Abstract
Acetaldehyde, a toxic byproduct of alcohol (i.e., ethanol) metabolism, has long been suspected of causing at least some of the central nervous system actions of ethanol. However, the data to support such a hypothesis have been difficult to obtain. One roadblock is the very low blood levels of acetaldehyde following ethanol intake and the finding that even elevated acetaldehyde levels in the blood do not easily gain access to the brain. The recent discovery of the oxidation of ethanol to acetaldehyde in the adult brain may help explain the acute effects of ethanol.
Keywords: Ethanol metabolism, ethanol-to-acetaldehyde metabolism, acetaldehyde, acetate, aldehyde dehydrogenase (ALDH), central nervous system, brain, catalase, cytochrome P450, alcohol dehydrogenase (ADH), ethanol oxidation, behavior, ethanol preference
This article reviews studies of a potential role for acetaldehyde, a toxic byproduct of alcohol (i.e., ethanol) metabolism, in ethanol’s effects in the central nervous system (CNS); the metabolism of ethanol to acetaldehyde in the brain; the metabolism of acetaldehyde in brain cells; the results of ethanol oxidation to form acetalde-hyde; and acetaldehyde’s effects on behavior. The studies cited primarily are those dealing with acute or very short-term administration of ethanol. The role of acetaldehyde in tolerance and dependence or in the peripheral effects of ethanol is not covered.
Acetaldehyde’s Role in Ethanol’s Effects
Acetaldehyde, a toxic byproduct of ethanol metabolism, may be at least partially responsible for ethanol’s actions in the CNS (Hunt 1996; Hashimoto et al. 1989; Bergamaschi et al. 1988; Zimatkin and Deitrich 1997; Thadani and Truitt 1977; Collins et al. 1988; Heap et al. 1995). However, several factors cast doubt on this hypothesis. First, avid metabolism of acetaldehyde by the liver keeps blood levels of acetaldehyde following ethanol ingestion extremely low (Sippel and Eriksson 1975). The levels of acetaldehyde in most people after ethanol ingestion are nearly undetectable in the blood, on the order of one micromole.1 Second, even if the blood acetaldehyde levels are significant, either because of genetic variation in alcohol-metabolizing enzymes or the presence of drugs that allow build-up of acetaldehyde, acetaldehyde does not seem to be able to penetrate blood vessels into the brain (i.e., the blood–brain barrier), and sub-stantial blood levels are required before acetaldehyde levels increase in the brain (Tabakoff et al. 1976; Westcott et al. 1980; Sippel 1974; Zimatkin and Pronko 1995). This is attributed primarily to the presence of the enzyme that converts acetaldehyde to acetate (i.e., aldehyde dehydrogenase [ALDH]) in the blood–brain barrier, which may help keep brain acetaldehyde levels low (Petersen 1985; Tampier et al. 1993). Third, although one could use the compound pyrazole to inhibit the reaction by which the enzyme alcohol dehydrogenase (ADH) breaks down ethanol (i.e., oxidation), and thus inhibit the formation of acetaldehyde, intoxication still would result, suggesting that acetaldehyde does not play a significant role in ethanol’s effects on the brain. Indeed, Goldstein and Zaechelein (1983) used pyrazole to study intoxication in mice using a vapor chamber method. In this method, the metabolism of inhaled ethanol is slowed, providing for more prolonged and consistent blood levels of ethanol, producing physical dependence in mice.
Metabolism of Ethanol to Acetaldehyde in the Brain
These considerations would be irrelevant if the brain could produce its own acetaldehyde from ethanol. Although there had been several reports of the oxidation of ethanol in the brain (Sutherland et al. 1958; Raskin 1973; Raskin and Sokoloff 1974; Raskin and Sokoloff 1968; Raskin and Sokoloff 1970a, b; Raskin and Sokoloff 1972a, b), this idea largely was dismissed by the findings of Mukherji and colleagues (1975), whose studies showed that ethanol did not break down to acetaldehyde in the brain.
Catalase
Catalase, the enzyme that facilitates the breakdown of hydrogen peroxide to oxygen and water, may play a role in the production of acetaldehyde from ethanol in the brain. Cohen and colleagues (1980) demonstrated that catalase in conjunction with hydrogen peroxide will oxidize ethanol in the brain. Although the authors did not directly demonstrate the production of acetaldehyde (and thus the metabolism of ethanol), in this system, they did provide impetus to other investigators’ attempts. Researchers initially were thwarted in their attempts to document metabolism of ethanol in the brain when they discovered nonenzymatic (i.e., artifactual) production of acetaldehyde. It was determined that the iron typically found in red blood cells (i.e., hemoglobin) caused this nonenzymatic formation of acetaldehyde from ethanol, thus masking any enzymatic production of acetaldehyde. Two groups, nearly simultaneously, overcame these problems. Aragon and colleagues (1992) demonstrated that acetaldehyde was produced from ethanol in rat brains with all blood removed. Gill and colleagues (1992) were able to prevent the artifactual formation of acetaldehyde and show the production of acetaldehyde from ethanol in rat brain tissue. In both studies, inhibitors of catalase also were effective in inhibiting the production of acetaldehyde. On the other hand, inhibitors of the enzymes cytochrome P450 and ADH—key enzymes involved in alcohol metabolism— were ineffective. Other investigators quickly confirmed these findings (Hamby-Mason et al. 1997; Aspberg et al. 1993; Zimatkin et al. 1999). In studies of cells from all parts of the brain (i.e., whole-brain homogenates), the intensity of ethanol oxidation is comparatively low but may be much higher in the specific structures and cells known for their increased catalase activity (Zimatkin and Lindros 1996).
Cytochrome P450
Cytochrome P450 enzymes, which are involved in ethanol metabolism in the liver, have been implicated in the metabolism of ethanol in the brain. First, Warner and Gustafsson (1994) demonstrated the presence of cytochrome P450 in rat brain and its induction by ethanol. Cytochrome P450 2E1, a variant of cytochrome P450 (i.e., isozyme) that is capable of oxidizing ethanol efficiently in other tissues, also was found in the brain (Hansson et al. 1990). Its protein, messenger RNA (mRNA), specific activity, and induction by ethanol were found in nerve cells (i.e., neurons) and support cells (i.e., glial cells) in the following brain regions: cerebellum, cerebral cortex, thalamus, and hippocampus (Sohda et al. 1993; Tindberg and Ingelman-Sundberg 1996; Upadhya et al. 2000). Cytochrome P450 also has been found in prenatal human brain cells (Khalighi et al. 1999). In addition, a recent study in rats and mice has reinforced the possibility that cytochrome P450 is involved in the brain’s metabolism of ethanol (Zimatkin et al. 2006).
ADH
The possible role of ADH in the metabolism of ethanol in the brain remains unclear. Research originally suggested that only the subtype ADH3 was expressed in the brain and that ethanol was not a good candidate for that enzyme to act on (i.e., it was a poor substrate) (Beisswenger et al. 1985; Kerr et al. 1989). Several groups (Kerr et al. 1989; Buhler et al. 1983) reported the presence of ADH1 in brain cells. Giri and colleagues (1989) found ADH3 expressed in rat brain, and Martinez and colleagues (2001) found mRNA for ADH1 and ADH4 in rat brain cells. Although the authors could not demonstrate ADH activity in analysis of whole-brain homogenates, they were able to detect ADH activity in specific neurons (i.e., granular cells and Purkinje cells) of the cerebellum. This shows that although the activity of ADH may be undetectable in whole homogenates, there may be sufficient activity in specific neurons to form acetaldehyde locally.
The Chemical Reactions Allowing Oxidation of Ethanol to Acetaldehyde
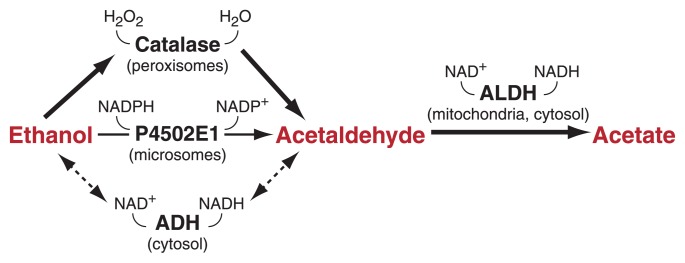
The oxidation of ethanol produces acetaldehyde (see Figure). The production of acetaldehyde by catalase is limited by the availability of hydrogen peroxide, a potentially harmful byproduct of ethanol metabolism by cytochrome P450. Hydrogen peroxide also can come from a number of other sources, including the enzyme monoamine oxidase, ascorbic acid (vitamin C), and other cytochrome P450 oxidations (Sandri et al. 1990; Simonson et al. 1993; Sinet et al. 1980).
Figure.
Pathways of ethanol metabolism in the brain. The oxidation of ethanol produces acetaldehyde. The production of acetaldehyde by the enzyme catalase (found in internal cell components called peroxisomes) requires hydrogen peroxide (H2O2). The enzyme cytochrome P4502E1 is present in brain cell structures in the smooth endoplasmic reticulum (microsomes). Alcohol dehydrogenase (ADH) is an enzyme found in the cell’s fluid or cytosol. The enzyme aldehyde dehydrogenase (ALDH), found in the cell’s mitochondria and cytosol, converts acetaldehyde to acetate.
All studies of the oxidation of ethanol to acetaldehyde depend on the ability to measure the accumulation of acetaldehyde. This can occur only if the rate of removal of acetaldehyde is slower than its rate of formation in the system under study. Substantial amounts of acetaldehyde are oxidized to acetate in these in vitro systems. This results in an underestimation of the rate of ethanol metabolism in the brain, because acetaldehyde is metabolized to acetate nearly as quickly as it is formed. Thus, only the net amount of acetaldehyde in the system is accounted for when only acetaldehyde accumulation is measured. Studies of the oxidation of ethanol to acetaldehyde in the brain therefore need to consider these limitations.
Metabolism of Acetaldehyde in Brain Cells
The metabolism of acetaldehyde in the brain is much less controversial than the metabolism of ethanol because ALDH enzymes have long been known to be present in brain cells (Deitrich 1966; Erwin and Deitrich 1966). The ALDH enzyme most likely to be responsible for the majority of the oxidation of acetaldehyde to acetate is ALDH2, the form found in the mitochondria, an internal component of the cell. This enzyme has a high affinity (i.e., a low Km)2 and rate of enzyme activity with acetaldehyde and is sufficient to remove most of the acetaldehyde. Several other forms of ALDH are expressed in brain cells as well (Sophos and Vasiliou 2003). The localization of the enzyme to specific cells or areas of the brain could greatly influence the local rate of removal of acetaldehyde. That is, acetaldehyde is only metabolized if it is present in an area of the brain that also has ALDH enzymes (Zimatkin et al. 1992). In a similar fashion, localization within the cell of the enzymes responsible for the production of acetaldehyde (catalase in internal cell components called peroxisomes and cytochrome P450 in a network of membranes within the cell called the endoplasmic reticulum or microsomes) and the enzyme of acetaldehyde removal—ALDH2 in the mitochondria—leave space and time for acetaldehyde to interact with other cellular elements before being converted to acetate. That is, if acetaldehyde is produced in separate cellular structures from where it is removed, it can have an effect on the cell before it is metabolized.
Acetaldehyde’s conversion to acetate has further implications for the cell. Acetate has significant CNS effects that are separate from those of ethanol (Carmichael et al. 1991; Cullen and Carlen 1992; Correa et al. 2003). Thus, administering sodium acetate in doses comparable with those observed after administering 1 to 2 g/kg ethanol produced a dose-dependent impairment of motor coordination (Carmichael et al. 1991). Because acetate’s effects can be blocked with the use of 8-phenyltheophylline, a substance that blocks receptors for adenosine (a byproduct in acetate breakdown), it has been suggested that acetate’s actions may be mediated by adenosine (Carmichael et al. 1991; Cullen and Carlen 1992). Moreover, administering low doses of acetate (0.35 to 2.8 micromolar) by injection into the brain through a small hole bored into the skull (i.e., intracerebroventricular [ICV] administration) produced a potent decrease in motor activity, similar to the effects of ethanol and acetaldehyde on motor activity (Correa et al. 2003).
Consequences of the Oxidation of Ethanol to Acetaldehyde
Ethanol oxidation to acetaldehyde has several consequences, which may be broken down into two broad categories. The first is the direct binding of acetaldehyde to proteins (Jennett et al. 1987; McKinnon et al. 1987; Nakamura et al. 2003; Zimatkin et al. 1992), nucleic acids (Wang et al. 2000), and a type of fat (i.e., lipid) containing phosphorus (i.e., phospholipids) (Trudell et al. 1990, 1991; Trudell et al. 1990; Kenney 1982, 1984). In total, the binding to these cellular components probably accounts for very little of the acetaldehyde that disappears, but the consequences of these interactions may be highly significant because the function of these cellular components can be compromised by this binding. The second category of ethanol oxidation consequences is indirect action. This occurs when the metabolism of other aldehydes that originate in the body (i.e., endogenous aldehydes) is inhibited through the presence of acetaldehyde. The aldehydes produced by the oxidation, by monoamine oxidase, of the brain chemicals (i.e., neurotransmitters) dopamine, norepinephrine, and serotonin, are particularly vulnerable to this reaction (Deitrich and Erwin 1980). One theory is that acetaldehyde or the aldehydes of dopamine, norepinephrine, or epinephrine condense with these same neurotransmitters to produce compounds called tetrahydroisoquinolines that may be responsible for some of ethanol’s CNS effects themselves. Acetaldehyde also may condense with serotonin to form compounds called tetrahydro-beta carbolines that may be active in the brain as well. Thus, these amine-aldehyde condensation products may be responsible for some of the behavioral actions of ethanol (Davis and Walsh 1970; Cohen and Collins 1970; Melchior and Myers 1977). Considerable controversy arose around this theory, especially because these compounds occur naturally in the diet, casting doubt on the relevance of their presence following ethanol ingestion (Collins et al. 1990).
Researchers recently have proposed that acetaldehyde may compete with malondialdehyde or 4-hydroxynonenal, the aldehyde products that result after the breakdown of lipids (i.e., lipid per-oxidation). These aldehydes also may inhibit the activity of ALDHs (Luckey et al. 1999; Mark et al. 1997; Meyer et al. 2004; Murphy et al. 2003). This would result in increased levels of acetaldehyde as well as of these toxic aldehydes.
The oxidation of ethanol to acetaldehyde may trigger various reactions that ultimately have behavior-related consequences, as described below.
Acetaldehyde and Behavior
Several studies have suggested that acetaldehyde is responsible for some of the behavioral effects (such as poor coordination [i.e., ataxia]) of ethanol (reviewed in Deitrich 2004 and Quertemont et al. 2005). Many of these studies measured the degree of relationship of the two variables (i.e., they were correlational). That is, the studies measured the behavioral effects of ethanol following presumed alteration of levels of acetaldehyde in the brain by inhibiting ALDH or inhibiting or activating catalase.
Acetaldehyde has been measured directly in the brain in only a few studies (Jamal et al. 2003, 2004, 2005). In those studies, ALDH was inhibited, resulting in relatively high levels of acetaldehyde in the brain. It is assumed that the proximal cause of these behavioral effects (such as ataxia or loss of the righting response) is the altered amount of acetaldehyde in the brains of the animals studied.
On the other hand, mice with about half the usual levels of catalase in the brain (i.e., acatalasemic mice), which should have less acetaldehyde from ethanol in the brain compared with control mice, had longer sleep times after ethanol intake than control mice (Aragon and Amit 1993; Vasiliou et al. 2004). This suggests that acetaldehyde does not influence ethanol-induced sleep times. Similar results were obtained using mice that had been genetically modified to have the ethanol-metabolizing enzyme CYP2E1 absent. The mice exhibited longer ethanol-induced sleep times, especially at higher ethanol doses; they also produced lower amounts of acetaldehyde following the incubation of ethanol with brain cell structures containing ethanol-metabolizing enzymes (i.e., microsomes) compared with control animals (Vasiliou et al 2004). In addition, induction of brain catalase activity resulted in decreased loss of righting reflex (LORR), which is used to estimate hypnotic sensitivity (a behavioral response) to ethanol, whereas reduced brain catalase activity resulted in increased LORR, showing involvement of brain catalase in the hypnotic effect of ethanol (Correa et al. 2001).
Ethanol Preference
Rats selectively bred to have either high or low preference for ethanol are useful animal models for the study of alcohol consumption. Alcohol-preferring (P) and nonpreferring (NP) rats have been shown to differ in hypnotic sensitivity to ethanol; thus, P rats are innately less sensitive to the effects of ethanol than NP rats (Lumeng et al. 1982).
Researchers have used P and NP pairs of rat lines or strains to study relationships between catalase, acetaldehyde, and ethanol preference. Although none of the studies below included direct measurements of brain ethanol oxidation in vitro, brain acetaldehyde levels in vivo, or acetaldehyde accumulation in vitro, their findings do offer some implications regarding the relationship between brain acetaldehyde and ethanol preference.
As discussed previously, catalase plays a role in the production of acetaldehyde in the brain. The catalase inhibitor aminotriazole attenuated ethanol preference in mice (Koechling and Amit 1994), suggesting that inhibiting catalase results in decreased levels of acetaldehyde and a decline in this particular ethanol-induced behavior. Consistent with these findings, Amit and Aragon (1988) found that blood catalase from rats naïve to ethanol correlated positively with ethanol preference in the animals (blood and brain catalase also correlated positively after exposure to ethanol). Increased catalase presumably would mean an increased rate of production and increased brain levels of acetaldehyde with resultant increases in preference. Similar studies have found that catalase correlates with alcohol intake in humans as well (Koechling and Amit 1992).
ALDH in the brain also positively correlates with ethanol preference. Indeed, Amir (1978) found that rats’ ethanol preference better correlated with brain ALDH than with liver ALDH. This would support the idea that blood levels of acetaldehyde (produced in the liver) are less important to establishing a preference for alcohol than brain levels of acetaldehyde. High blood acetaldehyde levels, resulting from a genetic defect in ALDH in Asian populations, do produce decreased ethanol intake (Harada et al. 1982), as does treatment with ALDH inhibitors such as disulfiram (Antabuse®) (Chick et al. 1992). High ALDH activity would presumably indicate a lower level of brain acetaldehyde because of increased rates of oxidation. Higher ALDH and lower acetaldehyde levels are not consistent with the positive correlation between catalase activity and ethanol preference. Also, acatalasemic mice have a higher preference for ethanol than do control mice (Aragon and Amit 1993). Vasiliou and colleagues (2006) reported that acatalasemic mice accumulate only about 50 percent as much acetaldehyde from ethanol in vitro as control mice and that they have about half the brain catalase activity as that in the brain of control mice. This would not totally explain the decreased ethanol metabolism rates because catalase is not the only enzyme capable of oxidizing ethanol in the brain. Previous studies on catalase contribution to ethanol oxidation were performed using catalase inhibitors capable of inhibiting the activity of other ethanol-metabolizing enzymes (P4502E1 and ALDH); therefore, this finding using genetics seems to be a more useful tool. Other researchers have conducted extensive research that provides further support for the involvement of brain catalase in ethanol-induced behavioral effects. This research also supports the notion that acetaldehyde may be produced directly in the brain by catalase and that it may be an important regulator of ethanol’s locomotor effects (for example, see Sanchis-Segura et al. 1999).
Acetaldehyde’s Actions in the Brain
Researchers also have studied the actions of acetaldehyde in the brain directly, usually with ICV infusions of acetaldehyde to bypass the metabolism of acetaldehyde by the liver. Smith and colleagues (1984) found that conditioned place preference3 could be induced by ICV infusions of acetaldehyde, suggesting that low levels of acetaldehyde have reinforcing properties. Brown and colleagues (1979) had found that rats would administer acetaldehyde, but not ethanol, intracerebroventricularly. Conversely, conditioned taste aversion can be induced by acetaldehyde. This action can be blocked by alpha-methyl-para-tyrosine, a substance that inhibits the neurotransmitters epinephrine (adrenaline) and dopamine—key brain chemicals involved in addiction. This indicated that perhaps the adrenergic system is involved in acetaldehyde’s action in the brain (Aragon et al. 1991). Rodd-Henricks and colleagues (2000, 2002) found that rats genetically predisposed to prefer alcohol would press a lever to infuse ethanol and acetaldehyde directly into the ventral tegmental area (VTA), located in the midbrain. Using similar techniques, it was found that rats would lever press for infusions of the condensation product between acetaldehyde and dopamine (i.e., salsolinol) directly into the nucleus accumbens, a collection of neurons involved in the brain’s reward system (McBride et al. 2002). Arizzi and colleagues (2003) studied the in vivo effects of intracerebroventricularly administered ethanol, acetaldehyde, and acetate on lever-pressing tasks. These studies showed that acetaldehyde appears to induce activating or disinhibiting effects and thus can produce at least some of the effects of ethanol, whereas acetate is more potent than the other substances at producing actions that lead to a suppression of lever pressing and locomotion and thus may be implicated in the motor impairments induced by ethanol. Unfortunately, the researchers gave the same dose of all three agents in spite of the large (1,000-fold) difference in their concentrations following ethanol administration peripherally.
Numerous studies show the possible pathophysiological effects of acetaldehyde. The theories of the condensation of acetaldehyde with biogenic amines to produce new compounds are reviewed above. Although this reaction certainly occurs in vivo, the importance of these condensation products to ethanol’s actions in the brain remains speculative. In a similar vein, acetaldehyde, by substrate competitive inhibition of ALDH, has been postulated to cause an increase in the aldehydes derived from biogenic amines (Deitrich and Erwin 1980). That is, ALDH can oxidize acetaldehyde and aldehydes derived from biogenic amines. When acetaldehyde is absent ALDH can oxidize other aldehydes. When acetaldehyde is present, acetaldehyde is bound to ALDH and is oxidized so other aldehydes are oxidized at a slower rate or not at all by ALDH. No studies have directly measured the purported increase in these aldehydes. However, these aldehydes do have suggested physiological actions. For example, indole-3acetaldehyde, a biogenic aldehyde, reacts with certain substances (i.e., phospholipids) to create specific physiological effects, as indicated by a change in spectrophotometric absorption4 (Nilsson and Tottmar 1985). This shows what can happen to biogenic aldehydes if they are not oxidized by ALDH. When aldehydes were directly applied to neurons, the biogenic aldehydes derived from dopamine and serotonin had a direct depressant effect on neurons in the neocortex and cerebellum (Palmer et al. 1986).
Many other studies of the direct actions of acetaldehyde are available. For example, large doses of acetaldehyde given ICV caused decreases of dopamine, serotonin, and a product of dopamine metabolism (i.e., a metabolite) (i.e., homovanillic acid [HVA]) and increases in a metabolite of serotonin (i.e., 5-hydroxyindoleacetic acid [5HIAA]) in an analysis of fluid from the nucleus accumbens (Ward et al. 1997). These and similar reports do not provide a consistent dataset from which likely mechanisms can be deduced. Part of the problem is that accurate measurements of acetaldehyde in the brain tissue have been difficult (see Westcott et al. 1980), and so no systematic correlation of acetaldehyde brain levels with behavioral effects has been carried out. Mascia and colleagues (2001) studied the effect of acetaldehyde on cloned neurotransmitter receptors in frog oocytes. Of those studied, only a receptor for the amino acid glycine was sensitive to acetaldehyde.
In summary, research has now provided ample evidence that ethanol is metabolized to acetaldehyde and then acetate in the brain. Several studies also suggest that the presence of acetaldehyde in the brain is responsible for at least some of the effects of ethanol.
The field will advance most rapidly by simultaneous measurement of acetaldehyde in the brain or brain areas and correlating these levels with specific behavioral actions.
Acknowledgements
Work was supported by National Institute on Alcohol Abuse and Alcoholism grants R21–AA12284 and R01–AA12650.
Footnotes
A micromole represents a concentration of 1/1,000,000 (one millionth) molecular weight per liter (mol/L).
Km is a measurement used to describe the activity of an enzyme. It describes the concentration of the substance upon which an enzyme acts at which the enzyme works at 50 percent capacity.
To induce conditioned place preference, an animal is injected with the drug being studied and is placed in a test chamber with distinctive environmental cues. This procedure is repeated for several days. During these conditioning trials, the animal develops an association between the subjective state produced by the drug and the environmental cues present during the drug state. When the subject is tested in an apparatus that contains the drug-related environmental cues in one compartment and neutral cues in another, it voluntarily moves toward the compartment containing the drug-related cues.
Spectrophotometry is a determination of the concentration of a material in a sample by measurement of the amount of light the sample absorbs.
Financial Disclosure
The authors declare that they have no competing financial interests.
References
- Amir S. Brain and liver aldehyde dehydrogenase activity and voluntary ethanol consumption by rats: Relations to strain, sex and age. Psychopharmacology. 1978;57:97–102. doi: 10.1007/BF00426964. [DOI] [PubMed] [Google Scholar]
- Amit Z, Aragon CM. Catalase activity measured in rats naive to ethanol correlates with later voluntary ethanol consumption: Possible evidence for a biological marker system of ethanol intake. Psychopharmacology. 1988;95:512–515. doi: 10.1007/BF00172965. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Abitbol M, Amit Z. Ethanol-induced CTA mediated by acetaldehyde through central catecholamine activity. Psychopharmacology. 1991;103:74–77. doi: 10.1007/BF02244077. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Amit Z. Differences in ethanol-induced behaviors in normal and acatalasemic mice: Systematic examination using a biobehavioral approach. Pharmacology, Biochemistry, and Behavior. 1993;44:547–554. doi: 10.1016/0091-3057(93)90165-p. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Rogan F, Amit Z. Ethanol metabolism in rat brain homogenates by a catalaseH2O2 system. Biochemical Pharmacology. 1992;44:93–98. doi: 10.1016/0006-2952(92)90042-h. [DOI] [PubMed] [Google Scholar]
- Arizzi MN, Correa M, Betz AJ, et al. Behavioral effects of intraventricular injections of low doses of ethanol, acetaldehyde, and acetate in rats: Studies with low and high rate operant schedules. Behavioral Brain Research. 2003;147:203–210. doi: 10.1016/s0166-4328(03)00158-x. [DOI] [PubMed] [Google Scholar]
- Aspberg A, Söderbäck M, Tottmar O. Increase in catalase activity in developing rat brain cell reaggregation cultures in the presence of ethanol. Biochemical Pharmacology. 1993;46:1873–1876. doi: 10.1016/0006-2952(93)90598-q. [DOI] [PubMed] [Google Scholar]
- Beisswenger TB, Holmquist B, Vallee BL. Chi-ADH is the sole alcohol dehydrogenase isozyme of mammalian brains: Implications and inferences. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:8369–8373. doi: 10.1073/pnas.82.24.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi S, Govoni S, Rius RA, Trabucchi M. Acute ethanol and acetaldehyde administration produce similar effects on L-type calcium channels in rat brain. Alcohol. 1988;5:337–340. doi: 10.1016/0741-8329(88)90076-6. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Rockman GE. Intraventricular self-administration of acetaldehyde, but not ethanol, in naive laboratory rats. Psychopharmacology (Berl) 1979;64:271–276. doi: 10.1007/BF00427509. [DOI] [PubMed] [Google Scholar]
- Buhler R, Pestalozzi D, Hess M, Von Wartburg JP. Immunohistochemical localization of alcohol dehydrogenase in human kidney, endocrine organs and brain. Pharmacology, Biochemistry, and Behavior. 1983;18(Suppl 1):55–59. doi: 10.1016/0091-3057(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Carmichael FJ, Israel Y, Crawford M, et al. Central nervous system effects of acetate: Contribution to the central effects of ethanol. Journal of Pharmacology and Experimental Therapeutics. 1991;259:403–408. [PubMed] [Google Scholar]
- Chick J, Gough K, Falkowski W, et al. Disulfiram treatment of alcoholism. British Journal of Psychiatry. 1992;161:84–89. doi: 10.1192/bjp.161.1.84. [DOI] [PubMed] [Google Scholar]
- Cohen G, Collins M. Alkaloids from catecholamines in adrenal tissue: Possible role in alcoholism. Science. 1970;167:1749–1751. doi: 10.1126/science.167.3926.1749. [DOI] [PubMed] [Google Scholar]
- Cohen G, Sinet PM, Heikkila R. Ethanol oxidation by rat brain in vivo. Alcoholism: Clinical and Experimental Research. 1980;4(4):366–370. doi: 10.1111/j.1530-0277.1980.tb04833.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Ung-Chhun N, Cheng BY, Pronger D. Brain and plasma tetrahydroisoquinolines in rats: Effects of chronic ethanol intake and diet. Journal of Neurochemistry. 1990;55:1507–1514. doi: 10.1111/j.1471-4159.1990.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Ung-Chhun NS, Raikoff K. Stable acetaldehyde adducts with brain proteins (Abstract) Alcohol and Alcoholism. 1988;23:31. [Google Scholar]
- Correa A, Arizzi MN, Betz A, et al. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Research Bulletin. 2003;62:197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Correa M, Sanchis-Segura C, Aragon CM. Brain catalase activity is highly correlated with ethanol-induced locomotor activity in mice. Physiology and Behavior. 2001;73:641–647. doi: 10.1016/s0031-9384(01)00511-x. [DOI] [PubMed] [Google Scholar]
- Cullen N, Carlen PL. Electrophysiological actions of acetate, a metabolite of ethanol, on hippocampal dentate granule neurons: Interactions with adenosine. Brain Research. 1992;588:49–57. doi: 10.1016/0006-8993(92)91343-d. [DOI] [PubMed] [Google Scholar]
- Davis VE, Walsh MJ. Alcohol, amines and alkaloids: A possible biochemical basis for alcohol addiction. Science. 1970;167:1005–1007. doi: 10.1126/science.167.3920.1005. [DOI] [PubMed] [Google Scholar]
- Deitrich RA. Tissue and subcellular distribution of mammalian aldehyde-oxidizing capacity. Biochemical Pharmacology. 1966;15:1911–1922. doi: 10.1016/0006-2952(66)90220-6. [DOI] [PubMed] [Google Scholar]
- Deitrich R. Acetaldehyde: Déjà vu du jour. Journal of Studies on Alcohol. 2004;65:557–572. doi: 10.15288/jsa.2004.65.557. [DOI] [PubMed] [Google Scholar]
- Deitrich R, Erwin V. Biogenic amine-aldehyde condensation products: Tetrahydroisoquinolines and tryptolines (beta-carbolines) Annual Review of Pharmacology and Toxicology. 1980;20:55–58. doi: 10.1146/annurev.pa.20.040180.000415. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Deitrich RA. Brain aldehyde dehydrogenase. Localization, purification, and properties. Journal of Biological Chemistry. 1966;241:3533–3539. [PubMed] [Google Scholar]
- Gill K, Menez JF, Lucas D, Deitrich RA. Enzymatic production of acetaldehyde from ethanol in rat brain tissue. Alcoholism: Clinical and Experimental Research. 1992;16:910–915. doi: 10.1111/j.1530-0277.1992.tb01892.x. [DOI] [PubMed] [Google Scholar]
- Giri PR, Linnoila M, O’Neill JB, Goldman D. Distribution and possible metabolic role of class III alcohol dehydrogenase in the human brain. Brain Research. 1989;481:131–141. doi: 10.1016/0006-8993(89)90493-9. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Zaechelein R. Time course of functional tolerance produced in mice by inhalation of ethanol. Journal of Pharmacology and Experimental Therapeutics. 1983;227:150–153. [PubMed] [Google Scholar]
- Hamby-Mason R, Chen JJ, Schenker S, et al. Catalase mediates acetaldehyde formation from ethanol in fetal and neonatal rat brain. Alcoholism: Clinical and Experimental Research. 1997;21:1063–1072. [PubMed] [Google Scholar]
- Hansson T, Tindberg N, Ingelman-Sundberg M, Kohler C. Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience. 1990;34:451–463. doi: 10.1016/0306-4522(90)90154-v. [DOI] [PubMed] [Google Scholar]
- Harada S, Agarwal DP, Goedde HW, et al. Possible protective role against alcoholism for aldehyde dehydrogenase isozyme deficiency in Japan. Lancet. 1982;9(8302):827. doi: 10.1016/s0140-6736(82)92722-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Ueha T, Kuriyama T, et al. Acetaldehyde-induced alterations in metabolism of monoamines in mouse brain. Alcohol and Alcoholism. 1989;24:91–99. doi: 10.1093/oxfordjournals.alcalc.a044889. [DOI] [PubMed] [Google Scholar]
- Heap L, Ward RJ, Abiaka C, et al. The influence of brain acetaldehyde on oxidative status, dopamine metabolism and visual discrimination task. Biochemical Pharmacology. 1995;50:263–270. doi: 10.1016/0006-2952(94)00539-x. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Role of acetaldehyde in the actions of ethanol on the brain: A review. Alcohol. 1996;13:147–151. doi: 10.1016/0741-8329(95)02026-8. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Kumihashi M, et al. Microdialysis for the determination of acetaldehyde and ethanol concentrations in the striatum of freely moving rats. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2003;798:155–158. doi: 10.1016/j.jchromb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Kumihashi M, et al. Failure of ethanol and acetaldehyde to alter in vivo norepinephrine release in the striatum and hippocampus of rats. Archives of Toxicology. 2004;78:723–727. doi: 10.1007/s00204-004-0600-1. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Wang W, et al. Inhibition of acetaldehyde metabolism decreases acetylcholine release in medial frontal cortex of freely moving rats. Brain Research. 2005;1039:90–96. doi: 10.1016/j.brainres.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Jennett RB, Sorrell MF, Johnson EL, Tuma DJ. Covalent binding of acetaldehyde to tubulin: Evidence for preferential binding to the alpha-chain. Archives of Biochemistry and Biophysics. 1987;256:10–18. doi: 10.1016/0003-9861(87)90420-6. [DOI] [PubMed] [Google Scholar]
- Kenney WC. Acetaldehyde adducts of phospholipids. Alcoholism: Clinical and Experimental Research. 1982;6:412–416. doi: 10.1111/j.1530-0277.1982.tb05000.x. [DOI] [PubMed] [Google Scholar]
- Kenney WC. Formation of Schiff base adduct between acetaldehyde and rat liver microsomal phosphatidylethanolamine. Alcoholism: Clinical and Experimental Research. 1984;8:551–555. doi: 10.1111/j.1530-0277.1984.tb05728.x. [DOI] [PubMed] [Google Scholar]
- Kerr JT, Maxwell DS, Crabb DW. Immunocytochemistry of alcohol dehydrogenase in the rat central nervous system. Alcoholism: Clinical and Experimental Research. 1989;13:730–736. doi: 10.1111/j.1530-0277.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Khalighi M, Brzezinski MR, Chen H, Juchau MR. Inhibition of human prenatal biosynthesis of all-trans-retinoic acid by ethanol, ethanol metabolites, and products of lipid peroxidation reactions: A possible role for CYP2E1. Biochemical Pharmacology. 1999;57:811–821. doi: 10.1016/s0006-2952(98)00362-1. [DOI] [PubMed] [Google Scholar]
- Koechling UM, Amit Z. Effects of 3amino-1,2,4-triazole on brain catalase in the mediation of ethanol consumption in mice. Alcohol. 1994;11:235–239. doi: 10.1016/0741-8329(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Koechling UM, Amit Z. Relationship between blood catalase activity and drinking history in a human population, a possible biological marker of the affinity to consume alcohol. Alcohol and Alcoholism. 1992;27:181–188. [PubMed] [Google Scholar]
- Luckey SW, Tjalkens RB, Petersen DR. Mechanism of inhibition of rat liver class 2 ALDH by 4-hydroxynonenal. Advances in Experimental Medicine and Biology. 1999;463:71–77. doi: 10.1007/978-1-4615-4735-8_9. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Waller MB, McBride WJ, Li TK. Different sensitivities to ethanol in alcohol-preferring and -nonpreferring rats. Pharmacology, Biochemistry, and Behavior. 1982;16:125–130. doi: 10.1016/0091-3057(82)90023-5. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, et al. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amy loid β-peptide. Journal of Neurochemistry. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Martinez SE, Vaglenova J, Sabria J, et al. Distribution of alcohol dehydrogenase mRNA in the rat central nervous system: Consequences for brain ethanol and retinoid metabolism. European Journal of Biochemistry. 2001;268:5045–5056. [PubMed] [Google Scholar]
- Mascia MP, Maiya R, Borghese CM, et al. Does acetaldehyde mediate ethanol action in the central nervous system? Alcoholism: Clinical and Experimental Research. 2001;25:1570–1575. [PubMed] [Google Scholar]
- McBride WJ, Li TK, Deitrich RA, et al. Involvement of acetaldehyde in alcohol addiction. Alcoholism: Clinical and Experimental Research. 2002;26:114–119. [PubMed] [Google Scholar]
- McKinnon G, De Jersey J, Shanley B, Ward L. The reaction of acetaldehyde with brain microtubular proteins: Formation of stable adducts and inhibition of polymerization. Neuroscience Letters. 1987;79:163–168. doi: 10.1016/0304-3940(87)90690-2. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Myers RD. Preference for alcohol evoked by tetrahydropapaveroline (THP) chronically infused in the cerebral ventricle of the rat. Pharmacology, Biochemistry, and Behavior. 1977;7:19–35. doi: 10.1016/0091-3057(77)90006-5. [DOI] [PubMed] [Google Scholar]
- Meyer MJ, Mosley DE, Amarnath V, Picklo MJ., Sr. Metabolism of 4-hydroxy-trans2-nonenal by central nervous system mitochondria is dependent on age and NAD+ availability. Chemical Research in Toxicology. 2004;9:1272–1279. doi: 10.1021/tx049843k. [DOI] [PubMed] [Google Scholar]
- Mukherji B, Kashiki Y, Ohyanagi H, Sloviter HA. Metabolism of ethanol and acetaldehyde by the isolated perfused rat brain. Journal of Neurochemistry. 1975;24:841–843. [PubMed] [Google Scholar]
- Murphy TC, Amarnath V, Picklo MJ., Sr. Mitochondrial oxidation of 4-hydroxy-2-nonenal in rat cerebral cortex. Journal of Neurochemistry. 2003;84:1313–1321. doi: 10.1046/j.1471-4159.2003.01628.x. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G. Intravenous self-administration of acetaldehyde in the rat as a function of schedule, food deprivation and photoperiod. Pharmacology, Biochemistry, and Behavior. 1982;17:807–811. doi: 10.1016/0091-3057(82)90364-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Iwahashi K, Furukawa A, et al. Acetaldehyde adducts in the brain of alcoholics. Archives of Toxicology. 2003;77:591–593. doi: 10.1007/s00204-003-0465-8. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Tottmar O. Biogenic aldehydes in brain: Characteristics of a reaction between rat brain tissue and indole-3-acetaldehyde. Journal of Neurochemistry. 1985;45:744–751. doi: 10.1111/j.1471-4159.1985.tb04055.x. [DOI] [PubMed] [Google Scholar]
- Palmer MR, Tottmar O, Deitrich RA. Electrophysiological effects of monoamine-derived aldehydes on single neurons in neocortex and cerebellum in rats. Alcoholism: Clinical and Experimental Research. 1986;10:682–685. doi: 10.1111/j.1530-0277.1986.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Petersen DR. Aldehyde dehydrogenase and aldehyde reductase in isolated bovine brain microvessels. Alcohol. 1985;2:79–83. doi: 10.1016/0741-8329(85)90020-5. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: A comprehensive review of animal studies. Progress in Neurobiology. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Raskin NH. Alcohol dehydrogenase in brain: A toxicologic role? Annals of the New York Academy of Sciences. 1973;215:49–53. doi: 10.1111/j.1749-6632.1973.tb28247.x. [DOI] [PubMed] [Google Scholar]
- Raskin NH, Sokoloff L. Enzymes catalysing ethanol metabolism in neural and somatic tissues of the rat. Journal of Neurochemistry. 1972a;19:273–282. doi: 10.1111/j.1471-4159.1972.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Raskin NH, Sokoloff L. Ethanol-induced adaptation of alcohol dehydrogenase activity in rat brain. Nature: New Biology. 1972b;236:138–140. doi: 10.1038/newbio236138a0. [DOI] [PubMed] [Google Scholar]
- Raskin NH, Sokoloff L. Adaptation of alcohol dehydrogenase activity in brain to chronic ethanol ingestion. Neurology. 1970a;20:391–392. [PubMed] [Google Scholar]
- Raskin NH, Sokoloff L. Alcohol dehydrogenase activity in rat brain and liver. Journal of Neurochemistry. 1970b;17:1677–1687. doi: 10.1111/j.1471-4159.1970.tb11392.x. [DOI] [PubMed] [Google Scholar]
- Raskin NH, Sokoloff L. Brain alcohol dehydrogenase. Science. 1968;162:131–132. doi: 10.1126/science.162.3849.131. [DOI] [PubMed] [Google Scholar]
- Raskin NH, Sokoloff L. Changes in brain alcohol dehydrogenase activity during chronic ethanol ingestion and withdrawal. Journal of Neurochemistry. 1974;22:427–434. doi: 10.1111/j.1471-4159.1974.tb07609.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, et al. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Melendez RI, Zaffaroni A, et al. The reinforcing effects of acetaldehyde in the posterior ventral tegmental area of alcohol-preferring rats. Pharmacology, Biochemistry, and Behavior. 2002;72:55–64. doi: 10.1016/s0091-3057(01)00733-x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Miquel M, Correa M, Aragon CM. The catalase inhibitor sodium azide reduces ethanol-induced locomotor activity. Alcohol. 1999;19:37–42. doi: 10.1016/s0741-8329(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Sandri G, Panfili E, Ernster Hydrogen peroxide production by monoamine oxidase in isolated rat-brain mitochondria: Its effect on glutathione levels and Ca2+ efflux. Biochimica et Biophysica Acta. 1990;1035:300–305. doi: 10.1016/0304-4165(90)90092-b. [DOI] [PubMed] [Google Scholar]
- Simonson SG, Zhang J, Canada AT, Jr, et al. Hydrogen peroxide production by monoamine oxidase during ischemia-reperfusion in the rat brain. Journal of Cerebral Blood Flow and Metabolism. 1993;13:125–134. doi: 10.1038/jcbfm.1993.15. [DOI] [PubMed] [Google Scholar]
- Sinet PM, Heikkila RE, Cohen G. Hydrogen peroxide production by rat brain in vivo. Journal of Neurochemistry. 1980;34:1421–1428. doi: 10.1111/j.1471-4159.1980.tb11222.x. [DOI] [PubMed] [Google Scholar]
- Sippel HW. The acetaldehyde content in rat brain during ethanol metabolism. Journal of Neurochemistry. 1974;23:451–452. doi: 10.1111/j.1471-4159.1974.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Sippel HW, Eriksson CJ. The acetaldehyde content in rat brain during ethanol oxidation. In: Lindros KO, Eriksson CJ, editors. The Role of Acetaldehyde in the Actions of Ethanol, Satellite Symposium 6th International Congress Pharmacology. Helsinki, Finland: The Finnish Foundation for Alcohol Studies; 1975. pp. 149–157. [Google Scholar]
- Smith BR, Amit Z, Splawinsky J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol. 1984;1:193–195. doi: 10.1016/0741-8329(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Sohda T, Shimizu M, Kamimura S, Okumura M. Immunohistochemical demonstration of ethanol-inducible P450 2E1 in rat brain. Alcohol and Alcoholism Supplement. 1993;1B:69–75. [PubMed] [Google Scholar]
- Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: The 2002 update. Chemico-biological Interactions. 2003;143–144:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]
- Sutherland VC, Burbridge TN, Simon A. Metabolism of C-114 ethanol to C14O2 by cerebral cortex in vitro. Federation Proceedings. 1958;17:413. [Google Scholar]
- Tabakoff B, Anderson RA, Ritzmann RF. Brain acetaldehyde after ethanol administration. Biochemical Pharmacology. 1976;25:1305–1309. doi: 10.1016/0006-2952(76)90094-0. [DOI] [PubMed] [Google Scholar]
- Tampier L, Cariz S, Quintanilla ME. Metabolism of acetaldehyde by rat isolated aortic rings: Does endothelial tissue contribute to its extrahepatic metabolism? Alcohol. 1993;10:203–206. doi: 10.1016/0741-8329(93)90036-n. [DOI] [PubMed] [Google Scholar]
- Thadani PV, Truitt EB., Jr Effect of acute ethanol or acetaldehyde administration on the uptake, release, metabolism and turnover of norepinephrine in rat brain. Biochemical Pharmacology. 1977;26:1147–1150. doi: 10.1016/0006-2952(77)90059-4. [DOI] [PubMed] [Google Scholar]
- Tindberg N, Ingelman-Sundberg M. Expression, catalytic activity, and inducibility of cytochrome P450 2E1 (CYP2E1) in the rat central nervous system. Journal of Neurochemistry. 1996;67:2066–2073. doi: 10.1046/j.1471-4159.1996.67052066.x. [DOI] [PubMed] [Google Scholar]
- Trudell JR, Ardies CM, Anderson WR. Cross-reactivity of antibodies raised against acetaldehyde adducts of protein with acetaldehyde adducts of phosphatidyl-ethanolamine: Possible role in alcoholic cirrhosis. Molecular Pharmacology. 1990;38:587–593. [PubMed] [Google Scholar]
- Trudell JR, Ardies CM, Green CE, Allen K. Binding of anti-acetaldehyde IgG antibodies to hepatocytes with an acetaldehyde-phosphatidylethanolamine adduct on their surface. Alcoholism: Clinical and Experimental Research. 1991;15:295–299. doi: 10.1111/j.1530-0277.1991.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Upadhya SC, Tirumalai PS, Boyd MR, et al. Cytochrome P4502E (CYP2E) in brain: Constitutive expression, induction by ethanol and localization by fluorescence in situ hybridization. Archives of Biochemistry and Biophysics. 2000;373:23–34. doi: 10.1006/abbi.1999.1477. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metabolism Reviews. 2004;36:279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Zeigler TL, Bludeau P, et al. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacognetics and Genomics. 2006;16(1):51–58. doi: 10.1097/01.fpc.0000182777.95555.56. [DOI] [PubMed] [Google Scholar]
- Wang M, McIntee EJ, Cheng G, et al. Identification of DNA adducts of acetaldehyde. Chemical Research in Toxicology. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Colantuoni C, Dahchour A, et al. Acetaldehyde-induced changes in the monoamine and amino acid extracellular microdialysate content of the nucleus accumbens. Neuropharmacology. 1997;36:225–232. doi: 10.1016/s0028-3908(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Warner M, Gustafsson JA. Effect of ethanol on cytochrome P450 in the rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1019–1023. doi: 10.1073/pnas.91.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott JY, Weiner H, Schultz J, Myers RD. In vivo acetaldehyde in the brain of the rat treated with ethanol. Biochemical Pharmacology. 1980;29:411–417. doi: 10.1016/0006-2952(80)90521-3. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Deitrich R. Ethanol metabolism in the brain. Addiction Biology. 1997;2:387–399. doi: 10.1080/13556219772444. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Lindros KO. Distribution of catalase in rat brain: Aminergic neurons as possible targets for ethanol effects. Alcohol and Alcoholism. 1996;31:167–174. doi: 10.1093/oxfordjournals.alcalc.a008128. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Liopo AV, Deitrich RA. Distribution and kinetics of ethanol metabolism in rat brain. Alcoholism: Clinical and Experimental Research. 1998;22:1623–1627. [PubMed] [Google Scholar]
- Zimatkin SM, Liopo AV, Deitrich RA. Oxidation of ethanol to acetaldehyde in brain and the possible behavioral consequences. Advances in Experimental Medicine and Biology. 1999;463:231–236. doi: 10.1007/978-1-4615-4735-8_28. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko PS. Levels of ethanol and acetaldehyde and morphological disturbances in brain following administration of alcohol and ALDH inhibitors. In: Jacob B, Bonte W, editors. International Association of Forensic Science. Verlag, Berlin: 1995. pp. 1–4. [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, et al. Enzymatic mechanisms of ethanol oxidation in the brain. Alcoholism: Clinical and Experimental Research. 2006;30:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Rout UK, Koivusalo M, et al. Regional distribution of low-Km mitochondrial aldehyde dehydrogenase in the rat central nervous system. Alcoholism: Clinical and Experimental Research. 1992;16:1162–1167. doi: 10.1111/j.1530-0277.1992.tb00713.x. [DOI] [PubMed] [Google Scholar]