Abstract
The chemical modification of structurally complex fermentation products, a process known as semisynthesis, has been an important tool for the discovery and manufacture of antibiotics for the treatment of various infectious diseases. However, many of the therapeutics obtained in this way are no longer efficacious, as bacterial resistance to many of these compounds has developed. In this manuscript, we describe a practical, fully synthetic route to macrolide antibiotics via the convergent assembly of simple chemical building blocks, with diverse structures not possible by traditional semisynthesis. More than 300 new macrolide antibiotic candidates, as well as the investigational drug solithromycin, were synthesized by our convergent approach. Evaluation of the novel compounds against a panel of pathogenic bacteria revealed many antibiotic candidates, with efficacy against strains resistant to macrolides in current use. The chemistry we describe herein provides a platform for the discovery of new macrolide antibiotics and may also serve as a basis for their manufacture.
Introduction
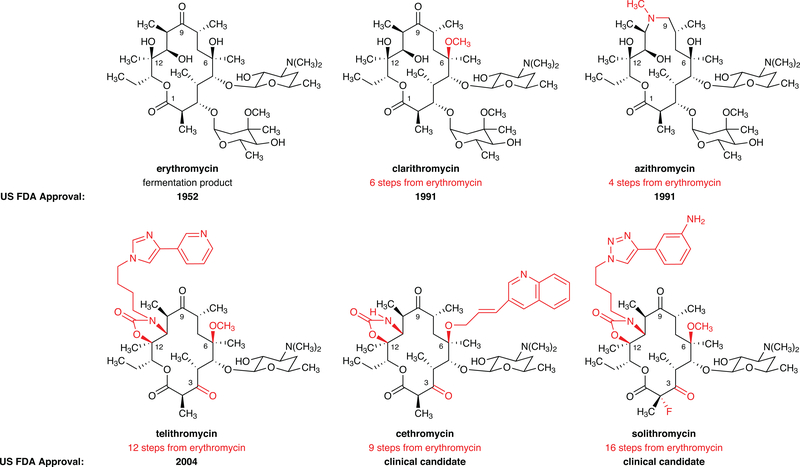
Natural products have provided critical starting points for the development of a majority of the antibacterial drugs listed as essential medicines by the World Health Organization. During the period ~1940–1960, sometimes described as the golden age of antibiotics research, academic and industrial laboratories identified the natural products that went on to define many of the major classes of antibiotics in our modern day armamentarium.1 Few if any natural products prove to be optimal for safety, efficacy, or oral bioavailability in humans, however, for these were likely not evolutionary pressures for the microbes in which they developed. For six decades a primary tool by which new antibiotics have been discovered and manufactured is semisynthesis, or chemically modifying natural products derived from fermentation. Semisynthesis is inherently limited because it is challenging to selectively modify structurally complex natural products, and typically few positions of any given scaffold can be modified effectively.2,3 Macrolide antibiotics (macrocyclic lactones with one or more pendant glycosidic residues), which have proven to be safe and effective for use in treating human infectious diseases such as community-acquired bacterial pneumonia, gonorrhea, and others, provide a compelling case in point. Since the discovery of erythromycin from a Philippine soil sample in 1949,4 in spite of extraordinary efforts leading to fully synthetic5–8 and modified biosynthetic routes9–12 to macrolide antibiotics, all members of this class approved or in clinical development for use in humans have been manufactured by chemically modifying erythromycin (erythromycin itself is unstable in the stomach, and rearranges to form a product with gastrointestinal side-effects).13 Thus, azithromycin is prepared from erythromycin in four steps,14 clarithromycin requires six steps,15 and the advanced clinical candidate solithromycin is currently produced from erythromycin by a 16-step linear sequence (Figure 1).16,17 New macrolides are badly needed, for resistance to approved macrolide antibiotics such as clarithromycin and azithromycin is now widespread in both hospitals and the community.18–20 Here we present a practical, fully synthetic platform for the preparation of macrolide antibiotics, providing both a discovery engine for structurally novel antibiotic candidates that would be difficult or impossible to obtain from erythromycin, as well as a basis for their eventual manufacture. Using simple building blocks and a highly convergent assembly process, we have prepared more than 300 structurally diverse macrolide antibiotic candidates, as well as the approved drug telithromycin and the clinical candidate solithromycin. We have identified molecules with diverse macrocyclic scaffolds that exhibit potent activities against bacterial strains resistant to erythromycin, azithromycin, and other current antibiotics of different classes.
Figure 1 |. Summary of macrolide antibiotic development by semisynthesis.
To date, all macrolide antibiotics are produced by chemical modification (semisynthesis) of erythromycin, a natural product produced on ton-scale by fermentation. Depicted are the approved semisynthetic macrolide antibiotics clarithromycin, azithromycin, and telithromycin along with the dates of their FDA approval and the number of steps for their synthesis from erythromycin. The previous ketolide clinical candidate cethromycin and the current clinical candidate solithromycin are also depicted. It is evident that increasingly lengthy sequences are being employed in macrolide discovery efforts.
Synthesis of 14-membered azaketolides
To illustrate our approach to the synthesis of macrolide antibiotics we begin by detailing a route to new and highly active hybrid antibiotics, 14-membered azaketolides (Figure 2), and then show how the approach can be easily expanded to subsume many other active macrolide scaffolds, with broad latitude for substitutional variation. Ketolides replace the C3-cladinose carbohydrate residue of erythromycin with a carbonyl group, a chemical modification that allows them to evade certain inducible forms of macrolide resistance,21,22 whereas the azalide azithromycin incorporates a nitrogen atom within an expanded (15-membered) macrolactone ring, features which have been proposed to impart favorable pharmacological properties, including increased efficacy against certain Gram-negative pathogens.23,24 The hybrid “azaketolides”, or macrolides that contain both a C3 carbonyl group and a nitrogen atom within the macrolactone ring, have been little explored; one such compound has been reported and was found to be essentially inactive.25 We were particularly interested in 14-membered azaketolides that would arise from formal replacement of the carbonyl group at position C9 of ketolides with an amino group, a transformation that would not be feasible by semisynthesis.
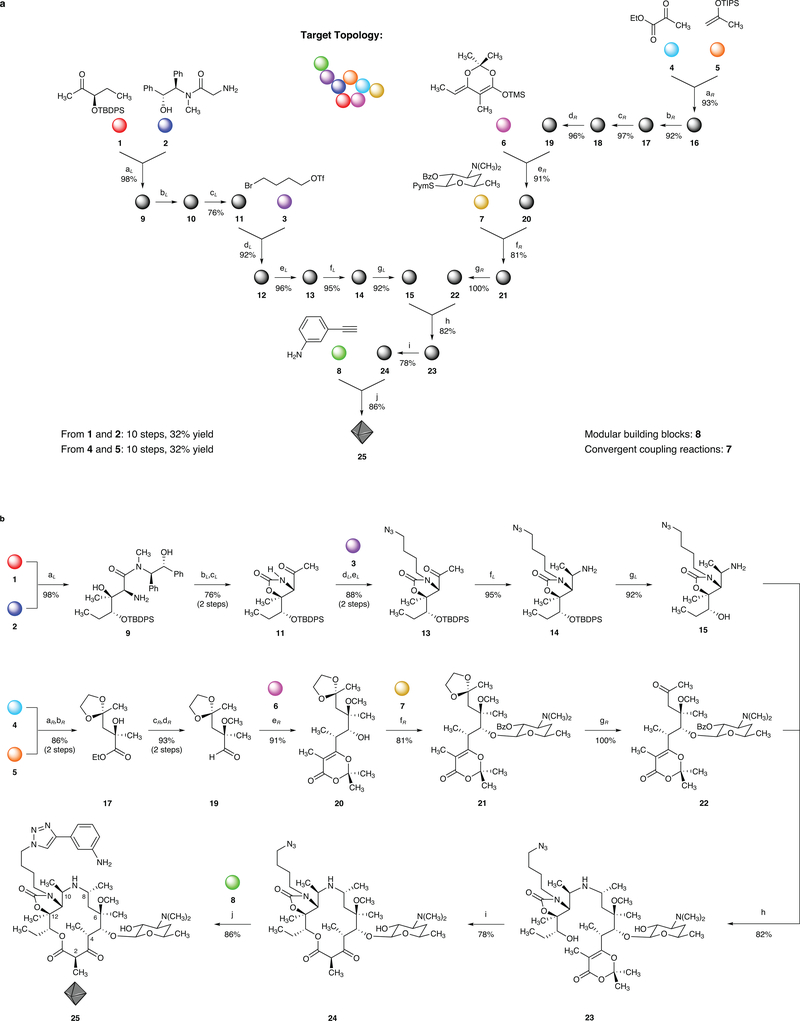
Figure 2 |. A convergent, fully synthetic route to the 14-membered azaketolide 25.
a, Graphical representation of the convergent synthesis of azaketolide 25 from eight variable building blocks (represented by colored spheres). Downward, Y-shaped arrows signify convergent coupling reactions. b, Synthesis of azaketolide 25, reagents and conditions (subscripts L and R indicate left and right halves, respectively): (aL) LiHMDS, LiCl, 98%; (bL) EtiPr2N, COCl2; (cL) iPrMgCl, CH3Li, 76% over 2 steps, 92% of recovered (R,R)-pseudoephenamine; (dL) NaHMDS; (eL) NaN3, 88% over 2 steps; (fL) NH3, Ti(OiPr)4, NaBH4, 95%; (gL) Bu4NF, 92%; (aR) Pd[(S)-SegPhos]Cl2, AgSbF6, 93%, 92% ee; (bR) ethylene glycol, PPTS, 92%; (cR) KH, MeI, 97%; (dR) (iBu)2AlH, 96%; (eR) Et3N, MgBr2•OEt2, 91%; (fR) AgOTf, 81%, 16:1 β:α; (gR) HCl, 100%; (h) NaCNBH3, 82%; (i) 132 °C, 1 mM, PhCl, 78%; (j) CuSO4, sodium L-ascorbate, 86%.
An overview is helpful as it emphasizes our synthetic strategy. Eight simple building blocks (1-8), two commercially available and six synthesized in 3–6-step sequences in 30–150-g amounts, are assembled in a highly convergent manner (Figure 2). As depicted, building blocks 1-3 are transformed by a seven-step sequence featuring two convergent coupling reactions to provide a key left-hand intermediate (15, an amine) in 57% yield, while building blocks 4–7 are transformed in a seven-step sequence featuring three convergent coupling reactions to form a key right-hand intermediate (22, a ketone) in 58% yield. The left- and right-hand intermediates are then joined in a highly stereoselective and efficient reductive coupling reaction (82% yield, d.r. > 20:1) that also serves to remove the benzoate protective group within the desosamine residue. The next step is the key macrocyclization reaction, for which we employ a method developed by Boeckman et al. in the context of their approaches toward the natural products diplodialide A (a 10-membered macrolactone), kromycin (a 14-membered macrolactone), and ikarugamycin (a 17-membered macrolactam).26,27 We found that thermolysis of intermediate 23 (1 mM in chlorobenzene) at 132 °C afforded the macrolactone 24 in 78% yield (1-g scale). There are a number of notable features of this transformation, not least its efficacy and, as we will show, generality. Many pioneering efforts to synthesize macrolide antibiotics failed at the stage of macrocyclization; successful macrocyclizations were shown to require carefully designed, rigidifying protective group strategies.28 We believe that the success of the present cyclization (which we note occurs in the presence of a free secondary amine as well as another secondary alcohol) is attributable in part to the rigid structuring induced by the cyclic carbamate function on the left-hand side of the molecule, which we suspect favorably brings the putative acyl ketene function (produced from the dioxolenone function upon thermolysis) and the reactive secondary alcohol into proximity. A single operation then transforms the product of macrocyclization into a fully synthetic antibiotic candidate (25) while incorporating the final building block using Sharpless’ optimized conditions for azide-alkyne dipolar cycloaddition29 (8, 86% yield). This final step is by design a convergent coupling reaction, and permits optimal late-stage diversification of a key side-chain residue that is known to occupy a novel pocket in the bacterial ribosome.30–34
Aspects of certain steps prior to the macrocyclization reaction merit brief discussion. The key aldol coupling reaction that unites building blocks 135 and 2,36 initiating the synthesis of the left-half intermediate, involves new methodology that was developed specifically for the present purpose (though it proves to be a general transformation) and proceeds in 98% yield on a 33-g scale to form a single, crystalline diastereomer.36 In a subsequent step, which transforms the pseudoephenamine amide function into a methyl ketone in one operation, the pseudoephenamine auxiliary is recovered by a simple aqueous extraction procedure in 92% yield. The convergent coupling reaction that unites building blocks 4 and 5 to begin the construction of the right-half intermediate is based on a palladium-catalyzed ene reaction developed by Mikami and coworkers, and proceeds in 93% yield and 92% ee.37 Two convergent coupling reactions follow three linear functional group interconversions, the first coupling being a Mukaiyama38 aldol reaction (to incorporate building block 639) whose diastereoselectivity was observed to be dependent upon the reaction conditions. The conditions employed here (magnesium bromide etherate as Lewis acid, triethylamine as base, dichloromethane as solvent, and a reaction temperature of –78 °C) provided maximum selectivity (d.r. >20:1, 91% yield) for the desired syn-aldol product. The next convergent coupling reaction introduced the protected desosamine carbohydrate residue and for this purpose we employed the thioglycoside 7,40,41 a donor very similar to that employed by Woodward et al. in their synthesis of erythromycin.5 In the present case, we found that the use of a benzoyl group to shield the secondary alcohol on the glycosidic donor in lieu of methoxycarbonyl improved the yield and diastereoselectivity of the glycosidation reaction. The overall yield of the fully synthetic azaketolide antibiotic candidate 25 was 32% from initial building blocks 1 and 2 (a 10-step sequence) or 32% from initial building blocks 4 and 5 (also a 10-step sequence).
Synthesis of 15-membered azaketolides
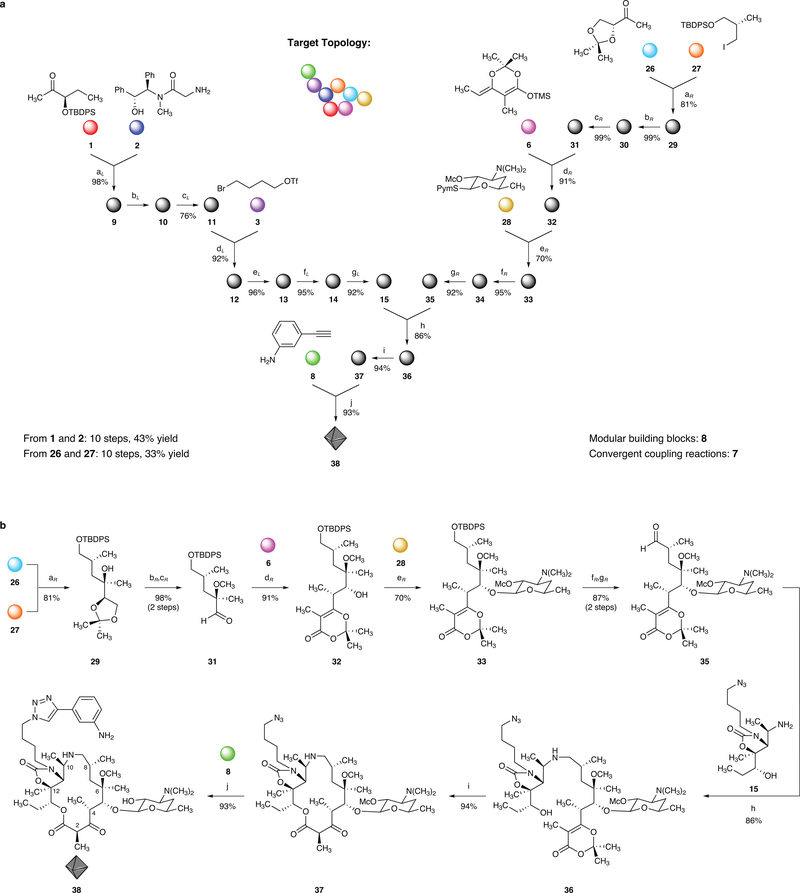
By design, the convergent building block strategy we employ enables rapid access to structurally related scaffolds; for example, synthesis of a 15-membered hybrid azaketolide scaffold comprising structural features of both the azalide antibiotic azithromycin and the ketolide clinical candidate solithromycin (Figure 3) required modification of just three of the eight building blocks that were used to assemble 14-membered azaketolides (Figure 2). The left-hand amine intermediate (15) was the same in both 14- and 15-membered azaketolide scaffold syntheses and thus construction of the 15-membered azaketolides simply required access to the homologated right-hand intermediate, aldehyde 35. Here, too, we employed a seven-step sequence (38% yield) featuring three convergent coupling reactions to assemble four simple building blocks. The first coupling, which unites building blocks 2642 and 2743,44 to form the tertiary alcohol 29, emerged only after extensive experimentation, in which we discovered that incorporating the large tert-butyldiphenylsilyl protective group within component 27 was key to achieve high diastereoselectivity. After two subsequent functional group interconversions, building blocks 6 (as before) and 28 (the original glycosyl donor of Woodward et al.,5 which proved to be effective in this scaffold synthesis) were incorporated by successive convergent coupling reactions. Two linear steps then provided the right-hand intermediate, aldehyde 35. Reductive coupling of the left-hand amine (15) and the right-hand aldehyde (35) in the presence of sodium cyanoborohydride proceeded without detectable epimerization to afford the macrocyclization precursor 36 in 86% yield. Thermolysis of 36 at 132 °C in chlorobenzene then afforded the 15-membered macrolide 37 in 94% yield on a 1.9-g scale. Incorporation of the final building block (8, as before) and cleavage of the methoxycarbonyl protective group were achieved in a single, final step to provide the azaketolide antibiotic candidate 38. The overall yield of the fully synthetic macrolide 38 (FSM-20707 in Figure 5) was 43% from initial building blocks 1 and 2 (a 10-step linear sequence) or 33% from initial building blocks 26 and 27 (also a 10-step linear sequence).
Figure 3 |. A convergent, fully synthetic route to the 15-membered azaketolide 38.
a, Graphical representation of the convergent synthesis of azaketolide 38 from eight variable building blocks (represented by colored spheres). Downward, Y-shaped arrows signify convergent coupling reactions. b, Synthesis of azaketolide 38, reagents and conditions (subscripts L and R indicate left and right halves, respectively): (aL) LiHMDS, LiCl, 98%; (bL) EtiPr2N, COCl2; (cL) iPrMgCl, CH3Li, 76% over 2 steps, 92% of recovered (R,R)-pseudoephenamine; (dL) NaHMDS; (eL) NaN3, 88% over 2 steps; (fL) NH3, Ti(OiPr)4, NaBH4, 95%; (gL) Bu4NF, 92%; (aR) tBuLi, MgBr2, 81%; (bR) KH, MeI, 99%; (cR) H5IO6, 99%; (dR) ZnCl2, 91%; (eR) AgOTf, 70%; (fR) HF (aq); (gR) Dess–Martin periodinane, 87% over 2 steps; (h) NaCNBH3, 86%; (i) 132 °C, 1 mM, PhCl, 94%; (j) CuSO4, sodium L-ascorbate, 93%.
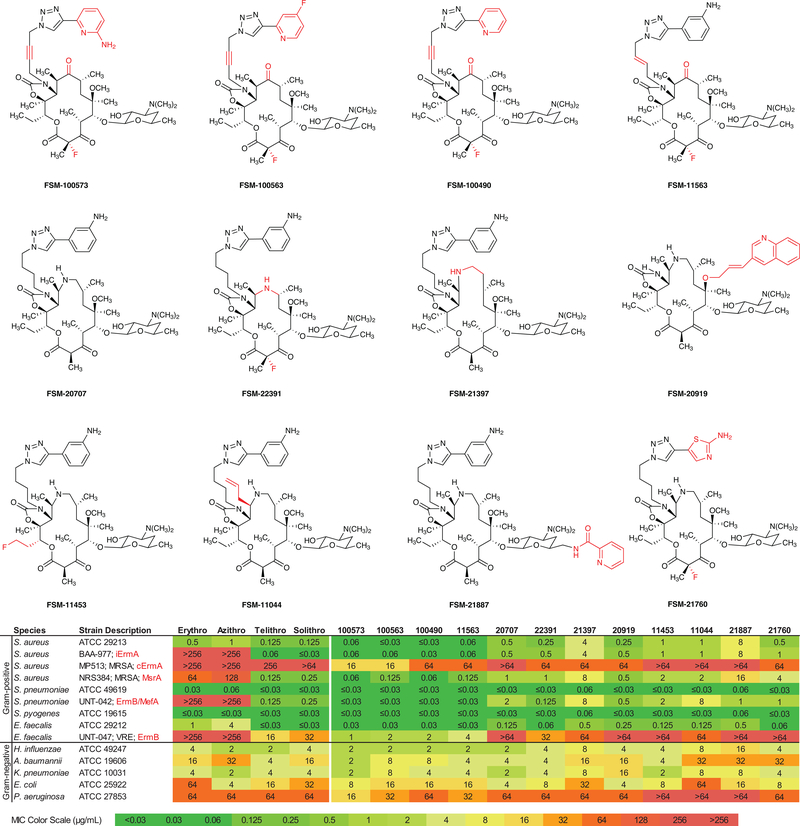
Figure 5 |. Minimum inhibitory concentrations (μg/mL) for selected analogs against 9 Gram-positive and 5 Gram-negative microorganisms.
iErmA – inducible erythromycin ribosome methyltransferase A; cErmA – constitutive erythromycin ribosome methyltransferase A; MsrA – macrolide streptogramin resistance efflux pump A; MefA – macrolide efflux protein A; ErmB – erythromycin ribosome methyltransferase B; Erythro – erythromycin; Azithro – azithromycin; Telithro – telithromycin; Solithro – solithromycin.
Synthesis of 14-membered ketolides
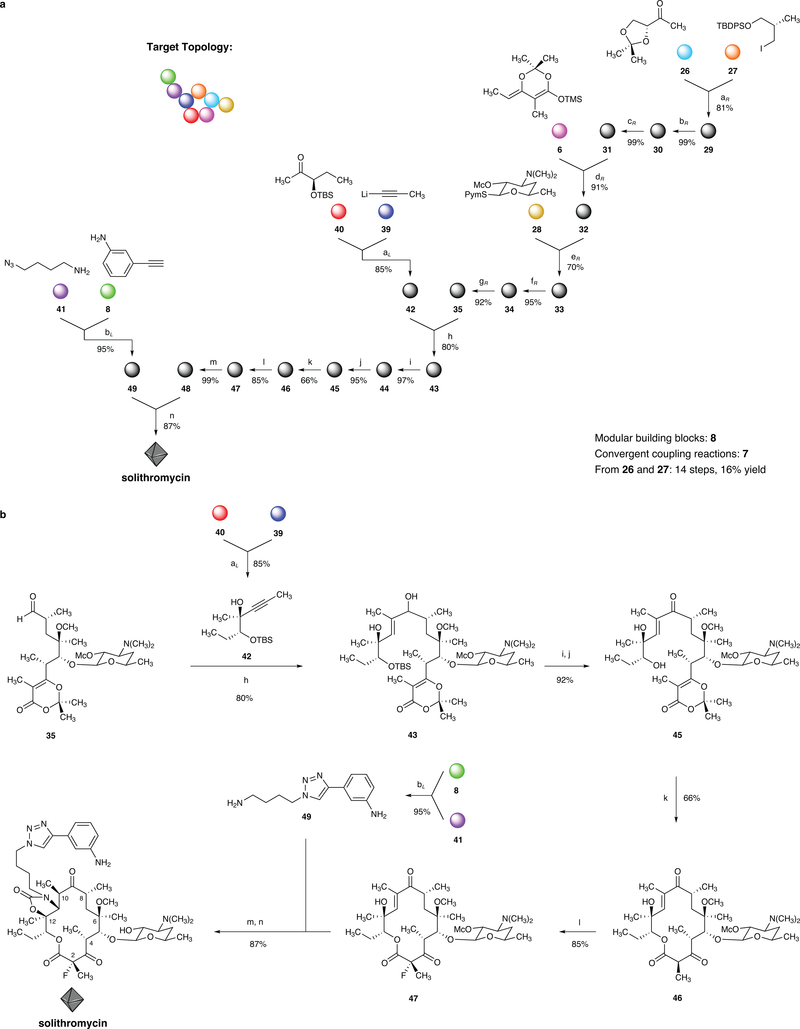
We next adapted our convergent assembly strategy to synthesize 14-membered ketolides, a class that includes the clinical candidate solithromycin as well as the approved drug telithromycin, with the goal of preparing diverse structural analogs. Toward this end we envisioned coupling of the same right-hand intermediate employed in the synthesis of 15-membered azaketolides (aldehyde 35) with a Grignard reagent derived from hydromagnesiation of the acetylenic alcohol 42. Intermediate 42 was obtained in one step (14:1 d.r., 85% yield) by the addition of 1-lithiopropyne (39) to ketone 4035 in the presence of lithium (1S,2R)-1-phenyl-2-(pyrrolidin-1-yl)-1-propanolate.45 Hydromagnesiation of 42 with cyclopentylmagnesium bromide and bis-cyclopentadienyltitanium dichloride46 proceeded regioselectively to furnish a vinyl Grignard reagent that was captured in situ with aldehyde 35. The resulting diastereomeric mixture of allylic alcohols (obtained in 80% yield on a 3-g scale) was oxidized directly (Dess-Martin periodinane, 97%) and the α,β-unsaturated ketone produced was desilylated (n-Bu4NF, 95%) to afford the keto alcohol 45. Macrocyclization, as before, then provided the 14-membered macrolactone 46 in 66% yield (1.7-g scale). Fluorination at position C2 proceeded in 85% yield.47 Transformation of the C12 tertiary alcohol within the latter intermediate (47) to the corresponding acyl imidazolide (Im2CO, DBU) and trapping with amine 49 then afforded fully synthetic solithromycin in one operation (87% yield, the methoxycarbonyl protective group was cleaved concomitantly under these conditions). The present route to solithromycin proceeds in 14 steps and 16% yield from building blocks 26 and 27, and differs from the semisynthetic route (16 steps from erythromycin, yields not reported16) in that the linker and its attached side-chain heterocycle are introduced in the final step of our synthesis and thus modification of these residues is quite facile, whereas the linker residue is introduced 6 steps prior to the penultimate step in the published semisynthetic route to solithromycin.16 We prepared a number of fully synthetic 14-membered ketolides by taking advantage of this feature of our synthesis to simultaneously vary the linker and side-chain heterocycle, and many of these products show potencies that are superior to solithromycin in microbiological assays (vide infra).
Construction of a Library of Macrolide Antibiotics
We prepared an initial library of >300 fully synthetic macrolide (FSM) antibiotic candidates by varying the building blocks in concert with modifying readily diversifiable elements (e.g., an azido group, an amino group, a β-keto lactone function, an allyl group, etc.) that we introduced into and around the macrolide ring, a powerful tactical combination. In addition to members of the three primary macrocyclic scaffolds discussed in detail above, we prepared a number of other unique scaffolds by modifying the principal coupling components (left- and right-halves) and their modes of coupling using straightforward alternative chemical transformations (see Supplementary Information for a complete list of structures synthesized and Extended Data Figures 1–10 for exemplary schemes for their preparation). This strategy not only provided novel scaffolds to explore, but also permitted deep-seated variations of positions within these scaffolds, thus enabling access to molecules that could not be prepared using semisynthetic methods.
Microbiological Testing
To evaluate our fully synthetic macrolides for antibiotic activity we screened 305 of them against a panel of pathogens comprising standardized Gram-positive and Gram-negative strains (See Supplementary Information). For the most promising antibiotic candidates the panel was expanded to include bacteria with genetically characterized resistance mechanisms to erythromycin as well as other antibiotics. These include 3 different strains of S. aureus, two of them clinically isolated methicillin-resistant (MRSA) pathogens, a clinically isolated strain of S. pneumoniae with both ribosome-modifying methyltransferase (ermB) and efflux (mefA) genes, and finally a clinically isolated strain of E. faecalis with an ermB gene, a strain resistant to all approved macrolides as well as vancomycin (VRE) (Figure 5). The data show that a majority of compounds in the library exhibit demonstrable antibiotic activity. For example, 83% of compounds in the collection displayed an MIC ≤4 μg/mL against wild-type S. pneumoniae, which is known to be highly susceptible to inhibition by macrolides (Supplementary Information). Among the most promising compounds (Figure 5) are represented variously 14-, 15-, and 16-membered azaketolide scaffolds (e.g., FSM-22391, FSM-20707, and FSM-21397, respectively), a 15-membered azacethromycin hybrid antibiotic (FSM-20919), and a number of fully synthetic 14-membered ketolides (most notably, FSM-100573 and FSM-100563). The latter two substances display superior potencies relative to any macrolide in current clinical use in the following extremely challenging strains: an S. pneumoniae with both ermB and mefA genes (MIC’s ≤0.03 μg/mL), a VRE with an ermB gene (MIC’s 1 and 2 μg/mL, respectively), a MRSA with a constitutively-expressed erythromycin ribosome methyltransferase (c-erm) gene (MIC’s 16 μg/mL), and P. aeruginosa (MIC’s 16 and 32 μg/mL, respectively). Clearly, further work will be required to drive potencies in the latter two strains to clinically efficacious levels, but we believe that demonstration of even modest activity in these challenging strains by a macrolide is significant. It is interesting and encouraging to note that FSM-100573 also displays improved Gram-negative activity relative to many other macrolides; further advances in this regard would address an important area of unmet medical need.48
Conclusion
Employing as a design strategy the multiply convergent assembly of simple chemical building blocks we have developed a platform of unprecedented versatility for the discovery and practical synthesis of novel macrolide antibiotics. Varying the building blocks as well as (at a later stage) diversifiable elements incorporated within them permits an almost exponential expansion of variability within any given scaffold. In addition, our work shows that variant scaffolds can be obtained by straightforward perturbations of our generalized assembly process. As with our earlier convergent synthesis of tetracycline antibiotics,49,50 we anticipate that many thousands of novel macrolide structures can be prepared for evaluation as potential antibiotics using the present synthetic platform. In light of this, it seems logical to conclude that developing similar convergent routes to other naturally occurring antibiotic families may accelerate the discovery of new therapeutic agents for human infectious disease.
Extended Data
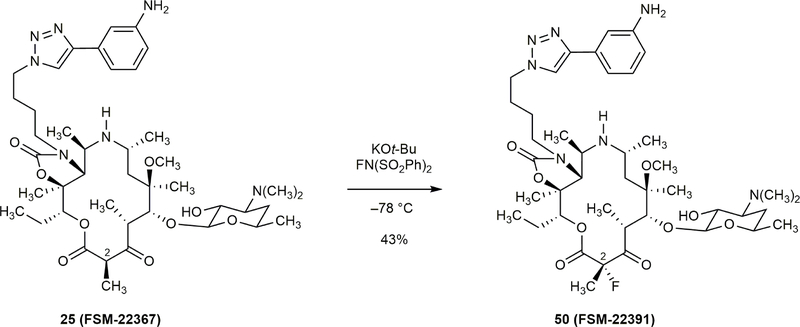
Extended Data Figure 1 |. Synthesis of a C2-fluoro 14-membered azaketolide by a late-stage fluorination reaction.
Subjection of β-keto lactone 25 to potassium tert-butoxide (1.0 equiv) at –78 °C followed by N-fluorobenzenesulfonimide (1.0 equiv) afforded FSM-22391 in 43% yield.
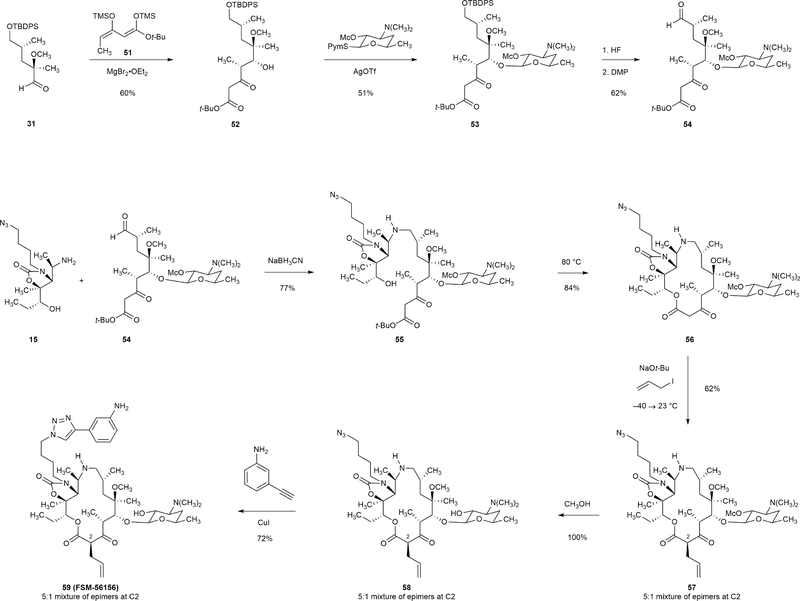
Extended Data Figure 2 |. Synthesis of a 15-membered azaketolide with a modified C2-substituent.
Thermolysis of a β-keto tert-butyl ester substrate (55) proceeded at a lower temperature (80 °C) and afforded a 15-membered macrocycle without substitution at C2 (56). This macrocycle served as a nearly ideal intermediate for preparation of macrolides with diverse C2-substitutions. For example, an allyl group was introduced at C2 by treatment of 56 with sodium tert-butoxide (1.1 equiv) and allyl iodide (1.1 equiv) at –40 °C followed by warming the reaction solution to 23 °C. The product 57 (obtained in 62% yield) was then transformed to FSM-56156 in two steps (72% yield).
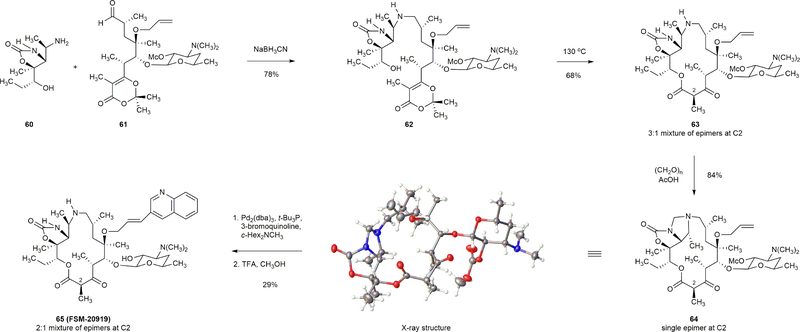
Extended Data Figure 3 |. Synthesis of a 15-membered azacethromycin hybrid.
Macrolactone 63 was prepared from amine 60 and aldehyde 61 in two steps by a reductive amination–macrocyclization sequence. Treatment of 63 with paraformaldehyde (6.0 equiv) and acetic acid (10.0 equiv) furnished adduct 64 as a crystalline solid (84% yield). The imidazolidine group within 64 served to protect both the secondary amine and the cyclic carbamate functions, and permitted the introduction of a quinoline heterocycle via a Heck reaction. Methanolysis (TFA, CH3OH) cleaved the imidazolidine group, affording FSM-20919 (29%, two-step yield).
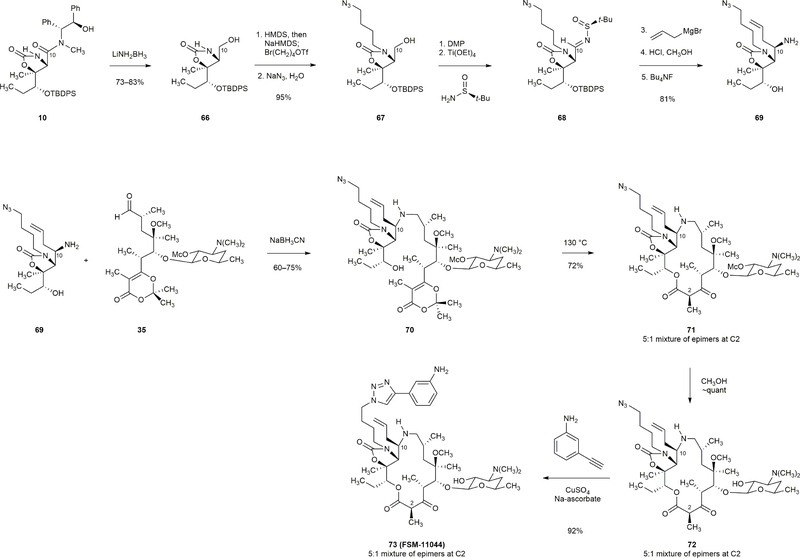
Extended Data Figure 4 |. Synthesis of a 15-membered azaketolide with a modified C10-substituent.
N-tert-butylsulfinyl imine 68 (prepared in five steps from amide 10) allowed for the stereocontrolled introduction of various C10-substituents. For example, addition of allylmagnesium bromide proceeded with >20:1 stereoselectively to furnish the adduct depicted; subsequent cleavage of the sulfinyl (HCl, CH3OH) and tert-butyldiphenylsilyl (Bu4NF) groups within the adduct then furnished left-hand intermediate 69 (81% yield). Amine 69 and aldehyde 35 were coupled by a reductive amination reaction (NaBH3CN, 60–75% yield). The product (70) was then transformed to FSM-11044 in a three-step sequence that consisted of a macrocyclization reaction (72% yield), a methanolysis reaction (quantitative yield) and lastly a [3+2] dipolar cycloaddition reaction (92% yield).
Extended Data Figure 5 |. Synthesis of a 15-membered azaketolide with a modified C13-substituent.
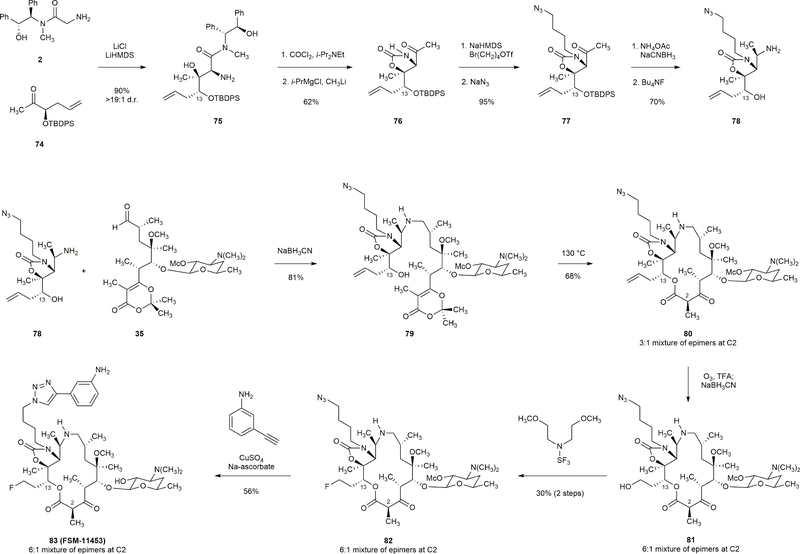
Modification of position C13 was achieved by modification of a single component, in this case the ketone building block 74 depicted above. Reductive coupling of 78 and 35 united the left- and right-halves; subsequent thermal macrocyclization provided macrolactone 80. The allyl group within intermediate 80 was cleaved upon ozonolysis (O3, trifluoroacetic acid); reductive workup with sodium cyanoborohydride afforded alcohol 81. Subjection of 81 to bis(2-methoxyethyl)aminosulfur trifluoride afforded the fluoroethyl-substituted macrocycle 82 (30%, two-step yield), which was transformed to FSM-11453 by a [3+2] dipolar cycloaddition reaction.
Extended Data Figure 6 |. Synthesis of a 15-membered azaketolide with a modified desosamine sugar residue.
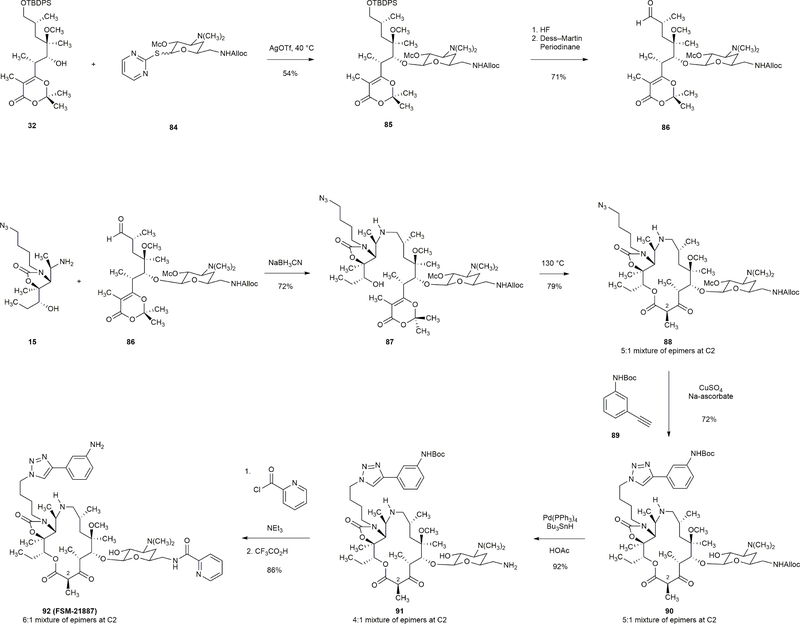
The 15-membered macrolactone 90 was synthesized using thioglycoside 84 and alkyne 89 as building blocks (in lieu of building blocks 28 and 8 used for the synthesis of 15-membered azaketolide FSM-20707). Treatment of 90 with tributyltin hydride (2.0 equiv), acetic acid (5.0 equiv), and tetrakis(triphenylphosphine)palladium (2 mol%) led to cleavage of the allyloxycarbonyl protective group, giving rise to amine 91 (92% yield). The latter intermediate has been transformed into a number of fully synthetic macrolides with modified desosamine sugar residues. For example, acylation of the primary amino group of intermediate 91 with pyridine-2-carbonyl chloride (2.0 equiv) in the presence of trimethylamine (3.0 equiv) followed by removal of the tert-butoxycarbonyl group afforded FSM-21887 (86%, two-step yield).
Extended Data Figure 7 |. Synthesis of a 16-membered azaketolide.
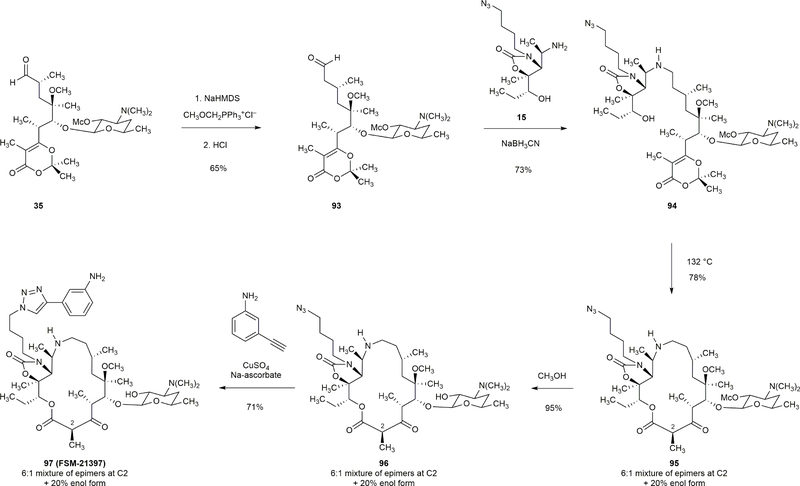
Homologation of aldehyde 35 was achieved by a Wittig olefination reaction (CH3OCH3PPh3+Cl–, NaHMDS) followed by hydrolysis of the resulting enol ether to afford aldehyde 93 in 65% yield. Reductive coupling of amine 15 and aldehyde 93 furnished macrocyclization precursor 94 (73% yield). The 16-membered macrolactone 95 was obtained in 78% yield upon thermolysis of 94 (1mM, 132 °C). Two additional steps transformed 95 to the 16-membered azaketolide FSM-21397.
Extended Data Figure 8 |. Synthesis of a 14-membered macrolide with a trans-olefin linkage.
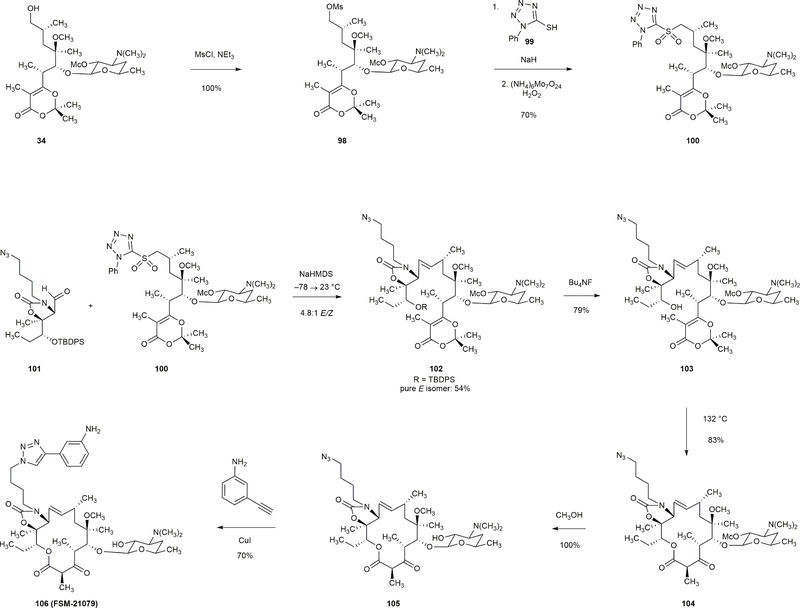
Mesylate 98 was prepared in quantitative yield by treatment of alcohol 34 with methanesulfonyl chloride (1.50 equiv) and triethylamine (2.0 equiv). Displacement of mesylate 98 with sodium 1-phenyl-1H-tetrazole-5-thiolate (2.0 equiv) followed by oxidation of the resulting thioether with ammonium molybdate (0.20 equiv)–hydrogen peroxide (100 equiv) afforded sulfone 100 in 70% yield. Aldehyde 101 and sulfone 100 were coupled in a Julia–Kocienski olefination reaction (NaHMDS, –78 → 23 °C) to provide a 4.8:1 mixture of E- and Z-olefin isomers. The E-isomer 102 was isolated and desilylated (Bu4NF, 79%). Thermolysis of the product 103 (1 mM, 132 °C) furnished the 14-membered macrocycle 104 in 83% yield. 104 was then transformed to FSM-21079 in two additional steps (methanolysis and [3+2] dipolar cycloaddition).
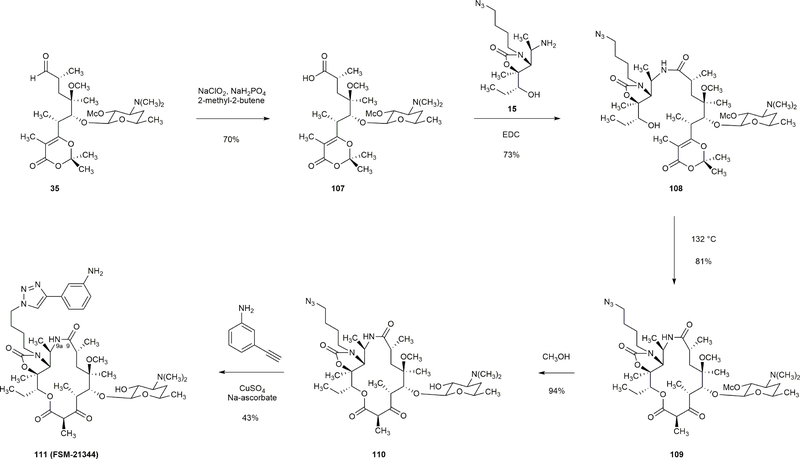
Extended Data Figure 9 |. Synthesis of a 15-membered macrolide with an amide linkage (C9-N9a).
Oxidation of aldehyde 35 with sodium chlorite (10.0 equiv) in the presence of sodium dihydrogen phosphate (10.0 equiv) and 2-methyl-2-butene (100 equiv) afforded carboxylic acid 107 in 70% yield. Acid 107 and amine 15 were coupled in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (2.00 equiv) to provide amide 108. Macrocyclization of 108 (1 mM, 132 °C) proceeded in 81% yield to afford macrolactam 109. Methanolysis and [3+2] dipolar cycloaddition then transformed 109 to FSM-21344 in two steps.
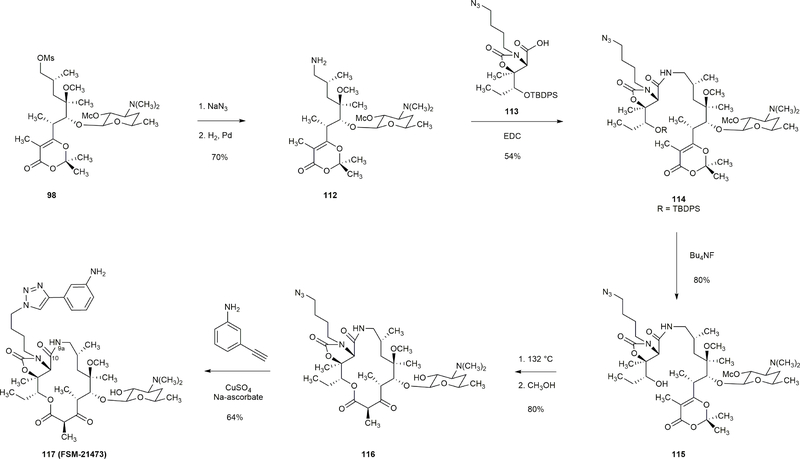
Extended Data Figure 10 |. Synthesis of a 15-membered macrolide with an amide linkage (C10-N9a).
Amine 112 was prepared in 70% yield by displacement of mesylate 98 with sodium azide followed by reduction of the resulting alkyl azide (H2, Pd). The coupling of amine 112 and acid 113 proceeded in 54% yield. The product, amide 114, was desilylated (Bu4NF, 80%) to afford the macrocyclization precursor 115. Thermal macrocyclization of 115 followed by cleavage of the methoxycarbonyl protective group afforded lactam 116 in 80% yield. Copper-catalyzed [3+2] dipolar cycloaddition then provided FSM-21473 in 64% yield.
Supplementary Material
Figure 4 |. A convergent, fully synthetic route to solithromycin.
a, Graphical representation of the convergent synthesis of solithromycin, which was previously only accessible by semisynthesis. This route has been adapted for the synthesis of >30 novel ketolide antibiotic candidates, as well as the FDA-approved ketolide telithromycin. Downward, Y-shaped arrows signify convergent coupling reactions. b, Synthesis of solithromycin, reagents and conditions (subscripts L and R indicate left and right portions, respectively): (aL) lithium (1S,2R)-1-phenyl-2-(pyrrolidin-1-yl)-1-propanolate, 85%; (bL) CuSO4, sodium L-ascorbate, 95%; (aR) tBuLi, MgBr2, 81%; (bR) KH, MeI, 99%; (cR) H5IO6, 99%; (dR) ZnCl2, 91%; (eR) AgOTf, 70%; (fR) HF (aq), 95%; (gR) Dess–Martin periodinane, 92%; (h) Cp2TiCl2, cyclopentylmagnesium bromide, 80%; (i) Dess–Martin periodinane, 97%; (j) Bu4NF, 95%; (k) 132 °C, 0.5 mM, PhCl, 66%; (l) KOtBu, FN(SO2Ph)2, 85%; (m) Im2CO, DBU; (n) imidazole hydrochloride, 60 °C, 87% over 2 steps.
Acknowledgements
We most gratefully acknowledge funding from Alastair and Celine Mactaggart, from a private family foundation, and from the Blavatnik Biomedical Accelerator Program at Harvard University. We thank NERCE (AI057159), W. Weiss (University of North Texas), and R. Alm and S. Lahiri (Macrolide Pharmaceuticals) for measuring MIC values, R. Alm and T. Dougherty (Harvard Medical School) for genetic characterization of a microbial resistance gene, and S.-L. Zheng (Harvard) for conducting X-Ray crystallographic analyses. I.B.S. acknowledges postdoctoral fellowship support from the National Institutes of Health (F32GM099233); Z.Z. is a Howard Hughes Medical Institute International Student Research fellow; A.L. acknowledges postdoctoral fellowship support from the Swiss National Science Foundation (PBGEPE2-139864) and the Novartis Foundation; D.T.H. is indebted to the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship (HO 5326/1-1); T.F. acknowledges Daiichi-Sankyo Co., Ltd. for financial support; Y.K. acknowledges support from the Engineering Promotion Fund of Gifu University.
Footnotes
Competing Financial Interests The authors I.B.S., Z.Z., and A.G.M. have filed three provisional patents and an international patent application: U.S. 62/061571, “14-Membered Ketolides and Methods of Their Preparation and Use”; U.S. 62/138198, “Macrolides with Modified Desosamine Sugars and Uses Thereof”; U.S. 62/214774, “Right Half Synthesis of 14-Membered Azaketolides”; PCT/US2014/033025, “Macrolides and Methods of Their Preparation and Use”. A.G.M. declares that he is a founder, board member, and chairman of the scientific advisory board of Macrolide Pharmaceuticals, and Z.Z. and I.B.S. declare that they serve as scientific consultants to Macrolide Pharmaceuticals.
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/nature.
Atomic coordinates and structure factors for the crystal structure reported have been deposited with the Cambridge Crystallographic Database under accession number CCDC 1440650.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Walsh C Antibiotics: Actions, Origins, Resistance. (American Society for Microbiology Press, 2003). [Google Scholar]
- 2.Fischbach MA & Walsh CT Antibiotics for emerging pathogens. Science 325, 1089–1093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright PM, Seiple IB & Myers AG The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed 53, 8840–8869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mcguire JM et al. Ilotycin, a new antibiotic. Antibiot. Chemother 2, 281–283 (1952). [PubMed] [Google Scholar]
- 5.Woodward RB et al. Asymmetric total synthesis of erythromycin. 3. Total synthesis of erythromycin. J. Am. Chem. Soc 103, 3215–3217 (1981). [Google Scholar]
- 6.Martin SF, Hida T, Kym PR, Loft M & Hodgson A The asymmetric synthesis of erythromycin B. J. Am. Chem. Soc 119, 3193–3194 (1997). [Google Scholar]
- 7.Kim HC & Kang SH Total synthesis of azithromycin. Angew. Chem 121, 1859–1861 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Andrade RB Total synthesis of desmethyl macrolide antibiotics. Synlett 26, 2199–2215 (2015). [Google Scholar]
- 9.Khosla C Harnessing the biosynthetic potential of modular polyketide synthases. Chem. Rev 97, 2577–2590 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Cane DE, Walsh CT & Khosla C Harnessing the biosynthetic code: combinations, permutations, and mutations. Science 282, 63–68 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Marsden AFA et al. Engineering broader specificity into an antibiotic-producing polyketide synthase. Science 279, 199–202 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Park S et al. Genetic engineering of macrolide biosynthesis: past advances, current state, and future prospects. Appl. Microbiol. Biotechnol 85, 1227–1239 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Kurath P, Jones PH, Egan RS & Perun TJ Acid degradation of erythromycin A and erythromycin B. Experientia 27, 362–362 (1971). [DOI] [PubMed] [Google Scholar]
- 14.Bright GM et al. Synthesis, in vitro and in vivo activity of novel 9-deoxo-9a-aza-9a-homoerythromycin A derivatives – a new class of macrolide antibiotics, the azalides. J. Antibiot 41, 1029–1047 (1988). [DOI] [PubMed] [Google Scholar]
- 15.Morimoto S, Takahashi Y, Watanabe Y & Omura S Chemical modification of erythromycins. 1. Synthesis and antibacterial activity of 6-O-methylerythromycins-A. J. Antibiot 37, 187–189 (1984). [DOI] [PubMed] [Google Scholar]
- 16.Fernandes PB Methods for treating gastrointestinal diseases. WO patent 2010/048599 (2010).
- 17.Putnam SD, Castanheira M, Moet GJ, Farrell DJ & Jones RN CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn. Microbiol. Infect. Dis 66, 393–401 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Leclercq R Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis 34, 482–492 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Gaynor M & Mankin AS Macrolide antibiotics: binding site, mechanism of action, resistance. Curr. Top. Med. Chem 3, 949–960 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Subramanian SL, Ramu H & Mankin AS in Antibiotic Discovery and Development (eds Dougherty TJ & Pucci MJ) 455–484 (Springer US, 2012). [Google Scholar]
- 21.Bryskier A Ketolides-telithromycin, an example of a new class of antibacterial agents. Clin. Microbiol. Infect. 6, 661–669 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Zhanel GG et al. The ketolides: a critical review. Drugs 62, 1771–1804 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Retsema J et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against Gram-negative organisms. Antimicrob. Agents Chemother 31, 1939–1947 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard AE et al. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob. Agents Chemother 31, 1948–1954 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denis A & Agouridas C Synthesis of 6-O-methyl-azithromycin and its ketolide analog via Beckmann rearrangement of 9(E)-6-O-methyl-erythromycin oxime. Bioorg. Med. Chem. Lett 8, 2427–2432 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Boeckman RK Jr. & Pruitt JR A new, highly efficient, selective methodology for formation of medium-ring and macrocyclic lactones via intramolecular ketene trapping: an application to a convergent synthesis of (–)-kromycin. J. Am. Chem. Soc. 111, 8286–8288 (1989). [Google Scholar]
- 27.Boeckman RK Jr. & Perni RB Studies directed toward the synthesis of naturally occurring acyltetramic acids. 2. Preparation of the macrocyclic subunit of ikarugamycin. J. Org. Chem 51, 5486–5489 (1986). [Google Scholar]
- 28.Woodward RB et al. Asymmetric total synthesis of erythromycin. 2. Synthesis of an erythronolide A lactone system. J. Am. Chem. Soc 103, 3213–3215 (1981). [Google Scholar]
- 29.Rostovtsev VV, Green LG, Fokin VV & Sharpless KB A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem 114, 2708–2711 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Berisio R et al. Structural insight into the antibiotic action of telithromycin against resistant mutants. J. Bacteriol 185, 4276–4279 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu D, Blaha G, Moore PB & Steitz TA Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121, 257–270 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Llano-Sotelo B et al. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother 54, 4961–4970 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulkley D, Innis CA, Blaha G & Steitz TA Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc. Natl. Acad. Sci. U.S.A 107, 17158–17163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyal Z et al. Structural insights into species-specific features of the ribosome from the pathogen Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A 112, E5805–E5814 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seiple IB, Hog DT & Myers AG Practical protocols for the preparation of highly enantioenriched silyl ethers of (R)-3-hydroxypentan-2-one, building blocks for the synthesis of macrolide antibiotics. Synlett 27, 57–60 (2016). [Google Scholar]
- 36.Seiple IB, Mercer JAM, Sussman RJ, Zhang Z & Myers AG Stereocontrolled synthesis of syn-β-hydroxy-α-amino acids by direct aldolization of pseudoephenamine glycinamide. Angew. Chem. Int. Ed 53, 4642–4647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikami K, Kawakami Y, Akiyama K & Aikawa K Enantioselective catalysis of ketoester-ene reaction of silyl enol ether to construct quaternary carbons by chiral dicationic palladium(II) complexes. J. Am. Chem. Soc 129, 12950–12951 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Mukaiyama T, Narasaka K & Banno K New aldol type reaction. Chem. Lett 2, 1011–1014 (1973). [Google Scholar]
- 39.Zhang Z, Kitamura Y & Myers AG An efficient directed Claisen reaction allows for rapid construction of 5,6-disubstituted 1,3-dioxin-4-ones. Synthesis 47, 2709–2712 (2015). [Google Scholar]
- 40.Hauske JR & Schulte GR Desosamino derivatives of macrolides as immunosuppressants and antifungal agents. WO patent 1993018042 (1993).
- 41.Zhang Z, Fukuzaki T & Myers AG Synthesis of D-desosamine and analogs by rapid assembly of 3-amino sugars. Angew. Chem. Int. Ed 55, 523–527 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Leyes AE & Poulter CD Synthesis of (R)-[2–2H]isopentenyl diphosphate and determination of its enantiopurity by 2H NMR spectroscopy in a lyotropic medium. Org. Lett 1, 1067–1070 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Tan Z & Negishi E -i. An efficient and general method for the synthesis of α,ω-difunctional reduced polypropionates by Zr-catalyzed asymmetric carboalumination: synthesis of the scyphostatin side shain. Angew. Chem. Int. Ed 43, 2911–2914 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Santaniello E, Ferraboschi P & Grisenti P An efficient chemo-enzymatic approach to the enantioselective synthesis of 2-methyl-1,3-propanediol derivatives. Tetrahedron Lett. 31, 5657–5660 (1990). [Google Scholar]
- 45.Thompson AS, Corley EG, Huntington MF & Grabowski EJJ Use of an ephedrine alkoxide to mediate enantioselective addition of an acetylide to a prochiral ketone: asymmetric synthesis of the reverse transcriptase inhibitor L-743,726. Tetrahedron Lett. 36, 8937–8940 (1995). [Google Scholar]
- 46.Sato F, Ishikawa H, Watanabe H, Miyake T & Sato M Specific hydromagnesiation of prop-2-ynylic alcohols. A simple and specific route to terpenoids. J. Chem. Soc., Chem. Commun, 718–720 (1981). [Google Scholar]
- 47.Ashley G et al. Ketolide antibacterials. US patent 6590083 (2003).
- 48.CDC’s Antibiotic Resistance Threats in the United States: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (2013).
- 49.Charest MG, Lerner CD, Brubaker JD, Siegel DR & Myers AG A convergent enantioselective route to structurally diverse 6-deoxytetracycline antibiotics. Science 308, 395–398 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Liu F & Myers AG Development of a platform for the discovery and practical synthesis of new tetracycline antibiotics. Curr. Opin. Chem. Biol, under review (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.