The authors provide an overview of pharmacological and psychosocial behavioral treatments for patients with cannabis use disorder, emphasizing aspects of clinical management unique to this patient population.
Abstract
Although the opioid epidemic may command more national headlines, the past two decades have seen a revolution in cannabis policy driven largely by the public and by business interests. As a result, the population of cannabis users nationwide—especially of heavy users—has grown yearly. Although less visible than patients admitted to the emergency department for opioid-related overdose or to treatment for opioid use disorder, an estimated 4.5–7 million individuals in the United States are thought to meet criteria for cannabis use disorder in a given year. This article focuses on the developmental pathways of cannabis use disorder, the epidemiology of cannabis use among adolescents and adults in the context of rapidly changing state laws, and evidence-based treatment for the general psychiatrist. The authors provide an overview of pharmacological and psychosocial behavioral treatments for patients with the disorder while emphasizing aspects of clinical management unique to this patient population.
Cannabis is the most commonly used illicit drug in the world, and in the United States, it is increasingly becoming a commonly used legal drug, at least under an expanding array of state-level laws. As of the November 2018 election, 10 states and Washington, DC, have passed laws allowing both medical and recreational use of cannabis (1). An additional 23 states (1) allow medical cannabis programs of varying design (2). Finally, since 2013, 13 states (some of which also allow medical cannabis) have authorized the use of cannabidiol (CBD), primarily for patients with seizure disorders and research studies. In this article, however, we do not address CBD, one of 100-plus cannabinoids in the cannabis plant, which does not have addictive liability when used alone.
Can Cannabis Be Addictive?
Remarkably, despite daily use of cannabis being at record high levels among high school students and college-age adults, many individuals, including clinicians, are unaware that cannabis can be addictive. Flawed drug awareness campaigns of the 1990s claimed that cannabis could be psychologically addictive but not physically addictive like other drugs. However, cannabis does predictably cause physical dependence—the hallmarks of which are tolerance and withdrawal—and heavy users may have great difficulty in attempting to reduce or end their use, leading to compulsive, continued use and related consequences (3, 4). In addition, although study findings have been mixed, it is apparent that at least for some individuals with vulnerabilities, heavy cannabis use is associated with increased risk of anxiety, depression, and psychosis (5). Early onset of cannabis use in particular has been associated with worse cognitive functioning and declines in IQ, although interpretations of findings and causality related to neuropsychological effects have been controversial (5, 6).
Historically, the addictions field has reported that among cannabis users (i.e., those who have used more than several times), the risk of developing cannabis use disorder is one in 11 for adults (approximately 9%) and rises to one in six for adolescent users (17%). However, the potency of tetrahydrocannabinol (THC), the primary psychoactive cannabinoid in cannabis, has increased dramatically since the 1990s, now often surpassing 12%-20%, approximately four times the strength of cannabis predating the 21st century (7, 8). As a result, how increased potency will affect the addictive liability of cannabis is unclear, especially among users who “dab” or use concentrates (“wax” or “shatter”) that can surpass 90% THC. Adding further confusion, cannabis-derived products, notably “edibles” such as candies and brownies, are now mass produced and commercially advertised for medical and recreational purposes. Because absorption and metabolism of orally ingested cannabis vary greatly by individual, studies have not yet been able to characterize the addictive profile of alternative forms of cannabis ingestion. Of great concern, recent large federally funded studies, such as the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III; data collected in 2012-2013), have suggested that among adult cannabis users (past year), upward of 30% meet diagnostic criteria for cannabis use disorder (see Table 1) (9).
TABLE 1.
Diagnostic criteria for cannabis use disorder and clinical correlates
| Diagnostic criteriona | Clinical presentation example |
|---|---|
| Excessive use | Smoking or vaping throughout the day, needing to get high to get out of bed or start the day, buying large quantities (i.e., excessive quantities for weeks at a time) |
| Excessive time spent obtaining, using, recovering | Stockpiling a supply of cannabis at home, always carrying cannabis when leaving home |
| Tolerance | Smoking or vaping the equivalent of five, 10, or even 20 joints daily, using throughout the day yet appearing sober or fully functioning |
| Withdrawal | Insomnia, anorexia, anxiety, irritability, restlessness, dysphoria, or physical pain and discomfort when using less than usual (may persist for weeks or months in attenuated form for long-time users) |
| Cravings | Preoccupation with obtaining or using cannabis, uncontrollable urge to use that interferes with other pursuits |
| Unsuccessful attempts to cut down | Making personal goals to use less or spend less on cannabis but repeatedly failing to make progress |
| Hazardous use | Using before driving or engaging in perilous activities |
| Use despite interpersonal trouble | Continuing to use despite concerns from family, prioritizing ongoing use over obligations to others |
| Use interfering with occupation | Delaying job seeking or settling for lower pay job while protecting time to get high or reducing responsibilities that would interfere with cannabis use |
| Use contributing to medical issues | Heavy cannabis use despite frequent respiratory infections (e.g., bronchitis) and shortness of breath with physical activity; frequent binge eating of junk food despite presence of obesity, diabetes, etc. |
| Use interfering with activities | Choosing to stay home alone and get high rather than meeting friends; giving up other hobbies and prioritizing cannabis use even if not a conscious decision |
Cannabis use disorder is specified as mild if meeting two or three of the 11 criteria, moderate if meeting four or five criteria, and severe if meeting six or more criteria according to DSM-5.
In 2010, the prevalence of cannabis use disorder (formerly termed cannabis abuse and cannabis dependence under DSM-IV-TR) among persons ages 12 years and older in the United States was estimated to be 1.8%, representing 4.5 million individuals (10). As of 2017, the prevalence of cannabis use disorder was estimated by some to be largely stable (11), but data from the 2012-2013 NESARC-III suggest a dramatic increase to approximately 7 million adults (9). Regardless, rates of adult use and daily adult use have risen substantially, likely reflecting the influence of broader access to legal cannabis among adults through both medical and recreational dispensaries (12).
Consistent with drug use generally, risk factors associated with cannabis use include a lifetime history of drug use disorder and past-year alcohol use disorder, both of which are associated with roughly a fivefold increase in the likelihood of cannabis use (10). Stressful life events and cigarette smoking have also been associated with lifetime cannabis use, and they may exert relatively greater influence on women’s likelihood of cannabis use (10).
Cannabis (and cannabinoid-derived product) intoxication can include relaxation and anxiolysis, euphoria, altered perception and awareness of external stimuli, and increased appetite (13). Symptoms of intoxication typically intensify over one to two hours of use, coinciding with peak plasma levels of THC (14), and these symptoms may be unpleasant for about 30% of individuals who report discomfort or anxiety when using cannabis. Common but undesirable changes after use may include impaired coordination and motor skills, memory loss (anterograde) and learning difficulties, and injected or reddened conjunctiva. Frequent cannabis users, however, tend to build a tolerance to many of these symptoms, especially cognitive disturbances.
A substantial body of research has identified a number of risk factors for the development of cannabis use disorder, including sexual abuse (especially during childhood), vulnerable family environments and family history of substance use disorders, antisocial behavior and impulsivity, early-onset anxiety disorders, and substance use, among others (10). Risk factors are interconnected and unlikely to act in isolation; for example, childhood traumas are well known to be related to later mood and anxiety disorders, and these disorders may mediate their eventual effects on substance use disorders.
In a review of Wave 2 NESARC data (collected in 2004–2005), Blanco and colleagues found that among cannabis users, risk factors for the development of cannabis use disorder include past-year alcohol use disorder (adjusted odds ratio [AOR]=4.09), impulsivity (AOR=2.18), greater number of axis I disorders (AOR=1.56), and social deviance (AOR=1.19). Factors that have been found to be protective against cannabis use disorder include religious service attendance and a strong support system (10). In their study, Blanco and colleagues found that 9% of adults reporting cannabis use (not necessarily active or daily users) transitioned to cannabis use disorder (per DSM-IV dependence criteria), with half developing cannabis use disorder within 4 years of cannabis initiation (10), relatively shorter than the 13 years observed among alcohol users who subsequently developed alcohol use disorder (15).
Notably, unlike other substance use disorders in which patients have more visible or outwardly disruptive consequences of heavy use (such as overdose or violent and injurious acts, as seen with opioids and stimulants), individuals with cannabis use disorder are often unaware of their addiction for many years and may continue frequent use without the notice of others, including health care providers. Similar to declines in functioning associated with the negative symptoms of schizophrenia or dementia, the slowly accumulating dysfunction resulting from cannabis use disorder can be hard to detect. Amotivational syndrome is a term that refers to the lack of motivation, declines in functioning and activity levels, and apathy common among heavy cannabis users (16). Given that rates of cannabis use disorder are higher among cannabis users with comorbid anxiety, mood, personality, psychotic, and attention-deficit disorders, it can be difficult to ascertain the primary drivers of functional decline for an individual patient.
Similar to patients presenting to treatment for other substance use disorders, those with cannabis use disorder are often referred via the criminal justice system (e.g., diversion programs, drug courts, probation officers; approximately 50%) or brought in by concerned family members. Cannabis is the drug most commonly reported in treatment admissions, according to the Substance Abuse and Mental Health Services Administration (17). It is not uncommon for patients seeking treatment to report that they did not realize they were addicted to cannabis until they needed to pass a pre-employment drug test or went on a trip without access to cannabis. On trying to stop and experiencing debilitating withdrawal symptoms (cardinal symptoms are insomnia, anorexia, anxiety, irritability, and restlessness), some patients realize they can no longer control their drug use and seek help. In hospital settings, acute cannabis withdrawal is almost universally missed by health care staff, yet it contributes to insomnia, anxiety, and irritability for regular users who are suddenly separated from active use.
Treatment of Cannabis Use Disorder
Assessment
Treatment begins with a comprehensive psychiatric assessment, ideally including collateral information from family, friends, coworkers, or previous providers and from medical records. For cannabis use disorder, the clinician should primarily assess patterns of substance use (both cannabis and other substances) and the patient’s reasoning for what the patient finds useful and problematic about these patterns. In general, best practices in addiction treatment emphasize a chronic management model in the outpatient setting—rather than residential rehabilitation or inpatient hospitalization—for acute and maintenance treatment for substance use disorder. Although some patients with severe cannabis use disorder may not be able to stabilize their use without being removed from their home environment, the outpatient setting is often the most appropriate level of care for patients with the disorder.
Addressing ambivalence is a universal aspect of management of substance use disorder. Once connected to specialty treatment, patients with cannabis use disorder often acknowledge spending an excessive amount of time or money on cannabis and a desire to reduce or stop use despite an ongoing attachment to the drug and a preference for how they feel while using compared with while not using. Starting with the initial assessment, the clinician can glean vocabulary, patient perspectives, and priorities that will subsequently allow for motivational work to reinforce patients’ “change talk” in a motivational interviewing framework (18).
A goal to immediately stop cannabis completely is unrealistic for the great majority of patients with a diagnosis of moderate to severe cannabis use disorder. Rather, frequent visits with intensive management for reducing frequency and amount of use in the direction of eventual abstinence is often more effective for this patient population. In general, patients make the most progress (in absolute volume) earlier in attempts to reduce the amount they consume because it is easier to go from a very large amount to a large amount than it is to go from a small amount to absolutely nothing, for both physiological and psychological reasons.
Often, patients who use cannabis heavily will report that it helps them with anxiety or insomnia irrespective of whether they have a common comorbid diagnosis such as general anxiety disorder, social anxiety disorder, posttraumatic stress disorder, or attention-deficit hyperactivity disorder. Although the short-term benefits of using cannabis may help with anxiolysis or treating early insomnia, in general cannabis, especially via rebound withdrawal symptoms, can worsen these underlying conditions over time (much like how patients with heavy alcohol use often develop worsening anxiety and irritability). In addition to managing these symptoms, identifying other patient goals, such as finding a new job, repairing relationships with parents, successfully finding a girlfriend or boyfriend, or passing a preemployment drug test, is key to being able to align with the patient and approach reducing cannabis use from a cooperative rather than a conflictual standpoint.
Pharmacotherapy
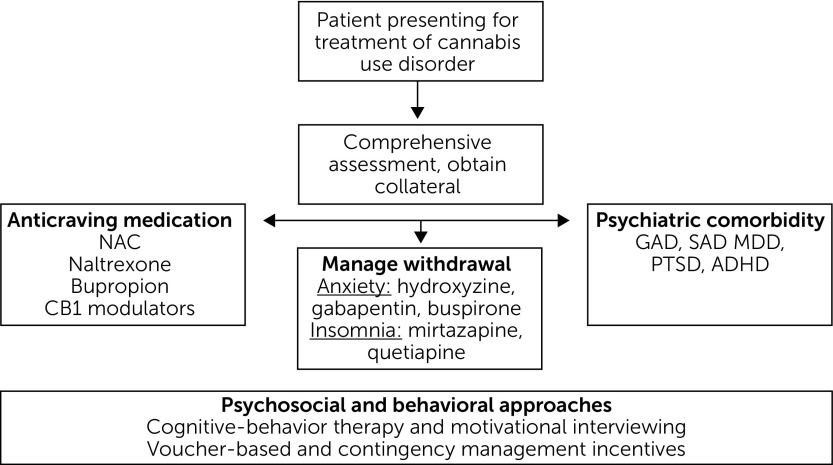
To date, no medications have been approved by the Food and Drug Administration (FDA) for the treatment of cannabis use disorder (19). Unlike opioid use disorder, for which three highly effective classes of medication have been approved (methadone, buprenorphine, and naltrexone) and benefit patients (with robust effect sizes), medications for cannabis use disorder (as with stimulant use disorder) have fallen short of the desired efficacy endpoint of continuous abstinence. Regardless, several medication options exist, some off-label, and fall into the following categories: treatment of cravings related to cannabis use disorder, treatment of cannabis-related withdrawal symptoms, and treatment of comorbid psychiatric disorders that may otherwise be contributing to ongoing cannabis use (Figure 1) (20). To aid medication development for substance use disorders, the FDA has recently announced that it will loosen criteria for clinical trials that have historically required continuous abstinence as the primary outcome for drug approval. To better reflect effective specialty clinical practice, and with the encouragement of the National Institute on Drug Abuse, the FDA has announced its intention to also consider reductions in subjective symptoms, such as craving, as sufficient to substantiate efficacy of medications for substance use disorders, a promising step forward for expanding patients’ access to effective treatment options.
FIGURE 1.
Pathway for the effective treatment of cannabis use disordera
aNAC, N-acetylcysteine; CB1, cannabinoid receptor type 1; GAD, generalized anxiety disorder; SAD, social anxiety disorder; MDD, major depressive disorder; PTSD, posttraumatic stress disorder; ADHD, attention-deficit hyperactivity disorder
Treatment of cravings related to cannabis use disorder.
Several medications have anticraving properties in addition to their primary mechanisms (19). Naltrexone, a mu-opioid receptor antagonist, has been approved by the FDA for alcohol and opioid use disorders as well as (in combination form with bupropion) binge eating disorder. A single daily dose of 50 mg of naltrexone can also generally help reduce cravings for other intoxicants and curb impulsive behaviors, such as those related to gambling. Naltrexone is currently under investigation specifically as a possible medication for cannabis use disorder as well as for stimulant use disorders (i.e., cocaine and crystal methamphetamine), although study findings at the group level among treatment seekers with cannabis use disorder and in preclinical human lab studies have thus far been mixed (21).
Additional medications that have demonstrated anticraving properties for some patients are bupropion and topiramate (20). Bupropion, often dosed between 150 and 450 mg daily, is a mildly stimulating antidepressant that works via dopamine and norepinephrine and is also FDA approved for smoking cessation. Given that bupropion and naltrexone have been demonstrated to reduce nicotine consumption and cigarette smoking, they may serve a dual role for patients with cannabis use disorder who are also frequent smokers. Topiramate is an antiepileptic drug also used for migraine prophylaxis via antagonism at sodium channels, N-methyl-d-aspartate receptors, and through gamma-aminobutyric acid augmentation. For many years, it has been studied for the treatment of alcohol use disorder. Although it has yet to receive FDA approval for that indication, clinicians who specialize in treating patients with alcohol use disorder have found that it can help reduce drinking while also improving depressive symptoms, mood reactivity, and stress. Topiramate, however, may be less well tolerated among adolescent patients with cannabis use disorder.
Several promising studies with adolescents have also been conducted with N-acetylcysteine (NAC), an anti-inflammatory supplemental amino acid that is hepato-protective and a glutamate modulator in the central nervous system. In the first published, fully powered clinical trial with positive results for pharmacotherapy for cannabis use disorder, Gray and colleagues initially found that youths and young adults (ages 15–21, N=116) dosed with 1,200 mg NAC twice a day along with contingency management had 2.4 times the odds of cannabis-negative urines as those assigned to placebo medication with contingency management (22). However, a follow-up study did not replicate their initial results among adult participants (23).
Most specific to the primary treatment of cannabis use disorder are medications that are active at the cannabinoid subtype 1 (CB-1) receptor, disproportionately found throughout the central nervous system (21). Dronabinol, a Schedule III synthetic THC for the treatment of nausea and cachexia, partially agonizes CB-1 receptors. In addition, a newer analog of THC, nabilone (Schedule II), has even stronger activity at the CB-1 receptor, potentially making it more reinforcing for patients through positive feel-good effects (20). Although dronabinol has demonstrated superior control of withdrawal symptoms in prospective randomized controlled trials and a few case studies, it has often fallen short of being associated with reductions in cannabis use (21). Further research on nabilone is needed, but it remains a promising candidate that could be helpful for individual patients. A recent small pilot study demonstrated the safety and feasibility of nabilone as pharmacotherapy for cannabis use disorder (24). New compounds such as second-generation cannabinoid receptor antagonists and other related inhibitors, such as CBD, a cannabinoid that acts as a negative allosteric modulator, are also being explored as potential candidates.
Treatment of cannabis-related withdrawal.
Drug use, of cannabis or another drug, is often colloquially associated with euphoria and other positive reinforcing effects, and by the time patients are heavy regular users, negative reinforcement (the relief of unpleasant distressing feelings such as anxiety or insomnia due to withdrawal or the fear of withdrawal) often disproportionately drives compulsive ongoing drug use. As a result, especially in the outpatient setting, aggressive management of withdrawal symptoms is key to minimizing the risk of returning to active drug use (20). As indicated in Table 1, protracted cannabis withdrawal symptoms, primarily anxiety and insomnia, can persist for weeks or even months beyond the last use of cannabis. Widely familiar medications for anxiety that have limited addictive liability (selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, hydroxyzine, alpha-2 agonists, and gabapentin) can be instrumental, especially in the first month or two of cannabis cessation (or clinically significant reductions in use, which may be more common). Medications for sleep are often needed for an even longer period of several months. Studies have been conducted with sedating mood stabilizers and antidepressants such as quetiapine and mirtazapine (20). Although they have been associated with subjective benefits at the individual level, to date they have not demonstrated improvements in continuous abstinence at the group level and must be used off label for the treatment of cannabis use disorder.
Treatment of comorbid psychiatric disorders.
The importance of treating comorbid conditions is often overlooked because of structural barriers in the mental health and behavioral health fields that often separate patients into treatment environments for either mental illness or substance use disorders. Quality care requires simultaneous treatment of both. Although some outdated opinions suggest that drug use must cease before depression or anxiety can be effectively treated (such as with psychotherapy), patients with substantial comorbid psychiatric conditions often face the greatest obstacles to successful cannabis cessation if such conditions remain untreated. The critical role of simultaneous treatment of comorbid psychiatric conditions underscores the necessity of a comprehensive psychiatric evaluation when individuals with cannabis use disorder first enter treatment. Paradoxically, patients with substance use disorders are often hesitant to take new medications while attempting to reduce or stop drug use, likely for a variety of reasons related to a desire to be completely drug free, internalized stigma and shame, and unfamiliarity with the benefits of psychopharmacology. Working with this resistance can be an important part of treatment and requires the clinician’s patience.
Psychosocial Treatments
As with most psychiatric conditions, patients would ideally not need to choose between pharmacotherapy or psychotherapy; a combination approach is ideal (Figure 1). Given the limitations on the efficacy of medications for treating patients with cannabis use disorder, intensive psychosocial approaches take on additional importance. Medications often help retain patients in care long enough for them to better respond to psychosocial and behavioral interventions for substance use disorders.
A Cochrane Review found that cognitive-behavioral therapy (CBT; one study with 134 participants) and motivational enhancement therapy (MET; six studies with 928 participants), especially in combination (six studies with 971 participants), had the most consistent evidence for assisting with initial reductions in cannabis use and severity of dependence (25). High-intensity treatment approaches, such as more than four sessions delivered over more than a month, were superior to lower intensity treatment. However, no particular intervention was consistently effective at nine-month follow-up or later. In addition, several studies demonstrated superior outcomes when contingency management was also added, such as voucher-based incentives for cannabis-negative urine tests. Consistent with treatment trials for other substance use disorders, abstinence rates were relatively low overall, with only 25% of participants fully abstinent at final follow-up endpoints (25). Overall, in comparison with minimal treatment controls or treatment as usual, psychosocial intervention was shown to reduce both frequency of cannabis use and severity of cannabis use disorder, especially when assessed over shorter time frames. To summarize, among studied interventions, an intensive intervention based on a combination of MET and CBT with abstinence-based incentives (i.e., contingency management) demonstrated the best results.
Psychotherapy for patients with cannabis use disorder should focus on reasons for change, goal commitments, emotion and impulsivity regulation, skills for coping with distress, and conquering denial and self-deception about the effects of drug use (26). CBT techniques can be used to analyze different thought processes associated with different smoking or use situations, challenge automatic thoughts regarding reasons for continued use, enable a potential role transition to no longer being an active cannabis user, and reflect on what it means to be in recovery. Additional behavioral approaches can help patients reengage with old hobbies and find new distracting and stimulating activities to occupy their time. As with any substance use disorder, working with patients to avoid the people, places, and things associated with drug use is key to minimizing environments and triggers for returning to drug use. This work may include coaching patients on the importance of deleting and blocking numbers for drug dealers and distancing themselves from friends with whom they often used. Especially for patients with comorbid psychiatric illness such as mood or anxiety disorders, intensive work to develop distress tolerance skills is often critical to reducing the likelihood that patients will continue to return to drug use to manage uncomfortable feelings and thoughts. Although some patients with cannabis use disorder may benefit from peer-support 12-step groups such as Marijuana Anonymous, the literature on the effectiveness of these groups for individuals whose primary drug of choice is cannabis is limited.
Conclusion
With rapidly changing state laws expanding adult access to cannabis for both medical and recreational purposes, rates of adult use and especially of heavy use (i.e., daily or near-daily use) have increased substantially in recent years. Although large epidemiological studies on the impact of these laws have reported mixed results regarding changes in adult rates of cannabis use disorder attributable to the laws themselves, there have been well-documented increases in the number of adults with cannabis use disorder as cannabis use has become more socially acceptable. Millions more adults now meet criteria for cannabis use disorder in a given year, and psychiatrists and other mental health professionals have a vital role to play in improving clinical management of patients with cannabis use disorder. Despite a lack of FDA-approved medications, several evidence-based pharmacological and psychotherapeutic treatments are available to practitioners in all care settings.
Footnotes
Dr. Williams reports no financial relationships with commercial interests. Dr. Hill reports receipt of royalties from Hazelden Publishing.
References
- 1.State Medical Marijuana Laws. Washington, DC, National Council of State Legislators, 2018; http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed Nov 28, 2018
- 2.Williams AR, Olfson M, Kim JH, et al. : Older, less regulated medical marijuana programs have much greater enrollment rates than newer “medicalized” programs. Health Aff 2016; 35:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haney M, Ward AS, Comer SD, et al. : Abstinence symptoms following smoked marijuana in humans. Psychopharmacology 1999; 141:395–404 [DOI] [PubMed] [Google Scholar]
- 4.Budney AJ, Hughes JR, Moore BA, et al. : Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 2004; 161:1967–1977 [DOI] [PubMed] [Google Scholar]
- 5.Halah MP, Zochniak MP, Barr MS, et al. : Review of cannabis and psychiatric disorders. Curr Addict Rep 2016; 3:450–462 [Google Scholar]
- 6.Scott JC, Slomiak ST, Jones JD, et al. : Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry 2018; 75:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElSohly MA, Mehmedic Z, Foster S, et al. : Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry 2016; 79:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehmedic Z, Chandra S, Slade D, et al. : Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci 2010; 55:1209–1217 [DOI] [PubMed] [Google Scholar]
- 9.Hasin DS, Saha TD, Kerridge BT, et al. : Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry 2015; 72:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco C, Rafful C, Wall MM, et al. : Towards a comprehensive developmental model of cannabis use disorders. Addiction 2014; 109:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu LT, Zhu H, Mannelli P, et al. : Prevalence and correlates of treatment utilization among adults with cannabis use disorder in the United States. Drug Alcohol Depend 2017; 177:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AR, Santaella-Tenorio J, Mauro C, et al. : The effect of medical marijuana laws and regulations on adolescent and adult recreational marijuana use and cannabis use disorder. Addiction 2017; 112:1985–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper Z, Williams AR: Cannabis and cannabinoid intoxication and toxicity; in Cannabis Use Disorder. Edited by Montoya I, Weiss S. Cham, Switzerland, Springer Nature Switzerland, 2019, pp 103–111 [Google Scholar]
- 14.Crippa JAS, Derenusson GN, Chagas MHN, et al. : Pharmacological interventions in the treatment of the acute effects of cannabis: a systematic review of literature. Harm Reduct J 2012; 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. : Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2011; 115:120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palamar JJ, Fenstermaker M, Kamboukos D, et al. : Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse 2014; 40:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treatment Episode Data Set (TEDS): 2004-2014. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-84, HHS Publication No. (SMA) 16-4986. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2016
- 18.Levounis P, Bachaar A, Marienfeld C: Handbook of Motivation and Change: A Practical Guide for Clinicians. Washington, DC, American Psychiatric Publishing Inc, 2010 [Google Scholar]
- 19.Bobb AJ, Hill KP: Behavioral interventions and pharmacotherapies for cannabis use disorder. Curr Treat Options Psychiatry 2014; 1:163–174 [Google Scholar]
- 20.Brezing CA, Levin FR: The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 2018; 43:173–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandrey R, Haney M: Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs 2009; 23:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray KM, Carpenter MJ, Baker NL, et al. : A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry 2012; 169:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray KM, Sonne SC, McClure EA, et al. : A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend 2017; 177:249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill KP, Palastro MD, Gruber SA, et al. : Nabilone pharmacotherapy for cannabis dependence: a randomized, controlled pilot study. Am J Addict 2017; 26:795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database of Systematic Reviews 2016; 5: CD005336. doi: 10.1002/14651858.CD005336.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgins DC, Stea JN: Insights from individuals successfully recovered from cannabis use disorder: natural versus treatment-assisted recoveries and abstinent versus moderation outcomes. Addict Sci Clin Pract 2018; 13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]