ABSTRACT
Chronic non-bacterial prostatitis (CNP) is a common urologic disease that is linked to the development of prostate cancer. Long non-coding RNA (lncRNA) GAS5 has been identified to mediate cell proliferation in prostate cancer, although its role in CNP is still unclear. Human prostate epithelial cell line RWPE-1 was induced by lipopolysaccharide (LPS) to mimic CNP model in vitro. Real-time PCR was performed to determine the expression of GAS5 and COX-2, while western blotting was used to evaluate the protein expression of COX-2. The interaction between GAS5 and COX-2 was determined using RNA pull-down and RNA immunoprecipitation (RIP). Cell proliferation was determined using MTT assay. The expression of GAS5 was decreased, while COX-2 was increased in prostatitis tissues and in LPS-induced RWPE-1 cells. The overexpression of GAS5 suppressed the protein level of COX-2, and inhibited cell proliferation of LPS-induced RWPE-1 cells and HPECs, which was rescued by the co-transfection with pcDNA-GAS5 and pcDNA-COX-2. GAS5 was confirmed to promote the ubiquitination of COX-2, and the in vivo GAS5-overexpressed CNP rat model decreased the motor scores, the volume of prostate tissues, the average number of inflammatory cells, prostatic proliferation, and COX-2 expression. Our findings revealed that overexpression of GAS5 inhibited cell proliferation via negatively regulating the expression of COX-2, thus alleviating the progression of CNP.
KEYWORDS: Cell proliferation, chronic non-bacterial prostatitis, COX-2, long non-coding RNA GAS5, ubiquitination
Introduction
Chronic non-bacterial prostatitis (CNP) is a common urologic disease that mainly appeared in older men, and is identified as the most important risk for the development of prostate cancer. Patients suffered from CNP often feel genitourinary pain in the presence of uropathogenic bacteria or malignancy. Statistics revealed that the morbidity of CNP accounts for about 70%-90% of all prostatitis patients and with an increased ratio in recent years [1,2]. To date, much research has reported that the non-coding RNAs (ncRNAs), proteins might affect the development of CNP, while the potential mechanism was still unclear. Additionally, the diagnosis of CNP is based on the prostate secretion in the nowadays, while the potential biomarkers for CNP diagnosis and therapy were still not available.
COX, including COX-1 and COX-2 isoforms, is an enzyme that is responsible for the production of prostaglandins from arachidonic acid. COX-2 is identified as an inducible protein and is up-regulated under stimulation [3]. It has been reported that the increased COX-2 is involved in prostate cancer [4]. In addition, the chronic inflammation could result in an up-regulation of COX-2, while the increased COX-2 promoted cell proliferation of human prostate epithelial cells [5]. While whether COX-2 mediates cell proliferation in CNP was still unclear.
LncRNA is a set of ncRNAs with a length of more than 200 nucleotides. Mounting studies have reported that lncRNA plays a key role in various cellular events. For example, lncRNA NEAT1 was involved in the ovarian cancer cell metastasis [6]. LncRNA MALAT-1 regulated EMT in non-small-cell lung cancer [7]. LncRNA MEG3 was mediated in chronic myeloid leukemia cells [8]. In the inflammation response, lncRNA XIST is involved in the female cell under inflammation [9]. LncRNA MALAT1 serves as an epigenetic regulator in diabetic retinopathy [10]. The growth arrest-specific 5 (GAS5), a single-stranded lncRNA, was originally found to be accumulated in growth-arrested cells [11]. It has been reported that GAS5 is involved in tumor proliferation, migration, inflammation, as well as epithelial-to-mesenchymal transition (EMT) in various diseases, such as cancers, neurogenic diseases, endocrine disorders [12–14]. A recent study revealed that GAS5 suppressed prostate cancer cell proliferation and development by targeting miR-103 via AKT/mTOR signaling pathway [15]. Another study demonstrated that GAS5 was decreased in autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus [16]. However, whether GAS5 was involved in the CNP has not been reported.
In the present study, the in vivo and in vitro study was employed to examine the role of COX-2 and GAS5 in CNP. In addition, the interaction between COX-2 and GAS5, and the potential mechanism of COX-2 and GAS5 in cell proliferation were also explored in order to evaluate the validity of COX-2 and GAS5 in CNP.
Materials and methods
Real-time PCR (RT-PCR)
Total RNA was isolated from tissues or cells using the TRIzol reagent kit (Invitrogen, Shanghai, China). The quality of the RNA was determined using 1% agarose gel electrophoresis. The 1 μg cDNA was synthesized using the RNA and the reverse transcription kit (Bio-Rad, USA). Real-time PCR was performed using real-time PCR reagents on an ABI7500 Real-Time PCR System (Applied Biosystems, USA). The relative gene expressions were determined using (ΔΔCt) method on three individual experimental times.
Western blot
Cells or tissues were lysed using RIPA lysis buffer (Beyotime, Beijing, China). The protein concentration was determined using the Bradford Protein Assay (BCA) method. Proteins were applied at 10% SDS-PAGE, and equal amount of protein was transferred onto PVDF membrane, and incubated with the primary antibodies (anti-COX-2, 1:1000, Catalogue number: ab179800, Abcam, Shanghai, China; anti-β-actin, 1:1000, Catalogue number: ab8226, Abcam) at 4°C for 24 h. Then, the PVDF membrane was clearly washed using PBS and incubated with the second antibody (horseradish peroxidase-conjugated goat anti-goat, Catalogue number: A0181, Beyotime) at room temperature for 1 h. The bands were visualized using ECL reagent.
RNA pull-down
Biotin-labeled full-length GAS5 was transcribed in 293T cells with a Biotin RNA Labeling Mix Kit and T7 RNA polymerase (Invitrogen, Shanghai, China). Then, they were isolated with an RNeasy Mini kit and purified by using magnetic beads. The RNA was detected through qRT-PCR analysis, and the proteins were detected by western blotting.
RNA immunoprecipitation (RIP)
RIP experiments were performed using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) according to the manufacturer’s instructions. Briefly, a sum of 2 μg of cell extract was mixed with TLR4 antibody (Abcam) precipitated agarose beads. Beads were washed three times with GLB+ lysis, and the retrieved protein was detected using western blotting. The co-precipitated RNAs were determined using real-time PCR.
Cell culture
The human prostate epithelial cell line (RWPE-1) was purchased from American Type Culture Collection (ATCC) and cultured in Keratinocyte Serum Free Medium (Invitrogen) with 5% CO2 at 37°C. Primary human prostate epithelial cells (HPECs) from American ScienCell Laboratory were purchased through Enzyme-Linked Biotechnology Co. (Shanghai, China) and cultured in RPMI-1640 medium supplemented with 10% FBS. After they were passaged at 80% confluence, cells were centrifuged and washed using PBS. Then, they were stimulated by using lipopolysaccharide (LPS) (10 μ/ml) for 24 h and were centrifuged and collected.
Cell transfection
The RWPE-1 cells and HPECs were seeded in a 24-well plate for 24 h. Lipofectamine2000 (Invitrogen) were used for cell transfection. The overexpression or the down-regulation of the genes and their negative control were all synthesized in Invitrogen (Shanghai, China).
Cell proliferation
Cell proliferation was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium cell viability assay (MTT assay). Briefly, RWPE-1 cells and HPECs were planted in 96-well plates at a density of 2.5 × 104 cells/well and cultures with RPMI plus 10% FBS for 24 h. Prior to measuring the viability, the media were removed and replaced with 200 μl of fresh RPMI plus 10% FBS medium and then 10 μL of MTT reagent (5 mg/ml, Sigma Aldrich) was added to incubate cells for 4 h at 37°C. Cells were centrifuged and the supernatant was discarded. Samples were dissolved using DMSO, and the absorbance was recorded at 490 nm in a VERSAmax microplate reader to determine the concentration, which is proportional to the number of live cells.
Establishment of CNP rat model
A total of 36 male Sprague-Dawley (SD) rats, weighing 240–260 g (4–6 weeks old) were purchased from Experimental Animals center of Shanghai and housed in a ventilated cage at 25 ± 5°C under a 12-h light/dark cycle in the humid environment. The rats were randomly divided into six groups, and each group included six rats. Experimental protocols were approved by the Animal Ethical Committee of the First Affiliated Hospital of Soochow University, and all procedures were performed in accordance with the guidelines for the laboratory animal use.
To establish the CNP model, rats were injected with 20 μL 1% carrageenan into the prostate under anesthesia. They were also injected with the lentivirus carrying pcDNA-GAS5 (5 × 107 TU/mL) to overexpress GAS5. The control group received the same volume of saline and the lentivirus carrying empty plasmid pcDNA. The locomotion score was defined using the scale of 1–5; 1 referred to no parameter was affected compared with baseline behavior, and 5 referred to a maximum severity of symptoms.
Then, the animals were sacrificed and the prostate was isolated. The prostates were divided into two parts: One was used for hematoxylin-eosin; the rest was stored for further usage. The inflammatory cells were quantified as density per unit area by randomly counting on four spots under high-power fields (HPF, 400magnification). The positive cells of rat ventral prostate were estimated in 20 microscopic fields, at a magnification of 6400, randomly selected from two different histological sections per animal. The cell proliferation was calculated using MTT. At least 2000 cells were counted in each group.
Statistical analysis
Data were presented as mean ± SD. Statistical analysis was performed by using student’s t-test with two-tailed value in the GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA) for two-group comparisons, while one-way ANOVA was used for multi-group comparisons. p < 0.05 was considered a significant difference. All experiments were repeated for three independent times.
Results
The expression of GAS5 and COX-2 in prostatitis tissues
The long non-coding RNA GAS5 has been reported to be involved in tumor proliferation, migration, inflammation in various diseases [12–14], and it was also demonstrated to suppress prostate cancer cell proliferation [15]. To determine the expression pattern of GAS5 and COX-2 in prostatitis tissues, we established the rat model of CNP by 20 μL 1% carrageenan injection in the prostate. As shown in Figure 1, the expression of GAS5 was significantly decreased in prostatitis tissues (Figure 1(a), p < 0.05), while the mRNA and protein levels of COX-2 were dramatically increased in prostatitis tissues (Figure 1(b), p < 0.05).
Figure 1.

The expression of GAS5 and COX-2 in prostatitis tissues. The rat model of CNP was established by 20 μL 1% carrageenan injection in the prostate. The sham group was injected with the same volume of saline. There were seven rats in each group. (a) The expression of GAS5 was determined using RT-PCR. (b) The expression of COX-2 was determined using real-time PCR and western blot. (*p < 0.05 vs sham). CNP, chronic non-bacterial prostatitis.
The expression of GAS5 and COX-2 in LPS induced cell lines
-To further identify the expression pattern of GAS5 and COX-2 in prostatitis cells, we stimulated the human prostate epithelial cell line (RWPE-1) by using LPS to induce CNP in vitro, and the expression of GAS5 and COX-2 was determined by RT-PCR and western blotting analysis. The results showed that the expression of GAS5 was markedly decreased in LPS-induced RWPE-1 cells (Figure 2(a), p < 0.05), but both mRNA level and protein level of COX-2 were significantly enhanced after LPS stimulation (Figure 2(b), p < 0.05).
Figure 2.
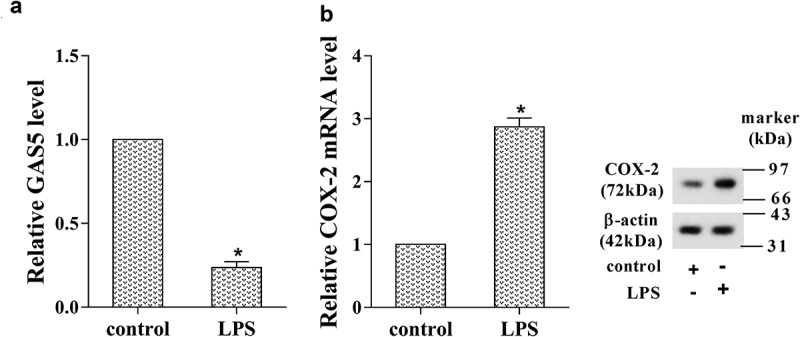
The expression of GAS5 and COX-2 in the prostatitis cell line. The human prostate epithelial cell line (RWPE-1) was stimulated by LPS to induce CNP in vitro. (a) The expression of GAS5 was determined using RT-PCR. (b) The expression of COX-2 was determined using RT-PCR and western blot. (*p < 0.05 vs control). LPS, lipopolysaccharide.
The interaction between GAS5 and COX-2
To determine the interaction between GAS and COX-2, RWPE-1 cells and HPECs were explored. GAS5 was considered to regulate the expression of COX-2 by RNA pull-down and RIP assay (Figure 3(a,b), p < 0.05). GAS5 knockdown was performed in RWPE-1 cells and HPECs by si-GAS5 transfection, and the down-regulation of GAS5 was confirmed by RT-PCR (Figure 3(c), p < 0.05). The protein level of COX-2 was significantly increased after the knockdown of GAS5 in LPS-induced RWPE-1 cells and HPECs, while the COX-2 level was markedly decreased after the overexpression of GAS5 in LPS-induced RWPE-1 cells and HPECs (Figure 3(c)). However, the up-regulation or down-regulation of GAS5 did not affect the mRNA expression of COX-2 (Figure 3(d), p > 0.05). Overexpressed GAS5 in LPS-induced RWPE-1 cells and HPECs significantly promoted proteolytic degradation after it was conducted by cycloheximide (CHX) (Figure 3(e), p < 0.05). Furthermore, the COX-2 protein binding to ubiquitin was significantly increased after GAS5 overexpression, indicating that up-regulation of GAS5 could reduce the COX-2 protein level by promoting ubiquitination (Figure 3(e), Supplement Figure 1).
Figure 3.

The interaction between GAS5 and COX-2. (a) The protein of the GAS5 pull-down compound. (b) The level of GAS5 in the protein sample of TLR4. (c) The expression of GAS5 and COX-2 in LPS-induced RWPE-1 cells and HPECs after the down-regulation or the up-regulation of GAS5 by RT-PCR and western blotting. (d) The expression of COX-2 mRNA in LPS-induced RWPE-1 cells and HPECs after the down-regulation or the up-regulation of GAS5 by RT-PCR. (e) Relative COX-2 level in LPS-induced RWPE-1 cells and HPECs co-treated with GAS5 and CHX, an inhibitor of protein synthesis. The ubiquitin level after overexpression of GAS5 was detected by western blotting. (*p < 0.05 vs NC, si-control, or pcDNA). NC, negative control; LPS, lipopolysaccharide; CHX, cycloheximide.
Overexpression of GAS5 suppressed COX-2 and inhibited proliferation of prostatitis cells
To explore the potential role of GAS5 in prostatitis cells, LPS-induced RWPE-1 and HPECs cells were transfected with pcDNA-GAS5. The results of RT-PCR confirmed the up-regulation of GAS5 after transfection (Figure 4(a), p < 0.05). The RT-PCR and western blotting results indicated a significant reduction of COX-2 (Figure 4(b), p < 0.05). We also detected the prostaglandin E2 (PGE2) level after GAS5 overexpression. As shown in Figure 4(c), the PGE2 level was dramatically reduced after GAS5 overexpression, indicating the protective effect of GAS5 in prostatitis cells (p < 0.05).
Figure 4.
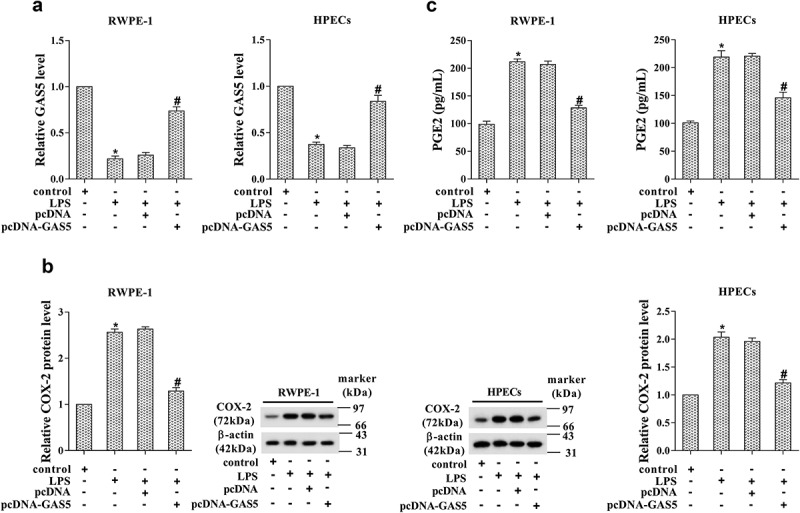
Overexpression of GAS5 suppressed COX-2 expression. (a) The expression of GAS5 in LPS-induced RWPE-1 cells and HPECs was determined by RT-PCR after pcDNA-GAS5 transfection. (b) The protein level of COX-2 after pcDNA-GAS5 transfection was determined by western blotting. (c) The level of PGE2 in LPS-induced RWPE-1 cells and HPECs was detected after pcDNA-GAS5 transfection. (*p < 0.05 vs control; #p < 0.05 vs LPS+pcDNA). LPS, lipopolysaccharide; PGE2, prostaglandin E2.
In cell proliferation, overexpression of GAS5 suppressed LPS-induced RWPE-1 and HPECs cell proliferation, which was reversed by co-transfection with pcDNA-GAS5 and pcDNA-COX-2 (Figure 5, p < 0.05). Taken together, our findings indicated that GAS5 suppressed prostatitis cell proliferation by targeting COX-2.
Figure 5.
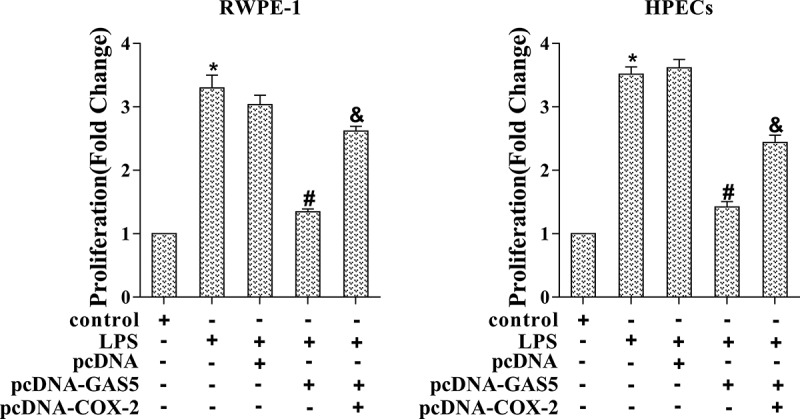
Prostatitis cell proliferation was regulated via GAS5/COX-2. LPS-induced RWPE-1 cells and HPECs were transfected with pcDNA-GAS5 or co-transfected with pcDNA-GAS5 and pcDNA-COX-2. Cell proliferation was determined by MTT assay. (*p < 0.05 vs control, #p < 0.05 vs LPS+pcDNA, &p < 0.05 vs LPS+pcDNA-GAS5). LPS, lipopolysaccharide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium cell viability assay.
Overexpressed GAS5 alleviated the injury of CNP in vivo
To further identify the role of GAS5 on the CNP, the GAS5-overexpressed CNP rat model was established by the injection with the lentivirus carrying pcDNA-GAS5. After the injection, the locomotion score, prostate volume, inflammatory cell count at high-power fields (HPF), and PCNA positive cells were dramatically decreased, indicating the protective effect of GAS5 in the CNP rat model (Figure 6(a-d), p < 0.05). The prostate tissues were obtained after the sacrifice of CNP rats, and the level of GAS5 and COX-2 was determined by RT-PCR and western blotting. As shown in Figure 6(e), GAS5 was confirmed to be up-regulated, and the level of COX-2 was markedly decreased in prostatitis tissues (p < 0.05).
Figure 6.
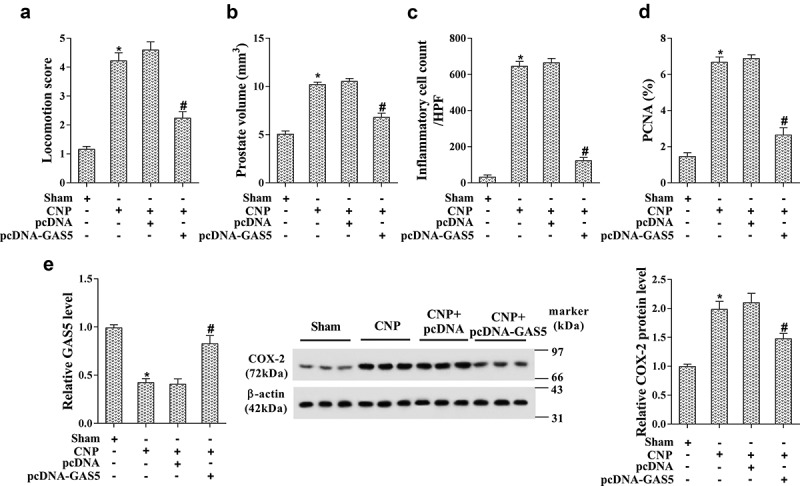
Overexpressed GAS5 alleviated the injury of CNP in vivo. The GAS5-overexpressed CNP rat model was established by the injection with the lentivirus carrying pcDNA-GAS5. The sham group was injected with the lentivirus carrying empty plasmid pcDNA. There were six rats in each group. (a) The locomotion scores. (b) The prostate volume (c) The average number of inflammatory cells in the prostate. (d) The cell proliferation was determined by the number of PCNA-positive cells in prostate tissue. (e) The expression of GAS5 and COX-2 was detected by RT-PCR and western blotting. (*p < 0.05 vs sham, #p < 0.05 vs CNP+pcDNA). CNP, chronic non-bacterial prostatitis; HPF, high power field; PCNA, proliferating cell nuclear antigen.
Discussion
CNP is a global public health problem that severely threatens men’s mental and physical wellness. Nevertheless, the understanding of the mechanism underlying CNP is still limited. Therefore, to identify the potential biomarkers in CNP seemed important for its clinical therapy. It has been suggested that prostate disorders, such as CNP, showed elevated cell proliferation in prostate epithelial cells [17]. In the present study, we found that the LPS stimulated prostate epithelial cell line RWPE-1 showed an increased cell proliferation. Additional studies also proved that LPS stimulation could induce prostatic inflammation [18,19].
Various studies have demonstrated that COX-2 was increased under the condition of diseases [20]. It has been reported that COX-2 expression was markedly increased in prostate cancer tissues and cells [21], and COX-2 showed positive role on prostate carcinogenesis [5]. In the present study, the LPS-induced RWPE-1 cells also showed an increased expression of COX-2, as compared with the normal cells, indicating that COX-2 might be involved in the development of CNP.
GAS5 has been identified in many diseases, as well as in the autoimmune, inflammatory, and infectious diseases. It has previously been reported that Gas5 gene is involved in the development of glomerulonephritis, the expression of GAS5 was varied in different diseases, for example, Gas5 level was down-regulated in CD4 T-cells and B-cells in patients with rheumatoid arthritis, while it was elevated in patients with multiple sclerosis [16]. The expression of GAS5 was also different in patients under sarcoidosis, tuberculosis or virus infection; however, it is still not clear whether GAS5 is involved in CNP. It is possible that the alteration of GAS5 levels modulates tissue actions of prostate cancer, and it acts as a tumor repressor in prostate cancer [15]. In the present study, GAS5 was decreased in LPS-induced RWPE-1 cells compared with the control, indicating that the down-regulated GAS5 might promote the development and progression of CNP. What is more, down-regulated GAS5 reduced the YBX1 protein level by binding YBX1 to decrease the expression of YBX1-transactivated p21 and further abolish G1 phase cell cycle arrest in stomach cancer [22]. Herein, COX-2 expression was altered along with the LPS treatment. The RNA pull-down and RIP assay confirmed that GAS5 directly regulates the expression of COX-2. In addition, the expression level of COX-2 was suppressed by GAS5, indicating that GAS5 regulated COX-2 expression by directly binding with it.
Prostaglandin E2 (PGE2) is the product of COX-2, it has been reported that Ketorolac is the inhibitor of PGE2 [23]. The increased COX-2 also resulted in a higher level of PGE2 in carrageenan-induced CNP rat model [24]. In our present study, the level of PGE2 was increased in LPS treated RWPE-1 cells, while pcDNA-GAS5 transfection significantly decreased the PGE2 level. The expression pattern of PGE2 was in accordance with the level of COX-2, indicating that the level of PGE2 might also the biomarker of CNP, which deserved further investigation.
In conclusion, the present study identified that the expression of GAS5 was decreased, while COX-2 was increased in prostatitis tissues and cells. GAS5 inhibited prostatitis cell proliferation by negatively regulating the expression of COX-2. The in vivo experiment confirmed that overexpressed GAS5 could alleviate the injury of CNP. The present study uncovered the mechanism of cell proliferation in CNP, which will provide a novel target for the therapy of CNP.
Funding Statement
This paper was not funded.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
This study was approved by the Institute Research Medical Ethics Committee of The First Affiliated Hospital of Soochow University. All animal-treatment operations were executed according to the Soochow University Ethical Guidelines for Animal Experiment.
Supplemental material
Supplemental data for this article can be accessed here.
References
- [1].Fei X, Jin W, Hua S, Song Y.. Prospective study on association of prostatic calcifications with clinical symptoms and results of treatment in men with type iii prostatitis. Sci Rep. 2017;7(1):5234–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nickel JC, Freedland Stephen J, Castro-Santamaria R, Moreira Daniel M. Chronic prostate inflammation predicts symptom progression in patients with chronic prostatitis/chronic Pelvic Pain. J Urol. 2017;198(1):122–128. [DOI] [PubMed] [Google Scholar]
- [3].Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299(1):125–140. [DOI] [PubMed] [Google Scholar]
- [4].Bieniek J, Childress C, Swatski MD, et al. COX-2 inhibitors arrest prostate cancer cell cycle progression by down-regulation of kinetochore/centromere proteins. Prostate. 2014;74(10):999–1011. [DOI] [PubMed] [Google Scholar]
- [5].Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61(1):60–72. [DOI] [PubMed] [Google Scholar]
- [6].Liu Y, Wang Y, Fu X, et al. Long non-coding RNA NEAT1 promoted ovarian cancer cells’ metastasis via regulating of miR-382-3p/ROCK1 axial. Cancer Sci. 2018;109(7):2188–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu J, Weng Y, He F, et al. LncRNA MALAT-1 competitively regulates miR-124 to promote EMT and development of non-small-cell lung cancer. Anticancer Drugs. 2018;29(7):628–636. [DOI] [PubMed] [Google Scholar]
- [8].Li Z, Yang L, Liu X, et al. Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomed Pharmacother. 2018;104:181–192. [DOI] [PubMed] [Google Scholar]
- [9].Shenoda BB, Tian Y, Alexander GM, et al. miR-34a-mediated regulation of XIST in female cells under inflammation. J Pain Res. 2018;11:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Biswas S, Thomas AA, Chen S, et al. MALAT1: an epigenetic regulator of inflammation in diabetic retinopathy. Sci Rep. 2018;8(1):6526–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54(6):787–793. [DOI] [PubMed] [Google Scholar]
- [12].Liu B, Wu S, Ma J, et al. lncRNA GAS5 reverses EMT and tumor stem cell-mediated gemcitabine resistance and metastasis by targeting miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yue Q, Zhao C, Wang Y, et al. Downregulation of growth arrestspecific transcript 5 alleviates palmitic acidinduced myocardial inflammatory injury through the miR26a/HMGB1/NFkappaB axis. Mol Med Rep. 2018;18(6):5742–5750. [DOI] [PubMed] [Google Scholar]
- [14].Dai SP, Jin J, Li WM. Diagnostic efficacy of long non-coding RNA in lung cancer: a systematic review and meta-analysis. Postgrad Med J. 2018;94(1116):578–587. [DOI] [PubMed] [Google Scholar]
- [15].Xue D, Zhou C, Lu H, et al. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol. 2016;37(12):16187–16197. [DOI] [PubMed] [Google Scholar]
- [16].Mayama T, Marr AK, Kino T. Differential expression of glucocorticoid receptor noncoding RNA repressor Gas5 in autoimmune and inflammatory diseases. Horm Metab Res. 2016;48(8):550–557. [DOI] [PubMed] [Google Scholar]
- [17].Dang T, Liou GY. Macrophage cytokines enhance cell proliferation of normal prostate epithelial cells through activation of ERK and Akt. Sci Rep. 2018;8(1):7718–7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dos Santos Gomes FO, Oliveira AC, Ribeiro EL, et al. Intraurethral injection with LPS: an effective experimental model of prostatic inflammation. Inflamm Res. 2018;67(1):43–55. [DOI] [PubMed] [Google Scholar]
- [19].Locatelli M, Macchione N, Ferrante C, et al. Graminex pollen: phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules. 2018;23(5):1145–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–266. [DOI] [PubMed] [Google Scholar]
- [21].Gupta S, Srivastava M, Ahmad N, et al. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42(1):73–78. [DOI] [PubMed] [Google Scholar]
- [22].Liu Y, Zhao J, Zhang W, et al. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci Rep. 2015;5:10159–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bucci FA Jr., Waterbury LD. Prostaglandin E2 inhibition of ketorolac 0.45%, bromfenac 0.09%, and nepafenac 0.1% in patients undergoing phacoemulsification. Adv Ther. 2011;28(12):1089–1095. [DOI] [PubMed] [Google Scholar]
- [24].Han P, Lai YJ, Chen J, et al. Protective potential of the methanol extract of Macrothelypteris oligophlebia rhizomes for chronic non-bacterial prostatitis in rats. Pak J Pharm Sci. 2016;29(4):1217–1221. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


