Abstract
Background
Primary prevention strategies to mitigate the burden of heart failure (HF) are urgently needed. However, no validated risk prediction tools are currently in use.
Objectives
We sought to derive 10-year risk equations of developing incident HF.
Methods
Race- and sex-specific 10-year risk equations for HF were derived and validated from individual-level data from 7 community-based cohorts with at least 12 years of follow-up. Participants who were recruited between 1985-2000, between 30 to 80 years, and were free of cardiovascular disease at baseline were included to create a pooled cohort (PC) and randomly split for derivation and internal validation. Model performance was also assessed in 2 additional cohorts.
Results
In the derivation sample of the PC (n=11771), 58% were women, 22% were black with a mean age 52±12 years, and HF occurred in 1339 participants. Predictors of HF included in the race-sex specific models were age, blood pressure (treated or untreated), fasting glucose (treated or untreated), body mass index, cholesterol, smoking status, and QRS duration. The PC equations to Prevent HF (PCP-HF) model had good discrimination and strong calibration in internal and external validation cohorts. A web-based tool was developed to facilitate clinical application of this tool.
Conclusions
We present a contemporary analysis from 33,010 men and women demonstrating the utility of the sex and race-specific 10-year PCP-HF risk score, which integrates clinical parameters readily available in primary care settings. This tool can be useful in risk-based decision making to determine who may merit intensive screening and/or targeted prevention strategies.
Keywords: Heart Failure, primary prevention, risk factor, epidemiology
Condensed Abstract
There is an is urgent need to identify strategies to prevent heart failure given the substantial morbidity, mortality, and cost associated with HF. We developed the Pooled Cohort equations to Prevent HF (PCP-HF) to estimate 10-year risk of incident HF among participants aged 30-80 years from 5 population-based cohorts. We assessed model performance internally and validated it externally in 2 large cohorts. The PCP-HF risk score demonstrated good discrimination and calibration. The PCP-HF risk score is a novel tool that integrates clinical risk factor data and may be useful in individual level risk-based decision making and population-level prevention of HF.
Introduction
Heart failure (HF) is highly prevalent, affecting more than 6 million US adults. (1,2) Over the lifetime, the risk for developing HF is estimated to range from 20 to 46% in white and black middle-aged men and women.(3) In the context of the growing aging population, the increasing burden of HF risk factors (e.g. obesity, diabetes), and advances in secondary prevention treatments for coronary heart disease, prevalence of HF has continued to increase (1,4,5). If current trends continue, HF is estimated to affect more than 8 million people by 2030 and total healthcare costs projected to exceed $70 billion.(6) The burden of HF continues to grow globally as well with a 23% increase in prevalence in the UK from 2002 to 2014.(7) Because HF is associated with significant morbidity, mortality, and cost of care, focusing on prevention is of utmost importance to address the public health and economic burden associated with HF, both nationally and globally (8).
Current guidelines emphasize the need for a standardized screening strategy to identify individuals with higher likelihood of developing HF.(9) Whereas risk prediction for atherosclerotic cardiovascular disease (ASCVD) has been widely implemented in clinical practice to guide preventive strategies for coronary heart disease and stroke, no validated race and sex-specific risk prediction tools for incident HF are available to guide similar personalized risk-based decision making.(10) Existing HF risk prediction models have limtations, including derivation in non-representative, non-contemporary or restricted higher-risk samples (e.g. history of coronary heart disease, valvular heart disease) with limited ethnic diversity.(11) Further, many risk models lack extensive external validation, limiting their applicability and implementation in a general population without prevalent cardiovascular disease (CVD).
Thus, using pooled individual level data from five diverse population-based cohorts, we sought to develop equations to estimate the 10-year risk of incident HF utilizing routinely available clinical data in a broad general population (30-80 years) of white and black adults without prevalent CVD. The Pooled Cohort equations to Prevent HF (PCP-HF) were also assessed for external validity in an additional two large population-based cohorts.
Methods
For derivation, we included pooled individual-level data from 5 large, racially and geographically diverse, National Heart Lung and Blood Institute (NHLBI) sponsored cohort studies, including: the Atherosclerosis Risk in Communities (ARIC) Study, Coronary Artery Risk Development in Young Adults (CARDIA) Study, Cardiovascular Health Study (CHS), Framingham Heart Study Offspring Cohort (FOF), and Multi-Ethnic Study of Atherosclerosis (MESA). The study designs of each cohort have been previously described.(11–18) All cohorts represent community-based or population-based samples with direct measurement of risk factors, surveillance and adjudication for incident HF, and had at least 12 years of follow-up. Black and white participants from the pooled cohorts (PC) were included in the analysis if they were recruited between 1985-2000, were between the ages of 30 to 80 years old, and free from CVD at baseline. External validation was performed in white participants from the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) cohort and in black participants from the JHS cohorts. In the JHS validation, participants who were included in the derivation or internal validation samples as part of the ARIC cohort were removed.
The protocols used to obtain data and ascertainment of vital status and HF for the Lifetime Risk Pooling Project, which includes the PC (ARIC, CARDIA, CHS, FOF, MESA), JHS, and PREVEND have been previously described.(12–19) The study protocol was approved by the Institutional Review Board at Northwestern University. Height, weight, blood pressure, and fasting glucose and cholesterol were measured directly in all participants; smoking status was self-reported. QRS duration was available from standardized automated ECG analysis. Incident HF events were adjudicated with the use of strategies selected by each cohort’s investigator group with available clinical records and pre-specified criteria (Detailed descriptions are provided in the Online Appendix).
All statistical analyses were performed with the use of SAS statistical software version 9.2 (SAS institute) and R version 3.1.2. Individual participant-level data were pooled to create the PC and the dataset was randomly split into two equal samples for derivation and internal validation in distinct participants. We chose this approach as opposed to bootstrapping to determine the beta-coefficeints with the entire dataset as our sample size was large. The baseline 10-year risk equations were developed from sex and race specific proportional hazards models that included HF risk factors: age, systolic blood pressure (SBP), anti-hypertension medication usage, body mass index (BMI), total and high-density cholesterol levels (TC; HDL-C), current smoking status, fasting glucose, diabetic medication usage, and electrocardiogram measurement of QRS duration. A variable for lipid therapy was evaluated, but did not remain in the final model, because prevalence of lipid treatment was relatively low in our cohorts (4.2%) and a statistical significant association with outcomes was lacking. Multiple Cox regression models were conducted to estimate the equation parameters. Likelihood statistics and Akaike information criterion were applied to optimize the regression model similar to the previously published methods for the Pooled Cohort Equations for ASCVD.(20) Specifically, interactions with age were tested for each risk factor and were retained in final models if the p value for the interaction term was less than .01, or the p value was .01 to .05 and the continuous net reclassification improvement for nonevents was 15 percent or greater, or the integrated discrimination improvement index was statistically significant. End points were censored at 12 years. Non-HF related death in follow-up was censored. Discrimination was assessed using Harrell’s C-statistic with 95% confidence intervals (CI). Greenwood-Nam-D’Agostino statistic was applied to evaluate the calibration of the model in derivation and validation samples. In order to demonstrate the utility of HF-specific risk estimation, we also assessed the discrimination and calibration in the prediction of HF using non-HF specific CVD risk scores: Adult Treatement Panel (ATP) III Framingham Risk Score, General Cardiovascular Risk Profile CVD Score, and the Pooled Cohort Equations for ASCVD.(21–23).
Results
Baseline characteristics of white and black adults from the derivation sample of the PC and the external validation cohorts (JHS, and PREVEND) are shown in Table 1. In the derivation cohort, 58% were women, 22% black, with mean age 52±12 years. Prevalence of risk factors were similar in the derivation and validation cohorts in each sex-race group with higher mean total cholesterol and lower prevalence of diabetes in the PREVEND cohort (Online Tables 1 and 2). Mean BMI and SBP were higher among black men and women compared with white men and women. Higher prevalence of treatment for risk factors of hypertension and diabetes was also present in black adults compared with white adults.
Table 1.
Baseline Characteristics among Black and White Men and Women in the Pooled Cohort Derivation and the External Validation Samples from 7 Population-Based Cohorts
| Black | White | |||
|---|---|---|---|---|
| PC | JHS | PC | PREVEND | |
| Derivation | Validation | Derivation | Validation | |
| N=2561 | N=2770 | N=9210 | N=6699 | |
| Mean age, years (SD) | 49.3 (12.8) | 49.9 (11.6) | 53.6 (11.6) | 49.3 (12.2) |
| Female, n (%) | 1628 (64) | 1704 (62) | 5213 (57) | 3493 (52) |
| Diabetes, n (%) | 290 (11) | 302 (11) | 522 (6) | 232 (4) |
| Mean fasting glucose, mg/dL (SD) | 102 (35) | 97 (31) | 99 (25) | 88 (21) |
| Diabetes treatment, n (%) | 176 (7) | 225 (9) | 193 (2) | 99 (2) |
| Current smoking, n (%) | 656 (26) | 349 (13) | 1873 (20) | 2552 (38) |
| Mean systolic blood pressure, mm Hg (SD) | 124 (20) | 125 (16) | 120 (18) | 129 (20) |
| Hypertension treatment, n (%) | 935 (37) | 1057 (41) | 2200 (24) | 933 (14) |
| Mean total cholesterol, mg/dL (SD) | 197 (44) | 197 (39) | 208 (41) | 220 (44) |
| Mean HDL cholesterol, mg/dL (SD) | 54 (15) | 51 (14) | 52 (16) | 52 (16) |
| Mean BMI, kg/m2 | 29.8 (6.3) | 31.9 (7.4) | 26.7 (4.7) | 26.1 (4.2) |
| Mean QRS duration, msec (SD) | 89 (13) | 93 (13) | 88 (13) | 98 (13) |
PC indicates pooled cohort, which includes ARIC, CARDIA, CHS, FOF, and MESA; JHS indicates Jackson Heart Study; PREVEND indicates Prevention of REnal and Vascular ENd-stage Disease cohort
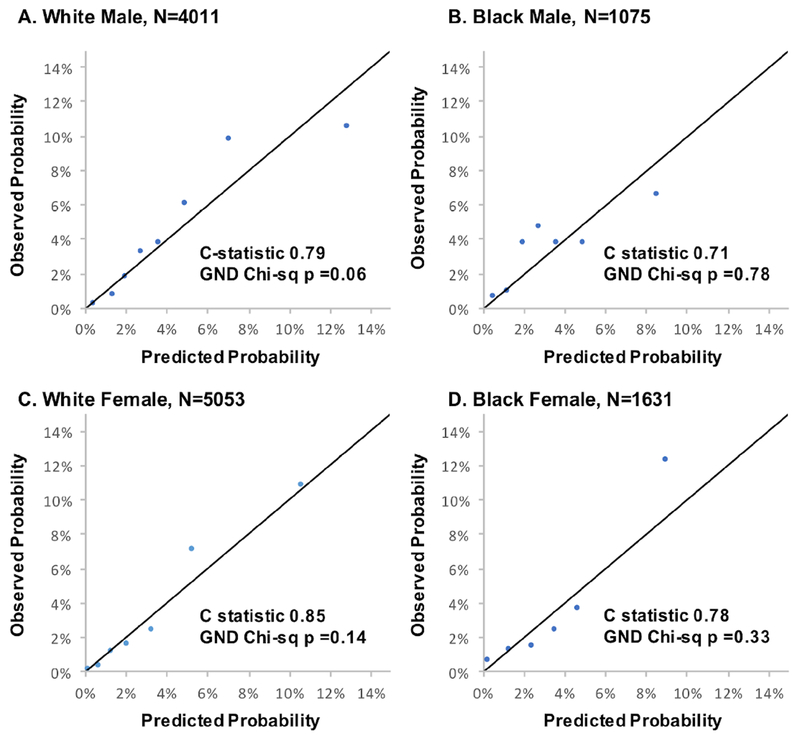
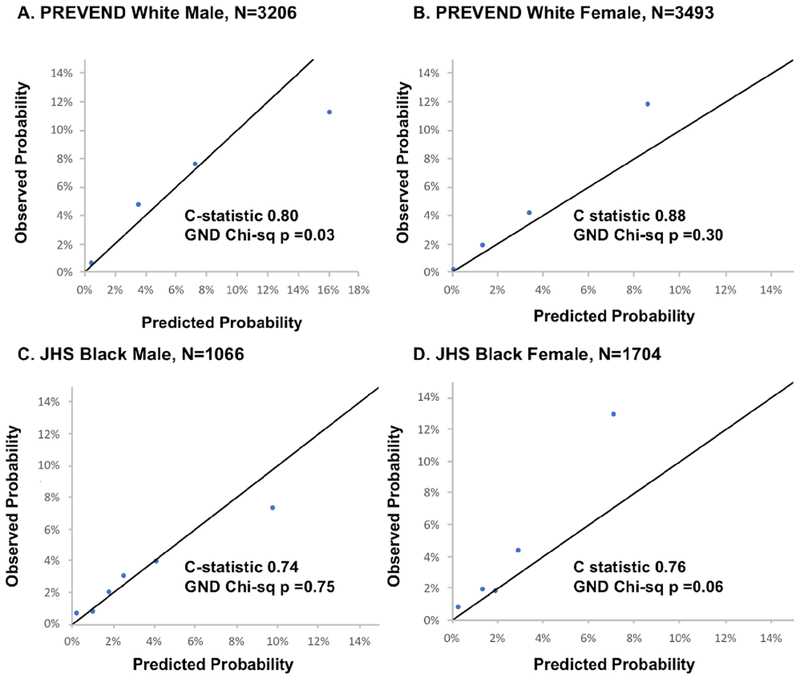
Over a follow-up of 10 years, 1339 HF events occurred in the derivation cohort. Variables and coefficients for the risk scores are presented in Table 2 and Online Table 3. Discrimination and calibration metrics of the 10-year risk equation, PCP-HF, are shown in Table 3 for the derivation and validation cohorts. The PCP-HF risk score had good to excellent discrimination in the derivation (Online Figure 1) and internal validation cohorts (PC) as well as externally (JHS and PREVEND). Among white men and women and black men and women in the PC internal validation sample, the C-statistic (95% confidence interval [CI]) in each sex-race group was 0.79 (95% CI: 0.76, 0.81), 0.71 (0.63, 0.80), 0.85 (0.82, 0.88), and 0.78 (0.71, 0.85), respectively (Figure 1). The PCP-HF risk score was also well-calibrated as assessed by the GND chi-square statistic in the PC internal validation sample (p>0.05 for all). External validation performed in the JHS and PREVEND samples demonstrated good discrimination (C-statistic ranging from 0.71-0.88 in black and white men and women) and strong calibration (Figure 2). For comparison, the c-statistics for age alone in prediction of 10-year risk of HF was 0.70 (0.68, 0.74) and 0.69 (0.61, 0.76) in white and black men and 0.74 (0.71, 0.89) and 0.65 (0.59, 0.71) in white and black women, respectively.
Table 2.
Race- and Sex-Specific Equation Parameters for Estimation of 10-year Risk of HF erived from the Pooled Cohort
| White Male | White Female | Black Male | Black Female | |
|---|---|---|---|---|
| Ln Age (y) | 41.94 | 20.55 | 2.88 | 51.75 |
| Ln Age, Squared | −0.88 | N/A | N/A | N/A |
| Ln Treated Systolic BP (mm Hg) | 1.03 | 12.95 | 2.31 | 29.0 |
| Ln Age×Ln Treated Systolic BP | N/A | −2.96 | N/A | −6.59 |
| Ln Untreated Systolic BP (mm Hg) | 0.91 | 11.86 | 2.17 | 28.18 |
| Ln Age×Ln Untreated Systolic BP | N/A | −2.73 | N/A | −6.42 |
| Current Smoker (1=Yes, 0=No) | 0.74 | 11.02 | 1.66 | 0.76 |
| Ln Age×Current Smoker | N/A | −2.50 | −0.25 | N/A |
| Ln Treated glucose (mg/dL) | 0.90 | 1.04 | 0.64 | 0.97 |
| Ln Untreated glucose (mg/dL) | 0.78 | 0.91 | 0.58 | 0.80 |
| Ln Total Cholesterol (mg/dL) | 0.49 | N/A | N/A | 0.32 |
| Ln HDL–C (mg/dL) | −0.44 | −0.07 | −0.81 | N/A |
| Ln BMI (Kg/m2) | 37.2 | 1.33 | 1.16 | 21.24 |
| Ln Age× Ln BMI | −8.83 | N/A | N/A | −5.0 |
| Ln QRS duration (msec) | 0.63 | 1.06 | 0.73 | 1.27 |
| Mean Coefficient× Value (MeanCV) | 171.5 | 99.73 | 28.73 | 233.9 |
| Baseline Survival (S0) | 0.98752 | 0.99348 | 0.98295 | 0.99260 |
N/A indicates not applicable; HF indicates heart failure; BP indicates blood pressure; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; and Ln, natural logarithm.
Table 3.
Numbers of Events, Discrimination and Calibration Statistics of the 10-year Pooled Cohort Equations to Prevent Heart Failure for Each Race and Sex Group in the Derivation and Validation Samples
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Derivation | Validation | Derivation | Validation | |||
| White | PC | PREVEND | PC | PREVEND | ||
| Total N | 3997 | 4011 | 3206 | 5213 | 5053 | 3493 |
| Events | 525 | 515 | 92 | 560 | 540 | 66 |
| C statistics (95% CI) | 0.83 (0.80, 0.86) | 0.79 (0.76, 0.81) | 0.80 (0.76, 0.84) | 0.84 (0.81, 0.88) | 0.85 (0.82, 0.88) | 0.88 (0.84, 0.91) |
| GND Chi-sq× (P value) | 9.45 (0.15) | 13.4 (0.06) | 8.7 (0.03) | 7.64 (0.17) | 9.76 (0.14) | 3.7 (0.30) |
| Derivation | Validation | Derivation | Validation | |||
| Black | PC | JHS | PC | JHS | ||
| Total N | 933 | 1075 | 1066 | 1628 | 1631 | 1704 |
| Events | 106 | 104 | 29 | 148 | 162 | 66 |
| C statistics (95% CI) | 0.83 (0.78, 0.87) | 0.71 (0.63, 0.80) | 0.74 (0.61, 0.86) | 0.80 (0.73, 0.86) | 0.78 (0.71, 0.85) | 0.76 (0.66, 0.85) |
| GND Chi-sq (P value) | 3.12 (0.68) | 3.18 (0.78) | 2.67 (0.75) | 3.45 (0.75) | 5.80 (0.33) | 10.9 (0.06) |
PC indicates pooled cohort, which includes ARIC, CARDIA, CHS, FOF, and MESA; JHS indicates Jackson Heart Study; PREVEND indicates_Prevention of Renal and Vascular End-stage Disease; CI represents confidence interval; GND represents Greenwood-Nam-D’Agostino
Figure 1. Sex- and Race-Specific Calibration Plots in the Validation Sample of the Pooled Cohort (n=11,770) with Discrimination and Calibration Statistics.
Calibration of the Pooled Cohort equations to Prevent Heart Failure (PCP-HF) risk score in the randomly split validation sample of the pooled cohort, which includes the ARIC, CARDIA, CHS, FOF, and MESA cohorts. Participants are grouped into deciles (collapsed when fewer than 2 events were observed in any group) according to increasing predicted risk and average predicted risk is compared with average observed risk in each category in each sex-race group. The model demonstrated good to excellent discrimination with c-statistics ranging from 0.71-0.85 and strong calibration (chi-sq p>0.05 for all sex-race groups).
Figure 2. Calibration Plots in the Jackson Heart Study and PREVEND Validation Samples with Discrimination and Calibration Statistics.
Calibration of the Pooled Cohort equations to Prevent Heart Failure (PCP-HF) risk score in 2 large population-based cohorts of White (PREVEND) and Black (Jackson Heart Study) adults aged 30-80 years after excluding individuals with prevalent CVD. The remaining participants were grouped into deciles (collapsed when fewer than 2 events were observed in any group) according to increasing predicted risk and average predicted risk is compared with average observed risk in each sex-race group. The model demonstrated good discrimination with c-statistics ranging from 0.74-0.79 and strong calibration (chi-sq <20 with p>0.05 for all sex-race groups except white men p=0.03).
We also examined C-indices and GND chi-squared test for the prediction of HF in the internal validation subset of the PC when using non-HF specific CVD risk scores (Online Table 4). C-statistic for the ATP III Framingham Risk Score, General Cardiovascular Risk Score, and the ASCVD Pooled Cohort Equations were simliar (>0.70 for all sex-race groups). However, calibration was poor for all of the non-HF specific CVD risk scores (GND chi-sq p<0.01 for all). We were unable to specifically assess the discrimination and calibration of previously published HF-specific risk scores (Online Table 5) due to lack of available data on biomarkers or cardiovascular imaging in all participants, similar to the real-world primary care setting.
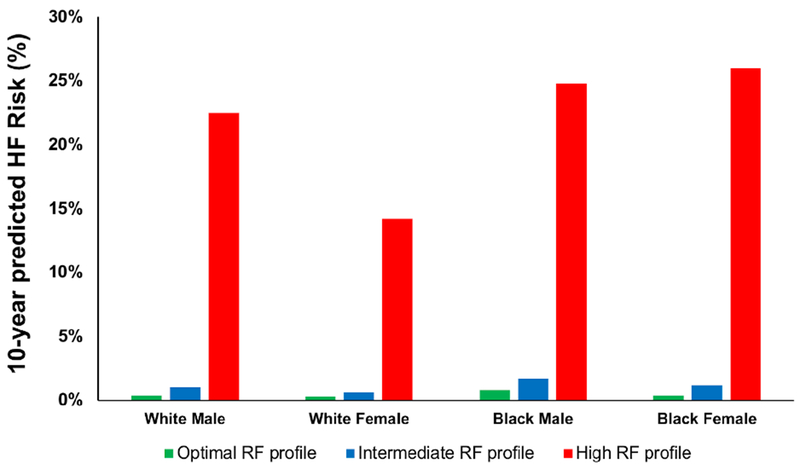
Observed and predicted mean 10-year risk of HF in sex-race groups are shown in Online Figure 2. Notably, participants in the highest group of predicted risk were at significantly higher risk compared with other strata and experienced the majority of the observed events in all groups. White men in the highest decile of predicted HF risk were more likely to be older, have hypertension, and have diabetes as shown in Online Table 6. Varying risk factor profiles and estimated risk of HF are demonstrated in Online Table 7 for white and black men and women. For example, estimated absolute 10-year HF risk is 23% and 25%, for a 55-year old white man and black man and 14% and 26% for a 55-year old white and black woman, respectively, with a high risk profile compared with <1% risk in all sex-race groups with an optimal risk factor profile (Figure 3).
Figure 3. Sex- and Race-Specific 10-year Heart Failure Risk Estimates for a 50-year-old White and Black Man and Woman based on Optimal, Intermediate, and High Risk Profiles.
Heart failure risk estimated by the Pooled Cohort equations to Prevent Heart Failure varies significantly based on risk factor profile among race-sex groups. For an optimal RF profile (never smoker, systolic blood pressure of 120mm Hg, fasting glucose of 90mg/dL, no treatment for hypertension or diabetes, BMI of 22kg/m2, total cholesterol of 180 mg/dL, HDL cholesterol of 55 mg/dL, and QRS duration of 90ms), 10-year risk is greatest among black men (0.8%) and lowest among white women (0.3%). Among participants with a high risk profile (current smoker, systolic blood pressure of 150mm Hg, fasting glucose of 126 mg/dL, treatment for hypertension and diabetes, BMI of 35 kg/m2, total cholesterol of 250 mg/dL, HDL cholesterol of 30 mg/dL, and QRS duration of 120ms), 10-year predicted risk varies greatly ranging from lowest risk among white women (14%) to highest risk among black men (24%) and women (26%).
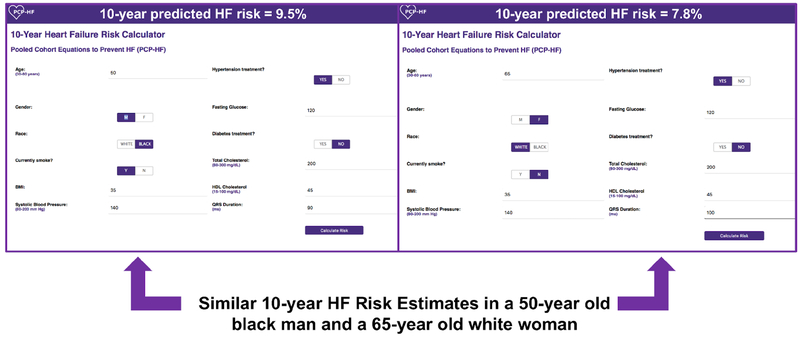
To facilitate clinical application of the PCP-HF risk tool, a web-based tool was developed and provides an estimated 10-year risk of HF for adults aged 30-80 years without prevalent CVD (Central Illustration). An example of how to calculate the HF risk score for a 50-year-old individual with BMI 28kg/m2, untreated SBP 130mm Hg, untreated fasting glucose 100 mg/dL, TC 200mg/dL, HDL-C 50 mg/dL, and QRS duration of 120 ms is shown in Online Table 8.
Central Illustration: Examples of predicted 10-year heart failure risk using the online PCP-HF tool in a 50-year-old black man and 65-year old white woman.
Ten-year predicted HF risk is high and similar for a 50-year black man and a 65-year old white woman with the following risk profile: treated systolic blood pressure 140 mm Hg, body mass index 35 kg/m2, untreated fasting glucose 120mg/dL, total cholesterol 200mg/dL, HDL-C 45 mg/dL, current smoker, and QRS of 90ms.
Discussion
In this study, we developed and validated sex- and race-specific 10-year risk prediction models for incident HF from 5 population-based cohorts using clinical variables routinely available in the primary care setting. The PCP-HF tool was derived from a sample of participants representative of the general US population without prevalent CVD. We validated this model in 2 additional population-based cohorts from the US and Europe, where it confirmed the clinical utility of the PCP-HF tool in risk assessment in a general primary prevention population. Lifetime risk of hospitalized HF has been estimated to be greater than that of incident CHD and stroke in both white and black adults in data from the ARIC cohort (24). Ample evidence supports successful prevention or postponement of HF development through modification or prevention of risk factors.(25,26) Therefore, focused efforts on prevention of HF are now of paramount importance and have been highlighted in the research priorities of the National Heart, Lung, and Blood Institute as an overarching strategic goal.(27)
While practice guidelienes for HF from national and international societies, including the American College of Cardiology (ACC)/American Heart Association (AHA), Heart Failure Society of America, and the European Society of Cardiology place continued emphasis on primary prevention of HF, identifying prevention and/or intervention strategies has been limited by well-validated risk prediction models in asymptomatic individuals without prevalent CVD. The ACC/AHA currently recommend a classification scheme to stage patients as follows: stage 0, no HF risk factors; stage A, HF risk factors; B, asymptomatic cardiac structural disease; C, symptomatic HF, and stage D, end-stage HF.(28) This construct emphasizes the progressive nature of HF and emphasizes the need for focused upstream efforts toward primordial and primary prevention and identification of individuals at risk for symptomatic HF (Stage 0/A/B). Individuals in these at-risk categories (0/A/B) represent the vast majority of the general population with estimates of 8-fold increase in mortality once Stage C/D HF develops underscoring the importance of intervening prior to this transition (29–31).
The present study confirms known individual risk factors as significant predictors of HF in at-risk participants and integrates these clinical variables into a reliable tool to provide a summary estimate of a persons’ likelihood of experiencing symptomatic HF (ACC/AHA Stage C/D) over a 10-year period. The PCP-HF tool provides a framekwork to guide risk assessment on an individual level for clinician-patient risk discussions and population level to promote research to identify optimal prevention and/or intervention strategies (e.g. randomized primary prevention trials) and policy approaches (e.g. reduction of dietary sodium intake) to reduce the burden of HF. For example, individuals who achieve greater than a 5% 10-year absolute risk estimate of HF (or highest decile of risk) may benefit from more intensive blood pressure lowering, use of specific therapies (e.g. sodium-glucose co-transporter 2 inhibitors), and more frequent surveillance.(32,33)
Our findings build upon prior HF risk prediction algorithms from individual cohorts, including the FHS, ARIC, Healthy Aging and Body Composition (Health ABC) study, and MESA.(34–37) The FHS risk score is derived from a mostly white population that included participants with prevalent CVD, and likely over-estimates risk in a general healthy population (Stage 0/A).(35,38) Both the MESA and ARIC risk scores integrate the use of N-terminal pro-brain natriuretic peptide (BNP), which is not routinely obtained in asymptomatic individuals without prevalent CVD.(34,36) The Health ABC study included 5-year risk estimates in elderly participants between the ages of 70-79 limiting the generalizability in young and middle-aged adults.(35) The International Collaboration on Heart Failure Subtypes pooled 3 community based cohorts (FHS, CHS, PREVEND) and developed risk scores for HF with preserved and reduced ejection fraction with external validation in MESA.(39) However, the majority of participants in the derivation subset were white (95%) and individuals with prior coronary heart disease were included (9%). In contrast, the PCP-HF risk score derivation included a broad age range of white and black men and women free of CVD at baseline from 5 population-based cohorts to allow for robust sex- and race- specific personalized 10-year estimates of HF from young adulthood to older age.
The PCP-HF score is an appealing and inexpensive initial screening tool to identify individuals in the primary care setting who are at higher risk for development of incident HF and may benefit from additional screening measures to refine and personalize risk assessment akin to the description of “risk enhancers” in the 2018 ACC/AHA Primary Prevention of ASCVD guidelines (40). Sequential screening for HF could integrate traditional and novel biomarkers (e.g. brain natriuretic peptide, galectin-3, soluble ST2 receptor) and advanced imaging (e.g. speckle tracking echocardiography, cardiac magnetic resonance imaging) in a stepwise, individualized, and cost-effective approach.(41) Studies on generalized use of brain natriuretic peptide (BNP) biomarkers for screening in asymptomatic adults have yielded mixed results.(42,43) In participants with known HF risk factors in the St. Vincent’s Screening to prevent Heart Failure Study, 36% of individuals were identified to have a BNP of 50ng/L or higher. Use of the PCP-HF tool, which is intended for a broader population may help to identify individuals at high 10-year risk who may also benefit from targeted BNP screening.(42) In addition, while imaging tools, such as speckle tracking echocardiography may be limited in their applicability for widespread screening in the general population due to cost, sequential screening strategies following identification of high risk indivdiauls to target with more intensive screening may provide the most effective and cost-efficient approach (44).
Significant health disparities in HF risk factors and incidence of HF exist across gender and race/ethnic groups. In the Coronary Artery Risk Development in Young Adults Study, premature development of incident HF before the age of 50 years occurred more commonly among Blacks than Whites.(45) In the MESA study of participants aged 45-84 years at baseline, the highest risk of developing incident HF was in Blacks, followed by Hispanic, White, and Chinese Americans (4.6, 3.5, 2.4, and 1.0 per 1000 person-years, respectively).(46) Differences in HF risk may be related to differences in prevalence of HF risk factors of obesity, diabetes, and hypertension.(47) Therefore, sex- and race-specific 10 year risk estimates in the PCP-HF tool were derived to provide absolute risk estimates specific to black and white men and women.
Key strengths of our study include derivation in 5 large population-based cohorts with inclusion of a diverse, contemporary sample that have representative proportion of women and blacks allowing for sex- and race-specific equations, broad age range from 30 to 80 years, and systematic adjudication of HF. Our study also has several limitations to note. The present observational study design cannot predict the effect of specific antihypertensive or antidiabetic therapies on risk for incident HF. Nevertheless, our estimates of risk for HF based on these clinical covariates may help guide clinicians and patients in decision-making regarding prevention strategies and/or intensification of therapies in individuals at higher risk for HF (e.g. aggressive blood pressure lowering). The current model is not able to discriminate between HF with systolic dysfunction or preserved ejection fraction. Nevertheless, the PCP-HF tool demonstrated good discrimination and calibration for HF events of all subtypes and allows for initiation of preventive strategies for shared risk factors for HF with preserved and reduced ejection fraction (e.g. choice of sodium-glucose cotransporter 2 inhibitors for DM management). This will allow broader upstream utility and generalizability, especially in the context of equally poor outcomes observed in patients with both HF with preserved and reduced ejection fraction.(5,48) Adjudication of HF events across cohorts differed, but all used pre-specified well-validated criteria. Finally, the PCP-HF tool is not able to provide accurate risk estimates for individuals from racial/ethnic groups other than non-Hispanic whites and non-Hispanic blacks. However, use of the equation versions for whites of the same sex can be considered and will need to be studied further.
In summary, we present a contemporary analysis from 33,010 men and women from 7 population-based cohorts describing the development and validation of novel sex- and race-specific estimates of 10-year risk of incident HF. Use of factors readily accessible in the primary care setting support implementation of the PCP-HF score in clinical practice to quantify absolute risk estimates for HF and identify individuals with higher likelihood of developing HF who may merit more intensive screening, targeted prevention, and/or enrollment in future primary prevention trials. The PCP-HF tool has excellent discrimination and calibration among a diverse group of US and European adults enhancing its broad generalizability and may be a valuable tool in risk assessment of HF on an individual and population level.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Systems-Based Practice:
Race- and sex-specific equations derived from community-based cohorts with long-term follow-up identified age, body mass index, blood pressure, fasting blood glucose and cholesterol levels, smoking status, and QRS duration as predictors of the risk of developing heart failure over 10 years.
Translational Outlook:
Prospective studies are needed to evaluate the clinical utility of specific preventive strategies in patients identified by this risk model.
Acknowledgements:
We thank the investigators of all the cohort studies including in this analysis for their hard work and dedication in collecting the underlying data, and especially the study participants, whose time and commitment have transformed our understanding of health and disease.
Funding: Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424. Supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute to Dr. Lloyd-Jones (R21 HL085375) and the Netherlands Heart Foundation (CVON DOSIS, grant 2014-40 and CVON RED-CVD, grant 2017-11 to Dr. de Boer and CVON SHE-PREDICTS-HF, grant 2017-21 to Drs de Boer and Suthahar). The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD).
Relationships with industry not relevant to this study: The UMCG, which employs Dr. De Boer and Dr. Suthahar, has received research grants and/or fees from AstraZeneca, Abbott, Bristol-Myers Squibb, Novartis, Roche, Trevena, and ThermoFisher GmbH. Dr. de Boer is a minority shareholder of scPharmaceuticals, Inc. Dr. de Boer received personal fees from MandalMed Inc, Novartis, and Servier. Sanjiv J Shah has received research funding from Actelion, AstraZeneca, Corvia, and Novarits, and consulting fees from Actelion, AstraZeneca, Amgen, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, Tenax, and United Therapeutics.
Abbreviations
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARIC
Atherosclerosis Risk in Communities
- ASCVD
Atherosclerotic cardiovascular disease
- ATP
Adult Treatment Panel
- BMI
Body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CHS
Cardiovascular Health Study
- CI
Confidence Interval
- FOF
Framingham Heart Study Offspring Cohort
- HDL-c
High-density cholesterol
- Health ABC
Healthy Aging and Body Composition
- HF
Heart Failure
- JHS
Jackson Heart Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- NHLBI
National Heart Lung and Blood Institute
- PC
Pooled Cohort
- PCP-HF
Pooled Cohort Equations to Prevent Heart Failure
- PREVEND
Prevention of REnal and Vascular ENd-stage Disease
- SBP
Systolic blood pressure
- TC
Total cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no relationships with industry relevant to the current study to report. All potential conflicts of interest have been explicitly described in the appendix.
Publisher's Disclaimer: Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Tweet: @HeartDocSadiya “Robust tool estimating HF risk frames the patient-centered discussion. Time to shift our focus to prevention and risk reduction.”
References
- 1.Benjamin EJ, Virani SS, Callaway CW et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman MD, Berry JD, Ning H et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013;61:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber Y, Weston SA, Redfield MM et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao CW, Lyass A, Enserro D et al. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich PA, Albert NM, Allen LA et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad N, Judge A, Tran J et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schocken DD, Benjamin EJ, Fonarow GC et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 2008;117:2544–65. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 10.Karmali KN, Lloyd-Jones DM. Implementing Cardiovascular Risk Prediction in Clinical Practice: The Future Is Now. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L et al. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail 2015;8:438–47. [DOI] [PubMed] [Google Scholar]
- 12.Wilkins JT, Karmali KN, Huffman MD et al. Data Resource Profile: The Cardiovascular Disease Lifetime Risk Pooling Project. Int J Epidemiol 2015;44:1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 15.Diercks GF, Janssen WM, van Boven AJ et al. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]). Am J Cardiol 2000;86:635–8. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 19.Taylor HA Jr., Wilson JG, Jones DW et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005;15:S6-4-17. [PubMed] [Google Scholar]
- 20.Goff DC Jr., Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino RB Sr., Vasan RS, Pencina MJ et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- 22.Karmali KN, Goff DC Jr., Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol 2014;64:959–68. [DOI] [PubMed] [Google Scholar]
- 23.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 24.Avery CL, Loehr LR, Baggett C et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol 2012;60:1640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler J Primary prevention of heart failure. ISRN Cardiol 2012;2012:982417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Hong Y, Labarthe D et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 27.Lauer MS, Kiley JP, Mockrin SC et al. National Heart, Lung, and Blood Institute (NHLBI) strategic visioning: setting an agenda together for the NHLBI of 2025. J Am Coll Cardiol 2015;65:1130–3. [DOI] [PubMed] [Google Scholar]
- 28.Hunt SA, American College of C, American Heart Association Task Force on Practice G. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46:e1–82. [DOI] [PubMed] [Google Scholar]
- 29.Ammar KA, Jacobsen SJ, Mahoney DW et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563–70. [DOI] [PubMed] [Google Scholar]
- 30.Kovell LC, Juraschek SP, Russell SD. Stage A Heart Failure Is Not Adequately Recognized in US Adults: Analysis of the National Health and Nutrition Examination Surveys, 2007-2010. PLoS One 2015;10:e0132228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xanthakis V, Enserro DM, Larson MG et al. Prevalence, Neurohormonal Correlates, and Prognosis of Heart Failure Stages in the Community. JACC Heart Fail 2016;4:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlof B, Devereux RB, Kjeldsen SE et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 33.Group SR, Wright JT Jr., Williamson JD et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal SK, Chambless LE, Ballantyne CM et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail 2012;5:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler J, Kalogeropoulos A, Georgiopoulou V et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail 2008;1:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chahal H, Bluemke DA, Wu CO et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999; 159:1197–204. [DOI] [PubMed] [Google Scholar]
- 38.D’Agostino RB Sr., Grundy S, Sullivan LM, Wilson P, Group CHDRP. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 2001;286:180–7. [DOI] [PubMed] [Google Scholar]
- 39.Ho JE, Enserro D, Brouwers FP et al. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lloyd-Jones DM, Braun LT, Ndumele CE et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease. A Special Report From the American Heart Association and American College of Cardiology 2018:25711. [DOI] [PubMed] [Google Scholar]
- 41.de Boer RA, Daniels LB, Maisel AS, Januzzi JL, Jr. State of the Art: Newer biomarkers in heart failure. Eur J Heart Fail 2015;17:559–69. [DOI] [PubMed] [Google Scholar]
- 42.Ledwidge M, Gallagher J, Conlon C et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 43.Vasan RS, Benjamin EJ, Larson MG et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA 2002;288:1252–9. [DOI] [PubMed] [Google Scholar]
- 44.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med 2003;138:907–16. [DOI] [PubMed] [Google Scholar]
- 45.Bibbins-Domingo K, Pletcher MJ, Lin F et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahrami H, Kronmal R, Bluemke DA et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008;168:2138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009;169:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies MJ, D’Alessio DA, Fradkin J et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi EY, Rosen BD, Fernandes VR et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psaty BM, Kuller LH, Bild D et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995;5:270–7. [DOI] [PubMed] [Google Scholar]
- 51.Ives DG, Fitzpatrick AL, Bild DE et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–85. [DOI] [PubMed] [Google Scholar]
- 52.Brouwers FP, de Boer RA, van der Harst P et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.