Abstract
In the phase III study RESONATE, ibrutinib reduced the risk of progression and improved overall survival versus ofatumumab in previously treated patients with CLL/SLL. In this novel analysis of patient well-being including patient-reported outcomes, ibrutinib reduced disease burden while preserving parameters of hematologic and immunologic function in RESONATE. These results suggest that ibrutinib can improve quality of life while prolonging survival.
Background:
Ibrutinib compared with ofatumumab significantly improves progression-free and overall survival in patients with previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Patients and Methods:
Measures of well-being were assessed in RESONATE, where previously treated patients with CLL/SLL were randomized to receive ibrutinib 420 mg/day (n = 195) or ofatumumab (n = 196) for up to 24 weeks. Endpoints included hematologic function, Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-F), disease-related symptoms, European Organization for Research and Treatment of Cancer Quality of Life Questionnaires Core 30 (EORTC QLQ-C30), and medical resource utilization.
Results:
With up to 24 months’ follow-up (median, 16.4 months), 79% of cytopenic patients showed sustained hematologic improvement (82% with improved platelet count, 69% with improved hemoglobin) on ibrutinib versus 43% on ofatumumab (P < .0001). Higher rates of clinically meaningful improvement were demonstrated with ibrutinib versus ofatumumab for FACIT-F and EORTC global health. Greater improvement was observed in disease-related weight loss, fatigue, night sweats, and abdominal discomfort with ibrutinib versus ofatumumab. Hospitalizations in the first 30 days occurred less frequently with ibrutinib than ofatumumab (0.087 vs. 0.184 events/patient; P = .0198). New-onset diarrhea was infrequent with ibrutinib after the first 6 months (47% at ≤6 months vs. 5% at 12-18 months). With ibrutinib, grade ≥ 3 hypertension occurred in 6%, grade ≥ 3 atrial fibrillation in 4%, major hemorrhage in 2%, and tumor lysis syndrome in 1% of patients.
Conclusion:
Ibrutinib led to significant improvements in hematologic function and disease symptomatology versus ofatumumab, and can restore quality of life while prolonging survival in relapsed/refractory CLL/SLL.
Keywords: Bruton’s tyrosine kinase, Disease-related symptoms, Fatigue, Quality of life, Relapsed/refractory CLL/SLL
Introduction
Chronic lymphocytic leukemia (CLL) is a common leukemia in the United States1; manifestations of progressive disease and indications for treatment include lymphadenopathy, splenomegaly, bone marrow failure (anemia, thrombocytopenia), and significant disease-related symptoms.2 CLL complications and toxicities associated with therapy can profoundly impact quality of life (QoL) and well-being, particularly in older patients.3,4 Patient-reported outcome (PRO) scores of emotional well-being tend to be lower in patients with CLL than in patients with other malignancies,4 and studies have shown significant QoL improvements with effective CLL treatment including chemotherapy, as well as in prior small non-randomized studies with ibrutinib.5–11
Ibrutinib, an oral, first-in-class, once-daily inhibitor of Bruton’s tyrosine kinase, has shown substantial single-agent efficacy and tolerability in CLL/small lymphocytic lymphoma (SLL).12–15 In the phase III study (RESONATE) comparing ibrutinib with ofatumumab in previously treated patients with CLL/SLL, ibrutinib reduced risk of progression as assessed by an independent review committee (IRC) by 78% and risk of death by 57% with a median follow-up of 9.4 months at primary analysis.14 Measures of patient well-being including parameters of hematologic and immunologic function, and QoL are reported from the RESONATE study. These data are the first to show sustained improvements in PRO, hematologic function, and disease symptoms associated with ibrutinib in a randomized study.
Patients and Methods
Patients
Patients with CLL/SLL who had received ≥ 1 prior therapy and had active disease with an indication for therapy per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines were enrolled.2 Eligibility criteria have been described previously.14 All patients provided written informed consent.
Study Design
Patients were randomized to once-daily oral ibrutinib (420 mg) or intravenous ofatumumab as previously described.14 Ofatumumab data beyond 6 months were limited by maximum treatment duration (6 months) and a median progression-free survival (PFS) of 8.1 months,14 affecting the efficacy and safety variables in this updated analysis. Further, 122 patients randomized to ofatumumab had crossed over to ibrutinib after disease progression (PD).
Study Endpoints
PFS, overall survival (OS), and overall response rate (ORR) have been previously reported.2,14 Secondary efficacy endpoints include sustained hematologic improvement (see Supplemental Methods [in the online version] for definition) in patients with cytopenia(s) at baseline, and PRO as measured by Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). FACIT-F is a validated instrument that assesses tiredness, weakness, and difficulty conducting daily activities owing to fatigue. Scores range from 0 to 52, with high scores indicating less fatigue.16 A change in FACIT-F score of ≥ 3 points17 was considered clinically meaningful. FACIT-F forms and other PRO instruments were generally completed by patients prior to any study procedures. Per protocol, patients were not required to complete the questionnaire following PD/crossover or after completing treatment if assigned to the ofatumumab arm.
Prespecified exploratory endpoints included improvement of investigator-assessed disease-related symptoms (National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [CTCAE] grade decrease of ≥ 1 post-baseline, reported for ≥ 2 consecutive assessments of weight loss, fatigue, fever, night sweats, abdominal discomfort owing to splenomegaly, and/or anorexia), PRO as measured by European Organization for Research and Treatment of Cancer Quality of Life Questionnaires Core 30 (EORTC QLQ-C30; clinically meaningful change defined as > 10 points18), and medical resource utilization associated with therapy including number of hospitalizations, any blood product transfusions (red blood cells, platelets, whole blood), and use of any hematopoietic growth factors. Compliance rates for FACIT-F and EORTC QLQ-C30 questionnaire completion were monitored (see Supplemental Table 1 in the online version).
To evaluate disease burden, lymph nodes and splenic enlargement were quantified by IRC-assessed computed tomography; reduction was defined per iwCLL criteria.2 The effect of ibrutinib versus ofatumumab on white blood cell count and differential was evaluated over time. Lymphocyte subsets (B cells, T cells, natural killer [NK] cells [see Supplemental Methods in the online version]) were evaluated by flow cytometry for patients with baseline samples. Serum immunoglobulin levels (IgA, IgG, IgM) were quantitated.
Adverse event (AE) severity was graded using CTCAE, except for hematologic toxicities, which were graded based on iwCLL criteria.2 The treatment-emergent period for AE collection was defined as the time from the first dose of study drug to 30 days after the last dose of study drug or initiation of subsequent antineoplastic therapy, whichever comes first. Treatment-emergent AEs were summarized by time to event onset and by prevalence. (See Supplemental Methods in the online version, for details regarding statistical methods used in the study.)
Results
Baseline Characteristics and Patient Disposition
As described previously, 391 patients with previously treated CLL/SLL were randomized to ibrutinib (n = 195; median 3 prior therapies [range, 1-12]) or ofatumumab (n = 196; median 2 prior therapies [range, 1-13]).14 Baseline characteristics were balanced across both treatment groups and have been previously published.14 In general, the enrolled population was elderly (over 20% of patients were aged ≥ 75 years), and comorbidities were common (such as reduced creatinine clearance and cytopenias at baseline). The median follow-up was 9.4 months at the time of primary analysis. This analysis reflects data at a median follow-up of 15.0 months (16.4 for ibrutinib, 11.9 for ofatumumab; up to 24 months maximum).
Sustained Hematologic Improvement
In cytopenic patients treated with ibrutinib, 69% showed sustained improvement in hemoglobin, 82% in platelets, and 68% in absolute neutrophil count, all of which were statistically significant versus changes with ofatumumab (Table 1). Of ibrutinib patients with any baseline cytopenia, 79% showed sustained improvement in blood counts with this longer follow-up compared with 69% at the primary analysis, where it was significantly higher versus ofatumumab (43%; P < .0001). Figure 1A, B demonstrates early improvement in median hemoglobin and platelet levels, respectively, which were sustained during the first 6 months in patients in both treatment arms with baseline cytopenias, and through 16 months on the ibrutinib arm.
Table 1.
Baseline Cytopenias and Sustained Hematologic Improvementa
| Baseline Cytopenia(s) | Sustained Hematologic Improvement in Patients With Specified Cytopenia(s) at Baseline, n/N (%) | ||||
|---|---|---|---|---|---|
| Ibrutinib (n = 195) | Ofatumumab (n = 196) | Ibrutinib | Ofatumumab | P Value | |
| Thrombocytopenia, n (%) | 74 (38) | 64 (33) | 61/74 (82) | 14/64 (22) | < .0001 |
| Median platelet count, × 109/L (range) | 65.5 (20-99) | 72 (23-100) | |||
| Neutropenia, n (%) | 41 (21) | 38 (19) | 28/41 (68) | 12/38 (32) | .0011 |
| Median neutrophil count, × 109/L (range) | 1.1 (0.4-1.5) | 1.1 (0.3-1.5) | |||
| Anemia, n (%) | 89 (46) | 86 (44) | 61/89 (69) | 32/86 (37) | < .0001 |
| Median hemoglobin, g/dL (range) | 9.8 (6.5-11) | 9.8 (6.2-11) | |||
| Any cytopenia, n (%) | 124 (64) | 123 (63) | 98/124 (79) | 53/123 (43) | < .0001 |
Sustained improvement ≥ 56 days without transfusions or growth factors. Blood counts assessed by central laboratory.
Figure 1.
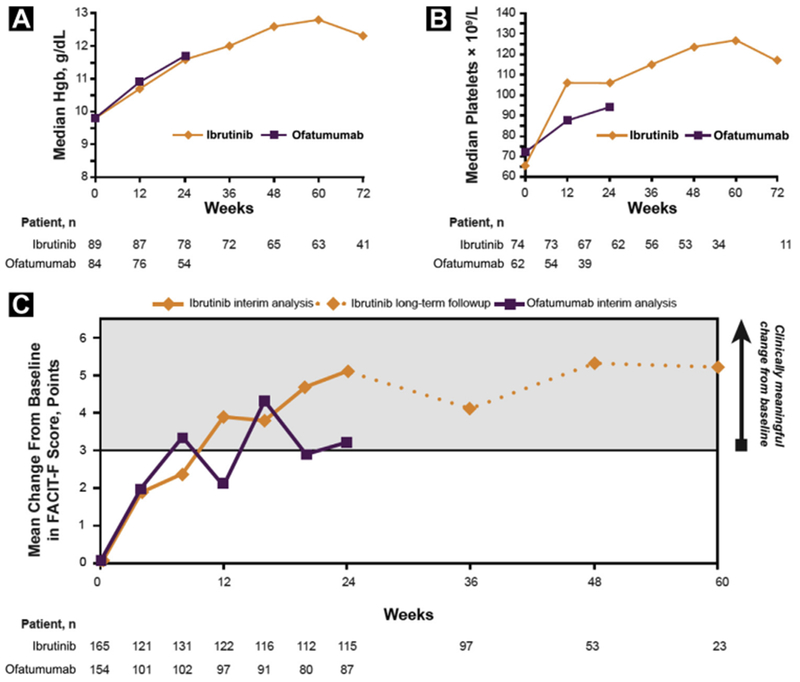
Change Over Time by Treatment Arm in Median Hgb Levels (A), Median Platelet Count (B), and Improvement in FACIT-F Including Long-Term Extended Follow-Up (C)
Abbreviations: FACIT-F = Functional Assessment of Chronic Illness Therapy-Fatigue; Hgb = hemoglobin.
Patient-Reported Outcomes
FACIT-F.
Baseline FACIT-F scores for ibrutinib and ofatumumab were similar (mean, 35.7 vs. 34.5, respectively). More patients achieved clinically meaningful improvement in FACIT-F with ibrutinib versus ofatumumab (56% vs. 43%; odds ratio [OR], 1.69; P = .0101); clinically meaningful improvement with ibrutinib was sustained through 60 weeks of follow-up (Figure 1C).
EORTC QLQ-C30.
At baseline, ibrutinib and ofatumumab patients had similar mean scores for EORTC QLQ-C30 global health status (60.0 vs. 58.3, respectively). Greater improvement in global health status mean score was seen at 24 weeks with ibrutinib versus ofatumumab (9.0 vs. 5.8, respectively) (see Supplemental Table 2 in the online version). Of the 15 EORTC domains assessed at baseline, nausea/vomiting, diarrhea, and constipation had the lowest severity, and global health status had the highest impairment. At week 24, patients treated with ibrutinib improved on 13 (all but nausea/vomiting and diarrhea) versus 12 domains (all but cognitive functioning, pain, or constipation) with ofatumumab (see Supplemental Table 2 in the online version). A significantly greater proportion of patients on ibrutinib compared with ofatumumab reported an improvement in cognitive functioning, including concentration and recall (32% vs. 20%; P = .008). Overall, the mean change in scores from baseline to week 24 across the EORTC QLQ-C30 domains appeared comparable between treatment arms. An improvement in the EORTC Fatigue Subscale score from baseline to week 24 was seen with ibrutinib (n = 117; median 11-point improvement), whereas no improvement was seen with ofatumumab (n = 87; median 0-point improvement).
Reduction in Disease Burden
Disease burden was assessed by independent review prior to discontinuation for the primary analysis with 9.4 months median follow-up, similar to the median PFS for the ofatumumab arm (8.1 months). At least a 50% reduction in lymph node size was observed more frequently with patients on ibrutinib versus ofatumumab (92% vs. 14%; P < .0001) (Figure 2A); the median time to reduction was 2.6 versus 5.3 months with ibrutinib and ofatumumab, respectively. Reduction in spleen size was more common in ibrutinib-treated than ofatumumab-treated patients (85% vs. 54%; P < .0001) (Figure 2B); the median time to reduction was 2.6 months with both ibrutinib and ofatumumab. These improvements are consistent with recently published updates to PFS and OS showing continued benefit for patients randomized to ibrutinib (median follow-up 19 months: PFS, hazard ratio [HR], 0.106; P < .0001; 18-month landmark OS, HR, 0.361; 95% confidence interval [CI], 0.208-0.628).14,19
Figure 2.
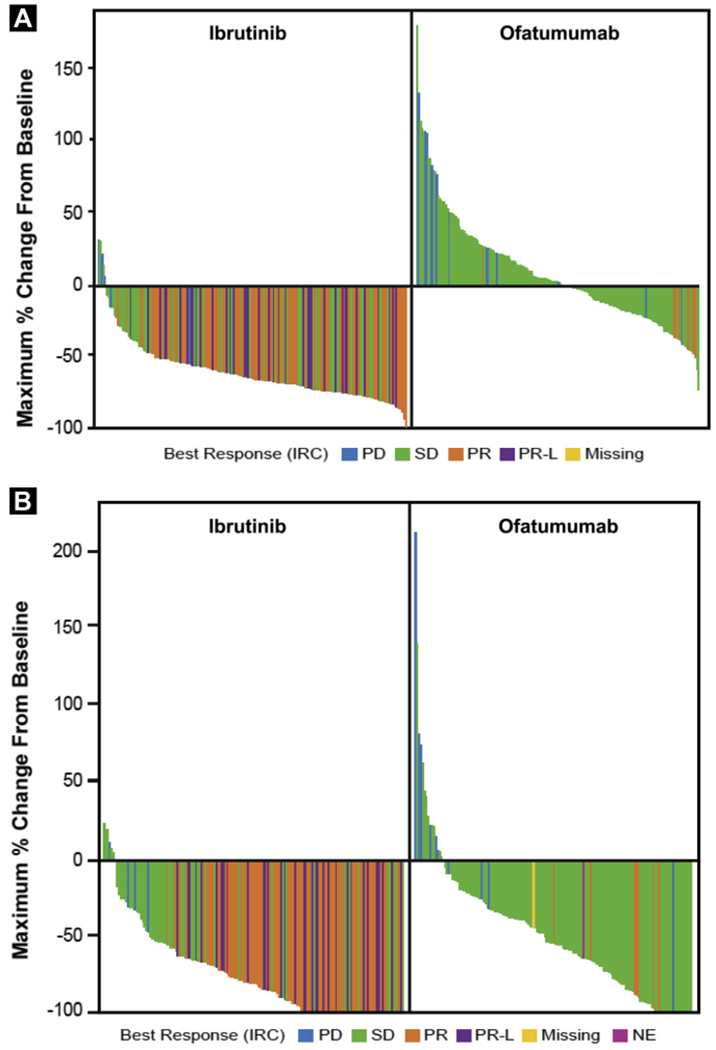
Reduction in Lymph Nodes* (A) and Splenic Enlargement† (B) (Primary Analysis)
*Sum of the products of multiple lymph nodes (as evaluated by computed tomography scans) or the longest diameter of one target lymph node. †Patients who experienced resolution of splenic enlargement by IRC are considered to have 100% reduction.
Abbreviations: IRC = independent review committee; NE = not evaluable; PD = progressive disease; PR = partial response; PR-L = PR with lymphocytosis; SD = stable disease.
Disease-Related Symptoms and Medical Resource Utilization
Consistent with findings at the primary analysis, improvement in disease-related symptoms were assessed by investigators more frequently with ibrutinib than with ofatumumab for weight loss (100% vs. 87%), fatigue (79% vs. 64%), night sweats (89% vs. 77%), abdominal pain/discomfort (96% vs. 75%), and anorexia (100% vs. 64%), but not fever (88% vs. 100%). Hospitalizations in the first 30 days occurred less frequently with ibrutinib than ofatumumab (0.087 vs. 0.184 events/patient20; P = .0198); however, no significant differences were observed through 6 months. Growth factor usage after day 30 occurred slightly more frequently with ofatumumab than ibrutinib (5%-9% vs. 2%-4% each month through month 6, respectively). Transfusion rates with ibrutinib and ofatumumab (30% vs. 24%) were not notably different through 6 months.
White Blood Cell Count, Lymphocyte Counts, and Serum Immunoglobulin Levels
In both arms, the white blood cell differential trended to normal with an increase in the proportion of neutrophils and a decrease in lymphocyte proportions versus baseline (see Supplemental Figure 1A in the online version). In the ibrutinib arm, elevated baseline absolute lymphocyte count (ALC) declined over time to a median of 50% below baseline at 24 weeks and continued to decrease to normal (< 4 × 109/L) at later time points (after the expected initial transient increase with ibrutinib) (see Supplemental Figure 1B, C in the online version). Supplemental Table 3 (in the online version) shows distributions of peak ALC in ibrutinib-treated patients relative to baseline ALC.
The primarily malignant B cell counts were stable with ibrutinib and decreased with ofatumumab at week 12 (Figure 3 and Supplemental Figure 2 [in the online version]). After week 12, B cell counts in the ibrutinib arm also decreased significantly over time, with median count approaching the upper limit of normal at week 72. Median T cell (including CD3+, CD4+, and CD8+ subsets) and NK cell counts remained within the limits of normal throughout testing, whereas the relative proportion of T cells (including CD4+ and CD8+ subsets) and NK cells progressively increased over time. For 57 patients with available baseline samples, median counts for CD3+ and CD4+ T cells (but not CD8+) and NK cells were significantly decreased at week 72, remaining above the lower limits of normal (LLN). The median regulatory T cell counts significantly decreased over time on ibrutinib, dropping from the upper limit of normal to the LLN (see Supplemental Figure 3 in the online version).
Figure 3.
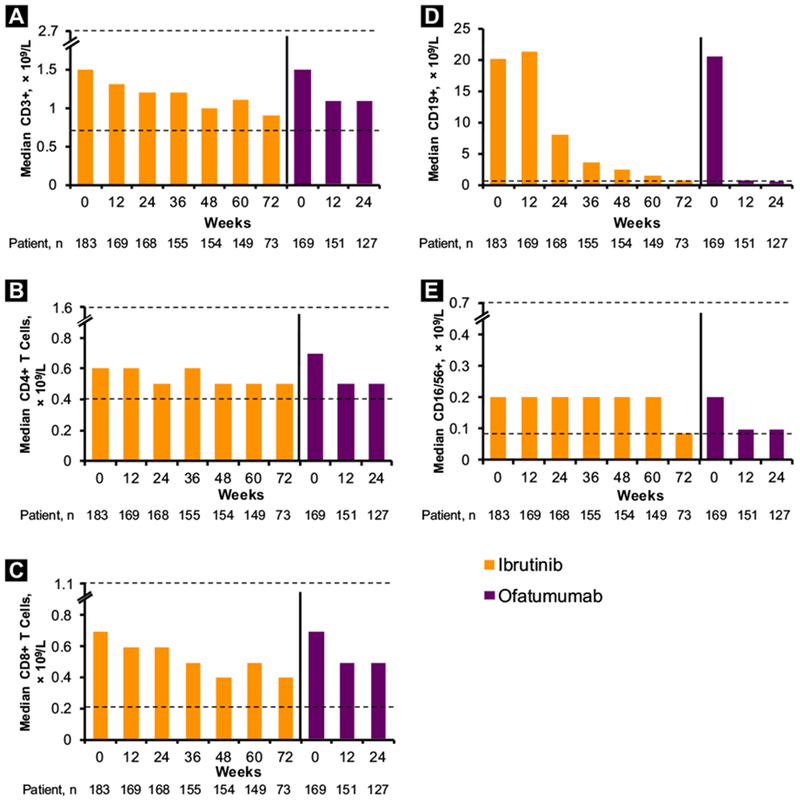
Absolute Counts Over Time of CD3+ (A), CD4+ (B), CD8+ (C), CD19+ (D), and CD16/56+ (E) Cells. ULN and LLN (× 109/L): CD3+, 0.723-2.737; CD4 + , 0.404-1.612; CD8+, 0.22-1.129; CD19+ , 0.08-0.724; and CD16/56+, 0.084-0.724. In Each Chart, ULN and LLN are Shown by Dashed Lines (With Axis Breaks); Only Uln is Shown for CD19+ Cells
Abbreviations: LLN = lower limit of normal; ULN = upper limit of normal.
Analysis of serum Immunoglobulin levels (excluding the 32% and 22% of ibrutinib and ofatumumab patients on intravenous Immunoglobulin replacement) showed a significant increase in IgA levels with ibrutinib, rising to the LLN at week 12, which was maintained through week 72. Median IgG levels were stable with up to 1.4 years on ibrutinib, rising to the LLN at week 72. Median IgM level changes were nonsignificant and varied over time from just above to just below the LLN from week 36 to week 60 (Figure 4). For those with baseline levels below the LLN, a similar increase was observed for IgA without significant decrement in IgG or IgM over time (data not shown).
Figure 4.
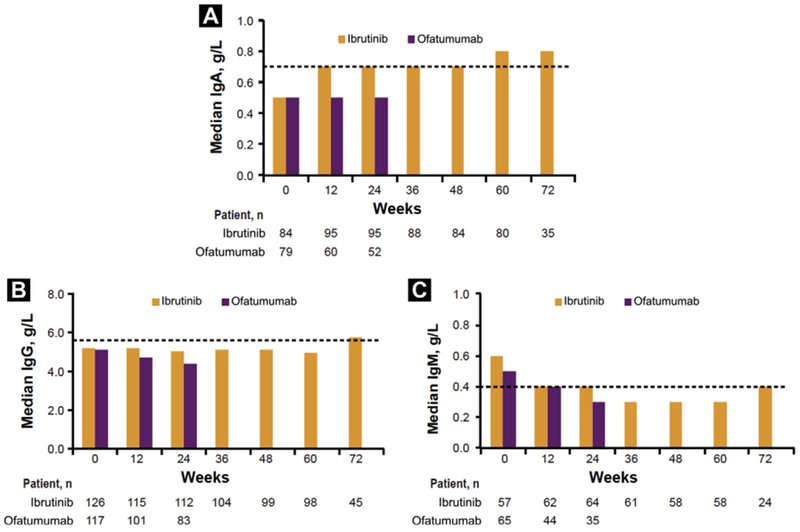
Median Levels* Over Time of IgA (A), IgG (B), and IgM (C). LLN for Baseline IgA, IgG, and IgM are 0.7 g/L, 5.65 g/L, and 0.4 g/L, Respectively, and are Shown by Dashed Lines
*Excluding patients on intravenous Ig.
Abbreviations: Ig = immunoglobin; LLN = lower limit of normal.
Adverse Events
Most AEs were grade 1 in severity. The most frequently observed AEs among ibrutinib patients at any time were diarrhea (37% grade 1, 10% grade 2, 4% grade 3), fatigue (19% grade 1, 12% grade 2, 3% grade 3), and nausea (24% grade 1, 6% grade 2, 2% grade 3), of which none were grade 4. Except for diarrhea (all grade), rates of common AEs in the first 6 months were similar between the treatment arms (Figure 5A). New onset of these events was less frequent after the first 6 months such as diarrhea (all grades) rates, which declined over time from 47% at 0 to 6 months to 5% at 12 to 18 months (Figure 5B).
Figure 5.
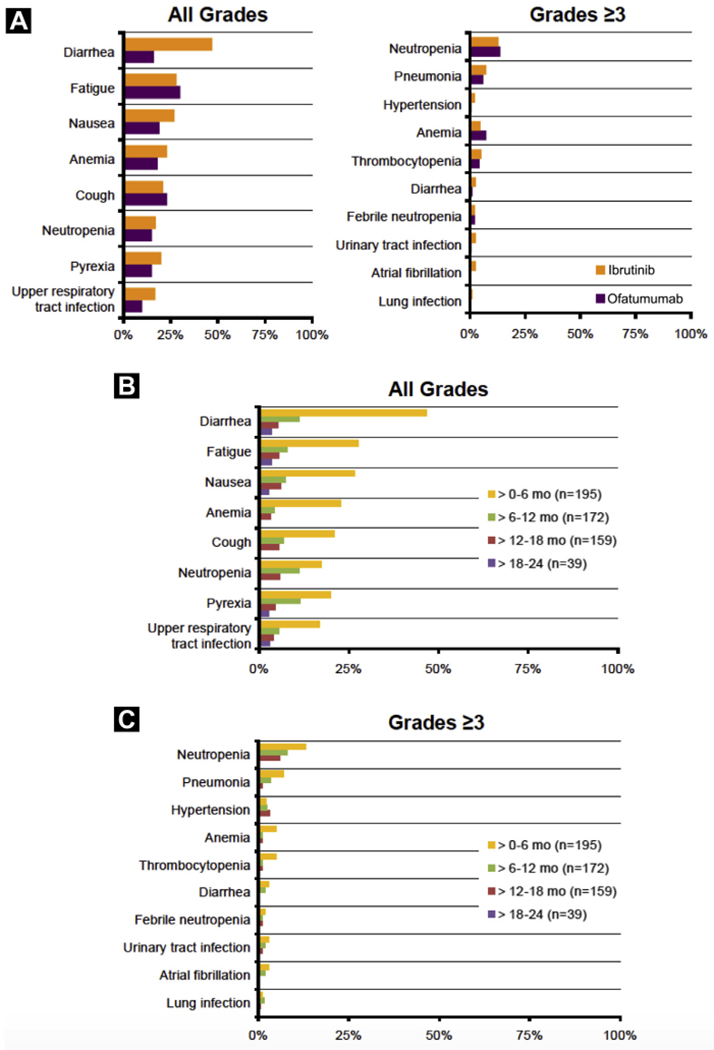
Incidence of Common (≥ 20% for all Grade, ≥ 3% for Grade ≥ 3 at Any Time) Treatment-Emergent Adverse Events that Occurred in the First 6 Months of Treatment in the Ibrutinib and Ofatumumab Arms (A), for all Grades by Time to Event Onset (B), and for Grades ≥ 3 by Time to Event Onset in the Ibrutinib Arm (C). New Events are Presented Only During the Period of Time Where Event Began. Note, No Grade ≥ 3 Events Started at > 18 Months
With prolonged ibrutinib treatment, the most frequent grade ≥ 3 AEs at any time were neutropenia (18%), pneumonia (11%), hypertension (6%), and thrombocytopenia (6%). The incidences of grade ≥ 3 infections and cytopenias were similar for ibrutinib and ofatumumab during the first 6 months of therapy (Figure 5A). Except for hypertension, the incidences of other frequent grade ≥ 3 AEs decreased or were similar over time (Figure 5C). Prevalence of grade ≥ 3 infections at any time was 30% for ibrutinib and 20% for ofatumumab (see Supplemental Figure 4 in the online version). Exposure-adjusted all-grade infection rate with ibrutinib versus ofatumumab was 14.7 versus 18.4 per 100 patient-months (grade ≥ 3 was 2.5 vs. 5.7, respectively). Upper respiratory tract infection was the most common all-grade infection for both the overall ibrutinib cohort (24%) and those with compromised Ig status at baseline (25% for IgA < LLN, 26% for IgG < LLN).
Atrial fibrillation occurred at any time in 13 (7%) patients, including grade ≥ 3 in 7 (4%) patients, resolving in 6 of 7 patients and leading to ibrutinib discontinuation in 1 patient, who was subsequently noted to have PD; the majority of grade ≥ 3 atrial fibrillation events were reported in the first 6 months of ibrutinib therapy. Major hemorrhage occurred in 4 patients (2%): grade 2 subdural hematoma, grade 3 post-procedural hemorrhage, grade 3 spontaneous hematoma, and grade 4 subdural hematoma (see Supplemental Table 4 in the online version). Tumor lysis syndrome occurred 5 and 11 days after ibrutinib discontinuation in 2 patients with PD (study days 534 and 242, respectively) who had not yet started any next line therapy for CLL.
At a median treatment duration of 16 months for ibrutinib versus 5 months for ofatumumab, 13 ibrutinib patients and 7 ofatumumab patients discontinued study drug primarily due to AEs. In total, 23 and 16 patients, respectively, had AEs associated with discontinuation, most frequently pneumonia; among patients who discontinued ibrutinib due to AEs, longer duration of therapy appears to be associated with increased time to progression (see Supplemental Table 5 in the online version). AEs leading to ibrutinib discontinuation occurred in 12 of 195, 9 of 172, 1 of 159, and 1 of 39 patients at 0 to 6, 7 to 12, 13 to 18, and 19 to 24 months, respectively. AEs leading to ibrutinib dose reduction occurred in 7 of 195, 4 of 172, and 2 of 159 patients at 0 to 6, 7 to 12, and 13 to 18 months, respectively.
Discussion
Ibrutinib is a selective first-in-class irreversible inhibitor of Bruton’s agammaglobulinemia tyrosine kinase with an important role in multiple cellular functions including B-cell antigen receptor signaling. A high frequency of durable responses and acceptable safety associated with ibrutinib in the previously treated population with CLL has been demonstrated in the RESONATE study with enhanced PFS, OS, and ORR as compared with ofatumumab, including in high-risk subgroups.14,19 This report demonstrates that in addition to its known efficacy, prolonged administration of ibrutinib confers patients additional benefits as assessed by multiple measures of well-being, including preservation of parameters of hematologic and immunologic function. At a median of 16.4 months follow-up, the present data are among the first reports from a randomized phase III study to demonstrate significant improvement in hematologic and immunologic parameters in parallel with QoL improvements. In the majority of patients with baseline cytopenias, ibrutinib led to early and sustained platelet and hemoglobin improvements (82% and 69%, respectively), which were markedly better than for the patients randomized to ofatumumab.
With up to 2 years of follow-up, the positive hematologic effects of ibrutinib resulted in sustained normalization of hemoglobin in 69% and of platelet count in 82% of patients with baseline anemia and thrombocytopenia, respectively. Transient increase followed by sustained decrease in percentage of lymphocytes over time is expected given the known pharmacodynamic ‘redistribution’ phenomenon of B-cell receptor inhibitor treatment-related lymphocytosis. Malignant B cell counts decreased dramatically with ibrutinib and approached normal limits by week 72, and median CD4+, CD8+, and NK cell counts remained within normal limits throughout treatment. Reduction in regulatory T cell counts to the LLN was observed in ibrutinib-treated patients consistent with preclinical studies of ibrutinib and other clinical data sets.21–23 Future analyses discriminating normal from neoplastic B cells would be beneficial to ascertain any modulatory effects of ibrutinib. An increase in IgA levels was also observed with ibrutinib over time, without significant decrement in IgG or IgM.
In a National Institutes of Health study of patients with CLL ≥ 65 years of age or with TP53 aberration, the infection rate overall was lower in treatment-naive (TN) versus relapsed/refractory (R/R) patients, but rates declined over time in both TN and R/R populations24; data that suggest clinically relevant improvement in humoral immunity with ibrutinib treatment. In an integrated analysis of patients from the RESONATE and RESONATE-2 (ibrutinib vs. chlorambucil in older patients with TN CLL/SLL) trials, infection rates were also shown to decrease with time on ibrutinib.25 Although the decreased incidence of various infections over time seen during ibrutinib treatment in this and other reports may reflect these positive hematologic effects on lymphocytes and immunoglobulin levels, future detailed analyses of these infections may be warranted, given recent descriptions of opportunistic infections occurring during ibrutinib treatment.26,27
PROs, including health-related QoL, are increasingly assessed in clinical trials as a measure of effectiveness and to help with decision-making. Analysis of FACIT-F outcomes showed that a clinically meaningful improvement from baseline was reached in < 24 weeks with ibrutinib and ofatumumab, with mean improvement sustained through 60 weeks of follow-up on ibrutinib. Similarly, fatigue was improved as measured with the EORTC fatigue subscale PRO as well as investigator-assessed disease-related symptoms. Additionally, 100% of patients treated with ibrutinib experienced improvements in weight loss and anorexia. The magnitude of improvement (mean change from baseline to week 24) in EORTC QLQ-C30 was greater with ibrutinib compared with ofatumumab for domains including global health status, physical functioning, fatigue, dyspnea, and constipation, whereas the improvement was greater with ofatumumab for role functioning, social functioning, and appetite loss. When considering the threshold for clinically meaningful improvement, only dyspnea was improved with ibrutinib (mean change, −11.0) and appetite loss with ofatumumab (mean change, −13.0). Notably, comparative data between treatment groups are limited due to the ofatumumab arm fixed duration of treatment (maximum 6 months) and relatively short PFS (median, 8.1 months),14 but the overall effects of ibrutinib on QoL reflect clinically meaningful gains that were sustained throughout the 60 weeks of follow-up. Irrespective of this disparity in drug exposure time, the majority of positive ibrutinib benefits were also observed at the time of the primary analysis when treatment durations were comparable.14 For safety and medical resource utilization data, this imbalance in follow-up would generally favor ofatumumab. Despite this, medical resource utilization as a prespecified exploratory endpoint, including hospitalizations in the first 30 days and growth factor usage after day 30, was higher in the ofatumumab group versus the ibrutinib group. Cost implications were not analyzed, although one recent real-world analysis of front line CLL treatment showed reduced medical resource utilization and reduced costs of care are incurred with ibrutinib compared with chemoimmunotherapy.28 That analysis did not evaluate pharmacy costs, and it remains unknown how these will contribute to the balance of costs for ibrutinib versus chemoimmunotherapy options. Such evaluations will be of further interest in the real-world setting.
No new safety signals were identified in this study versus earlier phase II studies,12 and new events of diarrhea, certain infections, and cytopenias, including grade ≥ 3 events, were observed less frequently over time. These data suggest that, except for the appearance of grade 3 hypertension, the likelihood of new onset treatment-emergent AEs may be reduced with long-term ibrutinib therapy.
Conclusion
Clinical data describing psycho-oncologic endpoints in studies of patients with CLL treated with new small targeted molecules or biologics are sparse. These novel RESONATE findings are among the first randomized data to assess multiple measures of overall patient well-being with treatment, including PROs, disease burden, hematologic function, and benefits to patients with regard to disease management and reduced requirement for supportive care. A survival benefit with ibrutinib, together with sustained improvements in hematologic endpoints and patient-reported outcomes, suggest that ibrutinib can improve QoL while prolonging survival.
Supplementary Material
Clinical Practice Points.
Complications associated with CLL and toxicities of therapy can profoundly affect QoL and patient well-being.
These analyses of RESONATE are the first to show significant psycho-oncologic improvements with ibrutinib in R/R CLL, in addition to the known benefits of longer PFS, OS, and improved ORR compared with anti-CD20 therapy.
Sustained hematologic improvement, clinically meaningful improvement in fatigue and overall QoL, and fewer early hospitalizations were observed with ibrutinib compared with ofatumumab while maintaining a reduction in disease burden.
No new safety signals with ibrutinib were identified in this study, and the onset of treatment-emergent AEs decreased over time.
Hematologic function, QoL, and overall patient well-being are a vital part of medical decision-making in addition to the clinical benefits of prolonged survival in patients with R/R CLL/SLL.
Acknowledgments
The authors would like to thank the patients who participated in the study and their supportive families, and the investigators and clinical research staff from the study centers. This study was sponsored by Pharmacyclics LLC, an AbbVie Company. Jacqueline Barrientos’s work is supported in part by National Institutes of Health/National Center for Advancing Translational Sciences (grant #UL1TR00457) and the 2015 American Society of Hematology—Harold Amos Medical Faculty Development Program fellowship. Clara Plascencia assisted with collection of data. Editorial support was provided by Stacey Rose, PhD, and funded by Pharmacyclics LLC, an AbbVie Company.
Disclosure
J.C.B. declares consultancy/advisory role for AbbVie, Gilead, Janssen, Pharmacyclics LLC, an AbbVie Company; and research funding from AbbVie, Gilead, Pharmacyclics LLC, an AbbVie Company. S.O’B. declares consultancy/advisory role for and honoraria from AbbVie, Janssen, and Pharmacyclics LLC, an AbbVie Company; and research funding from Pharmacyclics LLC, an AbbVie Company. J.R.B. declares consultancy/advisory role for Gilead, Pharmacyclics LLC, an AbbVie Company, Janssen, Sun Biopharma, AstraZeneca, Redx, Astellas, AbbVie, Pfizer, and TG Therapeutics; and research funding from Gilead and Sun Biopharma. N.E.K. declares consultancy/advisory role with Gilead, Morphosys, Agios, Infinity Pharma, Celgene, and Cytomx Therapeutics; and research funding from Genentech, Pharmacyclics LLC, an AbbVie Company, Celgene, Tolero Pharmaceutical, and Acerta. N.M.R. declares consultancy/advisory role for Celgene, Gilead, AbbVie, and BMS; and research funding from BMS. S.C. declares consultancy/advisory role for AbbVie, Gilead, Novartis, Celgene, Janssen, Pharmacyclics LLC, an AbbVie Company; and research funding from AbbVie, Pharmacyclics LLC, an AbbVie Company, Gilead, Celgene, Novartis. C.T. declares honoraria, consultancy/advisory role, and research funding from Janssen. S.M. declares consultancy/advisory role, honoraria, and speakers bureau for Roche, AbbVie, Janssen, and Gilead; consultancy/advisory role and honoraria from GlaxoSmithKline; and research funding from Roche, AbbVie, and Janssen. U.J. declares honoraria, consultancy/advisory role, and travel expenses for Gilead, Novartis, and AbbVie. S.D. declares honoraria and consultancy/advisory role for Janssen, Gilead, AbbVie, and MSD; speakers bureau for Janssen and Gilead; and travel expenses from Janssen and Gilead. C.P. declares honoraria from Janssen and Gilead; consultancy/advisory role with Takeda and Celgene; and travel accommodations from Gilead. T.R. declares honoraria, consultancy/advisory role, and research funding from Janssen and AbbVie; research funding from Pharmacyclics LLC, an AbbVie Company; and travel expenses from AbbVie. S.J.S. declares consultancy/advisory role with Celgene, Merck, Pharmacyclics, an AbbVie Company, Novartis, Genentech, Seattle Genetics, Janssen, Nordic Nanovector; research funding from Celgene, Merck, Pharmacyclics LLC, an AbbVie Company, Novartis, Genentech, Seattle Genetics, Janssen, Adaptive, BMS; and honoraria from Celgene, Merck, Pharmacyclics LLC, an AbbVie Company, Novartis, Genentech, Seattle Genetics, and Janssen. A.S. declares consultancy/advisory role for Roche, Gilead, Janssen, AbbVie, Pharmacyclics LLC, an AbbVie Company, Novartis; and research funding with Gilead, AbbVie; travel expenses with Gilead. D.G. declares travel expenses and speakers bureau for Janssen. A.B. declares consultancy/advisory role with AbbVie; speakers bureau for AbbVie; leadership role at The Doctors Laboratory; honoraria from AbbVie, Janssen, and Roche; and travel expenses for AbbVie and Gilead. C.D. declares consultancy/advisory role and honoraria from Roche, Gilead, Janssen, Sanofi, and AbbVie; travel expenses for Roche and Gilead; and expert testimony for Gilead. C.M. declares consultancy/advisory role for Janssen, AbbVie, and Gilead; and research funding from Gilead and Roche. G.C. declares research funding from BeiGene; and travel expenses from Amgen and Takeda. M.H. declares honoraria from Roche; research funding from Pharmacyclics, an AbbVie Company, and Janssen; and travel expenses from Novartis. J.A.J. declares employment with Celgene; consultancy/advisory role with Pharmacyclics LLC, an AbbVie Company, AbbVie, Janssen, Morphosys, and Gilead; research funding from Pharmacyclics LLC, an AbbVie Company, AbbVie, Janssen, Genentech, Gilead, and Acerta; and honoraria from Janssen and Acerta. E.H. declares employment with Pharmacyclics LLC, an AbbVie Company; and stock ownership with AbbVie. D.F.J. declares employment with Pharmacyclics LLC, an AbbVie Company; stock ownership with AbbVie (husband: employment and stock ownership with AbbVie); and patents/royalties/other intellectual property with AbbVie. K.E. declares employment with, patents/royalties/other intellectual property and travel expenses from Pharmacyclics LLC, an AbbVie Company; and stock ownership with AbbVie. I.G.S. declares employment and travel accommodations from Pharmacyclics LLC, an AbbVie Company; and stock ownership with AbbVie. S.S. declares employment and stock ownership with Iovance Biotherapeutics Inc; and consulting/advisory role for Pharmacyclics LLC, an AbbVie Company. J.C. Byrd declares research funding from Genentech, Acerta, and Pharmacyclics LLC, an AbbVie Company. P.H. declares honoraria, consultancy/advisory role, and speakers bureau with Janssen, Gilead, AbbVie, and Acerta; and research funding from AbbVie, Pharmacyclics LLC, an AbbVie Company, Janssen, Roche, GlaxoSmithKline, and Gilead.
Footnotes
Supplemental Data
Supplemental data, figures, and tables accompanying this article can be found in the online version at https://doi.org/10.1016/j.clml.2018.08.007.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holzner B, Kemmler G, Kopp M, et al. Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol 2004; 72:381–9. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Bowen D, Venkat C, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 2007; 139:255–64. [DOI] [PubMed] [Google Scholar]
- 5.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol 2014; 15:1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007; 370:230–9. [DOI] [PubMed] [Google Scholar]
- 7.Eichhorst BF, Busch R, Obwandner T, Kuhn-Hallek I, Herschbach P, Hallek M Health-related quality of life in younger patients with chronic lymphocytic leukemia treated with fludarabine plus cyclophosphamide or fludarabine alone for first-line therapy: a study by the German CLL Study Group. J Clin Oncol 2007; 25:1722–31. [DOI] [PubMed] [Google Scholar]
- 8.Else M, Cocks K, Crofts S, et al. Quality of life in chronic lymphocytic leukemia: 5-year results from the multicenter randomized LRF CLL4 trial. Leuk Lymphoma 2012; 53:1289–98. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Janssens A, Babu KG, et al. Health-related quality of life and patient-reported outcomes of ofatumumab plus chlorambucil versus chlorambucil monotherapy in the COMPLEMENT 1 trial of patients with previously untreated CLL. Acta Oncol 2016; 55:1115–20. [DOI] [PubMed] [Google Scholar]
- 10.Kutsch N, Busch R, Bahlo J, et al. FCR front-line therapy and quality of life in patients with chronic lymphocytic leukemia. Leuk Lymphoma 2017; 58:399–407. [DOI] [PubMed] [Google Scholar]
- 11.Van den Broek EC, Oerlemans S, Nijziel MR, Posthuma EF, Coeberg JW, van de Poll-Franse LV. Impact of active surveillance, chlorambucil, and other therapy on health-related quality of life in patients with CLL/SLL in the Netherlands. Ann Hematol 2015; 94:45–56. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol 2014; 15:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella D, Lai JS, Stone A. Self-reported fatigue: one dimension or more? Lessons from the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire. Support Care Cancer 2011; 19:1441–50. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage 2002; 24:547–61. [DOI] [PubMed] [Google Scholar]
- 18.Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLQ-C30 Scoring Manual (3rd Edition). Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 19.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 2018; 32:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui O Statistical methods to analyze adverse events data of randomized clinical trials. J Biopharm Stat 2009; 19:889–99. [DOI] [PubMed] [Google Scholar]
- 21.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122:2539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long M, Beckwith KA, Maddocks K, et al. Ibrutinib treatment reduces both T-regulatory cells and B-regulatory cell phenotype in malignant B cells in chronic lymphocytic leukemia patients. Blood 2015; 126:2940. [Google Scholar]
- 23.Kondo K, Burger JA, Micheal K, et al. Ibrutinib can modulate the T cell response in chronic lymphocytic leukemia by reducing PD1/PDL1 interactions. Blood 2015; 126:1737.26450953 [Google Scholar]
- 24.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood 2015; 126:2213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coutre S, Byrd JC, Hillmen P, et al. Integrated and long-term safety analysis of ibrutinib in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Blood 2016; 128:4383. [Google Scholar]
- 26.Rogers KA, Luay M, Zhao Q, et al. Incidence and type of opportunistic infections during ibrutinib treatment at a single academic center. Blood 2017; 130:830. [Google Scholar]
- 27.Ruchlemer R, Ami RB, Bar-Meir M, et al. Ibrutinib: a risk factor for invasive fungal infections? Blood 2017; 130:4323. [Google Scholar]
- 28.Nabhan C, Chung J, Mato AR, et al. Comparison of costs and health care resource utilization (HRU) in chronic lymphocytic leukemia (CLL) patients treated with front-line ibrutinib or chemoimmunotherapy. Blood 2017; 130:2111.28864813 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


