Abstract
The activation of hepatic stellate cells (HSCs) is an important step in the progress of liver fibrosis. Fibrosis can be impeded by HSC reversion to a quiescent state or HSC clearance through apoptosis. To investigate the apoptotic effects of hsian‐tsao (Mesona procumbens Hemsl) on human HSCs, the expression levels of cleaved caspase‐3, p38, and c‐Jun N‐terminal kinase (JNK) were assessed using Western blotting, and the caspase‐3 activity was measured using caspase‐3/CPP32 colorimetric assay kit. Hsian‐tsao extract (HTE) increased the activity of caspase‐3 and the level of activated caspase‐3, indicating the activation of apoptosis. The intracellular reactive oxygen species (ROS) level increased in a dose‐dependent manner. This increase was prevented by an antioxidant, suggesting that HTE induces ROS accumulation. In addition, we found that HTE induced the phosphorylation of the mitogen‐activated protein kinases JNK and p38. These collective data indicate that HTE induces apoptosis via ROS production through the p38, JNK, and caspase‐3‐dependent pathways. HTE may decrease HSC activation in liver fibrosis and may have a therapeutic potential.
Keywords: hepatic stellate cell, hsian‐tsao, reactive oxygen species, apoptosis
1. INTRODUCTION
Liver fibrosis is associated with severe morbidity and significant mortality (Bonis, Friedman, & Kaplan, 2001), and it involves the activation of hepatic stellate cells (HSCs) (Friedman, 2008a, 2008b). In liver fibrosis, the activated HSCs undergo proliferation, which can result in the inhibition of apoptosis, the accumulation of extracellular matrix (ECM), and the production of proinflammatory proteins (Friedman, 2010; Murphy et al., 2002). Therefore, HSCs are believed to be the key target for fibrosis treatment (Fallowfield, 2011; Friedman, 2008b). It has been reported that decreasing the survival rate of activated HSCs can be achieved via the inhibition of cell proliferation or by triggering apoptosis; in addition, this can be achieved by the suppression of excessive ECM deposition (Fan et al., 2013). Thus, the idea to decrease the activated HSC survival rate by using natural products could be effective in the treatment of liver fibrosis.
Mesona procumbens is a natural drink and is the main component of grass jelly in Taiwan (Huang et al., 2012). M. procumbens has therapeutic potential in the treatment of inflammation‐associated disorders (Huang et al., 2012). The inhibition of monosodium urate‐induced xanthine oxidase activity in human acute monocytic leukemia THP‐1 cells by a 50% ethanol extract of M. procumbens has been demonstrated (Jhang et al., 2016); this highlights the potential to improve hyperuricemia by the downregulation of xanthine oxidase activity in vivo. Aqueous extracts of hsian‐tsao have been reported to protect the myocardium in streptozotocin‐induced diabetic rats (Yang et al., 2008). Analysis of the serum levels of hepatic enzymes in experimental animal models revealed that the aqueous extracts of hsian‐tsao protect against tertiary butyl hydroperoxide‐induced acute hepatic damage and reduce oxidative stress (Yen, Yeh, & Chen, 2004). Many reports have indicated that reactive oxygen species (ROS) play a key role in the regulation of the activation of mitogen‐activated protein kinases (MAPKs), such as p38 and c‐Jun N‐terminal kinase (JNK) (Chuang & Chen, 2004; Jia et al., 2007; Junttila, Li, & Westermarck, 2008; McCubrey, Lahair, & Franklin, 2006; Son, Kim, Chung, & Pae, 2013).
However, the pharmacological effects and the mechanism of action of HTE on the inhibition of liver fibrosis are still unknown.
Herein, we report that a hsian‐tsao extract from M. procumbens Hemsl has an apoptotic effect on activated HSCs via ROS and the p38 MAPK, JNK, and caspase‐3‐dependent pathways.
2. MATERIALS AND METHODS
2.1. Materials
Dried hsian‐tsao leaves were purchased from BioWisdom. Water extraction of hsian‐tsao was performed as described by Yang et al. (2008). Finally, the final extract was collected and used for the experiments.
2.2. Reagents
A WST‐1 kit was purchased from Roche Applied Sciences. A caspase‐3/CPP32 colorimetric assay kit was purchased from BioVision. The antiphospho‐JNK and JNK antibodies were purchased from Cell Signaling Technology. The antiphospho‐p38, p38 MAPK, and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibodies were purchased from Santa Cruz Biotechnology. Sigma‐Aldrich was the manufacturer of all other chemicals.
2.3. Cell culture
Human primary hepatic stellate cells (hHSCs) were obtained from ScienCell Research Laboratories and were cultured according to the manufacturer's instructions. Briefly, the cells were seeded into poly‐l‐lysine‐coated T‐25 flasks in Stellate Cell Medium (ScienCell Research Laboratories) containing 2% fetal calf serum (FCS) and stellate cell growth supplement (ScienCell Research Laboratories).
2.4. Detection of cell viability and caspase‐3 activity
We used a WST‐1 cell proliferation assay kit and caspase‐3/CPP32 colorimetric assay kit to detect the cell viability and caspase‐3 activity in this study, respectively. The protocols were supplied by the manufacturer and were modified according to our previous study (Kuo et al., 2014).
2.5. Intracellular ROS analysis
Fluorescence‐activated cell sorting (FACS, BD Biosciences) was used to detect the relative ROS levels after the cells were stained with the reagent 2′,7′‐dichlorofluorescein diacetate (DCF‐DA; Sigma‐Aldrich).
2.6. Western blotting
The cell was harvested and lyzed according to a protocol from our previous study (Kuo et al., 2014). The primary antibodies were used and are mentioned in the above section (2.2. Reagents).
2.7. Statistical analyses
All data were examined by one‐way or two‐way analysis of variance (ANOVA). Additionally, the Bonferroni post hoc test was used in this study. A p‐value <0.05 was reported as statistically significant. *p < 0.05, **p < 0.01.
3. RESULTS
3.1. HTE treatment decreased the cell viability via apoptosis
The cell viability was inhibited in a dose‐dependent manner with HTE treatment (10–100 μg/ml) (Figure 1a). To assess the apoptotic effects on HSCs, we evaluated the level of activated caspase‐3 (cleaved caspase‐3) and the activity of caspase‐3 by Western blotting and enzyme‐linked immunosorbent assays, respectively. The data demonstrate that both levels were significantly increased in cells treated with HTE (30 and 100 μg/ml) for the indicated time periods (Figure 2a–c). We hypothesize that HTE can induce apoptosis via a caspase‐3‐dependent pathway.
Figure 1.

Hsian‐tsao extract (HTE) decreases the cell viability (a) Cells were treated with the indicated concentrations of hsian‐tsao for 24 hr; the control cells were not treated. (b). N‐acetyl‐cysteine (NAC) (3 mM) reversed hsian‐tsao‐induced cell death. The experiments were independently repeated three times (n = 3). C: means control group. *p < 0.05, **p < 0.01
Figure 2.
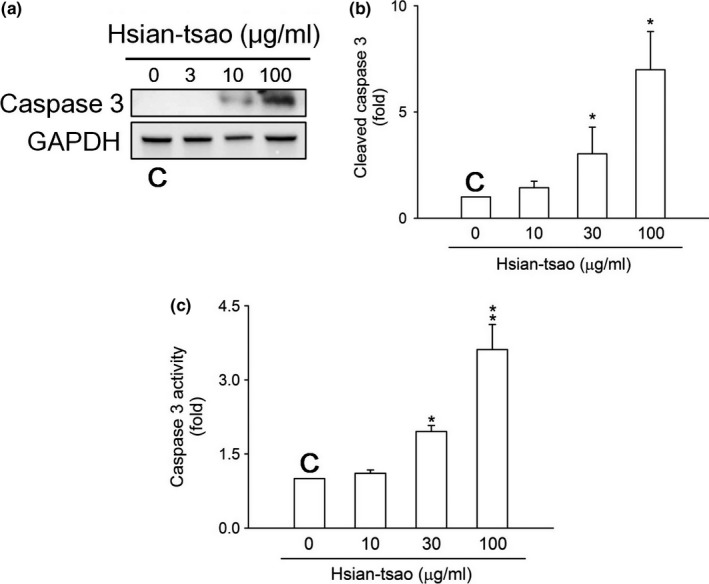
Hsian‐tsao extract (HTE) induces cell death via caspase‐3 activation (a) Cells were treated with the indicated concentrations of HTE or were left untreated (control) for 24 hr. The level of activated caspase‐3 (cleaved caspase‐3) was analyzed using Western blotting. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as a loading control. (b) The expression of specific proteins was quantified using ImageJ. (c) Cells were untreated (control) or treated with the indicated concentrations of hsian‐tsao for 24 hr. C: means control group. The experiments were independently repeated three times (n = 3)
3.2. Intracellular ROS production was induced under HTE treatment
DCF‐DA staining showed that HTE (100 μg/ml) significantly induced ROS production (Figure 3a). Furthermore, treatment with the ROS scavenger N‐acetyl‐cysteine (NAC, 3 mM) attenuated HTE‐induced ROS production (Figure 3b) and cell death (Figure 1b).
Figure 3.
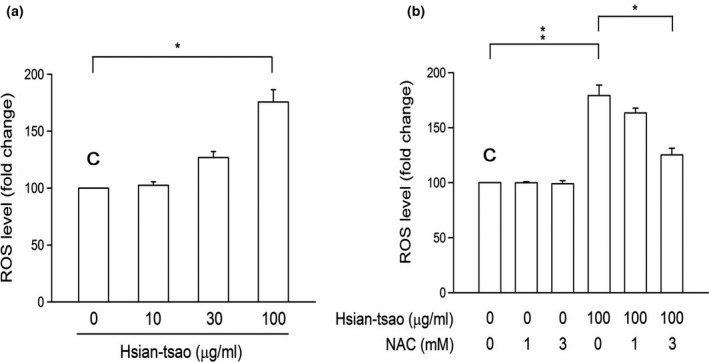
Hsian‐tsao extract (HTE) induces reactive oxygen species (ROS) production (a) Cells were treated with hsian‐tsao at the indicated concentrations for 24 hr. After DCF‐DA staining, fluorescence‐activated cell sorting (FACS) detected and quantitated the fluorescence signal. (b) N‐acetyl‐cysteine (NAC) (3 mM) reversed the induced ROS overproduction. The experiments were independently repeated three times (n = 3). C: means control group
3.3. Effect of HTE on the phosphorylation of JNK and p38 MAPK
The JNK and p38 pathways have been implicated in cell apoptosis (Troeger et al., 2012). To examine whether JNK and p38 phosphorylation is associated with HTE ‐induced apoptosis, the expression and phosphorylation of both proteins were measured by Western blotting. HTE rapidly induced JNK and p38 activation in time‐ and concentration‐dependent manners (Figure 4a,b). This phosphorylation was reversed by treatment with 3 mM NAC (Figure 5a,b). The increases in the cleaved caspase‐3 expression level and in caspase‐3 activity were also reversed after NAC treatment (Figure 6). These results suggest that HTE causes cell apoptosis due to ROS overproduction.
Figure 4.

Hsian‐tsao extract (HTE) induces the phosphorylation of JNK and p38. The induced phosphorylation of (a) JNK and (b) p38 in a dose‐dependent manner. The phosphorylation of JNK and p38 was quantitated using antibodies against the phosphorylated and total protein. Densitometric analysis was carried out by normalizing the total protein levels (lower panels of a and b). The experiments were independently repeated three times (n = 3). C: means control group
Figure 5.

N‐acetyl‐cysteine (NAC) reverses the hsian‐tsao extract (HTE)‐induced phosphorylation of JNK and ERK. The NAC reversal of the HTE‐induced phosphorylation of (a) JNK and (b) p38. Cells were pretreated with NAC (3 mM) for 1 hr before HTE treatment. The expression levels of phospho‐JNK, JNK, phospho‐p38, and p38 were detected with Western blotting. Densitometric analysis of all samples was carried out by normalizing the total protein levels. The experiments were independently repeated three times (n = 3). C: means control group
Figure 6.
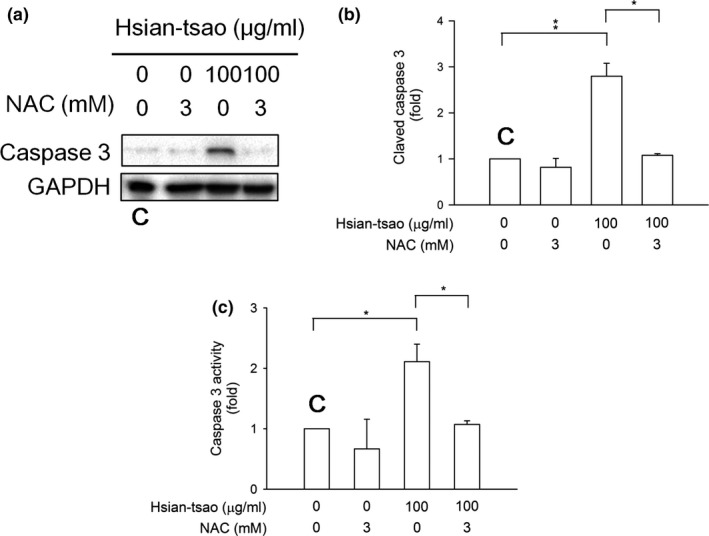
N‐acetyl‐cysteine (NAC) reverses hsian‐tsao‐induced caspase‐3 activation. (a) Cells were pretreated with NAC (3 mM) for 1 hr before the addition of hsian‐tsao extract (HTE). The Western blot analysis showed the level of activated caspase‐3 (cleaved caspase‐3). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as a loading control. (b) The expression of specific proteins was quantified using ImageJ. (c) Caspase‐3 activity was detected. The experiments were independently repeated three times (n = 3). C: means control group
4. DISCUSSION
The activation of HSCs is a key role in liver fibrosis (Franco & Cidlowski, 2012). Therefore, increasing the apoptotic levels of activated HSCs and decreasing the growth rate of activated HSCs may be good strategies for solving the problem of liver fibrosis (Friedman, 2010; Kuo et al., 2014; Mederacke et al., 2013; Puche, Saiman, & Friedman, 2013; Ray, 2014; von Schwarzenberg & Vollmar, 2013). Drugs that inhibit hepatic fibrogenesis might be obtained from marine natural products. To the best of our knowledge, this is the first study to demonstrate an apoptotic effect of the natural product HTE on HSCs via ROS accumulation.
We investigated the pharmacological effects of HTE on HSCs using multiple approaches and found that it induces apoptosis through ROS production via the p38, JNK, and caspase‐3‐dependent pathways. Many studies have demonstrated the role of HSC activation in liver fibrosis and have highlighted the significance of HSC‐induced apoptosis in the pathogenesis of liver fibrosis (Friedman, 2010; Issa et al., 2001; Jia et al., 2015; Kuo et al., 2014; Mederacke et al., 2013; Puche et al., 2013; von Schwarzenberg & Vollmar, 2013; Xie, Fujii, Zhao, Shinohara, & Matsukura, 2016). Therefore, we hypothesize that hsian‐tsao may potently inhibit HSC viability through ROS production via the JNK and p38 MAPK pathways.
N‐acetyl‐cysteine can prevent JNK phosphorylation in human gastric carcinoma MKN45 cells (Guo et al., 2016) and can reverse the overexpression of p38‐associated pathways in vascular endothelial cells (Bhattacharya, Halder, Mukhopadhyay, & Giri, 2009) and human melanoma cells (Bell et al., 2010). It has been reported that Pin was isolated from the gorgonian coral Pinnigorgia sp., which triggered the activated HSCs to undergo apoptosis via ROS‐ERK/JNK‐caspase‐3 signaling and probably caused the clearance of HSCs (Kuo et al., 2018). Another study suggested that the traditional Chinese medicine Fuzheng Huayu attenuates hepatic fibrosis by inhibiting tumor necrosis factor‐alpha‐induced hepatocyte apoptosis and by activating HSCs in mice treated with carbon tetrachloride (Tao et al., 2014). Curcumol is a guaiane‐type sesquiterpenoid hemiketal extracted from the roots of the herb Rhizoma Curcumae. It has been shown to target receptor‐interacting serine/threonine‐protein kinase‐1/‐3 and to induce necroptosis in HSCs via JNK1/2‐ROS signaling cascades (Jia et al., 2018). These data clearly show that natural products can protect against liver fibrosis through the deactivation of HSCs or the induction of cell death.
Accumulating evidence has suggested that ROS play a critical role in the progression of liver fibrosis formation (Ceni, Mello, & Galli, 2014; Parola & Robino, 2001; Poli & Parola, 1997; Siegmund et al., 2007). ROS accumulation caused cell death via apoptosis in activated HSCs in both humans and rats (Brunati, Pagano, Bindoli, & Rigobello, 2010). To further characterize the HTE‐induced cell apoptotic pathway, we studied the effect of HTE on ROS‐induced cell death and determined the origin of ROS production. The 1‐glutathione precursor NAC reversed the apoptosis induced by HTE and the cell death resulting from ROS accumulation (Figures 1b, 3b, and 6). Thus, we hypothesize that HTE induces apoptosis by ROS overproduction and through l‐glutathione depletion. These findings are consistent with the present and prior findings (Dunning et al., 2009, 2013; Gao et al., 2012; Kuo et al., 2014; Runchel, Matsuzawa, & Ichijo, 2011; Zarubin & Han, 2005).
ERK, p38 kinase, JNK, and MAPK are members of the MAPK family and are important for the response to oxidative stress (Chowdhury et al., 2013; Huang, Wu, Tashiro, Onodera, & Ikejima, 2008; Zarubin & Han, 2005). It has been reported that the survival of activated HSCs is mediated by the MAPK signaling pathway (Jia et al., 2018; Szuster‐Ciesielska, Mizerska‐Dudka, Daniluk, & Kandefer‐Szerszen, 2013). However, Yu et al. (2012) found that the continuous generation of hydrogen peroxide may result in the inhibition of the growth of human gingival fibroblasts and that this effect is independent of MAPK activation. Therefore, the mechanism underlying the MAPK‐mediated apoptosis of HSCs induced by oxidative stress is still unclear.
Currently, phenolic compounds (kaempferol, apigenin, caffeic acid, protocatechuic acid, syringic acid, vanillic acid, and p‐hydrobenzoic acid) were extracted from hsian‐tsao (Yeh, Huang, & Yen, 2009). Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of an EGFR‐related pathway (Lee & Kim, 2016). Apigenin has health‐promoting effects or therapeutic functions for chronic diseases, including diabetes, amnesia, Alzheimer's disease, depression, insomnia, and cancer (Salehi et al., 2019). Caffeic acid has anti‐inflammatory, anticancer, and antiviral activities (Touaibia, Jean‐Francois, & Doiron, 2011). On the other hand, protocatechuic acid displays notable atheroprotective effects via the regulation of the transition of M1‐ and M2‐type macrophages (Liu et al., 2019). The protective effect of syringic acid on myocardial infarction (MI) caused by isoproterenol (ISO) has been reported (Shahzad et al., 2019). Vanillic acid has potential as an agent for the treatment of sickle cell anemia and chronic liver injuries (Itoh et al., 2009; Safo & Kato, 2014). p‐Hydrobenzoic acid has antimicrobial, antialgal, antimutagenic, antiestrogenic, hypoglycemic, anti‐inflammatory, antiplatelet aggregating, nematicidal, antiviral, and antioxidant functions; in addition, it is widely used in cosmetic products (Azam, Dharanya, Mehta, & Sachdeva, 2013). Therefore, these phenolic compounds extracted from hsian‐tsao have antioxidant and anti‐inflammatory effects.
Herein, JNK and p38 were activated significantly after HTE treatment (Figure 4). Consistent with the previous results, the apoptotic effect of shikonin was determined in leukemia cells via ROS and the JNK pathway (Mao, Yu, Li, & Li, 2008). Panaxydol induces cellular apoptosis via the JNK/ROS pathway (Kim et al., 2011). Thus, the present study shows that the signaling pathway underlying HTE‐induced apoptosis in HSCs involves ROS production and JNK and p38 activation.
5. CONCLUSIONS
We have shown a significant induction of apoptosis in HSCs by hsian‐tsao treatment. These apoptotic effects are mediated through multiple mechanisms: the increased accumulation of ROS and the activation of JNK and p38. The structures of the bioactive compounds in HTE remain to be elucidated. In conclusion, our study makes a significant contribution toward the identification of pathways which can be pharmaceutical targets for the development of novel therapeutic approaches in the treatment of liver fibrosis.
CONFLICT OF INTEREST
None declared.
ETHICAL STATEMENT
This study does not involve any human or animal experiments.
ACKNOWLEDGMENTS
This study was supported by Show Chwan and Chan Bing Show Chwan Memorial Hospital in Changhua, Taiwan (RD107035), and by Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation in New Taipei City (TCRD‐TPE‐108‐25).
Yeh Y‐H, Liang C‐Y, Chen M‐L, et al. Apoptotic effects of hsian‐tsao (Mesona procumbens Hemsley) on hepatic stellate cells mediated by reactive oxygen species and ERK, JNK, and caspase‐3 pathways. Food Sci Nutr. 2019;7:1891–1898. 10.1002/fsn3.1046
Yung‐Hsiang Yeh and Chun‐Ya Liang equally contributed to this work.
Contributor Information
Jiunn‐Sheng Wu, Email: wujs.medoc@gmail.com.
Chan‐Yen Kuo, Email: cykuo863135@gmail.com.
REFERENCES
- Azam, M. A. , Dharanya, L. , Mehta, C. C. , & Sachdeva, S. (2013). Synthesis and biological evaluation of some novel pyrazolopyrimidines incorporating a benzothiazole ring system. Acta Pharmaceutica, 63(1), 19–30. 10.2478/acph-2013-0001 [DOI] [PubMed] [Google Scholar]
- Bell, L. N. , Temm, C. J. , Saxena, R. , Vuppalanchi, R. , Schauer, P. , Rabinovitz, M. , … Mattar, S. G. (2010). Bariatric surgery‐induced weight loss reduces hepatic lipid peroxidation levels and affects hepatic cytochrome P‐450 protein content. Annals of Surgery, 251, 1041–1048. 10.1097/SLA.0b013e3181dbb572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya, U. , Halder, B. , Mukhopadhyay, S. , & Giri, A. K. (2009). Role of oxidation‐triggered activation of JNK and p38 MAPK in black tea polyphenols induced apoptotic death of A375 cells. Cancer Science, 100, 1971–1978. 10.1111/j.1349-7006.2009.01251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis, P. A. , Friedman, S. L. , & Kaplan, M. M. (2001). Is liver fibrosis reversible? New England Journal of Medicine, 344, 452–454. 10.1056/NEJM200102083440610 [DOI] [PubMed] [Google Scholar]
- Brunati, A. M. , Pagano, M. A. , Bindoli, A. , & Rigobello, M. P. (2010). Thiol redox systems and protein kinases in hepatic stellate cell regulatory processes. Free Radical Research, 44, 363–378. 10.3109/10715760903555836 [DOI] [PubMed] [Google Scholar]
- Ceni, E. , Mello, T. , & Galli, A. (2014). Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World Journal of Gastroenterology, 20, 17756–17772. 10.3748/wjg.v20.i47.17756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, A. A. , Chaudhuri, J. , Biswas, N. , Manna, A. , Chatterjee, S. , Mahato, S. K. , … Bandyopadhyay, S. (2013). Synergistic apoptosis of CML cells by buthionine sulfoximine and hydroxychavicol correlates with activation of AIF and GSH‐ROS‐JNK‐ERK‐iNOS pathway. PLoS ONE, 8, e73672 10.1371/journal.pone.0073672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, J. I. , & Chen, T. H. (2004). Effect of melatonin on temporal changes of reactive oxygen species and glutathione after MPP(+) treatment in human astrocytoma U373MG cells. Journal of Pineal Research, 36, 117–125. 10.1046/j.1600-079X.2003.00107.x [DOI] [PubMed] [Google Scholar]
- Dunning, S. , Hannivoort, R. A. , de Boer, J. F. , Buist‐Homan, M. , Faber, K. N. , & Moshage, H. (2009). Superoxide anions and hydrogen peroxide inhibit proliferation of activated rat stellate cells and induce different modes of cell death. Liver International, 29, 922–932. 10.1111/j.1478-3231.2009.02004.x [DOI] [PubMed] [Google Scholar]
- Dunning, S. , Ur Rehman, A. , Tiebosch, M. H. , Hannivoort, R. A. , Haijer, F. W. , Woudenberg, J. , … Moshage, H. (2013). Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide‐induced cell death. Biochimica Et Biophysica Acta, 1832, 2027–2034. 10.1016/j.bbadis.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Fallowfield, J. A. (2011). Therapeutic targets in liver fibrosis. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 300, G709–G715. 10.1152/ajpgi.00451.2010 [DOI] [PubMed] [Google Scholar]
- Fan, H. N. , Wang, H. J. , Yang‐Dan, C. R. , Ren, L. , Wang, C. , Li, Y. E. , & Deng, Y. (2013). Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Molecular Medicine Reports, 7, 247–253. 10.3892/mmr.2012.1153 [DOI] [PubMed] [Google Scholar]
- Franco, R. , & Cidlowski, J. A. (2012). Glutathione efflux and cell death. Antioxidants & Redox Signaling, 17, 1694–1713. https://doi.org/10.org/1074/jbc.M703091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, S. L. (2008a). Preface. Hepatic fibrosis: Pathogenesis, diagnosis, and emerging therapies. Clinical Liver Disease, 12, xiii–xiv. 10.1016/j.cld.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Friedman, S. L. (2008b). Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews, 88, 125–172. 10.1152/physrev.00013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, S. L. (2010). Evolving challenges in hepatic fibrosis. Nature Reviews Gastroenterology & Hepatology, 7, 425–436. 10.1038/nrgastro.2010.97 [DOI] [PubMed] [Google Scholar]
- Gao, F. H. , Liu, F. , Wei, W. , Liu, L. B. , Xu, M. H. , Guo, Z. Y. , … Wu, Y. L. (2012). Oridonin induces apoptosis and senescence by increasing hydrogen peroxide and glutathione depletion in colorectal cancer cells. International Journal of Molecular Medicine, 29, 649–655. 10.3892/ijmm.2012.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C. , Yang, M. , Jing, L. , Wang, J. , Yu, Y. , Li, Y. , … Sun, Z. (2016). Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species‐mediated MAPK/Bcl‐2 and PI3K/Akt/mTOR signaling. International Journal of Nanomedicine, 11, 5257–5276. 10.2147/IJN.S112030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, G. J. , Liao, J. C. , Chiu, C. S. , Huang, S. S. , Lin, T. H. , & Deng, J. S. (2012). Anti‐inflammatory activities of aqueous extract of Mesona procumbens in experimental mice. Journal of the Science of Food and Agriculture, 92, 1186–1193. 10.1002/jsfa.4682 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Wu, L. , Tashiro, S. , Onodera, S. , & Ikejima, T. (2008). Reactive oxygen species mediate oridonin‐induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. Journal of Pharmacological Sciences, 107, 370–379. 10.1254/jphs.08044FP [DOI] [PubMed] [Google Scholar]
- Issa, R. , Williams, E. , Trim, N. , Kendall, T. , Arthur, M. J. , Reichen, J. , … Iredale, J. P. (2001). Apoptosis of hepatic stellate cells: Involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut, 48, 548–557. 10.1136/gut.48.4.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, A. , Isoda, K. , Kondoh, M. , Kawase, M. , Kobayashi, M. , Tamesada, M. , & Yagi, K. (2009). Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a‐induced liver injury. Biological and Pharmaceutical Bulletin, 32(7), 1215–1219. 10.1248/bpb.32.1215 [DOI] [PubMed] [Google Scholar]
- Jia, Y. T. , Wei, W. , Ma, B. , Xu, Y. , Liu, W. J. , Wang, Y. , … Xia, Z. F. (2007). Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress‐induced gastric mucosal injury. Journal of Immunology, 179, 7808–7819. 10.4049/jimmunol.179.11.7808 [DOI] [PubMed] [Google Scholar]
- Jhang, J. J. , Ong, J. W. , Lu, C. C. , Hsu, C. L. , Lin, J. H. , Liao, J. W. , & Yen, G. C. (2016). Hypouricemic effects of Mesona procumbens Hemsl. through modulating xanthine oxidase activity in vitro and in vivo. Food Function, 7, 4239–4246. 10.1039/c6fo00822d [DOI] [PubMed] [Google Scholar]
- Jia, D. , Duan, F. , Peng, P. , Sun, L. , Ruan, Y. , & Gu, J. (2015). Pyrroloquinoline‐quinone suppresses liver fibrogenesis in mice. PLoS ONE, 10, e0121939 10.1371/journal.pone.0121939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Y. , Wang, F. , Guo, F. Q. , Li, M. , Wang, L. , Zhang, Z. , … Zheng, S. (2018). Curcumol induces RIPK1/RIPK3 complex‐dependent necroptosis via JNK1/2‐ROS signaling in hepatic stellate cells. Redox Biology, 19, 375–387. 10.1016/j.redox.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila, M. R. , Li, S. P. , & Westermarck, J. (2008). Phosphatase‐mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. The FASEB Journal, 22, 954–965. 10.1096/fj.06-7859rev [DOI] [PubMed] [Google Scholar]
- Kim, J. Y. , Yu, S. J. , Oh, H. J. , Lee, J. Y. , Kim, Y. , & Sohn, J. (2011). Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase‐dependent generation of reactive oxygen species. Apoptosis, 16, 347–358. 10.1007/s10495-010-0567-8 [DOI] [PubMed] [Google Scholar]
- Kuo, L. M. , Kuo, C. Y. , Lin, C. Y. , Hung, M. F. , Shen, J. J. , & Hwang, T. L. (2014). Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules, 19, 3327–3344. 10.3390/molecules19033327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, L. M. , Chen, P. J. , Sung, P. J. , Chang, Y. C. , Ho, C. T. , Wu, Y. H. , & Hwang, T. L. (2018). The bioactive extract of Pinnigorgia sp. induces apoptosis of hepatic stellate cells via ROS‐ERK/JNK‐Caspase‐3 signaling. Marine Drugs, 16, E19 10.3390/md16010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , & Kim, J. H. (2016). Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR‐related pathway in vitro. PLoS ONE, 11(5), e0155264 10.1371/journal.pone.0155264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Wang, X. , Pang, J. , Zhang, H. , Luo, J. , Qian, X. , & Ling, W. (2019). Protocatechuic acid attenuates atherosclerosis by inhibiting M1 and promoting M2 macrophage polarization. Journal of Agriculture and Food Chemistry, 67(3), 807–818. 10.1021/acs.jafc.8b05719 [DOI] [PubMed] [Google Scholar]
- Mao, X. , Yu, C. R. , Li, W. H. , & Li, W. X. (2008). Induction of apoptosis by shikonin through a ROS/JNK‐mediated process in Bcr/Abl‐positive chronic myelogenous leukemia (CML) cells. Cell Research, 18, 879–888. 10.1038/cr.2008.86 [DOI] [PubMed] [Google Scholar]
- McCubrey, J. A. , Lahair, M. M. , & Franklin, R. A. (2006). Reactive oxygen species‐induced activation of the MAP kinase signaling pathways. Antioxidants & Redox Signaling, 8, 1775–1789. 10.1089/ars.2006.8.1775 [DOI] [PubMed] [Google Scholar]
- Mederacke, I. , Hsu, C. C. , Troeger, J. S. , Huebener, P. , Mu, X. , Dapito, D. H. , … Schwabe, R. F. (2013). Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nature Communications, 4, 2823 10.1038/ncomms3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, F. R. , Issa, R. , Zhou, X. , Ratnarajah, S. , Nagase, H. , Arthur, M. J. , … Iredale, J. P. (2002). Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase‐1 is mediated via effects on matrix metalloproteinase inhibition: Implications for reversibility of liver fibrosis. Journal of Biological Chemistry, 277, 11069–11076. 10.1074/jbc.M111490200 [DOI] [PubMed] [Google Scholar]
- Parola, M. , & Robino, G. (2001). Oxidative stress‐related molecules and liver fibrosis. Journal of Hepatology, 35, 297–306. 10.1016/S0168-8278(01)00142-8 [DOI] [PubMed] [Google Scholar]
- Poli, G. , & Parola, M. (1997). Oxidative damage and fibrogenesis. Free Radical Biology & Medicine, 22, 287–305. 10.1016/S0891-5849(96)00327-9 [DOI] [PubMed] [Google Scholar]
- Puche, J. E. , Saiman, Y. , & Friedman, S. L. (2013). Hepatic stellate cells and liver fibrosis. Comprehensive Physiology, 3, 1473–1492. 10.1002/cphy.c120035 [DOI] [PubMed] [Google Scholar]
- Ray, K. (2014). Liver: Hepatic stellate cells hold the key to liver fibrosis. Nature Reviews Gastroenterology & Hepatology, 11(2), 74 10.1038/nrgastro.2013.244 [DOI] [PubMed] [Google Scholar]
- Runchel, C. , Matsuzawa, A. , & Ichijo, H. (2011). Mitogen‐activated protein kinases in mammalian oxidative stress responses. Antioxidants & Redox Signaling, 15, 205–218. 10.1089/ars.2010.3733 [DOI] [PubMed] [Google Scholar]
- Safo, M. K. , & Kato, G. J. (2014). Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin. Hematology/Oncology Clinics of North America, 28(2), 217–231. 10.1016/j.hoc.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, B. , Venditti, A. , Sharifi‐Rad, M. , Kregiel, D. , Sharifi‐Rad, J. , Durazzo, A. , & Martins, N. (2019). The therapeutic potential of apigenin. International Journal of Molecular Sciences, 20(6), E1305 10.3390/ijms20061305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund, S. V. , Qian, T. , de Minicis, S. , Harvey‐White, J. , Kunos, G. , Vinod, K. Y. , … Schwabe, R. F. (2007). The endocannabinoid 2‐arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. FASEB Journal, 21, 2798–2806. 10.1096/fj.06-7717com [DOI] [PubMed] [Google Scholar]
- Son, Y. , Kim, S. , Chung, H. T. , & Pae, H. O. (2013). Reactive oxygen species in the activation of MAP kinases. Methods in Enzymology, 528, 27–48. 10.1016/B978-0-12-405881-100002-1 [DOI] [PubMed] [Google Scholar]
- Shahzad, S. , Mateen, S. , Naeem, S. S. , Akhtar, K. , Rizvi, W. , & Moin, S. (2019). Syringic acid protects from isoproterenol induced cardiotoxicity in rats. European Journal of Pharmacology, 849, 135–145. 10.1016/j.ejphar.2019.01.056 [DOI] [PubMed] [Google Scholar]
- Szuster‐Ciesielska, A. , Mizerska‐Dudka, M. , Daniluk, J. , & Kandefer‐Szerszen, M. (2013). Butein inhibits ethanol‐induced activation of liver stellate cells through TGF‐beta, NFκB, p38, and JNK signaling pathways and inhibition of oxidative stress. Journal of Gastroenterology, 48, 222–237. 10.1007/s00535-012-0619-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y. Y. , Yan, X. C. , Zhou, T. , Shen, L. , Liu, Z. L. , & Liu, C. H. (2014). Fuzheng Huayu recipe alleviates hepatic fibrosis via inhibiting TNF‐α induced hepatocyte apoptosis. BMC Complementary and Alternative Medicine, 14, 449 10.1186/1472-6882-14-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touaibia, M. , Jean‐Francois, J. , & Doiron, J. (2011). Caffeic acid, a versatile pharmacophore: An overview. Mini Reviews in Medicinal Chemistry, 11(8), 695–713. 10.2174/138955711796268750 [DOI] [PubMed] [Google Scholar]
- Troeger, J. S. , Mederacke, I. , Gwak, G. Y. , Dapito, D. H. , Mu, X. , Hsu, C. C. , … Schwabe, R. F. (2012). Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology, 143, 1073–1083.e1022. 10.1053/j.gastro.2012.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwarzenberg, K. , & Vollmar, A. M. (2013). Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Letters, 332, 295–303. 10.1016/j.canlet.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Yang, M. , Xu, Z. , Zhang, R. , Zhang, P. , Weng, Y. , Shen, Y. , & Zhang, X. (2008). Protection of myocardium in streptozotocin‐induced diabetic rats by water extracts of Hsian‐tsao (Mesona procumbens Hemsl.). Asia Pacific Journal of Clinical Nutrition, 17, 23–29. 10.6133/apjcn.2008.17.1.04 [DOI] [PubMed] [Google Scholar]
- Yeh, C. T. , Huang, W. H. , & Yen, G. C. (2009). Antihypertensive effects of Hsian‐tsao and its active compound in spontaneously hypertensive rats. Journal of Nutritional Biochemistry, 20(11), 866–875. 10.1016/j.jnutbio.2008.07.015 [DOI] [PubMed] [Google Scholar]
- Yen, G. C. , Yeh, C. T. , & Chen, Y. J. (2004). Protective effect of Mesona procumbens against tert‐butyl hydroperoxide‐induced acute hepatic damage in rats. Journal of Agricultural and Food Chemistry, 52, 4121–4127. 10.1021/jf049840d [DOI] [PubMed] [Google Scholar]
- Yu, J. Y. , Lee, S. Y. , Son, Y. O. , Shi, X. , Park, S. S. , & Lee, J. C. (2012). Continuous presence of H(2)O(2) induces mitochondrial‐mediated, MAPK‐ and caspase‐independent growth inhibition and cytotoxicity in human gingival fibroblasts. Toxicology in Vitro, 26, 561–570. 10.1016/j.tiv.2012.01.022 [DOI] [PubMed] [Google Scholar]
- Xie, P. , Fujii, I. , Zhao, J. , Shinohara, M. , & Matsukura, M. (2016). A novel polysaccharide derived from algae extract induces apoptosis and cell cycle arrest in human gastric carcinoma MKN45 cells via ROS/JNK signaling pathway. International Journal of Oncology, 49, 1561–1568. 10.3892/ijo.2016.3658 [DOI] [PubMed] [Google Scholar]
- Zarubin, T. , & Han, J. (2005). Activation and signaling of the p38 MAP kinase pathway. Cell Research, 15, 11–18. 10.1038/sj.cr.7290257 [DOI] [PubMed] [Google Scholar]


