Abstract
Objectives:
To control increasing pharmaceutical expenditures, Taiwan’s National Health Insurance has implemented a series of drug reimbursement price reductitablons since 2000. This study examined changes in use and expenditures of oral antidiabetic medications following the price regulation in November 2006.
Methods:
We obtained claims data between January 2006 and August 2007 from Taiwan’s National Health Insurance Research Database. We categorized oral antidiabetic products as affected by the reimbursement reduction (“targeted”) or not (“non-targeted”), by level of relative price reduction, and by manufacturer type (international vs. local manufacturers). We used an interrupted time series design and segmented regression models to estimate changes in monthly per capita prescribing rate, volume, and insurance reimbursement expenditures following the policy.
Results:
The majority (129/178; 72.5%) of oral antidiabetic products were targeted by this round of price reductions. There was a relative reduction of 9.5% [95%CI: −12.68, −6.32] in total expenditures at ten months post-policy compared to expected rates. For targeted products, there were 2.04% [95%CI: −4.15, 0.07] and 13.26% [95%CI: −16.64, −9.87] relative reductions in prescribing rate and expenditures, respectively, at ten months post-policy. Non-targeted products increased significantly (22% [95%CI: 10.49,33.51] and 22.85% [95%CI: 11.69,34.01] relative increases in prescribing rate and expenditures respectively). Larger reimbursement cuts led to greater reductions in prescribing rate, volume, and insurance reimbursement expenditures of targeted products. Prescribing rates of both targeted and non-targeted products by international manufacturers declined after the policy while rates of prescribing non-targeted products by local manufacturers increased.
Conclusions:
While total government expenditures for oral antidiabetic medications were contained by the policy, our results indicate that prescribing shifted at the margin from targeted to non-targeted products and from international to local products. Further research is warranted to understand how changes in medication use due to price regulation policies affect medication adherence and patient health outcomes.
Keywords: Drug reimbursement reduction, Oral hypoglycemic medications, Interrupted time series
1. Introduction
The rapid growth of health care expenditure, especially pharmaceutical costs, is a challenge for many countries [1,2]. Aging populations, escalating drug prices, increasing rates of drug use, and new pharmaceutical products contribute to rising pharmaceutical expenditures [3,4]. In Taiwan, pharmaceutical expenditures accounted for 25% of total health care expenditures paid by the Bureau of the National Health Insurance (BNHI) in 2009. Outpatient drugs were a major component of expenditures in medical centers (50%), regional hospitals (38%), and district hospitals (30%).
To control increasing pharmaceutical expenditures, the BNHI has implemented seven waves of reimbursement rate adjustments since 2000 to close the gap between procurement and BNHI reimbursement prices for prescription drugs. These were implemented in April 2000, April 2001, March 2003, September 2005, November 2006, September 2007, October 2009, and December 2011. Because institutions procure large quantities of medicines, procurement prices are typically lower than the amount reimbursed by BNHI and the differences constitutes a profit for hospitals [5].
To assess procurement prices, the BNHI conducted surveys and obtained drug wholesale prices from pharmaceutical companies and procurement prices from hospitals. Reimbursements were adjusted if there was a difference of 30% or more between the average procurement price and the BNHI reimbursed price. Prices were subsequently monitored and adjusted on an annual basis for a maximum of five years.
Some information exists about effects of drug reimbursement price reductions in Taiwan. Lee et al. examined the effects of six drug price policies and found that they reduced pharmaceutical expenditures, especially for outpatient medications and for hospitals (compared with clinics) [6]. Chen et al. found that reimbursement price adjustments reduced the daily medical use and expenditures for targeted cardiovascular medications, but did not affect non-targeted products [5]. Chu et al. focused on anti-hypertensive drugs and found that reimbursement price adjustments may have created an incentive for physicians to prescribe drugs with higher profit margins, and to increase prescription duration or the number of drug items per prescription [7]. Hsiao et al. did not find a significant association between reimbursement price adjustments and drug utilization and expenditures during 2001−2004 [8]. Chu et al. studied the short-term effects of reimbursement price reductions on outpatient hypertension treatment among the elderly. They found that the average cost per prescription increased slightly, and that physicians tended to substitute drugs whose prices were not reduced for those subject to price reductions [9].
Little is known, however, about changes in use following price adjustments of targeted (affected by the policy) and non-targeted (not affected by the policy) products, differential effects due to the magnitude of price changes, and changes in use of products made by international versus local manufacturers. This longitudinal study examines the effects of drug reimbursement price adjustments on the utilization and expenditures of oral antidiabetic medications in Taiwan. We focused on oral antidiabetic medications because diabetes is one of the most common chronic illnesses in Taiwan. We chose to focus on the fifth price reduction, implemented in 2006, because a large number of oral antidiabetic drugs were affected by this policy, including products from all drug classes of oral antidiabetic medications. Within each drug class, there were non-targeted products clinically interchangeable with targeted products. Similarly, clinical substitutes existed between small and large price cut products, and between products made by international and local manufacturers. We examined impacts of the price regulation policy separately within each class of oral antidiabetic medication. We also compared policy impacts between targeted and non-targeted groups, by relative price reduction, and between products from international versus local manufacturers. We hypothesized that reimbursement price reductions would be associated with changes in prescribing rates, drug utilization and expenditures because institutions or physicians would change some procurement or prescribing decisions in response to the policy in order to maintain profits.
2. Methods
2.1. Data source
We obtained a 0.2% random sample of monthly claims for all antidiabetic drugs in the ambulatory care setting from the Taiwan National Health Insurance Research Database (NHIRD).
2.2. Outcome measures
We analyzed 51,109 prescriptions for 178 oral antidiabetic drug products. We categorized oral antidiabetic drugs based on the World Health Organization’s Anatomical Therapeutic Chemical (ATC) drug classification system into biguanides (BG), sulfonylureas (SU), alpha glucosidase inhibitors (AGI), thiazolidinediones (TZD), fixed-dose combination products, dipeptidyl peptidase 4 (DPP-4) inhibitors, and others. The first four classes (BG, SU, AGI, and TZD) accounted for 96.8% of volume and 93.3% of oral antidiabetic expenditures in November 2006, and the study focused on these classes. The products were divided into targeted and non-targeted groups. We also divided targeted products into those experiencing small (<20%) versus large (≥20%) price reductions. We also categorized all products by manufacturer type (local vs. international, non-Taiwanese pharmaceutical manufacturers).
We used the monthly number of diabetes-related doctor visits as the denominator for all outcome measures. Study measures were prescribing rate (number of prescribed medicines per patient visit per month), volume in defined daily doses (DDDs per patient visit per month) [10] and insurance reimbursement expenditures (amount reimbursed per patient visit per month) for each class of oral antidiabetic drugs.
2.3. Study design and study period
We used an interrupted time series design, the strongest quasi-experimental method, to examine impacts of the reimbursement price regulation on prescribing rates, volumes, and expenditures [11]. This method adjusts for baseline level and trend of the outcomes before the price regulation, allowing us to infer that observed changes were likely attributable to the policy. We restricted the study period to January 2006 through August 2007 to exclude effects of other price regulations. This study period included a 10-month observation period before the fifth reimbursement price regulation (implemented in November 2006) and a 10-month follow-up period after the policy, ending the study period before the sixth reimbursement price regulation in September 2007. After reviewing the literature and public documents, we did not identify other major events or interventions during this period that might have influenced the use of oral antidiabetic medications.
2.4. Statistical analysis
We used segmented linear regression models to estimate post-policy changes in both the level and trend of each outcome measure [12]. This method uses baseline trends and levels to project future monthly outcomes with the assumption that these values reflect what would have happened without the policy (the counterfactual).
The basic model included terms to estimate the baseline level for each outcome (intercept), baseline trend (slope), change in the level of the outcome measure immediately after policy implementation, and change in trend post-policy [11]. We tested and corrected the models for possible serial autocorrelation [13]. To identify the most parsimonious models we used backward elimination and excluded nonsignificant terms (p > 0.2). To summarize results in a single metric, we estimated absolute and relative changes (with 95% CIs) [14] in outcomes at ten months post-policy compared to projected rates. All analyses were carried out with SAS, Version 9.2 (SAS Institute, Cary, NC).
3. Results
The fifth drug price regulation policy affected 129 (targeted products) of 178 oral antidiabetic products (72.5%) marketed at that time in Taiwan. Relative reimbursement price reductions were less than 20% for 66 targeted products and larger than or equal to 20% for 63 targeted products. Among targeted products, 101 were locally manufactured by Taiwanese manufacturers and 28 were imported; among non-targeted products, 41 were locally manufactured and 8 were imported.
3.1. Overall analysis
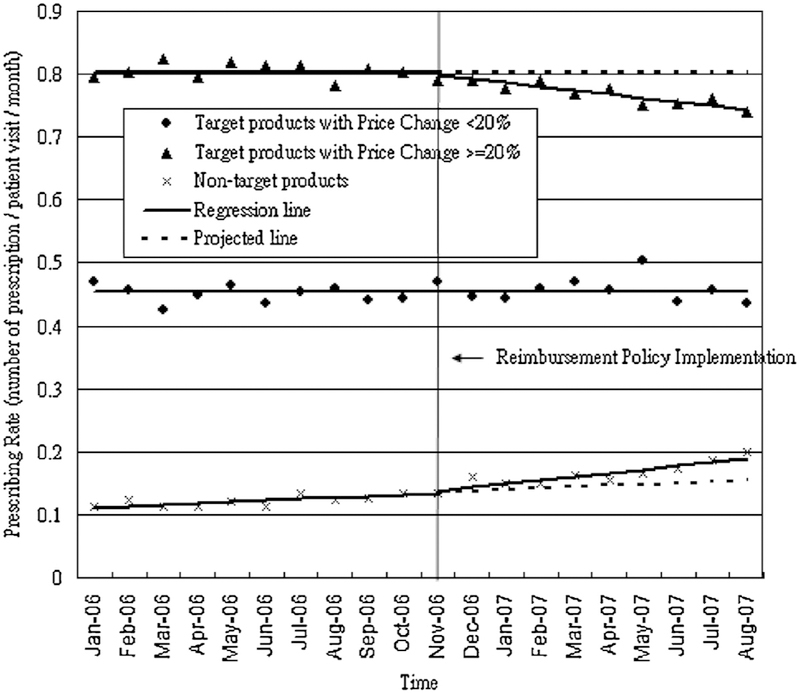
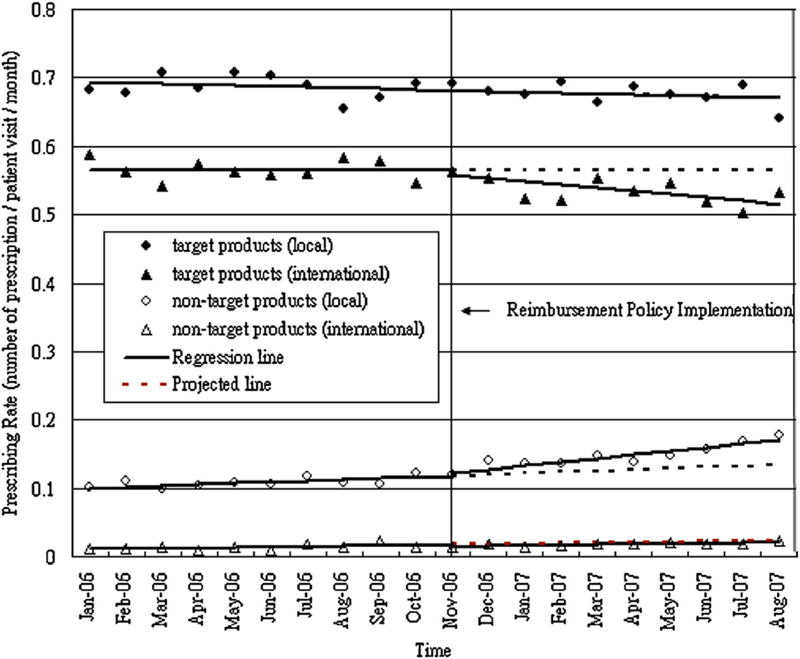
We report estimated relative changes in prescribing rates, volume in DDDs, and expenditures at ten months post-policy, compared to projected rates. Table 1 details the parameter estimates from segmented regression models for these measures, while Fig. 1 shows the monthly prescribing rates of targeted and non-targeted products overtime and Fig. 2 shows those of local and international products over time.
Table 1.
Estimated changes (with 95% confidence interval) in prescribing rates, volumes, and expenditures of oral antidiabetic products following the fifth reimbursement price reduction.
| No. of products |
Prescribing rates |
||||||
| Intercept | Baseline trend | Level change | Trend change | Absolute change |
Relative change | ||
| All drugs | 178 | 1.70 | 0.00 (−0.00, 0.01) | 0.03 | 1.50% (−0.15%, 3.16%) | ||
| Targeted | 129 | 0.97 | −0.00 (−0.00, −0.00) | 0.01 (0.00, 0.02) | −0.00 (0.00, 0.00) | −0.02 | −2.04% (−4.15%: 0.07%) |
| Non-Targeted | 49 | 0.11 | 0.00(0.00, 0.00) | 0.00(0.00, 0.00) | 0.03 | 22.00% (10.49%. 33.51%) | |
| Price change <20% | 66 | 0.45 | 0.00 | 0.0% | |||
| Price change ≥20% | 63 | 0.80 | −0.01(−0.01, 0.00) | −0.06 | −7.60% (−9.27%. −5.92%) | ||
| Target | |||||||
| Local | 101 | 0.70 | −0.00 (−0.00, −0.00) | 0.00 | 0.0% | ||
| International | 28 | 0.56 | −0.00 (−0.01, 0.00) | −0.05 | −8.62%(−12.13%.−5.11%) | ||
| Non-target | |||||||
| Local | 41 | 0.10 | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.01) | 0.04 | 27.17% (14.67%, 39.65%) | |
| International | 8 | 0.01 | 0.00 (0.00, 0.00) | −0.00 (−0.01, 0.00) | 0.00 | −12.73% (−23.94%, −1.52%) | |
| No. of products |
Volumes |
||||||
| Intercept | Baseline trend | Level change | Trend change | Absolute change |
Relative change | ||
| All drugs | 178 | 52.6 | −0.1 (−0.21,0.00) | 0.00 | 0.0% | ||
| Targeted | 129 | 47.9 | −0.3 (−0.35, −0.15) | 0.00 | 0.0% | ||
| Non-Targeted | 49 | 5.1 | 0.01(−0.02, 0.14) | 0.2 (0.0, 0.3) | 1.6 | 24.56% (−1.61%, −50.71%) | |
| Price change <20% | 66 | 19.0 | −0.2 (0.36, 0.02) | 0.3 (−0.0, 0.6) | 2.9 | 18.81% (−4.26%, −41.88%) | |
| Price change ≥20% | 63 | 28.6 | −1.3 (−2.6,0.1) | −0.3 (−0.4, 0.1) | −3.7 | −13.04% (−17.01%. −9.07%) | |
| Target | |||||||
| Local | 101 | 26.5 | −0.14(−0.21,−0.07) | 0.00 | 0.0% | ||
| International | 28 | 21.4 | −0.11 (−0.18,−0.04) | 0.00 | 0.0% | ||
| Non-target | |||||||
| Local | 41 | 5.2 | 0.2 (0.2, 0.3) | 2.3 | 22.94% (14.75%, 96.48%) | ||
| International | 8 | 0.2 | 0.02 (0.01, 0.03) | −0.1 (−0.2, −0.0) | −0.1 | −18.79% (−29.02%, −8.50%) | |
| No. of products |
Expenditures |
||||||
| Intercept | Baseline trend | Level change | Trend change | Absolute change |
Relative change | ||
| All drugs | 178 | 606.1 | −57.6(−77.8,−37.4) | −57.6 | −9.50% (−12.68%. −6.32%) | ||
| Targeted | 129 | 562.2 | −74.5(−99.9, −54.2) | −74.5 | −13.26% (−16.64%, −9.87%) | ||
| Non-Targeted | 49 | 39.0 | 1.00(0.64, 1.28) | 1.32 (0.8, 1.8) | 13.2 | 22.85% (11.69%, 34.01%) | |
| Price change <20% | 66 | 356.1 | −1.2 (−2.80, 0.33) | 0.00 | 0.0% | ||
| Price change >20% | 63 | 213.8 | −0.3 (−0.74, 0.05) | −57.2(−61.6, −52.8) | −57.2 | −27.26% (−29.01%. −26.23%) | |
| Target | |||||||
| Local | 101 | 171.7 | −39.0 (−44.1, −34.0) | −39.0 | −22.73% (−25.38%, −20.09%) | ||
| International | 28 | 390.4 | −35.5 (−53.8, −17.2) | −35.5 | −9.09% (−13.57%, −4.60%) | ||
| Non-target | |||||||
| Local | 41 | 33.9 | 0.70 (0.11,1.29) | 1.6 (0 6, 2.6) | 16.4 | 34.14% (7.61%, 60.67%) | |
| International | 8 | 3.9 | 0.40(0.21,0.60) | −1.8 (−3.5, −0.2) | −0.3 (−0.5,0.0) | −4.3 | −36.09% (−53.01%, −19.17%) |
All terms with p ≤ 0.20 were retained in models; bold = p ≤ 0.05.
Absolute difference and relative change were estimated at 10 months post-policy, compared to projected rates based on pre-existing level and trend. Prescribing rate = number of prescriptions per patient visit per month; volume = DDDs per patient visit per month; expenditures = amount reimbursed per patient visit per month.
Interpretation example: Targeted products were prescribed at 0.97 prescriptions per patient visit per month in the beginning of the study period without significant growth during the baseline. Immediately post-policy, there was a small increase (0.01 prescription per patient visit per month) with no significant, sustained change. At 10 months post-policy, there was an absolute difference of −0.02 prescriptions per patient visit per month, which reflects a −2% relative reduction in prescribing rate.
Fig. 1.
Monthly prescribing rates of targeted and non-targeted oral antidiabetic products (01/2006–08/2007).
Fig. 2.
Monthly prescribing rates of oral antidiabetic products by local and international manufacturers (01/2006–08/2007).
Overall prescribing rates and volumes of oral antidiabetic products did not change significantly after the price regulation policy, but there was a significant relative reduction of 9.5% [95%CI: −12.68, −6.32] in total expenditures at ten months post-policy compared to projected rates (level change: −57.6 NT$ per patient per month, [95%CI: −77.8, −37.4]).
For targeted products, prescribing rates and expenditures decreased by 2.04% [95%CI: −4.15, 0.07] and 13.26% [95%CI: −16.64, −9.87] (level change: −74.5 NT$ per patient per month, [95%CI: −99.9, −54.2]) respectively, at ten months post-policy compared to projected rates, but volume of use did not change.
For targeted products with small price cuts, prescribed volume decreased slightly before the policy, but there was a small increase in trend following the policy (0.3 DDDs per patient per month, [95%CI: −0.0, 0.6]), which resulted in an estimated increase of 18.81% [95%CI: −4.26, 41.88] at ten months post-policy compared to projected rates. However, for targeted products with large price cuts, there were marked, statistically significant drops in prescribing rate (−7.6% [95%CI: −9.27, −5.92]), volume (−13.04% [95%CI: −17.01, −9.07]) and expenditures (−27.26% [95%CI: −29.01, −26.23]) at ten months post-policy compared to projected rates.
Within targeted products, there was a significant reduction in prescribing rate of products by international manufacturers (−8.62%) [95%CI: −12.13, −5.11] but no marked change for products by local manufacturers.
For non-targeted products, there were significant increases in prescribing rate (22% [95%CI: 10.49, 33.51]), volume (24.56% [95%CI: −1.61, 50.71]) and expenditures (22.85% [95%CI: 11.69, 34.01]) at ten months post-policy compared to projected rates. For non-targeted products by international manufacturers, there were large reductions in prescribing rate (−12.73% [95%CI: −23.94, −1.52]), volume (−18.79% [95%CI: −29.02, −8.5]) and expenditures (−36.09% [95%CI: −53.01, −19.17]). In contrast, there were substantial increases in prescribing rate (27.17% [95%CI: 14.67, 39.65]), volume (22.94% [95%CI: 14.75, 96.48]) and expenditures (34.14%) [95%CI: 7.61,60.67]) for products by local manufacturers.
3.2. Analyses by antidiabetic drug class
Table 2 summarizes the estimated changes based on segmented regression models in prescribing rates, volumes in DDDs and expenditures for specific antidiabetic drug classes. After the reimbursement price regulation, prescribing rate of biguanides increased (7.11% [95%CI: 4.57, 9.64]) at ten months post-policy, as did volume in DDDs (4.02% [95%CI: 1.1, 6.95]), but reimbursed expenditures fell by 17.1%. [95%CI: −20.08, −14.13]. For sulfonylureas, both prescribing rate and expenditures decreased (−2.42% [95%CI: −3.68, −1.15] and −2.5% [95%CI: −14.01,9.01]respectively at ten months post-policy). No changes were detected for alpha glucosidase inhibitors or thiazolidinediones.
Table 2.
Estimated changes (with 95% confidence interval) in prescribing rate, volume, and expenditures following the fifth reimbursement price reduction by antidiabetic drug classes.
| No. of products |
Prescribing rates | ||||||
| Intercept | Baseline trend | Level change | Trend change | Absolute change |
Relative change | ||
| Total | |||||||
| BG | 50 | 0.68 | −0.00 (−0.00, 0.00) | 0.01 (0.00, 0.02) | 0.00 (0.00, 0.00) | 0.05 | 7.11% (4.57%, 9.64%) |
| SU | 99 | 0.79 | −0.00 (−0.00,−0.00) | −0.02 (−0.03, −1.01) | −0.02 | −2.42% (−3.68%, −1.15%) | |
| AGI | 9 | 0.1 | 0.00 (−0.00, 0.00) | 0.00 | 0.0% | ||
| TZD | 3 | 0.13 | 0.00 (0.00, 0.00) | 0.00 | 0.0% | ||
| Targeted and non-targeted | |||||||
| BG | |||||||
| Target | 37 | 0.67 | −0.00 (−0.01, 0.01) | 0.00 (0.00, 0.00) | −0.01 | −1.87% (−4.14%, 0.39%) | |
| Non-targeted | 13 | 0.016 | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.04 | 247.14% (191.41%, 303.65%) | |
| SU | |||||||
| Target | 75 | 0.71 | −0.00 (−0.01. −0.00) | −0.00 (−0.01.0.00) | −0.03 | −5% ((−10.70%, 0.65%) | |
| Non-target | 24 | 0.08 | 0.00 (0.00, 0.00) | −0.01 (−0.01, 0.00) | 0.00 (0.00, 0.00) | 0.01 | 12.2% (1.18%, 23.23%) |
| AGI | |||||||
| Target | 8 | 0.1 | 0.00 (0.00, 0.00) | 0.00 | 0.0% | ||
| TZD | |||||||
| Target | 2 | 0.13 | 0.00 | 0.0% | |||
| Level of price change | |||||||
| BG | |||||||
| 0% < PC < 20% | 16 | 0.04 | 0.00 (−0.01, 0.00) | 0.00 | −10.5% (−23.93%, 2.89%) | ||
| 20% < PC | 21 | 0.62 | −0.00 (0.00, 0.00) | −0.03 | −4.39% (−6.60%, −2.19%) | ||
| SU | |||||||
| 0% < PC < 20% | 33 | 0.24 | −0.00 (−0.01, −0.00) | 0.02 (0.00, 0.04) | 0.00 (0.00, 0.01) | 0.05 | 27.46% (−0.06%, 54.91%) |
| 20% < PC | 42 | 0.47 | −0.02 (−0.04, −0.01) | −0.01 (−0.01, 0.00) | −0.09 | −19.58% (−22.79%, −16.39%) | |
| AGI | |||||||
| 0% < PC < 20% | 8 | 0.1 | 0.00 (0.00.0.00) | 0.00 | 0.0% | ||
| TZD | |||||||
| 0% < PC < 20% | 2 | 0.13 | 0.00 | 0.0% | |||
| Local vs. international | |||||||
| BG | |||||||
| TL | 26 | 0.52 | 0.00 | 0.0% | |||
| TI | 11 | 0.14 | −0.02 (−0.03, −0.01) | −0.02 | −13.16% (−18.56%, −7.79%) | ||
| NL | 9 | 0.01 | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.03 | 228.79% (180.42%, 276.81%) | |
| NI | 4 | 0.00 | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 | 38.07% (−44.35%, 169.14%) | |
| SU | |||||||
| TL | 65 | 0.37 | −0.00 (−0.00, −0.00) | 0.00 | 0.0% | ||
| TI | 10 | 0.35 | −0.00 (−0.00, −0.00) | 0.00 | 0.0% | ||
| NL | 24 | 0.08 | 0.00 (0.00, 0.00) | −0.01 (−0.01, 0.00) | 0.00 (0.00, 0.00) | 0.01 | 12.2% (1.18%, 23.23%) |
| AGI | |||||||
| TL | 7 | 0.2 | 0.00 (0.00, 0.00) | 0.00 | 0.0% | ||
| TZD | |||||||
| TI | 2 | 0.13 | 0.00 | 0.0% | |||
| No. of products |
volumes | ||||||
| Intercept | Baseline trend | Level change | Trend change | Absolute change |
Relative change | ||
| Total | |||||||
| BG | 50 | 12.08 | −0.34 (−0.74, 0.05) | 0.08 (0.03, 0.14) | 0.49 | 4.02% (1.10%, 6.95%) | |
| SU | 99 | 34.75 | −0.13 (−0.21, −0.05) | 0.00 | 0.0% | ||
| AGI | 9 | 1.3 | 0.01 (0.01, 0.02) | 0.00 | 0.0% | ||
| TZD | 3 | 2.83 | 0.00 | 0.0% | |||
| Targeted and non-targeted | |||||||
| BG | |||||||
| Target | 37 | 11.74 | −0.35 (−0.55, −0.15) | −0.35 | −3% ((−4.66%, −1.33%) | ||
| Non-targeted | 13 | 0.35 | 0.08 (0.07, 0.09) | 0.81 | 231.84% (170.14%, 293.55%) | ||
| SU | |||||||
| Target | 75 | 30.5 | −0.22 (−0.30, −0.15) | 0.00 | 0.0% | ||
| Non-target | 24 | 4.77 | 0.16 (0.11, 0.21) | 1.6 | 33.43% (21.92%, 44.94%) | ||
| AGI | |||||||
| Target | 8 | 1.31 | 0.01 (0.00, 0.02) | 0.00 | 0.0% | ||
| TZD | |||||||
| Target | 2 | 2.83 | 0.00 | 0.0% | |||
| Level of price change | |||||||
| BG | |||||||
| 0% < PC < 20% | 16 | 0.85 | 0.00 | 0.0% | |||
| 20% < PC | 21 | 10.98 | −0.03 (−0.04, −0.01) | 0.00 | 0.0% | ||
| SU | |||||||
| 0% < PC < 20% | 33 | 12.56 | −0.17 (−0.31, −0.03) | 0.3 (0.06, 0.54) | 2.97 | 32.51% (−0.38%, 65.42%) | |
| 20% < PC | 42 | 17.75 | −1.06 (−2.19, 0.07) | −0.23 (−0.40, −0.07) | −3.41 | −19.19% (−24.6%, −13.78%) | |
| AGI | |||||||
| 0% < PC < 20% | 8 | 1.31 | 0.01 (0.00, 0.02) | 0.00 | 0.0% | ||
| TZD | |||||||
| 0% < PC < 20% | 2 | 2.83 | 0.00 | 0.0% | |||
| Local vs. international | |||||||
| BG | |||||||
| TL | 26 | 9.07 | 0.00 | 0.0% | |||
| TI | 11 | 2.66 | −0.35 (−0.53, −0.17) | −0.35 | −13.07% (−19.4%, −6.74%) | ||
| NL | 9 | 0.32 | 0.06 (0.05, 0.08) | 0.64 | 199.94% (140.57%, 259.07%) | ||
| NI | 4 | 0.03 | 0.02 (0.01, 0.02) | 0.17 | 561.29% (206.68%, 917.77%) | ||
| SU | |||||||
| TL | 65 | 17.18 | −0.15 (−0.20, −0.10) | 0.00 | 0.0% | ||
| TI | 10 | 13.33 | −0.08 (−0.13, −0.02) | 0.00 | 0.0% | ||
| NL | 24 | 4.77 | 0.16(0.11, 0.21) | 1.6 | 33.43%(21.92%, 44.94%) | ||
| AGI | |||||||
| TL | 7 | 0.2 | 0.00 (−0.00, 0.01) | 0.06 (−0.01, 0.12) | 0.06 | 19.58% (−7.97%, 47.14%) | |
| TZD | |||||||
| TI | 2 | 2.83 | 0.00 | 0.0% | |||
| No. of products |
Expenditures | ||||||
| Intercept | Baseline trend | Level change | Trend change | Absolute change |
Relative change | ||
| Total | |||||||
| BG | 50 | 118.28 | −31.09 (−35.21, −26.97) | 1.09 (0.48,1.69) | −20.23 | −17.1% (−20.08%, −14.13%) | |
| SU | 99 | 247.67 | −1.21 (−2.88, 0.47) | −30.46 (−44.16, 16.76) | 2.49 (0.12, 4.86) | −5.58 | −2.5% (−14.01%, 9.01%) |
| AGI | 9 | 44.19 | 0.28 (−0.01, 0.58) | 0.00 | 0.0% | ||
| TZD | 3 | 160.37 | 0.00 | 0.0% | |||
| Targeted and non-targeted | |||||||
| BG | |||||||
| Target | 37 | 113.82 | −31.3 (−33.13, −29.47) | −31.3 | −27.5% (−28.9%, −26.1%) | ||
| Non-targeted | 13 | 3.51 | 0.18 (−0.04, 0.39) | 0.8 (0.43, 1.16) | 7.95 | 112.87% (15.09%, 210.66%) | |
| SU | |||||||
| Target | 75 | 214.91 | −1.07 (−2.34, 0.19) | −27.16 (−41.75, −12.57) | −27.16 | −14.04% (−20.31%, −7.76%) | |
| Non-target | 24 | 29.2 | 0.45 (0.01, 0.90) | 0.93 (0.18, 1.68) | 9.3 | 24.3% (0.83%, −47.78%) | |
| AGI | |||||||
| Target | 8 | 44.44 | 0.23 (−0.07, 0.53) | 0.00 | 0.0% | ||
| TZD | |||||||
| Target | 2 | 160.37 | 0.00 | 0.0% | |||
| Level of price change | |||||||
| BG | |||||||
| 0% < PC < 20% | 16 | 9.88 | −1.36 (−3.06, 0.03) | −1.36 | −13.78% (−29.8%, 2.23%) | ||
| 20% < PC | 21 | 103.93 | −29.94 (−31.75, −28.12) | −29.94 | −28.8% (−30.32%, −27.29%) | ||
| SU | |||||||
| 0% < PC < 20% | 33 | 110.73 | −1.77 (−2.89, −0.65) | 1.94 (0.05, 3.82) | 19.36 | 25.7% (−4.47%, 55.86%) | |
| 20% < PC | 42 | 108.09 | −29.29 (−32.02, −26.55) | −0.3 (−0.72, 0.11) | −32.31 | −29.89% (−32.00%, −27.78%) | |
| AGI | |||||||
| 0% < PC < 20 | 8 | 44.44 | 0.23 (−0.07, 0.53) | 0.00 | 0.0% | ||
| TZD | |||||||
| 0% < PC <20 | 2 | 160.37 | 0.00 | 0.0% | |||
| Local vs. international | |||||||
| BG | |||||||
| TL | 26 | 84.74 | −21.91 (−24.21, −19.62) | −21.91 | −25.86% (−28.24%, −23.48%) | ||
| TI | 11 | 29.08 | −9.39 (−11.27, −7.50) | −9.39 | −32.28% (−37.81%, −26.75% | ||
| NI | 9 | 4.14 | 0.87 (0.72, 1.01) | 8.66 | 71.49% (−30.41%, 173.36%) | ||
| NL | 4 | 0.06 | 0.07 (0.01, 0.12) | 0.1 (0.01, 0.19) | 0.99 | 208.84% (146.81%, 270.86%) | |
| SU | |||||||
| TL | 65 | 82.72 | −0.37 (−0.89, 0.15) | −16.08 (−22.11, −10.05) | −16.08 | −21.34% (−27.33%, −15.36%) | |
| TI | 10 | 128.34 | −18.11 (−24.37, −11.84) | −18.11 | −14.11% (−18.66%, −9.56%) | ||
| NL | 24 | 29.2 | 0.45 (0.01, 0.90) | 0.93 (0.18, 1.68) | 9.3 | 24.3% (0.83%, 47.78%) | |
| AGI | |||||||
| TL | 7 | 5.47 | 0.12 (−0.03, 0.26) | 1.45 (−0.20, 3.11) | 1.45 | 18.62% (−7.69%, 44.93%) | |
| TZD | |||||||
| TI | 2 | 160.37 | 0.00 | 0.0% | |||
BG = Biguanides; SU = Sulfonylureas; AGI = Alpha-glucosidase inhibitors; TZD = Thiazolidinediones; TL = targeted local; TI = targeted international; NL = non-targeted local; NI = non-targeted international.
Only categories with two or more products are presented in this table.
All terms with p ≤ 0.20 were retained in models; bold = p ≤ 0.05.
Absolute difference and relative change were estimated at 10 months post-policy, compared to projected rates based on pre-existing level and trend. Prescribing rate = number of prescriptions per patient visit per month; Volume = DDDs per patient visit per month; Expenditures = amount reimbursed per patient visit per month.
For targeted biguanides, all three outcome measures decreased at ten months post-policy (prescribing rate: −1.87% [95%CI: −4.14, 0.39]; volume: −3% [95%CI: −4.66, −1.33]; expenditures: −27.5% [95%CI: −28.9, −26.1]). There were immediate nonsignificant post-policy reductions in prescribing rates of targeted biguanides that varied by magnitude of the price reductions (−4.39% [95%CI: −6.6, 2.19] for products with small price cuts versus −10.5% [95%CI: −23.93, 2.89] for products with larger price cuts).
Utilization and expenditures for targeted sulfonylurea products also declined after the price reductions (−5% [95%CI: −10.7, 0.65] in prescribing rate, −14.04% [95%CI: −20.31, −7.76] in expenditures at ten months post-policy). For targeted sulfonylurea products with large price cuts, there were significant reductions in prescribing rate and volume (−19.58% [95%CI: −22.79, −16.39] and −19.19% [95%CI: −24.6, −13.78] respectively at ten months post-policy). In contrast, for products with smaller price cuts, there were marginally significant increases in prescribing rate and volume (27.46% [95%CI: −0.06, 54.91] and 32.51% [95%CI: −0.38, 65.42] respectively) at ten months post-policy.
For the non-targeted biguanide and sulfonylurea products, there were also large estimated increases in utilization and expenditures at ten months post-policy (for biguanide: 247.14% [95%CI: 191.41, 303.65] in prescribing rate, 231.84% [95%CI: 170.14, 293.55] in volume, 112.87% [95%CI: 15.09, 210.66] in expenditures; for sulfonylurea: 12.2% [95%CI: 1.18, 23.23] in prescribing rate, 33.43% [95%CI: 21.92, 44.94] in volume, 24.3% [95%CI: 0.83, 47.78] in expenditures).
Our analyses by manufacturer type found significant decreases in the prescribing rate and volume for targeted biguanide products made by international manufacturers (−13.16% [95%CI: −18.56, −7.79] and −13.07% [95%CI: −19.4, 6.74] respectively), but no significant changes for those by local manufacturers. In contrast, for non-targeted biguanides, prescribing rate and volume increased dramatically for products by both local and international manufacturers. Use of targeted sulfonylurea medications produced either by local or international manufacturers did not change significantly after the policy; however, there were increases in both prescribing rate and volume for non-targeted sulfonylurea products by local manufacturers (12.2% [95%CI: 1.18,23.23] and33.43% [95%CI: 21.92,44.94] respectively). For targeted alpha glucosidase inhibitors, the prescribing volume and expenditures for products by local manufacturers increased by 19.58% [95%CI: −7.97, −47.14] and 18.62% [95%CI: −7.69, 44.93] respectively, with no change in use for products by international manufacturers.
4. Discussion
This is the first study to evaluate the effects of Taiwan’s reimbursement price regulation policy on utilization and reimbursed expenditures for oral antidiabetic drugs using longitudinal data and a rigorous quasi-experimental design.
The main purpose of the reimbursement price regulation policy was to control the rising pharmaceutical expenditures by narrowing the range of profits among different pharmaceutical products. After a series of price adjustments since 2000, the reimbursement prices of many products have been reduced. Our findings suggest that these policies might have been effective in containing drug expenditures of oral antidiabetic medications, consistent with previous studies of other therapeutic classes [5,6]. However, although overall changes were in the intended direction, we found substantial reductions in utilization of targeted oral antidiabetic products, which were offset by increases in non-targeted products following the policy, especially for biguanides and sulfonylureas. We also found that the magnitude of price reductions was influential in determining response to the price reductions. For example, among targeted sulfonylurea products, we found marked declines in prescribing rates, volumes and expenditures of products with price cuts larger than 20%, whereas the volume of medications experiencing smaller price cuts actually increased. These findings suggest that hospitals and/or physicians might have changed procurement or prescribing decisions to some degree in response to the policy in order to maintain profits [7,9]. For instance, physicians might have shown greater tendency to prescribe non-targeted drugs instead of targeted products, or hospitals might have reduced procurement of targeted drugs or even excluded them from their formulary [15]. There were no major changes observed in the use of alpha glucosidase inhibitors and thiazolidinediones, possibly because of smaller price adjustments and the limited number of non-targeted alternatives.
Our study also provides the first empirical evidence that the price regulation policy led to reductions in the use of products made by international manufacturers (the majority of which are brand name drugs) while increasing use of products by local manufacturers (largely generic drugs). We observed significant reductions in the use of targeted products by international manufacturers following the policy, while use of targeted local products did not change markedly. One possible reason is that hospitals may have negotiated to obtain lower prices from local manufacturers in order to maintain or increase their profit margin. There were large increases in the use of non-targeted, predominantly locally manufactured products. This suggests that the price regulation policy negatively impacted sales by international manufacturers in the Taiwan market; these manufacturers have previously expressed concerns about the possible negative impacts of such policies [15]. One possible unintended consequence of price reductions might be withdrawal of products from the Taiwan market by international companies, potentially decreasing the availability of some newer medications with clinical benefits; the shift away from internationally manufactured products may even reduce quality of care if it has negative effects on product availability or adherence. Policy makers should consider such unintended consequences on the pharmaceutical market when designing policies.
Our study has several limitations. We concentrated on estimating the effects of the 2006 drug price regulation policy on utilization and expenditures for oral antidiabetic medications. Changes in our study outcomes may have differed for other rounds of price reductions or for other therapeutic classes. We also did not have information in the claims data used for the study to assess how the price regulation policy may have impacted adherence to therapy and important clinical outcomes such as serum glucose levels or to assess the appropriateness of medication use or switches. Further research is needed to determine whether changes in medication use due to price regulation policies generalize to other times and therapeutic classes, affect medication adherence, or impact patient health outcomes.
In conclusion, our study provides empirical evidence on how the fifth drug price regulation policy has affected utilization, prescribing volume, and insurance system expenditure for oral antidiabetic medicines in Taiwan. While the price regulation policy had some intended monetary benefits for the insurance system, our results suggest that the policy also induced unintended substitution from targeted to non-targeted oral antidiabetic products, and a shift from products by international manufacturers to locally manufactured products.
Acknowledgements
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes.
Funding support
Dr. Hsu was supported by the Taiwan National Science Council Fellowship [Fellowship ID 100-2917-I-002-031], a Fulbright Grant (Grant ID 15110789), and the Harvard Medical School Fellowship in Pharmaceutical Policy Research. Dr. Ross-Degnan is supported in part by the Health Delivery Systems Center for Diabetes Translational Research (HDS-CDTR) (NIDDK grant 1P30-DK092924).
References
- [1].Mullins CD, Wang J, Palumbo FB, Stuart B. The impact of pipeline drugs on drug spending growth. Health Affairs (Millwood) 2001;20:210–5. [DOI] [PubMed] [Google Scholar]
- [2].Berndt ER. Pharmaceuticals in U.S. health care: determinants of quantity and price. Journal of Economic Perspectives 2002;16:45–66. [DOI] [PubMed] [Google Scholar]
- [3].Levit K, Smith C, Cowan C, Sensenig A, Catlin A. Health spending rebound continues in 2002. Health Affairs (Millwood) 2004;23:147–59. [DOI] [PubMed] [Google Scholar]
- [4].Hsieh CR, Sloan FA. Adoption of pharmaceutical innovation and the growth of drug expenditure in Taiwan: is it cost effective? Value Health 2008;11:334–44. [DOI] [PubMed] [Google Scholar]
- [5].Chen CL, Chen L, Yang WC. The influences of Taiwan’s generic grouping price policy on drug prices and expenditures: evidence from analysing the consumption of the three most-used classes of cardiovascular drugs. BMC Public Health 2008;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee YC, Yang MC, Huang YT, Liu CH, Chen SB. Impacts of cost containment strategies on pharmaceutical expenditures of the National Health Insurance in Taiwan, 1996–2003. Pharmacoeconomics 2006;24:891–902. [DOI] [PubMed] [Google Scholar]
- [7].Chu HL, Liu SZ, Romeis JC. Changes in prescribing behaviors after implementing drug reimbursement rate reduction policy in Taiwan: implications for the medicare system. Journal of Health Care Finance 2008;34:45–54. [PubMed] [Google Scholar]
- [8].Hsiao FY, Tsai YW, Huang WF. Price regulation, new entry, and information shock on pharmaceutical market in Taiwan: a nationwide data-based study from 2001 to 2004. BMC Health Services Research 2010;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chu HL, Liu SZ, Romeis JC. Assessing the effects of drug price reduction policies on older people in Taiwan. Health Services Management Research 2011;24:1–7. [DOI] [PubMed] [Google Scholar]
- [10].Oslo_WHO_Collaborating_Center. Accessed at http://www.whocc.no/atc_ddd_index/
- [11].Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of Clinical Pharmacy and Therapeutics 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- [12].Lu CY, Ross-Degnan D, Stephens P, Liu B, Wagner AK. Changes in use of antidiabetic medications following price regulations in China (1999–2009). Journal of Pharmaceutical Health Service Research 2013;4:3–11. [Google Scholar]
- [13].Shadish W, Cook T, Campbell D. Experimental and quasiexperimental designs for generalized causal inference. Boston, MA: Houghton Mifflin; 2002. [Google Scholar]
- [14].Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. Journal of Clinical Epidemiology 2009;62:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chien YS.A case study of business strategy for transnational pharmaceutical companies in Taiwan. Master thesis, Feng Chia University; 2010. [Google Scholar]


