ABSTRACT
The microRNA miRNA-1225-5p (miR-1225) is known as an essential modulator of the development of multiple cancers and other biological reactions. However, the understanding of its contribution to pancreatic cancer (PC) is insufficient. The effects of miR-1225 on PC cell survival and tumorigenesis in vivo as well as on the modulation of cell apoptosis were investigated. The expression of miR-1225 was upregulated in 20 human LC samples from acute myeloid leukemia patients with adverse prognosis and poor responses to therapy as well as in several human PC cell lines, as compared to that in healthy tissues, normal tissues, and normal pancreatic cells. In contrast, Janus kinase 1 (JAK1) expression was downregulated in human-derived PC samples and PC cell lines. EdU staining demonstrated that the aberrant expression of miR-1225 impaired the proliferation and survival of these two PC cell lines. The depletion of miR-1225 expression increased the apoptosis of both PANC-1 and AsPC-1 cells, as revealed by the TdT-mediated dUTP nick end labeling (TUNEL) staining and flow cytometry results. The results of dual-luciferase reporter assay indicated that miR-1225 targeted the 3′-untranslated region of JAK1 for silencing. Silencing of JAK1 expression counteracted the suppressive influence of miR-1225 depletion in PC cells. Thus, these results offer an insight into the biological and molecular mechanisms underlying the development of PC and provide potential strategies for PC treatment.
KEYWORDS: Pancreatic cancer, miR-1225-5p, apoptosis, JAK1
Introduction
Pancreatic cancer (PC) has an extremely low 5-year survival rate (2% to 9%) and ranks among the deadliest tumors. It is the seventh leading cause of cancer-related death worldwide [1]. The last few decades have observed a rapid increase in the incidence of PC in China [2,3]. Despite the improvement in the comprehension of PC, its prognosis remains unsatisfactory, mainly owing to non-specific symptoms, lack of curative treatments, and delayed diagnosis [4,5]. Hence, the discovery of new-generation PC biomarkers is desirable to provide more reliable and accurate treatment options.
Some risk factors associated with PC include environmental and genetic factors as well as smoking [6]. Micro-RNAs (miRNAs) are non-coding RNA molecules (length: <22 to 25 nucleotides) that exert post-transcriptional effects on the expression of specific genes [7]. Increasing evidences have indicated the essential role of miRNAs in various biological events, including apoptosis. For instance, miR-34a is downregulated in tumors and participates in many cellular events such as cell differentiation, apoptosis, and proliferation [8–10]. miR-1225 is thought to repress the expression of insulin receptor substrate-1 and activate the signaling of beta-catenin to inhibit the proliferation and metastasis of gastric carcinomas [11]. miR-1225 is thought to interfere with type I interferon (IFN) function through IFN/Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway. The infections caused by Newcastle disease virus, Sendai virus, hepatitis C virus, and many other IFN-susceptible viruses may be suppressed through the silencing of the endogenous miR-1225 expression [12]. In adult renal progenitor cells (ARPCs), the expressions of paired box 2 (PAX2), CD133, and other key renal progenitor markers are regulated by miR-1225 and miR-1915, and the upregulated expression of Toll-like receptor 2 (TLR2) in ARPCs was attributed to the downregulated miR-1225-5p level [13].
Here, we evaluate the influence of miR-1225 expression on PC generation and progression. Expression of miR-1225 was upregulated in PC cells and in specimens from patients suffering from PC. Furthermore, miR-1225 inhibition impaired the proliferation of PC cells in vitro and in vivo and suppressed the tumor growth. The depletion of miR-1225 expression resulted in the induction of apoptosis of PC cells. Our results suggest that PC cells employ miR-1225 to inhibit apoptosis through the abatement of JAK1 expression.
Materials and methods
Experimental samples
The subjects were selected from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Paired cancer tissues and adjacent normal tissues collected from these patients were used in the study. Informed consent in written form was provided by all subjects. The present study was carried out based on the declaration of Helsinki under the Ethical Approval of the Ethics Review Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Cell culture and transfection
Dulbecco’s modified Eagle’s medium (DMEM) was used in this study. This medium contained glutamine (1%) and penicillin/streptomycin (1%) and was supplemented with fetal bovine serum (FBS; 10%) and used for the cultivation of PC cell lines (AsPC-1 and PANC-1 cells), and normal pancreatic cells (MIA PaCa-2 cells). These two cell types were transfected with a miR-1225 inhibitor (50 nmol/L) and a negative control (NC) (Ambion, Austin, TX) using Lipofectamine RNAiMAX (Life Technologies), as per the manufacturer’s instructions. The short hairpin RNA (shRNA, 5′-GGT TAG AAG ACC TGA TCG A-3′) targeting JAK1 was located at +825 to +998 relative to the transcription start site. However, the NC of this shRNA (5′-GCG ATC TAC TCA AGT CAA A-3′) was not specific to any mammalian genomic sequence.
miR-1225 inhibitor preparation
The miR-1225 inhibitor (5′-GUG GGU ACG GCC CAG UGG GGG G-3′) and NC inhibitor (5′-CUC CCA CUG CUU CAC UUG ACU A-3′), were obtained from Creative Biogene. After cell transfection, the mature endogenous miR-1225 was inhibited by the miR-1225 inhibitor. The miR-1225 inhibitor is a double-stranded miRNA, which is chemically modified. Sodium chloride (NaCl; 0.9%) was used for the preparation of miR-1225 and NC inhibitors at a final concentration finally of 10 mg/mL.
Analysis of cell proliferation
The proliferation of cells was assayed using the cell-light 5-ethynyl-20-deoxyuridine (EdU) Apollo Imaging Kit (RiboBio, China). A fluorescence microscope was used to measure the percentage of EdU-positive cells.
Western blot analysis
After sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the cell lysates (20 g), separated protein bands were transferred onto polyvinylidene fluoride membranes. The membranes were blocked and treated with primary and secondary antibodies. Beta-actin and CADM1 antibodies were obtained from Abcam. The Amersham ECL Western Blotting detection system was used for signal detection.
RNA extraction and quantitative polymerase chain reaction (qPCR)
The extraction of total RNA was performed with TRIzol reagent using tissues or treated cells, as per the instructions of the manufacturer. Roche Light-Cycler 480 Real-Time PCR system (Roche, Germany) and SYBR Green were used to assay the levels of miR-206, with glyceraldehyde-3-phosphate (GAPDH) as an internal control. Sequences of primers for detection of indicated mRNAs are showing as follows: miR-1225: 5′-GTG GGT ACG GCC CAG TGG GGG G-3; JAK1 F: 5′-ATT GGA GAC TTC GGC CTG AC-3′; JAK1 R: 5′-GGG TGT TGC TTC CCA GCA TC-3′; GAPDH F 5′-TGC ACC ACC AAC TGC TTA GC-3′; GAPDH R: 5′-AGC TCA GGG ATG ACC TTG CC-3′. Using the SYBR Green PCR Master Mix, quantitative real-time PCR was carried out at 95°C in 20 μL reaction volume for 10 min, followed by 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The endogenous reference was used for normalization and the amount of target (2−ΔΔCT) was calculated relative to a calibrator (mean of the controls).
Colony generation assay
Cells were transfected using various reagents. Cells were resuspended in DMEM supplemented with 10% FBS after 2 days of transfection and plated on an 8-mm layer of 0.4% top agar, followed by transfer into 12-well plates containing 0.5 mL of 0.5% bottom agar. After 14 days, four regions were randomly chosen from each plate and colonies were quantified.
TdT-mediated dUTP nick end labeling (TUNEL) assay
The TUNEL assay was carried out to measure the apoptosis-related changes in cells; the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:5000; Beyotime, China) and cells were stained using TUNEL fluorescence kit (Roche, Basel, Switzerland). In addition, we evaluated the apoptosis of cells under a laser scanning confocal microscope (SP8, Leica, Japan) by counting the number of TUNEL-positive cells.
Analysis of apoptosis
Annexin V/propidium iodide (PI) staining and flow cytometry (FC) assay were performed for the characterization of apoptotic cells [14].
Dual-luciferase reporter assay (DLRA)
The target gene of miR-1225 was confirmed by 3′-untranslated region (UTR) luciferase reporter assay, wherein the wide-type (WT, 5′-GAC CCA TGG TCT CCC GAA CCC AT-3′) and mutant (MU, 5′-ATG GGT AGG TCT CCC GAA CCC AT-3′) 3′-UTR of JAK1 were used. The reporter luminescence (Rluc) was studied using the sequence of Renilla luciferase, while the calibration luminescence (Luc) was assayed using the firefly luciferase sequence. The vectors and miRNA mimic were added into cells, and the cells were cultured for 24 h. A DLRA system was used to study the luciferase activity.
Animal tests
BALB/c-nu mice (female, aged 5 weeks) were purchased from Vital River (Beijing, China). AsPC-1 cells (2 × 106) were stably transfected with shRNA-1225 or NC. All mice were subcutaneously injected with these cells through the right oxter following acclimatization for 3 days. The weights of tumors were expressed in grams, and the formula (L × W2)/2 was used to calculate the volume of tumors. On day 30 after injection, these mice were sacrificed. All animal tests were carried out under the approval of the Institutional Animal Care and Use Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Statistical analysis
The data obtained in this study are represented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to study the comparisons between two test groups, along with the two-tailed Student’s t-test. A value of P < 0.05 was considered to indicate statistically significant differences between groups.
Results
miR-1225 is upregulated in PC tissue and cell lines
We examined the expression of miR-1225 in PC tissues using qPCR. As per the National Cancer Center Network (NCCN) guidelines, three groups of patients were selected as follows: better, medium, and poor prognosis groups. As shown in (Figure 1(a)) the poor and medium prognosis groups showed significantly upregulated miR-1225 expression in PCs compared to the better prognosis group and the normal control group. In comparison to the surrounding normal pancreatic tissues, tumors showed an increase in the expression of miR-1225 (Figure 1(b)). In addition, AsPC-1 and PANC-1 cells exhibited increased miR-1225 level as compared with the normal pancreatic cells (Figure 1(c)). Therefore, the expression of miR-1225 was higher in pancreatic tumors, suggesting that the prognostic effect was poor and the therapeutic efficacy was unsatisfactory.
Figure 1.
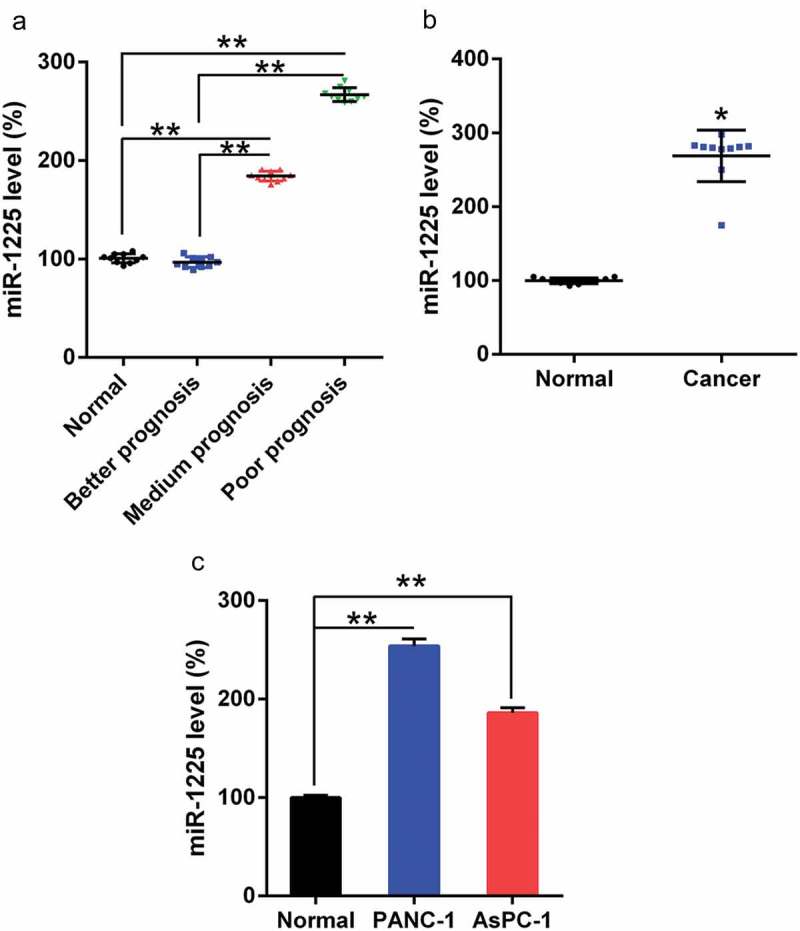
miR-1225 expression in PC tissues of patients and PC cell lines. (a) miR-1225 expression in pancreatic tumors cells from better prognosis (n = 10), medium prognosis (n = 10), and poor prognosis (n = 10) patients with AML and normal volunteer (n = 10), as analyzed using qPCR. (b) miR-1225 expression was compared between PC tumors cells and normal pancreatic tissues from volunteers. (c) miR-1225 expression in PC cell lines and normal pancreatic cells. Data are represented in the form of mean ± SD. *P< 0.05, **P< 0.01.
miR-1225 inhibition represses tumorigenesis of PC in mice
To determine the effect of miR-1225 on xenograft pancreatic tumor formation, AsPC-1 cells transfected with Lentiviral miR-1225 inhibitor or Lentiviral NC inhibitor were subcutaneously injected into BALB/c mice, and the tumor growth was monitored daily. The expression of miR-1225 was detected in the pancreatic tissue of mice from each group. miR-1225 expression was confirmed to be silenced in mice from miR-1225 silencing group (Figure 2(a)). These mice were killed on day 28 after injection, and the pancreatic tissues were excised and weighed (Figure 2(b)). miR-1225-silenced tumors grew at a much slower rate than the tumors from the control group. Furthermore, miR-1225-silenced tumors showed an obviously lower mean tumor weight than the tumors from the control group (Figure (2c, d)).
Figure 2.

miR-1225 silencing inhibits xenograft pancreatic tumor formation. (a) Expression of miR-1225 in the pancreatic tissue of each group was examined by qPCR analysis. (b) AsPC-1 cells infected with Adenovirus-miR-1225 inhibitor (Inhibitor) or control cells (NC) were subcutaneously injected into mice (n = 10 per group). Mice were sacrificed, and tumors were weighed at day 28 post-inoculation. Tumors were weighed after excision from each group. (c) Representative photographs of nude mice at day 30 post-inoculation. (d) Tumor growth curve during 28 days post-inoculation. Data represent the mean ± SD. *P< 0.05, **P< 0.01.
miR-1225 inhibition suppresses the proliferation of PC cells and induces their apoptosis
The effect of miR-1225 on PC cells was investigated. In PANC-1 and AsPC-1 cells, the transfection of the miR-1225 inhibitor resulted in a significant reduction in miR-1225 level (Figure 3(a, b)). EdU assay results revealed a significant decrease in the proliferation of these cell types at 24–72 h post-transfection of miR-1225 inhibitor as compared with cells from the normal and NC groups (Figure 3(c, d)). The soft agar colony formation assay results showed that the aberrant expression of miR-1225 also caused a noticeable reduction in the number of colonies formed by these two cells types, while transfection with NC mimic had no effect on colony numbers (Figure 3(e, f)).
Figure 3.
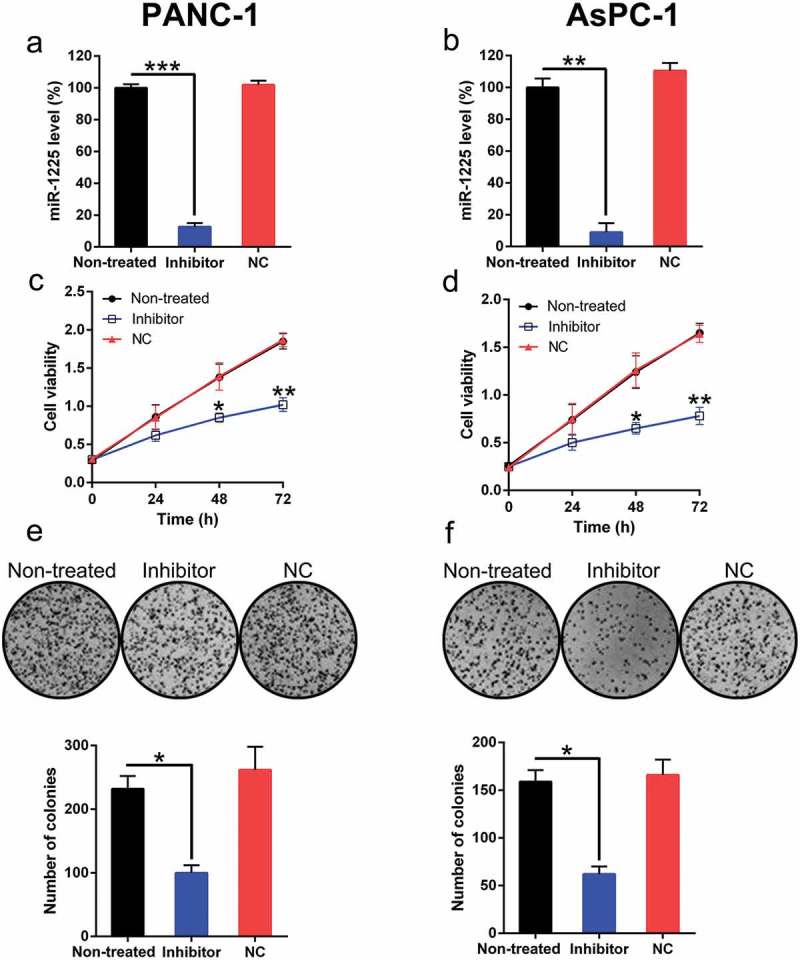
Effect of miR-1225 expression on the proliferation of PANC-1 and AsPC-1 cells. (a, b) Expression of miR-1225 in PANC-1 and AsPC-1 cells transfected with the miR-1225 inhibitor (Inhibitor) or NC inhibitor (NC), as measured by qPCR. (c, d) Proliferation rate of PANC-1 and AsPC-1 cells was measured at 24, 48, and 72 h post-transfection using the EdU assay. (e, f) Soft agar colony formation assay of the PANC-1 and AsPC-1 cells transfected with the miR-1225 inhibitor or NC inhibitor. The lower panel indicates the number of colonies formed in each group. Data are represented in the form of mean ± SD. *P < 0.05, **P < 0.01, ***P< 0.001.
Both the viability and proliferation of these two cell types were impaired following the inhibition of miR-1225 expression. Therefore, we hypothesized that miR-1225 depletion exerted a stimulatory effect on apoptosis in PC cell lines. Both TUNEL staining (Figure 4(a, b)) and FC (Figure 4(c, d)) assay results indicated an obvious increase in the apoptosis of PC cells transfected with the miR-1225 inhibitor as compared to that of cells from the normal and non-transfected groups.
Figure 4.
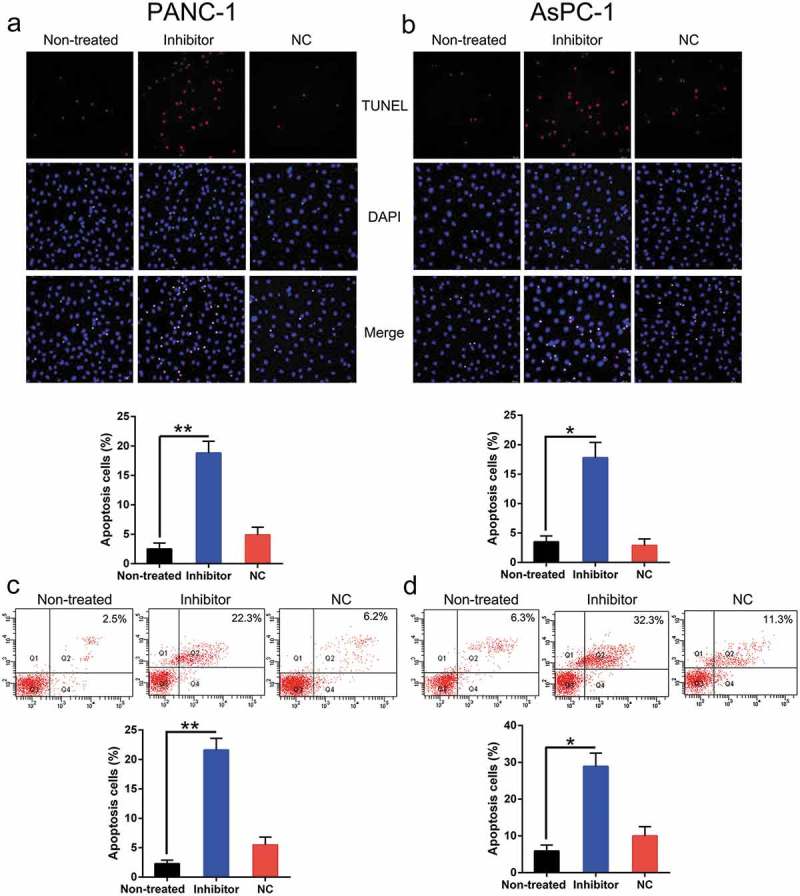
Aberrant expression of miR-1225 enhances the apoptosis of PC cell lines. PANC-1 and AsPC-1 cells were transfected with the miR-1225 inhibitor (Inhibitor) or NC inhibitor (NC). (a, b) TUNEL staining was carried out to examine the number of apoptotic cells in each group of PANC-1 and AsPC-1 cells. Magnification, ×200. Positive Hoechst 33,342 staining in each group of these cells is displayed in the lower panel. (c, d) Annexin V-FITC/PI staining and FC were performed to evaluate the number of apoptotic cells. The upper right quadrant of each plot represents early apoptotic cells. Apoptotic rate analysis in PANC-1 and AsPC-1 cells from each group, as shown in the lower panel. Data are represented in the form of mean ± SD. *P < 0.05, **P < 0.01 versus control group.
miR-1225 targets the 3′-UTR of JAK1
Bioinformatic analyses have shown that miR-1225 may target the 3′-UTR of JAK1 (Figure 5(a)). The direct relationship between miR-1225 and 3′-UTR of JAK1 was investigated using DLRA (Figure 5(b)). The function of luciferase was inhibited by 50% in cells transfected with the miR-1225 mimic fused to the 3′-UTR of JAK1 as compared to that in cells from the control groups. We evaluated the expression of miR-1225 in PC tissues and PC cells. As shown in (Figure 5(c)), pancreatic tumors showed decreased JAK1 expression, as well as its downstream sensor STAT3, comparing to the surrounding normal pancreatic tissues. JAK1 level in these two PC cell lines was reduced as compared to that observed in normal pancreatic cells (Figure 5(d, e)). The effects of miR-1225 inhibitor on the expression of JAK1 in these two PC cell lines were investigated by western blotting and qPCR. Protein and mRNA levels of JAK1 were increased following the transfection of cells with the miR-1225 inhibitor (Figure 5(f-i)). Meanwhile, STAT3 expression was also increased after miR-1225 inhibition. These results demonstrate that JAK1 expression increased after the silencing of miR-1225 expression and that the 3′-UTR of JAK1 gene was targeted by miR-1225.
Figure 5.

JAK1 is a direct target of miR-1225. (a) Graphical representation of the conserved miR-1225 binding motif at the 3′-UTR of JAK1. (b) Luciferase activity displayed by the luciferase reporter constructs carrying either the wild-type (WT) or mutated (MU) human JAK1 3′-UTR after miR-1225 mimic transfection. The luciferase activity was normalized to the activity of β-galactosidase. miR-1225 mimic markedly decreased the relative luciferase activity in cells carrying the WT 3′-UTR but not in those MU 3′-UTR. PANC-1 and AsPC-1 cells were transfected with the miR-1225 inhibitor (Inhibitor) or NC inhibitor (NC). Western blotting (c) showed the expression of JAK1 and STAT3 in PC cell lines. qPCR analysis (d, e) was used to confirm the downregulation in JAK1 expression in PC tumors and PC cell lines. Western blotting (f, g) and qPCR (h, i) were performed to evaluate JAK1 and STAT3 protein and mRNA expressions, respectively, after transfection of PANC-1 and AsPC-1 cells with miR-1225 inhibitor and NC inhibitor. Data are represented in the form of mean ± SD. *P < 0.05, **P < 0.01, ***P< 0.001.
JAK1 silencing diminishes the influence of miR-1225 on the proliferation and apoptosis of PC cell lines
To determine whether JAK1 silencing suppresses the influence of miR-1225 on PC proliferation and apoptosis, JAK1 transcription was silenced in the two PC cell lines. Western blotting and qPCR analyses were used to verify the changes in the expression of JAK1 (Figure 6(a–d)). The silencing of JAK1 also caused an inhibited expression of downstream STAT3. The depletion in the expression of JAK1 resulted in the recovery of the proliferation of these two cell lines inhibited by miR-1225 inhibitor, as evident from the results of EdU assay (Figure 6(e, f)). FC was performed to evaluate the apoptosis of PC cell lines. Aberrant expression of JAK1 led to a noticeable decrease in the number of apoptotic cells following transfection with the miR-1225 inhibitor (Figure 6(g, h)). The number of apoptotic cells noticeably decreased among cells subjected to CDC14B silencing as well as normal PC cells. Meanwhile, our data also showed that apoptosis-related proteins Caspase-3 and its cleavage were increased due to miR-1225 transfection, while silencing of JAK1 led to a reduction of Caspase-3 activity (Figure 6(a, b)). Thus, JAK1 silencing was sufficient to restore the proliferation and inhibit the apoptosis of cells.
Figure 6.

Silencing of JAK1 expression restores the suppressive effect of miR-1225 on the characteristics of PC cell lines. PANC-1 and AsPC-1 cells were co-transfected with the miR-1225 inhibitor (Inhibitor) and shRNA-JAK1 (JAK1i)/shRNA-NC (NCi). (a-d) Expression of JAK1, STAT3, Caspase-3, and cleaved Caspase-3 (cCaspase-3) was examined in PANC-1 and AsPC-1 cells at both protein and mRNA levels following transfection with different agents. JAK1i represent RNA interference of CDC14B. (e, f) JAK1 silencing induced the proliferation of PANC-1 and AsPC-1 cells. (g, h) JAK1 knockdown eliminated the apoptotic effect of the miR-1225 inhibitor. Annexin V-FITC/PI staining and FC were carried out to evaluate the number of early apoptotic PANC-1 and AsPC-1 cells after 48 h of transfection. Results are presented as mean ± SD. *P < 0.05, **P < 0.01.
Discussion
Tumorigenesis is a synergetic complex process associated with several oncogenes and anti-oncogenes. Although PC is well studied, the molecular mechanisms underlying PC development are partially understood. In the present study, miR-1225 known to exhibit increased expression in PC cells and tumor specimens suppressed the expression of JAK1, a key modulator belonging to the JAK/STAT signal pathway. Inhibition of miR-1225 resulted in the suppression of cell viability and proliferation and promoted apoptosis of both PANC-1 and AsPC-1 cells. Further investigation revealed the direct binding of miR-1225 to the 3′-UTR of JAK1 gene, resulting in the abrogation of JAK1 expression. The knockout of JAK1 expression may reverse the effect of miR-1225 on PC proliferation. Our findings strongly suggest that miR-1225 performs an oncogenic function by targeting the JAK1 gene and provide evidence for the use of miR-1225 as a potential agent for PC therapy.
The regulating effects of miRNAs on the expression of downstream target genes have been studied in different cancer types. Multiple target genes could negatively regulate the translation or trigger the cleavage of molecules by facilitating the binding of miRNAs to their 3′-UTR. Therefore, we aimed to evaluate the expression of miR-1225-regulated downstream target genes in the present study. Through bioinformatic tools and DLRA, JAK1 was confirmed as the target of miR-1225. According to our study, miR-1225 played a significant role in PC tumorigenesis as well as in the proliferation of the two PC cell lines, at least in part, by targeting the 3′-UTR of JAK1. The JAK/STAT pathway (from cytokine receptors to the nucleus) is known to be activated after receptor ligation and has been recognized as an essential apoptosis-associated signaling pathway [15,16]. The activation of the cytokine receptors may facilitate the stimulation of JAK. As a consequence, the STAT family of transcription factors was phosphorylated and activated. Activated STAT could facilitate the transcription of Bcl-XL, wherein the interaction between Bax and Bcl-XL or Bcl-2 was prevented and apoptosis was promoted [17,18]. Here, we demonstrated that the JAK signaling was deactivated in both PC tumors and PC cell lines and that JAK1 activation was significantly increased following transfection with an miR-1225 inhibitor. These data suggest that miR-1225 exerts its anti-apoptotic effect in PC cells partially through the JAK/STAT pathway. JAK/STAT signaling was also reported to be a modulator of PC in many other studies. In patients with locally advanced or metastatic PC, desirable therapeutic effects were observed after the administration of erlotinib and gemcitabine. According to Chen et al., the phosphorylation levels of STAT and JAK may be regulated with this combination treatment, thereby inhibiting the recurrent tumor growth and facilitating cell apoptosis in vivo. In the subsequent step, the expressions of caspase-3 and caspase-9 were upregulated owing to the downregulation of hypoxia-inducible factor 1-alpha (HIF1α) and cyclin D1 (downstream proteins) [19]. To increase the survival rate of patients with PC, the blockage of PD-1/PD-L1 pathway and other new-generation cancer immunotherapy approaches are still being studied. As reported by Doi et al., a dose-dependent reduction in the upregulation of PD-L1 by anticancer agents was observed after the treatment with a JAK2 inhibitor. Therefore, the involvement of JAK2/STAT1 pathway in the transcription of PD-L1 mediated by anticancer agents was suggested [20]. In addition, the antitumor effects of an indole-3-carbinol derivative (OSU-A9) on PC cell lines were studied in vitro and in vivo, and it was found that apoptosis was induced by OSU-A9, which further downregulated protein kinase B (Akt) phosphorylation, upregulated p38 as well as STAT3 and JAK phosphorylation. These authors suggested the modulating effect of OSU-A9 treatment on the p38-JAK-STAT3 pathway, resulting in cytotoxicity to PC cells [21]. These reports, together with the results of the present study, suggest the involvement of the JAK/STAT3 pathway in PC progression and its potential application for anti-PC treatment.
In conclusion, our study proposes the function of miR-1225 as a tumor oncogene during the progression of PC. The reduced expression of miR-1225 in PC cells and tumors is highly associated with tumor cell growth impairment, suggestive of its potential role as an excellent biomarker with favorable diagnostic and prognostic effects.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81572436]; Fundamental Research Funds for the Central Universities [2017KFYXJJ239]; Key Laboratory Open Project Fund of Hubei Province [02.03.2017-223].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [2].Long J, Luo GP, Xiao ZW, et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett. 2014;346(2):273–277. [DOI] [PubMed] [Google Scholar]
- [3].Lin Q-J, Yang F, Jin C, et al. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21(26):7988–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bosetti C, Bertuccio P, Negri E, et al. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51(1):3–13. [DOI] [PubMed] [Google Scholar]
- [5].Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. [DOI] [PubMed] [Google Scholar]
- [6].Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129(7):1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- [8].Ding N, Wu H, Tao T, et al. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther. 2017;10:4905–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luo Z, Zheng K, Fan Q, et al. Hyperthermia exposure induces apoptosis and inhibits proliferation in HCT116 cells by upregulating miR-34a and causing transcriptional activation of p53. Exp Ther Med. 2017;14(6):5379–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang B, Li D, Kovalchuk I, et al. miR-34a directly targets tRNAi(Met) precursors and affects cellular proliferation, cell cycle, and apoptosis. Proc Natl Acad Sci U S A. 2018;115(28):7392–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng H, Zhang F, Lin X, et al. MicroRNA-1225-5p inhibits proliferation and metastasis of gastric carcinoma through repressing insulin receptor substrate-1 and activation of beta-catenin signaling. Oncotarget. 2016;7(4):4647–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng M, Niu Y, Fan J, et al. Interferon down-regulation of miR-1225-3p as an antiviral mechanism through modulating Grb2-associated binding protein 3 expression. J Biol Chem. 2018;293(16):5975–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sallustio F, Serino G, Costantino V, et al. miR-1915 and miR-1225-5p regulate the expression of CD133, PAX2 and TLR2 in adult renal progenitor cells. PLoS One. 2013;8(7):e68296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peng DY, Song H, Liu LB. Resveratrol-downregulated phosphorylated liver kinase B1 is involved in senescence of acute myeloid leukemia stem cells. J Huazhong Univ Sci Technolog Med Sci. 2015;35(4):485–489. [DOI] [PubMed] [Google Scholar]
- [15].Steelman LS, Pohnert SC, Shelton JG, et al. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218. [DOI] [PubMed] [Google Scholar]
- [16].Kile BT, Nicola NA, Alexander WS. Negative regulators of cytokine signaling. Int J Hematol. 2001;73(3):292–298. [DOI] [PubMed] [Google Scholar]
- [17].Maurer U, Charvet C, Wagman AS, et al. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21(6):749–760. [DOI] [PubMed] [Google Scholar]
- [18].Putcha GV, Le S, Frank S, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38(6):899–914. [DOI] [PubMed] [Google Scholar]
- [19].Chen L, Zhou D, Liu Z, et al. Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway. Oncol Rep. 2018;39(3):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doi T, Ishikawa T, Okayama T, et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017;37(3):1545–1554. [DOI] [PubMed] [Google Scholar]
- [21].Tsai WC, Bai LY, Chen YJ, et al. OSU-A9 inhibits pancreatic cancer cell lines by modulating p38-JAK-STAT3 signaling. Oncotarget. 2017;8(17):29233–29246. [DOI] [PMC free article] [PubMed] [Google Scholar]


