Abstract
Studies have reported associations between cortical thickness (CT) and socioeconomic status (SES), as well as between CT and cognitive outcomes. However, findings have been mixed as to whether CT explains links between SES and cognitive performance. In the current study, we hypothesized that this inconsistency may have arisen from the fact that socioeconomic factors (family income and parental education) may moderate the relation between CT and neurocognitive skills. Results indicated that associations between CT and cognitive performance did vary by SES for both language and executive function (EF) abilities. Across all ages, there was a negative correlation between CT and cognitive skills, with thinner cortices associated with higher language and EF scores. Similarly, across all cognitive skills, children from higher-SES homes outperformed their age-matched peers from lower-SES homes. Moderation analyses indicated that the impact of SES was not constant across CT, with SES more strongly predictive of EF skills among children with thicker cortices and more strongly predictive of language skills among children with thinner cortices. This suggests that socioeconomic advantage may in some cases buffer against a neurobiological risk factor for poor performance. These findings suggest that links between brain structure and cognitive processes vary by family socioeconomic circumstance.
Keywords: Cortical thickness, Socioeconomic status, Cognition
1. Introduction
Extensive research has demonstrated socioeconomic disparities in brain structure and function (Brito & Noble, 2014; Hackman & Farah, 2009; Hackman, Farah, & Meaney, 2010; Lawson, Duda, Avants, Wu, & Farah, 2013; Luby et al., 2013; Menary et al., 2013; Noble, Grieve, et al., 2012; Noble, Houston, Kan, &Sowell, 2012; Noble et al., 2015; Noble, Korgaonkar, Grieve, & Brickman, 2013). However, it remains largely unknown how socioeconomic status (SES) shapes the development of brain structures and consequently affects cognitive performance. As brain development follows a nonlinear path (Giedd et al., 1999; Gogtay et al., 2004; Shaw et al., 2006), and varies from one cortical region to the next (Sowell et al., 2003), the association between socioeconomic status (SES) and children’s cognitive development may be more pronounced at different stages of neural development and may relate more strongly to some cognitive processes relative to others (Casey, Giedd, & Thomas, 2000; Hackman & Farah, 2009). Several lines of evidence point to this possibility.
First, reported links between socioeconomic disparities and brain structure vary depending upon the morphometric property being studied. The evidence for associations among socioeconomic factors and cortical volume are mixed and discrepant, depending in part on which brain regions are investigated (Brito & Noble, 2014). Some studies report no associations (Brain Development Cooperative Group, 2012; Gianaros et al., 2007; Lange, Froimowitz, Bigler, Lainhart, & Brain Development Cooperative Group, 2010) whereas others do observe relations (Butterworth, Cherbuin, Sachdev, & Anstey, 2012; Cavanagh et al., 2013; Hair, Hanson, Wolfe, & Pollak, 2015; Hanson, Chandra, Wolfe, & Pollak, 2011; Hanson et al., 2013; Jednoróg et al., 2012; Luby et al., 2013; Noble, Grieve, et al., 2012; Noble, Houston, et al., 2012; Raizada, Richards, Meltzoff, & Kuhl, 2008; Staff et al., 2012). However, cortical volume is a composite measure of cortical surface area (SA) and cortical thickness (CT), two distinct properties of the cortex that have different cellular and genetic bases (Raznahan et al., 2011; Winkler et al., 2012). Both SA and CT have a dynamic and heterogeneous development throughout the lifespan and vary depending on a multitude of factors including an individual’s age, IQ, and SES (Brito & Noble, 2014; Lawson et al., 2013; Mackey et al., 2015; Noble et al., 2015; Raznahan et al., 2011; Schnack et al., 2014; Shaw et al., 2006). Therefore, it is most appropriate to study these two cortical measures separately, as the composite metric of volume may oversimplify nuances in structural brain development. One study has reported a positive association between socioeconomic factors and SA (Noble et al., 2015), whereas the findings linking socioeconomic factors and CT have been mixed (Lawson et al., 2013; Mackey et al., 2015; Noble et al., 2015; Piccolo, Merz, He, Sowell, & Noble, 2016).
Secondly, the links between structural brain development and cognitive skills are also variable (Shaw et al., 2006). Cortical thickness has been related to cognitive outcomes, but not consistently, and associations are commonly specific to the particular brain region or cognitive function of interest. Indeed, longitudinal studies have demonstrated that it is the trajectory of cortical thickness, rather than the absolute value at any one time-point, that is most closely related to intelligence. In childhood, thinner cortices tend to be associated with higher IQ, whereas, in late childhood and beyond, thicker cortices tend to be related to higher IQ (Schnack et al., 2014; Shaw et al., 2006). Further, some studies have reported age-related localized cortical thickening in frontal and temporal regions during childhood and adolescence (Shaw et al., 2008; Sowell et al., 2004). In relation to brain and behavior, studies have observed an association between thicker cortices in the inferior frontal gyrus and better visual-motor processing in late adolescents/young adults (Menary et al., 2013), as well as an association between thicker cortices in the middle frontal gyrus and increased long-term verbal memory in adults (Walhovd et al., 2006). On the other hand, frontal lobe gray matter thinning has been predictive of better verbal learning (Sowell, 2001), verbal fluency (Porter, Collins, Muetzel, Lim, & Luciana, 2011), and working memory scores (Kharitonova, Martin, Gabrieli, & Sheridan, 2013; Tamnes et al., 2010) in children and adolescents.
Finally, when studies have examined whether SES and CT independently account for differences in cognitive performance, findings have been mixed. For example, Mackey et al. (2015) analyzed data from a sample of 58 adolescents and observed that higher family income was associated with greater CT in all lobes of the brain, with greater CT associated with better standardized test performance in reading and math. In contrast, in a sample of 1099 children and adolescents, Noble et al. (2015) did not find associations between either parental education or family income and CT. Further, although these authors reported that cortical surface area mediated links between family income and specific neurocognitive skills, cortical thickness did not.
These inconsistencies could potentially be explained if the relation between CT and cognition varies as a function of socioeconomic factors, as opposed to CT and SES each accounting for independent, unique variance in cognition. Indeed, past work has suggested that SES may serve as a moderating role in explaining age-related differences in brain structure (Noble, Grieve, et al., 2012; Piccolo et al., 2016) as well as in explaining links between brain function and behavior (Noble, Wolmetz, Ochs, Farah, & McCandliss, 2006). Therefore, using the same dataset as Noble et al. (2015), we hypothesized that, rather than CT mediating links between SES and cognitive performance, SES may moderate (either buffer or amplify) associations among brain structures and neurocognitive skills during childhood. In addition, we hypothesized that SES moderation may be most prominent in certain regions of interest (ROIs) in which CT has previously been related to cognitive function (Kharitonova et al., 2013; Lawson et al., 2013; Mackey et al., 2015; Menary et al., 2013; Porter et al., 2011; Walhovd et al., 2006). These included regions associated with vocabulary and oral reading (left inferior frontal gyrus, left superior temporal gyrus, and left fusiform gyrus), as well as those associated with working memory, attention/inhibition and cognitive flexibility (middle frontal gyrus and anterior cingulate cortex).
2. Methods
2.1. Participants
Data used in this study were collected as part of the multi-site Pediatric Imaging, Neurocognition, and Genetics (PING) study and obtained from the PING Study database (http://ping.chd.ucsd.edu). Participants were recruited through a combination of web-based, word-of-mouth, and community advertising at nine university-based data collection sites in and around the cities of Los Angeles, San Diego, New Haven, Sacramento, San Diego, Boston, Baltimore, Honolulu, and New York. Participants were excluded if they had a history of neurological, psychiatric, medical, or developmental disorders. In this study, analyses were conducted on the 1091 participants, ranging from 3 to 20 years old (M = 11.9, SD = 4.9). All participants and their parents gave their informed written consent/assent to participate in all study procedures, including whole genome SNP genotype, demographic and developmental history questionnaires, and high-resolution brain MRI (see Table 1 for demographics). Each data collection site’s Office of Protection of Research Subjects and Institutional Review Board approved the study.
Table 1.
Sample demographics (N = 1091).
| Mean (SD; Range) or N (%) | |
|---|---|
| Age (years) | 11.9 (4.9; 3.0–20.9) |
| Sex | |
| Male | 562 (51.5%) |
| Female | 529 (48.5%) |
| Parental education (years) | 15.57 (2.23; 6–18) |
| Family Income | $97,878 ($76,756; $4,500–325,000) |
| Genetic ancestry | |
| African | 0.12 (0.26; 0–1) |
| American Indian | 0.05 (0.11; 0–1) |
| Central Asian | 0.02 (0.12; 0–1) |
| East Asian | 0.16 (0.31; 0–1) |
| European | 0.64 (0.37; 0–1) |
| Oceanic | 0.01 (0.03; 0–1) |
Note. GAF data show mean, standard deviation, and range across all subjects of the estimated proportion of genetic ancestry for each reference population.
2.2. Measures
2.2.1. Socioeconomic status
Parents were asked to report the level of educational attainment for all parents in the home. Parents were also asked to report the total yearly family income. Data were not collected on the number of adults and children in the home, and therefore income-to-needs ratios were unable to be calculated. Both parental education and family income data were originally collected in bins, which were recoded as the means of each bin (Noble et al., 2015). Family income was natural log-transformed for all analyses due to the typically observed positive skew.
2.2.2. Genetic collection and analysis
Saliva samples were sent to Scripps Translational Research Institute for analysis. Once extracted, genomic DNA was genotyped with Illumina Human660W-Quad BeadChip. Replication and quality control filters (i.e., sample call rate > 99, call rates > 95%, minor allele frequency > 5%) were performed. To assess genetic ancestry and admixture proportions in the PING participants, a supervised clustering approach implemented in the ADMIXTURE software was used (Alexander & Lange, 2011). A genetic ancestry factor (GAF) was developed for each participant, representing the proportion of ancestral descent for each of six major continental populations: African, Central Asian, East Asian, European, Native American and Oceanic. Information on PING genetic collection and analysis is described in detail in Fjell et al. (2012) and Akshoomoff et al. (2014).
2.2.3. Image acquisition and processing
Each site administered a standardized high-resolution structural MRI (3D Tl-weighted scan) protocol (Fjell et al., 2012 for pre- and post-processing techniques information). Image analyses were performed using a modified Freesurfer software suite (http://surfer.nmr.mgh.harvard.edu/) to obtain vertex-wise CT (Fischl & Dale, 2000). Neuroimaging data was submitted to a standardized quality-image check, with no manual editing of images that were deemed acceptable for inclusion in the database (see Jernigan, Brown, Bartsch, & Dale, 2016 for details).
2.2.4. Cognitive measures
Performance on vocabulary, reading, working memory, attention/inhibition and cognitive flexibility tasks were evaluated using NIH Toolbox® Cognitive Function Battery (Weintraub et al., 2013), as described below.
2.2.4.1. Picture vocabulary test
This measure of receptive vocabulary was administered in a computerized adaptive format. The participant was presented with an auditory recording of a word and four high-resolution color photos on the computer screen. Then, they were instructed to touch the image that most closely represents the meaning of the auditory word. Each participant was given two practice trials and 25 test trials. Participant performance was converted to a theta score (ranging from −4 to 4), based on item response theory.
2.2.4.2. Oral reading recognition test
In this reading test, participants were asked to read aloud a word or letter presented on the computer screen. Items were presented in an order of increasing difficulty. Responses were recorded as correct or incorrect by the examiner. In order to assess the full range of reading ability across multiple ages, modifications were made and letters or multiple-choice ‘pre-reading’ items were presented to young children or participants with low literacy levels. The oral reading score ranged from 1 to 281.
2.2.4.3. List sorting working memory test
This working memory task requires immediate recall and sequencing of visually and orally presented stimuli (Tulsky et al., 2013). Participants were presented with a series of pictures of different animals and food on a computer screen and heard the name of the object from a speaker. The test was divided into the One-List and Two-List conditions. In the One-List condition, participants were told to remember a series of objects (food or animals) and repeat them in order, from smallest to largest. In the Two-List condition, participants were told to remember a series of objects (food and animals, intermixed) and then again report the food in order of size, followed by animals in order of size. Working memory scores consisted of combined total items correct on both conditions, with a max of 28 points.
2.2.4.4. Flanker inhibitory control and attention test
The NIH Toolbox Cognition Battery version of the flanker task was adapted from the Attention Network Test (ANT) (Rueda et al., 2004). Participants were asked to focus on a given stimulus, presented on the center of a computer screen and were required to indicate the left-right orientation while inhibiting attention to the flankers (surrounding stimuli: fish for ages 3–7 or arrows for ages 8–21). On some trials the orientation of the flankers was congruent with the orientation of the central stimulus and on the other trials the flankers were incongruent. The test consisted of a block of 25 fish trials (designed to be more engaging and easier for children) and a block of 25 arrow trials, with 16 congruent and nine incongruent trials in each block, presented in pseudorandom order. All children age 9 and above received both the fish and arrows blocks regardless of performance. The NIH Toolbox flanker vector score incorporates both the congruent and incongruent trials. A two-vector method was used that incorporated both accuracy and reaction time (RT) for participants who maintained a high level of accuracy (> 80% correct), and accuracy only for those who did not meet this criterion. Each vector score ranged from 0 to 5, for a maximum total score of 10.
2.2.4.5. Dimensional change card sort cognitive flexibility task
The DCCS is a measure of cognitive flexibility or set shifting. Participants are shown two target pictures, one on each side of the screen, which varies along two dimensions (e.g., shape and color). Participants are asked to match a series of bivalent test pictures (e.g., yellow trucks and red balls) to the target pictures, first according to one dimension (e.g., color) and then, after a number of trials, according to the other dimension (e.g., shape). “Switch” trials are employed in which the participant must change the dimension being matched. For example, after 4 straight trials matching on shape, the participant may be asked to match on color on the next trial and then go back to shape, thus requiring the cognitive flexibility to quickly choose the correct stimulus. A two-vector scoring method was used that incorporated both accuracy and reaction time (RT) for participants who maintained a high level of accuracy (> 80% correct), and accuracy only for those who did not meet this criterion. Each vector score ranged from 0 to 5, for a maximum total score of 10.
2.3. Analysis plan
Analyses were conducted on the 1091 participants for whom complete data were available on all relevant independent variables (age, sex, parental educational attainment, family income, genetic ancestry, scanner model, test site, and CT) and at least one dependent cognitive measure from the NIH Toolbox Cognitive Function Battery (Akshoomoff et al., 2014; Weintraub et al., 2013). Descriptive statistics and sample sizes for each NIH Toolbox cognitive variable are shown in Table 2. All measures were normally distributed (±2 values for skewness and kurtosis) except for Flanker and DCCS scores; therefore, these values were log-transformed prior to analyses. Family income was natural-log transformed and all outcome variables were winsorized to adjust for outliers. Additionally, for moderation analyses, Mahalanobis distance, Cook’s distance, and leverage values were calculated to determine dependent variable (cognitive skills) outliers based on independent variables of interest (CT and SES). Observations deemed highly influential or extreme were excluded from analyses. All cognitive values are represented in z-scores to enable comparison across tasks. All analyses were conducted in IBM SPSS (version 23).
Table 2.
NIH toolbox cognitive measures.
| Mean (SD; Range) | |
|---|---|
| Vocabulary | |
| Picture Vocabulary Test (n = 1090) | 0.67 (1.41; −3.5 to 4) |
| Oral Reading | |
| Oral Recognition Test (n = 1076) | 125.85 (68.25; 1–281) |
| Working Memory | |
| List Sorting Task (n = 1084) Flanker | 17.71 (5.39; 0–28) |
| Flanker | |
| Flanker Task Congruent & Incongruent Trials (n = 1074) | 7.67 (1.85; 0.8–9.9) |
| Cognitive Flexibility | |
| Dimensional Change Card Sort Task (n = 985) | 7.68 (1.52; 2–10) |
Associations between SES variables, cognitive skills, and CT controlled for age, age-squared, sex, scanner model, test site, and genetic ancestry (GAF). Our CT ROIs included the left inferior frontal gyrus (IFG), left superior temporal gyrus (STG), and left fusiform gyrus (which support vocabulary and oral reading) and the middle frontal gyrus (MFG; both left and right) and anterior cingulate cortex (ACC) (which support working memory, attention/inhibition and cognitive flexibility), as these regions have been implicated in past studies of CT and cognitive function (Kharitonova et al., 2013; Lawson et al., 2013; Mackey et al., 2015; Menary et al., 2013; Porter et al., 2011; Walhovd et al., 2006).
Moderation analyses were conducted using PROCESS (Hayes, 2015) to examine CT * SES interactions, controlling for all variables specified above. To probe interactions, the Johnson-Neyman (JN) technique (Bauer & Curran, 2005; Johnson & Neyman, 1936) was utilized. Unlike simple slopes analyses (Aiken & West, 1991), the JN technique does not require the selection of arbitrary moderator values to estimate the conditional effect of X on Y. This technique allows us to visualize the points along the continuum of the moderator where the conditional effect of X on Y transitions between statistically significant and not significant at the alpha (0.05) level of significance (Hayes, 2015). Standardized coefficients are reported for all multiple regression analyses and unstandardized coefficients (with heteroskedasticity-consistent standard errors) are reported for all analyses reporting interactions. All multiple comparisons (number of ROIs and cognitive tasks for each domain of interest) were verified using False Discovery Rate (FDR) analyses.
3. Results
3.1. SES is associated with cognitive skills but not CT
As previously reported (Noble et al., 2015), family income and parental education were not significant predictors of whole-brain average CT (p’s > 0.22). Additionally, no significant associations were found when examining associations between SES factors and CT for specific brain regions of interest (Left IFG, Left STG, MFG, or ACC). Both SES measures did, however, predict performance on all cognitive skills of interest. Higher family income was significantly related to higher vocabulary (β = 0.12, p < 0.001, Adj. R2= 0.75), reading (β = 0.08, p < 0.001, Adj. R2= 0.78), working memory (β = 0.08, p < 0.001, Adj. R2 = 0.70), flanker (β = 0.03, p =0.09, Adj. R2= 0.60) and cognitive flexibility (β = 0.06, p =0.006, Adj. R2= 0.65) scores. Higher parental education was also related to higher vocabulary (β = 0.14, p < 0.001, Adj. R2 =0.76), reading, (β = 0.11, p < 0.001, Adj. R2= 0.79), working memory (β = 0.09, p < 0.001, Adj. R2 = 0.70), flanker (β =0.06, p = 0.01, Adj. R2 = 0.59), and cognitive flexibility (β = 0.06, p= 0.002, Adj. R2 =0.65) scores.
3.2. Links between CT and cognitive skills vary by SES
3.2.1. CT associations with EF are moderated by family income
Next, we examined whether family income moderated the associations between whole-brain average CT and EF measures. The income * average (whole-brain) CT interaction term was significant for flanker (B = 0.38, p= 0.02, R2= 0.61) and DCCS (B =0.43, p = 0.01, R2= 0.66) (Table 3). Examining specific brain regions of interest, family income * CT interactions were found in MFG for flanker (B = 0.28, p= 0.01, R2 = 0.60) and DCCS (B = 0.32, p = 0.02, R2= 0.66), and in ACC for DCCS (B = 0.26, p =0.04, R2= 0.66), but none passed FDR corrections.
Table 3.
SES * CT interactions for cognitive outcomes.
| WM | Flanker | DCCS | Vocabulary | Reading | |||||||||||
| R2 | 0.71 |
0.61 |
0.66 |
0.76 |
0.79 |
||||||||||
| B | t | p | B | t | p | B | t | p | B | t | p | B | t | p | |
| Family Income Models | |||||||||||||||
| Age | 0.57 | 27.41 | <0.001 | 0.61 | 17.23 | < 0.001 | 0.62 | 16.89 | <0.001 | 0.36 | 19.34 | <0.001 | 0.38 | 22.67 | <0.001 |
| Age-Squared | −0.02 | −21.93 | <0.001 | −0.02 | −15.90 | < 0.001 | −0.12 | −15.01 | <0.001 | −0.01 | −11.74 | <0.001 | −0.01 | −12.07 | <0.001 |
| Sex | −0.06 | −1.71 | 0.08 | 0.01 | 0.17 | 0.87 | 0.15 | 3.79 | <0.001 | −0.06 | −1.82 | 0.07 | −0.05 | −1.56 | 0.12 |
| GAF African | −0.35 | −4.60 | <0.001 | −0.18 | −2.18 | 0.03 | −0.21 | −2.80 | 0.005 | −0.59 | −8.29 | <0.001 | −0.32 | −4.80 | <0.001 |
| GAF American Indian | −0.26 | −1.31 | 0.19 | −0.09 | −0.55 | 0.58 | −0.25 | −1.33 | 0.18 | −0.67 | −3.85 | <0.001 | −0.17 | −1.21 | 0.23 |
| GAF East Asian | −0.10 | −1.42 | 0.16 | 0.06 | 0.73 | 0.46 | 0.04 | 0.58 | 0.56 | 0.02 | 0.28 | 0.78 | 0.10 | 1.54 | 0.12 |
| GAF Oceanic | −0.24 | −0.38 | 0.70 | −1.53 | −1.16 | 0.24 | −1.54 | −1.34 | 0.18 | −1.60 | −3.31 | −001 | −0.62 | −1.55 | 0.12 |
| GAF Central Asian | 0.10 | 0.85 | 0.39 | 0.14 | 1.86 | 0.06 | 0.08 | 0.97 | 0.33 | −0.01 | −0.09 | 0.93 | −0.10 | −0.70 | 0.48 |
| CT | 0.04 | 0.21 | 0.83 | −0.57 | −2.50 | 0.01 | −0.16 | −0.74 | 0.46 | −0.01 | −0.01 | 0.99 | 0.30 | 1.96 | 0.05 |
| Family Income (ln) | 0.08 | 4.06 | <0.001 | 0.05 | 1.95 | 0.05 | 0.07 | 2.43 | 0.02 | 0.12 | 6.73 | <0.001 | 0.07 | 4.65 | <0.001 |
| Family Income * CT | 0.02 | 0.17 | 0.87 | 0.38 | 2.26 | 0.02 | −0.43 | 2.46 | 0.01 | −0.17 | −1.79 | 0.07 | −0.20 | −2.05 | 0.04 |
| WM | Flanker | DCCS | Vocabulary | Reading | |||||||||||
| R2 | 0.71 |
0.60 |
0.66 |
0.76 |
0.79 |
||||||||||
| B | t | p | B | t | p | B | t | p | B | t | p | B | t | p | |
| Parental Education Models | |||||||||||||||
| Age | 0.58 | 28.27 | <0.001 | 0.62 | 17.39 | < 0.001 | 0.63 | 16.79 | <0.001 | 0.37 | 20.08 | <0.001 | 0.38 | 23.39 | <0.001 |
| Age-Squared | −0.02 | −22.56 | <0.001 | −0.02 | −16.02 | < 0.001 | −0.19 | −14.96 | <0.001 | −0.01 | −12.24 | <0.001 | −0.01 | −12.59 | <0.001 |
| Sex | −0.06 | −1.87 | 0.06 | 0.01 | 0.06 | 0.95 | 0.14 | 3.67 | <0.001 | −0.06 | −2.12 | 0.03 | −0.05 | −1.78 | 0.07 |
| GAF African | −0.37 | −5.17 | <0.001 | −0.17 | −2.16 | 0.03 | −0.22 | −3.09 | 0.002 | −0.61 | −9.30 | <0.001 | −0.31 | −5.05 | <0.001 |
| GAF American Indian | −0.20 | −0.99 | 0.32 | −0.04 | −0.27 | 0.78 | −0.23 | −1.24 | 0.22 | −0.54 | −3.07 | 0.002 | −0.03 | −0.23 | 0.82 |
| GAF East Asian | −0.10 | −−1.48 | 0.14 | 0.06 | 0.83 | 0.40 | 0.05 | 0.08 | 0.54 | 0.01 | 0.20 | 0.84 | 0.10 | 1.60 | 0.11 |
| GAF Oceanic | −0.34 | 0.62 | 0.58 | −1.55 | −1.20 | 0.23 | −1.68 | −1.47 | 0.14 | −1.67 | −3.61 | <0.001 | −−0.62 | −1.54 | 0.12 |
| GAF Central Asian | 0.08 | 0.66 | 0.51 | 0.12 | 1.58 | 0.11 | 0.05 | 0.62 | 0.53 | −0.06 | −0.52 | 0.60 | −0.16 | −1.16 | 0.25 |
| CT | 0.05 | 0.30 | 0.76 | −0.55 | −2.44 | 0.01 | −0.14 | −0.62 | 0.53 | 0.01 | 0.08 | 0.93 | 0.31 | 2.04 | 0.04 |
| Parental Education | 0.04 | 4.39 | <0.001 | 0.03 | 2.32 | 0.02 | 0.03 | 2.83 | 0.005 | 0.06 | 8.34 | <0.001 | 0.05 | 7.03 | <0.001 |
| Parental Education * CT | −0.02 | −0.54 | 0.58 | 0.09 | 1.25 | 0.21 | 0.07 | 1.07 | 0.28 | −0.09 | −2.42 | <0.001 | −0.14 | −3.52 | <0.001 |
Note. GAF = Genetic Ancestry Factor; CT = Cortical Thickness. All analyses were adjusted for both testing site and scanner model.
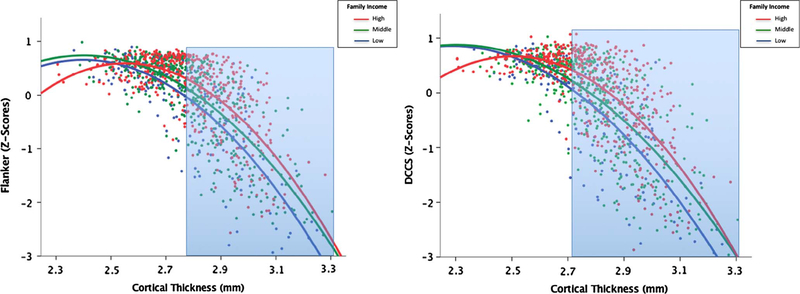
Probing the income * average CT interaction, Fig. 1 shows that, in general, thicker cortices were associated with worse EF scores. Among children with thinner overall cortices, children tended to perform well, regardless of family socioeconomic background. However, among children with thicker cortices, there were more pronounced differences as a function of family income, with higher family income buffering the effect of greater cortical thickness. Put another way, the link between CT and EF was more robust for children from lower income homes. Johnson-Neyman results indicated that this interaction was significant for cortical thickness values greater than 2.79 mm for flanker scores and greater than 2.72 mm for DCCS scores.
Fig. 1.
Left Panel: associations among family income, cortical thickness, and flanker scores. Overall there was a negative association between CT and EF scores, but among children with thinner average cortices, children tended to perform well regardless of family income. However, among children with thicker cortices there were more pronounced differences as a function of family income, specifically for cortical thickness values greater than 2.79 mm (area highlighted in blue). Right Panel: associations among family income, cortical thickness, and DCCS scores. Among children with thinner average cortices, children tended to perform well regardless of family income. However, among children with thicker cortices there were more pronounced differences as a function of family income, specifically for cortical thickness values greater than 2.72 mm (area highlighted in blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. CT associations with oral reading are moderated by family income
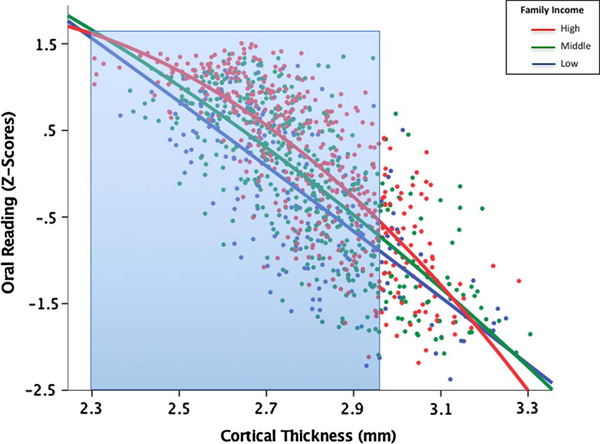
The income * average (whole-brain) CT interaction term was significant for oral reading (B = −0.20, p= 0.04, R2= 0.79) only (Table 3). The family income * CT interaction was significant for Left IFG (B =−0.15, p= 0.03, R2= 0.79), but did not pass FDR correction. Like the EF skills, results yielded an overall negative association between CT and language scores, with thinner cortices predictive of higher language scores. Fig. 2 shows that, unlike EF skills, family income moderated this association for children with thinner cortices. Probing the income * average CT interaction for oral reading, Johnson-Neyman results indicated that the interaction was significant for cortical thickness values less than 2.96 mm.
Fig. 2.
Associations among family income, cortical thickness, and oral reading scores. Overall there was a negative association between CT and language scores, with thinner cortices predictive of higher language scores. Family income moderated this association, specifically for cortical thickness values less than 2.96 mm (area highlighted in blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Impact of age on CT and income interactions
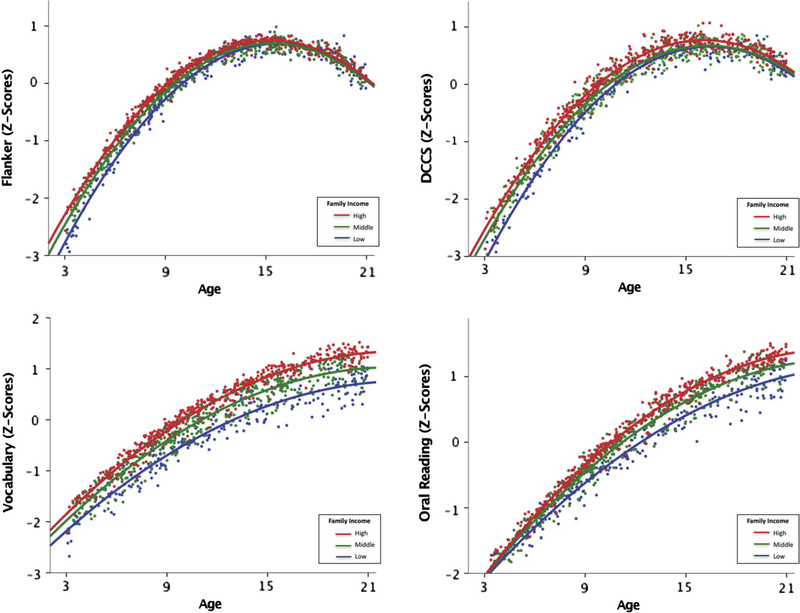
To probe the finding that family income moderated the above brain-behavior links for differing levels of cortical thickness, we examined whether associations between cortical thickness and cognition varied with age. Like past studies (Giedd et al., 1999), cortical thickness varied quadratically with age. We thus investigated whether there might be a three-way interaction of age-squared, CT and family income. Three-way interactions were not significant for vocabulary (p= 0.38) or reading (p =0.31), but approached significance for WM (p =0.07), flanker (p =0.05), and DCCS (p = 0.05). Although no significant CT * age-squared interactions were found for any of the EF skills, significant family income * age-squared interactions were found for flanker (p =0.01) and DCCS (p =0.01). No two-way interactions of age-squared were found for the language skills.
The family income * age-squared interactions for EF, but not language, could possibly account for the differences between moderation findings for EF and language. Higher family income was associated with better language scores across all ages, whereas the effect of income was attenuated for EF scores at the older ages (above 16 years; see Fig. 3). As older children are more likely to have both thinner cortices and higher EF scores, this may account for the reduced impact of income at lower levels of CT.
Fig. 3.
Top Panels: associations among family income, age and EF scores (flanker & DCCS). Three-way interactions approached significance for EF tasks and significant income * age-squared interactions were found for both flanker and DCCS. Effect of income was more attenuated for EF scores, particularly at the older ages (above 16 years). Bottom Panels: associations among family income, age and langauge scores (vocabulary & oral reading). Clear income effects for language scores across all ages and no three-way interactions or two-way interactions (income * age-squared) were found for language skills.
3.2.4. CT associations with vocabulary and reading moderated by parental education
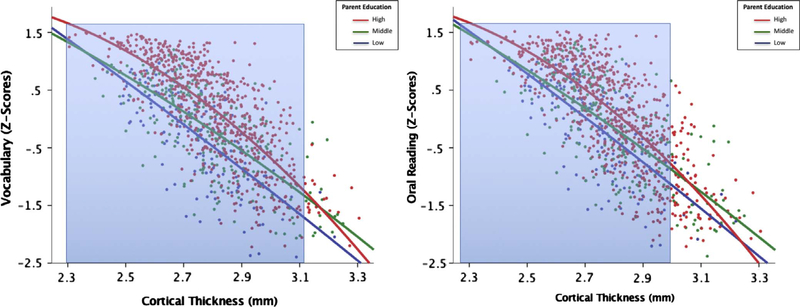
Finally, examining whether parental education moderated links between whole-brain average CT and specific neurocognitive skills, the education * average CT interaction term was significant for vocabulary (B = −0.09, p =0.02, R2 =0.76) and oral reading (B = −0.14, p < 0.001, R2= 0.79) only (Table 3, Fig. 4). Examining specific brain regions of interest, in predicting oral reading, parental education * left IFG CT (B = −0.09, p = 0.003, R2= 0.79), parental education * left STG CT (B =−0.12, p =0.001, R2= 0.79), and parental education * left fusiform CT (B = −0.09, p= 0.01, R2 =0.79) interactions survived FDR correction. No ROIs for vocabulary passed FDR correction.
Fig. 4.
Left Panel: associations among parental educational attainment, cortical thickness, and vocabulary scores. Overall there was a negative association between CT and language scores, but this relation was more robust for cortical thickness values less than 3.12 mm (highlighted in blue). Right Panel: associations among parental educational attainment, cortical thickness, and oral reading scores. Again, this association was more robust for cortical thickness values less than 2.99 mm (highlighted in blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Probing the education * CT interaction for oral reading, Fig. 4 shows that, similar to income, overall there was a negative association between CT and language scores, with thinner cortices associated with higher language scores. However, parental education magnified this association, particularly for children whose parents were the least educated. Johnson-Neyman results indicated that the interaction was significant for cortical thickness values less than 3.12 mm for vocabulary and values less than 2.99 mm for oral reading. No significant associations were found among parental education, CT, and EF scores.
4. Discussion
Although cortical thickness has been reported to mediate the association between family income and school achievement during adolescence (Mackey et al., 2015), our previous analysis of the PING dataset found that only cortical surface area, and not CT, partially explained the association between family income and executive function skills during childhood (Noble et al., 2015). In the current analysis, after adjusting for a wide range of individual characteristics (age, sex, scanner, site and genetic ancestry), we found that both family income and parental education moderate the association between CT and specific neurocognitive skills across the whole brain. In other words, CT does not explain the link between SES and cognitive skills; rather, socioeconomic disadvantage can exaggerate links between brain st ructure and cognitive performance, and socioeconomic advantage may mitigate these links.
Overall, children from higher SES homes tended to outperform their more disadvantaged peers on measures of vocabulary and reading, at all levels of cortical thickness. However, across all levels of CT, the association between CT and language skills was attenuated for children from higher-SES families. One possible interpretation of this finding is that the resources available to children from socioeconomically advantaged homes – higher quality childcare and schools, better housing in better neighborhoods, higher quality and quantity of linguistic stimulation in the home (Duncan & Magnuson, 2012; Duncan, Magnuson, & Votruba-Drzal, 2014; Kalil, Duncan, & Ziol-Guest, 2016) – lead to successful language and reading skill acquisition, even in the face of a neurobiological “risk factor” – namely, reduced cortical thickness in language-supporting regions. The particular experiences, and the neural mechanism(s) explaining this buffering process, remain to be investigated.
The findings concerning EF scores followed a slightly different pattern. Family income had very little impact on EF scores for children with thinner cortices, but significant differences were found by income level for children with thicker cortices. The disparate findings across language and EF domains may be related to differences in the impact of family income on cognitive skills across age. Unlike language scores where an income effect was consistent across all ages, there were no income effects for flanker or DCCS scores for older children (over 16 years). As older children are also more likely than younger children to have thinner cortices and higher EF scores, this may help to explain the diminished influence of income at lower levels of CT. Like language scores, the link between CT and EF scores was robust for children from lower-income homes and diminished for children from higher-income homes. One speculative explanation for this finding is that the negative experiences associated with socioeconomic disadvantage – higher family stress, exposure to environmental toxicants, fewer routines and greater chaos in the home (Kalil et al., 2016) – may accentuate difficulties in developing attention and EF skills. The precise mechanisms involved remain unknown, but it has been hypothesized that the hardships and challenges of disadvantaged environments may increase stress and reduce the resources for experience-dependent brain development (Callaghan & Tottenham, 2016). Again, future work will be necessary to explore this possibility, and to uncover the particular experiences and neural mechanism(s) explaining these processes.
Our investigation was concentrated in language and executive functioning skills, two complex cognitive functions that have prolonged maturation and have been associated with socioeconomic disparities (Casey et al., 2000; Craik & Bialystok, 2006; Farah et al., 2006; Hackman & Farah, 2009; Hulme & Snowling, 2009; Romine & Reynolds, 2005; Rueda et al., 2004; Snowling, Goulandris, & Defty, 1996). Studies in humans and animals have shown that environmental experiences interact with genetic and biological mechanisms, either exaggerating or attenuating effects of those variables into phenotypes, reflecting differences in synaptic plasticity, cognitive functions, and psychiatric disorders (Gräff & Mansuy, 2008). The results from our study support the notion that environmental experiences may buffer or amplify brain-behavior relations.
We found that differences in family income moderated links between CT and both EF and language skills, whereas differences in parental education only moderated links between CT and children’s language abilities. It has been suggested that these SES factors have differential effects on child development (Duncan & Magnuson, 2012; Duncan et al., 2014). Specifically, family income may be more strongly related to resource availability (access to quality foods, neighborhoods, and materials), while parental educational attainment may impact parenting style and cognitive stimulation within the home (Chou, Liu, Grossman, & Joyce, 2010; Duncan et al., 2014; Fletcher & Frisvold, 2009). As these different SES measures impact brain structures differently, these constructs should continue to be evaluated independently when examining the impact of SES on brain and behavior.
The current study is not without its limitations. Even with large sample sizes, cross-sectional studies allow for limited interpretation of developmental trajectories. Our findings suggest that family income and parental education may differentially impact structure-behavior relations in specific brain regions. Taking into account the heterogeneous pattern of maturation in different parts of the brain, the effects of SES on children’s cognition may vary at different stages of neural development. Past studies have suggested that decreases in cortical gray matter may reflect a shift to more efficient cortical networks, where cortical thinning could reflect either synaptic pruning or increased myelination (Giedd et al., 1999; Gogtay et al., 2004; Shaw et al., 2006; Sowell et al., 2004). However, developmental findings have been inconsistent concerning patterns of cerebral cortex maturation (for review see Walhovd, Fjell, Giedd, Dale, & Brown, 2016). Demonstrating some consistency with past research (Mackey et al., 2015), our results indicate that, after controlling for covariates, increased cortical thickness was associated with higher language scores, but overall the association between CT and cognition was negative and particularly robust for children from lower-SES homes. Discrepancies between the present results and past studies (Kharitonova et al., 2013; Mackey et al., 2015; Menary et al., 2013; Porter et al., 2011; Sowell et al., 2004; Tamnes et al., 2010) could be due to our careful consideration of socioeconomic differences, differences in CT maturation by ROI, or changes in cognitive capacities across ages. As relations between CT and some aspects of cognitive abilities change between early and late childhood (Shaw et al., 2006), longitudinal data could be more informative for understanding specific timing effects in relation to how SES impacts CT, and subsequently cognition.
Additionally, like all correlational studies, we cannot directly infer that thinner or thicker cortices in regions identified within this study are necessarily causing improved neurocognitive abilities. Likewise, although we are observing associations between CT and behavior, the measurement of CT does not directly reflect functional activation during neurocognitive task performance; future studies would benefit from convergence of structure and function methodologies in order to better understand neural substrates and mechanisms. Nonetheless, this study adds to the nascent literature examining associations among SES, CT, and neurocognitive performance during childhood (Lawson et al., 2013; Mackey et al., 2015; Noble et al., 2015) and may help to elucidate the significant role of SES in shaping both brain development and cognitive functioning.
Acknowledgements
This publication was supported by the Sackler Parent-Infant Project Fellowship to NHB, the Brazilian Coordination for the Improvement of Higher Education Personnel Grant (CAPES: #99999.000882/2015–01) to LRP, and funding from Teachers College, Columbia University. The authors have no conflict of interest to declare.
References
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. London, Sage: Newbury Park. [Google Scholar]
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, … Gruen JR (2014). The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology, 28(1), 1–10. 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DH, & Lange K (2011). Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics, 12, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, & Curran PJ (2005). Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research, 40(3), 373–400. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group (2012). Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI study of normal brain development. Cerebral Cortex, 22(1), 1–12. 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth P, Cherbuin N, Sachdev P, & Anstey KJ (2012). The association between financial hardship and amygdala and hippocampal volumes: Results from the PATH through life project. Social Cognitive and Affective Neuroscience, 7, 548–556. 10.1093/scan/nsr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences. 7, 76–81. 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, & Thomas KM (2000). Structural and functional brain development and its relation to cognitive development. Biological Psychology, 54(1–3), 241–257. 10.1016/S0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Krishnadas R, Batty GD, Burns H, Deans KA, Ford I, … Sattar N (2013). Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterized population sample. Cerebellum, 12(6), 882–891. 10.1007/s12311-013-0497-4. [DOI] [PubMed] [Google Scholar]
- Chou SY, Liu JT, Grossman M, & Joyce T (2010). Parental education and child health: Evidence from a natural experiment in Taiwan. American Economic Journal: Applied Economics, 2(1), 33–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FM, & Bialystok E (2006). Cognition through the lifespan: Mechanisms of change. TRENDS in Cognitive Sciences, 10(3), 131–138. 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, & Magnuson K (2012). Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science, 3(3), 377–386. 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K, & Votruba-Drzal E (2014). Boosting family income to promote child development. Future of Children, 24(1), 99–120. 10.1353/foc.2014.0008. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, … Hurt H (2006). Childhood poverty: Specific associations with neurocognitive development. Brain Research, 1110(1), 166–174. 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, … Akshoomoff N (2012). Multimodal imaging of the self-regulating developing brain. Proceedings of the National Academy of Sciences, 109(48), 19620–19625. 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, & Frisvold DE (2009). Higher education and health investments: Does more schooling affect preventive health care use? Journal of Human Capital, 3(2), 144 10.1086/645090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, … Hariri AR (2007). Perigenual anterior cingulate morphology covaries with perceived social standing. Social cognitive and affective neuroscience, 2(3), 161–173. 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2, 861–863. 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, … Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. PNAS, 101(21), 8174–8179. 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, & Mansuy IM (2008). Epigenetic codes in cognition and behaviour. Behavioural Brain Research, 192(1), 70–87. 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651–659. 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, & Pollak SD (2015). Association of child poverty, brain development, and academic achievement. JAMA Pediatrics, 169(9), 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between income and the hippocampus. PLoS ONE, 6, e18712 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, & Pollak SD (2013). Family poverty affects the rate of human infant brain growth. PLoS ONE, 8, e80954 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50, 1–22. [DOI] [PubMed] [Google Scholar]
- Hulme C, & Snowling MJ (2009). Developmental disorders of language, learning and cognition. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, … Ramus F (2012). The influence of socioeconomic status on children’s brain structure. PLoS ONE, 7, e42486 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Bartsch H, & Dale AM (2016). Toward an integrative science of the developing human mind and brain: Focus on the developing cortex. Dev Cogn Neurosci, 18, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PO, & Neyman J (1936). Tests of certain linear hypotheses and their application to some educational problems. Statistical research memoirs, 1, 57–93. [Google Scholar]
- Kalil A, Duncan GJ, & Ziol-Guest KM (2016). Early childhood poverty: Short and long-run consequences over the lifespan In Shanahan JM, Mortimer TJ, & Johnson M. Kirkpatrick (Vol. Eds.), Handbook of the Life Course. vol 2, (pp. 341–354). Cham: Springer International Publishing. [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JD, & Sheridan MA (2013). Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Developmental Cognitive Neuroscience, 6, 61–71. 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, & Lainhart JE Brain Development Cooperative Group. (2010). Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Developmental Neuropsychology, 35(3), 296–317. 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science, 16(5), 641–652. 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167(12), 1135–1142. 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AP, Finn AS, Leonard JA, Jacoby-Senghor DS, West MR, Gabrieli CF, & Gabrieli JD (2015). Neuroanatomical correlates of the income-achievement gap. Psychological Science, 26(6), 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, … Luciana M (2013). Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence, 41(5), 597–606. 10.1016/j.intell.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, … Brickman AM (2012a). Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience, 9(6), 307 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NB, Bartsch H, Kan E, Kuperman JM, … Sowell ER (2015). Family income, parental education and brain development in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012b). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–527. 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, & Brickman AM (2013). Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science, 16(5), 653–664. 10.1111/desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, & McCandliss BD (2006). Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 9(6), 642–654. 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Piccolo LR, Merz E, He X, Sowell E, & Noble KG (2016). Age-related Differences in Cortical Thickness Vary by Socioeconomic Status. PLoS ONE, 11(9), e0162511 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Collins PF, Muetzel RL, Lim KO, & Luciana M (2011). Associations between cortical thickness and verbal fluency in childhood, adolescence, and young adulthood. Neuroimage, 55(4), 1865–1877. 10.1016/j.neuroimage.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RD, Richards TL, Meltzoff A, & Kuhl PK (2008). Socioeconomic status predicts hemispheric specialization of the left inferior frontal gyrus in young children. Neuroimage, 40(3), 1392–1401. 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, … Giedd JN (2011). How does your cortex grow? The Journal of Neuroscience, 31(19), 7174–7177. 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romine CB, & Reynolds CR (2005). A model of the development of frontal lobe functioning: Findings from a meta-analysis. Applied Neuropsychology, 12(4), 190–201. 10.1207/s15324826an1204_2. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, … Posner MI (2004). Development of attentional networks in childhood. Neuropsychologia, 42(8), 1029–1040. 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, … Pol HEH (2014). Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cerebral Cortex, 25(6), 1608–1617. 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, … Giedd J (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440, 676–679. 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, … Giedd JN (2008). Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience, 28(14), 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling MG, Goulandris N, & Defty N (1996). A longitudinal study of reading development in dyslexic children. Journal of Educational Psychology, 88(4), 653–669. 10.1037/0022-0663.88.4.653. [DOI] [Google Scholar]
- Sowell ER (2001). Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. Journal of the International Neuropsychological Society, 7(3), 312–322. 10.1017/S135561770173305X. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, & Toga AW (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6, 309–315. 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PS, Leonard CM, Welcome SE, Kan E, & Toga AW (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience, 24(38), 8223–8231. 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, & Whalley LJ (2012). Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology, 71(5), 653–660. 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, & Fjell AM (2010). Neuroanatomical correlates of executive functions in children and adolescents: A magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia, 48, 2496–2508. [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi NE, Chevalier N, Espy KA, Beaumont JL, & Mungas D (2013). NIH toolbox cognition battery (CB): Measuring working memory. Monographs of the Society for Research in Child Development, 78(4), 70–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, … Reinvang I (2006). Regional cortical thickness matters in recall after months more than minutes. Neuroimage, 31(3), 1343–1351. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Giedd J, Dale AM, & Brown TT (2016). Through thick and thin: A need to reconcile contradictory results on trajectories of human cortical development. Cerebral Cortex, 1–10. 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikman SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, … Gershon R (2013). Cognition assessment using the NIH toolbox. Neurology, 80(Suppl. 3), S54–S64. 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Sabuncu MR, Yeo BTT, Fischl B, Greve DN, Kochunov P, … Glahn DC (2012). Measuring and comparing brain cortical surface area and other areal quantities. NeuroImage, 61(4), 1428–1443. 10.1016/j.neuroimage.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]