Abstract
Everyday life is defined by goal states that are continuously reprioritized based on available, often affective information. To pursue these goals, individuals need to process and maintain goal-relevant information, while ignoring potentially salient information that distracts resources from these goals. Empirically, this ability has typically been operationalized as working memory (WM) capacity. A growing body of research is investigating the impact of information’s affective salience on WM capacity. In the present review we address this question by exploring the potential differential impact of affective compared with neutral information on WM, and the underlying neural substrates. One-hundred and 65 studies (N = 7,433) were included in the meta-analysis. Results showed negligible to small (d̂ = −.07–.20) effects of affective information on behavioral measures of WM in healthy individuals (n = 4,936) that varied as a function of valence and task-relevance. Heterogeneity analyses were significant, demonstrating the need to identify further study-specific factors and individual differences that moderate affective WM. At the neural level (33 studies; n = 683), processing affective versus neutral material during WM tasks was associated with more frequent recruitment of the vlPFC, the amygdala, and the temporo-occipital cortex. In contrast to healthy individuals, across behavioral studies those suffering from mental health problems (n = 2,041) showed impaired WM accuracy (d̂ = −0.21) in the presence of affective material. These findings highlight the importance of integrating behavioral and neural levels of analysis. Finally, these findings suggest that affective WM capacity may be a transdiagnostic mechanism associated with poor mental health.
Keywords: working memory, emotion, mental health, frontoparietal control network, salience network
Public Significance Statement
The behavioral and neuroimaging meta-analyses showed that in psychologically healthy individuals there was limited support for behavioral working memory (WM) performance to be affected by affective information, whereas at the neural level WM in the presence of affective relative to neutral information was associated with differential recruitment of the salience network and the fronto-parietal control network. These findings highlight the importance of combining behavioral and neuroimaging research syntheses. Second, in individuals with mental health problems WM was significantly impaired by affective material. This suggests that WM performance on tasks including affective compared with neutral information may be a sensitive and transdiagnostic cognitive marker of mental health status.
Working memory1 (WM) constitutes a capacity-limited resource that temporally maintains and stores information (Baddeley, 2003) in the service of higher cognitive functions (fluid intelligence, for instance; Kane, Hambrick, & Conway, 2005). The vicissitudes of daily life frequently require such cognitive functions to operate in affectively laden contexts where much of the goal-relevant and goal-irrelevant information being processed has affective characteristics. Despite this, the impact of affective information on WM and the mechanisms through which that impact is realized remain poorly understood (Baddeley, 2003, 2013; Pessoa, 2009). Indeed, a consideration of WM in relation to affective phenomena has only recently attracted concerted discussion (Baddeley, 2013; Barrett, Tugade, & Engle, 2004; Okon-Singer, Hendler, Pessoa, & Shackman, 2015). Here, we review the literature and synthesize the research data comparing the impact of affective versus neutral information on WM, and the underlying neural substrates.
WM in the Laboratory Versus WM in the Outside World
Traditionally, WM has been experimentally assessed using paradigms that require individuals to update affectively neutral information such as numbers, letters or shapes in their memory store while simultaneously trying to minimize interference from other affectively neutral irrelevant material (e.g., Conway et al., 2005; Owen, McMillan, Laird, & Bullmore, 2005). These “affect-neutral” tasks are traditionally conducted in laboratory settings allowing extreme precision in the goal-demands placed on participants. However, in real-world contexts WM is deployed in the face of ever-changing goal-demands where the information that needs to be updated and maintained in WM to meet current goals can shift rapidly. Dynamic reprioritization of active goal-states typically occurs as salient and/or novel representations are selectively attended to (Corbetta & Shulman, 2002; Klink, Jentgens, & Lorteije, 2014). Salience can be perceptual (Corbetta & Shulman, 2002) or experience-driven (e.g., affect-neutral pictures and words attract attention when they are relevant to current task-goals; Vogt, De Houwer, & Crombez, 2011; Vogt, De Houwer, Crombez, & Van Damme, 2013).
Another source of salience concerns the affective properties of encountered information. Affective significance can be conferred by: learned associations (e.g., repeated exposure to an object’s rewarding properties; Gallagher & Holland, 1994; Gottfried, O’Doherty, & Dolan, 2003; Schoenbaum & Roesch, 2005), evolutionarily transmitted predispositions, for instance species-specific survival threats (i.e., biological preparedness or “inherent goal states”; LeDoux, 2012; Mobbs, Hagan, Dalgleish, Silston, & Prévost, 2015), as well as perceiver-based categorizations and appraisals (Barrett, 2006; Scherer, Dan, & Flykt, 2006). These perceiver-based conceptual pathways have the potential to overwrite inherent or learned associations about a stimulus’ affective impact (e.g., Ochsner, Bunge, Gross, & Gabrieli, 2002). Salience attribution to affective properties is likely to have developed phylogenetically in humans as a function of threat/reward detection mechanisms (Dolan, 2002; LeDoux & Brown, 2017) and more broadly as a heuristic for accelerating goal-directed behavior (Al-Shawaf, Conroy-Beam, Asao, & Buss, 2016; Barrett, 2013).
Imagine the case of a fire alarm going off during dinner preparations which involve the maintenance of necessary cooking steps in WM. The fire alarm will immediately lead to reprioritization of the goal of cooking as entirely insignificant, while the new goal of exiting the building with kin becomes the dominant priority in the goal hierarchy. This reprioritization occurs because of the alarm’s strong learned association with danger allied with the perceiver’s appraisals of how events are likely to unfold if no action is taken (Amo et al., 2014; Gilmartin, Balderston, & Helmstetter, 2014; Moscarello & Maren, 2018).
Outside the rarefied setting of the laboratory, information processed in WM, then, is evaluated in terms of its relative facilitation versus interference of the pursuit of current goal-states (Barrett, 2005; Clore & Huntsinger, 2007; Fox, 2008; Power & Dalgleish, 2015), and the affective significance of encountered information has the potential to initiate the overriding of currently active goals in order to prioritize other goal-states (Barrett, 2013; Krieglmeyer, Deutsch, De Houwer, & De Raedt, 2010) due to their salience to, for example, survival (LeDoux, 2012) or self-identity (Kendzierski, Ritter, Stump, & Anglin, 2015), or other domains central to the welfare of the organism.
Theories About the Impact of Affective Properties on WM
Despite this almost ubiquitous requirement for WM in the real world to operate in affective contexts, we currently lack a compelling unified theory of the different ways in which the processing of affective information can impact on WM. Instead, most theoretical work has focused on the role of acute or trait affective states on WM processing (for reviews, see: depression, Baddeley, 2013; mood, Mitchell & Phillips, 2007; anxiety, Moran, 2016). Other theories have offered frameworks about the impact of affective information within other domains of cognition including attention (Mather & Sutherland, 2011; Vuilleumier, 2005; Vuilleumier & Huang, 2009; Wells & Matthews, 2015) and memory (Hamann, 2001; Phelps, 2004, 2006; Talmi, 2013) and some of these theories make specific predictions regarding WM (e.g., Mather & Sutherland, 2011). Common to these diverse models is the proposal that affective properties of encountered information modulate the strength of its resultant cognitive and neural representations. This can then facilitate or impair goal-directed behavior, depending on whether the affective information is relevant to the goal at-hand or to an alternative competing goal, respectively. A theoretical framework that enshrines this common component across models is Pessoa’s dual competition framework (DCF; Pessoa, 2009). Specifically, the DCF proposes that affective properties of encountered information can compete for processing resources within the cognitive system either at the level of perceptual processing or at the level of executive control. Thus, at any one time, cognitive resources devoted to the processing of affective properties become temporarily unavailable to all other goal-relevant properties, thereby interfering with goal-directed behavior that depends on these other properties.
Investigating the Impact of Affective Properties on WM in the Laboratory and the Brain Scanner
How can we evaluate with some precision the impact of affective context on WM? Prototypically, the impact of affective properties on WM is tested by populating standard experimental tasks, administered in the laboratory, with affective stimuli. One way to do this is to solicit and use personally relevant affective information from participants. However, this tends to introduce sources of variance across participants regarding stimulus attributes that are unrelated to their affective properties (e.g., word length). Researchers therefore more commonly opt for “standardized” affective stimuli that pertain to prototypical affective goals presumed to be more or less relevant to all participants (e.g., survival motives). These can include words (Bradley & Lang, 1999), faces (Tottenham et al., 2009), and other affective images (Lang, Bradley, & Cuthbert, 2008). However, the potential downside of using such standardized stimuli is that their affective significance—their positive or negative value to a healthy research participant—will usually be relatively low (Pessoa, 2009). That is, while these generic stimuli are still likely to receive some preferential processing within the cognitive system, their modulating effect on current task performance is proposed to be limited—they are given what the DCF calls soft prioritization (Pessoa, 2009). At the behavioral level this relatively weak impact on prioritization is likely to be both difficult to detect and replicate, as well as being subject to strong influences from study-specific factors such as WM load. To translate this to our aforementioned real-world example of preparing dinner, imagine seeing news footage about a building on fire instead of hearing an alarm go off in your building. The footage may mildly interfere with the updating of the individual cooking steps in WM (e.g., forgetting to add salt), but is unlikely to have a fundamental effect on the priority of your goal to prepare dinner.
At the neural level, however, the effects of soft prioritization of standardized affective information should be easier to assess because the neural impact of the processing of affective information will be detectable even in situations where there has been no marked effect on overt behavior. Affective compared with neutral stimuli are proposed to have stronger perceptual representations in the brain’s visual cortices (Vuilleumier, 2005) and other sensory cortices for nonvisual stimuli (Satpute et al., 2015). This increased strength of representation is in part proposed to be a function of amygdalergic projections to cortical sensory areas (Amaral, Behniea, & Kelly, 2003; Sah, Faber, Lopez De Armentia, & Power, 2003) and has the potential to modulate executive competition by prioritizing attention toward affective compared with neutral stimuli. A second neural route through which executive competition can be impacted as a function of a stimulus’ affective significance is through the direct processing of affective information in the fronto-parietal control network (Okon-Singer et al., 2015; Pessoa, 2009). This would mean that executive resources are occupied by the processing of the affective information and thus no longer available for executive control- (here, WM-) demanding activities (Eysenck, Derakshan, Santos, & Calvo, 2007). Specifically, processing of affective information includes a wide range of potential processes including but not limited to valuation/appraisal of affective material with neural substrates distributed across the prefrontal cortex including a hub in the orbitofrontal cortex (Dixon, Thiruchselvam, Todd, & Christoff, 2017), and affect regulation involving multiple regions in the fronto-parietal control network including the lateral as well as the medial prefrontal and parietal cortices (Buhle et al., 2014; Etkin, Büchel, & Gross, 2015). Moreover, affective distractors and targets are likely to engage both overlapping and separate components of the frontoparietal control network (Dolcos, Katsumi, Denkova, & Dolcos, 2017). In sum, then, perceptual competition from affective (relative to neutral) material during the performance of a WM task should be associated with increased neural activation within the visual cortex (for affective visual stimuli) as well as within the brain’s “salience network” (Seeley et al., 2007; cf. ventral attention; Corbetta & Shulman, 2002), including the amygdala (Barrett & Satpute, 2013). Executive competition should also be reflected in augmented activation of the salience network and additionally with enhanced recruitment of the fronto-parietal control network (Pessoa, 2008, 2009).
This analysis suggests then that the behavioral and neural effects of affective stimuli on WM may be “dissociable.” It is hypothesized that there will small behavioral effects, because the stimuli prototypically used in the laboratory ultimately have low affective significance and only attract soft prioritization, allied to clear neural effects representing the analysis of the stimuli’s affective significant in preparation for any prioritization in the domain of behavior. A growing body of literature suggests that the impact of affective material on WM performance vary depending on the stimuli’s task-relevance (i.e., opposing effects of task-relevant material vs. task-irrelevant distractors) and in some cases the stimuli’s affective valence (Dolcos et al., 2017; Okon-Singer et al., 2015; Pessoa, 2009).
Task-Relevance
Preferential allocation of perceptual and executive processing resources to task-relevant affective stimuli is proposed to improve behavioral performance on the task at hand.2 This affective enhancement effect is well-established in the long-term memory literature (for reviews of laboratory and neuroimaging studies, see Buchanan & Adolphs, 2002; Hamann, 2001; LaBar & Cabeza, 2006; Phelps, 2004) with individuals remembering affective information and events better compared with neutral information. Similarly, research on “emotional attention” shows reliable affective processing biases with individuals being faster to detect affective information in visual searches (Vuilleumier & Huang, 2009). Evidence from behavioral research on WM in healthy individuals appears more mixed with some studies showing an enhancement of WM for affective compared with neutral information (e.g., Xie et al., 2017), others showing no effect (e.g., Grissmann, Faller, Scharinger, Spüler, & Gerjets, 2017; M. Li et al., 2018; Nejati, Salehinejad, & Sabayee, 2018), WM impairment (e.g., Garrison & Schmeichel, 2018; Hur, Iordan, Dolcos, & Berenbaum, 2017; Tamm, Kreegipuu, Harro, & Cowan, 2017; Yoon, Kutz, LeMoult, & Joormann, 2017), or complex interactions with task-design features (e.g., trial type; Levens, Armstrong, Orejuela-Dávila, & Alverio, 2017; Quinlan, Yue, & Cohen, 2017). Meta-analytic synthesis of the relevant evidence is therefore required to elucidate the potential impact(s) of affective memoranda on WM.
Greater perceptual- and executive-level prioritization of task-irrelevant (henceforth, distractors) affective, relative to neutral, stimuli is hypothesized to impair behavioral WM performance (e.g., Derakshan & Eysenck, 2009; Okon-Singer et al., 2015; Pessoa, 2009). This is in line with evidence from tasks assessing executive control in processes other than WM (e.g., dichotic listening tasks, modified Stroop tasks or spatial attention tasks; Schupp, Flaisch, Stockburger, & Junghöfer, 2006; Yiend, 2010). The literature on the impact of affective distractors on WM performance again is mixed, showing no effect (Jenness et al., 2018) or impaired behavioral (Ladouceur, Schlund, & Segreti, 2018; Stout, Shackman, Pedersen, Miskovich, & Larson, 2017; Tollenaar, Ruissen, Elzinga, & de Bruijn, 2017; Wingert, Blais, Ball, & Brewer, 2018) performance.
At the neural level, the inferior PFC is considered critical to selecting task-relevant targets and inhibiting responses and attention to task-irrelevant distractors (Aron, Robbins, & Poldrack, 2004; Miller & Cohen, 2001). However, recent reviews of the literature on the neural substrates of affect-cognition interactions suggest that the inhibition of attention and responses to, as well as the regulation of, affective distractors may recruit a wider network in the ventral stream of the fronto-parietal control network (Iordan & Dolcos, 2017; Okon-Singer et al., 2015), this includes the inferior PFC but is not limited to it. WM tasks performed in the presence of affective distractors have similarly shown greater recruitment of the ventral PFC (e.g., Dolcos & McCarthy, 2006), though some studies have also shown the involvement of more dorsal and medial parts of the frontoparietal control network (García-Pacios, Garcés, Del Río, & Maestú, 2015, 2017). The current neuroimaging meta-analysis allows us to investigate the relative contributions of these different brain regions to the interference from affective compared with neutral distractors.
Valence
The vast majority of the experimental literature on WM in affective contexts, to-date, focuses on the impact of negatively valenced information (usually threat-related). However, comparable theoretical arguments to those articulated above can be made for the effects of positive stimuli, neutral stimuli with high-arousal associations (Mourão-Miranda et al., 2003), and novel stimuli. Except in some circumstances (e.g., erotic stimuli, see below) the positive stimuli prototypically used in laboratory studies are considered very low in affective significance (Pereira et al., 2006; Pessoa, 2009) and are thus unlikely to elicit robust behavioral effects let alone reprioritize current goals. Studies on the temporal course of peripheral physiological responses to affective information in laboratory contexts support this notion with responses to negative material being faster (N. K. Smith, Cacioppo, Larsen, & Chartrand, 2003)3 and more protracted than for positive stimuli (Brosschot & Thayer, 2003; Taylor, 1991). Similarly, while both pleasant and unpleasant stimuli engage overlapping parts of the brain’s salience network, neural responses are nevertheless less reliable for pleasant than unpleasant stimuli in the amygdala and insula (Lindquist, Satpute, Wager, Weber, & Barrett, 2016). Evidence from WM appears to show a comparable pattern, with positive stimuli having a lower impact on performance compared with negative stimuli, though the effect of valence may be stronger for WM reaction time (RT) data compared with accuracy data (e.g., Colligan & Koven, 2015). Furthermore, there may be developmental differences with performance being more affected by rewarding stimuli in adolescence (Cromheeke & Mueller, 2016). The reviewed work then suggests that the impact of affective material as evaluated in laboratory WM tasks will be greater for negative compared with positive material.
The Impact of Affective Information on WM Beyond Young Psychologically Healthy Adults
Theoretically, stimuli high in affective significance are proposed to have pronounced effects on behavioral performance through the recruitment of common executive control resources in the service of processing these affectively laden stimuli—consider our real-world fire alarm example. Pessoa (2009) terms this hard prioritization. Such hard prioritization is difficult to investigate in the laboratory with psychologically healthy individuals as the standardized stimuli used in such studies, as discussed above, are low in affective significance. Indeed, to our knowledge, no study has systematically modulated stimuli’s affective significance to investigate the nature of the relationship between affective significance and WM performance.
However, one way that prioritization can be investigated experimentally is to work with populations—such as samples characterized by mental health difficulties—for whom standardized stimuli are evaluated as relatively high in affective significance. Affective information, it is proposed, gains harder prioritization in individuals suffering from mental health problems because it is critical to the individual’s perpetually activated affect-related concerns. That is, many mental health difficulties (including mood and anxiety disorders, schizophrenia, and attention deficit and hyperactivity disorder; Aleman & Kahn, 2005; Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Cubillo, Halari, Smith, Taylor, & Rubia, 2012; Gotlib & Joormann, 2010; Mathews & MacLeod, 2005) are associated with: preferential processing of affective, particularly negative, information; slowed disengagement from affective information (e.g., depression; Koster, De Lissnyder, Derakshan, & De Raedt, 2011); and maladaptive regulation of affective material (Aldao, Nolen-Hoeksema, & Schweizer, 2010). The impact of affective material on WM then is likely to be increased in individuals suffering from mental health problems compared with healthy controls. This is also likely to be the case for affectively positive stimuli. For example, individuals with eating-related mental health (Wagner et al., 2015) and physiological weight-related problems (Boutelle et al., 2014) show increased activation of the amygdala in response to standardized food-related stimuli compared with healthy individuals.
Related to this, another possible moderator of perceived affective significance is age. There is a wealth of evidence that for older adults positive stimuli may carry greater affective significance due to the age-related positivity effect—the finding that in older-age individuals preferentially process positive information across a range of cognitive domains and stimulus types (Carstensen, 2006; Mather & Carstensen, 2005). Comparing WM performance for affective information across age then may reveal dissociable effects for positive and negative stimuli.
To summarize, broadly speaking the effects of affective material on WM processing are hypothesized to vary as a function of the material’s affective significance, valence, and task-relevance. Furthermore, the behavioral and neural levels of analysis are predicted to show dissociable effects for prototypical studies involving standardized stimuli with low-affective significance administered to unselected or psychologically healthy populations. In the sections that follow, we outline specific hypotheses within each of these sets of circumstances before reviewing the relevant data.
The Present Reviews
The primary aim of the current reviews was to evaluate both the behavioral impact of affective information on WM performance and the neural substrates of those putative effects, through a pair of meta-analyses of the extant literatures. To this end we reviewed behavioral and functional MRI (fMRI) studies published up until February 28, 2017, that investigated the effect of affective material on WM functioning.
Guided by the definition of WM as comprising one or more storage components alongside an executive control component, the present meta-analytic work included studies employing three types of tasks as measures of WM (see Figure 1 for task schematics): (a) tasks that require continuous updating of WM content through the sequential presentation of memoranda—these include simple span tasks and n-back tasks (Figure 1A; Cohen et al., 1997; Owen et al., 2005); (b) delayed-match-to-sample tasks (Figure 1B) that require the recall of memoranda following a delay interval during which participants are either presented with distractors or some other form of secondary task-demand4 (Courtney, Petit, Maisog, Ungerleider, & Haxby, 1998; Jiang, Haxby, Martin, Ungerleider, & Parasuraman, 2000; Sawaguchi & Goldman-Rakic, 1991); and (c) complex span tasks (Figure 1C) which comprise an operation task (e.g., solving a mathematical problem) and a storage task (e.g., remembering words; Conway et al., 2005). For a given study to be included in our analyses these tasks needed to present affective stimuli as either task-relevant memoranda (targets) or task-irrelevant distractors.
Figure 1.
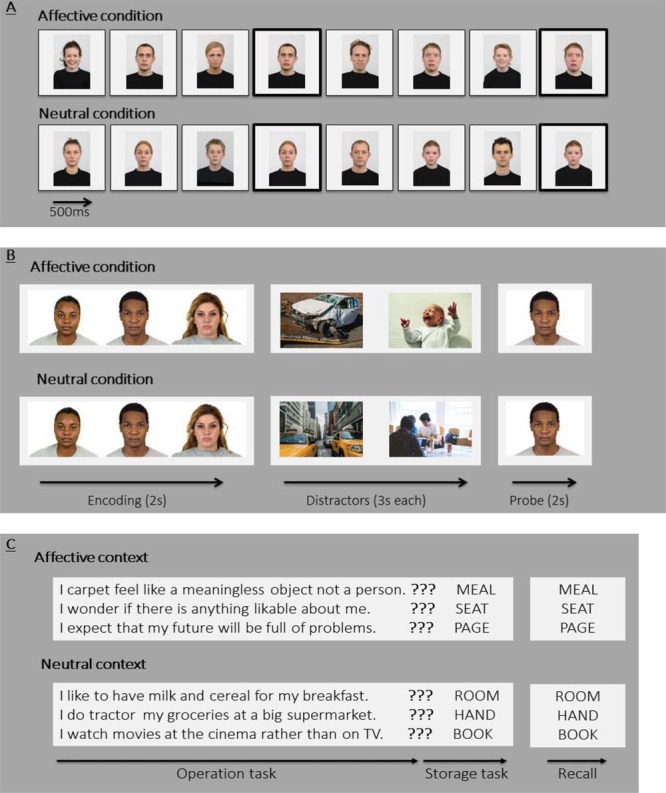
Schematics of three prototypical WM tasks, presented in affective and neutral contexts. The figure depicts three prototypical WM tasks capturing the range of paradigms included in the present meta-analyses. 1A shows an n-back task (where in this case n = 2) that requires participants to continuously update the content of their active WM representations. In the figure, trials with a bold black border indicate target trials. In the affective context the images that have to be matched across trials are negative in valence and in the neutral context the target stimuli are neutral. 1B depicts an example of a delayed-match-to-sample task. In this task, participants are required to match the emotional expression of a probe face with the expression in one of three presented memoranda. During the retention interval participants see either two negative distractor images (affective context) or two valence-neutral images (neutral context). 1C provides an illustration of a complex span task, which comprises an operation component and a storage component. The example depicts an affective reading span task where participants make judgments about the semantic accuracy of self-statements. In the affective context the first sentence requires a “no” response as it is semantically meaningless, while the other sentences are semantically correct. In the neutral context the second sentence is incorrect and the others are semantically meaningful. For the storage component participants have to recall the words in upper case that are presented at the end of each sentence. The recall happens at the end of each block, with block lengths typically varying between three and seven trials.
Behavioral Meta-Analysis
In line with the previous theoretical discussion, the behavioral meta-analysis examined the following hypotheses:
Hypothesis A: In the context of a proposed dissociation between behavioral and neural levels of analysis in psychologically healthy individuals (or unselected), for the behavioral meta-analysis we predicted at most small effects of affective, relative to neutral, material on WM performance due to affective stimuli’ low affective significance and the predicted moderating and interacting effects of valence (A1) and task relevance (A2). Specifically, we hypothesized that:
Hypothesis A1: Positive stimuli have a smaller effect on WM performance compared with negative stimuli, and;
Hypothesis A2: Affective distractors and targets have opposing effects on WM performance, with affective distractors impairing WM performance relative to neutral distractors and affective targets enhancing WM performance relative to neutral targets.
Hypothesis B1: Affective stimuli have a greater impact on behavioral WM performance in individuals with mental health problems for whom it is proposed they have greater affective significance compared with healthy individuals.
Hypothesis B2: The impact of positive, but not negative, stimuli, relative to neutral stimuli, on WM performance increases as a function of age in line with the age-related positivity bias.
Methods for the Behavioral Meta-Analysis
Identification of Studies for Inclusion5
The literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Moher, Liberati, Tetzlaff, & Altman, 2009) guidelines (for the PRISMA Checklist, see online supplementary materials). There was no review protocol. All searches were executed in the databases PubMed and PsycINFO with the following search parameter delimitations—publication language: English, human participants, publication date: 01.01.1900 (default in PubMed)–28.02.2017 (for the electronic search strategy please see the online supplementary materials). The search term combinations entered were: Combination I6 = “emotion* OR affective AND executive* function*; Combination II = “emoti* OR affective AND cogniti* function*”; Combination III = “emoti* OR affective AND working memory”; Combination IV = “emoti* OR affective AND n-back”; and Combination V = “emoti* OR affective AND delayed-match-to-sample.” In addition to the articles yielded by the database search we also checked the reference lists of those articles.
Screening
After removing all duplicates, review articles, and theoretical papers, articles generated in the identification stage were screened. Inclusion criteria in the screening phase were: Article titles needed to refer to two separate components: (a) the word “emotional” (or synonyms thereof) or a “mental health disorder,” as well as (b) the word “cognitive” or terms referring to “executive functioning.” Abstracts needed to mention the use of one or more memory tasks or refer to executive functioning tasks. This led to a set of full-text articles, which were assessed in the final step.
Eligibility
Eligibility was assessed by checking the full-text articles for the following components: (a) They needed to report at least one empirical study in humans. (b) The studies also had to report accuracy performance and/or RTs on a measure of WM, which required the recall of affective and neutral task-relevant memoranda in WM, or contained affective and neutral task-irrelevant distractors which had to be ignored. If these data were not reported in the paper authors were contacted with a request for these data (denoted with data request [DR] in Table 1; studies that met all inclusion criteria but for which no data was made available are reported in the relevant online supplementary materials section). Studies that used mood induction or naturally occurring mood states (e.g., mania) as an emotion manipulation were excluded as this was beyond the scope of the present reviews.
Table 1. Included Study Samples and Task Descriptions.
| Author | N | Age | Data | Population | Task | Valence | Task-relevance | Imaging |
|---|---|---|---|---|---|---|---|---|
| Note. The effect sizes for the studies represented in Table 1 are summarized in two separate forest plots (Figure S1A and B) in the online supplementary materials. Age = Average age of the study participants; Data = type of data extracted from the study (i.e., accuracy, RT = reaction time, or both); Population = Participant sample included (i.e., healthy, psychopathology, denoted as 1; or neurological disorder and altered neurological state group, denoted as 2); Task = task design reported in the study; Valence = valence of the affective stimuli (i.e., negative and/or positive); Task-relevance = task-relevance of affective stimuli (i.e., task-irrelevant distractors or task-relevant targets); Imaging = this column states “Reported” for studies that included neuroimaging data on the affective WM task and an asterisk (*) indicates that the study was included in the neuroimaging meta-analysis. DR = data requested from the authors; PTSD = posttraumatic stress disorder; PD = personality disorder; DMTS = delayed-match-to-sample task; S. span = simple span tasks; C. span = complex span tasks; D. n-back = dual n-back; + means that study included several experiments with different tasks including the n-back task, AX-CPT = AX continuous performance task required the continuous updating of cue and probes with distractors presented in between the presentation of cues and distractors. | ||||||||
| a Women with reported child abuse were included in the psychopathology and analogue group due to high levels of depression and anxiety symptoms. b Women with reported childhood stressors (other than abuse) were included in the healthy group due to nonclinical levels of self-reported depression and anxiety symptoms. c We included these participants in the psychopathology and analogue group, however, as they were currently asymptomatic we also ran all analyses with them classified as healthy, which did not change the pattern of results. d Authors had no longer access to the behavioral data. e In consultation with the authors no accuracy data were included for studies administering the internal shift task as this measure assess accuracy on a block rather than trial basis leading to frequent missing data and accuracy scores that are not directly comparable with other measures of WM (correspondence with Koster is available upon request). f We included only the neuroimaging data from this study as the authors did not reply to our request for behavioral data before our analysis deadline. g The study compared WM for face and houses with faces constituting the emotional condition. h There was no distraction in the delay interval; however, the probe was only an extract of the sample requiring active reconstruction of the original stimulus and was therefore included. i RT data included in Pallesen, Brattico, Bailey, Korvenoja, and Gjedde (2009). | ||||||||
| Amir and Bomyea (2011) | 32 | 31 | RT | Generalized and social phobia | C. span | Negative | Task-relevant | |
| 30 | 29 | Healthy | ||||||
| Anticevic, Repovs, and Barch (2010)DR | 21 | 25 | Both | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Anticevic, Repovs, Corlett, and Barch (2011) | 24 | 37 | Both | Healthy | DMTS | Negative | Task-irrelevant | |
| 28 | 36 | Schizophrenia1 | ||||||
| Artuso, Palladino, and Ricciardelli (2012)DR | 20 | 23 | Both | Healthy | S. span | Negative and positive | Task-relevant | |
| Bakvis, Spinhoven, Putman, Zitman, and Roelofs (2010)DR | 20 | 31 | Accuracy | Healthy | n-back | Negative and positive | Task-irrelevant | |
| 19 | 35 | Psychogenic seizures2 | ||||||
| Becerril and Barch (2011)DR | 32 | 36 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| 38 | 37 | Schizophrenia1 | ||||||
| Beckwé, Deroost, Koster, De Lissnyder, and De Raedt (2014) | 84 | 19 | RTe | Healthy | S. span | Negative | Task-relevant | |
| Belham et al. (2013) | 27 | 21 | Both | Healthy | S. span | Negative and positive | Task-relevant | |
| 25 | 70 | Healthy | ||||||
| Beneventi, Barndon, Ersland, and Hugdahl (2007)DR | 12 | 24 | Imagingd | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| Berger et al. (2015) | 12 | 78 | Both | Healthy | n-back | Negative | Task-irrelevant | Reported |
| 12 | 74 | Alzheimer’s disease2 | ||||||
| Bertocci et al. (2012) | 23 | 32 | Both | Depression1 | n-back | Negative and positive | Task-relevant | Reported |
| 18 | 30 | Bipolar disorder1 | ||||||
| 16 | 33 | Healthy | ||||||
| Bertocci et al. (2014) | 22 | 14 | Both | Mixed diagnoses with high emotional dysregulation1 | n-back | Negative and positive | Task-relevant | Reported |
| 39 | 14 | Mixed diagnoses with low emotional dysregulation1 | ||||||
| 24 | 13 | Healthy | ||||||
| Borella, Carretti, Grassi, Nucci, and Sciore (2014) | 93 | 69 | Accuracy | Healthy | C. span | Negative and positive | Task-relevant | |
| 63 | 26 | Healthy | ||||||
| Borg, Leroy, Favre, Laurent, and Thomas-Antérion (2011)DR | 28 | 53 | Accuracy | Healthy | S. span | Negative | Task-relevant | |
| 14 | 81 | Alzheimer’s disease2 | ||||||
| Brunyé, Howe, Walker, and Mahoney (2013)DR | 13 | 20 | Both | Healthy | DMTS | Negative | Task-irrelevant | |
| Buratto, Pottage, Brown, Morrison, and Schaefer (2014) | 40 | 24 | Both | Healthy | n-back | Negative medium and high intensity | Task-irrelevant | |
| 40 | 20 | Healthy | ||||||
| Burhan et al. (2016) | 10 | 73 | Both | Mild cognitive impairment2 | DMTS | Negative | Task-irrelevant | Reported |
| 12 | 66 | Healthy | ||||||
| Chuah et al. (2010) | 24 | 22 | Both | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| 24 | 22 | Sleep-deprived2 | ||||||
| Colligan and Koven (2015) | 93 | 19 | RT | Healthy | S. span | Negative and positive | Task-relevant | |
| Cromheeke, Herpoel, and Mueller (2014) | 17 | 20 | Both | Childhood abuse1,a | DMTS | Negative and positive | Task-relevant | |
| 17 | 20 | Childhood stressorsb | ||||||
| 17 | 20 | Healthy | ||||||
| Cromheeke and Mueller (2016)DR | 33 | 14 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 37 | 21 | Healthy | ||||||
| Dai, Rahman, Lau, Sook Kim, and Deldin (2015) | 34 | 19 | RT | Healthy | n-back | Negative and positive | Task-relevant | |
| 33 | 19 | Dysphoric | ||||||
| De Lissnyder, Koster, and De Raedt (2012) | 37 | 20 | RTe | Healthy | S. span | Negative | Task-relevant | |
| De Lissnyder et al. (2012) | 20 | 45 | RTe | Healthy | S. span | Negative | Task-relevant | |
| 20 | 40 | Depression1 | S. span | Negative | Task-relevant | |||
| De Lissnyder et al. (2012)DR | 50 | 19 | RTe | Healthy | S. span | Negative | Task-relevant | |
| Demeyer, De Lissnyder, Koster, and De Raedt (2012) | 30 | 47 | RTe | Depression remitted1, c | S. span | Negative | Task-relevant | |
| Denkova et al. (2010) | 18 | 23 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Diaz et al. (2011)DR | 17 | 24 | Both | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| 11 | 33 | Schizophrenia1 | ||||||
| Doallo, Holguín, and Cadaveira (2006)DR | 10 | 26 | Both | Healthy | DMTS | Negative | Task-irrelevant | |
| Döhnel et al. (2008)DR | 16 | 63 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| 16 | 63 | Mild cognitive impairment2 | ||||||
| Dolcos and McCarthy (2006)DR | 18 | 22 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Dolcos, Kragel, Wang, and McCarthy (2006) | 15 | 22 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Dolcos, Diaz-Granados, Wang, and McCarthy (2008) | 14 | 25 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Dolcos et al. (2013)DR | 17 | 27 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Edelstein (2006) | 255 | — | Accuracy | Healthy | C. span | Negative and positive (mixed) and attachment-related words | Task-relevant | |
| Erk, Kleczar, and Walter (2007) | 12 | 25 | Both | Healthy | Sternberg | Negative | Task-irrelevant | Reported* |
| Evans, Craig, Oliver, and Drobes (2011) | 24 | 30 | RT | Smokers | n-back | Smoking cues | Task-relevant | |
| 16 | 28 | Healthy | ||||||
| Fairfield, Mammarella, and Di Domenico (2015)DR | 40 | 22 | Accuracy | Healthy | S. span | Negative and positive | Task-relevant | |
| Fairfield et al. (2015) | 35 | 25 | Both | Healthy | n-back | Positive | Task-relevant | |
| 35 | 70 | Healthy | ||||||
| Fales, Becerril, Luking, and Barch (2010)DR | 29 | 36 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| Ferré (2002) | 21 | 22 | Accuracy | Healthy | S. span | Negative and positive (mixed) | Task-relevant | |
| García-Pacios, Del Río, and Maestú (2014)DR | 34 | 22 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | |
| García-Pacios, Del Río, Villalobos, Ruiz-Vargas, and Maestú (2015)DR | 30 | 21 | Both | Healthy | DMTS | Negative and positive | Task-irrelevant | |
| 43 | 22 | Healthy | ||||||
| 26 | 21 | Healthy | ||||||
| García-Pacios et al. (2015)DR | 15 | 20 | Accuracy | Healthy | DMTS | Negative and positive | Task-irrelevant | |
| Gathmann, Pawlikowski, Schöler, and Brand (2014) | 194 | 27 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| Giles et al. (2015) | 36 | 20 | Both | Healthy | DMTS | Negative | Task-irrelevant | |
| 36 | 20 | Healthy | ||||||
| Gläscher, Rose, and Büchel (2007)DR | 23 | 24 | Both | Healthy | n-back | Negative | Task-relevant | Reported* |
| González-Garrido, López-Franco, Gómez-Velázquez, Ramos-Loyo, and Sequeira (2015) | 20 | 26 | Both | Healthy | S. span | Negative and positive | Task-relevant | |
| Gonzalez-Garrido et al. (2007) | 14 | 29 | Both | Healthy | DMTS | Negative and positive | Task-irrelevant | |
| Gooding and Tallent (2003) | 39 | 19 | Both | Healthy | DMTS | Negative | Task-relevant | |
| 43 | 19 | Social anhedonia1,c | ||||||
| Gooding and Tallent (2004) | 36 | 58 | Both | Schizophrenia and | DMTS | Negative | Task-relevant | |
| Schizoaffective disorder1 | ||||||||
| 29 | 41 | Healthy | ||||||
| Goolsby, Shapiro, and Raymond (2009) | 34 | 24 | Both | Healthy | DMTS | g | Task-relevant | |
| Gotoh (2012) | 26 | 21 | Accuracy | Healthy | S. span | Negative and positive | Task-relevant | |
| Grecucci, Soto, Rumiati, Humphreys, and Rotshtein (2010)DR | 12 | 24 | Both | Healthy | DMTS | Negative and positive | Task-irrelevant | |
| Grimm et al. (2015)DR | 541 | 46 | Accuracy | Healthy | n-back | Negative and positive | Task-relevant | |
| Grimm, Weigand, Kazzer, Jacobs, and Bajbouj (2012)DR | 20 | 24 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| Hadley and MacKay (2006) | 28 | 20 | Accuracy | Healthy | S. span | Negative | Task-relevant | |
| Han et al. (2016) | 20 | 26 | Both | Obsessive-compulsive disorder | DMTS | Negative | Task-irrelevant | Reported* |
| 21 | 23 | Healthy | ||||||
| Hubbard, Hutchison, Hambrick, and Rypma (2016)DR | 53 | Accuracy | Healthy | C. span | Negative | Task-irrelevant | ||
| 31 | Dysphoric1, c | |||||||
| Hubbard et al. (2016)DR | 45 | Accuracy | Healthy | C. span | Negative | Task-irrelevant | ||
| 30 | Dysphoric1, c | |||||||
| Iordan, Dolcos, Denkova, and Dolcos (2013)DR | 36 | 23 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| Joormann, Levens, and Gotlib (2011) | 27 | 38 | RT | Healthy | S. span | Negative and positive | Task-relevant | |
| 26 | 47 | Depression1 | ||||||
| Kellermann et al. (2012) | 36 | 25 | Accuracy | Healthy | S. span | Negative and positive | Task-irrelevant | Reported* |
| Kensinger and Corkin (2003) | 178 | 24 | Both | Healthy | + | Negative and positive | Task-relevant | |
| Kerestes et al. (2012) | 20 | 36 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| 19 | 34 | Remitted depression1, c | ||||||
| Kessel et al. (2016) | 23 | 22 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| King and Schaefer (2011) | 73 | 22 | Both | Healthy | DMTS | Negative | Task-irrelevant | |
| Kopf, Dresler, Reicherts, Herrmann, and Reif (2013) | 30 | 24 | Accuracy | Healthy | n-back | Negative and positive | Task-relevant | |
| Koster, De Lissnyder, and De Raedt (2013)DR | 30 | 21 | RTe | Healthy | S. span | Negative | Task-relevant | |
| 40 | 19 | Healthy | S. span | Negative | Task-relevant | |||
| Krämer, Mohammadi, Doñamayor, Samii, and Münte (2010) | 17 | 28 | Imaging | Healthy | DMTS | Negative | Task-relevant | Reported* |
| Krause-Utz et al. (2012) | 22 | 28 | Both | Borderline PD1 | Sternberg | Negative | Task-irrelevant | Reported* |
| 22 | 27 | Healthy | ||||||
| Krause-Utz et al. (2014) | 28 | 31 | Both | Borderline PD1 | Sternberg | Negative | Task-irrelevant | |
| 28 | 31 | Healthy | ||||||
| Ladouceur et al. (2005) | 17 | 12 | Both | Anxiety disorders1 | n-back | Negative and positive | Task-irrelevant | |
| 16 | 15 | Depression1 | ||||||
| 24 | 13 | Anxiety and depression1 | ||||||
| 18 | 12 | Healthy | ||||||
| Ladouceur et al. (2009) | 26 | 16 | Both | Healthy | n-back | Negative and positive | Task-irrelevant | |
| 31 | 15 | High trait anxiety1 | ||||||
| Ladouceur et al. (2013) | 16 | 14 | Both | High risk bipolar disorder1 | n-back | Negative and positive | Task-irrelevant | Reported* |
| 15 | 14 | Healthy | ||||||
| Laier, Schulte, and Brand (2013) | 28 | 26 | Both | Healthy | n-back | Negative, positive high arousal and positive low arousal | Task-relevant | |
| Lamm, Pine, and Fox (2013)DR | 32 | 20 | Both | Healthy | AX-CPT | Negative | Task-irrelevant | |
| LeMoult, Carver, Johnson, and Joormann (2015) | 167 | 18 | Accuracy | Healthy | C. span | Negative | Task-relevant | |
| Levens and Gotlib (2010) | 29 | 37 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 29 | 42 | Depression1 | ||||||
| Levens and Gotlib (2012) | 40 | — | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| Levens and Gotlib (2015) | 24 | 37 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 23 | 41 | Remitted depression1, c | ||||||
| Li, Li, and Luo (2006)DR | 15 | 22 | Both | Healthy | n-back | Negative | Task-irrelevant | |
| Li et al. (2009) | 23 | 15 | Imaging | Healthy | n-back | Negative | Task-irrelevant | Reported* |
| 33 | 15 | Prenatal cocaine exposure2 | ||||||
| Lim, Bruce, and Aupperle (2014) | 38 | 31 | Accuracy | Healthy | DMTS | Negative | Task-relevant | |
| Lindström and Bohlin (2011) | 55 | 24 | Accuracy | Healthy | n-back | Negative and positive | Task-relevant | |
| Luciana, Burgund, Berman, and Hanson (2001) | 19 | 22 | Accuracy | Healthy | DMTSh | Negative | Task-relevant | |
| Luksys et al. (2015) | 1,239 | — | Accuracy | Healthy | S. span | Negative and positive (DR) | Task-relevant | |
| 526 | 22 | Healthy | ||||||
| MacLean, Nichols, LeBreton, and Wilson (2016) | 17 | 27 | Accuracy | Healthy | DMTS | Positive | Task-irrelevant | |
| MacNamara, Ferri, and Hajcak (2011) | 45 | — | Both | Healthy | S. span | Negative | Task-irrelevant | |
| MacNamara, Schmidt, Zelinsky, and Hajcak (2012)DR | 16 | — | Accuracy | Healthy | S. span | Negative | Task-irrelevant | |
| MacNamara and Proudfit (2014)DR | 35 | 24 | Accuracy | Healthy | S. span | Negative | Task-irrelevant | |
| 71 | 24 | Generalized anxiety disorder1 | ||||||
| Mammarella et al. (2012) | 22 | 46 | Accuracy | Schizophrenia | C. span | Negative and positive | Task-relevant | |
| 22 | 44 | Healthy | ||||||
| Mammarella, Borella, Carretti, Leonardi, and Fairfield (2013)DR | 35 | 25 | Accuracy | Healthy | C. span | Negative and positive | Task-relevant | |
| 37 | 65 | Healthy | ||||||
| 37 | 78 | Healthy | ||||||
| Mammarella et al. (2016) | 91 | 21 | Accuracy | Healthy | C. span | Negative and positive | Task-relevant | |
| 121 | 20 | Healthy | ||||||
| Mano et al. (2013) | 60 | 22 | RT | Healthy | DMTS | Negative and positive | Task-irrelevant | |
| 29 | 19 | Healthy | ||||||
| Marx et al. (2011) | 40 | 25 | Both | Healthy | n-back | Negative | Task-irrelevant | |
| 39 | 29 | ADHD1 | ||||||
| Marx, Krause, Berger, and Häßler (2014)DR | 30 | 30 | Both | Healthy | n-back | Negative | Task-irrelevant | |
| 16 | 33 | Alcohol dependence1 | ||||||
| 22 | 28 | ADHD1 | ||||||
| Mather et al. (2006)DR | 46 | 18 | Both | Healthy | S. span | Negative | Task-relevant | Reported* |
| Meule, Skirde, Freund, Vögele, and Kübler (2012) | 56 | 24 | Both | Healthy | n-back | Positive | Task-relevant | |
| Mikels, Larkin, Reuter-Lorenz, and Cartensen (2005) | 40 | 48 | Accuracy | Healthy | n-back | Negative and positive | Task-relevant | |
| Mikels, Reuter-Lorenz, Beyer, and Fredrickson (2008)DR | 64 | 20 | Accuracy | Healthy | n-back | Negative | Task-relevant | |
| Mitchell, Mather, Johnson, Raye, and Greene (2006) | 19 | 21 | Accuracy | Healthy | S. span | Negative | Task-relevant | Reported* |
| Moon and Jeong (2015) | 18 | 37 | Both | Generalized anxiety disorder | DMTS | Negative | Task-irrelevant | Reported |
| 18 | 37 | Healthy | ||||||
| Moreno et al. (2015) | 20 | 27 | RTe | Healthy | S. span | Negative | Task-relevant | |
| 20 | 32 | Depression1 | ||||||
| 20 | 27 | Healthy after tDCS2 | ||||||
| 20 | 32 | Depression after tDCS1 | ||||||
| Morey et al. (2009)DR | 20 | 34 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported* |
| 22 | 34 | PTSD1 | ||||||
| Morey et al. (2011)DR | 20 | 37 | Both | Healthy | Reported | |||
| 22 | 31 | PTSD1 | ||||||
| Mueller et al. (2015)DR | 22 | 13 | Both | Healthy | DMTS | Negative | Task-relevant | |
| 33 | 12 | Anxiety disorder | ||||||
| Mullin et al. (2012) | 22 | 32 | Both | Bipolar disorder1 | n-back | Negative and positive | Task-irrelevant | Reported |
| 19 | 33 | Healthy | ||||||
| Neta and Whalen (2011)DR | 18 | 19 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| Noreen and Ridout (2010)DR | 22 | 23 | Accuracy | Healthy | DMTS | Negative and positive | Task-relevant | |
| 29 | 23 | Dysphoric1 | ||||||
| Oei, Tollenaar, Spinhoven, and Elzinga (2009) | 27 | 20 | Both | Healthy | Sternberg | Negative | Task-irrelevant | |
| 27 | 22 | Hydrocortisone administration2 | ||||||
| Oei, Tollenaar, Elzinga, and Spinhoven (2010) | 27 | 20 | Both | Healthy | Sternberg | Negative | Task-irrelevant | |
| 27 | 20 | Propranolol administration2 | ||||||
| Oei et al. (2012) | 16 | 24 | Both | Healthy | Sternberg | Negative | Task-irrelevant | Reported* |
| 16 | 24 | Stress induction2 | ||||||
| Onraedt and Koster (2014) | 45 | 21 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| Osaka, Yaoi, Minamoto, and Osaka (2013)DR | 26 | 24 | Imagingf | Healthy | S. span | Negative and positive | Task-relevant | Reported* |
| Pallesen, Brattico, Bailey, Korvenoja, and Gjedde (2009)DR | 10 | 25 | Accuracyi | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| Pallesen et al. (2010) | 21 | 25 | RT | Healthy | n-back | Negative and positive | Task-relevant | |
| Park, Kim, Jeong, Chung, and Yang (2016) | 15 | 36 | Accuracy | Generalized anxiety disorder | DMTS | Negative | Task-irrelevant | Reported |
| Passarotti, Sweeney, and Pavuluri (2010)DR | 19 | 13 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported* |
| 23 | 13 | Bipolar disorder1 | ||||||
| 14 | 13 | ADHD1 | ||||||
| Passarotti, Sweeney, and Pavuluri (2011) | 13 | 14 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported |
| 17 | 14 | Bipolar disorder1 | ||||||
| Passarotti, Ellis, Wegbreit, Stevens, and Pavuluri (2012) | 41 | 14 | Both | Healthy | n-back | Negative | Task-relevant | Reported |
| 16 | 15 | Bipolar disorder1 | ||||||
| Pavuluri, Passarotti, Fitzgerald, Wegbreit, and Sweeney (2012)DR | 15 | 14 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported |
| 21 | 13 | ADHD1 | ||||||
| Pehlivanoglu, Jain, Ariel, and Verhaeghen (2014) | 21 | 71 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 21 | 20 | Healthy | ||||||
| Perlstein, Elbert, and Stenger (2002) | 10 | 25 | Imagingd | Healthy | S. span | Negative and positive | Task-relevant | Reported* |
| Phillips et al. (2011)DR | 21 | 26 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 22 | 25 | First degree relative with schizophrenia1c | ||||||
| Prehn et al. (2013) | 17 | 29 | Both | Healthy | n-back | Negative | Task-irrelevant | |
| 15 | 28 | Antisocial and borderline PD1 | ||||||
| Putman, Hermans, and van Honk (2007) | 18 | 20 | Accuracy | Healthy | S. span | Negative and positive | Task-relevant | |
| 18 | 20 | Hydrocortisone administration2 | ||||||
| Rebetez, Rochat, Billieux, Gay, and Van der Linden (2015) | 88 | 23 | Both | Healthy | S. span | Negative and positive | Task-relevant | |
| Reinecke, Rinck, and Becker (2006) | 23 | 21 | Accuracy | Spider fearful1 | S. span | Negative | ||
| 23 | 21 | Healthy | ||||||
| Reinecke, Becker, and Rinck (2009) | 23 | 21 | Accuracy | Spider fearful1 | S. span | Negative | ||
| 24 | 22 | Healthy | ||||||
| 18 | 21 | Spider fearful1 | ||||||
| 19 | 21 | Healthy | ||||||
| Reinecke, Soltau, Hoyer, Becker, and Rinck (2012)DR | 29 | 31 | Accuracy | Spider fearful1 | S. span | Negative | ||
| Richter et al. (2013) | 18 | 24 | Both | Healthy | n-back | Negative | Task-relevant | Reported |
| 16 | 24 | Healthy | ||||||
| Robinaugh, Crane, Enock, and McNally (2016) | 30 | 26 | Both | Healthy | S. span | Negative and positive | Task-relevant | |
| 30 | 26 | Healthy | ||||||
| Román et al. (2015)DR | 51 | 20 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 52 | 20 | Healthy | ||||||
| Sabharwal et al. (2016)DR | 46 | 45 | Both | Psychotic disorders1 | DMTS | Negative | Task-irrelevant | Reported |
| 23 | 45 | Currently psychotic1 | ||||||
| 27 | 47 | Healthy | ||||||
| Schenkel, Passarotti, Sweeney, and Pavuluri (2012) | 31 | 13 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 23 | 13 | Bipolar disorder I1 | ||||||
| 16 | 15 | Bipolar disorder II1 | ||||||
| Schweizer and Dalgleish (2011) | 33 | 47 | Accuracy | Healthy | C. span | Negative | Task-irrelevant | |
| 20 | 46 | PTSD1 | ||||||
| Schweizer and Dalgleish (2016) | 31 | 23 | Accuracy | Healthy | C. span | Negative | Task-irrelevant | |
| 59 | 45 | Healthy | ||||||
| 15 | 46 | Healthy | ||||||
| 12 | 46 | PTSD1 | ||||||
| Schweizer et al. (2011) | 45 | 25 | Accuracy | Healthy | D. n-back | Negative | Task-relevant | |
| Schweizer et al. (2013) | 34 | 23 | Accuracy | Healthy | D. n-back | Negative | Task-relevant | Reported |
| Schweizer et al. (2018) | 123 | 41 | Accuracy | Healthy | C. span | Negative | Task-relevant | |
| 14 | 51 | Healthy | ||||||
| 21 | 52 | Depression1 | ||||||
| 20 | 37 | Healthy | ||||||
| 27 | 44 | Depression1 | ||||||
| 23 | 51 | Remitted depression1c | ||||||
| Segal, Kessler, and Anholt (2015)DR | 34 | 23 | Accuracy | Healthy | n-back | Negative and positive | Task-relevant | |
| 31 | 23 | High social anxiety1 | ||||||
| Shi, Gao, and Zhou (2014) | 53 | 20 | Accuracy | High test anxiety1 | C. span | Negative | Task-irrelevant | |
| 58 | 20 | Healthy | ||||||
| Simione et al. (2014)DR | 22 | 23 | Both | Healthy | DMTS | Negative and positive | Task-relevant | |
| 20 | 27 | Both | Healthy | DMTS | Negative and positive | Task-relevant | ||
| Simon et al. (2015)DR | 18 | 27 | Both | Healthy | n-back | Negative and positive | Task-irrelevant | Reported* |
| 18 | 27 | Sleep-deprived2 | ||||||
| Stiernströmer, Wolgast, and Johansson (2016) | 38 | 26 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| Tamm, Kreegipuu, Harro, and Cowan (2017)DR | 507 | 25 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| Tavares, Logie, and Mitchell (2016) | 39 | 22 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | |
| 53 | 21 | Healthy | ||||||
| Tavitian et al. (2014)DR | 22 | 16 | Accuracy | Depression1 | n-back | Negative and positive | Task-irrelevant | |
| 21 | 15 | Healthy | ||||||
| Tempesta, De Gennaro, Presaghi, and Ferrara (2014)DR | 25 | 24 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| 25 | 24 | Sleep-deprived2 | ||||||
| Terfehr et al. (2011) | 27 | 34 | Accuracy | Depression1 | S. span | Negative | Task-relevant | |
| 30 | 34 | Depression after hydrocortisone administration1 | ||||||
| 29 | 32 | Healthy | ||||||
| 27 | 32 | Healthy after hydrocortisone administration2 | ||||||
| Truong and Yang (2014) | 36 | 20 | Both | Healthy | DMTS | Negative and positive | Task-relevant | |
| 36 | 73 | Healthy | ||||||
| Uher, Brooks, Bartholdy, Tchanturia, and Campbell (2014) | 31 | 25 | Accuracy | Healthy | n-back | Negative and positive | Task-relevant | |
| Vanderhasselt, Brunoni, Loeys, Boggio, and De Raedt (2013) | 22 | 22 | Accuracy | Healthy | S. span | Negative | Task-relevant | |
| 22 | 22 | Healthy after tDCS2 | ||||||
| Vermeulen, Niedenthal, Pleyers, Bayot, and Corneille (2014)DR | 33 | 19 | Both | Healthy | n-back | Negative and positive | Task-relevant | |
| Visu-Petra, Ţincaş, Cheie, and Benga (2010) | 30 | 6 | Accuracy | Healthy | S. span | Negative and positive (mixed) | Task-relevant | |
| 30 | 6 | Healthy | ||||||
| Wanmaker, Geraerts, and Franken (2015) | 49 | 46 | RTe | Depression and anxiety1 | S. span | Negative | Task-relevant | |
| 49 | 47 | Depression and anxiety1 | ||||||
| Weigand et al. (2013) | 26 | 26 | Both | Healthy | n-back | Negative | Task-relevant | |
| Weigand et al. (2013) | 15 | 25 | Both | Healthy | n-back | Negative | Task-relevant | |
| Wilson, Sayette, Fiez, and Brough (2007) | 23 | 34 | Accuracy | Smokers | DMTS | Smoking-related cues | ||
| Xin and Lei (2015)DR | 33 | 22 | Both | Healthy | n-back | Negative and positive | Task-relevant | Reported |
| Yang, Wang, Jin, and Li (2015) | 14 | 24 | Accuracy | Healthy | DMTS | Negative and positive | Task-irrelevant | |
| Zhang et al. (2013) | 20 | 32 | Accuracy | Healthy | DMTS | Negative | Task-irrelevant | Reported |
| 20 | 33 | PTSD1 | ||||||
To ensure that the search was performed in accordance to the search strategy outlined above 30% of all hits at the screening stage were checked by CH and MB in addition to the first author who completed the search for all entries. Interrater agreement was 89%. All conflicts for this stage were resolved in discussion between the first author and the two additional raters. Finally, all full text studies included in the final stage were checked by the twos additional raters. For this stage there was 100% independent interrater agreement.
Analytic Approach
Behavioral analyses
Publication bias
The presence of publication biases (Rothstein, Sutton, & Borenstein, 2005) was tested in two steps. An approximation for multilevel analyses of the standard regression test (Egger, Davey Smith, Schneider, & Minder, 1997) examined whether the standard errors were significant predictors of the observed effect sizes. Considering that publication bias can vary as a function of study characteristics (Coburn & Vevea, 2015) and that we investigated several such characteristics as moderators of interest the regression analysis was supplemented with a regression test for multilevel implementation, where study variance is included as a moderator before running the rank correlation test (thereby approximating the Egger test for multilevel data).
Effect sizes
Effect sizes (Cohen’s d̂) were calculated using the escalc function in the metafor (Version 1.9–5; Viechtbauer, 2010) software package in R (Version 2.15.0; R Core Team, 2013) by dividing the mean difference of WM performance/response time in an affective versus neutral context by the unbiased estimates of the sampling variance. Unbiased estimates of the sampling variance are computed by applying a correction to the pooled standard deviation to correct for a slight positive bias within the standard error function (for a detailed discussion of the positive bias see: Hedges, 1982, p. 492; 1989).
Hypothesis testing
To test our hypotheses investigating the effects of affective context on WM performance (accuracy and response time) we conducted a random effects model analysis on the effect sizes of studies that directly compared WM in affective versus neutral contexts. This analysis was based on the premise that differences in methods and samples across the studies included in the meta-analysis would introduce variance (heterogeneity) among the true effects, which could be incorporated into the study weights (Hedges & Vevea, 1998).
The predicted moderating effects of task-relevance (target vs. distractor) and valence (positive vs. negative) were tested in the sample of healthy participants. Affective significance as a function of study population (healthy individuals vs. individuals suffering from psychopathology7) was investigated in the total sample. The association of WM and age was investigated with correlation analyses. All hypothesized moderator effects were investigated in a series of planned moderation analyses using multilevel models in which effect size (Level 1) is nested within the study (Level 2) estimated using the rma.mv() function in the metafor package (Version 1.9–5; Viechtbauer, 2010). This approach enabled the models to include multiple effect sizes from the same study. We fitted a random intercepts model, allowing effects sizes to be free to vary across studies. We chose to apply multivariate random- and mixed-effects models, because fixed-effects models have been shown to be too liberal, overestimating true effect sizes (Field, 2003; Hunter & Schmidt, 2000), whereas random-effects models are thought to provide better estimates of the true effect investigated (Schmidt, Oh, & Hayes, 2009). It should be noted, however, that random-effects models with relatively small sample sizes provide only approximations of the true effect (Schmidt et al., 2009).
All analyses were performed twice, once with WM accuracy as the outcome measure and once with WM RT.
Results of the Behavioral Meta-Analysis
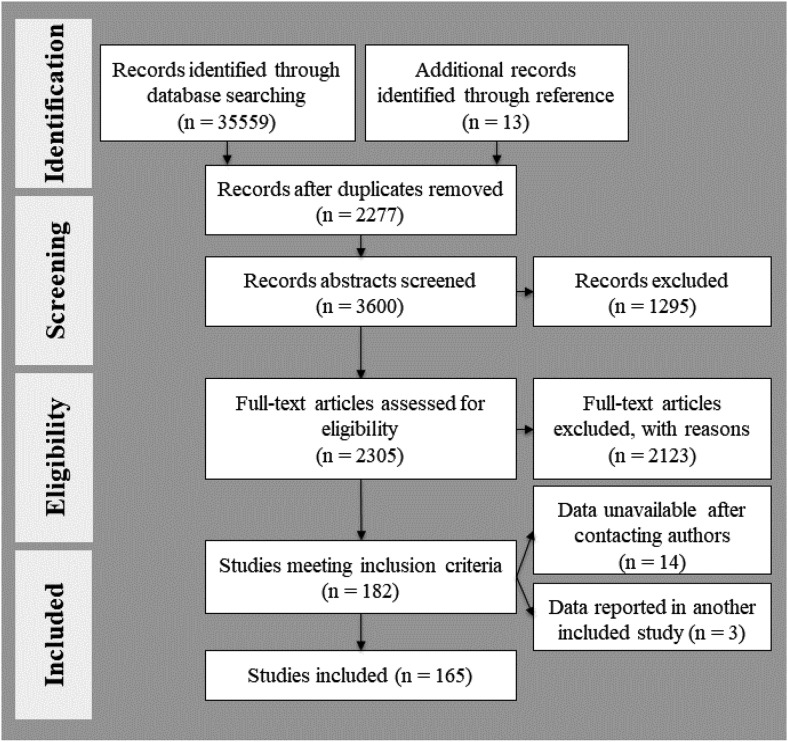
Figure 2 provides a schematic overview of the search results for the behavioral meta-analysis (PRISMA flow diagram; Moher et al., 2009). One-hundred and 65 data sets were included in the present meta-analyses. Table 1 provides a list of the included studies together with an overview of their task designs, participant samples, task-relevance and valence of the included affective stimuli, and whether the studies reported functional neuroimaging data.
Figure 2.
PRISMA flow-diagram for the behavioral meta-analysis. Reasons for exclusion are detailed in the online supplementary materials. For those meeting inclusion, data were unavailable due to departmental, personnel move, or data storage issues (de Almeida et al., 2012; DeYoung, Shamosh, Green, Braver, & Gray, 2009; Lindström & Bohlin, 2012; Maat et al., 2014; Mirabolfathi, Moradi, & Bakhtiari, 2016) and we did not receive replies from the following authors (Chen, Feng, Wang, Su, & Zhang, 2016; Diwadkar et al., 2012; Fan, Hsu, & Cheng, 2013; Gotoh, 2008; Liu, Wang, Wang, & Jiang, 2016; Luo et al., 2014; Mackay et al., 2004; Pecchinenda & Heil, 2007; Shi, Gao, & Zhou, 2015). The following publications were included as part of other citations included in the analysis (García-Pacios, Garcés, Del Río, & Maestú, 2017; Krause-Utz, Elzinga, Oei, Paret et al., 2014; Luksys et al., 2014).
Publication Bias
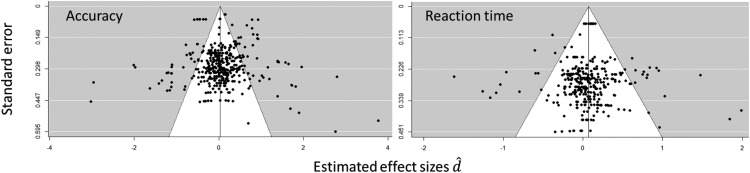
The funnel plots (see Figure 3) for accuracy and RT show the distribution of the standardized mean difference (observed outcome) between accuracy and RT for affective compared with neutral WM across the standard error distribution. The regression test of the publication bias was nonsignificant for both accuracy (z = −1.33, p = .184) and RT (z = −1.64, p = .101). The regression test for multilevel implementation was also non-significant for both accuracy (Kendall’s τ = −0.02, p = .523) and reaction time (Kendall’s τ = −0.02, p = .518), which suggests that there was no significant publication bias in the set of studies included in the meta-analytic review.
Figure 3.
Funnel plots for studies reporting accuracy and RT for the behavioral meta-analysis. For WM accuracy the plot in the left box shows, from left to right on the x-axis, studies where WM performance is impaired by the presence of affective compared with neutral stimuli through to studies where WM is more accurate in the presence of affective relative to neutral stimuli. In the right-hand box the RT plot shows the distribution of effect sizes for studies showing faster response times for affective compared with neutral from left to the right of the middle line, from which point onward studies showed slowed RTs for affective compared with neutral stimuli.
Overall Effect of Affective Context on WM in Psychologically Healthy Individuals (Hypothesis A)
Consistent with Hypothesis A, although the multivariate random effects analysis in healthy individuals showed that RTs for affective compared with neutral material in WM were significantly slowed, the effect size was of trivial magnitude k = 317, d̂ = 0.07, 95% CI [0.03, .12], SEM = 0.02, p = .002. Furthermore, there was no significant effect of affective information on WM accuracy, k = 391, d̂ = 0.03, 95% CI [−0.05, 0.12], SEM = 0.04, p = .438. In addition to the effect sizes, we report the omnibus Q-tests of heterogeneity, because the statistic is less disposed to Type I errors than other tests of heterogeneity (Viechtbauer, 2007). The estimated heterogeneities in the overall effect sizes accounted for by the differential effect of affective compared with neutral material on both WM accuracy, Q(390) = 2609.79, p ≤ .0001, σ2 = 0.21; and RT, Q(311) = 529.19, p ≤ .0001, σ2 = 0.02, were significant. That is, for both WM accuracy and RT a significant amount of variance is likely to be accounted for by variations in study-specific factors.
The moderating effects of valence (Hypothesis A1), task-relevance (Hypothesis A2), and mental health status (Hypothesis B1) and age (Hypothesis B2) are tested below. However, given the substantial amount of heterogeneity in the results we additionally explored the potentially moderating effects of emotion-type (fear, anger, sad, happy) and WM task load. Differential influences of emotion-type might partially account for the heterogeneity in the results because threat-related stimuli might be more arousing, and thus impact WM, more compared with sadness-related stimuli (Saxton, Myhre, Siyaguna, & Rokke, 2018; Vuilleumier, 2002). The rationale for WM load as an additional moderator is that it has been shown to influence attentional control (Lavie, Hirst, de Fockert, & Viding, 2004). The load theory of selective attention and cognitive control (Lavie et al., 2004) would suggest that the impact of affective relative to neutral material is greatest for lower levels of WM load. The results showed that WM RT, but not WM accuracy, was moderated by emotion type. For RT the moderating effect of emotion type reflected the valence effect (Hypothesis A1) reported below, with RTs being faster in the context of happiness-related versus neutral stimuli, whereas all negative emotions were associated with relatively slower RTs (see SM6 for a full set of statistics and results). For WM load there was no main effect of load on either WM accuracy or RT, p’s > .648. WM load did, however, interact with task-relevance, indicating that WM accuracy (not RT) for task-relevant affective, relative to neutral, targets improved across load, r(130) = .24, 95% CI [.07, .39], p = .006 (see SM6 for a full set of statistics and results).
Effects of Task-Relevance and Valence on WM Performance in Psychologically Healthy Individuals (Hypotheses A1 and A2 and Their Interaction)
For WM accuracy there were significant moderating effects of valence, k = 385, d̂ = 0.14, 95% CI [0.10, 0.19], SEM = 0.02, p ≤ .0001, QM(1) = 38.29, p ≤ .0001; and task-relevance, k = 391, d̂ = −0.24, 95% CI [−0.40, −0.07], SEM = 0.08, p = .004, QM(1) = 8.15, p = .004. The valence effect was due to positive stimuli, k = 117, d̂ = 0.12, p = .02, having a greater enhancement effect on WM accuracy compared with negative stimuli, k = 268, d̂ = 0.04, p = .38 (see Table S2 for full statistics). The effect of task-relevance was due to task-relevant affective targets, k = 257, d̂ = 0.08, p = .15, improving WM performance and task-irrelevant affective distractors impairing performance, k = 134, d̂ = −0.04, p = .55, though both effects considered alone were trivial in magnitude and neither was significant (Table S2).
These main effects on WM accuracy were qualified by a significant interaction of valence and task-relevance, k = 385, d̂ = −0.52, 95% CI [−0.70, −0.33], SEM = 0.09, p ≤ .0001, QM(3) = 78.62, p ≤ .0001. Univariate analyses (see supplemental results, SM7, for the moderating effect of valence in task-relevant and task-irrelevant stimuli separately) revealed that task-relevant targets improved WM irrespective of valence (Table 2). In contrast, negative and positive task-irrelevant distractors had opposing effects with positive distractors improving, and negative distractors impairing, performance (Table 2). However, neither of these separate effects in the context task-irrelevant distractors was significant alone.
Table 2. Effect Sizes for Each Type of Stimulus Across Task-Relevance (Task-Relevant and Irrelevant) and Valence (Positive and Negative) for WM Accuracy and WM Reaction Time.
| Stimulus type | k | d̂ | 95% CI [LB, UB] | SEM | Q |
|---|---|---|---|---|---|
| Note. The table reports effect sizes on WM accuracy and reaction time of the comparison between affective stimuli of a certain task-relevance and valence and neutral stimuli of the same task-relevance. | |||||
| † ≤ .10. * p < .05. ** p < .01. *** p ≤ .001. | |||||
| Accuracy | |||||
| Task-irrelevant distractors | |||||
| Positive | 22 | 0.11 | −0.10, 0.51 | 0.16 | 85.23*** |
| Negative | 112 | −0.07 | −0.22, 0.07 | 0.07 | 547.71*** |
| Task-relevant targets | |||||
| Positive | 95 | 0.09† | −0.00, 0.20 | 0.05 | 454.76*** |
| Negative | 156 | 0.11* | 0.00, 0.23 | 0.06 | 1350.91*** |
| Reaction time | |||||
| Task-irrelevant distractors | |||||
| Positive | 22 | 0.11* | 0.01, 0.21 | 0.05 | 39.06*** |
| Negative | 81 | 0.05 | −0.03, 0.13 | 0.04 | 123.26*** |
| Task-relevant targets | |||||
| Positive | 86 | −0.04 | −0.14, 0.06 | 0.05 | 172.03*** |
| Negative | 116 | 0.11** | 0.03, 0.18 | 0.04 | 175.41*** |
For RT neither main effects were significant, p ≥ .20. Unlike WM accuracy there was no significant heterogeneity, QM(3) = 6.80, p = .079. However, there was a significant interaction of valence and task relevance, k = 309, d̂ = −0.16, 95%CI [−0.30, .03], SEM = 0.07, p = .023. Univariate analyses showed a significantly moderating effect of valence only for targets not task-irrelevant distractors (SM7). The significant effect in targets was due to significantly slowed WM RT for negative targets, which was not observed for positive targets, which showed a non-significant speeding effect (Table 2).
Variations in Affective Significance as a Function of Mental Health Status (Hypothesis B1)
As a test of affective significance—the difference between the predicted hard prioritization afforded highly significant material versus soft prioritization (Pessoa, 2009)—we hypothesized (Hypothesis B1) that, overall, affective information will have a greater behavioral impact on WM processing in individuals suffering from mental health problems compared with psychologically healthy individuals.
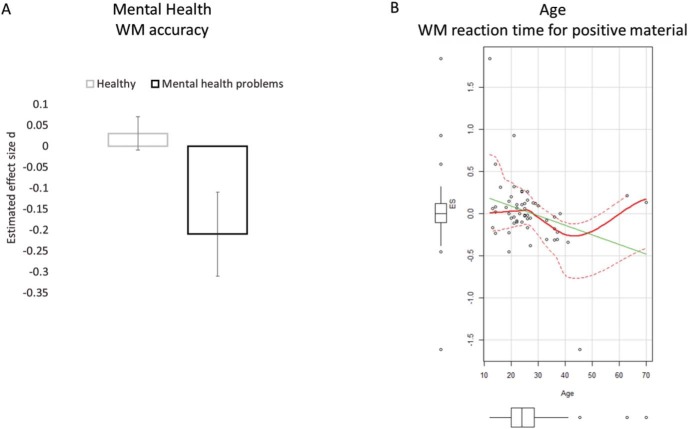
In line with Hypothesis B1, results showed that WM accuracy was significantly more impaired by affective material in those experiencing mental health problems compared with healthy individuals, k = 505, d̂ = −0.17, 95% CI [−0.26, −0.09], SEM = 0.04, p ≤ .0001, QM(1) = 17.49, p ≤ .0001 (Figure 4A) with affective stimuli having the predicted larger effect on WM performance in individuals suffering from mental health difficulties, k = 114, d̂ = −0.21, 95% CI [−0.42, −0.01], SEM = 0.10, p = .041, QM(113) = 790.96, p ≤ .0001, compared with healthy individuals (see results for Hypothesis A for a characterization of healthy performance).
Figure 4.
Affective significance across mental health status (A) and age (B). (A) The left panel depicts the effect sizes (d̂) of the difference between WM accuracy for affective compared with neutral stimuli in healthy individuals (light gray) and those suffering from mental health problems (black). (B) The right panel illustrates the association between ES = the effects size of the difference in WM RT for positive relative to neutral stimuli and age.
As in the healthy individuals (see results for Hypotheses A1 and A2), the effect of affective material in those with mental health problems was moderated by main effects of valence, k = 114, d̂ = 0.21, 95% CI [0.06, 0.36], SEM = 0.08, p = .006, QM(1) = 7.71, p = .006; and task-relevance, k = 114, d̂ = −0.59, 95% CI [−0.92, −0.27], SEM = 0.17, p = .0004, QM(1) = 12.70, p = .0004. Univariate analyses showed impairing effects on WM accuracy of similar magnitude for negative (d̂ = −0.20) and positive (d̂ = −0.25) material, although only in the case of negative stimuli was this statistically significant (Table S3). In individuals with mental health problems, task-irrelevant distractors (d̂ = −0.24) showed a greater impairing effect on WM accuracy compared with task-relevant targets (d̂ = −0.05), which did not significantly impair WM accuracy (Table S3). There were insufficient studies including positive materials across the two conditions of task-relevance to investigate the interacting effects between task-relevance and valence.
There was no effect of mental health status for WM RT, k = 409, d̂ = 0.08, 95% CI [−0.06, 0.08], SEM = 0.04, p = .774, QM(1) = 0.08, p = .774 nor was there a moderating effect of valence or task relevance, k = 95, p’s > .114.
Variations in Affective Significance Across Age (Hypothesis B2)
A second source of variation in affective significance is age, with the age-related positivity effect in attention and memory (Scheibe & Carstensen, 2010) leading to the prediction that with increasing age individuals become better at processing positive information in WM. WM accuracy showed small positive associations with age for both negative, r(143) = .17, 95% CI [0.00, 0.32], p = .045; and positive stimuli, r(73) = .15, 95% CI [−0.08, 0.37], p = .186, with older individuals remembering more affective relative to neutral material. For WM RT there was a small to moderate size significant association with RT for positive relative to neutral stimuli decreasing across age, r(53) = −.31, 95% CI [−0.53, −0.05], p = .023. That is, older individuals were faster to respond to WM tasks when the tasks included positive relative to neutral stimuli. There was no significant association between WM RT and age for negative stimuli, r(96) = .13, 95% CI [−0.07, 0.32], p = .202.
Interim Discussion: The Behavioral Meta-Analysis
In line with our Hypothesis A, in psychologically healthy individuals, although WM RTs in the presence of affective, relative to neutral, stimuli were significantly slower, the effect size was trivial in magnitude (d̂ = 0.07). We also found no significant overall effect of affective material (d̂ = 0.03) on WM accuracy. These negligible effect sizes are in line with the DCF’s assertion that the kinds of stimuli typically employed in laboratory experiments—affective words and pictures—will be afforded low affective significance and only elicit a “soft prioritization” in the system. This will result in correspondingly minimal behavioral effects, that are modulated by other study-specific and individual-differences factors beyond affective significance such as WM load, age, and the nature of the affective stimuli (e.g., words, vs. images) and interactions between them (King & Schaefer, 2011; Mano et al., 2013; Mikels, Larkin, Reuter-Lorenz, & Cartensen, 2005; Rypma & D’Esposito, 2000; Sander, Lindenberger, & Werkle-Bergner, 2012; Scheibe & Carstensen, 2010). Previous work showed opposing effects of affective distractors compared with task-relevant information on a range of cognitive processes (for a review, see Dolcos et al., 2017). In line with this work we predicted that study-specific sources of variation would be affective stimuli task-relevance and valence.
Task-Relevance Interacts With the Valence of Affective Stimuli to Impact on WM Performance
There were small significant effects of valence and task-relevance on WM accuracy in line with our Hypotheses A1 and A2. The valence effect was due to positive stimuli enhancing WM accuracy compared with negative stimuli. The effect of task-relevance was due to task-relevant affective targets improving WM performance while task-irrelevant affective distractors impaired WM performance. Importantly, there was also a moderate to large significant interaction between task-relevance and valence on WM accuracy, with task-irrelevant positive and negative distractors having no significant effects on WM accuracy (but in opposite directions), while task-relevant affective (irrespective of valence) targets significantly improved WM performance, although effects were small. Interestingly, in the absence of accuracy effects there was a trivial to small slowing effect of negative targets on WM RT. These facilitation effects suggest that relative to neutral targets, task-relevant affective targets may confer a small advantage in terms of perceptual competition. The neuroimaging meta-analysis may further elucidate this point if affective information does show a related activation increase within the brain’s attention network.
The well-documented affective enhancement effect in long-term memory is proposed to be associated with enhanced early encoding of the affective memory trace, which is then consolidated over time (Murty, Ritchey, Adcock, & LaBar, 2010). The mediation model of emotional memory (Talmi, Schimmack, Paterson, & Moscovitch, 2007) argues that the mnemonic enhancement effect is the product of three types of interrelated and interacting processes: first, the above noted prioritizing of affective information within the context of limited attentional resources (Pourtois, Schettino, & Vuilleumier, 2013; Vuilleumier, 2005); second distinctiveness, the notion that encoding of affective information is prioritized because affective relative to neutral information stands out (cf. the notion of “impact”; Ewbank, Barnard, Croucher, Ramponi, & Calder, 2009); and finally, shared thematic links (organization), which Talmi, Schimmack, Paterson, and Moscovitch (2007) argue are more easily formed between affective compared with neutral information further assisting memory encoding. These processes could similarly account for the small affective advantage observed for task-relevant affective material here in WM and could usefully be systematically explored in future research.
The small, nonsignificant enhancing effect (d̂ = 0.20) of positive distractors and the negligible impairing effect of negative distractors (lower accuracy d̂ = −0.07, slowed WM RT d̂ = 0.11) suggest that competition for perceptual or executive resources from affective distractors do not markedly affect WM performance over and above that of neutral distractors. However, the nonsignificant enhancement effect of positive distractors needs to be considered in the context of the small number of effect sizes (k = 28) from 13 studies contributing to this effect. If this small enhancing effect does replicate across a larger number of future studies, it could arguably be interpreted as reflecting the motivational impact of positive information (H. Yang, Yang, & Isen, 2013). Specifically, one could argue that positive stimuli related to reward and motivations of affiliation may focus executive processes due to the increased—relative to neutral—motivational salience of the context in which WM is engaged (Stussi, Pourtois, & Sander, 2018). To further explore the role of motivational salience in WM and executive control more broadly, careful consideration should be given to the nature of the positive and negative stimuli used in research. The type of stimuli should be theory-driven and tap into affective concerns relevant to the study population under investigation (e.g., social stimuli in adolescence; Mueller, Cromheeke, Siugzdaite, & Boehler, 2017; or negative self-referential processing in depression Schweizer et al., 2018) and the construct under investigation (e.g., survival relevance; Lindström & Bohlin, 2012).
Affective Significance
We hypothesized that the effect of affective stimuli on WM performance would vary as a function of their affective significance. Affective significance was proposed to vary as a function of both mental health status (Hypothesis B1) and age (Hypothesis B2).
Mental health status
Supporting Hypothesis B1, we found a significantly greater effect of affective relative to neutral material on WM accuracy in individuals suffering from mental health problems (d̂ = −0.21) compared with healthy individuals (d̂ = 0.03). In individuals suffering from mental health problems performance was impaired relative to neutral by both negative (d̂ = −0.20) and positive stimuli (d̂ = −0.25). Though the effect was significant only for negative stimuli. The lack of significance for positive material may reflect a power issue as only 26 effect sizes were included. Showing that both positive and negative information have an effect of similar magnitude highlights the importance of recent developments toward the investigation of hedonic processing and reward learning in individuals with psychopathology (e.g., Admon & Pizzagalli, 2015; Husain & Roiser, 2018) to complement research into the processing of negative information. The relatively greater impairment in WM performance for affective relative to neutral material is remarkable considering that this is over and above the substantial impairments in performance on affectively neutral task measures of executive functions (including WM) found in most types of mental health problems. For example, compared with healthy individuals those with depression (d̂ = 0.32–0.97; Snyder, 2013), attention-deficit and hyperactivity disorder (d̂ = 0.60–0.89; Boonstra, Oosterlaan, Sergeant, & Buitelaar, 2005), and posttraumatic stress disorder (d̂ = 0.46–0.62; Scott et al., 2015) show moderate to large impairments in executive functioning in tasks populated with neutral material.
In individuals suffering from mental health problems there was a significant effect of task-relevance, with task-irrelevant distractors having a greater impairing impact (d̂ = −0.24) compared with task-relevant targets (d̂ = −0.05). This effect of task relevance in those with mental health difficulties is in line with theories emphasizing the importance of attentional control with respect to cognitive vulnerabilities to mental health problems. Reduced inhibition of negative, especially threat-related, information in anxiety is likely to account for increased attentional resources drawn to the affective distractors that become unavailable to task-relevant processing (Bar-Haim et al., 2007; Derakshan & Eysenck, 2009; Reinholdt-Dunne, Mogg, & Bradley, 2013). In depression, the inability to disengage attention from affective distractors may similarly limit the attentional resources available to processing task-relevant information in WM (De Raedt & Koster, 2010; Everaert, Koster, & Derakshan, 2012). Affective WM tasks then may be sensitive transdiagnostically to individual differences in mental health status. While attentional control capacity has been shown to be predictive of the onset of depressive and anxiety symptoms prospectively (Kertz, Belden, Tillman, & Luby, 2016), little is known about the development of attentional control over affective information specifically (Peterson & Welsh, 2014; Prencipe et al., 2011), which may identify those at risk for mental health problems across a range of disorders.
Disorder-specific variation
A cautionary note is warranted when interpreting these findings of course because, as with psychologically healthy individuals, tests of heterogeneity for all of these effects in individuals with mental health problems were significant. It is worth rehearsing two caveats related to using mental health status as a proxy for affective significance that may partly account for this heterogeneity. First, the status “mental health” here included a wide range of mental health problems (including schizophrenia, attention deficit and hyperactivity disorder, depression, and posttraumatic stress disorder). Second, the impact of affective material is potentially and likely not uniform across this diversity of mental health conditions or across other syndrome-specific sources of variation such as phase of the syndrome (e.g., acute vs. remitted), although these remain empirical questions.
Age
The effects discussed above were limited to WM accuracy. The moderating effect of age, however, was strongest on WM RTs with effects on accuracy being trivial-to-small and unreliable. The RT results showed that, with increasing age, individuals respond more quickly on WM tasks in the context of positive information (r = −.31) with this effect being nonsignificant in the reverse direction in the context of negative information (r = .15). This is in line with the positivity effect that characterizes socioemotional selectivity theory (Carstensen, 2006), whereby older adults preferentially process positive information due to age-related motivational shifts (Mather, 2016; Mather & Carstensen, 2005). Kensinger (2008) interestingly showed that the age-related positivity effect may be particularly marked for low arousing material, whereas items high in arousal hijack attentional resources irrespective of valence. This argument is also in line with Labouvie-Vief, Grühn, and Studer’s (2010) equilibrium model, which argues that with increasing age the spectrum of acceptable emotions shrinks, in particular for negative emotions. As noted above, with one exception, all of the positive stimuli included in the current meta-analysis are arguably low in arousal and may therefore be particularly sensitive to the age-related positivity effect.
We turn next to the neuroimaging review and revisit the results of this behavioral meta-analysis in the General Discussion in light of the results of imaging data synthesis.
Neuroimaging Meta-Analysis
Functional neuroimaging studies and research in lesion patients have provided good evidence for the involvement of a fronto-parietal control network (Figure 5) in WM and other higher-order cognitive functions such as fluid intelligence (for reviews, see Duncan, 2006, 2010; Nee et al., 2013). Specifically, neural models of WM capacity have implicated this network in the active maintenance of representations and goal-states in WM, and in the control of task-related attention (Constantinidis & Klingberg, 2016; Duncan & Owen, 2000; Miller, 2000; Miller & Cohen, 2001; Nee et al., 2013; Nee, Wager, & Jonides, 2007; Owen et al., 2005; Postle, 2016). The major nodes of the frontoparietal control network include the bilateral dorsolateral prefrontal cortex (dlPFC) and the inferior parietal lobe.
Figure 5.
Fronto-parietal control network (blue) and salience network (red).
In addition to the fronto-parietal network (e.g., Coull, Frith, Frackowiak, & Grasby, 1996; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), WM, especially in the presence of affective information, may recruit portions of the so-called salience network and ventral attention network (Barrett & Satpute, 2013; Corbetta, Patel, & Shulman, 2008; Eckert et al., 2009; Seeley et al., 2007; see Figure 5), which include nodes in the anterior cingulate cortex (ACC), anterior insula, and amygdala (Seeley et al., 2007; Wilson-Mendenhall, Barrett, & Barsalou, 2013). Both of these networks are predicted by the theories reviewed above to be sensitive to the perceptual and executive competition created by affective (relative to neutral) stimuli in WM tasks (e.g., Pessoa, 2008, 2009).
Neural Substrates of Affective WM Processing
The hypothesized involvement of the amygdala during affective WM is in line with research showing that attentional capture from affective information (e.g., Pessoa & Ungerleider, 2004) is associated with increased activation of the amygdala (LeDoux, 2012; Öhman, Flykt, & Esteves, 2001; Vuilleumier, 2005; Vuilleumier & Huang, 2009). The interactive connections of the amygdala with sensory processing regions show that these effects appear early in processing and implicate the amygdala in the biasing of processing toward affective salience at a preconscious stage (Pessoa, 2005; Phelps, 2006; Whalen & Phelps, 2009).
This prioritized processing of affective information reliably shows greater recruitment of visual brain areas for affective compared with neutral stimuli (Sabatinelli et al., 2011; Satpute et al., 2015), irrespective of whether the stimuli are attended or unattended (for reviews, see Tamietto & de Gelder, 2010; Vuilleumier, 2005; Vuilleumier & Huang, 2009).
The elicited affective experience in turn may engender affect-regulatory processes that recruit components from the fronto-parietal control network (for reviews, see Buhle et al., 2014; Kalisch, 2009). Models of affect regulation have implicated the ventrolateral node (i.e., inferior frontal gyrus) of the fronto-parietal control network in the regulation of affective responses as the neural substrate of processes involved in the selection of alternative semantic interpretations of the affective material, as well as of more generic inhibitory processes (Dillon & Pizzagalli, 2007; Elliott & Deakin, 2005; Ochsner, Silvers, & Buhle, 2012). Cognitive control models of emotion regulation also implicate dorsal nodes of the fronto-parietal control network, including the dlPFC, posterior PFC and inferior parietal regions, because of their likely role underpinning the directing of selective attention and the updating of WM (Ochsner et al., 2012). Given these regions’ involvement in WM per se, however, it seems unlikely that they will be more activated during the processing of affective material relative to neutral information. Indeed, we suggest below that the opposite (greater involvement for neutral over affective) may be the case.
Neural Substrates of WM Processing of Affectively Neutral Information Relative to Affective Information
At the neural level, the theories of emotion-cognition interactions do not offer a specific prediction regarding this “reverse contrast”—the neural substrates that are recruited more frequently during WM tasks in the presence of neutral versus affective information. The DCF does however predict that, through perceptual and executive competition, affective information draws resources away from task-related processing. Consequently, it seems plausible that task-relevant brain regions should be recruited more frequently in the absence of affective material. In the case of WM, these task-related regions include the more dorsal regions of the fronto-parietal network, especially the dlPFC (Nee et al., 2013). This putative dissociation between the ventral and the dorsal streams of the fronto-parietal control network is supported by recent reviews of the neuroimaging literature showing greater involvement of the dorsal stream of the network for neutral compared with affective distractor material presented in executive functioning tasks, including WM tasks (Iordan & Dolcos, 2017; Okon-Singer et al., 2015). In contrast, and in line with our hypotheses derived from the DCF, affective distractors are associated with more frequent recruitment of the ventral stream of the network (Iordan & Dolcos, 2017; Okon-Singer et al., 2015).
To summarize, we hypothesized that:
Hypothesis C: Compared with the processing of neutral stimuli, the processing of affective stimuli would be associated with more frequent activation within the visual cortices, portions of the salience network, including the amygdala, and the ventrolateral prefrontal node within the fronto-parietal control network.
Hypothesis D: And that the reverse contrast—differential activation when processing neutral compared with affective stimuli (neutral > affective)—would be associated with more frequent neural activation within task-related regions in the dorsal components of the fronto-parietal control network.
In line with the behavioral analyses, we explored the neural correlates of affective versus neutral stimuli’s task-relevance.8 However, we were unable to explore the effects of valence or interactions between valence and task-relevance within the fMRI data because insufficient neuroimaging studies included these contrasts. Moreover, it was not possible to explore the neural correlates of the effects of affective significance because the few studies which reported neuroimaging data for individuals with mental health problems ranged across various disorders that arguably present with both overlapping and distinct anatomical and functional anomalies (Davidson et al., 2002; Dickstein, Bannon, Castellanos, & Milham, 2006; Elzinga & Bremner, 2002; Menon, 2011; Shenton, Dickey, Frumin, & McCarley, 2001), thus precluding useful data synthesis at this stage.
Methods for the Imaging Meta-Analysis
Identification and Screening of Studies
The identification and screening stages were conducted in tandem with the behavioral meta-analysis according to PRISMA guidelines.
Eligibility
We checked the full-texts of the identified studies to ascertain whether they reported fMRI data associated with the effects of affective material on WM in healthy individuals (Table 1). To be included, studies had to report functional imaging contrasts comparing neutral and affective information. Specifically, we included the contrasts examining regions showing greater activation for neutral versus affective stimuli during WM task performance and the reverse contrast identifying regions that reported greater activation for affective versus neutral stimuli during a WM task (for reasons for exclusion see SM8). The studies had to report BOLD response data on these contrasts either across the whole brain or in specified regions of interest using normalized stereotactic spaces (i.e., Montreal Neurological Institute and Hospital [MNI] or Talairach space). For each contrast, peak activations were included that were reported in the individual studies. It should be noted here that while we tested specific anatomical hypotheses (Hypotheses C and D) about the correlates of WM tasks including affective versus neutral information, the imaging meta-analytic approach we adopted was agnostic to these hypotheses and conducted across the whole brain.
Analytic Approach
We coded contrasts based on the affective qualities of the stimuli (e.g., affective vs. neutral, neutral vs. affective) and on the task-relevance (i.e., task-relevant targets vs. task-irrelevant distractors). Based on these codes, we computed multikernel density maps (procedures described below) that corresponded with the behavioral analysis. First, we examined the brain regions that were frequently engaged during affective versus neutral stimulus conditions (28 contrasts), and neutral versus affective stimulus conditions (19 contrasts). To investigate differential effects of affective material depending on task-relevance of the affective stimuli, multikernel density analysis (MKDA) maps were calculated separately for task-relevant affective targets (10 contrasts) and task-irrelevant affective distractors (18 contrasts).
Multikernel density analysis (MDKA)
The contrast maps were submitted to a MKDA, as described in detail and validated by Wager, Lindquist, and Kaplan (2007; see Kober et al., 2008; Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012) and implemented in Matlab software using the NeuroElf toolbox (www.neuroelf.net). A MKDA nests activation points within contrast maps and thereby limits the undue influence of studies that report many more activation points than others. Coordinates reported in Talairach space were transformed to MNI space using the “mni2tal” estimation procedure provided by M. Brett (http://imaging.mrc-cbu.cam.ac.uk/imaging/CbuImaging). An indicator map was generated for each study contrast by setting voxels in a 10-mm sphere surrounding each reported peak activation point to 1. Contrasts were weighted by the square root of the sample size. For each voxel, a point estimate of the probability of contrasts that activated the voxel was computed. To determine significance, for each comparison a Monte Carlo simulation (5,000 iterations) was performed that preserved the number of contrasts and coordinates within contrasts, but randomly assigned the coordinate locations to gray matter regions of the brain, and for a voxel-level threshold of p < .01, a k-extent cluster-level threshold was obtained to meet a whole-brain family wise error rate (FWER) statistical correction of p < .05.
Results of the Neuroimaging Meta-Analysis
Included Studies
Of the 165 studies identified in the behavioral meta-analysis, 52 studies included fMRI data. Of these, 19 were excluded from the neuroimaging meta-analysis (see supplementary results for reasons for exclusion and the PRISMA diagram in Figure S2). The final sample included 683 participants, 456 coordinates, and 63 contrasts from 33 studies (denoted with an asterisk in the column titled “Imaging” in Table 1). See Table S5 for an overview of the specific contrasts and number of peak activation points (i.e., coordinates) included across studies. The tasks included in the analyses are described in Table 1 with the most frequently used tasks in the neuroimaging studies reviewed being n-back (n = 13) and delayed-match-to-sample (n = 12) tasks.
Brain Regions Consistently Engaged During Affective Compared With Neutral Stimulus Conditions in WM Tasks (Hypotheses C and D)
The neuroimaging meta-analytic results supported our first neural hypothesis (Hypothesis C) that the processing of affective stimuli during WM tasks, relative to neutral stimuli, would be associated with more frequent activation across the brain’s salience network, specifically the bilateral amygdalae, and also within the ventrolateral prefrontal cortex (Figure 6A). We also found support for our second neural hypothesis (Hypothesis D), with the contrast comparing neutral with affective stimulus material being associated with more frequent activation in brain regions commonly associated with WM task performance in the dorsal stream of the fronto-parietal control network—a large node in the right dlPFC—as well as the precuneus in the inferior parietal cortex (Figure 6B). For a full list of significant clusters comparing affective with neutral stimuli, see Table S6. Given the proposed greater affective significance of negative compared with positive stimuli (Hypothesis A1) we explored the corresponding neural effects for negative and positive stimuli separately. The contrast comparing negative with neutral stimuli (Figure 6C–D; Table 3) showed the same pattern of results as the overall affective effect. In contrast, no brain regions were significantly more recruited when comparing positive with neutral stimuli.
Figure 6.
Affective stimuli as distractors or targets in WM tasks. Each panel shows brain regions that were more frequently engaged for WM contrasts comparing: (A) affective > neutral stimuli; (B) neutral > affective stimuli; (C) negative > neutral stimuli; (D) neutral > negative stimuli; (E) affective task-irrelevant distractor > affective task-relevant targets in WM tasks; and (F) task-relevant compared with irrelevant affective stimuli, for which there were no reliable activations. The color gradation in the figure indicates the frequency of recruitment of a specific region. That is, the lighter the yellow, the more frequently the region was recruited during the contrast of interest. Colored areas represent activation frequencies at p < .05, FWER corrected.
Table 3. Brain Regions Consistently Engaged During Affective Compared With Neutral Stimulus Conditions in WM Tasks.
| Region | L/R | Cluster size (voxels) | Subcluster size (voxels) | Maximum | x/y/z |
|---|---|---|---|---|---|
| Note. Table 3 reports brain regions that were significantly more frequently activated in response to one condition compared with another. Peak activations for each (sub)cluster are reported as well as the maximum statistic, which reflects the analysis of the distribution of maximum values corrected for multiple comparisons at a FWER of .05 (Salimi-Khorshidi, Smith, Keltner, Wager, and Nichols, 2009; Wager, Lindquist, and Kaplan, 2007). dl = dorsolateral; vl = ventrolateral; PFC = prefrontal cortex; OFC = orbitofrontal cortex; L = left; R = right; Maximum = maximum of the z-field. The negative > neutral comparison was based on 211 coordinates from 24 contrasts; the neutral > negative comparison was based on 144 coordinates from 20 contrasts. | |||||
| Negative > Neutral Stimuli | |||||
| vlPFC/OFC | L | 228 | .31 | −39/33/−6 | |
| 71 | −39/36/3 | ||||
| Amygdala | L | 338 | .35 | −27/−3/−18 | |
| 103 | −24/−12/−18 | ||||
| 80 | −18/0/−18 | ||||
| 46 | −21/−6/−6 | ||||
| Temporal lobe (including amygdalo-hippocampal complex) | R | 419 | .50 | 21/−6/−18 | |
| 48 | 36/0/−24 | ||||
| 51 | 39/−3/−15 | ||||
| Temporo-occipital lobe (including fusiform gyrus) | L | 648 | .29 | −39/−57/−12 | |
| 192 | −42/−78/0 | ||||
| 93 | −45/−75/12 | ||||
| 110 | −36/−51/−21 | ||||
| 36 | −51/−63/6 | ||||
| 34 | −48/−51/3 | ||||
| Temporo-occipital lobe (including fusiform gyrus) | R | 512 | .33 | 42/−54/−18 | |
| 110 | 39/−75/−12 | ||||
| 87 | 42/−45/−12 | ||||
| 70 | 42/−72/0 | ||||
| 45 | 48/−69/−12 | ||||
| 33 | 42/−54/−6 | ||||
| 60 | 54/−69/6 | ||||
| Neutral > Negative stimuli | |||||
| dlPFC | R | 505 | .49 | 36/42/30 | |
| 180 | 36/33/33 | ||||
| 34 | 27/51/12 | ||||
| 51 | 33/54/18 | ||||
| 44 | 33/39/42 | ||||
| 21 | 27/30/51 | ||||
Task-Relevance: An Exploratory Analysis of Brain Regions More Consistently Engaged When the Affective Information Is the Task-Relevant Target Versus the Task-Irrelevant Distractor9
Next, we computed differences in MKDA maps to explore which neural regions were more frequently engaged during the processing of affective relative to neutral information, separately for task-relevant targets and task-irrelevant distractors. Interestingly, given the behavioral results which showed a significant effect of affective material only for task-relevant targets, the neuroimaging effects were driven by the task-irrelevant distractors. Relative to neutral distractors affective distractors more frequently activated the bilateral vlPFC, amygdalo-hippocampal complex, and left temporo-occipital lobe (including the fusiform gyrus; see Figure 6C). The reverse contrast showed greater activation in the dlPFC for neutral compared with affective distractors. For a full list of the clusters and peak activations see Table S7. There were no significant differences in MKDA maps between contrasts that included affective compared with neutral targets.
Finally, looking at only the affective conditions comparing MKDA maps for affective task-irrelevant distractors with affective task-relevant targets revealed more frequent activation of a cluster in the right temporal lobe and subcortical regions including the amygdalo-hippocampal complex, k = 476, maxima = .71, peak coordinate = 24/0/–24 (see Figure 6E) for task-irrelevant distractors. The reverse contrast showed no brain regions to be more significantly activated for task relevant affective target compared with irrelevant distractors.
Interim Discussion of the Neuroimaging Meta-Analysis
The meta-analysis of the brain regions recruited during WM performance in the presence of affective compared with neutral stimuli confirmed our hypothesis (Hypothesis C) that affective stimuli would recruit regions from both the larger salience network (including the amygdalo-hippocampal complex) as well as ventral components of the fronto-parietal control network (i.e., vlPFC), in addition to regions in the temporo-occipital lobe, in particular the fusiform gyrus, a brain region involved in the processing of faces (Bressler & Menon, 2010; Ishai, 2008). In line with our second neural prediction (Hypothesis D), the MKDA map for brain regions that were more frequently activated for WM tasks performed with neutral (relative to affective) stimuli revealed two clusters in dorsal components of the fronto-parietal control network: one in the right dlPFC and a second cluster in the precuneus. Our exploratory analyses showed that contrasting regions activated more frequently in the presence of affective distractors compared with task-relevant targets yielded more frequent activation in the right amygdalo-hippocampal complex.
Neural Substrates of Affective WM and the Dual Competition Framework
Before discussing the meta-analytic findings, a note of caution is warranted. Any interpretations of findings from neuroimaging at the level of cognitive theory are subject to the concerns surrounding reverse inference (Poldrack, 2011). In the present case, a particular contrast, for example the presence of affective compared with neutral stimulus material in WM tasks, may be associated with more frequent activation of a given brain region. At the same time, in the wider literature, a particular cognitive process (e.g., attentional capture through salience) may have been previously putatively linked to that same region in other studies. Through a process of reverse inference, evidence supporting activation of that region in the present meta-analysis could be taken to mean that that particular cognitive process is also engaged by this contrast (Poldrack, 2006). However, of course, most brain regions and networks support a multitude of cognitive functions and so any such assumptions that the implicated processes across studies or sets of studies are the same, and specific, must only be tentative. That said, it would be remiss not to interpret the present findings within the context of the wider extant literature and so we have sought an appropriate balance of informed discussion and inferential caution.
Affective versus neutral material
Interestingly in light of negligible behavioral effects of affective information on WM performance in psychologically healthy individuals, the neuroimaging data shows that robust effects exist at the neural level of processing. The more frequent activation evident within the amygdala and the temporo-occipital lobe including the fusiform gyrus, arguably reflect the allocation of greater processing resources toward these affective, often facial, stimuli within the included studies. The more frequent activation of the amygdala is in line with the well-documented role of the amygdala in salience processing (Adolphs, 2010; Pessoa & Adolphs, 2010; Whalen & Phelps, 2009). The involvement of the inferior temporal gyrus, which has reliably been implicated in emotion regulation (for a review, see Buhle et al., 2014) may be indicative of individuals’ affect regulatory efforts in response to affective stimuli.
Increased activation frequency observed in the vlPFC has been implicated in inhibitory processing (D’Esposito, Postle, Ballard, & Lease, 1999; E. E. Smith & Jonides, 1999; Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998) and has been proposed to reflect individuals capacity to cope with the greater affective responses elicited by affective relative to neutral stimuli (Denkova et al., 2010; Dolcos, Kragel, Wang, & McCarthy, 2006). Indeed, Dolcos and McCarthy (2006) showed a clear association (r = −.74) between vlPFC activation and individuals’ ratings of affective stimuli’s distractibility during a WM task, but not for neutral distractors r = .13. The vlPFC’s role in cognitive control over affective responses has also been related to the deployment of cognitively engaging affect regulatory strategies such as reappraisal (Buhle et al., 2014; Ochsner et al., 2009, 2012). The present finding may therefore also in part reflect the implicit emotion regulation of affective material that participants likely engage in when performing tasks that contain such material. These regulatory processes may be enacted more specifically through the retrieval and/or selection of relevant semantic (Badre & Wagner, 2005, 2007) or social (Satpute, Badre, & Ochsner, 2014) information.
Task-relevance
Our exploration of the neural substrates of a stimulus’ task-relevance revealed that task-irrelevant distractors showed greater activation frequency, relative to task-relevant targets in the vlPFC, amygdalo-hippocampal complex, and temporo-occipital complex, whereas neutral distractors recruited the dlPFC more reliably. This dissociation has been observed in reviews of the neural substrates of affective distractors included in cognitive paradigms beyond WM (Dolcos & Denkova, 2014; Iordan, Dolcos, & Dolcos, 2013) and indeed has been proposed by Dolcos and colleagues across a series of studies on the impact of affective distractors on WM (e.g., Dolcos, Diaz-Granados, Wang, & McCarthy, 2008; Dolcos & McCarthy, 2006; Iordan & Dolcos, 2017). Specifically, the review by Iordan, Dolcos, Denkova, and Dolcos (2013) noted a dissociation between what they termed a ‘cold’ dorsal executive system (including the dlPFC reported in the present meta-analysis) that was recruited for neutral over affective distractors and the “hot” ventral system that includes all the areas that showed greater activation during WM tasks, including affective compared with neutral distractors, in the present meta-analysis (i.e., vlPFC, amygdala, fusiform gyrus, and visual cortex).
More recently, Dolcos and colleagues’ dorsal executive and ventral attention systems have been linked to specific functional networks to offer a systems-level dissociation between the two (e.g., Dolcos & McCarthy, 2006; Iordan & Dolcos, 2017). In particular they highlight the dorsal executive system’s integration within the wider fronto-parietal network and the ventral attention system’s overlap with the salience network for the vlPFC and amygdala (Iordan & Dolcos, 2017). As Iordan and Dolcos (2017) note, this functional dissociation extends beyond a simplistic attribution of bottom-up processes to a ventral system and top-down executive functions to a dorsal system (cf., Pfeifer & Allen, 2012), instead emphasizing the contribution of both systems in emotion processing (e.g., showing valence specific effects in the lateral parietal cortex of the fronto-parietal network/dorsal executive system; Iordan & Dolcos, 2017; Iordan, Dolcos, & Dolcos, 2018) and control.
Finally, of the neuroimaging studies investigating the effects of task-irrelevant distractors, 60% (nine of 15) were studies including variants of the delayed-match-to-sample task (e.g., Dolcos & McCarthy, 2006). These tasks introduce a temporal latency between the distractors and memoranda (Daniel, Katz, & Robinson, 2016), which might place greater demands on executive compared with perceptual competition.
General Discussion
Our aim with these two meta-analytic reviews was to help advance understanding of how human cognition operates in affectively laden environments, by synthesizing data on the impact of affective information on WM and the neural correlates of this effect. WM is implicated in virtually all day-to-day cognition (Barrett et al., 2004; Engle & Kane, 2004; Miyake & Shah, 1999) and much of its operation takes place in affective contexts, ranging from the overt manipulation of affective information to the performance of relatively neutral tasks in the context of affectively laden goals and plans. The studies reviewed here have tried to measure these forms of interplay by looking at WM in affective versus comparatively affect-neutral contexts within the laboratory and scanner using carefully controlled tasks.10 The challenge inherent in these tasks is to pursue the relatively neutral task goals while dealing with affectively laden contexts of different types as a proxy for the challenges faced in day-to-day cognition.
Our findings show that neural and behavioral data reviews and syntheses can complement each other; in this case with evidence for widespread neural engagement that arguably reflects broader cognitive engagement than the resultant behavioral data reveal (Barrett, 2009). This is a vindication of models such as Pessoa’s (2009) DCF and others (e.g., the conceptual act theory, Barrett, 2014; the model of the cognitive control of emotions, Ochsner et al., 2012) that seek to generate and integrate sets of both behavioral and neural predictions. These complementary insights from the current set of reviews further highlight the importance for future data synthesis endeavors of including, where possible, measures of behavioral performance as well as functional neuroimaging data. While this conclusion appears self-evident there is a surprising lack of meta-analytic reviews that integrate findings in this manner.
Dissociable Behavioral and Neural Effects of Affective Information Across Task-Relevance
The dissociation between behavioral and neural findings in healthy individuals was strongest for the moderating effect of task-relevance. Interestingly, across the behavioral studies affective targets had a negligible-to-small enhancing effect on WM accuracy, whereas the effect of affective distractors was small and dependent on valence. The neuroimaging meta-analysis, however, showed that affective distractors led to more frequent recruitment of the predicted brain regions (including, amygdala, vlPFC) whereas affective targets did not. Moreover, task-irrelevant affective distractors had a greater impairing effect on WM accuracy compared with affective targets in individuals with mental health issues.
The differential behavioral and neural effects of affective stimuli on WM in healthy individuals arguably evidence the efficiency of the cognitive control system in mitigating any impact of affective information on performance. The increased recruitment of the vlPFC may reflect the organism’s effort to inhibit attention and responses toward these distractors and regulate any affective experience elicited by the distractor. This is particularly adaptive in our contemporary environments that are populated with myriads of affective distractors (e.g., phone alerts). This dissociation of behavioral and neural results observed in healthy individuals is in line with research into the interaction between affect and other types of cognition including long-term memory (Erk, von Kalckreuth, & Walter, 2010). This dissociation appears to be maintained across time (Erk et al., 2010), with behavioral memory performance for affective material being unaffected by whether individuals had been instructed to regulate their affective responses to the memoranda at encoding 12 months prior (in line with Dolcos, Labar, & Cabeza, 2005). At the neural level, however, amygdala activation during encoding of affective items that were viewed without attempts to downregulate affective experiences was stronger than amygdala activation to items encoded 12 months prior while individuals were attempting to regulate their affective responses. The reviewed evidence further suggests that it is in particular the connectivity between this vlPFC node and the amygdalo-hippocampal complex that reflects the efficacy of healthy individuals in controlling any potential interference from affective information in WM (Krause-Utz, Elzinga, Oei, Paret et al., 2014; Ladouceur et al., 2013; Ziaei, Salami, & Persson, 2017). Interestingly, Ladouceur et al. (2013) showed reduced downregulation of amygdala reactivity by the vlPFC in response to negative and positive distractors in young people with a parent suffering from bipolar disorder compared with a healthy age-matched sample. This differential pattern of neural activation across groups was observed in the absence of behavioral performance differences. Altered functional connectivity during WM performance in the presence of affective compared with neutral material may therefore constitute a sensitive marker for mental health problems before the behavioral differences that were observed in the current behavioral meta-analysis emerge. Together the studies lend support to the argument that competition for resources from affective information is being routinely resolved in the vlPFC.
In mental ill health, however, maladaptive behavioral responses and involuntary attentional engagement with affective distractors are characteristic of many disorders (e.g., anxiety disorders; Bar-Haim et al., 2007). WM performance and its neural substrates in the presence of affective distractors may therefore constitute a source of individual differences associated with mental health problems. In line with this argument, Menon’s (2011) triple neural network model of mental health proposes that weak mapping from the salience network is involved (among other things) in “[. . .] aberrant bottom-up detection of salient events, [and] aberrant control signals to other large-scale networks that facilitate access to attention and working memory resources, [. . .]” (Menon, 2011, p. 501). That is, mental health problems may be associated with particularly impaired WM performance in the presence of affective distractors due to both aberrant salience attribution to affective information at the perceptual level of competition as well as impaired control at the executive level of competition.
Affective Significance in Mental Health and Across the Life Span
A critical prediction, although somewhat underresearched in the literature, is the impact of stimuli’s degree of affective significance on executive performance. Here we used age and mental health status as proxies for affective significance. In line with our predictions older people were faster to respond to positive material and WM performance in individuals with mental health problems was significantly impaired by affective information.
The changing impact of affective information on WM performance across the life span
The age results were in line with the age-related positivity effect shown in the attention and memory literature (for a meta-analytic review, see Reed, Chan, & Mikels, 2014). However, little is known about the development of WM in affective contexts from childhood through into adulthood. Of the included studies fewer than 10% (n = 14) were conducted in children and/or adolescents (Bertocci et al., 2014; Cromheeke & Mueller, 2016; Ladouceur et al., 2005, 2013, 2009; Mueller et al., 2015; Passarotti, Ellis, Wegbreit, Stevens, & Pavuluri, 2012, 2010, 2011; Pavuluri, Passarotti, Fitzgerald, Wegbreit, & Sweeney, 2012; Schenkel, Passarotti, Sweeney, & Pavuluri, 2012; Tavitian et al., 2014; Visu-Petra, Ţincaş, Cheie, & Benga, 2010; Z. Li et al., 2009) and there was no study of the typical development of affective WM. This is particularly surprising given that affective WM in developmental samples may provide evidence for those at risk for emotional disorders by virtue of problems with affective control capacity. Moreover, interventions that augment executive control in affective contexts may constitute efficient forms of prevention, especially when administered early in development (Wass, Porayska-Pomsta, & Johnson, 2011).
Pathways to competition from affective information in individuals with mental health problems
There are likely to be variations in the pathways through which the effects of affective significance create perceptual and executive competition across different mental health disorders. Arguably, differences in affective significance may exert their impact on perceptual competition in a similar way across diverse forms of psychopathology, whereas the intersection of affective significance and executive competition may rely upon different mechanisms across disorders. For example, engaging in cognitively costly emotion regulation strategies (including rumination in depression, or suppression in anxiety disorders; Aldao et al., 2010) in response to affective stimuli versus increased executive competition due to resources deployed to disambiguate affective information in schizophrenia (Kohler, Walker, Martin, Healey, & Moberg, 2010). Similarly, there are likely to be variances in the relative affective significance of the stimuli included in standard experimental paradigms across disorders. Despite these potential differences, all of the mental health disorders included in the current behavioral meta-analysis have been shown to be associated with affective dysregulation: alcohol dependence (Cheetham, Allen, Yücel, & Lubman, 2010); anxiety disorders (Cisler, Olatunji, Feldner, & Forsyth, 2010); attention-deficit and hyperactivity disorder (Graziano & Garcia, 2016; Shaw, Stringaris, Nigg, & Leibenluft, 2014); borderline personality disorder (Carpenter & Trull, 2013); mood disorders (Hofmann, Sawyer, Fang, & Asnaani, 2012; Townsend & Altshuler, 2012); obsessive–compulsive disorder (Calkins, Berman, & Wilhelm, 2013); PTSD (Frewen & Lanius, 2006); and schizophrenia (Horan, Kring, & Blanchard, 2006; Trémeau, 2006). Poor WM performance in the presence of affective material then may be a transdiagnostic marker of dysregulated affect across these disorders.
At the neural level the paucity of available studies means that the current analysis cannot speak to finer-grained questions concerning the neural substrates of the effects of affective significance across disorders. As and when further evidence emerges on affective WM from each disorder, future meta-analyses should investigate the interaction between behavioral and neuroimaging findings in these clinical populations. We currently know little about the neural substrates associated with individual differences in affective WM and potentially different pathways to interference from affective information across mental health problems. As with the posited cognitive-level pathways, the neural signatures are argued to be both overlapping and distinct across different types of mental health problems. Interestingly, the networks proposed in Menon’s (2011) triple neural network model of mental health overlap with the neural networks shown in the current meta-analysis to be associated with the effects of affective stimuli on WM performance (i.e., the salience and fronto-parietal control networks). Future research is warranted to explore the neural substrates of affective WM both within and across disorders.
Future Directions
These behavioral and neural reviews focus on the interplay and integration between affective and cognitive processing. Here we offer some suggestions for potential next steps in this endeavor. A primary aim, we submit, should be to refine and provide empirical evaluation of neurobehavioral models of cognitive functioning in both intrinsic (e.g., affective states) and/or extrinsic (e.g., facial expression) affective contexts. Empirical support of, or challenges to, these models are currently typically offered by experimental tasks performed in laboratory settings, such as the affective WM tasks reviewed here. Empirical evidence for the influence of affective material in the real world, however, is scarce. That said, preliminary, yet critical, attempts have recently been made to embed and relate the findings from these laboratory measures to exerting affective control in everyday environments (Pe, Brose, Gotlib, & Kuppens, 2016; Pe, Raes, Koval et al., 2013; Pe, Raes, & Kuppens, 2013; Quinn & Joormann, 2015a, 2015b). For example, in an experience sampling study (N = 95), Pe, Koval, and Kuppens (2013) showed that affective WM updating ability predicted individuals’ ability to down-regulate high-arousal negative affective states (e.g., experiencing anger), but not low-arousal negative affective states (e.g., dysphoria). The study further showed differential associations between WM updating ability and self-reported tendencies to use rumination and reappraisal as emotion regulation strategies. These types of studies provide important insights into how executive control, as measured on laboratory tasks, may be sensitive to some but not all types of executive control over affective input in daily life. Similarly, the current analyses were limited in exploring only the impact of externally presented affective material. In daily life, however, executive control is often taxed and arguably impacted on by internally generated affective information (e.g., thoughts, memories). In a recent study, Iordan, Dolcos, and Dolcos (2018) show that autobiographical memories processed with an emotion-focus, compared with a context-focus, impair WM performance.
Inherently linked to the notion of embedding findings from tasks assessing executive control over affective information in our understanding of quotidian human cognition is the construct of emotion regulation. WM in affective contexts has been posited as central to contemporary models of emotion regulation (Etkin, Büchel, & Gross, 2015). A recent example stems from a meta-analysis, which showed that repetitive negative thinking, a maladaptive emotion regulation strategy (Ehring & Watkins, 2008; McEvoy, Mahoney, & Moulds, 2010) commonly observed in mood and anxiety disorders (Klemanski, Curtiss, McLaughlin, & Nolen-Hoeksema, 2017; Spinhoven, Drost, van Hemert, & Penninx, 2015), is selectively associated with difficulties in discarding task-irrelevant material from WM (Zetsche, Bürkner, & Schulze, 2018). Similarly, the current study showed that task-irrelevant distractors impair WM performance (d̂ = −0.24), unlike task-relevant information (d̂ = −0.05), in individuals with mental health problems.
Investigating the association between emotion regulation at diverse levels of analysis (from self-report to experience sampling in everyday life) and performance on affective WM tasks could advance our understanding of the role of higher-order cognitive control in emotion regulation and open new avenues for intervention (Engen & Kanske, 2013; Schweizer & Dalgleish, 2013). Preliminary studies have shown that training affective WM can improve individuals’ executive control over affective stimuli across executive functions (e.g., on an affective Stroop task; Schweizer, Hampshire, & Dalgleish, 2011) as well as their emotion regulation capacity (Schweizer et al., 2013). However, to optimize the success of such endeavors, we require mechanistic accounts of the role of cognitive control in mental health, beyond merely showing deficits in specific processes (Grahek, Everaert, Krebs, & Koster, 2018). In their important opinion article Grahek, Everaert, Krebs, and Koster (2018) propose that, in order to advance our understanding of the role of cognitive control in mental health, we require a multifaceted approach integrating the affective, cognitive and motivational domain rather than viewing them as separate processes merely interacting with each other.
Finally, all analyses showed considerable remaining heterogeneity. That is, the moderators included (i.e., valence, task-relevance, mental health status, age, emotion type, and WM load) accounted for only part of the variance in the effect of affective relative to neutral information on WM performance. Understanding the effects of other individual difference variables (e.g., factors that influence affective processing including gender and personality; Fischer, Kret, & Broekens, 2018; Hamann & Canli, 2004; Kret & De Gelder, 2012; Speed et al., 2015) not modeled in the current analyses will therefore constitute an important next step in elucidating the impact of affective information on WM performance. Characterizing the relation between these individual differences and the impact of affective information on cognition is especially relevant in the context of recent findings showing that self-relevant information may particularly tax executive resources (Dai, Rahman, Lau, Sook Kim, & Deldin, 2015; Hubbard, Hutchison, Hambrick et al., 2016; Hubbard, Hutchison, Turner et al., 2016; Iordan et al., 2018; though see Schweizer et al., 2018, Experiment 3).
Conclusions
The present meta-analyses support theoretical proposals concerning the complex interplay between affective information and WM performance. Based on the current state of science, affective information has only a negligible effect on behavioral measures of WM in healthy individuals. At the neural level, however, processing affective versus neutral material during WM is associated with more frequent recruitment of the vlPFC, the amygdala, and the temporo-occipital cortex. The behavioral impact of affective information appears to be augmented in individuals for whom affective stimuli carry greater affective significance. Compared with healthy individuals, those suffering from mental health problems show a small and reliable impairment of WM accuracy in the presence of affective material and older adults show faster RTs in WM tasks including positive material. These findings suggest that investigating the impact of affective information on executive performance can provide an important window into the understanding of individuals’ cognitive functioning in affectively valenced everyday environments.
Supplementary Material
Footnotes
There is a multitude of competing theories of WM, generating vibrant debate around definitions and underlying mechanisms (cf. Miyake & Shah, 1999) which we acknowledge. An indebt discussion of these debates, however, is beyond the scope of this review.
It should be noted that the arousal-biased competition model (Mather & Sutherland, 2011), qualifies as an exception to this enhancement. This theory suggests that WM performance for multiple task-relevant items will be impaired for affective compared with neutral targets. The rationale is that, while maintaining affective stimuli in WM, the prioritized attentional processing afforded to the active affective item increases the costs for the competing stimuli more than for less arousing neutral items.
However, see Brosch, Sander, Pourtois, and Scherer (2008) for highly similar patterns of attentional capture for both negative and positive pictures.
Studies that simply presented a delay interval without distractors or a secondary task were not included as we consider them to be better conceptualized as perceptual, short-term or long-term memory tasks depending on the delay interval.
There is no published review protocol for the two meta-analyses reported in this article.
The asterisks in the search term combinations denote so-called wild cards in the database search. That is, every permutation of the term is entered and searched within the database (e.g., emoti* performs searches including emotive, emotion, emotional, etc., . . .).
Some studies included neuropsychological populations. Data from these samples were excluded from the moderation analyses, as they were not part of the investigation of interest. The healthy control samples from those studies, however, were included in the moderation analyses.
It was not possible to look at the comparisons between neutral and affective target stimuli, because there were insufficient studies reporting the contrast neutral > affective.
There were insufficient contrasts including neutral memoranda to look at the effects of affective versus neutral stimuli across task-relevant and task-irrelevant material.
It should be noted here that theorists have argued that there is no affect-free cognition (Barrett, 2006, 2009; Lindquist, 2013) and seemingly well-validated “neutral” stimuli elicit significant amounts of ambivalence, which is related to arousal (Schneider, Veenstra, van Harreveld, Schwarz, & Koole, 2016).
References
- Admon R., & Pizzagalli D. A. (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114–118. 10.1016/j.copsyc.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. 10.1111/j.1749-6632.2010.05445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A., Nolen-Hoeksema S., & Schweizer S. (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30, 217–237. 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Aleman A., & Kahn R. S. (2005). Strange feelings: Do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Progress in Neurobiology, 77, 283–298. 10.1016/j.pneurobio.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Allen R. J., Schaefer A., & Falcon T. (2014). Recollecting positive and negative autobiographical memories disrupts working memory. Acta Psychologica, 151, 237–243. 10.1016/j.actpsy.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Alonso-Recio L., Martín-Plasencia P., Loeches-Alonso Á., & Serrano-Rodríguez J. M. (2014). Working memory and facial expression recognition in patients with Parkinson’s disease. Journal of the International Neuropsychological Society, 20, 496–505. 10.1017/S1355617714000265 [DOI] [PubMed] [Google Scholar]
- Al-Shawaf L., Conroy-Beam D., Asao K., & Buss D. M. (2016). Human emotions: An evolutionary psychological perspective. Emotion Review, 8, 173–186. 10.1177/1754073914565518 [DOI] [Google Scholar]
- Amaral D. G., Behniea H., & Kelly J. L. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118, 1099–1120. 10.1016/S0306-4522(02)01001-1 [DOI] [PubMed] [Google Scholar]
- Amir N., & Bomyea J. (2011). Working memory capacity in generalized social phobia. Journal of Abnormal Psychology, 120, 504–509. 10.1037/a0022849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo R., Fredes F., Kinoshita M., Aoki R., Aizawa H., Agetsuma M., et al. Okamoto H. (2014). The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron, 84, 1034–1048. 10.1016/j.neuron.2014.10.035 [DOI] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., & Barch D. M. (2010). Resisting emotional interference: Brain regions facilitating working memory performance during negative distraction. Cognitive, Affective & Behavioral Neuroscience, 10, 159–173. 10.3758/CABN.10.2.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Corlett P. R., & Barch D. M. (2011). Negative and nonemotional interference with visual working memory in schizophrenia. Biological Psychiatry, 70, 1159–1168. 10.1016/j.biopsych.2011.07.010 [DOI] [PubMed] [Google Scholar]
- Aron A. R., Robbins T. W., & Poldrack R. A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–177. 10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Aronen E. T., Vuontela V., Steenari M. R., Salmi J., & Carlson S. (2005). Working memory, psychiatric symptoms, and academic performance at school. Neurobiology of Learning and Memory, 83, 33–42. 10.1016/j.nlm.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Artuso C., Palladino P., & Ricciardelli P. (2012). How do we update faces? Effects of gaze direction and facial expressions on working memory updating. Frontiers in Psychology, 3, 362 10.3389/fpsyg.2012.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti E.-M., Torheim H. K., & Melinder A. (2014). The effect of emotional facial expressions on children’s working memory: Associations with age and behavior. Child Neuropsychology, 20, 86–105. 10.1080/09297049.2012.749225 [DOI] [PubMed] [Google Scholar]
- Avery R. E., Smillie L. D., & de Fockert J. W. (2013). The role of working memory in achievement goal pursuit. Acta Psychologica, 144, 361–372. 10.1016/j.actpsy.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Baddeley A. (2003). Working memory: Looking back and looking forward. Nature Reviews Neuroscience, 4, 829–839. 10.1038/nrn1201 [DOI] [PubMed] [Google Scholar]
- Baddeley A. D. (2013). Working memory and emotion: Ruminations on a theory of depression. Review of General Psychology, 17, 20–27. 10.1037/a0030029 [DOI] [Google Scholar]
- Badre D., & Wagner A. D. (2005). Frontal lobe mechanisms that resolve proactive interference. Cerebral Cortex, 15, 2003–2012. 10.1093/cercor/bhi075 [DOI] [PubMed] [Google Scholar]
- Badre D., & Wagner A. D. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. 10.1016/j.neuropsychologia.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Bakvis P., Spinhoven P., Putman P., Zitman F. G., & Roelofs K. (2010). The effect of stress induction on working memory in patients with psychogenic nonepileptic seizures. Epilepsy & Behavior, 19, 448–454. 10.1016/j.yebeh.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Balodis I. M., Johnsrude I. S., & Olmstead M. C. (2007). Intact preference conditioning in acute intoxication despite deficient declarative knowledge and working memory. Alcoholism, Clinical and Experimental Research, 31, 1800–1810. 10.1111/j.1530-0277.2007.00482.x [DOI] [PubMed] [Google Scholar]
- Banks J. B., Tartar J. L., & Tamayo B. A. (2015). Examining factors involved in stress-related working memory impairments: Independent or conditional effects? Emotion, 15, 827–836. 10.1037/emo0000096 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M. J., & van IJzendoorn M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133, 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Barrett L. F. (2005). Feeling is perceiving: Core affect and conceptualization in the experience of emotion In Barrett L. F., Niedenthal P. M., & Winkielman P. (Eds.), Emotion and consciousness (pp. 255–284). New York, NY: Guilford Press. [Google Scholar]
- Barrett L. F. (2006). Valence is a basic building block of emotional life. Journal of Research in Personality, 40, 35–55. 10.1016/j.jrp.2005.08.006 [DOI] [Google Scholar]
- Barrett L. F. (2009). The future of psychology: Connecting mind to brain. Perspectives on Psychological Science, 4, 326–339. 10.1111/j.1745-6924.2009.01134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L. F. (2013). Psychological construction: The Darwinian approach to the science of emotion. Emotion Review, 5, 379–389. 10.1177/1754073913489753 [DOI] [Google Scholar]
- Barrett L. F. (2014). The conceptual act theory: A précis. Emotion Review, 6, 292–297. 10.1177/1754073914534479 [DOI] [Google Scholar]
- Barrett L. F., & Satpute A. B. (2013). Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23, 361–372. 10.1016/j.conb.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L. F., Tugade M. M., & Engle R. W. (2004). Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin, 130, 553–573. 10.1037/0033-2909.130.4.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer I. E., Jordan G., Soares J. C., & Meyer T. D. (2015). The role of negative mood induction on working memory capacity in individuals putatively at risk for bipolar disorder: A pilot study. Journal of Affective Disorders, 185, 60–66. 10.1016/j.jad.2015.05.068 [DOI] [PubMed] [Google Scholar]
- Becerril K., & Barch D. (2011). Influence of emotional processing on working memory in schizophrenia. Schizophrenia Bulletin, 37, 1027–1038. 10.1093/schbul/sbq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwé M., Deroost N., Koster E. H. W., De Lissnyder E., & De Raedt R. (2014). Worrying and rumination are both associated with reduced cognitive control. Psychological Research, 78, 651–660. 10.1007/s00426-013-0517-5 [DOI] [PubMed] [Google Scholar]
- Belham F. S., Satler C., Garcia A., Tomaz C., Gasbarri A., Rego A., & Tavares M. C. (2013). Age-related differences in cortical activity during a visuo-spatial working memory task with facial stimuli. PLoS ONE, 8, e75778 10.1371/journal.pone.0075778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneventi H., Barndon R., Ersland L., & Hugdahl K. (2007). An fMRI study of working memory for schematic facial expressions. Scandinavian Journal of Psychology, 48, 81–86. 10.1111/j.1467-9450.2007.00536.x [DOI] [PubMed] [Google Scholar]
- Bennett D. S., Mohamed F. B., Carmody D. P., Malik M., Faro S. H., & Lewis M. (2013). Prenatal tobacco exposure predicts differential brain function during working memory in early adolescence: A preliminary investigation. Brain Imaging and Behavior, 7, 49–59. 10.1007/s11682-012-9192-1 [DOI] [PubMed] [Google Scholar]
- Berger C., Erbe A.-K., Ehlers I., Marx I., Hauenstein K., & Teipel S. (2015). Effects of task-irrelevant emotional stimuli on working memory processes in mild cognitive impairment. JAD, 44, 439–453. 10.3233/JAD-141848 [DOI] [PubMed] [Google Scholar]
- Bergmann H. C., Rijpkema M., Fernández G., & Kessels R. P. (2012). The effects of valence and arousal on associative working memory and long-term memory. PLoS ONE, 7, e52616 10.1371/journal.pone.0052616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. G., Nee D. E., Casement M., Kim H. S., Deldin P., Kross E., et al. Jonides J. (2011). Neural and behavioral effects of interference resolution in depression and rumination. Cognitive, Affective & Behavioral Neuroscience, 11, 85–96. 10.3758/s13415-010-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci M. A., Bebko G. M., Mullin B. C., Langenecker S. A., Ladouceur C. D., Almeida J. R. C., & Phillips M. L. (2012). Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychological Medicine, 42, 1417–1428. 10.1017/S003329171100242X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci M. A., Bebko G., Olino T., Fournier J., Hinze A. K., Bonar L., et al. Phillips M. L. (2014). Behavioral and emotional dysregulation trajectories marked by prefrontal-amygdala function in symptomatic youth. Psychological Medicine, 44, 2603–2615. 10.1017/S0033291714000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A. M., Oosterlaan J., Sergeant J. A., & Buitelaar J. K. (2005). Executive functioning in adult ADHD: A meta-analytic review. Psychological Medicine, 35, 1097–1108. 10.1017/S003329170500499X [DOI] [PubMed] [Google Scholar]
- Borella E., Carretti B., Grassi M., Nucci M., & Sciore R. (2014). Are age-related differences between young and older adults in an affective working memory test sensitive to the music effects? Frontiers in Aging Neuroscience, 6, 298 10.3389/fnagi.2014.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg C., Leroy N., Favre E., Laurent B., & Thomas-Antérion C. (2011). How emotional pictures influence visuospatial binding in short-term memory in ageing and Alzheimer’s disease? Brain and Cognition, 76, 20–25. 10.1016/j.bandc.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Boutelle K. N., Wierenga C. E., Bischoff-Grethe A., Melrose A. J., Grenesko-Stevens E., Paulus M. P., & Kaye W. H. (2014). Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. International Journal of Obesity, 39, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B. P., & Lang P. J. (1999). Affective Norms for English Words (ANEW): Instruction manual and affective ratings. Gainesville, FL: The Center for Research Psychophysiology, University of Florida. [Google Scholar]
- Bressler S. L., & Menon V. (2010). Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14, 277–290. 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Brosch T., Sander D., Pourtois G., & Scherer K. R. (2008). Beyond fear: Rapid spatial orienting toward positive emotional stimuli. Psychological Science, 19, 362–370. 10.1111/j.1467-9280.2008.02094.x [DOI] [PubMed] [Google Scholar]
- Brose A., Lövdén M., & Schmiedek F. (2014). Daily fluctuations in positive affect positively co-vary with working memory performance. Emotion, 14, 1–6. 10.1037/a0035210 [DOI] [PubMed] [Google Scholar]
- Brose A., Schmiedek F., Lövdén M., & Lindenberger U. (2012). Daily variability in working memory is coupled with negative affect: The role of attention and motivation. Emotion, 12, 605–617. 10.1037/a0024436 [DOI] [PubMed] [Google Scholar]
- Brosschot J. F., & Thayer J. F. (2003). Heart rate response is longer after negative emotions than after positive emotions. International Journal of Psychophysiology, 50, 181–187. 10.1016/S0167-8760(03)00146-6 [DOI] [PubMed] [Google Scholar]
- Brunyé T. T., Howe J. L., Walker L. A., & Mahoney C. R. (2013). Acute bouts of endurance exercise increase distractibility to emotional stimuli. International Journal of Sport Psychology, 44, 471–492. [Google Scholar]
- Buchanan T. W., & Adolphs R. (2002). The role of the human amygdala in emotional modulation of long-term declarative memory. Advances in Consciousness Research, 44, 9–34. 10.1075/aicr.44.02buc [DOI] [Google Scholar]
- Buckert M., Kudielka B. M., Reuter M., & Fiebach C. J. (2012). The COMT Val158Met polymorphism modulates working memory performance under acute stress. Psychoneuroendocrinology, 37, 1810–1821. 10.1016/j.psyneuen.2012.03.014 [DOI] [PubMed] [Google Scholar]
- Buhle J. T., Silvers J. A., Wager T. D., Lopez R., Onyemekwu C., Kober H., et al. Ochsner K. N. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratto L. G., Pottage C. L., Brown C., Morrison C. M., & Schaefer A. (2014). The effects of a distracting N-back task on recognition memory are reduced by negative emotional intensity. PLoS ONE, 9, e110211 10.1371/journal.pone.0110211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhan A. M., Anazodo U. C., Chung J. K., Arena A., Graff-Guerrero A., & Mitchell D. G. (2016). The effect of task-irrelevant fearful-face distractor on working memory processing in mild cognitive impairment versus healthy controls: An exploratory fMRI study in female participants. Behavioural Neurology. Advance online publication 10.1155/2016/1637392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins A. W., Berman N. C., & Wilhelm S. (2013). Recent advances in research on cognition and emotion in OCD: A review. Current Psychiatry Reports, 15, 357 10.1007/s11920-013-0357-4 [DOI] [PubMed] [Google Scholar]
- Cao H., Plichta M. M., Schäfer A., Haddad L., Grimm O., Schneider M., et al. Tost H. (2014). Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. NeuroImage, 84, 888–900. 10.1016/j.neuroimage.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Carpenter R. W., & Trull T. J. (2013). Components of emotion dysregulation in borderline personality disorder: A review. Current Psychiatry Reports, 15, 335 10.1007/s11920-012-0335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen L. L. (2006). The influence of a sense of time on human development. Science, 312, 1913–1915. 10.1126/science.1127488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A., Allen N. B., Yücel M., & Lubman D. I. (2010). The role of affective dysregulation in drug addiction. Clinical Psychology Review, 30, 621–634. 10.1016/j.cpr.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Chen X., Feng Z., Wang T., Su H., & Zhang L. (2016). Internal switching and backward inhibition in depression and rumination. Psychiatry Research, 243, 342–348. 10.1016/j.psychres.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Chen Y., Norton D., McBain R., Ongur D., & Heckers S. (2009). Visual and cognitive processing of face information in schizophrenia: Detection, discrimination and working memory. Schizophrenia Research, 107, 92–98. 10.1016/j.schres.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah L. Y. M., Dolcos F., Chen A. K., Zheng H., Parimal S., & Chee M. W. L. (2010). Sleep deprivation and interference by emotional distracters. Sleep, 33, 1305–1313. 10.1093/sleep/33.10.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J. M., Olatunji B. O., Feldner M. T., & Forsyth J. P. (2010). Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathology and Behavioral Assessment, 32, 68–82. 10.1007/s10862-009-9161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. L., & Huntsinger J. R. (2007). How emotions inform judgment and regulate thought. Trends in Cognitive Sciences, 11, 393–399. 10.1016/j.tics.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn K. M., & Vevea J. L. (2015). Publication bias as a function of study characteristics. Psychological Methods, 20, 310–330. 10.1037/met0000046 [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Perlstein W. M., Braver T. S., Nystrom L. E., Noll D. C., Jonides J., & Smith E. E. (1997). Temporal dynamics of brain activation during a working memory task. Nature, 386, 604–608. 10.1038/386604a0 [DOI] [PubMed] [Google Scholar]
- Colligan S. M., & Koven N. S. (2015). Interference resolution in emotional working memory as a function of alexithymia. The American Journal of Psychology, 128, 337–345. 10.5406/amerjpsyc.128.3.0337 [DOI] [PubMed] [Google Scholar]
- Constantinidis C., & Klingberg T. (2016). The neuroscience of working memory capacity and training. Nature Reviews Neuroscience, 17, 438–449. 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- Conway A. R. A., Kane M. J., Bunting M. F., Hambrick D. Z., Wilhelm O., & Engle R. W. (2005). Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review, 12, 769–786. 10.3758/BF03196772 [DOI] [PubMed] [Google Scholar]
- Cook I. A., Bookheimer S. Y., Mickes L., Leuchter A. F., & Kumar A. (2007). Aging and brain activation with working memory tasks: An fMRI study of connectivity. International Journal of Geriatric Psychiatry, 22, 332–342. 10.1002/gps.1678 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., & Shulman G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58, 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., & Shulman G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Coull J. T., Frith C. D., Frackowiak R. S. J., & Grasby P. M. (1996). A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia, 34, 1085–1095. 10.1016/0028-3932(96)00029-2 [DOI] [PubMed] [Google Scholar]
- Courtney S. M., Petit L., Maisog J. M., Ungerleider L. G., & Haxby J. V. (1998). An area specialized for spatial working memory in human frontal cortex. Science, 279, 1347–1351. 10.1126/science.279.5355.1347 [DOI] [PubMed] [Google Scholar]
- Cromheeke S., Herpoel L.-A., & Mueller S. C. (2014). Childhood abuse is related to working memory impairment for positive emotion in female university students. Child Maltreatment, 19, 38–48. 10.1177/1077559513511522 [DOI] [PubMed] [Google Scholar]
- Cromheeke S., & Mueller S. C. (2016). The power of a smile: Stronger working memory effects for happy faces in adolescents compared to adults. Cognition and Emotion, 30, 288–301. 10.1080/02699931.2014.997196 [DOI] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Smith A., Taylor E., & Rubia K. (2012). A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex, 48, 194–215. 10.1016/j.cortex.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Curci A., Lanciano T., Soleti E., & Rimé B. (2013). Negative emotional experiences arouse rumination and affect working memory capacity. Emotion, 13, 867–880. 10.1037/a0032492 [DOI] [PubMed] [Google Scholar]
- Dai Q., Rahman S., Lau B., Sook Kim H., & Deldin P. (2015). The influence of self-relevant materials on working memory in dysphoric undergraduates. Psychiatry Research, 229, 858–866. 10.1016/j.psychres.2015.07.068 [DOI] [PubMed] [Google Scholar]
- Daniel T. A., Katz J. S., & Robinson J. L. (2016). Delayed match-to-sample in working memory: A BrainMap meta-analysis. Biological Psychology, 120, 10–20. 10.1016/j.biopsycho.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. J., Lewis D. A., Alloy L. B., Amaral D. G., Bush G., Cohen J. D., et al. Peterson B. S. (2002). Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry, 52, 478–502. 10.1016/S0006-3223(02)01458-0 [DOI] [PubMed] [Google Scholar]
- Deckersbach T., Rauch S. L., Buhlmann U., Ostacher M. J., Beucke J.-C., Nierenberg A. A., et al. Dougherty D. D. (2008). An fMRI investigation of working memory and sadness in females with bipolar disorder: A brief report. Bipolar Disorders, 10, 928–942. 10.1111/j.1399-5618.2008.00633.x [DOI] [PubMed] [Google Scholar]
- De Lissnyder E., Koster E. H. W., & De Raedt R. (2012). Emotional interference in working memory is related to rumination. Cognitive Therapy and Research, 36, 348–357. 10.1007/s10608-011-9352-4 [DOI] [Google Scholar]
- De Lissnyder E., Koster E. H. W., Everaert J., Schacht R., Van den Abeele D., & De Raedt R. (2012). Internal cognitive control in clinical depression: General but no emotion-specific impairments. Psychiatry Research, 199, 124–130. 10.1016/j.psychres.2012.04.019 [DOI] [PubMed] [Google Scholar]
- De Lissnyder E., Koster E. H. W., Goubert L., Onraedt T., Vanderhasselt M. A., & De Raedt R. (2012). Cognitive control moderates the association between stress and rumination. Journal of Behavior Therapy and Experimental Psychiatry, 43, 519–525. 10.1016/j.jbtep.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Demeyer I., De Lissnyder E., Koster E. H. W., & De Raedt R. (2012). Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: A prospective study in remitted depressed adults. Behaviour Research and Therapy, 50, 292–297. 10.1016/j.brat.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Denkova E., Wong G., Dolcos S., Sung K., Wang L., Coupland N., & Dolcos F. (2010). The impact of anxiety-inducing distraction on cognitive performance: A combined brain imaging and personality investigation. PLoS ONE, 5, e14150 10.1371/journal.pone.0014150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt R., & Koster E. H. W. (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience, 10, 50–70. 10.3758/CABN.10.1.50 [DOI] [PubMed] [Google Scholar]
- Derakshan N., & Eysenck M. W. (2009). Anxiety, processing efficiency, and cognitive performance: New developments from attentional control theory. European Psychologist, 14, 168–176. 10.1027/1016-9040.14.2.168 [DOI] [Google Scholar]
- D’Esposito M., Postle B. R., Ballard D., & Lease J. (1999). Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition, 41, 66–86. 10.1006/brcg.1999.1096 [DOI] [PubMed] [Google Scholar]
- DeYoung C. G., Shamosh N. A., Green A. E., Braver T. S., & Gray J. R. (2009). Intellect as distinct from Openness: Differences revealed by fMRI of working memory. Journal of Personality and Social Psychology, 97, 883–892. 10.1037/a0016615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. T., He G., Gadde S., Bellion C., Belger A., Voyvodic J. T., & McCarthy G. (2011). The influence of emotional distraction on verbal working memory: An fMRI investigation comparing individuals with schizophrenia and healthy adults. Journal of Psychiatric Research, 45, 1184–1193. 10.1016/j.jpsychires.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein S. G., Bannon K., Castellanos F. X., & Milham M. P. (2006). The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. Journal of Child Psychology and Psychiatry, 47, 1051–1062. 10.1111/j.1469-7610.2006.01671.x [DOI] [PubMed] [Google Scholar]
- Dillon D. G., & Pizzagalli D. A. (2007). Inhibition of action, thought, and emotion: A selective neurobiological review. Applied & Preventive Psychology, 12, 99–114. 10.1016/j.appsy.2007.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth-Bart J., Poehlmann J., Hilgendorf A. E., Miller K., & Lambert H. (2010). Maternal scaffolding and preterm toddlers’ visual-spatial processing and emerging working memory. Journal of Pediatric Psychology, 35, 209–220. 10.1093/jpepsy/jsp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar V. A., Pruitt P., Zhang A., Radwan J., Keshavan M. S., Murphy E., et al. Zajac-Benitez C. (2012). The neural correlates of performance in adolescents at risk for schizophrenia: Inefficiently increased cortico-striatal responses measured with fMRI. Journal of Psychiatric Research, 46, 12–21. 10.1016/j.jpsychires.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. L., Thiruchselvam R., Todd R., & Christoff K. (2017). Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin, 143, 1033–1081. 10.1037/bul0000096 [DOI] [PubMed] [Google Scholar]
- Doallo S., Holguín S. R., & Cadaveira F. (2006). Attentional load affects automatic emotional processing: Evidence from event-related potentials. NeuroReport, 17, 1797–1801. 10.1097/01.wnr.0000246325.51191.39 [DOI] [PubMed] [Google Scholar]
- Döhnel K., Sommer M., Ibach B., Rothmayr C., Meinhardt J., & Hajak G. (2008). Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia, 46, 37–48. 10.1016/j.neuropsychologia.2007.08.012 [DOI] [PubMed] [Google Scholar]
- Dolan R. J. (2002). Emotion, cognition, and behavior. Science, 298, 1191–1194. 10.1126/science.1076358 [DOI] [PubMed] [Google Scholar]
- Dolcos F., & Denkova E. (2014). Current emotion research in cognitive neuroscience: Linking enhancing and impairing effects of emotion on cognition. Emotion Review, 6, 362–375. 10.1177/1754073914536449 [DOI] [Google Scholar]
- Dolcos F., Diaz-Granados P., Wang L., & McCarthy G. (2008). Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia, 46, 326–335. 10.1016/j.neuropsychologia.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Dolcos F., Iordan A. D., Kragel J., Stokes J., Campbell R., McCarthy G., & Cabeza R. (2013). Neural correlates of opposing effects of emotional distraction on working memory and episodic memory: An event-related FMRI investigation. Frontiers in Psychology, 4, 293 10.3389/fpsyg.2013.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Katsumi Y., Denkova E., & Dolcos S. (2017). Factors influencing opposing effects of emotion on cognition: A review of evidence from research on perception and memory In Opris I. & Casanova M. F. (Eds.), The physics of the mind and brain disorders: Integrated neural circuits supporting the emergence of mind (pp. 297–341). Cham, Switzerland: Springer International Publishing; 10.1007/978-3-319-29674-6_14 [DOI] [Google Scholar]
- Dolcos F., Kragel P., Wang L., & McCarthy G. (2006). Role of the inferior frontal cortex in coping with distracting emotions. NeuroReport, 17, 1591–1594. 10.1097/01.wnr.0000236860.24081.be [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K. S., & Cabeza R. (2005). Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences of the United States of America, 102, 2626–2631. 10.1073/pnas.0409848102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., & McCarthy G. (2006). Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience, 26, 2072–2079. 10.1523/JNEUROSCI.5042-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorahy M. J., Irwin H. J., & Middleton W. (2004). Assessing markers of working memory function in dissociative identity disorder using neutral stimuli: A comparison with clinical and general population samples. The Australian and New Zealand Journal of Psychiatry, 38, 47–55. 10.1177/000486740403800101 [DOI] [PubMed] [Google Scholar]
- Dowson J. H., Blackwell A. D., Turner D. C., Harvey E., Malhotra T., Robbins T. W., & Sahakian B. J. (2007). Questionnaire ratings of attention-deficit/hyperactivity disorder (ADHD) in adults are associated with spatial working memory. European Psychiatry, 22, 256–263. 10.1016/j.eurpsy.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Drapier D., Surguladze S., Marshall N., Schulze K., Fern A., Hall M. H., et al. McDonald C. (2008). Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biological Psychiatry, 64, 513–520. 10.1016/j.biopsych.2008.04.038 [DOI] [PubMed] [Google Scholar]
- Dretsch M. N., & Tipples J. (2008). Working memory involved in predicting future outcomes based on past experiences. Brain and Cognition, 66, 83–90. 10.1016/j.bandc.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Duncan J. (2006). EPS Mid-Career Award 2004: Brain mechanisms of attention. Quarterly Journal of Experimental Psychology: Human Experimental Psychology, 59, 2–27. 10.1080/17470210500260674 [DOI] [PubMed] [Google Scholar]
- Duncan J. (2010). The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14, 172–179. 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Duncan J., & Owen A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23, 475–483. 10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Eckert M. A., Menon V., Walczak A., Ahlstrom J., Denslow S., Horwitz A., & Dubno J. R. (2009). At the heart of the ventral attention system: The right anterior insula. Human Brain Mapping, 30, 2530–2541. 10.1002/hbm.20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein R. S. (2006). Attachment and emotional memory: Investigating the source and extent of avoidant memory impairments. Emotion, 6, 340–345. 10.1037/1528-3542.6.2.340 [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M., & Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Journal of Medicine, 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T., & Watkins E. R. (2008). Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy, 1, 192–205. 10.1521/ijct.2008.1.3.192 [DOI] [Google Scholar]
- El-Hage W., Gaillard P., Isingrini M., & Belzung C. (2006). Trauma-related deficits in working memory. Cognitive Neuropsychiatry, 11, 33–46. 10.1080/13546800444000164 [DOI] [PubMed] [Google Scholar]
- Elliott R., & Deakin B. (2005). Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology, 65, 89–116. 10.1016/S0074-7742(04)65004-5 [DOI] [PubMed] [Google Scholar]
- Elzinga B. M., & Bremner J. D. (2002). Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders, 70, 1–17. 10.1016/S0165-0327(01)00351-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard I. M., van den Hout M. A., & Smeets M. A. M. (2011). Taxing working memory reduces vividness and emotional intensity of images about the Queen’s Day tragedy. Journal of Behavior Therapy and Experimental Psychiatry, 42, 32–37. 10.1016/j.jbtep.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Engen H., & Kanske P. (2013). How working memory training improves emotion regulation: Neural efficiency, effort, and transfer effects. The Journal of Neuroscience, 33, 12152–12153. 10.1523/JNEUROSCI.2115-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle R. W., & Kane M. J. (2004). Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychology of Learning and Motivation, 44, 145–200. 10.1016/S0079-7421(03)44005-X [DOI] [Google Scholar]
- Epperson C. N., Amin Z., Ruparel K., Gur R., & Loughead J. (2012). Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology, 37, 372–382. 10.1016/j.psyneuen.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Kleczar A., & Walter H. (2007). Valence-specific regulation effects in a working memory task with emotional context. NeuroImage, 37, 623–632. 10.1016/j.neuroimage.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Erk S., von Kalckreuth A., & Walter H. (2010). Neural long-term effects of emotion regulation on episodic memory processes. Neuropsychologia, 48, 989–996. 10.1016/j.neuropsychologia.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Etkin A., Büchel C., & Gross J. J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16, 693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Evans D. E., Craig C., Oliver J. A., & Drobes D. J. (2011). The smoking N-back: A measure of biased cue processing at varying levels of cognitive load. Nicotine & Tobacco Research, 13, 88–93. 10.1093/ntr/ntq214 [DOI] [PubMed] [Google Scholar]
- Everaert J., Koster E. H. W., & Derakshan N. (2012). The combined cognitive bias hypothesis in depression. Clinical Psychology Review, 32, 413–424. 10.1016/j.cpr.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Ewbank M. P., Barnard P. J., Croucher C. J., Ramponi C., & Calder A. J. (2009). The amygdala response to images with impact. Social Cognitive and Affective Neuroscience, 4, 127–133. 10.1093/scan/nsn048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M. W., Derakshan N., Santos R., & Calvo M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. 10.1037/1528-3542.7.2.336 [DOI] [PubMed] [Google Scholar]
- Fairfield B., Mammarella N., & Di Domenico A. (2015). Motivated goal pursuit and working memory: Are there age-related differences? Motivation and Emotion, 39, 201–215. 10.1007/s11031-014-9428-z [DOI] [Google Scholar]
- Fairfield B., Mammarella N., Di Domenico A., & Palumbo R. (2015). Running with emotion: When affective content hampers working memory performance. International Journal of Psychology, 50, 161–164. 10.1002/ijop.12101 [DOI] [PubMed] [Google Scholar]
- Fales C. L., Becerril K. E., Luking K. R., & Barch D. M. (2010). Emotional-stimulus processing in trait anxiety is modulated by stimulus valence during neuroimaging of a working-memory task. Cognition and Emotion, 24, 200–222. 10.1080/02699930903384691 [DOI] [Google Scholar]
- Fan Y.-T., Hsu Y.-Y., & Cheng Y. (2013). Sex matters: N-back modulates emotional mismatch negativity. NeuroReport, 24, 457–463. 10.1097/WNR.0b013e32836169b9 [DOI] [PubMed] [Google Scholar]
- Faridi N., Karama S., Burgaleta M., White M. T., Evans A. C., Fonov V., et al. Waber D. P. (2015). Neuroanatomical correlates of behavioral rating versus performance measures of working memory in typically developing children and adolescents. Neuropsychology, 29, 82–91. 10.1037/neu0000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P. (2002). Advantage for emotional words in immediate and delayed memory tasks: Could it be explained in terms of processing capacity? The Spanish Journal of Psychology, 5, 78–89. 10.1017/S1138741600005850 [DOI] [PubMed] [Google Scholar]
- Field A. P. (2003). The problems in using fixed-effects models of meta-analysis on real-world data. Understanding Statistics: Statistical Issues in Psychology, Education, and the Social Sciences, 2, 105–124. [Google Scholar]
- Fischer A. H., Kret M. E., & Broekens J. (2018). Gender differences in emotion perception and self-reported emotional intelligence: A test of the emotion sensitivity hypothesis. PLoS ONE, 13, e0190712 10.1371/journal.pone.0190712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. (2008). Emotion science: Cognitive and neuroscientific approaches to understanding human emotions. New York, NY: Palgrave Macmillan; 10.1007/978-1-137-07946-6 [DOI] [Google Scholar]
- Freeman D., Startup H., Dunn G., Černis E., Wingham G., Pugh K., et al. Kingdon D. (2013). The interaction of affective with psychotic processes: A test of the effects of worrying on working memory, jumping to conclusions, and anomalies of experience in patients with persecutory delusions. Journal of Psychiatric Research, 47, 1837–1842. 10.1016/j.jpsychires.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P. A., & Lanius R. A. (2006). Toward a psychobiology of posttraumatic self-dysregulation: Reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences, 1071, 110–124. 10.1196/annals.1364.010 [DOI] [PubMed] [Google Scholar]
- Fuge P., Aust S., Fan Y., Weigand A., Gärtner M., Feeser M., et al. Grimm S. (2014). Interaction of early life stress and corticotropin-releasing hormone receptor gene: Effects on working memory. Biological Psychiatry, 76, 888–894. 10.1016/j.biopsych.2014.04.016 [DOI] [PubMed] [Google Scholar]
- Gallagher M., & Holland P. C. (1994). The amygdala complex: Multiple roles in associative learning and attention. Proceedings of the National Academy of Sciences of the United States of America, 91, 11771–11776. 10.1073/pnas.91.25.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pacios J., Del Río D., & Maestú F. (2014). State anxiety in healthy people can increase their vulnerability to neutral but not to unpleasant distraction in working memory. Cliníca y Salud, 25, 181–185. 10.1016/j.clysa.2014.10.002 [DOI] [Google Scholar]
- García-Pacios J., Del Río D., Villalobos D., Ruiz-Vargas J. M., & Maestú F. (2015). Emotional interference-based forgetting in short-term memory. Cognitive inhibition of pleasant but not unpleasant biologically relevant distractors. Frontiers in Psychology, 6, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pacios J., Garcés P., Del Río D., & Maestú F. (2015). Early detection and late cognitive control of emotional distraction by the prefrontal cortex. Scientific Reports, 5, 10046 10.1038/srep10046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pacios J., Garcés P., Del Río D., & Maestú F. (2017). Tracking the effect of emotional distraction in working memory brain networks: Evidence from an MEG study. Psychophysiology, 54, 1726–1740. 10.1111/psyp.12912 [DOI] [PubMed] [Google Scholar]
- Garrison K. E., & Schmeichel B. J. (2018). Effects of emotional content on working memory capacity. Cognition and Emotion, 33, 370–377. 10.1080/02699931.2018.1438989 [DOI] [PubMed] [Google Scholar]
- Gärtner M., Rohde-Liebenau L., Grimm S., & Bajbouj M. (2014). Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology, 43, 105–113. 10.1016/j.psyneuen.2014.02.009 [DOI] [PubMed] [Google Scholar]
- Gathmann B., Pawlikowski M., Schöler T., & Brand M. (2014). Performing a secondary executive task with affective stimuli interferes with decision making under risk conditions. Cognitive Processing, 15, 113–126. 10.1007/s10339-013-0584-y [DOI] [PubMed] [Google Scholar]
- Giles G. E., Mahoney C. R., Urry H. L., Brunyé T. T., Taylor H. A., & Kanarek R. B. (2015). Omega-3 fatty acids and stress-induced changes to mood and cognition in healthy individuals. Pharmacology, Biochemistry, and Behavior, 132, 10–19. 10.1016/j.pbb.2015.02.018 [DOI] [PubMed] [Google Scholar]
- Gilmartin M. R., Balderston N. L., & Helmstetter F. J. (2014). Prefrontal cortical regulation of fear learning. Trends in Neurosciences, 37, 455–464. 10.1016/j.tins.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J., Rose M., & Büchel C. (2007). Independent effects of emotion and working memory load on visual activation in the lateral occipital complex. The Journal of Neuroscience, 27, 4366–4373. 10.1523/JNEUROSCI.3310-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohier B., Ferracci L., Surguladze S. A., Lawrence E., El Hage W., Kefi M. Z., et al. Le Gall D. (2009). Cognitive inhibition and working memory in unipolar depression. Journal of Affective Disorders, 116, 100–105. 10.1016/j.jad.2008.10.028 [DOI] [PubMed] [Google Scholar]
- Gokcen S., Bora E., Erermis S., Kesikci H., & Aydin C. (2009). Theory of mind and verbal working memory deficits in parents of autistic children. Psychiatry Research, 166, 46–53. 10.1016/j.psychres.2007.11.016 [DOI] [PubMed] [Google Scholar]
- González-Garrido A. A., López-Franco A. L., Gómez-Velázquez F. R., Ramos-Loyo J., & Sequeira H. (2015). Emotional content of stimuli improves visuospatial working memory. Neuroscience Letters, 585, 43–47. 10.1016/j.neulet.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garrido A. A., Ramos-Loyo J., Gomez-Velazquez F. R., Alvelais Alarcón M., & Moises de la Serna Tuya J. (2007). Visual verbal working memory processing may be interfered by previously seen faces. International Journal of Psychophysiology, 65, 141–151. 10.1016/j.ijpsycho.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Gooding D. C., & Tallent K. A. (2003). Spatial, object, and affective working memory in social anhedonia: An exploratory study. Schizophrenia Research, 63, 247–260. 10.1016/S0920-9964(02)00326-2 [DOI] [PubMed] [Google Scholar]
- Gooding D. C., & Tallent K. A. (2004). Nonverbal working memory deficits in schizophrenia patients: Evidence of a supramodal executive processing deficit. Schizophrenia Research, 68, 189–201. 10.1016/j.schres.2003.07.007 [DOI] [PubMed] [Google Scholar]
- Goolsby B. A., Shapiro K. L., & Raymond J. E. (2009). Distractor devaluation requires visual working memory. Psychonomic Bulletin & Review, 16, 133–138. 10.3758/PBR.16.1.133 [DOI] [PubMed] [Google Scholar]
- Gotlib I. H., & Joormann J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh F. (2008). Influence of affective valence on working memory processes. International Journal of Psychology, 43, 59–71. 10.1080/00207590701318306 [DOI] [PubMed] [Google Scholar]
- Gotoh F. (2012). Affective valence of words impacts recall from auditory working memory. Journal of Cognitive Psychology, 24, 117–124. 10.1080/20445911.2011.589380 [DOI] [Google Scholar]
- Gottfried J. A., O’Doherty J., & Dolan R. J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301, 1104–1107. 10.1126/science.1087919 [DOI] [PubMed] [Google Scholar]
- Grahek I., Everaert J., Krebs R. M., & Koster E. H. W. (2018). Cognitive control in depression: Toward clinical models informed by cognitive neuroscience. Clinical Psychological Science, 6, 464–480. 10.1177/2167702618758969 [DOI] [Google Scholar]
- Graziano P. A., & Garcia A. (2016). Attention-deficit hyperactivity disorder and children’s emotion dysregulation: A meta-analysis. Clinical Psychology Review, 46, 106–123. 10.1016/j.cpr.2016.04.011 [DOI] [PubMed] [Google Scholar]
- Grecucci A., Soto D., Rumiati R. I., Humphreys G. W., & Rotshtein P. (2010). The interrelations between verbal working memory and visual selection of emotional faces. Journal of Cognitive Neuroscience, 22, 1189–1200. 10.1162/jocn.2009.21276 [DOI] [PubMed] [Google Scholar]
- Grimm S., Gärtner M., Fuge P., Fan Y., Weigand A., Feeser M., et al. Bajbouj M. (2015). Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene modulates age effects on working memory. Journal of Psychiatric Research, 61, 57–63. 10.1016/j.jpsychires.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Grimm S., Weigand A., Kazzer P., Jacobs A. M., & Bajbouj M. (2012). Neural mechanisms underlying the integration of emotion and working memory. NeuroImage, 61, 1188–1194. 10.1016/j.neuroimage.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Grissmann S., Faller J., Scharinger C., Spüler M., & Gerjets P. (2017). Electroencephalography based analysis of working memory load and affective valence in an N-back task with emotional stimuli. Frontiers in Human Neuroscience. Advance online publication 10.3389/fnhum.2017.00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald M., Weiss T., Mueller S., & Rall L. (2014). EEG changes caused by spontaneous facial self-touch may represent emotion regulating processes and working memory maintenance. Brain Research, 1557, 111–126. 10.1016/j.brainres.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Habel U., Koch K., Pauly K., Kellermann T., Reske M., Backes V., et al. Schneider F. (2007). The influence of olfactory-induced negative emotion on verbal working memory: Individual differences in neurobehavioral findings. Brain Research, 1152, 158–170. 10.1016/j.brainres.2007.03.048 [DOI] [PubMed] [Google Scholar]
- Hadley C. B., & Mackay D. G. (2006). Does emotion help or hinder immediate memory? Arousal versus priority-binding mechanisms. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32, 79–88. 10.1037/0278-7393.32.1.79 [DOI] [PubMed] [Google Scholar]
- Haldane M., Jogia J., Cobb A., Kozuch E., Kumari V., & Frangou S. (2008). Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. European Neuropsychopharmacology, 18, 48–54. 10.1016/j.euroneuro.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Hamann S. (2001). Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences, 5, 394–400. 10.1016/S1364-6613(00)01707-1 [DOI] [PubMed] [Google Scholar]
- Hamann S., & Canli T. (2004). Individual differences in emotion processing. Current Opinion in Neurobiology, 14, 233–238. 10.1016/j.conb.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Han H. J., Jung W. H., Yun J.-Y., Park J. W., Cho K. K., Hur J.-W., et al. Kwon J. S. (2016). Disruption of effective connectivity from the dorsolateral prefrontal cortex to the orbitofrontal cortex by negative emotional distraction in obsessive-compulsive disorder. Psychological Medicine, 46, 921–932. 10.1017/S0033291715002391 [DOI] [PubMed] [Google Scholar]
- Hedges L. V. (1982). Estimation of effect size from a series of independent experiments. Psychological Bulletin, 92, 490–499. 10.1037/0033-2909.92.2.490 [DOI] [Google Scholar]
- Hedges L. V. (1989). An unbiased correction for sampling error in validity generalization studies. Journal of Applied Psychology, 74, 469–477. 10.1037/0021-9010.74.3.469 [DOI] [Google Scholar]
- Hedges L. V., & Vevea J. L. (1998). Fixed- and random-effects models in meta-analysis. Psychological Methods, 3, 486–504. 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- Hillary F. G., Chiaravalloti N. D., Ricker J. H., Steffener J., Bly B. M., Lange G., et al. DeLuca J. (2003). An investigation of working memory rehearsal in multiple sclerosis using fMRI. Journal of Clinical and Experimental Neuropsychology, 25, 965–978. 10.1076/jcen.25.7.965.16490 [DOI] [PubMed] [Google Scholar]
- Hofmann S. G., Sawyer A. T., Fang A., & Asnaani A. (2012). Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety, 29, 409–416. 10.1002/da.21888 [DOI] [PubMed] [Google Scholar]
- Hood A., Pulvers K., Spady T. J., Kliebenstein A., & Bachand J. (2015). Anxiety mediates the effect of acute stress on working memory performance when cortisol levels are high: A moderated mediation analysis. Anxiety, Stress, and Coping, 28, 545–562. 10.1080/10615806.2014.1000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W. P., Kring A. M., & Blanchard J. J. (2006). Anhedonia in schizophrenia: A review of assessment strategies. Schizophrenia Bulletin, 32, 259–273. 10.1093/schbul/sbj009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Tan S. P., Walsh S. C., Spriggens L. K., Neumann D. L., Shum D. H., & Chan R. C. (2014). Working memory dysfunctions predict social problem solving skills in schizophrenia. Psychiatry Research, 220, 96–101. 10.1016/j.psychres.2014.07.043 [DOI] [PubMed] [Google Scholar]
- Hubbard N. A., Hutchison J. L., Hambrick D. Z., & Rypma B. (2016). The enduring effects of depressive thoughts on working memory. Journal of Affective Disorders, 190, 208–213. 10.1016/j.jad.2015.06.056 [DOI] [PubMed] [Google Scholar]
- Hubbard N. A., Hutchison J. L., Turner M., Montroy J., Bowles R. P., & Rypma B. (2016). Depressive thoughts limit working memory capacity in dysphoria. Cognition and Emotion, 30, 193–209. 10.1080/02699931.2014.991694 [DOI] [PubMed] [Google Scholar]
- Hunter J. E., & Schmidt F. L. (2000). Fixed effects vs. random effects meta-analysis models: Implications for cumulative research knowledge. International Journal of Selection and Assessment, 8, 275–292. 10.1111/1468-2389.00156 [DOI] [Google Scholar]
- Hur J., Iordan A. D., Dolcos F., & Berenbaum H. (2017). Emotional influences on perception and working memory. Cognition and Emotion, 31, 1294–1302. 10.1080/02699931.2016.1213703 [DOI] [PubMed] [Google Scholar]
- Husain M., & Roiser J. P. (2018). Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nature Reviews Neuroscience, 19, 470–484. 10.1038/s41583-018-0029-9 [DOI] [PubMed] [Google Scholar]
- Iordan A. D., & Dolcos F. (2017). Brain activity and network interactions linked to valence-related differences in the impact of emotional distraction. Cerebral Cortex, 27, 731–749. [DOI] [PubMed] [Google Scholar]
- Iordan A. D., Dolcos S., Denkova E., & Dolcos F. (2013). Sex differences in the response to emotional distraction: An event-related fMRI investigation. Cognitive, Affective & Behavioral Neuroscience, 13, 116–134. 10.3758/s13415-012-0134-6 [DOI] [PubMed] [Google Scholar]
- Iordan A. D., Dolcos S., & Dolcos F. (2013). Neural signatures of the response to emotional distraction: A review of evidence from brain imaging investigations. Frontiers in Human Neuroscience, 7, 200 10.3389/fnhum.2013.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordan A. D., Dolcos S., & Dolcos F. (2018). Brain activity and network interactions in the impact of internal emotional distraction. Cerebral Cortex. Advance online publication 10.1093/cercor/bhy129 [DOI] [PubMed] [Google Scholar]
- Ishai A. (2008). Let’s face it: It’s a cortical network. NeuroImage, 40, 415–419. 10.1016/j.neuroimage.2007.10.040 [DOI] [PubMed] [Google Scholar]
- Jackson M. C., Linden D. E., & Raymond J. E. (2014). Angry expressions strengthen the encoding and maintenance of face identity representations in visual working memory. Cognition and Emotion, 28, 278–297. 10.1080/02699931.2013.816655 [DOI] [PubMed] [Google Scholar]
- Jeffries S., & Everatt J. (2004). Working memory: Its role in dyslexia and other specific learning difficulties. Dyslexia, 10, 196–214. 10.1002/dys.278 [DOI] [PubMed] [Google Scholar]
- Jenness J. L., Rosen M. L., Sambrook K. A., Dennison M. J., Lambert H. K., Sheridan M. A., & McLaughlin K. A. (2018). Violence exposure and neural systems underlying working memory for emotional stimuli in youth. Development and Psychopathology, 30, 1517–1528. 10.1017/S0954579417001638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A. P., Stanley E. A., Kiyonaga A., Wong L., & Gelfand L. (2010). Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion, 10, 54–64. 10.1037/a0018438 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Haxby J. V., Martin A., Ungerleider L. G., & Parasuraman R. (2000). Complementary neural mechanisms for tracking items in human working memory. Science, 287, 643–646. 10.1126/science.287.5453.643 [DOI] [PubMed] [Google Scholar]
- Jonides J., Smith E. E., Marshuetz C., Koeppe R. A., & Reuter-Lorenz P. A. (1998). Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences of the United States of America, 95, 8410–8413. 10.1073/pnas.95.14.8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., & Gotlib I. H. (2008). Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology, 117, 182–192. 10.1037/0021-843X.117.1.182 [DOI] [PubMed] [Google Scholar]
- Joormann J., Levens S. M., & Gotlib I. H. (2011). Sticky thoughts: Depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychological Science, 22, 979–983. 10.1177/0956797611415539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah M. R., Grant D. M., Lechner W. V., & Mills A. C. (2013). Working memory load moderates late attentional bias in social anxiety. Cognition and Emotion, 27, 502–511. 10.1080/02699931.2012.719490 [DOI] [PubMed] [Google Scholar]
- Kalisch R. (2009). The functional neuroanatomy of reappraisal: Time matters. Neuroscience and Biobehavioral Reviews, 33, 1215–1226. 10.1016/j.neubiorev.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Kane M. J., Brown L. H., McVay J. C., Silvia P. J., Myin-Germeys I., & Kwapil T. R. (2007). For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science, 18, 614–621. 10.1111/j.1467-9280.2007.01948.x [DOI] [PubMed] [Google Scholar]
- Kane M. J., Hambrick D. Z., & Conway A. R. A. (2005). Working memory capacity and fluid intelligence are strongly related constructs: Comment on Ackerman, Beier, and Boyle (2005). Psychological Bulletin, 13, 66–71. [DOI] [PubMed] [Google Scholar]
- Kellermann T. S., Sternkopf M. A., Schneider F., Habel U., Turetsky B. I., Zilles K., & Eickhoff S. B. (2012). Modulating the processing of emotional stimuli by cognitive demand. Social Cognitive and Affective Neuroscience, 7, 263–273. 10.1093/scan/nsq104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzierski D., Ritter R. L., Stump T. K., & Anglin C. L. (2015). The effectiveness of an implementation intentions intervention for fruit and vegetable consumption as moderated by self-schema status. Appetite, 95, 228–238. 10.1016/j.appet.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Kensinger E. A. (2008). Age differences in memory for arousing and nonarousing emotional words. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences, 63, 13–18. 10.1093/geronb/63.1.P13 [DOI] [PubMed] [Google Scholar]
- Kensinger E. A., & Corkin S. (2003). Effect of negative emotional content on working memory and long-term memory. Emotion, 3, 378–393. 10.1037/1528-3542.3.4.378 [DOI] [PubMed] [Google Scholar]
- Kerestes R., Ladouceur C. D., Meda S., Nathan P. J., Blumberg H. P., Maloney K., et al. Phillips M. L. (2012). Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychological Medicine, 42, 29–40. 10.1017/S0033291711001097 [DOI] [PubMed] [Google Scholar]
- Kerns J. G., & Becker T. M. (2008). Communication disturbances, working memory, and emotion in people with elevated disorganized schizotypy. Schizophrenia Research, 100, 172–180. 10.1016/j.schres.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertz S. J., Belden A. C., Tillman R., & Luby J. (2016). Cognitive control deficits in shifting and inhibition in preschool age children are associated with increased depression and anxiety over 7.5 years of development. Journal of Abnormal Child Psychology, 44, 1185–1196. 10.1007/s10802-015-0101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D., García-Rubio M. J., González E. K., Tapia M., López-Martín S., Román F. J., et al. Carretié L. (2016). Working memory of emotional stimuli: Electrophysiological characterization. Biological Psychology, 119, 190–199. 10.1016/j.biopsycho.2016.07.009 [DOI] [PubMed] [Google Scholar]
- King R., & Schaefer A. (2011). The emotional startle effect is disrupted by a concurrent working memory task. Psychophysiology, 48, 269–272. 10.1111/j.1469-8986.2010.01062.x [DOI] [PubMed] [Google Scholar]
- Klemanski D. H., Curtiss J., McLaughlin K. A., & Nolen-Hoeksema S. (2017). Emotion regulation and the transdiagnostic role of repetitive negative thinking in adolescents with social anxiety and depression. Cognitive Therapy and Research, 41, 206–219. 10.1007/s10608-016-9817-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemen J., Büchel C., Bühler M., Menz M. M., & Rose M. (2010). Auditory working memory load impairs visual ventral stream processing: Toward a unified model of attentional load. Journal of Cognitive Neuroscience, 22, 437–446. 10.1162/jocn.2009.21204 [DOI] [PubMed] [Google Scholar]
- Klink P. C., Jentgens P., & Lorteije J. A. M. (2014). Priority maps explain the roles of value, attention, and salience in goal-oriented behavior. The Journal of Neuroscience, 34, 13867–13869. 10.1523/JNEUROSCI.3249-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Barrett L. F., Joseph J., Bliss-Moreau E., Lindquist K., & Wager T. D. (2008). Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage, 42, 998–1031. 10.1016/j.neuroimage.2008.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C. G., Walker J. B., Martin E. A., Healey K. M., & Moberg P. J. (2010). Facial emotion perception in schizophrenia: A meta-analytic review. Schizophrenia Bulletin, 36, 1009–1019. 10.1093/schbul/sbn192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könen T., Dirk J., & Schmiedek F. (2015). Cognitive benefits of last night’s sleep: Daily variations in children’s sleep behavior are related to working memory fluctuations. Journal of Child Psychology and Psychiatry, 56, 171–182. 10.1111/jcpp.12296 [DOI] [PubMed] [Google Scholar]
- Kopf J., Dresler T., Reicherts P., Herrmann M. J., & Reif A. (2013). The effect of emotional content on brain activation and the late positive potential in a word n-back task. PLoS ONE, 8, e75598 10.1371/journal.pone.0075598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostandov E. A., Kurova N. S., Cheremushkin E. A., Petrenko N. E., Ashkinazi M. L., & Yakovenko I. A. (2009). Relationship between the plasticity of a set to an emotional facial expression and the load on working memory. Neuroscience and Behavioral Physiology, 39, 223–229. 10.1007/s11055-009-9126-6 [DOI] [PubMed] [Google Scholar]
- Koster E. H. W., De Lissnyder E., & De Raedt R. (2013). Rumination is characterized by valence-specific impairments in switching of attention. Acta Psychologica, 144, 563–570. 10.1016/j.actpsy.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Koster E. H. W., De Lissnyder E., Derakshan N., & De Raedt R. (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31, 138–145. 10.1016/j.cpr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Krämer U. M., Mohammadi B., Doñamayor N., Samii A., & Münte T. F. (2010). Emotional and cognitive aspects of empathy and their relation to social cognition—An fMRI-study. Brain Research, 1311, 110–120. 10.1016/j.brainres.2009.11.043 [DOI] [PubMed] [Google Scholar]
- Krause-Utz A., Elzinga B. M., Oei N. Y. L., Paret C., Niedtfeld I., Spinhoven P., et al. Schmahl C. (2014). Amygdala and dorsal anterior cingulate connectivity during an emotional working memory task in borderline personality disorder patients with interpersonal trauma history. Frontiers in Human Neuroscience, 8, 848 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4211399/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A., Elzinga B. M., Oei N. Y. L., Spinhoven P., Bohus M., & Schmahl C. (2014). Susceptibility to distraction by social cues in borderline personality disorder. Psychopathology, 47, 148–157. 10.1159/000351740 [DOI] [PubMed] [Google Scholar]
- Krause-Utz A., Oei N. Y. L., Niedtfeld I., Bohus M., Spinhoven P., Schmahl C., & Elzinga B. M. (2012). Influence of emotional distraction on working memory performance in borderline personality disorder. Psychological Medicine, 42, 2181–2192. 10.1017/S0033291712000153 [DOI] [PubMed] [Google Scholar]
- Kret M. E., & De Gelder B. (2012). A review on sex differences in processing emotional signals. Neuropsychologia, 50, 1211–1221. 10.1016/j.neuropsychologia.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Krieglmeyer R., Deutsch R., De Houwer J., & De Raedt R. (2010). Being moved: Valence activates approach-avoidance behavior independently of evaluation and approach-avoidance intentions. Psychological Science, 21, 607–613. 10.1177/0956797610365131 [DOI] [PubMed] [Google Scholar]
- LaBar K. S., & Cabeza R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7, 54–64. 10.1038/nrn1825 [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G., Grühn D., & Studer J. (2010). Dynamic integration of emotion and cognition: Equilibrium regulation in development and aging In Lerner R. M., Lamb M. E., & Freund A. M. (Eds.), The handbook of life-span development (Vol. 2, pp. 79–115). Hoboken, NJ: Wiley; Retrieved from 10.1002/9780470880166.hlsd002004 [DOI] [Google Scholar]
- Ladouceur C. D., Dahl R. E., Williamson D. E., Birmaher B., Ryan N. D., & Casey B. J. (2005). Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. Journal of Abnormal Child Psychology, 33, 165–177. 10.1007/s10802-005-1825-z [DOI] [PubMed] [Google Scholar]
- Ladouceur C. D., Diwadkar V. A., White R., Bass J., Birmaher B., Axelson D. A., & Phillips M. L. (2013). Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Developmental Cognitive Neuroscience, 5, 185–196. 10.1016/j.dcn.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C. D., Schlund M. W., & Segreti A.-M. (2018). Positive reinforcement modulates fronto-limbic systems subserving emotional interference in adolescents. Behavioural Brain Research, 338, 109–117. 10.1016/j.bbr.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C. D., Silk J. S., Dahl R. E., Ostapenko L., Kronhaus D. M., & Phillips M. L. (2009). Fearful faces influence attentional control processes in anxious youth and adults. Emotion, 9, 855–864. 10.1037/a0017747 [DOI] [PubMed] [Google Scholar]
- Laier C., Schulte F. P., & Brand M. (2013). Pornographic picture processing interferes with working memory performance. Journal of Sex Research, 50, 642–652. 10.1080/00224499.2012.716873 [DOI] [PubMed] [Google Scholar]
- Lamm C., Pine D. S., & Fox N. A. (2013). Impact of negative affectively charged stimuli and response style on cognitive-control-related neural activation: An ERP study. Brain and Cognition, 83, 234–243. 10.1016/j.bandc.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., & Cuthbert B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Technical report). Gainesville, FL: University of Florida. [Google Scholar]
- Lavie N., Hirst A., de Fockert J. W., & Viding E. (2004). Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General, 133, 339–354. 10.1037/0096-3445.133.3.339 [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2012). Rethinking the emotional brain. Neuron, 73, 653–676. 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. E., & Brown R. (2017). A higher-order theory of emotional consciousness. Proceedings of the National Academy of Sciences of the United States of America, 114, E2016–E2025. 10.1073/pnas.1619316114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Carver C. S., Johnson S. L., & Joormann J. (2015). Predicting change in symptoms of depression during the transition to university: The roles of BDNF and working memory capacity. Cognitive, Affective & Behavioral Neuroscience, 15, 95–103. 10.3758/s13415-014-0305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S. M., Armstrong L. M., Orejuela-Dávila A. I., & Alverio T. (2017). The two sides of adversity: The effect of distant versus recent adversity on updating emotional content in working memory. Cognition and Emotion, 31, 1243–1251. 10.1080/02699931.2016.1197099 [DOI] [PubMed] [Google Scholar]
- Levens S. M., Devinsky O., & Phelps E. A. (2011). Role of the left amygdala and right orbital frontal cortex in emotional interference resolution facilitation in working memory. Neuropsychologia, 49, 3201–3212. 10.1016/j.neuropsychologia.2011.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S. M., & Gotlib I. H. (2009). Impaired selection of relevant positive information in depression. Depression and Anxiety, 26, 403–410. 10.1002/da.20565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S. M., & Gotlib I. H. (2010). Updating positive and negative stimuli in working memory in depression. Journal of Experimental Psychology: General, 139, 654–664. 10.1037/a0020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S. M., & Gotlib I. H. (2012). The effects of optimism and pessimism on updating emotional information in working memory. Cognition and Emotion, 26, 341–350. 10.1080/02699931.2011.574110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S. M., & Gotlib I. H. (2015). Updating emotional content in recovered depressed individuals: Evaluating deficits in emotion processing following a depressive episode. Journal of Behavior Therapy and Experimental Psychiatry, 48, 156–163. 10.1016/j.jbtep.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S. M., & Phelps E. A. (2008). Emotion processing effects on interference resolution in working memory. Emotion, 8, 267–280. 10.1037/1528-3542.8.2.267 [DOI] [PubMed] [Google Scholar]
- Levens S. M., & Phelps E. A. (2010). Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. Journal of Cognitive Neuroscience, 22, 2790–2803. 10.1162/jocn.2010.21428 [DOI] [PubMed] [Google Scholar]
- Li M., Feng L., Liu X., Zhang M., Fu B., Wang G., et al. Hu B. (2018). Emotional working memory in patients with major depressive disorder. The Journal of International Medical Research, 46, 1734–1746. 10.1177/0300060518758225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Chan R. C., & Luo Y. J. (2010). Stage effects of negative emotion on spatial and verbal working memory. BMC Neuroscience, 11, 60 10.1186/1471-2202-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li X., & Luo Y. J. (2006). Differential influences of negative emotion on spatial and verbal working memory: Evidence from event-related potential and source current density analysis. NeuroReport, 17, 1555–1559. 10.1097/01.wnr.0000234744.50442.2b [DOI] [PubMed] [Google Scholar]
- Li Z., Coles C. D., Lynch M. E., Hamann S., Peltier S., LaConte S., & Hu X. (2009). Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicology and Teratology, 31, 342–348. 10.1016/j.ntt.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley S. A., Andrade J., Turpin G., Sabin-Farrell R., & Holmes E. A. (2009). Visuospatial working memory interference with recollections of trauma. British Journal of Clinical Psychology, 48, 309–321. 10.1348/014466508X398943 [DOI] [PubMed] [Google Scholar]
- Lim S.-L., Bruce A. S., & Aupperle R. L. (2014). The influence of a working memory task on affective perception of facial expressions. PLoS ONE, 9, e111074 10.1371/journal.pone.0111074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. E., Lancaster T. M., Wolf C., Baird A., Jackson M. C., Johnston S. J., et al. Thome J. (2013). ZNF804A genotype modulates neural activity during working memory for faces. Neuropsychobiology, 67, 84–92. 10.1159/000344001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S. C., Jackson M. C., Subramanian L., Healy D., & Linden D. E. J. (2011). Sad benefit in face working memory: An emotional bias of melancholic depression. Journal of Affective Disorders, 135, 251–257. 10.1016/j.jad.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K. A. (2013). Emotions emerge from more basic psychological ingredients: A modern psychological constructionist model. Emotion Review, 5, 356–368. 10.1177/1754073913489750 [DOI] [Google Scholar]
- Lindquist K. A., Satpute A. B., Wager T. D., Weber J., & Barrett L. F. (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–1922. 10.1093/cercor/bhv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K. A., Wager T. D., Kober H., Bliss-Moreau E., & Barrett L. F. (2012). The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences, 35, 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström B. R., & Bohlin G. (2011). Emotion processing facilitates working memory performance. Cognition and Emotion, 25, 1196–1204. 10.1080/02699931.2010.527703 [DOI] [PubMed] [Google Scholar]
- Lindström B. R., & Bohlin G. (2012). Threat-relevance impairs executive functions: Negative impact on working memory and response inhibition. Emotion, 12, 384–393. 10.1037/a0027305 [DOI] [PubMed] [Google Scholar]
- Liu D., Wang L., Wang Y., & Jiang Y. (2016). Conscious access to suppressed threatening information is modulated by working memory. Psychological Science, 27, 1419–1427. 10.1177/0956797616660680 [DOI] [PubMed] [Google Scholar]
- LoPresti M. L., Schon K., Tricarico M. D., Swisher J. D., Celone K. A., & Stern C. E. (2008). Working memory for social cues recruits orbitofrontal cortex and amygdala: A functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. The Journal of Neuroscience, 28, 3718–3728. 10.1523/JNEUROSCI.0464-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., MacLean P. C., Shaffer M. L., & Watterberg K. (2009). Early working memory in children born with extremely low birth weight: Assessed by object permanence. Journal of Child Neurology, 24, 410–415. 10.1177/0883073808324533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Burgund E. D., Berman M., & Hanson K. L. (2001). Effects of tryptophan loading on verbal, spatial and affective working memory functions in healthy adults. Journal of Psychopharmacology, 15, 219–230. 10.1177/026988110101500410 [DOI] [PubMed] [Google Scholar]
- Luksys G., Ackermann S., Coynel D., Fastenrath M., Gschwind L., Heck A., et al. deq Uervain D. (2014). BAIAP2 is related to emotional modulation of human memory strength. PLoS ONE, 9, e83707 10.1371/journal.pone.0083707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksys G., Fastenrath M., Coynel D., Freytag V., Gschwind L., Heck A., et al. deq Uervain D. J-F. (2015). Computational dissection of human episodic memory reveals mental process-specific genetic profiles. Proceedings of the National Academy of Sciences of the United States of America, 112, E4939–E4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Qin S., Fernández G., Zhang Y., Klumpers F., & Li H. (2014). Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Human Brain Mapping, 35, 5606–5616. 10.1002/hbm.22573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maat A., Cahn W., Gijsman H. J., Hovens J. E., Kahn R. S., & Aleman A. (2014). Open, randomized trial of the effects of aripiprazole versus risperidone on social cognition in schizophrenia. European Neuropsychopharmacology, 24, 575–584. 10.1016/j.euroneuro.2013.12.009 [DOI] [PubMed] [Google Scholar]
- MacKay D. G., Shafto M., Taylor J. K., Marian D. E., Abrams L., & Dyer J. R. (2004). Relations between emotion, memory, and attention: Evidence from taboo Stroop, lexical decision, and immediate memory tasks. Memory & Cognition, 32, 474–488. 10.3758/BF03195840 [DOI] [PubMed] [Google Scholar]
- MacLean R. R., Nichols T. T., LeBreton J. M., & Wilson S. J. (2016). Effects of cognitive load on neural and behavioral responses to smoking-cue distractors. Cognitive, Affective & Behavioral Neuroscience, 16, 588–600. 10.3758/s13415-016-0416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A., Ferri J., & Hajcak G. (2011). Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cognitive, Affective & Behavioral Neuroscience, 11, 321–331. 10.3758/s13415-011-0036-z [DOI] [PubMed] [Google Scholar]
- MacNamara A., & Proudfit G. H. (2014). Cognitive load and emotional processing in generalized anxiety disorder: Electrocortical evidence for increased distractibility. Journal of Abnormal Psychology, 123, 557–565. 10.1037/a0036997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A., Schmidt J., Zelinsky G. J., & Hajcak G. (2012). Electrocortical and ocular indices of attention to fearful and neutral faces presented under high and low working memory load. Biological Psychology, 91, 349–356. 10.1016/j.biopsycho.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Mammarella N., Borella E., Carretti B., Leonardi G., & Fairfield B. (2013). Examining an emotion enhancement effect in working memory: Evidence from age-related differences. Neuropsychological Rehabilitation, 23, 416–428. 10.1080/09602011.2013.775065 [DOI] [PubMed] [Google Scholar]
- Mammarella N., Fairfield B., De Leonardis V., Carretti B., Borella E., Frisullo E., & Di Domenico A. (2012). Is there an affective working memory deficit in patients with chronic schizophrenia? Schizophrenia Research, 138, 99–101. 10.1016/j.schres.2012.03.028 [DOI] [PubMed] [Google Scholar]
- Mammarella N., Fairfield B., Di Domenico A., D’Onofrio L., Stuppia L., & Gatta V. (2016). The modulating role of ADRA2B in emotional working memory: Attending the negative but remembering the positive. Neurobiology of Learning and Memory, 130, 129–134. 10.1016/j.nlm.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Mammarella N., Fairfield B., Frisullo E., & Di Domenico A. (2013). Saying it with a natural child’s voice! When affective auditory manipulations increase working memory in aging. Aging & Mental Health, 17, 853–862. 10.1080/13607863.2013.790929 [DOI] [PubMed] [Google Scholar]
- Mano Q. R., Brown G. G., Bolden K., Aupperle R., Sullivan S., Paulus M. P., & Stein M. B. (2013). Curvilinear relationship between phonological working memory load and social-emotional modulation. Cognition and Emotion, 27, 283–304. 10.1080/02699931.2012.712948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano Q. R., Brown G. G., Mirzakhanian H., Bolden K., Cadenhead K. S., & Light G. A. (2014). Not all distraction is bad: Working memory vulnerability to implicit socioemotional distraction correlates with negative symptoms and functional impairment in psychosis. Schizophrenia Research and Treatment. Advance online publication 10.1155/2014/320948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. N., & Chaudry A. (2014). Working memory performance and exposure to pleasant and unpleasant ambient odor: Is spatial span special? The International Journal of Neuroscience, 124, 806–811. 10.3109/00207454.2014.890619 [DOI] [PubMed] [Google Scholar]
- Marx I., Domes G., Havenstein C., Berger C., Schulze L., & Herpertz S. C. (2011). Enhanced emotional interference on working memory performance in adults with ADHD. The World Journal of Biological Psychiatry, 12, 70–75. 10.3109/15622975.2011.599213 [DOI] [PubMed] [Google Scholar]
- Marx I., Krause J., Berger C., & Häßler F. (2014). Dissociable patterns in the control of emotional interference in adults with attention-deficit/hyperactivity disorder (ADHD) and in adults with alcohol dependence. PLoS ONE, 9, e107750 10.1371/journal.pone.0107750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. (2016). The affective neuroscience of aging. Annual Review of Psychology, 67, 213–238. 10.1146/annurev-psych-122414-033540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., & Carstensen L. L. (2005). Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences, 9, 496–502. 10.1016/j.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Mather M., Mitchell K. J., Raye C. L., Novak D. L., Greene E. J., & Johnson M. K. (2006). Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience, 18, 614–625. 10.1162/jocn.2006.18.4.614 [DOI] [PubMed] [Google Scholar]
- Mather M., & Sutherland M. R. (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science, 6, 114–133. 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A., & MacLeod C. (2005). Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology, 1, 167–195. 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- Mattarella-Micke A., Mateo J., Kozak M. N., Foster K., & Beilock S. L. (2011). Choke or thrive? The relation between salivary cortisol and math performance depends on individual differences in working memory and math-anxiety. Emotion, 11, 1000–1005. 10.1037/a0023224 [DOI] [PubMed] [Google Scholar]
- McEvoy P. M., Mahoney A. E., & Moulds M. L. (2010). Are worry, rumination, and post-event processing one and the same? Development of the repetitive thinking questionnaire. Journal of Anxiety Disorders, 24, 509–519. 10.1016/j.janxdis.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Mehta M. A., Hinton E. C., Montgomery A. J., Bantick R. A., & Grasby P. M. (2005). Sulpiride and mnemonic function: Effects of a dopamine D2 receptor antagonist on working memory, emotional memory and long-term memory in healthy volunteers. Journal of Psychopharmacology, 19, 29–38. 10.1177/0269881105048889 [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Meule A., Skirde A. K., Freund R., Vögele C., & Kübler A. (2012). High-calorie food-cues impair working memory performance in high and low food cravers. Appetite, 59, 264–269. 10.1016/j.appet.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Mikels J. A., Larkin G. R., Reuter-Lorenz P. A., & Cartensen L. L. (2005). Divergent trajectories in the aging mind: Changes in working memory for affective versus visual information with age. Psychology and Aging, 20, 542–553. 10.1037/0882-7974.20.4.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels J. A., Reuter-Lorenz P. A., Beyer J. A., & Fredrickson B. L. (2008). Emotion and working memory: Evidence for domain-specific processes for affective maintenance. Emotion, 8, 256–266. 10.1037/1528-3542.8.2.256 [DOI] [PubMed] [Google Scholar]
- Miller E. K. (2000). The prefrontal cortex and cognitive control. Nature Reviews Neuroscience, 1, 59–65. 10.1038/35036228 [DOI] [PubMed] [Google Scholar]
- Miller E. K., & Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mirabolfathi V., Moradi A. R., & Bakhtiari M. (2016). Emotional working memory in post-traumatic stress disorder and depression. Advances in Cognitive Science, 17, 33–44. [Google Scholar]
- Mitchell K. J., Mather M., Johnson M. K., Raye C. L., & Greene E. J. (2006). A functional magnetic resonance imaging investigation of short-term source and item memory for negative pictures. NeuroReport, 17, 1543–1547. 10.1097/01.wnr.0000234743.50442.e5 [DOI] [PubMed] [Google Scholar]
- Mitchell R. L. C. (2007). fMRI delineation of working memory for emotional prosody in the brain: Commonalities with the lexico-semantic emotion network. NeuroImage, 36, 1015–1025. 10.1016/j.neuroimage.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Mitchell R. L. C., & Phillips L. H. (2007). The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia, 45, 617–629. 10.1016/j.neuropsychologia.2006.06.030 [DOI] [PubMed] [Google Scholar]
- Miyake A., & Shah P. (Eds.). (1999). Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 10.1017/CBO9781139174909 [DOI] [Google Scholar]
- Mobbs D., Hagan C. C., Dalgleish T., Silston B., & Prévost C. (2015). The ecology of human fear: Survival optimization and the nervous system. Frontiers in Neuroscience, 9, 55 10.3389/fnins.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., & Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6, e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort V., Bernardin F., Grosdemange A., Ducrocq X., Mathieu P., & Bolmont B. (2013). Paradoxical state anxiety and working memory in a patient with acute stroke. Cognitive and Behavioral Neurology, 26, 195–207. 10.1097/WNN.0000000000000010 [DOI] [PubMed] [Google Scholar]
- Moon C.-M., & Jeong G.-W. (2015). Functional neuroanatomy on the working memory under emotional distraction in patients with generalized anxiety disorder. Psychiatry and Clinical Neurosciences, 69, 609–619. 10.1111/pcn.12295 [DOI] [PubMed] [Google Scholar]
- Moore A. B., Clark B. A., & Kane M. J. (2008). Who shalt not kill? Individual differences in working memory capacity, executive control, and moral judgment. Psychological Science, 19, 549–557. 10.1111/j.1467-9280.2008.02122.x [DOI] [PubMed] [Google Scholar]
- Moran T. P. (2016). Anxiety and working memory capacity: A meta-analysis and narrative review. Psychological Bulletin, 142, 831–864. 10.1037/bul0000051 [DOI] [PubMed] [Google Scholar]
- Moreno M. L., Vanderhasselt M.-A., Carvalho A. F., Moffa A. H., Lotufo P. A., Benseñor I. M., & Brunoni A. R. (2015). Effects of acute transcranial direct current stimulation in hot and cold working memory tasks in healthy and depressed subjects. Neuroscience Letters, 591, 126–131. 10.1016/j.neulet.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Morey R. A., Dolcos F., Petty C. M., Cooper D. A., Hayes J. P., LaBar K. S., & McCarthy G. (2009). The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatric Research, 43, 809–817. 10.1016/j.jpsychires.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. A., Hariri A. R., Gold A. L., Hauser M. A., Munger H. J., Dolcos F., & McCarthy G. (2011). Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry, 11, 76 10.1186/1471-244X-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B., Terburg D., Thornton H. B., Stein D. J., & van Honk J. (2012). Paradoxical facilitation of working memory after basolateral amygdala damage. PLoS ONE, 7, e38116 10.1371/journal.pone.0038116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J., Koster E. H., & De Raedt R. (2014). The influence of working memory on visual search for emotional facial expressions. Journal of Experimental Psychology: Human Perception and Performance, 40, 1874–1890. 10.1037/a0037295 [DOI] [PubMed] [Google Scholar]
- Morra S., Parrella I., & Camba R. (2011). The role of working memory in the development of emotion comprehension. British Journal of Developmental Psychology, 29, 744–764. 10.1348/2044-835X.002006 [DOI] [PubMed] [Google Scholar]
- Moscarello J. M., & Maren S. (2018). Flexibility in the face of fear: Hippocampal-prefrontal regulation of fear and avoidance. Current Opinion in Behavioral Sciences, 19, 44–49. 10.1016/j.cobeha.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourão-Miranda J., Volchan E., Moll J., de Oliveira-Souza R., Oliveira L., Bramati I., et al. Pessoa L. (2003). Contributions of stimulus valence and arousal to visual activation during emotional perception. NeuroImage, 20, 1955–1963. 10.1016/j.neuroimage.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Mu Y. G., Huang L. J., Li S. Y., Ke C., Chen Y., Jin Y., & Chen Z. P. (2012). Working memory and the identification of facial expression in patients with left frontal glioma. Neuro-Oncology, 14, iv81–iv89. 10.1093/neuonc/nos215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Cromheeke S., Siugzdaite R., & Boehler C. (2017). Evidence for the triadic model of adolescent brain development: Cognitive load and task-relevance of emotion differentially affect adolescents and adults. Developmental Cognitive Neuroscience, 26, 91–100. 10.1016/j.dcn.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Shechner T., Rosen D., Nelson E. E., Pine D. S., & Ernst M. (2015). Incidental threat during visuospatial working memory in adolescent anxiety: An emotional memory-guided saccade task. Depression and Anxiety, 32, 289–295. 10.1002/da.22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder H., Pitchford N. J., & Marlow N. (2011). Inattentive behaviour is associated with poor working memory and slow processing speed in very pre-term children in middle childhood. The British Journal of Educational Psychology, 81, 147–160. 10.1348/000709910X505527 [DOI] [PubMed] [Google Scholar]
- Müller U., Mottweiler E., & Bublak P. (2005). Noradrenergic blockade and numeric working memory in humans. Journal of Psychopharmacology, 19, 21–28. 10.1177/0269881105048888 [DOI] [PubMed] [Google Scholar]
- Mullin B. C., Perlman S. B., Versace A., de Almeida J. R. C., Labarbara E. J., Klein C., et al. Phillips M. L. (2012). An fMRI study of attentional control in the context of emotional distracters in euthymic adults with bipolar disorder. Psychiatry Research: Neuroimaging, 201, 196–205. 10.1016/j.pscychresns.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V. P., Ritchey M., Adcock R. A., & LaBar K. S. (2010). fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia, 48, 3459–3469. 10.1016/j.neuropsychologia.2010.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee D. E., Brown J. W., Askren M. K., Berman M. G., Demiralp E., Krawitz A., & Jonides J. (2013). A meta-analysis of executive components of working memory. Cerebral Cortex, 23, 264–282. 10.1093/cercor/bhs007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee D. E., Wager T. D., & Jonides J. (2007). Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience, 7, 1–17. 10.3758/CABN.7.1.1 [DOI] [PubMed] [Google Scholar]
- Nejati V., Salehinejad M. A., & Sabayee A. (2018). Impaired working memory updating affects memory for emotional and non-emotional materials the same way: Evidence from post-traumatic stress disorder (PTSD). Cognitive Processing, 19, 53–62. 10.1007/s10339-017-0837-2 [DOI] [PubMed] [Google Scholar]
- Neta M., & Whalen P. J. (2011). Individual differences in neural activity during a facial expression vs. identity working memory task. NeuroImage, 56, 1685–1692. 10.1016/j.neuroimage.2011.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen S., & Ridout N. (2010). Short-term memory for emotional faces in dysphoria. Memory, 18, 486–497. 10.1080/09658211003762092 [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Bunge S. A., Gross J. J., & Gabrieli J. D. (2002). Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215–1229. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Gross J. J., Ochsner K. N., & Gross J. J. (2008). Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17, 153–158. 10.1111/j.1467-8721.2008.00566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N., Ray R. R., Hughes B., McRae K., Cooper J. C., Weber J., et al. Gross J. J. (2009). Bottom-up and top-down processes in emotion generation: Common and distinct neural mechanisms. Psychological Science, 20, 1322–1331. 10.1111/j.1467-9280.2009.02459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N., Silvers J. A., & Buhle J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei N. Y., Everaerd W. T., Elzinga B. M., van Well S., & Bermond B. (2006). Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress, 9, 133–141. 10.1080/10253890600965773 [DOI] [PubMed] [Google Scholar]
- Oei N. Y. L., Tollenaar M. S., Elzinga B. M., & Spinhoven P. (2010). Propranolol reduces emotional distraction in working memory: A partial mediating role of propranolol-induced cortisol increases? Neurobiology of Learning and Memory, 93, 388–395. 10.1016/j.nlm.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Oei N. Y. L., Tollenaar M. S., Spinhoven P., & Elzinga B. M. (2009). Hydrocortisone reduces emotional distracter interference in working memory. Psychoneuroendocrinology, 34, 1284–1293. 10.1016/j.psyneuen.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Oei N. Y. L., Veer I. M., Wolf O. T., Spinhoven P., Rombouts S. A. R. B., & Elzinga B. M. (2012). Stress shifts brain activation towards ventral ‘affective’ areas during emotional distraction. Social Cognitive and Affective Neuroscience, 7, 403–412. 10.1093/scan/nsr024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A., Flykt A., & Esteves F. (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130, 466–478. 10.1037/0096-3445.130.3.466 [DOI] [PubMed] [Google Scholar]
- Okon-Singer H., Hendler T., Pessoa L., & Shackman A. J. (2015). The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience. Advance online publication 10.3389/fnhum.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onraedt T., & Koster E. H. W. (2014). Training working memory to reduce rumination. PLoS ONE, 9, e90632 10.1371/journal.pone.0090632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opmeer E. M., Kortekaas R., van Tol M. J., van der Wee N. J., Woudstra S., van Buchem M. A., et al. Aleman A. (2013). Influence of COMT val158met genotype on the depressed brain during emotional processing and working memory. PLoS ONE, 8, e73290 10.1371/journal.pone.0073290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka M., Yaoi K., Minamoto T., & Osaka N. (2013). When do negative and positive emotions modulate working memory performance? Scientific Reports, 3, 1375 10.1038/srep01375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. M., McMillan K. M., Laird A. R., & Bullmore E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25, 46–59. 10.1002/hbm.20131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen K. J., Brattico E., Bailey C. J., Korvenoja A., & Gjedde A. (2009). Cognitive and emotional modulation of brain default operation. Journal of Cognitive Neuroscience, 21, 1065–1080. 10.1162/jocn.2009.21086 [DOI] [PubMed] [Google Scholar]
- Pallesen K. J., Brattico E., Bailey C. J., Korvenoja A., Koivisto J., Gjedde A., & Carlson S. (2010). Cognitive control in auditory working memory is enhanced in musicians. PLoS ONE, 5, e11120 10.1371/journal.pone.0011120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-I., Kim G.-W., Jeong G.-W., Chung G. H., & Yang J.-C. (2016). Brain activation patterns associated with the effects of emotional distracters during working memory maintenance in patients with generalized anxiety disorder. Psychiatry Investigation, 13, 152–156. 10.4306/pi.2016.13.1.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Gibson C., & McMichael T. (2006). Socioaffective factors modulate working memory in schizophrenia patients. Neuroscience, 139, 373–384. 10.1016/j.neuroscience.2005.06.034 [DOI] [PubMed] [Google Scholar]
- Passarotti A. M., Ellis J., Wegbreit E., Stevens M. C., & Pavuluri M. N. (2012). Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connectivity, 2, 320–334. 10.1089/brain.2012.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A. M., Sweeney J. A., & Pavuluri M. N. (2010). Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 1064–1080. 10.1016/j.jaac.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A. M., Sweeney J. A., & Pavuluri M. N. (2011). Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology, 216, 485–499. 10.1007/s00213-011-2243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly K., Seiferth N. Y., Kellermann T., Ruhrmann S., Daumann B., Backes V., et al. Habel U. (2010). The interaction of working memory and emotion in persons clinically at risk for psychosis: An fMRI pilot study. Schizophrenia Research, 120, 167–176. 10.1016/j.schres.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Pavuluri M. N., Passarotti A. M., Fitzgerald J. M., Wegbreit E., & Sweeney J. A. (2012). Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: A pharmacological functional magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 157–170.e5. 10.1016/j.jaac.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe M. L., Brose A., Gotlib I. H., & Kuppens P. (2016). Affective updating ability and stressful events interact to prospectively predict increases in depressive symptoms over time. Emotion, 16, 73–82. 10.1037/emo0000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe M. L., Koval P., & Kuppens P. (2013). Executive well-being: Updating of positive stimuli in working memory is associated with subjective well-being. Cognition, 126, 335–340. 10.1016/j.cognition.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Pe M. L., Raes F., Koval P., Brans K., Verduyn P., & Kuppens P. (2013). Interference resolution moderates the impact of rumination and reappraisal on affective experiences in daily life. Cognition and Emotion, 27, 492–501. 10.1080/02699931.2012.719489 [DOI] [PubMed] [Google Scholar]
- Pe M. L., Raes F., & Kuppens P. (2013). The cognitive building blocks of emotion regulation: Ability to update working memory moderates the efficacy of rumination and reappraisal on emotion. PLoS ONE, 8, e69071 10.1371/journal.pone.0069071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecchinenda A., Dretsch M., & Chapman P. (2006). Working memory involvement in emotion-based processes underlying choosing advantageously. Experimental Psychology, 53, 191–197. 10.1027/1618-3169.53.3.191 [DOI] [PubMed] [Google Scholar]
- Pecchinenda A., & Heil M. (2007). Role of working memory load on selective attention to affectively valent information. The European Journal of Cognitive Psychology, 19, 898–909. 10.1080/09541440601095388 [DOI] [Google Scholar]
- Pehlivanoglu D., Jain S., Ariel R., & Verhaeghen P. (2014). The ties to unbind: Age-related differences in feature (un)binding in working memory for emotional faces. Frontiers in Psychology, 5, 253 10.3389/fpsyg.2014.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. G., Volchan E., de Souza G. G. L., Oliveira L., Campagnoli R. R., Pinheiro W. M., & Pessoa L. (2006). Sustained and transient modulation of performance induced by emotional picture viewing. Emotion, 6, 622–634. 10.1037/1528-3542.6.4.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein W. M., Elbert T., & Stenger V. A. (2002). Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of the National Academy of Sciences of the United States of America, 99, 1736–1741. 10.1073/pnas.241650598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2005). To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology, 15, 188–196. 10.1016/j.conb.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9, 148–158. 10.1038/nrn2317 [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13, 160–166. 10.1016/j.tics.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., & Adolphs R. (2010). Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience, 11, 773–783. 10.1038/nrn2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., & Ungerleider L. G. (2004). Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in Brain Research, 144, 171–182. 10.1016/S0079-6123(03)14412-3 [DOI] [PubMed] [Google Scholar]
- Peterson E., & Welsh M. C. (2014). The development of hot and cool executive functions in childhood and adolescence: Are we getting warmer? In Goldstein S. & Naglieri J. A. (Eds.), Handbook of executive functioning (pp. 45–65). New York, NY: Springer New York; 10.1007/978-1-4614-8106-5_4 [DOI] [Google Scholar]
- Pfeifer J. H., & Allen N. B. (2012). Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences, 16, 322–329. 10.1016/j.tics.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A. (2004). Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology, 14, 198–202. 10.1016/j.conb.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Phelps E. A. (2006). Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. 10.1146/annurev.psych.56.091103.070234 [DOI] [PubMed] [Google Scholar]
- Phillips L. H., Channon S., Tunstall M., Hedenstrom A., & Lyons K. (2008). The role of working memory in decoding emotions. Emotion, 8, 184–191. 10.1037/1528-3542.8.2.184 [DOI] [PubMed] [Google Scholar]
- Phillips L. K., Giuliano A. J., Lee E. H., Faraone S. V., Tsuang M. T., & Seidman L. J. (2011). Emotion-cognition interaction in people at familial high risk for schizophrenia: The impact of sex differences. Journal of Abnormal Psychology, 120, 993–998. 10.1037/a0023542 [DOI] [PubMed] [Google Scholar]
- Pio de Almeida L. S., Jansen K., Köhler C. A., Pinheiro R. T., da Silva R. A., & Bonini J. S. (2012). Working and short-term memories are impaired in postpartum depression. Journal of Affective Disorders, 136, 1238–1242. 10.1016/j.jad.2011.09.031 [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10, 59–63. 10.1016/j.tics.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. (2011). Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron, 72, 692–697. 10.1016/j.neuron.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle B. R. (2016). How does the brain keep information “in mind”? Current Directions in Psychological Science, 25, 151–156. 10.1177/0963721416643063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G., Schettino A., & Vuilleumier P. (2013). Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology, 92, 492–512. 10.1016/j.biopsycho.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Power M. J., & Dalgleish T. (2015). Cognition and emotion: From order to disorder (3rd ed.). Hove, UK: Psychology Press; 10.4324/9781315708744 [DOI] [Google Scholar]
- Prehn K., Schulze L., Rossmann S., Berger C., Vohs K., Fleischer M., et al. Herpertz S. C. (2013). Effects of emotional stimuli on working memory processes in male criminal offenders with borderline and antisocial personality disorder. The World Journal of Biological Psychiatry, 14, 71–78. 10.3109/15622975.2011.584906 [DOI] [PubMed] [Google Scholar]
- Prencipe A., Kesek A., Cohen J., Lamm C., Lewis M. D., & Zelazo P. D. (2011). Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology, 108, 621–637. 10.1016/j.jecp.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Putman P., Hermans E. J., & van Honk J. (2007). Exogenous cortisol shifts a motivated bias from fear to anger in spatial working memory for facial expressions. Psychoneuroendocrinology, 32, 14–21. 10.1016/j.psyneuen.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Qi S., Ding C., & Li H. (2014). Neural correlates of inefficient filtering of emotionally neutral distractors from working memory in trait anxiety. Cognitive, Affective & Behavioral Neuroscience, 14, 253–265. 10.3758/s13415-013-0203-5 [DOI] [PubMed] [Google Scholar]
- Quinlan P. T., Yue Y., & Cohen D. J. (2017). The processing of images of biological threats in visual short-term memory. Proceedings. Biological Sciences. Advance online publication 10.1098/rspb.2017.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. E., & Joormann J. (2015a). Control when it counts: Change in executive control under stress predicts depression symptoms. Emotion, 15, 522–530. 10.1037/emo0000089 [DOI] [PubMed] [Google Scholar]
- Quinn M. E., & Joormann J. (2015b). Stress-induced changes in executive control are associated with depression symptoms examining the role of rumination. Clinical Psychological Science, 3, 628–636. 10.1177/2167702614563930 [DOI] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved from http://www.R-project.org
- Rebetez M. M. L., Rochat L., Billieux J., Gay P., & Van der Linden M. (2015). Do emotional stimuli interfere with two distinct components of inhibition? Cognition and Emotion, 29, 559–567. 10.1080/02699931.2014.922054 [DOI] [PubMed] [Google Scholar]
- Reed A. E., Chan L., & Mikels J. A. (2014). Meta-analysis of the age-related positivity effect: Age differences in preferences for positive over negative information. Psychology and Aging, 29, 1–15. 10.1037/a0035194 [DOI] [PubMed] [Google Scholar]
- Reinecke A., Becker E. S., & Rinck M. (2009). Selective visual working memory in fear of spiders: The role of automaticity and material-specificity. Journal of Anxiety Disorders, 23, 1053–1063. 10.1016/j.janxdis.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Reinecke A., Rinck M., & Becker E. S. (2006). Spiders crawl easily through the bottleneck: Visual working memory for negative stimuli. Emotion, 6, 438–449. 10.1037/1528-3542.6.3.438 [DOI] [PubMed] [Google Scholar]
- Reinecke A., Soltau C., Hoyer J., Becker E. S., & Rinck M. (2012). Treatment sensitivity of implicit threat evaluation, avoidance tendency and visual working memory bias in specific phobia. Journal of Anxiety Disorders, 26, 321–328. 10.1016/j.janxdis.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Reinholdt-Dunne M. L., Mogg K., & Bradley B. P. (2013). Attention control: Relationships between self-report and behavioural measures, and symptoms of anxiety and depression. Cognition and Emotion, 27, 430–440. 10.1080/02699931.2012.715081 [DOI] [PubMed] [Google Scholar]
- Richter S., Gorny X., Machts J., Behnisch G., Wüstenberg T., Herbort M. C., et al. Schott B. H. (2013). Effects of AKAP5 Pro100Leu genotype on working memory for emotional stimuli. PLoS ONE, 8, e55613 10.1371/journal.pone.0055613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinaugh D. J., Crane M. E., Enock P. M., & McNally R. J. (2016). Training the removal of negative information from working memory: A preliminary investigation of a working memory bias modification task. Cognition and Emotion, 30, 570–581. 10.1080/02699931.2015.1014312 [DOI] [PubMed] [Google Scholar]
- Robinson K. E., Pearson M. M., Cannistraci C. J., Anderson A. W., Kuttesch J. F. Jr., Wymer K., et al. Compas B. E. (2015). Functional neuroimaging of working memory in survivors of childhood brain tumors and healthy children: Associations with coping and psychosocial outcomes. Child Neuropsychology, 21, 779–802. 10.1080/09297049.2014.924492 [DOI] [PubMed] [Google Scholar]
- Román F. J., García-Rubio M. J., Privado J., Kessel D., López-Martín S., Martínez K., et al. Colom R. (2015). Adaptive working memory training reveals a negligible effect of emotional stimuli over cognitive processing. Personality and Individual Differences, 74, 165–170. 10.1016/j.paid.2014.10.014 [DOI] [Google Scholar]
- Rosenberg H., Dethier M., Kessels R. P., Westbrook R. F., & McDonald S. (2015). Emotion perception after moderate-severe traumatic brain injury: The valence effect and the role of working memory, processing speed, and nonverbal reasoning. Neuropsychology, 29, 509–521. 10.1037/neu0000171 [DOI] [PubMed] [Google Scholar]
- Rothstein H. R., Sutton A. J., & Borenstein M. (2005). Publication bias in meta-analysis. Chichester, UK: Wiley; 10.1002/0470870168 [DOI] [Google Scholar]
- Rypma B., & D’Esposito M. (2000). Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience, 3, 509–515. 10.1038/74889 [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E. E., Li Q., Siddiqui A., Krafft C., Oliver W. T., et al. Jeffries J. (2011). Emotional perception: Meta-analyses of face and natural scene processing. NeuroImage, 54, 2524–2533. 10.1016/j.neuroimage.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Sabharwal A., Szekely A., Kotov R., Mukherjee P., Leung H.-C., Barch D. M., & Mohanty A. (2016). Transdiagnostic neural markers of emotion-cognition interaction in psychotic disorders. Journal of Abnormal Psychology, 125, 907–922. 10.1037/abn0000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Faber E. S. L., Lopez De Armentia M., & Power J. (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83, 803–834. 10.1152/physrev.00002.2003 [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S. M., Keltner J. R., Wager T. D., & Nichols T. E. (2009). Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. NeuroImage, 45, 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Salvadore G., Cornwell B. R., Sambataro F., Latov D., Colon-Rosario V., Carver F., et al. Zarate C. A. Jr. (2010). Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology, 35, 1415–1422. 10.1038/npp.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M. C., Lindenberger U., & Werkle-Bergner M. (2012). Lifespan age differences in working memory: A two-component framework. Neuroscience and Biobehavioral Reviews, 36, 2007–2033. 10.1016/j.neubiorev.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Satpute A. B., Badre D., & Ochsner K. N. (2014). Distinct regions of prefrontal cortex are associated with the controlled retrieval and selection of social information. Cerebral Cortex, 24, 1269–1277. 10.1093/cercor/bhs408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute A. B., Kang J., Bickart K. C., Yardley H., Wager T. D., & Barrett L. F. (2015). Involvement of sensory regions in affective experience: A meta-analysis. Frontiers in Psychology, 6, 1860 10.3389/fpsyg.2015.01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N., Downham R., Turman B., Kropotov J., Clark R., Yumash R., & Szatmary A. (2015). Working memory training with tDCS improves behavioral and neurophysiological symptoms in pilot group with post-traumatic stress disorder (PTSD) and with poor working memory. Neurocase, 21, 271–278. 10.1080/13554794.2014.890727 [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., & Goldman-Rakic P. S. (1991). D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science, 251, 947–950. 10.1126/science.1825731 [DOI] [PubMed] [Google Scholar]
- Saxton B. T., Myhre S. K., Siyaguna T., & Rokke P. D. (2018). Do arousal and valence have separable influences on attention across time? Psychological Research. Advance online publication 10.1007/s00426-018-0995-6 [DOI] [PubMed] [Google Scholar]
- Schaefer A., Braver T. S., Reynolds J. R., Burgess G. C., Yarkoni T., & Gray J. R. (2006). Individual differences in amygdala activity predict response speed during working memory. The Journal of Neuroscience, 26, 10120–10128. 10.1523/JNEUROSCI.2567-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S., & Carstensen L. L. (2010). Emotional aging: Recent findings and future trends. The Journals of Gerontology: Series B, Psychological Sciences and Social Sciences, 65B, 135–144. 10.1093/geronb/gbp132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel L. S., Passarotti A. M., Sweeney J. A., & Pavuluri M. N. (2012). Negative emotion impairs working memory in pediatric patients with bipolar disorder type I. Psychological Medicine, 42, 2567–2577. 10.1017/S0033291712000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer K., Dan E., & Flykt A. (2006). What determines a feeling’s position in affective space? A case for appraisal. Cognition and Emotion, 20, 92–113. 10.1080/02699930500305016 [DOI] [Google Scholar]
- Schmeichel B. J., Volokhov R. N., & Demaree H. A. (2008). Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology, 95, 1526–1540. 10.1037/a0013345 [DOI] [PubMed] [Google Scholar]
- Schmidt F. L., Oh I. S., & Hayes T. L. (2009). Fixed- versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. British Journal of Mathematical & Statistical Psychology, 62, 97–128. 10.1348/000711007X255327 [DOI] [PubMed] [Google Scholar]
- Schneider I. K., Veenstra L., van Harreveld F., Schwarz N., & Koole S. L. (2016). Let’s not be indifferent about neutrality: Neutral ratings in the International Affective Picture System (IAPS) mask mixed affective responses. Emotion, 16, 426–430. 10.1037/emo0000164 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., & Roesch M. (2005). Orbitofrontal cortex, associative learning, and expectancies. Neuron, 47, 633–636. 10.1016/j.neuron.2005.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H. T., Flaisch T., Stockburger J., & Junghöfer M. (2006). Emotion and attention: Event-related brain potential studies. Progress in Brain Research, 156, 31–51. 10.1016/S0079-6123(06)56002-9 [DOI] [PubMed] [Google Scholar]
- Schweizer S., & Dalgleish T. (2011). Emotional working memory capacity in posttraumatic stress disorder (PTSD). Behaviour Research and Therapy, 49, 498–504. 10.1016/j.brat.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S., & Dalgleish T. (2013). What are the critical ingredients of affective working memory training? Comment on Engen and Kanske. The Journal of Neuroscience, 33, 12152–12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S., & Dalgleish T. (2016). The impact of affective contexts on working memory capacity in healthy populations and in individuals with PTSD. Emotion, 16, 16–23. 10.1037/emo0000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S., Grahn J., Hampshire A., Mobbs D., & Dalgleish T. (2013). Training the emotional brain: Improving affective control through emotional working memory training. The Journal of Neuroscience, 33, 5301–5311. 10.1523/JNEUROSCI.2593-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S., Hampshire A., & Dalgleish T. (2011). Extending brain-training to the affective domain: Increasing cognitive and affective executive control through emotional working memory training. PLoS ONE, 6, e24372 10.1371/journal.pone.0024372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S., Navrady L., Breakwell L., Howard R. M., Golden A.-M., Werner-Seidler A., & Dalgleish T. (2018). Affective enhancement of working memory is maintained in depression. Emotion, 18, 127–137. 10.1037/emo0000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. C., Matt G. E., Wrocklage K. M., Crnich C., Jordan J., Southwick S. M., et al. Schweinsburg B. C. (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141, 105–140. 10.1037/a0038039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., et al. Greicius M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A., Kessler Y., & Anholt G. E. (2015). Updating the emotional content of working memory in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 48, 110–117. 10.1016/j.jbtep.2015.02.012 [DOI] [PubMed] [Google Scholar]
- Sessa P., Luria R., Gotler A., Jolicœur P., & Dell’acqua R. (2011). Interhemispheric ERP asymmetries over inferior parietal cortex reveal differential visual working memory maintenance for fearful versus neutral facial identities. Psychophysiology, 48, 187–197. 10.1111/j.1469-8986.2010.01046.x [DOI] [PubMed] [Google Scholar]
- Shackman A. J., Sarinopoulos I., Maxwell J. S., Pizzagalli D. A., Lavric A., & Davidson R. J. (2006). Anxiety selectively disrupts visuospatial working memory. Emotion, 6, 40–61. 10.1037/1528-3542.6.1.40 [DOI] [PubMed] [Google Scholar]
- Sharbanee J. M., Stritzke W. G., Wiers R. W., Young P., Rinck M., & MacLeod C. (2013). The interaction of approach-alcohol action tendencies, working memory capacity, and current task goals predicts the inability to regulate drinking behavior. Psychology of Addictive Behaviors, 27, 649–661. 10.1037/a0029982 [DOI] [PubMed] [Google Scholar]
- Shaw P., Stringaris A., Nigg J., & Leibenluft E. (2014). Emotion dysregulation in attention deficit hyperactivity disorder. The American Journal of Psychiatry, 171, 276–293. 10.1176/appi.ajp.2013.13070966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton M. E., Dickey C. C., Frumin M., & McCarley R. W. (2001). A review of MRI findings in schizophrenia. Schizophrenia Research, 49, 1–52. 10.1016/S0920-9964(01)00163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Gao X., & Zhou R. (2014). Emotional working memory capacity in test anxiety. Learning and Individual Differences, 32, 178–183. 10.1016/j.lindif.2014.03.011 [DOI] [Google Scholar]
- Shi Z., Gao X., & Zhou R. (2015). Frontal theta activity during working memory in test anxiety. NeuroReport, 26, 228–232. 10.1097/WNR.0000000000000334 [DOI] [PubMed] [Google Scholar]
- Silver H., & Feldman P. (2005). Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. The Journal of Neuropsychiatry and Clinical Neurosciences, 17, 391–398. 10.1176/jnp.17.3.391 [DOI] [PubMed] [Google Scholar]
- Silver H., Feldman P., Bilker W., & Gur R. C. (2003). Working memory deficit as a core neuropsychological dysfunction in schizophrenia. The American Journal of Psychiatry, 160, 1809–1816. 10.1176/appi.ajp.160.10.1809 [DOI] [PubMed] [Google Scholar]
- Simione L., Calabrese L., Marucci F. S., Belardinelli M. O., Raffone A., & Maratos F. A. (2014). Emotion based attentional priority for storage in visual short-term memory. PLoS ONE, 9, e95261 10.1371/journal.pone.0095261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. B., Oren N., Sharon H., Kirschner A., Goldway N., Okon-Singer H., et al. Hendler T. (2015). Losing neutrality: The neural basis of impaired emotional control without sleep. The Journal of Neuroscience, 35, 13194–13205. 10.1523/JNEUROSCI.1314-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodol A. E., Oldham J. M., Bender D. S., Dyck I. R., Stout R. L., Morey L. C., et al. Gunderson J. G. (2005). Dimensional representations of DSM–IV personality disorders: Relationships to functional impairment. The American Journal of Psychiatry, 162, 1919–1925. 10.1176/appi.ajp.162.10.1919 [DOI] [PubMed] [Google Scholar]
- Smith E. E., & Jonides J. (1999). Storage and executive processes in the frontal lobes. Science, 283, 1657–1661. 10.1126/science.283.5408.1657 [DOI] [PubMed] [Google Scholar]
- Smith M. J., Horan W. P., Cobia D. J., Karpouzian T. M., Fox J. M., Reilly J. L., & Breiter H. C. (2014). Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophrenia Bulletin, 40, 824–834. 10.1093/schbul/sbt084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. K., Cacioppo J. T., Larsen J. T., & Chartrand T. L. (2003). May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia, 41, 171–183. 10.1016/S0028-3932(02)00147-1 [DOI] [PubMed] [Google Scholar]
- Snyder H. R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spachtholz P., Kuhbandner C., & Pekrun R. (2014). Negative affect improves the quality of memories: Trading capacity for precision in sensory and working memory. Journal of Experimental Psychology: General, 143, 1450–1456. 10.1037/xge0000012 [DOI] [PubMed] [Google Scholar]
- Speed B. C., Nelson B. D., Perlman G., Klein D. N., Kotov R., & Hajcak G. (2015). Personality and emotional processing: A relationship between extraversion and the late positive potential in adolescence. Psychophysiology, 52, 1039–1047. 10.1111/psyp.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P., Drost J., van Hemert B., & Penninx B. W. (2015). Common rather than unique aspects of repetitive negative thinking are related to depressive and anxiety disorders and symptoms. Journal of Anxiety Disorders, 33, 45–52. 10.1016/j.janxdis.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Spitzer B., Gloel M., Schmidt T. T., & Blankenburg F. (2014). Working memory coding of analog stimulus properties in the human prefrontal cortex. Cerebral Cortex, 24, 2229–2236. 10.1093/cercor/bht084 [DOI] [PubMed] [Google Scholar]
- Stegmayer K., Usher J., Trost S., Henseler I., Tost H., Rietschel M., et al. Gruber O. (2015). Disturbed cortico-amygdalar functional connectivity as pathophysiological correlate of working memory deficits in bipolar affective disorder. European Archives of Psychiatry and Clinical Neuroscience, 265, 303–311. 10.1007/s00406-014-0517-5 [DOI] [PubMed] [Google Scholar]
- Stiernströmer E. S., Wolgast M., & Johansson M. (2016). Effects of facial expression on working memory. International Journal of Psychology, 51, 312–317. 10.1002/ijop.12194 [DOI] [PubMed] [Google Scholar]
- Storbeck J., Davidson N. A., Dahl C. F., Blass S., & Yung E. (2015). Emotion, working memory task demands and individual differences predict behavior, cognitive effort and negative affect. Cognition and Emotion, 29, 95–117. 10.1080/02699931.2014.904222 [DOI] [PubMed] [Google Scholar]
- Storbeck J., & Watson P. (2014). Verbal makes it positive, spatial makes it negative: Working memory biases judgments, attention, and moods. Emotion, 14, 1072–1086. 10.1037/a0037327 [DOI] [PubMed] [Google Scholar]
- Stout D. M., Shackman A. J., Johnson J. S., & Larson C. L. (2015). Worry is associated with impaired gating of threat from working memory. Emotion, 15, 6–11. 10.1037/emo0000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout D. M., Shackman A. J., Pedersen W. S., Miskovich T. A., & Larson C. L. (2017). Neural circuitry governing anxious individuals’ mis-allocation of working memory to threat. Scientific Reports, 7, 8742 10.1038/s41598-017-08443-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T., Trippe R., Schmidt S., Weiss T., Hecht H., & Miltner W. H. (2011). Dissociation of acquisition and expression of fear conditioned responses under working memory load. Emotion, 11, 209–213. 10.1037/a0021157 [DOI] [PubMed] [Google Scholar]
- Strauss G. P., Lee B. G., Waltz J. A., Robinson B. M., Brown J. K., & Gold J. M. (2012). Cognition-emotion interactions are modulated by working memory capacity in individuals with schizophrenia. Schizophrenia Research, 141, 257–261. 10.1016/j.schres.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stussi Y., Pourtois G., & Sander D. (2018). Enhanced Pavlovian aversive conditioning to positive emotional stimuli. Journal of Experimental Psychology: General, 147, 905–923. 10.1037/xge0000424 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Yamada M., & Suhara T. (2012). Functional significance of central D1 receptors in cognition: Beyond working memory. Journal of Cerebral Blood Flow and Metabolism, 32, 1248–1258. 10.1038/jcbfm.2011.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D. (2013). Enhanced emotional memory: Cognitive and neural mechanisms. Current Directions in Psychological Science, 22, 430–436. 10.1177/0963721413498893 [DOI] [Google Scholar]
- Talmi D., Schimmack U., Paterson T., & Moscovitch M. (2007). The role of attention and relatedness in emotionally enhanced memory. Emotion, 7, 89–102. 10.1037/1528-3542.7.1.89 [DOI] [PubMed] [Google Scholar]
- Tamietto M., & de Gelder B. (2010). Neural bases of the non-conscious perception of emotional signals. Nature Reviews Neuroscience, 11, 697–709. 10.1038/nrn2889 [DOI] [PubMed] [Google Scholar]
- Tamm G., Kreegipuu K., Harro J., & Cowan N. (2017). Updating schematic emotional facial expressions in working memory: Response bias and sensitivity. Acta Psychologica, 172, 10–18. 10.1016/j.actpsy.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Tavares T. P., Logie K., & Mitchell D. G. V. (2016). Opposing effects of perceptual versus working memory load on emotional distraction. Experimental Brain Research, 234, 2945–2956. 10.1007/s00221-016-4697-2 [DOI] [PubMed] [Google Scholar]
- Tavitian L. R., Ladouceur C. D., Nahas Z., Khater B., Brent D. A., & Maalouf F. T. (2014). Neutral face distractors differentiate performance between depressed and healthy adolescents during an emotional working memory task. European Child & Adolescent Psychiatry, 23, 659–667. 10.1007/s00787-013-0492-9 [DOI] [PubMed] [Google Scholar]
- Taylor S. E. (1991). Asymmetrical effects of positive and negative events: The mobilization-minimization hypothesis. Psychological Bulletin, 110, 67–85. 10.1037/0033-2909.110.1.67 [DOI] [PubMed] [Google Scholar]
- Tempesta D., De Gennaro L., Presaghi F., & Ferrara M. (2014). Emotional working memory during sustained wakefulness. Journal of Sleep Research, 23, 646–656. 10.1111/jsr.12170 [DOI] [PubMed] [Google Scholar]
- Terfehr K., Wolf O. T., Schlosser N., Fernando S. C., Otte C., Muhtz C., et al. Wingenfeld K. (2011). Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology, 215, 71–79. 10.1007/s00213-010-2117-z [DOI] [PubMed] [Google Scholar]
- Thermenos H. W., Goldstein J. M., Milanovic S. M., Whitfield-Gabrieli S., Makris N., Laviolette P., et al. Seidman L. J. (2010). An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. American Journal of Medical Genetics: Part B, Neuropsychiatric Genetics, 153b, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos H. W., Makris N., Whitfield-Gabrieli S., Brown A. B., Giuliano A. J., Lee E. H., et al. Seidman L. J. (2011). A functional MRI study of working memory in adolescents and young adults at genetic risk for bipolar disorder: Preliminary findings. Bipolar Disorders, 13, 272–286. 10.1111/j.1399-5618.2011.00920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R., Hajcak G., & Gross J. J. (2012). Looking inward: Shifting attention within working memory representations alters emotional responses. Psychological Science, 23, 1461–1466. 10.1177/0956797612449838 [DOI] [PubMed] [Google Scholar]
- Thomas P. M., Jackson M. C., & Raymond J. E. (2014). A threatening face in the crowd: Effects of emotional singletons on visual working memory. Journal of Experimental Psychology: Human Perception and Performance, 40, 253–263. 10.1037/a0033970 [DOI] [PubMed] [Google Scholar]
- Tollenaar M. S., Ruissen M., Elzinga B. M., & de Bruijn E. R. A. (2017). Does oxytocin lead to emotional interference during a working memory paradigm? Psychopharmacology, 234, 3467–3474. 10.1007/s00213-017-4737-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Ernst T., Caparelli E. C., & Chang L. (2006). Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Human Brain Mapping, 27, 694–705. 10.1002/hbm.20211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J. W., Leon A. C., McCarry T., Nurse M., Hare T. A., et al. Nelson C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J., & Altshuler L. L. (2012). Emotion processing and regulation in bipolar disorder: A review. Bipolar Disorders, 14, 326–339. 10.1111/j.1399-5618.2012.01021.x [DOI] [PubMed] [Google Scholar]
- Trémeau F. (2006). A review of emotion deficits in schizophrenia. Dialogues in Clinical Neuroscience, 8, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise K., & Reeve R. A. (2014). Working memory, worry, and algebraic ability. Journal of Experimental Child Psychology, 121, 120–136. 10.1016/j.jecp.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Truong L., & Yang L. (2014). Friend or foe? Decoding the facilitative and disruptive effects of emotion on working memory in younger and older adults. Frontiers in Psychology, 5, 94 10.3389/fpsyg.2014.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C., & McNally R. J. (2014). Effects of emotionally valenced working memory taxation on negative memories. Journal of Behavior Therapy and Experimental Psychiatry, 45, 15–19. 10.1016/j.jbtep.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Uher R., Brooks S. J., Bartholdy S., Tchanturia K., & Campbell I. C. (2014). Increasing cognitive load reduces interference from masked appetitive and aversive but not neutral stimuli. PLoS ONE, 9, e94417 10.1371/journal.pone.0094417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hout M. A., Eidhof M. B., Verboom J., Littel M., & Engelhard I. M. (2014). Blurring of emotional and non-emotional memories by taxing working memory during recall. Cognition and Emotion, 28, 717–727. 10.1080/02699931.2013.848785 [DOI] [PubMed] [Google Scholar]
- van den Hout M. A., Engelhard I. M., Beetsma D., Slofstra C., Hornsveld H., Houtveen J., & Leer A. (2011). EMDR and mindfulness. Eye movements and attentional breathing tax working memory and reduce vividness and emotionality of aversive ideation. Journal of Behavior Therapy and Experimental Psychiatry, 42, 423–431. 10.1016/j.jbtep.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.-A., Brunoni A. R., Loeys T., Boggio P. S., & De Raedt R. (2013). Nosce te ipsum—Socrates revisited? Controlling momentary ruminative self-referent thoughts by neuromodulation of emotional working memory. Neuropsychologia, 51, 2581–2589. 10.1016/j.neuropsychologia.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Van Dillen L. F., & Derks B. (2012). Working memory load reduces facilitated processing of threatening faces: An ERP study. Emotion, 12, 1340–1349. 10.1037/a0028624 [DOI] [PubMed] [Google Scholar]
- Van Dillen L. F., & Koole S. L. (2007). Clearing the mind: A working memory model of distraction from negative mood. Emotion, 7, 715–723. 10.1037/1528-3542.7.4.715 [DOI] [PubMed] [Google Scholar]
- Vermeulen N., Niedenthal P. M., Pleyers G., Bayot M., & Corneille O. (2014). Emotion-specific load disrupts concomitant affective processing. Quarterly Journal of Experimental Psychology: Human Experimental Psychology, 67, 1655–1660. 10.1080/17470218.2014.905610 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. (2007). Accounting for heterogeneity via random-effects models and moderator analyses in meta-analysis. Journal of Psychology, 215, 104–121. [Google Scholar]
- Viechtbauer W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Vincent J. L., Kahn I., Snyder A. Z., Raichle M. E., & Buckner R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100, 3328–3342. 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visu-Petra L., Ţincaş I., Cheie L., & Benga O. (2010). Anxiety and visual-spatial memory updating in young children: An investigation using emotional facial expressions. Cognition and Emotion, 24, 223–240. 10.1080/02699930903387546 [DOI] [Google Scholar]
- Vogt J., De Houwer J., & Crombez G. (2011). Multiple goal management starts with attention: Goal prioritizing affects the allocation of spatial attention to goal-relevant events. Experimental Psychology, 58, 55–61. 10.1027/1618-3169/a000066 [DOI] [PubMed] [Google Scholar]
- Vogt J., De Houwer J., Crombez G., & Van Damme S. (2013). Competing for attentional priority: Temporary goals versus threats. Emotion, 13, 587–598. 10.1037/a0027204 [DOI] [PubMed] [Google Scholar]
- Vugs B., Hendriks M., Cuperus J., & Verhoeven L. (2014). Working memory performance and executive function behaviors in young children with SLI. Research in Developmental Disabilities, 35, 62–74. 10.1016/j.ridd.2013.10.022 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2002). Facial expression and selective attention. Current Opinion in Psychiatry, 15, 291–300. 10.1097/00001504-200205000-00011 [DOI] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–594. 10.1016/j.tics.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., & Huang Y.-M. (2009). Emotional attention: Uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Science, 18, 148–152. 10.1111/j.1467-8721.2009.01626.x [DOI] [Google Scholar]
- Vuontela V., Carlson S., Troberg A. M., Fontell T., Simola P., Saarinen S., & Aronen E. T. (2013). Working memory, attention, inhibition, and their relation to adaptive functioning and behavioral/emotional symptoms in school-aged children. Child Psychiatry and Human Development, 44, 105–122. 10.1007/s10578-012-0313-2 [DOI] [PubMed] [Google Scholar]
- Wager T. D., Lindquist M., & Kaplan L. (2007). Meta-analysis of functional neuroimaging data: Current and future directions. Social Cognitive and Affective Neuroscience, 2, 150–158. 10.1093/scan/nsm015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., Simmons A. N., Oberndorfer T. A., Frank G. K. W., McCurdy-McKinnon D., Fudge J. L., et al. Kaye W. H. (2015). Altered sensitization patterns to sweet food stimuli in patients recovered from anorexia and bulimia nervosa. Psychiatry Research: Neuroimaging, 234, 305–313. 10.1016/j.pscychresns.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., & Saudino K. J. (2013). Genetic and environmental influences on individual differences in emotion regulation and its relation to working memory in toddlerhood. Emotion, 13, 1055–1067. 10.1037/a0033784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanmaker S., Geraerts E., & Franken I. H. A. (2015). A working memory training to decrease rumination in depressed and anxious individuals: A double-blind randomized controlled trial. Journal of Affective Disorders, 175, 310–319. 10.1016/j.jad.2014.12.027 [DOI] [PubMed] [Google Scholar]
- Wass S., Porayska-Pomsta K., & Johnson M. H. (2011). Training attentional control in infancy. Current Biology, 21, 1543–1547. 10.1016/j.cub.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand A., Grimm S., Astalosch A., Guo J. S., Briesemeister B. B., Lisanby S. H., et al. Bajbouj M. (2013). Lateralized effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Experimental Brain Research, 227, 43–52. 10.1007/s00221-013-3483-7 [DOI] [PubMed] [Google Scholar]
- Weigand A., Richtermeier A., Feeser M., Guo J. S., Briesemeister B. B., Grimm S., & Bajbouj M. (2013). State-dependent effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Brain Stimulation, 6, 905–912. 10.1016/j.brs.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Wells A., & Matthews G. (2015). Attention and emotion (2nd ed.). New York, NY: Psychology Press. [Google Scholar]
- Whalen P. J., & Phelps E. A. (2009). The human amygdala. New York, NY: Guilford Press. [Google Scholar]
- Wilson S. J., Sayette M. A., Fiez J. A., & Brough E. (2007). Carry-over effects of smoking cue exposure on working memory performance. Nicotine & Tobacco Research, 9, 613–619. 10.1080/14622200701243144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Mendenhall C. D., Barrett L. F., & Barsalou L. W. (2013). Neural evidence that human emotions share core affective properties. Psychological Science, 24, 947–956. 10.1177/0956797612464242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert K. M., Blais C., Ball B. H., & Brewer G. A. (2018). Working memory cannot regulate overt emotional capture. Acta Psychologica, 185, 52–64. 10.1016/j.actpsy.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Wolf C., Jackson M. C., Kissling C., Thome J., & Linden D. E. J. (2011). Dysbindin-1 genotype effects on emotional working memory. Molecular Psychiatry, 16, 145–155. 10.1038/mp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C., Linden S., Jackson M. C., Healy D., Baird A., Linden D. E., & Thome J. (2011). Brain activity supporting working memory accuracy in patients with paranoid schizophrenia: A functional magnetic resonance imaging study. Neuropsychobiology, 64, 93–101. 10.1159/000323800 [DOI] [PubMed] [Google Scholar]
- Wolfe C. D., & Bell M. A. (2007). The integration of cognition and emotion during infancy and early childhood: Regulatory processes associated with the development of working memory. Brain and Cognition, 65, 3–13. 10.1016/j.bandc.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Xie W., Li H., Ying X., Zhu S., Fu R., Zou Y., & Cui Y. (2017). Affective bias in visual working memory is associated with capacity. Cognition and Emotion, 31, 1345–1360. [DOI] [PubMed] [Google Scholar]
- Xin F., & Lei X. (2015). Competition between frontoparietal control and default networks supports social working memory and empathy. Social Cognitive and Affective Neuroscience, 10, 1144–1152. 10.1093/scan/nsu160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Yang S., & Isen A. M. (2013). Positive affect improves working memory: Implications for controlled cognitive processing. Cognition and Emotion, 27, 474–482. 10.1080/02699931.2012.713325 [DOI] [PubMed] [Google Scholar]
- Yang P., Wang M., Jin Z., & Li L. (2015). Visual short-term memory load modulates the early attention and perception of task-irrelevant emotional faces. Frontiers in Human Neuroscience, 9, 490 10.3389/fnhum.2015.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiend J. (2010). The effects of emotion on attention: A review of attentional processing of emotional information. Cognition and Emotion, 24, 3–47. 10.1080/02699930903205698 [DOI] [Google Scholar]
- Yogo M., & Fujihara S. (2008). Working memory capacity can be improved by expressive writing: A randomized experiment in a Japanese sample. British Journal of Health Psychology, 13, 77–80. 10.1348/135910707X252440 [DOI] [PubMed] [Google Scholar]
- Yoon K. L., Kutz A. M., LeMoult J., & Joormann J. (2017). Working memory in social anxiety disorder: Better manipulation of emotional versus neutral material in working memory. Cognition and Emotion, 31, 1733–1740. 10.1080/02699931.2016.1257482 [DOI] [PubMed] [Google Scholar]
- Yoon K. L., LeMoult J., & Joormann J. (2014). Updating emotional content in working memory: A depression-specific deficit? Journal of Behavior Therapy and Experimental Psychiatry, 45, 368–374. 10.1016/j.jbtep.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Zetsche U., Bürkner P.-C., & Schulze L. (2018). Shedding light on the association between repetitive negative thinking and deficits in cognitive control. A meta-analysis. Clinical Psychology Review, 63, 56–65. 10.1016/j.cpr.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Zhang J.-N., Xiong K.-L., Qiu M.-G., Zhang Y., Xie B., Wang J., et al. Zhang J.-J. (2013). Negative emotional distraction on neural circuits for working memory in patients with posttraumatic stress disorder. Brain Research, 1531, 94–101. [DOI] [PubMed] [Google Scholar]
- Ziaei M., Peira N., & Persson J. (2014). Brain systems underlying attentional control and emotional distraction during working memory encoding. NeuroImage, 87, 276–286. 10.1016/j.neuroimage.2013.10.048 [DOI] [PubMed] [Google Scholar]
- Ziaei M., Salami A., & Persson J. (2017). Age-related alterations in functional connectivity patterns during working memory encoding of emotional items. Neuropsychologia, 94, 1–12. 10.1016/j.neuropsychologia.2016.11.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.