Abstract
Background: While haploidentical transplantation has led to the near-universal availability of donors, several challenges for this form of transplant still exist. This study sought to investigate the rates of infection-related mortality and other complications following haploidentical vs nonhaploidentical transplant.
Methods: We conducted a retrospective cohort study in adults with various malignant and benign hematological conditions who underwent allogeneic hematopoietic stem cell transplantation from 2011 to 2018. One hundred-day and 1-year overall survival were defined as survival from the time of transplant until 100 days or 1 year later.
Results: A total of 187 patients were included in this study, with 45 (24.1%) receiving transplants from haploidentical donors and 142 (75.9%) from nonhaploidentical donors. There were similar rates of acute graft-versus-host disease (GVHD) (40% vs 38% in haploidentical vs nonhaploidentical recipients, P=0.86) and chronic GVHD (44.4% vs 43.7%, P=1). Rates of 100-day and 1-year infection-related mortality were significantly higher in the haploidentical group compared to the nonhaploidentical group (8.9% vs 1.4% at 100 days, P=0.03, and 15.9% vs 3.8% at 1 year, P=0.01). There were also higher rates of cytomegalovirus infections (59.1% vs 23.8%, P<0.01), BK virus-associated hemorrhagic cystitis (40.9% vs 8.4%, P<0.01), and BK viremia (15.9% vs 0.8%, P<0.01) in haploidentical recipients.
Conclusions: Despite the use of identical antimicrobial prophylactic and treatment agents, haploidentical recipients were found to have significantly increased rates of 100-day and 1-year infection-related mortality as well as several other infectious complications.
Keywords: infection, survival, haploidentical stem cell transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative therapeutic modality for various malignant and nonmalignant hematological conditions. While previous studies showed that the best results of allogeneic HSCT were obtained when the stem cells from a human leukocyte antigen (HLA)-matched sibling were used, such a donor can only be found for approximately 30% of patients.1 Alternative sources of stem cells include HLA-matched unrelated donors and, in more recent times, HLA-haploidentical donors. The use of HLA-haploidentical donors has led to the near-universal availability of donors with an average of 2.7 potential donors among first-degree relatives.1 Prior studies have also suggested a stronger graft-versus-leukemia effect in high-risk acute leukemia patients following haploidentical transplants with a lower incidence of cumulative relapse and improved overall survival (OS).2,3
Within the past decade, there have been large improvements in the process of haploidentical transplantation with regard to decreasing morbidity and mortality; however, many challenges for this form of transplantation still exist. Historically, the major complication following haploidentical transplant was the high incidence of severe or fatal graft-versus-host disease (GVHD). A previous analysis of over 2,000 allogeneic HSCTs from 1985 to 1991 confirmed the high incidence of bidirectional alloreactivity, demonstrating higher rates of transplant-related mortality, graft failure, acute GVHD, and chronic GVHD compared to HLA-matched related donors.4 Subsequently, advances in transplantation methods such as the addition of T-cell depletion with antithymocyte globulin (ATG) to pharmacologic prophylaxis against GVHD have decreased the risks of graft failure and GVHD and made haploidentical transplantation an acceptable alternative to HLA-matched related and HLA-matched unrelated transplants.5
Another frequently encountered complication of haploidentical transplants is an increased rate of infectious complications thought to be due to a delay in immune reconstitution.6 A prior study involving 75 patients with hematological malignancies who underwent different forms of allogeneic HSCT demonstrated that there was a longer time to immune reconstitution, specifically of the T-cell subset and dendritic cell subgroup prior to day 90 after grafting, following haploidentical transplant compared to HLA-matched transplant.7 Of the modern haploidentical transplantation strategies, megadose T-cell depletion has been associated with the highest rate of infectious mortality at approximately 40%.8 Both the GIAC protocol (a T-cell-replete haploidentical HSCT protocol) and post-transplant cyclophosphamide (PTCy) have also led to slower rates of immune reconstitution after haploidentical transplant compared to matched related transplant, though without significant effects on rates of nonrelapse mortality.7,9 Our study further investigated the clinical implications of delayed immune reconstitution after haploidentical transplant by analyzing the rates of infection-related mortality and complications following each type of transplantation.
Materials and methods
Study design
This was a retrospective cohort study conducted at the Norris Comprehensive Cancer Center affiliated with the University of Southern California (USC), which analyzed the outcomes of patients aged >18 years who underwent allogeneic HSCT. The study was approved by the institutional review board at the Norris Comprehensive Cancer Center and patient consent was obtained for the reviewing of medical records. Patients included in the study had a variety of hematological conditions, both malignant and nonmalignant, and underwent transplant with haploidentical donor transplants, matched-related donor transplants, or matched-unrelated transplants from 2011 to 2018. Two cohorts were included in the analysis: those who underwent HLA-haploidentical transplantation and those who underwent other forms of transplantation (either matched-related donor or matched-unrelated donor transplants). Human progenitor cells were collected from either the bone marrow or peripheral blood of donors. Electronic medical records from the Norris Comprehensive Cancer Center provided all patient information including demographic data, conditioning regimens, infection prophylaxis, and clinical outcomes.
Transplant regimens
Conditioning regimens included total body irradiation (TBI) with cyclophosphamide (TBI 200 cGy twice daily for 6 doses on days −6 to −4 and cyclophosphamide 60 mg/kg i.v. daily on days −3 and −2), busulfan with cyclophosphamide (busulfan 0.9 mg/kg i.v. every 6 hrs on days −7 to −4 and cyclophosphamide 60 mg/kg i.v. daily on days −3 to −2), fludarabine with cyclophosphamide (fludarabine 30 mg/m2 i.v. daily on days −5 to −2 and cyclophosphamide 50 mg/kg i.v. daily on day −5), fludarabine with TBI (fludarabine 25 mg/m2 i.v. daily on days −6 to −4 and TBI 200 cGy twice daily for 6 doses on days −3 to −1), fludarabine with busulfan (fludarabine 30 mg/m2 i.v. daily and busulfan 0.9 mg/kg i.v. every 6 hrs on days −7 to −4), melphalan with fludarabine (melphalan 140 mg/m2 i.v. daily on day −6 and fludarabine 40 mg/m2 i.v. daily on days −5 to −2), and fludarabine with cyclophosphamide and TBI (fludarabine 30 mg/m2 i.v. daily and cyclophosphamide 14.5 mg/kg i.v. daily on days −6 to −2 and TBI 200cGy for 1 dose on day −1).
GVHD prophylaxis regimens included either the combination of cyclophosphamide, tacrolimus and mycophenolate mofetil (MMF) or tacrolimus and methotrexate (MTX). The former regimen included cyclophosphamide 50 mg/kg i.v. daily on days +3 and +4 administered with mesna 10 mg/kg i.v. given 3 hrs, 6 hrs, and 9 hrs after cyclophosphamide infusion; this was followed by MMF and tacrolimus starting day +5 (MMF 1 g three times daily by mouth and tacrolimus 0.03 mg/kg i.v. daily). MMF was continued until day +35, and tacrolimus was transitioned from intravenous to oral administration to be tapered outpatient. The regimen of tacrolimus and MTX included MTX 15 mg/m2 i.v. daily on day +1 and then 10 mg/m2 i.v. daily on days +3, +6, and +11. Tacrolimus 0.3 mg/kg i.v. daily was started on day −1 and then transitioned to oral administration to be tapered outpatient. When administered, rabbit-derived ATG was given either on a 3-day schedule of 3 mg/kg i.v. daily on days −3 to −1 or a 3-day schedule of 0.5 mg/kg i.v. daily on day −3, 1.5 mg/kg i.v. daily on day −2, and 2.5 mg/kg i.v. daily on day −1. The Keystone Consensus criteria were used in assessing grades of acute GVHD, with severity ranging from grade 1 (mild) up to grade 4 (most severe).10 Chronic GVHD was referred to as either limited, characterized by localized skin involvement or hepatic dysfunction without evidence of progressive hepatitis or cirrhosis on histology, or extensive, characterized by involvement of the eyes, salivary glands, oral mucosa, or any other target organ.
All patients received antiviral prophylaxis with acyclovir, antifungal prophylaxis with either micafungin or an azole (fluconazole, voriconazole, posaconazole, or isavuconazole), and pneumocystis pneumonia prophylaxis with trimethoprim-sulfamethoxazole. No antibacterial prophylaxis was used universally amongst all patients in the study; an exception is that levofloxacin was started when patients met neutropenic criteria, as indicated by an absolute neutrophil count (ANC) of <500 x 109 cells/L, and continued until neutrophil recovery.
Monitoring for infections
Routine monitoring for cytomegalovirus (CMV) viremia was conducted 3 times per week by qRT-PCR for the first 6 months after transplant in all patients, with CMV infection defined as detectable DNA by this assay. The frequency of CMV monitoring was subsequently decreased for the remainder of the 1-year post-transplant period. Patients otherwise underwent evaluation for specific bacterial, fungal, and viral infections as needed per clinical presentation as well as laboratory and imaging findings. Testing for BK viruria and viremia was performed by DNA urine RT-PCR and DNA plasma RT-PCR, respectively, in all patients who reported new-onset urinary symptoms such as dysuria and hematuria after transplant. BK virus infection was defined as detectable DNA by these assays.
Statistical analysis
One hundred-day survival was defined as survival from the time of allogeneic HSCT until 100 days post-transplant. Likewise, 1-year survival was the time from transplant until 1 year post-transplant. Time to neutrophil engraftment was defined as the time from HSCT until the first day of 3 consecutive days in which the ANC remained >500 neutrophils/μL. Time to platelet engraftment was defined as the time from HSCT until the first day of 7 consecutive days in which the platelet count remained above 20×109 platelets/μL. Statistical analysis was performed using the Mann–Whitney U Test and Pearson’s Chi-square test with two-tailed P-values <0.05 deemed significant.
Results
A total of 187 patients were eligible for analysis in this study. From these patients, 45 (24.1%) underwent transplants from haploidentical donors and 142 (75.9%) underwent transplant from nonhaploidentical donors (matched-related donors and matched-unrelated donors). Characteristics of sex, age at transplant, age groups, and race were similar between the two groups. However, there was a significant difference in time to both neutrophil and platelet engraftment, with neutrophil engraftment requiring a median of 17 days vs 15 days (P<0.01) and platelet engraftment requiring a median of 25 days vs 17 days (P<0.01) in those who underwent haploidentical transplants vs nonhaploidentical transplants, respectively. These characteristics are listed in Table 1. Patients underwent HSCT for a variety of malignant and nonmalignant hematological conditions, with the full list of diagnoses listed in Table 2. All patients with malignant hematological conditions underwent transplant while in complete remission.
Table 1.
Patient characteristics
| Variable | Haploidentical (n=45) | Nonhaploidentical (n=142) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.73 | ||
| Male | 22 (48.9%) | 74 (52.1%) | |
| Female | 23 (51.1%) | 68 (47.9%) | |
| Age at transplant (years) | 0.68 | ||
| Median | 47 | 47 | |
| Range | 23–67 | 19–75 | |
| Age group (years), n (%) | 0.73 | ||
| <40 | 21 (46.7%) | 61 (43%) | |
| >40 | 24 (53.3%) | 81 (57%) | |
| Race, n (%) | 0.76 | ||
| White | 14 (31.1%) | 43 (30.3%) | |
| Hispanic | 26 (57.8%) | 88 (62%) | |
| Other | 5 (11.1%) | 11 (7.7%) | |
| Time to neutrophil engraftment (days) | <0.01 | ||
| Median | 17 | 15 | |
| Range | 15–29 | 11–25 | |
| Time to platelet engraftment (days) | <0.01 | ||
| Median | 25 | 17 | |
| Range | 15–31 | 8–28 | |
| Human leukocyte antigen (HLA) matching | <0.01 | ||
| Median | 5/10 | 10/10 | |
| Range | 5/10–8/10 | 9/10–10/10 |
Note: Bold type indicates statistical significance.
Table 2.
Diagnoses of allogeneic hematopoietic stem cell transplant recipients
| Diagnosis | Haploidentical (n=45) | Nonhaploidentical (n=142) | P-value |
|---|---|---|---|
| Myeloid malignancies | |||
| Acute myeloid leukemia | 16 (35.6) | 64 (45.1) | 0.30 |
| Chronic myeloid leukemia | 0 (0) | 7 (4.9) | 0.20 |
| Chronic myelomonocytic leukemia | 1 (2.2) | 0 (0) | 0.24 |
| Acute myelomonocytic leukemia | 0 (0) | 1 (0.7) | 1 |
| Lymphoid malignancies | |||
| Acute lymphoblastic leukemia | 16 (35.6) | 45 (31.7) | 0.72 |
| Hodgkin’s lymphoma | 2 (4.4) | 2 (1.4) | 0.24 |
| Diffuse large B-cell lymphoma | 1 (2.2) | 0 (0) | 0.24 |
| Marginal zone lymphoma | 0 (0) | 1 (0.7) | 1 |
| Nonmalignant conditions | |||
| Aplastic anemia | 4 (8.9) | 7 (4.9) | 0.47 |
| Myelofibrosis | 1 (2.2) | 8 (5.6) | 0.46 |
| Myelodysplastic syndrome | 3 (6.7) | 3 (2.1) | 0.15 |
| Systemic mastocytosis | 1 (2.2) | 0 (0) | 0.24 |
| Hemophagocytic lymphohistiocytosis | 0 (0) | 2 (1.4) | 1 |
| Polycythemia vera | 0 (0) | 1 (0.7) | 1 |
| Chronic variable immunodeficiency | 0 (0) | 1 (0.7) | 1 |
Note: Values are presentend as n (%).
Graft-versus-host disease
The rates of acute GVHD were similar between the two groups, occurring in 18 (40%) haploidentical recipients and 54 (38%) nonhaploidentical recipients (P=0.86). Likewise, there were no significant differences in the grades of disease, with grade 1 acute GVHD occurring in 9 (20%) vs 25 (17.6%) patients (P=0.82), grade 2 in 5 (11.1%) vs 22 (15.5%) patients (P=0.63), grade 3 in 2 (4.4%) vs 4 (2.8%) patients (P=0.63), and grade 4 in 2 (4.4%) vs 3 (2.1%) patients (P=0.60) for haploidentical vs nonhaploidentical recipients, respectively. Rates of chronic GVHD were also similar between the two groups, occurring in 20 (44.4%) haploidentical recipients and 62 (43.7%) nonhaploidentical recipients (P=1). Limited chronic GVHD was seen in 9 (20%) haploidentical patients and 32 (22.5%) nonhaploidentical patients (P=0.84) while extensive chronic GVHD was noted in 11 (24.4%) vs 30 (21.1%) patients (P=0.68), again indicating no significant differences. Results of these findings are displayed in Table 3.
Table 3.
Rates of acute and chronic graft-versus-host disease (GVHD)
| Classification of GVHD | Haploidentical (n=45) | Nonhaploidentical (n=142) | P-value |
|---|---|---|---|
| Acute GVHD | |||
| Overall incidence | 18 (40) | 54 (38) | 0.86 |
| Grade 1 | 9 (20) | 25 (17.6) | 0.82 |
| Grade 2 | 5 (11.1) | 22 (15.5) | 0.63 |
| Grade 3 | 2 (4.4) | 4 (2.8) | 0.63 |
| Grade 4 | 2 (4,4) | 3 (2.1) | 0.60 |
| Chronic GVHD | |||
| Overall incidence | 20 (44.4) | 62 (43.7) | 1 |
| Limited | 9 (20) | 32 (22.5) | 0.84 |
| Extensive | 11 (24.4) | 30 (21.1) | 0.68 |
Note: Values are presentend as n (%).
Infection-related mortality
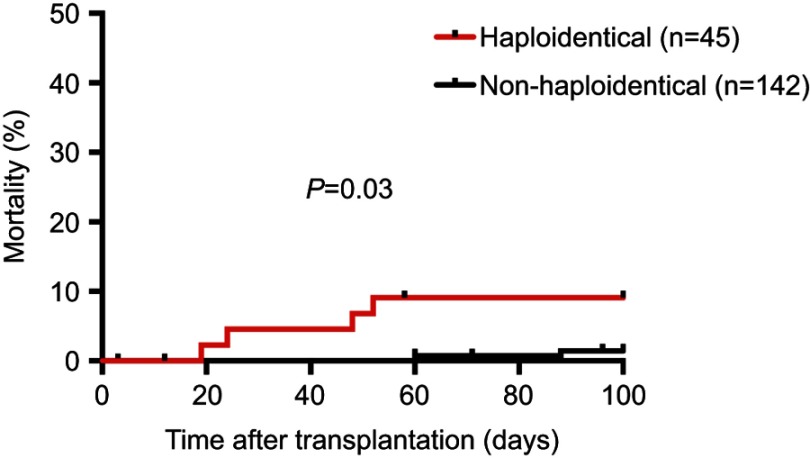
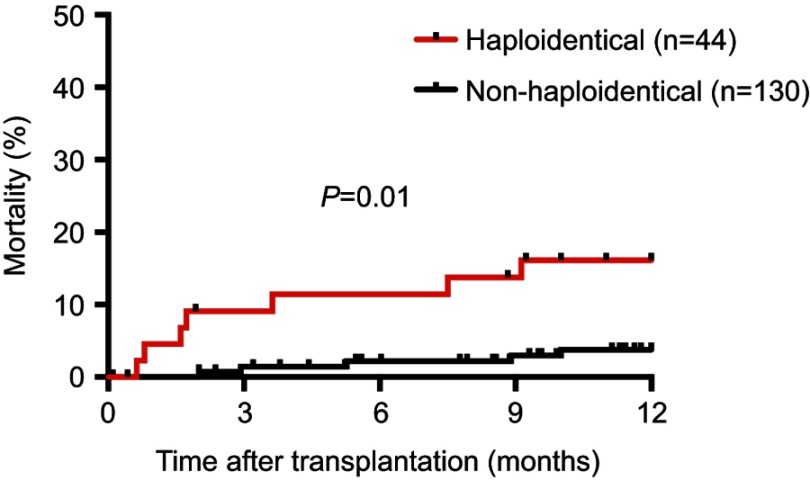
All 187 patients were eligible for 100-day survival analysis and 174 patients were eligible for 1-year analysis. When comparing the rates of infection-related mortality at different time points, the 100-day infection-related mortality rate was significantly higher in the haploidentical group at 8.9% compared to 1.4% in the nonhaploidentical group (P=0.03, Figure 1). In addition, the 1-year rate of infection-related mortality was also significantly increased at 15.9% vs 3.8% in the haploidentical vs nonhaploidentical groups, respectively (P=0.01, Figure 2). The full list of infections that occurred during this study is displayed in Table 4.
Figure 1.
100-day infection-related mortality.
Figure 2.
1-year infection-related mortality.
Table 4.
Incidence of infections within 1 year after transplant
| Type of infection | Haploidentical (n=44) | Nonhaploidentical (n=130) | P-value |
|---|---|---|---|
| Bacterial | |||
| Clostridium difficile | 8 (18.2) | 16 (12.3) | 0.44 |
| Bacteremia | 9 (20.5) | 12 (9.2) | 0.06 |
| Urinary tract infection | 8 (18.2) | 11 (8.5) | 0.09 |
| Pneumonia | 4 (9.1) | 9 (6.9) | 0.74 |
| Pyelonephritis | 1 (2.3) | 2 (1.5) | 1 |
| Typhlitis | 1 (2.3) | 1 (0.8) | 1 |
| Helicobacter pylori | 0 | 1 (0.8) | 1 |
| Cellulitis | 0 | 1 (0.8) | 1 |
| Sinusitis | 0 | 1 (0.8) | 1 |
| Orchitis | 0 | 1 (0.8) | 1 |
| Fungal | |||
| Coccidioidomycosis | 6 (13.6) | 8 (6.2) | 0.20 |
| Candidiasis | 1 (2.3) | 6 (4.6) | 0.68 |
| Mucormycosis | 1 (2.3) | 0 | 0.25 |
| Aspergillosis | 0 | 1 (0.8) | 1 |
| Tinea pedis | 0 | 1 (0.8) | 1 |
| Viral | |||
| Cytomegalovirus | 26 (59.1) | 31 (23.8) | <0.01 |
| BK viruria | 18 (40.9) | 11 (8.4) | <0.01 |
| BK viremia | 7 (15.9) | 1 (0.8) | <0.01 |
| Ebstein–Barr virus | 4 (9.1) | 7 (5.4) | 0.47 |
| Herpes zoster | 2 (4.5) | 4 (3.1) | 1 |
| Viral gastroenteritis | 1 (2.3) | 1 (0.8) | 1 |
| Herpes simplex virus | 0 | 2 (1.5) | 1 |
| Hepatitis B virus | 0 | 1 (0.8) | 1 |
Notes: Bold type indicates statistical significance. Values are presentend as n (%).
Incidence of infections
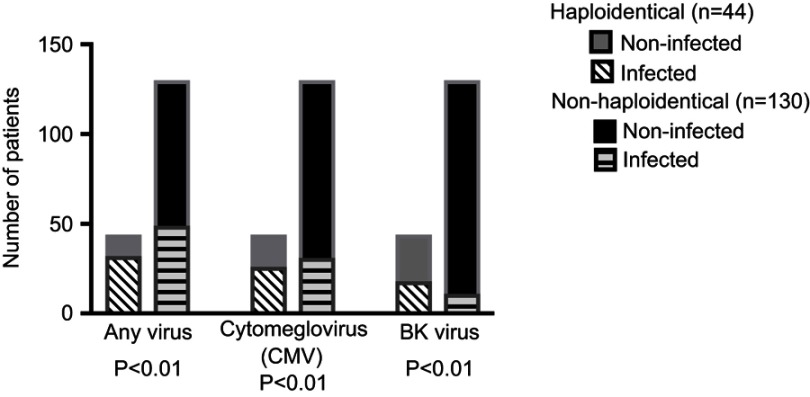
Within 1 year after transplant, the proportion of patients who experienced bacterial and fungal infections was comparable between the two groups, occurring in 40.9% vs 41.5% (P=1) for bacterial infections and 18.2% vs 12.3% (P=0.44) for fungal infections in haploidentical patients vs nonhaploidentical patients, respectively. On the other hand, the incidence of viral infections was significantly higher in the haploidentical group at 72.7% vs 38.5% in the nonhaploidentical group (P<0.01). Table 4 displays the rates of specific bacterial, fungal, and viral infections.
Specific viruses with increased rates of infection in the haploidentical vs nonhaploidentical population included CMV viremia (59.1% vs 23.8%, P<0.01) and BK viruria (40.9% vs 8.4%, P<0.01). In addition, there was a significantly higher overall incidence of BK viremia (15.9% vs 0.8%, P<0.01) in haploidentical recipients as well. Figure 3 displays rates of overall viral infections as well as CMV and BK virus specifically.
Figure 3.
Incidence of viral infections within 1 year after transplant.
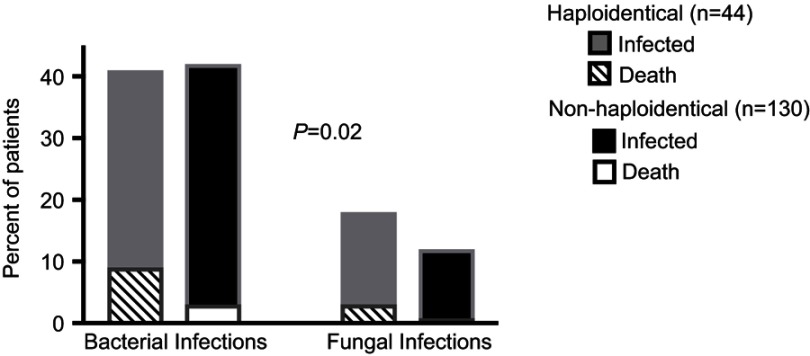
Despite the greater incidence of viral infections in the haploidentical population, all cases of 1-year infection-related mortality were due to bacterial and fungal infections rather than viral with 26.9% of bacterial or fungal infections, leading to patient death in haploidentical patients compared to 7.1% in nonhaploidentical patients (P=0.02). This finding is displayed in Figure 4.
Figure 4.
Incidence of bacterial and fungal infections and rates of subsequent mortality within 1 year after transplant.
Abbreviation: CMV, Cytomegalovirus.
Discussion
In recent years, HLA-haploidentical transplantation has become a reasonable therapeutic modality for malignant and nonmalignant hematological conditions. While previous analyses had suggested that higher rates of acute and chronic GVHD of all grades in haploidentical transplantation made it an inferior treatment option, our study showed that there was no significant difference in GVHD incidence between haploidentical and nonhaploidentical transplants with modern-day conditioning regimens and GVHD prophylaxis.4 This was an important finding that further supports haploidentical transplantation as a therapeutic alternative to matched-related and matched-unrelated donor transplants.
However, the results of our study did show that compared to nonhaploidentical transplant recipients, patients who underwent haploidentical transplants had significantly increased risks of both 100-day and 1-year mortality secondary to infectious complications. Given that the increased risk of infection-related mortality was not due to higher rates of acute or chronic GVHD, this implies that the increased risk of mortality could be due to haploidentical transplantation itself as opposed to increased amounts of immunosuppressive therapy. These findings were consistent with a prior report analyzing causes of mortality after different forms of allogeneic HSCT in over 1,400 patients, ultimately demonstrating increased infection-related mortality following haploidentical transplant compared to matched-related donor transplant at a rate of 21.2% vs 13.4% over a 10-year time period.11 This conclusion was also shown despite comparable rates of GVHD, similar to the results of our study.
As mentioned previously, this increased mortality risk could be attributed to the delayed time to immune recovery for certain T-cell subsets following haploidentical transplantation.7 In particular, haploidentical recipients were previously found to have lower CD4+ T cells prior to 90 days post-transplant as well as a significant delay in the reconstitution of dendritic cells.7 The impact of lymphocyte subset recovery on transplant outcomes was studied in a prior report as well, demonstrating that in 69 allogeneic transplant patients, more rapid CD4+ T-cell recovery to >2×108 cells/L at 3 months was associated with improved clinical outcomes.12 Subsequent studies again emphasized the effect of recovery CD4+ T lymphocytes, correlating more rapid recovery with a significant decrease in transplant-related mortality in 758 allogeneic HSCT patients.13 While our study did not include measurements of lymphocyte subsets nor times to CD4 T-cell or dendritic cell recovery, we found that patients who underwent haploidentical transplants required a significantly longer time to neutrophil and platelet engraftment, with haploidentical recipients reaching neutrophil engraftment at a median of 17 days compared to 15 days in nonhaploidentical recipients (P<0.01) as well as platelet engraftment at a median of 25 days compared to 17 days (P<0.01). Although these results were unlikely to have significantly contributed to the increased risk of 100-day and 1-year infection mortality in the haploidentical recipients, they are important to note for future studies as larger patient populations may lead to more drastic findings.
While there was a greater incidence rate of viral infections among haploidentical patients, no viral infections led to patient death in either transplant group. The increased rate of CMV viremia in haploidentical recipients has been previously described in other reports, particularly following the use of PTCy14 In a prior prospective study investigating immune reconstitution in 75 allogeneic HSCT patients, CD4+ T cells were shown to play a critical role in controlling CMV infection by mediating antiviral effector functions.15 The delayed reconstitution of CD4+ T cells in haploidentical patients would, therefore, correlate with the increased rates of CMV viremia in this population.
In contrast, while BK viruria has been reported as a frequent complication of HSCT that occurs in 50–90% of patients,16 the greater risk of BK viruria with associated hemorrhagic cystitis in the haploidentical population has not been thoroughly discussed in previous literature. The alloimmune reaction in allogeneic HSCT is suspected to play a role in the development of BK virus hemorrhagic cystitis as this condition is rarely seen in recipients of autologous transplants who receive similar myeloablative conditioning regimens.17 In addition, other reported risk factors include prior BK virus infections and the presence of acute GVHD following transplant.18,19 Nonetheless, there were again no significant differences in the rates of acute GVHD between the haploidentical and nonhaploidentical recipients in our study, implying that the delayed immune reconstitution following haploidentical transplant could contribute to the higher rates of BK virus infection in this population. It has also been suggested that the process of immune reconstitution leads to more severe presentations of BK virus infection, including hemorrhagic cystitis, due to viral antigens of the bladder wall being recognized by emerging, functioning lymphocytes.20
Of all the patients in our study who were found to have BK viruria, haploidentical recipients were at greater risk of developing BK viremia as well. Most often reported in kidney transplant patients, BK viremia can result in complications of progressive renal dysfunction and ureteral stenosis due to its tropism for the renourinary epithelum.21 Ureteral stenosis due to BK viremia has also been described in HSCT recipients in rare instances, with prior case studies reporting subsequent obstructive uropathy that was relieved with percutaneous nephrostomy catheter placements.22 Given the potential sequelae of BK viremia infections, further investigation is warranted regarding the mechanism by which BK virus infections occur in haploidentical patients and approaches to prophylaxis.
Despite similar rates of bacterial and fungal infections between haploidentical and nonhaploidentical recipients, these infections led to death more often in the haploidentical population. This was an important and surprising conclusion as patients in both groups received identical agents and duration of antimicrobial prophylaxis and treatment. As a result, future studies would be beneficial to investigate whether more intensive infectious management in the haploidentical population can decrease this discrepancy in infection-related mortality.
Conclusion
To conclude, while the process of haploidentical transplantation has achieved monumental advancements in GVHD prophylaxis and transplant technique, there still remain many areas for improvement in the management of infectious complications. Though any definitive conclusions from our study are limited by a small sample size and narrow window of follow-up, the results are important in considering future studies of haploidentical patients and approaches to infection prophylaxis and treatment in this population.
Acknowledgments
Portions of this data were displayed at the 2018 American Society of Hematology Annual Meeting as a poster presentation on December 3, 2018, in San Diego, California.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMoa1410490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000). Blood. 2003;102(4):1541–1547. doi: 10.1182/blood-2003-02-0430 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Liu D, Xu L, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant. 2011;17(6):821–830. doi: 10.1016/j.bbmt.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 4.Szydlo R, Goldman J, Klein J, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767–1777. doi: 10.1200/JCO.1997.15.5.1767 [DOI] [PubMed] [Google Scholar]
- 5.Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Efficacy of antithymocyte globulin for allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Leuk Lymphoma. 2017;58(8):1840–1848. doi: 10.1080/10428194.2016.1266624 [DOI] [PubMed] [Google Scholar]
- 6.Atilla E, Atilla P, Bozdağ S, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45(4):403–411. doi: 10.1007/s15010-017-1016-1 [DOI] [PubMed] [Google Scholar]
- 7.Chang Y, Zhao X, Huo M, et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol. 2012;32(2):268–280. doi: 10.1007/s10875-011-9630-7 [DOI] [PubMed] [Google Scholar]
- 8.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186–1193. doi: 10.1056/NEJM199810223391702 [DOI] [PubMed] [Google Scholar]
- 9.Raiola A, Dominietto A, Di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–1579. doi: 10.1016/j.bbmt.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 11.Yan C, Xu L, Wang F, et al. Causes of mortality after haploidentical hematopoietic stem cell transplantation and the comparison with HLA-identical sibling hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(3):391–397. doi: 10.1038/bmt.2015.306 [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Sohn S, Won D, Lee N, Suh J, Lee K. Rapid helper T-cell recovery above 200×10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37(12):1119–1128. doi: 10.1038/sj.bmt.1705381 [DOI] [PubMed] [Google Scholar]
- 13.Berger M, Figari O, Bruno B, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41(1):55–62. doi: 10.1038/sj.bmt.1705870 [DOI] [PubMed] [Google Scholar]
- 14.Al Malki MM, Dadwal S, Yang D, et al. High incidence of CMV reactivation after haploidentical donor hematopoietic cell transplantation using high-dose post-transplant cyclophosphamide, and its impact on transplant outcomes. Blood. 2017;130(Suppl 1):4494. [Google Scholar]
- 15.Casazza J, Betts M, Price D, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203(13):2865–2877. doi: 10.1084/jem.20052246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arthur R, Shah K, Charache P, Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158(3):563–569. [DOI] [PubMed] [Google Scholar]
- 17.Leung A, Mak R, Lie A, et al. Clinicopathological features and risk factors of clinically overt haemorrhagic cystitis complicating bone marrow transplantation. Bone Marrow Transplant. 2002;29(6):509–513. doi: 10.1038/sj.bmt.1703415 [DOI] [PubMed] [Google Scholar]
- 18.Leung A, Chan M, Cheng V, Lie A, Yuen K, Kwong Y. Polyoma BK viruria in patients undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(10):1029–1030. doi: 10.1038/sj.bmt.1704944 [DOI] [PubMed] [Google Scholar]
- 19.Seber A, Shu X, Defor T, Sencer S, Ramsay N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 1999;23(1):35–40. doi: 10.1038/sj.bmt.1701523 [DOI] [PubMed] [Google Scholar]
- 20.Cheng V, Yuen K, Chan W, Wong S, Ma E. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30(6):882–892. doi: 10.1086/313809 [DOI] [PubMed] [Google Scholar]
- 21.Hirsch H, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;179–188. doi: 10.1111/ajt.12110 [DOI] [PubMed] [Google Scholar]
- 22.Khan H, Oberoi S, Mahvash A, et al. Reversible ureteral obstruction due to polyomavirus infection after percutaneous nephrostomy catheter placement. Biol Blood Marrow Transplant. 2011;17(10):1551–1555. doi: 10.1016/j.bbmt.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]