aseptic technique is maintained throughout the procedure. However, a surgical assistant is not always required, and the need is largely based on the complexity of the procedure. The assistant can prepare the animal, monitor anesthesia, administer medications, monitor recovery, and maintain records. This transfer of duties allows surgeons to remain focused on their tasks, thus limiting the risk of breaking sterility in the surgical field, optimizing and increasing workflow, increasing the number of surgeries performed during each session, and reducing the risk of failure of asepsis.
At the end of surgery, surgeons should ask themselves each of the following questions. Was the model correctly induced? Did the animal experience pain or distress at levels less than or equal to those expected? Was there minimal occurrence of infection and other surgical complications? If the answer to each is ‘yes,’ then the surgery was a success.18
In summary, the combination of situated learning and instructional scaffolding is an ideal strategy for educational training of surgeons in model development. Rodent surgeries, in particular, are often performed by biomedical researchers or technicians, not veterinarians or physicians who have had formal surgical training.11 Because surgery involves acquired motor skills, it takes preparation, patience, and practice to master. Using our guide to create a robust and individually tailored training plan that incorporates instructional scaffolding in the context of a situated learning environment and that follows the stages of learning (cognitive, associative, and autonomous) will greatly improve the chance of achieving the desired surgical outcomes and optimizing animal welfare. We highly recommend that new surgeons have intensive hands-on mentoring with an experienced technician or research surgeon, a surgical research technical specialist (such as a technician certified by the Academy of Surgical Research), or veterinarian with appropriate surgical experience (for example, laboratory animal veterinarian, diplomate of the American College of Laboratory Animal Medicine or American College of Veterinary Surgeons) to complete the proposed training paradigm. With this type of training plan incorporated, novices should expect to spend as long as 6 mo in intensive training to develop a new small-animal surgical model and to achieve competency and proficiency in performing the model. However, note that training times and specific training benchmarks may vary. Timetables and appropriate goals should be determined and assessed by the mentor. The parameters we have presented are to serve as an implementation example and are based on our independent results. Therefore, every training plan will be unique and dependent on the desired outcome and model, prior experience of both trainee and mentor, and resources available.
Acknowledgments
This work was supported by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs (VA Merit Award to ACL, BX000333). We thank Kirsten Kelly for her aid and expertise in generating artwork and the Ralph H Johnson VMU staff for their tireless work and dedication to maintaining the animal facility at a high standard. All authors read and approved of the final manuscript prior to submission.
References
- 1.Academy of Surgical Research. 2009. Guidelines for training in surgical research with animals. Academy of Surgical Research. J Invest Surg 22:218–225. 10.1080/08941930902904542. [DOI] [PubMed] [Google Scholar]
- 2.[Anonymous]. 2016. ACLAM position statement on rodent surgery. J Am Assoc Lab Anim Sci 55:822–823. [PMC free article] [PubMed] [Google Scholar]
- 3.Baran SW, Johnson EJ, Kehler J, Hankenson FC. 2010. Development and implementation of multimedia content for an electronic learning course on rodent surgery. J Am Assoc Lab Anim Sci 49:307–311. [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JI, Hannafin M. 1995. Situated cognition and learning environments: roles, structures, and implications for design. Educ Technol Res Dev 43:53–69. 10.1007/BF02300472. [DOI] [Google Scholar]
- 5.Clifford P, Melfi N, Bogdanske J, Johnson EJ, Kehler J, Baran SW. 2013. Assessment of proficiency and competency in laboratory animal biomethodologies. J Am Assoc Lab Anim Sci 52:711–716. [PMC free article] [PubMed] [Google Scholar]
- 6.Custalow CB, Kline JA, Marx JA, Baylor MR. 2002. Emergency department resuscitative procedures: animal laboratory training improves procedural competency and speed. Acad Emerg Med 9:575–586. 10.1197/aemj.9.6.575. [DOI] [PubMed] [Google Scholar]
- 7.DiVincenti L, Jr, Westcott R, Lee C. 2014. Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J Am Assoc Lab Anim Sci 53:439–448. [PMC free article] [PubMed] [Google Scholar]
- 8.Galasko CS, Smith K. 1999. Ratio of basic surgical trainees to type 1 specialist registrar programmes 1999/2000/2001/2002. Ann R Coll Surg Engl 81:124–128. [PubMed] [Google Scholar]
- 9.Garcia P, Herwerth S, Matthys R, Holstein JH, Histing T, Menger MD, Pohlemann T. 2011. The LockingMouseNail—a new implant for standardized stable osteosynthesis in mice. J Surg Res 169:220–226. 10.1016/j.jss.2009.11.713. [DOI] [PubMed] [Google Scholar]
- 10.Gross RH, Wu Y, Bonthius DJ, Gross V, Smith A, McCrackin MA, Wolfe A, Helke K, Gallien T, Yao H. 2019. Creation of a porcine kyphotic model. Spin Deform 7:213–219. 10.1016/j.jspd.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoogstraten-Miller SL, Brown PA. 2008. Techniques in aseptic rodent surgery. Curr Protoc Immunol 82:1.12.1–1.12.14. 10.1002/0471142735.im0112s82. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 13.Kelly RR, Hying JV, Helke KL, LaRue AC, McCrackin MA. 2016. Flesh eating beetles produce cleaned rodent skeletons for teaching orthopedic animal models. Lab Animal Sci Prof December:40–43. [Google Scholar]
- 14.Margrath III GN. 2018. Development of a swine model of radiation–induced retinopathy for therapeutic drug testing. Presented at the 34th Annual meeting of Academy of Surgical Research; Charleston, South Carolina: 26–28 September 2018. ASR final program. p 43. [Google Scholar]
- 15.Mehrotra M, Rosol M, Ogawa M, Larue AC. 2010. Amelioration of a mouse model of osteogenesis imperfecta with hematopoietic stem cell transplantation: microcomputed tomography studies. Exp Hematol 38:593–602. 10.1016/j.exphem.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrotra M, Williams CR, Ogawa M, LaRue AC. 2013. Hematopoietic stem cells give rise to osteo-chondrogenic cells. Blood Cells Mol Dis 50:41–49. 10.1016/j.bcmd.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulton CA, Dubrowski A, Macrae H, Graham B, Grober E, Reznick R. 2006. Teaching surgical skills: what kind of practice makes perfect?: a randomized, controlled trial. Ann Surg 244:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pritchett-Corning KR, Luo Y, Mulder GB, White WJ. 2011. Principles of rodent surgery for the new surgeon. J Vis Exp 47:1–4. 10.3791/2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Public Health Service. 1986. Policy on humane care and use of laboratory animals. Bethesda (MD): U.S. Department of Health and Human Services. [Google Scholar]
- 20.Ridgway PF, Sheikh A, Sweeney KJ, Evoy D, McDermott E, Felle P, Hill AD, O'Higgins NJ. 2007. Surgical e-learning: validation of multimedia web-based lectures. Med Educ 41:168–172. 10.1111/j.1365-2929.2006.02669.x. [DOI] [PubMed] [Google Scholar]
- 21.Russell W, Burch R, Hume C. 1959. The principles of humane experimental technique. London (United Kingdom): Metheun Publishing. [Google Scholar]
- 22.Stevens CA, Dey ND. 2007. A program for simulated rodent surgical training. Lab Anim (NY) 36:25–31. 10.1038/laban1007-25. [DOI] [PubMed] [Google Scholar]
- 23.Sutkin G, Littleton EB, Kanter SL, Cianciolo AT, Chen XP, Cope A, Koschmann T. 2017. Teaching, learning, and performance in the surgical workplace: insights from the examination of intraoperative interactions. Teach Learn Med 29:378–382. 10.1080/10401334.2017.1384732. [DOI] [PubMed] [Google Scholar]
- 24.Tannenbaum J, Bennett BT. 2015. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci 54:120–132. [PMC free article] [PubMed] [Google Scholar]
- 25.Whitcomb TL, Taylor EW. 2014. Teaching laboratory rodent research techniques under the tenets of situated learning improves student confidence and promotes collaboration. J Am Assoc Lab Anim Sci 53:368–375. [PMC free article] [PubMed] [Google Scholar]