Abstract
Introduction.
We conducted a systematic umbrella review to evaluate the literature relating to effects of physical activity on pain, physical function, health-related quality of life (HRQoL), co-morbid conditions and osteoarthritis (OA) structural disease progression in individuals with lower extremity OA.
Methods.
Our primary search encompassed 2011 – 2/2018 for existing systematic reviews (SRs), meta-analyses (MAs) and pooled analyses dealing with physical activity including exercise (not mixed with any other intervention and compared to a no-activity control group). A supplementary search encompassed 2006-2/2018 for original research related to physical activity (including exercise) and lower limb OA progression. Study characteristics were abstracted and risk of bias was assessed.
Results.
Physical activity decreased pain and improved physical function (strong evidence) and improved HRQoL (moderate evidence) among people with hip or knee OA relative to less active adults with OA. There was no evidence to suggest accelerated OA progression for physical activity below 10,000 steps per day. Both physical activity equivalent to the 2008 Physical Activity Guidelines for Americans (150 minutes/week of moderate-intensity exercise in bouts ≥10 minutes) and lower levels of physical activity (at least 45 total minutes/week of moderate-intensity) were associated with improved or sustained high function. No SRs/MAs addressing co-morbid conditions in OA were found. Measurable benefits of physical activity appeared to persist for periods of up to 6 months following cessation of a defined program.
Conclusions.
People with lower extremity OA should be encouraged to engage in achievable amounts of physical activity, of even modest intensities. They can choose to accrue minutes of physical activity throughout the entire day, irrespective of bout duration, and be confident in gaining some health and arthritis-related benefits.
Keywords: Pain, Function, Quality of Life, Progression, Knee, Hip
INTRODUCTION
There are approximately 100 different arthritic conditions with a total of 54.4 million Americans estimated to have physician-diagnosed arthritis(1). Among these, osteoarthritis (OA) is the most common joint disorder in the US, affecting an estimated 30.8 million adults (13.4% of the civilian adult US population)(2). OA affects a broad spectrum of age groups in the US, including 2 million Americans under the age of 45 years with knee OA(3). By the year 2040, an estimated 78.4 million (25.9% of the projected total adult population) adults aged 18 years and older are expected to have physician-diagnosed arthritis(4), the majority of whom will have OA. Methodological issues, such as the current inability to reliably diagnose early non-radiographic OA and traditional accounting of OA in only a limited number joint sites (hip and knee), make it highly likely that the real burden of OA has been underestimated(5). The risk of mobility disability (defined as needing help walking or climbing stairs) attributable to knee OA alone is greater than that attributable to any other medical condition in people aged 65 years and older(6). As expected based on these prevalence and disability figures, OA is associated with an extremely great economic burden—by one national estimate equal to $185.5 billion in aggregate annual medical care expenditures(7).
To provide recommendations to the Department of Health and Human Services for updating the Physical Activity Guidelines for Americans, the Physical Activity Guidelines Advisory Committee (PAGAC) chose to investigate 7 chronic conditions, among them OA(8). The choice of OA was predicated on the large portion of the general population having this chronic condition, the high disability associated with OA(9), and the potential public health importance of physical activity in people with OA. The overall goal of this systematic umbrella review was to evaluate the literature relating to effects of physical activity on (1) pain, (2) physical function, (3) health-related quality of life (HRQoL), (4) disease progression and (5) risk of co-morbid conditions in individuals with existing lower limb (hip and/or knee) OA. As a secondary goal, we also evaluated the literature for evidence of variation in the relationship of physical activity and these outcomes based on a) the dose of physical activity exposure, b) age, sex, race/ethnicity, socio-economic status, or weight status, and c) frequency, duration, intensity, mode (type), or means of measuring physical activity. This paper represents the scientific research performed to inform the 2018 Physical Activity Guidelines for Americans(10) with an extension of the literature search by one year through 2/2018.
METHODS
The overarching methods used to conduct systematic reviews informing the 2018 Physical Activity Guidelines Advisory Committee Scientific Report (search strategy development, article triage, data abstraction, bias assessment, and quality control processes and methods for analysis) have been described in detail elsewhere(11). The searches were conducted of electronic databases (PubMed®, CINAHL, and Cochrane) and were supplemented by authors (experts in the area), to provide additional articles identified through their expertise and familiarity with the literature. The full search strategies are available online(12). The inclusion criteria were pre-defined and searches were registered in PROSPERO #CRD42018092365. Studies were included if they were published in English; were meta-analyses (MAs), systematic reviews (SRs) or pooled analyses published from 2011 through February 2018, and investigated individuals of all ages with preexisting OA of the hip or knee; the association between all types and intensities of physical activity, including exercise, not mixed with any other interventions (such as diet); and one of the health outcomes of interest (pain, physical function, HRQoL, disease progression or risk of co-morbid conditions). Physical activity was defined as bodily movement produced by skeletal muscles that results in energy expenditure. Exercise was defined as a form of physical activity that is planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health. Physical function was defined as the ability of a person to move around and to perform types of activity; in the studies included in this summary, this was most often measured by a standardized instrument used routinely in OA clinical trials, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)(13). HRQoL was defined as a multi-dimensional concept including domains related to physical, mental, emotional, and social functioning.
Studies of non-ambulatory adults, hospitalized patients, or animals were excluded. We also excluded studies of multimodal interventions not presenting data on physical activity alone and studies of single, acute sessions of physical activity. The titles, abstracts, and full-text of the identified articles were independently screened by two reviewers. Disagreement between reviewers was resolved by discussion or by a third member of the PAGAC committee.
The amended literature search yielded 20 MAs and SRs meeting the inclusion criteria for our analysis of OA and pain, physical function, and HRQoL outcomes(14–31); however, the studies identified included significant overlap. In an attempt to minimize redundancy, the Committee reviewed the overlap of studies within all the MAs/SRs; those with considerable overlap, with three or fewer unique additional studies, and that did not add additional information to the larger studies, were not retained for purposes of the final summary. This procedure resulted in retention of six MAs (14–16, 18, 22, 32) and three SRs for the purposes of the summary related to OA pain, physical function, and HRQoL(17, 33, 34) (Table 1); from the amended search, one additional MA and 2 additional SRs were added to the original search conducted as part of the governmental report.
Table 1.
Summary of included studies assessing the relationship of physical activity with pain, function and quality of life in individuals with lower extremity osteoarthritis(14–18, 22, 32–34).
| Author (year) | Type of Study; Joints; mean age and/or range | Sample Size & Number of studies | Type(s) of Physical Activity | Outcome Measures | Effect Sizes (95% CI)* |
|---|---|---|---|---|---|
| Bartels (2016)(14) | MA; Knee & Hip; 68 years | Total: 1190 participants from 13 studies Pain: 971 participants from 12 trials Physical Function: 1059 participants from 12 trials HRQoL: 971 participants from 10 trials |
Range of motion, strength training, and aerobic exercise in a therapeutic/heated indoor pool | Pain: WOMAC, VAS, SF-36, HAQ, Lequesne algofunctional index, AIMS, KSPS, McGill Pain Questionnaire, ASES, SES, and NRS |
Pain: SMD −0.31 (−0.47 to −0.15) |
| Physical Function: WOMAC, SF-36, PCS, HAQ, PDI, ASES |
Physical Function: SMD −0.32 (−0.47 to −0.17) |
||||
| HRQoL: SF-36/SF-12/SF-8, EuroQoL, KOOS Subscore: QOL, Quality of well-being, AIMS, others |
HRQoL: SMD −0.25 (−0.49 to −0.01) |
||||
| Bartholdy (2017)(33) | SR, Knee, 64 years | 4699 participants from 45 studies | ^ACSM Interventions (29% of included studies) and Non-ACSM Interventions | Strength: knee extension | Strength: SMD 0.448 (0.091 to 0.805) |
| Pain: WOMAC, NRS, KOOS, pain score, AIMS2, OASI, and VAS | Pain: SMD 0.106 (−0.239 to 0.451) | ||||
|
Physical Function: WOMAC, Lequesne Index, KOOS, functional incapacity score, SF-36 AIMS2, OASI, subjective rating of daily activity, and KOOS ADL |
Physical Function: SMD 0.153 (−0.243 to 0.549) | ||||
| Beumer (2016)(15) | SR/MA, Hip, 52-77 years | 1213 participants from 19 studies | Land- and aquatic-based therapeutic strength and aerobic exercises | Pain: VAS, WOMAC |
Pain: (WOMAC): SMD −0.53 (−0.96 to −0.10) (VAS): SMD −.49 (−0.70 to −0.29) |
| Chang (2016)(16) | SR/MA, Knee mean age of study participants ranged from 61.15 to 79.01 years |
508 participants from 11 studies | Tai Chi Chuan | Pain: WOMAC |
Pain (WOMAC): SMD −0.41 (−0.67 to −0.74) |
| Physical function: WOMAC, 6 min-walk test, stair climb test, and SAFE |
Physical Function (WOMAC): SMD −0.16 (−0.44 to −0.11) (6-min walking test): SMD −0.16 (−1.23 to 0.90) (Stair climb test): SMD −0.76 (−1.34 to 0.15) (SAFE): SMD −0.63 (−0.98 to −0.27) |
||||
| Escalante (2011)(17) | SR, Hip & Knee Mean age range 54.4 – 74 years (discoverable for 19 studies) | 2145 participants from 20 studies | Land-based (strength, tai chi, aerobic, and mixed) and aquatic exercise; | Physical function: 6 minute walk test |
Physical Function (6-minute walk test): Strength: SMD 0.31 (0.06 to 0.56) Pooled Tai Chi: SMD 0.66 (0.23 to 1.09) Pooled Aerobic: SMD 0.90 (0.70 to 1.10) Pooled Mixed: SMD 0.47 (.032 to .62) Pooled Hydrotherapy: SMD 0.00 (−0.38 to 0.39) |
| Fernandopulle, (2017)(32) | SR/MA, Hip & Knee, 54-73 years | 3233 participants from 27 studies | Land-based generic physical activity interventions on pain, physical function, and physical performance |
Pain: pain intensity, VAS, WOMAC; Physical Function: WOMAC, AIMS, KOOS, Japanese Knee OA measurement, customized measure of disability; Physical Performance: 6MWT, TUG, timed stair climbing |
Recreational Activity Physical Function (WOMAC) at 3 months from randomization: SMD −9.56 (−13.95 to −5.17) Conditioning Exercise Physical Performance (6MWT) 6 months from randomization: SMD 42.72 (27.28 to 57.66); Physical Function (WOMAC) 6 months from randomization: SMD −3.74 (−5.70 to −1.78); Physical Performance (Timed-stair climbing test) at 6 months randomization: SMD −2.29 (−4.65 to .006); Physical Performance (Timed-stair climbing test) at 18 months: SMD −0.49 (−0.75 to −0.23) Walking Physical Function at 6 months from randomization: SMD, −10.38 (−12.27 to −8.49); Physical Function at 12 months from randomization: SMD, −0.03 (−0.035 to 0.28); Physical Performance 6MWT at 12 months from randomization: SMD, −1.88 (−43.46 to 39.71) Pain 3 months from randomization: SMD 0.19 (−0.31 to 0.68) Pain 6 months from randomization: SMD, −1.55 (−3.62 to 0.52) |
| Fransen (2015)(18) | SR/MA, Knee Mean age range 55-73 years | 6345 participants from 54 studies Pain (immediately post-treatment): 3537 participants from 44 studies. Physical Function (immediately post-treatment): 3913 participants from 44 studies. HRQoL (immediately post-treatment): 1073 participants from 13 studies |
Land-based strength and aerobic exercises (muscle strengthening, balance training, aerobic walking, cycling, Tai Chi) | Pain: WOMAC, global pain scores, Lequesne Osteoarthritis Index (2-6 months in and >6 months) |
Pain: Pooled SMD 0.49 (0.39 to 0.59) ; 12 studies (1468 participants) SMD 0.24 (95% CI 0.14 to 0.35) at 2–6 months post-exercise training; 6 studies SMD 0.08 (95% CI −0.15 to 0.30) after more than 6 months post-exercise training |
| Physical function: WOMAC, global disability scores, Sickness Impact profile (2-6 and >6 months) |
Physical Function: Pooled SMD 0.52 (95% CI 0.39 to 0.64); 10 studies (1279 participants) SMD 0.15 (95% CI 0.04 to 0.26) at 2–6 months post-exercise training; 7 studies SMD 0.20 (95% CI 0.08 to 0.32) after more than 6 months post-exercise training |
||||
| HRQoL: SF-12 |
HRQoL: SMD 0.28 (0.15 to 0.40) |
||||
| Juhl (2014)(22) | SR/MA, Knee, 64.3 years | 4418 participants from 48 studies | Single or combination exercises (aerobic, resistance, and performance training) | Pain: WOMAC, VAS, SF-36, AIMS, BPI, KOOS, OASI |
Pain: Overall pooled SMD 0.50 (0.39 to 0.62); Aerobic Exercise SMD 0.67; Resistance Exercise SMD 0.62; Performance Exercise SMD 0.48 |
| Physical function: WOMAC, AIMS, Self-Reported from FAST, SF-36, KOOS, OASI |
Physical Function: Overall Pooled SMD 0.49 (0.35 to 0.63); Aerobic Exercise SMD 0.56; Resistance Exercise SMD 0.60; Performance Exercise SMD.56 |
||||
| Young (2018)(34) | SR, Knee, mean age range 54.4-70 years | 2173 participants from 24 studies (that included effect sizes related to knee OA) | To identify specific doses of exercise (aerobic, balance, and strength) related to improved outcomes of pain and function in individuals with common knee disorders. |
Pain: WOMAC, KOOS, VAS Physical Function: WOMAC, KOOS, Step Test, SF-36, 6MWT |
Pain (Land based): WOMAC: SMD 0.28 to SMD 1.39 KOOS: SMD 0.64 to SMD 0.76 VAS: SMD 0.48 to SMD 2.44 Physical Function (Land based): WOMAC: SMD 0.01 to SMD 1.83 KOOS: SMD 0.45 to SMD 0.99 Step Test: SMD 0.45 SF-36: SMD 0.68 to SMD 1.49 6MWT: SMD 0.58 Pain (aquatic based): KOOS: SMD 0.17 to SMD 0.58 VAS: SMD 1.78 to SMD 2.43 Physical Function (aquatic based): KOOS: SMD 1.19 SF-36: SMD 0.58 6MWT: SMD 0.72 |
statistically significant results favoring exercise are shown in bold font (note sign can be positive or negative depending upon coding of data within the study); MA=meta-analysis; HRQoL=health related quality of life; WOMAC=Western Ontario & McMaster Universities Osteoarthritis Index; VAS=visual analog scale for pain; SF=Short Form (−8, −12 or −36 item) Health Survey; HAQ= Health Assessment Questionnaire; AIMS=Arthritis Impact Measurement Scales; KSPS=Knee Specific Pain Scale; ASES=Arthritis Self-Efficacy Scale; SES=Schmerzempfindungsskala; NRS=Numeric Rating System (of pain); PCS=Physical Composite Score; PDI=Pain Disability Index; EuroQoL=an instrument for measuring HRQoL; KOOS=Knee Injury and Osteoarthritis Outcome score; SAFE= Survey of Activities and Fear of Falling in the Elderly; SMD=standardized mean difference; SR=systematic review; OASI= Osteoarthritis Screening Index; FAST=Fitness Arthritis and Senior Trial; KOOS ADL=KOOS scale of activities of daily living; 6MWT=6-minute walk test; TUG=timed up and go test; BPI=Brief Pain Inventory; FAST= Fitness Arthritis and Seniors Trial
ACSM Interventions=A voluntary contraction against an external resistance typically performed in especially designed equipment or with free weights. The external load should be above 40% of 1 repetition maximum (1RM) corresponding to very light to light intensity, and the exercises performed in 2–4 sets of 8–12 repetitions; preferably to contraction failure or muscular exhaustion. The exercise program should consist of at least 2–3 sessions per week. Non-ACSM interventions=Exercise interventions that in their description were considered not to follow all of the above definitions were categorized as “not-ACSM interventions”, and include all other types of interventions.
Additional Results for Juhl 2014
- When the studies that evaluated only a single exercise type were pooled, the SMD for pain 0.61 (95% CI 0.48, 0.75), and for the SMD for disability was 0.58 (95% CI 0.40, 0.75) but with large heterogeneity.
- Exercise programs that included a combination of resistance, aerobic, and performance exercise were not significantly better than control treatments in reducing pain (SMD 0.16 [95% CI ⫺0.04, 0.37], I2=44.0%) and had only a small effect in reducing disability (SMD 0.22 [95% CI 0.08, 0.37], I2=0%). The difference between exercise programs focusing on one type of exercise compared with programs mixing two or more types was significant for both outcomes (SMD for pain 0.45 [95% CI 0.20, 0.69], P < 0.001 and SMD for disability 0.36 [95% CI 0.13, 0.58], P < 0.002) in favor of using only one type of exercise.
- The effect of aerobic exercise on pain relief increased with an increased number of supervised sessions (slope 0.022 [95% confidence interval 0.002,0.043]).
- More pain reduction occurred with quadriceps-specific exercise than with lower limb exercise (SMD 0.85 versus 0.39; P = 0.005) and when supervised exercise was performed at least 3 times a week (SMD 0.68 versus 0.41; P = 0.017).
- Stratified analysis showed similar effects for pain in patients with severe knee OA (SMD 0.60 [95% CI 0.38, 0.82], I2 = 36.1%) and those with mild/moderate knee OA (SMD 0.66 [95% CI 0.34, 0.99], I2=77.0%) (P= 0.736).
- Although exercise therapy seemed to reduce patient-reported disability less in patients with severe knee OA (SMD 0.39 [95% CI 0.05, 0.74], I2= 73.6%) than in patients with mild/moderate knee OA (SMD 0.66 [95% CI 0.32, 0.99], I2= 84.6%) (P=0.282), the differences did not reach significance.
Upon completion of triage based on the MAs, SRs and pooled analyses, the authors observed a paucity of MAs and SRs dealing with physical activity and knee OA progression defined as structural worsening of OA based on imaging (radiographic or magnetic resonance (MRI)), worsening function (based on patient-reported outcomes or gait speed) or progression to total joint arthroplasty (replacement) for OA. Based on the paramount importance of the issue of disease progression for individuals with OA, we elected to perform a separate literature search, using the same search strategy, process, and inclusion/exclusion criteria used for the pain, physical function and HRQoL outcomes but including two additional specific criteria: only inclusion of original research published from 2006 through February 2018 and only include the outcome of OA progression. Of note, we did not identify any studies examining the effects of physical activity on progression based on systemic biomarkers associated with disease state.
The search for MAs, SRs and pooled analyses, and reports failed to identify any literature to address the question of the effects of physical activity on co-morbid conditions in OA. The term comorbid condition referred to any other existing chronic condition identified by a medical diagnosis (e.g., coronary heart disease) or by clinical events (e.g., cardiovascular mortality); therefore, this question was not pursued.
The quality of each MA, SR and pooled analysis, summarized in Supplemental Digital Content 1 (see Table, SDC 1, quality assessment chart), was assessed using AMSTARExBP(35), a modified version of “A Measurement Tool to Assess Systematic Reviews” (AMSTAR)(36, 37); the majority of the studies met 11 of the 18 AMSTAR criteria. Risk of bias, or internal validity was assessed for each original study using an adapted version of the USDA NEL Bias Assessment Tool (BAT)(38) as summarized in Supplemental Digital Content 2 (see Table, SDC 2, original research bias assessment chart); the majority of the studies met 8 of the 10 applicable criteria. The bias assessment of the original research and the full search strategy is available(12). Recently, the method of data extraction has been published in detail(11). Literature trees summarize the selection of MAs, SRs and pooled analyses and reports in Supplemental Digital Content 3 (see figure, SDC 3, providing details of literature tree search for reviews related to OA pain, physical function, HRQoL, progression and risk of co-morbid conditions) and original research related to OA progression in Supplemental Digital Content 4 (see figure, SDC 4, providing details of literature tree search for original research related to osteoarthritis progression).
RESULTS
OA and Pain, Physical Function, and Health-related Quality of Life as outcomes
Most of the retained MA (six) and SR (three) publications evaluated randomized controlled trials (RCTs) reviewing the effects of one or more modalities of exercise (land-based and aquatic, aerobic, muscle-strengthening, and Tai Chi) on knee and hip OA. Most used the Western Ontario and McMaster Arthritis Index (WOMAC) scale —common in the OA research arena — to assess pain and physical function, and SF-12 to assess HRQoL. One systematic review examined land-based exercise studies exclusively(18); another examined pool-based exercise effects only(14). In sum, these references encompassed 261 studies related to knee and/or hip OA involving 25,924 individuals with pain, physical function or HRQoL as an outcome. A total of 240 studies involving 24,583 participants included knee OA; a total of 52 studies involving 4803 participants included hip OA.
Taken together, the evidence demonstrated that physical activity reduces pain and improves physical function and HRQoL for persons with lower limb OA. The effect sizes (based on standardized mean differences, SMDs) favored exercise: maximal SMDs reported were 0.53 for pain(15), 0.76 for physical function(16) and 0.28 for HRQoL(18) (Table 1). For pain, physical function and HRQoL, the effect sizes for those with hip OA did not vary from those with knee OA only. Although there were some modest differences in effect sizes across different exposures, in general, the reviews were consistent in finding that physical activity is associated with reductions in pain and improvements in physical function and HRQoL for both knee and hip OA irrespective of the mode (aquatic versus land-based exercise), or muscle strengthening versus aerobic versus Tai Chi (Table 1). Following cessation of the intervention, the beneficial effects of physical activity persisted up to 6 months for pain, and beyond 6 months for physical function(18) (Table 1).
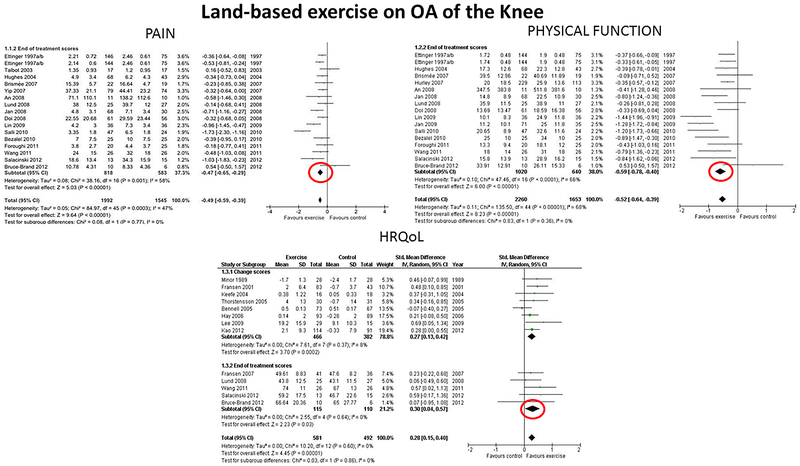
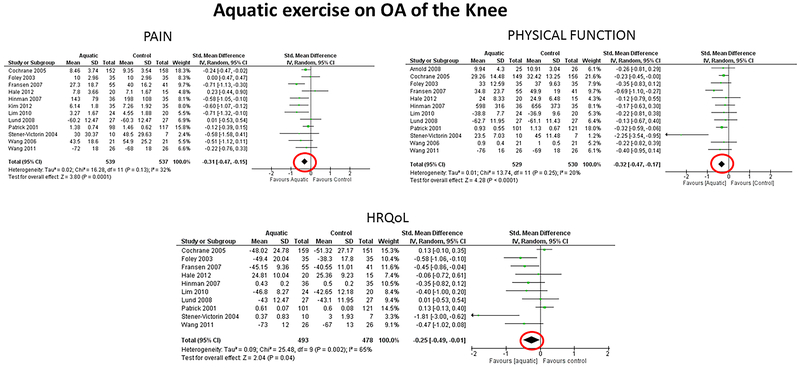
The findings on pain, physical function and HRQoL are illustrated in Figures 1 and 2, which present results from one review addressing land-based exercise effects on the knee (from Fransen et al.(18)) and one review addressing aquatic exercise effects on the knee (from Bartels et al.(14)). In Figure 1, the direction to the left favors exercise (decreased pain and improved physical function), whereas, improved HRQoL is to the right. In Figure 2, the direction to the left favors exercise (decreased pain, improved physical function and HRQoL).
Figure 1.
Effects of Land-based Exercise on Pain, Physical Function and Health-Related Quality of Life (QOL) in Knee OA. Reproduced from “Exercise for osteoarthritis of the knee: a Cochrane systematic review”, Marlene Fransen et al., 49, 2015 with permission from BMJ Publishing Group Ltd. Negative Standardized Mean Differences (SMDs) represent improvements in pain and physical function (lower scores represent better pain and/or physical function) whereas positive SMDs represent improvements in health-related quality of life (HRQoL, higher scores represent better HRQoL).
Figure 2.
Effects of Aquatic Exercise on Pain, Physical Function and Health-Related Quality of Life (HRQoL) in Knee OA. Reproduced from EM Bartels, “Aquatic exercise for the treatment of knee and hip osteoarthritis”, Cochrane Database of Systematic Reviews, John Wiley and Sons. Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd/. A multitude of measures were used in the included studies; standardized instruments used most often were WOMAC for pain and function and SF-36, SF-12 or SF-8 for physical function. Negative Standardized Mean Differences (SMDs) represented improvements in pain, physical function and/or HRQoL (lower scores mean better pain, physical function and/or HRQoL).
Mode and dose of exercise
Most studies of the effects of physical activity on pain, physical function and HRQoL were RCTs of one mode, intensity or duration; there was significant heterogeneity for these factors among the studies included within each MA/SR. Limited information was available on dose-response or different modes (types of exercise). Overall, the literature search revealed four MA/SR(22) addressing mode and/or dose of exercise for OA (Table 1). One MA/SR of 48 RCTs (4,028 patients with pain data)(22) observed similar pain reduction for aerobic, resistance, and performance exercise (practicing a specific activity with the lower extremity); single-type exercise programs were more efficacious than programs that included different exercise types. The effect of aerobic exercise on pain relief increased with an increased number of supervised sessions; overall, more pain reduction occurred when supervised exercise was performed at least three times a week. The authors recommended supervised exercise 3 times a week, noting that such programs have a similar effect, regardless of patient characteristics, including radiographic disease severity and baseline pain.
Another SR, encompassing 45 trials (4699 participants), addressed mode and dose of exercise for knee OA(33). This review concluded that knee extensor strength significantly improved following American College of Sports Medicine (ACSM) recommendations(39) (described in Table 1 footer) versus all other types (i.e., any that did not deliver the intervention according to the ACSM recommendation) of strength training for older or sedentary patients. Although a dose response association was identified between knee extensor strength gain and improvement in pain and physical function, there was no difference in pain and function outcomes comparing ACSM versus other types of exercise interventions.
A third SR, encompassing 24 trials (1747 participants), addressed dose of exercise for knee OA(34). Large differences among studies in the type, duration, and volume of exercise made it difficult to discern specific variables influencing the effects of treatment. A few generalizations based on self-reported pain and function were possible: 1) 24 or more total exercise sessions were most often related to large effect sizes (studies ranged from 3-108 sessions), 2) 8- and 12-week exercise durations most often exhibited larger effect sizes (studies ranged from 4 to 36 weeks), and 3) a frequency of 1 time per week exercise showed no effect.
A fourth MA/SR, encompassing 27 trials (3060 participants), addressed different modes of land-based exercise (recreational activities, walking or conditioning exercise consisting of a combination of strength training, flexibility and aerobic interventions)(32). In contrast to studies lasting 12 months, walking and conditioning exercise lasting 6 months had a significant impact on physical function and/or physical performance (6-minute walk test or timed stair climbing test) but not on pain. Conditioning exercise also had a moderate level of evidence for effectiveness on physical function in individuals with knee OA in both the short- (6 months) and longer- (18 months) term. Adherence to the interventions is very likely to have an effect on the significance of the results.
Although not a MA or SR, and therefore not utilized in the PAGAC report, we found one original research article worth noting related to dose of exercise and function. In this study, Dunlop et al.(40) assessed the association of accelerometer measured physical activity and physical function in 1647 participants with lower extremity symptoms in the Osteoarthritis Initiative (OAI) cohort. Moderate-to-vigorous physical activity (MVPA) was defined as greater than 2020 counts/min corresponding to 3 METs or a level of exertion corresponding to a ~3½ mile/hour walk(41). Physical function based on measured (gait speed) and self-reported (SF-12) function was assessed 2 years later. Improved or sustained high function was achieved by 34% of participants. Compared with participants performing ≤45 total minutes of MVPA per week (including bouts <10 minutes in duration), those performing >45 minutes/week were more likely to improve gait speed (relative risk (RR) 1.8, 95% CIs 1.6 to 2.1) and self-reported function (RR 1.4, 95% CIs 1.3 to 1.6). Individuals performing or exceeding the 2008 Physical Activity Guidelines for Americans of >150 minutes/week of MVPA in bouts lasting ≥10 minutes also improved gait speed (RR 1.4, 95% CIs 1.3 to 1.6) and self-reported function (RR 1.3, 95% CIs 1.2 to 1.4). Results were consistent across varying knee osteoarthritis severities. It is evident that important health improvements can be achieved even with levels of physical activity below those recommended by the 2008 Physical Activity Guidelines for Americans.
Demographic factors and weight status
Dunlop et al. determined that the results for the intermediate level of physical activity (≥45 minutes/week moderate-vigorous activity) were consistent across sex, body mass index and age(40). However, effect modifications by sex, age, race/ethnicity and socioeconomic status were not addressed in any of the MA/SR identified for this umbrella review. Although a relationship between BMI and OA is generally well recognized(42), to our knowledge, there are no meta-analyses evaluating whether BMI modifies the physical activity-OA relationship.
Osteoarthritis Disease Progression as an outcome
Existing Systematic Review and Meta-Analyses
A concern about the potential harm that high intensity and large amounts of weight-bearing exercise may cause for OA progression prompted a targeted review for this outcome.
We identified one SR/MA(29) that assessed the association of self-reported running or jogging (including running-related sports such as triathlon and orienteering) with knee OA onset or progression defined by any definition of diagnosed knee OA, radiographic or imaging markers of knee OA, knee arthroplasty for OA, knee pain and/or disability specifically associated with the knee (Table 2). Although this SR/MA included incident as well as progressive OA, the data are instructive for understanding the potential role of running in the development and/or progression of OA. With this evidence, the authors concluded that it was not possible to determine the role of running in knee OA. However, they noted that a key finding of their review was the result of their meta-analysis (2,172 individuals) of 3 case-control studies (two of the three controlled for joint injury), which suggested that runners (running for 1 year up to a lifetime) had around a 50% reduced odds of undergoing a total knee replacement for OA than non-runners (pooled odds ratio 0.46, P=0.0004, Table 2). Evidence relating to symptomatic outcomes was sparse and inconclusive. Because retrospective case-control studies are subject to several types of bias, these data have to be interpreted with caution; these biases include recall and observer bias, bias related to choice of control groups, and selection bias. Selection bias could occur if individuals with joint symptoms or injury ceased their participation in physical activity and went on to eventual joint replacement; therefore, individuals with total knee replacement would be identified as having engaged in less physical activity leading to an apparent protective effect of physical activity on knee replacement.
Table 2.
Summary of included studies assessing the relationship of physical activity and osteoarthritis progression in individuals with lower extremity osteoarthritis.(29, 40, 43, 45–49)
| Author (year) | Type of Study, Joints, Mean age and/or range | Sample Size & Number of Studies | Type of Physical Activity | Outcome Measures | Measures of Association (95% CIs) |
|---|---|---|---|---|---|
| Quicke (2015)(43) | SR, Knee, 45 years or older | 8,920 participants from 49 studies. 46 studies measured pain 43 measured physical function 3 measured TKR |
Three months or more of physical activity intervention or exposure | OA Progression (structural OA biomarker imaging and TKR) between three and thirty months of physical activity intervention | There was no evidence of progression of structural OA by imaging or increased TKR at a group level (n=8 TKRs within the physically active groups compared to n=10 TKRs in the non-physically active) The case control study concluded that increasing levels of regular physical activity was associated with lower risk of progression to TKR; OR 0.91 (0.31 to 2.63) and OR 0.56 (0.30 to 0.93) in men and women, respectively, with low cumulative hours of physical activity; OR 0.35 (0.12 to 0.95) and OR 0.56 (0.32 to 0.98) in men and women, respectively, with a high number of accumulative hours of physical activity. |
| Timmins (2017)(29) | SR/MA, Knee, 28 - 69 years | 15 studies: 110 cohort and 4 case-control | Any form of running or jogging | OA Progression defined by one of the following outcomes and a minimum of 1 year of running/jogging: 1. Any definition of diagnosed knee OA 2. Radiographic or imaging markers of knee OA 3. Knee arthroplasty for OA 4. Knee pain 5. Disability specifically associated with the knee |
Findings of studies with a diagnostic OA outcome were mixed. Some radiographic differences were observed in runners, but only at baseline within some subgroups. Meta-analysis suggested a protective effect of running against surgery due to OA: pooled OR 0.46 (0.30 to 0.71) |
| Kwee (2016)(48) | ORes, Knee, 62.2 years | 100 | Physical Activity Scale for the Elderly (PASE) | OA Progression was measured via cartilage damage progression in medial tibiofemoral compartment using 2-year follow-up MRI in participants with denuded areas of subchondral bone (dABs) at the central weight-bearing medial femur (cMF) at baseline MRI examination. | Standardized Regression Coefficient: Mean cMF.ThCtAB change: −0.15762 mm; cMF.dAB% change: 0.11479; MT.ThCtAB change: −0.08589; MT.dAB% change: 0.13846 |
| Lin (2013)(49) | ORes, Knee, 52.8 years | 205 participants from the OAI without symptomatic or radiographic evidence of OA | Physical Activity Scale for the Elderly (PASE) | OA Progression was measured with MRI T2 relaxation time over a 4-year period. | T2 progression was increased in the highest tertile of physical activity compared to the mid-tertile at the medial tibia (P = 0.041), patella (P = 0.019), and average T2 of all knee compartments combined (P = 0.033). Participants with the lowest 15% PASE scores showed significantly higher T2 progression compared to the mid-level physical activity group at the lateral femur (P = 0.025), lateral tibia (P = 0.043), medial femur (P = 0.044), tibiofemoral compartment (P = 0.017), patellofemoral compartment (P = 0.016), lateral compartments (P = 0.003), and average of all compartments (P = 0.043). |
| Felson (2013)(46) | ORes, Knee, 61 years | 2,073 participants (3,542 knees) | Physical Activity Scale for the Elderly (PASE) | OA Progression was measuring with long limb radiographs. Participants were followed for 30 months (in MOST) and 48 months (in OAI), respectively, with at least one of the following incident outcomes: Symptomatic tibiofemoral OA (radiographic OA and knee pain) Tibiofemoral narrowing. | Incident Tibiofemoral Symptomatic Knee OA: Lower 75%: OR 1.0 Upper 25%: OR 0.60 (0.03 to 1.3) Joint Space Loss: Lower 75%: OR 1.0 Upper 25%: OR 0.9 (0.05 to 1.5) |
| Oiestad (2015)(47) | ORes, Knee, 67 +/− 7.6 years | 1179 participants (2008 knees) | Steps/Day using accelerometer | OA Progression measured by radiographic and MRI assessment with a follow-up of 2 years. | Radiographic Worsening: <5859 steps/day: OR 0.91 (0.64 to 1.27); 5859-7846 steps/day: OR 1.0; >7846 steps/day: OR 0.99 (0.69 to 1.42) Intensity minutes: OR 1.01 (0.99 to 1.02); Lateral Cartilage Loss: <6078 steps/day: OR 0.82 (0.45 to 1.51) 6078-7938 steps/day: OR 1.0 >7938 steps/day; Medial Cartilage Loss: <6078 steps/day: OR 1.37 (0.81 to 2.32); 6078-7938 steps/day: OR 1.0; >7938 steps/day: 1.37 .80 to 2.33); MVPA minutes: OR 0.99 (0.97 to 1.01) |
| Dore (2013)(45) | ORes, Knee, 51-81 years | 405 participants | Steps/Day | OA Progression between objectively measured steps/day and knee structural change using MRI with approximately a 2.7-year follow-up | ≥10 000 steps/day increases BML: RR 1.97 (1.19 to 3.27) ≥10 000 steps/day had a 1.52 times (1.05 to 2.20) greater risk of increasing meniscal pathology score, which increased to 2.49 (1.05 to 3.93) in those with adverse meniscal pathology at baseline. ≥10 000 steps/day was associated with a greater risk of increasing cartilage defect score in those with prevalent BMLs at baseline: RR 1.36, (1.03 to 1.69). Steps/day was protective against volume loss in those with more baseline cartilage volume but led to increased cartilage loss in those with less baseline cartilage volume. (p=0.046 for interaction). |
SR=systematic review; MA=meta-analysis; TKR=total knee replacement; OR=odds ratio; ORes=original research; PASE=Physical Activity Scale for the Elderly; cMF.ThCtAB= mean cartilage thickness over total subchondral bone area at the central medial femur in mm; cMF.dAB%=% denuded area of subchondral bone (dAB) at the central medial femur; MT.ThCtAB= mean cartilage thickness over total subchondral bone area at the medial tibia in mm; MT.dAB%=% denuded area of subchondral bone (dAB) at the medial tibia; OAI=the Osteoarthritis Initiative cohort; MRI=magnetic resonance imaging; MOST=the Multicenter Osteoarthritis study; MVPA=moderate to vigorous physical activity; BML=bone marrow lesion (MRI measure); RR=relative risk; SF-12=Short Form (12 item) Health Survey; PRO=patient reported outcome;
We also identified one SR that included 49 studies(43) assessing the safety of physical activity in older adults with knee pain (summarized in Table 2). The systematic review(43) examined 49 longitudinal studies (comprising 48 RCTs and one case control study) of 8,614 total participants with knee pain and/or a diagnosis of knee OA ranging in radiographic severity from Kellgren Lawrence(44) grade 1 to 4. All physical activity interventions were low-impact, most often combining muscle-strengthening, stretching, and aerobic elements for 3 to 30 weeks. None of the primary literature studies in this SR dealt with hip OA. Comparing groups with greater amounts of low-impact physical activity to groups with the least amounts, this SR provided no evidence of serious adverse events defined as increased pain, decreased physical function, progression of structural OA on imaging or increased total knee replacement at a group level. In addition, although the total numbers were small and total follow-up brief, based on four RCTs (985 participants), there were no more total knee replacements over a 2- to 24-month observation period within physical activity groups compared to non-physical activity groups (n=8 vs n=10 total knee replacements, respectively).
Original Research
We identified five original research studies that examined the relationship between physical activity and disease progression(40, 45–49) (Table 2); no additional studies were identified as part of the extended search for this summary. All studies were prospective cohort studies (published 2013 to 2016). The analytical sample size ranged from 100(48) to 2,073(46); four were US studies(40, 46–49), one Australian(45). Three studies used self-reported physical activity via the Physical Activity Scale for the Elderly (PASE)(46, 48, 49). Two studies had device-measured physical activity via accelerometer or pedometer(40, 45, 47). The five included studies determined OA progression based on change in radiographic imaging(34), change in MRI imaging (cartilage loss)(45, 48, 49) or both(47). Collectively, these five studies focused on one of three longitudinal cohort studies: the Osteoarthritis Initiative (OAI)(40, 46, 48, 49), the Multicenter Osteoarthritis (MOST) study(34, 35) and a longitudinal cohort study of 405 community dwelling adults from Australia(33). The OAI assessed physical activity with the Physical Activity Scale for the Elderly (PASE) survey(34, 36, 37) and accelerometry(29); the MOST study and the Australian cohort assessed exposure by objective step count measures. Overall, the findings in these studies were mixed.
Three progression related studies quantified physical activity with PASE at baseline and quantified OA progression by imaging (radiographic or MRI) outcomes. Kwee et al.(48) assessed 2-year knee OA progression based on MRI of 100 participants in the OAI with symptomatic OA and baseline full-thickness cartilage defects of the knee; although OA progressed, there was no association of disease progression and levels of physical activity as measured by PASE (mean 2-year score 156, range 42-334). Lin et al.(49) assessed 4-year knee OA progression based on knee MRI (increasing T2 signal) of 205 asymptomatic individuals with (80%) and without (20%) risk factors for knee OA in the OAI. Greater OA progression was identified in the individuals with the 15% highest (score range 242-368) and 15% lowest (score range 31-120) PASE scores compared with the 70% mid-range (score range 153-207) scores of the reference group. The moderate activity mid-range group consistently showed the lowest (best) T2 values at baseline and 48-month follow-up. This study supported a potential U-shaped relationship of physical activity and OA progression for individuals at high risk for radiographic OA (80% with risk factors) or who had radiographic OA (Kellgren Lawrence grade 1), although the overall proportion of this subset was not reported. Potential interactions of baseline MRI lesion severity and physical activity for OA progression were not evaluated. Felson et al.(46) assessed 30 to 48-month knee OA progression based on radiograph or symptoms of 2,073 participants (3,542 knees, 50% symptomatic) with or at high risk of knee OA; there was no relation of quartiles of PASE scores with any OA progression outcomes (radiographic joint space loss or incident symptomatic knee OA) and no difference by degree of knee malalignment. The upper quartile of PASE scores (median score 250 for women, 300 for men) corresponded to regular work with some walking, “walks outside the home 1-2 hours a day occasionally”, light house or yard work in the prior 7 days but no extensive sports participation.
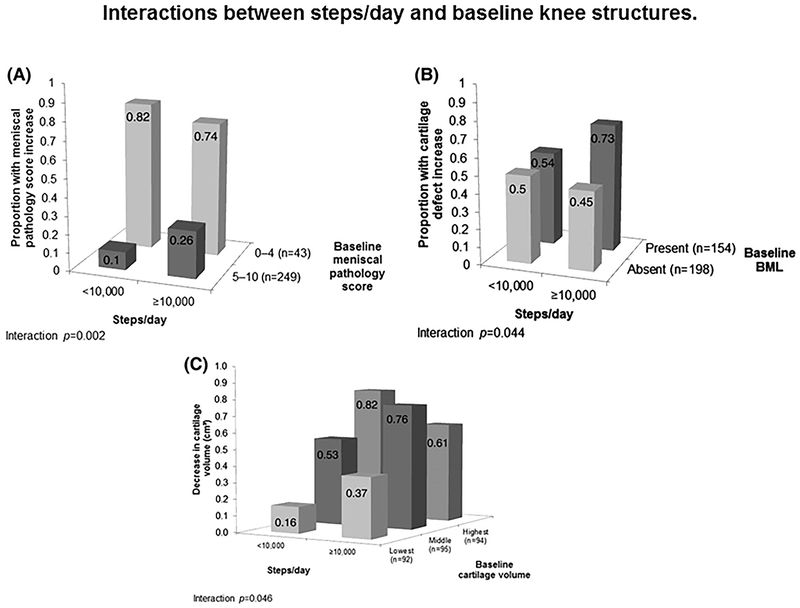
Two progression related studies quantified physical activity with pedometers or accelerometers at baseline and quantified OA progression by imaging (radiographic or MRI) outcomes. Oiestad et al.(47) assessed 2-year knee OA progression based on both knee radiographs (X-rays) and MRI (cartilage loss) of 1,179 participants in the MOST study, at risk of or with mild knee OA with physical activity measured at baseline by accelerometer (steps). There were no significant associations between daily walking or more time spent walking at a moderate to vigorous intensity with radiographic worsening or cartilage loss. Dore et al.(45) assessed ~2.7-year knee OA progression based on knee MRI (with four structural measures) of 405 Australian individuals (aged 50 – 80 years) in a community-based sample with physical activity measured at baseline by pedometer. There was no association of steps and OA progression for individuals with baseline MRI joint pathology performing fewer than 10,000 steps/day. However, in the context of baseline joint pathology at baseline, compared with the individuals performing fewer than 10,000 steps per day, there was greater OA progression (more meniscal pathology, more bone marrow lesions and/or lower cartilage volume by MRI) related to performing ≥10,000 steps/day (Figure 3). Thus, the effect of physical activity was modified by baseline OA status. When steps were analyzed as a continuous variable, there was a significant association of steps and risk of progression of cartilage defects and bone marrow lesions; there was also an interaction of steps and baseline severity of OA for MRI-based cartilage volume and meniscal pathology. Taken together, these data support a potential J-shaped relationship of physical activity and OA progression for those with pre-existing OA.
Figure 3.
Interaction of Underlying Joint Pathology by MRI and Ambulatory Physical Activity Amounts (Step Counts) on OA Progression, as Shown on MRI. Greater meniscal pathology scores, presence of bone mineral lesions (BML) and less cartilage volume all indicate more severe disease. BML are areas of increased signal adjacent to the subcortical bone at the medial tibial, medial femoral, lateral tibial, and lateral femoral sites and indicate more severe joint pathology. All figures show an interaction effect, wherein for those individuals with less baseline meniscal pathology, steps are not related to pathology score increases. In contrast, in adults with greater baseline pathology scores, a greater percent of adults with more than 10,000 steps per day show worsening of pathology scores over time (26%) compared to adults with fewer than 10,000 steps day (10%). Reproduced from “The association between objectively measured physical activity and knee structural change using MRI”, Dawn A Dore et al., 72, 2013 with permission from BMJ Publishing Group Ltd.
DISCUSSION and NEEDS FOR FUTURE RESEARCH
Over an entire week, as many as 40% of adults with lower extremity joint conditions do not engage in even a single session of moderate physical activity lasting 10 minutes(40). However, as is clear from our review, regular exercise at amounts up to those consistent with the 2008 Physical Activity Guidelines for Americans — 150 minutes/week of moderate-intensity aerobic exercise, 2 days per week of muscle-strengthening exercise — has a substantial beneficial impact on health of individuals with pre-existing knee and hip OA. The evidence suggests that up to 10,000 steps per day of activity does not accelerate OA progression in individuals with pre-existing OA. Land-based exercise appears to be as efficacious as water-based exercise for these outcomes. Benefits related to pain relief, physical function, and HRQoL appear to be applicable for aerobic exercise, muscle-strengthening exercise, and Tai Chi. Although not tested head to head, effect sizes for joint pain reduction by physical activity are comparable to those reported for analgesics(20). Although this review did not identify any MA/SR related to risk of co-morbid conditions, a recent large cohort study (16,362 individuals aged ≥55 years, median 13.5 years follow-up) demonstrated that the presence and burden of radiographic hip and/or knee OA was significantly associated with increased risk (16-25%) for incident diabetes (controlled for confounders) with 37-46% of this relationship explained by baseline limitations in walking(50). This excellent study begins to address the important question of physical activity and co-morbidities in OA and underscores the necessity of further studies to determine means of counteracting the incidence or reversing established serious co-morbidities, such as diabetes, in individuals with OA. A summary of the overall conclusions and grade of the evidence, based on a consensus of the 2018 PAGAC, are provided in Table 3.
Table 3.
Physical Activity Guidelines Advisory Committee (PAGAC) Recommendations.
| Research Question | Evidence Grade* |
|---|---|
| Amounts of physical activity and comorbidities in individuals with osteoarthritis | Not assignable |
| Amounts of physical activity and decreased pain and improved physical function in adults with osteoarthritis of the knee and hip | Strong (evidence is unlikely to be modified by more studies for these outcomes) |
| Amounts of physical activity and improved health-related quality of life in persons with osteoarthritis of the knee and hip | Moderate |
| Relationships vary by age, sex, race, ethnicity, socioeconomic status, or body mass index | Not assignable |
| Dose-response relationship between physical activity and improved pain, physical function and health-related quality of life in individuals with osteoarthritis | Not assignable |
| Intensity or duration of aerobic and muscle-strengthening physical activity is related to improvement in pain and functional capacity in individuals with osteoarthritis of the knee and hip | Limited |
| Dose-response relationship between physical activity and disease progression in individuals with osteoarthritis | Moderate for safety (the relationship appears to be U-shaped; up to the range of 10,000 steps per day, ambulatory physical activity does not accelerate osteoarthritis of the knee); Limited for adverse effects (range of more than 10,000 steps per day may have an adverse effect on progression of osteoarthritis of the knee in individuals with existing osteoarthritis of the knee) |
The 5 grading criteria serving as the determinants of the final Evidence Grade are described in Torres 2018 (Table 1); in brief they were 1) applicability, 2) generalizability (to the US population of interest), 3) risk of bias or study limitations (as determined by NEL BAT and/or AMSTARExBP), 4) quantity and consistency (of the results across the available studies) and 5) magnitude and precision of effect.
There are a number of barriers to physical activity for individuals with OA. For people with lower extremity joint symptoms, even 10-minute bouts of activity can be a challenge. Moreover, greater knee pain and BMI can both contribute to poorer compliance with exercise(51). One study suggested a potential U-shaped, and another a J-shaped, dose-response relationship of physical activity with OA progression(40, 45, 49). Interestingly, this U-shaped dose response relationship is supported by a MA of exercise studies in healthy animals(52).
Evidence addressing some of the barriers to physical activity for individuals with joint disease are provided by Dunlop et al. where an intermediate level of accumulated physical activity—minimum of 45 minutes/week of at least moderate intensity, irrespective of bouts—benefited function of individuals with lower extremity OA(40). Given the ready accessibility to the general public of mobile health devices—including individuals with arthritis—it is useful for patients and arthritis health related professionals to understand what is known about the relation of step counts to health outcomes in those with OA. The goal of 150 minutes/week of MVPA (walking at least 3.3 miles/hr) equates to ~2500 steps/day whereas the goal of 45 minutes/week of MVPA corresponds to ~750 steps/day. Considering a background of daily activity of 5,000 steps/day(53), a computed translation of these recommendations yields estimates of a total of ~7500 steps/day (corresponding to a ‘somewhat active’ lifestyle(54)), and ~5750 steps/day (also considered a ‘somewhat active’ lifestyle(54)), respectively. It is possible that background daily activity in some individuals with OA does not exceed basal activity levels of 2500 steps/day(54); under these circumstances, the corresponding minimal estimates of activity would be a total of ~5000 steps/day and ~3250 steps/day (considered a ‘sedentary’ lifestyle). Interestingly, all these goals fall within the apparent safe range for individuals with more severe lower limb OA of less than 10,000 steps/day. In a large (n=4,840) community-based sample, benefits are similar for both bouted and non-bouted physical activity(55, 56). Moreover, a marked mortality benefit accrues from as little as 40-80 minutes/day of moderate activity(56) defined as a threshold of 760 counts/min using a waist-worn accelerometer—roughly equivalent to the level of exertion of activities of daily living. Taken together, these new insights provide encouraging news for individuals with OA for whom non-bouted activity and intermediate levels of activity below US guideline amounts are likely to be more readily achieved on a regular basis.
Although umbrella reviews represent one of the highest levels of evidence synthesis currently available, they are subject to several limitations including: incomplete stratification of the evidence due to residual overlap within the included MAs/SRs; heterogeneity of exposures making it difficult to determine the exact relationships of physical activity and outcomes; and heterogeneity of studied populations potentially limiting the generalizability of results. In addition, this review was limited by the lack of studies related to HRQoL and OA progression and a lack of uniform definitions of OA—a current challenge to the OA research field as a whole. As a strength, this review has yielded insights into knowledge gaps that led us to formulate the recommendations described below for future research.
1. Conduct additional research to assess effect sizes of physical activity on OA to determine the clinical impact exercise may have on particular outcomes.
Rationale: There is a particular need to conduct prospective longer-term RCTs of physical activity to evaluate OA disease progression, with objective quantification of physical activity exposures with molecular and imaging disease status biomarkers as outcomes. In addition, more data are needed to address the critical issues of varying amounts and intensities of physical activity and their relationship to incidence and progression of OA (tibiofemoral and patellofemoral) in the absence of underlying injury. Because it often takes years for disease activity to result in structural, detectable radiographic changes in the joint, sophisticated imaging modalities, such as MRI, and biological biomarkers of disease activity (circulating systemic or intra-articular) are needed to measure the outcomes. Recently (after the timeframe of the searches for this review), the first meta-analysis of synovial fluid, serum and urine biomarkers in individuals with established knee OA was published(57). It concluded that 4-24 weeks of exercise therapy (strengthening and or aerobic) was not harmful as it did not increase the concentration of molecular biomarkers related to inflammation and cartilage turnover, associated with cartilage breakdown. The overall quality of evidence was graded as low because of the limited number of RCTs available underscoring the need for more biomarker research in this field.
2. Conduct research to clarify how OA progression is modified by baseline demographic and disease characteristics as well as pain responses to exercise.
Rationale: For the outcome of disease progression induced by physical activity, some evidence suggests that baseline disease status plays a role in modifying the effect of physical activity; but this role has not yet been fully explained. In addition, although a relationship between BMI and OA is generally recognized, no studies have investigated through MAs whether BMI modifies the physical activity-OA relationship. More studies on OA progression need to evaluate groups of individuals with clear evidence of OA (defined biochemically, by MRI or radiograph) at baseline as well as those “at risk” of OA.
3. Conduct direct head-to-head comparisons of the relative effectiveness of physical activity and analgesics for pain control in individuals with OA.
Rationale: Our review of the literature revealed that the effect sizes for pain control from exercise interventions is very similar to that of analgesics, including narcotic analgesics(20). If true, this would be a critical observation with profound implications for patient care, especially as the effects of physical activity on OA-related pain seem to be durable for up to 6 months following cessation of an intervention. Determining the comparative effects of physical activity and analgesics on OA pain could contribute greatly to effective clinical management of OA and potentially to greater third-party payment of exercise treatments for OA.
4. Conduct research to determine the optimal physical activity dose, mode, intensity, duration and frequency to optimize efficacy and sustainability of physical activity for different types and severity of OA.
Rationale: Different modalities or amounts of physical activity (using the same modality) have not been compared head-to-head to ascertain their relative effects on OA progression, as well as pain, physical function, and HRQoL. Dose-response investigations on the relationship of daily step counts and other device-based measures of physical activity and OA disease progression are particularly needed. Given that varying pain intensities and structural severities of OA have been associated with reduced compliance with exercise therapy, it is important to develop approaches to personalize physical activity prescriptions for individuals with OA to minimize discontinuation due to exacerbation of symptoms and/or disease progression.
5. Determine the capacity of individuals with OA to perform physical activity at intensities and amounts of exercise that are able to modify co-morbidities.
Rationale: Obesity is a risk factor for OA incidence and progression. Obesity is also a significant risk factor for OA-related co-morbidities, including diabetes, cardiovascular disease, and cancer. However, few to no data address the relationships of physical activity and modification of OA-related co-morbidities and mortality in those with OA. New longitudinal cohort studies, facilitated by device-based measures of physical activity, will be required to adequately address this question. In addition, more data are needed to determine whether those with advanced OA can safely exercise at intensities or amounts that are able to modify the risk of developing disease co-morbidities without subjecting themselves to a greater risk of disease progression.
6. Develop biomarkers of exercise responsiveness and trajectories for different types and severity of OA, to determine who is likely to respond favorably to physical activity interventions versus who is at risk of disease progression.
Rationale: As for many human conditions and physiologic states, even when controlling for possible effect modifiers, individuals with different OA characteristics (pain, physical function, HRQoL, and disease structural severity) demonstrate a range of individual responses to the same exercise exposure. Developing technologies (such as biomarkers) and approaches to better understand the demographic, physiologic, and molecular basis of disease will be valuable for predicting and monitor responses to exercise and thereby for developing the best exercise regimen to elicit specific responses at the individual level.
CONCLUSIONS
Physical activity decreases pain, improves physical function and HRQoL among people with hip and/or knee OA relative to less active adults with OA. Given the strength of the evidence (261 studies of various physical activity modes of exposure including land and pool, aerobic, resistance and flexibility), it is highly unlikely that the conclusions will be modified by more RCTs for these outcomes. There is currently no evidence to suggest accelerated progression of OA in individuals with pre-existing joint pathology for physical activity below 10,000 steps per day. A total of at least 45 minutes/week of moderate-vigorous physical activity can improve or sustain function of individuals with lower extremity OA. Thus, people with lower extremity OA should be encouraged to engage in achievable amounts of physical activity, of even modest intensities, accrued throughout the entire day, irrespective of bouts, and be confident of gaining some health and arthritis-related benefits.
Supplementary Material
Supplemental Digital Content 1. Quality assessment chart for Existing Systematic Reviews (SRs) and Meta-Analyses (MAs) using AMSTARExBP: SR/MA. The quality of each MA, SR and pooled analysis was assessed using a modified version of “A Measurement Tool to Assess Systematic Reviews” (AMSTAR); the majority of the studies met 11 of the 18 AMSTAR criteria.
Supplemental Digital Content 2. Bias assessment chart for Original Research related to osteoarthritis progression. The risk of bias, or internal validity, was assessed for each original study using an adapted version of the USDA NEL Bias Assessment Tool (BAT); the majority of the studies met 8 of the 10 applicable criteria.
Supplemental Digital Content 3. Literature tree of search strategy for systematic reviews, meta-analyses, pooled analyses and reports. PubMed, Cochrane database and CINAHL databases were searched for articles (existing systematic reviews, meta-analyses, pooled analyses and reports) published 2011 – 2/2018 addressing the primary questions of the effect of physical activity on (1) pain, (2) physical function, (3) health-related quality of life, (4) disease progression and (5) risk of co-morbid conditions in individuals with pre-existing lower extremity OA.
Supplemental Digital Content 4. Literature Tree search for original research related to osteoarthritis progression. Due to lack of sufficient meta-analyses and systematic reviews related to OA progression, a de novo supplementary search of PubMed, Cochrane database and CINAHL databases was conducted for original research articles published 2006-2/2018 related to physical activity and lower limb OA progression.
Acknowledgments
The authors gratefully acknowledge the assistance of Andrea Torres for her AMSTAR criteria review and other ICF librarians, abstractors, and additional support staff involved with the literature search and retrieval process.
Role of the Funder/Sponsor
HHS staff provided general administrative support to the Committee and assured that the Committee adhered to the requirements for Federal Advisory Committees. HHS also contracted with ICF, a global consulting services company, to provide technical support for the literature searches conducted by the Committee. HHS and ICF staff collaborated with the Committee in the design and conduct of the searches by assisting with the development of the analytical frameworks, inclusion/exclusion criteria, and search terms for each primary question; using those parameters, ICF performed the literature searches.
Footnotes
Conflicts of Interest and Source of Funding
The Committee’s work was supported by the U.S. Department of Health and Human Services (HHS). Committee members were reimbursed for travel and per diem expenses for the five public meetings; Committee members volunteered their time. The authors report no other potential conflicts of interest.
This paper is being published as an official pronouncement of the American College of Sports Medicine. This pronouncement was reviewed for the American College of Sports Medicine by members-at-large and the Pronouncements Committee. Disclaimer: Care has been taken to confirm the accuracy of the information present and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this publication and make no warranty, expressed or implied, with respect to the currency, completeness, or accuracy of the contents of the publication. Application of this information in a particular situation remains the professional responsibility of the practitioner; the clinical treatments described and recommended may not be considered absolute and universal recommendations.
REFERENCES
- 1.Barbour K, Helmick C, Boring M, Brady T. Vital signs: Prevalence of doctor-diagnosed athritis and arthritis-attributable activity limitation --- United States, 2013–2015. Morbidity and Mortality Weekly Report (MMWR). 2017;66(9):246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res (Hoboken). 2016;68(5):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande BR, Katz JN, Solomon DH et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken). 2016;68(12):1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015-2040. Arthritis & rheumatology (Hoboken, N.J.). 2016;68(7):1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross M, Smith E, Hoy D et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. [DOI] [PubMed] [Google Scholar]
- 6.Guccione AA, Felson DT, Anderson JJ et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–53. [DOI] [PubMed] [Google Scholar]
- 8.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. In. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- 9.Neogi T The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. In: USDoHaH Services; editor. Washington, D.C2018. [Google Scholar]
- 11.Torres A, Tennant B, Ribeiro-Lucas I, Vaux-Bjerke A, Piercy K, Bloodgood B. Umbrella and Systematic Review Methodology to Support the 2018 Physical Activity Guidelines Advisory Committee. J Physical Activity and Health. 2018;15:805–810. [DOI] [PubMed] [Google Scholar]
- 12.Physical Activity Guidelines Advisory Committee. Evidence Portfolio – Chronic Conditions Subcommittee, Question 2. 2018. [cited 2018]. Available from: https://health.gov/paguidelines/second-edition/report/supplementary_material/pdf/Chronic_Conditions_Q2_Osteoarthritis_Evidence_Portfolio.pdf.
- 13.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 14.Bartels EM, Juhl CB, Christensen R et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523.(doi): 10.1002/14651858.CD005523.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beumer L, Wong J, Warden SJ, Kemp JL, Foster P, Crossley KM. Effects of exercise and manual therapy on pain associated with hip osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2016;50(8):458–63. doi: 10.1136/bjsports-2015-095255. Epub 2015 Nov 26. [DOI] [PubMed] [Google Scholar]
- 16.Chang WD, Chen S, Lee CL, Lin HY, Lai PT. The Effects of Tai Chi Chuan on Improving Mind-Body Health for Knee Osteoarthritis Patients: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.(doi): 10.1155/2016/1813979. Epub 2016 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escalante Y, Garcia-Hermoso A, Saavedra JM. Effects of exercise on functional aerobic capacity in lower limb osteoarthritis: a systematic review. J Sci Med Sport. 2011;14(3):190–8. doi: 10.1016/j.jsams.2010.10.004. Epub Nov 25. [DOI] [PubMed] [Google Scholar]
- 18.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–7. doi: 10.136/bjsports-2015-095424. Epub 2015 Sep 24. [DOI] [PubMed] [Google Scholar]
- 19.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912. doi: 10.1002/14651858.CD007912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksen M, Hansen JB, Klokker L, Bliddal H, Christensen R. Comparable effects of exercise and analgesics for pain secondary to knee osteoarthritis: a meta-analysis of trials included in Cochrane systematic reviews. J Comp Eff Res. 2016;5(4):417–31. doi: 10.2217/cer-016-0007. Epub 2016 Jun 27. [DOI] [PubMed] [Google Scholar]
- 21.Jansen MJ, Viechtbauer W, Lenssen AF, Hendriks EJ, de Bie RA. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother. 2011;57(1):11–20. doi: 10.1016/S836-9553(11)70002-9. [DOI] [PubMed] [Google Scholar]
- 22.Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66(3):622–36. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Su Y, Chen S et al. The effects of resistance exercise in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil. 2016;30(10):947–59. doi: 10.1177/0269215515610039. Epub 2015 Oct 15. [DOI] [PubMed] [Google Scholar]
- 24.Regnaux JP, Lefevre-Colau MM, Trinquart L et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):Cd010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampath KK, Mani R, Miyamori T, Tumilty S. The effects of manual therapy or exercise therapy or both in people with hip osteoarthritis: a systematic review and meta-analysis. Clin Rehabil. 2016;30(12):1141–55. doi: 10.77/0269215515622670. Epub 2015 Dec 22. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka R, Ozawa J, Kito N, Moriyama H. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27(12):1059–71. doi: 10.177/0269215513488898. Epub 2013 Jul 4. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka R, Ozawa J, Kito N, Moriyama H. Effect of the Frequency and Duration of Land-based Therapeutic Exercise on Pain Relief for People with Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J Phys Ther Sci. 2014;26(7):969–75. doi: 10.1589/jpts.26.969. Epub 2014 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka R, Ozawa J, Kito N, Moriyama H. Does exercise therapy improve the health-related quality of life of people with knee osteoarthritis? A systematic review and meta-analysis of randomized controlled trials. J Phys Ther Sci. 2015;27(10):3309–14. doi: 10.1589/jpts.27.3309. Epub 2015 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmins KA, Leech RD, Batt ME, Edwards KL. Running and Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am J Sports Med. 2017;45(6):1447–57. doi: 10.177/0363546516657531. Epub 2016 Aug 20. [DOI] [PubMed] [Google Scholar]
- 30.Uthman OA, van der Windt DA, Jordan JL et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Huang L, Su Y, Zhan Z, Li Y, Lai X. The Effects of Traditional Chinese Exercise in Treating Knee Osteoarthritis: A Systematic Review and Meta-Analysis. PLoS One. 2017;12(1):e0170237. doi: 10.1371/journal.pone.. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandopulle S, Perry M, Manlapaz D, Jayakaran P. Effect of Land-Based Generic Physical Activity Interventions on Pain, Physical Function, and Physical Performance in Hip and Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am J Phys Med Rehabil. 2017;96(11):773–92. doi: 10.1097/PHM.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 33.Bartholdy C, Juhl C, Christensen R, Lund H, Zhang W, Henriksen M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Semin Arthritis Rheum. 2017;47(1):9–21. doi: 10.1016/j.semarthrit.2017.03.007. Epub Mar 18. [DOI] [PubMed] [Google Scholar]
- 34.Young JL, Rhon DI, Cleland JA, Snodgrass SJ. The Influence of exercise dosing on outcomes in patients With knee disorders: a systematic review. J Orthop Sports Phys Ther. 2018;48(3):146–61. [DOI] [PubMed] [Google Scholar]
- 35.Johnson BT, MacDonald HV, Bruneau ML Jr., et al. Methodological quality of meta-analyses on the blood pressure response to exercise: a review. J Hypertens. 2014;32(4):706–23. [DOI] [PubMed] [Google Scholar]
- 36.Shea BJ, Grimshaw JM, Wells GA et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Department of Agriculture (USDA). 2015 Dietary Guidelines Advisory Committee (DGAC) nutrition evidence library methodology. 2017. Available from: https://www.cnpp.usda.gov/sites/default/files/usda_nutrition_evidence_flbrary/2015DGAC-SR-Methods.pdf. .
- 38.Guidelines SRotD, Advisory Committee. Table C.2, NEL Grading Rubric. 2015. Available from: https://health.gov/dietaryguidelines/2015-scientific-report/05-methodology.asp.
- 39.Garber CE, Blissmer B, Deschenes MR et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. [DOI] [PubMed] [Google Scholar]
- 40.Dunlop D, Song J, Lee J et al. Physical Activity Minimum Threshold Predicting Improved Function in Adults With Lower-Extremity Symptoms. Arthritis Care Res (Hoboken). 2017;69(4):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 42.Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteo & Cartilage. 2017;12(1522–9653 (Electronic)):1926-41. [DOI] [PubMed] [Google Scholar]
- 43.Quicke Foster, Thomas. Is long-term physical activity safe for older adults with knee pain? A systematic review. Osteoarthritis Cartilage. 2015;23(9):1445–1456 [DOI] [PubMed] [Google Scholar]
- 44.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dore D, Winzenberg T, Ding C et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2013;72(7):1170–5 [DOI] [PubMed] [Google Scholar]
- 46.Felson D, Niu J, Yang T et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage. 2013;21(6):789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oiestad B, Banion MK, Quinn E et al. No Association between Daily Walking and Knee Structural Changes in People at Risk of or with Mild Knee Osteoarthritis. Prospective Data from the Multicenter Osteoarthritis Study. J Rheumatol. 2015;42(9):1685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwee R, Wirth W, Hafezi-Nejad N, Zikria B, Guermazi A, Demehri S. Role of physical activity in cartilage damage progression of subjects with baseline full-thickness cartilage defects in medial tibiofemoral compartment: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24(11):1898–904. [DOI] [PubMed] [Google Scholar]
- 49.Lin W, Alizai H, Joseph G et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21(10):1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kendzerska T, King L, Lipscombe L, Croxford R, Stanaitis I, Hawker G. The impact of hip and knee osteoarthritis on the subsequent risk of incident diabetes: a population-based cohort study. Diabetologia. 2018;61:2290–9. [DOI] [PubMed] [Google Scholar]
- 51.Rejeski W, Brawley L, Ettinger W, Morgan T, Thompson C. Compliance to exercise therapy in older participants with knee osteoarthritis: implications for treating disability. Med Sci Sports Exerc. 1997;29(8):977–85. [DOI] [PubMed] [Google Scholar]
- 52.Bricca A, Juhl CB, Grodzinsky AJ, Roos EM. Impact of a daily exercise dose on knee joint cartilage - a systematic review and meta-analysis of randomized controlled trials in healthy animals. Osteoarthritis Cartilage. 2017;25(8):1223–37. [DOI] [PubMed] [Google Scholar]
- 53.Tudor-Locke C, Bassett DR Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. [DOI] [PubMed] [Google Scholar]
- 54.Tudor-Locke C, Craig CL, Aoyagi Y et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saint-Maurice PF, Troiano RP, Matthews CE, Kraus WE. Moderate-to-Vigorous Physical Activity and All-Cause Mortality: Do Bouts Matter? J Am Heart Assoc. 2018;7(6).(pii):JAHA.117.007678. doi: 10.1161/JAHA.117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saint-Maurice PF, Troiano RP, Berrigan D, Kraus WE, Matthews CE. Volume of Light Versus Moderate-to-Vigorous Physical Activity: Similar Benefits for All-Cause Mortality? J Am Heart Assoc. 2018;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bricca A, Struglics A, Larsson S, Steultjens M, Juhl CB, Roos EM. Impact of exercise therapy on molecular biomarkers related to cartilage and inflammation in people at risk of, or with established, knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2018; doi 10.1002/acr.23786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Quality assessment chart for Existing Systematic Reviews (SRs) and Meta-Analyses (MAs) using AMSTARExBP: SR/MA. The quality of each MA, SR and pooled analysis was assessed using a modified version of “A Measurement Tool to Assess Systematic Reviews” (AMSTAR); the majority of the studies met 11 of the 18 AMSTAR criteria.
Supplemental Digital Content 2. Bias assessment chart for Original Research related to osteoarthritis progression. The risk of bias, or internal validity, was assessed for each original study using an adapted version of the USDA NEL Bias Assessment Tool (BAT); the majority of the studies met 8 of the 10 applicable criteria.
Supplemental Digital Content 3. Literature tree of search strategy for systematic reviews, meta-analyses, pooled analyses and reports. PubMed, Cochrane database and CINAHL databases were searched for articles (existing systematic reviews, meta-analyses, pooled analyses and reports) published 2011 – 2/2018 addressing the primary questions of the effect of physical activity on (1) pain, (2) physical function, (3) health-related quality of life, (4) disease progression and (5) risk of co-morbid conditions in individuals with pre-existing lower extremity OA.
Supplemental Digital Content 4. Literature Tree search for original research related to osteoarthritis progression. Due to lack of sufficient meta-analyses and systematic reviews related to OA progression, a de novo supplementary search of PubMed, Cochrane database and CINAHL databases was conducted for original research articles published 2006-2/2018 related to physical activity and lower limb OA progression.