Abstract
Purpose
Two-thirds of cancer patients report taste disorders during and after chemotherapy. Taste disorders impact on nutritional status which is highly relevant for treatment efficacy and overall prognosis. Improvement of taste disorder is of particular importance for cancer patients’ outcomes, thus the TASTE trial was conducted to improve taste disorders with a taste and smell training.
Methods
In this trial, patients undergoing chemotherapy were screened for taste disorders. Subsequently, patients were allocated based on the detection of taste disorders (≤8 taste strips points) to an intervention group with a taste and smell training at baseline and week 3–5 or were only followed up, if no taste disorder was detected (≥9 taste strips points) (non-intervention group). At baseline, all patients received a nutritional counseling. The primary endpoint was the minimal clinically relevant improvement of taste strips score by 2 taste strips points in at least 50% of the patients with taste disorders.
Results
The trial included 62 patients (48 women [77%], 14 male [23%], age 54.5±11.6 years) who had gastrointestinal (n=29), breast (n=31), or lung cancer (n=2). Taste disorders were more frequent in gastrointestinal than in breast cancer patients. Out of 62 patients screened, 30 patients showed taste disorders. The primary endpoint was met with 92% (n=23 of 25) of the patients completing the intervention. In the intervention group, the patients’ taste significantly improved from baseline (median taste strips: 7.0 points) to week 12 (median taste strips: 10.0 points) (P≤0.001). Patients of the non-intervention group who completed the reassessment (n=27 of 32) experienced no change in taste perception in the 3-month follow-up (P=0.897).
Conclusion
Intensified nutritional counseling with taste and smell training may improve taste perception of patients undergoing chemotherapy. A confirmatory randomized trial is planned.
Keywords: cancer patients, taste disorders, taste and smell training, nutritional intervention, nutritional counseling, malnutrition
Introduction
Patients with cancer can have various cancer-related problems and treatment-related side effects. Besides significantly improving prognosis, the increasing use of multi-modal treatment including aggressive systemic treatment, radiotherapy, and surgery may result in high rates of treatment-related acute and long-term side effects. During the last decades, the burden of gastrointestinal symptoms relevantly decreased due to improved supportive measures against nausea, vomiting, or mucositis. Nevertheless, malnutrition and weight loss remain the most challenging problems in the management of cancer patients negatively impacting on prognosis.1–3 Besides loss of appetite and obstruction, smell and taste perceptions are of particular importance for malnutrition and weight loss and are often compromised by chemotherapy- or radiotherapy-induced neurotoxicity or mucositis.
Smell and taste disorders can occur as acute side effects but may also remain for a long period after completion of treatment. Up to two-thirds of cancer patients report a reduced or overall lack of taste.4,5 Cohen et al observed taste disorders in about 30% of childhood cancer survivors even 12 years after treatment completion.6 The prevalence was significantly higher compared to the general population.
Taste qualities are affected differently and can have a different intensity and frequency.7 Most common taste disorder seem to affect the “bitter” and “sour” quality of taste.5,7–10 The study by Maes et al showed that patients who had head and neck radiotherapy 1–2 years ago had a taste loss of 41%, 50%, 27%, and 27% for the flavors bitter, salty, sweet, and sour, respectively.11 In addition to treatment-related side effects, insufficient oral hygiene, lack of saliva, and nicotine or alcohol abuse are possible factors for a reduced taste and smell perception.12–14
Several studies demonstrated that a change in taste perception affects the nutritional behavior of patients during and after therapy.10,15,16 In prospective interventional trials in adolescent and young adult cancer survivors aiming at improvement of nutritional behavior, patients reported a far higher sodium intake than recommended due to changes in their taste perception.17
Actually, no guidelines for the treatment of taste disorders specifically for cancer patients are available.
Different methods to measure changes in taste and smell are available, for example, “Whole Mouth Test”, “Tasting Tablets”, and “Three Drop Test”.18 The “Taste Strips” test is a validated method. It is based on filter paper strips (“Taste Strips”) impregnated with the four (sweet, sour, salty, and bitter) taste qualities in four different concentrations. To obtain an impression of taste function, the number of correctly identified tastes is being summed up for a “taste score”. This test is quick and easy to apply.18
Pathological differences in taste perception can be suspected with a score difference of ≥3 points between the left and right side of the tongue.18 To date, no definition of the relevant clinical improvement of taste perception with the taste strips test is available. Hence, in the current study we defined 2 points as the minimal clinically relevant improvement (primary endpoint) and additionally analyzed a 3-point improvement as secondary endpoint.
Despite the high medical need and the validated assessments, trials with interventions to improve smell and taste in cancer patients are rare. Although in patients with olfactory disorders a significant improvement in smell perception was noted with daily smell training using scented pencils of lemon and cloves.19 A smell training with the scented pencil of eucalyptus improved smell perception but not significantly.19 It is unknown whether it is helpful in chemotherapy-induced smell disorders.
Thus a clinical pilot trial was initiated to assess the potential short-term impact of taste and smell training on smell and taste by an intervention in outpatient cancer patients receiving chemotherapy.
Participants and methods
Study design and participants
In this single-center Phase II trial, patients (aged ≥18 years) with gastrointestinal, lung or breast cancer undergoing chemotherapy were consecutively recruited in the outpatient clinics at the University Medical Center Hamburg-Eppendorf from April 2017 to November 2017.
Inclusion criteria comprised application of chemotherapy for breast, lung or gastrointestinal cancer. Exclusion criteria were the presence of a genetic metabolic disorder, an eating disorder, an enteral or a parenteral nutrition, an irradiation of the head/neck area or pregnancy.
The trial was performed in accordance with the Declaration of Helsinki and has been approved by the local ethics committee “Ethik-Kommission der Ärztekammer Hamburg” on 4/4/2017 (reference number PV5471) and registered in the German Clinical Trials Register (DRKS, clinical trial identifier DRKS00012501). All patients provided written informed consent in this pilot trial before study inclusion.
Study assessments
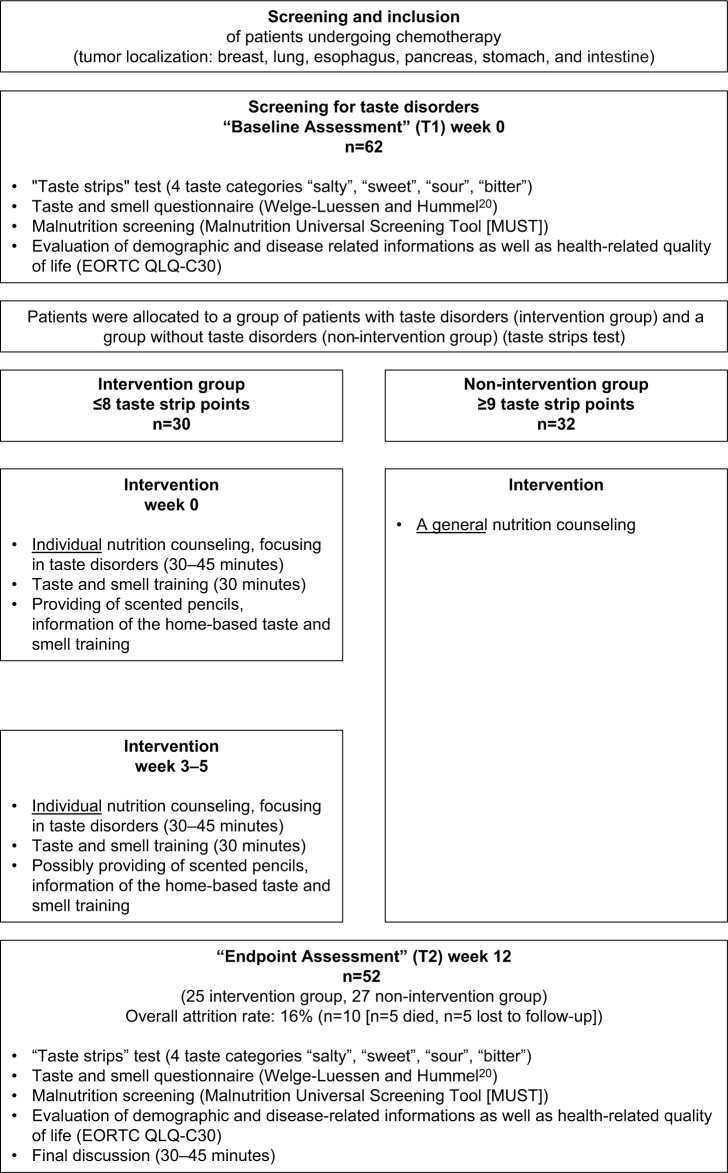
At baseline and in week 12 after inclusion, all consenting patients were assessed for taste disorders using the validated taste strips test (Taste Strips, Burghart Messtechnik GmbH, Tinsdaler Weg 175, D-22880 Wedel) impregnated with four different concentrations of each of the tastes “sweet” (strips A–D), “sour” (strips E–H), “salty” (strips I–L), and “bitter” (strips M–P) to be moved from the left to the right side of the tongue. Patients had to identify the taste from a list of four descriptors: sweet, sour, salty, and bitter (multiple forced-choice). Based on the answers of the patients, a taste score was calculated (0–16 points), used for the identification of taste disorders (≤8 points) and no taste disorders (≥9 points).18 The first strips in each category (A, E, I, M) have the highest and the subsequent strips have lower taste concentration. For a standardized and reproducible performance of the taste test, the order of taste strips has to be respected. At first taste strips with low taste concentrations were presented. According to the increasing concentrations in each category, the most concentrated taste strips were given at the end of the test.
In addition, body mass index (BMI), reported subjective taste (questionnaire by T. Hummel),20 health-related quality of life (HRQOL, measured by European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire),21 and malnutrition risk (measured by Malnutrition Universal Screening Tool [MUST])22 were assessed at baseline and after 12 weeks (refer to study flowchart in Figure 1). Demographic information and medical history were collected at baseline.
Figure 1.
Study flowchart.
Intervention
Based on the results of the taste strips, test patients were allocated to a group of patients with taste disorders (≤8 points measured by taste strips) (“intervention group”) and a group without taste disorders (≥9 points measured by taste strips) (“non-intervention group”). The intervention group received a taste and smell training and a single nutritional counseling at baseline and week 3–5. The group without taste disorders (non-intervention group) received only general nutritional information.
The individual face-to-face nutritional counseling of 60 minutes was performed by a dietitian and based on the recommendations of the World Cancer Research Fund International (WCRF)23 and the German Society of Nutritional Medicine Deutsche Gesellschaft für Ernährungsmedizin (DGEM).24 The main focus of the nutritional counseling was the use of flavor-enhancing nutrition, management of weight, and gastrointestinal side effects of the treatment. Depending on the dietary habits measured by a 24-hour recall protocol and reported nutritional problems, general recommendations were modified and tailored to the needs of the individual patient.
The 15-minute taste and smell training was conducted under the guidance of the dietitian. The patients tasted blindfolded specific drinks (eg, different fruit juices and teas) and foods (eg, pretzel sticks), smelled scented pencils (lemon and clove) to strengthen the olfactory nerves, and was educated in adequate oral hygiene.
In order to continue with the taste and smell training at home, all patients received a weekly schedule with detailed information on the conduction of home-based smell and taste training and summarized recommendations (eg, how to keep the tongue clean, try to drink at least 1.5–2 l per day, and to pay attention to a breathable nose). In addition, every patient received two odor probes (in the shape of pens, filled with lemon and clove odors) from the dietitian with the recommendation to smell them at least twice daily for 15 seconds.
Outcomes
The primary endpoint was the rate of patients with a minimal clinically relevant effect of 2-point improvement in taste strips scores after 12 weeks. Secondary endpoint was an at least 3-point improvement in taste strips score. Subjective taste perception20 was analyzed for both the cutoffs (2 and 3 points difference between baseline and week 12 thereafter).
Other secondary endpoints were changes in median taste strips scores, taste categories of “salty”, “sweet”, “sour”, and “bitter”, reported subjective taste (questionnaire by T. Hummel), quality of life (QoL) (EORTC QLQ-C30 questionnaire), and malnutrition risk (MUST) after 12 weeks.
Statistical analysis
The sample size calculation was conducted using G-Power version 3.1 applying an exact binominal test (one sample case) with the rate of patients with a 2-point improvement in taste strips after 12 weeks as primary endpoint. The rate of patients with an improvement of at least 2 points in taste strips 12 weeks after a general nutritional counseling with focus of a flavor-strengthening diet is expected to be around 25%. An intensified taste intervention with two nutritional counseling sessions and supervised as well as home-based taste and smell trainings should double this rate to 50% of patients to regard the intervention as meaningful. The probability to accept the intervention as promising (improvement rate ≥50% of patients), in spite of a true improvement rate of ≤25% only, was set at 0.1 (type I error). The probability to erroneously reject the intervention as not sufficiently efficient (≤25%), although the true improvement rate is meaningful (≥50%) was set at 0.2 (type II error, corresponding to a power of 80%).
According to these parameters and based on a one-sided test, 19 patients were required for statistical analysis in the intervention group. Based on the common attrition rate of about 30% in lifestyle interventional trials, overall 30 patients were planned to be included in the intervention group. The rate of taste disorders in the eligible patient population was estimated to be 50%. Thus, overall 60 patients were planned to be accrued.
Pre-post differences of the secondary endpoints were analyzed with Wilcoxon test for depended samples in an exploratory fashion.
Results
Patient characteristics
Sixty-two patients, 48 female (77%) and 14 male (23%), were included in the TASTE trial. Mean age at time of study inclusion was 54.5±11.6 years. Cancer diagnosis included gastrointestinal cancer (47% [n=29]), breast cancer (50% [n=31]), and lung cancer (3% [n=2]). As required by the inclusion criteria, all patients received chemotherapy, either single agent taxane, platinum, anthracycline or fluoropyrimidin (19% [n=12]), or combination chemotherapy (81% [n=50]). Platinum-based combination chemotherapy was applied in 22 patients (35%), mainly gastrointestinal cancer patients (n=15) (Table 1). The rate of platinum-based chemotherapy was numerically higher in patients with taste disorders (intervention group). Full baseline characteristics of intervention and non-intervention group are shown in Table 1.
Table 1.
Baseline characteristics
| Patient characteristics | All patients (n=62) | Intervention group (n=30) | Non-intervention group (n=32) |
|---|---|---|---|
|
| |||
| Gender: male/female | 14/48 | 8/22 | 6/26 |
| Age (years; mean ± SD) | 54.5±11.6 | 52.7±12.9 | 54.3±9.8 |
| Cancer type, n (%) | |||
| Gastrointestinal | 29 (47%) | 17 (59%) | 12 (41%) |
| Breast | 31 (50%) | 11 (37%) | 20 (63%) |
| Lung | 2 (3%) | 2 (7%) | 0 (0%) |
| Treatment, n (%) | |||
| Single agent of taxane, platinum, anthracycline, or fluoropyrimidin | 12 (19%) | 6 (20%) | 6 (19%) |
| Combination chemotherapy combination with platinum | 50 (81%) 22 (35%) |
23 (77%) 15 (50%) |
27 (84%) 7 (22%) |
Baseline results showed that patients with gastrointestinal cancer (median taste strips: 8.0 points) seem to generally have lower taste scores than breast cancer patients (median taste strips: 10.0 points). Expectedly, patients with gastrointestinal cancer seem to have a higher risk for taste disorders than patients with breast cancer.
The compliance rate decreased from 100% (n=62) at baseline to 84% (n=52) at week 12. The overall attrition rate was lower than expected at 16% (n=10, with n=5 patients died and n=5 patients lost to follow-up) during the 3 months of intervention. Overall 52 patients (25 intervention group, 27 non-intervention group) completed the 12-week assessment and were thus evaluable for the final analysis (Figure 1).
Primary endpoint
The primary endpoint was met with 92% (n=23 of 25) of the patients in the intervention group improving their taste by at least two taste strips points from baseline to week 12. Thus, the intensified and individualized nutritional counseling with taste and smell training appeared to be effective (Table 2).
Table 2.
Changes in the rate of improvement by two TS points and median TS points
| Intervention group (n=25) | |||
|---|---|---|---|
| Rate of improvement | 92% (n=23) | ||
| Time points | week 0 | week 12 | P-value |
| TS points (median value) | 7.0 | 10.0 | ≤0.001 |
Note: Analyzed with Wilcoxon test.
Abbreviation: TS, taste strip.
Secondary endpoints
Taste strip points and taste categories
About 64% (n=16 of 25) of the patients in the intervention group had an at least 3-point improvement in taste strips score. In the non-intervention group, 30% (n=8 of 27) and 26% (n=7 of 27) improved their taste perception by 2 and ≥3 points, respectively.
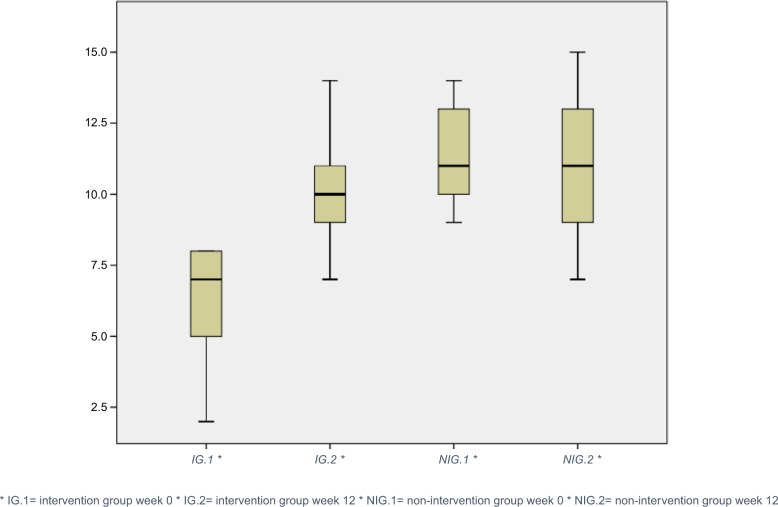
Patients had a significant improvement of median taste strip score in the intervention group over time, whereas the non-intervention group remained at stable levels (Table 2, Figure 2).
Figure 2.
Changes in median TS points.
Abbreviation: TS, taste strip.
There was a significant improvement in all taste categories after 12 weeks of intervention in patients with taste disorders (Table 3). At baseline, patients reached in the categories “sweet”, “sour”, and “bitter” median 2.0 points out of possible 4.0 points, but all improved to an average of 2.4 or 3.0 points after 12 weeks. Notably, the taste category “salty” had the lowest median value of all taste categories at baseline (median 1.0 points) and at week 12 (median 2.0 points). This suggests that the taste categories are affected in varying degrees.
Table 3.
Change in median TS points in taste categories
| Intervention group (n=25) | |||
|---|---|---|---|
| Time points | Week 0 | Week 12 | P-value |
| TS points in taste categories (median value) | |||
| Sweet | 2.0 | 3.0 | ≤0.001 |
| Sour | 2.0 | 2.4 | ≤0.001 |
| Salty | 1.0 | 2.0 | 0.002 |
| Bitter | 2.0 | 3.0 | 0.004 |
Note: Analyzed with Wilcoxon test.
Abbreviation: TS, taste strip.
Questionnaires for subjective taste and QoL
Subjective taste disorders according to questionnaire were reported by 77% (n=48) of the patients. In addition, 25% (n=15) reported smell disorders. About one-third of patients (n=20) reported impairments by these taste and/or smell disorders.
Furthermore, 13% (n=8) of patients suffered from burning, 37% (n=23) from xerostomia (dry mouth), 5% (n=3) from a foreign body sensation, 19% (n=12) from a constantly bitter taste, and 5% (n=3) from a constantly sour taste in the mouth (Table 4). The results show that patients of the intervention group with objective taste disorders reported numerically more subjective taste disorders than the non-intervention group with no objective taste disorders. Nonetheless, patients of the non-intervention group also had subjective taste disorders. Furthermore, this group reported a high rate of smell disorders and xerostomia (dry mouth).
Table 4.
Rate of patients of the intervention group and the non-intervention group in selected items of the taste and smell questionnaire in week 0
| Selected items of the questionnaire20 | Taste disorders | Smell disorders | Burning (mouth) | Xerostomia (dry mouth) | Constantly bitter taste (mouth) |
|---|---|---|---|---|---|
| All patients (n=62) | 77% (n=48) | 24% (n=15) | 13% (n=8) | 37% (n=23) | 19% (n=12) |
| Intervention group (n=30) | 93% (n=28) | 17% (n=5) | 20% (n=6) | 40% (n=12) | 23% (n=7) |
| Non-intervention group (n=32) | 75% (n=24) | 38% (n=12) | 16% (n=5) | 50% (n=16) | 16% (n=5) |
After 12 weeks, patients were questioned about their nasal congestion, smell perception, perception of flavors, taste perception, and mouth/tongue burning compared to their baseline consultation. Regarding the most frequent answers in the intervention group, 78% (n=18) had the same nasal congestion, 61% (n=14) the same smell perception, 43% (n=10) the same perception of flavors, 35% (n=8) the same taste perception, and 39% (n=9) never had a problem with mouth/tongue burning. In contrast to the improvement of taste disorders according to the taste strips (objective taste) in the intervention group as compared to the non-intervention group, the results of the questionnaires after 12 weeks were largely similar in both groups. Notably, rates of improved perception of taste (sweet, sour, bitter, salty) seem to favor the intervention group (Table S1).
A detailed analysis of the intervention group with patients of an improvement of 2 points and patients with an improvement of ≥3 points was conducted to see whether there were differences of changes in subjective taste perception. Results showed that patients with an improvement of ≥3 points had a higher rate of a better perception of flavors and taste perception (sweet, sour, bitter, salty) than the patients with an improvement of 2 points (Table S2).
To determine the potential impact of taste disorders on QoL, the global health status/quality of life (GHS/QOL) score of the EORTC QLQ C-30 questionnaire was determined. Median GHS/QOL score in the intervention group did not change significantly and was 50.0 points (range: 41.7–83.3 points) at week 0 and 58.3 points (range: 50.0–75.0 points) at week 12 (P=0.811). Similarly in the non-intervention group, median GHS/QOL score did not change and was 58.3 points (range: 50.0–75.0 points) at week 0 and 66.7 points (range: 50.0–75.0 points) at week 12 (P=0.438). A clinically relevant improvement of GHS/QOL score (≥10 points) was seen in 24% (n=6) in the intervention group and 30% (n=8) in the non-intervention group.
Malnutrition risk
Of 62 patients, 34 patients had a high risk of malnutrition, seven patients had a moderate risk of malnutrition, and 21 patients had a low risk of malnutrition according to the MUST score. Notably, patients with gastrointestinal cancer had a higher risk of malnutrition compared to breast cancer patients (Figure S1). The malnutrition risk was similar in both groups and interestingly rather improved in both groups after 12 weeks. This suggests that the malnutrition risk does not correlate with taste disorders but rather with the tumor type and the respective treatment.
Discussion
Despite the knowledge of the high risk for taste and smell disorders and the subsequent increased risk of malnutrition and weight loss in cancer patients, taste and smell training are not included in general nutritional counseling yet. Thus, this pilot trial was conducted to evaluate the impact of a specific taste and smell training accompanied by individual nutritional counseling in this vulnerable patient group. This first, prospective trial in patients undergoing chemotherapy demonstrated the feasibility and preliminary efficacy of this approach.
Notably, the majority of intervention patients completed the trial. An improvement of >2 points was noted in 92% (n=23) of the patients in the intervention group. Thus, the primary endpoint of this study was met. In addition, the median taste test strips score changed significantly from a median score 7.0 points (taste disorder defined as ≤8 taste strips points) to a score of 10.0 points which indicates normal taste perception (≥9 taste strips points).
In detail, all analyzed taste categories improved significantly. Interestingly, the taste category “salty” was the lowest at baseline (median 1.0 points). Previous studies in breast cancer patients or young cancer survivors noted a relevantly higher sodium intake potentially related to taste changes induced by chemotherapy.17,25 Particularly in cancer survivors this may be a relevant issue, regarding the association between high sodium intake and cardiovascular morbidity.26
The taste and smell questionnaire20 showed that patients of both group(s) have almost similar results of change in perception of nasal congestion, smell perception, perception of flavors, and mouth/tongue burning compared to their baseline assessment. Of note, in the intervention group a numerically higher number of patients reported an improved perception of taste (sweet, sour, bitter, salty), potentially related to the intervention applied in the TASTE trial.
So far, there is no exact definition of improvement of taste perception with taste strips test. The detailed analysis of the questionnaire showed that patients with an improvement of ≥3 points had a higher rate of improved subjective taste than patients with an improvement of two points. Thus, the ≥3 points cutoff might be more clinically relevant than the 2 point cutoff, although further trials are clearly needed to defined the best cutoff.
Beside the efficacy of the intervention, this study shed some light on the incidence and risk factors of taste and smell disorders and malnutrition in cancer patients undergoing chemotherapy. In the general (non-cancer) population, it is assumed that about 5% of people have taste disorders.27 Above the age of 50 years about 25% of people have an impaired sense of smell.28,29 The data obtained in this study clearly demonstrate the by far higher rate in cancer patients, calling for supportive interventions. Notably, diagnosis of a gastrointestinal cancer and the use of a platinum-based chemotherapy seem to be associated with an increased risk for taste disorders.5 Thus, particularly patients with gastrointestinal cancer with about 60% risk for taste disorders and 50% risk for malnutrition should be assessed and receive respective supportive measures like the intervention applied in this trial.
Due to the relatively short intervention and follow-up period of 12 weeks, the risk for malnutrition according to the MUST score, which is mainly determined by BMI and weight loss, remained largely unchanged. The noted trend showing some improvement in both groups maybe induced by taking part in a nutritional intervention trial.
The HRQOL scores remained similar during the observed 12-week period in both groups. HRQOL assessment refers to a multidimensional construct, considering the subjective perceptions of disease symptoms, treatment side effects as well as physical, emotional, social, and cognitive functions. Expectedly, there was no impact on HRQOL by the improvement of taste perception due to the relatively short period of time in a relatively small and heterogeneous patient population.
Limitations of the TASTE trial are primarily the uncontrolled single-arm design and a relatively small, heterogenous patient number. Due to the lack of a control group, determination of efficacy is limited. The non-intervention group could not serve as control as this group had no objective taste disorders at baseline. Patients’ oncological disease, change in chemotherapeutic regimen and gastrointestinal-related symptoms (eg, nausea, vomiting) may affect their taste perception, thus a larger sample size is required to account for these individual differences. The TASTE trial was conducted within a short follow-up period of 3 months in the individual patient. Thus, no data on the sustainability of the intervention are available.
Based on the short-term efficacy noted by applying the 12-week TASTE intervention, there might be a meaningful way to move forward. To clearly define the effect of this intervention with nutritional counseling and taste and smell training objectively, a randomized trial in patients with taste disorders with a longer follow-up period is currently planned.
Conclusion
Patients with cancer undergoing chemotherapy are at high risk for taste and smell disorders. These risks are associated with specific types of cancer and different chemotherapy regimens. The intervention applied in the TASTE trial with intensified nutritional counseling with taste and smell training is feasible and seems to improve short-term taste perception of patients undergoing chemotherapy and may thus potentially reduce the long-term risk for therapy-related taste disorders, further deterioration of nutritional status, and subsequently weight loss. A confirmatory randomized controlled trial is required.
Supplementary materials
Malnutrition risk according to group and tumor type at baseline and changes over time.
Table S1.
Rate of patients of the intervention group and the non-intervention group changes over time in items of the taste and smell questionnaire
| Items of the questionnaire1 | Answer options | Intervention group (n=25) | Non-intervention group (n=27) |
|---|---|---|---|
| 1. How would you describe your nasal congestion compared to your last visit? | Worse | 0% (n=0) | 11% (n=3) |
| Equal | 76% (n=19) | 44% (n=12) | |
| A little better | 16% (n=4) | 11% (n=3) | |
| Much better | 0% (n=0) | 15% (n=4) | |
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | |
| I never had a problem with it | 8% (n=2) | 19% (n=5) | |
| 2. How would you describe your smell perception compared to your last visit? | Worse | 0% (n=0) | 15% (n=4) |
| Equal | 60% (n=15) | 48% (n=13) | |
| A little better | 12% (n=3) | 19% (n=5) | |
| Much better | 20% (n=5) | 11% (n=3) | |
| I have no problem with that anymore | 8% (n=2) | 0% (n=0) | |
| I never had a problem with it | 0% (n=0) | 7% (n=2) | |
| 3. How would you describe your perception of flavors compared to your last visit? | Worse | 4% (n=1) | 30% (n=8) |
| Equal | 44% (n=11) | 30% (n=8) | |
| A little better | 16% (n=4) | 22% (n=6) | |
| Much better | 36% (n=9) | 19% (n=5) | |
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | |
| I never had a problem with it | 0% (n=0) | 0% (n=0) | |
| 4. How would you describe your taste perception (sweet, sour, bitter, salty) compared to your last visit? | Worse | 4% (n=1) | 30% (n=8) |
| Equal | 32% (n=8) | 33% (n=9) | |
| A little better | 28% (n=7) | 22% (n=6) | |
| Much better | 36% (n=9) | 15% (n=4) | |
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | |
| I never had a problem with it | 0% (n=0) | 0% (n=0) | |
| 5. How would you describe the mouth/tongue burning compared to your last visit? | Worse | 4% (n=1) | 11% (n=3) |
| Equal | 20% (n=5) | 15% (n=4) | |
| A little better | 16% (n=4) | 0% (n=0) | |
| Much better | 4% (n=1) | 4% (n=1) | |
| I have no problem with that anymore | 12% (n=3) | 4% (n=1) | |
| I never had a problem with it | 44% (n=11) | 67% (n=18) |
Table S2.
Rate of patients with 2 and ≥3 points of the intervention group changes over time in items of the taste and smell questionnaire
| Items of the questionnaire1 | Answer options | Intervention group TS points of 2 points (n=7) | Intervention group TS points of ≥3 points (n=16) | |
|---|---|---|---|---|
| 1. How would you describe your nasal congestion compared to your last visit? | Worse | 0% (n=0) | 0% (n=0) | |
| Equal | 71% (n=5) | 81% (n=13) | ||
| A little better | 29% (n=2) | 13% (n=2) | ||
| Much better | 0% (n=0) | 0% (n=0) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 6% (n=1) | ||
| 2. How would you describe your smell perception compared to your last visit? | Worse | 0% (n=0) | 0% (n=0) | |
| Equal | 43% (n=3) | 69% (n=11) | ||
| A little better | 43% (n=3) | 19% (n=3) | ||
| Much better | 14% (n=1) | 13% (n=2) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 0% (n=0) | ||
| 3. How would you describe your perception of flavors compared to your last visit? | Worse | 0% (n=0) | 6% (n=1) | |
| Equal | 71% (n=5) | 31% (n=5) | ||
| A little better | 0% (n=0) | 19% (n=3) | ||
| Much better | 29% (n=2) | 44% (n=7) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 0% (n=0) | ||
| 4. How would you describe your taste perception (sweet, sour, bitter, salty) compared to your last visit? | Worse | 0% (n=0) | 6% (n=1) | |
| Equal | 71% (n=5) | 19% (n=3) | ||
| A little better | 14% (n=1) | 31% (n=5) | ||
| Much better | 14% (n=1) | 44% (n=7) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 0% (n=0) | ||
| 5. How would you describe the mouth/tongue burning compared to your last visit? | Worse | 0% (n=0) | 6% (n=1) | |
| Equal | 29% (n=2) | 19% (n=3) | ||
| A little better | 43% (n=3) | 6% (n=1) | ||
| Much better | 0% (n=0) | 6% (n=1) | ||
| I have no problem with that anymore | 14% (n=1) | 13% (n=2) | ||
| I never had a problem with it | 14% (n=1) | 8% (n=50) | ||
Abbreviation: TS, taste strip.
Reference
- 1.Welge-Lüssen A, Hummel T. Praktisches Vorgehen bei Riech- und Schmeckstörungen. In: Hummel T, Welge-Lüssen A, editors. Riech- und Schmeckstörungen. Stuttgart: Georg Thieme Verlag; 2009. pp. 3–10. [Google Scholar]
Acknowledgments
We want to thank all patients who participated in this study, all participating clinicians who included patients, and all the staff engaged in this study. The study and manuscript was prepared without any funding or contribution of persons not mentioned in the authors’ section.
Footnotes
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data availability
Whether the authors intend to share individual deidentified participant data? No.
What specific data they intend to share? Not available.
What other study-related documents will be made available? Not available.
How the data will be accessible? Not applicable.
When and for how long they will be made available? Not applicable.
References
- 1.Capra S, Ferguson M, Ried K. Cancer: impact of Nutrition Intervention outcome-nutrition issues for patients. Nutrition. 2001;17(9):769–772. doi: 10.1016/s0899-9007(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 2.Laviano A, Renvyle T, Yang ZJ. From laboratory to bedside: new strategies in the treatment of malnutrition in cancer patients. Nutrition. 1996;12(2):112–122. doi: 10.1016/0899-9007(96)90709-1. [DOI] [PubMed] [Google Scholar]
- 3.Sarhill N, Mahmoud F, Walsh D, et al. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer. 2003;11(10):652–659. doi: 10.1007/s00520-003-0486-0. [DOI] [PubMed] [Google Scholar]
- 4.Ravasco P. Aspects of taste and compliance in patients with cancer. Eur J Oncol Nurs. 2005;9(Suppl 2):S84–S91. doi: 10.1016/j.ejon.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Wickham RS, Rehwaldt M, Kefer C, et al. Taste changes experienced by patients receiving chemotherapy. Oncol Nurs Forum. 1999;26(4):697–706. [PubMed] [Google Scholar]
- 6.Cohen J, Laing DG, Wilkes FJ, Chan A, Gabriel M, Cohn RJ. Taste and smell dysfunction in childhood cancer survivors. Appetite. 2014;75:135–140. doi: 10.1016/j.appet.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Hong JH, Omur-Ozbek P, Stanek BT, et al. Taste and odor abnormalities in cancer patients. J Support Oncol. 2009;7(2):58–65. [PubMed] [Google Scholar]
- 8.McLaughlin L. Taste dysfunction in head and neck cancer survivors. Oncol Nurs Forum. 2013;40(1):E4–E13. doi: 10.1188/13.ONF.E4-E13. [DOI] [PubMed] [Google Scholar]
- 9.Kokal WA. The impact of antitumor therapy on nutrition. Cancer. 1985;55(1 Suppl):273–278. doi: 10.1002/1097-0142(19850101)55:1+<273::aid-cncr2820551312>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.DeWys WD, Walters K. Abnormalities of taste sensation in cancer patients. Cancer. 1975;36(5):1888–1896. doi: 10.1002/1097-0142(197511)36:5<1888::aid-cncr2820360546>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Maes A, Huygh I, Weltens C, et al. De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol. 2002;63(2):195–201. doi: 10.1016/s0167-8140(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JB, Barasch A. Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol. 2010;46(2):77–81. doi: 10.1016/j.oraloncology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255(8):1121–1126. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JB, Smutzer G, Doty RL. Understanding the impact of taste changes in oncology care. Support Care Cancer. 2016;24(4):1917–1931. doi: 10.1007/s00520-016-3083-8. [DOI] [PubMed] [Google Scholar]
- 15.Farmer MN, Raddin RS, Roberts JD. The relationship between taste, olfaction, and nutrition in the cancer population. J Support Oncol. 2009;7(2):70–72. [PubMed] [Google Scholar]
- 16.Trant AS, Serin J, Douglass HO. Is taste related to anorexia in cancer patients? Am J Clin Nutr. 1982;36(1):45–58. doi: 10.1093/ajcn/36.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Quidde J, von Grundherr J, Koch B, et al. Improved nutrition in adolescents and young adults after childhood cancer-INAYA study. BMC Cancer. 2016;16(1):872. doi: 10.1186/s12885-016-2896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis BN, Welge-Lüssen A, Brämerson A, et al. “Taste Strips” – a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256(2):242–248. doi: 10.1007/s00415-009-0088-y. [DOI] [PubMed] [Google Scholar]
- 19.Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119(3):496–499. doi: 10.1002/lary.20101. [DOI] [PubMed] [Google Scholar]
- 20.Welge-Lüssen A, Hummel T. Praktisches Vorgehen bei Riech- und Schmeckstörungen. In: Hummel T, Welge-Lüssen A, editors. Riech- und Schmeckstörungen. Stuttgart: Georg Thieme Verlag; 2009. pp. 3–10. [Google Scholar]
- 21.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 23.Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund/American Institute for Cancer Research; 2007. [Google Scholar]
- 24.Arends J, Bertz H, Bischoff SC, et al. DGEM Steering Committee S3-Leitline der Deutschen Gesellschaft für Ernährungsmedizin e. V. (DGEM) in Kooperation mit der Deutschen Gesellschaft für Hämatologie und Onkologie e. V. (DGHO), der Arbeitsgemeinschaft “Supportive Maßnahmen in der Onkologie, Rehabilitation und Sozialmedizin” der Deutschen Krebsgesellschaft (ASORS) und der Österreichischen Arbeitsgemeinschaft für klinische Ernährung (AKE). Klinische Ernährung in der Onkologie. Aktuel Ernährungsmed. 2015;40(5):e1–e74. [Google Scholar]
- 25.Ceccatto V, Faria di Pietro P, Nogueira Previdelli A, et al. Brazilian healthy eating index revised (BHEI-R) of women before and during adjuvant treatment for breast cancer. Nutr Hosp. 2014;30(5):1101–1109. doi: 10.3305/nh.2014.30.5.7439. [DOI] [PubMed] [Google Scholar]
- 26.Mente A, O’Donnell M, Rangarajan S, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392(10146):496–506. doi: 10.1016/S0140-6736(18)31376-X. [DOI] [PubMed] [Google Scholar]
- 27.Welge-Lüssen A, Dörig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol. 2011;258:386–392. doi: 10.1007/s00415-010-5763-5. [DOI] [PubMed] [Google Scholar]
- 28.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 29.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Malnutrition risk according to group and tumor type at baseline and changes over time.
Table S1.
Rate of patients of the intervention group and the non-intervention group changes over time in items of the taste and smell questionnaire
| Items of the questionnaire1 | Answer options | Intervention group (n=25) | Non-intervention group (n=27) |
|---|---|---|---|
| 1. How would you describe your nasal congestion compared to your last visit? | Worse | 0% (n=0) | 11% (n=3) |
| Equal | 76% (n=19) | 44% (n=12) | |
| A little better | 16% (n=4) | 11% (n=3) | |
| Much better | 0% (n=0) | 15% (n=4) | |
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | |
| I never had a problem with it | 8% (n=2) | 19% (n=5) | |
| 2. How would you describe your smell perception compared to your last visit? | Worse | 0% (n=0) | 15% (n=4) |
| Equal | 60% (n=15) | 48% (n=13) | |
| A little better | 12% (n=3) | 19% (n=5) | |
| Much better | 20% (n=5) | 11% (n=3) | |
| I have no problem with that anymore | 8% (n=2) | 0% (n=0) | |
| I never had a problem with it | 0% (n=0) | 7% (n=2) | |
| 3. How would you describe your perception of flavors compared to your last visit? | Worse | 4% (n=1) | 30% (n=8) |
| Equal | 44% (n=11) | 30% (n=8) | |
| A little better | 16% (n=4) | 22% (n=6) | |
| Much better | 36% (n=9) | 19% (n=5) | |
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | |
| I never had a problem with it | 0% (n=0) | 0% (n=0) | |
| 4. How would you describe your taste perception (sweet, sour, bitter, salty) compared to your last visit? | Worse | 4% (n=1) | 30% (n=8) |
| Equal | 32% (n=8) | 33% (n=9) | |
| A little better | 28% (n=7) | 22% (n=6) | |
| Much better | 36% (n=9) | 15% (n=4) | |
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | |
| I never had a problem with it | 0% (n=0) | 0% (n=0) | |
| 5. How would you describe the mouth/tongue burning compared to your last visit? | Worse | 4% (n=1) | 11% (n=3) |
| Equal | 20% (n=5) | 15% (n=4) | |
| A little better | 16% (n=4) | 0% (n=0) | |
| Much better | 4% (n=1) | 4% (n=1) | |
| I have no problem with that anymore | 12% (n=3) | 4% (n=1) | |
| I never had a problem with it | 44% (n=11) | 67% (n=18) |
Table S2.
Rate of patients with 2 and ≥3 points of the intervention group changes over time in items of the taste and smell questionnaire
| Items of the questionnaire1 | Answer options | Intervention group TS points of 2 points (n=7) | Intervention group TS points of ≥3 points (n=16) | |
|---|---|---|---|---|
| 1. How would you describe your nasal congestion compared to your last visit? | Worse | 0% (n=0) | 0% (n=0) | |
| Equal | 71% (n=5) | 81% (n=13) | ||
| A little better | 29% (n=2) | 13% (n=2) | ||
| Much better | 0% (n=0) | 0% (n=0) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 6% (n=1) | ||
| 2. How would you describe your smell perception compared to your last visit? | Worse | 0% (n=0) | 0% (n=0) | |
| Equal | 43% (n=3) | 69% (n=11) | ||
| A little better | 43% (n=3) | 19% (n=3) | ||
| Much better | 14% (n=1) | 13% (n=2) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 0% (n=0) | ||
| 3. How would you describe your perception of flavors compared to your last visit? | Worse | 0% (n=0) | 6% (n=1) | |
| Equal | 71% (n=5) | 31% (n=5) | ||
| A little better | 0% (n=0) | 19% (n=3) | ||
| Much better | 29% (n=2) | 44% (n=7) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 0% (n=0) | ||
| 4. How would you describe your taste perception (sweet, sour, bitter, salty) compared to your last visit? | Worse | 0% (n=0) | 6% (n=1) | |
| Equal | 71% (n=5) | 19% (n=3) | ||
| A little better | 14% (n=1) | 31% (n=5) | ||
| Much better | 14% (n=1) | 44% (n=7) | ||
| I have no problem with that anymore | 0% (n=0) | 0% (n=0) | ||
| I never had a problem with it | 0% (n=0) | 0% (n=0) | ||
| 5. How would you describe the mouth/tongue burning compared to your last visit? | Worse | 0% (n=0) | 6% (n=1) | |
| Equal | 29% (n=2) | 19% (n=3) | ||
| A little better | 43% (n=3) | 6% (n=1) | ||
| Much better | 0% (n=0) | 6% (n=1) | ||
| I have no problem with that anymore | 14% (n=1) | 13% (n=2) | ||
| I never had a problem with it | 14% (n=1) | 8% (n=50) | ||
Abbreviation: TS, taste strip.
Data Availability Statement
Whether the authors intend to share individual deidentified participant data? No.
What specific data they intend to share? Not available.
What other study-related documents will be made available? Not available.
How the data will be accessible? Not applicable.
When and for how long they will be made available? Not applicable.