ABSTRACT
Daratumumab is an anti-CD38 fully human IgG1 mAb approved for multiple myeloma treatment. One of the proposed mechanisms of action is the induction of antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells. NK cells acquire surface CD137 expression in the presence of solid-phase-attached daratumumab and when encountering a daratumumab-coated CD38+ tumor cell line. In this setting, addition of the agonist anti-CD137 mAb urelumab enhances NK-cell activation increasing CD25 expression and IFNɣ production. However, in vitro ADCC is not increased by the addition of urelumab both in 4h or 24h lasting experiments. To study urelumab-increased daratumumab-mediated ADCC activity in vivo, we set up a mouse model based on the intravenous administration of a luciferase-transfected multiple myeloma cell line of human origin, human NK cells and daratumumab to immuno-deficient NSG mice. In this model, intravenous administration of urelumab 24h after daratumumab delayed tumor growth and prolonged mice survival.
KEYWORDS: NK cells, ADCC, multiple myeloma, CD137, daratumumab
Introduction
CD38 is a transmembrane protein expressed on all malignant plasma cells in multiple myeloma.1 The anti-CD38 IgG1 human monoclonal antibody daratumumab is used in combination with other drugs in relapsed or refractory multiple myeloma patients2 and has been recently approved in combination with bortezomib, melphalan, and prednisone for newly diagnosed multiple myeloma.3 Its clinical activity is contingent on antibody dependent-cellular cytotoxicity (ADCC) as mediated by NK cells and macrophages4,5 and probably the additional poorly understood effects of CD38 ligation on immune system cells and on myeloma cells themselves.6 Complement-mediated cytotoxicity and reductions of myeloid-derived suppressor cells in the myeloma microenvironment have also been observed.4,7 CD137 (4-1BB) is a TNFR family surface glycoprotein expressed on activated T and NK cells.8 Agonist monoclonal antibodies directed to CD137, such as urelumab, enhance T-cell mediated antitumor immunity9 and have been shown to enhance anti-tumor ADCC as mediated by rituximab,10 cetuximab,11 and trastuzumab.12 The mechanism proposed is that CD16 (FcRɣIIIa) ligation by a cell surface-bound IgG1 Fc leads to the induction of CD137 on the surface of NK cells. Once on the NK surface, if CD137 is stimulated by a CD137 agonist antibody, it enhances the survival and cytotoxic performance of NK cells in terms of more efficient ADCC.10–12 These preclinical studies support the combined use of daratumumab with urelumab in multiple myeloma including evidence in NK-humanized mice.
Results and discussion
We hypothesized that daratumumab could synergize with urelumab and perhaps other CD137 agonists in its activity against multiple myeloma. We first found that plastic-bound daratumumab, as any other human IgG1 antibody, induces in 16 hours surface expression of CD137 on resting NK cells freshly isolated from peripheral blood of healthy donors (Figure 1(a)). Of note, such an activity was not further increased by incubation with known NK-stimulating cytokines such as IL-2, IL-15 or IL-12 (Figure 1(a)). Consistent with this, if the CD38+ multiple myeloma-derived cell line MM1S-GFP-Luc (Supp. Figure 1) was coated with daratumumab but not with irrelevant human IgG, it induced CD137 surface expression on cocultured resting NK cells (Figure 1(b)). This effect was not enhanced by the addition of cytokines to the 16-hour cocultures. These results were recapitulated with the multiple myeloma cell line KMS28-BM coated with daratumumab (Supp. Figure 2A). NK cells that upregulated CD137 on the membrane were mostly CD56dim. Moverover, NK cells positive for CD25, CD94, NKG2D and NKG2A expressed more frequently CD137 on the membrane than those NK cells that did not expressed these markers (Figure 1(c)). However, CD16+ cells expressed less frequently CD137 than CD16− NK cells, as had been previously descrided.12 Importantly, gated NK cells in seven bone-marrow aspirates from multiple myeloma patients upregulated CD137 in short-term cultures when daratumumab was added (Figure 1(d)). CD38 expression on normal and clonal plasma cells as well and on other lymphoid and myeloid populations of one of the patient bone marrow was studied, confirming expression not only on plasma cells but also on a variety of lymphoid and myeloid cells including NK cells (Supp. Figure 3).
Figure 1.
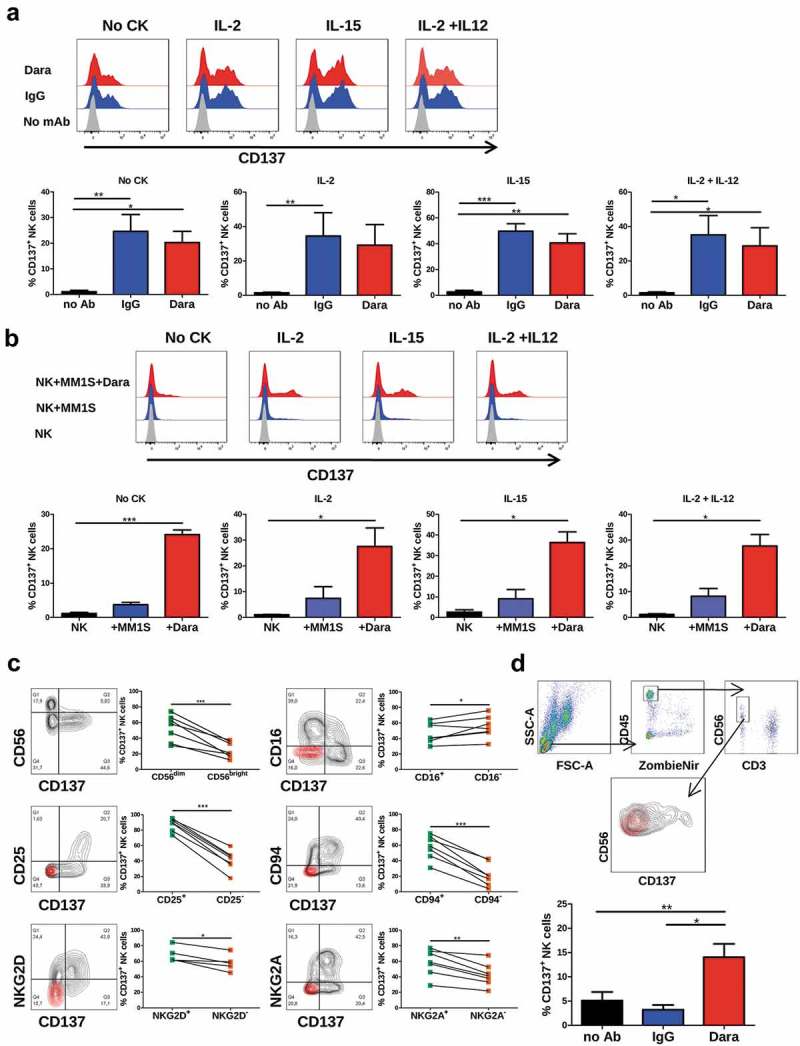
CD137 expression on NK cells is induced by immobilized monoclonal antibodies. (a) CD137 expression on NK cells cultured during 16h in plates coated with human IgG or daratumumab in the presence or absence of indicated cytokines. Bar graphs show data using NK cells from 4 unrelated subjects. Cultures were set up in the presence of the indicated cytokines. (b) CD137 expression on NK cells cocultured during 16h with the multiple myeloma MM1S-GFP-Luc cell line with or without daratumumab in the presence or absence of different cytokines. Bar graphs represent data from three independent experiments with NK cells from different donors. (c) CD137 co-expression with CD56, CD16, CD25, CD94, NKG2D and NKG2A on NK cells cultured during 16h in plates coated with human IgG. Paired dots in the graphs indicate data from each subject. (d) FACS-Gating strategy and CD137 expression on NK cells from bone marrow aspirates taken from multiple myeloma patients, that were cultured during 16h with daratumumab or irrelevant human IgGs. A representative contour plot and a bar graph with data from 7 subjects are shown. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001 in a paired T-test or one-way ANOVA test.
To ascertain if CD137 ligation by urelumab costimulated NK cells, we exposed freshly isolated human NK cells to plate-bound human IgG1 in order to upregulate surface CD137 and harvested these cells 24h later to be exposed to MM1S-GFP-Luc cells coated with daratumumab in the presence or absence of soluble urelumab that was added to these 24 and 48h cultures. Figure 2(a) shows that urelumab increased the level of CD25 surface expression, as well as the intracellular content of IFNɣ (Figure 2(c)). In these co-cultures, CD137 was co-expressed with CD25 on NK cells (Figure 2(b)), and the fraction of NK cells expressing a higher level of intracellular IFNɣ, co-expressed more frequently CD137 (Figure 2(e)). Co-expression of other NK cell markers was also studied in this setting (Supp. Figure 4A-F). NK cells that expressed more PD-L1, LAG-3, NKG2D, CD94 and NKG2A on the membrane, expressed more frequently CD137 than their negative counterparts. In the same way, NK cells containing more Granzyme B exhibited a higher proportion of CD137+ cells. Moreover, the supernatants of such cultures showed higher concentrations of secreted IFNɣ in the presence of urelumab (Figure 2(d)). These results were also seen in independent series of coculture experiments with the KMS28-BM cell line (Supp. Figure 2B-D) that also express daratumumab-recognizable CD38 (Supp. Figure 1). The anti-SLAMF7 mAb elotuzumab, approved for multiple myeloma treatment13 showed similar effects upregulating CD137 expression upon co-culture of NK cells with multiple myeloma cell lines expressing SLAMF7 (Supp. Figure 5A-B) resulting in enhanced of CD25 expression and IFNɣ production when urelumab was added to the cultures (Supp- Figure 5C-D).
Figure 2.
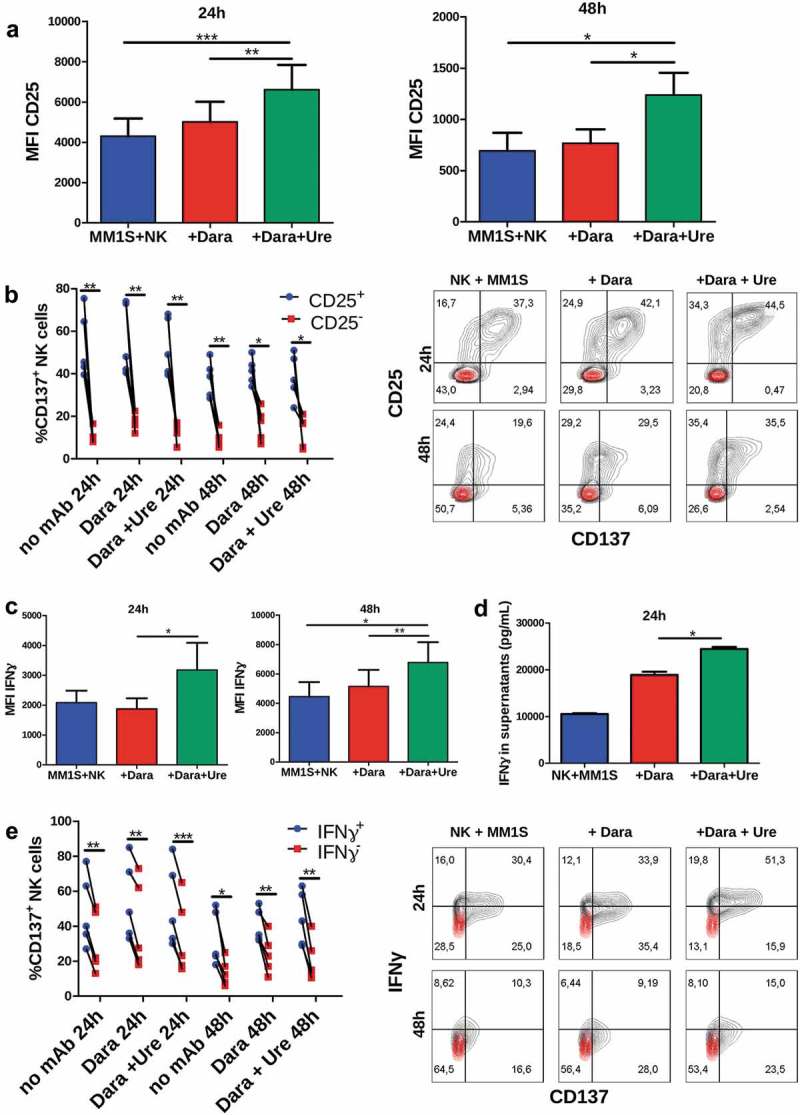
Urelumab increases daratumumab-mediated NK cell activation. (a) Urelumab increases CD25 expression on NK cells cultured during 16h with IL-2 and immobilized IgG and then cocultured 24h/48h with MM1S-GFP-Luc cells, daratumumab, and urelumab. (b) Percentage of CD137+ NK cells among the CD25+ and CD25− cell fractions of the corresponding co-cultures of NK cells as in A. A representative FACS contour plot is shown. (c) Urelumab increases IFNɣ intracellular expression in NK cells cultured as in A. (d) Urelumab increases IFNɣ release to the supernatant by NK cells cultured as in A. This graph shows data of one experiment from three independently performed with similar results. (e) Percentage of CD137+ NK cells among the IFNɣ+ and IFNɣ− cell fractions of the corresponding co-cultures of NK cells as in A. A representative FACS contour plot is shown. Cytometry results are representative of five different donors. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001 in a paired t-Test or one-way ANOVA test.
We repeatedly tried to see if such CD137-stimulated NK cells would perform better in ADCC assays.10–12 However, in these types of cultures such an enhancement of the cytotoxicity triggered by daratumumab could not be observed (Figure 3(a)). In line with this, CD16 engagement by myeloma cell-coated daratumumab resulted in downregulation of surface CD16 expression but again, this effect was not modified by urelumab (Figure 3(b)). NK-cell degranulation was also studied in such co-cultures, where only minor increases of CD107a surface expression on NK cells could be observed under the influence of urelumab (Figure 3(c)). Thinking that this negative result was due to insufficient CD137 induction, we performed experiments with NK cells that had been co-cultured for a week in the presence of an irradiated lymphoblastoid cell line plus recombinant IL-2, which showed high levels of CD137 expression and overall activation (Supp. Figure 6A). The purity of isolated NK cells was verified in each case (Supp. Figure 7B). ADCC mediated by activated and expanded NK cells was of far greater intensity than that performed by freshly harvested NK cells (Supp. Figure 7C). However, even when using these activated NK cells, the effect of adding urelumab to ADCC cytotoxicity cultures against daratumumab–coated MM1S-GFP-Luc cells was again negligible (Supp. Figure 6B-C). Our data indicate that at least in this myeloma model, enhancement of NK mediated ADCC by urelumab is not mediating any prominent effect, albeit CD137 ligation clearly augmented NK-cell production of IFNɣ.
Figure 3.
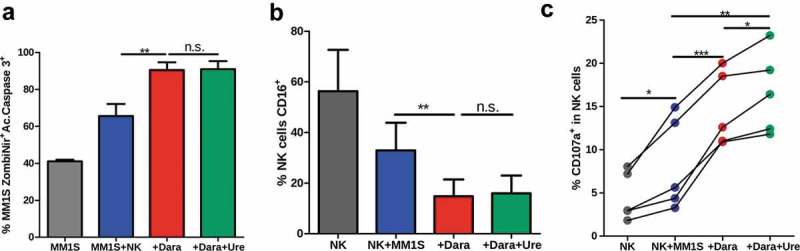
Urelumab does not increase daratumumab-mediated ADCC in culture (a) Urelumab does not increase direct ADCC effects exerted by NK cells cultured as in Figure 2(a), that was measured as the % of MM1S-GFP-Luc dead cells at 1:1 E:T ratio. (b) Percentage of NK cells expressing CD16 when cultured as in Figure 2(a). (a and b) include results from seven different donors. (c) Surface CD107a expression on NK cells cultured as in Figure 2(a) corresponding to five different healthy donors. * = p < 0.05, ** = p < 0.01 and *** = p < 0.001 in a paired t-Test or one-way ANOVA test.
To model in vivo the effects of urelumab on NK antitumor activity upon daratumumab treatment, we used a model of NSG mice reconstituted with human NK cells. In this setting, mice intravenously injected with luciferase-transfected MM1S-GFP-Luc cells were given 2 × 106 human NK cells and daratumumab i.v. on the same day. Twenty-four hours later, mice were dosed with urelumab, once having given time for CD137 induction. A purity check of the administered NK cells is shown in supplementary figure 7A. Twenty four hours after administration, MM1S-GFP-Luc and NK cells were present in bone marrow. Human NK cells could also be found in blood, liver and spleen of the mice (Supp. Figure. 8).
Comparing the different treatment groups by measuring tumor-emitted bioluminescence and survival, it was clear that the daratumumab and NK + daratumumab groups showed disease control over the untreated group and the group receiving NK cells. Importantly, the group given triple treatment with NK transfer, daratumumab and urelumab had significantly better tumor control (Figure 4(a-b)) and survival advantage over the daratumumab + NK group (Figure 4(c)). Similar experiments were carried out with the lymphoblastoid cell line+IL-2 activated NK cells. In these experiments, the group co-treated with urelumab again showed a certain degree of survival advantage (Supp. Figure 9A). Of note, daratumumab as a single agent without NK cell transfer exerted some antitumor effects that could be related to ADCC performed by murine macrophages, but this effect is not enhanced by urelumab in ausence of human NK cells (Supp. Figure 9B).
Figure 4.
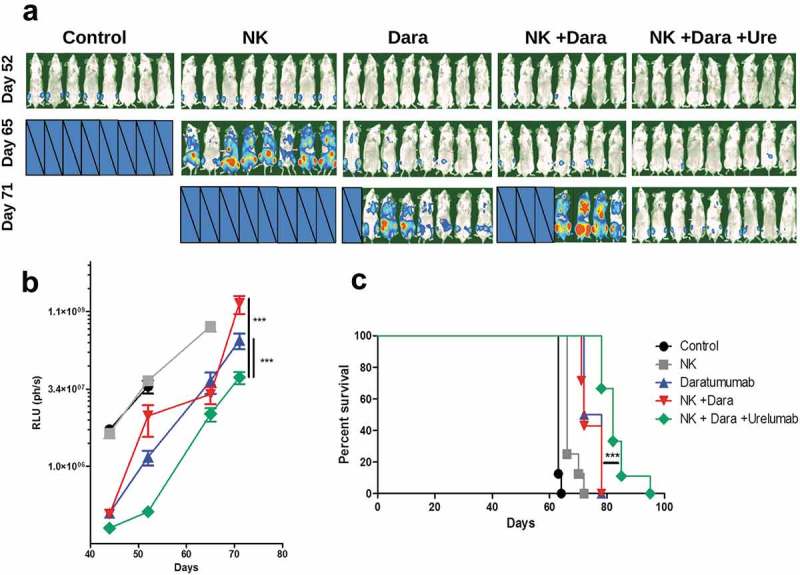
Urelumab increases the antitumoral effect of daratumumab in immunodeficient mice inoculated with a MM1S-GFP-Luc multiple myeloma-derived cell line and reconstituted with human NK cells (a and b) Tumor burden of mice monitored by bioluminescence. Censored pictures indicate mice which had died due to myeloma progression. RLU represents relative light units from regions of interest. *** = p < 0.001 in a linear regression comparison test of Dara and [NK + Dara] vs. [NK + Dara + Ure] groups. (c) Kaplan-Meier curves representing survival. *** = p < 0.001 in a Long-Rank test, comparing of [NK + Dara] vs. [NK + Dara + Ure] groups. Graphs show data of one experiment from two performed with similar results.
Considering these results as a whole, we conclude that even in the absence of effects on CD8 T cells,14 addition of urelumab to daratumumab treatment may improve therapeutic outcomes. Given the fact that urelumab at full doses causes liver inflammation in a subset of patients,15 testing other safer or targeted CD137 agonists in the same setting will be important. It is surprising that the pharmacological interaction mechanism between daratumumab and urelumab seems not to be a mere enhancement of the effector phase of ADCC as proposed by H. Kohrt et al.10–12 but probably involves a higher level of intricacy. This could involve the immunoregulatory and proinflammatory functions of NK cells. We cannot exclude agonist effects of CD38 ligation on NK cells themselves that could potentially further foster their activation.16 Indeed, we confirmed the binding of daratumumab to a large fraction of NK cells (data not shown).
Furthermore, in immunocompetent patients, anti-CD137 and anti-CD38 mAb may act on their target molecules on T lymphocytes thereby potentially enhancing and consolidating therapeutic efficacy through amplification of adaptive immunity. In this regard, we have previously reported enhancement of CD8+ T-cell immunity by agonist anti-CD137 mAbs against syngeneic myeloma mouse models.17 Our model system in NSG mice permits us to focus on NK-mediated mechanisms since complement activation and macrophage functions are deficient in this mouse model. Additionally, urelumab decreases antibody responses through a route dependent on T follicular helper cells.18 This may help reduce anti-drug antibodies.
Clinical trials are ongoing testing the combination of low-dose urelumab with elotuzumab, another anti-myeloma ADCC-inducing antibody (NTC02252263). Effects of the daratumumab + urelumab combination in a xenografted multiple myeloma model advocate for a clinical trial to sustain and enhance with CD137 agonists the outstanding effects produced by daratumumab against myeloma.
Material and methods
Tumor cell lines
MM1S-GFP-Luc19,20 and KMS28-BM21 cell lines were kindly provided by Dr. Maiso and Dr. Agirre (CIMA, Universidad de Navarra). RPMI8866 were gifted by Dr. Lopez-Botet (IMIM, Barcelona). Cell lines were maintained at 37°C in 5% CO2 and were grown in RPMI medium (RPMI 1640) with Glutamax (Gibco, Invitrogen) containing 10% heat-inactivated FBS (Gibco, Invitrogen), 100 IU/mL penicillin and 100 g/mL streptomycin (Biowhittaker). MM1S-GFP-Luc cells were also cultured in the presence of 500µg/mL of G418 (Sigma-Aldrich).
Isolation and activation of NK cells
Blood samples were obtained from healthy volunteers through the Banco de Sangre y Tejidos de Navarra while the fulfilling the requirements of the Clinical Research Ethics Committee of the Navarre Government following the 2016.143 protocol. PBMCs from buffy coats were isolated after gradient centrifugation with Ficoll-Paque (GE Healthcare), and NK cells were isolated by negative selection using magnetic beads and LS selection columns following the manufacturer’s protocol (Miltenyi Biotec).
When indicated, NK cells were cultured during 16h in plastic culture plates coated by daratumumab or irrelevant human IgG. For this, a solution of antibody (10µg/mL) in DPBS without calcium or magnesium (Gibco, Invitrogen) was added to culture plates and maintained at least 4h at 37ºC. Then, plates were washed at least three times with fresh DPBS. NKs were cultured in antibody-coated plates with the different treatments in NK MACS Medium (Miltenyi Biotec) supplemented with 5% human male AB serum (Sigma-Aldrich) and 500 IU/mL of IL-2 (Aldesleukin, Novartis) for 16h. Then, cells were harvested and used for study by flow cytometry or for subsequent experiments.
For NK long-term activation and expansion, NK cells were cultured with allogeneic 20Gy irradiated PBMCs in a 10:1 ratio and 20Gy irradiated RPMI8866 lymphoblastoid cells in a 4:1 ratio in RPMI 1640 medium with glutamax (Gibco, Invitrogen). The culture medium was also supplemented with 10% of heat-inactivated FBS, 10% human male AB serum, 1mM non-essential aminoacids, 1mM sodium pyruvate, 2x10−5M beta-mercaptoethanol, 50U/mL of IL-2, 1µg/mL PHA (Sigma-Aldrich), 100 IU/mL penicillin and 100 g/mL streptomycin. Cultures were maintained during 7–14 days and resulting activated NK cells were freshly used or cryopreserved. NK purity was checked in every experiment and found to be over 98%.
Cocultures of NKs and tumor cells
Activated NK cells were cocultured with MM1S-GFP-Luc or KMS28-BM cells in a 1:1 ration in the presence or absence of daratumumab and urelumab (10µg/mL). At 24h, part of the supernatants was collected and frozen for subsequent IFNɣ concentration determination by commercial ELISA (R&D). Part of the cells was used to study the tumor cell lysis percentage by flow cytometry, and the rest of the culture was maintained with brefeldin A during 4h. Cytotoxicity was measured as the percentage of non-viable (ZombieNir+ and/or active caspase 3+) versus viable (ZombieNir− and active caspase 3−) cells among GFP+ myeloma cells. At this point, cells were harvested, and CD25, IFNɣ and other molecules expression were studied by flow cytometry. Daratumumab was acquired from Janssen. Elotuzumab and urelumab was obtained from Bristol-Myers Squibb. Beriglobin P (CSL Behring) was used as irrelevant human IgG.
Flow cytometry
Cells were pretreated with 10µg/mL of human IgG to reduce nonspecific staining. Monoclonal antibodies to the human antigens were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), PerCP-Cy5.5, allophycocyanin (APC), AlexaFluor 488, Alexa Fluor 647, Brillant Violet 510, Pacific Blue or Brillant Violet 421. Anti-CD137 (4B4-1), anti-PD-L1 (29E.2AE), anti-Granzyme B (6B11), anti-LAG3 (3DS223H), anti-mCD11b (M1/70) and anti-CD45 (HI30) were purchased from Biolegend. Anti-CD56 (NCAM16.2), CD16 (3G8) and active Caspase 3 (C92-605) were obtained from BD. Anti-CD3 (UCHT1), anti CD25 (BC96) and anti-IFNɣ (4SB3) were from eBioscience and anti-NKG2A (131411) was purchased from R&D System. To study the expression of CD137 on NK cells previously treated with urelumab, a biotilinated mAb developed by our group was used (5D1). This mAb does not compete with urelumab in binding to CD137.22 The anti-CD38 (Cy38F2) mAb was from Cytognos. Intracellular staining (to study active Caspase 3 and anti-IFNɣ expression) was performed with the BD Cytofix/Cytoperm™ solution following the manufacturer’s protocol. Dead cells were identified using the ZombieNIR™ kit (Biolegend). FACSCanto II cytometer was used for cell acquisition, and data analysis was performed using FACS DiVa (BD Biosciences) and FlowJo 7.2.1 (Tree Star Inc., San Carlos, CA).
In vivo model
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratory and bred in the animal facility of CIMA of the University of Navarra under the guidelines of Ethics Committee of the center following the 001–17 protocol. For the xenograft model, 3 × 106 MM1S-GFP-Luc cells were inoculated i.v. via the caudal vein together with 2 × 106 freshly isolated NK cells and 50µg of daratumumab per mice. The following day, 100µg of urelumab were injected i.v. In the activated NK cell model, 5 × 106 MM1S-GFP-Luc cells were injected together with 4 × 106 activated NK cells, 50µg of daratumumab and 100µg of urelumab per mice at the same time.
Six weeks following tumor inoculation, bioluminescence was measured once every week. For this purpose, mice were anesthetized using ketamine/xylazine and injected with 100 μl d-luciferine i.p. (Xenogen) at a concentration of 30 mg/ml. Mice were placed in the imaging chamber of the PhotonIMAGER Optima system (Biospace labs). A color-scale photograph of the animals was acquired, followed by bioluminescent acquisition. Regions of interest were drawn over the animal image, as well as over regions of no signal, which were used as background readings. Light intensity was quantified using photons/s (RLU). The color-scale photograph and data images from all studies were superimposed using M3 Vision software (Biospace labs).
Statistical analysis
Prism software (GraphPad Software) was used for statistical analysis. For in vitro experiments, differences between groups were verified with t-student, paired T tests or one-way ANOVA tests. Linear regression was used to compare photon emission in bioluminescence experiments. In vivo luciferase expression was analyzed by differences in linear regression after logarithmic transformation of the data. Mice survival was studied with Log-Rank (Mantel-Cox) test. *p < 0.05, **p < 0.01, ***p < 0.001.
Funding Statement
This work was supported by Asociación Española contra el Cancer (Fundación AECC), Foundation for Applied Medical Research (FIMA), Worldwide Cancer Research (AIRC) and Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional (FEDER) under Grant [PI16/00668]. This project has received funding from the European Union´s Horizon 2020 research and innovation programme (grant agreement [n° 635122 – PROCROP]. P.B. was supported by a Miguel Servet II [CPII15/00004] contract from Instituto de Salud Carlos III;
Disclosure of Potential Conflicts of Interest
I.M. is a consultant for Bristol-Myers Squibb, Boehringer Inghelheim, AstraZeneca, Roche-Genentech, Merck Serono, Medimmune, Alligator, Tusk, Genmab, F-Star, Molecular Partners, Bioncotech and Bayer. Research grants have been received from Alligator, Bioncotech, Pfizer, Roche and Bristol-Myers-Squibb. The other authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Lin P1, Owens R, Tricot G, Wilson CS.. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121(4):482–488. doi: 10.1309/74R4-TB90-BUWH-27JX:. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, et al. Daratumumab, Bortezomib, and Dexamethasone for multiple Myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 3.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, et al. Daratumumab plus Bortezomib, Melphalan, and prednisone for untreated Myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 4.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, et al. Daratumumab, novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 5.Overdijk MB, Jh J, Nederend M, Jj LVB, Groen RW, Parren PW, Leusen JH, Boross P.. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via fcγ receptor-mediated cross-linking. J Immunol. 2016;197(3):807–813. doi: 10.4049/jimmunol.150135. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, Villalobos PA, Cascone T, Liu X, Tan L. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1. Cancer Discov. 2018:pii: CD-17–1033. doi: 10.1158/2159-8290.CD-17-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overdijk MB, Verploegen S, Bögels M, van Egmond M, Jj Lammerts van Bueren, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321. doi: 10.1080/19420862 2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190(2):167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 9.Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131(1):49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 10.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117(8):2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124(6):2668–2682. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122(3):1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H, et al. Elotuzumab therapy for relapsed or refractory multiple Myeloma. N Engl J Med. 2015;373(7):621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 14.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. [DOI] [PubMed] [Google Scholar]
- 15.Segal NH, Logan TF, Hodi FS, McDermott D, Melero I, Hamid O, Schmidt H, Robert C, Chiarion-Sileni V, Ascierto PA, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2017;23(8):1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 16.Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW, Malavasi F, Segal DM. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–3871. [PubMed] [Google Scholar]
- 17.Murillo O, Arina A, Hervas-Stubbs S, Gupta A, McCluskey B, Dubrot J, Palazón A, Azpilikueta A, Ochoa MC, Alfaro C, et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008;14(21):6895–6906. doi: 10.1158/1078-0432.CCR-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaro C, Echeveste JI, Rodriguez-Ruiz ME, Solorzano JL, Perez-Gracia JL, Idoate MA, Lopez-Picazo JM, Sanchez-Paulete AR, Labiano S, Rouzaut A. Functional expression of CD137 (4-1BB) on T helper follicular cells. Oncoimmunology. 2015;4(12):e1054597. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T, Anderson KC, Rosen ST. Characterization of the MM.1 human multiple myeloma (MM) cell lines: A model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Experimental Hematology. 2003;31:271–282. [DOI] [PubMed] [Google Scholar]
- 20.Wu KD, Zhou L, Burtrum D, Ludwig DL, Moore MA. Antibody targeting of the insulin-like growth factor I receptor enhances the anti-tumor response of multiple myeloma to chemotherapy through inhibition of tumor proliferation and angiogenesis. Cancer Immunol Immunother. 2007;56(3):343–357. doi: 10.1007/s00262-006-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue J, Otsuki T, Hirasawa A, Imoto I, Matsuo Y, Shimizu S, Taniwaki M, Inazawa J. Overexpression of PDZK1 within the 1q12-q22 amplicon is likely to be associated with drug-resistance phenotype in multiple myeloma. Am J Pathol. 2004;165(1):71–81. doi: 10.1016/S0002-9440(10)63276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Forero I, Azpilikueta A, Bolaños-Mateo E, Nistal-Villan E, Palazon A, Teijeira A, Perez-Chacon G, Morales-Kastresana A, Murillo O, Jure-Kunkel M, et al. cell costimulation with anti-CD137 monoclonal antibodies is mediated by K63-polyubiquitin-dependent signals from endosomes. J Immunol. 2013;190(12):6694–6706. doi: 10.4049/jimm-unol.1203010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


