ABSTRACT
Tumor-infiltrating lymphocytes (TILs) are associated with prognosis in various tumors. However, it remains controversial whether the presence of TILs is related to an improved prognosis in melanoma. This meta-analysis confirmed the favorable prognostic role of the CD3+, CD4+, CD8+, FOXP3+, and CD20+ TILs in the overall survival of melanoma patients and found an association between the TILs present and improved overall survival. Additionally, subgroup analysis demonstrated that brisk TILs were obviously associated with OS, RFS and DSS/MSS. Thus, TILs may be a predictive biomarker in melanoma. This analysis will provide more insight into the study of TILs and predictive biomarker.
KEYWORDS: Melanoma, prognostic biomarkers, systematic review, tumor-infiltrating lymphocytes (TILs)
Introduction
Despite the advances in global healthcare, malignant tumors remain a major threat to human health. Malignant melanoma is the most aggressive form of skin cancer with unpredictable behavior. Although multiple approaches have been used to treat melanoma, the mortality of melanoma patients has barely improved over the last decades.1–3 Thus, in addition to Breslow thickness, Clark level, Sentinel lymph node biopsy and serum LDH level, early prediction of the patient prognosis using biomarkers is also a powerful weapon against melanoma.
Melanoma is considered a highly immunogenic tumor, responsive to immunologic manipulation, and the role of the immune response in melanoma has gained much attention in recent years.4,5 To identify the accurately predictive biomarkers, much focus has been on the biologic properties of cancer cells and immune response in their microenvironment.6 Tumor-infiltrating lymphocytes (TILs) are a local histopathological reflection of the host’s immune response against cancer cells. Currently, TILs have gained increasing attention in the treatment and prognosis prediction of melanoma.7–9
Many studies have indicated that TILs are a favorable prognostic factor for melanoma patients, and the presence of TILs might lead to a better prognosis.10,11 However, some studies did not reveal a significant association between TILs and melanoma prognosis, different TIL responses or subsets have different functions in melanoma.8,9 Thus, there is currently no conclusive evidence regarding whether TILs are a robust prognostic factor in melanoma. The conflicting reports on the prognostic value of TILs in melanoma may be due to the heterogeneity of clinical and histologic subtypes, different patient populations, and different TIL grading systems.
Some studies have indicated a distinct correlation between different TIL intensities or grades and different prognosis values; the higher presence of TILs or brisk TILs predict a lower rate of sentinel lymph node (SLN) metastases or favorable survival in melanoma.11 Considering the great heterogeneity of TILs and their number and distribution within the tumor, most groups have quantified TILs as absent, non-brisk and brisk,12–16 while others have quantified TILs as absent, mild or scanty, moderate and marked,11,17,18 moreover, as the TIL grade increases, the hazard ratio of melanoma patient survival is different. However, different subsets of TILs have different or even opposing functions in the tumors.
As a heterogenous group, TILs are implicated in not only effector T cells but also in functionally exhausted T cells, tolerogenic or T regulatory (Treg) cells, dendritic cells (DCs), natural killer (NK) cells, myeloid-derived suppressor cells (MDSCs), macrophages, and other immune cell types.8,19 As the main antitumor effector cells,20 CD8+ T lymphocytes comprise the majority of TILs and have been linked to a better prognosis in several types of cancer.21–23 According to previous reports, CD4+ T lymphocytes exert different functions.24 Both CD4+ Th1 cells and CD4+ Th2 are related to anti-tumor immunity;25 however, CD4+ regulatory T cells were observed to inhibit an effective anti-tumor immune response.26 On the other hand, in the adaptive response, the MHC class I and II molecules processed the tumor-associated antigens (TAA) from APCs to the specific receptors of CD8+ and CD4+ T-cells for their activation.27 Additionally, CD3+ T-cell infiltration into the primary tumor has also been observed as an excellent early predictor of longer survival in metastatic melanoma patients receiving DC-based immunotherapy.28 Thus, better understanding of the TIL phenotype and function in tumor or the tumor microenvironment is crucial for early prognosis prediction.
Treg cells are the main population involved in maintaining peripheral tolerance.26 As the most specific Treg marker,29 FOXP3 expression has been shown to correlate with a poor prognosis in various types of human cancer, including breast cancer and gastrointestinal cancers.30,31 However, a better prognostic value of Tregs has also been observed in head and neck squamous cell carcinoma.32,33 For melanoma, most previous studies have suggested that FOXP3+ cells are associated with a favorable prognosis;34 inconsistently, the controversial results have also been observed by others.35,36
In this study, we systematically reviewed the articles for publication about the prognostic roles of TIL responses and CD3+, CD4+, CD8+, FOXP3+, and CD20+ TIL subsets in the prognosis of melanoma. We aimed to include all studies that assessed tumor infiltration with TIL grades, CD3+, CD4+, CD8+ and FOXP3+ lymphocytes between these markers as a prognostic biomarker in melanoma.
Methods
Search strategy
A computerized search was performed that included the domain (“melanoma”), the determinant (“Tumor-infiltrating cells”, “TIL”), their synonyms, and a filter for prognostic studies.37 The publications were gained by searching the PubMed, EMBASE, Sino Med, Springer, Science Direct, The Cochrane Library, and Web of Science databases from the inception of each database to July 6, 2018. There was no restriction on the publication status; however, non-English language studies were excluded. The search strategy, as well as predetermined inclusion and exclusion criteria was based on a previous study,32 and two researchers independently screened the titles and abstracts based on the criteria. The final selection was made by full-text reading of the selected studies. Discrepancies between the two researchers were resolved by discussion and consensus from another or more researchers. The markers were assessed in two or more studies, and the reference lists of the retrieved articles were also reviewed for sufficient trials.
Inclusion criteria
According to a previous study,32 studies were included in which the prognostic value of TILs and CD3+, CD4+, CD8+, FOXP3+, and CD20+ lymphocytes was investigated in patients with melanoma. For TIL response markers, the studies in which TILs were quantified as absent, non-brisk and brisk (focal TIL infiltrate and when TILs present across the entire base or throughout the substance of the tumor)14–16 as well as absent, mild or scanty, moderate and marked (focal, multifocal, or diffuse across the entire extent of the tumor)11,17,18 were included. In this study, both the non-brisk TILs and mild or scanty TILs were defined as non-brisk TILs, the brisk TILs and marked TILs were defined as brisk TILs, but the moderate TILs were analyzed alone. There was no restriction on the detection methods, only the publications concerning lymphocytes in the tumor epithelium were included, and the studies that only investigated lymphocytes in the tumor stroma were excluded. The prognostic value had to be investigated by time-to-event survival analysis with either overall survival (OS), disease-free survival (DFS), relapse/recurrence free survival (RFS), disease-specific survival (DSS) or melanoma-specific survival (MSS). Animal studies, case reports, and commentaries were excluded.
Data extraction
The data including the author and title, year of publication, biomarker(s), sample size, tumor subsite, tumor stage, scoring methods, cutoffs, and finally outcome of univariate and multivariate analysis defined by the hazard ratio (HR), 95% confidence interval (CI), and p-values were obtained from each included publication. When these parameters were not mentioned in the article but the Kaplan-Meier curves were available, the data from the Kaplan-Meier curves were extracted and digitized using the open-source Engauge Digitizer software (http://digitizer.sourceforge.net/), and the univariate HR was estimated.38,39 When HRs were not mentioned, Kaplan-Meier curves were not available, or HRs did not match the shown Kaplan-Meier curves, the studies were excluded from the meta-analysis.
Assessment of study quality
This study is compliant with the PRISMA checklist.40 All the relevant publications were appraised for the risk of bias using the Quality and Prognosis Studies (QUIPS) criteria, a validated and useful tool for systematic reviewers for the critical appraisal of study quality.41 According to the previous study,32 the risk of bias was scored as low, moderate or high for six different domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis, and reporting. For this systematic review, there was no restriction on the treatment modality, and studies that mentioned treatment methods were rare. The risk of bias was assessed by two researchers independently. Differences were resolved by discussion.
Statistical analysis
According to a previous study,32 HRs were used that described the risk of events for high TILs versus low TILs. If the study reported the HR for low TILs vs. high TILs, the reciprocal was taken. The meta-analysis and creation of the forest plots was performed in Stata11.0 software.
Results
Study selection and basic characteristics
The PubMed, EMBASE, Sino Med, Springer, Science Direct, The Cochrane Library, and Web of Science databases search gained 5,492 hits and yielded 3,303 hits after removing duplicates (Figure 1). Of the 298 publications that remained after the screening of titles and abstract, 6 were non-English, 47 were reviews or commentaries, 4 were unavailable, and 169 were mismatched with our domain, determinant and outcomes. Therefore, 72 full-text articles were assessed, among which 41 met our inclusion criteria and were included in the final analysis.4,10–13,15–18,28,34–36,42–70 Of the excluded literature, 11 studies reported no hazard ratios or Kaplan-Meier curves; 9 studies reported the outcome only in terms of metastasis survival or metastasis-free survival; 6 studies reported the prognostic role of TILs in multiple tumors, including melanoma, and the hazard ratios or Kaplan-Meier curves in melanoma were not available; 5 studies evaluated FOXP3 expression in tumor cells instead of TILs or TILs in tumor stroma. The study characteristics of the remaining 41 studies that were included in the final meta-analysis are shown in Table 1. All the enrolled studies in our study detected TILs at the same time of pathological diagnosis or used the tissue samples of pathological diagnosis to detect TILs. Almost all studies investigated TILs by immunohistochemistry or hematoxylin- and eosin (H&E)-stained sections in paraffin-embedded tissue except the study by Knol et al.
Figure 1.
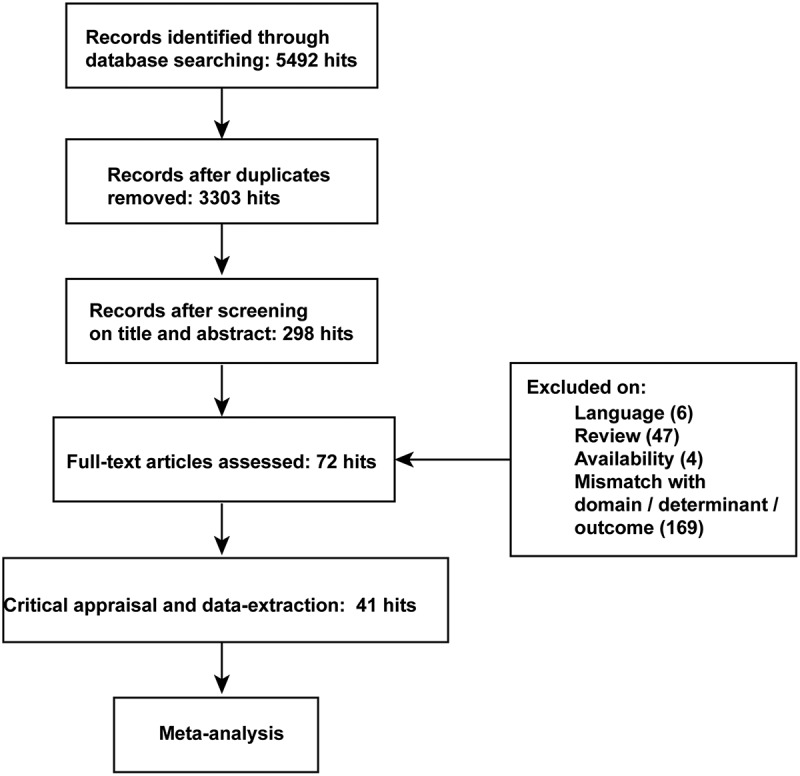
The flow diagram of studies selection and identification.
Table 1.
Study characteristics of studies included in meta-analysis. RT (Radiotherapy), CT (Chemotherapy), CRT (Chemoradiotherapy), BRAFi (BRAF inhibitor), IM (Immunotherapy), DC-V (DC vaccinations), BCT (Biochemotherapy), OS (Overall Survival), DFS (Disease-free survival), RFS (Relapse/Recurrence free survival), DSS (Disease-specific survival), MFS (Metastasis-free survival), MSS (Melanoma-specific survival), PFS (Progression-free survival), CSS(Cancer-specific survival), BMS (Brain metastases survival), CM (cutaneous melanoma), ALM (acral lentiginous melanoma), UM (uveal melanomas), AM (acral melanoma), Melanoma (M) was defined as the type of melanoma that is not explicitly stated in the studies.
| Study | Sample size | Stage | Breslow thickness | Clark level | Treatment | Biomarkers | Survival | Material | Technique | Melanoma |
|---|---|---|---|---|---|---|---|---|---|---|
| Sinnamon AJ 201816 | 1367 | ? | ≥0.76mm | ? | ? | TILs (nonbrisk, brisk) | OS | FFPE | H&E | CM |
| Letca AF 201811 | 173 | ? | all | ? | ? | TILs (Mild, Moderate, Marked) | OS, DFS | FFPE | H&E | CM |
| Balatoni T 201743 | 30 | III/IV | ? | ? | CT/CRT/BRAFi+IM | CD4, CD8, FoxP3, CD16, CD68 | OS | FFPE | IHC | CM |
| Lee WJ 201813 | 122 | all | all | ? | ? | TILs, PD1, PD-1+ TILs | OS | FFPE | IHC | CM |
| Melsted WN 201761 | 200 | ? | all | ? | ? | FoxP3 | OS, DFS, MSS | FFPE | IHC | CM |
| Castaneda CA 201744 | 537 | all | all | I-V | ? | TILs (Mild, Moderate, Marked) | OS | FFPE | H&E | ALM |
| Tas F 201765 | 750 | all | all | I-V | ? | TILs (Mild, Moderate, Marked) | OS, RFS | FFPE | H&E | CM |
| Weiss SA 201670 | 1241 | ? | all | ? | ? | TILs (nonbrisk, brisk), CD3, CD45, FoxP3 |
OS, RFS | FFPE | IHC/H&E | CM |
| Massi D 201759 | 64 | M1 | ? | ? | BRAFi | CD8, CD103, FoxP3 | OS, PFS | FFPE | IHC | M |
| Saldanha G 201764 | 655 | I/II | ? | ? | ? | TILs (nonbrisk, brisk) | OS, DSS, MFS | FFPE | H&E | CM |
| Melief SM 201760 | 73 | IV | ? | ? | ? | CD4, CD8, FoxP3 | OS | FFPE | IHC/IF | M |
| Lagouros E 200935 | 44 | ? | ? | ? | ? | CD3, CD4, CD25, FoxP3 | OS | FFPE | IHC | UM |
| Vasaturo A 201628 | 77 | III/M1 | all | ? | DC-V, CT | CD3 | OS | FFPE | IHC | CM |
| Kakavand H 201552 | 60 | III/IV | ? | III-V | ? | CD3, CD4, CD8, FoxP3 | OS, RFS | FFPE | IHC | M |
| de Moll EH 201510 | 94 | II-III | ? | ? | ? | TILs (nonbrisk, brisk), CD2 | OS, RFS | FFPE | IHC/IF | CM |
| Massi D 201558 | 80 | M1 | ? | ? | BRAFi | TILs (present) | OS, PFS, MSS | FFPE | IHC/H&E | M |
| Fortes C 201518 | 4133 | ? | all | ? | ? | TILs (scanty, moderate, marked) | OS | FFPE | H&E | CM |
| Donizy P 201546 | 104 | all | all | I-V | ? | TILs (nonbrisk, brisk) | OS, CSS, DFS | FFPE | IHC/H&E | CM |
| Madore J 201556 | 51 | IV | ? | ? | ? | TILs (mild, moderate, marked) | MSS | FFPE | IHC/H&E | CM |
| Thomas NE 201367 | 2845 | all | all | ? | ? | TILs (nonbrisk, brisk) | OS, MSS | FFPE | H&E | CM |
| Cintolo JA 201345 | 161 | T4 | ≥4mm | ? | ? | TILs (present) | MSS | FFPE | IHC/H&E | M |
| Lee SJ 201355 | 90 | ? | all | ? | ? | TILs (nonbrisk) | OS | FFPE | IHC/H&E | AM |
| Grotz TE 201348 | 250 | all | all | II-V | ? | TILs (nonbrisk, brisk) | RFS, DFS, MSS | FFPE | H&E | CM |
| Knol AC 201253 | 47 | III | ? | ? | TIL | TILs (present) | OS, RFS | Fozen | FACS/IHC | M |
| Azimi F 201242 | 1865 | all | ≥0.75mm | II-V | ? | TILs grade | RFS, MSS | FFPE | H&E | CM |
| Holtan SG 201251 | 118 | III/IV | ? | ? | ? | CD20 | OS | FFPE | IHC | M |
| Erdag G 201247 | 147 | III/IV | ? | ? | ? | CD45, CD3, CD8, CD20, CD138 | OS | FFPE | IHC | M |
| Knol AC 201134 | 102 | III | all | ? | ? | FoxP3 | OS, PFS | Fresh | qRT-PCR | M |
| Burton AL 201112 | 515 | all | ≥1mm | II-V | ? | TILs (nonbrisk, brisk) | OS, DFS | FFPE | H&E | CM |
| Rao UN 201063 | 293 | T4 | all | ? | ? | TILs (nonbrisk, brisk) | OS, RFS | FFPE | H&E | CM |
| Mougiakakos D 201036 | 100 | ? | ? | ? | ? | FoxP3, CD4 | OS | FFPE | IHC | UM |
| Bogunovic D 200917 | 38 | III/IV | ? | ? | RT, IM, CT, ? | CD3, TILs grade | OS | FFPE | IHC/H&E | M |
| Mandalà M 200957 | 1251 | I-II | all | I-V | ? | TILs (nonbrisk, brisk) | OS, DFS | FFPE | H&E | CM |
| Tuomaala S 200768 | 85 | ? | ? | ? | ? | TILs (few, moderate, many) | OS | FFPE | H&E | CM |
| Hillen F 200850 | 58 | ? | all | ? | ? | CD3, CD16, CD20, CD69, FoxP3 | OS | FFPE | IHC | CM |
| Taylor RC 200766 | 887 | all | all | I-V | ? | TILs (nonbrisk, brisk) | DFS | FFPE | H&E | CM |
| Piras F 200562 | 47 | I/II | ? | II-V | ? | CD8 | OS | FFPE | IHC | CM |
| Ladányi A 200454 | 76 | ? | all | ? | ? | CD25, OX40 | OS | FFPE | IHC | CM |
| Håkansson A 200149 | 41 | ? | ? | ? | BCT | CD4 | OS, PFS | FFPE | IHC | M |
| Tuthill RJ 200269 | 259 | ? | all | ? | ? | TILs (nonbrisk, brisk) | OS, PFS | FFPE | H&E | CM |
| Clemente CG 199615 | 285 | ? | all | ? | ? | TILs (nonbrisk, brisk) | OS | FFPE | H&E | CM |
| Kluger HM 20154 | 95 | IV | ? | ? | IM, ? | CD3, CD4, CD8, FoxP3 | OS, BMS | FFPE | IF | M |
Furthermore, we also paid attention to the definition of time-to-event variables. In a few studies which mentioned the specific treatment,28,43,58,59 OS was defined as the time from the date of start of the treatment to death or last follow-up, DFS was defined as the time from the date of start of the treatment to the date of first recurrence and/or disease progression (regional or distant metastases) or last follow-up, RFS was defined as the time from the date of start of the treatment to the date of the clinical recurrence. In two studies involved in Sentinel lymph node biopsy (SLNB),16,52 RFS and OS were calculated from SLNB to an event (either recurrence or death) or until last follow-up. In the remaining studies, OS was defined as the time from pathological diagnosis to the time of death or last follow-up, DFS was defined as the time from pathological diagnosis to the date of first recurrence and/or disease progression (regional or distant metastases) or last follow-up, RFS was defined as the time from pathological diagnosis to the date of the recurrence. DSS or MSS was calculated from the date of pathological diagnosis to the date of death from melanoma or last follow-up.
Summary of the quality and risk of bias of the included studies
According to the QUIPS criteria system, the risk of bias of the 41 studies remaining after full-text screening was critically evaluated, and all the literature was evaluated by 2 independent reviewers; if a difference in evaluation arose, it was solved by discussion. Consistent with previous studies in head and neck squamous carcinoma,32 very few articles mentioned information about patients who were lost to follow up or study attrition, and most studies did not use consecutive cohorts. Because no consensus exists on cut-offs, several studies were unclear about their scoring methods and data-dependent cut-offs. The complete quality assessment of the publications included in the meta-analysis is shown in Supplement Table 1.
TILs as prognostic biomarkers and the correlation between different TIL grades and prognosis value
Many studies have reported that TILs are a prognostic factor for melanoma patients and have indicated a distinct correlation between different TIL intensities or grades and prognosis value. Thus, the prognostic value of TILs present was assessed in 8 studies that were eligible for inclusion in the meta-analysis, among which 1 study analyzed the prognostic roles of TILs in two different subtypes of melanoma (nail unit melanoma and non-nail unit acral melanoma). As shown in Figure 2, although no significant difference was found, the pooled meta-analysis showed an advantage trend for TILs present [pooled HR: 0.65 (0.42–1.00)] for OS. Additionally, we assessed the prognostic roles of different TIL grades for the OS of melanoma patients, and the results indicated that an advantageous prognostic role for non-brisk TILs [HR: 0.71 (0.55–0.90)] and brisk TILs [HR: 0.61 (0.52–0.72)] but not moderate TILs [HR: 0.81 (0.32–2.07)] for OS (Figure 3).
Figure 2.
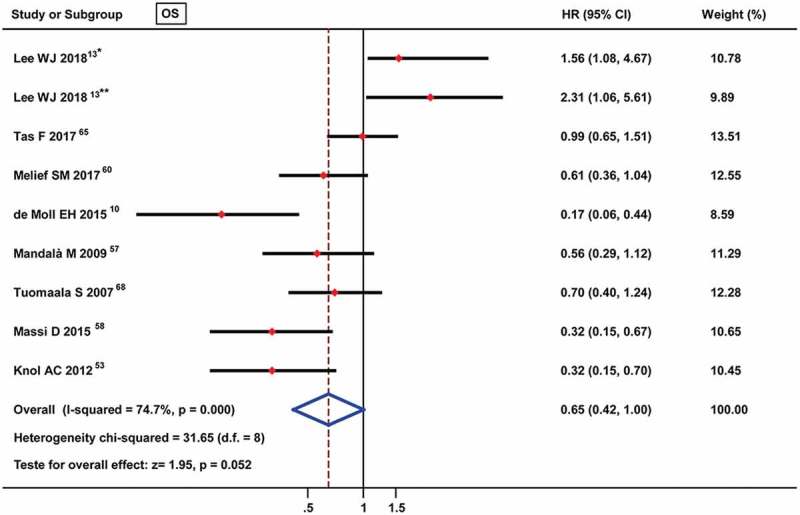
Forest plots of prognostic value of TILs present on overall survival in melanoma patients. * nail unit melanoma, ** non-nail unit acral melanoma.
Figure 3.
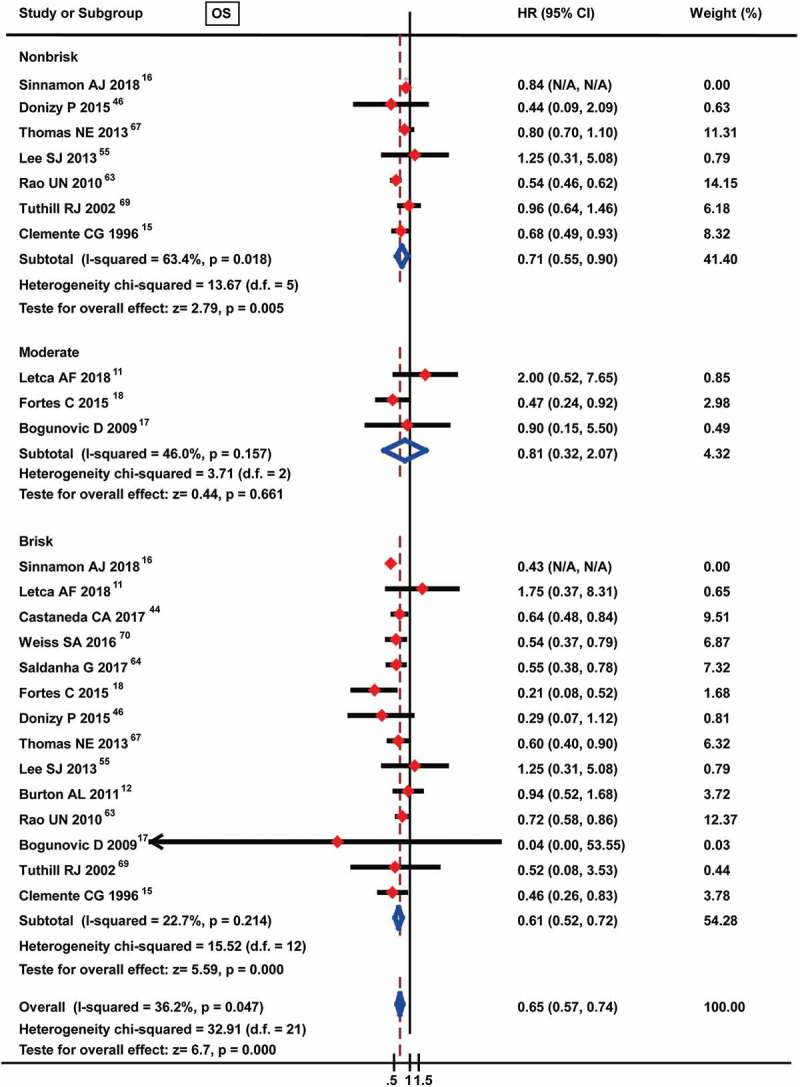
Forest plots of prognostic value of different TIL grades on overall survival in melanoma patients.
For the prognostic roles of TILs for DFS, no study mentioned the prognostic roles of TILs present for DFS; thus, we directly assessed the correlation between different TIL grades and DFS. The results of the meta-analysis showed no obvious predictive roles of TILs for DFS, among which non-brisk TILs were assessed in 2 studies [HR: 0.82 (0.59–1.12)], moderate TILs were assessed in only 1 study [HR: 0.88 (0.32–2.41)], and brisk TILs were assessed in 5 studies [HR: 0.74 (0.34–1.62)] (Figure 4).
Figure 4.
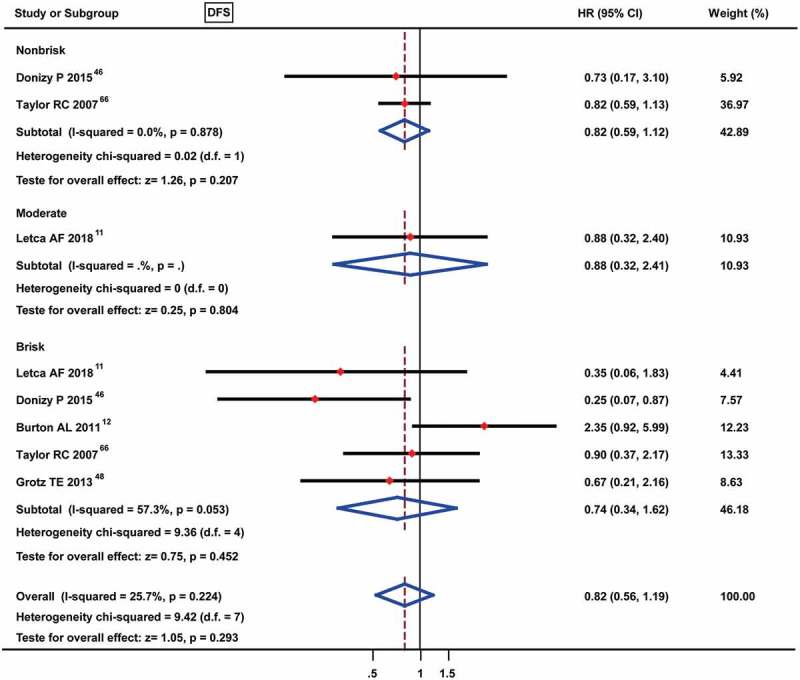
Forest plots of prognostic value of different TIL grades on disease-free survival in melanoma patients. No data were available for the TILs present and only 1 study was met the moderate TILs.
For the prognostic roles of TILs for RFS, 3 studies were eligible for inclusion in the meta-analysis of TILs present, and the results showed an advantageous prognostic value [HR: 0.72 (0.58–0.90)] (Figure 5(a)). No study mentioned the prognostic value of moderate TILs for RFS, and only one study involved the prognostic role of non-brisk TILs for RFS [HR: 0.57 (0.32–1.01)] (Figure 5(b)). The prognostic value of brisk TILs was assessed in 3 studies, and the meta-analysis demonstrated brisk TILs to be a favorable prognostic factor for RFS [HR: 0.50 (0.27–0.94)] (Figure 5(b)).
Figure 5.
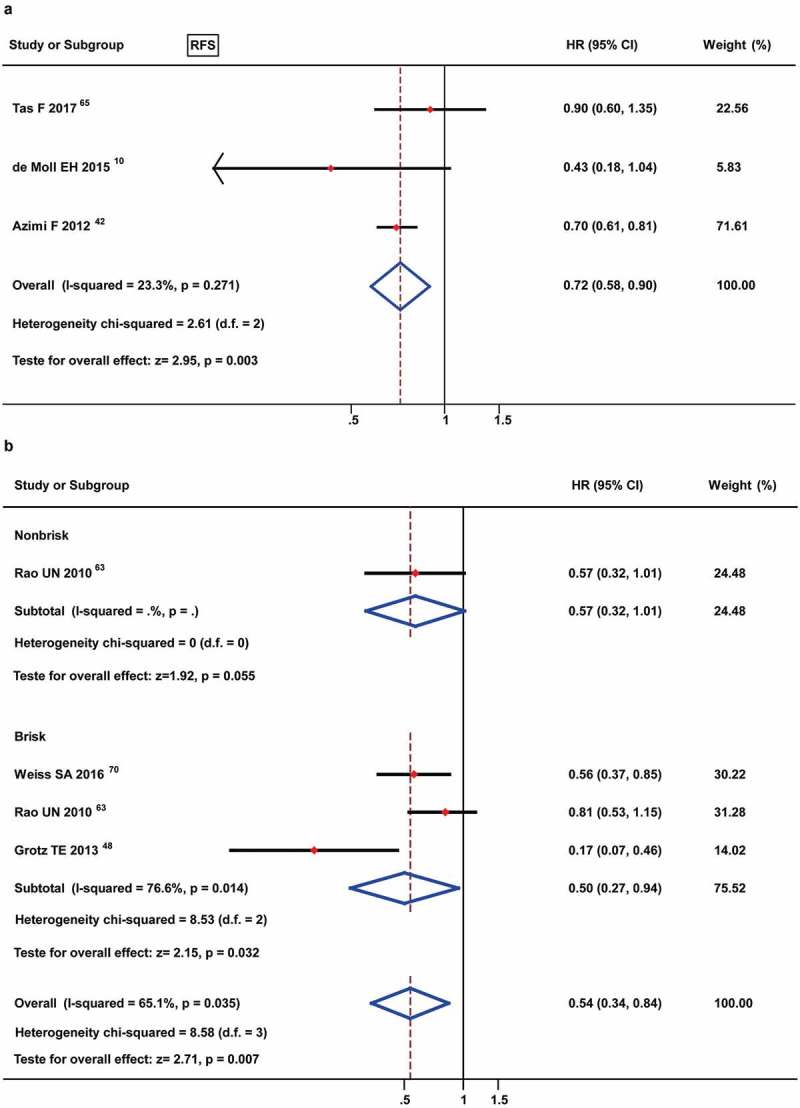
Forest plots of prognostic value of TILs present (A) and different TIL grades (B) on progression-free survival in melanoma patients. Only 1 study was met the non-brisk TILs and no data were available for the moderate TILs.
The prognostic value of TILs present for DSS/MSS was analyzed in 4 studies by meta-analysis, and the results showed an obviously favorable prognostic role [HR: 0.46 (0.30–0.70)] (Figure 6(a)). Furthermore, the 2 studies that reported non-brisk TILs also yielded a conclusive result [HR: 0.69 (0.49–0.97)], the 4 studies that involved brisk TILs showed a better prognostic role [HR: 0.31 (0.19–0.52)] (Figure 6(b)).
Figure 7.
Funnel plot of of TILs present on overall survival.
Figure 6.
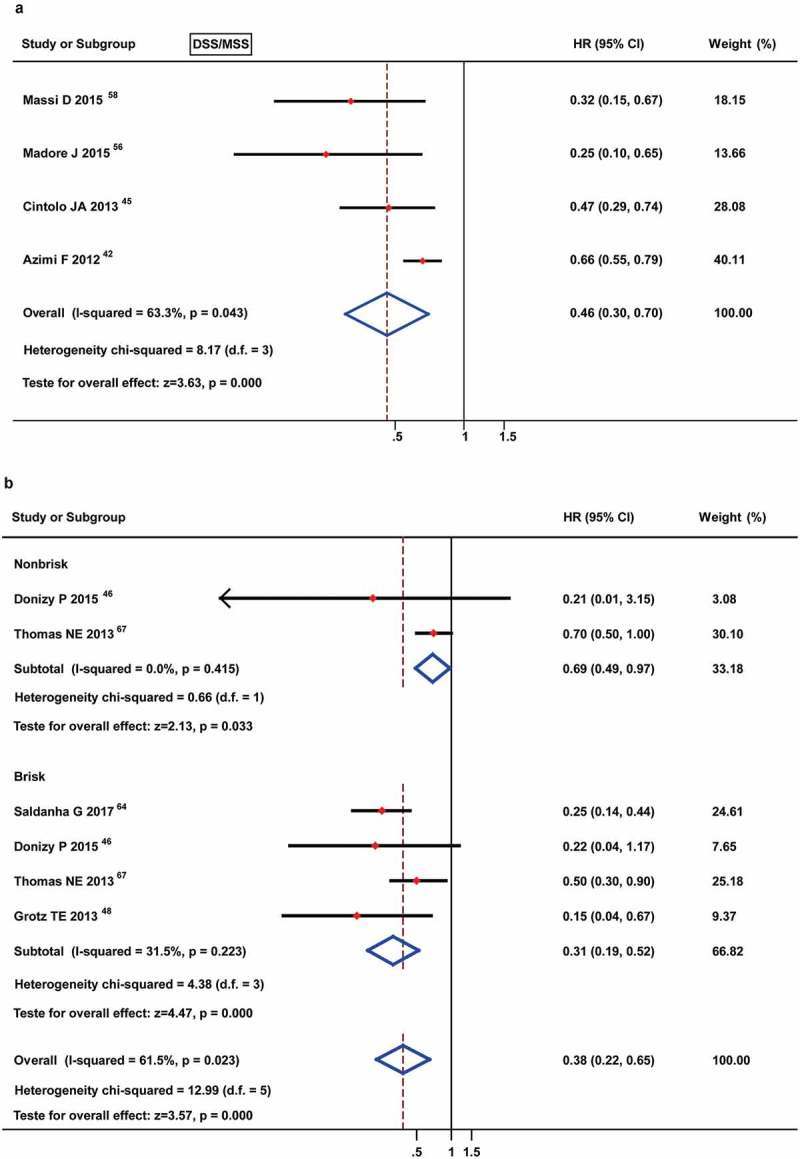
Forest plots of prognostic value of TILs present (A) and different TIL grades (B) on disease-specific survival or melanoma-specific survival in melanoma patients. No data were available for the moderate TILs.
Subsequently, the prognosis value of different TIL grades in different types of melanoma was analyzed. For OS, TILs present showed a favorable prognostic role in unspecified melanoma patients (that was defined as the type of melanoma that is not explicitly stated in the studies) [HR: 0.43 (0.27–0.67)], but not in cutaneous melanoma (P= 0.475) (Table 2, Supplement Figure 1). Interestingly, brisk TILs showed an advantageous prognostic value in cutaneous melanoma from 12 studies [HR: 0.61 (0.52–0.72)] and only one study involved the OS in unspecified melanoma (Table 2, Supplement Figure 3). Consistent with the pooled analysis, moderate TILs did not show a favorable prognostic role in cutaneous melanoma and unspecified melanoma (Table 2, Supplement Figure 2). For DSS/MSS, only TILs present were involved in 4 studies and showed a good prognostic value in unspecified melanoma but not cutaneous melanoma (Table 2, Supplement Figure 4). For DFS and RFS, all the studies included in this meta-analysis were performed in cutaneous melanoma.
Table 2.
Summary of the prognostic value of different TIL status on OS and DSS/MSS in different types of melanoma. CM (cutaneous melanoma), UM (uveal melanoma), Melanoma (M) was defined as the type of melanoma that is not explicitly stated in the studies.
| OS |
DSS/MSS |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TIL status | Subgroup | No of studies | I2 (P) | HR (95% CI) | P | No of studies | I2 (P) | HR (95% CI) | P |
| Present | CM | 6 | 75.7% (0.001) | 0.82 (0.47–1.42) | 0.475 | 2 | 74.9% (0.046) | 0.45 (0.18–1.15) | 0.095 |
| M | 3 | 27.5% (0.252) | 0.43 (0.27–0.67) | <0.001 | 2 | 0% (0.393) | 0.42 (0.28–0.63) | <0.001 | |
| Moderate | CM | 2 | 72% (0.059) | 0.86 (0.21–3.48) | 0.830 | ||||
| M | 1 | 0.9 (0.15–5.45) | 0.909 | ||||||
| Brisk | CM | 12 | 26.8% (0.181) | 0.61 (0.52–0.72) | <0.001 | ||||
| M | 1 | 0.04 (0–92.56) | 0.415 | ||||||
CD3+ TILs as a prognostic biomarker
The prognostic value of CD3+ TILs was assessed in 8 studies, among which 7 were included in the analysis and 1 was excluded from this meta-analysis because of the lack of a survival curve and 95% confidence interval (CI). The results showed an advantage for high CD3 TIL infiltration [HR: 0.57 (0.41–0.79)] for OS. Only one study that reported RFS also yielded a similar conclusive result [HR: 0.36 (0.17–0.76)] (Table 3, Supplement Figure 5). However, no study analyzed the DFS and DSS/MSS. For the subgroup analysis, CD3+ TILs indicated a favorable prognostic role for OS in unspecified melanoma patients in 4 studies [HR: 0.55 (0.4–0.77)], but not in cutaneous melanoma and uveal melanoma (Table 4, Supplement Figure 10).
Table 3.
Summary of the prognostic value of different TIL phenotypes on overall survival (OS) and relapse/recurrence free survival (RFS) in melanoma patients.
| OS |
RFS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | No of studies | I2 (P) | HR (95% CI) | P | No of studies | I2 (P) | HR (95% CI) | P |
| CD3+ TILs | 7 | 27.9% (0.215) | 0.57 (0.41–0.79) | 0.001 | 1 | 0.36 (0.17–0.76) | 0.007 | |
| CD4+ TILs | 6 | 62.6% (0.02) | 0.56 (0.37–0.85) | 0.006 | 1 | 0.34 (0.15–0.77) | 0.01 | |
| CD8+ TILs | 7 | 44.3% (0.096) | 0.5 (0.37–0.69) | <0.001 | 1 | 0.42 (0.21–0.84) | 0.015 | |
| FOXP3+ TILs | 9 | 46.4% (0.061) | 0.57 (0.4–0.82) | <0.001 | 1 | 0.6 (0.31–1.17) | 0.015 | |
| CD20+ TILs | 3 | 0% (0.748) | 0.49 (0.34–0.71) | <0.001 | ||||
Table 4.
Summary of the prognostic value of different TIL phenotypes on OS in different types of melanoma. CM (cutaneous melanoma), UM (uveal melanoma), Melanoma (M) was defined as the type of melanoma that is not explicitly stated in the studies.
| TIL phenotypes | Subgroup | No of studies | I2 (P) | HR (95% CI) | P |
|---|---|---|---|---|---|
| CD3+ TILs | CM | 3 | 51% (0.130) | 0.93 (0.27–3.15) | 0.906 |
| UM | 1 | 5.5 (N/A- N/A) | 0.03 | ||
| M | 4 | 28.4% (0.241) | 0.55 (0.4–0.77) | <0.001 | |
| CD4+ TILs | CM | 1 | 0.22 (0.09–0.55) | 0.001 | |
| UM | 1 | 0.80 (0.50–1.27) | 0.346 | ||
| M | 4 | 55.6% (0.080) | 0.60 (0.37–0.97) | 0.039 | |
| CD8+ TILs | CM | 2 | 0.0% (0.352) | 0.38 (0.19–0.75) | 0.005 |
| M | 5 | 50.4% (0.089) | 0.53 (0.37–0.75) | <0.001 | |
| FOXP3+ TILs | CM | 4 | 0% (0.785) | 0.58 (0.36–0.95) | 0.032 |
| UM | 1 | 1.14 (0.68–1.91) | 0.617 | ||
| M | 4 | 47.6% (0.126) | 0.45 (0.28–0.73) | 0.001 | |
| CD20+ TILs | CM | 1 | 0.42 (0.06–2.91) | 0.380 | |
| M | 2 | 0% (0.456) | 0.49 (0.34–0.72) | <0.001 |
CD4+ TILs as a prognostic biomarker
Similar to the studies of CD3+ TILs, 7 studies were assessed for CD4+ TILs: 6 articles were finally included in the meta-analysis, and 1 was excluded because of the lacking of a survival curve and CI values. As shown in Figure 8, high CD4+ TILs are a favorable prognostic factor for the OS of melanoma patients according to the HR [0.56 (0.37–0.85)]. For RFS, only one study was reported and showed a similar favorable prognostic role of the HR [0.34 (0.15–0.77)] (Table 3, Supplement Figure 6). No study has reported on the DFS and DSS/MSS. For the subgroup analysis, CD4+ TILs showed an advantage prognostic role for OS in cutaneous melanoma in only one study [HR: 0.22 (0.09–0.55)] and in unspecified melanoma in 4 studies [HR: 0.6 (0.37–0.97)], but not in uveal melanoma with only one study (Table 4, Supplement Figure 11).
CD8+ TILs as a prognostic biomarker
For the prognostic value of CD8+ TILs, we searched 7 studies that met the enrollment requirements for OS and analyzed by meta-analyses. The results showed a favorable prognostic value for CD8+ TIL infiltration [HR: 0.50 (0.37–0.69)] (Figure 9). Only one study reported the RFS and indicated an advantageous prognostic value [HR: 0.42 (0.21–0.84)] (Table 3, Supplement Figure 7). Consistent with the studies on CD3+ and CD4+ TILs, no study has reported on the DFS and DSS/MSS of CD8+ TILs in melanoma. For the subgroup analysis, CD8+ TILs showed a good prognostic value for OS in cutaneous melanoma in 2 studies [HR: 0.38 (0.19–0.75)] and unspecified melanoma in 5 studies [HR: 0.53 (0.37–0.75)], but no study has reported in uveal melanoma (Table 4, Supplement Figure 12).
FOXP3+ TILs as a prognostic biomarker
Ten studies reported results on the prognostic value of FOXP3+ TILs, among which 1 lacked survival curve and 95% CI values, and the remaining 9 studies were included in the meta-analysis. The results indicated a better OS in melanoma patients with high FOXP3 TIL infiltration [HR: 0.57 (0.40–0.82)] (Table 3, Supplement Figure 8). A comparable trend was observed for RFS with only one study [HR: 0.60 (0.31–1.17)]. No study reported on DFS and DSS/MSS. For the subgroup analysis, FOXP3+ TILs showed a favorable prognostic role for OS in cutaneous melanoma patients in 4 studies [HR: 0.58 (0.36–0.95)] and unspecified melanoma in 4 studies [HR: 0.45 (0.28–0.73)], but not in uveal melanoma with only one study (Table 4, Supplement Figure 13).
CD20+ TILs as a prognostic biomarker
For the prognostic value of CD20+ TILs, we searched 3 studies that met the enrollment requirements for OS. The meta-analysis showed a markedly favorable prognostic value for patients with high CD20 TIL infiltration [HR: 0.49 (0.34–0.71)] (Table 3, Supplement Figure 9). However, no study has reported on the DFS, RFS and DSS/MSS of CD20+ TILs in melanoma. For the subgroup analysis, CD20+ TILs indicated a good prognostic value for OS in unspecified melanoma in 2 studies [HR: 0.49 (0.34–0.72)], but no in cutaneous melanom with only one study, and no study has reported in uveal melanoma (Table 4, Supplement Figure 14).
Discussion
Melanoma is the most aggressive skin cancer. Thus, accurate prognostic prediction is paramount for the selection of appropriate therapies and management of melanoma patients; however, robust biomarkers are currently lacking. Considering that melanoma is considered an “immunogenic” tumor, the principal goal of our study was to investigate the prognostic role of the TIL response and different grades and subsets of TILs to predict the outcome of melanoma.
First, we investigated the prognostic efficacy of the TIL response by meta-analysis to provide a quantitative synthesis of several studies. Our analysis indicated that TILs present correlated with a favorable prognosis for the OS, RFS and DSS/MSS of melanoma. This finding was consistent with a hypothesis proposed in 1978 that concluded from a retrospective study including 669 melanoma patients that a dense lymphocytic infiltrate in primary tumors improved their survival rates.71 This is also supported by the findings in subsequent studies of melanoma10 and fit with studies in other tumors.32
A distinct correlation is found between different TIL intensities or grades and different prognosis values in melanoma. Thus, the establishment of a graded system to categorize the extent of TIL involvement within a tumor has become the first requirement to assess of the role of TILs in prognostication. Currently, two main comprehensive classification systems are commonly used in melanoma, and most groups quantify TILs as absent, non-brisk and brisk (focal TIL infiltrate and when TILs present across the entire base or throughout the substance of the tumor),14–16 while others quantify TILs as absent, mild or scanty, moderate and marked (focal, multifocal, or diffuse across the entire extent of the tumor).17,18 In this study, both the non-brisk TILs and mild or scanty TILs were defined as non-brisk TILs, the brisk TILs and marked TILs were defined as brisk TILs, and the moderate TILs were analyzed alone. The meta-analysis demonstrated a favorable prognostic role of non-brisk TILs [HR: 0.71 (0.55–0.90) for OS, HR: 0.57 (0.32–1.01) for RFS, and HR 0.69 (0.49–0.97) for DSS/MSS]. Noteworthy, a better favorable prognostic value of TILs was observed in the brisk TIL grade with HR 0.61 (0.52–0.72) for OS, HR 0.50 (0.27–0.94) for RFS, and HR 0.31 (0.19–0.52) for DSS/MSS. However, TILs of any grade showed no obviously predictive effect on DFS. Thus, our study draws a conclusion that TILs are a favorable prognostic factor in melanoma, and the larger is the infiltration area of TILs, the better is the prognosis of melanoma patients. This finding is consistent with the reports of Clemente et al and Clark et al,14,15 both of whom observed that the survival rate of melanoma patients with a brisk TIL response compared with those with a non-brisk response was higher, and the TIL response was an independent prognostic indicator. The possible reason for the favorable role of TILs in the survival of melanoma patients is that, TILs is not just inflammatory cells but rather antitumor effector cells that are recruited to the tumor as a part of the specific immune response. In addition, the presence of TILs would decrease the involvement of regional lymph nodes and distant metastasis to lead a positive outcome.65
Considering the great heterogeneity of the TIL phenotype, the phenotype of TIL cells from different tumor sources varies greatly. Histologically, most TILs express CD3 and CD3 as a general TIL marker.72,73 Although some studies have reported that the CD3-TCR complex should be helpful in the regulation of the signal transduction and CD3 is usually used as a pan-T cell marker,74–76 the exact function of CD3 TILs in the prognosis of melanoma patients is currently unclear. In this study, the prognostic value of CD3+ TILs was assessed in 7 studies and found that CD3 TIL infiltration is a favorable prognostic factor for overall survival in melanoma. This finding is consistent with the results of meta-analysis in squamous cell carcinoma of the head and neck.32 The specific mechanism of CD3+ TILs as favorable prognostic factor is unclear, and may be related to its regulation on cell signal transduction.
T cells present in tumors are mixtures of CD8+ and CD4+ cells, and CD8+ TILs and CD4+ TILs are the main cell populations of the TIL treatment system, in which CD8+ TILs kill tumor cells and CD4+ TILs show immunomodulatory effects.77,78 The proportion of CD4+ and CD8+ TILs in different tumor tissues was different, reflecting the changes in the body’s immune status.79 The prognostic value of CD8+ TILs was most frequently assessed in various tumors. For the prognostic role of CD8+ TILs in melanoma, we assessed 7 studies by meta-analysis and showed a better favorable prognostic value for the OS of melanoma patients compared with CD3+ TIL infiltration. This may be closely related to the directly killing effect of CD8+ TILs on tumors cells.
Recently, the role of CD4+ T cells in tumor immunity has received extensive attention; CD4+ T cells play an important role in the initiation of cytotoxic T lymphocytes (CTLs) as well as in the formation and functional maintenance of memory CD8+ T cells. In tumors, after CD4+ T cells are activated, CTLs can be activated by activated CD4+ T cells through various mechanisms to maintain and enhance CTL’s anti-tumor response.80,81 Except for the immunomodulatory effects, CD4+ TILs function as effector cells to aid the killing of tumor cells by CD8+ TILs and even independently clear tumor cells that are resistant to CD8+ cells.81–83 Currently, the prognostic role of CD4+ TILs has gain much attention. Our study enrolled 6 studies into the meta-analysis and showed that CD4+ TILs are a favorable prognostic factor for the OS of melanoma patients. Thus, the favorable role of CD4+ TILs in the survival of melanoma patients is probably closely related to above-mentioned functions of CD4+ TILs.
Generally, the infiltration of Treg cells into tumors inhibits antitumor immune responses, and the depletion of Treg cells enhance anticancer treatments.84 Thus, understanding the markers of Treg cells is essential for the studies of Treg cells in tumors. It has been confirmed that CD25 and transcription factor FOXP3 are highly expressed in Treg cells and FOXP3 is the most specific marker.29 Several studies have found a close association between FOXP3 expression and the prognosis of patients in various tumors.30,31 However, the controversial results have always existed in melanoma. Most previous studies have suggested that FOXP3+ cells are associated with a favorable prognosis,34,43 while others have shown that FOXP3+ cells are a poor prognosis factor.35,36 Therefore, 9 studies were included in our meta-analysis for the prognostic value of FOXP3+ TILs and demonstrated that FOXP3 TIL infiltration is a beneficial prognosis factor in melanoma. This is inconsistent with the standpoint that the infiltration of Treg cells inhibits the antitumor immune response.
The first possible explanation for this inconsistency is that, Tregs suppress the inflammatory response and disturb metabolism which are associated with tumor progression by secreting anti-inflammatory cytokines and growth factor such as IL-10, IL-35 and tumor growth factor-(TGF-) β.84 Another possible reason is that the FoxP3+ TILs could be just a basic reflection of Tregs, the ratio of FOXP3+ Tregs to other lymphocyte subsets and CD8+/FOXP3+ T cell ratio are the potential effective indicator of the quantity of Tregs.32,33 However, almost no study assessed these ratios in melanoma, only one study assessed the prognostic role of CD8/Tregs ratio (Tregs were defined as CD3+ CD8-FOXP3+) HR 1.637 (0.956–2.801) for OS.60 The last possible explanation for the favorable role of FOXP3+ TILs is that, FOXP3 is not specific for activated Tregs, it is needed to conjoint assess FOXP3 and additional markers, such as CD25. Regretfully, no study assessed this combination in melanoma, only two studies assessed the prognostic role of CD25 for OS by HR 1.8 (no 95% CI) (P= 0.68)35 and two almost identical survival curves (P= 0.802).54
CD20 is a marker of TILs, and the prognostic role of CD20+ TILs was rarely assessed in various tumors. For the prognostic value of CD20+ TILs, we searched 3 studies analyzed by meta-analysis and showed a markedly favorable prognostic value for the patients with high CD20 TIL infiltration in melanoma. The specific mechanism of CD3+ TILs as favorable prognostic factor is unclear, and additional large-sample studies are needed to confirm the results due to the small number of enrolled studies.
Certain limitations of our meta-analysis should be described. First, although the literature search was performed in 7 electronic databases, databases and literature published in other languages except English were not included in our study. Thus, some relevant publications might have been missed.
The main limitation of this study is the heterogeneity within the tumor subgroups included in our meta-analysis. Although most studies reported in our study were cutaneous melanoma that included many subgroups, very few articles reported the information about patients with uveal melanomas. The prognostic value of biomarkers may be different between different tumor subgroups and tumor stages. Furthermore, most studies did not frequently allow stratification for these different groups. Therefore, the categorizations of patients and tumors could strengthen the conclusions on prognostic biomarkers and provide more insight into the differences between patient or tumor subgroups.
Another limitation of this study is the heterogeneity of the scoring methods and data-dependent cut-offs of biomarkers. Because no consensus exists on cut-offs, several studies were unclear about their scoring methods and data-dependent cut-offs, and different scoring systems and thresholds may lead to different results. Thus, the consensus of the scoring methods and data-dependent cut-offs of TILs would strengthen the conclusions on prognostic biomarkers and provide more insight into the study of TILs.
The heterogeneity in the treatment modality is also a key limitation. Because very few studies mentioned the treatment modality in their reports, our analysis did not consider treatment modalities. However, different treatment modalities have different mechanisms, and the prognostic value of TILs is likely to depend on the given therapy. Therefore, more prognostic studies with different treatment modalities among patient cohorts are needed.
Because the number of retrieved studies was not sufficient to be analyzed depending on the detection methods of biomarkers, no restriction was placed on the detection methods. However, different detection methods may have different TILs, and the prognostic value of TILs is likely to depend on the detection methods. Therefore, additional prognostic studies with H&E staining and immunohistochemistry of TILs are needed in the future.
In conclusion, our meta-analysis confirmed the favorable prognostic role of CD3+, CD4+, CD8+, FOXP3+, and CD20+ TILs in the overall survival of melanoma patients. Brisk TILs are obviously associated with the OS, RFS and DSS/MSS. Thus, TILs showed predictive value for the prognosis of melanoma patients, and detection of TILs status and phenotype in pathological diagnosis would be helpful to guide the treatment and prognosis of patients. However, to incorporate different T-cell markers as predictive biomarkers in clinical practice, studies using homogeneous patient cohorts according to tumor subsite, stage and treatment are necessary.
Funding Statement
This study was supported by China Postdoctoral Science Foundation [No.2017M613008]; National Natural Science Foundation of China [No.81702295, No.81760554, No.81602029, No.81660389, No.81460356].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Davar D, Wang H, Chauvin JM, Pagliano O, Fourcade JJ, Ka M, Menna C, Rose A, Sander C, Borhani AA, et al. Phase Ib/II study of pembrolizumab and pegylated-interferon alfa-2b in advanced melanoma. J Clin Oncol. 2018. Oct 25:JCO1800632. doi: 10.1200/JCO.18.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadpour A, Derakhshan M, Darabi H, Hedayat P, Momeni M.. Melanoma: where we are and where we go. J Cell Physiol. 2019 Apr;234(4):3307–3320. doi: 10.1002/jcp.27286. [DOI] [PubMed] [Google Scholar]
- 4.Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VL, Sznol M, Rimm DL, Chen L, Jilaveanu LB. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015;21:3052–3060. doi: 10.1158/1078-0432.CCR-14-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. [DOI] [PubMed] [Google Scholar]
- 6.Tuccitto A, Shahaj E, Vergani E, Ferro S, Huber V, Rodolfo M, Castelli C, Rivoltini L, Vallacchi V. Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Arch. 2018. doi: 10.1007/s00428-018-2477-z. [DOI] [PubMed] [Google Scholar]
- 7.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34:2389–2397. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee N, Zakka LR, Mihm MC Jr., Schatton T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology. 2016;48:177–187. doi: 10.1016/j.pathol.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Schatton T, Scolyer RA, Thompson JF, Mihm MC Jr.. Tumor-infiltrating lymphocytes and their significance in melanoma prognosis. Methods Mol Biol. 2014;1102:287–324. doi: 10.1007/978-1-62703-727-3_16. [DOI] [PubMed] [Google Scholar]
- 10.de Moll EH, Fu Y, Qian Y, Perkins SH, Wieder S, Gnjatic S, Remark R, Bernardo SG, Moskalenko M, Yao J, et al. Immune biomarkers are more accurate in prediction of survival in ulcerated than in non-ulcerated primary melanomas. Cancer Immunol Immunother. 2015;64:1193–1203. doi: 10.1007/s00262-015-1726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letca AF, Ungureanu L, Senila SC, Grigore LE, Pop S, Fechete O, Vesa ŞC, Cosgarea R. Regression and Sentinel Lymph Node Status in Melanoma Progression. Med Sci Monit. 2018;24:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton AL, Roach BA, Mays MP, Chen AF, Ginter BA, Vierling AM, Scoggins CR, Martin RCG, Stromberg AJ, Hagendoorn L, et al. Prognostic significance of tumor infiltrating lymphocytes in melanoma. Am Surg. 2011;77:188–192. [PubMed] [Google Scholar]
- 13.Lee WJ, Lee YJ, Shin HJ, Won CH, Chang SE, Choi JH, Lee MW. Clinicopathological significance of tumor-infiltrating lymphocytes and programmed death-1 expression in cutaneous melanoma: a comparative study on clinical subtypes. Melanoma Res. 2018;28:423–434. doi: 10.1097/CMR.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 14.Clark WH Jr., Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. [DOI] [PubMed] [Google Scholar]
- 15.Clemente CG, Mihm MC Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Sinnamon AJ, Sharon CE, Song Y, Neuwirth MG, Elder DE, Xu X, Chu EY, Ming ME, Fraker DL, Gimotty PA, et al. The prognostic significance of tumor-infiltrating lymphocytes for primary melanoma varies by sex. J Am Acad Dermatol. 2018;79:245–251. doi: 10.1016/j.jaad.2018.02.066. [DOI] [PubMed] [Google Scholar]
- 17.Bogunovic D, O’Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortes C, Mastroeni S, Mannooranparampil TJ, Passarelli F, Zappala A, Annessi G, Marino C, Caggiati A, Russo N, Michelozzi P. Tumor-infiltrating lymphocytes predict cutaneous melanoma survival. Melanoma Res. 2015;25:306–311. doi: 10.1097/CMR.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 19.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–S84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 20.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–751. doi: 10.1172/JCI74894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL.. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3):pii:dju435. doi: 10.1093/jnci/dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–2713. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol Res. 2014;2:91–98. doi: 10.1158/2326-6066.CIR-13-0216. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res. 2016;2016:1–13. doi: 10.1155/2016/4273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasaturo A, Halilovic A, Bol KF, Verweij DI, Blokx WA, Punt CJ, Groenen PJTA, van Krieken JHJM, Textor J, de Vries IJM, et al. T-cell landscape in a primary melanoma predicts the survival of patients with metastatic disease after their treatment with dendritic cell vaccines. Cancer Res. 2016;76:3496–3506. doi: 10.1158/0008-5472.CAN-15-3211. [DOI] [PubMed] [Google Scholar]
- 29.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (New York, NY). 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Liao H, Zhang Y, Yuan R, Wang F, Gao Y, Wang P, Du Z, Seno M. Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: a meta analysis. PLoS One. 2014;9:e94376. doi: 10.1371/journal.pone.0094376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, Koura K, Takahashi R, Otsuka H, Takahashi H, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1:625–632. doi: 10.3892/mco.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1356148. doi: 10.1080/2162402X.2017.1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knol AC, Nguyen JM, Quereux G, Brocard A, Khammari A, Dreno B. Prognostic value of tumor-infiltrating Foxp3+ T-cell subpopulations in metastatic melanoma. Exp Dermatol. 2011;20:430–434. doi: 10.1111/j.1600-0625.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 35.Lagouros E, Salomao D, Thorland E, Hodge DO, Vile R, Pulido JS. Infiltrative T regulatory cells in enucleated uveal melanomas. Trans Am Ophthalmol Soc. 2009;107:223–228. [PMC free article] [PubMed] [Google Scholar]
- 36.Mougiakakos D, Johansson CC, Trocme E, All-Ericsson C, Economou MA, Larsson O, Seregard S, Kiessling R. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer. 2010;116:2224–2233. doi: 10.1002/cncr.24999. [DOI] [PubMed] [Google Scholar]
- 37.Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in medline to enhance systematic reviews. PLoS One. 2012;7:e32844. doi: 10.1371/journal.pone.0032844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deva S, Jameson M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database Syst Rev. 2012;15(8):CD007814. doi: 10.1002/14651858.CD007814.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 42.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 43.Balatoni T, Mohos A, Papp E, Sebestyen T, Liszkay G, Olah J, Varga A, Lengyel Z, Emri G, Gaudi I, et al. Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol Immunother. 2018;67:141–151. doi: 10.1007/s00262-017-2072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castaneda CA, Torres-Cabala C, Castillo M, Villegas V, Casavilca S, Cano L, Sanchez J, Dunstan J, Calderon G, De La Cruz M, et al. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin Transl Oncol. 2017;19:1478–1488. doi: 10.1007/s12094-017-1685-3. [DOI] [PubMed] [Google Scholar]
- 45.Cintolo JA, Gimotty P, Blair A, Guerry D, Elder DE, Hammond R, Elenitsas R, Xu X, Fraker D, Schuchter LM, et al. Local immune response predicts survival in patients with thick (t4) melanomas. Ann Surg Oncol. 2013;20:3610–3617. doi: 10.1245/s10434-013-3086-3. [DOI] [PubMed] [Google Scholar]
- 46.Donizy P, Kaczorowski M, Halon A, Leskiewicz M, Kozyra C, Matkowski R. Paucity of tumor-infiltrating lymphocytes is an unfavorable prognosticator and predicts lymph node metastases in cutaneous melanoma patients. Anticancer Res. 2015;35:351–358. [PubMed] [Google Scholar]
- 47.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grotz TE, Vaince F, Hieken TJ. Tumor-infiltrating lymphocyte response in cutaneous melanoma in the elderly predicts clinical outcomes. Melanoma Res. 2013;23:132–137. [DOI] [PubMed] [Google Scholar]
- 49.Hakansson A, Gustafsson B, Krysander L, Hjelmqvist B, Rettrup B, Hakansson L. Biochemotherapy of metastatic malignant melanoma. Br J Cancer. 2001;85:1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hillen F, Baeten CI, van de Winkel A, Creytens D, van der Schaft DW, Winnepenninckx V, Griffioen AW. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother. 2008;57:97–106. doi: 10.1007/s00262-007-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holtan SG, Mansfield AS, Creedon DJ, Nevala WK, Haluska P, Leontovich AA, Markovic SN. An organ system based approach to prognosis in advanced melanoma. Front Biosci. 2012;4:2723–2733. [DOI] [PubMed] [Google Scholar]
- 52.Kakavand H, Vilain RE, Wilmott JS, Burke H, Yearley JH, Thompson JF, Hersey P, Long GV, Scolyer RA. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015;28:1535–1544. doi: 10.1038/modpathol.2015.110. [DOI] [PubMed] [Google Scholar]
- 53.Knol AC, Nguyen JM, Pandolfino MC, Quereux G, Brocard A, Peuvrel L, Saint-Jean M, Saiagh S, Khammari A, Dréno B, et al. Tissue biomarkers in melanoma patients treated with TIL. PLoS One. 2012;7:e48729. doi: 10.1371/journal.pone.0048729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladanyi A, Somlai B, Gilde K, Fejos Z, Gaudi I, Timar J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10:521–530. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Lim HJ, Choi YH, Chang YH, Lee WJ, Kim DW, Yoon GS. The clinical significance of tumor-infiltrating lymphocytes and microscopic satellites in acral melanoma in a korean population. Ann Dermatol. 2013;25:61–66. doi: 10.5021/ad.2013.25.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 57.Mandala M, Imberti GL, Piazzalunga D, Belfiglio M, Labianca R, Barberis M, Marchesi L, Poletti P, Bonomi L, Novellino L, et al. Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer. 2009;45:2537–2545. doi: 10.1016/j.ejca.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 58.Massi D, Brusa D, Merelli B, Falcone C, Xue G, Carobbio A, Nassini R, Baroni G, Tamborini E, Cattaneo L, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann Oncol. 2015;26:1980–1987. doi: 10.1093/annonc/mdv255. [DOI] [PubMed] [Google Scholar]
- 59.Massi D, Romano E, Rulli E, Merelli B, Nassini R, De Logu F, Bieche I, Baroni G, Cattaneo L, Xue G, et al. Baseline beta-catenin, programmed death-ligand 1 expression and tumour-infiltrating lymphocytes predict response and poor prognosis in BRAF inhibitor-treated melanoma patients. Eur J Cancer. 2017;78:70–81. doi: 10.1016/j.ejca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Melief SM, Visconti VV, Visser M, van Diepen M, Kapiteijn EH, van Den Berg JH, Haanen JBAG, Smit VTHBM, Oosting J, van der Burg SH, et al. Long-term survival and clinical benefit from adoptive T-cell transfer in stage IV melanoma patients is determined by a four-parameter tumor immune signature. Cancer Immunol Res. 2017;5:170–179. doi: 10.1158/2326-6066.CIR-16-0288. [DOI] [PubMed] [Google Scholar]
- 61.Melsted WN, Johansen LL, Lock-Andersen J, Behrendt N, Eriksen JO, Bzorek M, Scheike T, Hviid TVF. HLA class Ia and Ib molecules and FOXP3+ TILs in relation to the prognosis of malignant melanoma patients. Clin Immunol. 2017;183:191–197. doi: 10.1016/j.clim.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C, Corbu A, Perra MT, Sirigu P. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer. 2005;104:1246–1254. doi: 10.1002/cncr.21283. [DOI] [PubMed] [Google Scholar]
- 63.Rao UN, Lee SJ, Luo W, Mihm MC Jr., Kirkwood JM. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133:646–653. doi: 10.1309/AJCPTXMEFOVYWDA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saldanha G, Flatman K, Teo KW, Bamford M. A novel numerical scoring system for melanoma tumor-infiltrating lymphocytes has better prognostic value than standard scoring. Am J Surg Pathol. 2017;41:906–914. doi: 10.1097/PAS.0000000000000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tas F, Erturk K. Tumor infiltrating lymphocytes (TILs) may be only an independent predictor of nodal involvement but not for recurrence and survival in cutaneous melanoma patients. Cancer Invest. 2017;35:501–505. doi: 10.1080/07357907.2017.1351984. [DOI] [PubMed] [Google Scholar]
- 66.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 67.Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, Gruber SB, Gallagher RP, Zanetti R, Rosso S, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013;31:4252–4259. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuomaala S, Toivonen P, Al-Jamal R, Kivela T. Prognostic significance of histopathology of primary conjunctival melanoma in Caucasians. Curr Eye Res. 2007;32:939–952. doi: 10.1080/02713680701648019. [DOI] [PubMed] [Google Scholar]
- 69.Tuthill RJ, Unger JM, Liu PY, Flaherty LE, Sondak VK. Risk assessment in localized primary cutaneous melanoma: a Southwest oncology group study evaluating nine factors and a test of the Clark logistic regression prediction model. Am J Clin Pathol. 2002;118:504–511. doi: 10.1309/WBF7-N8KH-71KT-RVQ9. [DOI] [PubMed] [Google Scholar]
- 70.Weiss SA, Han SW, Lui K, Tchack J, Shapiro R, Berman R, Zhong J, Krogsgaard M, Osman I, Darvishian F. Immunologic heterogeneity of tumor-infiltrating lymphocyte composition in primary melanoma. Hum Pathol. 2016;57:116–125. doi: 10.1016/j.humpath.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsen TE, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 3. The relation between the tumour-associated lymphocyte infiltration and age and sex, tumour cell type, pigmentation, cellular atypia, mitotic count, depth of invasion, ulceration, tumour type and prognosis. APMIS Sec A Pathol. 1978;86A:523–530. [PubMed] [Google Scholar]
- 72.Kovacsovics-Bankowski M, Chisholm L, Vercellini J, Tucker CG, Montler R, Haley D, Newell P, Ma J, Tseng P, Wolf R, et al. Detailed characterization of tumor infiltrating lymphocytes in two distinct human solid malignancies show phenotypic similarities. J Immunother Cancer. 2014;2:38. doi: 10.1186/s40425-014-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vose BM, Moore M. Human tumor-infiltrating lymphocytes: a marker of host response. Semin Hematol. 1985;22:27–40. [PubMed] [Google Scholar]
- 74.Finke JH, Zea AH, Stanley J, Longo DL, Mizoguchi H, Tubbs RR, Wiltrout RH, O’Shea JJ, Kudoh S, Klein E. Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 75.Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin JL, Vivier E, Anderson P, Kiessling R. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 76.Ullman KS, Northrop JP, Verweij CL, Crabtree GR. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- 77.de Wolf C, van de Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human T cells used in anti-tumor immunotherapy. Cytotherapy. 2018;20:601–622. doi: 10.1016/j.jcyt.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang H, Hao S, Li F, Ye Z, Yang J, Xiang J. CD4+ Th1 cells promote CD8+ Tc1 cell survival, memory response, tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007;120:148–159. doi: 10.1111/j.1365-2567.2006.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci USA. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu J, Ren X, Cao S, Zhang W, Hao X. Th1 polarization and apoptosis-inducing activity of CD4+ T -cells in cytokine-induced killers might favor the antitumor cytotoxicity of cytokine-induced killers in vivo. Cancer Biother Radiopharm. 2006;21:276–284. doi: 10.1089/cbr.2006.21.276. [DOI] [PubMed] [Google Scholar]
- 83.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 84.Lee GR. Phenotypic and functional properties of tumor-infiltrating regulatory T cells. Mediators Inflamm. 2017;2017:1–9. doi: 10.1155/2017/5458178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


