Abstract
Background/Aim:
Helicobacter pylori (H. pylori) infection is a well-known risk factor for gastric cancer. Toll-like receptor 9 (TLR9) plays an important role in many cancers and is important for immunity to H. pylori infection. Thus, the present study aimed to evaluate the influence of H. pylori on TLR9 and explore its roles in gastric cancer.
Materials and Methods:
TLR9 expression in MKN45 cells, which were cocultured with or without H. pylori or H. pylori DNA, was detected using quantitative reverse transcription-polymerase chain reaction and Western blot assays. Then, TLR9 was knocked down through RNA interference technology in MKN45 cells. Cell Counting Kit-8 assay was performed to investigate cell proliferation, and the Transwell system was established to test the migrative and invasive abilities of MKN45 cells.
Results:
H. pylori infection or H. pylori DNA level was positively correlated with TLR9 upregulation in MKN45 cells. In vitro, H. pylori DNA significantly accelerated cell proliferation and promoted the migration and invasion in MKN45 cells. In contrast, the knockdown of TLR9 significantly suppressed cell proliferation and inhibited the migration and invasion in MKN45 cells.
Conclusions:
The present results suggest that the H. pylori DNA/TLR9-signaling pathway plays an important role in gastric cancer, which might be a potential therapeutic target.
Keywords: Gastric cancer, H. pylori, invasion, proliferation, toll-like receptor 9
INTRODUCTION
As a common gram-negative pathogen, the consistent infection of Helicobacter pylori (H. pylori) has been demonstrated to be closely correlated with chronic gastritis and peptic ulcers, and a strong cause of gastric cancer.[1] According to previous reports, approximately 80% of gastric cancers and 5.5% of all malignancies could be attributed to H. pylori infection.[2] Thus, the World Health Organization has classified H. pylori as one of the class I carcinogens in 1994.[3] At present, gastric cancer remains one of the most common malignancies and the leading cause of cancer-related deaths.[4] Unfortunately, the mechanism underlying gastric cancer caused by H. pylori infection has not been fully uncovered.
Toll-like receptors (TLRs) are evolutionarily conserved transmembrane proteins that can orchestrate host immune responses targeting pathogens via selective recognition of pathogen-associated molecules and mediate pathogen–epithelium interactions.[5,6] In human beings, researchers have found 10 TLRs that participate in the progress of microbial pathogen recognition and innate immunity initiation.[7] TLR9 is an endosome-bound, transmembrane receptor that can recognize and target hypo-methylated CpG motifs (being abundant in the DNA of bacteria and viruses).[8] When infection occurs, the DNA can be released actively, or due to the degradation from invading microbes or injured host cells.[9] By recognizing these aberrant DNAs, TLR9 is activated and triggers alterations in cellular redox balance, as well as the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa-B. These increase the production of inflammatory mediators, thereby causing a much higher risk for chronic inflammatory diseases and cancer.[10]
Recently, a large body of evidence indicated that TLRs play an important role in tumorigenesis and metastasis. For example, TLR2 and TLR9 have been proven to be able to promote the migration of breast cancer cells.[11,12] Moreover, TLR9 signaling could also promote cellular migrative and invasive abilities in prostate cancer and oral cancer.[13,14]
In gastric cancer, several studies have reported that the upregulation of TLR9 may play a role in the progression of gastric cancer.[15,16,17] However, there is limited knowledge on the mechanism of the upregulation of TLR9 and the roles of TLR9 in gastric cancer. Thus, the present study aimed to evaluate the influence of H. pylori on the expression and functions of TLR9 in gastric cancer.
MATERIALS AND METHODS
Culture and isolation of Helicobacter pylori DNA
H. pylori 26695 (ATCC) was cultured in Columbia agar (Guangdong Huankai Microbial Technology Co., Ltd.) with the addition of 5% sheep blood (Oxoid). Bacterial cells were cultured in a microaerophilic chamber, containing 10% CO2, 5% O2, and 85% N2, at 37°C. After 48-h culture on plates coated with Columbia agar, bacterial cells were carefully washed with phosphate buffer solution (PBS) and collected. Finally, these bacterial cells were centrifugated and resuspended in PBS buffer (pH 7.4) for use on the following experiments. A Wizard genomic DNA purification kit (Promega, Madison, WI, USA) was used to extract the genomic DNA of H. pylori 26695. In order to remove the bacterial lipopolysaccharide, the genomic DNA was treated with polymyxin B (Sigma-Aldrich) (5 mg/ml of DNA) for 1 h at room temperature.
Cell culture
MKN-45 (an immortalized human gastric cancer cell line) was obtained from the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences (Beijing, China). MKN45 cells were cocultured with H. pylori or H. pylori DNA (5 μg/ml) for 12 h in antibiotics-free RPMI1640 (GIBCO, Grand Island, NY, USA) with the addition of 10% fetal bovine serum (GIBCO). The multiplicity of infection was set as 150:1. Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C.
Knockdown of TLR9 in MKN45 cells
Four precursor microRNA (pre-miRNA) sequences targeting TLR9 (Gene Bank accession no. NM_138688) were designed using Internet-based application (Invitrogen, USA; Table 1). The double-stranded DNA oligonucleotides encoding these four pre-miRNAs were inserted into the pcDNATM6.2-GW/EmGFP-miR expression vector (Invitrogen) and named as pcDNA-TLR9-miR 1#, 2#, 3#, and 4#, respectively. The negative control (pcDNA-TLR9-miR-neg) with no TLR9-targeting sequence inserted was constructed. The transient transfection was performed using Lipofectamine 2000 (Invitrogen), according to manufacturer's instructions. After 48 h, the efficacy of the transfection was observed using a fluorescent microscope. Then, the mRNA and protein expression of TLR9 were detected using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot.
Table 1.
MiRNA sequences targeting TLR9
| TLR9-miR-F1 | TGCTGAGAACTGTCCTTCAACACCAGGTTTTGGCCACTGACTGACCTGGTGTTAGGACAGTTCT |
| TLR9-miR-R1 | CCTGAGAACTGTCCTAACACCAGGTCAGTCAGTGGCCAAAACCTGGTGTTGAAGGACAGTTCTC |
| TLR9-miR-F2 | TGCTGAGAAGATGCCGTGCATGTCCAGTTTTGGCCACTGACTGACTGGACATGCGGCATCTTCT |
| TLR9-miR-R2 | CCTGAGAAGATGCCGCATGTCCAGTCAGTCAGTGGCCAAAACTGGACATGCACGGCATCTTCTC |
| TLR9-miR-F3 | TGCTGTAGAGGTCCAGCTTATTGTGGGTTTTGGCCACTGACTGACCCACAATACTGGACCTCTA |
| TLR9-miR-R3 | CCTGTAGAGGTCCAGTATTGTGGGTCAGTCAGTGGCCAAAACCCACAATAAGCTGGACCTCTAC |
| TLR9-miR-F4 | TGCTGAAAGAAGGCCAGGTAATTGTCGTTTTGGCCACTGACTGACGACAATTATGGCCTTCTTT |
| TLR9-miR-R4 | CCTGAAAGAAGGCCATAATTGTCGTCAGTCAGTGGCCAAAACGACAATTACCTGGCCTTCTTTC |
Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from MKN45 cells using Trizol reagent (Invitrogen), according to manufacturer's instructions. The primer sequences were as follows: TLR9: forward: 5′- CACGAGCACTCATTCACGG-3′, reverse: 5′- GACAAGTCCAGCCAGATCAAA-3′; β-actin (internal control): forward: 5′- GGCACTCTTCCAGCCTTCC-3′, reverse: 5′- GAGCCGCCGATCCACAC-3′. These results were analyzed using the comparative CT (ΔΔCT) method to establish the relative expression curves.
Western blot
Total protein was extracted from MKN45 cells using a radio-immunoprecipitation assay lysis buffer (Beyotime Biotechnology, Shanghai, China), and the protein concentrations were determined using a BCA Protein Assay kit (Beyotime). The samples were resolved by 5% sodium dodecyl–sulfate polyacrylamide gel electrophoresis and transferred onto PVDF (polyvinylidene fluoride) membranes (EMD Millipore, Billerica, MA, USA). Then, these membranes were blocked with 5% nonfat milk and reacted with the primary antibody of TLR9 (Abcam, UK) overnight at 4°C. Thereafter, the secondary antibody (Jackson) was added and allowed to further react for 1 h at room temperature. The specific bands were detected using a Tanon-4200 automatic chemiluminescence image analysis system (Tanon, Shanghai, China). The results were analyzed using ImageJ software.
Cell Counting Kit-8 assay
Cell proliferation was determined using Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan). In brief, 4 × 103 cells per well were seeded into a 96-well plate (NEST). After 1 h of incubation, 5 μg of DNA was added and cocultured with normal medium for 12 h. Then, 10 μl of the CCK-8 solution was added into each well, and absorbance at 450 nm was measured on a microplate reader (Thermo) after 2 h.
Transwell assay
Cellular migration and invasion were detected using the Transwell system (Corning Incorporated, CA, USA). In brief, after 48 h of posttransfection with miRNA, 600 μl of RPMI 1640 plus 10% FBS was added into the lower chamber. Then, the upper chamber, which was coated with (to measure cellular invasion) or without (to measure cellular migration) a Matrigel layer (BD Biosciences, CA, USA), was added with 100 μl of MKN45 cell suspension (2 × 105 cells/ml, containing H. pylori DNA at concentrations of 0 and 5 μg/ml). After 72 h, noninvading cells in the filter were removed. Then, cells on the underside of the filter were fixed with 4% paraformaldehyde for 15 min at room temperature, stained with 1% crystal violet (Genemed, No. GMS10007) for 10 min at room temperature, and photographed. For the quantitative assessment, five visual fields per filter were randomly selected, and the number of invading cells was counted using a microscope. Finally, the number of migrating or invading cells stained with crystal violet was lysated and detected at 570 nm on a plate reader (Thermo).
Statistical analysis
SPSS software version 19.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. All data were expressed as mean ± standard deviation. t-Test and analysis of variance were used for comparisons between two groups and multiple groups, respectively. P < 0.05 was considered statistically significant.
RESULTS
H. pylori DNA increased the expression of TLR9 in MKN45 cells
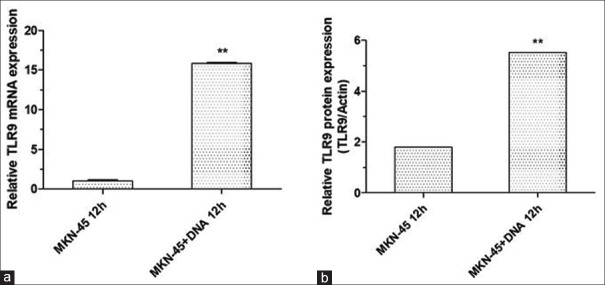
First, the expression of TLR9 in MKN45 cells was investigated using qRT-PCR and Western blot. As shown in the present data, TLR9 was abundantly expressed in MKN45 cells, and the treatment with H. pylori significantly increased the level of TLR9 [Figure 1]. Since H. pylori DNA was reported to be a ligand of TLR9, the effect of H. pylori DNA on TLR9 expression was further evaluated. It was found that the 12-h treatment of H. pylori DNA notably increased the expression of TLR9 in MKN45 cells [Figure 2].
Figure 1.
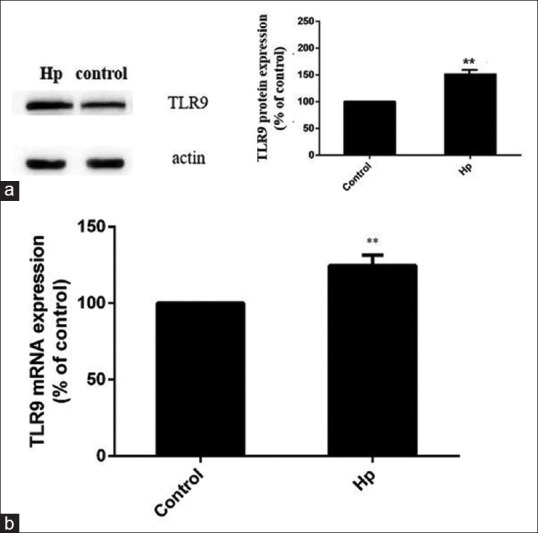
Helicobacter pylori (H. pylori) upregulates the expression of TLR9 in MKN45 cells. MKN45 cells were stimulated with H. pylori for 12 h at a bacterium-to-cell ratio of 150:1, and the expression of TLR9 was detected using Western blot (a) and qRT-PCR (b). **P < 0.01, compared with controls
Figure 2.
Helicobacter pylori (H. pylori) DNA increased the expression of TLR9 in MKN45 cells. After culturing with 5 μg/ml of DNA for 12 h, the mRNA (a) and protein (b) expression of TLR9 were significantly upregulated in MKN-45 cells. **P < 0.01, compared with that in MKN45 cells treated without DNA
Identification of specific and efficient miRNA sequences that could silence TLR9
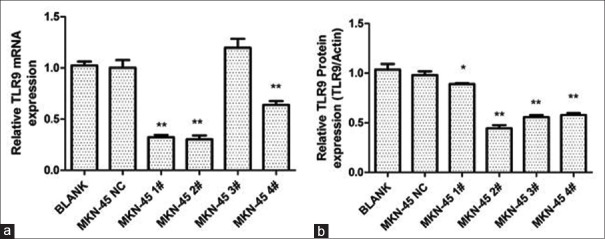
As shown in Figure 3, the DNA sequencing results confirmed the successful insertion of the four pre-miRNA sequences targeting TLR9, and no mutations were detected. At 48 h after transfection, qRT-PCR and Western blot assays were used to validate the knockdown of TLR9. As shown in Figure 4, MKN45 cells transfected with pcDNA-TLR9-miR 2# had the lowest expression of TLR9.
Figure 3.
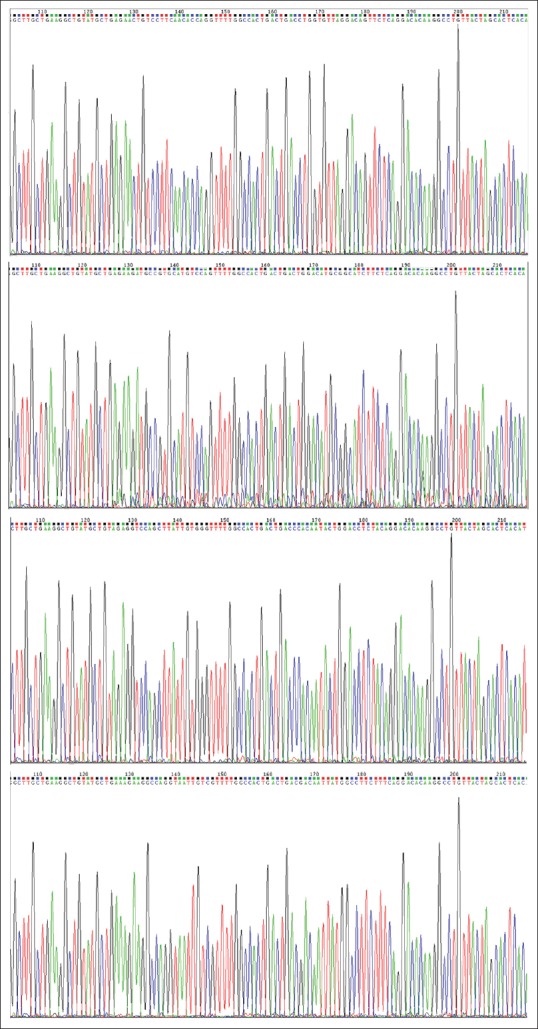
The construction of recombinant plasmids containing the miRNA insert fragments targeting TLR9. The DNA sequencing results revealed that the inserts are correct, and no mutation was found in the recombinants
Figure 4.
Knockdown of TLR9 by the specific miRNA. The silencing effect of TLR9 miRNA was evaluated by RT-PCR (a) and Western blot (b) in MKN-45 cells. Blank: untreated; NC: cells transfected with pcDNA-TLR9-miR-neg with no TLR9-targeting sequences inserted. **P < 0.01, compared with the blank group. *P < 0.05
H. pylori DNA enhanced cell proliferation, migration, and invasion by upregulating TLR9 expression
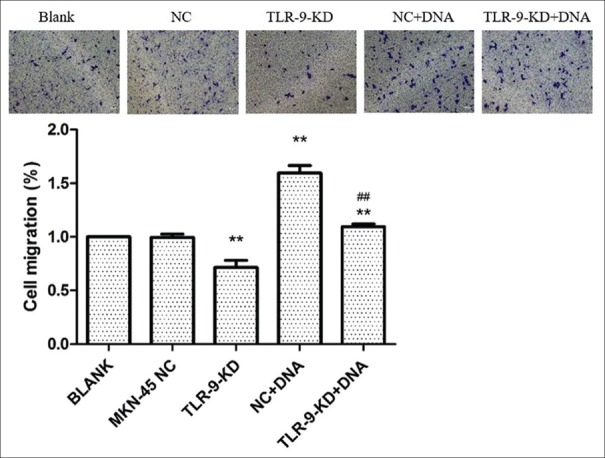
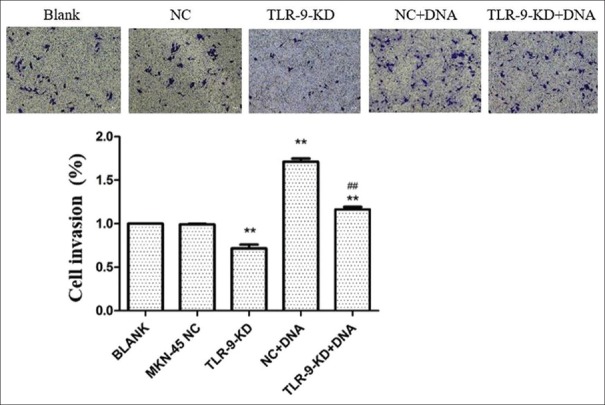
The effects of TLR9 silencing on cellular proliferation were first examined. As revealed by the CCK-8 assay, MKN45 cells with TLR9 silencing exhibited significantly less viability, when compared with cells in the control group (P < 0.01, Figure 5). Next, the influence of H. pylori DNA on cell proliferation was evaluated, and it was found that H. pylori DNA could promote cell proliferation, while MKN45 cells with TLR9 downregulation exhibited a significantly lower viability, when compared to parental cells. Moreover, the Transwell assay revealed that both the migration and invasion of MKN45 cells treated by H. pylori DNA significantly increased, when compared with controls (P < 0.01 and P < 0.01; Figures 6 and 7). In addition, the effects of TLR9 on tumor migration and invasion were also examined. According to the present data, the migration and invasion of MKN45 cells were markedly impaired following the knockdown of TLR9 [Figures 6 and 7]. Collectively, these results indicate that H. pylori DNA could enhance proliferation, migration, and invasion by activating TLR9 in human gastric cancer cells.
Figure 5.
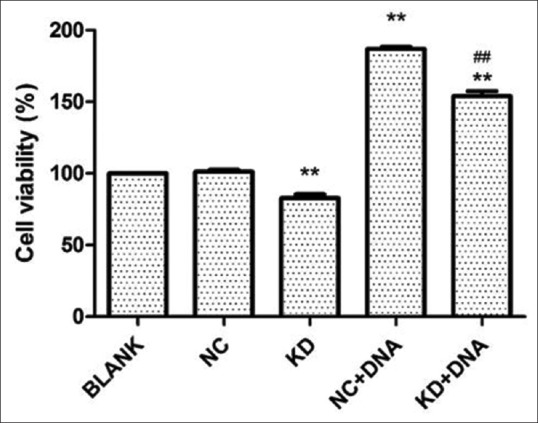
The silencing of TLR9 suppressed proliferation, whereas Helicobacter pylori (H. pylori) DNA induced cell proliferation in MKN45 cells. Cell growth viability was investigated in TLR9-knockdown cells with or without H. pylori DNA treatment. **P < 0.01, compared with NC;##P< 0.01, compared with NC + DNA
Figure 6.
The silencing of TLR9 inhibits migration, whereas Helicobacter pylori (H. pylori) DNA promotes cell migration in MKN45 cells. MKN45 cells were transfected with the scrambled miRNA or indicated miRNA and cocultured with DNA, respectively. Cells that migrated into the lower wall were stained and counted under a microscope. For quantitative assessment, the number of migrating cells stained with crystal violet was lysated and detected at 570 nm on a plate reader. **P < 0.01, compared with the blank group; ##P < 0.01, compared with the NC + DNA group
Figure 7.
The silencing of TLR9 inhibits invasion, whereas Helicobacter pylori (H. pylori) DNA promotes cell invasion in MKN45 cells. MKN45 cells were transfected with the scrambled miRNA or indicated miRNA and cocultured with H. pylori DNA, respectively. **P < 0.01, compared with the blank group; ##P < 0.01, compared with the NC + DNA group
DISCUSSION
Recently, growing evidence has revealed that TLR9 plays an important role during the initiation and progression of various cancers, such as breast cancer, prostate cancer, and lung cancer.[11,12,13,18] At present, little is known about the functions of TLR9 in gastric cancer. Kauppila et al.[19] found that H. pylori DNA could promote the invasion in AGS (a human gastric adenocarcinoma cell line) gastric cancer cells, whereas chloroquine (a nonspecific TLR9 inhibitor) could inhibit H. pylori DNA-induced invasion. However, these did not directly demonstrate that these effects were TLR9-mediated. Hence, the investigators called for these needs to be further confirmed through the controlled downregulation of TLR9 in other studies. In the present study, it was demonstrated that H. pylori infection and H. pylori DNA could upregulate the expression of TLR9 and enhance cellular proliferation, migration, and invasion in human gastric cancer cells. Furthermore, the knockdown of TLR9 significantly inhibited cellular proliferation, migration, and invasion. These results strongly propose that TLR9 is involved in the tumorigenesis and progression of gastric cancer.
TLRs have been found to play important roles in host cell responses toward pathogens and result in the increased production of inflammatory mediators, which eventually led to higher risk of chronic inflammatory diseases. As it is known, chronic inflammation plays a crucial role in the development of many human cancers. In previous studies, the positive expression of TLR9 was detected in epithelial cells of the human stomach.[16,17,20] In addition, TLR9 could recognize the existence of H. pylori DNA and induce pro-inflammatory responses.[21,22] Interestingly, another study reported that interactions between H. pylori DNA and TLR9 could significantly enhance the production of interleukin (IL)-8.[23] Similarly, there is a correlation between IL-8 mRNA expression and the severity of tissue damage in the gastric wall.[24] Moreover, it was found that the overexpression of IL-8 was associated with the development of gastric cancer.[25] Recently, substantial evidence has suggested that cyclooxygenase-2 (COX-2) and matrix metalloproteinase (MMP) might serve as downstream targets and regulate cellular migration and invasion in gastric cancer. Chang et al. reported that TLR2/TLR9 signaling could activate MAPKs and promote a panel of transcriptional factors to bind with the cAMP response element and activator protein 1 elements within the COX-2 promoter. Then, prostaglandin E2 is released to promote invasion and angiogenesis in gastric cancer.[26] Kauppila et al. reported that the stimulation of TLR9 with its agonist increased the expression of MMP13 in gastric cancer cells.[19] Consistently, Ilvesaro et al. found that TLR9 increased the invasion of human prostate cancer cells via upregulating MMP13.[27] In the future, we intend to conduct a microarray analysis to further explore the regulation network of H. pylori DNA and TLR9 signaling in gastric cancer. We also will use GES (a human immortalized gastric epithelial cell line), AGS, SGC7901 gastric cells, and a xenograft model to strengthen the significance of the present study.
CONCLUSION
In conclusion, we demonstrated that H. pylori DNA can enhance proliferation, migration and invasion by activating TLR9 in gastric cancer. These findings propose TLR9 as a therapeutic target for gastric cancer. The application of the TLR9 inhibitor or neutralizing antibody may provide an alternative therapy to reduce the development of H. pylori-mediated gastric cancer after controlling its targeting, specifically to gastric cancer cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by the Applied Basic Research Fund of Changzhou, China (Grant no. CJ20140022), the High-level Medicine Talents Training Project (2016CZBJ022), and the Youth Science and Technology Project of Changzhou Health and Family Planning Commission (QN201601).
REFERENCES
- 1.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Amieva M, Peek RM., Jr Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Ribaldone DG, Pellicano R, Actis GC. Inflammation: A highly conserved, Janus-like phenomenon-a gastroenterologist’ perspective. J Mol Med (Berl) 2018 doi: 10.1007/s00109-018-1668-z. doi: 10.1007/s00109-018-1668-z. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 7.De Nardo D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine. 2015;74:181–9. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 9.Varga MG, Peek RM. DNA Transfer and toll-like receptor modulation by Helicobacter pylori. Curr Top Microbiol Immunol. 2017;400:169–93. doi: 10.1007/978-3-319-50520-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–6. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Xie W, Wang Y, Huang Y, Yang H, Wang J, Hu Z. Toll-like receptor 2 mediates invasion via activating NF-kappaB in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2009;379:1027–32. doi: 10.1016/j.bbrc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, Rosenthal E, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4:437–47. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y, Jiang QW, Wu JY, Qiu JG, Zhang WJ, Mei XL, et al. Regulation of migration and invasion by Toll-like receptor-9 signaling network in prostate cancer. Oncotarget. 2015;6:22564–74. doi: 10.18632/oncotarget.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan M, Zhang Z, Li S, Yan M, Liu S, Yang W, et al. Activation of toll-like receptor-9 promotes cellular migration via up-regulating MMP-2 expression in oral squamous cell carcinoma. PLoS One. 2014;9:e92748. doi: 10.1371/journal.pone.0092748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Zhang Y, Li Y, Li Y, Li R, Ma Y, Wang H, et al. Chloroquine inhibits MGC803 gastric cancer cell migration via the Toll-like receptor 9/nuclear factor kappa B signaling pathway. Mol Med Rep. 2015;11:1366–71. doi: 10.3892/mmr.2014.2839. [DOI] [PubMed] [Google Scholar]
- 16.Wang TR, Peng JC, Qiao YQ, Zhu MM, Zhao D, Shen J, et al. Helicobacter pylori regulates TLR4 and TLR9 during gastric carcinogenesis. Int J Clin Exp Pathol. 2014;7:6950–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Schmausser B, Andrulis M, Endrich S, Müller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: An implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179–85. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Jiang DS, Wang YW, Jiang J, Li SM, Liang SZ, Fang HY. MicroRNA-26a involved in Toll-like receptor 9mediated lung cancer growth and migration. Int J Mol Med. 2014;34:307–12. doi: 10.3892/ijmm.2014.1764. [DOI] [PubMed] [Google Scholar]
- 19.Kauppila JH, Karttunen TJ, Saarnio J, Nyberg P, Salo T, Graves DE, et al. Short DNA sequences and bacterial DNA induce esophageal, gastric, and colorectal cancer cell invasion. APMIS. 2013;121:511–22. doi: 10.1111/apm.12016. [DOI] [PubMed] [Google Scholar]
- 20.Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Müller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–6. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga MG, Shaffer CL, Sierra JC, Suarez G, Piazuelo MB, Whitaker ME, et al. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated cag type IV secretion system. Oncogene. 2016;35:6262–9. doi: 10.1038/onc.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rad R, Ballhorn W, Voland P, Eisenächer K, Mages J, Rad L, et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–57. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Arellano L, Cortés-Reynosa P, Sánchez-Zauco N, Salazar E, Torres J, Maldonado-Bernal C. TLR9 and NF-κB are partially involved in activation of human neutrophils by Helicobacter pylori and its purified DNA. PLoS One. 2014;9:e101342. doi: 10.1371/journal.pone.0101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S. A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics. 2008;9:361–74. doi: 10.2174/138920208785699544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 26.Chang YJ, Wu MS, Lin JT, Chen CC. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol. 2005;175:8242–52. doi: 10.4049/jimmunol.175.12.8242. [DOI] [PubMed] [Google Scholar]
- 27.Ilvesaro JM, Merrell MA, Swain TM, Davidson J, Zayzafoon M, Harris KW, et al. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro . Prostate. 2007;67:774–81. doi: 10.1002/pros.20562. [DOI] [PubMed] [Google Scholar]