Abstract
Poor oral health, including caries, tooth loss, and periodontitis are ubiquitous worldwide, and are potentially treatable and preventable. Like adverse oral health conditions, Alzheimer disease and related disorders are also very common among aging populations. Established risk factors for Alzheimer disease include cerebrovascular disease and its vascular risk factors, many of which share associations with evidence of systemic inflammation also identified in periodontitis and other poor oral health states. In this review, we present epidemiologic evidence of links between poor oral health and both prevalent and incident cognitive impairment, as well as review plausible mechanisms linking these conditions including evidence from compelling animal models. Considering that a large etiologic fraction of dementia remains unexplained, these studies argue for further multidisciplinary research between oral health conditions including translational, epidemiologic, and possibly clinical treatment studies.
Keywords: Periodontitis, Periodontal disease, Tooth loss, Oral health, Caries, Dentures, Alzheimer disease, Cerebrovascular disease, Vascular dementia, Dementia, Epidemiology
INTRODUCTION
Dementia is a common disorder among the elderly that becomes more prevalent with advancing age. It is typically medically refractory, reduces life expectancy, and diminishes quality of life for patients and their caregivers. Clinical Alzheimer Disease (AD) is the most common type of dementia, representing 60–70% of all cases. The prevalence increases with age from 5% in the seventh decade to 50% by the tenth decade of life1, 2. In the US alone, an estimated 5.1 million had AD in 2010 and this number is expected to increase to 13.2 million by 2050 3. Sporadic, late onset AD (LOAD) represents 98% of all AD cases and is likely due to a complex interaction of environmental, vascular, and genetic risk factors4, 5. However, the population attributable risk (PAR) associated with each of the vascular or environmental risk factors for AD does not exceed 15%, and approximately half of the risk for AD remains unexplained6. Thus, a search for additional, potentially causal risk factors is warranted.
Cognition and dental health may be related in several ways: A compelling and rather straight-forward argument can be made for cognitive impairment leading to poor dental health. That is, persons with impaired cognition may be expected to be inattentive to oral hygiene or may have restricted access to routine oral care as cognitive disease progresses,7 and thus have worse dental health. Conversely, worth considering is whether poor oral health could instead be an antecedent condition, possibly contributing to subsequent cognitive impairment as a causal exposure, rather than as an outcome. Irrespective on the directionality of the association, here we briefly review the literature associating poor dental health and stroke, as it has been more fully reviewed elsewhere. Our focus is on the relationship between poor dental health and cognitive impairment, dementia, and Alzheimer disease, particularly emphasizing periodontal disease given its associations with systemic health.
EPIDEMIOLOGY OF POOR ORAL HEALTH AND PERIODONTAL DISEASE
Poor oral health, including periodontitis, caries, edentulism, and infrequent preventive care, become more prevalent among older people8, 9.Edentulism is a global health problem in the elderly with prevalence as high as 78% in some European countries; persons with low socioeconomic status are disproportionately affected10, 11. Caries and periodontitis are thought to be two predominant causes of tooth loss, often co-occurring within individuals, and share several risk factors including poor oral hygiene, low socioeconomic status, and inattention to care. However, of these oral health states, periodontitis is more common among adults and progresses with age.12, 13
Worldwide estimates in the prevalence of periodontitis vary, partly because of substantial heterogeneity in definitions of the disease14. Clinical and serological markers of disease indicate that moderate to severe periodontitis may be prevalent in more than 50% of US adults.15, 16 Exposure to established periodontal pathogens appears to begin as early as at 2 years of age, with a large proportion of the population exposed by adolescence17, often by vertical and horizontal transmission patterns among family members.18 While clinical markers of periodontitis may vary with time or treatment, periodontal serum IgG levels typically remain stable.19
POOR DENTAL HEALTH AND CEREBROVASCULAR DISEASE
Prior to first explorations relating dental health to frank cognitive impairment, a number of studies explored the relationship between history of periodontal disease and incident stroke,20, 21 with associations identified as ranging from no adverse risk to more than double. Epidemiological evidence supports an association between the level of serum antibodies to periodontal pathogens and stroke 22–24 and accelerated aortic atherogenesis,25 while high levels of colonization by specific periodontal pathogens has been associated with increased carotid artery intimal-medial thickness.26 To date, no treatment trials addressing mitigation of periodontal disease and incident stroke have been performed.
Many of the risk factors associated with cerebrovascular disease are also associated with dementia. For example, vascular risk factors27, 28 including diabetes mellitus,28–33 dyslipidemia,34 hypertension,35 atrial fibrillation,36 smoking,28, 37–39 hyperhomocysteinemia,40 and obesity41–45 have been associated with the development of dementia, including AD. Up to 33% of dementia patients with AD pathology have concomitant stroke46 and patients may be more likely to become demented when both AD pathology and cerebrovascular disease are present47, 48.
POOR DENTAL HEALTH AND COGNITIVE IMPAIRMENT
Poor oral hygiene
Inattention to oral health care may be a precursor to many oral health diseases and could be a longstanding habituation among individuals, or alternatively could change in advancing age for various reasons including impaired physical movements, with or without cognitive impairment being implicated. In a subgroup of the Geriatric Multidisciplinary Strategy for the Good Care of the Elderly (Gems), drawn from a population of those aged 75 years and above in eastern Finland, AD was associated with both poor oral hygiene (OR=12.2 [1.9–77.0]) and poor denture hygiene (OR=2.9 [1.1–7.8]).49 In the Aichi Gerontological Evaluation Study (AGES) Project, among older Japanese aged 65 years and above, those without regular dental visits were more likely to have incident dementia (HR=1.44 [1.04–2.01 ]).50 Exploratory analyses within a small clinical trial examining the effect of dental care on incident pneumonia in a group of elderly nursing home residents demonstrated a 1.5 point significantly slower decline in Folstein Mini Mental Status Exam score after 2 years in oral care recipients51. However, from this study one cannot determine whether the effect specifically related to the dental hygiene intervention, or was instead simply related to greater frequency of attention to general health needs in the intervention subjects.
Caries
Caries is the most common cause of tooth loss in younger patients and thought to be caused by acid-producing oral microbiota, in individuals with poor oral hygiene and frequent intake sugar-rich diet the context of chronic poor attention to dental hygiene.52, 53 In contrast to periodontitis, however, caries is not typically thought to cause a systemic host inflammatory response. Several case-control studies have identified caries in older adults and cross-sectional associations with impaired cognition; 54, 55 these findings have been corroborated in larger community based cohorts. In the Gems cohort, caries was associated with Alzheimer disease (RR=2.8 [1.8–4.5] as well as non-AD dementia (RR=3.4 [1.9–6.4]);49 another Finnish cohort of older adults identified similar patterns of increased caries rates being associated with cognitive impairment.56 A case-control study of elderly Australians found that dementia patients were more likely to have declining oral health including worsening caries when followed longitudinally.57
Tooth loss
Tooth loss reflects the end stage of a several oral diseases, alone or in combination, including caries, periodontal disease, and endodontic infections. Thus, tooth loss is a marker of cumulative morbidity of pathologic oral inflammatory conditions including periodontitis, and has been associated with prevalent cognitive impairment in several cross-sectional studies. In the Health Survey for England 2000, an association between edentulism and cognitive impairment among population aged 65 years and older was strong (OR=2.61 [1.49–4.28]), although it was primarily driven by community based subjects, as no relationship was identified among those residing in nursing homes.58 The Study of Health in Pomerania in northeast Germany suggested that women may be at greater risk for having tooth loss associated with prevalent cognitive impairment.59 Several studies drawn from Asian populations have found similar associations between tooth loss and prevalent cognitive impairment, including a small Japanese case-control study of late-life tooth loss.60 In the Fujiwara-kyo study, a large community-based study of persons aged 65 years and older, those with the fewest teeth had the highest risk of cognitive impairment (OR=1.71 [1.05–2.78]); in addition, there was a significant trend for fewer teeth predicting cognitive impairment.61 However, other cross-sectional studies exploring dental health and cognition have either failed to identify edentulism as a risk for dementia49 or found only a weak association with cognitive impairment.62
Several studies have identified tooth loss as a risk for incident cognitive impairment. In the VA Dental Longitudinal Study, community-dwelling men with tooth loss were more likely to have impaired cognitive test performance, with those over the age of 45 being more significantly affected.63 In the HARMONY Swedish twin registry, dementia was associated with mid-life tooth loss (OR=1.49 [1.14–1.95]).64 In the United States, in the Religious Orders Study, fewer teeth in adulthood were significantly associated with incident dementia (HR 2.2 (1.1–4.5).65 APOE-£4 appeared to be a significant effect modifier in this relationship, with impaired memory developing at a younger age among APOE-ε4 carriers, particularly those with fewer teeth in adulthood.66
Impaired chewing ability and dentures
Patients with tooth loss, even when given dentures, have inadequate chewing capacity (low masticatory efficiency); a reported maximum load on natural teeth during chewing is 8 to 15 kilograms 67 while a typical load by sustained dentures is less than 2 kilograms.68 In the Swedish Panel Study of Living Conditions of the Oldest Old people (SWEOLD), a national sample of elders aged 77 and above, those with impaired chewing ability were more likely to be cognitively impaired (OR=1.72 [1.05–2.80]).69 In a prospective study of community dwelling elderly residents in Kwangju, South Korea, those persons with tooth loss and no dentures were most likely to develop dementia (OR=1.61, [1.02–2.49])70 and this was additionally corroborated in AGES (HR=1.85, [1.04–3.31]).50
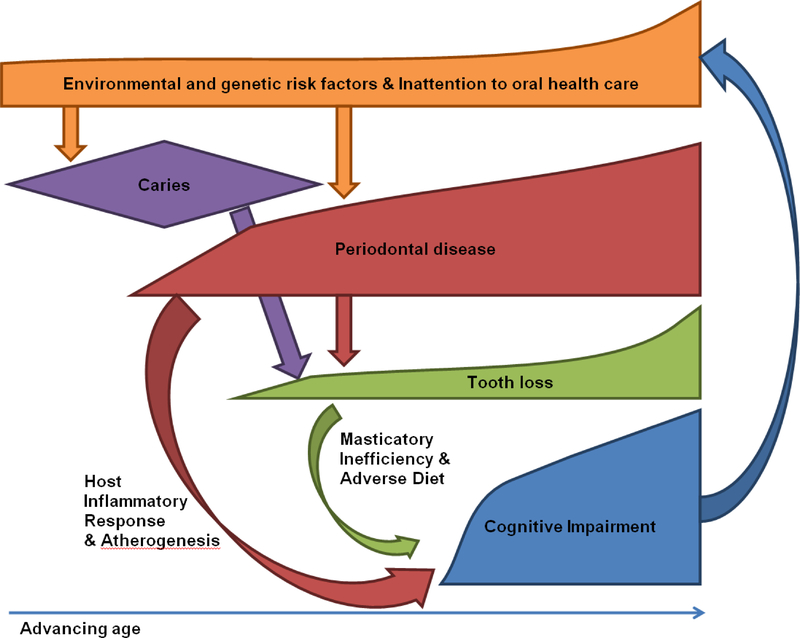
Persons with low masticatory efficiency may have to adapt to eat diets low in fiber and essential micronutrients,71 and high in saturated fats and cholesterol, possibly due to ease of chewing these foods relative to fiber-rich foods.72 Such dietary changes, adaptive to low masticatory efficiency, could potentially increase the risk for stroke and dementia by making difficult adherence to diets thought to be protective against AD such as the “Mediterranean diet” 73, 74. However, dietary habits may instead be confounded by lifelong influences of taste, economics, social norms, or unhealthy lifestyle decisions associated with tooth loss.75, 76 Micronutrient deficiencies, such as vitamin B12 and thiamine deficiency, may also develop as a result of edentulism 77 and may contribute to cognitive impairment (Fig 1).
Figure 1.
Proposed pathway associating poor oral health and cognitive impairment.
Periodontitis
Periodontitis is a chronic oral biofilm-mediated infection78, 79 that is strongly associated with tooth loss in adults. Several studies have associated periodontitis with prevalent and incident cognitive impairment. In the VA Dental Longitudinal Study, for each tooth lost, pocket depth progression and alveolar bone loss progression were additionally associated with impaired cognitive test performance, with the strongest associations observed in individuals over 45 years of age.63 We identified a cross-sectional association between a serological marker of a common periodontitis pathogen (Porphyromonas gingivalis) and poor cognitive test performance among patients aged >60 years in NHANES-III.80 Our group additionally identified serum antibodies to several periodontal pathogens were associated with incident AD in a complex relationship including adverse or protective risk, depending upon the pathogen studied.81 Serum antibodies to several other periodontal organisms have been identified as potential risk markers for AD and mild cognitive impairment.82 In an age-matched case-control study of AD patients and healthy elders, serum levels of TNF-alpha and IgG antibodies to periodontal bacteria discriminated between the two groups at the time of cognitive diagnosis.83
Pathophysiologic links between periodontitis and cognitive impairment
From a neuropathological standpoint, AD is a progressive neurodegenerative process initially related to accumulation of excess brain amyloid-beta (Aβ) protein and subsequent tau deposition84. Amyloid metabolism is complex and is influenced by local and systemic host inflammatory mediators. These include interactions between advanced glycation end products (AGEs) and their receptor RAGE that affect transduction of extra-cellular Aβ and influx of vascular Aβ, leading to increased intracellular Aβ84.
Periodontitis is currently considered to impact overall health via complex mechanisms mediated through a state of enhanced systemic inflammation21. Periodontitis is associated with both a local85 and a systemic inflammatory response characterized by elevation in multiple serum cytokines including interleukin-1 (IL-1),86 IL-6,87, 88 C-reactive protein (CRP),87, 89 and TNF-alpha90–92 and generation of serum antibodies to common periodontal organisms15, 93. Several hundred periodontal pathogens have been implicated in causing periodontal disease,94 although only a small fraction of these hundreds of pathogens are cultivable and have been demonstrably associated with establishing a progressively pathogenic milieu at the biofilm interface.78 Gingival tissues of patients with periodontitis express high levels of AGEs and RAGE95, and AGE-RAGE interactions are one of the key mechanisms underlying the observed accelerated periodontal tissue breakdown in patients with diabetes96. Importantly, treatment of periodontitis has been associated with significant reduction in serum levels of IL-687, IL-6 soluble receptor97 and CRP98, and a substantial improvement in vascular endothelial function97, 99–101. Thus, although several specific periodontal pathogens have been specifically studied in the relationship between periodontitis and stroke or cognitive impairment, it is more likely that downstream systemic inflammatory responses affect this relationship, rather than individual pathogens.
In addition to stroke, periodontitis has been associated with risk factors for cardiovascular disease25, 102–105 and diabetes.106–108 Risk factors for stroke and dementia, including diabetes,109, 110 obesity,111 and smoking112 have a similar systemic inflammatory profile to periodontitis,112, 113 suggesting that inflammatory markers may contribute to a final common pathway of impaired cognition, perhaps mediated through a cascade of atherogenesis114, 115 related to systemic inflammation.113 Interestingly, high levels of CRP have also been reported to act as an effect modifier of the association between carriage of the APOE e4 allele and memory impairment in patients without dementia.116
ANIMAL MODELS RELATING POOR ORAL HEALTH AND COGNITIVE IMPAIRMENT
As detailed above, numerous human epidemiologic studies have identified both cross sectional and longitudinal relationships between poor oral health and cognitive impairment. Although these reports can perhaps validate hypotheses of associations, one must turn to other studies, such as animal, biochemical, and pathologic models to begin to understand possible causal links. Moreover, given overlapping adverse oral health conditions, animal models may provide an opportunity to determine which adverse oral health condition may be most specifically associated with cognitive impairment, as well as identify possible treatment paradigms.
To date, several animal models have started to explore these associations in both experimental tooth loss and periodontitis. Young rats made surgically edentulous and fed nutritionally identical powder, rather than pelletized foods, were significantly more likely than dentate rats to have poor spatial memory and decreased stimulated acetylcholine release in the parietal cortex,117 the mechanisms for this relationship were unclear but proposed to relate to decreased mastication-induced sensory stimulation leading to degeneration of secondary neurons in the spatial pathway of the alveolar and trigeminal nerves,118, 119 and through downstream cortical-brainstem circuits, contribute to diminished cortical cholinergic function. A subsequent rat model which found similar clinical findings validated some elements of this earlier model, through identification of hippocampal neuronal loss and decreased trkB-mRNA expression suggesting decreased hippocampal synaptic transmission.120
Subsequent amyloidogenic mouse models have suggested experimental edentulism induces hippocampal neural cell loss,121, 122 alters astroglial behavior in the hippocampus, 121, 123 and changes hippocampal gene expression.124 Experimental tooth loss in another mouse model over-expressing amyloid precursor protein has been associated with decreased numbers of pyramidal cells in CA1 and CA3 hippocampal subregions, but without significant associated changes in histopathologic or soluble amyloid.125 More recently, an amyloidogenic mouse model with experimental P. gingivalis periodontitis (non-edentulous mice) developed impaired memory and significantly increased hippocampus and whole brain amyloid plaque loads, as well as elevated brain IL-1β and TNF-α;126 peripheral measures of systemic inflammation or periodontal antibodies were not measured. Taken together, these models shed light on plausible causal links between poor oral health dementia, including the possibility of different causal pathways between tooth loss and periodontitis and cognitive impairment.
CONCLUSIONS
Poor oral health, including tooth loss, caries, and periodontal disease, may be an unrecognized risk factor contributing to the development of cognitive impairment through dietary changes, malnutrition, and a systemic inflammatory response associated with increased risk of stroke and AD. This growing body of evidence justifies further, multimodal exploration including periodontal disease burden, clinical oral health markers, and systemic host response to better understand the possible contribution of clinical, microbiologic and serologic markers of periodontal infection to a potential causal pathway for cognitive impairment among the elderly.
Acknowledgements
James M. Noble has received grant support from Taub Institute (internal funding mechanism) as some of the prior research described was supported through this grant. This manuscript was not directly supported by any means; he also received grant support from NIDCR; as co-PI An R56/R01 study has been under review by the NIDCR since May 2012, proposing to study links between periodontitis and AD longitudinally, but no funding as yet.
Nikolaos Scarmeas has received grant support from US Federal NIA grant AG028506, and Alzheimer’s Association grant IIRG-04-1353.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
James M. Noble has received honoraria from Barclays for a single consulting event regarding IVIg as an AD therapeutic (trial has since been reported as failure).
Nikolaos Scarmeas declares that he has no conflict of interest.
Panos N. Papapanou declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 1999;14:481–493. [PubMed] [Google Scholar]
- 2.Mayeux R Epidemiology of neurodegeneration. Annu Rev Neurosci 2003;26:81–104. [DOI] [PubMed] [Google Scholar]
- 3.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60:1119–1122. [DOI] [PubMed] [Google Scholar]
- 4.Mayeux R Apolipoprotein E, Alzheimer disease, and African Americans. Arch Neurol 2003;60:161–163. [DOI] [PubMed] [Google Scholar]
- 5.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 1998;279:751–755. [DOI] [PubMed] [Google Scholar]
- 6.Barnes DE, Yaffe K The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819–828.• This review places into perspective the etiologic fraction of several lifestyle and vascular risk factors for AD and underscores the impact that idenfication and elimiation of others factors, such as oral health, could have on disease prevalence.
- 7.Noble JM, Scarmeas N. Cognitive Impairment In: Lamster IB, Northridge ME, eds. Improving Oral Health for the Elderly: Springer Books; USA, 2008. [Google Scholar]
- 8.Miller AJ, Brunelle JA, Carlos JP, Brown LJ, Loe H. Oral Health of United States Adults. Bethesda, MD: U.S. Department of Health & Human Services, Public Health Service, National Institutes of Health, 1987. [Google Scholar]
- 9.Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol 1990;61:521–528. [DOI] [PubMed] [Google Scholar]
- 10.Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol 2003;31 Suppl 1:3–23. [DOI] [PubMed] [Google Scholar]
- 11.Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol 2005;33:81–92. [DOI] [PubMed] [Google Scholar]
- 12.Machtei EE, Hausmann E, Dunford R, et al. Longitudinal study of predictive factors for periodontal disease and tooth loss. Journal of clinical periodontology 1999;26:374–380. [DOI] [PubMed] [Google Scholar]
- 13.Rosling B, Serino G, Hellstrom MK, Socransky SS, Lindhe J. Longitudinal periodontal tissue alterations during supportive therapy. Findings from subjects with normal and high susceptibility to periodontal disease. Journal of clinical periodontology 2001;28:241–249. [DOI] [PubMed] [Google Scholar]
- 14.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000 2010;53:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol 2007;78:1911–1925. [DOI] [PubMed] [Google Scholar]
- 16.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012;91:914–920.• A thorough appraisal of current epidemiolgic estimates of periodontitis.
- 17.Kulekci G, Leblebicioglub B, Keskina F, Ciftcia S, Badurc S. Salivary detection of periodontopathic bacteria in periodontally healthy children. Anaerobe 2007. [DOI] [PubMed] [Google Scholar]
- 18.Preus HR, Zambon JJ, Dunford RG, Genco RJ. The distribution and transmission of Actinobacillus actinomycetemcomitans in families with established adult periodontitis. J Periodontol 1994;65:2–7. [DOI] [PubMed] [Google Scholar]
- 19.Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, Dahlen G. Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. Journal of clinical periodontology 2004;31:985–990. [DOI] [PubMed] [Google Scholar]
- 20.Joshipura K, Zevallos JC, Ritchie CS. Strength of evidence relating periodontal disease and atherosclerotic disease. Compend Contin Educ Dent 2009;30:430–439. [PMC free article] [PubMed] [Google Scholar]
- 21.Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!”-- epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res 2010;89:879–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis 2007;193:222–228. [DOI] [PubMed] [Google Scholar]
- 23.Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. Antibodies to periodontal pathogens and stroke risk. Stroke 2004;35:2020–2023. [DOI] [PubMed] [Google Scholar]
- 24.Johansson A, Johansson I, Eriksson M, Ahren AM, Hallmans G, Stegmayr B. Systemic antibodies to the leukotoxin of the oral pathogen Actinobacillus actinomycetemcomitans correlate negatively with stroke in women. Cerebrovasc Dis 2005;20:226–232. [DOI] [PubMed] [Google Scholar]
- 25.Ford PJ, Gemmell E, Timms P, Chan A, Preston FM, Seymour GJ. Anti-P. gingivalis response correlates with atherosclerosis. J Dent Res 2007;86:35–40. [DOI] [PubMed] [Google Scholar]
- 26.Desvarieux M, Demmer RT, Rundek T, et al. Periodontal microbiota and carotid intimamedia thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 2005;111:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer’s disease. Curr Atheroscler Rep 2004;6:261–266. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 1997;145:301–308. [DOI] [PubMed] [Google Scholar]
- 30.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999;53:1937–1942. [DOI] [PubMed] [Google Scholar]
- 31.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262. [DOI] [PubMed] [Google Scholar]
- 32.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 2001;154:635–641. [DOI] [PubMed] [Google Scholar]
- 33.Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 2011;31:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet 2000;356:1627–1631. [DOI] [PubMed] [Google Scholar]
- 35.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 36.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 1997;349:151–154. [DOI] [PubMed] [Google Scholar]
- 37.Ott A, Slooter AJ, Hofman A, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet 1998;351:1840–1843. [DOI] [PubMed] [Google Scholar]
- 38.Merchant C, Tang MX, Albert S, Manly J, Stern Y, Mayeux R. The influence of smoking on the risk of Alzheimer’s disease. Neurology 1999;52:1408–1412. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident Alzheimer’s disease in a biracial urban community population. Neuroepidemiology 2006;26:140–146. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002;346:476–483. [DOI] [PubMed] [Google Scholar]
- 41.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med 2003;163:1524–1528. [DOI] [PubMed] [Google Scholar]
- 42.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr., Yaffe K Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj 2005;330:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 2005;165:321–326. [DOI] [PubMed] [Google Scholar]
- 44.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol 2000;20:2255–2260. [DOI] [PubMed] [Google Scholar]
- 45.Gorospe EC, Dave JK. The risk of dementia with increased body mass index: a systematic review. Age Ageing 2006. [DOI] [PubMed] [Google Scholar]
- 46.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology 2005;64:494–500. [DOI] [PubMed] [Google Scholar]
- 47.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA 2004;292:2901–2908. [DOI] [PubMed] [Google Scholar]
- 48.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Annals of neurology 2007;61:403–410. [DOI] [PubMed] [Google Scholar]
- 49.Syrjala AM, Ylostalo P, Ruoppi P, et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology 2012;29:36–42. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association between selfreported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) Project. Psychosom Med 2012;74:241–248. [DOI] [PubMed] [Google Scholar]
- 51.Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc 2002;50:430–433. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res 2011;90:294–303. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res 2008;42:409–418. [DOI] [PubMed] [Google Scholar]
- 54.Ship JA. Oral health of patients with Alzheimer’s disease. J Am Dent Assoc 1992;123:53–58. [DOI] [PubMed] [Google Scholar]
- 55.Jones JA, Lavallee N, Alman J, Sinclair C, Garcia RI. Caries incidence in patients with dementia. Gerodontology 1993;10:76–82. [DOI] [PubMed] [Google Scholar]
- 56.Syrjala AM, Ylostalo P, Sulkava R, Knuuttila M. Relationship between cognitive impairment and oral health: results of the Health 2000 Health Examination Survey in Finland. Acta Odontol Scand 2007;65:103–108. [DOI] [PubMed] [Google Scholar]
- 57.Chalmers JM, Carter KD, Spencer AJ. Caries incidence and increments in communityliving older adults with and without dementia. Gerodontology 2002;19:80–94. [DOI] [PubMed] [Google Scholar]
- 58.Stewart R, Hirani V. Dental health and cognitive impairment in an English national survey population. J Am Geriatr Soc 2007;55:1410–1414. [DOI] [PubMed] [Google Scholar]
- 59.Grabe HJ, Schwahn C, Volzke H, et al. Tooth loss and cognitive impairment. Journal of clinical periodontology 2009;36:550–557. [DOI] [PubMed] [Google Scholar]
- 60.Kondo K, Niino M, Shido K. A case-control study of Alzheimer’s disease in Japan--significance of life-styles. Dementia 1994;5:314–326. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto N, Morikawa M, Okamoto K, et al. Tooth loss is associated with mild memory impairment in the elderly: the Fujiwara-kyo study. Brain Res 2010;1349:68–75. [DOI] [PubMed] [Google Scholar]
- 62.Avlund K, Holm-Pedersen P, Morse DE, Viitanen M, Winblad B. Tooth loss and caries prevalence in very old Swedish people: the relationship to cognitive function and functional ability. Gerodontology 2004;21:17–26. [DOI] [PubMed] [Google Scholar]
- 63.Kaye EK, Valencia A, Baba N, Spiro A 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc 2010;58:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gatz M, Mortimer JA, Fratiglioni L, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement 2006;2:110–117. [DOI] [PubMed] [Google Scholar]
- 65.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc 2007;138:1314–1322; quiz 1381–1312. [DOI] [PubMed] [Google Scholar]
- 66.Stein PS, Kryscio RJ, Desrosiers M, Donegan SJ, Gibbs MB.•• Tooth loss, apolipoprotein E, and decline in delayed word recall. J Dent Res 2010;89:473–477. A rather compelling epidemiologic study of tooth loss predicting memory loss, including APOE effect modification, within a well described aging cohort (Religious Orders Study).
- 67.Anderson DJ. Measurement of stress in mastication. I. J Dent Res 1956;35:664–670. [DOI] [PubMed] [Google Scholar]
- 68.Yurkstas A The effect of masticatory exercise on the maximum force tolerance of individual teeth. J Dent Res 1953;32:322–327. [DOI] [PubMed] [Google Scholar]
- 69.Lexomboon D, Trulsson M, Wardh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc 2012;60:1951–1956. [DOI] [PubMed] [Google Scholar]
- 70.Kim JM, Stewart R, Prince M, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry 2007;22:850–855. [DOI] [PubMed] [Google Scholar]
- 71.Sheiham A, Steele J. Does the condition of the mouth and teeth affect the ability to eat certain foods, nutrient and dietary intake and nutritional status amongst older people? Public Health Nutr 2001;4:797–803. [DOI] [PubMed] [Google Scholar]
- 72.Walls AW, Steele JG, Sheiham A, Marcenes W, Moynihan PJ. Oral health and nutrition in older people. J Public Health Dent 2000;60:304–307. [DOI] [PubMed] [Google Scholar]
- 73.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean Diet, Alzheimer Disease, and Vascular Mediation. Arch Neurol 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Annals of neurology 2006;59:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krall EA, Garvey AJ, Garcia RI. Alveolar bone loss and tooth loss in male cigar and pipe smokers. J Am Dent Assoc 1999;130:57–64. [DOI] [PubMed] [Google Scholar]
- 76.Services USDoHaH. Oral Health in America: A Report of the Surgeon General. In: Services DoHaH, ed. Rockville, MD: National Institutes of Health, National Institute of Dental and Craniofacial Research, 2000. [Google Scholar]
- 77.Hutton B, Feine J, Morais J. Is there an association between edentulism and nutritional state? J Can Dent Assoc 2002;68:182–187. [PubMed] [Google Scholar]
- 78.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. Journal of clinical periodontology 1998;25:134–144. [DOI] [PubMed] [Google Scholar]
- 79.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews 2002;15:167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noble JM, Borrell LN, Papapanou PN, Elkind MS, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry 2009;80:1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noble J, Scarmeas N, Celentia R, et al. Serum Antibodies to Periodontal Pathogens are associated with Incident Alzheimer Disease [ABSTRACT]. Alzheimer’s & Dementia 2012;8:498. [Google Scholar]
- 82.Sparks Stein P, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement 2012;8:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamer AR, Craig RG, Pirraglia E, et al. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol 2009;216:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med 2010;362:329–344. [DOI] [PubMed] [Google Scholar]
- 85.Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res 2007;86:306–319. [DOI] [PubMed] [Google Scholar]
- 86.Bodet C, Chandad F, Grenier D. Porphyromonas gingivalis-induced inflammatory mediator profile in an ex vivo human whole blood model. Clin Exp Immunol 2006;143:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res 2004;83:156–160. [DOI] [PubMed] [Google Scholar]
- 88.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 2000;71:1528–1534. [DOI] [PubMed] [Google Scholar]
- 89.Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol 2000;151:273–282. [DOI] [PubMed] [Google Scholar]
- 90.Roberts FA, McCaffery KA, Michalek SM. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res 1997;76:1833–1839. [DOI] [PubMed] [Google Scholar]
- 91.Murata T, Miyazaki H, Senpuku H, Hanada N. Periodontitis and serum interleukin-6 levels in the elderly. Jpn J Infect Dis 2001;54:69–71. [PubMed] [Google Scholar]
- 92.Bretz WA, Weyant RJ, Corby PM, et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc 2005;53:1532–1537. [DOI] [PubMed] [Google Scholar]
- 93.Dye BA, Choudhary K, Shea S, Papapanou PN. Serum antibodies to periodontal pathogens and markers of systemic inflammation. Journal of clinical periodontology 2005;32:1189–1199. [DOI] [PubMed] [Google Scholar]
- 94.Colombo AP, Boches SK, Cotton SL, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol 2009;80:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmidt AM, Weidman E, Lalla E, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res 1996;31:508–515. [DOI] [PubMed] [Google Scholar]
- 96.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 2011;7:738–748. [DOI] [PubMed] [Google Scholar]
- 97.Elter JR, Hinderliter AL, Offenbacher S, et al. The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J 2006;151:47. [DOI] [PubMed] [Google Scholar]
- 98.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C- reactive protein in relation to periodontitis. Journal of clinical periodontology 2008;35:277–290. [DOI] [PubMed] [Google Scholar]
- 99.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007;356:911–920. [DOI] [PubMed] [Google Scholar]
- 100.Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 2005;149:1050–1054. [DOI] [PubMed] [Google Scholar]
- 101.Mercanoglu F, Oflaz H, Oz O, et al. Endothelial dysfunction in patients with chronic periodontitis and its improvement after initial periodontal therapy. J Periodontol 2004;75:1694–1700. [DOI] [PubMed] [Google Scholar]
- 102.Armitage GC. Periodontal infections and cardiovascular disease--how strong is the association? Oral Dis 2000;6:335–350. [DOI] [PubMed] [Google Scholar]
- 103.Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:559–569. [DOI] [PubMed] [Google Scholar]
- 104.Cueto A, Mesa F, Bravo M, Ocana-Riola R. Periodontitis as risk factor for acute myocardial infarction. A case control study of Spanish adults. J Periodontal Res 2005;40:36–42. [DOI] [PubMed] [Google Scholar]
- 105.Behle JH, Papapanou PN. Periodontal infections and atherosclerotic vascular disease: an update. Int Dent J 2006;56:256–262. [DOI] [PubMed] [Google Scholar]
- 106.Takeda M, Ojima M, Yoshioka H, et al. Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. J Periodontol 2006;77:15–20. [DOI] [PubMed] [Google Scholar]
- 107.Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 1990;121:532–536. [DOI] [PubMed] [Google Scholar]
- 108.Lalla E, Kaplan S, Chang SM, et al. Periodontal infection profiles in type 1 diabetes. Journal of clinical periodontology 2006;33:855–862. [DOI] [PubMed] [Google Scholar]
- 109.Craft S Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiology of aging 2005;26 Suppl 1:65–69. [DOI] [PubMed] [Google Scholar]
- 110.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS letters 2008;582:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation 2007;14:375–387. [DOI] [PubMed] [Google Scholar]
- 112.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest 2007;131:1557–1566. [DOI] [PubMed] [Google Scholar]
- 113.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 114.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annual review of pathology 2006;1:297–329. [DOI] [PubMed] [Google Scholar]
- 115.DeGraba TJ. Immunogenetic susceptibility of atherosclerotic stroke: implications on current and future treatment of vascular inflammation. Stroke 2004;35:2712–2719. [DOI] [PubMed] [Google Scholar]
- 116.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C- reactive protein with cognitive impairment. Arch Neurol;67:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kato T, Usami T, Noda Y, Hasegawa M, Ueda M, Nabeshima T. The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behav Brain Res 1997;83:239–242. [DOI] [PubMed] [Google Scholar]
- 118.Gobel S An electron microscopic analysis of the trans-synaptic effects of peripheral nerve injury subsequent to tooth pulp extirpations on neurons in laminae I and II of the medullary dorsal horn. J Neurosci 1984;4:2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gobel S, Binck JM. Degenerative changes in primary trigeminal axons and in neurons in nucleus caudalis following tooth pulp extirpations in the cat. Brain Res 1977;132:347–354. [DOI] [PubMed] [Google Scholar]
- 120.Yamazaki K, Wakabayashi N, Kobayashi T, Suzuki T. Effect of tooth loss on spatial memory and trkB-mRNA levels in rats. Hippocampus 2008;18:542–547. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe K, Tonosaki K, Kawase T, et al. Evidence for involvement of dysfunctional teeth in the senile process in the hippocampus of SAMP8 mice. Exp Gerontol 2001;36:283–295. [DOI] [PubMed] [Google Scholar]
- 122.Onozuka M, Watanabe K, Mirbod SM, et al. Reduced mastication stimulates impairment of spatial memory and degeneration of hippocampal neurons in aged SAMP8 mice. Brain Res 1999;826:148–153. [DOI] [PubMed] [Google Scholar]
- 123.Onozuka M, Watanabe K, Nagasaki S, et al. Impairment of spatial memory and changes in astroglial responsiveness following loss of molar teeth in aged SAMP8 mice. Behav Brain Res 2000;108:145–155. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe K, Ozono S, Nishiyama K, et al. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav Brain Res 2002;128:19–25. [DOI] [PubMed] [Google Scholar]
- 125.Oue H, Miyamoto Y, Okada S, Koretake K, Michikawa M, Akagawa Y. Tooth loss induces memory impairment and neuronal cell loss in APP transgenic mice. Behav Brain Res 2013. [DOI] [PubMed] [Google Scholar]
- 126.Ishida N, Ishihara Y, Ishida K, et al. Periodontitis Induced by Bacterial Infection Exacerbates Features of Alzheimer’s disease in Transgenic Mice [ABSTRACT]. In. Alzheimer’s Association International Conference, Boston MA, July 17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]