Abstract
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression primarily at the post-transcriptional level. Emerging evidence supports a regulatory role for miRNAs in the immune response and autoimmunity. In this work, we investigated the implication of miR-21 in the experimentally inducible bm12 → B6 cGVHD model of systemic lupus erythematosus (SLE). cGVHD host mice deficient in miR-21 show a 2-fold reduction in splenomegaly, significantly reduced autoantibody titers and down-regulated components of the CD40:CD40L and CD28:CD80/86 co-stimulation pathways. Furthermore, we demonstrate that miR-21-deficient hosts have reduced CD4+ IL-17+ cell populations and an expanded CD4+ CD25+ FoxP3+ cell compartment. We propose that miR-21 has a pluripotent role, serving to link distinct lymphocyte signaling pathways and acting as a “rheostat” for signals that promote B and T cell activation in lupus. Collectively, our experiments demonstrate that miR-21 deficiency in cGVHD host mice is sufficient to protect from lupus-like autoimmunity.
1. Introduction
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression primarily at a post-transcriptional level [1]. Emerging evidence supports a regulatory role for microRNAs (miRNAs) in the immune response and autoimmunity [2]. We have previously shown that microRNA-21 (miR-21) is differentially regulated in mouse lupus B and T lymphocytes and that its expression correlates with disease severity [3]. We have also shown that in vivo inhibition of miR-21 in the spontaneous, tricongenic model Sle123 via antisense oligonucleotides alleviates autoimmune splenomegaly.
Other studies have also shown miR-21 upregulation in mouse and human SLE lymphocytes [4, 5]. Furthermore, miR-21 is detected in higher concentration in plasma from newly diagnosed, treatment-naive lupus patients compared to healthy individuals [6, 7] and increased concentration of urinary miR-21 may predict a high risk of renal function loss [8]. In lupus prone mice, miR-21 expression in T cells, pDCS, PBMCs and urine is reduced after treatment with hydroxychloroquine [9].
Although the exact role of miR-21 in the immune system and autoimmunity remains elusive, several studies highlight its regulatory function in signaling pathways involved in lymphocyte activation via diverse mechanisms that include direct targeting of PDCD4 [10], PTEN [11], FOXO3 [12, 13] and SMAD7 [14, 15]. It was recently shown that miR-21 is required for Th17 cell differentiation and its deficiency confers resistance to experimental autoimmune encephalitis [15]. The role of miR-21 in Th17 differentiation is of special interest since recent evidence underscores an important role for Th17 in renal injury in lupus nephritis.
Having previously shown that lymphoproliferative manifestations in the Sle123 model are miR-21-dependent, we sought to investigate broader application of our findings to other models of lupus. In this work, we investigated the implication of miR-21 in the experimentally inducible bm12 → B6 cGVHD model of SLE. A chronic GVH reaction in this model is characterized by systemic autoimmunity similar to human SLE [16, 17]. In the bm12 → B6 model, a lupus-like syndrome is induced by transfer of splenocytes from non-autoimmune B6.C-H2bm12/KhEg (bm12) donors into recipient mice on a C57BL/6 (B6) background. Autoreactivity in this model depends on recognition of the host B cell MHC class II by donor T cells [18]. Although the donor bm12 MHC-II (I-A) differs from the B6 recipient by three amino acids, this is sufficient to elicit an autoimmune reaction characterized by splenomegaly, periarteritis, proteinuria, glomerulonephritis, increased titers of IgG and autoantibodies against nuclear antigens and double-stranded DNA.
We induced cGVHD reactions in B6 (WT) and B6.miR-21−/− (miR-21−/−) hosts and report a statistically significant reduction of splenomegaly in miR-21−/− mice, consistent with our results from in vivo miR-21 inhibition in the Sle123 model. MiR-21−/− hosts had significantly reduced autoantibody titers, a statistically significant reduction in proteinuria and down-regulated components of the CD28:CD80/86 and CD40:CD40L co-stimulation pathways. Furthermore, we detected an expanded the CD25+ FoxP3+ Treg compartment in miR-21−/− hosts which was accompanied by reduced CD4+ IL-17+ expression. Collectively, our experiments in a model of lupus that depends upon allogeneic T and B cell interactions, indicate that miR-21 deficiency in the host is sufficient to protect from lupus-like autoimmunity.
2. Materials and Methods
2.1. Animals
C57BL/6 (B6), B6.129S6-Mir21tm1Yoli (miR-21−/−) and B6.C-H2bm12/KhEg (bm12) mice were originally purchased from The Jackson Laboratory and subsequently bred and maintained in our mouse colony at Thomas Jefferson University. Recipient and donor mice were sex- and age- matched within each independent experiment. All of the experimental procedures performed on these animals were conducted according to the guidelines of the Thomas Jefferson University Institutional Animal Care and Use Committee.
2.2. Induction of cGVHD
cGVHD reactions were induced in 8- to 12- weeks old female B6 or miR-21−/− recipient mice by injecting intraperitoneally (i.p.) single-cell suspensions of 100 x 106 donor (bm12) splenocytes. Splenocyte suspensions were prepared under aseptic conditions by pressing donor spleens through a 70 um mesh screen into HBSS followed by incubation in 1 ml ACK buffer (150 mM NH4Cl; 10 mM KHCO3; 0.1 mM EDTA) per spleen for 5 minutes to remove red blood cells. The cells were washed twice with HBSS and resuspended at 500 x 106 cells/ml.
2.3. Blood and urine collection
Blood and urine specimens were collected from each animal pre-cGVHD induction and weekly thereafter until experiment termination. Blood samples were collected by submandibular venous puncture using Goldenrod animal lancets (Braintree Scientific, Braintree, MA) into 1.5 ml microcentrifuge tubes. Blood allowed to clot by incubation for 30 minutes at room temperature. The serum was decanted into fresh tubes, adjusted to 0.1% sodium azide and stored at 4 °C for up to one week or frozen at −20 °C for long-term storage. Urine samples were collected by gentle depression of the animal’s caudal abdomen until urination. Samples free of fecal contamination were collected in 1.5 ml microcentrifuge tubes and centrifuged at 10,000 x g for 10 minutes. Samples were diluted 1:10, adjusted to 0.1% sodium azide and filtered through a 0.22 um filter.
2.4. Assessment of disease progression
Renal disease progression was evaluated weekly by qualitative evaluation of urine protein and blood urea nitrogen (BUN) concentrations. Urine protein concentration was assessed using Uristix 4 reagent test strips (Siemens Diagnostics). The strips were wetted with fresh urine and immediately scored according to color change. BUN concentration was assessed with Azostix reagent test strips (Siemens Diagnostics) according to the manufacturer’s directions.
2.5. Evaluation of total urine protein
Quantitative evaluation of total urine protein concentration was performed using a BCA protein assay kit (Pierce Biotechnology) by following the Manufacturer’s instructions for the microplate procedure (Sample to WR ratio = 1:8). Urine samples were quantitatively diluted 1:10, 1:50 and 1:100 and triplicate reactions prepared for each dilution by mixing 25 ul sample with 200 ul BCA working reagent. Plates were incubated for 30 minutes at 37 °C and absorbance recorded at 562 nm in a SpectraMax microplate reader (Molecular Devices). Protein concentration was determined by comparison to a BSA standard curve assayed in parallel.
2.6. Detection of autoantibodies
Serum levels of double stranded DNA (dsDNA) antibodies were detected by solid-phase enzyme-linked immunosorbent assays (ELISA) as previously described [19]. Briefly, calf thymus DNA (Sigma-Aldrich, St. Louis, MO) was treated with S1 nuclease at 37 °C for 45 minutes to remove the single stranded segments of DNA, and then extracted by phenol-chloroform. Polyvinylchloride (PVC) flat bottom microtiter plates (Thermo Electron Corp., Milford, MA) were treated with 0.01% poly-L-lysine (Sigma-Aldrich, St. Louis, MO) for 1 hour at room temperature. After washing plates with ddH2O and letting them air-dry, the plates were coated with 100 μl of 2.5 μg/ml calf thymus dsDNA at 4 °C overnight. The dsDNA was diluted in BBS (0.2 M borate buffed solution, pH 8.2). Coated plates were blocked with BBT (0.5% bovine serum albumin and 0.4% Tween-80 in BBS) at room temperature for 2 hours. Serum samples and controls were diluted in BBT, added to plates, and incubated at 4 °C overnight. Alkaline phosphatase labeled detecting antibodies were diluted in BBT to 50 ng/ml, added to plates and incubated at room temperature for 1 hour, before the addition of 1 mg/ml pNPP (Sigma-Aldrich, St. Louis, MO) in 10 mM diethanolamine. Anti-dsDNA total IgG was detected with alkaline phosphatase-labeled goat anti-mouse IgG F(ab′)2γ specific (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). A pooled serum standard from 5 to 9 month-old MRL/lpr mice with a high dsDNA antibody titer was used as experimental reference and positive control. One-month old B6.RAG1KO mouse serum without detectable anti-dsDNA was used as a negative control. The serum samples and controls were diluted 1:200 for IgG detection. Optical density at 405 nm of each well was determined at various time points using a microplate reader (E-Max, Molecular Devices Corp., Sunnyvale, CA) and analyzed with Microsoft Excel 2011. The levels of serum anti-dsDNA were reported as O.D.
2.7. Immunostaining and flow cytometry
The following antibodies were purchased from BioLegend (San Diego, California): APC anti-IL-17 (TC11-18H10.1), APC anti-CD28 (37.51), APC anti-CD80, (16-10A1), FITC anti-CD25 (3C7), FITC anti-CD44 (IM7), FITC anti-CD19 (MB19-1), FITC anti-CD40 (3/23), PE anti-CD4 (RM4-4), PE anti-FoxP3 (150D), PE anti-CD154 (MR1), PE anti-CD86 (GL-1), PerCp anti-CD4 (GK1.5) and PerCp anti-CD19 (6D5). Cell surface marker staining was routinely performed on 1 x 106 splenocytes in 0.1 ml staining buffer (0.5% BSA/PBS). Cells were blocked with purified anti-mouse CD16/CD32 (BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions, incubated with appropriate fluorophore-conjugated antibodies for 30 minutes on ice then washed twice with staining buffer. Cells were fixed by incubation in 1 ml PBS containing 1% paraformaldehyde and analyzed on an Accuri C6 flow cytometer (BD Biosciences). For intracellular staining of FoxP3 and IL-17, surface markers were stained as described above then fixed with Fix/Perm buffer (Biolegend cat. no. 421403) for 20 minutes, washed twice with permeabilization wash buffer (Biolegend, cat. no. 421002) then stained with appropriate fluorophore-conjugated antibodies for 30 min. Cells were washed, fixed in PBS containing 1% paraformaldehyde and analyzed on an Accuri C6 flow cytometer. Resulting data were analyzed with FlowJo flow cytometry software.
2.8. Statistical analysis
Statistical analysis was performed using a Mann–Whitney unpaired Student’s t test. A value of p < 0.05 was considered to be significant.
3. Results
3.1. MiR-21 deficiency ameliorates cGVHD-induced splenomegaly
In previous work [3], we used small LNA antimiR to silence miR-21 in vivo in the Sle123 lupus model and reported a significant reduction of splenomegaly in treated mice compared to controls. In this work, we induced cGVHD reactions in age- and sex-matched miR-21-deficient (B6.129S6-Mir21tm1Yoli; miR-21−/−) and control (C57BL/6; WT) mice (hosts) by in vivo transfer of 100 x 106 purified bm12 (donor) splenocytes.
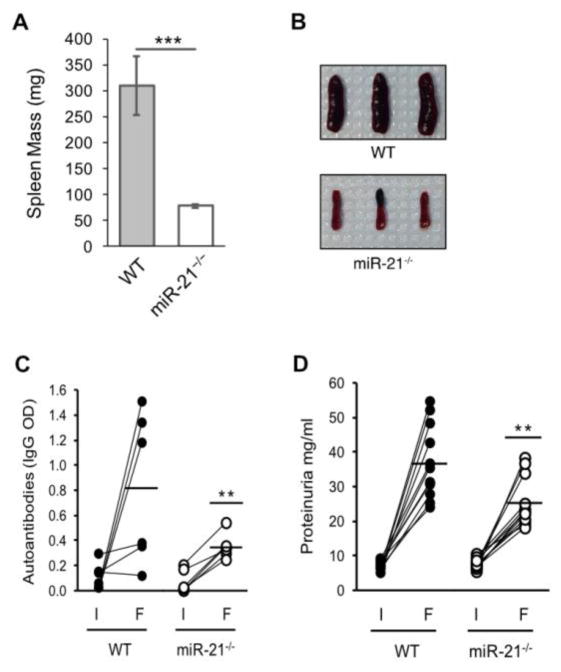
Consistent with our previous report in the spontaneous Sle123 model, host mice deficient in miR-21 showed a significant amelioration of splenomegaly (Figure 1A and B). Average spleen weights were 78.4 mg ± 4.0 SEM for the miR-21−/− hosts and 306.9 mg ± 56.6 SEM for the WT controls.
Figure 1.
MiR-21 deficiency alleviates cGVHD-induced autoimmunity. cGVH reactions were induced in age- and sex matched wild-type (WT) and miR-21-deficient (miR-21−/−) hosts by in vivo transfer of 100 x 106 purified bm12 splenocytes. (A) MiR-21 deficiency ameliorates cGVH-induced splenomegaly. Mean WT and miR-21−/− spleen masses 6-weeks post-induction. n = nine independent experiments (B) representative spleen images demonstrating ameliorated splenomegaly in miR-21−/− host mice. (C) MiR-21 regulates autoantibody production. Blood samples were collected pre-induction (I) and at 6-weeks post-cGVH induction (F) and the sera assayed by ELISA for anti-double-stranded DNA antibodies as described in Materials and Methods. n = 6 independent experiments and (D) MiR-21 deficiency reduces immune complex-mediated nephrotic proteinuria. Urine samples were collected pre-induction (I) and at 6-weeks post-cGVH induction (F) and assayed for total urine protein as described in Materials and Methods. n = nine independent experiments. *p < 0.05 and **p < 0.01 by Mann–Whitney unpaired t test.
3.2. MiR-21 regulates autoantibody production
Initiation of a cGVHD reaction in the bm12 → B6 model is dependent on allogeneic recognition of host (B6) B cells by activated donor (bm12) T cells [20]. The autoimmune phenotype in this model is characterized by production of high levels of autoantibodies directed against nuclear antigens and double-stranded DNA that closely resemble the antigenic specificities characteristic of SLE [18].
To investigate the effect of host miR-21 deficiency on autoantibody production, we collected blood samples from cGVHD mice pre- and six weeks post-induction and quantified the anti-dsDNA titers by ELISA. As shown in Figure 1C, dsDNA autoantibody titers were reduced ~ 60% (0.352 ± 0.041 SEM IgG OD) in miR-21 deficient hosts as compared to WT controls (0.80 ± 0.24 SEM).
3.3. MiR-21 deficiency reduces immune complex-mediated nephrotic proteinuria
cGVHD recipient mice develop advanced immune complex-mediated glomerulonephritis with associated elevated urine protein levels (proteinuria) [21]. To assess the implication of miR-21 deficiency on the development of cGVHD-induced kidney disease, we collected urine samples from recipient mice pre-cGVHD induction and at six weeks post-induction and quantified urine protein concentrations as described in Materials and Methods.
As shown in Figure 1D, urine protein concentrations in miR-21−/− recipients at 6-weeks post cGVHD induction were reduced 1.5 fold relative to WT controls (25.1 mg/ml ± 2.04 SEM vs. 36.6 mg/ml ± 3.02 SEM).
3.4. MiR-21 deficiency down-regulates components of the CD28:CD80/86 and CD40:CD154 co-stimulatory pathways
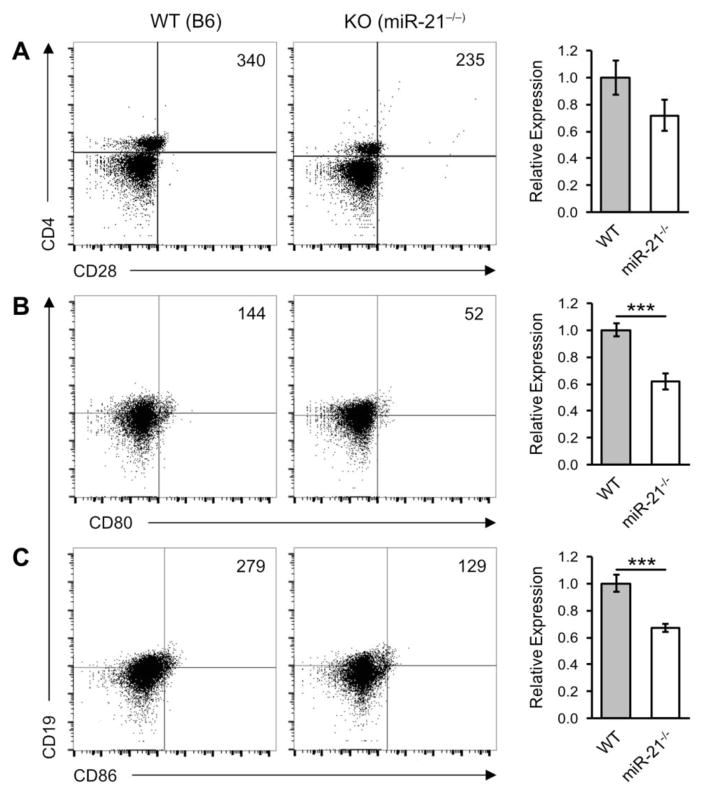
The CD28:CD80/86 and CD40:CD154 (CD40L) axes are important co-stimulatory pathways that regulate the initiation and magnitude of the immune response. In the bm12 B6 cGVHD model, activation and terminal differentiation of host B cells into antibody producing cells in the bm12 → B6 model results almost entirely from direct cognate T:B cell interactions [22]. To investigate the implication of miR-21-deficiency on expression of key cognate T- B cell receptor/ligands, we induced cGVHD reactions in miR-21−/− and WT mice and performed flow cytometry analysis for markers of the CD28:CD80/86 and CD40:CD154 co-stimulation pathways. As shown in Figure 2, miR-21−/− hosts had reduced B cell CD19+ CD80+ (0.62 ± 0.062 SEM) and CD19+ CD86+ (0.67 ± 0.029 SEM) expression relative to WT controls. We detected an overall, but not statistically significant, reduction of CD4+ CD28+ expression.
Figure 2.
MiR-21 deficiency down-regulates components of the CD28:CD80/86 co-stimulatory axis. cGVH reactions were induced in wild-type (WT) and miR-21-deficient (miR-21−/−) hosts as described in Figure 1. Six weeks post-induction host splenocytes were analyzed by flow cytometry for components of the CD28:CD80/86 co-stimulatory pathway. Bar graphs show mean expression of (A) CD4+ CD28+ (B) CD19+ CD80+ and (C) CD19+ CD86+ in miR-21−/− recipients relative to WT controls. Scatter plots shown are representative of six independent experiments. Live/dead cell gates were drawn on the basis of forward-scatter/side scatter. Numbers in the panels represent cell counts of the indicated population in the lymphocyte gate. *p < 0.05 and ***p < 0.005 by Mann–Whitney unpaired Student’s t test with n = 9 independent experiments. Error bars represent the standard error of the mean.
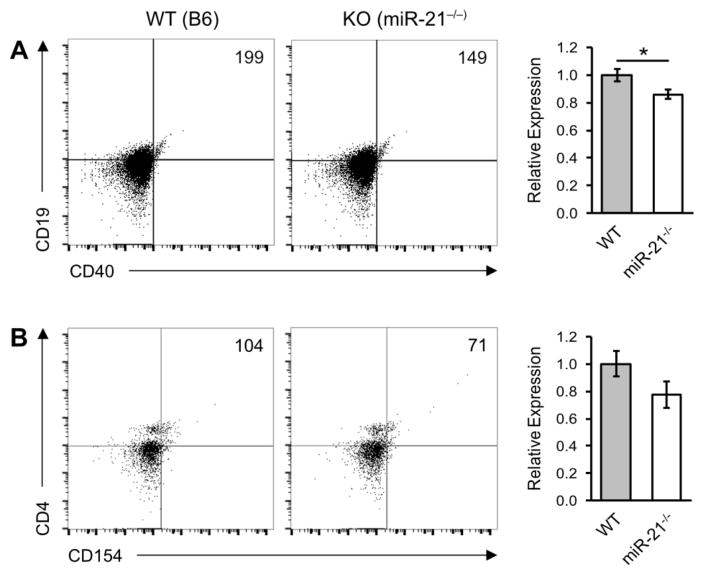
Components of the CD40:CD154 axis were down-regulated in miR-21−/− recipients (Figure 3). B cell CD19 + CD40+ expression was reduced (0.86 ± 0.034 SEM) and expression of its cognate T cell ligand, CD4+ CD154, was down-regulated (0.77 ± 0.095 SEM) although not statistically significant.
Figure 3.
MiR-21 deficiency down-regulates the CD40:CD154 co-stimulatory pathway. cGVH reactions were induced in wild-type (WT) and miR-21-deficient (miR-21−/−) hosts as described in Figure 1. Six weeks post-induction host splenocytes were subjected to flow cytometry analysis for activated B cell markers. Bar graphs show mean expression of (A) CD19+ CD40+ and (B) CD4+ CD154+ cell markers in miR-21−/− recipients relative to WT controls. Scatter plots shown are representative of six independent experiments. Live/dead cell gates were drawn on the basis of forward-scatter/side scatter. Numbers in the panels represent cell counts of the indicated population in the lymphocyte gate. *p < 0.05 and **p < 0.01 by Mann–Whitney unpaired Student’s t test with n = 9 independent experiments. Error bars represent the standard error of the mean.
3.5. MiR-21 regulates Treg and Th17 differentiation
Naive murine CD4+ T helper cells (Th) can be induced to differentiate along mutually exclusive pathways favoring either the development of Th17 or Treg cells [23, 24]. Furthermore, it has been proposed that skewing of Th lineage commitment towards Th17 and away from Treg (and Th2) may be responsible for the development and progression of autoimmune disease [25, 26].
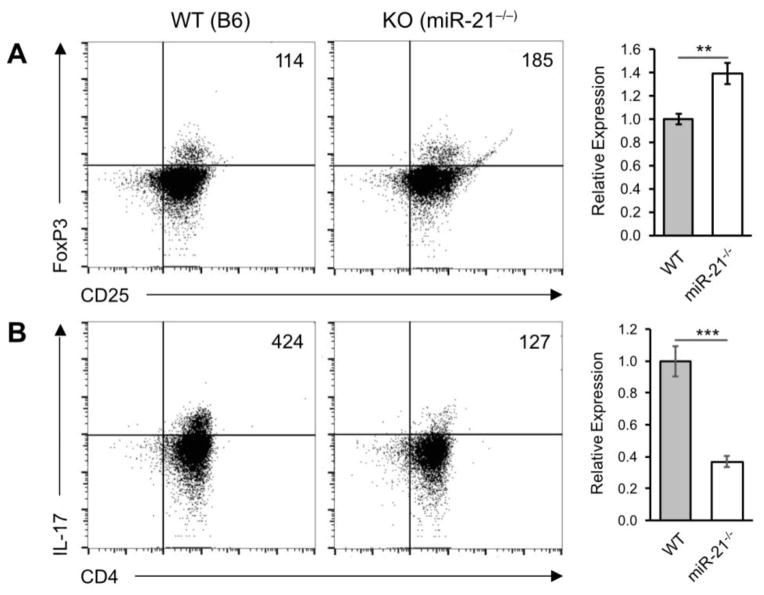
Our results demonstrate that miR-21 deficiency may protect from autoimmunity in the cGVHD model by biasing T cell responses toward Treg at the expense of Th17. As shown in Figure 4, the CD4+ CD25+ FoxP3+ Treg compartment in miR-21−/− host mice was expanded 1.39 ± 0.092 SEM relative to WT controls and that this expansion was accompanied by a 0.37 ± 0.035 SEM reduction of CD4+ IL-17+ expressing Th17 cells.
Figure 4.
miR-21 regulates Treg and Th17 differentiation. cGVH reactions were induced in wild-type (WT) and miR-21-deficient (miR-21−/−) hosts as described in Figure 1. Six weeks post-induction host splenocytes were subjected to flow cytometry analysis for expression of (A) CD4+ CD25+ FoxP3+ T-regulatory (Treg) and (B) CD4+ IL-17+ T-helper 17 (Th17) cell markers. Scatter plots shown are representative of six independent experiments. Live/dead cell gates were drawn on the basis of forward-scatter/side scatter. Numbers in the panels represent (C) CD25+ FoxP3+ cell counts in the lymphocyte → CD4+ cell gate and (D) CD4+ IL-17+ cell counts in the lymphocyte gate. ***p < 0.005 by Mann–Whitney unpaired Student’s t test with n = 9 independent experiments. Error bars represent the standard error of the mean.
4. Discussion
MiR-21 is emerging as an important regulator of lymphocyte signaling [27, 28] and has been shown to be up-regulated in several autoimmune diseases including SLE [4–6, 29], scleroderma [30], psoriasis [31] and multiple sclerosis [32]. MiR-21 is detected in higher concentration in plasma from newly diagnosed, treatment-naive lupus patients relative to healthy individuals [6], suggesting that elevated urinary miR-21 may predict a high risk of renal function loss [29]. In lupus mice, miR-21 expression decreases after treatment with hydroxychloroquine, an immunomodulator commonly used for treatment of various SLE manifestations [9].
In previous work, we quantified miR-21 expression in the spontaneous tri-congenic lupus model Sle123 [3]. We showed that miR-21 expression is elevated in Sle123 B and T lymphocytes and that its expression positively correlates with lymphoproliferative and renal disease severity. We then used an LNA inhibitor to silence miR-21 in vivo and showed that miR-21 inhibition reduces significantly the severe splenomegaly seen in this model.
In the present work, we investigated the implication of miR-21 deficiency in the inducible bm12 → B6 chronic graft- vs. host disease (cGVHD) model, which depends on allorecognition of host B cells by MHC class II incompatible donor T cells. The lupus-like immunopathology exhibited in this model includes splenomegaly, autoantibodies and diffuse proliferative glomerulonephritis.
Our results demonstrate that miR-21 deficiency protects cGVHD host mice from autoimmunity. Consistent with our previous results we report a significant reduction of splenomegaly in miR-21−/− recipients, with a ~4-fold reduction in mean spleen mass in the cGVHD mice (Figure 1A). In addition, we show that miR-21 deficient hosts have significantly reduced dsDNA autoantibody titers (Figure 1C), reduced proteinuria (Figure 1D), down-regulated components of the CD28:CD80/86 and CD40:CD154 co-stimulation pathways (Figures 2 and 3) and increased Treg/Th17 ratios (Figure 4) that negatively correlate with disease severity.
Regulation and maintenance of immune self-tolerance involves a complex interaction network of effector cells with co-stimulatory and co-inhibitory molecules. FoxP3-expressing T regulatory cells (Treg), a subset of effector CD4+ T cells, function as potent suppressors of autoimmunity [33]. Defects in Treg function and/or a reduced Treg population has been associated with a number of autoimmune diseases including SLE [34], whereas rescue of Treg function and/or number can both prevent and reverse autoimmune responses [35]. Th17 cells produce IL-17, a potent pro-inflammatory cytokine with critical protective roles in host defense against bacterial infection. Dysregulated IL-17 production has been implicated in the pathogenesis of SLE [36]. In an autoimmune context, sustained overproduction of IL-17 by autoreactive Th17 cells can lead to chronic inflammation and tissue damage [37].
A key role played by miR-21 in the regulation of T cell survival, differentiation and function is beginning to emerge. MiR-21 has been shown to regulate T cell activation [38], apoptosis [31, 39] and Th1/Th2 differentiation [40]. Recently, miR-21 was found to be up-regulated in Th17 cells and its inhibition via LNA antisense oligonucleotides reduced the Th17 compartment and attenuated experimental autoimmune encephalomyelitis (EAE) [15]. Consistent with these results, we detected a 2.7-fold reduction of CD4+ IL-17+ Th17 cells in miR-21−/− cGVHD host mice (Figure 4B). However, in contrast to Murugaiyan, et. al. who report unaltered levels of CD4+ CD25+ FoxP3+ Treg cells, the reduction in Th17 cell numbers in miR-21−/− cGVHD host mice was accompanied by a ~1.4-fold increase in the CD4+ CD25+ FoxP3+ Treg compartment (Figure 4A). These data support the concept of functional antagonism between “pathogenic” Th17 and “protective” Treg lineages and the notion that Th → Treg/Th17 differentiation involves reciprocal and, possibly, mutually-exclusive pathways [41–43]. Furthermore, the correlation between increased Treg/Th17 ratios and protection from autoimmunity in miR-21−/− cGVHD hosts is consistent with studies demonstrating a negative relationship between Treg/Th17 ratios and SLE disease severity [44]. The differences in Treg differentiation in response to miR-21 deficiency in the EAE and cGVHD models may be due to differences in the experimental systems, mode of miR-21 depletion (LNA inhibition versus genetic knockout) or both, and warrants further investigation.
Many critical interactions between effector CD4+ T cells and B cells are known to be contact mediated. The best characterized B-T cell interaction pathways are the CD28 CD80/86 and CD4-CD154 (CD40L) co-stimulatory axes that have essential yet opposing functions in the regulation of adaptive immunity [45]. CD40-CD154 signaling promotes T cell activation and stimulates B cell proliferation, isotype switching and terminal plasma cell differentiation [46], while signaling through the CD28-CD80/86 axis regulates T cell activation, controls the B cell response and establishes T cell tolerance [47].
In the bm12 → B6 cGVHD model, activation and terminal differentiation of host B cells into antibody producing cells in the bm12 → B6 model results almost entirely from direct cognate T:B cell interactions [22]. We found that components of both co-stimulatory axes were down-regulated in miR-21−/− recipients (Figures 2 and 3). The decrease in CD19+ CD80/86 expression (Figure 2B and C) accompanied by an expansion of the Treg compartment (Figure 4A) is in accord with recent evidence showing that a CD4+ CD25+ FoxP3+ Treg population with suppressive function resides in the B cell follicle and regulates germinal center formation and proliferation [48]. The T follicular regulatory (Tfr) cells derive from the natural Treg population and function by depleting B cell surface CD80/86 [49], either directly or through regulation of T follicular helper (Tfh) cells [50]. Without CD80/86, the CD28-CD80/86 co-stimulation axis is broken and B cells cannot receive the T cell help required to mount an antibody response. The reduction of B cell CD80/86 in miR-21−/− recipients is consistent with the reduced anti-dsDNA antibody titers in these mice (Figure 1C). For our experiments, we induced cGVHD by injection of a total donor splenocyte preparation and our measurements of B cell CD80/86 expression did not differentiate between donor and host sources. However, studies have confirmed that the source of serum autoantibodies in the cGVHD model are produced entirely by bone marrow derived conventional B cells [51] suggesting that host B cells are the major pathogenic species. Together, these results suggest that miR-21 may indirectly regulate autoantibody production in the cGVHD model by skewing the Treg/Th17 ratio toward the Th17 phenotype thereby relieving Treg (Thr) suppression of the B cell germinal center response allowing terminal plasma cell differentiation and autoantibody production.
We detected an overall down-regulation of components of the CD40:CD154 co-stimulation axis (Figure 3). The reduced expression of B cell CD40+ in miR-21−/− recipients is consistent with the measured reduction of CD80/86-expressing B cells (Figure 2B and C). Our analysis of T cell CD154 expression did not differentiate between donor and recipient and likely contains contributions from both sources. We hypothesize that reduced CD154 expression in miR-21−/− recipients is not due to direct miR-21 regulation of CD154 induction but is rather a downstream effect of miR-21 regulation of Th17 polarization.
Lupus nephritis is the most common severe target organ manifestation in human SLE. Glomerulonephritis (GN) in human and mouse lupus is mediated by immune complex deposition on the glomerular membrane and likely, direct insult from nephritogenic antibodies. In the cGVHD model, the GN is membranous [21] or membranous and glomerular/mesangial proliferative nephritis [21, 52] closely recapitulating the membranous and membranoproliferative nephritis seen in patients with lupus. WT cGVHD recipient mice develop avanced immune complex-mediated glomerulonephritis accompanied by elevated nephrotic proteinuria [21]. In our experiments, we measured a statistically significant 1.5-fold reduction of proteinuria in miR-21−/− recipients that positively correlated with reduced autoantibody titers in these mice (Figure 1D). We extrapolate that in the setting of active lupus nephritis, miR-21 inhibition may derepress mechanisms that modulate active inflammation in kidneys and halt disease progression. Future studies in animal models of lupus nephritis will allow us to test this hypothesis.
In our previous studies in the Sle123 model, we showed that miR-21 is differentially regulated in B and T cell subsets. The results of our recent studies in the cGVHD model may implicate a role for miR-21 in B and T cell activation in lupus. We propose that miR-21 serves a pluripotent role (1) as a link between distinct signaling pathways, namely the SMAD/TGF-B, PTEN/FOXO, and PDCD4/IL-10 axes and (2) acting as a “rheostat” for components of the CD28:CD80/86 and CD40:CD154 co-stimulation pathways resulting in an attenuated disease phenotype. The cGVHD mouse, while not “intrinsically” autoimmune, serves as an excellent model to investigate the role of miR-21 and its targets in the activation of T and B lymphocytes, under conditions promoting autoreactivity and autoimmunity in vivo.
Highlights.
MiR-21 deficiency in the cGVHD lupus model protects from autoimmunity.
MiR-21-deficiency alleviates autoimmune splenomegaly.
MiR-21-deficient hosts have reduced proteinuria and autoantibody titers.
MiR-21-deficiency down-regulates components of the CD28:CD80/86 and CD40:CD154 co-stimulatory pathways.
MiR-21-deficient hosts have reduced Th17 cell populations and an expanded Treg compartment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 3.Garchow BG, Bartulos Encinas O, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, Obad S, Petri A, Kauppinen S, Kiriakidou M. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3:605–615. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184:6773–6781. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- 5.Stagakis E, Bertsias G, Verginis P, Nakou M, Hatziapostolou M, Kritikos H, Iliopoulos D, Boumpas DT. Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann Rheum Dis. 2011;70:1496–1506. doi: 10.1136/ard.2010.139857. [DOI] [PubMed] [Google Scholar]
- 6.Tang ZM, Fang M, Wang JP, Cai PC, Wang P, Hu LH. Clinical relevance of plasma miR-21 in new-onset systemic lupus erythematosus patients. J Clin Lab Anal. 2014;28:446–451. doi: 10.1002/jcla.21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Peng W, Ouyang X, Li W, Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160:198–206. doi: 10.1016/j.trsl.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC. Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol. 2012;36:412–418. doi: 10.1159/000343452. [DOI] [PubMed] [Google Scholar]
- 9.Chafin CB, Regna NL, Hammond SE, Reilly CM. Cellular and urinary microRNA alterations in NZB/W mice with hydroxychloroquine or prednisone treatment. Int Immunopharmacol. 2013;17:894–906. doi: 10.1016/j.intimp.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D’Esposito M, Di Lauro R, Verde P. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 11.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarilyo G, La Cava A. miRNA in systemic lupus erythematosus. Clin Immunol. 2012;144:26–31. doi: 10.1016/j.clim.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Li PF. Foxo3a regulates apoptosis by negatively targeting miR-21. J Biol Chem. 2010;285:16958–16966. doi: 10.1074/jbc.M109.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 15.Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S, Yosef N, Vaidya VS, Weiner HL. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125:1069–1080. doi: 10.1172/JCI74347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg R. The chronic graft-versus-host model of systemic autoimmunity. Curr Dir Autoimmun. 2003;6:228–244. doi: 10.1159/000066864. [DOI] [PubMed] [Google Scholar]
- 17.Gleichmann E, Van Elven EH, Van der Veen JP. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur J Immunol. 1982;12:152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- 18.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Allotype-specific immunoregulation of autoantibody production by host B cells in chronic graft-versus host disease. J Immunol. 1990;144:916–922. [PubMed] [Google Scholar]
- 19.Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181:7770–7777. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- 20.Morris SC, Cohen PL, Eisenberg RA. Experimental induction of systemic lupus erythematosus by recognition of foreign Ia. Clin Immunol Immunopathol. 1990;57:263–273. doi: 10.1016/0090-1229(90)90040-w. [DOI] [PubMed] [Google Scholar]
- 21.Gleichmann H, Gleichmann E, Andre-Schwartz J, Schwartz RS. Chronic allogeneic disease. 3. Genetic requirements for the induction of glomerulonephritis. J Exp Med. 1972;135:516–532. doi: 10.1084/jem.135.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J Exp Med. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells- origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 26.Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87–102. doi: 10.1111/j.1600-065X.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 27.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson LJ, Ansel KM. MicroRNA regulation of lymphocyte tolerance and autoimmunity. J Clin Invest. 2015;125:2242–2249. doi: 10.1172/JCI78090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeto CC. Urine miRNA in nephrotic syndrome. Clin Chim Acta. 2014;436:308–313. doi: 10.1016/j.cca.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Li Y, Qu S, Luo H, Zhou Y, Wang Y, Zhao H, You Y, Xiao X, Zuo X. MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol. 2012;32:514–522. doi: 10.1007/s10875-011-9647-y. [DOI] [PubMed] [Google Scholar]
- 31.Meisgen F, Xu N, Wei T, Janson PC, Obad S, Broom O, Nagy N, Kauppinen S, Kemeny L, Stahle M, Pivarcsi A, Sonkoly E. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol. 2012;21:312–314. doi: 10.1111/j.1600-0625.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 32.Fenoglio C, Cantoni C, De Riz M, Ridolfi E, Cortini F, Serpente M, Villa C, Comi C, Monaco F, Mellesi L, Valzelli S, Bresolin N, Galimberti D, Scarpini E. Expression and genetic analysis of miRNAs involved in CD4+ cell activation in patients with multiple sclerosis. Neurosci Lett. 2011;504:9–12. doi: 10.1016/j.neulet.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Bin Dhuban K, Kornete M, SME, Piccirillo CA. Functional dynamics of Foxp3(+) regulatory T cells in mice and humans. Immunol Rev. 2014;259:140–158. doi: 10.1111/imr.12168. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 35.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 36.Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193:540–543. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 37.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, He L, Zhang R, Liu X, Ren Y, Liu Z, Zhang X, Cheng W, Hua ZC. Regulation of T lymphocyte activation by microRNA-21. Mol Immunol. 2014;59:163–171. doi: 10.1016/j.molimm.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Ruan Q, Wang P, Wang T, Qi J, Wei M, Wang S, Fan T, Johnson D, Wan X, Shi W, Sun H, Chen YH. MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2. Cell Death Dis. 2014;5:e1095. doi: 10.1038/cddis.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, Orkin SH, Aronow BJ, Rothenberg ME. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Korn T, Bettelli E, Oukka M, Kuchroo VK. Annu Rev Immunol. United States: 2009. IL-17 and Th17 Cells; pp. 485–517. [DOI] [PubMed] [Google Scholar]
- 43.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29:1251–1258. doi: 10.1007/s10067-010-1510-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 48.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Reap EA, Sobel ES, Jennette JC, Cohen PL, Eisenberg RA. Conventional B cells, not B1 cells, are the source of autoantibodies in chronic graft-versus-host disease. J Immunol. 1993;151:7316–7323. [PubMed] [Google Scholar]
- 52.Bruijn JA, van Elven EH, Hogendoorn PC, Corver WE, Hoedemaeker PJ, Fleuren GJ. Murine chronic graft-versus-host disease as a model for lupus nephritis. Am J Pathol. 1988;130:639–641. [PMC free article] [PubMed] [Google Scholar]