Abstract
A number of studies have shown the bifidogenic effects of either probiotic bifidobacteria or inulin, and this bifidogenic shift in the composition of the colonic microbiota is likely the basis for their positive impact on human health. This study aimed to evaluate the effects of synbiotics containing the probiotic bacterium Bifidobacterium animalis subsp. lactis (B. lactis) GCL2505 and inulin on the levels of intestinal bifidobacteria compared with B. lactis GCL2505 alone. A randomized, double‐blind, placebo‐controlled, crossover trial was carried out involving 60 healthy subjects with a tendency for constipation using fermented milk containing B. lactis GCL2505 and inulin (synbiotic), only B. lactis GCL2505 (probiotic), and placebo. Fecal samples were collected at the end of each 2‐week intervention period, and the bifidobacterial count was analyzed by quantitative real‐time PCR. The numbers of total bifidobacteria and B. lactis in feces were significantly increased during the probiotic and synbiotic intake periods compared with the placebo intake period. Furthermore, the numbers of total bifidobacteria and endogenous bifidobacteria were significantly higher in the synbiotic intake period compared with the probiotic intake period, while there was no difference in the number of B. lactis. These results suggested that the synbiotics containing B. lactis GCL2505 and inulin had a greater effect on the number of bifidobacteria than a drink containing probiotics alone and could be useful for the improvement of the intestinal environment.
Keywords: Bifidobacterium animalis subsp. lactis, intestinal bifidobacteria, randomized trial, synbiotics
1. INTRODUCTION
Several hundred different types and over 100 trillion bacteria inhabit the human intestinal tract, and they form a complex community of microbes (Finegold, Sutter, & Matheisen, 1983). In recent years, many studies have indicated that there is a relationship between microbiota and various diseases of the host human, with an individual's microbiota playing a role in diseases such as obesity, diabetes, inflammation, and autism spectrum disorder (Blander, Longman, Iliev, Sonnenberg, & Artis, 2017; Larsen et al., 2010; Ley, Turnbaugh, Klein, & Gordon, 2006; van De Sande, van Buul, & Brouns, 2014). It has also been reported that changes in the microbiota, including a reduction of diversity and shifts in the composition ratio of intestinal bacteria, play a key role in host health (Ley et al., 2005; Manichanh et al., 2006; Rajilic‐Stojanovic et al., 2011).
Members of the genus Bifidobacterium are one of the most predominant organisms in the human intestinal tract and are important for general health, which means that their diversity and abundance provide markers for measuring the stability of the human intestinal microbiota, as well as the overall intestinal environment (Mitsuoka, 1984; Tanaka, 1995). Since bifidobacteria, regardless of the species, have various positive features for host health, including the production of vitamins, polyphenols, conjugated linoleic acids, and lactate/acetate as well as the enhancement of gut barrier function and the immune system (Rivière, Selak, Lantin, Leroy, & De Vuyst, 2016), many attempts have been made to increase the number of intestinal bifidobacteria. An increase in the number of bifidobacteria is thought to improve the intestinal microflora and environment, which not only prevents the deterioration of fecal characteristics, constipation, and diarrhea, but also benefits systemic health, such as metabolic disease, atopic disease, or irritable bowel syndrome (Kalliomäki et al., 2001; Kerckhoffs et al., 2009; Malinen et al., 2005; Schwiertz et al., 2010). Therefore, it can be said that the improvement of the intestinal environment by maintaining a high number of bifidobacteria in the intestine is important for the maintenance and promotion of good health, and numerous attempts to increase the number of intestinal bifidobacteria in the human intestinal tract have been made.
A definition of probiotics has been proposed as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). Many probiotic strains, mainly bifidobacteria and lactic acid bacteria, have been studied and demonstrated to exert various health benefits. Especially, some probiotic strains increase the number of intestinal bifidobacteria, which contribute to the improvement of intestinal disorders such as constipation (Matsumoto et al., 2010; Yaeshima et al., 1997; Yamano et al., 2006). To induce a greater effect on the number of intestinal bifidobacteria, another approach has been proposed that combines probiotics with prebiotics. Prebiotics are nonviable food components that confer a health benefit on the host associated with modulation of the gut microbiota (Pineiro et al., 2008), and are expected to be utilized by endogenous bifidobacteria and to increase their number in the intestinal tract. The combination of both probiotics and prebiotics is called synbiotics (Gibson & Roberfroid, 1995). The ingestion of synbiotics is also expected to lead to a large increase in the number of bifidobacteria in the intestinal tract (Childs et al., 2014; Krumbeck et al., 2018; Macfarlane, Cleary, Bahrami, Reynolds, & Macfarlane, 2013; Shioiri et al., 2006). Unfortunately, because previous reports evaluated the bifidogenic effects of synbiotics compared with only placebo, not with probiotics directly, and studies did not result in a significant increase in the number of bifidobacteria compared with probiotics, it has not been shown definitively that synbiotics are clearly better than probiotics or prebiotics at increasing the number of bifidobacteria in the gut.
Bifidobacterium animalis subsp. lactis (B. lactis) GCL2505 is a probiotic strain derived from healthy human intestines. We previously revealed that B. lactis GCL2505 has some positive effects on health, such as an improvement of defecation frequency and a reduction of visceral fat (Aoki et al., 2016; Ishizuka et al., 2012; Takahashi et al., 2016; Tanaka et al., 2015). It is thought that these effects are attributable to a unique feature of B. lactis GCL2505, which can reach the intestine in a viable form and is able to proliferate after a single ingestion. This leads to an increase in the number of intestinal bifidobacteria. Combining B. lactis GCL2505 with prebiotics was expected to be more effective in increasing intestinal bifidobacteria, which would then lead to an enhancement of health benefits such as an improvement of defecation frequency. The purpose of the present study was to evaluate the effects of synbiotics using B. lactis GCL2505 on changes in intestinal bifidobacteria counts and defecation frequency, compared with those of B. lactis GCL2505 alone. In this study, we used inulin as a prebiotic, which is a fructan‐type, soluble dietary fiber (Mensink, Frijlink, van der Voort Maarschalk, & Hinrichs, 2015), and has a bifidogenic effect by which it is assimilated selectively into bifidobacteria in the intestine (Bouhnik et al., 2007; Rao, 2001). We designed a placebo‐controlled randomized, double‐blind, three‐group crossover study in healthy adults with a tendency for constipation to investigate the changes in the counts of B. lactis and nine different endogenous bifidobacteria in feces by quantitative real‐time PCR with Bifidobacterium species‐ and subspecies‐specific primers as the primary outcome and the frequency of defecation as the secondary outcome.
2. MATERIALS AND METHODS
2.1. Test food
The test products were fermented milk containing inulin (Orafti GR; BENEO GmbH, Mannheim, Germany) and B. lactis GCL2505 (synbiotic drink), only B. lactis GCL2505 (probiotic drink), or placebo. The inulin content of the synbiotic drink was 2.0 g/100 g, and the viable cell count of B. lactis GCL2505 in the synbiotic drink and probiotic drink was 1 × 1010 colony‐forming units (cfu)/100 g. The placebo was prepared with the same ingredients and adjusted for flavor and pH by adding food‐grade acetic acid and lactic acid similarly to the other test products. Their basic ingredients were skim milk powder, high‐fructose corn syrup, apple juice, starch syrup, yeast extract, flavor, acidulant, stabilizer, and sweetener. The nutritional details of the test drinks are shown in Table 1.
Table 1.
Nutritional details of the test drinks
| Placebo | Probiotic drink | Synbiotic drink | |
|---|---|---|---|
| Functional substance | — | B. lactis GCL2505a | B. lactis GCL2505a and inulinb |
| Nutritional components (values for 100 g test drink) | |||
| Energy (kcal) | 42 | 42 | 45 |
| Protein (g) | 3.1 | 3.1 | 3.1 |
| Fat (g) | 0 | 0 | 0 |
| Carbohydrate (g) | 7.3 | 7.3 | 9.2 |
| NaCl (g) | 0.14 | 0.14 | 0.14 |
| Ca (mg) | 104 | 104 | 104 |
1 × 1010 cfu/100 g test drink.
2.0 g/100 g test drink.
2.2. Study design
The study was designed as a randomized, double‐blind, placebo‐controlled, three‐group crossover intervention trial. It was conducted according to the Consolidated Standards of Reporting Trials statement (Schulz, Altman, & Moher, 2011), the principles of the Declaration of Helsinki, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare of Japan. The study protocol was approved by the institutional review board of the Ethics Committee of Nihonbashi Cardiology Clinic (Tokyo, Japan). The subjects provided written informed consent before initiation of the study. This study was performed by a contract research organization, KSO Corporation (Tokyo, Japan), from February to April 2018 at the Shinagawa Season Terrace Health Care Clinic (Tokyo, Japan) and registered as UMIN000031101 in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry.
2.3. Subjects
Healthy Japanese participants aged 20–64 years with a tendency for constipation (3–5 days a week) were recruited. The exclusion criteria were as follows: (a) regular use of intestinal drugs and laxatives; (b) regular intake of healthy food to relieve constipation; (c) intake of food containing viable bacteria, such as lactic acid bacteria, bifidobacteria, and natto bacteria, and/or enhanced with oligosaccharides and dietary fiber, and/or healthy food to relieve constipation (including Food for Specified Health Uses [FOSHU]), and/or containing a large amount of sugar alcohol; (d) use of medicine that affects digestion and absorption such as antibiotics; (e) food allergy; (f) pregnancy, lactation, or planning to become pregnant; (g) serious diseases requiring urgent treatment or with severe complications; (h) a medical history of diseases or surgeries affecting digestion, absorption, and bowel movements; (i) a current or history of drug dependence and/or alcoholism; (j) participation in other clinical trials; (k) judgment of unsuitability for this study based on the subject's responses to a lifestyle questionnaire; and (l) judgment of unsuitability for this study by the principal investigator on the basis of clinical laboratory test results. Eligible subjects kept a daily record of defecation, ingestion of healthy food, usage of medicine, and physical condition before the screening test, in which the subjects received anthropometric measurements, clinical laboratory tests, and a medical interview. After analyzing the daily record and screening test, a total of 60 subjects were selected. An independent clinician randomly allocated the subjects into three groups (20 subjects/group) stratified for age, sex, and days of defecation according to the information obtained until the screening test. The groups were defined as group A: intake of the test drinks in the order of placebo, probiotic drink, and synbiotic drink; group B: intake of the test drinks in the order of probiotic drink, synbiotic drink, and placebo; and group C: intake of the test drinks in the order of synbiotic drink, placebo, and probiotic drink. The link between identification number and treatment group was kept in a sealed document by the allocation officer. The investigators, subjects, and study statistician were blinded to the allocation of the treatment groups until after all data analyses were completed.
2.4. Study protocol
This study consisted of six periods of 2 weeks each, and the periods were as follows: observation period, ingestion period I, washout I, ingestion period II, washout II, and ingestion period III (Figure 1). During the ingestion periods, each subject consumed 100 g of the indicated test drink every day without setting a time for ingestion. The test drinks were delivered every week and stored in a refrigerator by the subjects. Throughout the study, the subjects were instructed to: (a) keep a daily record of test drink consumption, defecation, fecal status, ingestion of healthy food, usage of medicine, and physical condition; (b) maintain their regular lifestyle such as food and exercise (to avoid undereating, overeating, and overexercising); (c) avoid the overconsumption of alcohol; (d) use as few medicines as possible that affect digestion and absorption such as antibiotics; and (e) avoid the intake of food containing viable bacteria, such as lactic acid bacteria, bifidobacteria, and natto bacteria, and/or enhanced with oligosaccharides and dietary fiber, and/or healthy food to relieve constipation (including FOSHU), and/or containing a large amount of sugar alcohol. Clinical surveys were conducted at the start of each ingestion period and at the end of the study, in which the subjects received anthropometric measurements, clinical laboratory tests, and a medical interview. Feces were sampled at the end of each period and delivered to the laboratory in a refrigerated, anaerobic state using an AnaeroPack Kenki (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). These samples were diluted 10‐fold with phosphate‐buffered saline (pH 7.4) and homogenized. Suspensions were kept at −80°C until required for analysis.
Figure 1.
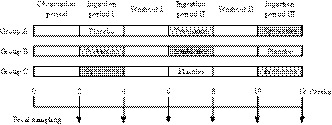
Study design
2.5. Gut bifidobacteria
Bacterial DNA was extracted from 10‐fold dilutions of the fecal samples, and the number of gut bifidobacteria was subsequently determined by quantitative real‐time PCR using Bifidobacteria species‐ and subspecies‐specific primers according to a procedure described previously (Tanaka et al., 2015). Total counts of bifidobacteria in the fecal samples are represented as the sum of 10 species (B. longum subsp. longum, B. adolescentis, B. catenulatum, B. pseudocatenulatum, B. breve, B. bifidum, B. longum subsp. infantis, B. dentium, B. angulatum, and B. lactis). Endogenous bifidobacteria were regarded as the sum of nine species, without B. lactis. The detection limit of each species or subspecies was 2.0 × 105 cells per gram of feces.
2.6. Statistical analysis
From our previous studies, we assumed that the mean of differences in total bifidobacteria counts after intake of each test drink was 0.3 logarithm with a standard deviation of 0.5 logarithm. It was calculated that a sample size of 59 subjects would provide 80% power to detect a significant difference in means assuming that the common standard deviation was 0.5 logarithm using a t test with a 0.05 two‐sided significance level after the Bonferroni correction. On the basis of this calculation, we selected a total of 60 subjects in this crossover study.
The number of each Bifidobacterium species was expressed and analyzed after common logarithmic transformation. All measured values are expressed as the mean ± standard deviation. For the validation of the crossover, we carried out analysis of variance with the number of total bifidobacteria in the feces and evaluated the timing effect and order effect. Between‐group comparisons were conducted by Student's paired t test. Statistical significance was determined as p < 0.05 after the Bonferroni correction. All statistical analyses were performed using IBM SPSS Statistics for Windows software version 24 (IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Subjects
The study outline is shown in Figure 2. From among the 103 participants who provided written informed consent and completed the screening test, we selected 60 subjects who had relatively low defecation frequencies (3–5 days a week) and who did not meet the exclusion criteria. The background characteristics of the subjects are shown in Table 2. The subjects were allocated randomly to three groups, among which there was no significant difference in any baseline data (data not shown). All subjects completed the study and were incorporated into the efficacy analysis. No adverse events were observed in any subject throughout this study.
Figure 2.
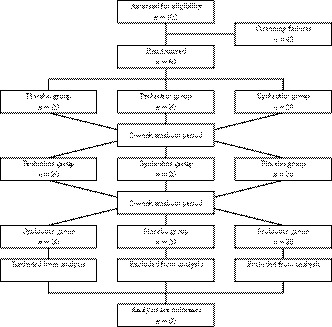
Flow diagram of the study showing numbers of participants
Table 2.
Baseline characteristics of the subjects
| Total | |
|---|---|
| N | 60 |
| Male/female | 17/43 |
| Age (years) | 45.0 ± 8.9 |
| Height (cm) | 162.1 ± 6.5 |
| Body weight (kg) | 59.2 ± 9.5 |
| Body mass index (kg/m2) | 22.5 ± 3.0 |
| Systolic blood pressure (mmHg) | 119.9 ± 13.7 |
| Diastolic blood pressure (mmHg) | 75.0 ± 9.5 |
| Pulse rate (beats/min) | 76.4 ± 11.5 |
| Bifidobacterium (log cells/g feces) | 9.72 ± 0.89 |
| Number of defecations (/week) | 3.72 ± 0.63 |
| Days of defecation (/week) | 3.57 ± 0.58 |
Values are expressed as the mean ± standard deviation.
3.2. Gut bifidobacteria
The time effect and order effect of each ingestion period for the number of total bifidobacteria in the intestinal tract were not statistically significant (p = 0.597 and p = 0.403, respectively). Thus, we concluded that the carryover effect could be ignored and the results obtained from the crossover design of the present study could be evaluated appropriately.
The number of gut bifidobacteria is shown in Table 3. In the probiotic intake period, there was a significant increase in the total count of bifidobacteria compared with the placebo intake period. Regarding the individual bifidobacteria species, the counts of B. lactisin the probiotic and synbiotic intake periods were significantly increased compared to the placebo intake period, while the number of endogenous bifidobacteria and B. longum was significantly decreased. In the synbiotic intake period, the amount of total bifidobacteria was increased significantly compared to the placebo and probiotic intake periods, and the number of B. longum, B. adolescentis, and endogenous bifidobacteria was increased compared with the probiotic intake period.
Table 3.
Number of intestinal bifidobacteria
| Total bifidobacteria | lac | lon | ado | cat | bre | bif | inf | den | ang | Endogenous bifidobacteria | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 9.78 ± 0.79 | 5.69 ± 0.87 | 9.12 ± 1.22 | 7.92 ± 2.09 | 7.95 ± 1.98 | 5.80 ± 0.95 | 6.10 ± 1.35 | 5.66 ± 0.87 | 5.54 ± 0.70 | n.d. | 9.76 ± 0.84 |
| Probiotics | 10.01 ± 0.46** | 9.39 ± 0.67*** | 8.98 ± 1.27* | 7.77 ± 1.99 | 7.79 ± 1.99 | 5.70 ± 0.88 | 6.07 ± 1.32 | 5.63 ± 0.81 | 5.63 ± 0.79 | n.d. | 9.62 ± 0.91** |
| Synbiotics | 10.11 ± 0.42*** # | 9.27 ± 0.65*** | 9.20 ± 1.24## | 8.06 ± 2.11## | 7.86 ± 2.00 | 5.80 ± 0.98 | 6.15 ± 1.40 | 5.69 ± 0.89 | 5.59 ± 0.73 | n.d. | 9.84 ± 0.87### |
Values are expressed as the mean ± standard deviation of common logarithms of the number of bacteria per 1 g feces.
lac: B. animalis subsp. lactis; lon: B. longum subsp. longum; ado: B. adolescentis group; cat: B. catenulatum or B. pseudocatenulatum; bre: B. breve; bif: B. bifidum; inf: B. longum subsp. infantis; den: B. dentium; ang: B. angulatum.
n.d.: not detected. The detection limit of quantitative PCR was 2.0 × 105 cells per g of feces.
Comparisons were conducted using a paired Student's t test with Bonferroni's correction after logarithmic transformation of the number of each bacterial species.
p < 0.001,
p < 0.01,
p < 0.05 (vs. placebo);
p < 0.001,
p < 0.01,
p < 0.05 (vs. probiotics).
Table 4 shows the number of gut bifidobacteria in the tertile of the subjects (n = 20) who had lower B. lactis counts during the probiotic intake period. There was no significant difference in any of the species including total bifidobacteria, except for B. lactis, in the probiotic intake period compared with the placebo intake period. On the other hand, in the synbiotic intake period, the number of total bifidobacteria was significantly increased compared with that of the placebo and probiotic intake periods, respectively, and the number of endogenous bifidobacteria tended to increase compared with the probiotic intake period (p = 0.067).
Table 4.
Number of intestinal bifidobacteria in the tertile of subjects with a smaller number of B. lactis during the probiotic intake period
| Total bifidobacteria | lac | lon | ado | cat | bre | bif | inf | den | ang | Endogenous bifidobacteria | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 9.45 ± 1.24 | 5.69 ± 0.95 | 8.68 ± 1.84 | 6.58 ± 2.06 | 7.24 ± 2.17 | 5.82 ± 1.15 | 5.96 ± 1.35 | 5.52 ± 0.67 | 5.63 ± 0.83 | n.d. | 9.41 ± 1.33 |
| Probiotics | 9.76 ± 0.57 | 8.70 ± 0.64*** | 8.50 ± 1.78 | 6.50 ± 1.93 | 7.28 ± 2.18 | 5.76 ± 1.08 | 5.93 ± 1.29 | 5.52 ± 0.59 | 5.48 ± 0.60 | n.d. | 9.25 ± 1.38 |
| Synbiotics | 9.97 ± 0.55* # | 9.08 ± 0.60*** | 8.88 ± 1.75# | 6.56 ± 1.97 | 7.33 ± 2.19 | 5.83 ± 1.16 | 5.97 ± 1.38 | 5.54 ± 0.69 | 5.60 ± 0.81 | n.d. | 9.48 ± 1.36 |
Values are expressed as the mean ± standard deviation of common logarithms of the number of bacteria per 1 g feces.
lac: B. animalis subsp. lactis; lon: B. longum subsp. longum; ado: B. adolescentis group; cat: B. catenulatum or B. pseudocatenulatum; bre: B. breve; bif: B. bifidum; inf: B. longum subsp. infantis; den: B. dentium; ang: B. angulatum.
n.d.: not detected. The detection limit of quantitative PCR was 2.0 × 105 cells per g of feces.
Comparisons were conducted using a paired Student's t test with Bonferroni's correction after logarithmic transformation of the number of each bacterial species.
p < 0.001,
p < 0.05 (vs. placebo);
p < 0.05 (vs. probiotics).
3.3. Frequency of defecation
The number and days of defecation are shown in Table 5. Both parameters in all periods were significantly increased compared with those in the baseline period (Table 2). However, there was no significant difference in either parameter among any of the periods.
Table 5.
Defecation states
| Number of defecations (/week) | Days of defecation (/week) | |
|---|---|---|
| Placebo | 4.56 ± 1.23 | 4.23 ± 0.97 |
| Probiotics | 4.56 ± 1.23 | 4.27 ± 1.04 |
| Synbiotics | 4.68 ± 1.22 | 4.37 ± 1.04 |
Values are expressed as the mean ± standard deviation.
4. DISCUSSION
In this study, we demonstrated that a synbiotic drink containing B. lactis GCL2505 and inulin significantly increased the total number of intestinal bifidobacteria compared with a probiotic drink containing B. lactis GCL2505 alone or a placebo in healthy adults with mild constipation after 2 weeks of ingestion. In the synbiotic intake period, compared to the probiotic intake period, significant increases were observed in the number of endogenous bifidobacteria. Moreover, in the tertile of the subjects (n = 20) who had lower B. lactis counts during the probiotic intake period, the number of total bifidobacteria increased significantly in the synbiotic intake period compared to the placebo and probiotic intake periods, while that in the probiotic intake period was not significantly different from the placebo intake period.
Bifidobacterium animalis subsp. lactis is considered to be a subspecies of nonendogenous bifidobacteria in the human gut (Kato et al., 2017; Turroni et al., 2009). In this study, the number of B. lactis was above the detection limit in the feces of only five subjects during the observation period and their levels were very low. Therefore, it was thought that most of the B. lactis detected during the test drink intake periods was B. lactis GCL2505. In this study, the ingestion of a probiotic drink containing B. lactis GCL2505 alone led to a significant increase in the number of total bifidobacteria in feces compared to the placebo intake period. Previously, we revealed that B. lactis GCL2505 reaches the intestine alive and increases the number of bifidobacteria in the intestinal tract (Ishizuka et al., 2012). In the present study, we reconfirmed the effect of B. lactis GCL2505 ingestion on the number of intestinal bifidobacteria.
Synbiotics, a synergistic combination of probiotics and prebiotics, are expected to have a greater effect on the health of the host than when each is used alone, and many clinical studies of synbiotics have been conducted (Asemi, Khorrami‐Rad, Alizadeh, Shakeri, & Esmaillzadeh, 2014; Childs et al., 2014; Stenman et al., 2016; Waitzberg et al., 2013). However, these reports have not shown that synbiotics have a clearly stronger effect on the numbers of intestinal bifidobacteria compared with the use of probiotics or prebiotics alone. The present study showed a greater increase in the number of total bifidobacteria by ingesting a synbiotic drink combining B. lactis GCL2505 and inulin than ingesting B. lactis GCL2505 alone or placebo. In terms of each bacterial species, the counts of B. longum, B. adolescentis, and total endogenous bifidobacteria showed a significant increase when compared with the probiotic food intake period, while no difference was observed in the number of B. lactis. It was reported that inulin was assimilated by some species or strains of bifidobacteria including B. longum and B. adolescentis (Roberfroid, Van Loo, & Gibson, 1998; Rossi et al., 2005). Furthermore, it was also reported that the ingestion of inulin increased the number of B. longum and B. adolescentis in the intestinal tract (Ramirez‐Farias et al., 2009). The inulin contained in the synbiotic drink in this study was thought to contribute to the increase in endogenous bifidobacteria, including B. longum and B. adolescentis. On the other hand, it was reported that B. lactis has relatively lower assimilability of inulin than other bifidobacteria species (Roberfroid et al., 1998). Therefore, inulin might be assimilated by some species or strains of endogenous bifidobacteria rapidly and preferentially, but did not contribute to the growth of B. lactis GCL2505 directly. It also means that inulin might not interfere with the proliferation of B. lactis GCL2505 in the gut. From these facts, it is suggested that B. lactis GCL2505 and inulin have little influence on each other's ability to increase the number of intestinal bifidobacteria when they are ingested concomitantly.
The amount of probiotics that arrive in the gut and their health effects often differ from one individual to another because of the individual differences in the intestinal microbiota (Mackie, Sghir, & Gaskins, 1999). This has been shown through the difference in the proliferative rates of B. lactis GCL2505 in the intestinal tracts of different subjects (Ishizuka et al., 2012). We hypothesized that ingestion of synbiotics containing B. lactis GCL2505 and inulin would increase the number of intestinal bifidobacteria, even for those subjects with low effects of probiotics. In the present study, a stratified analysis of the subjects who had low numbers of B. lactis subspecies during the probiotic intake period showed a significant increase in the total number of bifidobacteria in the synbiotic intake period compared with the placebo and probiotic intake periods. However, there was no significant difference in the number of total bifidobacteria between the probiotic and placebo intake periods. These results suggest that the synbiotics containing both B. lactis GCL2505 and inulin can increase the number of intestinal bifidobacteria even in the subjects who demonstrated modest effects from the intake of B. lactis GCL2505 alone. Therefore, this synbiotic approach may benefit a higher number of people compared with the usual probiotic approach, considering the variations in human intestinal microbiota.
An improvement of the intestinal microbiota resulting from the intake of probiotics or prebiotics, especially an increase in the number of intestinal bifidobacteria, often leads to a higher frequency of defecation (Matsumoto et al., 2010; Yamano et al., 2006). The greater presence of bifidobacteria in the intestinal tract promotes the production of short‐chain fatty acids, such as acetate (Aoki et al., 2017; Igarashi et al., 2017), which stimulate colonic motility (Fukumoto et al., 2003; Ono, Karaki, & Kuwahara, 2004). Some strains of B. lactis, including B. lactis strain GCL2505, were also reported to improve defecation frequency (Flach et al., 2018; Ishizuka et al., 2012; Tanaka et al., 2015; Yang et al., 2008). However, in the present study, no differences were found in a comparison between placebo and probiotic/synbiotic intake periods. Compared with baseline (Table 2), defecation frequency was significantly increased in all ingestion periods including the placebo intake period (all p < 0.001). Since the degree of change in the number of defecations between the baseline and each intake period at 2 weeks after ingestion was about the same level as that after the intake of B. lactis in previous studies (Eskesen et al., 2015; Ishizuka et al., 2012; Matsumoto et al., 2001), it was likely that B. lactis GCL2505 or the combination of B. lactis GCL2505 and inulin could reasonably exert their improvement effects on defecation frequency. On the other hand, high defecation frequency after intake of placebo was sometimes observed in past clinical studies using B. lactis (Eskesen et al., 2015; Nishida et al., 2004). For that reason, we determined that there was a high placebo effect for bowel movements in this study. However, the main outcome measure in the present study was the number of intestinal bifidobacteria. Bifidobacteria is one of the most beneficial bacteria for host health, and maintaining a high number of intestinal bifidobacteria is considered to be important (Rivière et al., 2016). Because the combination of B. lactis GCL2505 and inulin exerted a synbiotic effect on the number of intestinal bifidobacteria in this study, it is likely that synbiotics have stronger health effects than B. lactis GCL2505 alone.
In conclusion, the present results indicate that the ingestion of a synbiotic drink containing B. lactis GCL2505 and inulin increased the amount of total bifidobacteria in the human intestine compared with the ingestion of a placebo drink or a probiotic drink containing B. lactis GCL2505 alone. Furthermore, this synbiotic drink increased the number of total bifidobacteria in subjects who had a relatively small increase in B. lactis GCL2505 after the ingestion of B. lactis GCL2505 alone. The present findings suggest that the synbiotics containing B. lactis GCL2505 and inulin could be useful for the improvement of the intestinal environment of more poeple by increasing the number of bifidobacteria in the gut, which may contribute to health benefits.
CONFLICT OF INTEREST
D. Anzawa, T. Mawatari, Y. Tanaka, M. Yamamoto, T. Genda, S. Takahashi, T. Nishijima, H. Kamasaka, and T. Kuriki are employees of Ezaki Glico Co., Ltd., which produces products using B. lactis GCL2505. S. Suzuki declares no conflict of interest.
ETHICAL REVIEW
The study protocol was approved by the institutional review board of the Ethics Committee of Nihonbashi Cardiology Clinic (Tokyo, Japan).
INFORMED CONSENT
Written informed consent was obtained from all study participants.
ACKNOWLEDGMENTS
KSO Corporation (Tokyo, Japan) supported the work of this clinical study as a contract research organization. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Anzawa D, Mawatari T, Tanaka Y, et al. Effects of synbiotics containing Bifidobacterium animalis subsp. lactis GCL2505 and inulin on intestinal bifidobacteria: A randomized, placebo‐controlled, crossover study. Food Sci Nutr. 2019;7:1828–1837. 10.1002/fsn3.1033
REFERENCES
- Aoki, R. , Kamikado, K. , Suda, W. , Takii, H. , Mikami, Y. , Suganuma, N. , … Koga, Y. (2017). A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Scientific Report, 7, 43522 10.1038/srep43522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, R. , Tsuchida, S. , Arai, Y. , Ohno, K. , Nishijima, T. , Mawatari, T. , … Ushida, K. (2016). Effect of Bifidobacterium animalis subsp. lactis GCL2505 on the physiological function of intestine in a rat model. Food Science & Nutrition, 4(6), 782–790. 10.1002/fsn3.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemi, Z. , Khorrami‐Rad, A. , Alizadeh, S. A. , Shakeri, H. , & Esmaillzadeh, A. (2014). Effects of synbiotic food consumption on metabolic status of diabetic patients: A double‐blind randomized cross‐over controlled clinical trial. Clinical Nutrition, 33(2), 198–203. 10.1016/j.clnu.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Blander, J. M. , Longman, R. S. , Iliev, I. D. , Sonnenberg, G. F. , & Artis, D. (2017). Regulation of inflammation by microbiota interactions with the host. Nature Immunology, 18(8), 851–860. 10.1038/ni.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhnik, Y. , Raskine, L. , Champion, K. , Andrieux, C. , Penven, S. , Jacobs, H. , & Simoneau, G. (2007). Prolonged administration of low‐dose inulin stimulates the growth of bifidobacteria in humans. Nutrition Research, 27(4), 187–193. 10.1016/j.nutres.2007.01.013 [DOI] [Google Scholar]
- Childs, C. E. , Röytiö, H. , Alhoniemi, E. , Fekete, A. A. , Forssten, S. D. , Hudjec, N. , … Gibson, G. R. (2014). Xylo‐oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: A double‐blind, placebo‐controlled, randomised, factorial cross‐over study. British Journal of Nutrition, 111(11), 1945–1956. 10.1017/S0007114513004261 [DOI] [PubMed] [Google Scholar]
- Eskesen, D. , Jespersen, L. , Michelsen, B. , Whorwell, P. J. , Müller‐Lissner, S. , & Morberg, C. M. (2015). Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB‐12, on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: A randomised, double‐blind, placebo‐controlled, parallel‐group trial. British Journal of Nutrition, 114(10), 1638–1646. 10.1017/S0007114515003347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold, S. M. , Sutter, V. L. , & Matheisen, G. E. (1983). Normal indigenous flora In Hentges D. J. (Ed.), Human intestinal microflora in health and disease (pp. 3–31). New York, NY: Academic Press. [Google Scholar]
- Flach, J. , van der Waal, M. B. , Kardinaal, A. F. M. , Schloesser, J. , Ruijschop, R. M. A. J. , & Claassen, E. (2018). Probiotic research priorities for the healthy adult population: A review on the health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB‐12. Cogent Food & Agriculture, 4(1), 1452839 10.1080/23311932.2018.1452839 [DOI] [Google Scholar]
- Fukumoto, S. , Tatewaki, M. , Yamada, T. , Fujimiya, M. , Mantyh, C. , Voss, M. , … Takahashi, T. (2003). Short‐chain fatty acids stimulate colonic transit via intraluminal 5‐HT release in rats. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 284(5), 1269–1276. 10.1152/ajpregu.00442.2002 [DOI] [PubMed] [Google Scholar]
- Gibson, G. R. , & Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. The Journal of Nutrition, 125(6), 1401–1412. 10.1093/jn/125.6.1401 [DOI] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G. R. , Merenstein, D. J. , Pot, B. , … Sanders, M. E. (2014). The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11, 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Igarashi, H. , Ohno, K. , Matsuki, N. , Fujiwara‐Igarashi, A. , Kanemoto, H. , Fukushima, K. , … Tsujimoto, H. (2017). Analysis of fecal short chain fatty acid concentration in miniature dachshunds with inflammatory colorectal polyps. Journal of Veterinary Medical Science, 79(10), 1727–1734. 10.1292/jvms.17-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka, A. , Tomizuka, K. , Aoki, R. , Nishijima, T. , Saito, Y. , Inoue, R. , … Ikeda, T. (2012). Effects of administration of Bifidobacterium animalis subsp. lactis GCL2505 on defecation frequency and bifidobacterial microbiota composition in humans. Journal of Bioscience and Bioengineering, 113(5), 587–591. 10.1016/j.jbiosc.2011.12.016 [DOI] [PubMed] [Google Scholar]
- Kalliomäki, M. , Kirjavainen, P. , Eerola, E. , Kero, P. , Salminen, S. , & Isolauri, E. (2001). Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. Journal of Allergy and Clinical Immunology, 107(1), 129–134. 10.1067/mai.2001.111237 [DOI] [PubMed] [Google Scholar]
- Kato, K. , Odamaki, T. , Mitsuyama, E. , Sugahara, H. , Xiao, J. Z. , & Osawa, R. (2017). Age‐related changes in the composition of gut Bifidobacterium species. Current Microbiology, 74(8), 987–995. 10.1007/s00284-017-1272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs, A. P. , Samsom, M. , van der Rest, M. E. , de Vogel, J. , Knol, J. , Ben‐Amor, K. , & Akkermans, L. M. (2009). Lower Bifidobacteria counts in both duodenal mucosa‐associated and fecal microbiota in irritable bowel syndrome patients. World Journal of Gastroenterology, 15(23), 2887–2892. 10.3748/wjg.15.2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbeck, J. A. , Rasmussen, H. E. , Hutkins, R. W. , Clarke, J. , Shawron, K. , Keshavarzian, A. , & Walter, J. (2018). Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome, 6, 121 10.1186/s40168-018-0494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, N. , Vogensen, F. K. , van den Berg, F. W. J. , Nielsen, D. S. , Andreasen, A. S. , Pedersen, B. K. , … Jakobsen, M. (2010). Gut microbiota in human adults with type 2 diabetes differs from non‐diabetic adults. PLoS ONE, 5(2), e9085 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R. E. , Bäckhed, F. , Turnbaugh, P. , Lozupone, C. A. , Knight, R. D. , & Gordon, J. I. (2005). Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 11070–11075. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R. E. , Turnbaugh, P. J. , Klein, S. , & Gordon, J. I. (2006). Microbial ecology: Human gut microbes associated with obesity. Nature, 444(7122), 1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Macfarlane, S. , Cleary, S. , Bahrami, B. , Reynolds, N. , & Macfarlane, G. T. (2013). Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double‐blind, placebo‐controlled crossover study. Alimentary Pharmacology & Therapeutics, 38(7), 804–816. 10.1111/apt.12453 [DOI] [PubMed] [Google Scholar]
- Mackie, R. I. , Sghir, A. , & Gaskins, H. R. (1999). Developmental microbial ecology of the neonatal gastrointestinal tract. The American Journal of Clinical Nutrition, 69(5), 1035S–1045S. 10.1093/ajcn/69.5.1035s [DOI] [PubMed] [Google Scholar]
- Malinen, E. , Rinttilä, T. , Kajander, K. , Mättö, J. , Kassinen, A. , Krogius, L. , … Palva, A. (2005). Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real‐time PCR. American Journal of Gastroenterology, 100(2), 373–382. 10.1111/j.1572-0241.2005.40312.x [DOI] [PubMed] [Google Scholar]
- Manichanh, C. , Rigottier‐Gois, L. , Bonnaud, E. , Gloux, K. , Pelletier, E. , Frangeul, L. , … Dore, J. (2006). Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut, 55(2), 205–211. 10.1136/gut.2005.073817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, K. , Takada, T. , Shimizu, K. , Moriyama, K. , Kawakami, K. , Hirano, K. , … Nomoto, K. (2010). Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. Journal of Bioscience and Bioengineering, 110(5), 547–552. 10.1016/j.jbiosc.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Matsumoto, M. , Imai, T. , Hironaka, T. , Kume, H. , Watanabe, M. , & Benno, Y. (2001). Effect of yogurt with Bifidobacterium lactis LKM 512 in improving fecal microflora and defecation of healthy volunteers. Journal of Intestinal Microbiology, 14(2), 97–102. 10.11209/jim1997.14.97 [DOI] [Google Scholar]
- Mensink, M. A. , Frijlink, H. W. , van der Voort Maarschalk, K. , & Hinrichs, W. L. J. (2015). Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydrate Polymers, 130(5), 405–419. 10.1016/j.carbpol.2015.05.026 [DOI] [PubMed] [Google Scholar]
- Mitsuoka, T. (1984). Taxonomy and ecology of bifidobacteria. Bifidobacteria and Microflora, 3(1), 11–28. 10.12938/bifidus1982.3.1_11 [DOI] [Google Scholar]
- Nishida, S. , Gotou, M. , Akutsu, S. , Ono, M. , Hitomi, Y. , Nakamura, T. , & Iino, H. (2004). Effect of yogurt containing Bifidobacterium lactis BB‐12 on improvement of defecation and fecal microflora of healthy female adults. Milk Science, 53(2), 71–80. 10.11465/milk.53.71 [DOI] [Google Scholar]
- Ono, S. , Karaki, S. , & Kuwahara, A. (2004). Short‐chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. The Japanese Journal of Physiology, 54(5), 483–493. 10.2170/jjphysiol.54.483 [DOI] [PubMed] [Google Scholar]
- Pineiro, M. , Asp, N. G. , Reid, G. , Macfarlane, S. , Morelli, L. , Brunser, O. , & Tuohy, K. (2008). FAO technical meeting on prebiotics. Journal of Clinical Gastroenterology, 42, S156–S159. 10.1097/MCG.0b013e31817f184e [DOI] [PubMed] [Google Scholar]
- Rajilic‐Stojanovic, M. , Biagi, E. , Heilig, H. G. , Kajander, K. , Kekkonen, R. A. , Tims, S. , & de Vos, W. M. (2011). Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology, 141(5), 1792–1801. 10.1053/j.gastro.2011.07.043 [DOI] [PubMed] [Google Scholar]
- Ramirez‐Farias, C. , Slezak, K. , Fuller, Z. , Duncan, A. , Holtrop, G. , & Louis, P. (2009). Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii . British Journal of Nutrition, 101(4), 541–550. 10.1017/S0007114508019880 [DOI] [PubMed] [Google Scholar]
- Rao, V. A. (2001). The prebiotic properties of oligofructose at low intake levels. Nutrition Research, 21(6), 843–848. 10.1016/S0271-5317(01)00284-6 [DOI] [Google Scholar]
- Rivière, A. , Selak, M. , Lantin, D. , Leroy, F. , & De Vuyst, L. (2016). Bifidobacteria and butyrate‐producing colon bacteria: Importance and strategies for their stimulation in the human gut. Frontiers in Microbiology, 7, 979 10.3389/fmicb.2016.00979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid, M. B. , Van Loo, J. A. E. , & Gibson, G. R. (1998). The bifidogenic nature of chicory inulin and its hydrolysis products. The Journal of Nutrition, 128(1), 11–19. 10.1093/jn/128.1.11 [DOI] [PubMed] [Google Scholar]
- Rossi, M. , Corradini, C. , Amaretti, A. , Nicolini, M. , Pompei, A. , Zanoni, S. , & Matteuzzi, D. (2005). Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Applied and Environmental Microbiology, 71(10), 6150–6158. 10.1128/AEM.71.10.6150-6158.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, K. F. , Altman, D. G. , & Moher, D. ;CONSORT Group (2011). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. International Journal of Surgery, 9(8), 672–677. 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Schwiertz, A. , Taras, D. , Schäfer, K. , Beijer, S. , Bos, N. A. , Donus, C. , & Hardt, P. D. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring), 18(1), 190–195. 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- Shioiri, T. , Yahagi, K. , Nakayama, S. , Asahara, T. , Yuki, N. , Kawakami, K. , … Totani, M. (2006). The effects of a synbiotic fermented milk beverage containing Lactobacillus casei strain Shirota and transgalactosylated oligosaccharides on defecation frequency, intestinal microflora, organic acid concentrations, and putrefactive metabolites of sub‐optimal health state volunteers: A randomized placebo‐controlled cross‐over study. Bioscience and Microflora, 25(4), 137–146. 10.12938/bifidus.25.137 [DOI] [Google Scholar]
- Stenman, L. K. , Lehtinen, M. J. , Meland, N. , Christensen, J. E. , Yeung, N. , Saarinen, M. T. , … Lahtinen, S. (2016). Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults ‐ randomized controlled trial. EBioMedicine, 13, 190–200. 10.1016/j.ebiom.2016.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, S. , Anzawa, D. , Takami, K. , Ishizuka, A. , Mawatari, T. , Kamikado, K. , … Nishijima, T. (2016). Effect of Bifidobacterium animalis ssp. lactis GCL2505 on visceral fat accumulation in healthy Japanese adults: A randomized controlled trial. Bioscience of Microbiota, Food and Health, 35(4), 163–171. 10.12938/bmfh.2016-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, R. (1995). Clinical effects of bifidobacteria and lactobacilli In Fuller R., Heidt P. J., Rusch V., & Waaij D. V. D. (Eds.), Probiotics: Prospects of use in opportunistic infections, Old Herborn University seminar monograph 8 (pp. 141–157). Herborn‐Dill, Germany: Institute for Microbiology and Biochemistry. [Google Scholar]
- Tanaka, Y. , Takami, K. , Nishijima, T. , Aoki, R. , Mawatari, T. , & Ikeda, T. (2015). Short‐ and long‐term dynamics in the intestinal microbiota following ingestion of Bifidobacterium animalis subsp. lactis GCL2505. Bioscience of Microbiota, Food and Health, 34(4), 77–85. 10.12938/bmfh.2015-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni, F. , Foroni, E. , Pizzetti, P. , Giubellini, V. , Ribbera, A. , Merusi, P. , … Ventura, M. (2009). Exploring the diversity of the bifidobacterial population in the human intestinal tract. Applied and Environmental Microbiology, 75(6), 1534–1545. 10.1128/AEM.02216-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van De Sande, M. M. , van Buul, V. J. , & Brouns, F. J. (2014). Autism and nutrition: The role of the gut‐brain axis. Nutrition Research Reviews, 27(2), 199–214. 10.1017/S0954422414000110 [DOI] [PubMed] [Google Scholar]
- Waitzberg, D. L. , Logullo, L. C. , Bittencourt, A. F. , Torrinhas, R. S. , Shiroma, G. M. , Paulino, N. P. , & Teixeira‐da‐Silva, M. L. (2013). Effect of synbiotic in constipated adult women – A randomized, double‐blind, placebo‐controlled study of clinical response. Clinical Nutrition, 32(1), 27–33. 10.1016/j.clnu.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Yaeshima, T. , Takahashi, S. , Matsumoto, N. , Ishibashi, N. , Hayasawa, H. , & Iino, H. (1997). Effect of yogurt containing Bifidobacterium longum bb536 on the intestinal environment, fecal characteristics and defecation frequency. Bioscience and Microflora, 16(2), 73–77. 10.12938/bifidus1996.16.73 [DOI] [Google Scholar]
- Yamano, T. , Iino, H. , Takada, M. , Blum, S. , Rochat, F. , & Fukushima, Y. (2006). Improvement of the human intestinal flora by ingestion of the probiotic strain Lactobacillus johnsonii La1. British Journal of Nutrition, 95(2), 303–312. 10.1079/BJN20051507 [DOI] [PubMed] [Google Scholar]
- Yang, Y. X. , He, M. , Hu, G. , Wei, J. , Pages, P. , Yang, X. H. , & Bourdu‐Naturel, S. (2008). Effect of a fermented milk containing Bifidobacterium lactis DN‐173010 on Chinese constipated women. World Journal of Gastroenterology, 14(40), 6237–6243. 10.3748/wjg.14.6237 [DOI] [PMC free article] [PubMed] [Google Scholar]


