ABSTRACT
Purpose: Exosomes, small extracellular vesicles (EVs) derived from the endocytic compartment of their parent cells, are present in plasma of cancer patients and may serve as non-invasive biomarkers of disease outcome. Here, we asked whether tumor-derived (TEX) and/or T-cell derived exosomes can predict outcome in head and neck squamous cell carcinoma (HNSCC) patients treated with oncological therapy.
Materials and Methods: 18 HNSCC patients enrolled in phase I clinical trial and receiving a combination of cetuximab, ipilimumab and radiation therapy were serially monitored for TEX and T cell-derived exosomes. Exosomes isolated from plasma by size exclusion chromatography were fractionated into TEX and CD3 + T cell-derived exosomes by immunocapture. Exosome-associated proteins were quantified by on-bead flow cytometry. Exosome molecular cargos of patients whose tumors recurred within 2 years (N = 5) were compared to cargos of patients who remained disease free at 2 years (N = 13) after therapy.
Results: The predictive value of the exosome molecular cargo for disease recurrence was evaluated pre-, during and post therapy. In patients whose disease recurred, total exosome proteins, TEX/total exosome ratios, total CD3+, CD3(-)PD-L1+ and CD3 + 15s+ (Treg-derived) exosomes increased from the baseline levels. In patients who remained disease free, total exosome protein and TEX levels decreased, CD3+ and CD3+ CD15s+ exosomes stabilized and CD3+ CTLA4+ exosomes declined after ipilimumab therapy.
Conclusion: TEX and T cell-derived circulating exosomes instead of immune cells were used for monitoring of patients’ responses to oncological therapy. The results support the potential role of exosomes as a non-invasive tumor and immune cell biomarkers in cancer.
KEYWORDS: Exosomes, T cell-derived exosomes, tumor derived exosomes, head and neck cancer, immunotherapy
Introduction
Head and neck squamous cell carcinomas (HNSCC) are among the most immunosuppressive malignancies. HNSCC are characterized by impaired functions of immune effector cells, dysregulated cytokine profiles and an increased frequency of intra-tumoral and circulating regulatory T cells (Treg).1-4 Furthermore, Treg in HNSCC patients express high levels of immune checkpoint ligands such as CTLA4 and effectively downregulate anti-tumor functions of cytotoxic T cells.5,6 Immune therapies aiming to rejuvenate patients’ immune responses are expected to have beneficial effects on disease control6 and represent a critical addition to the standard of care therapy for patients with HNSCC.
An EGFR-specific monoclonal antibody, cetuximab, is an established immunotherapy for HNSCC patients.4,7 Cetuximab improves survival when added to radiation therapy in locally advanced HNSCC or cytotoxic chemotherapy in recurrent/metastatic HNSCC.8-10 However, the response rate for cetuximab monotherapy in HNSCC patients is less than 15%, and our studies suggest this might be due to diverse effects of cetuximab on immune cells that simultaneously enhance cytotoxic functions of effector T and NK cells and promote the expansion of CTLA4+ Treg.11 Therefore, we hypothesized that the addition of a CTLA4 inhibitor, such as ipilimumab, to a cetuximab-based regimen might promote the ablation of Treg and contribute to a better clinical response. Based on this rationale, we conducted a phase I clinical trial in which patients with locally advanced HNSCC received a curative-intent combination of cetuximab, ipilimumab and radiation therapy (RT). Immune recovery was a secondary objective of the trial, and thus patients were immunologically monitored using standard immune measures. The availability of serially collected plasma specimens provided an opportunity for the isolation and molecular characterization of circulating exosomes.
Exosomes are membrane-bound nano-sized (30–150nm) extracellular vesicles (EVs) that are derived from the endocytic compartments of parental cells and are present in all body fluids.12,13 Among a broad variety of surface molecules exosomes carry are immune checkpoint receptors and ligands, and in their lumen are nucleic acids (mRNA, miRNAs, DNA) as well as numerous soluble factors and enzymes.14 The luminal content of exosomes is protected from external enzymatic degradation and thus can be safely conveyed to recipient cells. Because exosome content partly recapitulates that of parent tumor cells, 15 and because T cell-derived exosomes have been reported to reflect the molecular attributes of parent T lymphocytes, 16 plasma-derived exosomes may serve as biomarkers of prognosis, response to therapy and outcome in cancer.15,16
We reported earlier that relative to healthy donors, HNSCC patients with active disease had significantly increased levels of circulating exosomes, with a high enrichment in tumor-derived exosomes (TEX) and in immune suppressive factors.17-19 The levels of CTLA4 and PD-L1 ligands these exosomes carried on their surface correlated with disease activity, lymph node involvement and tumor stage.20,21 Recently, we have succeeded in the separation by immunocapture of CD3(+)T cell-derived exosomes and CD3(-) TEX-enriched exosomes from HNSCC patients’ plasma.22 This antibody-based separation of exosome subsets allowed for the identification of the cargo of exosomes derived from different parent cells (i.e., T cells vs. tumor cells) as also described by us for melanoma.23 Interestingly, TEX, as well as T cell-derived exosomes, carried similarly high levels of immunosuppressive markers in patients with advanced HNSCC.21 Moreover, T cell-derived exosomes in HNSCC patients with stage III/IV disease carried significantly higher levels of CD15s, a marker of functional Treg, 24 than did T cell-derived exosomes in patients with stage I/Il disease.21 These results suggested that plasma-derived exosomes of patients with HNSCC could serve as an easily accessible, non-invasive “liquid biopsy” of disease activity and stage. In this study, we used plasma samples from the patients enrolled in phase I clinical trial for the isolation of exosomes, separation of TEX from T cell-derived exosomes and evaluation of their potential role as biomarkers of response to therapy.
Results and discussion
This study is the first to investigate the use of exosomes and their subsets in the monitoring of HNSCC patients’ responses to oncological therapy. Of the 18 HNSCC patients treated with combination therapy (cetuximab, ipilimumab, and IMRT) in this clinical trial, five (28%) experienced tumor recurrence during the first 24 months of follow-up. Thirteen (72%) patients had no evidence of tumor progression during the same 24-month period. The study objective was to evaluate the predictive value of plasma-derived CD3(+) exosomes as surrogates for T lymphocytes and of CD3(-)TEX-enriched exosomes as surrogates for tumor cells. Instead of monitoring isolated lymphocytes or obtaining tumor biopsies, the profiling of exosomes derived from 1 mL of plasma was used to distinguish patients who benefited from oncological therapy from those who did not. By comparing the changes in exosome loads and cargos prior to and during therapy in patients who recurred vs those who remained NED in the 2-year follow-up period, it was possible to evaluate the potential role of plasma exosomes as biomarkers of the tumor as well as immune cells in cancer. Remarkably, despite the small patient numbers, which were limited to patients participating in the clinical trial, the results reached statistical significance in many, albeit not all, measurements of exosome molecular cargos.
Time points for exosome monitoring
Exosomes were studied in plasma obtained prior to surgery (at baseline) and at weeks 5 and 14 of therapy. These time points were selected since patients received cetuximab-RT for 5 weeks, then began treatment with ipilimumab given in four doses for 12 weeks and completed it at week 14 (Supplemental Figure 1). Specimens for week 14 for two patients (#7 and #8) were not available for analysis.
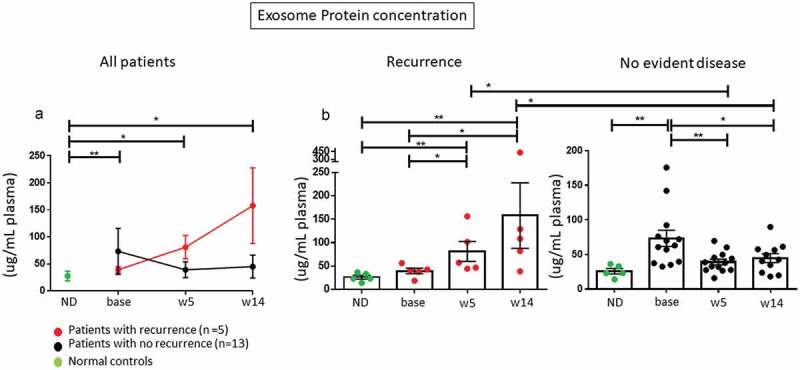
Exosome protein levels correlate with response to therapy
Exosome protein levels in patients’ plasma were higher at baseline in all patients relative to healthy donors, as also previously reported, 17 but increased during therapy only in patients who experienced a recurrence within the 24-month follow-up period. In contrast, plasma exosome levels in 13 patients with NED decreased relative to pre-therapy levels (Figure 1(a)). At baseline and throughout therapy, exosome plasma levels were higher in HNSCC patients than in healthy volunteers. Changes in exosome plasma levels from baseline to weeks 5 and 14 were significant for both groups (p < 0.05). However, patients with recurrence had higher levels of plasma exosomes at weeks 5 and 14 compared to lower levels in patients without disease (Figure 1(b) and Supplemental Table 1). The decrease from baseline in plasma exosome levels at week 5 in patients with no evident disease was highly significant (**p < 0.005). These changes in plasma exosome protein levels could be potentially related to the decreased production of exosomes by the tumor responding to therapy. In contrast, in patients with recurrence increased levels of total plasma exosomes suggest more active exosome production due to progressive disease.
Figure 1.
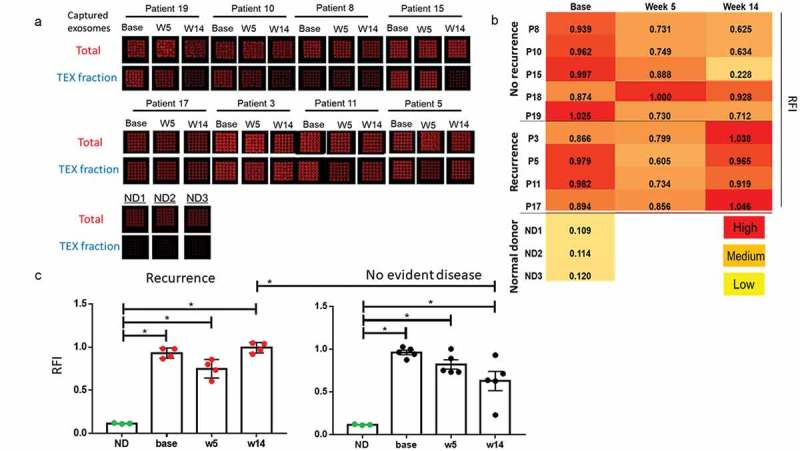
Changes in protein concentrations of circulating exosomes for HNSCC patients prior to and during therapy. (a): A decrease in plasma exosome protein concentrations in plasma is seen between baseline and weeks 5 and 14 in patients who remained disease free, while an increase is evident for patients whose disease progressed. (b): Individual exosome protein levels are shown for patients who progressed (n = 5) and those who did not (n = 13). Only patients with recurrent disease showed an overall increase in exosome protein levels during therapy (p < 0.05). Exosome protein levels decreased in patients who did not progress at week 5 (p < 0.005) and remained low at week 14 (p < 0.05) relative to baseline values. At week 14, patients who recurred had significantly higher exosome protein levels in plasma than patients who did not recur (p < 0.05). *p < 0.05 or **p < 0.005. In A the data are presented as mean values with connecting lines and error bars. In (b and c) and Figure 3–6, the data are presented as boxplots.
Plasma-derived exosomes in HNSCC patients are enriched in TEX
The bioprinted Ab microarrays containing a mix of Abs to antigens overexpressed in HNSCCs (Figure 2(a)) provided an opportunity to identify TEX and establish the ratios of TEX/total exosomes. The latter were captured on arrays bioprinted using anti-tetraspanin Abs (anti-CD63, -CD9, and -CD81;25). In Figure 3(a), TEX-enriched fractions from the plasma of four patients with recurrence (pts #3, 5, 11, and 17) and from the plasma of four NED patients (pts #8, 10, 15, and 19) collected at all time points gave a positive signal. The signal intensity varied among exosomes of different patients, suggesting that the TEX/total exosome ratios were different for each patient. No TEX were seen in total exosomes obtained from plasma of healthy volunteers.
Figure 2.
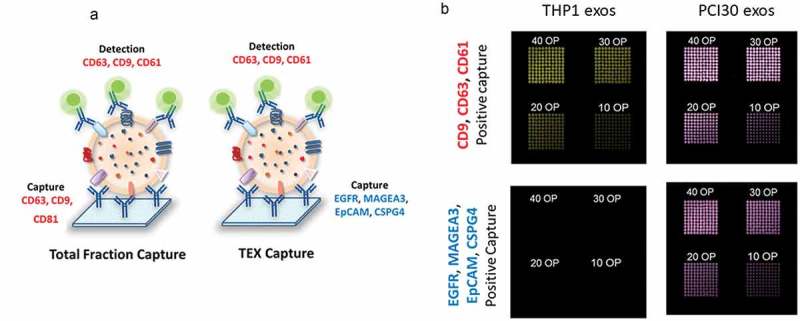
The microarray system developed for the capture of total exosomes or tumor-derived exosomes (TEX) from plasma. (a): Schematic depiction of the method. A cocktail of mAbs was bioprinted on a microarray plate, using anti-CD63, anti-CD9, and anti-CD81Abs for the capture of total exosomes and anti-EGFR, anti-MAGEA3, anti-EpCAM and anti-CSPG-4 Abs for TEX capture. The Abs are specific for antigens overexpressed on HNSCC and carried by HNSCC-derived exosomes. Total exosomes isolated from plasma by SEC were applied to the microarray and visualized with a detection cocktail of labeled anti-CD63, anti-CD9 and anti-CD61 Abs. (b): Evaluation of the method and titration of the capture Abs. As expected, THP1-derived exosomes did not bind to the microarray bioprinted using the Abs specific for antigens expressed by HNSCC. In contrast, PCI30-derived exosomes (TEX) bound to microarrays capturing total exosomes as well as microarrays capturing TEX. To optimize capture, different concentrations of primary Abs were tested as numbers of over prints (OPs), where 10 is the lowest OP, 40 is the highest OP, with the best results obtained at 40 OP.
Figure 3.
Microarray analysis of total exosomes and TEX isolated from plasma of the patients enrolled in the trial. (a): Images of the microarrays for total captured exosomes and the captured TEX fractions for seven patients at baseline, week 5, and week 14 and for three normal donors (ND). The images for four patients with recurrence are shown in the lower row. Note the variation in levels of captured TEX between the patients in higher and lower rows. (b): The same results are presented as a heatmap with superimposed values for the ratios of TEX RFI/total exosome RFI (RFI = relative fluorescence intensity). All patients showed similarly high TEX levels at baseline, with decreases at week 5. However, in the five patients with recurrence, TEX levels increased at week 14 (p = 0.03). C: Statistical analysis of data presented in (a and b). The RFI values of patients at all time points are significantly higher than of normal donors. At week 14 RFI values of patients with no evident disease are significantly lower than in patients with recurrence (*p < 0.05).
Changes in TEX levels during therapy
Total exosomes collected at all time points during therapy were captured on the Ab microarrays containing either the anti-tumor Ab cocktail or the Ab cocktail for capturing total exosomes (Figure 3(a)). The ratios of TEX/total exosomes were thus determined. While all patients had high TEX/total exosome ratios at baseline (Figure 3(b)), the ratios measured at weeks 5 and 14 varied among patients. Nearly all patients showed decreased ratios at week 5. However, the TEX/total exosome ratios of patients who remained disease-free remained low at week 14. In contrast, these ratios increased (p = 0.03) in four patients who recurred (Figure 3(c)). The data suggest that changes in TEX plasma levels during therapy reflect patients’ responses to therapy.
Changes in CD3(+) T cell-derived exosomes during therapy
As re-activated T cells could be a source of exosomes in plasma of patients treated with immunotherapy, CD3(+) T cell-derived exosomes were separated from CD3(-) TEX-enriched fractions by immunocapture, and changes in levels of CD3(+) exosomes were monitored throughout therapy. At baseline, CD3(+) exosome levels were higher (p < 0.05) in patients who later responded to therapy (Figure 4(a)), a finding potentially consistent with greater T-cell activation and lower immunosuppression in these patients. These changes in CD3(+) exosome levels were not evident when the entire patient cohort was evaluated, but in patients who recurred, the CD3(+) exosome frequency increased at week 5 only to return to the lower baseline level at week 14 (Figure 4(a)). In patients who remained disease free, levels of CD3(+) exosomes remained unchanged from baseline throughout therapy.
Figure 4.
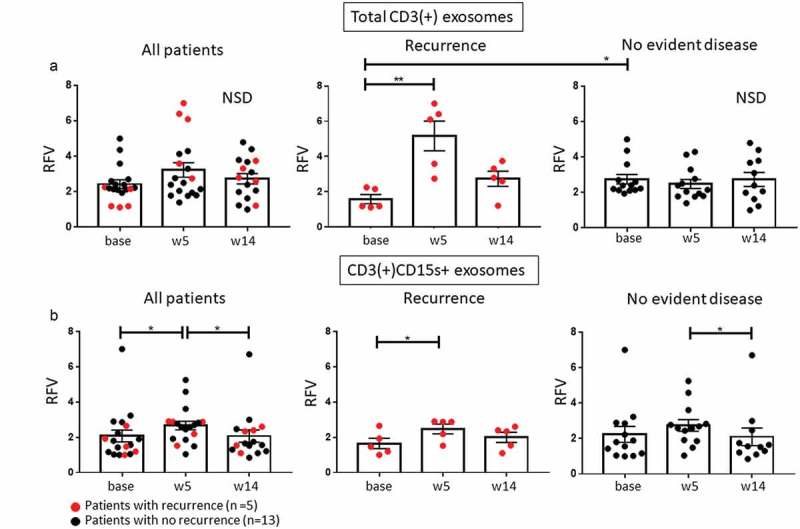
Changes in levels of CD3(+) exosomes and Treg-derived exosomes. (a): No significant change was seen in levels of CD3(+) T cell-derived exosomes at weeks 5 and 14 relative to baseline for all patients (NSD). However, at baseline, CD3(+) median exosome level was lower (p < 0.05) in patients with recurrence than in NED patients. (b): CD3(+)CD15s+ exosomes, presumably derived from Treg, increased significantly at week 5 in all patients but then normalized to baseline at week 14 in patients with no recurrence. Note the mild but sustained increase in CD3(+)CD15s+ exosomes in patients with recurrence (**p < 0.005; *p < 0.05).
Changes in CD3(+)CD15s+ exosomes derived from treg
Circulating CD3(+) exosomes carrying CD15s, considered to be a reliable marker of human effector Treg (eTreg;24), increased at week 5 (prior to the use of ipilimumab) in all patients (Figure 4(b)), implying a Treg expansion after cetuximab treatment (p < 0.05), in line with our previous report.11 However, a moderate expansion of CD3(+)CD15s+ exosomes at week 5 was only seen in patients with recurrence (p = 0.05). At week 14, levels of CD3(+)CD15s+ exosomes tended to normalize in both recurrent and NED patients. It should be emphasized that CD15s (sialyl Lewis x) is specific for highly activated and most suppressive FOXP3high subset of eTreg24 and CD15s+ exosomes only reflect changes in eTreg, not all Treg, in the patients’ circulation. Nevertheless, it should be noted that the moderate increase in eTreg-derived (CD3(+)CD15s+) exosomes in patients with recurrence at week 5 shown in Figure 4(b), paralleled increased levels of total CD3(+) exosomes (see Figure 4(a)). This parallel increase suggested that eTreg expansion contributes to the increased total CD3(+) exosome fraction in response to cetuximab in patients with recurrence. In contrast, in patients with NED after therapy, the CD3(+) total exosomes, as well as CD15s+ eTreg exosomes, remained at the baseline levels. Taken together, these data imply that cetuximab-driven increases from baseline in levels of total CD3+ and CD3+ CD15s+ exosomes as measured on week 15 of therapy were more pronounced in patients with recurrence than in patients with NED.
Changes in levels of CD3(+) exosomes carrying PD-L1 and/or CTLA4
Expression of checkpoint inhibitory ligands, PD-L1 and CTLA4, on T cells has been associated with upregulated suppression in Treg26 and with negative signaling of T cells in the TME.22 At baseline, levels of CD3(+)PD-L1+ exosomes were higher (p < 0.05) in patients who later recurred vs patients who remained disease free (Figure 5(a)). These levels declined at weeks 5 (p < 0.005; after cetuximab). No changes were seen in CD3(+)PD-L1+ exosomes of patients with NED.
Figure 5.
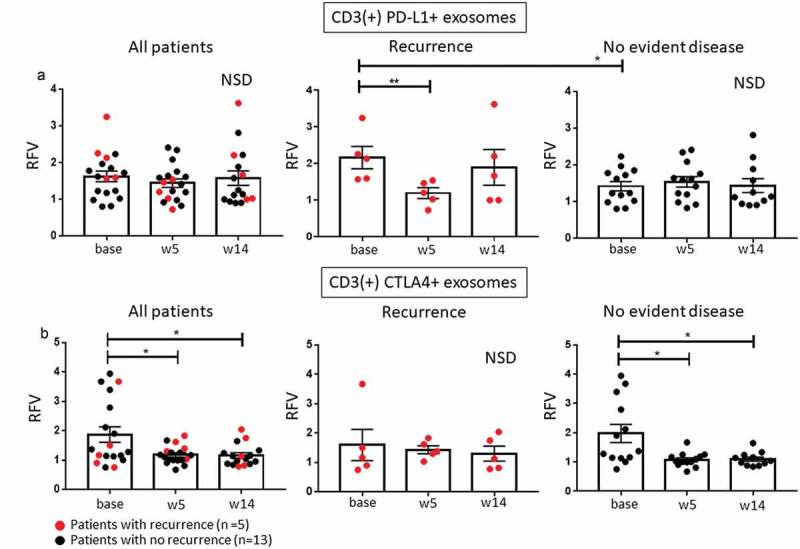
Changes in CD3(+) and CD3(-) exosome subsets carrying PD-L1 or CTLA4. (a): Changes in CD3(+)PD-L1 + T cell-derived exosomes. Results for all patients showed no changes during therapy. In patients who had a recurrence, levels of CD3(+)PD-L1+ exosomes were elevated at baseline (p < 0.05) but decreased at week 5 (p < 0.005). (b): Changes in CD3(+)CTLA4+ exosomes. Only patients with NED after therapy had elevated levels of CD3(+)CTLA4+ exosomes at baseline and showed a decrease in levels of these exosomes during therapy. Note that four-fifths patients with recurrence had low baseline levels of CD3(+)CTLA4+ exosomes. *p < 0.05, **p < 0.005; NSD: no significant difference for overall comparison of changes from baseline.
The levels of CD3(+)CTLA4+ exosomes were elevated at baseline in NED patients vs those who recurred but declined (p < 0.05) at weeks 5 and 14 (Figure 5(b)). The five patients with recurrence had low levels of circulating CD3(+)CTLA4+ exosomes at all time points except for one patient with high baseline levels. The data imply that patients with high baseline levels of CD3+ CTLA4 + T cells may selectively benefit from immunotherapy with CTLA4 antagonists such as ipilimumab. As both PD-L1 and CTLA4 are expressed on Treg and tumors, 27 it could be that exosomes carrying these ligands reflect responses of Treg or tumor cells to ipilimumab. However, it could also be that ipilimumab binds to circulating CTLA4+ exosomes, as has been reported for other therapeutic Abs, 28 and interferes with the accuracy of detection of CD3(+)CTLA4+ exosomes during and after therapy.
Changes in CD3(-) TEX-enriched exosomes carrying PD-L1 or CTLA4
In HNSCC patients, the CD3(-) exosomes are expected to be enriched in TEX. Changes in levels of CD3(-)CTLA4+ and CD3(-)PD-L1+ TEX were of interest because of our previous data linking the PD-L1 and CTLA4 exosome cargos to tumor-induced immune suppression.20-22 Figure 6(a and b) show that at baseline, these TEX-enriched subsets were enlarged (p < 0.05) only in patients who remained NED after therapy. In two-fifths of patients with recurrence, CD3(-)PD-L1+ exosomes were elevated (p = 0.056) at weeks 5 and 14 (Figure 6(a)). In contrast, levels of CD3(-)PD-L1+ TEX declined (p < 0.005) at week 5 relative to baseline in NED patients (Figure 6(a)). During therapy, CD3(-)CTLA4+ TEX declined only in disease-free patients (p < 0.05), whereas in patients with recurrence these levels remained unchanged (Figure 6(b)). The data imply that patients with a high content of CD3(-)PD-L1+ and CD3(-)CTLA4+ exosomes at baseline might benefit from immunotherapy, as also suggested by a therapy-induced decrease in levels of these exosomes only in patients with NED.
Figure 6.
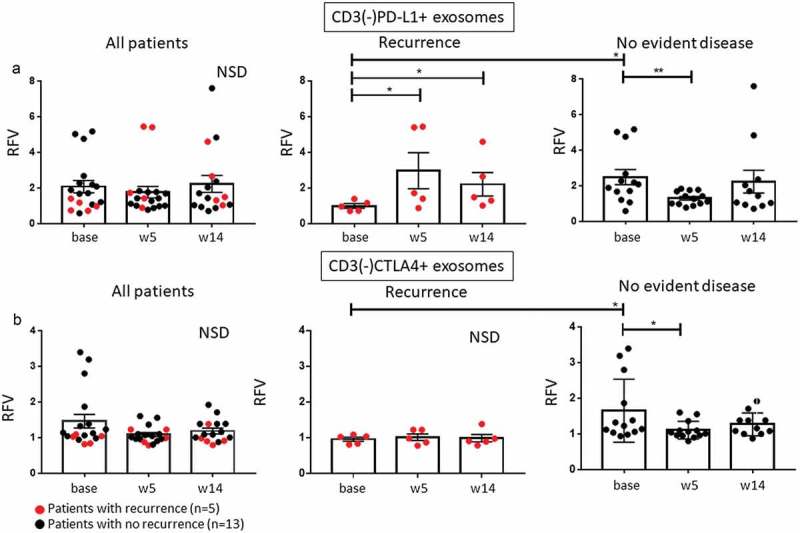
Changes in CD3(-)PD-L1+ TEX during therapy. (a): Patients who did not have recurrence had significantly increased levels of these exosomes at baseline relative to patients with recurrence with a significant decrease at week 5. Also, in patients with recurrence, there was a significant increase of CD3(-)PD-L1+ TEX at weeks 5 and 14 relative to baseline. (b): Changes in CD3(-)CTLA4+ TEX during therapy. Note that in patients with recurrence, the baseline levels of these exosomes were significantly lower than those in patients with NED and remained low throughout therapy, whereas in patients with no evident disease decreasing levels of CD3(-)CTLA4+ exosomes are visible during therapy. *p < 0.05, **p < 0.005; NSD: no significant difference for overall comparison of changes from baseline.
The results of this study support the predictive role of plasma exosomes as potential biomarkers in cancer. Advantages of using plasma exosomes in place of immune cells for non-invasive monitoring of the tumor and immune cell responses to immunotherapies are obvious. In this “proof of principle” study, HNSCC patients whose cancers recurred vs patients who remained disease free in the 2-year follow-up period had measurable and distinctly recognizable changes in exosome molecular cargos at baseline and during therapy. Despite the small patient cohort in this clinical trial, total exosome levels, TEX/total exosome ratios, phenotypic characteristics of TEX or of T cell-derived exosomes were shown to discriminate patients who responded or did not respond to oncological therapy. It is important to note that exosomes were monitored in the context of a clinical trial, where therapy and clinical events were strictly recorded and correlated. While the reported data await validation of the role of exosomes as biomarkers of response to oncological therapy, the exosome-based monitoring platform emerges as an advantageous strategy that allows for non-invasive acquisition of data that can be used for establishing correlations between immune and clinical endpoints.
Materials and methods
Study design
This study used specimens collected in phase I clinical trial designed to identify the recommended stage II dose of ipilimumab when administered with a standard combination of cetuximab and IMRT for high- or intermediate-risk patients with locally advanced HNSCC (see: NCT01935921). Patients with previously untreated AJCCv.7 stage III to IVb were included. The patients received combinatorial therapy consisting of an EGFR mAb, cetuximab; an anti-CTLA-4 mAb, ipilimumab; and standard IMRT (35 fractions of 2Gy delivered over 7 weeks). A phase I dose de-escalation design was used. Supplemental Figure 1 presents the study design.
Complying with ethics of experimentation
The phase I clinical trial was performed at the UPMC Hillman Cancer Center with the IRB approval # PRO13020549. Specimens for research studies were collected from patients or healthy volunteers who signed an IRB approved consent forms (approvals IRB#960279, IRB#0403105, and IRB#991206).
Study population
From July 2013 to May 2016, 18 patients were enrolled in the trial. Five patients had a tumor localized in the larynx, three in the hypopharynx, and ten in the oropharynx with three HPV(-) and seven HPV(+) patients. Two patients had stage III, 13 had stage IVa, and 3 had AJCC IVb tumors. Fourteen of the 18 patients were ever smokers. All patients were followed for disease progression, and the median follow up was 24 months (range 5–56 months). At >2 years from treatment termination, 13 patients had no evidence of disease (NED) as determined by clinical evaluation and scans in accordance with RECIST 1.1 criteria. One patient who died within 2 years of treatment termination was disease free. Additionally, peripheral blood of five healthy donors was obtained and plasma-derived exosomes were evaluated. All five healthy donors were male, and the mean age was 40 yrs.
Plasma specimens
Peripheral blood specimens were obtained from all patients at the designated time points before (baseline), during (week 5) and after (week 14) treatment (Supplemental Figure 1). Blood samples collected into heparinized tubes were centrifuged at 1,000xg for 10 min, and plasma specimens were stored in 1 mL aliquots at −80°C. Specimens were thawed immediately prior to exosome isolation.
Additionally, peripheral blood was obtained from three healthy male donors (IRB approval #04–001) to serve as exosome controls.
Cell cultures
For studies of exosomes from tumor cell supernatants, PCI30, an HNSCC HPV(-) cell line established in our laboratory29 and THP1, a monocytic cell line purchased from the American Tissue Culture Collection (Manassas, VA) were used. Cells were cultured in 150 cm2 culture flasks in 25 ml of DMEM for PCI30 (or RPMI for THP1) supplemented with 5% (v/v) penicillin and streptomycin and 10% (v/v) exosome-depleted fetal bovine serum (FBS, Fisher Scientific, Pittsburgh, PA) at 37°C in an atmosphere of 5% CO2 in air. The supernatants were harvested for exosome isolation at the confluency of 60% to 80%.
Exosome isolation by mini size-exclusion chromatography (mini-SEC)
Exosomes were isolated from plasma or cell supernatants by the mini-SEC method optimized in our laboratory (EV-TRACK ID: EV160007). Briefly, thawed plasma samples were centrifuged at 2,000xg for 10 min at room temperature (RT) and then for 30 min at 10,000xg at 4°C. After ultrafiltration (0.22 μm filter, EMD Millipore, Billerica, MA), 1 mL of plasma was placed on a mini-SEC column and eluted with PBS. The 4th 1mL-void volume fraction (i.e., fraction #4) contained the majority of intact and functionally active exosomes and was, therefore, collected as previously described.30
Characteristics of plasma-derived exosomes
Exosomes isolated by mini-SEC were evaluated for their size by qNano (Izon) and for morphology by transmission electron microscopy.30 The protein concentration of the isolated exosome fraction #4 was determined using a BCA protein assay kit (Pierce Biotechnology, Rockford, lL). Exosomes were concentrated using Vivaspin 500 (VS0152, 300,000 MWCO, Sartorius, Göttingen, Germany). For flow cytometry analysis, 10μg of exosome protein in 100μL PBS were used.
Exosome separation into CD3(+) and CD3(-) fractions
Exosomes isolated from plasma of each patient by mini-SEC (fraction #4) were separated into CD3(+) and CD3(-) subsets by immunoaffinity-based capture using anti-CD3 Abs as previously reported22 and described in Supplemental Methods.
Detection of proteins carried by exosomes
The on-bead flow cytometry was used for detection of proteins carried by exosomes as previously reported20-22 and described in Supplemental Methods. The following detection Abs were used: anti-PD-L1 PE (12–5983-42, e-Bioscience) or anti-PD-1 PE (12–2799-42, e-Bioscience), anti-CTLA4-APC (349908, Biolegend), anti-CD15s-PE (sc-32243, Santa Cruz), anti-CD3-PE (12–0037-42, e-Bioscience). Isotype control Abs were used in all experiments.
Detection of exosome cargo components was performed using the Gallios flow cytometer equipped with Kaluza 1.0 software (Beckman Coulter, Krefeld, Germany). Samples were run for 2 min and around 10,000 events were acquired. Gates were set on the bead fraction visible in the forward/sideward light scatter. Reproducibility of this flow cytometry-based detection assay was established as described in Supplemental Methods. Flow cytometry results of a representative experiment are shown in Supplemental Figure 3.
Bioprinting of antibodies for exosome capture on microarrays
Printing of Abs to be used for exosome capture in microarrays was performed as described in Supplemental Methods. The following Abs were used for capture of total exosomes: anti-CD9 (ab58989, Abcam, Cambridge, UK), anti-CD63 (NBP2-42225AF647, Novusbio), anti-CD81 (ab59477, Abcam). The Abs cocktail for the capture of tumor-enriched exosomes (TEX) consisted of anti-EGFR1 (ab52894, Abcam), anti-MAGEA3 (ab38496, Abcam), anti-EpCAM (ab75813, Abcam) and anti-CSPG4 (a gift from Dr. Soldano Ferrone, Harvard Medical School). As positive and negative controls, human IgG and PBS were used. Figure 2(a) is a graphic illustrating the strategy used for capture of TEX from plasma-derived total exosomes. Titrations of optimal Ab concentrations for TEX capture and testing of microarrays specificity for TEX are illustrated in Figure 2(b) and described in Supplemental Methods.
Capture of TEX from plasma-derived exosomes by microarrays
Aliquots of total exosomes (5ug protein) isolated from plasma specimens of HNSCC patients or of normal donors were applied to the microarray and processed as described in Supplemental Methods. The results are presented as relative fluorescence intensity (RFI) ratios of TEX/total exosomes. The captured exosomes were eluted from microarrays with 100 mM of glycine-HCl, pH 2.4 for 30 min and analyzed by dynamic light scattering (Supplemental Figure 2(a)) and electron microscopy (Supplemental Figure 2(b)) to verify their identity. Reproducibility of TEX capture was determined as described in Supplemental Methods.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 7, GraphPad Software, La Jolla, CA 92037 USA). The data are presented as scatter plots depicting means and SEM. Since this is a small cohort (n = 18) the distribution of normality was tested with the Kolmogorov-Smirnov test and no normal distribution was visible. For comparison between different time points of one group (either in patients with recurrence or in patients with no evident disease), the paired, non-parametric Wilcoxon matched-pairs signed rank test was used. For comparison between time points in recurrence vs non-recurrence groups or between normal donors and different time points of patients the non-parametric, unpaired Mann–Whitney test was used. A p-value of <0.05 was considered significant.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft [TH 2172/1-1]; National Institutes of Health [P50CA097190]; National Institutes of Health [RO-1CA 168628]; National Institutes of Health [R21-C205644].
Disclosure of Potential Conflict of Interest
No potential conflicts of interests were disclosed.
Financial Support
This work has been supported in part by NIH grants RO-1CA 168628 and R21-C205644 to TLW, P50CA097190 to RLF, and by the Deutsche Forschungsgemeinschaft to M-N Theodoraki (research fellowship # TH 2172/1-1).
Author Contributions
MNT performed all experiments, analyzed the data and wrote the manuscript. YS performed microarray experiments. WG consulted on statistical analyses. JO, AB and JEB conducted the clinical trial. RLF designed and conducted the clinical trial, supervised collection and processing of specimens, read and edited the manuscript. TLW designed the study, interpreted results and edited the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33:3293–3304. doi: 10.1200/jco.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteside TL. Head and neck carcinoma immunotherapy: facts and hopes. Clin Cancer Res. 2018;24:6–13. doi: 10.1158/1078-0432.CCR-17-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/jco.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauman JE, Ferris RL. Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer. 2014;120:624–632. doi: 10.1002/cncr.28380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argiris A, Heron DE, Smith RP, Kim S, Gibson MK, Lai SY, Branstetter BF, Posluszny DM, Wang L, Seethala RR, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28:5294–5300. doi: 10.1200/jco.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer. 2015;3:54. doi: 10.1186/s40425-015-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 10.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 11.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RL. CTLA-4(+) regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015;75:2200. doi: 10.10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017;189:259–267. doi: 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atay S, Godwin AK. Tumor-derived exosomes: A message delivery system for tumor progression. Commun Integr Biol. 2014;7:e28231. doi: 10.4161/cib.28231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert Rev Mol Diagn. 2015;15:1293–1310. doi: 10.1586/14737159.2015.1071666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23:4843–4854. doi: 10.1158/1078-0432.ccr-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126:1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 20.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24:896–905. doi: 10.1158/1078-0432.ccr-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theodoraki MN, Hoffmann TK, Jackson EK, Whiteside TL. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin Exp Immunol. 2018;194:67–78. doi: 10.1111/cei.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theodoraki MN, Hoffmann TK, Whiteside TL. Separation of plasma-derived exosomes into CD3((+)) and CD3((-)) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin Exp Immunol. 2018;192:271–283. doi: 10.1111/cei.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, Whiteside TL. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7:1435138. doi: 10.1080/20013078.2018.1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, Claer L, Hingorani R, Balderas R, Rohrer J, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci U S A. 2015;112:7225–7230. doi: 10.1073/pnas.1508224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteside TL. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. 2018;22:353–363. doi: 10.1080/14728222.2018.1451514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Fala F, Fabbi M, Kato T, Lucarelli E, Donati D, Polito L, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 28.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 29.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, Ferris RL, Lai SY. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29:163–188. [DOI] [PubMed] [Google Scholar]
- 30.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5:29289. doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


