Abstract
Vacuolar-type H+-ATPase (V-ATPase) is a highly conserved proton pump responsible for acidification of intracellular organelles and potential drug target. It is a multisubunit complex comprising a cytoplasmic V1 domain responsible for ATP hydrolysis and a membrane-embedded Vo domain that contributes to proton translocation across the membrane. Saccharomyces cerevisiae V-ATPase is composed of 14 subunits, deletion of any one of which results in well-defined growth defects. As the structure of V-ATPase and the function of each subunit have been well-characterized in yeast, this organism has been recognized as a preferred model for studies of V-ATPases. In this study, to assess the functional relatedness of the yeast and human V-ATPase subunits, we investigated whether human V-ATPase subunits can complement calcium- or pH-sensitive growth, acidification of the vacuolar lumen, assembly of the V-ATPase complex, and protein sorting in yeast mutants lacking the equivalent yeast genes. These assessments revealed that 9 of the 13 human V-ATPase subunits can partially or fully complement the function of the corresponding yeast subunits. Importantly, sequence similarity was not necessarily correlated with functional complementation. We also found that besides all Vo domain subunits, the V1 F subunit is required for proper assembly of the Vo domain at the endoplasmic reticulum. Furthermore, the human H subunit fully restored the level of vacuolar acidification, but only partially rescued calcium-sensitive growth, suggesting a specific role of the H subunit in V-ATPase activity. These findings provide important insights into functional homologies between yeast and human V-ATPases.
Keywords: vacuolar ATPase, yeast genetics, vacuole, Saccharomyces cerevisiae, human
Introduction
Vacuolar-type H+-ATPase (V-ATPase)3 is an evolutionally conserved multisubunit complex responsible for acidification of intracellular organelles such as the Golgi and the lysosome/vacuole (1, 2). In mammalian cells, acidification of organelles in the biosynthetic pathway (e.g. trans-Golgi compartment, pH ∼6.0; secretory vesicle, pH ∼5.5) is thought to be essential for post-transcriptional processing and trafficking of newly synthesized proteins (3, 4). Similarly, in the endocytic pathway, progressive acidification of organelles (e.g. early endosome, pH ∼6.3; late endosome, pH ∼5.5; lysosome/vacuole, pH ∼4.7) is important for trafficking and recycling of internalized membrane proteins such as cell-surface receptors and transporters (3, 5–7). In yeast, cytoplasmic pH has been shown to be ∼6.7, whereas pH in the vacuole is reportedly ∼5.6, when cells are grown in media with a pH of 5.5 (8–12). In contrast, yeast mutants lacking each of the V-ATPase subunits have alkalinized vacuoles and acidified cytosol because of an inability to transport protons from the cytosol to the vacuole (12). Similar to mammalian cells, acidification of organelles in yeast is required for the protein sorting and recycling pathways.
Previous studies have also suggested a role for V-ATPase in various physiological processes, including sperm maturation, urine acidification, and bone remodeling (13–15). Targeted disruption of the c subunit of the murine Vo domain, which has one gene copy in the mouse genome, causes complete loss of V-ATPase function, which is embryonically lethal (16). In contrast, loss of a tissue-specific V-ATPase subunit, such as the a1, a3, or B1 subunit, has been reported to cause human disease, including osteoporosis, sensorineural deafness, distal renal tubular acidosis, or diabetes (15, 17–19). Overexpression and/or ectopic expression of V-ATPase is also reportedly associated with numerous diseases, including cancer (20). For instance, V-ATPases generate an acid microenvironment that influences cancer progression, metastasis, and chemoresistance (21, 22). V-ATPase has therefore been recognized as a valuable drug target (21).
V-ATPase is expressed ubiquitously in various organisms, and in most eukaryotic cells complete loss of V-ATPase function is lethal. However, the yeast Saccharomyces cerevisiae can survive lacking functional V-ATPase and exhibits well-defined conditionally lethal phenotypes, including sensitivity to elevated pH and calcium concentrations, inability to grow on nonfermentable carbon sources, and sensitivity to a variety of heavy metals (23–25). Furthermore, in yeast, each subunit of V-ATPase is encoded by a single gene, except for the VPH1 and STV1 genes, which encode two isoforms of a subunit (Vph1p and Stv1p) and target V-ATPase to the vacuole or the Golgi, respectively (26, 27). These features make yeast a preferred model organism for the study of V-ATPases.
V-ATPase comprises two functional domains: a cytoplasmic V1 domain responsible for ATP hydrolysis, and the membrane-embedded Vo domain that functions in proton translocation across the membrane (2). The V1 domain contains eight different subunits (A–H) with three copies of the catalytic A and B subunits, three copies of the stator subunits E and G, and one copy of the regulatory C and H subunits, whereas the Vo domain contains at least five transmembrane subunits, including a ring of proteolipid subunits (c and c″ for mammals; c, c′, and c″ for yeast) that are adjacent to subunits a and e (2). The structure of V-ATPase and the function of each of the subunits have been well-characterized in S. cerevisiae (28, 29), and these provide novel insights into human V-ATPase. Using the structure of yeast V-ATPase as a template, it is possible to build a homology model of human V-ATPase. However, it is important to determine the ability of the human subunits to functionally replace their yeast equivalents.
In this study, we expressed human V-ATPase subunits in yeast mutants lacking the equivalent subunits and examined the level of functional conservation of each subunit. These assessments revealed that the cytosolic subunits of human V-ATPase, except for the B and G subunits, were able to complement the function of the corresponding yeast subunits, but the proteolipid subunits were mostly unable to do so. These proteolipid subunits became mis-localized to the endoplasmic reticulum (ER), indicating that assembly of the Vo domain at the ER is deficient in these mutants. We also demonstrated that the human H subunit largely complemented the vacuolar acidification and localization of the V1 domain but only partially rescued the growth of yeast lacking the native H subunit, suggesting a specific role of the human H subunit in V-ATPase activity.
Results
Complementation of yeast genes encoding V-ATPase subunits with equivalent human genes
To investigate functional conservation between yeast and human V-ATPase subunits, we replaced yeast genes with their human equivalents (Fig. 1A). For expression of human genes, we utilized two different promoters: one was the promoter of yeast equivalent genes (native promoter), expressing human genes at the same level as its equivalent yeast subunit (Fig. 1B), and the other was the yeast triose-phosphate isomerase (TPI1) gene promoter, which has constitutive and powerful promoter activity that increases the expression to ∼3.8-fold compared with the VMA1 promoter (Fig. 1B) (30). After expressing the human gene in a mutant lacking the equivalent yeast gene, we evaluated four cell phenotypes: 1) growth ability on media containing a high-calcium concentration and elevated pH; 2) vacuolar acidification; 3) localization of the V1 and Vo subunits; and 4) endocytic recycling of Wsc1–3GFP (Fig. 1A).
Figure 1.
Overview of the experimental design for the complementation test. A, yeast mutants, in which gene encoding each V-ATPase subunit was replaced with the KanMX marker, were obtained from GE Dharmacon. For expression of human genes, two different promoters, the promoter of yeast equivalent genes (PVMA) and the yeast triose-phosphate isomerase gene promoter (PTPI1), were utilized. After expressing the human gene in a mutant lacking the equivalent yeast gene, the following four cell phenotypes, 1) growth ability, 2) vacuolar acidification, 3) localization of the V1 and Vo subunits, and 4) endocytic recycling of Wsc1–3GFP, were evaluated. B, immunoblots showing the expression of GFP-tagged Vma1p and its human equivalent subunit (ATP6V1A) from the VMA1 gene or the TPI1 gene promoter in the vma1Δ mutants. 10 μg of whole-cell extracts from each strain were loaded per lane and immunoblotted with an anti-GFP antibody or anti-glyceraldehyde-3-phosphate dehydrogenase antibody. The bar graphs represent the relative expression levels of these proteins. Data show the mean ± S.D. of three experiments. **, p value < 0.01, one-way ANOVA with Tukey's test. n.s., not statistically significant.
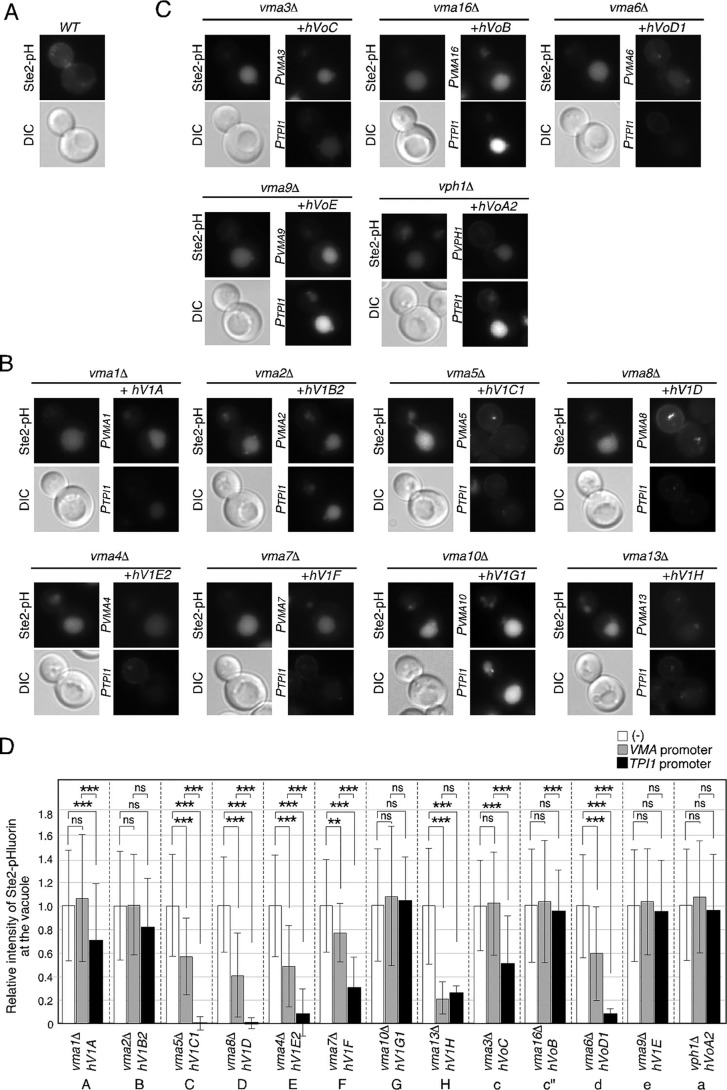
Yeast V-ATPase comprises 14 subunits, including a catalytic V1 domain of peripherally associated proteins (Vma1p, 2p, 4p, 5p, 7p, 8p, 10p, and 13p) and a proton-translocating Vo domain of integral membrane proteins (Vma3p, 6p, 9p, 11p, 16p, and Vph1p) (see Fig. 7C and Table 1) (2, 31). Previous studies have shown that cells lacking any of the ATPase subunit genes exhibited an identical set of vma phenotypes, including sensitivity to elevated pH and high extracellular Ca2+ concentration (24). Whereas in yeast all of the V-ATPase subunits are encoded by single genes with the exception of the a subunit, six of the V-ATPase subunits (subunits B, C, E, G, a, and d) have multiple subunits in mammalian cells (2). In each case, one isoform is expressed ubiquitously, whereas the other isoforms are often expressed in a specific tissue (32). For this reason, we used ubiquitously expressed isoforms in the following experiments. To investigate functional conservation between the yeast and human V-ATPase subunits, we expressed each human subunit from the native promoter (Fig. 2A) or the TPI1 promoter (Fig. 2B) in cells lacking the equivalent yeast subunit. Before beginning the complementation tests, we confirmed that expression of each human V-ATPase subunit from the TPI1 promoter does not affect the growth of WT yeast on media containing 100 mm CaCl2 or elevated pH (pH 7.0) (Fig. S1). Next, we examined the growth ability of the strains after replacing the yeast V1 subunit genes with their human equivalents on these media. Among strains expressing the human gene from the native promoter, two strains expressing the human C1 (V1C1) or D (V1D) subunit completely recovered their ability to grow in medium containing 100 mm Ca2+ concentration or elevated pH (Fig. 2A and Table 1), suggesting functional conservation of these subunits between yeast and human. Two strains expressing the human E2 (V1E2) or F (V1F) subunit showed partial growth rescue when expressed from the native promoter (Fig. 2A and Table 1), and they showed almost complete recovery when expressed from the TPI1 promoter (Fig. 2B and Table 1), suggesting that these human subunits partially complement the functions of the yeast subunits. The strain expressing the human H subunit(s) exhibited partial rescue of growth when expression was from either the native or the TPI1 promoter (Fig. 2, A and B, and Table 1).
Figure 7.
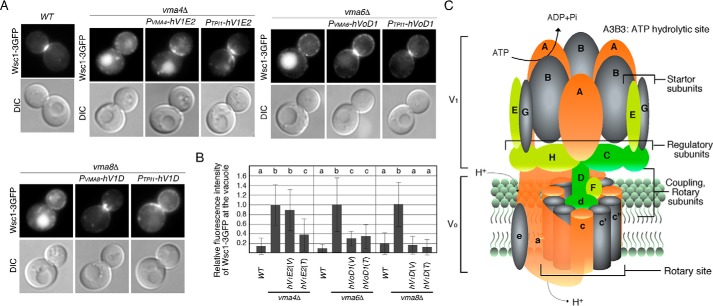
Recycling of Wsc1–3GFP in yeast V-ATPase subunit mutants expressing the equivalent human gene. A, localization of Wsc1–3GFP in WT and vma mutants. Cells expressing Wsc1–3GFP were grown to early logarithmic phase in YPD medium at 25 °C and observed by epifluorescence and differential interference contrast (DIC) microscopy. B, quantification of the fluorescence intensity of Wsc1–3GFP at the vacuole in WT and mutant cells. The fluorescence intensity of Wsc1–3GFP at the vacuole was calculated by subtracting average fluorescence intensity in the cytosol from that in the vacuole. For the relative fluorescence intensity in mutants, the average fluorescence intensity in mutant cells was divided by the average fluorescence intensity in WT cells. Data show the mean ± S.D., with >50 cells counted for each strain. Different letters indicate significant difference at p < 0.01 (one-way ANOVA with Tukey's post-hoc test). C, schematic representation of the subunit structure of yeast V-ATPase (2, 28). The subunits whose function were mostly complemented by the corresponding human subunits are shown in green, partially complemented are shown in yellow, and slightly complemented are shown in orange. The subunits that were not complemented are shown in gray.
Table 1.
Functional complementation between yeast and human V-ATPase subunits
The following abbreviations are used: acid., acidification: local., localization: recycl., recycling; ND, not determined.
| Yeast | Human | Similarity/identity | Growth |
Vacuolar acid. |
Vph1p local. |
Vma1p local. |
Wsc1p recycl. |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (PVMA) | (PTPI1) | (PVMA) | (PTPI1) | (PVMA) | (PTPI1) | (PVMA) | (PTPI1) | (PVMA) | (PTPI1) | ||||
| % | |||||||||||||
| V1 domain | |||||||||||||
| A | VMA1 | V1A | 45/37 | − | +/− | − | +/− | ++ | ++ | − | +/− | ND | ND |
| B | VMA2 | V1B2 | 78/71 | − | +/− | − | − | ++ | ++ | − | − | ND | ND |
| C | VMA5 | V1C1 | 53/35 | ++ | ++ | + | ++ | ++ | ++ | + | ++ | ND | ND |
| D | VMA8 | V1D | 70/51 | ++ | ++ | + | ++ | ++ | ++ | + | ++ | ++ | ++ |
| E | VMA4 | V1E2 | 57/35 | +/− | ++ | + | ++ | ++ | ++ | − | +/− | − | + |
| F | VMA7 | V1F | 64/50 | +/− | ++ | + | ++ | − | + | +/− | + | ND | ND |
| G | VMA10 | V1G1 | 53/36 | − | − | − | − | ++ | ++ | − | − | ND | ND |
| H | VMA13 | V1H | 39/21 | + | + | ++ | ++ | ++ | ++ | +/− | ++ | ND | ND |
| Vo domain | |||||||||||||
| a | VPH1 | VoA2 | 53/36 | − | + | − | − | ND | ND | +/− | +/− | ND | ND |
| c | VMA3 | VoC | 80/66 | − | +/− | − | +/− | − | + | − | +/− | ND | ND |
| c′ | VMA11 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| c″ | VMA16 | VoB | 63/48 | − | − | − | − | − | − | − | − | ND | ND |
| d | VMA6 | VoD1 | 64/45 | ++ | ++ | + | ++ | ++ | ++ | + | ++ | + | + |
| e | VMA9 | VoE | 38/20 | − | − | − | − | − | − | − | − | ND | ND |
Figure 2.
Growth complementation assays of yeast V-ATPase (V1 subunit) mutants by the equivalent human gene. A and B, growth assays of yeast V-ATPase (V1 subunit) mutants expressing the human equivalent genes from the native promoter (A) or the TPI1 gene promoter (B). 10-Fold dilutions of cells were spotted on YPD plates containing 0, 30, and 100 mm CaCl2 or pH 7.0. YPD plates were buffered with 50 mm KH2PO4 and 50 mm K2HPO4 and incubated at 25 °C for 72 h. The white dashed line shows boundaries in the composite images. WT and mutant cells in a composite image were spotted and grown on the same plate. After imaging the plate, images of cells spotted in a distant place were cropped separately and combined to create a composite image.
We also examined the growth ability of strains in which the yeast Vo subunit genes had been replaced with their human equivalents. Among the five subunits, we found that only the human d1 (VoD1) subunit rescued the ability to grow in medium containing 100 mm Ca2+ or elevated pH (Fig. 3, A and B, and Table 1). Yeast has two genes, VPH1 and STV1, which encode the a subunit (26, 27). A previous study has shown that yeast lacking the VPH1 gene is able to grow in medium containing 100 mm Ca2+ or elevated pH but is unable to survive in the presence of 3.5 mm Zn2+ (27). Accordingly, we examined the growth ability of the strain expressing the human a1 subunit (VoA2) on media containing 4 mm Zn2+. Expression of the VoA2 subunit partially rescued the growth of vph1Δ cells only when expressed from the TPI1 promoter (Fig. 3, A and B, and Table 1), suggesting that the function of the a subunit is partially conserved between yeast and human.
Figure 3.
Growth complementation assays of yeast V-ATPase (Vo subunit) mutants by the equivalent human gene. A and B, growth assays of yeast V-ATPase (Vo subunit) mutants expressing the human equivalent genes from the native promoter (A) or the TPI1 gene promoter (B). 10-Fold dilutions of cells were spotted on YPD plates containing 0, 30, and 100 mm CaCl2 or 4 mm ZnCl2, pH 7.0, YPD plates were buffered with 50 mm KH2PO4 and 50 mm KH2PO4 and incubated at 25 °C for 72 h.
Complementation analysis based on the level of vacuolar acidification
We next determined whether expression of the human V-ATPase subunits could recover vacuolar acidification in vma/vph1 mutants by observing the fluorescence of a pH-sensitive GFP variant, pHluorin, whose fluorescence is quenched at pH values below 6.0 (33). Previous studies have demonstrated that the pHluorin-fused pheromone receptors Ste2p or Ste3p can be used as effective quantitative reporters of vacuolar acidification (8, 34). Although Ste2–pHluorin, which is constitutively internalized and transported to the vacuole lumen, was almost invisible in the vacuoles of WT cells (Fig. 4A), all of the vma mutants, including vhp1Δ, exhibited strong fluorescence in their vacuoles (Fig. 4, B and C, and Table 1). Consistent with these growth phenotypes, expression of the V1C1, V1D, V1E2, V1F, V1H, or VoD1 subunit in the vma mutant efficiently restored vacuolar acidification, although V1C1, V1D, or VoD1 expressed from the native promoter did not have complete restoration function (Fig. 4, B and C, and Table 1). Quantitative analysis revealed that, in the yeast strain whose V1 C subunit (Vma5p) had been replaced by its human equivalent, the fluorescence of Ste2–pHluorin decreased to ∼56.7 or ∼0.4%, when expressed from the native or the TPI1 promoter, respectively (Fig. 4D). In the strain whose V1 D subunit (Vma8p) had been replaced, the fluorescence of Ste2–pHluorin decreased to ∼40.4% (native promoter) and ∼1.4% (TPI1 promoter), whereas replacement of the Vo d subunit resulted in a decrease to ∼59.7% (native promoter) and ∼8.4% (TPI1 promoter). Growth of these strains expressing human subunits from the native promoter showed almost complete recovery, but vacuolar acidification showed only partial recovery, suggesting that evaluation of complementation using the fluorescence intensity of Ste2–pHluorin is more accurate than that based on growth phenotype.
Figure 4.
Functional complementation of yeast V-ATPase subunit mutants by the equivalent human gene. A–C, localization and fluorescence of Ste2–pHluorin in WT (A), yeast V-ATPase V1 subunit mutants (B), and Vo subunit mutants (C) expressing the human equivalent genes. Cells expressing Ste2–pHluorin were grown to early logarithmic phase in YPD medium at 25 °C and observed by epifluorescence and differential interference contrast (DIC) microscopy. The pHluorin is sensitive in pH and loses its fluorescence when transported to the acidic vacuole. D, relative intensities of Ste2–pHluorin at the vacuole in yeast V-ATPase mutants. The fluorescence intensity of vacuoles in the vma mutants, including vhp1Δ was calculated by subtracting the average cytosolic background signal from the fluorescence intensity of the individual vacuole. For the relative fluorescence intensity in mutants, the average fluorescence intensity of the vacuole in mutant cells was divided by the average fluorescence intensity of that in WT cells (n = 50). The fluorescence intensities were analyzed by using the program ImageJ Version 1.44. Data show the mean ± standard deviation (S.D.). **, p value < 0.001; ***, p value < 0.0001, one-way ANOVA with Tukey's test. n.s., not statistically significant.
Localization of the Vo/V1 domains in a yeast mutant expressing the equivalent human gene
We next examined localization of the Vo subunit in the vma/vph1 mutant expressing the human equivalent. As a marker of the Vo domain, we used GFP-tagged Vph1p, which is transported from the Golgi to the vacuole via the VPS pathway (36). GFP tag is inserted into the end of the VPH1 gene at its chromosomal locus to place expression of Vma1–GFP under the control of the native promoter (see “Experimental procedures”). Previous studies reported that endogenous V-ATPase is localized at the vacuolar membrane (27, 37), and GFP-tagged Vph1p exhibits similar vacuolar membrane localization (38). Consistent with these studies, we observed that GFP-tagged Vph1p is localized to the vacuolar membrane in WT cells (Fig. 5A). We expected that Vph1–GFP would be localized at the vacuolar membrane in the yeast V1 subunit mutants because the Vo domain is assembled at the ER and Golgi, independently of the V1 domain (2). As expected, we observed localization of Vph1–GFP at the vacuolar membrane in all of the V1 subunit mutants, except for the vma7Δ mutant in which Vph1p was mis-localized at the ER labeled by the mCherry–HDEL ER marker, which contains the tetrapeptide ER-targeting signal (Fig. 5A and Fig. S2) (39). Quantitative analysis categorizing Vph1–GFP localization as vacuolar membrane only, vacuolar membrane and ER, or ER (including intravacuolar localization) revealed that vma7Δ mutation causes complete mis-localization of Vph1–GFP to the ER, whereas other V1 subunit mutants exhibit normal vacuolar membrane localization (Fig. 5C). Because Vma7p (ScF) is thought to be a subunit of the V1 domain, this result was surprising and suggested a role for the F subunit in the assembly of the Vo domain. Consistent with the growth phenotype, expression of the V1F subunit partially restored the localization of Vph1–GFP to the vacuolar membrane (Fig. 5, B and D).
Figure 5.
Localization of Vo subunit in yeast V-ATPase subunit mutants expressing the equivalent human gene. A and B, localization of Vph1–GFP in yeast V-ATPase V1 subunit mutants (A) and Vo subunit mutants (B) expressing the human equivalent genes. Cells expressing Vph1–GFP and HDEL–mCherry (mCH) were grown to early logarithmic phase in YPD medium at 25 °C and observed by epifluorescence and differential interference contrast (DIC) microscopy. All images were taken with 1-s exposure time under the same conditions. C, quantification of localization of Vph1–GFP. Data show the mean of at least two experiments, with >50 Vph1–GFP-labeled cells counted per experiment (green, vacuolar membrane (Vac); yellow, vacuolar membrane and endoplasmic reticulum (Vac. and ER); red, ER including intravacuolar localization). D, localization of GFP-tagged human VoC protein. Cells expressing GFP-tagged protein and HDEL–mCherry (mCH) were grown and observed as described above. E and F, localization and expression of V5-tagged human VoC protein in the vma3Δ mutant. E, cells were grown to early logarithmic phase, fixed with paraformaldehyde, converted to spheroplasts, and stained with anti-V5 tag antibody. V5-tagged proteins were subsequently visualized with Alexa Fluor 488-conjugated anti-mouse IgG. F, 10 μg of whole-cell extracts from each strain were loaded per lane and immunoblotted with an anti-V5 tag antibody.
A previous study reported that Vph1p becomes a short-lived ER membrane protein and is rapidly degraded when the Vo complex cannot be properly assembled due to the loss of Vma3p or Vma12p (40). Consistent with this observation, we found that in all strains lacking Vo subunits Vph1–GFP was mis-localized to the ER (Fig. 5B). This indicates that each Vo subunit is necessary for proper assembly of the Vo domain at the ER and for subsequent transport to the vacuolar membrane. We found that expression of VoB or VoE from the native or the TPI1 promoter did not restore Vph1–GFP localization, although VoC was able to partially restore localization when expressed from the TPI1 promoter (Fig. 5, B and C). In addition to the ER localization, we observed that Vph1–GFP is localized at the vacuole in vma3Δ, vma7Δ, vma9Δ, and vma16Δ mutants (Fig. 5, A and B), indicating that Vph1–GFP is degraded in these strains, as reported previously (40). To confirm that each human subunit is expressed in these strains, we tagged each human subunit with GFP and observed the resulting localization. A previous study showed that the C-terminal pHluorin tagging of human VoC expresses as a functional subunit in mouse hippocampal neuron (41). Because pHluorin is a GFP variant, GFP fusion to the human VoC subunit appears to be functional in human cell. When being expressed in the vma3Δ mutant, GFP-tagged VoC was ectopically localized to the ER and the vacuole (Fig. 5D), similar to Vph1–GFP. Although the functionalities of GFP-tagged VoB and VoD have not been reported in the human cell, GFP-tagged VoB and VoE also showed similar localization to VoC–GFP (Fig. S2B). Thus, these human subunits were translated and targeted to the ER. To further confirm the expression and localization of the human VoC subunit in yeast, we expressed the C-terminally V5-epitope–tagged VoC in the vma3Δ mutant, because it was reported that the V5-tagged VoC is functionally expressed in human MDA–MB231 breast cancer cells (42). Immunostaining of V5-tagged human VoC showed similar fluorescent localization to VoC–GFP (Fig. 5E), and immunoblot analysis indicated the correct size of VoC is expressed in the vma3Δ mutant (Fig. 5F). These results further suggest that human VoC, VoB, and VoE were expressed in yeast but were unable to complement the functions of each equivalent yeast subunit. Importantly, in the vma6Δ mutant, expression of the VoD1 subunit restored the localization of Vph1–GFP almost completely upon expression from both the native and the TPI1 promoter (Fig. 5, B and C), consistent with the growth phenotypes (Fig. 2, A and B).
We also examined localization of the V1 domain by tagging Vma1p, yeast subunit A, with GFP. A previous study had shown that the V1 domain is assembled in the cytosol and then becomes attached to the assembled Vo domain at the vacuolar membrane. In WT cells, Vma1–GFP was localized at the vacuolar membrane (Fig. 6A), as reported previously (43), whereas it was localized in the cytosol in all of the vma/vph1 mutants (Fig. 6, B and C). Among these mutants, those with deletion of Vma5p (ScC) or Vma13p (ScH), both of which are stator subunits, showed partial localization of Vma1–GFP at the vacuolar membrane, in addition to the cytosol (Fig. 6B). Consistent with evaluation using Ste2–pHluorin, strains with replacement of the V1C1, V1D, V1H, or VoD1 subunit showed partial recovery of Vma1–GFP localization at the vacuolar membrane when expressed from the native promoter and almost complete recovery when expressed from the TPI1 promoter (Fig. 6, B and D). Similarly, in the strains with replacement of the V1E2 or V1F subunit, localization of Vma1–GFP was partially restored (Fig. 6, B and D). In contrast, in the strains with replacement of V1B2, V1G1, VoB, or VoE, the Vma1–GFP was mostly diffused in the cytosol (Fig. 6, B–D), consistent with defective growth and the level of vacuolar acidification.
Figure 6.
Localization of V1 subunit in yeast V-ATPase subunit mutants expressing the equivalent human gene. A–C, localization of Vma1–GFP or Vma2–GFP in WT (A), yeast V-ATPase V1 subunit mutants (B), and Vo subunit mutants (C) expressing the human equivalent genes. Cells expressing Vma1–GFP or Vma2–GFP were grown to early logarithmic phase in YPD medium at 25 °C and observed by epifluorescence and differential interference contrast (DIC) microscopy. D, quantification of localization of Vma1–GFP or Vma2–GFP. Data show the mean of at least two experiments, with >50 Vph1–GFP-labeled cells counted per experiment (green, vacuolar membrane (Vac); yellow, vacuolar membrane and cytosol (Vac and Cyt); red, cytosol (Cyt)).
Functional complementation between yeast and human V-ATPase subunits for endocytic recycling of Wsc1p
We then investigated whether replacement of yeast subunits with their human equivalents would be able to complement the function of V-ATPase in the protein-recycling pathway. In our previous study, we found that deletion of V-ATPase subunits led to mis-localization of Wsc1p from the plasma membrane to the vacuole (34). Wsc1p is a major cell wall sensor protein localized at the polarized cell surface, and its localization is maintained by endocytosis and recycling from endosomes back to the cell surface (44). For this purpose, we chose two V1 subunit-replaced strains that exhibited different complementation levels: one strain with replacement of the E subunit exhibits moderate complementation, and the other with replacement of the D subunit exhibits high complementation. We also chose one strain with replacement of the Vo subunit (d subunit), which exhibits high complementation. As reported previously, Wsc1p was localized at the plasma membrane in WT cells, but it was mis-localized to the vacuole in the vma4Δ, vma6Δ, or vma8Δ mutant (Fig. 7A). Consistent with the results based on the fluorescence intensity of Ste2–pHluorin, localization in the vma4Δ mutant was slightly restored when expressed from the native promoter and was mostly restored when expressed from the TPI1 promoter (Fig. 7, A and B). Similarly, in the vma6Δ and vma8Δ mutants, expression of each human subunit mostly or almost completely led to recovery of Wsc1–3GFP localization at the plasma membrane (Fig. 7, A and B). These results clearly indicated that human subunits were able to complement the physiological function of yeast V-ATPase subunits in the protein-recycling pathway.
Discussion
In this study, we evaluated functional complementation between yeast and human subunits of V-ATPase in terms of 1) growth ability on media containing a high-calcium concentration or elevated pH values; 2) vacuolar acidification; 3) localization of the V1 and Vo subunits, and 4) endocytic recycling of Wsc1–3GFP. Overall, our results were well-correlated with growth ability, although evaluation in terms of vacuolar acidification or Vma1–GFP localization appeared to be more sensitive. A previous study using yeast vma1 mutants showed that mutant vma1–40 possessing ∼25% of WT V-ATPase activity was able to grow on media buffered to pH 7.5 (45). The vma5 mutants, which possess as little as 35% of WT V-ATPase activity, were also reported to be capable of growth on media containing 60 mm CaCl2 (46). These observations, taken together with our present results, indicate that although comparison of growth ability is reliable and valid, it is not sufficiently precise for evaluation of V-ATPase function. As a more sensitive method for assessment of V-ATPase function in yeast, the lipophilic fluorescent amine quinacrine, which crosses membranes in uncharged form and is retained in the vacuole upon protonation, has been widely used (47). Quinacrine staining is the simplest method for assessment of vacuolar acidity, but it appears unsuitable for quantitative analysis because it is not visible in vacuoles with increased pH values (47). In this study, we therefore used the pHluorin-fused Ste2p as an effective quantitative reporter of vacuolar acidification (8, 34). In contrast to quinacrine, super-ecliptic pHluorin displays a reversible excitation ratio change between pH 7.5 and 5.5, and it gradually loses its fluorescence as the pH value is lowered (33). Vacuolar pH in yeast has been shown to vary from ∼5 to 6.5 in WT cells and ∼6.6 to 7.2 in the vma2Δ mutant depending on growth conditions (12, 48), and therefore, pHluorin is suitable for quantitative monitoring of vacuolar pH.
As an alternative method, we expressed GFP-tagged Vma1p in each of the strains. In WT cells, GFP–Vma1p was localized at the vacuolar membrane, whereas in a mutant lacking functional V-ATPase, it was localized in the cytosol, suggesting that each of the individual V-ATPase subunits is necessary for Vma1p localization at the vacuole. Previous studies have revealed that the V1 complex is assembled independently of the Vo complex and then binds to the fully assembled Vo domain at the Golgi (1, 49, 50). Therefore, although Vma1–GFP showed cytosolic localization in mutants lacking Vo subunits, Vma1p was able to form a complex with other V1 subunits. Localization of Vma1–GFP at the vacuolar membrane is mostly consistent with the level of vacuolar acidification, suggesting that evaluation of V-ATPase function in terms of Vma1–GFP localization is as sensitive as evaluation using Ste2–pHluorin.
In addition to its role in the vacuolar membrane, V-ATPase activity is necessary for several different steps of intracellular vesicle transport. For example, acidification of sorting endosomes by V-ATPase triggers uncoupling of internalized ligand–receptor complexes and facilitates recycling of unoccupied receptors back to the plasma membrane (2). In addition, a recent study has demonstrated that V-ATPase–dependent luminal acidification appears to be critical for cargo sorting at early–to–late endosomes. Here, we demonstrated that disruption of V-ATPase subunits caused defects of Wsc1p trafficking from the endosome to the plasma membrane (34), whereas expression of the V1H, VoD1, or V1E2 subunit in each yeast mutant restored the endocytic recycling of Wsc1p. This observation suggests that V-ATPase–dependent luminal acidification in early–to–late endosomes in vma mutants is also restored by expression of equivalent human subunits.
ATP hydrolysis occurs at catalytic sites located at the interface of the A and B subunits (Fig. 7C) (51, 52), three copies of which are present per complex and are arranged in an alternating manner in a ring. In this study, we substituted the ScA or the ScB subunit with each human equivalent, but neither was able to complement the function. Sequence comparison between ScB and V1B2 or ScA and V1A revealed that although the overall identity of the A subunit is only 37%, that of the B subunit is significantly high (71%). Additionally, the ScA subunit contains an extra 450 amino acids that form an extra β-sheet structure (28, 53), compared with the V1A subunit, in the central region, and therefore it seems reasonable that the V1A subunit would not form a proper complex with the ScB subunit. Accordingly, simultaneous substitution of the V1A and V1B2 subunits might be able to complement the function of the yeast catalytic domain.
Consistent with a previous study showing that overexpression of the V1H subunit complemented deletion of the H subunit in yeast (54), we found that expression of the V1H subunit from either the native or the TPI1 promoter largely complemented the vacuolar acidification and localization of the V1 domain. However, we demonstrated that expression of the V1H subunit, and even its overexpression, was unable to complement fully the growth of the vma13Δ mutant. One possible explanation might be that regulation of V-ATPase activity is disrupted when the ScH subunit is substituted by the V1H subunit. The ScH subunit comprises two domains: the N-terminal domain, which is required for activation of the complex, and the C-terminal domain, which is required for coupling of ATP hydrolysis to proton translocation (55). The H subunit also inhibits MgATPase activity in membrane-detached yeast V1 (56). The molecular mechanism by which the H subunit silences MgATPase activity in free V1 is still not well-understood, but a recent study has revealed that the inhibitory interactions between the H subunit and the catalytic core are mediated by a loop in the C-terminal region of H (29). Interestingly, a chimeric H subunit construct containing ScHNT and HsHct complemented the ScH deletion phenotype, but it failed to silence MgATPase activity (29), probably because the loop present in the ScH subunit is absent in the V1H subunit. Additionally, this ScH subunit loop mutant complemented the growth phenotype of the strain with the H subunit deletion, but it did not silence MgATPase activity. These observations indicate that the nonconserved (yeast-specific) loop is essential for silencing of isolated ScV1. Therefore, chimera yeast V-ATPase containing the V1H subunit is highly activated, causing vacuolar acidification, even though growth is not fully complemented.
Subunits of the human Vo domain, except for the d subunit, were unable to complement their equivalent yeast subunits. We demonstrated that all of the human c, c″, and e subunits were mislocalized to the ER membrane. Because the Vo domain is assembled at the ER membrane and then transported to the Golgi, and then to the vacuolar membrane, our results suggest that assembly or transport of the Vo domain at the ER is defective in these mutants. In the process of Vo assembly, several assembly factors, such as Vma12p, Vma21p, Vma22p, and Pkr1p, are known to be required (2). Vma21p promotes assembly of the proteolipid subunits into a ring and binding of subunit d to the cytoplasmic side of this ring, and Vma12p and Vma22p interact transiently with subunit a and mediate its assembly with the proteolipid ring (31). Human orthologs of these assembly factors, VMA21, TMEM199, and CCDC115, have been identified (57, 58), and co-expression of these proteins with human Vo subunits might be needed for proper assembly of the chimeric Vo domain at the ER in yeast.
Experimental procedures
Yeast strains, growth conditions, and plasmids
The yeast strains used in this study are listed in Table S1. All strains were grown in standard rich medium (YPD) or synthetic medium (SM) supplemented with 2% glucose and appropriate amino acids. PCR-based integration of GFP gene at the 3′ end of VMA1, VPH1, or WSC1 gene was used to generate strains expressing a C-terminal GFP fusion protein under the control of its native promoter (59). Expression plasmids containing the promoter of the TPI1 gene or yeast V-ATPase subunit genes were created as follows: 500-bp of STE2 3′UTR was amplified by PCR with a forward primer containing EcoRI and BamHI sites and a reverse primer containing XhoI site, and was cloned into the EcoI and XhoI sites of pRS316 (pRS316–STE2 3′UTR). To create the expression plasmid containing the TPI1 gene promoter, 412 bp of the TPI1 5′ UTR was amplified by PCR with a forward primer containing the SacI site and a reverse primer containing the EcoRI site and was cloned into the SacI and EcoRI sites of pRS316–STE2 3′UTR (pRS316–TPI1 5′UTR-(EcoRI, BamHI)-STE2 3′UTR). To create the expression plasmids containing the yeast V-ATPase gene promoters, the 5′UTR fragments of the yeast V-ATPase subunit gene were amplified by PCR with primers listed in Table S2 and were cloned into SacI and EcoRI sites of pRS316–STE2 3′UTR (pRS316–VMA 5′UTR-(EcoRI, BamHI)-STE2 3′UTR). Then, gene fragments of human V-ATPase subunits were amplified by PCR with primers listed in Table S2 and were cloned into the EcoRI, BamHI, or EcoRI and BamHI-digested pRS316–TPI1 5′UTR-(EcoRI, BamHI)-STE2 3′UTR or pRS316–VMA 5′UTR-(EcoRI, BamHI)-STE2 3′UTR.
Fluorescence microscopy
Fluorescence microscopy was performed using an Olympus IX81 microscope equipped with a ×100/NA 1.40 (Olympus) objective and an Orca-AG cooled CCD camera (Hamamatsu), using Metamorph software (Universal Imaging). Simultaneous imaging of red and green fluorescence was performed using an Olympus IX81 microscope, as described above, and an image splitter (Dual-View; Optical Insights) that divided the red and green components of the images with a 565-nm dichroic mirror and passed the red component through a 630/50-nm filter and the green component through a 530/30-nm filter. FM4-64 staining was performed as described previously (35).
Author contributions
M. A., M. S., A. T., S. S., K. U., H. S., and M. N. data curation; M. A., M. S., A. T., S. S., K. U., H. S., M. N., J. Y. T., and J. T. formal analysis; M. A., M. N., and J. T. investigation; M. A. and J. T. methodology; J. Y. T. and J. T. conceptualization; J. Y. T. and J. T. supervision; J. Y. T. and J. T. funding acquisition; J. Y. T. and J. T. writing-original draft; J. T. validation.
Supplementary Material
Acknowledgments
We thank Beverly Wendland for kindly providing the pHluorin plasmid. We thank Dr. Ichiro Yamato for helpful advice and the members of the Toshima lab for sharing materials and for helpful discussions.
This work was supported by JSPS KAKENHI Grant 18K06229, the Takeda Science Foundation, the Novartis Foundation, Japan (to J. Y. T.), JSPS KAKENHI Grant 6K07303, the Uehara Memorial Foundation, and the Takeda Science Foundation (to J. T.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1 and S2 and Tables S1 and S2.
- V-ATPase
- vacuolar-type H+-ATPase
- ER
- endoplasmic reticulum
- ANOVA
- analysis of variance.
References
- 1. Kane P. M. (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol. Mol. Biol. Rev. 70, 177–191 10.1128/MMBR.70.1.177-191.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- 3. Mellman I., Fuchs R., and Helenius A. (1986) Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55, 663–700 10.1146/annurev.bi.55.070186.003311 [DOI] [PubMed] [Google Scholar]
- 4. Anderson R. G., and Pathak R. K. (1985) Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell 40, 635–643 10.1016/0092-8674(85)90212-0 [DOI] [PubMed] [Google Scholar]
- 5. Mellman I. (1996) Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 10.1146/annurev.cellbio.12.1.575 [DOI] [PubMed] [Google Scholar]
- 6. Orlowski J., and Grinstein S. (2004) Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 447, 549–565 10.1007/s00424-003-1110-3 [DOI] [PubMed] [Google Scholar]
- 7. Mellman I. (1992) The importance of being acid: the role of acidification in intracellular membrane traffic. J. Exp. Biol. 172, 39–45 [DOI] [PubMed] [Google Scholar]
- 8. Prosser D. C., Whitworth K., and Wendland B. (2010) Quantitative analysis of endocytosis with cytoplasmic pHluorin chimeras. Traffic 11, 1141–1150 10.1111/j.1600-0854.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peña A., Ramírez J., Rosas G., and Calahorra M. (1995) Proton pumping and the internal pH of yeast cells, measured with pyranine introduced by electroporation. J. Bacteriol. 177, 1017–1022 10.1128/jb.177.4.1017-1022.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plant P. J., Manolson M. F., Grinstein S., and Demaurex N. (1999) Alternative mechanisms of vacuolar acidification in H+-ATPase-deficient yeast. J. Biol. Chem. 274, 37270–37279 10.1074/jbc.274.52.37270 [DOI] [PubMed] [Google Scholar]
- 11. Brett C. L., Tukaye D. N., Mukherjee S., and Rao R. (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 16, 1396–1405 10.1091/mbc.e04-11-0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez-Muñoz G. A., and Kane P. (2008) Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J. Biol. Chem. 283, 20309–20319 10.1074/jbc.M710470200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyomura T., Murata Y., Yamamoto A., Oka T., Sun-Wada G. H., Wada Y., and Futai M. (2003) From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J. Biol. Chem. 278, 22023–22030 10.1074/jbc.M302436200 [DOI] [PubMed] [Google Scholar]
- 14. Pietrement C., Sun-Wada G. H., Silva N. D., McKee M., Marshansky V., Brown D., Futai M., and Breton S. (2006) Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol. Reprod. 74, 185–194 10.1095/biolreprod.105.043752 [DOI] [PubMed] [Google Scholar]
- 15. Karet F. E., Finberg K. E., Nelson R. D., Nayir A., Mocan H., Sanjad S. A., Rodriguez-Soriano J., Santos F., Cremers C. W., Di Pietro A., Hoffbrand B. I., Winiarski J., Bakkaloglu A., Ozen S., Dusunsel R., et al. (1999) Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat. Genet. 21, 84–90 10.1038/5022 [DOI] [PubMed] [Google Scholar]
- 16. Inoue H., Noumi T., Nagata M., Murakami H., and Kanazawa H. (1999) Targeted disruption of the gene encoding the proteolipid subunit of mouse vacuolar H+-ATPase leads to early embryonic lethality. Biochim. Biophys. Acta 1413, 130–138 10.1016/S0005-2728(99)00096-1 [DOI] [PubMed] [Google Scholar]
- 17. Thudium C. S., Jensen V. K., Karsdal M. A., and Henriksen K. (2012) Disruption of the V-ATPase functionality as a way to uncouple bone formation and resorption–a novel target for treatment of osteoporosis. Curr. Protein Pept. Sci. 13, 141–151 10.2174/138920312800493133 [DOI] [PubMed] [Google Scholar]
- 18. Smith A. N., Skaug J., Choate K. A., Nayir A., Bakkaloglu A., Ozen S., Hulton S. A., Sanjad S. A., Al-Sabban E. A., Lifton R. P., Scherer S. W., and Karet F. E. (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat. Genet. 26, 71–75 10.1038/79208 [DOI] [PubMed] [Google Scholar]
- 19. Sun-Wada G. H., Toyomura T., Murata Y., Yamamoto A., Futai M., and Wada Y. (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J. Cell Sci. 119, 4531–4540 10.1242/jcs.03234 [DOI] [PubMed] [Google Scholar]
- 20. Sennoune S. R., Bakunts K., Martínez G. M., Chua-Tuan J. L., Kebir Y., Attaya M. N., and Martínez-Zaguilán R. (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am. J. Physiol. Cell Physiol. 286, C1443–C1452 10.1152/ajpcell.00407.2003 [DOI] [PubMed] [Google Scholar]
- 21. Fais S., De Milito A., You H., and Qin W. (2007) Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 67, 10627–10630 10.1158/0008-5472.CAN-07-1805 [DOI] [PubMed] [Google Scholar]
- 22. Sennoune S. R., Luo D., and Martínez-Zaguilán R. (2004) Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem. Biophys. 40, 185–206 10.1385/CBB:40:2:185 [DOI] [PubMed] [Google Scholar]
- 23. Nelson H., and Nelson N. (1990) Disruption of genes encoding subunits of yeast vacuolar H+-ATPase causes conditional lethality. Proc. Natl. Acad. Sci. U.S.A. 87, 3503–3507 10.1073/pnas.87.9.3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohya Y., Umemoto N., Tanida I., Ohta A., Iida H., and Anraku Y. (1991) Calcium-sensitive cls mutants of Saccharomyces cerevisiae showing a Pet− phenotype are ascribable to defects of vacuolar membrane H+-ATPase activity. J. Biol. Chem. 266, 13971–13977 [PubMed] [Google Scholar]
- 25. Yamashiro C. T., Kane P. M., Wolczyk D. F., Preston R. A., and Stevens T. H. (1990) Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol. Cell. Biol. 10, 3737–3749 10.1128/MCB.10.7.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawasaki-Nishi S., Nishi T., and Forgac M. (2001) Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J. Biol. Chem. 276, 17941–17948 10.1074/jbc.M010790200 [DOI] [PubMed] [Google Scholar]
- 27. Manolson M. F., Wu B., Proteau D., Taillon B. E., Roberts B. T., Hoyt M. A., and Jones E. W. (1994) STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J. Biol. Chem. 269, 14064–14074 [PubMed] [Google Scholar]
- 28. Zhao J., Benlekbir S., and Rubinstein J. L. (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521, 241–245 10.1038/nature14365 [DOI] [PubMed] [Google Scholar]
- 29. Oot R. A., Kane P. M., Berry E. A., and Wilkens S. (2016) Crystal structure of yeast V1-ATPase in the autoinhibited state. EMBO J. 35, 1694–1706 10.15252/embj.201593447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawamura S., Nagano M., Toshima J. Y., and Toshima J. (2014) Analysis of subcellular localization and function of the yeast Rab6 homologue, Ypt6p, using a novel amino-terminal tagging strategy. Biochem. Biophys. Res. Commun. 450, 519–525 10.1016/j.bbrc.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 31. Graham L. A., Flannery A. R., and Stevens T. H. (2003) Structure and assembly of the yeast V-ATPase. J. Bioenerg. Biomembr. 35, 301–312 10.1023/A:1025772730586 [DOI] [PubMed] [Google Scholar]
- 32. Toei M., Saum R., and Forgac M. (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49, 4715–4723 10.1021/bi100397s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miesenböck G., De Angelis D. A., and Rothman J. E. (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 10.1038/28190 [DOI] [PubMed] [Google Scholar]
- 34. Ueno K., Saito M., Nagashima M., Kojima A., Nishinoaki S., Toshima J. Y., and Toshima J. (2014) V-ATPase-dependent luminal acidification is required for endocytic recycling of a yeast cell wall stress sensor, Wsc1p. Biochem. Biophys. Res. Commun. 443, 549–555 10.1016/j.bbrc.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 35. Toshima J., Toshima J. Y., Martin A. C., and Drubin D. G. (2005) Phosphoregulation of Arp2/3-dependent actin assembly during receptor-mediated endocytosis. Nat. Cell Biol. 7, 246–254 10.1038/ncb1229 [DOI] [PubMed] [Google Scholar]
- 36. Toshima J. Y., Nishinoaki S., Sato Y., Yamamoto W., Furukawa D., Siekhaus D. E., Sawaguchi A., and Toshima J. (2014) Bifurcation of the endocytic pathway into Rab5-dependent and -independent transport to the vacuole. Nat. Commun. 5, 3498 10.1038/ncomms4498 [DOI] [PubMed] [Google Scholar]
- 37. Raymond C. K., Howald-Stevenson I., Vater C. A., and Stevens T. H. (1992) Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3, 1389–1402 10.1091/mbc.3.12.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finnigan G. C., Hanson-Smith V., Houser B. D., Park H. J., and Stevens T. H. (2011) The reconstructed ancestral subunit a functions as both V-ATPase isoforms Vph1p and Stv1p in Saccharomyces cerevisiae. Mol. Biol. Cell 22, 3176–3191 10.1091/mbc.e11-03-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gomord V., Denmat L. A., Fitchette-Lainé A. C., Satiat-Jeunemaitre B., Hawes C., and Faye L. (1997) The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J. 11, 313–325 10.1046/j.1365-313X.1997.11020313.x [DOI] [PubMed] [Google Scholar]
- 40. Jackson D. D., and Stevens T. H. (1997) VMA12 encodes a yeast endoplasmic reticulum protein required for vacuolar H+-ATPase assembly. J. Biol. Chem. 272, 25928–25934 10.1074/jbc.272.41.25928 [DOI] [PubMed] [Google Scholar]
- 41. Bodzȩta A., Kahms M., and Klingauf J. (2017) The presynaptic v-ATPase reversibly disassembles and thereby modulates exocytosis but is not part of the fusion machinery. Cell Rep. 20, 1348–1359 10.1016/j.celrep.2017.07.040 [DOI] [PubMed] [Google Scholar]
- 42. Cotter K., Capecci J., Sennoune S., Huss M., Maier M., Martinez-Zaguilan R., and Forgac M. (2015) Activity of plasma membrane V-ATPases is critical for the invasion of MDA–MB231 breast cancer cells. J. Biol. Chem. 290, 3680–3692 10.1074/jbc.M114.611210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tabke K., Albertmelcher A., Vitavska O., Huss M., Schmitz H. P., and Wieczorek H. (2014) Reversible disassembly of the yeast V-ATPase revisited under in vivo conditions. Biochem. J. 462, 185–197 10.1042/BJ20131293 [DOI] [PubMed] [Google Scholar]
- 44. Piao H. L., Machado I. M., and Payne G. S. (2007) NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol. Biol. Cell 18, 57–65 10.1091/mbc.e06-08-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu J., and Kane P. M. (1996) Mutational analysis of the catalytic subunit of the yeast vacuolar proton-translocating ATPase. Biochemistry 35, 10938–10948 10.1021/bi9608065 [DOI] [PubMed] [Google Scholar]
- 46. Curtis K. K., Francis S. A., Oluwatosin Y., and Kane P. M. (2002) Mutational analysis of the subunit C (Vma5p) of the yeast vacuolar H+-ATPase. J. Biol. Chem. 277, 8979–8988 10.1074/jbc.M111708200 [DOI] [PubMed] [Google Scholar]
- 47. Weisman L. S., Bacallao R., and Wickner W. (1987) Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J. Cell Biol. 105, 1539–1547 10.1083/jcb.105.4.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Padilla-López S., and Pearce D. A. (2006) Saccharomyces cerevisiae lacking Btn1p modulate vacuolar ATPase activity to regulate pH imbalance in the vacuole. J. Biol. Chem. 281, 10273–10280 10.1074/jbc.M510625200 [DOI] [PubMed] [Google Scholar]
- 49. Doherty R. D., and Kane P. M. (1993) Partial assembly of the yeast vacuolar H+-ATPase in mutants lacking one subunit of the enzyme. J. Biol. Chem. 268, 16845–16851 [PubMed] [Google Scholar]
- 50. Tomashek J. J., Sonnenburg J. L., Artimovich J. M., and Klionsky D. J. (1996) Resolution of subunit interactions and cytoplasmic subcomplexes of the yeast vacuolar proton-translocating ATPase. J. Biol. Chem. 271, 10397–10404 10.1074/jbc.271.17.10397 [DOI] [PubMed] [Google Scholar]
- 51. Liu Q., Leng X. H., Newman P. R., Vasilyeva E., Kane P. M., and Forgac M. (1997) Site-directed mutagenesis of the yeast V-ATPase A subunit. J. Biol. Chem. 272, 11750–11756 10.1074/jbc.272.18.11750 [DOI] [PubMed] [Google Scholar]
- 52. Liu Q., Kane P. M., Newman P. R., and Forgac M. (1996) Site-directed mutagenesis of the yeast V-ATPase B subunit (Vma2p). J. Biol. Chem. 271, 2018–2022 10.1074/jbc.271.4.2018 [DOI] [PubMed] [Google Scholar]
- 53. Maher M. J., Akimoto S., Iwata M., Nagata K., Hori Y., Yoshida M., Yokoyama S., Iwata S., and Yokoyama K. (2009) Crystal structure of A3B3 complex of V-ATPase from Thermus thermophilus. EMBO J. 28, 3771–3779 10.1038/emboj.2009.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu X., Yu H., Liu S. H., Brodsky F. M., and Peterlin B. M. (1998) Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8, 647–656 10.1016/S1074-7613(00)80569-5 [DOI] [PubMed] [Google Scholar]
- 55. Liu M., Tarsio M., Charsky C. M., and Kane P. M. (2005) Structural and functional separation of the N- and C-terminal domains of the yeast V-ATPase subunit H. J. Biol. Chem. 280, 36978–36985 10.1074/jbc.M505296200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parra K. J., Keenan K. L., and Kane P. M. (2000) The H subunit (Vma13p) of the yeast V-ATPase inhibits the ATPase activity of cytosolic V1 complexes. J. Biol. Chem. 275, 21761–21767 10.1074/jbc.M002305200 [DOI] [PubMed] [Google Scholar]
- 57. Miles A. L., Burr S. P., Grice G. L., and Nathan J. A. (2017) The vacuolar-ATPase complex and assembly factors, TMEM199 and CCDC115, control HIF1α prolyl hydroxylation by regulating cellular iron levels. Elife 6, e22693 10.7554/eLife.22693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramachandran N., Munteanu I., Wang P., Ruggieri A., Rilstone J. J., Israelian N., Naranian T., Paroutis P., Guo R., Ren Z. P., Nishino I., Chabrol B., Pellissier J. F., Minetti C., Udd B., et al. (2013) VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy. Acta Neuropathol. 125, 439–457 10.1007/s00401-012-1073-6 [DOI] [PubMed] [Google Scholar]
- 59. Longtine M. S., McKenzie A. 3rd., Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., and Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.