Abstract
Purpose
We investigate whether small incision lenticule extraction (SMILE) is associated with less ectasia than laser-assisted in-situ keratomileusis (LASIK) and whether concomitant collagen cross-linking (CXL) is protective in SMILE Xtra and LASIK Xtra.
Methods
Using an established LASIK rabbit ectasia model, we performed −5 diopter (D) LASIK on six eyes and −5 D SMILE on six eyes; five eyes had −5 D LASIK Xtra, five eyes −5 D SMILE Xtra. Anterior segment optical coherence tomography and corneal topography were performed preoperatively and 2, 4, and 6 weeks postoperatively. Mean (standard deviation [SD]) values of postoperative keratometry (K), maximum posterior elevation (MPE) and minimum corneal thickness (CT) were compared to preoperatively and among the surgical groups (paired t-test, analysis of variance).
Results
Mean (SD) K values decreased significantly following SMILE, SMILE Xtra, LASIK, and LASIK Xtra. The MPE increased significantly (P < 0.05) following LASIK, SMILE, and SMILE Xtra, but not following LASIK Xtra (P = 0.12). The MPE was less following SMILE than LASIK, but not statistically significant (week 2, 17.73 [5.77] vs. 22.75 [5.05] μm; P = 0.13); post-LASIK Xtra MPE was less than that following LASIK (week 2. 13.39 [3.05] vs. 22.75 [5.05] μm; P < 0.001). CT decreased significantly in all surgical groups; no differences were detected among the groups.
Conclusions
SMILE may have less potential than LASIK to induce ectasia. LASIK Xtra and SMILE Xtra showed the smallest increase in MPE.
Translational Relevance
Concomitant CXL may be protective following keratorefractive surgery and may reduce further the risk of ectasia.
Keywords: collagen cross-linking, ectasia, LASIK, SMILE, LASIK Xtra
Introduction
Laser-assisted in-situ keratomileusis (LASIK) is the most common excimer refractive procedure, due to its fast and painless visual rehabilitation.1 It is very safe, achieves excellent visual outcomes and has very high patient satisfaction rates.1–3 Vision-threatening complications are rare; infection, the most devastating complication, occurs in approximately 1:3000 to 1:9000 cases.4,5 Post-LASIK ectasia, however, is more common and can develop as frequently as in 1:151 to 1:496 cases with microkeratome LASIK.6,7 A similar incidence of 1 in 398 was reported recently with femtosecond LASIK.8 Ectasia may develop because the lamellar bed ablation and predominantly the vertical cut of the LASIK flap cause structural weakening of the cornea.9
Two contemporary developments in the cornea and refractive fields have been smile incision lenticule extraction (SMILE)10,11 and collagen cross-linking (CXL).12,13 SMILE involves creation of an intrastromal lenticule exclusively with the femtosecond laser.10,14 Advantages over LASIK include less postoperative dry eye, less subbasal corneal nerve damage, and no flap-related complications.15–17 Ocular response analyzer and dynamic high-speed Scheimpflug imaging studies have shown that SMILE may better preserve corneal biomechanical properties than LASIK,18,19 and, thus, may have a lower ectasia risk. However, ectasia has been reported following SMILE.20,21 This area clearly requires further research.
Collagen cross-linking, first reported in a clinical study in 2003, aims to restore the stiffness of a pathologic ectatic cornea,22 and has an excellent record in halting progression of ectasia in keratoconus12 and post-LASIK ectasia.13,23 Recently, CXL has been combined with either LASIK or SMILE to reduce the risk of postoperative keratectasia;24,25 these procedures have been termed LASIK Xtra and SMILE Xtra, respectively. Concomitant CXL may potentially strengthen the cornea following keratorefractive surgery and make up for the biomechanical weakening caused by either LASIK or SMILE. Although evidence to support LASIK Xtra and SMILE Xtra is emerging,24–26 to our knowledge there is no proof of the biomechanical benefit of concomitant CXL.
We previously established an animal model of post-LASIK ectasia, with which ectasia can be induced in the rabbit cornea following a −5 diopter (D) LASIK treatment.27 In the current study, using this animal model of ectasia, we investigated whether SMILE was associated with less ectasia than LASIK, and whether concomitant CXL with SMILE or LASIK was protective compared to the standard procedures.
Methods
Animals and Study Design
Fourteen New Zealand white rabbits (3–4 kg body weight) were procured from the National University of Singapore and housed at Singapore Eye Research Institute, as described previously.27–29 They were treated according to guidelines of the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmic and Vision Research. The study was approved by the institutional animal care and use committee of SingHealth (ref 2014/SHS/900).
The rabbits were anesthetized preoperatively and during examinations with xylazine hydrochloride (5 mg/kg intramuscularly; Troy Laboratories, Smithfield, Australia) and ketamine hydrochloride (50 mg/kg intramuscularly; Parnell Laboratories, Alexandria, Australia). At conclusion of the study, they were euthanized under anesthesia by overdose with intracardiac injection of sodium pentobarbitone (Jurox, Rutherford, Australia).
We previously established that ectasia can be induced in the rabbit cornea following −5 D LASIK treatment.27 Using controlled laboratory conditions, we compared ectasia development between −5 D LASIK and −5 D SMILE treatments and also investigated the effect of concomitant CXL. The rabbit eyes were allocated randomly to four surgical groups. After exclusion of one rabbit that suffered corneal infection, SMILE was performed on six eyes, SMILE Xtra on five eyes, LASIK on six eyes, and LASIK Xtra on five eyes. Bilateral surgery was permitted, as LASIK and SMILE do not disrupt the animals' daily activities by causing visual disability. The rabbits were examined under anesthesia preoperatively, and 2, 4, and 6 weeks postoperatively.
Surgery
LASIK and SMILE Procedure to Induce Ectasia
The SMILE and LASIK flap procedures were performed with the 500 kHz Visumax Femtosecond Laser (VisuMax, Carl Zeiss Meditec, Jena, Germany) as described previously.27–29 In brief, the laser settings for SMILE were 120 μm cap thickness, 7.5 mm cap diameter, 6.5 mm lenticule diameter, and 170 nJ laser energy. The spot distance and tracking spacing were 4.5 μm for the cap and lenticule and 2.5 μm for the side cuts; the side cut angle was 90°. The incision was positioned at 120° and was 2.5 mm wide. The maximum lenticule thickness was 94 μm. The LASIK flap settings were 120 μm thickness, 7.9 mm diameter, 170 nJ laser energy; spot distance and tracking spacing were 4.8 and 4.8 μm, respectively, for the lamellar cut, and 2 and 2 μm, respectively, for the flap side cut. Once the flap was lifted, a 6.5 mm optical zone was ablated using a Technolas excimer laser (Technolas; Bausch & Lomb, Rochester, NY); settings were spot size 2.0 mm diameter, fluence 120 mJ/cm2, and repetition rate 50 Hz. The maximum ablation depth was 101 μm. Finally, the LASIK flap and eyelids were sutured with 10-0 nylon and 6-0 silk sutures, respectively. The sutures were removed 4 days later.
Collagen Cross-Linking in SMILE Xtra and LASIK Xtra
The Vibex Xtra protocol was used for cases with concomitant CXL. After lenticule extraction for SMILE Xtra and excimer ablation for LASIK Xtra cases, Vibex Xtra (Avedro, Inc., Waltham, MA) 0.25% riboflavin with saline drops was applied in the pocket or the stromal bed, respectively, for 60 seconds. The excess nonabsorbed riboflavin then was rinsed off with balanced salt solution (BSS). The flap then was repositioned in LASIK Xtra cases and the wounds dried in position for LASIK Xtra and SMILE Xtra cases. Ultraviolet A (UVA) radiation was applied with the Avedro KXL system (Avedro, Inc.) with an intensity of 30 mW/cm2 for 90 seconds, delivering a total 2.7 Joules/cm2 energy. Finally, the LASIK flaps were sutured and the lids closed temporarily, as described above.
Investigations
A lid speculum was used to keep the rabbit eye open during corneal imaging and the cornea was kept wet regularly with BSS.
Slit Lamp Biomicroscopy Photography
Slit-lamp photos (Righton, Tokyo, Japan) were taken preoperatively, and on day 1 and weeks 2, 4, and 6 postoperatively. They were examined for the presence of conjunctival redness, corneal infiltration, and corneal scarring.
Anterior Segment Optical Coherence Tomography (AS-OCT)
Three anterior segment AS-OCT scans (RTVue; Optovue, Inc., Fremont, CA) were performed at each time point through the center of the cornea at the 180° axis, preoperatively and at weeks 2, 4, and 6 postoperatively. Corneal thickness (CT) was measured in the center of each AS-OCT image. The mean CT of the three measurements was calculated and then analyzed serially in the postoperative period.
Corneal Topography
ATLAS and Visante Omni (Carl Zeiss Meditec) topography were performed preoperatively and at weeks 2, 4, and 6 postoperatively. Three scans were performed each time and the mean value of the three measurements was calculated. Mean simulated keratometry (K) values, measured in D, were examined for change in the postoperative period and also compared among the four surgical groups. Astigmatism, also measured in D, was calculated from the simulated K values and examined for serial change and among the surgical groups.
The maximum posterior elevation (MPE), measured in μm, was recorded on posterior surface topography and examined for serial change with time, and compared among the four surgical groups. Minimum CT, recorded from the pachymetry maps, also was examined serially and between groups.
In Vivo Confocal Microscopy
In vivo confocal microscopy (IVCM) was performed with the corneal module of the HRT3 (Heidelberg Engineering GmbH, Heidelberg, Germany) 2 weeks postoperatively. A carbomer gel was applied on the confocal lens and used as the immersion fluid.
Each cornea was examined centrally with a z-axis scan throughout the entire corneal thickness. Three areas of the cornea were selected for reflectivity analysis: the laser interface, 20 to 30 μm above the interface, and 20 to 30 μm below the interface. Keratocytes per frame were counted at a depth of 50 to 60 μm below the interface. At each examination, three micrographs were selected for analysis and mean values were calculated. Reflectivity was analyzed by semi-quantifying the mean gray value of reflectivity using Image J (available in the public domain at http://imagej.nih.gov/ij/; National Institutes of Health, Bethesda, MD).29 Mean values were calculated and normalized to the mean value of controls.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Data normality was assessed by Shapiro-Wilk statistics and histograms. All data showed a normal distribution; statistical comparison among different groups was performed using 1-way analysis of variance (ANOVA), paired t-tests and Bonferroni post hoc analysis. Statistical significance was P < 0.05; analysis was performed with StatPlus:mac (AnalystSoft, Inc., Walnut, CA) for Mac OS (Version v6). Comparisons among the four groups are presented consistently in the order: SMILE, SMILE Xtra, LASIK, and LASIK Xtra.
Results
Anterior Surface Topography
Keratometry Values
Mean (SD) K values decreased following all four procedures (Table 1). There was a significant difference in preoperative K values among SMILE, SMILE Xtra, LASIK, and LASIK Xtra (44.78 [1.95] vs. 47.94 [1.18] vs. 43.52 [1.04] vs. 43.44 [1.22] D; P < 0.001). SMILE Xtra had the greatest K values (Bonferroni P < 0.001). At weeks 2, 4, and 6, the reduction in K values compared to preoperative values was significantly different among the four groups (week 6, −3.67 [1.35] vs. −5.23 [0.50] vs. −1.82 [1.71] vs. −2.26 [1.90] D; P < 0.001). SMILE Xtra showed a greater reduction than LASIK (P < 0.001) and LASIK Xtra (P = 0.02). At week 6, there was no significant difference in K values among the groups (41.11 [0.95] vs. 42.71 [0.80] vs. 41.70 [1.21] vs. 41.18 [1.46] D; P = 0.07).
Table 1.
Change in K values, Posterior Elevation, and Corneal Thickness Following SMILE, SMILE Xtra, LASIK, and LASIK Xtra
|
Parameter |
Simulated K Values, D |
Reduction in K Values, D |
MPE, μm |
Minimum CT, μm |
Central CT, μm |
| SMILE | |||||
| SMILE Preoperative | 44.78 (1.95) | 7.77 (1.48) | 347.95 (21.70) | 373.19 (42.34) | |
| SMILE week 2 | 42.10 (1.41) | −2.67 (0.92) | 17.73 (5.77) | 309.7 (36.6) | 308.9 (43.6) |
| SMILE week 4 | 41.69 (1.44) | −3.09 (1.04) | 15.44 (8.11) | 287.22 (31.83) | 306.7 (41.97) |
| SMILE week 6 | 41.11 (0.95) | −3.67 (1.35) | 18.77 (5.03) | 292.33 (25.46) | 311 (37.6) |
| P value | <0.001 | NA | <0.001 | <0.001 | 0.02 |
| SMILE Xtra | |||||
| SMILE Xtra Preoperative | 47.94 (1.18) | 8.54 (1.96) | 339.12 (22.75) | 355.13 (21.13) | |
| SMILE Xtra week 2 | 43.91 (1.02) | −4.04 (0.91) | 14.44 (6.01) | 275.72 (20.56) | 291.2 (26.0) |
| SMILE Xtra week 4 | 43.21 (0.63) | −4.73 (1.11) | 12.71 (2.38) | 283.54 (23.25) | 314.87 (31.46) |
| SMILE Xtra week 6 | 42.71 (0.80) | −5.23 (0.50) | 17.76 (7.67) | 298.52 (18.02) | 314.5 (34.4) |
| P value | <0.001 | NA | 0.04 | <0.001 | <0.001 |
| LASIK | |||||
| LASIK Preoperative | 43.52 (1.04) | 8.09 (2.32) | 368.67 (27.51) | 388.87 (42.59) | |
| LASIK week 2 | 42.79 (1.21) | −0.74 (1.15) | 22.75 (5.05) | 315.37 (40.02) | 329.8 (40.4) |
| LASIK week 4 | 42.16 (1.13) | −1.36 (1.04) | 19.43 (8.54) | 298.75 (31.49) | 309.95 (33.77) |
| LASIK week 6 | 41.70 (1.21) | −1.82 (1.71) | 20.25 (4.70) | 300.15 (31.25) | 340.8 (52.4) |
| P value | 0.06 | NA | <0.001 | <0.001 | 0.03 |
| LASIK Xtra | |||||
| LASIK Xtra Preoperative | 43.44 (1.22) | 9.34 (1.19) | 341.28 (20.27) | 367.43 (39.23) | |
| LASIK Xtra week 2 | 42.05 (2.05) | −1.39 (2.09) | 13.39 (3.05) | 266.58 (18.17) | 285.8 (34.0) |
| LASIK Xtra week 4 | 40.77 (0.85) | −2.67 (0.87) | 14.08 (2.81) | 269.18 (16.98) | 288.43 (28.01) |
| LASIK Xtra week 6 | 41.18 (1.46) | −2.26 (1.90) | 11.59 (4.57) | 281.18 (20.08) | 307 (36) |
| P value | 0.05 | NA | 0.12 | <0.001 | 0.04 |
NA, not applicable.
Astigmatism
Preoperative corneal astigmatism was not significantly different among the groups (1.41 [0.59] vs. 1.51 [0.60] vs. 1.33 [0.63] vs. 0.86 [0.34] D; P = 0.26). There was no significant difference among the groups at week 4 (1.99 [1.41] vs. 1.40 [0.66] vs. 2.20 [1.25] vs. 2.18 [1.39] D; P = 0.65) and week 6 (1.89 [0.85] vs. 1.13 [0.25] vs. 2.06 [0.72] vs. 2.00 [1.04] D; P = 0.22). Astigmatism did not change with time in any group.
Posterior Surface Topography
Following SMILE, MPE was significantly different between preoperatively and weeks 2, 4, and 6 (7.77 [1.48] vs. 17.73 [5.77] vs. 15.44 [8.11] vs. 18.77 [5.03] μm; P < 0.001). Bonferroni post hoc analysis compared to preoperatively showed a significant increase at week 6 (P = 0.04). Following SMILE Xtra, there also was a significant change in MPE (8.54 [1.96] vs. 14.44 [6.01] vs. 12.71 [2.38] vs. 17.76 [7.67] μm; P = 0.04). The MPE increase became significant at week 6 (P = 0.04).
A significant increase was detected following LASIK (8.09 [2.32] vs. 22.75 [5.05] vs. 19.43 [8.54] vs. 20.25 [4.70] μm; P < 0.001). Bonferroni analysis compared to preoperatively showed a significant increase at week 2 (P = 0.01). Finally, there was no significant difference following LASIK Xtra (9.34 [1.19] vs. 13.39 [3.05] vs. 14.08 [2.81] vs. 11.59 [4.57] μm; P = 0.12). Post hoc analysis did not show any significant change compared to preoperatively.
The increase in MPE following the four procedures is illustrated in Figure 1.
Figure 1.
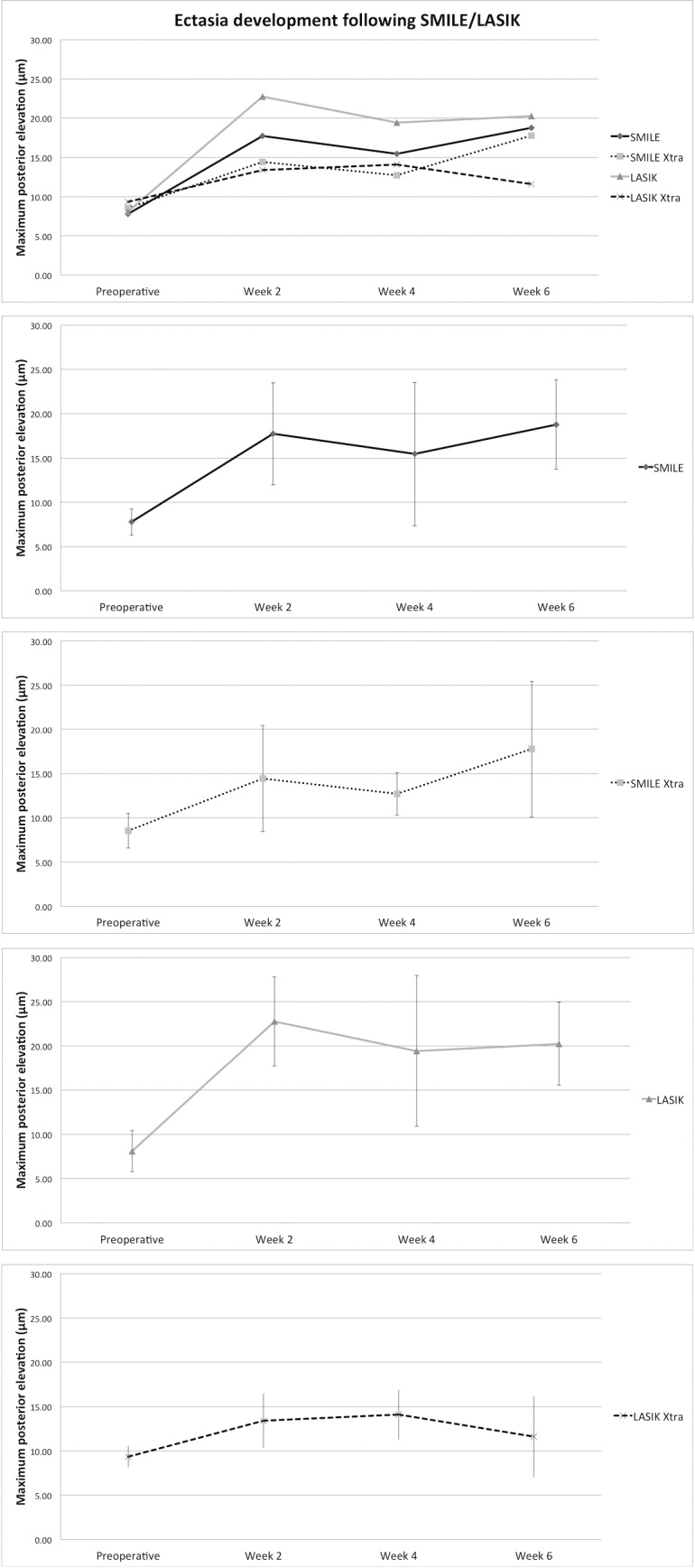
MPE following SMILE, SMILE Xtra, LASIK, and LASIK Xtra. LASIK cases had the greatest posterior elevation postoperatively, whereas LASIK Xtra and SMILE Xtra had the smallest.
There was no significant difference in MPE between SMILE and LASIK at weeks 2, 4, and 6 (P = 0.13, 0.41, and 0.60. respectively) or at any time point comparison. The MPE was significantly lower following LASIK Xtra than LASIK at weeks 2 (P < 0.001) and 6 (P = 0.01), but not at week 4 (P = 0.22); it also was significantly lower for LASIK Xtra compared to SMILE at week 6 (P = 0.03). There was no significant MPE difference between SMILE and SMILE Xtra, nor between SMILE Xtra and LASIK Xtra cases at any time point.
Corneal Thickness
Following SMILE, minimum CT decreased significantly (P < 0.001); the reduction became significant at weeks 4 (Bonferroni P < 0.001) and 6 (P = 0.02).
Following SMILE Xtra, LASIK, and LASIK Xtra, minimum CT also decreased significantly (P < 0.001); the reduction was significant at weeks 2 and 4 (week 4 Bonferroni, P < 0.001 for SMILE Xtra, LASIK Xtra; P ≤ 0.04 for LASIK), but borderline significant for the SMILE Xtra group at week 6 (P = 0.11 for SMILE Xtra; P = 0.01 for LASIK, and P < 0.001 for LASIK Xtra). Postoperative corneal thickness change is shown in Table 1 and Figure 2.
Figure 2.
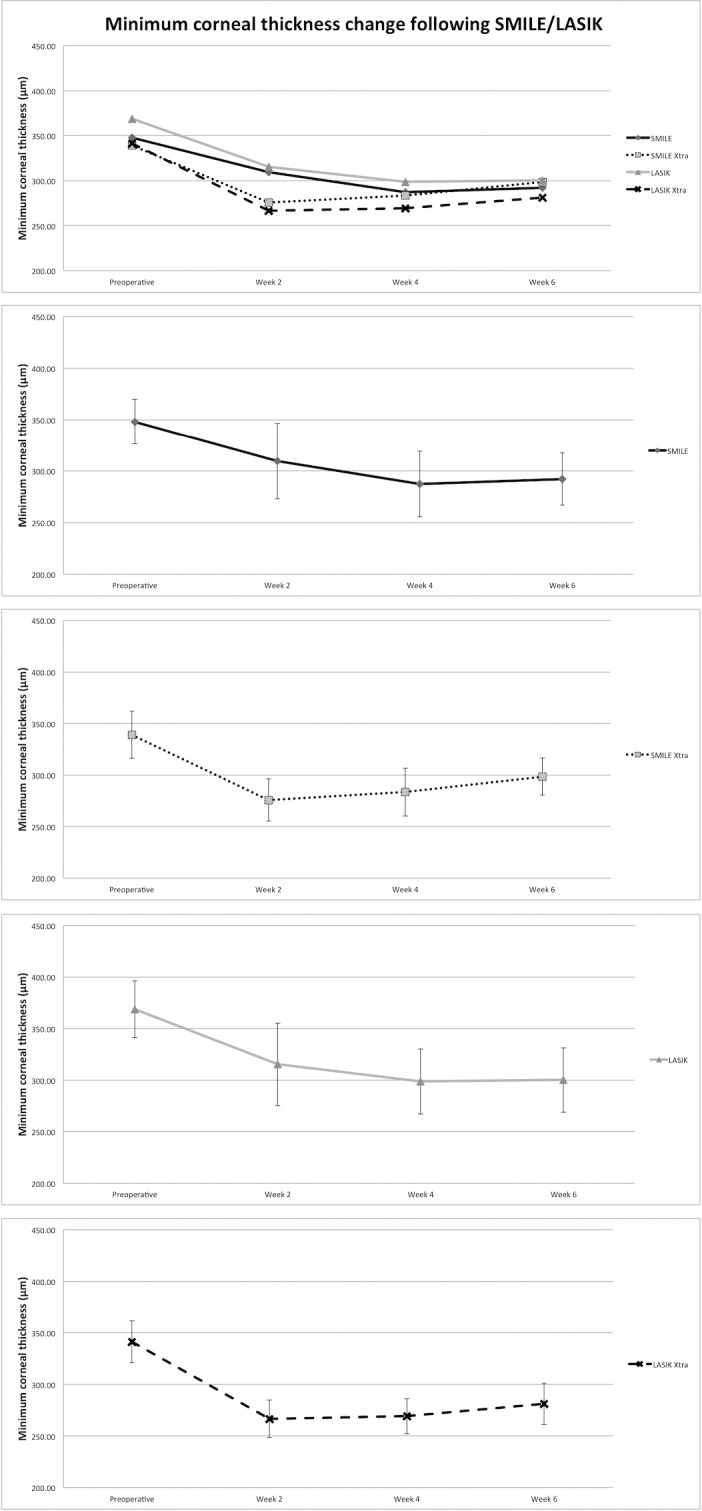
Minimum CT following SMILE, SMILE Xtra, LASIK, and LASIK Xtra.
There was no significant difference in preoperative minimum CT among the surgical groups (347.95 [21.70] vs. 339.12 [22.75] vs. 368.67 [27.51] vs. 341.28 [20.27] μm; P = 0.18). At week 2, there was a borderline difference in minimum CT among the groups (P = 0.07), but post hoc analysis did not show significant differences between specific groups. At weeks 4 and 6, there was no significant difference between the groups.
There was no significant difference in preoperative mean (SD) central CT among SMILE, SMILE Xtra, LASIK, and LASIK Xtra (373.19 [42.34] vs. 355.13 [21.13] vs. 388.87 [42.59] vs. 367.43 [39.23] μm; P = 0.49). There was a significant reduction in central CT following all four surgical procedures (P = 0.02, P < 0.001, P = 0.03, and P = 0.04, respectively) (Table 1, Fig. 3). There was no significant difference in central CT among SMILE, SMILE Xtra, LASIK, and LASIK Xtra at weeks 2 (P = 0.24), 4 (P = 0.77), and 6 (311 [37.6] vs. 314.5 [34.4] vs. 340.8 [52.4] vs. 307 [36] μm; P = 0.60).
Figure 3.
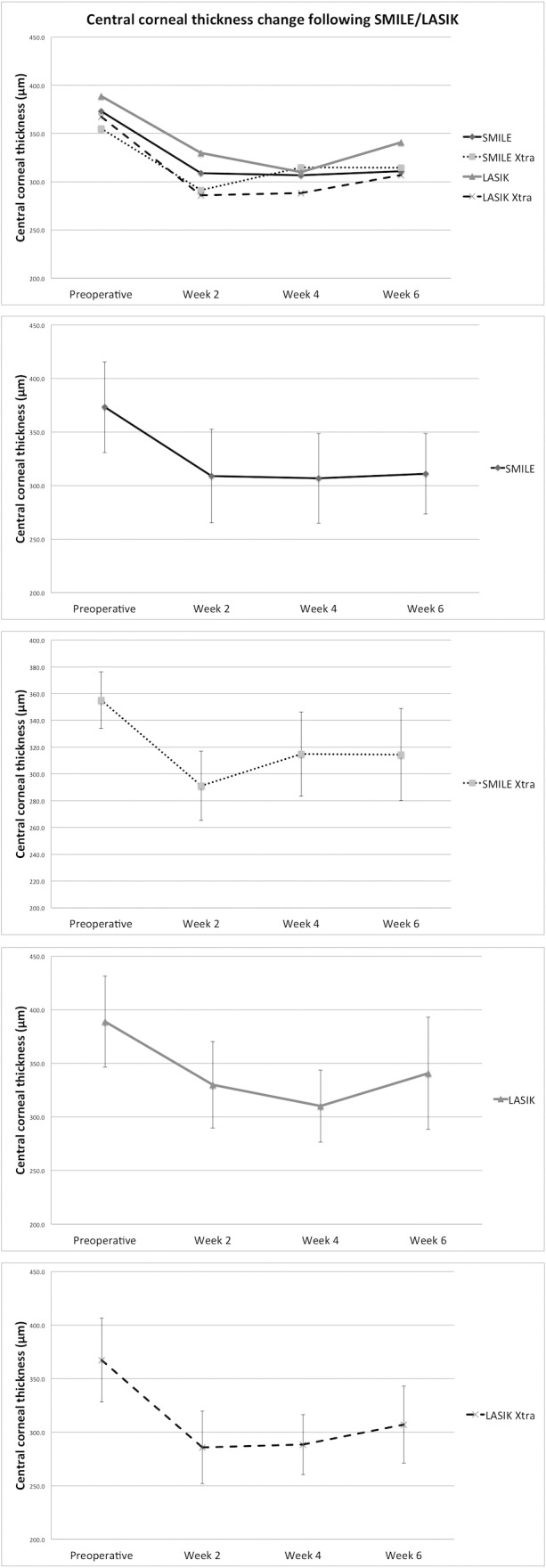
Central CT following SMILE, SMILE Xtra, LASIK, and LASIK Xtra. LASIK Xtra and SMILE Xtra had the thinnest central cornea in the postoperative period, although the difference was not statistically significant.
In Vivo Confocal Microscopy
The interface showed the greatest reflectivity, with no difference in reflectivity scores among the four groups (P = 0.63). Reflectivity anterior to the interface was less than at the interface. SMILE showed the least reflectivity and LASIK Xtra the greatest. Reflectivity scores were lower in SMILE than LASIK (P = 0.05), and also in SMILE than LASIK Xtra (P = 0.05).
The stroma posterior to the interface showed the least reflectivity (Figs. 4, 5). A significant difference was detected only among SMILE Xtra and LASIK Xtra, reflectivity being greater in SMILE Xtra (P = 0.02). The keratocytes per frame, measured 50 to 60 μm below the interface, were least in the LASIK Xtra group (SMILE vs. SMILE Xtra vs. LASIK vs. LASIK Xtra, 24 [4] vs. 22 [2.83] vs. 18.5 [6.36] vs. 13.5 [7.05], P = 0.10); a borderline significant difference was present between SMILE and LASIK Xtra (P = 0.07).
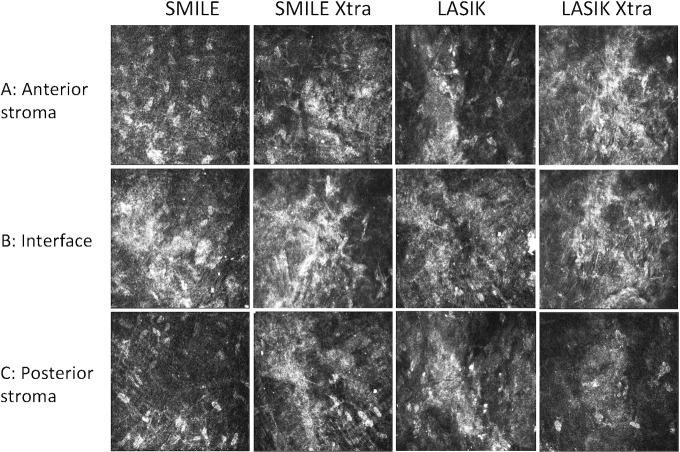
Figure 4.
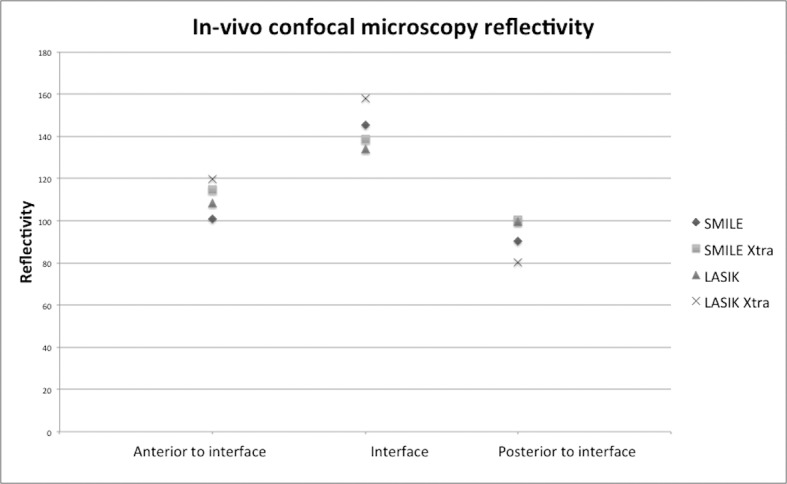
In vivo confocal microscopy reflectivity at the laser interface, anterior to the interface, and posterior to the interface 2 weeks following SMILE, SMILE Xtra, LASIK, and LASIK Xtra. The reflectivity was greatest for all procedures at the interface and the least posterior to the interface.
Figure 5.
Representative in vivo confocal microscopy images following SMILE, SMILE Xtra, LASIK, and LASIK Xtra. (A) Reflectivity 20 to 30 μm above the interface was greatest following LASIK Xtra and least following SMILE. (B) The interface was acellular and small bright particles, which may represent inflammatory cells, were observed following all four procedures. The interface was characteristically more reflective than the anterior and posterior stroma. (C) The stroma 20 to 30 μm deep to the interface had a low keratocyte population in LASIK Xtra; the stromal architecture was relatively normal in SMILE.
Discussion
Using an established post-LASIK ectasia model,27 we found that SMILE and SMILE Xtra also demonstrated a significant increase in posterior surface elevation with the same −5 D myopic correction; LASIK Xtra did not. The largest elevation was observed following LASIK (22.75 μm), and the least following LASIK Xtra (14.08 μm) and SMILE Xtra (17.76 μm).
Ectasia developed in LASIK and SMILE cases; following LASIK, the MPE became significantly increased at week 2 compared to week 6 for SMILE cases. Although the difference was not statistically significant, SMILE showed a smaller MPE than LASIK (17.73 vs. 22.75 μm at week 2). This was a consistent finding at weeks 2, 4, and 6, despite preoperative corneal thickness being greater in the LASIK group (368.67 vs. 347.95 μm). The postoperative status at week 2 most likely reflects the true biomechanical weakening effect of surgery, as in this early phase the weakening effect would be expected to be decoupled from the healing response. Although the long-term effect of healing on ectasia is not known and the small number of cases may limit statistical analysis, our findings suggest that SMILE may have less potential for inducing ectasia than LASIK.
Ocular response analyzer studies have shown that corneal hysteresis, a parameter of viscous damping, and corneal resistance factor, a parameter indicative of overall viscous damping and elastic resistance, decrease following SMILE and LASIK.18,30,31 Although no significant postoperative difference has been shown between SMILE and LASIK,30 two studies have found a greater decrease in corneal hysteresis and corneal resistance factor following LASIK than SMILE for cases with a spherical equivalent greater than −6 D.18,31 A further study showed that the percentage reduction in corneal hysteresis and corneal resistance factor was greater following LASIK than SMILE.17 These studies suggest a biomechanical advantage to SMILE, which could translate into a smaller ectasia risk, as found in our rabbit study.
Postoperative deformation amplitude and applanation time, measured with dynamic high-speed Scheimpflug imaging, have been shown not to be different between the two procedures,19,32 although increased and reduced, respectively, following SMILE and LASIK.32,33 Shetty et al.,33 in support of a preferential biomechanical recovery or healing response for SMILE, found that corneal deformation with higher forces returned to near preoperative levels by month 6 following SMILE, but not following LASIK. In addition, Mastropasqua et al.34 found no significant postoperative change in deformation amplitude and applanation time 30 and 90 days following SMILE. A biomechanical advantage may be inferred for SMILE performed on healthy corneas; however, reports of post-SMILE ectasia have shown that a biomechanically weak cornea, such as in forme fruste or manifest keratoconus, can suffer ectasia.20,21
LASIK Xtra cases consistently maintained a significantly lower MPE than LASIK cases. Although SMILE Xtra cases also showed a lower MPE than SMILE at all time points, the difference was not significant. The small number of study cases may be a contributing factor, potentially unmasking small inherent differences in corneal elasticity among the groups. Thus, concomitant CXL may reduce the risk of post-keratorefractive surgery ectasia, due to a biomechanical stabilizing effect on the cornea. This stabilizing effect was similarly manifest in LASIK Xtra and SMILE Xtra from week 2 postoperatively and up to week 4. At week 6, the SMILE Xtra group had an increase in MPE that was not significantly different to LASIK Xtra. However, as our study aimed to induce ectasia and was time-limited in follow-up, the long-term biomechanical benefit of concomitant CXL cannot be presumed. Clinical studies on LASIK Xtra and SMILE Xtra have been promising. In a randomized comparison study, unaided and corrected visual acuities at 6 months were identical in LASIK and LASIK Xtra, although 4.16% of cases in the LASIK Xtra group lost one or more lines of corrected distance visual acuity (CDVA) due to haze.35 Two independent studies, however, have shown that LASIK Xtra was not associated with visual loss.24,26 Similarly for SMILE Xtra, one study found that 33% of cases lost 1 line of CDVA at 6 months,36 whereas Ganesh et al.25 showed no loss of CDVA at 12 months. Larger prospective studies are required to investigate the effect of concomitant CXL on vision, safety and refractive outcomes. Our findings, however, suggested that biomechanically it has an important stabilizing effect on the cornea.
Corneal thickness decreased significantly in the postoperative period for all procedures, as removal of the lenticule in SMILE and excimer ablation in LASIK result in tissue loss. The SMILE Xtra and LASIK Xtra groups had a lower postoperative corneal thickness than the SMILE and LASIK groups, respectively. It is well documented that CXL is associated with an immediate postoperative reduction in corneal thickness and a gradual return toward baseline in the subsequent 3 to 12 months.12 Stromal compaction, dehydration, or epithelial changes may account for this.37
Confocal microscopy showed that the interface of all four procedures was hypocellular and with similar levels of increased reflectivity 2 weeks postoperatively. LASIK Xtra, and to an extent SMILE Xtra, had less keratocytes than standard LASIK and SMILE 50 to 60 μm deep to the interface, most likely due to the apoptotic effect of CXL on keratocytes; this may account for the lowest posterior stromal reflectivity observed following LASIK Xtra. We found that SMILE had less anterior stromal reflectivity than LASIK and LASIK Xtra. SMILE has been shown, with IVCM and immunohistochemistry, to induce less stromal inflammation than LASIK.38 LASIK Xtra and SMILE Xtra have not been studied in detail with IVCM; however a hypocellular interface and keratocyte apoptosis to a depth of 60 μm below the interface have been found following LASIK Xtra and SMILE Xtra.39,40
In conclusion, to our knowledge this is the first study to show that, for the same myopic refractive correction, SMILE may have less potential than LASIK to induce ectasia. Concomitant CXL in this biological animal model had a protective effect, as LASIK Xtra cases showed the least potential for ectasia. Combined with the novelty of the procedure, this exciting finding advocates further clinical research to investigate the refractive effect, haze development, and safety of LASIK and SMILE when combined with CXL.
Acknowledgments
Supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Programme (NMRC/TCR/008-SERI/2013) and administered by the Singapore Ministry of Health's National Medical Research Council.
Disclosure: A. Konstantopoulos, None; Y.-C. Liu, None; E.P. Teo, None; C.L. Nyein, None; G.H. Yam, None; J.S. Mehta, None
References
- 1.Shortt AJ, Allan BD, Evans JR. Laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia. Cochrane Database Syst Rev. 2013;31:CD005135. doi: 10.1002/14651858.CD005135.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Wen D, McAlinden C, Flitcroft I, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. 2017;178:65–78. doi: 10.1016/j.ajo.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and satisfaction of patients in the patient-reported outcomes with laser in situ keratomileusis (PROWL) studies. JAMA Ophthalmol. 2017;135:13–22. doi: 10.1001/jamaophthalmol.2016.4587. [DOI] [PubMed] [Google Scholar]
- 4.Ortega-Usobiaga J, Llovet-Osuna F, Djodeyre MR, Llovet-Rausell A, Beltran J, Baviera J. Incidence of corneal infections after laser in situ keratomileusis and surface ablation when moxifloxacin and tobramycin are used as postoperative treatment. J Cataract Refract Surg. 2015;41:1210–1216. doi: 10.1016/j.jcrs.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Llovet F, de Rojas V, Interlandi E, et al. Infectious keratitis in 204 586 LASIK procedures. Ophthalmology. 2010;117:232–238. doi: 10.1016/j.ophtha.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1796–1802. doi: 10.1016/s0886-3350(01)01090-2. [DOI] [PubMed] [Google Scholar]
- 7.Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. 2004;20:718–722. doi: 10.3928/1081-597X-20040903-18. [DOI] [PubMed] [Google Scholar]
- 8.Moshirfar M, Smedley JG, Muthappan V, Jarsted A, Ostler EM. Rate of ectasia and incidence of irregular topography in patients with unidentified preoperative risk factors undergoing femtosecond laser-assisted LASIK. Clin Ophthalmol. 2014;8:35–42. doi: 10.2147/OPTH.S53370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knox Cartwright NE, Tyrer JR, Jaycock PD, Marshall J. Effects of variation in depth and side cut angulations in LASIK and thin-flap LASIK using a femtosecond laser: a biomechanical study. J Refract Surg. 2012;28:419–425. doi: 10.3928/1081597X-20120518-07. [DOI] [PubMed] [Google Scholar]
- 10.Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg. 2011;37:127–137. doi: 10.1016/j.jcrs.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Vestergaard A, Ivarsen AR, Asp S, Hjortdal JØ. Small-incision lenticule extraction for moderate to high myopia: predictability, safety, and patient satisfaction. J Cataract Refract Surg. 2012;38:2003–2010. doi: 10.1016/j.jcrs.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121:812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Tong JY, Viswanathan D, Hodge C, Sutton G, Chan C, Males JJ. Corneal collagen crosslinking for post-LASIK ectasia: an Australian study. Asia Pac J Ophthalmol (Phila) 2017;6:228–232. doi: 10.22608/APO.2016197. [DOI] [PubMed] [Google Scholar]
- 14.Reinstein DZ, Archer TJ, Gobbe M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye Vis (Lond) 2014;1:3. doi: 10.1186/s40662-014-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denoyer A, Landman E, Trinh L, Faure JF, Auclin F, Baudouin C. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. 2015;122:669–676. doi: 10.1016/j.ophtha.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed-Noriega K, Riau AK, Lwin NC, Chaurasia SS, Tan DT, Mehta JS. Early corneal nerve damage and recovery following small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK) Invest Ophthalmol Vis Sci. 2014;55:1823–1834. doi: 10.1167/iovs.13-13324. [DOI] [PubMed] [Google Scholar]
- 17.Osman IM, Helaly HA, Abdalla M, Shousha MA. Corneal biomechanical changes in eyes with small incision lenticule extraction and laser assisted in situ keratomileusis. BMC Ophthalmol. 2016;16:123. doi: 10.1186/s12886-016-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Liu M, Chen Y, et al. Differences in the corneal biomechanical changes after SMILE and LASIK. J Refract Surg. 2014;30:702–707. doi: 10.3928/1081597X-20140903-09. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Chen Z, Knorz MC, Li M, Zhao J, Zhou X. Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J Refract Surg. 2014;30:310–318. doi: 10.3928/1081597X-20140422-01. [DOI] [PubMed] [Google Scholar]
- 20.El-Naggar MT. Bilateral ectasia after femtosecond laser-assisted small-incision lenticule extraction. J Cataract Refract Surg. 2015;41:884–888. doi: 10.1016/j.jcrs.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Cui C, Li Z, et al. Corneal ectasia 6.5 months after small-incision lenticule extraction. J Cataract Refract Surg. 2015;41:1100–1106. doi: 10.1016/j.jcrs.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 23.Yildirim A, Cakir H, Kara N, et al. Corneal collagen crosslinking for ectasia after laser in situ keratomileusis: long-term results. J Cataract Refract Surg. 2014;40:1591–1596. doi: 10.1016/j.jcrs.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Seiler TG, Fischinger I, Koller T, Derhartunian V, Seiler T. Superficial corneal crosslinking during laser in situ keratomileusis. J Cataract Refract Surg. 2015;41:2165–2170. doi: 10.1016/j.jcrs.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Ganesh S, Brar S. Clinical outcomes of small incision lenticule extraction with accelerated cross-linking (ReLEx SMILE Xtra) in patients with thin corneas and borderline topography. J Ophthalmol. 2015;2015:263412. doi: 10.1155/2015/263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanellopoulos AJ, Asimellis G. Combined laser in situ keratomileusis and prophylactic high-fluence corneal collagen crosslinking for high myopia: two-year safety and efficacy. J Cataract Refract Surg. 2015;41:1426–1433. doi: 10.1016/j.jcrs.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 27.Liu YC, Konstantopoulos A, Riau AK, et al. Repeatability and reproducibility of corneal biometric measurements using the visante omni and a rabbit experimental model of post-surgical corneal ectasia. Transl Vis Sci Technol. 2015;4:16. doi: 10.1167/tvst.4.2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YC, Teo EP, Lwin NC, Yam GH, Mehta JS. Early corneal wound healing and inflammatory responses after SMILE: comparison of the effects of different refractive corrections and surgical experiences. J Refract Surg. 2016;32:346–353. doi: 10.3928/1081597X-20160217-05. [DOI] [PubMed] [Google Scholar]
- 29.Riau AK, Liu YC, Lwin NC, et al. Comparative study of nJ- and μJ-energy level femtosecond lasers: evaluation of flap adhesion strength, stromal bed quality, and tissue responses. Invest Ophthalmol Vis Sci. 2014;55:3186–3194. doi: 10.1167/iovs.14-14434. [DOI] [PubMed] [Google Scholar]
- 30.Agca A, Ozgurhan EB, Demirok A, et al. Comparison of corneal hysteresis and corneal resistance factor after small incision lenticule extraction and femtosecond laser-assisted LASIK: a prospective fellow eye study. Cont Lens Anterior Eye. 2014;37:77–80. doi: 10.1016/j.clae.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Zhang Z, Naidu RK, et al. Comparison of the change in posterior corneal elevation and corneal biomechanical parameters after small incision lenticule extraction and femtosecond laser-assisted LASIK for high myopia correction. Cont Lens Anterior Eye. 2016;39:191–196. doi: 10.1016/j.clae.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Sefat SM, Wiltfang R, Bechmann M, Mayer WJ, Kampik A, Kook D. Evaluation of changes in human corneas after femtosecond laser-assisted LASIK and small-incision lenticule extraction (SMILE) using non-contact tonometry and ultra-high-speed camera (Corvis ST) Curr Eye Res. 2016;41:917–922. doi: 10.3109/02713683.2015.1082185. [DOI] [PubMed] [Google Scholar]
- 33.Shetty R, Francis M, Shroff R, et al. Corneal biomechanical changes and tissue remodeling after SMILE and LASIK. Invest Ophthalmol Vis Sci. 2017;58:5703–5712. doi: 10.1167/iovs.17-22864. [DOI] [PubMed] [Google Scholar]
- 34.Mastropasqua L, Calienno R, Lanzini M, et al. Evaluation of corneal biomechanical properties modification after small incision lenticule extraction using Scheimpflug-based noncontact tonometer. Biomed Res Int. 2014;2014:290619. doi: 10.1155/2014/290619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Tian L, Wang LQ, Huang YF. Efficacy and Safety of LASIK combined with accelerated corneal collagen cross-linking for myopia: six-month study. Biomed Res Int. 2016;2016:5083069. doi: 10.1155/2016/5083069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng AL, Chan TC, Cheng GP, et al. Comparison of the early clinical outcomes between combined small-incision lenticule extraction and collagen cross-linking versus SMILE for myopia. J Ophthalmol. 2016;2016:2672980. doi: 10.1155/2016/2672980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontadakis GA, Ginis H, Karyotakis N, et al. In vitro effect of corneal collagen cross-linking on corneal hydration properties and stiffness. Graefes Arch Clin Exp Ophthalmol. 2013;251:543–547. doi: 10.1007/s00417-012-2082-9. [DOI] [PubMed] [Google Scholar]
- 38.Riau AK, Angunawela RI, Chaurasia SS, Lee WS, Tan DT, Mehta JS. Early corneal wound healing and inflammatory responses after refractive lenticule extraction (ReLEx) Invest Ophthalmol Vis Sci. 2011;52:6213–6221. doi: 10.1167/iovs.11-7439. [DOI] [PubMed] [Google Scholar]
- 39.Mazzotta C, Balestrazzi A, Traversi C, Caragiuli S, Caporossi A. In vivo confocal microscopy report after LASIK with sequential accelerated corneal collagen cross-linking treatment. Case Rep Ophthalmol. 2014;5:125–131. doi: 10.1159/000362327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Liu M, Zhang T, et al. In vivo confocal laser microscopy of morphologic changes after small incision lenticule extraction with accelerated cross-linking (SMILE Xtra) in patients with thin corneas and high myopia. Graefes Arch Clin Exp Ophthalmol. 2018;256:199–207. doi: 10.1007/s00417-017-3811-x. [DOI] [PubMed] [Google Scholar]